ABSTRACT
Standard methods for enumerating Mycobacterium tuberculosis in patient sputum can miss large populations of viable M. tuberculosis cells that are unable to grow either on solid medium or in liquid medium unless the medium has been extensively diluted. Because these bacteria can be detected in liquid medium after limiting dilution, they have been termed differentially culturable or differentially detectable M. tuberculosis (DD-Mtb). Treatment with isoniazid (H), rifampin (R), pyrazinamide (Z), and ethambutol (E) (HRZE) for 1 to 2 weeks has been shown to increase the representation of DD-Mtb in the sputum of drug-sensitive (DS) tuberculosis (TB) patients. However, little is known about DD-Mtb after longer periods of treatment with HRZE or in patients with drug-resistant (DR) TB who receive second-line therapies. Here, we measured the proportion of DD-Mtb cells in the sputum of 47 subjects, 29 with DS TB and 18 with DR TB, before initiation of treatment and at 2 weeks and 2 months thereafter. Prior to treatment, DD-Mtb cells represented the majority of M. tuberculosis cells in the sputum of 21% of subjects with DS TB, and this proportion rose to 65% after 2 weeks of treatment with first-line drugs. In subjects with DR TB, DD-Mtb cells were found in the sputum of 29% of subjects prior to treatment initiation, and this proportion remained steady at 31% after 2 weeks of treatment with second-line drugs. By 2 months, DD-Mtb cells were detected in the sputum of only 2/15 (13.3%) subjects with DS TB and in 0/15 of subjects with DR TB. One of the DS subjects whose sputum was positive for DD-Mtb at month 2 later experienced treatment failure.
KEYWORDS: Mycobacterium tuberculosis, diagnostics, multidrug resistance, pulmonary infection, viable but nonculturable
INTRODUCTION
In 2019, 10 million cases of tuberculosis (TB) led to approximately 1.4 million deaths, making TB the leading cause of death from a single infectious agent prior to COVID-19 (1). TB exerts a devastating toll in the developing world, where more than 95% of deaths occur, and is projected to lead to an economic loss of U.S. $1 trillion from 2015 to 2030 (2). New drug regimens are needed to shorten the currently required 6-month treatment protocol for drug-sensitive (DS) TB and to combat drug-resistant (DR) TB.
To evaluate the efficacy of new drug regimens in phase I/II early bactericidal activity (EBA) trials, viable Mycobacterium tuberculosis cells are quantified from the sputum of pulmonary TB patients during the first 2 to 8 weeks of therapy. The gold-standard method for enumerating M. tuberculosis is culturing sputum on solid agar and counting the number of CFU. This approach is often supplemented or replaced by assessing time to positivity (TTP) of sputum samples in an automated liquid culture system that measures the rate of depletion of oxygen from culture medium as a proxy for M. tuberculosis growth and quantity. These techniques may miss subpopulations of M. tuberculosis that are incapable of growing on solid medium or in liquid medium if the suspension has not been extensively diluted (3–12). These bacteria have been called by the interchangeable terms “viable but nonculturable” (VBNC-Mtb) (13), “differentially culturable” (DCTB) (10), and “differentially detectable” M. tuberculosis (DD-Mtb) (8). Here, we refer to these bacteria as “DD-Mtb” because they are often culturable and because they can sometimes be identified by methods other than culture, such as by inoculation into an experimental host. The role of limiting dilution in detection of DD-Mtb has been linked to imposition of a delay in onset of replication, which allows for repair of oxidative injury (K. Saito, S. Mishra, T. Warrier, N. Cicchetti, J. Mi, E. Weber, J. Roberts, A. Gouzy, E. Kaplan, C. D. Brown, B. Gold, and C. Nathan, unpublished data). Notably, DD-Mtb cells show profound phenotypic tolerance to anti-TB chemotherapy (8, 14, 15).
DD-Mtb bacteria were suspected to exist in humans when lung homogenates prepared at autopsy detected no M. tuberculosis based on CFU, but aliquots of the same homogenates caused TB in guinea pigs (16). These suspicions were validated in 2010 when Mukamolova et al. (9) reported that DD-Mtb constituted 80 to 99.9% of the viable M. tuberculosis bacteria in sputa from 20 of 25 (80%) treatment-naive TB patients, as revealed by comparing CFU counts to the most probable number (MPN) estimation from liquid medium using a limiting dilution (LD) assay. Treatment with rifampin for 7 to 11 days reduced the number of M. tuberculosis bacteria detected by CFU in the sputa to a much greater extent than it reduced M. tuberculosis bacteria detected by the MPN-LD method, indicating proportionate enrichment of DD-Mtb populations during therapy (9). Similarly, Chengalroyen et al. (10) reported that DD-Mtb represented the majority of viable M. tuberculosis cells in the sputa of >70% of 110 treatment-naive TB patients in South Africa. In both studies, DD-Mtb populations were detectable in some specimens only when the LD assay was performed in medium supplemented with culture filtrate (CF), that is, cell-free medium from a growing culture of a laboratory strain of M. tuberculosis. However, in the studies of Chengalroyen et al. (10) and McAulay et al. (11), MPN-LD assays performed with CF impaired the detection of DD-Mtb from some sputum samples (17).
With methodological and statistical methods designed to increase stringency (8), sputum samples from 21% of treatment-naive Haitian subjects with TB were found to be dominated by DD-Mtb, and this proportion increased to 69% after 2 weeks of isoniazid (H), rifampin (R), pyrazinamide (Z), and ethambutol (E) (HRZE) treatment (11). That study left unanswered the questions of how long DD-Mtb populations persist during treatment and how treatments other than HRZE may affect their persistence and/or generation.
Collectively, these findings suggest that not taking DD-Mtb into account could complicate interpretation of EBA trials of investigational TB drugs and contribute to the ambiguous clinical significance of sputum conversion—the apparent elimination of viable M. tuberculosis bacteria— after 2 months of treatment (5, 8, 9, 11, 14, 15). The present study is meant to help lay the groundwork for filling the following knowledge gaps about DD-Mtb. Does their prevalence differ between specimens from subjects with DS or DR TB? Is the impact of HRZE on their prevalence different from the impact of other treatment regimens? What happens to DD-Mtb bacteria in sputum when measured at 2 weeks and at 2 months of treatment, at a later time point than those in earlier studies? To address these questions, we used the methods of Saito et al. (8) and McAulay et al. (11) to study additional subjects with DS TB and those with DR TB, to perform DD-Mtb tests before treatment and at 2 weeks and 2 months after the onset of treatment, and to monitor the clinical outcome at 6 months.
RESULTS
Patient characteristics.
Between May 2018 and September 2020, we enrolled 29 participants with DS TB and 18 participants with DR TB. Their characteristics are described in Table 1. The prevalences of bilateral disease, dyspnea (grade 2+), and prior treatment with first-line drugs were significantly higher at time of enrollment for participants with DR versus those with DS TB (Table 1).
TABLE 1.
Characteristics of the drug-sensitive and drug-resistant TB cohorts at time of enrollment
| Characteristicb | Cohortc |
P | |
|---|---|---|---|
| DS (n = 29) | DR (n = 18) | ||
| Male (% [n]) | 62.1 (18) | 50 (9) | 0.546 |
| HIV+ (% [n])a | 3.4 (1) | 22.2 (4) | 0.063 |
| Presence of cavities (% [n]) | 48.3 (14) | 44.4 (8) | 1.0 |
| Bilateral disease (% [n]) | 31.0 (9) | 72.2 (13) | 0.008 |
| Prior first-line TB treatment (% [n]) | 0 (0) | 83.3 (15) | <0.001 |
| Blood-tinged sputum (% [n]) | 0 (0) | 11.1 (2) | 0.142 |
| Pleuritic chest pain (% [n]) | 34.5 (10) | 55.6 (10) | 0.226 |
| Fever (% [n]) | 34.5 (10) | 11.1 (2) | 0.095 |
| Age in yrs (median [IQR]) | 33 (28–37) | 32 (28–41) | 0.793 |
| Hemoglobin, g/dl (median [IQR]) | 10.7 (9.8–11.3) | 11.0 (10.3–11.5) | 0.456 |
| Creatinine, mg/dl (median [IQR]) | 0.7 (0.6–0.8) | 0.6 (0.5–0.7) | 0.159 |
| Pulse oximetry, % (median [IQR]) | 97 (95–98) | 98 (97–99) | 0.113 |
| BMI (median [IQR]) | 19.0 (17.1–21.0) | 17.7 (16.8–19.6) | 0.381 |
| Dyspnea (% [n]) | <0.001 | ||
| Not present | 44.8 (13) | 16.7 (3) | |
| Grade 1 | 55.2 (16) | 22.2 (4) | |
| Grade 2 | 0 (0) | 55.6 (10) | |
| Grade 3 | 0 (0) | 5.6 (1) | |
| Cough (% [n]) | 0.074 | ||
| Not present | 0 (0) | 5.6 (1) | |
| Grade 1 | 89.7 (26) | 66.7 (12) | |
| Grade 2 | 10.3 (3) | 22.2 (4) | |
| Grade 3 | 0 (0) | 5.6 (1) | |
| Xpert positivity (% [n]) | 0.948 | ||
| Very low | 3.4 (1) | 0 (0) | |
| Low | 13.8 (4) | 11.1 (2) | |
| Medium | 37.9 (11) | 50 (9) | |
| High | 44.8 (13) | 38.9 (7) | |
All HIV+ participants were on antiretroviral therapy at time of enrollment and during the duration of this study.
