Abstract
Muscle afferent nerve-activated reflex sympathetic nervous and blood pressure responses are exaggerated during exercise in patients with peripheral artery diseases (PAD) and in PAD rats induced by femoral artery occlusion. However, the precise signaling pathways and molecular mediators responsible for these abnormal autonomic responses in PAD are poorly understood. A-type voltage-gated K+ (KV) channels are quintessential regulators of cellular excitability in the various tissues. Among KV channels, KV4 (i.e., KV4.1 and KV4.3) in primary sensory neurons mainly participate in physiological functions in regulation of mechanical and chemical sensation. However, little is known about the role of KV4 in regulating neuronal activity in muscle afferent neurons of PAD. In addition, bradykinin (BK) is considered as a muscle metabolite contributing to the exaggerated exercise pressor reflex in PAD rats with femoral artery occlusion. Our data demonstrated that 1) KV4 currents are attenuated in dorsal root ganglion (DRG) neurons innervating the hindlimb muscles of PAD rats, along with decreasing threshold of action potential firing; 2) KV4 currents are inhibited by application of BK onto muscle DRG neurons of PAD rats to a greater degree; and 3) expression of KV4.3 is downregulated in DRGs of PAD rats and KV4.3 channel is a major contributor to the activity of KV4 currents in muscle DRG neurons. In conclusion, data suggest that femoral artery occlusion induced-limb ischemia and/or ischemia induced-metabolites (i.e., BK) inhibit the activity of KV4 channels in muscle afferent neurons likely leading to the exaggerated exercise pressor reflex observed in PAD.
Keywords: peripheral artery disease, A-type voltage-gated K+ channels, bradykinin, dorsal root ganglion
Introduction
Peripheral artery disease (PAD) is a common and disabling disease that affects over 200 million worldwide and 12 to 20% of Americans over age 60 (Criqui & Aboyans, 2015; Fowkes et al., 2017). It is due to atherosclerotic vascular disease that results in progressive narrowing of the lower extremity conduit vasculature. PAD can lead to severe limb ischemia. Thus, the classic discomfort syndrome of PAD is termed “intermittent claudication” which is characterized by pain in lower limbs that occurs with walking and is relieved by rest. This in turn limits walking tolerance. Importantly, PAD patients are at high risk of myocardial infarctions, cerebral vascular accidents and all-cause mortality (Ouriel, 2001; Anand et al., 2018; Bauersachs et al., 2019) with a death rate similar to that seen in patients with coronary or cerebral vascular disease.
Relative to the major advances seen in the management of other cardiovascular diseases, i.e, coronary artery disease and systolic heart failure, therapeutic options other than surgery for PAD remain extremely limited (Hirsch et al., 2006). A number of drugs have been evaluated for use in patients with claudication symptoms, but efficacy has only been reported for cilostazol and anti-platelet agents (Clagett et al., 2004; Kitrou et al., 2017). In fact, exercise training (advice to walk more often) is commonly recommended for PAD patients and supervised treadmill exercise is supported by studies (Brendle et al., 2001; Hamburg & Balady, 2011; McDermott, 2018). However, the implementation of exercise into the daily lives of PAD patients is met with significant challenges (e.g. pain in the legs upon exertion). In addition, during exercise the arterial blood pressure (BP) response is exaggerated in PAD patients (Baccelli et al., 1999; Ritti-Dias et al., 2011). Moreover, the BP response during the exercise with the “diseased” limb is greater than that during the exercise with the “non-diseased” limb (Lorentsen, 1972). Recent human studies further indicate that BP and renal vasoconstriction responses during plantar flexion exercise are accentuated in PAD patients (Muller et al., 2012; Drew et al., 2013). Taken together, it is accepted that an exaggerated exercise pressor reflex is a major determinant of why BP rises with exercise in PAD (Ritti-Dias et al., 2011).
It is important to note that augmented BP response during exercise is associated with higher incidence of cardiovascular diseases (Lewis et al., 2008; Thanassoulis et al., 2012). Also, PAD is associated with the reduced functional capacity, increased risk for cardiovascular diseases and reduced quality of life (Anand et al., 2018). The exaggerated sympathetic nervous activity (SNA)-regulated BP response during exercise contributes to poor clinical outcomes (Piepoli et al., 1999; Ponikowski et al., 2001). Moreover, the leg revascularization attenuates the exaggerated BP and heart rate (HR) responses to exercise in PAD patients and improves coronary blood velocity (Miller et al., 2018). Remarkably, epidemiological evidence indicates that an exaggerated BP response to exercise is associated with decreased survival in both asymptomatic normotensive subjects (Weiss et al., 2010) and in PAD patients (de et al., 2008). Thus, identifying the molecular mediators alleviating the exaggerated BP response to exercise in PAD is clinically significant.
The sympathetic nervous system is activated during exercise, which contributes to the increases in BP, HR, myocardial contractility and peripheral vasoconstriction (Victor et al., 1988; Sinoway et al., 1989). There are two basic mechanisms in contribution to sympathetic engagement during exercise. The first termed “Central Command” (Waldrop et al., 1996), suggests that motor and sympathetic activation occur in parallel, i.e. there is a volitional signal emanating from central motor areas leading to increased SNA during exercise. This system is linked to skeletal muscle metabolic needs via parallel brain activation of motor and autonomic centers (Waldrop et al., 1996). The second termed the “Exercise Pressor Reflex” (Coote et al., 1971; Mitchell et al., 1983) suggests that afferents arising from contracting skeletal muscle are engaged and an autonomic reflex is initiated. This system responds to metabolic stimulation (i.e. “metaboreceptor” stimulation) and to mechanical deformation of the muscle afferents receptive field (i.e. “mechanoreceptor stimulation”) (Kaufman & Forster, 1996). Group IV afferents are predominantly metabosensitive and Group III afferents are predominantly mechanically sensitive (Kaufman & Forster, 1996). When these receptors are stimulated, thin fiber muscle afferent nerves are engaged, cardiovascular nuclei in the brainstem are activated, SNA increases and BP rises (Mitchell et al., 1983). Additionally, activation of muscle afferent fibers modulates the baroreflex functions in involvement of the SNA and cardiovascular responses to exercise (Drew et al., 2008). The baroreflex mechanism is also important because impairment in this reflex is likely to contribute to an abnormal neural-hemodynamic responses to exercise in PAD (Chehuen et al., 2017).
Femoral artery occlusion in rats has been used to study human PAD (Waters et al., 2004). In particular, in occluded rats, the blood flow limitation is observed in the occluded limb during exercise while the resting blood flow is maintained (Waters et al., 2004). This makes it appropriate to investigate exercise physiology in PAD. Importantly, in a rat model of PAD induced by 3-day femoral artery occlusion, the exaggerated SNA and BP responses are observed during muscle contraction or stimulation of muscle metabolic receptors in the occluded limb, but not in the opposing control limb of the same rats (Li & Xing, 2012; Stone & Kaufman, 2015). Among those muscle metabolite, bradykinin (BK) has been reported to play a role in regulating the exaggerated exercise pressor reflex in PAD rats (Leal et al., 2013; Lu et al., 2013). Nonetheless, signaling pathways and molecular mediators involved in the exaggerated SNA and BP responses to stimulation of mechanically/chemically sensitive muscle afferents during exercise in PAD need to be studied.
A-type voltage-gated K+ (KV) channels are regulators of cellular excitability in the primary sensory nerves (neurons) (Barnett & Larkman, 2007). Among KV channels, KV4 (i.e., KV4.1 and KV4.3) in primary sensory neurons mainly participate in sensation functions associated with mechanical, chemical and thermal stimulation (Chien et al., 2007; Conner et al., 2016; Viatchenko-Karpinski et al., 2018). BK also modulates cell excitability by inhibiting K+ channels through kinin B1 and B2 receptors in sensory neurons (Jones et al., 1995).
Thus, the purpose of the present report was to characterize KV channels in muscle afferent neurons-dorsal root ganglion (DRG) neurons of PAD rats and further to examine if BK inhibits the activities of KV4 channels in muscle afferent neurons of PAD rats induced by femoral artery occlusion likely via B1 and/or B2 receptor. We hypothesized that femoral artery occlusion attenuates the activities of KV4.1 and/or KV4.3 channels in muscle DRG neurons and muscle metabolic BK participates in the inhibitory effects of femoral artery occlusion on the activities of KV4.
Materials and Methods
Ethical Approval
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Penn State College of Medicine (Protocol#: PRAMS201147671) and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (4–6 weeks old) were housed in accredited temperature and ventilation controlled facilities with a 12:12 hours-light-dark cycle and ad libitum access to standard rat chow and water.
Femoral Artery Occlusion
The rats were anaesthetized by inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen). The femoral artery on one limb was surgically exposed, dissected and ligated ~3 mm distal to the inguinal ligament as previously described (Li et al., 2020). As the control, the contralateral limb was dealt with the same procedure except the suture below the femoral artery not tied. After the surgery, all the rats were returned to the cage for regular housing for 3 days before experiments. Note that buprenorphine hydrochloride (0.05 mg/kg, subcutaneously) was administered prior to the surgery for post-operative pain relief. Following the surgery, the animals were kept in the surgery room for 2–3 h for observation, and then returned to the animal facility.
Electrophysiology
Labeling of hindlimb muscle afferent DRG neurons:
Briefly as described (Li et al., 2020), 2 days before femoral artery ligation was performed an incision in the calf area of one limb was made and the gastrocnemius muscle was exposed following rats were anaesthetized. The lipophilic dye 1, 1’-dioctadecyl-3, 3, 3’, 3’-tetramethylindocarbocyanine perchlorate (DiI, 60 mg/ml) was injected into the white portion of the gastrocnemius muscle. A total volume of 1 μl DiI tracer was administered at different locations, with the needle left in the muscle for 1 min to prevent the tracer leakage. Then the rats were returned to their cages to wait for the fluorescent DiI retrograde transported to DRG to label muscle DRG neurons.
Culture of DRG neurons:
As described (Li et al., 2020), the rats were euthanatized and decapitated, rat L4 – L6 DRGs in both control limb and occluded limb were removed and dissected, immediately transferred into ice-cold Hank’s balanced salt solution. After being freed from the connective tissues, the ganglia were enzymatically digested and dissociated in Earle’s balanced salt solution (Sigma-Aldrich) containing collagenase Type D (0.6 mg/ml; Roche), trypsin (0.30 mg/ml; Worthington), and DNase (0.1 mg/ml; Alfa Aesar), followed by shaking for 40 min at 34°C. The dissociated neurons were seeded on 10% poly-L-lysine–coated coverslips (Dia# 8mm) in 35-mm culture dish containing 2ml DMEM medium (Thermo) supplemented with 10% FBS, 1% glutamine, and 1% penicillin-streptomycin. Then the neurons were cultured at 37°C with 5% CO2, 95% air in a cell culture incubator (VWR).
Recording of K+ currents and action potential (AP) firing:
Muscle DRG neurons (Dil-positive neurons) were firstly identified under an inverted microscope with a fluorescent filter (Nikon TE2000), and images were displayed on a video monitor. K+ currents and AP firing of rat muscle DRG neurons (cell diameters≤ 35 μM) were recorded in the whole-cell configuration using a MultiClamp 700B amplifier supplied with Digitizer 1440A (Axon Inc). Signals were acquired with pClamp10.1 and analyzed with pClampfit10.7 software. All experiments were performed at 20°C-22°C.
The extracellular solution contained (in mM): 110 choline chloride, 5 KOH, 1 MgCl2, 20 TEA, 10 HEPES, 2 CdCl2, and 10 D-glucose (pH 7.4, osmolality 310 mosm). The electrode was filled with a solution containing (in mM): 120 KCl, 2.5 MgCl2, 10 EGTA, 10 HEPES, 0.3 Li-GTP, 2 MgATP, and 1 CaCl2 (pH 7.3 adjusted with KOH, osmolality 290 mosm).
Holding at −65 mV, after the seals (2~8 GΩ) obtained with 2~4 MΩ resistance of glass electrodes filled with internal solution, the whole-cell configuration was applied. In voltage-clamp mode, two separate protocols were used to record tetraethylammonium (TEA) resistant A-type-K+ channel currents (TEA-R-IKA) in muscle DRG neurons as previously reported (Phuket & Covarrubias, 2009; Qian et al., 2009). Total K+ currents (IKAtotal) were recorded with the protocol 1: a 1-sec conditioning pulse of −100 mV prior to 500 ms step depolarizations from −100 mV to 60 mV with 10 mV increments per step. The protocol 2 was similar to protocol 1 except of 1-sec conditioning pulse of – 30 mV for the delayed rectifier K+ currents (IKADR). The recording current was filtered at 2 kHz and sampled at 10 kHz. Voltage errors were minimized by 80% series resistance compensation and linear leak subtraction was used for all recordings. All signals were acquired with pClamp10.1 and analyzed with Clampfit10.7 software. TEA-R-IKA = IKAtotal − IKADR was obtained by pClampfit10.7. 20 μM TEA was applied to the external solution to block the TEA sensitive K+ channels.
On the other hand, AP of muscle DRG neurons was determined by the whole cell current-clamp recording as reported previously (Xing et al., 2012). The extracellular solution contained (in mM): 150 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 10 D-glucose (pH 7.4 adjusted with NaOH, osmolality 330 mosm). Electrodes were filled with a solution containing (in mM): 135 K+- gluconate, 5 KCl, 2 MgCl2, 2 CaCl2, 5 EGTA, 10 HEPES, 0.3 Na-GTP, 2 MgATP (pH 7.3 adjusted with KOH, osmolality 310 mosm). In voltage-clamp mode, the whole-cell configuration was applied, then switched to current-clamp mode, and AP generation was stimulated with a 1-sec step currents protocol: −50pA to 500pA with an increments of 10pA. All signals were acquired with pClamp10.1 and analyzed with Clampfit10.7 software and then the AP firing threshold and frequency were analyzed.
Chemicals stored in the stock solutions were diluted in extracellular solution immediately before being used and individually held in a series of independent syringes of the pressurized VC3–8MP perfusion system (ALA). The distance from the outlet tip mouth to the neuron examined was within 100 μm. The neurons in the recording chamber were continuously bathed in the extracellular solution.
Western Blot Analysis
A total protein of rat L4 – L6 DRG tissue of both control limb (n=6) and occluded limb (n=6) was extracted. 10 μg of protein was loaded in 10% Mini-Protean TGX Precast gels (Bio-Rad) after being boiled at 95°C for 5 min in SDS sample buffer, then electrophoretically transferred to polyvinylidene difluoride (PVDF) membrane. After blocking with 5% non-fat milk in 0.1% Tween-TBS buffer (TBST) for 1 hr, the membrane was incubated with rabbit anti-KV4.1 (1:500, Alomone #APC-119) and anti-KV4.3 (1:500, Alomone #APC-017) primary antibody overnight at 4°C, respectively. Then, a HRP-conjugated anti-rabbit secondary antibody (1:3000, Abcam #ab6721) was incubated at room temperature for 1 hr, and the immunoreactivity was visualized using an enhanced chemiluminescence system (Cell Signaling Technology #6883). The membrane was then stripped and incubated with a mouse anti-β-actin primary antibody (1:3000, Abcam #ab8227) as the internal expression control. The optical densities of target bands were analyzed using the NIH Image J Software.
Statistical Analysis
All data in this study were presented as mean ± SD (standard deviation). A paired t test was used for the experiments of current density and AP frequency using AmmTX3 and BK vs. vehicle control. An unpaired t test was used for the comparison in other experiments. One-way ANOVA was applied to compare the difference in inhibition of TEA-R-IKA (%), and as appropriate, post-hoc analysis with Tukey’s tests were applied to compare the difference between specific groups. In the event, the distribution of mean changes was not normally distributed, the Non-parametric test was used to analyze the data. All statistical analyses were performed using SPSS v26, and the significant differences were considered at P < 0.05.
Results
Identifying activity of Kv4 channels in rat muscle DRG neurons
Two voltage protocols were used to identify A-type K+ currents (Figure 1A). Total K+ currents (IKtotal) were recorded with the protocol holding at −65mV, and a 1-sec conditioning pulse to −100mV was delivered prior to 500ms step depolarizations from −100mV to 60mV with 10mV increments per step. This condition fully activates KV channels. The conditioning pulse permitted ~90% recovery of the total K+ channels that inactivated at −65 mV. The delayed rectifier K+ currents (IKDR) was recorded with the similar condition except 1-sec conditioning pulse to −30mV (Phuket & Covarrubias, 2009). This depolarization voltage was sufficient to inactivate the subthreshold KV channels. Thus, the remaining outward current evoked by subsequent step depolarizations was mostly IKDR and TEA-R-IKA was obtained by subtracting IKDR from IKtotal (TEA-R-IKA = IKtotal− IKDR).
Figure 1. Kv4 channels are involved in AP firing in rat muscle DRG neurons (DiI-labeled).

(A): Kv4 currents in muscle neurons. Protocol 1 was used to record the total TEA-resistant K+ currents (IKtotal), and protocol 2 to record the delayed rectifying current (IKDR), TEA-R-IKA = IKtotal− IKDR. Traces 1 & 2 were TEA-R-IKA of a muscle DRG neuron before and after AmmTX3 (inhibitor to Kv4 channels). Application of 2 μM AmmTX3 for 5 min blocked ~80% of the density of TEA-R-IKA in DRG neurons. *P < 0.05, comparing to untreated neurons (n=8 in each group). (B): In muscle DRG neurons (silent), AP firing was seen when currents were injected. After its application for 5 min, 2 μM of AmmTX3 enhanced the firing frequency of DRG neurons compared with controls using the same current when step currents were injected from −50 pA to 500 pA in 10 pA increments per step, and the protocol was shown with 50 pA increments per step from 150 pA to 200 pA. Averaged data showing that the frequency of AP firing was increased by AmmTx3 with 200 pA injected current. *P < 0.05, comparing to untreated neurons (n=8 in each group).
TEA-R-IKA in Dil-positive DRG neurons (cell dimeter ≤ 35mm) was examined. According to the previous reports (Vacher et al., 2002; Maffie et al., 2013), 2 μM of AmmTX3 toxin (a specific blocker to Kv4 channels) was used to block the activity of Kv4 channels in rat DRG neurons. As shown in Figure 1A, with treatment of 2 μM of AmmTX3 for 5 min, the current density of TEA-R-IKA when depolarizing to 60mV from −100mV (Densitymax), was reduced by 76 ± 6% from 376 ± 131 pA/pF to 87 ± 36 pA/pF (P < 0.05 between two groups, n=8 before and after AmmTX3). Also, ~80% TEA-R-IKA amplitude of currents was blocked. This indicates that TEA-R-IKA mainly reflected the activity of Kv4 channels in muscle DRG neurons, estimated as TEA resistant KV4 currents in this report.
KV4 channels contribute to AP firing in muscle DRG neurons
In addition, with the whole cell current-clamp mode, AP firing of muscle afferent neurons was examined (Figure 1B). Generally, this method is used to assess the excitability of cells, and the cells with increased frequency and/or decreased threshold of AP firing are considered more excitable and sensitive to a stimulus (Barnett & Larkman, 2007). Figure 1B shows that the AP firing frequency in muscle DRG neurons was increased after 2 μM of AmmTX3 application (P < 0.05, before vs. after AmmTX3; n=6). The data suggested that Kv4 channels mainly contributed to a component of TEA-R-IKA in muscle DGR neurons (Kv4 currents) and that a blockade of Kv4 channels enhanced the excitabilities of muscle DRG neurons. In a general consistence with the previous reports (Phuket & Covarrubias, 2009; Yunoki et al., 2014; Zheng et al., 2019), our data demonstrated that the activities of KV4 channels participated in AP generation in muscle sensory neurons in contribution to the neuronal excitabilities.
Decreases in KV4 currents and threshold of AP firing in muscle DRG neurons of occluded rats
Moreover, KV4 currents and threshold of AP firing in muscle afferent neurons of control rats and occluded rats were examined (Figure 2). After 3 days of femoral artery occlusion, KV4 current density in muscle neurons of occluded rats was significantly reduced when depolarizing voltage stepped over −30mV from −100mV (Figure 2A&B). i.e., Densitymax was decreased over 50% (376 ± 131 pA/pF in control and 181 ± 64 pA/pF in occlusion, P < 0.05 between two groups; n=22 in each group). Also, the threshold of AP firing was decreased to 53±40 pA in DRG neurons of occluded rats (n=27) from 98±66 pA in neurons of controls (n=45; P<0.05 between two groups, Figure 2C&D). Our data suggest that inhibition of KV4 channel activities is likely to participate in the signal transits in muscle afferent neurons of occluded rats.
Figure 2. Decreases in Kv4 currents and threshold of AP firing in muscle DRG neurons of occluded rats.

(A): The representatives of TEA resistant Kv4 currents in muscle afferent neurons of both control rats and PAD rats. Protocol 1 was for TEA-resistant IKtotal, and protocol 2 for IKDR. (B): I-V curve of Kv4 current in rat DRG neurons. After 3 days of femoral artery occlusion, the density of Kv4 currents in DRG neurons was decreased when depolarizing voltage stepped over −30mV from −100mV. *P<0.05 between control and occlusion (n=22 in each group) at the same depolarizing voltage. (C): The representatives of AP firing in rat muscle DRG neurons when step currents were injected from −50pA to 500pA in 10pA increments per step. (D): Averaged threshold of AP was decreased in muscle DRG neurons of occluded rats compared with control rats. *P<0.05 between control (n=45) and occlusion (n=27). (E): The density of TEA-R-IKA in muscle DRG neurons of both control rats and occluded rats when 2 μM AmmTX3 was applied. *P<0.05 between 2 μM AmmTX3 treatment and vehicle control in each group; #P <0.05 between control rats (n=8) and occluded rats (n=7). (F): A histogram showing % inhibitory efficiency of 2 μM AmmTX3 on Kv4 in muscle DRG neurons of both the control rats and the occluded rats. *P <0.05, comparing to the control animals.
Furthermore, 2 μM AmmTX3 significantly blocked TEA-R-IKA in both control and occluded rat muscle DRG neurons (Figure 2E&F). After blocking Kv4, Densitymax was very similar in both groups (87 ± 37 pA/pF in control/n=8 and 97 ± 22 pA/pF in occlusion/n=7). However, % inhibitory efficiency in the control group was higher than that in the occlusion group. i.e., 76 ± 6% in control and 54 ± 20 % in occlusion (P < 0.05 between two groups). This indicates that the decrease of TEA-R-IKA current density in muscle DRG neurons induced by femoral occlusion is likely due to the reduction of Kv4 expression, which is linked to the excitabilities of muscle DRG neurons.
BK decreases KV4 currents and increases AP firing in muscle DRG neurons
We further found that 1μM of BK decreased the density of KV4 currents in muscle DRG neurons of both control and occluded rats after it was applied for 5 min (Figure 3A&B). The Densitymax was 251 ± 109 pA/pF in control (n=9) and 112 ± 74 pA/pF in occlusion (n=8; P < 0.05 vs. control). A significant inhibition appeared when depolarizing voltage stepped over −20mV from −100 mV in occluded rats, while it was over −30mV in control rats (Figure 3A&B). Meanwhile, as shown in Figure 3C&D, BK enhanced AP firing frequency in DRG neurons of both control and occlusion groups with injected currents. Note that BK enhanced AP firing frequency to a greater degree in occluded rats when the injected current was below 200pA. These data suggest that BK is likely to inhibit KV4 activities and enhance the excitability of rat muscle afferent neurons, and the alteration of these signaling mechanisms is likely to lead to an increase in sensory inputs in DRG neurons of occluded rats.
Figure 3. The effect of BK on Kv4 currents and AP firing in rat muscle afferent neurons.
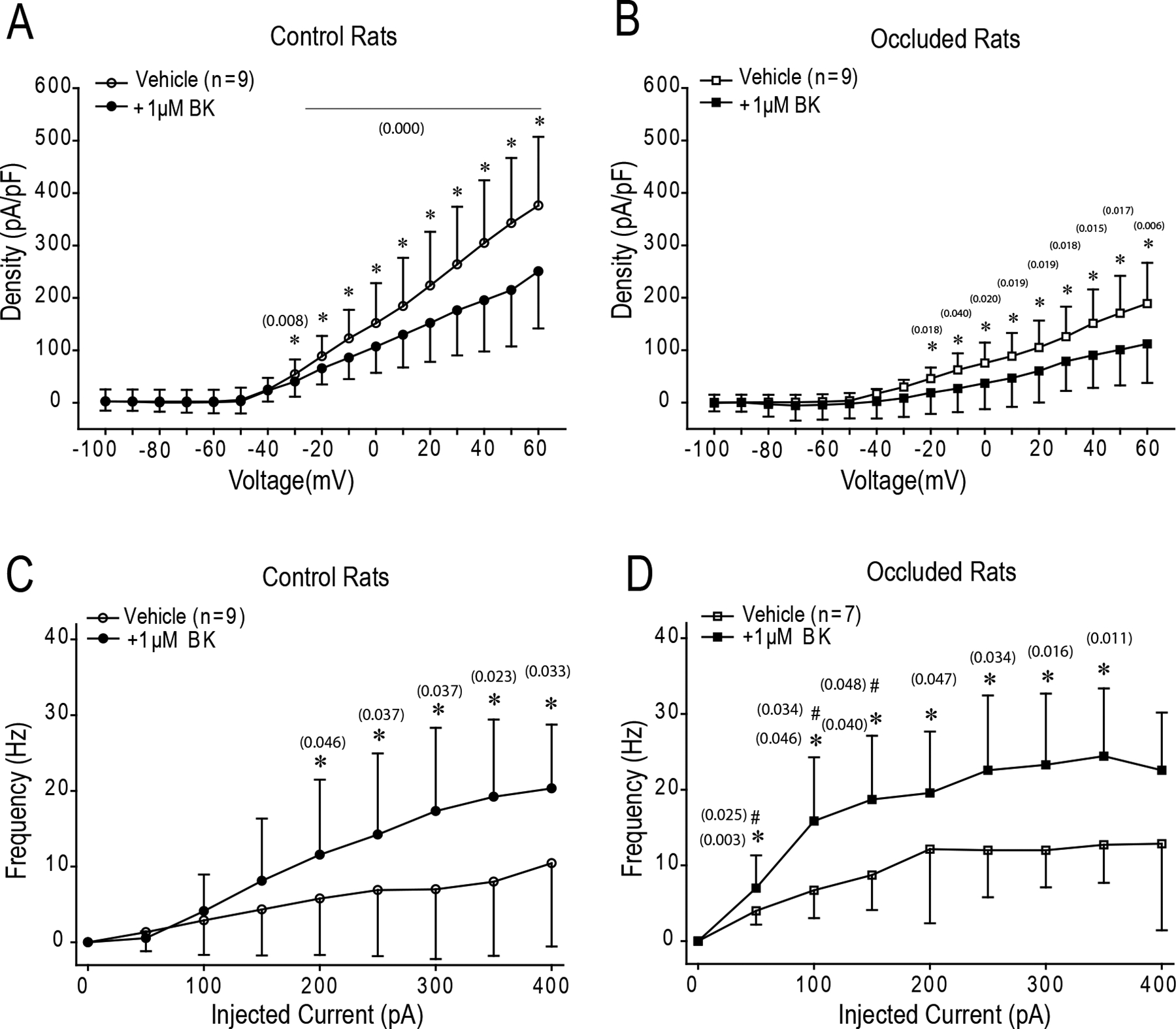
After 1μM BK of application for 5 min, Kv4 currents were reduced in DRG neurons of control rats when depolarizing voltage stepped over 10mV from −100mV (A), and in DRG neurons of occluded rats when depolarizing voltage stepped over −30mV (B) *P<0.05, BK (n=8) vs. control without BK (n=9). (C) & (D): Showing AP firing frequency in rat DRG neurons after current injections. BK enhanced AP firing frequency in DRG neurons of both control and PAD *P<0.05, BK vs. control without BK. # P<0.05 vs. control rats.
Thus, in this report, if BK B1 and/or B2 was involved in the role played by BK in regulating the activities of KV4 currents in muscle DRG neurons was also determined (Figure 4). As shown in Figure 4A, pre-incubated with 1μM R-715 (a specific blocker to B1 receptor) for 20 min, the inhibitory efficiency of 1μM BK on KV4 currents in muscle DRG neurons was decreased to 4.30 ± 10.1% (n=10 in control rats) and 8.07 ± 9.1% (n=7 in occluded rats), respectively. No significant difference was observed in % inhibitory efficiency on KV4 currents as compared with application of 1μM R-715 alone. i.e., 4.36 ± 8.5% in control rats (n=10) and 6.25 ± 12.3% in occluded rats (n=9). Figure 4B also shows that pre-incubated with 200 nM HOE140 (a specific blocker to B2 receptor) for 20 min, 1μM BK was still observed to inhibit KV4 currents in muscle DRG neurons of both control rats and occluded rats. There was no significant difference in % inhibitory efficiency on KV4 currents when compared to the effect of 1μM BK alone in each group. % inhibitory efficiency on KV4 currents was 34.38 ± 10.9% in control rats (n=8; P > 0.05 vs. 1μM BK alone) and 36.71 ± 11.5% in occluded rats (n=7; P > 0.05 vs. 1μM BK alone), respectively. Note that pre-incubation of 1μM R-715 or 200nM HOE 140 had no effects on KV4 currents in both control rats and occluded rats (Figure 4A&B).
Figure 4. BK receptors involved in the effects of BK on Kv4 currents in rat muscle afferent neurons.

All DRG neurons were pre-incubated with 1 μM R-715 or 200 nM HOE 140 for 20 min before recording. (A): The inhibitory efficiency of 1 μM R-715 on TEA-R-IKA in muscle DRG neurons. *P <0.05, respectively comparing with 1 μM BK alone in the control or occluded groups. No significant differences were seen in % inhibitory efficiency between 1 μM R-715 and 1 μM R-715 plus 1 μM BK (P >0.05 for control and occluded groups). (B): The inhibitory efficiency of 200 nM HOE 140 on TEA-R-IKA in muscle DRG neurons. *P <0.05, respectively comparing with 1 μM BK alone and 200 nM HOE 140 plus 1 μM BK in the control or occluded groups.
KV4 subunits KV4.1/KV4.3 expression and contribution to Kv4 currents in muscle DRG neurons
In this study, we specifically distinguished the distribution and potential roles of KV4 subunits KV4.1 and KV4.3 in muscle DRG neurons and further determined their characteristics in muscle DRG neurons of occluded rats and control rats (n=6 in each group). Expression of KV4.3 appeared to be less in DRGs of occluded rats (Figure 5A&B).
Figure 5. Expression of KV4.1 and KV4.3 channels of DRGs and the effects of blocking individual KV4.1 and KV4.3 channel on the activities of KV currents.

(A): Representative bands and (B): averaged data, showing that the total protein levels of KV4.3 in DRGs were less in occluded rats (n=6) than in control rats (n=6). *P<0.05 vs control. KV4.1 expression tended to be lower in DRGs of occluded rats. Dots indicate individual data. Represented lanes on the bands indicate DRGs protein sample from individual rats. β-actin was used as the internal protein control. (C): representative traces and (D): averaged data showing that blocking KV4.3 subunits (500 nM of PaTx1) had a less inhibitory effect on KV4 currents in DRG neurons of occluded rats than its effect in DRG neurons of control rats. The representative traces of TEA-R-IKA in the rat muscle DRG neurons: the black traces and red traces indicate before and after Kv4.1 and Kv4.3 inhibitors were applied for 5 min. *P < 0.05 vs. control rats (n=14 in each group). There were no significant differences observed in inhibitory effects of blocking KV4.1 subunit (2 μM of JZX-XII) on KV4 currents in DRG neurons of control rats (n=8) and occluded rats (n=10; P > 0.05 between control rats and occluded rats).
In addition, the effects of antagonist to KV4.1 and KV4.3 subunits on the activities of KV4 currents were examined (Figure 5C&D). Results further showed that blocking KV4.3 subunits using 500 nM of PaTx1 (a specific antagonist to Kv4.3 channels) had a less inhibitory effect on KV4 currents in DRG neurons of occluded rats than its effect in DRG neurons of control rats. % inhibitory efficiency of PaTX1 was 16 ± 15 % (n = 14) in muscle DRG neurons of occluded rats and 32 ± 18 % (n = 14) in DRG neurons of control rats (P < 0.05 between two groups). There were no significant differences observed in inhibitory effects of blocking KV4.1 subunit using 2 μM of JZX-XII (a specific antagonist to Kv4.1 channels) on KV4 currents in DRG neurons of control rats and occluded rats. % inhibitory efficiency of JZX-XII was 23 ± 9 % in the control group (n = 8) and 25 ± 21 % in the occlusion group (n = 10; P < 0.05 between two groups). This suggests a contribution of the downregulation of Kv4.3 subunit to the decrease of Kv4 current activity in muscle DRG neurons of occluded rats.
Discussion
In the present study, we took advantage of multidisciplinary approaches used in animals to study human PAD. In particular, we examined cellular signals. i.e., Western blot analysis was used to examine the protein levels of afferent nerves’ KV4 channels; and whole cell patch clamp was performed to examine KV4 current activities of muscle DRG neurons. Using a PAD rat model induced by 3-day of formal artery ligation, we demonstrated that 1) femoral artery occlusion decreases amplitude of Kv4 currents in rat DRG neurons; 2) smaller amplitude of Kv4 currents induced by femoral artery occlusion leads to a greater AP frequency and a lower threshold evoking AP in muscle DRG neurons of PAD rats; 3) BK inhibits the activity of Kv4 currents in muscle DRG neurons likely via B1 receptor; and 4) a decrease in expression of KV4.3 subunits is a main contribution to the attenuated activities of Kv4 channels in DRG neurons of PAD rats.
KV channel subtypes in sensory nerves.
A-type KV channels appear in various tissues of mammalian animals and are transmembrane channels for potassium and sensitive to voltage changes in the cell membrane potential with fast inactivation and time-dependent properties. They play a crucial role in returning the depolarized cell to a resting state during AP and are quintessential regulators of neuronal excitability (Barnett & Larkman, 2007). The importance of A-type KV channels (KV1.4, KV3.3, KV3.4, KV4s) in DRG neurons is highlighted (Chien et al., 2007; Cao et al., 2010; Sun et al., 2011; Conner et al., 2016; Viatchenko-Karpinski et al., 2018), suggesting that dysfunction of KV channels is associated with persistent pain sensitization. Particularly, KV4 subtypes contain KV4.1, KV4.2 and KV4.3α subunits, and KChiP and DPPL β subunits, assembled together to generate KV4 channel complex. Upon activated, KV4 channels evoke a rapid transmembrane K+ efflux to generate a transient outward A-type-K+ current, KV4 current, which is resistant to TEA, but sensitive to 4-aminopyridine (4-AP). KV4 currents are also involved in the regulation of rest membrane potential (RMP) and AP firing, thereby influence neuronal excitability and signal integration (Du & Gamper, 2013). KV4.1 and KV4.3 subunits are predominantly distributed in DRG neurons of Aδ and C fibers and especially KV4.3 is engaged in nociceptive sensation (Phuket & Covarrubias, 2009). i.e., nerve injury reduces the expression and distribution of KV4 in DRG neurons and KV4.3 is decreased by 40% (Kim et al., 2002; Park et al., 2003; Chien et al., 2007; Furuta et al., 2012; Conner et al., 2016). There are robust decreases in KV4 currents and expression in rat’s nociceptive neurons after neuropathic pain induced by streptozotocin or oxiliplatin (Grabauskas et al., 2011; Viatchenko-Karpinski et al., 2018). Knockdown of KV4.3 subunit in rat spinal cord with antisense oligonucleotides induces mechanical allodynia and hypersensitivities to vibration (Chien et al., 2007; Conner et al., 2016).
Nonetheless, there are lacking for characteristics of KV4 channels in thin fiber of DRG neurons innervating the hindlimb muscles in PAD rats. Because the exercise pressor reflex is mediated by thin-myelinated group III and unmyelinated group IV fibers arising from contracting skeletal muscle (Kaufman et al., 1984), which mostly correspond to the small to medium size of DRG neurons (Lawson & Waddell, 1991; Basbaum & Woolf, 1999), in the current study, we determined KV4 currents and AP in muscle DRG neurons with diameter ≤ 35 μm. We found that femoral artery occlusion decreases amplitude of KV4 currents and threshold of AP firing in muscle DRG neurons. We further provided evidence for distribution of KV4.1 and KV4.3 subunits in DRG neurons and their contribution to KV4 currents, suggesting the potential cellular mechanisms of KV4.3 channels responsible for the role played by KV4 in PAD.
The role of BK in regulating the activity of KV4 channels in muscle sensory nerves.
BK is an autacoid produced within the interstitium of most tissue, and synthesized from its precursor kininogen via activation of the enzyme, kallikrein (Björkqvist et al., 2013). BK can modulate cell excitability by inhibiting K+ channels through kinin B1 and/or B2 receptor in peripheral neurons (Jones et al., 1995). BK also inhibits activity of K+ channels in rat sensory neurons to induce the nociceptive signals, which is accompanied with the intracellular Ca2+ increase (Lu et al., 2013; Ambrosino et al., 2019).
BK production in skeletal muscle is increased with exercise and BK produced in active muscle (Stebbins et al., 1990; Langberg et al., 2002; Scott et al., 2002), plays a role in engagement of the exercise pressor reflex by stimulating and/or sensitizing muscle afferents responding to muscle contraction, due to kinin B2 receptors (Pan et al., 1993). In PAD rats, B2 receptors are up-regulated in DRGs after femoral occlusion (Lu et al., 2013). Furthermore, HOE-140, a B2 receptor antagonist, can attenuate SNA and BP responses to muscle tendon stretch in PAD rats to a greater extent than in control animals (Lu et al., 2013). A previous report also indicates that the response of group III afferents to contraction is attenuated by HOE-140 (Leal et al., 2013). In a general agreement, through the upregulated B2 receptor in muscle afferents, the increased BK in exercising muscles sensitizes afferents responding to active muscle to a greater degree, and in turn, exaggerates SNA and BP responses during activation of the exercise pressor reflex in PAD.
In the current study, our data showed that BK attenuated KV4 currents and decreased threshold of AP firing in muscle DRG neurons and the effects of BK appeared to be a greater degree in DRG neurons of occluded rats. However, our also data showed that the inhibitory effects of BK on KV4 currents in DRG neurons were decreased by R-715, a B1 receptor antagonist, but not by HOE-140. It should be noted that there are general differences in the results of in vitro and in vivo experiments. Intra-arterial injection of HOE-140 attenuated the exaggerated BP response in occluded rats, but failed to change the effect of BK on KV4 currents in DRG neurons of either control rats or occluded rats. This result may be explained by which there is likely an afferent nerve’s signaling pathway responsible for BK-KV4 activation in regulating of the exercise pressor reflex in PAD. In contrast, R-715 was not observed to attenuate the exaggerated BP response in occluded rats, but it attenuated the effect of BK on KV4 currents in DRG neurons of either control rats or occluded rats. The differences were likely due to that endogenous activation of BK-B1 signal pathway was lacking in vivo whole animal experiment. However, in our current patch-clamp experiments, application of BK onto muscle DRG neurons could directly stimulate B1 signal pathways linked to the activities of KV4 channels.
Study Limitation.
In the present study, our focus was to determine the effects of BK on the activities of Kv4 signaling pathways in muscle DRG neurons of PAD rats at a cellular level. A major limitation is that we did not investigate the effects of blocking and stimulating Kv4 channels on the SNA and BP responses to muscle contraction in PAD rats using the whole animal preparations. Thus, care should be taken when extrapolating the findings of the current study to the exercise pressor reflex. Another limitation of this study is that only male animals were included because sex discrepancies were beyond the scope of our current study. To address those remaining issues requires additional studies.
Perspectives.
There is poorly known about the effects of KV4 in muscle afferent nerves on autonomic functions in PAD. To determine the role of BK-KV4 pathways in regulating the exercise pressor reflex in the future, it would be considered to examine 1) the resting levels of interstitial BK in the hindlimb muscles of PAD rats and its response contracting muscles; and 2) the effects of blocking and stimulating KV4 channels on the SNA and BP responses to muscle contraction and to B1/B2 activation. These results may provide insights regarding the role of K+ channels in muscle afferent nerves in adjusting cardiovascular control during exercise in PAD. In addition, Kv4 channels are likely to play a role in regulating the activities of DRG neurons in other cardiovascular diseases, such as heart failure and type-II diabetes since an augmented pressor response to exercise is observed in experimental models of these diseases (Smith et al., 2005; Grotle & Stone, 2019).
Conclusions.
In a rat model of PAD, femoral artery occlusion induced-limb ischemia decreases the protein expression levels of KV4 channels subunit KV4.3 in DRGs and inhibits the activities of KV4 in muscle DRG neurons. Ischemia induced-metabolite BK also participates in the inhibitory effects of femoral artery occlusion on the activities of KV4. Overall, data suggest that attenuation of KV4 channels in muscle afferent nerves is likely to lead to the exaggerated exercise pressor reflex observed in PAD.
Supplementary Material
Key Points.
During exercise, bradykinin (BK), a muscle metabolite in ischemic muscles exaggerates autonomic responses to activation of muscle afferent nerves in peripheral artery disease (PAD).
We examined if BK inhibits activity of KV4 channels in muscle afferent neurons of PAD rats induced by femoral artery occlusion.
We demonstrated that 1) femoral occlusion attenuates KV4 currents in dorsal root ganglion (DRG) neurons innervating the hindlimb muscles and decreases threshold of action potential firing; 2) BK has a greater inhibitory effect on KV4 currents in muscle DRG neurons of PAD rats; and 3) expression of KV4.3 is downregulated in DRGs of PAD rats and inhibition of KV4.3 significantly decreases activity of KV4 currents in muscle DRG neurons.
Femoral artery occlusion induced-limb ischemia and/or ischemia induced-metabolites (i.e., BK) inhibit activity of KV4 channels in muscle afferent neurons and this is likely involved in the exaggerated exercise pressor reflex in PAD.
Acknowledgements
The authors greatly thank Chunying Yang for her excellent technical assistance.
Grant support
This study was supported by NIH P01 HL134609 and R01 HL141198.
Biography

Qin Li received his PhD from Southern Medical University of China in 2010. Dr. Li is currently a research associate at the Heart and Vascular Institute of Penn State college of Medicine at Hershey. His long-term research goal is to examine the channelopathies in the cardiovascular disease, especially the neuro control of ion channels in the circulation during exercise, to improve the interventions on the abnormal circulation responses to exercise in cardiovascular diseases.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Ambrosino P, Soldovieri MV, Di Zazzo E, Paventi G, Iannotti FA, Mosca I, Miceli F, Franco C, Canzoniero LMT & Taglialatela M. (2019). Activation of Kv7 Potassium Channels Inhibits Intracellular Ca(2+) Increases Triggered By TRPV1-Mediated Pain-Inducing Stimuli in F11 Immortalized Sensory Neurons. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand SS, Caron F, Eikelboom JW, Bosch J, Dyal L, Aboyans V, Abola MT, Branch KRH, Keltai K, Bhatt DL, Verhamme P, Fox KAA, Cook-Bruns N, Lanius V, Connolly SJ & Yusuf S. (2018). Major Adverse Limb Events and Mortality in Patients With Peripheral Artery Disease: The COMPASS Trial. J Am Coll Cardiol 71, 2306–2315. [DOI] [PubMed] [Google Scholar]
- Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S & Catalano M. (1999). The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50, 361–374. [DOI] [PubMed] [Google Scholar]
- Barnett MW & Larkman PM. (2007). The action potential. Pract Neurol 7, 192–197. [PubMed] [Google Scholar]
- Basbaum AI & Woolf CJ. (1999). Pain. Curr Biol 9, R429–431. [DOI] [PubMed] [Google Scholar]
- Bauersachs R, Zeymer U, Brière JB, Marre C, Bowrin K & Huelsebeck M. (2019). Burden of Coronary Artery Disease and Peripheral Artery Disease: A Literature Review. Cardiovasc Ther 2019, 8295054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkqvist J, Jämsä A & Renné T. (2013). Plasma kallikrein: the bradykinin-producing enzyme. Thrombosis and haemostasis 110, 399–407. [DOI] [PubMed] [Google Scholar]
- Brendle DC, Joseph LJ, Corretti MC, Gardner AW & Katzel LI. (2001). Effects of exercise rehabilitation on endothelial reactivity in older patients with peripheral arterial disease. The American journal of cardiology 87, 324–329. [DOI] [PubMed] [Google Scholar]
- Cao XH, Byun HS, Chen SR, Cai YQ & Pan HL. (2010). Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J Neurochem 114, 1460–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehuen M, Cucato GG, Carvalho CRF, Ritti-Dias RM, Wolosker N, Leicht AS & Forjaz CLM. (2017). Walking training at the heart rate of pain threshold improves cardiovascular function and autonomic regulation in intermittent claudication: A randomized controlled trial. Journal of science and medicine in sport 20, 886–892. [DOI] [PubMed] [Google Scholar]
- Chien LY, Cheng JK, Chu D, Cheng CF & Tsaur ML. (2007). Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci 27, 9855–9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett GP, Sobel M, Jackson MR, Lip GYH, Tangelder M & Verhaeghe R. (2004). Antithrombotic Therapy in Peripheral Arterial Occlusive Disease. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126, 609S–626S. [DOI] [PubMed] [Google Scholar]
- Conner LB, Alvarez P, Bogen O & Levine JD. (2016). Role of Kv4.3 in Vibration-Induced Muscle Pain in the Rat. J Pain 17, 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH, Hilton SM & Perez-Gonzalez JF. (1971). The reflex nature of the pressor response to muscular exercise. J Physiol 215, 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criqui MH & Aboyans V. (2015). Epidemiology of peripheral artery disease. Circ Res 116, 1509–1526. [DOI] [PubMed] [Google Scholar]
- de L II, Hoeks SE, van Gestel YR, Bax JJ, Klein J, van Domburg RT & Poldermans D. (2008). Usefulness of hypertensive blood pressure response during a single-stage exercise test to predict long-term outcome in patients with peripheral arterial disease. The American journal of cardiology 102, 921–926. [DOI] [PubMed] [Google Scholar]
- Drew RC, Bell MP & White MJ. (2008). Modulation of spontaneous baroreflex control of heart rate and indexes of vagal tone by passive calf muscle stretch during graded metaboreflex activation in humans. Journal of applied physiology (Bethesda, Md : 1985) 104, 716–723. [DOI] [PubMed] [Google Scholar]
- Drew RC, Muller MD, Blaha CA, Mast JL, Heffernan MJ, Estep LE, Cui J, Reed AB & Sinoway LI. (2013). Renal vasoconstriction is augmented during exercise in patients with peripheral arterial disease. Physiol Rep 1: e00154, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X & Gamper N. (2013). Potassium channels in peripheral pain pathways: expression, function and therapeutic potential. Curr Neuropharmacol 11, 621–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK & Criqui MH. (2017). Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol 14, 156–170. [DOI] [PubMed] [Google Scholar]
- Furuta S, Watanabe L, Doi S, Horiuchi H, Matsumoto K, Kuzumaki N, Suzuki T & Narita M. (2012). Subdiaphragmatic vagotomy increases the sensitivity of lumbar Adelta primary afferent neurons along with voltage-dependent potassium channels in rats. Synapse 66, 95–105. [DOI] [PubMed] [Google Scholar]
- Grabauskas G, Heldsinger A, Wu X, Xu D, Zhou S & Owyang C. (2011). Diabetic visceral hypersensitivity is associated with activation of mitogen-activated kinase in rat dorsal root ganglia. Diabetes 60, 1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotle AK & Stone AJ. (2019). Exaggerated exercise pressor reflex in type 2 diabetes: Potential role of oxidative stress. Autonomic neuroscience : basic & clinical 222, 102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburg NM & Balady GJ. (2011). Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 123, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr., White CJ, White J, White RA, Antman EM, Smith SC Jr., Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL & Riegel B. (2006). ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol 17, 1383–1397; quiz 1398. [DOI] [PubMed] [Google Scholar]
- Jones S, Brown DA, Milligan G, Willer E, Buckley NJ & Caulfield MP. (1995). Bradykinin excites rat sympathetic neurons by inhibition of M current through a mechanism involving B2 receptors and G alpha q/11. Neuron 14, 399–405. [DOI] [PubMed] [Google Scholar]
- Kaufman MP & Forster HV. (1996). Reflexes controlling circulatory, ventilatory and airway responses to exercise. Chapter 10. In Handbook of Physiology - Section 12, Exercise: Regulation and Integration of Multiple Systems, ed. Rowell LB & Shepherd JT, pp. 381–447. Oxford University Press, New York. [Google Scholar]
- Kaufman MP, Rybicki KJ, Waldrop TG & Ordway GA. (1984). Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol Respir Environ Exerc Physiol 57, 644–650. [DOI] [PubMed] [Google Scholar]
- Kim DS, Choi JO, Rim HD & Cho HJ. (2002). Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Brain Res Mol Brain Res 105, 146–152. [DOI] [PubMed] [Google Scholar]
- Kitrou P, Katsanos K, Karnabatidis D, Reppas L, Brountzos E & Spiliopoulos S. (2017). Current Evidence and Future Perspectives on Anti-platelet and Statin Pharmacotherapy for Patients with Symptomatic Peripheral Arterial Disease. Curr Vasc Pharmacol 15, 430–445. [DOI] [PubMed] [Google Scholar]
- Langberg H, Bjorn C, Boushel R, Hellsten Y & Kjaer M. (2002). Exercise-induced increase in interstitial bradykinin and adenosine concentrations in skeletal muscle and peritendinous tissue in humans. J Physiol (London) 542, 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN & Waddell PJ. (1991). Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol 435, 41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal AK, Stone AJ, Yamauchi K, McCord JL & Kaufman MP. (2013). Blockade of B2 receptors attenuates the responses of group III afferents to static contraction. Neurosci Lett 555, 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GD, Gona P, Larson MG, Plehn JF, Benjamin EJ, O’Donnell CJ, Levy D, Vasan RS & Wang TJ. (2008). Exercise blood pressure and the risk of incident cardiovascular disease (from the Framingham Heart Study). The American journal of cardiology 101, 1614–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J & Xing J. (2012). Muscle afferent receptors engaged in augmented sympathetic responsiveness in peripheral artery disease. Front Physiol 3, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Qin L & Li J. (2020). Enhancement by TNF-alpha of TTX-resistant NaV current in muscle sensory neurons after femoral artery occlusion. Am J Physiol Regul Integr Comp Physiol 318, R772–R780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentsen E (1972). Systemic arterial blood pressure during exercise in patients with atherosclerosis obliterans of the lower limbs. Circulation 46, 257–263. [DOI] [PubMed] [Google Scholar]
- Lu J, Xing J & Li J. (2013). Bradykinin B2 receptor contributes to the exaggerated muscle mechanoreflex in rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol 304, H1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffie JK, Dvoretskova E, Bougis PE, Martin-Eauclaire MF & Rudy B. (2013). Dipeptidyl-peptidase-like-proteins confer high sensitivity to the scorpion toxin AmmTX3 to Kv4-mediated A-type K+ channels. J Physiol 591, 2419–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM. (2018). Exercise Rehabilitation for Peripheral Artery Disease: A REVIEW. J Cardiopulm Rehabil Prev 38, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Luck JC, Kim DJ, Leuenberger UA, Aziz F, Radtka JF 3rd, Sinoway LI & Muller MD. (2018). Peripheral revascularization attenuates the exercise pressor reflex and increases coronary exercise hyperemia in peripheral arterial disease. J Appl Physiol (1985) 125, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP & Iwamoto GA. (1983). The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45, 229–242. [DOI] [PubMed] [Google Scholar]
- Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB & Sinoway LI. (2012). Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590, 6237–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouriel K (2001). Peripheral arterial disease. Lancet 358, 1257–1264. [DOI] [PubMed] [Google Scholar]
- Pan HL, Stebbins CL & Longhurst JC. (1993). Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol (1985) 75, 2061–2068. [DOI] [PubMed] [Google Scholar]
- Park SY, Choi JY, Kim RU, Lee YS, Cho HJ & Kim DS. (2003). Downregulation of voltage-gated potassium channel alpha gene expression by axotomy and neurotrophins in rat dorsal root ganglia. Mol Cells 16, 256–259. [PubMed] [Google Scholar]
- Phuket TR & Covarrubias M. (2009). Kv4 Channels Underlie the Subthreshold-Operating A-type K-current in Nociceptive Dorsal Root Ganglion Neurons. Front Mol Neurosci 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A & Coats AJ. (1999). A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am Heart J 137, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ & Piepoli MF. (2001). Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation 104, 2324–2230. [DOI] [PubMed] [Google Scholar]
- Qian AH, Liu XQ, Yao WY, Wang HY, Sun J, Zhou L & Yuan YZ. (2009). Voltage-gated potassium channels in IB4-positive colonic sensory neurons mediate visceral hypersensitivity in the rat. Am J Gastroenterol 104, 2014–2027. [DOI] [PubMed] [Google Scholar]
- Ritti-Dias RM, Meneses AL, Parker DE, Montgomery PS, Khurana A & Gardner AW. (2011). Cardiovascular responses to walking in patients with peripheral artery disease. Med Sci Sports Exerc 43, 2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AC, Wensel R, Davos CH, Kemp M, Kaczmarek A, Hooper J, Coats AJ & Piepoli MF. (2002). Chemical mediators of the muscle ergoreflex in chronic heart failure: a putative role for prostaglandins in reflex ventilatory control. Circulation 106, 214–220. [DOI] [PubMed] [Google Scholar]
- Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R & Zelis R. (1989). Muscle Acidosis during Static Exercise Is Associated with Calf Vasoconstriction. J Appl Physiol 66, 429–436. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Naseem RH & Garry MG. (2005). Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation 112, 2293–2300. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Carretero OA, Mindroiu T & Longhurst JC. (1990). Bradykinin release from contracting skeletal muscle of the cat. J Appl Physiol 69, 1225–1230. [DOI] [PubMed] [Google Scholar]
- Stone AJ & Kaufman MP. (2015). The exercise pressor reflex and peripheral artery disease. Auton Neurosci 188, 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Maffie JK, Lin L, Petralia RS, Rudy B & Hoffman DA. (2011). DPP6 establishes the A-type K(+) current gradient critical for the regulation of dendritic excitability in CA1 hippocampal neurons. Neuron 71, 1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanassoulis G, Lyass A, Benjamin EJ, Larson MG, Vita JA, Levy D, Hamburg NM, Widlansky ME, O’Donnell CJ, Mitchell GF & Vasan RS. (2012). Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the Framingham Heart Study. Circulation 125, 2836–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher H, Alami M, Crest M, Possani LD, Bougis PE & Martin-Eauclaire MF. (2002). Expanding the scorpion toxin alpha-KTX 15 family with AmmTX3 from Androctonus mauretanicus. European journal of biochemistry 269, 6037–6041. [DOI] [PubMed] [Google Scholar]
- Viatchenko-Karpinski V, Ling J & Gu JG. (2018). Down-regulation of Kv4.3 channels and a-type K(+) currents in V2 trigeminal ganglion neurons of rats following oxaliplatin treatment. Mol Pain 14, 1744806917750995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Bertocci L, Pryor S & Nunnally R. (1988). Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82, 1301–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop TG, Eldridge FL, Iwamoto GA & Mitchell JH. (1996). Central neural control of respiration and circulation during exercise. Chapter 9. In Handbook of Physiology - Section 12, Exercise: Regulation and Integration of Multiple Systems, ed. Rowell LB & Shepherd JT, pp. 333–380. Oxford University Press, New York. [Google Scholar]
- Waters RE, Terjung RL, Peters KG & Annex BH. (2004). Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol (1985) 97, 773–780. [DOI] [PubMed] [Google Scholar]
- Weiss SA, Blumenthal RS, Sharrett AR, Redberg RF & Mora S. (2010). Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation 121, 2109–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Lu J & Li J. (2012). Acid-sensing ion channel subtype 3 function and immunolabelling increases in skeletal muscle sensory neurons following femoral artery occlusion. J Physiol 590, 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunoki T, Takimoto K, Kita K, Funahashi Y, Takahashi R, Matsuyoshi H, Naito S & Yoshimura N. (2014). Differential contribution of Kv4-containing channels to A-type, voltage-gated potassium currents in somatic and visceral dorsal root ganglion neurons. J Neurophysiol 112, 2492–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Liu P, Bai L, Trimmer JS, Bean BP & Ginty DD. (2019). Deep Sequencing of Somatosensory Neurons Reveals Molecular Determinants of Intrinsic Physiological Properties. Neuron 103, 598–616.e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


