ABSTRACT
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to spread rapidly worldwide. This study is the first to report the tolerability, safety, pharmacokinetics (PK), and immunogenicity of a recombinant human anti-SARS-CoV-2 monoclonal antibody, etesevimab (CB6, JS016, LY3832479, or LY-CoV016), in healthy adults. This paper describes a randomized, double-blind, placebo-controlled, phase 1 study. A total of 40 participants were enrolled to receive a single intravenous dose of either etesevimab or placebo in one of four sequential ascending intravenous dose cohorts. All 40 participants completed the study. Seventeen (42.5%) participants experienced 22 treatment emergent adverse events (TEAEs) that were drug-related, and the rates of these TEAEs among different dose cohorts were numerically comparable. No difference was observed between the combined etesevimab group and the placebo group. The exposure after etesevimab infusion increased in an approximately proportional manner as the dose increased from 2.5 to 50 mg/kg. The elimination half-life (t1/2) value did not differ among different dose cohorts and was estimated to be around 4 weeks. Etesevimab was well tolerated after administration of a single dose at a range of 2.5 mg/kg to 50 mg/kg in healthy Chinese adults. The PK profiles of etesevimab in healthy volunteers showed typical monoclonal antibody distribution and elimination characteristics. (This study has been registered at ClinicalTrials.gov under identifier NCT04441918.)
KEYWORDS: COVID-19, JS016, LY3832479, SARS-CoV-2, anti-spike neutralizing antibodies, etesevimab, neutralizing antibodies, pharmacokinetics
INTRODUCTION
More than 141 million individuals have been infected with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) globally, and the cumulative deaths have exceeded 3 million by 20 Apr 2021 (https://www.who.int/). Despite the acceleration of development of vaccines and therapeutic treatments all over the world, a specific treatment for coronavirus disease 2019 (COVID-19) remains unavailable for broad clinical use (1, 2). SARS-CoV-2 continues to spread rapidly worldwide and results in largely unmet medical needs. The development of optimal antiviral drugs is urgently needed worldwide.
Etesevimab (also known as CB6, JS016, LY3832479, or LY-CoV016) is a recombinant neutralizing human immunoglobulin G1 (IgG1)κ monoclonal antibody (MAb) to the spike protein of SARS-CoV-2, with amino acid substitutions in the fragment crystallizable (Fc) region (L234A, L235A) to reduce effector function. Etesevimab binds the spike protein with a dissociation constant (Kd) of 6.45 nM and blocks spike protein attachment to the human angiotensin converting enzyme 2 (ACE2) receptor with a 50% inhibitory concentration (IC50) value of 0.32 nM (0.046 μg/ml). Thus, etesevimab blocks viral cell fusion to prevent SARS-CoV-2 invasion of human cells and inhibits viral replication (3). In a SARS-CoV-2 challenge study conducted in rhesus macaques, 50 mg/kg etesevimab exhibited favorable effects for both prophylactic and therapeutic venues against SARS-CoV-2 infection. Therefore, etesevimab is expected to have high potential as a treatment for COVID-19.
Globally, multiple clinical developments of SARS-CoV-2 neutralizing antibodies are in fierce competition (https://clinicaltrials.gov/). Several interim analyses have revealed promising results regarding the viral load, symptoms, and related hospitalization in patients with COVID-19 (4–6). Although especially important for accumulating safety information for prophylactic use, no report on healthy volunteers has been released to date. Herein, we report the tolerability, safety, PK, and immunogenicity of etesevimab in healthy Chinese adults, which were obtained from a randomized, double-blind, placebo-controlled, first-in-human phase 1 study (JS016-001-I).
RESULTS
Participant characteristics.
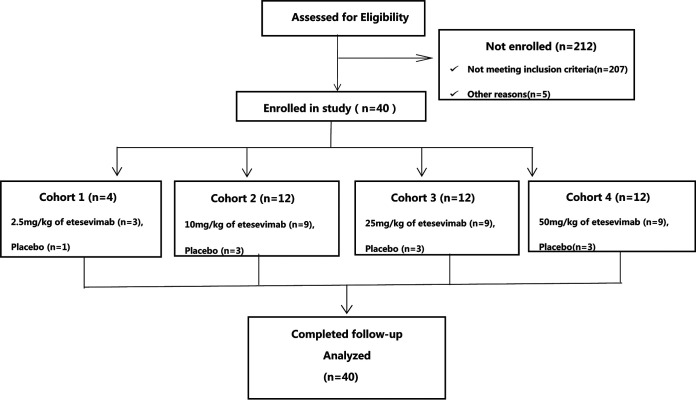
A total of 40 participants were randomly included in the study (Fig. 1). The demographic information and baseline characteristics of the participants are listed in Table 1. All the healthy adults are Chinese. Thirty participants were male (75%). The average age (min, max) was 28.7 (19, 30) years old, and the average body mass index (BMI) (min, max) was 23.2 (18.6, 27.6) kg/m2. The baseline information across the dose cohorts was numerically comparable, and no difference was found between the etesevimab combined group and the placebo group.
FIG 1.
CONSORT flow diagram of the JS016-001-I study. Forty healthy adults were allocated to four different dose cohorts. All participants received a single dose of the one dose level. In each dose cohort, the participants were randomly allocated to receive etesevimab or placebo at a ratio of 3:1. The randomization scheme was generated using an interactive web response system (IWRS). All participants completed the study at the time of submission of the manuscript.
TABLE 1.
Demographic characteristics of the study participants
| Parametera | Etesevimab |
Placebo (n = 10) | Overall (n = 40) | ||||
|---|---|---|---|---|---|---|---|
| 2.5 mg/kg (n = 4) | 10 mg/kg (n = 12) | 25 mg/kg (n = 12) | 50 mg/kg (n = 12) | Combined (n = 30) | |||
| Age in yrs (median [range]) | 30 (23–34) | 29 (26–35) | 30 (20–33) | 25 (19–39) | 29.5 (19–39) | 26 (21–38) | 29 (19–39) |
| Men, n (%) | 3 (100) | 8 (88.9) | 5 (55.6) | 8 (88.9) | 24 (80.0) | 6 (60.0) | 30 (75.0) |
| Women, n (%) | 0 (0) | 1 (11.1) | 4 (44.4) | 1 (11.1) | 6 (20.0) | 4 (40.0) | 10 (25.0) |
| Wt in kg, mean (SD) | 66.17 (4.366) | 66.20 (7.707) | 63.84 (9.77) | 62.64 (6.62) | 64.42 (7.649) | 64.53 (6.635) | 64.45 (7.325) |
| Ht in cm, mean (SD) | 170.83 (3.502) | 166.46 (9.776) | 166.12 (9.274) | 166.58 (8.457) | 166.83 (8.517) | 166.56 (7.762) | 166.76 (8.238) |
| Body mass index in kg/m2, mean (SD) | 22.77 (0.907) | 23.78 (1.482) | 22.99 (2.877) | 22.53 (2.877) | 23.07 (1.89) | 23.21 (1.573) | 23.1 (1.797) |
SD, standard deviation; Wt, weight; Ht, height.
Safety.
All 40 participants completed the scheduled safety follow-up. During the study, no dose limiting events (DLEs), serious adverse events (SAEs), or adverse events of special interest (AESIs), such as infusion related/allergic reactions, was observed. No discontinuation due to adverse event (AE) occurred.
A total of 173 treatment emergent adverse events (TEAEs) were reported by the 40 (100%) participants; 122 of TEAEs experienced by 36 (90%) participants were lab abnormalities. No differences were observed among different dose groups or between the etesevimab and placebo group (Table 2). Among all TEAEs, 22 were judged to be drug-related by the investigators. Drug-related TEAEs that occurred in ≥5% of participants included upper respiratory infection in 9 participants (22.5% total; 6 in etesevimab, 20%; 3 in placebo, 30%); C-reaction protein elevation in 4 participants (10% total; 3 in etesevimab, 10%; 1 in placebo, 10%); alanine aminotransferase (ALT) elevation in 2 participants (5.0%, in etesevimab); and gastrointestinal signs/symptoms in 2 participants (5.0% total; 1 in etesevimab, 5%; 1 in placebo, 10%).
TABLE 2.
Safety summary after a single dose of intravenous infusion of etesevimab
| Effect | Etesevimab |
Placebo (n = 10) |
Overall (n = 40) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 mg/kg (n = 4) |
10 mg/kg (n = 12) |
25 mg/kg (n = 12) |
50 mg/kg (n = 12) |
Combined (n = 30) |
||||||||||
| No. events | No. subjects (%) | No. events | No. subjects (%) | No. events | No. subjects (%) | No. events | No. subjects (%) | No. events | No. subjects (%) | No. events | No. subjects (%) | No. events | No. subjects (%) | |
| Treatment emergent adverse events (TEAEs) | 6 | 3 (100%) | 39 | 9 (100%) | 50 | 9 (100%) | 30 | 9 (100%) | 125 | 30 (100%) | 48 | 10 (100%) | 173 | 40 (100%) |
| Drug-related TEAEs | 0 | 0 | 5 | 4 (44.4%) | 8 | 5 (55.6%) | 4 | 4 (44.4%) | 17 | 13 (43.3%) | 5 | 4 (40.0%) | 22 | 17 (42.5%) |
| Drug related TEAEs (≥5%) | ||||||||||||||
| Upper respiratory infection | 0 | 0 | 4 | 4 (44.4%) | 0 | 0 | 2 | 2 (22.2%) | 6 | 6 (20.0%) | 3 | 3 (30.0%) | 9 | 9 (22.5%) |
| C-reactive protein elevation | 0 | 0 | 0 | 0 | 1 | 1 (11.1%) | 2 | 2 (22.2%) | 3 | 3 (10.0%) | 1 | 1 (10.0%) | 4 | 4 (10.0%) |
| Alanine aminotransferase elevation | 0 | 0 | 1 | 1 (11.1%) | 1 | 1 (11.1%) | 0 | 0 | 2 | 2 (6.7%) | 0 | 0 | 2 | 2 (5.0%) |
| Gastrointestinal symptoms | 0 | 0 | 0 | 0 | 1 | 1 (11.1%) | 0 | 0 | 1 | 1 (3.3%) | 1 | 1 (10.0%) | 2 | 2 (5.0%) |
| Severity of TEAE | ||||||||||||||
| Mild (grade 1) | 3 (100%) | 7 (77.8%) | 5 (55.6%) | 8 (88.9%) | 23 (76.7%) | 7 (70.0%) | 30 (75.0%) | |||||||
| Moderate (grade 2) | 0 | 2 (22.2%) | 0 | 1 (11.1%) | 3 (10.0%) | 3 (30.0%) | 6 (15.0%) | |||||||
| Severe (grade 3) | 0 | 0 | 4 (44.4%) | 0 | 4 (13.3%) | 0 | 4 (10.0%) | |||||||
| Life threatening (grade 4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Dose limiting events (DLE) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Serious adverse event (SAE) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| TEAE of special interest (AESI) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| TEAE leading to treatment discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
There were 4 TEAEs experienced by 4 participants that were judged as grade 3. All 4 participants were randomly assigned to receive single intravenous infusion of etesevimab 25 mg/kg. Three participants (01137, 01148, and 01166) experienced an increase in cholesterol to 1.13×, 1.14×, and 1.17× the upper limit of normal (ULN), and 1 participant (01175), male, experienced an abnormal urinalysis result, with an increased red blood cell count and positive urine occult blood (BLD) without any signs/symptoms. All of the above-described TEAEs recovered or stabilized without any intervention within 1 to 2 week(s) follow-up. None of the four grade-3 TEAEs were judged as related to the investigational product.
Pharmacokinetics.
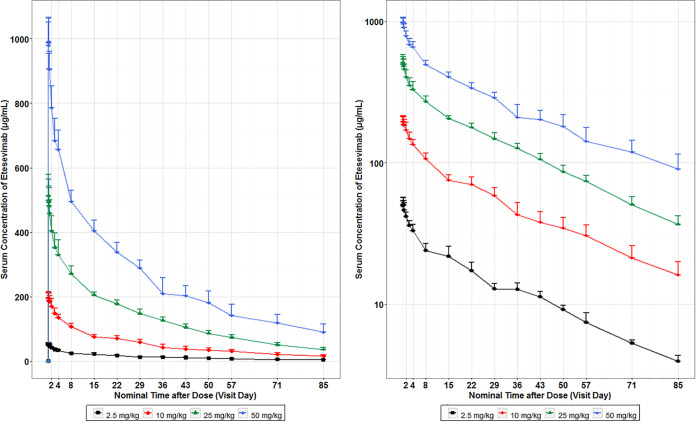
As shown in Fig. 2, the mean serum concentrations of etesevimab in the healthy volunteers increased as the dose increased, ranging from 2.5 mg/kg to 50 mg/kg.
FIG 2.
Mean (± standard deviation [SD]) of etesevimab concentration-time profiles in healthy participants following single-dose intravenous infusions. PK samples were collected on day 1 (dosing) and days 2, 3, 4, 8, 15, 22, 29, 36, 57, 71, and 85 postdose, or at early discontinuation. The left side of the figure represents a linear scale; the right side of the figure represents a semi-log scale. At each time point, the error bars represent the SD. Concentration values less than the lower limit of quantitation (LLQQ) were set as 0 on the linear scale plot and were omitted on the semi-log plot. The mean serum concentrations of etesevimab in healthy participants increased as the dose increased from 2.5 to 50 mg/kg.
The PK parameters of etesevimab are summarized descriptively by dose in Table 3. Following a single intravenous infusion of etesevimab, the inter-individual variation in exposure parameters of etesevimab were low, with coefficient of variation in percentage (CV%) of the maximum concentration of drug in serum (Cmax) being ∼6% to ∼11% and CV% of the area under the curve (AUC) being ∼4% to ∼13%. The median time to maximum concentration of drug in serum (Tmax) was similar (1 to 3 h), and the mean half-life (t1/2) was 26.8 days (643 h) to 29.8 (716 h) days across dose groups.
TABLE 3.
Summary of PK parameters of etesevimab after a single-dose intravenous infusiona
| Dose (mg/kg) | Cmax (mean μg/ml ± SD; CV%) | Tmax median h (min, max) | AUC0–last (mean μg·h/ml ± SD; CV%) | Tlast median h (min, max) | AUC0–∞ (mean μg·h/ml ± SD; CV%) | CL (mean ml/h ± SD; CV%) | Vz (mean ml ± SD; CV%) | t1/2 (mean h ± SD; CV%) | MRT0–last (mean h ± SD; CV%) | MRT0–∞ (mean h ± SD; CV%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 (n = 3) | 51.8 ± 5.52; −11% | 3 (2.00,5.00) | 26,700 ± 2,850; −11% | 2,041.82 (2,039.90, 2,041.92) | 30,800 ± 3,070; −10% | 5.40 ± 0.493; −9% | 5,580 ± 649; −12% | 716 ± 29.8; −4% | 678 ± 13.7; −2% | 996 ± 33.6; −3% |
| 10 (n = 9) | 202 ± 19.4; −10% | 2 (1.00,5.00) | 102,000 ± 11,500; −11% | 2,015.07 (2,014.57, 2,017.23) | 119,000 ± 15,800; −13% | 5.63 ± 0.746; −13% | 5,700 ± 645; −11% | 708 ± 80.2; −11% | 657 ± 43.1; −7% | 989 ± 126; −13% |
| 25 (n = 9) | 526 ± 55.7; −11% | 1.03 (1.00,9.00) | 261,000 ± 13,000; −5% | 2,015.12 (2,014.98, 2,016.42) | 296,000 ± 12200; −4% | 5.39 ± 0.750; −14% | 4,940 ± 793; −16% | 643 ± 109; −17% | 653 ± 31.5; −5% | 925 ± 118; −13% |
| 50 (n = 9) | 1,020 ± 66.3; −6% | 2 (1.02,3.00) | 512,000 ± 40,300; −8% | 2,015.13 (2,014.73, 2,018.00) | 566,000 ± 57,000 (10%)b | 5.61 ± 0.674 (12%)b | 5,520 ± 1010 (18%)b | 684 ± 114 (17%)b | 672 ± 41.9; −6% | 947 ± 117; (12%)b |
n, number of subjects; Cmax, maximum concentration; Tmax, time to reach maximum concentration; AUC0–last, area under the serum concentration-time curve from time zero to the time of the last quantifiable concentration; Tlast, time of the last quantifiable concentration; AUC0–∞, area under the curve from time zero to infinity; CL, clearance; Vz, volume of distribution; t1/2, elimination half-life; MRT0–last, mean residence time from time zero to the time of the last quantifiable concentration; MRT0–∞, mean residence time from time zero to infinity. Note that pharmacokinetic parameters are presented as mean ± standard deviation (SD) (CV%). T max and Tlast are presented as median (minimum, maximum).
λz of 4 subjects in the 50 mg/kg group could not be calculated accurately; the number of subjects included in the analysis for AUC0–∞, CL, Vz, t1/2, and MRT0–∞ was 5.
A power model method was used to assess the dose proportionality based on the log- transformed Cmax, AUC0–last, and AUC0–∞. With the dose ranging from 2.5 to 50 mg/kg, the point estimates of Cmax, AUC0–last, and AUC0–∞ were 1.01, 1.01, and 0.99, respectively. The 90% confidence interval (CI) of the point estimates was completely within the calculated judgment interval. Therefore, exposure parameters (Cmax and AUC) were considered to be increased in an approximately proportional manner as the dose increased.
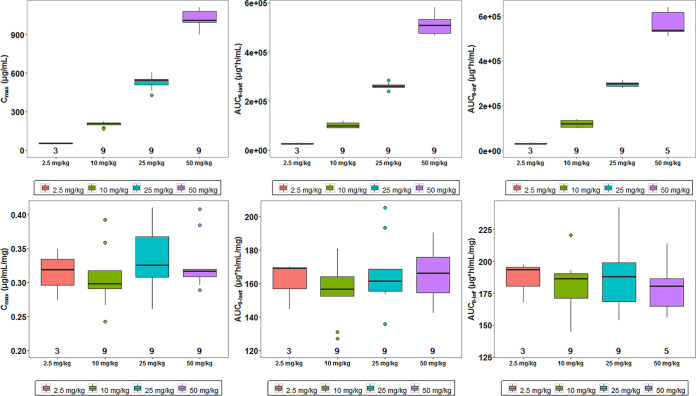
The individual exposure parameters (Cmax and AUC) of etesevimab were compared across different dose cohorts following single-dose intravenous infusion (Fig. 3). Dose-normalized individual exposure parameters of etesevimab were also compared across dose cohorts (Fig. 3). Results consistently showed that the exposure parameters (Cmax and AUC) increased in an approximately proportional manner as the dose increased from 2.5 to 50 mg/kg.
FIG 3.
Comparison of exposure parameters (the first row) or dose-normalized exposure parameters (the second row) of etesevimab across different dose cohorts. Cmax, maximum concentration; AUC0–last, area under the serum concentration-time curve from time zero to the time of the last quantifiable concentration; AUC0–∞, area under the curve from time zero to infinity. Note that in each boxplot, the horizontal line at the center of the box is the median, the box represents the interquartile distance, and whiskers represent ≤1.5 times the interquartile range (75th to 25th quartile). In the first row, the value below the boxplot is the number of participants included in the analysis. Hollow circles represent outliers. The exposure parameters (Cmax and AUC) increased in an approximately proportional manner as the dose increased from 2.5 to 50 mg/kg. In the second row, the value below the boxplot is the number of participants included in the analysis. Hollow circles represent outliers. After dose normalization of exposure parameters, all boxes (cohorts) were distributed on a uniform horizontal line, indicating the exposure parameters (Cmax and AUC) increased in an approximately proportional manner as the dose increased from 2.5 to 50 mg/kg.
Population pharmacokinetics.
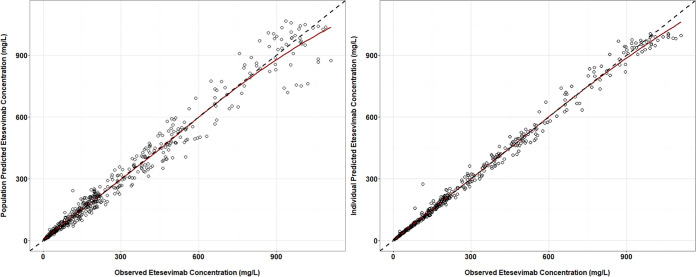
The goodness-of-fit plots for the population pharmacokinetic (PPK) model are shown in Fig. 4. The predicted concentrations of etesevimab were close to the actual data. The data points distributed evenly across the unit line. The R2 for population (PRED) or individual predicted concentration (IPRE) versus the observed concentration were 0.973 and 0.993, respectively.
FIG 4.
Goodness-of-fit plots for the population pharmacokinetic model of etesevimab. The black dashed line indicates the unit line. The red line indicates the locally weighted regression line (Loess).
The final parameter estimates for the PPK model are shown in Table 4. The typical values of clearance (CL) and volume of distribution in the central compartment (V1) were 0.129 liters/day and 3.27 liters, respectively. The interindividual variations were 13% and 12%, and their shrinkages were less than 1%. The typical values of the intercompartment clearance between the central and peripheral compartments (Q) and of the peripheral compartment (V2) were 0.47 liters/day and 2.06 liters, respectively.
TABLE 4.
Final parameter estimates for the population pharmacokinetic model of etesevimab
| Parameter | Unit | Typical value (RSE%)a | % IIV (RSE%)a | % shrinkage |
|---|---|---|---|---|
| CL | liters/day | 0.128 (2.3) | 12.8 (10.3) | <0.1 |
| V1 | liters | 3.26 (2.2) | 12.4 (12.4) | 0.7 |
| Q | liters/day | 0.47 (6.8) | ||
| V2 | liters | 2.04 (3.4) | 15.5 (14.4) | 16.2 |
| CV(residual) | % | 6.93 (4.0) | 7.5 |
IIV, interindividual variation; RSE, relative standard error.
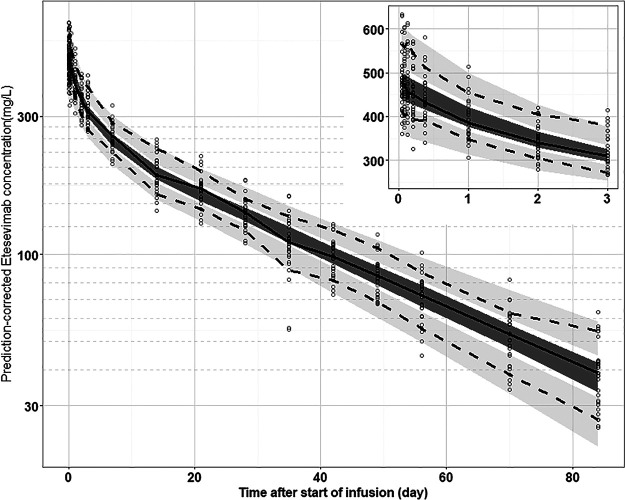
The prediction-corrected visual predictive check result for the PPK model is shown in Fig. 5. The median of observed data (solid line) was within the 95% CI of simulated data (deep shaded area). Similarly, the 90% and 10% percentile of observed data were also within the 95% confidence interval of simulated data (shallow shaded area). This indicated that the PPK model characterized time profiles of etesevimab quite well.
FIG 5.
Prediction-corrected visual predictive check (pcVPC) for final PPK model of etesevimab. The circles indicate the observed concentration corrected by prediction. The solid line is the median for prediction-corrected observed data, and the deep shaded area is the 95% confidence interval. The two dashed lines represent the 90% and 10% percentiles for prediction-corrected observed data, respectively, whereas the shallow shaded area corresponds to the 95% confidence interval. The inset graph shows the pcVPC results within 3 days following drug administration.
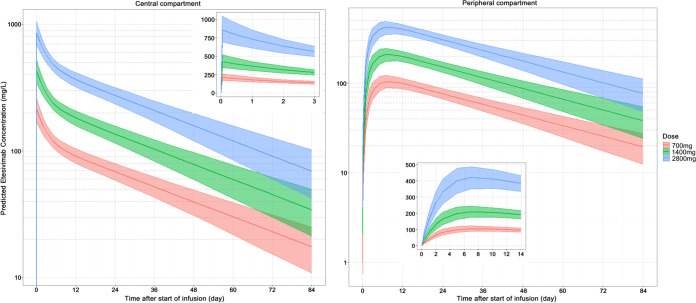
The simulation results for the PPK model of etesevimab are shown in Fig. 6. The linear characteristics of PK for etesevimab could be observed in the figure. For the central compartment, the AUC0–2016 for single doses of 700, 1,400, and 2,800 mg were 4,687, 9,305, and 18,833 mg·day/liter, respectively. The corresponding Cmax values were 212, 427, and 859 mg/liter, respectively. For the peripheral compartment, the Cmax values were 104, 209, and 422 mg/liter for doses of 700, 1,400, and 2,800 mg, respectively. The median of Tmax reached 6 to 8 days. The corresponding AUC0–2016 values were 4,583, 9,110, and 18,433 mg·day/liter, respectively.
FIG 6.
Simulation of a PPK model of etesevimab following a single administration. The left and right panels show the time profiles of etesevimab in the central and peripheral compartments, respectively. The doses are 700, 1,400, or 2,800 mg. The infusion time was 1 h. The solid line indicates the median of the predicted concentration and the shaded area indicates the 90% confidence interval.
Immunogenicity.
Three (7.5%) participants were anti-drug antibody (ADA) positive after a single-dose intravenous infusion of etesevimab, including 1 in the etesevimab 2.5 mg/kg group, 1 in the etesevimab 25 mg/kg group, and 1 in the placebo group (Table 5). The participant in the etesevimab 25 mg/kg group was ADA positive only on day 29, while the other 2 participants were ADA positive prior to randomization and remained positive throughout the study. The participant in the etesevimab 2.5 mg/kg group was ADA positive at day 181 after dosing, and the other participant in the placebo group refused to ADA testing after 6 months of dosing. No impact of ADA positivity was observed on the serum concentration of etesevimab or the safety profile.
TABLE 5.
Summary of anti-drug antibody after a single-dose intravenous infusion of etesevimab
| Etesevimab |
Placebo (n = 10) | Overall (n = 40) | |||||
|---|---|---|---|---|---|---|---|
| 2.5 mg/kg (n = 4) | 10 mg/kg (n = 12) | 25 mg/kg (n = 12) | 50 mg/kg (n = 12) | Combined (n = 30) | |||
| ADA test post dose | |||||||
| Negative | 2 (66.7%) | 9 (100%) | 8 (88.9%) | 9 (100%) | 28 (93.3%) | 9 (90.0%) | 37 (92.5%) |
| Positive | 1 (33.3%) | 0 | 1 (11.1%) | 0 | 2 (6.7%) | 1 (10.0%) | 3 (7.5%) |
| Time from randomization to initial ADA positive | |||||||
| Subject no. | 1 | 0 | 1 | 0 | 2 | 1 | 3 |
| Median (min, max) | 14 (14, 14) | 29 (29, 29) | 21.5 (14, 29) | 15 (15, 15) | 15 (14, 29) | ||
DISCUSSION
In the current phase 1 healthy Chinese volunteer study, etesevimab demonstrated a tolerable and safety drug profile following a single-dose intravenous infusion.
The amino acid sequence of etesevimab was derived from the human memory B cells of a COVID-19 convalescent patient. The formation of ADAs and related side effects are less likely since it has been screened by the human immune system (3). Etesevimab is an antibody of the IgG1 isotype. Considering that potential antibody-dependent enhancement (ADE) was observed in SARS-CoV (7, 8), point mutations (LALA mutation) were introduced into the Fc domain of the native human IgG1 antibody to potentially mitigate ADE, antibody-dependent cell-mediated cytotoxicity, and antibody-dependent cellular phagocytosis activities. In addition, the cytokine storm has been considered to play an important role in the deterioration of COVID-19 to severe and critical illness (9, 10). The introduction of LALA mutations to the Fc region of a monoclonal antibody has been shown to limit the activation of macrophages, which might lead to a cytokine storm and acute tissue damage (11). These provide the fundamentals a favorable safety profile of the drug.
The ability of etesevimab to reduce the viral load following preexposure prophylactic or postexposure therapeutic treatment was evaluated in vivo using a rhesus macaque SARS-CoV-2 infection model (study: RET-JS016-PC-0008-00). Nine male rhesus macaques were allocated to 3 experimental groups: control, treatment, and prophylaxis. All animals in the study were challenged with an inoculation dose of 1 × 105 50% tissue culture infectious dose (TCID50) SARS-CoV-2 virus via intratracheal inoculation. The control group was administered phosphate-buffered saline intravenously (i.v.) on days 1 and 3 postviral challenge. The treatment group received a 50 mg/kg i.v. dose of etesevimab on days 1 and 3 postchallenge. The prophylaxis group received an i.v. administration of etesevimab at 50 mg/kg 1 day prior to the viral challenge. Viral RNA load from oropharyngeal swabs was determined using quantitative reverse transcription-PCR and was monitored up to 7 days postchallenge. The study showed the viral load reached peak levels (approximately 106.5 RNA copies/ml) on day 4, and then declined thereafter in the control group; the viral load was lower on day 4 (averages of 103.5 and 102.1 RNA copies/ml) in the treatment and prophylaxis groups, respectively, compared with the control group. These data confirmed the in vivo ability of the test article to reduce the viral load under the experimental conditions of this study.
Eli Lilly and Company (Lilly) licensed etesevimab from Junshi Biosciences (Junshi) after it was jointly developed by Junshi Biosciences and the Institute of Microbiology, Chinese Academy of Science (IMCAS). Lilly has also successfully completed a phase 1 study (NCT04441931) of etesevimab (700, 2,800, and 7,000 mg) in healthy U.S. volunteers to evaluate the safety, tolerability, pharmacokinetics, and immunogenicity, but the results have not been published. In a Lilly-sponsored, phase 2 study (BLAZE-1), the efficacy results of etesevimab in COVID-19 patients together with another neutralizing antibody, bamlanivimab (LY3819253 or LY-CoV555), have been published and report promising efficacy in lowering the viral load, attenuating the symptoms of the illness, and decreasing the incidences of COVID-19-related hospitalization and emergency room (ER) visits (5). The safety profile of the combination therapy in patients with COVID-19 is comparable with that of the placebo. However, to date, no safety data or PK profile has been reported for healthy adults.
Throughout the safety follow-up period of up to 85 days after dosing in this study, no SAE, DLE, or AESI were experienced by any of the participants. Most of the TEAEs were mild, and the majority of them were lab abnormalities that recovered soon without any intervention. Based on their temporality and magnitude, these lab changes are more likely to reflect the participant variability rather than a legitimate safety signal. Theoretically, infusion of any protein product may result in injection-related topical reactions or hypersensitive reactions, including but not limited to injection site subcutaneous hemorrhage (erythema and bruise), pain, swelling, and pruritus and, in more serious cases, rashes, urticaria, angioedema, and anaphylactic shock (12, 13). During the study, no allergic reactions were observed, demonstrating the satisfactory safety of etesevimab.
We used the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (FDA, 2007) for AE reporting and severity classification. Compared with the commonly used CTCAE 5.0 grading scale, the current scale applied much stricter standards in reporting AEs and in grading their severity. Thus, the safety profile of etesevimab becomes even more favorable.
After a single intravenous infusion of etesevimab ranging from 2.5 mg/kg to 50 mg/kg, the mean t1/2 was around 27 to 30 days. The exposure parameters (Cmax, AUC0–last, and AUC0–∞) increased in a linearly proportional manner as the dose increased. The PK profiles of etesevimab in healthy volunteers showed typical monoclonal antibody distribution and elimination characteristics.
During the development of the PPK model, we attempted to construct both a two-compartment model and a three-compartment model. The objective function value for the three-compartment model was not significantly lower than that of the two-compartment model (3431.01 versus 3435.10), and the Akaike information criterion (AIC) was quite close (3453.01 versus 3453.10). Hence, we selected the two-compartment model as the base model.
The diagnostic plots of the PPK model showed that three data points had relative higher absolute value of conditional weighted residual error (CWRES) than that of other data points (the CWRES were below −4). We attempted to remove these three data points and repeated fitting. The results showed that parameter estimates were quite similar to that based on entire data set. For example, typical values of CL and V1 were 0.129 and 3.27, and they changed to 0.128 and 3.26, respectively, after removing three data points. Hence, we reported the PPK analysis results based on the entire data set.
ADA tested positive in 7.5% of the participants, including 2 in the etesevimab group and 1 in the placebo group. The ADA positivity did not impact on the serum concentration or safety profile of etesevimab. This should be confirmed with neutralizing antibody analysis in future studies. The vaccines have attracted great attention from the wider public (14–16). Although several types of vaccines have been approved for emergency use globally, their protection rates are reportedly approximately 50 to 70%, and large-scale vaccination requires a long time and strong public cooperation. The development of optimal antiviral drugs, such as etesevimab, is urgently needed worldwide.
This phase 1 study had some limitations. As we only evaluated healthy volunteers between 18 and 45 years in the study, no safety information is available for older adults with underlying conditions. Although etesevimab was demonstrated as a tolerable and safe product following a single-dose intravenous infusion in healthy adults, its antiviral efficacy could not be observed in this population. Based on the modeling-based pharmacologic activity of the virus’ inhibition capability, a single infusion of a relatively low dose of etesevimab was speculated to be sufficient for therapeutic and prophylactic treatment for SARS-CoV-2 infection, but this needs to be directly confirmed in patients. Currently, phase 2/3 studies to evaluate the efficacy of etesevimab monotherapy or etesevimab together with bamlanivimab in patients with COVID-19 are under way. More efficacy and safety data of etesevimab monotherapy or dual therapy with bamlanivimab in patients with COVID-19 are expected.
MATERIALS AND METHODS
Ethics statement.
The study was approved by the Ethics Committee of Huashan Hospital (no. 2020 Clinical Study Review no. 772). All participants were fully informed of the benefits, purpose, and risks of participating in this study and signed the informed consent form (ICF) prior to enrollment. This study was conducted in accordance with the guidelines of the National Medical Products Administration, Declaration of Helsinki, International Conference on Harmonization, and Good Clinical Practice.
Study design.
JS016-001-I was a single-center, randomized, double-blind, placebo-controlled, phase 1 study conducted in healthy Chinese volunteers to evaluate the tolerability, safety, PK, and immunogenicity of etesevimab following single intravenous ascending doses.
Four sequential ascending dose cohorts were included in this study: 2.5 mg/kg, 10 mg/kg, 25 mg/kg, and 50 mg/kg, and a total of 40 participants were enrolled randomly.
Randomization and blinding.
A randomization list was generated using SAS v9.4, and the randomization scheme was generated by an interactive web response system (IWRS) to randomly assign participants to receive a single dose of etesevimab or placebo (at a randomization ratio of 3:1) in one of four dose cohorts (2.5, 10, 25, and 50 mg/kg). Each antibody infusion preparation was provided under a coded, masked identification. The placebo (sterile saline) and active product preparations were indistinguishable. Nurses, study staff, investigators, and participants were blinded to the identity of the preparation.
Participants and drug administration.
Healthy Chinese males and nonpregnant, nonlactating females were eligible to enroll in the study if they were aged 18 to 45 years, had a body mass index (BMI) of 18 to 28 kg/m2, weight of ≥50 kg for males and ≥45 kg for females, and had no clinically relevant abnormalities. The complete inclusion and exclusion criteria are in the protocol summary. The investigational drug etesevimab or the matching placebo was administered by intravenous infusion for ≥60 min to the participants on day 1.
Safety assessment and dose escalation.
The study included a 12-week (85 days) safety follow-up period. Each participant underwent electrocardiograph monitoring within 2 h postdose. Physical examination, vital signs, laboratory tests, TEAEs, and concomitant medications were assessed at days 1, 2, 3, 4, 8, 15, 22, 29, 36, 43, 50, 57, 71, 85, or at early discontinuation. All TEAEs that occurred within 84 days after etesevimab administration, including all serious adverse events (SAEs), adverse events of special interest (AESIs), dose limiting events (DLEs), and new abnormal medical conditions were collected and assessed by the clinicians. In this study, AESIs refer to potential drug-induced liver injury (i.e., “Hy’s” rules for drug-induced liver injury/potential drug-induced liver injury) and injection related/allergic reactions. Adverse events were coded using The Medical Dictionary for Regulatory Activities and severity was graded using The Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (FDA, 2007). A DLE was defined as any drug-related TEAE graded as ≥3 according to this grading scale.
Escalation to a higher dose cohort was based upon safety evaluation of a lower dose cohort during a 14-day (2.5 mg/kg dose cohort) or a 7-day (other cohorts) DLE observation period. Dose-escalation rules are presented in the protocol (Text S1 in the supplemental material).
Pharmacokinetics and immunogenicity sample assessment.
PK samples were collected over 12 weeks within 4 h before dosing, within 5 min after the completion of dosing, and at the following postdose time points: 2, 3, 5, and 9 h postdose on day 1; 24, 48, and 72 h postdose on days 2, 3, and 4; and on days 8, 15, 22, 29, 36, 57, 71, and 85 postdose, or at early discontinuation. Immunogenicity samples were collected within 4 h before dosing, on days 15, 29, 57, and 85 postdose, or at early discontinuation. The participants with positive anti-drug antibody (ADA) at the last visit were asked to return to the study center for collection of immunogenicity samples at least 6 months (day 169) after dosing.
The serum concentrations of etesevimab were determined by a validated enzyme-linked immunosorbent assay (ELISA), the ELISA parameters are shown in Table 6. Anti-etesevimab antibodies in serum were detected with a validated bridging electrochemiluminescence immunoassay-affinity capture elution method based on the Meso Scale Discovery (MSD) platform. This method includes a tiered approach to first define positivity (tier 1), the specificity of the positivity assay (tier 2), and finally to titer the responses of samples confirmed to be specific (tier 3).
TABLE 6.
Parameters of the quantification methods of the serum concentration of etesevimaba
| Validation parameters | Result | Acceptance criteria |
|---|---|---|
| Method | Enzyme-linked immunosorbent assay (ELISA) analysis procedure | |
| Lower limit of quantitation | 78.1 ng/ml | |
| Upper limit of quantitation | 5,000 ng/ml | |
| Accuracy and precision | ULOQ and LLOQ: Intrabatch accuracy RE%: -6.9%∼11.1%; Intrabatch precision CV%: 1.7%∼10.2%; Interbatch accuracy RE%: 1.3%∼3.6%; Interbatch precision CV%: 8.2%∼10.2%; Total error between batches: 11.5%∼11.8% | Intra- and interbatch: ULOQ and LLOQ: CV%≤25%, RE% within ± 25%; QCs: CV%≤20%, RE% within ± 20%; Total error between batches; ULOQ and LLOQ: ≤40% QCs: ≤30% |
| QC measures: Intrabatch accuracy RE%: 9.0%∼7.5%; Intrabatch precision CV%: 1.3%∼6.5%; Interbatch accuracy RE%: -5.5%∼3.7%; Interbatch precision CV%: 5.9%∼17.6%; Total error between batches: 8.4%∼23.1% | ||
| Dilute the linear | 500 times, 50 times, 5 times and 2 times | At least 3/5 of the samples for liner verification of each level dilution should be within 20% of the accuracy (Re%). The precision of the samples with the same final concn and different dilution ratios should be ≤20% |
| Specificity | The addition of interfering agent 2019-nCoVS protein RBD or anti-JS016 polyclonal antibody did not meet the criteria | (i) The back-calculated concn of blank matrix was BQL; (ii) LLOQ and ULOQ: Re% within ±25%; (iii) If the above criteria cannot be met, the actual results can be reported |
ULOQ, upper limit of quantitation; LLOQ, lower limit of quantitation; QC, quality control; CV, coefficient of variation; RE, relative error; BQL, below the quantification limit; RBD, receptor binding domain.
Pharmacokinetic analysis.
The drug concentration data were analyzed to obtain the PK parameters by a noncompartmental analysis method using Phoenix WinNonlin software (v8.2, Certara USA, Inc., New Jersey, US). The actual sampling time was used for the calculation of PK parameters, including maximum concentration (Cmax), time to reach Cmax (Tmax), area under the serum concentration-time curve from time zero to the time of the last quantifiable concentration (AUC0–last). If the data permitted, the elimination rate constant, (kel [λz]), area under the serum concentration-time curve from time zero to infinity (AUC0–∞), elimination half-life (t1/2), clearance (CL), volume of distribution (Vz), and mean residence time (MRT) were further calculated. The calculation method of these parameters has been described previously (17).
A population pharmacokinetic (PPK) model was developed to characterize the time profiles of etesevimab in healthy Chinese participants. The base model was a two-compartment model, where both the distribution and elimination process were linear. The PK parameters include clearance (CL), distribution volume of the central compartment (V1) and peripheral compartment (V2), and the intercompartment clearance between central and peripheral compartment (Q). The interindividual variation for these PK parameters were assumed to be consistent with normal distribution where the mean was zero and the variance was ω2. Residual error was specified as proportional model, where the proportional error term was normally distributed with mean zero and variance δ2. Because the study object was healthy participants instead of patients, the value of covariates were narrow, so the covariate screening was not performed in the PPK study. Prediction-corrected visual predictive check (pcVPC) was used to validate the PPK model (18). Model simulation was performed 1,000 times using final estimates of parameter. The PPK model was developed based on first-order conditional estimation method using Intel Fortran Compiler (GCC, Ver 4.6.0, Free Software Foundation, Inc.), NONMEM (Ver 7.4.4, ICON Development Solutions, MD, USA), R (Ver 3.6.3, The R Foundation for Statistical Computing), and Xpose4 (ver 4.7.0 Uppsala University, Sweden).
To observe the effect of fixed dose on the time profiles of etesevimab, simulation of the PPK model was performed. The regimens were single administration with infusion time 1 h, and the doses included 700 mg, 1,400 mg, and 2,800 mg etesevimab. One thousand simulations for each subject were performed using final parameter estimates. Based on simulation data, the AUC0–2016 and Cmax were calculated using the noncompartmental analysis method (17).
Statistical analysis.
Descriptive statistics with SAS 9.4 were used to show the baseline demographic information and safety data of the participants. The sample size was determined based on the confidence intervals (CIs) observed around a range of adverse event (AE) rates; with an AE rate of 25%, the CI would be 11.5% to 43.4% with 40 participants, considering a 20% drop-out rate.
R software (v3.5.0, R core team [2018], R foundation for statistical computing, Vienna, Austria) was used for the generation of figures. For continuous variables, the number of nonmissing observations, mean (arithmetic mean, geometric mean), standard deviation (SD), coefficient of variation in percentage (CV%, including arithmetic CV% and geometric CV%), median, minimum, and maximum, and for categorical variables, frequency and percentage were carried out using SAS (v9.4). The time and proportion of etesevimab ADA positivity were analyzed in the dose cohort group and in total for immunogenicity. There was no imputation of missing data.
ACKNOWLEDGMENTS
We thank all the volunteers for their contribution and commitment to COVID-19 research. The authors also thank Acumen Medical Information & Technology Co., Ltd., for statistical analysis, and Linking Truth Technology Co., Ltd., for pharmacokinetic analysis. We also acknowledge the contributions of research team members not listed. Xiaojie Wu is the project manager for the JS016 (i.e., etesevimab) clinical trial, while Nanyang Li is the assistant project manager for the JS016 clinical trial.
This study was sponsored and funded by Shanghai Junshi Bioscience Co., Ltd., supported by Science and Technology Supporting Project by Shanghai Municipal Science and Technology Committee (20S11908200), the New Drug Creation and Manufacturing Program of the Ministry of Science and Technology of China (2017ZX09304005), and the National Natural Science Foundation of China (82041010).
Guoqin Wang was an employee of Junshi Bioscience Co., Ltd., before 8 Mar 2021.
Ning Li, Sheng Yao, Hui Feng, Wei Liu, and Juan Ma are employees of Junshi Bioscience Co., Ltd.
All other authors have no conflict of interest.
Contributor Information
Wenhong Zhang, Email: zhangwenhong@fudan.edu.cn.
Jing Zhang, Email: zhangj_fudan@aliyun.com.
REFERENCES
- 1.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, Mourão MPG, Brito-Sousa JD, Baía-da-Silva D, Guerra MVF, Hajjar LA, Pinto RC, Balieiro AAS, Pacheco AGF, Santos JDO, Jr, Naveca FG, Xavier MS, Siqueira AM, Schwarzbold A, Croda J, Nogueira ML, Romero GAS, Bassat Q, Fontes CJ, Albuquerque BC, Daniel-Ribeiro CT, Monteiro WM, Lacerda MVG, CloroCovid-19 Team. 2020. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 3:e208857. 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, et al. 2020. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382:1787–1799. 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi R, Shan C, Duan X, Chen Z, Liu P, Song J, Song T, Bi X, Han C, Wu L, Gao G, Hu X, Zhang Y, Tong Z, Huang W, Liu WJ, Wu G, Zhang B, Wang L, Qi J, Feng H, Wang FS, Wang Q, Gao GF, Yuan Z, Yan J. 2020. A human neutralizing antibody targets the receptor-binding site of SARS- CoV-2. Nature 584:120–124. 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- 4.Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Adams AC, Van Naarden J, Custer KL, Shen L, Durante M, Oakley G, Schade AE, Sabo J, Patel DR, Klekotka P, Skovronsky DM, BLAZE-1 Investigators. 2021. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 384:229–237. 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Klekotka P, Shen L, Skovronsky DM. 2021. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: a Randomized Clinical Trial. JAMA 325:632–644. 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, Perry C, Pan C, Hosain R, Mahmood A, Davis JD, Turner KC, Hooper AT, Hamilton JD, Baum A, Kyratsous CA, Kim Y, Cook A, Kampman W, Kohli A, Sachdeva Y, Graber X, Kowal B, DiCioccio T, Stahl N, Lipsich L, Braunstein N, Herman G, Yancopoulos GD, Trial Investigators. 2021. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 384:238–251. 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. 2016. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 19:181–193. 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, Tang H, Nishiura K, Peng J, Tan Z, Wu T, Cheung KW, Chan KH, Alvarez X, Qin C, Lackner A, Perlman S, Yuen KY, Chen Z. 2019. Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 4:e123158. 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellgrau D, Modiano JF. 2021. The cytokine storm—an appropriate, over-reactive response to SARS-CoV-2 or the wrong immune pathway? Scand J Immunol 93:e12979. 10.1111/sji.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asrani P, Hassan MI. 2021. SARS-CoV-2 mediated lung inflammatory responses in host: targeting the cytokine storm for therapeutic interventions. Mol Cell Biochem 476:675–687. 10.1007/s11010-020-03935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Peng Y, Wang R, Jiao S, Wang M, Huang W, Shan C, Jiang W, Li Z, Gu C, Chen B, Hu X, Yao Y, Min J, Zhang H, Chen Y, Gao G, Tang P, Li G, Wang A, Wang L, Zhang J, Chen S, Gui X, Yuan Z, Liu D. 2020. Characterization of neutralizing antibody with prophylactic and therapeutic efficacy against SARS-CoV-2 in rhesus monkeys. Nat Commun 11:5752. 10.1038/s41467-020-19568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. 2010. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 9:325–338. 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Li Y. 2014. The unexpected side effects and safety of therapeutic monoclonal antibodies. Drugs Today (Barc) 50:33–50. 10.1358/dot.2014.50.1.2076506. [DOI] [PubMed] [Google Scholar]
- 14.Shah DK, Betts AM. 2013. Antibody biodistribution coefficients: inferring tissue concentrations of monoclonal antibodies based on the plasma concentrations in several preclinical species and human. MAbs 5:297–305. 10.4161/mabs.23684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagotto G, Yu J, Barouch DH. 2020. Approaches and challenges in SARS-CoV-2 vaccine development. Cell Host Microbe 28:364–370. 10.1016/j.chom.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. 2019. COVID-19 vaccines. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines.
- 17.Wu XJ, Zhang J, Guo BN, Zhang YY, Yu JC, Cao GY, Chen YC, Zhu DM, Ye XY, Wu JF, Shi YG, Chang LW, Chang YT, Tsai CY. 2015. Pharmacokinetics and pharmacodynamics of multiple-dose intravenous nemonoxacin in healthy Chinese volunteers. Antimicrob Agents Chemother 59:1446–1454. 10.1128/AAC.04039-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]