SUMMARY
Background:
The number of persons on antiretroviral therapy (ART) requiring treatment monitoring in low-resource settings is rapidly increasing. Point-of-care (POC) testing for ART monitoring may alleviate burden on centralized laboratories and improve clinical outcomes, but its cost-effectiveness is unknown.
Methods:
We used cost and effectiveness data from the STREAM trial in South Africa, which evaluated POC testing for viral load, CD4 count, and creatinine, with task-shifting from professional to lower-cadre registered nurses compared to laboratory-based testing without task-shifting. We parameterized an agent-based network model, EMOD-HIV, to project the impact of implementing this intervention in South Africa. We assumed POC monitoring increased viral suppression by 9%, enrollment into community-based ART delivery by 25%, and switching to second-line ART by 1%, as reported in STREAM. We evaluated POC implementation in varying clinic sizes (10–50 patient initiating ART/month) over 20 years. We used a cost-effectiveness threshold of $500 USD/disability adjusted life year (DALY) averted for our main analysis.
Findings:
POC testing at 70% coverage of ART patients was projected to reduce HIV infections by 4.5% and HIV-related deaths by 3.9%. In clinics with 30 ART initiations/month, the intervention had an incremental cost-effectiveness ratio (ICER) of $197/DALY averted (90% model variability: −$27, $863); results remained cost-effective when varying background viral suppression, ART dropout, and intervention effectiveness. Assuming POC testing did not increase enrollment into community ART delivery produced an ICER of $1,149 (90% model variability: $184, $3,886), exceeding the cost-effectiveness threshold. At higher clinic volumes (≥40 ART initiations/month), POC testing was cost-saving and at lower clinic volumes (20 patients initiating ART/month) the ICER was $734 (90% model variability: $184, $3,886).
Interpretation:
POC testing is a promising strategy to cost-effectively improve patient outcomes in moderately-sized clinics in South Africa. Results are most sensitive to changes in intervention impact on enrollment into community-based ART delivery.
Funding:
National Institutes of Health
Keywords: Point-of-care testing, viral load testing, ART monitoring, sub-Saharan Africa, South Africa, cost-effectiveness, modeling
INTRODUCTION
The majority of the 37 million people living with HIV (PLHIV) globally reside in resource-limited settings. Successful scale-up of antiretroviral therapy (ART) through “test and treat” has transformed HIV from a terminal disease into a manageable chronic illness. PLHIV who are virally suppressed on ART have good clinical outcomes and a near normal life expectancy. Additionally, viral suppression virtually eliminates transmission to sexual partners, providing hope that treatment as prevention can help end the HIV epidemic. Currently, over 25 million individuals receive ART worldwide, and reaching UNAIDS’ 95–95-95 targets will dramatically increase number of persons on ART by 2030.(1)
Monitoring the millions of PLHIV in HIV care remains challenging in resource-limited settings. World Health Organization (WHO) recommends routine testing of HIV viral load (VL), CD4 count, and creatinine for patients receiving ART.(2) However, less than 50% of PLHIV on ART in sub-Saharan Africa (SSA) receive routine VL monitoring.(3) Most monitoring tests in SSA are processed in centralized laboratories requiring highly trained staff and specialized equipment. Scale-up is hindered by challenges of timely sample transportation, insufficient capacity to meet demand, and inadequate infrastructure.(4) Further, among patients receiving monitoring, a second clinic visit after several weeks is required to obtain results, which can cause delays in clinical decision making, non-delivery of results, or lack of adherence counseling provision.(3)
Decentralized point-of-care (POC) testing for ART monitoring is a promising strategy to expand testing coverage and improve clinical outcomes by decreasing turn-around time for results and reducing loss-to-follow-up among viremic patients. A recent randomized clinical trial (STREAM) evaluated the impact of POC testing for VL, creatinine, and CD4 combined with task-shifted care to lower-cadre nurses compared to standard lab-based testing without task-shifting among ART patients in South Africa. The intervention improved viral suppression and increased referral to ART differentiated service delivery (DSD).(5) However, the economic impact of implementing POC testing for lifelong ART management is uncertain. To inform policy discussions about ART monitoring guidelines, we utilized mathematical modeling to project the health and economic impact of scaling-up the STREAM intervention in South Africa.
METHODS
STREAM clinical trial
We utilized cost and effectiveness data from the STREAM (Simplifying HIV TREAtment and Monitoring) randomized clinical trial.(5) The trial was conducted from February 2017 to October 2018 at a large public clinic providing care for a diverse, mobile population in Durban, South Africa. Eligible participants were HIV-positive adults presenting for routine care six months post-ART initiation; individuals who were pregnant, diagnosed with active tuberculosis, or required acute medical care by a physician were excluded. Participants (N=390) were randomized to receive 1)POC testing for VL, creatinine, and CD4 count testing with same-day counseling and task-shifted care by an enrolled nurse or 2)standard-of-care laboratory testing and care by a professional nurse. All patients received treatment monitoring according to South African and WHO guidelines (Figure S1, page 9). Individuals with VL>1,000 copies/mL received enhanced adherence counselling and repeat VL testing after two months. If the repeat VL was >1,000 copies/mL, participants were offered to switch to second-line ART. Based on South African guidelines, participants on ART for ≥12 months with two consecutive VL<40 copies/mL and CD4 count>200 cells/mm3 were referred to DSD to collect ART at community pharmacies.
Costs
We conducted a detailed microcosting and time-and-motion observation of the STREAM trial to estimate costs of POC VL, CD4, and creatinine testing, described previously (and in the Appendix, page 8).(6) Since the equipment component of the test cost depends on number of tests conducted, we calculated POC test costs per patient at varying clinic volumes of 10–50 PLHIV initiating ART/month (Table 1). At higher volumes, instrument costs are spread over a greater number of tests and the cost per test is lower. Centralized laboratory costs were obtained from South African National Health Laboratory Service price lists. HIV tests, ART provision, and healthcare costs were obtained from literature and inflated to 2018 US dollars (USD).
Table 1:
HIV-related healthcare costs for South Africa *
| Parameter | Cost |
|---|---|
| Facility HIV test(32) | |
| HIV− test | $3.62 |
| HIV+ test | $5.62 |
| ART costs per year (including supply chain)(18) | |
| 1st line regimen* | $90 |
| 2nd line regimen§ | $318 |
| ART program costs per year (health facility) | $80 |
| ART program costs per year (DSD due to VL <1000) | $40 |
| Annual health-care for HIV-positive persons not in care(33) | |
| CD4 count >350 cells per μL | $11 |
| CD4 count >200–350 cells per μL | $39 |
| CD4 count ≤200 cells per μL | $142 |
| End of life care (per death) | $136 |
| Diagnostic tests (centralized laboratory)(6) | |
| CD4 | $6.03 |
| VL test | $25.98 |
| Creatinine | $3.41 |
| Diagnostic tests (POC) by monthly clinic volume(6) ¥ | |
| Clinic volume 10 | |
| CD4 | $24.41 |
| VL | $46.51 |
| Creatinine | $9.71 |
| Clinic volume 20 | |
| CD4 | $16.09 |
| VL | $33.31 |
| Creatinine | $9.14 |
| Clinic volume 30 | |
| CD4 | $13.32 |
| VL | $28.91 |
| Creatinine | $8.96 |
| Clinic volume 40 | |
| CD4 | $11.94 |
| VL | $26.71 |
| Creatinine | $8.86 |
| Clinic volume 50 | |
| CD4 | $11.11 |
| VL | $25.39 |
| Creatinine | $8.81 |
ART: antiretroviral therapy, DSD: differentiated service delivery, POC: point-of-care, VL: viral load. HIV test and diagnostic test costs refer to cost per test conducted.
1st line ART regimen consisting of tenofovir, lamivudine, and efavirenz
2nd line ART regimen consisting of lopinavir/ritonavir
Clinic volume refers to the number of patient initiating ART per month
Mathematical model
We adapted a previously developed microsimulation model, EMOD-HIV, described in detail at www.idmod.org/idmdoc and elsewhere.(7) EMOD-HIV is an open-source, stochastic, agent-based model integrating population demography, HIV disease progression, and network-based HIV transmission, configured to match age- and sex-specific propensities to form different sexual partnerships. The model incorporates detailed within-host HIV progression to simulate HIV health and transmission effects and the impact of ART on epidemic dynamics. EMOD-HIV includes a highly configurable HIV care cascade, including HIV testing, linkage and retention in care, time-varying treatment eligibility, ART dropout, and heterogeneity in treatment engagement by age and sex. Model validation is shown to reproduce age and sex-specific HIV incidence in KwaZulu-Natal, South Africa as confirmed by epidemiologic and phylogenetic studies.(8–10) The model tracks health outcomes including HIV infections and HIV-related deaths and healthcare utilization, enabling calculation of economic costs and disability-adjusted life years (DALYs). We simulated approximately 175,000 individuals per model run.
The model was parameterized with epidemiologic data from South Africa including fertility, mortality, voluntary male circumcision coverage, and health seeking behavior. We calibrated the model to South Africa data on age- and sex-specific HIV prevalence, ART coverage, population size and validated to HIV incidence (Appendix, page 12–20). Model calibration was performed using a parallel simultaneous perturbation optimization (PSPO) algorithm which maximizes the pseudo-likelihood of stochastic epidemiological models given observed data and identifies an optimal set of input parameters. We selected 250 model parameter sets using roulette resampling in proportion to the likelihood of each simulation.
Scenarios
In the standard-of-care scenario, we assumed individuals initiating ART received clinical management by a professional nurse and monitoring using centralized laboratory tests. Based on the literature for South Africa, we assumed 3% of ART patients were on second-line regimens, 20% of individuals on ART collected drugs through DSD,(11, 12) and 83% of ART patients were virally suppressed (VL<1000 copies/mL).(13) In the POC intervention scenario, we assumed 70% of individuals on ART received monitoring using POC testing and task-shifted care by an enrolled nurse, based on acceptance rates in the STREAM trial and the assumption that POC testing would not be uniformly rolled out in all HIV clinics in South Africa. The remaining 30% of patients received standard-of-care laboratory monitoring from a professional nurse and were assumed to have the same health outcomes as individuals in the standard-of-care scenario. Among those receiving POC monitoring, we assumed a 1% increase in switching to second-line treatment, 25% higher referral to DSD and 9% higher viral suppression on ART, for the duration of time on ART, as reported in STREAM. We assumed individuals on ART but not virally suppressed experience 1.96-times the mortality risk of those who are suppressed for the duration of time they are on ART.(14) Based on clinical data, we estimated that PLHIV on ART with VL<1,000 copies/mL experience a 96% reduction in HIV transmission compared to PLHIV not on ART.(15, 16) Individuals on ART with VL>1,000 copies/mL were estimated to have a 35% reduced risk of HIV transmission compared to those not on ART; due to uncertainty in this parameter, we varied it from 0–70% in sensitivity analyses(15, 16) (Appendix, page 10–11).
In all scenarios, we assumed PLHIV on ART received treatment monitoring according to WHO guidelines: 1) ART initiation: creatinine and CD4 count; 2) Month 3: creatinine; 3) Month 6: creatinine and VL; 4) Month 12: CD4 count, creatinine, VL; 5) Annual visit after first year: creatinine, VL. Individuals found to be unsuppressed (VL>1000) were assumed to have a repeat VL after 2 months with additional adherence counseling.(2) All scenarios were implemented for the duration of the modeled time horizon.
Cost effectiveness analysis
We calculated incremental cost-effectiveness ratios (ICERs) as difference in costs divided by difference in effects in intervention versus standard-of-care scenarios across each of the 250 parameter sets. We report the mean and 90% model variability of 250 ICERs. We utilized a 20-year time horizon for costs and DALYs and discounted both outcomes at 3% annually.(17) In light of ongoing deliberations regarding the appropriate threshold at which to identify efficient interventions, we utilized two cost-effectiveness thresholds: $500/DALY averted, frequently used in economic evaluations in SSA, and $1,175/ DALY averted, calculated as an appropriate threshold for South Africa by a panel of economic experts to reflect the opportunity costs of additional health investment.(18, 19) Cost-effectiveness analyses were conducted using R software (2019).
Budget impact analysis
We calculated the undiscounted total cost of implementing the POC testing intervention at varying clinic volumes compared to standard-of-care over 5 and 20 years, including intervention and healthcare costs, both incurred and averted.
Sensitivity analyses
In addition to evaluating parameter uncertainty across 250 good-fitting sets, we conducted extensive sensitivity analyses to assess influence of uncertain parameters. We varied ART dropout, HIV infectivity if unsuppressed on ART, background viral suppression, clinic size, infectivity on ART if not virally suppressed, intervention impact on viral suppression, intervention impact on switching to both second-line ART and DSD, ART costs, and discount rate.
RESULTS
Table 2 displays the health impact and cost-effectiveness of implementing POC testing with task-shifting for ART monitoring under varying assumptions. In the baseline scenario, assuming 70% coverage of ART patients, the intervention was projected to avert 4.5% of HIV infections and 3.9% of HIV-related deaths. The intervention was cost-saving at clinic volumes of ≥40 patients initiating ART/month. At clinic volumes of 30 patients initiating ART/month, ICERs fell below the $500/DALY averted threshold. At the higher threshold ($1,175/DALY averted), the intervention was cost-effective at clinic volumes of ≥20 monthly ART initiations. Assuming lower background viral suppression on ART (71% vs. 83%) yielded slightly lower HIV infections averted (4.0%) and higher ICERs, although cost-effectiveness results remained the same as the baseline scenario. Similarly, doubling the annual ART dropout rate from 5% to 10% resulted in lower health benefits: 3.8% and 3.7% infections and HIV-related deaths averted, respectively, but cost-effectiveness results remained the same as the baseline scenario. Assuming lower intervention effectiveness (5% increase in viral suppression) nearly halved the projected health benefits (2.2% of HIV-related deaths and HIV infections averted respectively); ICERs were considerably higher, particularly at clinic volumes of 10 and 20 monthly ART initiations, although cost-effectiveness results remained the same. Assuming a higher intervention effectiveness (15% increase in viral suppression) increased health benefits to 6.6% and 6.9% HIV-related deaths and infections averted, respectively; the intervention was considered cost-effective at clinic volumes of ≥20 at the $500/DALY averted threshold. Using the threshold of $1,175/DALY averted, the intervention was cost-effective at all clinic volumes assessed. Similarly, assuming PLHIV on ART with unsuppressed VL had the same HIV transmissibility as PLHIV not on ART resulted in greater infections averted compared to the baseline scenario; the intervention was cost-effective at the $500/DALY threshold at clinic volumes of ≥20 monthly ART initiations and all clinic volumes were cost-effective at the higher threshold. Conservatively assuming that PLHIV on ART with unsuppressed VL had a 2-fold higher reduction in HIV transmissibility (70% vs. 35%) compared to PLHIV not on ART, resulted in lower intervention health benefits and higher ICERs. Using both thresholds, the intervention was cost-effective at clinic volumes with ≥30 monthly ART initiations.
Table 2:
Health impact and cost-effectiveness of point-of care testing intervention across varying assumptions§
| Baseline | Lower background viral suppression on ART | 2X higher ART dropout | Lower bound intervention effectiveness | Upper bound intervention effectiveness | No reduction in HIV transmission among those on ART w/ VL>1000 | 2X higher reduction in HIV transmission among those on ART w/ VL>1000 | |
|---|---|---|---|---|---|---|---|
| Health Impact (%) | |||||||
| HIV infections averted | 4.5 (1.6, 7.6) | 4.0 (0.8, 7.1) | 3.8 (1.1, 6.4) | 2.2 (0.5, 5.2) | 6.9 (3.2, 10.2) | 5.6 (2.6, 8.6) | 2.6 (−1.0, 6.9) |
| HIV deaths averted | 3.9 (2.0, 6.0) | 3.8 (1.7, 5.9) | 3.6 (1.7, 5.3) | 2.2 (0.1, 4.1) | 6.6 (4.4, 8.6) | 4.2 (2.3, 6.0) | 3.6 (1.2, 5.6) |
| Cost-effectiveness ($ per DALY averted) by monthly clinic volume (number of ART initiations/month) | |||||||
| Clinic volume: 50 | −239 (−602, −96) | −229 (−721, −100) | −107 (−245, −36) | −209 (−582, −103) | −358 (−464, −103) | −161 (−281, −98) | −126 (−410, −18) |
| % under $500 threshold | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
| Clinic volume: 40 | −72 (−176, 26) | −50 (−142, 144) | 40 (−68, 279) | −45 (−145, 63) | −82 (−125, −79) | −75 (−122, −18) | −39 (−175, 140) |
| % under $500 threshold | 100% | 99% | 97% | 98% | 100% | 100% | 98% |
| Clinic volume: 30 | 197 (−27, 863) | 242 (−39, 1,051) | 280 (−2, 1079) | 319 (−14, 908) | 49 (−41, 276) | 63 (−36, 328) | 374 (−17, 1,373) |
| % under $500 threshold | 93% | 87% | 91% | 85% | 97% | 97% | 87% |
| Clinic volume: 20 | 734 (93, 2,569) | 824 (69, 3,190) | 757 (107, 2,546) | 1045 (111, 2,960) | 305 (58, 890) | 339 (63, 1,075) | 1,197 (117, 4,567) |
| % under $500 threshold | 72% | 66% | 68% | 49% | 88% | 85% | 58% |
| Clinic volume: 10 | 2,348 (436, 7,681) | 2,571 (380, 9,720) | 2,190 (421, 6,779) | 3,226 (494, 9,159) | 1,073 (344, 2,738) | 1,169 (341, 3,213) | 2,451 (503, 8,474) |
| % under $500 threshold | 9% | 13% | 10% | 5% | 28% | 22% | 5% |
Values in parenthesis represent 90% model variability across 250 simulations. Values in green represent scenarios where the mean ICER is considered cost-effective at both thresholds of $500 and $1,175 per DALY averted. Values in yellow represent scenarios where the mean ICER is considered cost-effective using only the threshold of $1,175 per DALY averted and values in red exceed both thresholds. VL: Viral load
Lower background viral suppression on ART: 71%; 2X higher ART dropout: 10% annual dropout; Lower bound intervention effectiveness: 5% increase in viral suppression compared to SOC; Upper bound intervention effectiveness: 15% increase in viral suppression compared to SOC; No reduction in HIV transmission among those on ART w/ VL>1000: Individuals on ART with VL>1000 have same HIV transmissibility as those not on ART; 2X higher reduction in HIV transmission among those on ART w/ VL>1000: Individuals on ART with VL>1000 have 70% reduction in HIV transmissibility compared to those not on ART
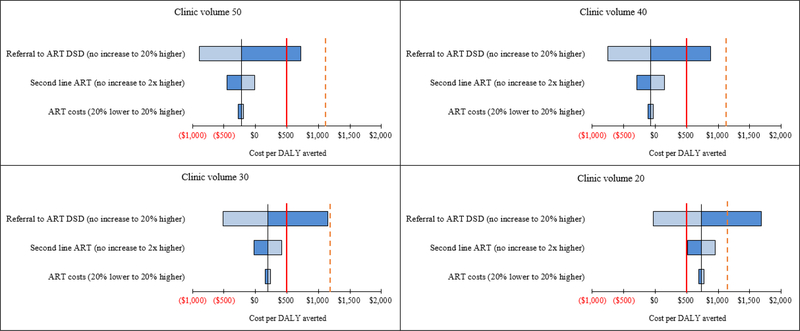
Figure 1 and Table S9 show the impact of varying healthcare costs on ICERs in the baseline scenario. Across clinic volumes, ICERs were most sensitive to changes in proportion of patients receiving ART through DSD, which was assumed to incur lower ART program costs than clinic-based ART delivery. Increasing the proportion of patients referred to DSD by the intervention by 20% (65% vs. 45%) resulted in cost-saving ICERs for clinic volumes of ≥20 monthly ART initiations. Conversely, assuming no intervention impact on DSD (i.e. 20% of patients referred to DSD in both standard-of-care and intervention), yielded ICERs that were no longer cost-saving and exceeded the $500/DALY averted across all clinics volumes. However, using the threshold of $1,175/DALY averted, the intervention was still cost-effective at clinic volumes of ≥30 monthly ART initiations. Increasing the proportion of patients on second-line ART in the intervention scenario (from 1% to 2% higher than standard-of-care) resulted in higher ICERs while assuming no increase in second-line ART by the intervention lowered the ICERs. Neither scenario altered cost-effectiveness results. Similarly, varying ART costs, discount rate (0–6%), POC testing coverage (60–80%), and conducting a probabilistic sensitivity analysis around POC intervention costs minimally impacted ICERs (Table S10). Across sensitivity analyses, ICERs for clinics with 10 monthly ART initiations exceeded both thresholds.
Figure 1: Influence of varying healthcare costs on ICERS by clinic volume.
ART, antiretroviral therapy; DALY, disability-adjusted life years; ICER, incremental cost-effectiveness ratio. Clinic volume is defined as number of ART initiations per month. The solid red line represents the cost-effectiveness threshold of $500 and per DALY averted and the dashed red line represents the threshold of $1,175 per DALY averted.
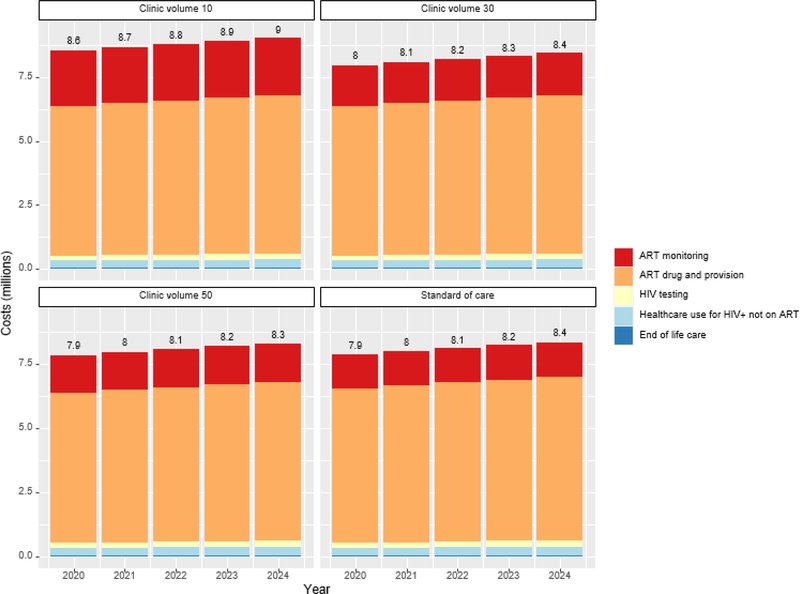
Figure 2 and Table S11 display the 5-year undiscounted healthcare costs of implementing the POC testing intervention at varying clinic volumes and standard-of-care testing for a population of 175,000 adults. Costs ranged from $40.4–44.0 million depending on scenario, with ART drugs and provision making up the majority of costs (>75%). POC ART monitoring costs increased with clinic volume, making up 18% of costs for clinic with 50 monthly ART initiations and 22% of costs at clinics with 20 ART initiations/month. At clinic with 40 ART initiations/month, the intervention cost approximately $54,000 more than standard-of-care, indicating that although ICERs show cost-savings over a 20-year time horizon, the intervention would not save costs in the near-term. Incremental costs compared to SOC were $423,000 at clinics with 30 ART initiations/month and rapidly increased with lower clinic volume, exceeding 1.16 million at clinic with 20 ART initiations/month. With a 20-year time horizon, annual costs increase over time (Figure S6) but intervention costs relative to standard of care decrease over time for all clinic volumes (Figure S7). At clinics with 30 ART initiations/month, the intervention becomes cheaper than standard-of-care by year 2031.
Figure 2: Budget impact.
Undiscounted annual healthcare costs of implementing the point-of-care testing intervention at varying clinic volumes and standard-of-care laboratory testing for a population of 175,000 adults. Clinic volume refers to the number of patient initiating ART per month.
DISCUSSION:
Our model-based analysis assessed the population-level impact of implementing POC testing for VL, CD4 count, and creatinine with task-shifted HIV care in South Africa. We find that the intervention can reduce HIV transmission and HIV-related mortality and is cost-effective in moderately-sized clinics in South Africa. In clinics with ≥30 ART initiations per month, the intervention fell below $500/DALY averted; results were robust to changes in background viral suppression on ART, treatment dropout, intervention effectiveness, and HIV transmissibility on ART. Using a higher threshold of $1,175/DALY averted, the intervention was considered cost-effective at lower clinic volumes of ≥20 monthly ART initiations, which was robust to most sensitivity analyses. At smaller clinic volumes with 10 monthly ART initiations, ICERs exceeded both thresholds in all but the most optimistic sensitivity analyses.
Cost-effectiveness results were most sensitive to changes in proportion of patients referred to DSD by the intervention, which was the main driver of the finding that the intervention resulted in cost-savings in higher clinic volumes. When assuming POC testing did not increase patient referrals to DSD, ICERs exceeded the conservative threshold for all clinic volumes, although the intervention was still cost-effective using the higher threshold ($1,175/DALY averted) at clinic volumes of ≥30 monthly ART initiations. Our results highlight the importance of decentralized services in increasing efficiency of ART delivery. DSD provides a client-centered alterative to standard clinical care that allows stable patients to obtain ART refills from local pharmacies or community groups at lower frequencies (every 3–6 months) than standard-of-care. This reduces patient time spent traveling and waiting for care while also decongesting busy clinics, reducing healthcare worker burnout, and allowing providers to focus on counseling patients most at risk of treatment failure.(20, 21) A systematic review found DSD can improve patient retention in care.(20) As the number of PLHIV on ART rapidly grows in resource-limited settings, decentralized approaches to ART care including DSD and POC testing are becoming increasingly vital to sustainably delivering high-quality care.
The 5-year budget impact analysis shows that, although cost-effective, implementing POC testing for VL, CD4 count, and creatinine requires substantial upfront investment. While the intervention is cost-saving in clinics with ≥50 monthly ART initiations, POC testing would cost approximately $420,000 more than standard of care over 5 years for clinic volumes of 30 monthly ART initiations and more than $1.16 million more for clinic volumes of 20 patients initiated on ART per month. These costs assume a population size of 175,000 so would increase rapidly with coverage of larger populations. However, annual costs compared to standard-of-care decline over time as clinical benefits of POC testing are realized and HIV infections and deaths averted result in costs savings to the healthcare system. At clinic volumes of ≥30 monthly ART initiations, the intervention eventually becomes cheaper than standard-of-care within a 20-year time horizon. ART drugs and provision made up the vast majority of the costs; however drug monitoring costs are also considerable.
The higher drug monitoring costs in the intervention scenarios relative to standard-of-care are largely driven by costs of POC creatinine, which is considerably more expensive as a POC test. While the cost of CD4 count testing is also higher when administered as POC, the frequency of CD4 testing is low compared to creatinine: WHO guidelines recommend CD4 monitoring only twice in the first year while creatinine testing is recommended 4 times in the first year and annually thereafter to monitor for declines in kidney function due to tenofovir containing ART regimens. However, there is ongoing debate about the value of universal creatinine testing in ART patients as clinically significant tenofovir-related kidney toxicity is uncommon.(22, 23) A cost-effectiveness analysis found that routine monitoring of asymptomatic patients on ART for renal toxicity was expensive and rarely improved clinical care in low-resource settings.(24) Instead, targeted monitoring of patients at high risk of renal decline due to clinical indications (e.g. low body mass index, diabetes, or hypertension) is shown to have similar clinical outcomes and is more cost-effective than routine creatinine testing.(25) In the STREAM trial, POC creatinine testing allowed for faster referral of patients to DSD. This intervention would likely become more cost-effective if future ART guidelines remove the requirement for creatinine testing before DSD referral while also endorsing targeted instead of routine creatinine monitoring.
Our analysis should be viewed in the context of several limitations. We used clinical effectiveness data from a randomized clinical trial; real-world intervention effectiveness may vary across clinics. For example, if provision of POC testing or delivery of same-day results are delayed in clinic settings, intervention effectiveness would likely be reduced. However, if a substantial portion of PLHIV do not receive routine VL testing from centralized laboratories in accordance with national guidelines, as suggested by surveillance data, POC testing may have a higher clinical benefit than that observed in the trial.(26) Additionally, the STREAM trial enrolled patients without co-morbidities at 6 months post-ART initiation, but our analysis assumes the intervention is provided at ART initiation to 70% of all patients on ART. Since patients are more likely to dropout of care in the first 6 months after initiating ART, it is possible that POC testing with immediate adherence counseling would have a greater impact than our modeled results.(27) Yet, it is also possible the intervention provides the highest benefit to patients on ART for at least 6 months and our results overestimate cost-effectiveness. However, our cost-effectiveness results were robust to changes in intervention effectiveness to the upper and lower bounds of the 95% confidence interval of the STREAM trial. Additionally, the STREAM trial tested a package of interventions including POC testing for VL, CD4 count and creatinine along with task-shifted care to an enrolled nurse, so it is not possible to tease out the clinical impact or cost-effectiveness of different parts of the intervention. However, our intervention package is in line with recent recommendations from a WHO expert consultation, which endorsed streamlined approaches for ART provision in limited resource settings, including task-shifting for clinic visits and diagnostic testing, point-of care testing, and community-based ART refill pick-up for stable clients.(28) A combination of evidence-based interventions will likely need to be implemented simultaneously to support high quality ART delivery in SSA. In addition, uncertainty exists in the surveillance data used for model calibration. Further, cost data were collected at one clinic in an urban region of South Africa yet we model average impact of intervention scale-up nationally in South Africa. Although our results provide insights into overall cost-effectiveness of implementing POC testing with task-shifted care in South Africa, we do not account for regional differences affecting costs. While we find that POC testing is cost-effective for moderate/high volume clinics, geospatial modeling of POC VL scale up in Zambia suggest that some low-volume rural clinics may be cost-efficient locations for POC instrument placement because of high costs of transporting samples to laboratories due to inadequate road infrastructure, long distances, and cold chain failures.(29) Future studies conducting detailed geospatial analyses, including transport networks, are needed to optimize POC testing placement in South Africa. Additionally, if POC instruments are used for other diseases, such as tuberculosis diagnostics, cost-effectiveness would increase. Further, our analysis is conducted from the payer perspective which underestimates economic benefits of POC monitoring at the societal level including cost-savings to patients from reduced transport costs and missed work associated with additional clinic visits for adherence counseling and reduced time, transport and waiting costs associated with increased coverage of DSD. Finally, our budget impact analysis does not assess the cost impact of implementing POC testing within the context of South Africa’s HIV budget; future studies are needed to fully assess intervention affordability.
Reaching the third 95 of UNAIDS global targets, 95% of individuals on ART virally suppressed in less than 10 years requires efficient mobilization of limited resources in an era of shrinking donor funding. We find that POC testing for VL, CD4, and creatinine with task-shifted care can avert substantial HIV-related morbidity and is cost-effective in moderate/high volume clinics in South Africa. We utilized a well-established network model and accounted for parameter uncertainty across 250 good-fitting parameter sets. Our results were robust to a range of sensitivity analyses and are similar to other modeling analyses showing POC diagnostics cost-effectively improve patient clinical outcomes in SSA.(30, 31) As countries strive to scale-up high-quality care to a growing number of patients, POC testing combined with client-centered care including referral of stable clients to DSD, can efficiently improve patient outcomes and reach UNAIDS ambitious treatment targets in SSA.
Supplementary Material
Research in context.
Evidence before this study
Point-of-care (POC) monitoring for ART care has been shown to improve patient retention in care and viral suppression in sub-Saharan Africa. However, its cost-effectiveness is uncertain and will depend on clinic volume, improvements in patient clinical outcomes, and prevention of onward HIV transmission. We searched PubMed for studies published through August 2, 2020 that assessed the cost-effectiveness of POC testing for ART monitoring using the terms: “point-of-care systems”, “point of care” and “hiv” and “cost-benefit analysis”, “cost”, “effectiveness”, “cost effectiveness”, “ICER”, “cost-benefit”, “cost benefit”, “cost-utility”, “economic evaluation.” We found several studies evaluating the cost-effectiveness of POC CD4 testing to determine eligibility for ART initiation, but these analyses were not conducted in the context of universal test and treat for ART. One modeling analysis assessing POC viral load (VL) testing for ART monitoring in South Africa found a large range of incremental cost-effectiveness ratios (ICERs) ranging from cost-saving to not cost-effective depending on assumptions used. Another static model found that POC VL testing in Kenya was cost-effective for adults and children. A cost model evaluating POC instrument placement in South Africa found that POC VL testing may reduce costs of expanding VL testing to the hardest-to-reach populations. We did not find any dynamic mathematical models evaluating the cost-effectiveness of POC testing for ART monitoring informed by clinical trial data on patient outcomes.
Added value of this study
To our knowledge, this is the first study to assess the health and economic impact of POC testing for ART monitoring using a dynamic transmission model that projects both patient clinical outcomes and population-level impacts (HIV-related deaths and HIV infections averted) and cost-effectiveness across clinic volumes. We find that POC testing for CD4 count, VL, and creatinine with task-shifted care can reduce HIV-related infections by 4.5% and HIV-related deaths by 3.9% compared to centralized laboratory monitoring and is cost-effective in moderately-sized clinics in South Africa.
Implications of all the available evidence
Monitoring the rapidly growing number of individuals on ART is challenging in resource-limited settings with inadequate infrastructure to scale-up centralized laboratory testing. POC testing is a promising strategy to efficiently increase coverage of ART monitoring and improve patient outcomes. Our analysis provides a quantitative assessment of the health and economic impact of POC testing for ART monitoring to provide information to policymakers creating guidelines for ART monitoring.
ACKNOWLEDGEMENTS:
We thank the STREAM trial study team all the patients who participated in the study. We gratefully acknowledge the developers of the EMOD disease modeling framework and its associated scripts and calibration capabilities, especially Daniel Bridenbecker, Daniel Goes, Daniel J. Klein, and Clark Kirkman IV. We thank Allen Roberts for providing statistical support.
Funding sources: This study was funded by the U.S. National Institute of Allergy and Infectious Diseases (R21AI124719 and R01AI147752). MS received support from NIMH K01MH115789. The funders had no role in study design, data collection, analysis, writing of the report, nor the decision to submit for publication.
DECLARATION OF INTERESTS:
Dr. Paul K Drain reports being a member of the Scientific Advisory Board for Alveo Technologies, and receiving research support from the NIH, CDC, Gilead Sciences, and the Bill and Melinda Gates Foundation during the conduct of the study. Drs. Sharma and Abdool Karim report grants from the NIH during the conduct of the study. The other authors have no conflicts of interests to declare. The funders had no role in data collection, analysis, interpretation nor the decision to submit the manuscript for publication. The authors did not receive payment for writing this article and the corresponding author had full access to all the data and final responsibility for the decision to submit for publication.
Footnotes
DATA SHARING
All data used for model calibration are publicly available and can be found in summary tables in the appendix. EMOD-HIV is open-source and available online: www.idmod.org/idmdoc. The STREAM trial results are published and publicly available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.UNAIDS: Fast-track, Ending the AIDS epidemic by 2030. Accessed on 1/16/2020 from: https://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf.
- 2.WHO Consolidated ARV Guidelines. Accessed from https://www.who.int/hiv/pub/guidelines/arv2013/treatment/en/ on 3/4/2019.
- 3.Lecher S, Williams J, Fonjungo PN, Kim AA, Ellenberger D, Zhang G, et al. Progress with Scale-Up of HIV Viral Load Monitoring - Seven Sub-Saharan African Countries, January 2015-June 2016. MMWR Morbidity and mortality weekly report. 2016;65(47):1332–5. [DOI] [PubMed] [Google Scholar]
- 4.Nichols BE, Girdwood SJ, Crompton T, Stewart-Isherwood L, Berrie L, Chimhamhiwa D, et al. Monitoring viral load for the last mile: what will it cost? Journal of the International AIDS Society. 2019;22(9):e25337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drain PK, Dorward J, Violette LR, Quame-Amaglo J, Thomas KK, Samsunder N, et al. Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): findings from an open-label, non-inferiority, randomised controlled trial. The lancet HIV. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simeon K, Sharma M, Dorward J, Naidoo J, Dlamini N, Moodley P, et al. Comparative cost analysis of point-of-care versus laboratory-based testing to initiate and monitor HIV treatment in South Africa. PloS one. 2019;14(10):e0223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bershteyn A, Gerardin J, Bridenbecker D, Lorton CW, Bloedow J, Baker RS, et al. Implementation and applications of EMOD, an individual-based multi-disease modeling platform. Pathog Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bershteyn A, Klein DJ, Eckhoff PA. Age-dependent partnering and the HIV transmission chain: a microsimulation analysis. J R Soc Interface. 2013;10(88):20130613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akullian A, Bershteyn A, Klein D, Vandormael A, Barnighausen T, Tanser F. Sexual partnership age pairings and risk of HIV acquisition in rural South Africa. AIDS (London, England). 2017;31(12):1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Oliveira T, Kharsany AB, Graf T, Cawood C, Khanyile D, Grobler A, et al. Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: a community-wide phylogenetic study. The lancet HIV. 2017;4(1):e41–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venter F, The Dolutegravir Dilemma: South African perspective. Accessed from: https://sahivsoc.org/FileUpload/20181022%20at%2011h50%20Venter.pdf on July 31, 2019.
- 12.Wilkinson L, Harley B, Sharp J, Solomon S, Jacobs S, Cragg C, et al. Expansion of the Adherence Club model for stable antiretroviral therapy patients in the Cape Metro, South Africa 2011–2015. Tropical medicine & international health : TM & IH. 2016;21(6):743–9. [DOI] [PubMed] [Google Scholar]
- 13.Johnson LF, Dorrington RE, Moolla H. Progress towards the 2020 targets for HIV diagnosis and antiretroviral treatment in South Africa. South Afr J HIV Med. 2017;18(1):694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JS, Cole SR, Richardson DB, Dittmer DP, Miller WC, Moore RD, et al. Incomplete viral suppression and mortality in HIV patients after antiretroviral therapy initiation. AIDS (London, England). 2017;31(14):1989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes JP, Baeten JM, Lingappa JR, Magaret AS, Wald A, de Bruyn G, et al. Determinants of percoital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. The Journal of infectious diseases. 2012;205(3):358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization Statistical Information System: CHOICE (Choosing Interventions that are Cost Effective). Last accessed 2 February 2019 from: https://www.who.int/choice/cost-effectiveness/en/
- 18.Phillips AN, Venter F, Havlir D, Pozniak A, Kuritzkes D, Wensing A, et al. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. The lancet HIV. 2019;6(2):e116–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods B, Revill P, Sculpher M, Claxton K. Country-Level Cost-Effectiveness Thresholds: Initial Estimates and the Need for Further Research. Value Health. 2016;19(8):929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mutasa-Apollo T, Ford N, Wiens M, Socias ME, Negussie E, Wu P, et al. Effect of frequency of clinic visits and medication pick-up on antiretroviral treatment outcomes: a systematic literature review and meta-analysis. Journal of the International AIDS Society. 2017;20(Suppl 4):21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bochner AF, Meacham E, Mhungu N, Manyanga P, Petracca F, Muserere C, et al. The rollout of Community ART Refill Groups in Zimbabwe: a qualitative evaluation. Journal of the International AIDS Society. 2019;22(8):e25393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamkuemah M, Kaplan R, Bekker LG, Little F, Myer L. Renal impairment in HIV-infected patients initiating tenofovir-containing antiretroviral therapy regimens in a Primary Healthcare Setting in South Africa. Tropical medicine & international health : TM & IH. 2015;20(4):518–26. [DOI] [PubMed] [Google Scholar]
- 23.De Waal R, Cohen K, Fox MP, Stinson K, Maartens G, Boulle A, et al. Changes in estimated glomerular filtration rate over time in South African HIV-1-infected patients receiving tenofovir: a retrospective cohort study. Journal of the International AIDS Society. 2017;20(1):21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koenig SP, Schackman BR, Riviere C, Leger P, Charles M, Severe P, et al. Clinical impact and cost of monitoring for asymptomatic laboratory abnormalities among patients receiving antiretroviral therapy in a resource-poor setting. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51(5):600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Team DT, Mugyenyi P, Walker AS, Hakim J, Munderi P, Gibb DM, et al. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010;375(9709):123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascoe S, Huber A, Murphy J, MacLeod W, Bor J, White C, et al. Identifying gaps in viral load monitoring: Results from an evaluation of viral load reporting at primary health care facilities in South Africa. AIDS Conference - Amsterdam. 2018. [Google Scholar]
- 27.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Tropical medicine & international health : TM & IH. 2010;15 Suppl 1:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford N, Geng E, Ellman T, Orrell C, Ehrenkranz P, Sikazwe I, et al. Emerging priorities for HIV service delivery. PLoS medicine. 2020;17(2):e1003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girdwood SJ, Nichols BE, Moyo C, Crompton T, Chimhamhiwa D, Rosen S. Optimizing viral load testing access for the last mile: Geospatial cost model for point of care instrument placement. PloS one. 2019;14(8):e0221586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estill J, Egger M, Blaser N, Vizcaya LS, Garone D, Wood R, et al. Cost-effectiveness of point-of-care viral load monitoring of antiretroviral therapy in resource-limited settings: mathematical modelling study. AIDS (London, England). 2013;27(9):1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyle EP, Jani IV, Lehe J, Su AE, Wood R, Quevedo J, et al. The clinical and economic impact of point-of-care CD4 testing in mozambique and other resource-limited settings: a cost-effectiveness analysis. PLoS medicine. 2014;11(9):e1001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer-Rath G, van Rensburg C, Chiu C, Leuner R, Jamieson L, Cohen S. The per-patient costs of HIV services in South Africa: Systematic review and application in the South African HIV Investment Case. PloS one. 2019;14(2):e0210497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eaton JW, Menzies NA, Stover J, Cambiano V, Chindelevitch L, Cori A, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. The Lancet Global health. 2014;2(1):e23–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.