IQR, interquartile range; BMI, body mass index.
DS, drug susceptible; DR, drug resistant.
Of the 18 DR participants, 15 had prior treatment with first-line anti-TB drugs. All isolates were resistant to rifampin (with isolates from 14 subjects showing resistance to rifabutin as well); 17 also showed resistance to isoniazid, and 15 were resistant to additional drugs besides rifamycins and isoniazid (see Table S1 in the supplemental material).
Proportion of TB subjects with DD-Mtb before and after initiation of therapy.
M. tuberculosis numbers were quantified from the sputa of DS and DR TB subjects in three different ways, as follows: (i) CFU counting using solid agar plates, (ii) MPN using a limiting dilution (MPN-LD) assay without culture filtrate (MPN−CF), and (iii) MPN-LD assay with 50% sterile culture filtrate (MPN+CF). DD-Mtb was said to be present when the CFU value was less than the lower bound of the 95% confidence interval of the MPN−CF or MPN+CF value. Some sample collections were missed because of civic unrest and the COVID-19 pandemic, and some samples were lost to contamination, so that day 0 and week 2 data were both available for 23/29 and 17/18 subjects with DS and DR TB, respectively; and month 2 data were available for 15/29 and 15/18 subjects with DS and DR TB, respectively.
The proportion of participants whose sputa were DD-Mtb positive was comparable before onset of therapy in treatment-naive subjects with DS TB (20.7%) and in those with DR TB before administration of second-line therapy (29.4%) (Fig. 1 and Table 2). This was apparent when performing the MPN-LD assay without CF (17.2% versus 22.2% positivity for DD-Mtb in the sputa of subjects with DS and DR TB, respectively), although when the MPN-LD assay was performed with CF, a 2.3-fold higher proportion of subjects with DR TB showed presence of DD-Mtb in their sputa in comparison to subjects with DS TB (10.3% versus 23.5% for sputa from DS and DR TB subjects, respectively) (Table 2). In comparison, only 1/29 (3.4%) subjects with DS TB and 1/18 (5.6%) of those with DR TB provided sputum with a CFU value higher than the upper bound of the 95% confidence interval of the MPN−CF or MPN+CF value.
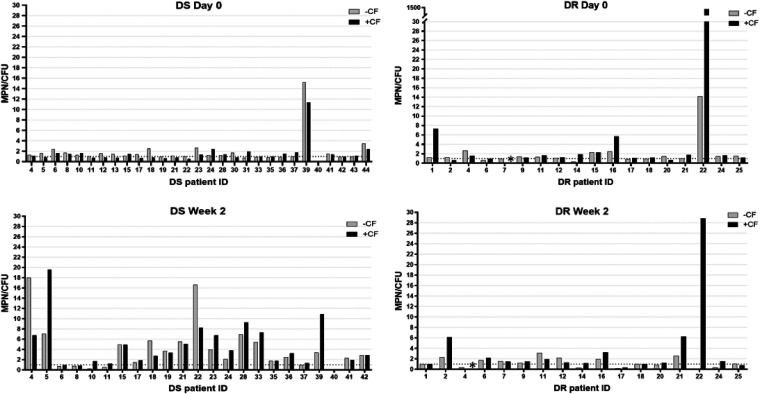
FIG 1.
Ratio of most probable number (MPN) to CFU (MPN/CFU), with and without culture filtrate (CF), for sputum from subjects with drug-sensitive (DS) and drug-resistant (DR) TB at day 0 and week 2 post initiation of therapy. The dashed line represents an MPN/CFU ratio of 1. Raw numbers can be found in Table S4 in the supplemental material. An asterisk (*) indicates data not available due to contamination.
TABLE 2.
Proportion of subjects with drug-sensitive or drug-resistant TB whose sputum was positive for DD-Mtb before and after initiation of therapy
| Culture filtrate status | Time pointb | % (n) of subjects positive for DD-Mtb in cohortc: |
Pa | |
|---|---|---|---|---|
| DS | DR | |||
| All | Day 0 | 20.7 (6/29) | 29.4 (5/17) | 0.726 |
| Wk 2 | 65.2 (15/23) | 31.3 (5/16) | 0.054 | |
| Mo 2 | 13.3 (2/15) | 0 (0/15) | NA | |
| −CF | Day 0 | 17.2 (5/29) | 22.2 (4/18) | 0.716 |
| Wk 2 | 65.2 (15/23) | 17.6 (3/17) | 0.004 | |
| Mo 2 | 0 (0/15) | 0 (0/15) | NA | |
| +CF | Day 0 | 10.3 (3/29) | 23.5 (4/17) | 0.397 |
| Wk 2 | 60.9 (14/23) | 25.0 (4/16) | 0.049 | |
| Mo 2 | 13.3 (2/15) | 0 (0/15) | NA | |
Comparing DS to DR. NA, not applicable.
Day 0 was prior to initiation of therapy.
Positivity was defined as when the CFU value was less than the lower bound of the 95% confidence interval of the MPN value (with or without culture filtrate [CF]). DD, differentially detectable.
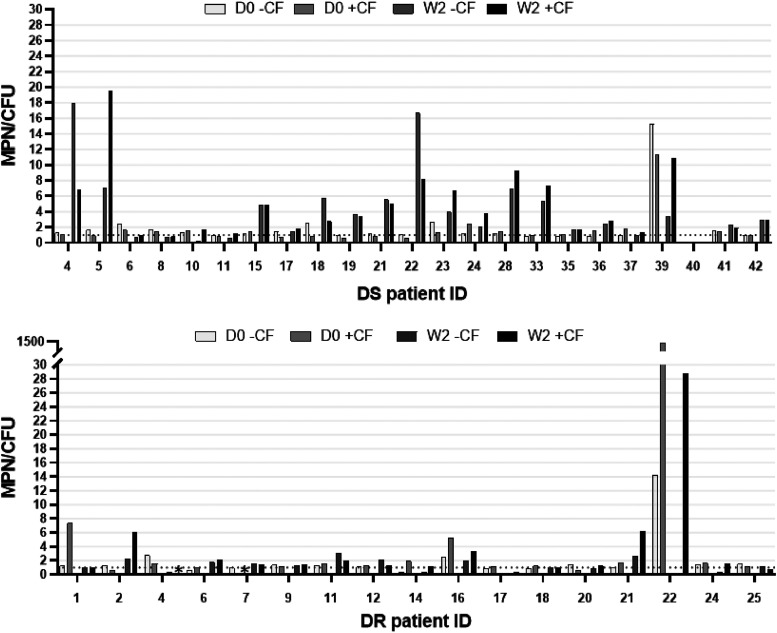
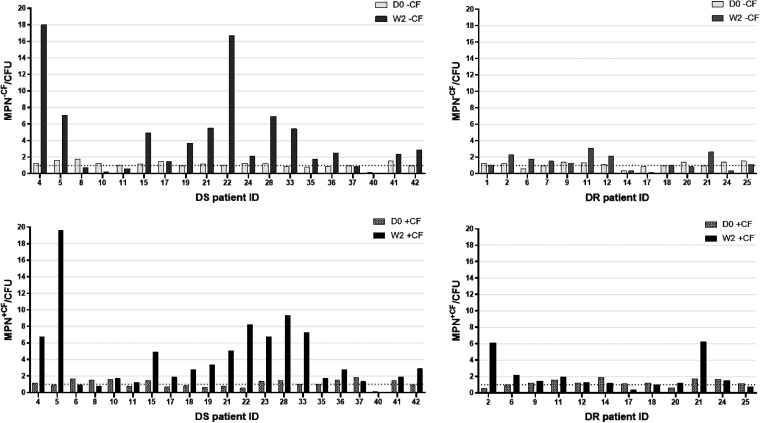
At 2 weeks post initiation of therapy, the proportion of subjects with DS TB whose sputa were DD-Mtb positive increased by about 3-fold, to 65.2% (15/23) and 60.9% (14/23) when the MPN-LD assay was performed without or with CF, respectively (Fig. 1 and Table 2). This, too, is similar to what was observed in an earlier study (69%) that examined a similar cohort of subjects with DS TB at the same study site after 2 weeks of treatment with HRZE (11). Of 5 subjects with DS TB whose sputa were positive for DD-Mtb at day 0, 4 (80%) had sputa that remained positive for DD-Mtb at week 2 (Fig. 2). Of the subjects with DS TB whose sputa lacked DD-Mtb at day 0, approximately 61% showed DD-Mtb positivity after 2 weeks of therapy with first-line drugs (Fig. 3 and Table 3).
FIG 2.
The MPN to CFU ratio (MPN/CFU), with and without culture filtrate (CF), for sputum from subjects with drug-sensitive (DS) and drug-resistant (DR) TB where data for both day 0 (DO) and week 2 (W2) samples were available. The dashed line represents an MPN/CFU ratio of 1. Raw numbers can be found in Table S4. An asterisk (*) indicates data not available due to contamination.
FIG 3.
The MPN to CFU ratio (MPN/CFU), with and without culture filtrate (CF), at day 0 (D0) and week 2 (W2) for sputum from subjects with drug-sensitive (DS) and drug-resistant (DR) TB whose sputa showed no DD-Mtb at day 0 (D0). The dashed line represents an MPN/CFU ratio of 1. Raw numbers can be found in Table S4.
TABLE 3.
Proportion of subjects with drug-sensitive or drug-resistant TB whose sputum showed apparent selection or induction of DD-Mtb after initiation of treatmenta
| Culture filtrate status | % (n) of subjects showing selection/induction in cohort: |
Pb | |
|---|---|---|---|
| DS | DR | ||
| All | 61.1 (11/18) | 25.0 (3/12) | 0.072 |
| −CF | 63.2 (12/19) | 21.4 (3/14) | 0.033 |
| +CF | 57.1 (12/21) | 16.7 (2/12) | 0.033 |
Defined as their sputum having no DD-Mtb cells at day 0 but showing DD-Mtb at week 2 post initiation of therapy, as determined by comparing MPN with or without culture filtrate (CF) to CFU.
Comparing DS to DR cohorts.
In contrast, the sputa of subjects with DR TB showed comparable levels of DD-Mtb both before (29.4%) and after 2 weeks of therapy with second-line drugs (31.3%) (Fig. 1 and Table 2). This was apparent when the MPN-LD assays were performed both with or without CF (Table 2). Of the four participants in this cohort who had DD-Mtb-positive sputa at day 0 and from whom a week 2 sample was also available, only 2 remained positive for DD-Mtb at week 2 (Fig. 2). Of the DR TB subjects whose sputa lacked DD-Mtb at day 0, only 25% showed DD-Mtb positivity after 2 weeks of therapy (Fig. 3 and Table 3).
By month 2, the sputa of 84% (26/31) of all subjects showed no detectable M. tuberculosis growth, and the median count of viable M. tuberculosis in the remaining samples was very low (<20 M. tuberculosis/ml) (see Tables S2 and S3 in the supplemental material). None of the 9 subjects with DS TB whose sputa were positive for DD-Mtb at week 2 (including 4 whose sputa were positive for DD-Mtb at both day 0 and week 2) showed any M. tuberculosis growth at month 2 (Table S2). In contrast, of the 4 subjects with DR TB whose sputa were DD-Mtb positive at week 2, two showed M. tuberculosis growth at month 2 (although neither was positive for DD-Mtb) (Table S3). In neither cohort was DD-Mtb positivity at day 0 associated with sputum positivity for M. tuberculosis at month 2 (see Table S4 in the supplemental material).
Of the 5 sputa that showed detectable M. tuberculosis growth at 2 months, two were positive for DD-Mtb, but only when comparing MPN+CF to CFU, and both belonged to the DS TB cohort. Neither subject’s sputum was positive for DD-Mtb at day 0 (week 2 samples were lacking for both). Therefore, the proportion of subjects with DS TB who had DD-Mtb-positive sputum fell from 65.2% at week 2 to 13.3% at month 2, and in subjects with DR TB it fell from 31.3% at week 2 to 0% at month 2 (Table 2).
Relationship of DD-Mtb to clinical characteristics and outcomes.
We assessed whether baseline characteristics at enrollment were associated with the occurrence of DD-Mtb at either week 0 or week 2 (see Table S5 in the supplemental material). No patient characteristics were associated with the presence of DD-Mtb in the sputa of DS TB subjects. HIV positivity (P = 0.015), lower hemoglobin (P = 0.014), and lower body mass index (BMI; P = 0.040) were positively associated with DD-Mtb for the cohort with DR TB.
When limiting analyses to presence of DD-Mtb only at day 0 (prior to initiation of treatment), no association with patient characteristics was found in the DS cohort. In the DR cohort, HIV positivity (P = 0.044) and lack of bilateral disease (P = 0.008) were positively associated with DD-Mtb. No patient characteristics were associated with the presence of DD-Mtb in the sputa of either cohort at week 2 (Table S5).
All 29 DS participants completed 6 months of therapy; 28 achieved either treatment completion or cure, while 1 participant had treatment failure (positive acid-fast bacillus [AFB] smear at month 5) as defined by WHO guidelines. Presence of DD-Mtb at month 2 was associated with the only treatment failure of this study (DS no. 12).
Of the 18 participants with DR TB, the median time to culture conversion to negative was 2 months, and mean weight gain at 2 months was 2 kg. The mean time to discharge from the inpatient service was 4 months, and 2 of 18 patients required rehospitalization after initial discharge. There was no significant difference in these parameters between subjects whose sputa were DD-Mtb positive versus those who were negative. It is of note that the DR patient with the longest hospitalization (6 months) and persistent symptoms after discharge, which resulted in a rehospitalization, was one of the 5 DR patients with DD-Mtb at week 2 (DR no. 21).
Relative proportions of DD-Mtb bacteria in sputum from subjects with DS and DR TB.
For all DD-Mtb-positive samples in this study, DD-Mtb constituted the majority (>50%) of the total M. tuberculosis bacteria present in the sputum (as defined by an MPN/CFU ratio of >2-fold) (Fig. 1; see also Table S6 in the supplemental material). The median MPN/CFU ratio for DD-Mtb-positive samples was 4.4 (interquartile range [IQR], 2.7 to 7.0), meaning that DD-Mtb cells comprised a median of nearly 80% of the viable M. tuberculosis cells in the sputa of these participants. When samples from subjects with DS and DR TB were compared, the median MPN/CFU ratios at day 0 were 2.6 and 4.2, respectively; whereas at week 2, the median MPN/CFU ratio increased to 5.0 in sputa from subjects with DS TB but held nearly steady at 3.3 for those with DR TB (Table S6).
Subjects with DS and DR TB showed comparable levels of M. tuberculosis in their sputum prior to initiation of therapy and at 2 weeks post initiation (see Table S7 in the supplemental material). Consistent with this, the rate of decline of M. tuberculosis from day 0 to week 2 was also similar for both cohorts (see Table S8 in the supplemental material). However, as seen in Table S7, at day 0, DD-Mtb-positive sputa from the cohort with DR TB showed a 389-fold lower median CFU (P = 0.018), a 550-fold lower median MPN−CF (P = 0.011), and a 10-fold lower median MPN+CF value (P = 0.067) in comparison to those of DD-Mtb-positive sputa from the DS cohort. By week 2, these differences were absent. At day 0, DD-Mtb-positive sputa from the cohort with DR TB also showed a 1,259-fold lower median CFU (P = 0.004), a 550-fold lower median MPN−CF (P = 0.007), and a 27-fold lower median MPN+CF value (P = 0.011) in comparison to those of DD-Mtb-negative sputa from the same cohort. This distinction in levels of viable M. tuberculosis bacteria according to presence or absence of DD-Mtb was not seen in sputa from the cohort with DS TB.
DS- and DR-Mtb samples show differing responses to CF.
The median MPN+CF/MPN−CF ratio was 1.6-fold higher for sputa from subjects with DR TB than for those with DS TB at day 0 (P = 0.032). This ratio was 3.2-fold when the comparison was limited to DD-Mtb-positive samples (Table S7). When the comparison was made between MPN+CF and MPN−CF values for individual sputa that contained DD-Mtb, the MPN+CF assay detected a 2.3-fold higher median DD-Mtb number for samples from subjects with DR TB but a 1.5-fold lower median DD-Mtb number for samples from subjects with DS TB. In other words, on average, CF appeared to promote the growth of DD-Mtb bacteria from the DR cohort while inhibiting the growth of DD-Mtb bacteria in sputa from the DS cohort at day 0.
DISCUSSION
Here, we report that subjects with DS and DR TB show differing incidence of DD-Mtb in their sputa after initiation of therapy. We found that similar proportions of subjects with DS and DR TB—20.7% and 29.4%, respectively—presented with sputum positive for DD-Mtb prior to therapy. These proportions resemble what was reported earlier (21%) for subjects with DS TB by a different experimental investigator applying the same methods at the same study site (11). After 2 weeks of HRZE, the proportion of subjects whose sputa were DD-Mtb-positive more than tripled, again mirroring earlier observations (11). In contrast, the proportion of subjects with DR TB whose sputa were DD-Mtb positive changed little after 2 weeks on a multidrug regimen that included pyrazinamide but lacked rifampin, isoniazid, and ethambutol. These observations are consistent with the hypotheses that HRZE or one of its components, perhaps rifampin (8), promotes an increased proportion of DD-Mtb bacteria in the sputum by selective elimination of M. tuberculosis bacteria that are not DD-Mtb or by induction of DD-Mtb (and second-line therapy does not do so), or that the more extensive disease or prior treatment in most subjects receiving second-line therapy (along with any potential accompanying resistance-associated mutations) limits selection or induction of DD-Mtb.
Studies of DD-Mtb cells generated in vitro reveal that they are bacteria that have sustained oxidative damage at a sublethal level and can regain the capacity to form colonies on agar if their replication is delayed until they undergo repair (Saito et al., unpublished). While disengaged from replication, DD-Mtb are profoundly antibiotic resistant (8, 14, 15). While intermediate levels of oxidative damage give rise to DD-Mtb, more extensive oxidative damage kills them (Saito et al., unpublished). Perhaps the exposure of subjects with DR TB to oxidant-generating drugs like clofazimine (18) damages a higher proportion of their M. tuberculosis bacteria beyond the point of repair.
The notion that sublethal damage can promote formation of DD-Mtb cells is consistent with the finding that HIV-positive (HIV+) subjects with TB who had peripheral blood CD4 counts of >200 cells/ml were more likely to have DD-Mtb in their sputum than those with CD4 counts of <200 cells/ml (10). In the present study, subjects with DS TB who had DD-Mtb-positive sputum at day 0 had a 3.5-fold lower median CFU count in their sputum than those whose sputum lacked DD-Mtb (see Table S7 in the supplemental material). Perhaps there is a level of host immunity that suffices to reduce but not eliminate M. tuberculosis and inflicts the intermediate level of damage that favors formation of DD-Mtb. Similarly, subjects with DR TB who had DD-Mtb-positive sputum at day 0 had a >1,000-fold lower median CFU count in their sputum than that of their DD-Mtb-negative counterparts, perhaps highlighting the additive effects of host immunity and previous exposure to first-line therapies. Consistent with this, Xpert positivity indicative of very low or low M. tuberculosis counts in sputum was positively associated with the presence of DD-Mtb (P = 0.014) (see Table S5 in the supplemental material).
DD-Mtb populations in the sputum did not persist beyond the first 2 months of therapy for most subjects. Specifically, 9/9 subjects with DS TB and 7/7 with DR TB whose sputum previously contained DD-Mtb bacteria lacked evidence of DD-Mtb at 2 months. Nonetheless, DD-Mtb was detected at month 2 in the sputa of 2 subjects with DS TB whose sputa did not show DD-Mtb at day 0 (week 2 samples were missing for both). More generally, sputa from most subjects showed no evidence of viable M. tuberculosis cells at 2 months by any of the assays used, and when M. tuberculosis was detected at 2 months, the levels were very low. These findings are consistent with a report that found no M. tuberculosis growth after 3 months of treatment in the sputum of 17 subjects with DS TB using assays similar to those employed here (5). Therefore, detection of DD-Mtb at an earlier time point did not foretell sputum positivity by any assay used at 2 months of treatment.
Nonetheless, those subjects whose sputum contains detectable DD-Mtb at some point before or during their treatment may face a more challenging clinical course than those whose sputum does not. The two subjects who responded most poorly to treatment in each cohort, namely, the subject with DS TB whose treatment failed and the subject with DR TB who required rehospitalization, both had DD-Mtb detected during treatment. However, the number of subjects who did poorly was too small for statistical assessment, highlighting a limitation of the current study. Larger studies with longer follow-up times will be needed to draw firm conclusions about the role of DD-Mtb in treatment outcome. Beltran et al. (6) identified DD-Mtb in the sputum of two subjects with DS TB who subsequently relapsed, while Almeida Júnior et al. (5) found no association between DD-Mtb and treatment failure or relapse during the first 6 months of treatment for DS TB. While CF had a variable effect on M. tuberculosis growth in this and previous studies (10–12, 17), for both subjects for whom DD-Mtb was associated with poorer clinical outcome, CF markedly improved M. tuberculosis recovery in the MPN-LD assay, similarly to what Beltran et al. (6) observed in their subjects who went on to relapse.
Additionally, CF tended to promote recovery of DD-Mtb in sputum from subjects with DR TB but tended to suppress recovery of DD-Mtb in sputum from subjects with DS TB. The latter effect was alleviated in sputa from the cohort with DS TB after initiation of drug treatment, suggesting that the prior drug exposure experienced by subjects with DR TB may have contributed to this difference. What components of CF exert these effects and by what mechanisms remain unknown (12), making it difficult to draw biologic inferences. As a practical matter, it is advisable to perform MPN-LD assays both with and without CF to maximize detection of DD-Mtb.
In the future, a rapid, quantitative, and culture-independent means of estimating the number of DD-Mtb cells in clinical specimens could help address the many open questions that remain regarding DD-Mtb. Given that culture conversion is already known to be a poor predictor of TB cure, there is a need for the development of diagnostics that more accurately reflect total viable M. tuberculosis populations in sputa, which can be used to screen investigational anti-TB drugs and identify individuals who qualify for shorter treatments, with the aim of ultimately improving TB outcomes.
MATERIALS AND METHODS
Study design and patient populations.
This prospective observational study was performed at the Groupe Haïtien d’Étude du Sarcome de Kaposi et des Infectieuses Opportuniste (GHESKIO) centers in Port au Prince, Haiti, and approved by the institutional review boards of both GHESKIO and Weill Cornell Medicine. All participants provided written informed consent and scored at least 90% on an assessment of understanding quiz before enrollment. HIV+ subjects from both cohorts were on antiretroviral therapy at the time of enrollment and remained on treatment during the period of the study.
The DS cohort met the following criteria: the participants were ≥ 18 years of age; had been diagnosed as having active pulmonary TB based on clinical symptoms and signs, chest radiography, and Xpert Mtb/RIF assay (Cepheid, Sunnyvale, CA) positivity, without indication of rifampin resistance; were free of extrapulmonary manifestations of TB or concomitant illnesses that might interfere with treatment in the opinion of the participant’s care provider; had no history of TB or its treatment; and were planning to initiate treatment at the GHESKIO clinic. The DR cohort met the same criteria, except that the Xpert test indicated rifampin resistance and participants were not excluded if they had previously been treated for drug-sensitive TB. Sputum was collected from both cohorts prior to initiation of therapy, and at 2 weeks and 2 months after initiation of therapy. TB symptoms (cough, dyspnea, hemoptysis, fever, and pleuritic chest pain) were graded according to the Division of AIDS (DAIDS) grading system (19).
Participants with DS TB were followed in the GHESKIO outpatient clinic for the duration of their directly observed therapy (DOT). Participants received isoniazid (H), rifampin (R), ethambutol (E), and pyrazinamide (Z) for 2 months, then HR for 4 months. During the first 2 months, participants had clinical visits every 2 weeks at which time signs and symptoms were recorded, and then every month for the remainder of their treatment regimen. Treatment outcome was determined based on Haitian and WHO guidelines—spot sputum samples were collected during clinical visits for AFB smear at months 2, 5, and 6 and underwent culture only if AFB positive. Subjects also provided overnight sputum samples on day 0 and at week 2 and month 2 for the laboratory analyses performed in this study. One month after completion of therapy, participants had a final clinical visit and were counseled to return if their condition worsened. Participants were not actively followed after completion of treatment, and so the relationship with posttreatment recurrence could not be assessed.
Participants with DR TB were hospitalized in GHESKIO’s inpatient multidrug-resistant (MDR)-TB hospital for approximately the first 4 months of treatment. They were discharged once they demonstrated clinical improvement and negative M. tuberculosis cultures and had designated family support to ensure treatment adherence upon discharge. Treatment was in accordance with Haitian and WHO guidelines, with DOT regimens comprised of bedaquiline, levofloxacin, linezolid, clofazimine, and pyrazinamide. Bedaquiline was discontinued after 6 months and linezolid after 12 months, with the remaining drugs continued to complete 20 months of therapy. Once discharged, participants were followed in GHESKIO’s outpatient MDR-TB clinics with monthly outpatient follow-up. Spot sputum samples were provided monthly for AFB smear and monthly for culture during months 1 to 8, every other month during months 9 to 18, and then monthly for the final 3 months of treatment. For the current study, we documented the following outcomes: length of hospitalization and any readmission, change in weight by month 2, persistent TB symptoms, and time to culture conversion.
Sputum processing and microbiological assays.
Subjects collected overnight sputum into a cool box with ice packs (4°C) from 5 p.m. to 9 a.m. Samples reached the GHESKIO laboratory the same morning, with >79% of samples being processed the same day, >92% being processed within 2 days, and all being processed within 5 days. Experimental work took place in a biosafety level 3 laboratory with appropriate safety guidelines and personal protective equipment.
Decontamination of sputum, preparation of culture filtrate (CF), and CFU and MPN-LD assays were conducted as reported previously (11). