Abstract
Muscle function is dependent on innervation by the correct motor nerves. Motor nerves are composed of motor axons which extend through peripheral tissues as a compact bundle, then diverge to create terminal nerve branches to specific muscle targets. As motor nerves approach their targets, they undergo a transition where the fasciculated nerve halts further growth then after a pause, the nerve later initiates branching to muscles. This transition point is potentially an intermediate target or guidepost to present specific cellular and molecular signals for navigation. Here we describe the navigation of the oculomotor nerve and its association with developing muscles in mouse embryos. We found that the oculomotor nerve initially grew to the eye three days prior to the appearance of any extraocular muscles. The oculomotor axons spread to form a plexus within a mass of cells, which included precursors of extraocular muscles and other orbital tissues and expressed the transcription factor Pitx2. The nerve growth paused in the plexus for more than two days, persisting during primary extraocular myogenesis, with a subsequent phase in which the nerve branched out to specific muscles. To test the functional significance of the nerve contact with Pitx2+ cells in the plexus, we used two strategies to genetically ablate Pitx2+ cells or muscle precursors early in nerve development. The first strategy used Myf5-Cre-mediated expression of diphtheria toxin A to ablate muscle precursors, leading to loss of extraocular muscles. The oculomotor axons navigated to the eye to form the main nerve, but subsequently largely failed to initiate terminal branches. The second strategy studied Pitx2 homozygous mutants, which have early apoptosis of Pitx2-expressing precursor cells, including precursors for extraocular muscles and other orbital tissues. Oculomotor nerve fibers also grew to the eye, but failed to stop to form the plexus, instead grew long ectopic projections. These results show that neither Pitx2 function nor Myf5-expressing cells are required for oculomotor nerve navigation to the eye. However, Pitx2 function is required for oculomotor axons to pause growth in the plexus, while Myf5-expressing cells are required for terminal branch initiation.
Keywords: Oculomotor nerve, axon guidance, extraocular muscle, muscle precursor, neuromuscular targeting, terminal branching
Graphical Abstract
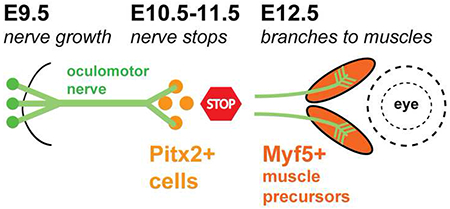
Introduction
During embryonic development, motor axons project out from the spinal cord and brain stem into peripheral tissues as large fasciculated nerves which supply muscles of the limbs or organs. However, upon reaching a peripheral target region, specific subpopulations of motor axons must halt further growth, defasciculate from the main nerve trunk and reorient as nerve branches toward specific muscles. The cues and tissues that govern nerve transition between growth and branching to specific muscles remain poorly understood.
The oculomotor nerve, along with the trochlear and abducens nerves, innervates the extraocular muscles. During development in mouse and chick embryos, oculomotor axons extend from the ventral midbrain toward the developing eye, and upon reaching a “decision region,” reorient to form specific branches toward four extraocular muscle targets (Cheng et al., 2014; Michalak et al., 2017). Similarly in zebrafish, oculomotor axon growth cones transition from exploratory behavior to axon subgroups which align to project to individual muscles (Clark et al., 2013). Interestingly, several human mutations that result in mis-innervation cause nerve errors in this decision region in animal models (Chilton and Guthrie, 2017; Clark et al., 2013; Park et al., 2016; Whitman and Engle, 2017)
The tissue substrates for the extending oculomotor nerve are likely important for guidance and branching decisions. However, to date, no substrate capable of influencing nerve growth and branching prior to muscle formation has been described for this nerve. Historic studies in chick embryos describe a mass of cells near the eye and the tip of the developing nerve (Carpenter, 1906), potentially coinciding with the decision region, although the identity of this cell mass has not been molecularly defined. Similarly, spinal nerves pause at a mass of mesodermal cells before entering the limb (Hollyday and Morgan-Carr, 1995; Tosney and Landmesser, 1985a, b; Wang and Scott, 2000). This mesodermal mass coincides with a decision region where axons pause before making distinct pathway choices (Lance-Jones and Landmesser, 1981; Tosney and Landmesser, 1985a, b).
In grasshopper and fly, motor nerves interact with muscle precursor cells (Ball et al., 1985; Ho et al., 1983). In grasshoppers, a single muscle precursor is sufficient to cause an entire nerve to branch (Landgraf et al., 1999), and the removal of muscle precursors prevents nerve branching (Prokop et al., 1996). Similarly in zebrafish, muscle precursors act as intermediate targets that halt motor axon extension (Melancon et al., 1997). Together these findings suggest that axons navigate by contacts with muscle precursors.
A previous study described mouse oculomotor nerve development patterns focusing on branching and innervation (Michalak et al., 2017). This study ablated extraocular muscles using a Myf5Cre gene insertion predicted to disrupt the function of both the muscle differentiation transcription factors Myf5 and Mrf4. It found that while the oculomotor nerve projected to the eye, three of four terminal branches failed to form in the periorbital region and most of the oculomotor neurons died within several days (Michalak et al., 2017). This study did not describe the initial outgrowth of pioneer motor axons, nor their potential contacts with extraocular muscle precursor cells.
In the developing oculomotor system, the transcription factor Pitx2 is an early marker for precursors for extraocular muscles (Zacharias et al., 2011), as well as other orbital tissues. Pitx2+ cells form a thick wedge in the mesenchyme ventrolateral to the eye early in development (Zacharias et al., 2011). This suggests that a mass of Pitx2+ cells may precede nerve projections. In our present study, we sought to determine whether Pitx2+ cells regulate oculomotor nerve pathfinding and branching by ablating this potential intermediate guidance target.
Materials and Methods
Mouse embryos
Animal experiments were approved by the UNRIACUC, following NIH guidelines. Timed pregnancies used the convention of embryonic day E0.5 as noon of the day when a vaginal plug was observed. Wild type CD-I mice were purchased from Charles River Laboratories (Wilmington, MA USA). Pitx2 mutant and control litter mate embryos were produced by crossing Pitx2 heterozygotes (Zacharias et al., 2011). Myf5-DTA embryos were obtained by crossing a Myf5-Cre line (JAX 007893 B6.129S4-Myf5tm3(cre)Sor/J) (Tallquist et al., 2000) to a second transgenic floxed DTA line that allows diphtheria toxin A expression in a Cre-dependent manner for cell type specific ablation (JAX 09669 B6.129P2-Gt(ROSA)26Sortm1(DTA)Lky/J) (Voehringer et al., 2008). Embryos were genotyped by PCR against the Cre and DTA genes. Embryos were collected and fixed in 4% paraformaldehyde (PFA) in 0.1M P04 buffer, as described (Bjorke et al., 2016). Embryos were then either labeled as whole mounts by antibody labeling or axon tracing, or were embedded for cryosectioning and antibody labeling. For each label type and embryonic stage, the data shown is representative of three (or more) biological replicates.
Immunofluorescence labeling of sections and whole mounts.
Antibody labeling was as described on cryosections and on E10.5-11.5 whole mounts (Farmer et al., 2008). Primary antibodies included Pitx2 (1:1000, rabbit anti-rodent/human Pitx2 peptide antigen, Capra Science), MyoD (Dako, clone 5.8A), MyoHC (1:5, MF 20, Developmental Studies Hybridoma Bank), beta-III-tubulin (1:2000, TuJ1, mouse anti-rat beta-III-tubulin, Biolegend). The Pitx2 antibody labeling pattern was validated by noting matching expression in precursors as seen by an independent Pitx2 antibody reported by Zacharias, et al. (2011), and the specificity of that antibody was confirmed by noting a complete loss of labeling in sections of Pitx2 null embryos (PJG, data not shown). Secondary antibodies included anti-rabbit and anti-mouse Alexa 488 and Alexa 555 (Invitrogen). Each image shown was representative of an analysis of serial sections, and of successful labels of three (or more) biological replicates, from two or three litters of embryos.
Immunofluorescence labeling of vibratome sections.
Vibratome sections were used on E12.5 to make thick sections to map motor nerve patterns around the eye in Pitx2 and Myf5-DTA embryos. Fixed embryo heads were washed in 0.1M P04 buffer, then embedded in 1% agarose in 0.1M P04 buffer. 400 micron sections were cut with a Leica Vibratome. A modified iDISCO labeling protocol was used, based on the 2014 iDISCO version (https://idisco.info/idisco-protocol/update-history/). Briefly, E12.5 embryos were collected in 0.1M P04 buffer, and the heads removed and fixed overnight in 4% PFA. The head tissue was washed overnight in 0.1M PO4 buffer, then embedded in warm 1% agarose in 0.1M PO4. A Leica Vibratome was used to cut 400 micron sections in a sagittal plane, resulting in about 4 sections with oculomotor nerves. From the iDISCO protocol steps of “Sample Pretreatment with Methanol”, the sections were dehydrated through a methanol series, bleached in 5% H2O2 in 20% DMSO/methanol overnight at 4C, washed in methanol, then rehydrated to lxPBS with 0.2% TritonX-100. For the “Immunolabeling” protocol steps, sections were incubated overnight at 37C in PBS/0.2% TritonX-100/20% DMSO/0.3M glycine, then blocked overnight at 37C in PBS/0.2% TritonX-100/10% DMSO/6% normal goat serum. The sections were washed twice for 1 hour in PBS/0.2% Tween-20/10 μg/mL heparin (PTwH), then incubated in primary antibody in PTwH/5% DMSO/3% normal goat serum for two days. Primary antibodies were anti β-III-tubulin made in rabbit (1:2000; 802001, Biolegend, San Diego, CA); and mouse actin α-smooth muscle directly conjugated to Cy3 (1:1000; C6198, Sigma, St. Louis, MO). Sections were washed several times then overnight in PTwH, then incubated in secondary antibodies of anti-rabbit and anti-mouse Alexa 488 and Alexa 555 at (1:1000; Invitrogen) in PTwH/3% normal goat serum for 2 days. Sections were washed several times and overnight with PTwH. Vibratome sections were cleared in 80% glycerol, mounted under coverslips with Vaseline dots at the comers as spacers, and sealed with fingernail polish. The sections were imaged for nerve and muscle patterns using a Leica SP8 confocal with a hybrid detector and resonance scanner in 300-400 thick z-stacks.
ColorPop tracing of nerves through confocal z stacks.
To depict the 3D nerve and muscle patterns in thick vibratome sections as 2D images for publication, we developed a strategy to manually color annotate the oculomotor and trochlear nerves in the confocal z-stacks. Using ImageJ, the z-stack for red and green channels was opened separately, and max z-projection images created. Z-projections used ZProject which was sufficient in some z-stacks or subsets of z-stacks to track a specific nerve. Other stacks were opened in VolumeViewer to collect a more thorough z-stack projection using the Snapshot function. The z-projections were then saved as RGB .tif files. From the green nerve RGB image, the channels were split, then merged in combinations to make three separate RGB files with gray, green, and magenta versions. Brightness and contrast were adjusted to optimally show fine nerve fibers. The green and magenta nerve images were then opened in the ColorPop program (Enventico, via Microsoft), which creates a gray overlay with tools to selectively reveal underlying colors along nIII or nIV nerves. Using the full z-stack in ImageJ as a guide, at high magnification and with tools at small pixel size, the segments of each nerve visible in the z-projection were colored. This manual coloring was done in a conservative way, with only clearly identifiable nerve segments colored. Ambiguous segments were left gray, and were frequently seen in mutants, where errors led the motor axons to fasciculate into trigeminal branches, so the colored nerve images are only subsets of the true nerve projections. The resulting files with colored nerves were opened in Photoshop, and the false color selected using the Select/Color range tool. Each nerve color was pasted as a layer on the gray nerve z-projection to show the green nIII and magenta nIV nerve trajectories on a gray background of gV and ambiguous nerves.
Axon tracing
To label the oculomotor nerve, the lipophilic dye, DiI (Invitrogen/Thermo Fisher Scientific) was crushed onto the oculomotor nerve, as described (Bjorke et al., 2016). The dye tracer was allowed to diffuse by incubation in 4% PFA for 1 day at 37 degrees C. Labeled embryos were cleared in 50% glycerol for a few hours before mounting under a coverslip for imaging. Images were obtained by combining confocal images via Z-stack on a Leica SP8 confocal microscope. Data shown are representative images of the stated numbers of embryos labeled; Investigators were not blinded to the embryo genotypes; Labels were considered successful when the diI tracer reached the end of the axons (growth cones), and excluded if diI transferred from the nerve into adjacent tissues. Unfortunately, the incubation in PFA at high temperature to allow diI transport prevented subsequent antibody labeling, such as to label other nerve or muscle markers.
Results
The oculomotor nerve initially grows to a mass of Pitx2-expressing precursor cells next to the eye
To investigate whether Pitx2+ precursors were in position to regulate nerve growth and branching, we labeled motor nerves and Pitx2 during initial nerve development from E9.5-14.5. Motor nerves were labeled by driving GFP under the Islet promoter (Lewcock et al., 2007), and embryos or sections with Pitx2 antibody. On E9.5, motor axons began to exit from the oculomotor nucleus in the ventral midbrain into peripheral mesenchyme. On E9.5, Pitx2 antibody did not label the oculomotor nucleus, or the mesenchyme traversed by the motor axons (Fig. 1A). However, by E10.0, oculomotor axons reached an area ventrolateral to the eye that was diffusely populated with Pitx2+ cells (Fig. 1A). Thus, the first target of the oculomotor nerve is Pitx2+ precursor cells, not the eye itself or the later appearing muscle fibers. The nerve fanned out at the tip to form a plexus within the mass of Pitx2+ cells (Fig. 1C, D).
Figure 1. The initial developmental target of the oculomotor nerve is a mass of Pitx2+ cells.
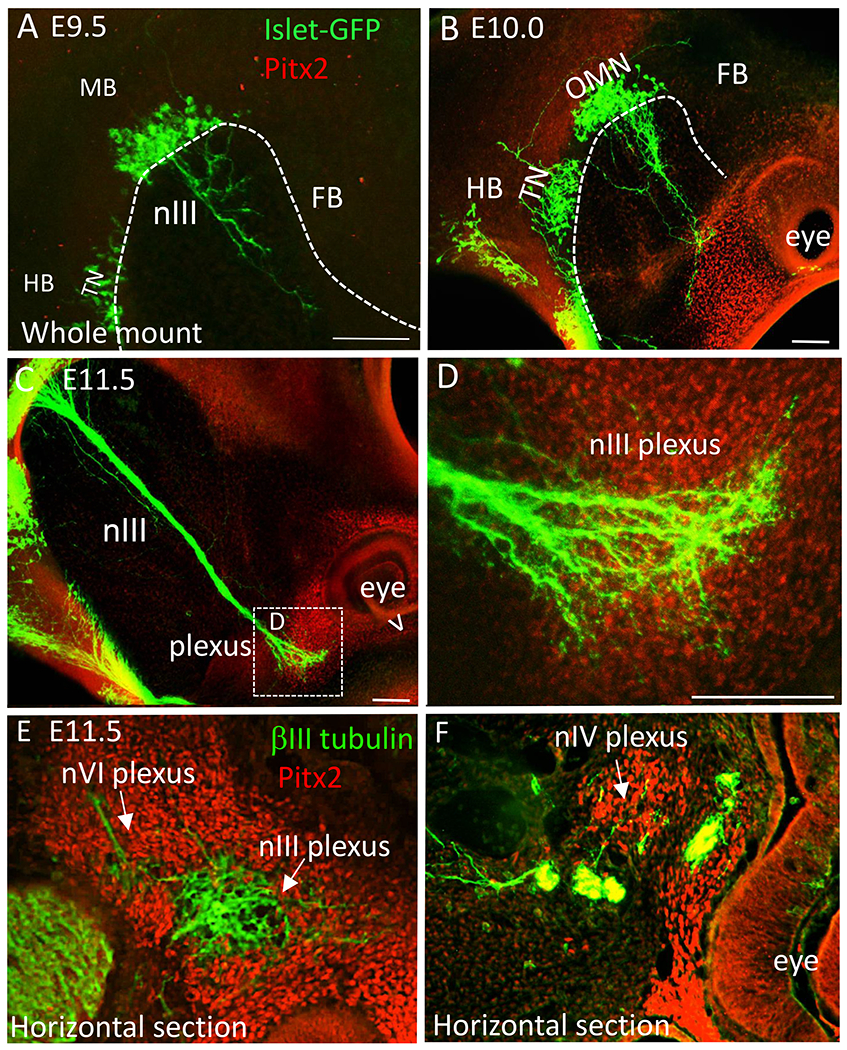
The developing oculomotor nerve projections were compared with the location of Pitx2+ cells in both whole mounts (confocal z-stack projections) (A-D) and sections (E,F). Motor neurons were labeled with an Isl-GFP transgene (green), and muscle and tendinous precursor cells with Pitx2 antibody (red). A. On E9.5, oculomotor nerve fibers projected into peripheral mesenchyme towards the eye. Neither the oculomotor neurons nor the mesenchyme were labeled with Pitx2. B. On E10.0, oculomotor nerve fibers first contacted with Pitx2+ cells ventrolateral to the eye. C, D. On E11.5, the compact oculomotor nerve fanned out to form a plexus within the Pitx2+ mass. V indicates the ventral side of the eye. E. The abducens nerve fibers interacted with Pitx2+ cells near the oculomotor plexus. F. The trochlear nerve also contacted more dorsal Pitx2+ cells to form a plexus. Abbreviations: nIII, oculomotor nerve; TN, trochlear nIV nucleus; MB, midbrain; HB, hindbrain; FB, forebrain; D, dorsal; V, ventral; L, lateral; M, medial; nVI, abducens nerve; nIV, trochlear nerve. Scale bars, 100 μm.
By E11.0, two smaller nerve plexuses were also present in the Pitx2+ mass. These were traced across adjacent sections and identified as the trochlear nerve (originating from r1 isthmus) and abducens (originating from r5/6). The fasciculated abducens nerve terminated ventrolateral and deep to the oculomotor plexus, while the less fasciculated trochlear nerve contacted Pitx2+ cells dorsal to the oculomotor plexus (Fig. 1E, F). Therefore, sub-regions within the Pitx2+ mass are contacted by the three nerves that innervate the extraocular muscles.
Axons of the developing oculomotor nerve pause during primary myogenesis
We carried out a time course of oculomotor nerve projections as extraocular muscles differentiated and were innervated, using a different imaging method but confirming a similar time course described in a previous publication (Michalak et al., 2017). Extraocular muscles derive from prechordal mesoderm and cranial paraxial mesoderm (Gage et al., 2005; Lescroart et al., 2010; Noden and Francis-West, 2006). Like typical somite-derived trunk muscles, extraocular muscles s undergo a characteristic progression of primary myogenesis: individual muscle precursors differentiate to myocytes, which later fuse to form multinucleated myotubes.
A close association between the oculomotor nerve and Pitx2+ precursors prior to primary myogenesis suggests that the nerve maintains contact with Pitx2+ precursors as they progress through development. However, we found that the oculomotor nerve did not maintain contact with Pitx2+ cells as the muscle precursors moved toward the orbit and underwent myogenesis defined by the appearance of myofibers (Fig. 2; Summary diagram of development time course of muscles and nerves in Fig 3). Instead, the oculomotor nerve maintained a plexus in the central location with Pitx2+ cells, which was devoid of myofibers as marked by myosin heavy chain antibody (Fig. 2A, B). The nerve did form small dorsal axon projections on E12.5 to initiate the superior branch of the oculomotor nerve (thin arrow in Fig. 2B) and likely the branch to the inferior oblique (bold arrow in Fig. 2B).
Figure 2. Oculomotor nerve fibers wait before branching to innervate distinct muscle targets.
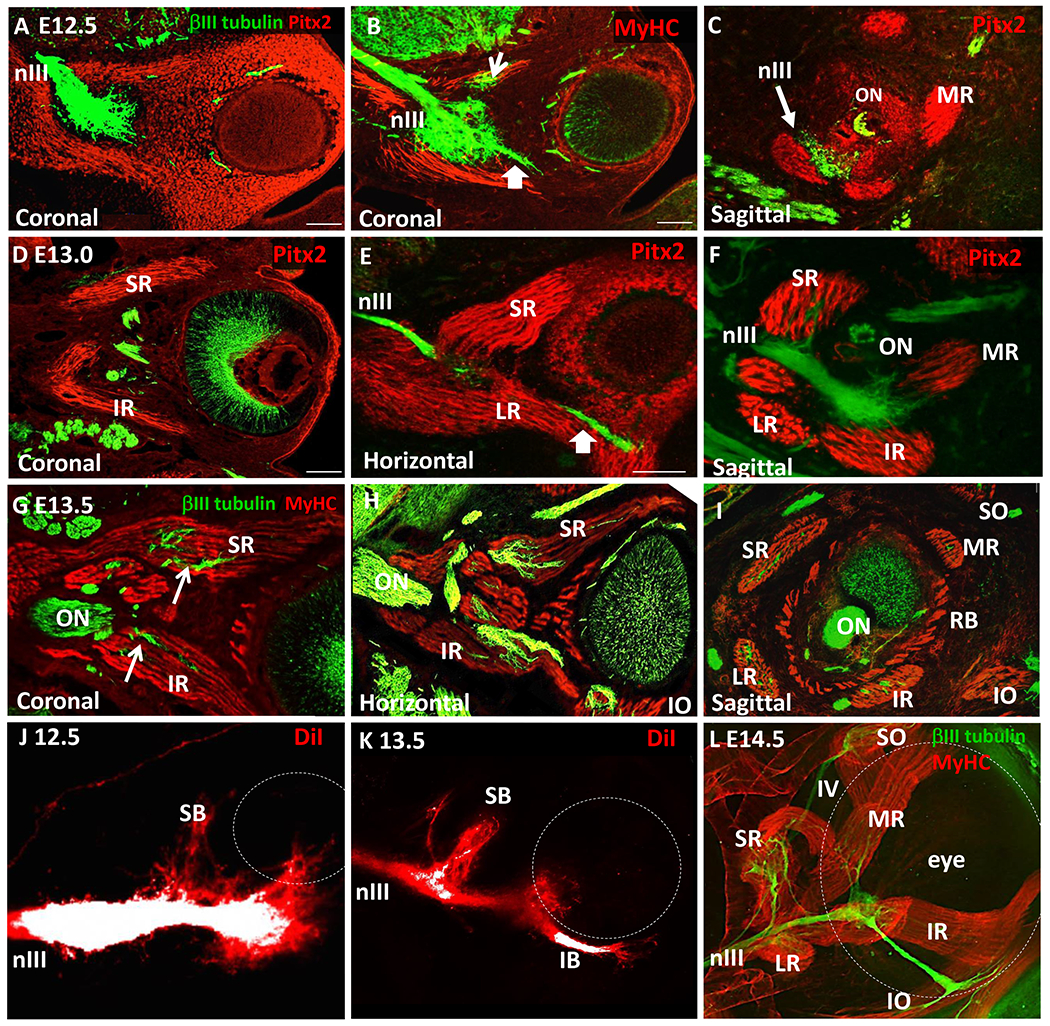
(A-I) Sections labeled with βIII–tubulin (nerve, green), and either Pitx2 or Myosin Heavy Chain (MyHC, muscle, red). A-C. Coronal sections, E12.5. Pitx2+ cells streamed away from the plexus around the eye, followed by MyHC+ fiber formation. B. E12.5. A small branch projected ventrally (arrow), likely toward the inferior oblique. C. E12.5 sagittal section, showing typical muscles with no nerve fibers yet inserted. ON, optic nerve; MR, medial rectus. D-F. E13.0, Distinct Pitx2+ muscle fibers around the eye formed, but generally were not penetrated by nerves, except the superior rectus (SR) by the superior branch (SB). IR, inferior rectus; LR, lateral rectus; RB, retrobulbar. G-I. E13.5, nerves contacted and began to penetrate each muscle. IO, inferior oblique; SO, superior oblique. J, K. Whole mount DiI tracing of the oculomotor nerve and confocal z-stack projection revealed a superior nerve branch forming, branching out proximal to the plexus; distinct superior (SB) and inferior (IB) branches were apparent on E13.5. DiI labels were over-exposed in the main nerve (white) to reveal smaller branches (red); confocal z-stack projection. L. Whole mount E14.5 eyes were dissected out, immuno-labelled, and imaged by confocal z-stack projection from behind the eye, showing that each muscle received innervation. Scale bars, 100 μm.
Figure 3. Time course of oculomotor development in mouse embryos.
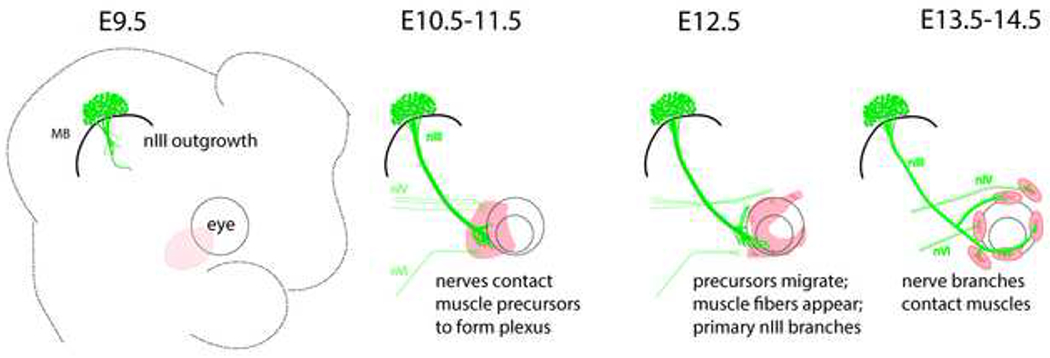
Schematic summarizing the main events in the development of the oculomotor nerves and extraocular muscles. Before E10.5, the nIII nerve contacts a mass of Pitx2+ cells (pink) at the ventro-lateral side of the eye (dashed circles), spreading to form a plexus. The trochlear nIV and abducens nVI nerves arrive a day later to contact separate areas within the Pitx2+ mass. On E12.5, the muscle precursors migrate around the eye and begin to form muscle fibers at coordinate positions, as the main superior and inferior branches extend. On E13.5 to E14.5, the branches contact muscles.
Despite the initial formation of the superior and inferior branches, these nerves did not yet contact the nascent myofibers on E12.5 (Fig. 2C, n=4). By E13.0, the EOM myofibers established their stereotypical coordinate positions around the eye (Fig. 2D, E), extending from the eye to the orbit. At this point the superior rectus (and possibly other muscles) was contacted by axons (Fig. 2D–F, n=5). Nerve invasion into the remaining muscles was more extensive on E13.5 (Fig. G-I, n=5), and occurred at the belly of each muscle. The timing of branches was verified by applying the lipophilic dye diI to the oculomotor nerve on E12.5 and E13.5, although muscles could not be co-labeled (Fig. 2J, K). We observed that the motor nerves formed an adult pattern by E14.5 in whole mount embryos viewed deep to the eye (Fig. 2L).
Taken together, these observations show that the oculomotor nerve pauses to make prolonged contact with Pitx2+ precursor cells, with the plexus maintained through primary myogenesis. Figure 3 shows a summary of the time course of the development of the oculomotor nerves, including nerve growth, positions of Pitx2+ precursors, and differentiation of muscles.
Ablation of Myf5-expressing cells allows motor nerve navigation to the eye, but disrupts terminal branch formation.
The early and prolonged contact between the nerve plexus and the Pitx2+ cells suggests that the precursor mass regulates nerve growth and branching. To determine the functional significance of the early nerve interaction with the Pitx2+ mass, we used a genetic approach to block muscle formation by targeted ablation of Myf5-expressing cells.
Our first approach to disrupting muscle development was to study motor nerve development in Myf5-Cre; DTA mutants. Myf5 is a muscle regulatory factor which activates around E10.5, downstream of Pitx2 (Zacharias et al., 2011). In this model, a heterozygous Myf5-Cre insertion allele was crossed to a Cre-activated diphtheria toxin transgene, the expression of which triggered cell death in muscle precursor cells (Haldar et al., 2007; Haldar et al., 2008). However, some cells may be spared and subsequently proliferate to generate delayed extraocular muscles (Comai et al., 2014; Haldar et al., 2008).
We note that this Myf5-DTA ablation strategy differed from the previous publication of Michalak, et al. That study used Myf5 Cre/Cre homozygotes to disrupt Myf5 (and likely Mrf4) gene expression, which results in a lack of differentiated extraocular muscles, although precursors were not examined. The oculomotor nerve reached the periorbital area in those mutants, implying the first step in navigation was not disrupted by ablation of muscle precursor cells at E10.5. After E12.5, the oculomotor nerve failed to form branches away from the decision region (plexus). No branches formed in the positions expected for superior rectus, inferior rectus, or medial rectus, and Only a very thin branch extended toward the inferior oblique. The nIV reached its expected dorsal location on E12.5, but by E14.5 formed multiple abnormal and thin branches.
In contrast to Michalak, et al, our Myf5-DTA ablation strategy should DTA-mediated apoptosis of muscle precursors when they initiated Myf5 transcription, which we predicted would result in an ablation of a subset of the Pitx2+ mass and remove extraocular muscles. We verified a depletion of Pitx2+ cells in the precursor mass in Myf5-DTA embryos on E10.5, consistent with the initiation of Myf5 transcription about E10.5 (Fig 4A,B; n=3). The mass ventrolateral to the eye contained fewer Pitx2+ cells. The remaining Pitx2+ cells spread over a larger area than in control embryos. These cells may represent another population of Pitx2+ cells with non-muscle fates, a set of muscle precursors spared from ablation, or muscle precursors that have not yet undergone apoptosis. The Myf5-DTA strategy was largely effective at disrupting muscle formation by E12.5, as shown by the absence of large Pitx2+ condensations in the position of muscle primordia (Fig 4C,D). Significant numbers of Pitx2+ cells remained, emphasizing that Pitx2+ cell populations contribute to non-muscle tissues. In whole mount labels, smooth muscle antibody labeling in four of five whole mount embryos showed no muscle fibers, although blood vessel labeling remained (Fig 4E,F). One embryo showed a few scattered actin-positive muscle fibers, but no condensed extraocular muscles (not shown).
Figure 4. Motor axon guidance is disrupted by the loss of extraocular muscles in Myf5-DTA mutants.
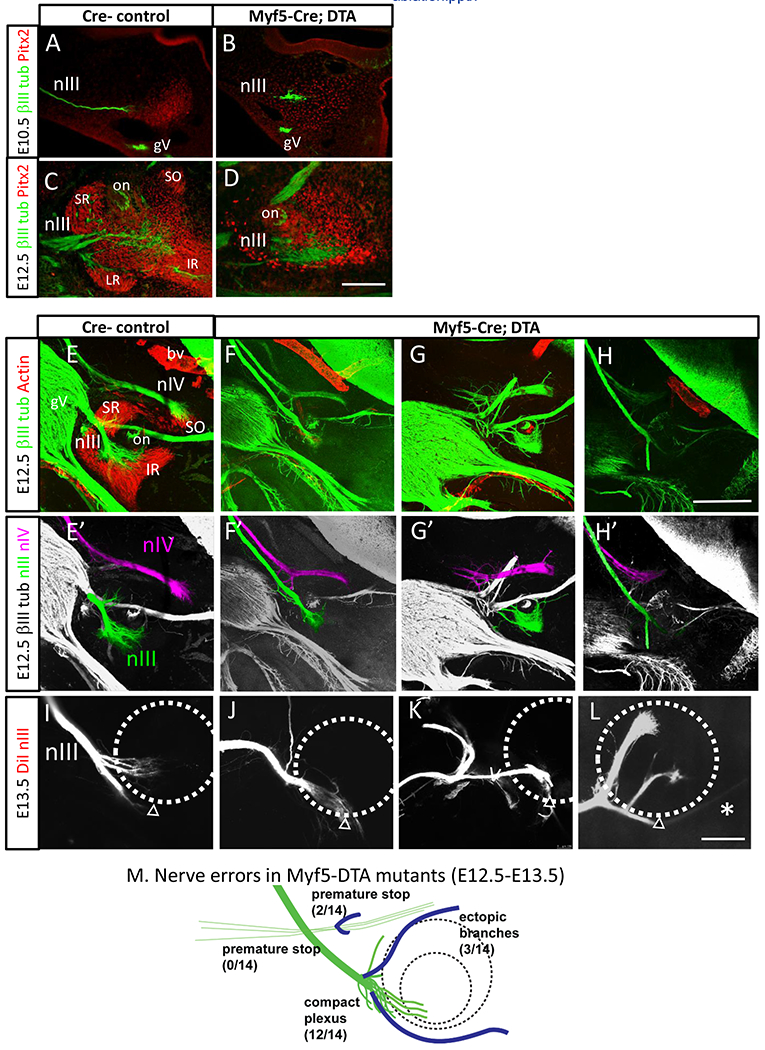
Oculomotor axon projections on E10.5, E12.5, and E13.5 in Myf5-DTA mutants and littermate controls. A, B. E10.5 oculomotor nerve projection to the eye. Nerve (green) and Pitx2 (red) antibody labeling on sections, cut roughly parallel to the oculomotor nerve. A. In Myf5-Cre-minus control littermates, the oculomotor nerve terminated when projecting into the mass of Pitx2+ cells ventrolateral to the eye. B. E10.5 Myc-DTA embryo. The Pitx2+ antibody labeling showed fewer Pitx2+ cells, which appeared to be less condensed than in controls. The oculomotor nerve arrived in this region in a normal position.
C, D. E12.5 Pitx2 antibody labeling, sagittal 20 μm cryostat sections. Note that these thin sections clearly showed Pitx2 antibody labeling patterns, but show a small part of the nIII plexus. C. In control embryos, Pitx2 labeling showed condensing muscles beginning to form in an array around the optic nerve. Muscles marked are superior rectus (SR), inferior rectus (IR), lateral rectus (LR), superior oblique (SO); on, optic nerve. Additional Pitx2+ cells formed layers around the optic nerve and cup. Pitx2 antibody signal varied between sections, so the reduced signal in B appeared to be due to a reduced number and less dense mass of Pitx2+ cells. D. In Myf5-DTA embryos, the Pitx2+ masses in the expected muscle positions were much smaller, and less densely packed, while Pitx2+ layers remained around the optic nerve and cup.
E, E’. E12.5 smooth muscle actin (SMA, red) and βIII-tubulin (green) labels of sagittal vibratome sections. Maximum projection of confocal z-stacks. Smooth muscle actin antibody (red) labels the primordial muscles beginning to form, including superior rectus (SR), inferior rectus (IR), and a few muscle fibers of the superior oblique (SO). SMA also labels blood vessels (bv). βIII tubulin label (green) labels motor nerves (nIII, nIV) and trigeminal ganglion (gV). Note that these vibratome sections were behind the orbit to show the oculomotor nerve plexus and the nIV nerve terminus. The sections vary in showing the proximal nIII nerve bundle, or other landmarks such as gV branches. E’. Guided by the confocal Z stacks, the trajectories of nIII and nIV nerves colored in z-stack projections.
F, F’, G, G’, H, H’ Three examples of E12.5 Myf5-DTA mutants, showing axon labels (βIII tubulin, gray) of sagittal vibratome sections, with colored oculomotor and trochlear nerves. These nerve branches, particularly in mutants, frequently merged into trigeminal branches. Ambiguous nerve branches were uncolored, so the mutant images do not show the complete pattern of axon errors. Oculomotor errors included abnormal branching around the eye; trochlear errors included premature stopping. Myf5-DTA mutant embryos usually lacked any muscle fiber labeling, although 1 of 5 Myf5-DTA embryos had small numbers of scattered muscle fibers.
I, J, K, L. E13.5 control and Myf5-DTA embryos, showing oculomotor tracing with diI. In Myf5-DTA embryos (n=5) compared to controls (n=3), large random branches formed generally dorsal to the main nerve, including abnormal branches before the eye, and around the eye. In one case, a small branch extended past the eye (L).
M. Summary of nerve errors in Myf5-DTA mutants, combined from E12.5 and 13.5 samples. (n=14; 1 nerve had a compact plexus, plus an ectopic branch.)
Scale bars: 100 μm in B (applies to A-B); 300 μm in F (applies to E-I); 300 μm in J (applies to J-M).
The motor nerves showed largely normal guidance to the eye in Myf5-DTA mutants. On E10.5, the oculomotor nerve navigated to the periocular area, stopping in contact with the remnant Pitx2 mass (Fig 4A,B). Therefore the initial navigation signals to the eye appeared intact, consistent with the Myf5Cre/Cre mutants previously reported.
Myf5-DTA embryos were examined for motor nerve branch patterns on E12.5 with whole mount labels by bill-tubulin labeling (Fig 4E–M). Motor nerve errors were apparent in thick sections, including long ectopic branches. To show these more clearly, we collected Z stacks with confocal microscopy, and used the Z stacks to guide manual coloring of motor nerves. Most commonly on E12.5 (n=12 of 14), the oculomotor nerve ended in a plexus in the expected position near the eye (Fig 4F’, G’). It did not form a branch at the expected superior branch position and several disorganized axons were observed wandering out of the plexus. The trochlear nerve was also visible on E12.5, and navigated to its dorsal terminus as expected. On E12.5, one Myf5-DTA embryo had more severe errors bilaterally, including long oculomotor nerve projections, and premature stopping of the trochlear nerve (Fig 4H), suggesting some variability in nerve navigation between embryos.
Later-stage DiI tracing of the oculomotor nerve on E13.5 showed a relatively compact plexus without major branches (Fig 4J), which was consistent with the absent branching observed in Myf5Cre/Cre embryos. We did note two cases in which large ectopic branches emerged from varying positions, including proximal to the eye, next to the eye, and projecting under the eye (Fig 4K, L). An abnormally long branch was also present in one case (Fig 4L).
Overall, the initial growth of the oculomotor nerve to the eye indicates that nerve navigation was independent of differentiated muscle cells, but terminal branch formation was altered. These results agree largely with the previous study of Myf5Cre/Cre mutants, although ectopic projections were observed in a few cases of Myf5-DTA embryos.
Pitx2 loss results in highly abnormal navigation and branch formation around the eye.
Our second approach to disrupt muscle development used Pitx2 mutant embryos. Pitx2 mutants lack extraocular muscles, as previously characterized (Zacharias et al., 2011). Pitx2+-mutant muscle precursor cells are ablated early in development, as demonstrated by the loss of the proliferation marker Ki67+ on E9.5, and extensive TUNEL+ apoptotic labeling on E10.5 (Zacharias et al., 2011). The pro-myogenic transcription factors Myf5 and MyoD, also fail to be expressed after the loss of Pitx2 (Zacharias et al., 2011). Other orbital tissues are also derived from Pitx2+ cells, and may be lost in Pitx2 mutants. This prior work defines Pitx2 as a key regulator upstream of, and required for the coordinated assembly of extraocular muscles and associated tissues (summarized in Fig 5A).
Figure 5. Motor axon guidance is disrupted by the loss of extraocular muscles in Pitx2 mutants.
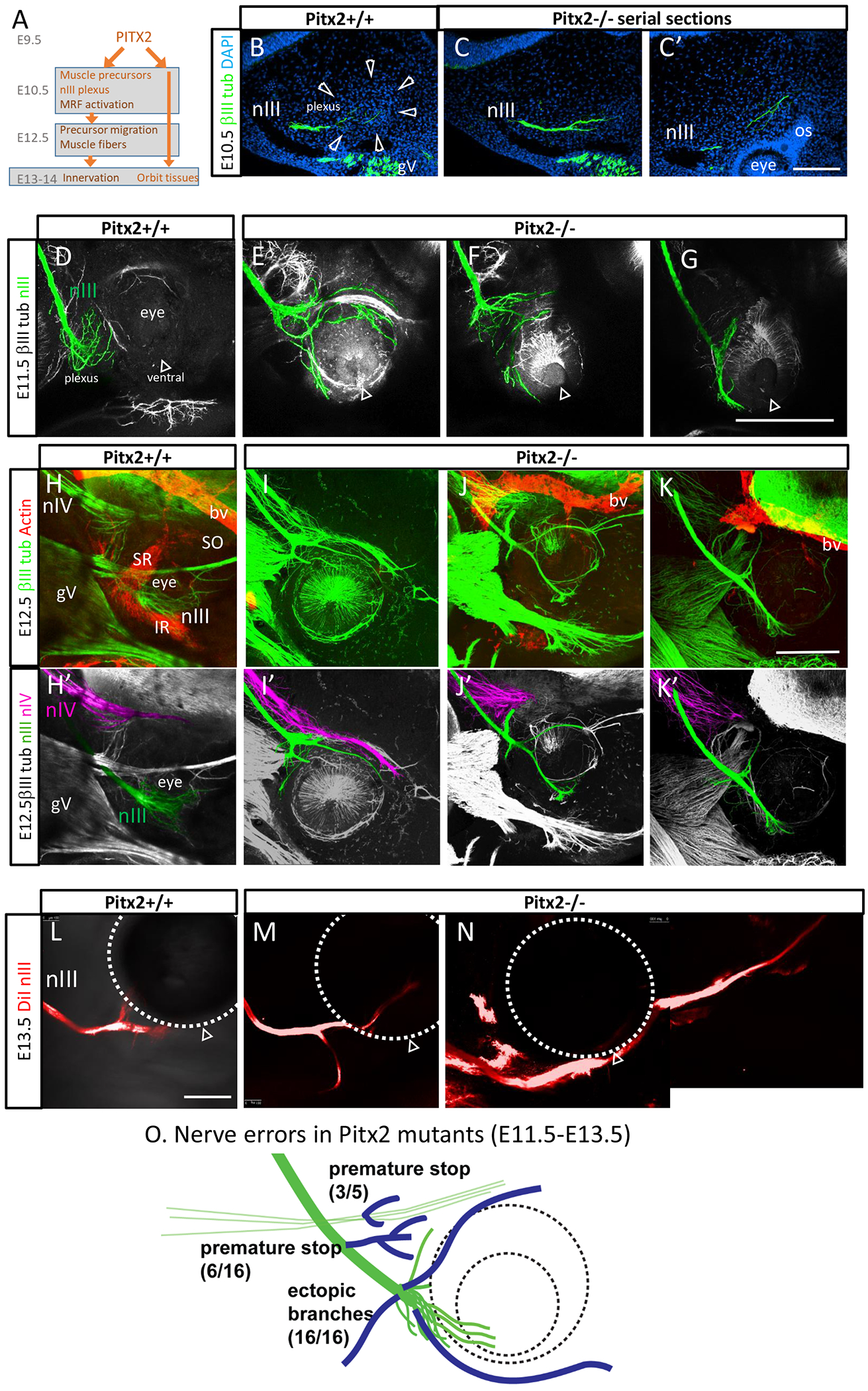
Oculomotor axon projections from E10.5 to E13.5 in Pitx2 mutants and littermate controls. A. Summary of the steps of Pitx2 expression and extraocular muscle differentiation. Pitx2 function is required for precursor cell survival, and activation of muscle regulatory factors, including Myf5, that promote muscle fiber differentiation (Zacharias, et al, 2011). Pitx2 mesodermal cells lead to additional cell lineages.
B. E10.5 Pitx2+/+ control. Transverse section parallel to the oculomotor nerve, 20 μm thick, labeled with βIII-tubulin antibody (green) and DAPI staining of nuclei (blue). Serial sections revealed the oculomotor nerve pioneers projecting out to the eye. The nerve ending was within a dense mass of cells (outlined by arrowheads), ventrolateral to the eye.
C, C’. Pitx2−/− mutant. Serial sections show that the oculomotor nerve projected to the ventrolateral area of the eye, but this area of mesenchyme failed to form the dense cell mass. Note the abnormal split in the nerve, instead of defasciculated axons in wild type. C’ shows an example of oculomotor nerve fibers that projected past their normal stopping point, instead reaching close to the optic stalk. The oculomotor axon trajectory was traced through several serial sections.
D-G. E11.5 Pitx2 control and mutants, βIII-tubulin labeling of whole mounts. Maximum projection of confocal z-stacks; nIII nerve projections were colored, guided by z-projections, with ambiguous axon segments left uncolored, such as where they merged with gV bundles. The open arrowhead shows the ventral side of the eye (choroid fissure). Control embryos had a plexus of defasciculated oculomotor axons in a compact area. Mutant embryos had widely projecting oculomotor axons, projecting abnormally both dorsal and ventral around the eye.
H-K. E12.5 Pitx2 +/+ control and Pitx2−/− mutant embryos; 400 μm vibratome section in sagittal plane. Maximum projection of confocal z-stacks; nIII and nIV nerve projections were colored, guided by z-projections; ambiguous axon segments were left uncolored.
H, H’ E12.5 control, 400 μm vibratome section in sagittal plane. Maximum projection of confocal z-stacks. Smooth muscle actin antibody (red) labeled the primordial muscles beginning to form, including superior rectus (SR), inferior rectus (IR), and a few muscle fibers of the superior oblique (SO). SMA also labeled blood vessels (bv). βIII tubulin label (green) labels motor nerves (nIII, nIV) and trigeminal ganglion (gV).
I, I’, J, J’ K, K’ Three examples of nIII and nIV errors in E12.5 Pitx2−/− mutants. Smooth muscle actin antibody labeled blood vessels, but no muscle fibers were seen in any Pitx2−/− embryos (n=5). The nerve branches, particularly in mutants, frequently merged into trigeminal branches. Ambiguous nerve branches were left uncolored, so these images do not show the complete pattern of axon errors. The oculomotor nerve often stopped prematurely, forming abnormal branches before the eye, while other branches projected around the dorsal and ventral eye. The trochlear nerve projected abnormally close to the eye, or appeared to terminate prematurely. Trochlear axons, which were normally more defasciculated, did not form prominent ectopic branches, but many trochlear axons strayed to fasciculate with trigeminal or oculomotor nerve bundles.
L. E13.5 control embryo. Tracing of oculomotor nerve with the lipophilic tracer diI. The diI was placed in the nerve near the midbrain, and allowed to transport to the ends of the axons. The open arrowhead indicates the ventral eye. Typical pattern at this stage was a simple pattern of a large inferior branch, and a smaller superior branch.
M, N. Two examples of Pitx2 mutants. Tracing of the oculomotor nerve with diI confirmed ectopic branches, and an example of a long axon bundle growing past the eye.
O. Summary of motor nerve errors in Pitx2 mutants. Oculomotor nerve errors included premature stop with associated branch sites, ectopic branches, and overshooting. Trochlear errors included premature stop. Scale bars: 100 μm in C’ (applies to B, C); 300 μm in G (applies to D-F); 300 μm in K’ (applies to H-K’); 300 μm in L (applies to M, N).
We examined the development of the oculomotor nerve in Pitx2 mutant embryos (Figure 5). In E10.5 control littermates (n=3), around 32-34 somites, the first oculomotor axons arrived at the eye and spread out within the dense precursor mass (Fig 5B). In E10.5 Pitx2−/− mutants (n=3), the oculomotor nerve was compact as it arrived at the correct location ventro-lateral to the eye (Fig 5C). At this early stage, we attempted antibody labeling for muscle regulatory factors (e.g. MyoD), but labeling was not detectable. The precursor cells visible in wild type controls were missing in Pitx2−/− embryos. By tracing the nerve through serial sections, the oculomotor nerve appeared longer, with axons extended abnormally close to the optic stalk (Fig 5C’).
To confirm that the oculomotor axons grew abnormally long instead of stopping, oculomotor nerve patterns were examined on E11.5 in whole mount labels. In wild type controls, branching does not occur until E12.5. At E11.5 in wildtype littermates, the oculomotor nerve maintained a relatively compact plexus, with axon fibers defasciculating but remaining close to the ventrolateral eye (Fig 5D). However, consistent with the longer axons observed on E10.5, Pitx2 mutants had extended axons growing around the dorsal and ventral aspects of the eye (Fig 5E–G). These nerves projected in patterns which varied between embryos, as well as bilaterally between left and right sides of the same embryo. Ectopic projections extended abnormally around the dorsal and ventral sides of the eye. The ectopic axons projected earlier and in disordered patterns compared to the normal branches initiating on E12.5. Furthermore, ectopic projections were highly variable from the wildtype control, with much longer and thicker bundles. In a few cases, there appeared to be an abnormally placed or premature plexus formed at the dorsal orbit. The trochlear nerve also made errors, although at lower frequency. Some trochlear nerves reached the approximate correct location of their target, but the nerves in other mutants projected too close and contacted the eye (Fig 5I), or stopped far short (Fig 5J, K). The abducens could not be consistently detected at this E12.5 stage, particularly in the mutant litters, so it was not further analyzed.
In the E12.5 Pitx2 mutants, it was difficult to trace nIII ectopic branches because they frequently fasciculated with the branches of the trigeminal nerve (which also took abnormal trajectories around the eye). We therefore used diI tracing of the oculomotor nerve in E13.5 embryos (Fig 5L–N; n=7). This tracing strategy was specific for the oculomotor nerve and enabled further characterization of mutant branching. It revealed that some of the ectopic branches projected ventral to and far beyond the eye. When co-imaged with a tubulin label, ectopic oculomotor branches appeared to fasciculate with the trigeminal branches toward the nose.
Overall, the axon errors in Pitx2 mutants suggests that Pitx2+ cells provide a signal required to pause motor nerve growth in the plexus, and to prevent premature growth around the eye.
Discussion
The ocular motor system is a relatively simple model for understanding the mechanisms of motor axon guidance. The precise navigation of motor nerves to the eye is followed by the formation of terminal branches to innervate specific extraocular muscles (EOMs). Oculomotor migration occurs surprisingly early in embryonic development, with the nerve growth preceding the appearance of differentiated extraocular muscles.
We identified a mass of Pitx2+ cells as the first target of initial oculomotor nerve growth toward the orbit, and showed that the nerve plexus pauses in contact with these Pitx2+ extraocular muscle precursor cells. We then manipulated nerve formation by genetically blocking EOM formation with Myf-DTA and Pitx2 mutant mouse embryos. The motor nerves grew to the eye accurately in both mutants, showing that navigation to the eye is independent of either Pitx2 function or Myf5-expressing cells. Myf5-expressing cells were subsequently required for the oculomotor nerve to initiate terminal branches. In contrast, Pitx2 function was required to regulate motor nerve growth, as motor axons fail to pause to form a plexus in Pitx2 mutants, and instead grew abnormally to form long ectopic branches around the eye.
From the midbrain to the orbit
The initial outgrowth of the oculomotor nerve to the eye is precocious, preceding muscle differentiation by more than two days. It was previously observed that the abducens nerve contacts distinct subsets of mesenchymal condensations, presumably lateral rectus and other facial muscle precursors (Wahl et al., 1994). Our study elaborates on these findings by showing that all three ocular motor nerves contact a Pitx2-expressing cell mass, with each of the three nerves localized to a separate region. This suggests a subdivision and pre-patterning of Pitx2+ cells, which presumably find their appropriate nerve via secreted or contact-mediated cues which remain unknown.
As a whole, the Pitx2+ cell mass is an intermediate target and potentially the source of an attractive long-range signal. However, oculomotor nerve guidance to the periorbital area is clearly independent of Pitx2 function, because the nerve navigated accurately in Pitx2 mutants. It was also independent of Myf5 function or the presence of Myf5-expressing cells.
Both our study as well as Michalak et al., suggest that extraocular muscle precursors are not required to attract the oculomotor nerve to the eye, because the nerve fibers navigated correctly to the eye after genetic disruption of the precursors. However, our results do not rule out the possibility that the muscle precursors provided attractive signals during the earlier period of E9-E10. Interestingly, Pitx2-driven LacZ expression is broadly expressed across the cranial mesoderm as early as E8.5 (Shih et al., 2007), and these cells may play some early role. We could not detect Pitx2 antibody labeling on E9.5 in cranial tissues, and Pitx2 labeling localized only in the condensed cell mass on E10.0-10.5, which suggests local, contact-mediated guidance functions for motor axons. Several potential molecular cues are expressed around the developing chick eye (Ferrario et al., 2012; Lerner et al., 2010; Ojeda et al., 2013). Future research should investigate their influence on muscle precursor cells and motor axons.
The factors that guide the initial oculomotor nerve trajectory from the midbrain remain unknown, but could include signals produced by the mesenchyme traversed by the axon pioneers. The nerve trajectory is also flanked by the ventral midline of the midbrain/hindbrain on one side, and the forebrain on the other, so long range cues such as floor plate signals may also contribute to guide the oculomotor nerve trajectory.
Plexus formation and terminal branching
Once the oculomotor nerve arrived at the eye, Pitx2 function was required for the nerve to stop to form a plexus. In the absence of Pitx2 function, the pioneer oculomotor axons continued to grow, abnormally approaching the eye and optic stalk on E10.5 and forming long disordered branches around the eye over the next few days. This suggests that one key Pitx2 function is to provide an early stop signal to inhibit further growth of the oculomotor nerve.
Based on the early abnormal growth seen in E10.5 Pitx2 mutants, random errors by the first pioneering axons may mislead the many following axons. The normal oculomotor nerve was remarkably compact in its projection, perhaps due to the strong tendency of later axons to fasciculate with and closely follow the pioneers. The variety of errors in Pitx2 mutants implies a growth-promoting environment lacking secondary “back up” guidance signals when Pitx2+ cells are missing. In a subset of cases, particularly in Pitx2 mutants, the oculomotor and/or trochlear motor nerves stopped prematurely. The inconsistency of this type of error suggests a random pioneer axon decision, or abnormal tissue boundaries arising from the apoptotic loss of Pitx2-expressing cells.
Oculomotor terminal branches were also disrupted in the Myf5-DTA and Pitx2 −/− mutants. These branches form by axonal subpopulations diverging from the main nerve to their final extraocular muscle targets. The mechanism for branch formation are not well understood. Once the motor axons reached the periocular area, they paused in a plexus at the decision region while myogenic precursors migrated to orbital attachment points. This suggests that terminal branching is an independent guidance step which occurs after nerve extension to the periocular tissues.
The oculomotor nerve consists of at least four subpopulations of motor axons sharing a main nerve. Terminal branches might occur by a combination of selective defasciculation and selective attraction toward a specific muscle. Terminal branch patterns may require changes in adhesion molecules, and also receptors to respond to potential muscle-derived guidance cues such as CXCL12, HGF and Sema3 (Chilton and Guthrie, 2004; Ferrario et al., 2012; Lerner et al., 2010). The almost complete lack of branches in Myf5Cre/Cre mutants reported by Michalak, et al., and the similar reduced branching in most cases of Myf5-DTA mutants that we show here, suggests that the failure of muscles to differentiate leads to the loss of muscle-derived cues required to initiate branching.
In contrast, Pitx2 loss led to an earlier and more severe defect in the motor axons: failure to stop and form a plexus. We interpreted these errors as aberrant axon growth patterns caused by the loss of the plexus stop signal, rather than defects in terminal branch guidance.
Several human mutations lead to congenital cranial dysinnervation disorders (CCDDs) (Graeber et al., 2013; Whitman and Engle, 2017). Mouse and zebrafish studies of these genes suggest that a critical decision point where oculomotor axons stall, reorient toward muscle targets and initiate branching may be particularly sensitive to disruption (Cheng et al., 2014; Clark et al., 2013; Ferrario et al., 2012; Miyake et al., 2008). Our observations suggest that errors in the ‘waiting zone’ or ‘distal decision zone’ may involve axons receiving signals from Pitx2+ precursor cells.
Acknowledgements
We thank Claudia Garcia Pena for comments in editing the manuscript, and her expert advice in analyzing oculomotor development. Several individuals contributed technical assistance in genotyping, and assistance in optimizing antibody labeling including Minkyung Kim, Thomas Kidd, and Hillary Price.
Funding sources
Funding was provided by RO1 EY 025205 and RO1 NS114219 to GSM, RO1 EY014126 to PJG, and R21 NS 107922 to TWG. Further support for core facilities at the University of Nevada was provided by NIH COBREs RR024210, GM103650, GM103554, and the Nevada INBRE 8 P20 GM103440-11, P20 GM103554. The funding bodies had no roles in the study, collection, analysis, interpretation of data, nor in writing the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Ethics approval
Animal experiments were approved by the UNR IACUC, following NIH guidelines, with the approved protocol #2015-00435.
References
- Ball EE, Ho RK, Goodman CS, 1985. Development of neuromuscular specificity in the grasshopper embryo: guidance of motoneuron growth cones by muscle pioneers. J Neurosci 5, 1808–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorke B, Shoja-Taheri F, Kim M, Robinson GE, Fontelonga T, Kim K-T, Song MR, Mastick GS, 2016. Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling. Neural Development 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter F, 1906. The development of the oculomotor nerve, the ciliary ganglion, and the abducent nerve in the chick. Cambridge, Mass., Bulletin of the museum of comparative zoology. [Google Scholar]
- Cheng L, Desai J, Miranda CJ, Duncan JS, Qiu W, Nugent AA, Kolpak AL, Wu CC, Drokhlyansky E, Delisle MM, Chan WM, Wei Y, Propst F, Reck-Peterson SL, Fritzsch B, Engle EC, 2014. Human CFEOM1 Mutations Attenuate KIF21A Autoinhibition and Cause Oculomotor Axon Stalling. Neuron 82, 334–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton JK, Guthrie S, 2004. Development of oculomotor axon projections in the chick embryo. Journal of Comparative Neurology 472, 308–317. [DOI] [PubMed] [Google Scholar]
- Chilton JK, Guthrie S, 2017. Axons get ahead: Insights into axon guidance and congenital cranial dysinnervation disorders. Dev Neurobiol 77, 861–875. [DOI] [PubMed] [Google Scholar]
- Clark C, Austen O, Poparic I, Guthrie S, 2013. α2-Chimaerin regulates a key axon guidance transition during development of the oculomotor projection. J Neurosci 33, 16540–16551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai G, Sambasivan R, Gopalakrishnan S, Tajbakhsh S, 2014. Variations in the efficiency of lineage marking and ablation confound distinctions between myogenic cell populations. Dev Cell 31, 654–667. [DOI] [PubMed] [Google Scholar]
- Farmer WT, Altick AL, Nural HF, Dugan JP, Kidd T, Charron F, Mastick GS, 2008. Pioneer longitudinal axons navigate using floor plate and Slit/Robo signals. Development 135, 3643–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario JE, Baskaran P, Clark C, Hendry A, Lerner O, Hintze M, Allen J, Chilton JK, Guthrie S, 2012. Axon guidance in the developing ocular motor system and Duane retraction syndrome depends on Semaphorin signaling via alpha2-chimaerin. Proc Natl Acad Sci U S A 109, 14669–14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T, 2005. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci 46, 4200–4208. [DOI] [PubMed] [Google Scholar]
- Graeber CP, Hunter DG, Engle EC, 2013. The genetic basis of incomitant strabismus: consolidation of the current knowledge of the genetic foundations of disease. Semin Ophthalmol 28, 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar M, Hancock JD, Coffin CM, Lessnick SL, Capecchi MR, 2007. A conditional mouse model of synovial sarcoma: insights into a myogenic origin. Cancer Cell 11, 375–388. [DOI] [PubMed] [Google Scholar]
- Haldar M, Karan G, Tvrdik P, Capecchi MR, 2008. Two cell lineages, myf5 and myf5-independent, participate in mouse skeletal myogenesis. Dev Cell 14, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho RK, Ball EE, Goodman CS, 1983. Muscle pioneers: large mesodermal cells that erect a scaffold for developing muscles and motoneurones in grasshopper embryos. Nature 301, 66–69. [DOI] [PubMed] [Google Scholar]
- Hollyday M, Morgan-Carr M, 1995. Chick wing innervation. II. Morphology of motor and sensory axons and their growth cones during early development. J Comp Neurol 357, 254–271. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C, Landmesser L, 1981. Pathway selection by chick lumbosacral motoneurons during normal development. Proc R Soc Lond B Biol Sci 214, 1–18. [DOI] [PubMed] [Google Scholar]
- Landgraf M, Baylies M, Bate M, 1999. Muscle founder cells regulate defasciculation and targeting of motor axons in the Drosophila embryo. Curr Biol 9, 589–592. [DOI] [PubMed] [Google Scholar]
- Lerner O, Davenport D, Patel P, Psatha M, Lieberam I, Guthrie S, 2010. Stromal Cell-Derived Factor-1 and Hepatocyte Growth Factor Guide Axon Projections to the Extraocular Muscles. Developmental Neurobiology 70, 549–564. [DOI] [PubMed] [Google Scholar]
- Lescroart F, Kelly RG, Le Garrec JF, Nicolas JF, Meilhac SM, Buckingham M, 2010. Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development 137, 3269–3279. [DOI] [PubMed] [Google Scholar]
- Lewcock JW, Genoud N, Lettieri K, Pfaff SL, 2007. The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron 56, 604–620. [DOI] [PubMed] [Google Scholar]
- Melancon E, Liu DW, Westerfield M, Eisen JS, 1997. Pathfinding by identified zebrafish motoneurons in the absence of muscle pioneers. J Neurosci 17, 7796–7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak SM, Whitman MC, Park JG, Tischfield MA, Nguyen EH, Engle EC, 2017. Ocular Motor Nerve Development in the Presence and Absence of Extraocular Muscle. Invest Ophthalmol Vis Sci 58, 2388–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake N, Chilton J, Psatha M, Cheng L, Andrews C, Chan WM, Law K, Crosier M, Lindsay S, Cheung M, Allen J, Gutowski NJ, Ellard S, Young E, Iannaccone A, Appukuttan B, Stout JT, Christiansen S, Ciccarelli ML, Baldi A, Campioni M, Zenteno JC, Davenport D, Mariani LE, Sahin M, Guthrie S, Engle EC, 2008. Human CHN1 mutations hyperactivate alpha 2-chimaerin and cause Duane’s retraction syndrome. Science 321, 839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM, Francis-West P, 2006. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn 235, 1194–1218. [DOI] [PubMed] [Google Scholar]
- Ojeda AF, Munjaal RP, Lwigale PY, 2013. Expression of CXCL12 and CXCL14 during eye development in chick and mouse. Gene Expr Patterns 13, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JG, Tischfield MA, Nugent AA, Cheng L, Di Gioia SA, Chan WM, Maconachie G, Bosley TM, Summers CG, Hunter DG, Robson CD, Gottlob I, Engle EC, 2016. Loss of MAFB Function in Humans and Mice Causes Duane Syndrome, Aberrant Extraocular Muscle Innervation, and Inner-Ear Defects. Am J Hum Genet 98, 1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A, Landgraf M, Rushton E, Broadie K, Bate M, 1996. Presynaptic development at the Drosophila neuromuscular junction: assembly and localization of presynaptic active zones. Neuron 17, 617–626. [DOI] [PubMed] [Google Scholar]
- Shih HP, Gross MK, Kioussi C, 2007. Expression pattern of the homeodomain transcription factor Pitx2 during muscle development. Gene Expr Patterns 7, 441–451. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, Weismann KE, Hellstrom M, Soriano P, 2000. Early myotome specification regulates PDGFA expression and axial skeleton development. Development 127, 5059–5070. [DOI] [PubMed] [Google Scholar]
- Tosney KW, Landmesser LT, 1985a. Development of the major pathways for neurite outgrowth in the chick hindlimb. Dev Biol 109, 193–214. [DOI] [PubMed] [Google Scholar]
- Tosney KW, Landmesser LT, 1985b. Specificity of early motoneuron growth cone outgrowth in the chick embryo. J Neurosci 5, 2336–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D, Liang HE, Locksley RM, 2008. Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice. J Immunol 180, 4742–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl CM, Noden DM, Baker R, 1994. Developmental relations between sixth nerve motor neurons and their targets in the chick embryo. Dev Dyn 201, 191–202. [DOI] [PubMed] [Google Scholar]
- Wang G, Scott SA, 2000. The “waiting period” of sensory and motor axons in early chick hindlimb: its role in axon pathfinding and neuronal maturation. J Neurosci 20, 5358–5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman MC, Engle EC, 2017. Ocular congenital cranial dysinnervation disorders (CCDDs): insights into axon growth and guidance. Hum Mol Genet 26, R37–R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias AL, Lewandoski M, Rudnicki MA, Gage PJ, 2011. Pitx2 is an upstream activator of extraocular myogenesis and survival. Dev Biol 349, 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]


