ABSTRACT
Antitoxin is currently the only approved therapy for botulinum intoxications. The efficacy of antitoxin preparations is evaluated in animals. However, while in practice antitoxin is administered to patients only after symptom onset, in most animal studies, it is tested in relation to time postintoxication. This may be attributed to difficulties in quantitating early botulism symptoms in animals. In the current study, a novel system based on high-resolution monitoring of mouse activity on a running wheel was developed to allow evaluation of postsymptom antitoxin efficacy. The system enables automatic and remote monitoring of 48 mice simultaneously. Based on the nocturnal activity patterns of individual naive mice, two criteria were defined as the onset of symptoms. Postsymptom treatment with a human-normalized dose of antitoxin was fully protective in mice exposed to 4 50% lethal doses (LD50s) of botulinum neurotoxin serotype A (BoNT/A) and BoNT/B. Moreover, for the first time, a high protection rate was obtained in mice treated postsymptomatically, following a challenge with BoNT/E, the fastest-acting BoNT. The running wheel system was further modified to develop a mouse model for the evaluation of next-generation therapeutics for progressive botulism at time points where antitoxin is not effective. Exposure of mice to 0.3 LD50 of BoNT/A resulted in long-lasting paralysis and a reduction in running activity for 16 to 18 days. Antitoxin treatment was no longer effective when administered 72 h postintoxication, defining the time window to evaluate next-generation therapeutics. Altogether, the running wheel systems presented herein offer quantitative means to evaluate the efficacy of current and future antibotulinum drugs.
KEYWORDS: animal models, antitoxin, botulinum
INTRODUCTION
Botulinum neurotoxins (BoNTs), produced by the anaerobic bacterium Clostridium botulinum, are the most potent toxins known in nature, with an estimated human 50% lethal dose (HLD50) of 1 ng/kg of body weight (1). There are eight different BoNT serotypes, of which types A (BoNT/A), B, E, and rarely F are responsible for most cases of human botulism (2). BoNTs block acetylcholine transmission across neuromuscular junctions and cause peripheral flaccid muscle paralysis that eventually may end in respiratory failure and possible death if not treated (3, 4). Widespread outbreaks of foodborne botulism might involve dozens of infected people (5–7). In addition, due to their extreme potency, BoNTs are classified as category A biothreat agents (8).
Postsymptomatic administration of botulinum antitoxin is currently the only approved therapy for botulism (9). In severe cases, intensive supportive care by means of mechanical ventilation is required, which may pose a significant concern for health authorities. Antitoxin consists of polyclonal antibodies purified from vaccinated horses. These preparations are labeled to be injected only after manifestation of clear botulism symptoms due to safety issues concerning the high load of foreign protein administered. In the United States, a human-derived antitoxin with a much safer profile is available for infant botulism (Baby BIG) (10). In a paradoxical manner, the time window for antitoxin treatment is limited not only by the earliest time but also by the latest time it can be administered. This is due to the mechanism of neutralization, in which antibodies encounter only BoNTs found in circulation and cannot follow the toxin into nerve cells (11). Notably, while antitoxin is administered to patients only after symptom onset, its efficacy evaluation in animal studies has been mostly related to time postintoxication regardless of symptoms (12–18).
The main reason for testing antitoxin efficacy in a time-dependent manner rather than a symptom-dependent manner is the difficulty of identifying and quantifying relevant clinical symptoms of botulism in animals at early stages of the disease. Human patients report early botulism symptoms such as blurred vision, dry mouth, and diplopia or just odd feelings long before the appearance of observed signs, such as ptosis and difficulty speaking (19). Animals, especially rodents, however, do not present such facial symptoms and obviously cannot report their situation. Furthermore, in the few studies where attempts have been made to administer antitoxin after symptom onset, the symptoms were based on subjective observations (20, 49), and therefore, their onset may vary substantially among different studies.
Recently, we established a novel quantitative and objective rabbit model for the evaluation of postsymptom antitoxin efficacy (PSAE). This model relies on spirometry detection of early respiratory symptoms, which are also the most predominant symptom in human botulism and are the leading cause of mortality (21, 22). The profile of antitoxin efficacy observed in the rabbit spirometry model was highly correlated with that observed in humans (23). Other reported quantitative botulinum models include the digit abduction score (DAS) (24), rotarod (25), and complex wheel (26). However, these models are labor-intensive, intrusive, and allow only low-resolution monitoring. To the best of our knowledge, these models have not been applied to study postsymptom antitoxin efficacy.
The aim of the current study was to develop a mouse model for objective and quantitative evaluation of the efficacy of antibotulism therapies using a voluntary running wheel system. The use of the voluntary running wheel to assess physical activity in rodents is common, and the system is used to monitor behavioral and physical changes that yield important information regarding drug treatment efficacy, disease state, etc. (27, 28). We hypothesized that the voluntary nature of the running activity may serve as a predictive tool for early detection of botulism that will precede visual symptoms, such as difficulty breathing. The experimental system developed in the current study relies on continuous high-resolution monitoring of individual mouse motor activity. The system was set to allow real-time detection of objective and quantitative symptoms from numerous animals in parallel and from remote locations without disturbing the natural behavior of the mice. The system was used to demonstrate the high efficacy of postsymptomatic antitoxin treatment. Additionally, the running wheel system was further modified to allow evaluation of next-generation therapeutics for late stages of botulism, where antitoxin is no longer effective. In this model, animals are exposed to a sublethal dose of BoNTs, simulating the long-lasting spontaneous recovery that characterizes human botulism. Taken together, the novel models developed in this study provide tools for evaluating current and future therapeutics for the different stages of botulism.
RESULTS
Development of quantitative tools for real-time detection of botulism symptoms.
The aim of the first part of the current study was to develop an experimental system for evaluation of postsymptom antitoxin efficacy in mice challenged with a lethal dose of BoNTs. As BoNTs target the neuromuscular junction and inhibit motor function, we chose to use mouse activity on a running wheel as the experimental system for detection of botulism symptoms.
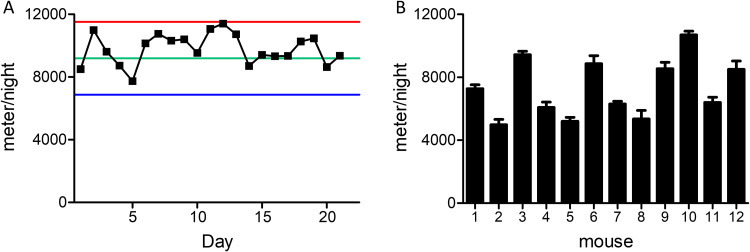
Figure 1 presents basic characteristics of mouse activity on the running wheel. Mice tended to run an individually characteristic distance per night that was kept constant for at least 3 weeks (Fig. 1A) (averaged relative standard deviation [SD] of 12%). Individual mouse activity was kept constant and stable, despite variance in the nocturnal running distances between different mice (Fig. 1B).
FIG 1.
Consistency of nocturnal mouse activity on the running wheel. (A) Representative activity profile of a single mouse over 21 days. (The average running distance per night was 9.7 km, with an RSD of 10%.) Green line, average distance per night; red line, average distance per night plus 2 standard deviations (SDs); blue line, average distance per night −2 SD. (B) Averaged nocturnal running distance of 12 mice over 10 nights. Error bars represent the standard error (SE) of the mean.
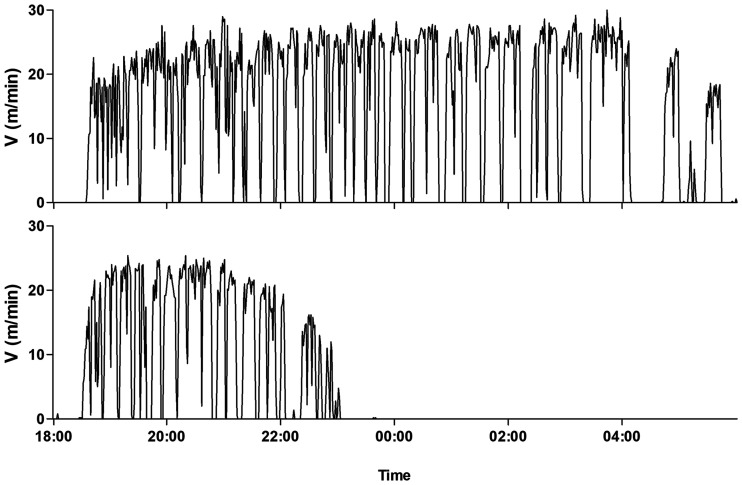
Following administration of a lethal dose of BoNT to mice, depending on toxin serotype and dose, death can occur within 24 to 48 h of exposure. Qualitative botulinum symptoms, such as the wasp waist, begin at approximately 7.5 and 9.5 h from exposure to 4 intramuscular (i.m.) LD50s of BoNT/A and -B, respectively (29). For mice exposed to BoNT/E, wasp waist symptoms are observed in only some of the animals. The symptom begins 5 to 7 h after intoxication, and postsymptom antitoxin treatment fails to protect the animals, as death occurs after 10 h. Therefore, to develop a quantitative tool to study postsymptom antitoxin efficacy in animals exposed to lethal doses of BoNTs, high-resolution monitoring of activity is required. Figure 2 shows the nocturnal running activity of a mouse before (upper panel) and after (lower panel) intoxication with 4 i.m. LD50 of BoNT/A at high resolution (1-min resolution). Following toxin exposure, a latent period was observed at the beginning of the night until 6 h from toxin exposure, where the mouse activity was normal. At approximately 6 h after exposure, the toxin began to affect mouse activity, as running speed gradually decelerated. Although running activity was still observed, the mouse could not maintain its characteristic running pace (>20 m/min). This gradual decrease in activity lasted about an hour until complete halt. It should be noted that injection of saline into the gastrocnemius muscle did not affect mouse running.
FIG 2.
Representative high-resolution monitoring of mouse running activity before (upper panel) and after (lower panel) exposure to 4 i.m. LD50s of BoNT/A. The exposure time was 14:30.
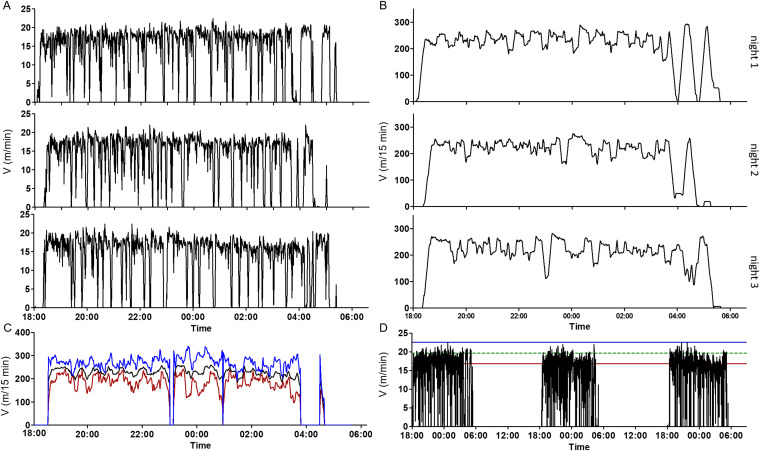
The high-resolution activity profile presented in Fig. 2 allowed us to evaluate the time of symptom onset only in retrospect. We wished to develop an online system that would allow real-time symptom detection in multiple parallel cages and would be defined by clear statistically based quantitative measures of deviation from individual normal mouse activity. To this end, the normal activity of naive mice was individually characterized at high resolution. Figure 3A shows the normal running activity of a single representative mouse over three consecutive nights. A general common pattern of nocturnal activity was presented. The mice tended to run at a constant pace throughout most of each night. Every 10 to 40 min, the activity stopped for a few minutes of rest and was restored afterwards to the typical mouse-specific pace. Occasionally, longer breaks in activity were observed (for example, at 05:00 on night 2), which appeared mostly in the second half of the night. Based on the individual normal activity of each mouse, a model of the predicted activity at any given time point throughout the night was established. First, the high-resolution activity data, which are of a sawtooth pattern, were transformed into a smoother parameter in which every minute value represents the total distance the mouse ran over the former 15 min (Fig. 3B). The predicted activity of the mouse at any given moment was then calculated by averaging the distance the mouse ran over the former 15 min for at least three consecutive nights. This average with its statistical confidence interval (±2 standard deviations) represented the predicted boundaries of the normal activity for any time point (Fig. 3C).
FIG 3.
A prediction model of individual mouse running activity. (A) Running speed of a naive mouse over three consecutive nights at a 1-min resolution. (B) Accumulating 15 min of running distance. Data were calculated for each 1-min interval. (C) A model of the predicted activity at any given time point throughout the night based on the three previous nights. Black line, average running distance over the former 15 min; blue and red lines, upper and lower limits of the predicted activity, respectively. (D) The running pace of an individual naive mouse is between the borders of the Vmax parameter. Black line, running pace of a naive mouse over 3 nights; green line, Vmax; blue and red lines, upper and lower limits of the Vmax parameter. A deviation of 15 min from the lower limits of the predicted activity model or the Vmax parameter was defined as the onset of botulism symptoms.
At the time points at which mice presented long breaks in activity in at least one of the three reference nights (relative standard deviation [RSD] of >30% between the 3 nights), a predicted value was not generated by the model. To still be able to detect symptoms of botulism at these points, a complementary parameter was defined, designated Vmax. This parameter relied on two fundamental observations collected from dozens of healthy mice. First, each mouse ran at a typical relatively constant pace. Second, in a healthy mouse, fatigue was reflected by a complete stop in running activity rather than in attenuation from its typical running pace. To calculate the Vmax parameter, the activity hours, from at least 3 nights, were divided into intervals of 15 min. For each interval in which the mouse was active, the highest speed (per minute) was recorded. Vmax was defined as the average of the highest speeds recorded over all active 15-min intervals. According to the Vmax parameter, a symptom was defined by two criteria: (i) the mouse was running, and (ii) a reduced running pace (at least 2 SDs below its characteristic average maximal velocity) was observed.
Evaluation of the system with healthy mice showed that short deviations from the parameters could occur spontaneously for short periods—more often in the first hour of darkness. Hence, to avoid false-positive events, we defined the onset of botulism symptoms as deviation in either of these parameters that persisted for at least 15 min.
To test the compatibility of the developed system as a means to detect early symptoms of botulism, mice were exposed i.m. to lethal doses of either BoNT/A, BoNT/B, or BoNT/E, and the onset of botulism symptoms was determined (Table 1). The times to symptom (TTS) were 6.7 ± 0.9 and 8.3 ± 1.3 h postexposure to 4 i.m. LD50s of BoNT/A and BoNT/B, respectively. Symptom onsets in mice exposed to BoNT/E were detected at 4.7 ± 1.5 and 3.5 ± 1.3 h postexposure to 2 and 4 i.m. LD50s of BoNT/E, respectively. The order of symptom manifestation between the three BoNT serotypes was fully compatible with that reported in human cases of botulism, where serotype E is the fastest to affect and serotype B is the slowest-acting toxin of these three human clinically relevant serotypes (30). Detection of symptom manifestation using the running wheel system significantly preceded symptom onset, as reflected by the wasp waist symptom. The serotypic orders of symptom onset were identical in both systems, confirming that the new monitoring system is validated for the detection of botulinum symptoms and for the evaluation of postsymptom antitoxin efficacy.
TABLE 1.
Times to symptom detection for BoNT/A, BoNT/B, and BoNT/E using the running wheel system
| BoNT serotype | Toxin dose (i.m. LD50) | Avg TTS ± SD (h) | No. of mice | No. of expts |
|---|---|---|---|---|
| A | 4 | 6.7 ± 0.9 | 59 | 5 |
| B | 4 | 8.3 ± 1.3 | 21 | 2 |
| E | 4 | 3.5 ± 1.3 | 32 | 2 |
| 2 | 4.7 ± 1.5 | 44 | 3 |
Evaluation of postsymptom antitoxin efficacy in mice exposed to a lethal dose of BoNT/A, BoNT/B, or BoNT/E.
To evaluate the efficacy of antitoxin following botulism symptom onset by the running wheel system, mice were exposed to 4 i.m. LD50s of botulinum neurotoxin A, B, or E, and running activity was monitored. Immediately following symptom manifestation, mice were individually treated with a body-weight-normalized human dose of botulinum antitoxin.
Postsymptom antitoxin treatment was fully protective in mice exposed to lethal doses of type A and type B botulinum toxins (Table 2). Moreover, a high survival rate of 88% was also achieved in mice exposed to 4 i.m. LD50s of BoNT/E and treated with antitoxin after symptom onset. The survival rate was higher (96%) in mice exposed to a reduced toxin dose of 2 i.m. LD50s BoNT/E. No survival was observed in any untreated control group (n = 4 per group).
TABLE 2.
Evaluation of postsymptom antitoxin efficacy using the running wheel system
| BoNT serotype | Toxin dose (i.m. LD50) | No. of mice surviving/tested (%) | Avg TTS ± SD (h) |
|---|---|---|---|
| A | 4 | 11/11 (100) | 6.8 ± 0.8 |
| B | 4 | 13/13 (100) | 8.4 ± 1.5 |
| E | 4 | 15/17 (88) | 3.5 ± 1.2 |
| 2 | 26/27 (96) | 5.3 ± 1.5 |
A mouse model for evaluation of next-generation antibotulinum therapeutics.
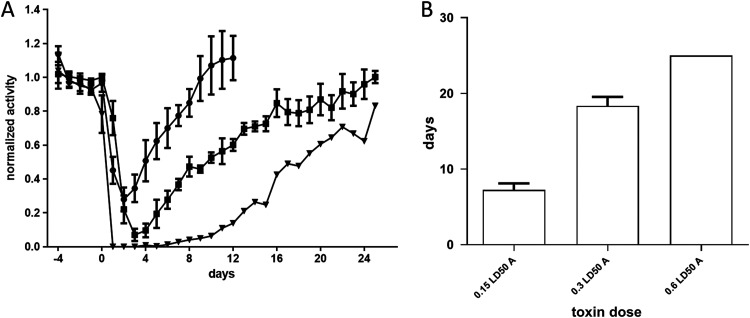
The efficacy of antitoxin therapy is limited to a narrow time window postsymptom manifestation, in which botulinum neurotoxins are still traveling in the bloodstream (11). Following entrance of the toxin into target neural cells, immunoglobulin therapy is no longer effective, and botulism patients require mechanical ventilation—often for a prolonged period (31). Next-generation therapeutics for botulism are mainly intended to address this chronic phase by restoring neuronal function via intracellular neutralization of BoNT’s light chain (32, 33). Currently, no animal model is available for evaluation of next-generation therapeutics that are effective at the chronic phase of botulism. The time interval between symptom onset and death in mice exposed to a lethal dose of BoNT is too short to enable potency evaluation of drugs intended to be effective at the chronic phase. Mechanical ventilation that may extend survival is highly challenging to conduct in animals, and the number of animals that can be ventilated is extremely limited. However, as shown in Fig. 4 and previously demonstrated by others (26, 34), sublethal doses of BoNTs induce long-lasting paralysis followed by spontaneous recovery in mice. This pattern may be utilized as a means to simulate the chronic phase of botulinum intoxication and to enable evaluating new therapeutics at times where antitoxins are no longer effective.
FIG 4.
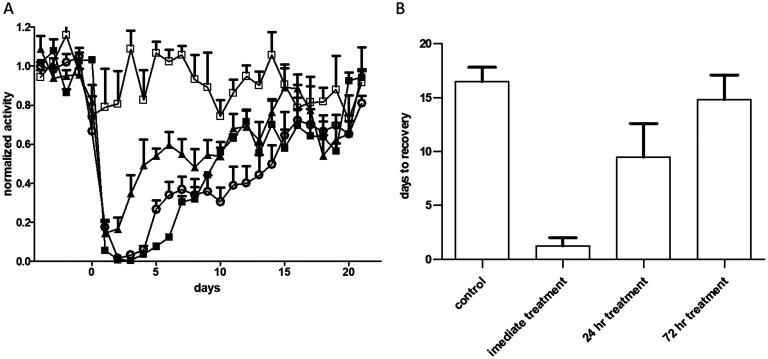
Spontaneous recovery following exposure to a sublethal dose of BoNT/A. (A) Groups of mice (n = 3) were injected i.m. with various doses of BoNT/A on day 0. The activity of the animals on each night was normalized to its average activity on the 4 nights preceding toxin exposure. Each point represents the average of the group activity. The toxin doses were 0.15 (circles), 0.3 (squares), and 0.6 (triangles) i.m. LD50. (B) Average days to recovery in mice exposed to the respective doses of BoNT/A. Recovery was defined as return of mouse’s activity to its individual average nocturnal running distance prior to toxin exposure − 2 SDs.
Administration of sublethal doses of BoNT/A to mice resulted in decreased running distance per night. The durations of decreased running distance correlated with the toxin dose and lasted 7.25 ± 1.7, 18.3 ± 2, and 25 nights for 0.15, 0.3, and 0.6 i.m. LD50s, respectively. Exposure to 0.3 i.m. LD50 caused a prolonged and robust reduction in mouse activity without causing death, which was observed in some of the mice exposed to 0.6 i.m. LD50. Therefore, 0.3 i.m. LD50 was chosen as the optimal dose to be used in this model.
Next, the latest time point at which antitoxin was still effective in the chronic botulism model was determined (Fig. 5). Treatment of intoxicated animals with antitoxin on the day of intoxication prevented most of the toxic effect, as only a slight reduction in running was observed (Fig. 5). Delayed treatment administered 24 h postintoxication had a partial therapeutic effect that significantly (P = 0.02) shortened the duration of the disease from 16.5 ± 1.3 days in untreated mice to 9.5 ± 3.1 days. Administration of antitoxin 3 days after intoxication did not shorten the disease duration: 14.8 ± 2.3 days versus 16.5 ± 1.3 days in the untreated mouse group (P = 0.59). Thus, this model, in which mice are exposed to a sublethal dose of BoNT and treated 3 days postintoxication or later, can serve as a means for evaluating antibotulinum intracellular drugs intended for the chronic phase of botulism. Molecules that will shorten the paresis period in comparison to an untreated control group will be considered good candidates and should be further evaluated for the treatment of botulism.
FIG 5.
Determination of the time window for next-generation antibotulinum therapeutics in the sublethal model. Mice were exposed to 0.3 i.m. LD50 of BoNT/A on day 0 and treated with a body-weight-normalized human dose of antitoxin at different time points. (A) Normalized activity in relation to the averaged activity over the 4 days preceding BoNT exposure. The antitoxin treatment time points are as follows: immediate treatment (open squares), 24 h from intoxication (closed triangles), or 72 h (open circles) from intoxication (closed squares, untreated group). (B) Average times to recovery for the different groups.
DISCUSSION
In the current study, a real-time, quantitative, and continuous system for detecting botulism symptoms in mice was developed. The system allows remote automated data collection from a large number of animals, facilitating powerful statistical analysis, without intervention in the normal behavior of the mice. The motor parameter monitored by the system is running, which depends on the transmission of neural signals from neurons to muscle cells at the neuromuscular junction, the target site for botulinum toxins. In addition, running is a voluntary behavior. Meijer et al. (27) placed running wheels in nature and recorded extensive running activity of wild mice. Human patients report early botulism symptoms long before the appearance of observed signs such as ptosis and difficulty speaking (19). On the other hand, animals, especially rodents, do not present such facial symptoms and obviously cannot report their situation. The voluntary decision of a mouse to run may reflect the same capabilities of human patients to report early botulism-related symptoms.
Previous studies have used a running wheel system to characterize botulinum toxin effects when administered at sublethal doses. Keller compared the lengths of paralysis induced by various sublethal doses of BoNT/A, BoNT/B, and BoNT/E (34). Kutschenko et al. used an irregularly spaced crossbar running wheel to compare three pharmaceutical preparations of botulinum A (26, 35). In subsequent studies, a running wheel system was used to evaluate the potency of a BoNT/AB hybrid (36). In all of these studies, the activity was analyzed retrospectively at a resolution of nights and without any treatment. In the current study, we wished to evaluate postsymptom antitoxin efficacy in a clinically relevant scenario. Thus, intoxication with a lethal dose of BoNT was mandatory. Times to death (TTD) in mice exposed to 4 i.m. LD50s of BoNT/A, BoNT/B, and BoNT/E ranged between 10 and 40 h, depending on toxin serotype. It is expected that symptoms corresponding to the disease in such kinetics will manifest within several hours of exposure. Accordingly, a high-resolution analysis system was required.
The development of a high-resolution monitoring system for detection of disease symptoms presents several challenges arising from the high variability associated with voluntary mouse running. In the current study, a series of tools were developed to overcome variability and enable objective symptom detection. These tools consisted of a gradual acclimation protocol, comparing the activity of each mouse to its own preexposure activity, use of a moving average to overcome the noncontinuous nature of voluntary running activity, and designation of a minimal time period of deviation from normal activity as a symptom.
Using the running wheel system, the TTS following exposure to 4 i.m. LD50s of BoNT/A, BoNT/B, and BoNT/E, which account for the vast majority of human botulism cases, were 6.7, 8.3, and 3.5 h, respectively. This pattern coincides with the reported pathogenesis of human botulism. In mice, the onset of wasp waist symptoms was observed first for serotype E and then for serotypes A and B (29). Notably, symptom detection using the running wheel system preceded wasp waist symptoms for all serotypes. In another study, rabbits that were exposed to 4 LD50s of BoNT/A and BoNT/B presented respiratory symptoms after approximately 41 h postexposure (22), and rabbits exposed to 2 LD50s of BoNT/E demonstrated respiratory symptoms 18 h after exposure (21). Most importantly, the order of symptom onset by serotype in the running wheel system correlated well with reported human botulism cases. In an analysis of epidemiologic data collected between 1975 and 1988 from clinical human botulism cases, it was found that type E patients had the shortest incubation periods, while type B patients had the longest incubation periods (30). These observations support the high-resolution running system as a clinically relevant tool for the evaluation of postsymptom treatments.
Administration of a human-normalized dose of antitoxin after symptom onset was fully protective in mice exposed to a lethal dose of either BoNT/A or BoNT/B. A high protection rate was also achieved in mice challenged with a lethal dose of BoNT/E (88 and 96% for challenge with 4 and 2 i.m. LD50s, respectively). Notably, for serotype E only a very few reports are available regarding antitoxin efficacy (37, 38). The model developed in the current study enabled for the first time, successful evaluation of postsymptom anti-BoNT/E efficacy in mice. The mouse running wheel model, together with the rabbit spirometry model previously developed in our lab, allows objective postsymptom efficacy evaluation of antitoxin, or other therapeutics, in two animal species.
Following entrance of the lethal concentration (LC) of botulinum toxin into neurons, it cannot be neutralized by antitoxin. Inside the neurons, the LC may persist for extended periods (39), leading to muscle atrophy and endplate silencing (40, 41), both delaying recovery. Intoxicated humans can remain paralyzed for months and often require mechanical ventilation for weeks (42). Thus, there have been long-lasting attempts to develop next-generation therapeutics, aiming to shorten paralysis periods by inhibiting intracellular LC. These include small molecule inhibitors (31), uncleavable SNAP derivatives (43), and molecular vehicles delivering drugs into the cytosol (44). In vivo examination of these therapeutics used lethal models, and treatment was given simultaneously with the toxin or in a time-dependent manner.
To address the long-lasting paralysis stage of botulism, we developed a sublethal exposure model. According to this model, a robust and long-lasting reduction in nocturnal running activity is induced by exposure of mice to sublethal doses of BoNTs (without causing mortality), followed by prolonged spontaneous recovery. Exposure of mice to 0.15, 0.3, and 0.6 i.m. LD50s of BoNT/A resulted in a reduction in nocturnal running for averages of 7.25, 18.3, and 25 days, respectively. A dose of 0.6 i.m. LD50 resulted in the death of some animals in the group, and therefore, although the reduced activity effect was longer for this dose, it was incompatible. Antitoxin treatment was effective when administered immediately or 24 h postexposure to 0.3 i.m. LD50 of BoNT/A. This observation indicates that toxin entrance into target cells is a prolonged process, as even at 24 h post-toxin exposure, a considerable amount of toxin is still in the circulation and amenable to antitoxin neutralization. On the other hand, administration of antitoxin 72 h post-toxin exposure did not significantly accelerate animal recovery, indicating complete toxin entrance at this time point. Hence, next-generation therapeutics, aiming to decrease the time of paralysis in BoNT-intoxicated patients and to be effective beyond the therapeutic window of antitoxin, can be evaluated using the running wheel model by exposure of mice to 0.3 i.m. LD50 of BoNT/A and treating them 72 h from exposure or later.
Overall, the model system described in the current study pertains to various aspects of the clinical features of botulinum intoxication. It allows the transition of complex voluntary behavior to quantitative and objective models, each of which is especially relevant to the question at hand. The lethal model, which entails an a priori construction of predicted individual behavior, is highly suited for high-resolution online detection of symptoms and efficacy testing of current and novel treatments for the outer cell phase of the intoxication process. The sublethal model reflects the chronic phase of the disease in humans, where prolonged paralysis is prominent, owing to inner cell persistence of the toxin. In this scenario, delayed successful treatment, beyond the efficacy window of antitoxin, can serve as a relevant screening phase for novel therapeutics.
MATERIALS AND METHODS
Ethics statement.
All animal experiments were performed in accordance with Israeli law and were approved by the ethics committee for animal experiments at the Israel Institute for Biological Research (protocol no. M-15-15, M-25-15, M-31-16, M-06-17, and M-29-18). Animals reaching endpoint (loss of righting reflex) were euthanized by cervical dislocation. All efforts were made to minimize animal suffering.
Bacteria and toxins.
Clostridium botulinum A, B, and E strains were obtained from the Israel Institute for Biological Research (IIBR) collection (A198, B592 and E450, respectively). A sequence analysis revealed compliance of the neurotoxin genes with serotypes 62A (GenBank accession no. M30196), Danish (GenBank accession no. M81186), and NCTC 11219 (accession no. X62683) of C. botulinum types A1, B1, and E, respectively.
Toxins were prepared from the concentrated supernatant of cultures grown for 6 days in anaerobic culture tubes. Botulinum E was used in its activated form throughout the study (45). Activation was performed by treatment with 0.1% (wt/vol) trypsin (37°C, 1 h). Toxin potency was determined by serial dilutions of toxin preparations in gelatin-phosphate buffer (0.2% [wt/vol] gelatin in phosphate buffer, pH 6.4) and injection of 0.1 ml into the gastrocnemius musculature of the right hind leg, followed by survival monitoring for 96 h. The median lethal dose (LD50) was calculated by the Spearman-Karber method (46).
Antitoxin.
Equine anti-BoNT/A, -B, and -E antitoxins were obtained from IIBR (47). The neutralizing antibody concentrations of the antitoxin preparations were determined according to the European Pharmacopeia (48).
Mouse running system. (i) Hardware.
The system consisted of a standard mouse cage, top fitted with a metal running wheel with a diameter of 12.5 cm (purchased from a local pet shop). A magnetic detector (di-soric DCC 18M 12 PSK-IBSL; di-soric GmbH, Urbach, Germany) was fixed into the cage wall to sense wheel revolutions. The detectors were connected to a custom-made controller that was able to simultaneously handle 48 sensors (Litvak Electrical Engineering and Control, Ltd., Israel). The controller was connected to a computer (running a Microsoft Windows operating system) with control software (PCIM 7.7 SP4; Afcon Control & Automation), which was set up to collect the wheel revolution data and export the data online (at a 1-min interval resolution) into a text file. The system was set to create a backup file every hour. The controller and computer were connected to a uninterruptible power supply (UPS).
(ii) Software.
For high-resolution activity monitoring following exposure to lethal toxin doses, online (at a 1-min resolution) data were used, as exported from the system. Custom-made software was created (Visual Basic) to handle these data and perform all calculations as described in the Results section. Briefly, mouse running was described by two quantitative parameters calculated to characterize individual mouse-specific expected running. One parameter is time specific, and one is performance specific. The parameters were calculated based on specific data from the naive mouse prior to the experiment. During an experiment, deviations from the expected performance were formulated into a rule set and defined as symptom onset.
For experiments in which mice were exposed to sublethal doses of toxin, the total running distance per night for each individual mouse was measured. Basic calculations were done using Microsoft Excel. Data generation, creation of graphs, and statistical analysis were performed using GraphPad Prism.
Animals.
Mice (CD-1) were from Charles River, Canada. Mice were kept under 12-h light/12-h dark cycles. Acclimation of animals was done gradually. Initially, 3 to 4 mice were housed per cage, with access to 1 or 2 running wheels for 4 to 7 days. Afterwards, mice were split into running-monitored cages, with one mouse per cage, for at least 5 days. During these days, running was monitored for consistency as to the total running per night and the specific running patterns for each mouse. Mice showing consistent and robust running (as further explained in the Results section) were used in subsequent experiments.
Antitoxin efficacy studies.
Naive mice (CD-1; Charles River, Canada), previously acclimated and monitored for individual running performance, were injected intramuscularly (i.m.) into the gastrocnemius musculature of the right hind limb with the indicated dose of BoNTs in 0.1 ml gelatin-phosphate buffer. For each toxin serotype and dose, the exposure time was adjusted to allow symptom manifestation within the robust running time frame, which is usually at the beginning of the dark cycle hours. The antitoxin treatment doses were normalized to the human recommended treatment dose on a weight basis as follows: 215 IU/kg of body weight for BoNT/A and BoNT/B and 120 IU/kg for BoNT/E. Antitoxins A and B were injected i.m. in a volume of 100 μl to the left hind leg. Antitoxin E was injected intraperitoneally in a volume of 0.5 ml to facilitate fast distribution. Activity monitoring was performed in a remote room, and the minimal resolution of entry into the room was half an hour. To minimize disturbance to mouse activity, the room was illuminated with red light while antitoxin treatment was administered.
REFERENCES
- 1.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K, Working Group on Civilian Biodefense. 2001. Botulinum toxin as a biological weapon: medical and public health management. JAMA 285:1059–1070. 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 2.Pirazzini M, Rossetto O, Eleopra R, Montecucco C. 2017. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev 69:200–235. 10.1124/pr.116.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dembek ZF, Smith LA, Rusnak JM. 2007. Botulism: cause, effects, diagnosis, clinical and laboratory identification, and treatment modalities. Disaster Med Public Health Prep 1:122–134. 10.1097/DMP.0b013e318158c5fd. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama H. 1980. Clostridium botulinum neurotoxin. Microbiol Rev 44:419–448. 10.1128/MR.44.3.419-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kongsaengdao S, Samintarapanya K, Rusmeechan S, Wongsa A, Pothirat C, Permpikul C, Pongpakdee S, Puavilai W, Kateruttanakul P, Phengtham U, Panjapornpon K, Janma J, Piyavechviratana K, Sithinamsuwan P, Deesomchok A, Tongyoo S, Vilaichone W, Boonyapisit K, Mayotarn S, Piya-Isragul B, Rattanaphon A, Intalapaporn P, Dusitanond P, Harnsomburana P, Laowittawas W, Chairangsaris P, Suwantamee J, Wongmek W, Ratanarat R, Poompichate A, Panyadilok H, Sutcharitchan N, Chuesuwan A, Oranrigsupau P, Sutthapas C, Tanprawate S, Lorsuwansiri J, Phattana N, Thai Botulism Study Group. 2006. An outbreak of botulism in Thailand: clinical manifestations and management of severe respiratory failure. Clin Infect Dis 43:1247–1256. 10.1086/508176. [DOI] [PubMed] [Google Scholar]
- 6.Weber JT, Hibbs RG, Jr, Darwish A, Mishu B, Corwin AL, Rakha M, Hatheway CL, El Sharkawy S, El-Rahim SA, Al-Hamd MFS, Sarn JE, Blake PA, Tauxe RV. 1993. A massive outbreak of type E botulism associated with traditional salted fish in Cairo. J Infect Dis 167:451–454. 10.1093/infdis/167.2.451. [DOI] [PubMed] [Google Scholar]
- 7.McCarty CL, Angelo K, Beer KD, Cibulskas-White K, Quinn K, de Fijter S, Bokanyi R, St Germain E, Baransi K, Barlow K, Shafer G, Hanna L, Spindler K, Walz E, DiOrio M, Jackson BR, Luquez C, Mahon BE, Basler C, Curran K, Matanock A, Walsh K, Slifka KJ, Rao AK. 2015. Large outbreak of botulism associated with a church potluck meal—Ohio, 2015. MMWR Morb Mortal Wkly Rep 64:802–803. 10.15585/mmwr.mm6429a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2018. Bioterrorism agents/diseases. https://emergency.cdc.gov/agent/agentlist-category.asp.
- 9.Ni SA, Brady MF. 2021. Botulism antitoxin. StatPearls, Treasure Island, FL. [PubMed] [Google Scholar]
- 10.Arnon SS, Schechter R, Maslanka SE, Jewell NP, Hatheway CL. 2006. Human botulism immune globulin for the treatment of infant botulism. N Engl J Med 354:462–471. 10.1056/NEJMoa051926. [DOI] [PubMed] [Google Scholar]
- 11.Sobel J. 2005. Botulism. Clin Infect Dis 41:1167–1173. 10.1086/444507. [DOI] [PubMed] [Google Scholar]
- 12.Franz DR, Pitt LM, Clayton MA, Hanes MA, Rose KJ. 1993. Efficacy of prophylactic and therapeutic administration of antitoxin for inhalation botulism, p 473–476. In Das-Gupta BR (ed), Botulinum and tetanus neurotoxins: neurotransmission and biomedical aspects. Plenum Press, New York, NY. [Google Scholar]
- 13.Habermann E, Bernath S. 1975. Preparation, measurement and possible use of human antitoxin against Cl. botulinum A, B, and E toxins. Med Microbiol Immunol 161:203–210. 10.1007/BF02121011. [DOI] [PubMed] [Google Scholar]
- 14.Iida H, Ono T, Karashimada T. 1970. Experimental studies on the serum therapy of type E botulism: the relationship between the amount of toxin in the blood and the effect of antitoxic serum. Jpn J Med Sci Biol 23:344–347. [PubMed] [Google Scholar]
- 15.Iida H, Ono T, Karashimada T, Ando Y. 1970. Studies on the serum therapy of type E botulism: absorption of toxin from the gastrointestinal tract. Jpn J Med Sci Biol 23:282–285. [PubMed] [Google Scholar]
- 16.Ono T, Karashimada T, Iida H. 1969. Studies on the serum therapy of type E botulism. II. J Infect Dis 120:534–538. 10.1093/infdis/120.5.534. [DOI] [PubMed] [Google Scholar]
- 17.Ono T, Karashimada T, Iida H. 1970. Studies of the serum therapy of type E botulism. III. Jpn J Med Sci Biol 23:177–191. 10.7883/yoken1952.23.177. [DOI] [PubMed] [Google Scholar]
- 18.Lewis GE, Jr, Metzger JF. 1979. Studies on the prophylaxis and treatment of botulism. Toxicon 17:102. [Google Scholar]
- 19.Mottate K, Yokote H, Mori S, Horita A, Miyatsu Y, Torii Y, Kozaki S, Iwaki M, Takahashi M, Ginnaga A. 2016. Retrospective survey to evaluate the safety and efficacy of Japanese botulinum antitoxin therapy in Japan. Toxicon 110:12–18. 10.1016/j.toxicon.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Barker D, Gillum KT, Niemuth NA, Kodihalli S. 2019. Therapeutic efficacy of equine botulism heptavalent antitoxin against all seven botulinum neurotoxins in symptomatic guinea pigs. PLoS One 14:e0222670. 10.1371/journal.pone.0222670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamant E, Pass A, Rosen O, Ben David A, Torgeman A, Barnea A, Tal A, Rosner A, Zichel R. 2018. A novel rabbit spirometry model of type E botulism and its use for the evaluation of postsymptom antitoxin efficacy. Antimicrob Agents Chemother 62:e02379-17. 10.1128/AAC.02379-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torgeman A, Schwartz A, Diamant E, Baruchi T, Dor E, Ben David A, Pass A, Barnea A, Tal A, Rosner A, Rosen O, Zichel R. 2018. Studying the differential efficacy of postsymptom antitoxin treatment in type A versus type B botulism using a rabbit spirometry model. Dis Model Mech 11:dmm035089. 10.1242/dmm.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Horo JC, Harper EP, El Rafei A, Ali R, DeSimone DC, Sakusic A, Abu Saleh OM, Marcelin JR, Tan EM, Rao AK, Sobel J, Tosh PK. 2018. Efficacy of antitoxin therapy in treating patients with foodborne botulism: a systematic review and meta-analysis of cases, 1923–2016. Clin Infect Dis 66:S43–S56. 10.1093/cid/cix815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donald S, Elliott M, Gray B, Hornby F, Lewandowska A, Marlin S, Favre-Guilmard C, Perier C, Cornet S, Kalinichev M, Krupp J, Fonfria E. 2018. A comparison of biological activity of commercially available purified native botulinum neurotoxin serotypes A1 to F1 in vitro, ex vivo, and in vivo. Pharmacol Res Perspect 6:e00446. 10.1002/prp2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellett S, Tepp WH, Whitemarsh RC, Bradshaw M, Johnson EA. 2015. In vivo onset and duration of action varies for botulinum neurotoxin A subtypes 1–5. Toxicon 107:37–42. 10.1016/j.toxicon.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kutschenko A, Reinert MC, Klinker F, Paulus W, Hesse S, Liebetanz D. 2011. Botulinum toxin-induced focal paresis in mice is unaffected by muscle activity. Muscle Nerve 44:930–936. 10.1002/mus.22210. [DOI] [PubMed] [Google Scholar]
- 27.Meijer JH, Robbers Y. 2014. Wheel running in the wild. Proc Biol Sci 281:20140210. 10.1098/rspb.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seward T, Harfmann BD, Esser KA, Schroder EA. 2018. Reinventing the wheel: comparison of two wheel cage styles for assessing mouse voluntary running activity. J Appl Physiol 124:923–929. 10.1152/japplphysiol.00880.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mimran A, Chay Y, Barnea A, Halperin G, Reuveny S, Zichel R. 2012. Evaluating the efficacy of post-clinical symptom anti-toxin therapy in a mouse model of botulism A and B. Presented at the 49th Interagency Botulism Research Coordinating Committee Meeting (IBRCC), Baltimore, MD.
- 30.Woodruff BA, Griffin PM, McCroskey LM, Smart JF, Wainwright RB, Bryant RG, Hutwagner LC, Hatheway CL. 1992. Clinical and laboratory comparison of botulism from toxin types A, B, and E in the United States, 1975–1988. J Infect Dis 166:1281–1286. 10.1093/infdis/166.6.1281. [DOI] [PubMed] [Google Scholar]
- 31.Duplantier A, Kane C, Bavari S. 2016. Searching for therapeutics against botulinum neurotoxins: a true challenge for drug discovery. Curr Top Med Chem 16:2330–2349. 10.2174/1568026616666160413135630. [DOI] [PubMed] [Google Scholar]
- 32.Tsai YC, Maditz R, Kuo CL, Fishman PS, Shoemaker CB, Oyler GA, Weissman AM. 2010. Targeting botulinum neurotoxin persistence by the ubiquitin-proteasome system. Proc Natl Acad Sci U S A 107:16554–16559. 10.1073/pnas.1008302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin L, Olson ME, Eubanks LM, Janda KD. 2019. Strategies to counteract botulinum neurotoxin A: nature's deadliest biomolecule. Acc Chem Res 52:2322–2331. 10.1021/acs.accounts.9b00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller EJ. 2006. Recovery from botulinum neurotoxin poisoning in vivo. Neuroscience 139:629–637. 10.1016/j.neuroscience.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 35.Kutschenko A, Manig A, Reinert MC, Mönnich A, Liebetanz D. 2016. In-vivo comparison of the neurotoxic potencies of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA. Neurosci Lett 627:216–221. 10.1016/j.neulet.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Kutschenko A, Reinert MC, Krez N, Liebetanz D, Rummel A. 2017. BoNT/AB hybrid maintains similar duration of paresis as BoNT/A wild-type murine running wheel assay. NeuroToxicology 59:1–8. 10.1016/j.neuro.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Kondo H, Kondo S, Murata R, Sakaguchi G. 1963. Potency test and therapeutic effect of type E botulinus antitoxin. Jpn J Med Sci Biol 16:310–311. [PubMed] [Google Scholar]
- 38.Iida H. 1963. Specific antitoxin therapy in type E botulism. Jpn J Med Sci Biol 16:311–313. [PubMed] [Google Scholar]
- 39.Whitemarsh RC, Tepp WH, Johnson EA, Pellett S. 2014. Persistence of botulinum neurotoxin A subtypes 1–5 in primary rat spinal cord cells. PLoS One 9:e90252. 10.1371/journal.pone.0090252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossetto O, Pirazzini M, Montecucco C. 2014. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nat Rev Microbiol 12:535–549. 10.1038/nrmicro3295. [DOI] [PubMed] [Google Scholar]
- 41.Tighe AP, Schiavo G. 2013. Botulinum neurotoxins: mechanism of action. Toxicon 67:87–93. 10.1016/j.toxicon.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro RL, Hatheway C, Swerdlow DL. 1998. Botulism in the United States: a clinical and epidemiologic review. Ann Intern Med 129:221–228. 10.7326/0003-4819-129-3-199808010-00011. [DOI] [PubMed] [Google Scholar]
- 43.Kumar G, Swaminathan S. 2015. Recent developments with metalloprotease inhibitor class of drug candidates for botulinum neurotoxins. Curr Top Med Chem 15:685–695. 10.2174/1568026615666150309150338. [DOI] [PubMed] [Google Scholar]
- 44.Vazquez-Cintron EJ, Vakulenko M, Band PA, Stanker LH, Johnson EA, Ichtchenko K. 2014. Atoxic derivative of botulinum neurotoxin A as a prototype molecular vehicle for targeted delivery to the neuronal cytoplasm. PLoS One 9:e85517. 10.1371/journal.pone.0085517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duff JT, Wright GG, Yarinsky A. 1956. Activation of Clostridium botulinum type E toxin by trypsin. J Bacteriol 72:455–460. 10.1128/JB.72.4.455-460.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irwin JO, Cheeseman EA. 1939. On an approximate method of determining the median effective dose and its error, in the case of a quantal response. J Hyg (Lond) 39:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torgeman A, Mador N, Dorozko M, Lifshitz A, Eschar N, White MD, Wolf DG, Epstein E. 2017. Efficacy of inactivation of viral contaminants in hyperimmune horse plasma against botulinum toxin by low pH alone and combined with pepsin digestion. Biologicals 48:24–27. 10.1016/j.biologicals.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 48.European Directorate for the Quality of Medicines and Healthcare. 2014. European pharmacopoeia, 8th ed, vol 1, p 1029. EDQM Council of Europe, Strasbourg, France. [Google Scholar]
- 49.Emanuel A, Qiu H, Barker D, Takla T, Gillum K, Neimuth N, Kodihalli S. 2019. Efficacy of equine botulism antitoxin in botulism poisoning in a guinea pig model. PLoS One 14:e0209019. 10.1371/journal.pone.0209019. [DOI] [PMC free article] [PubMed] [Google Scholar]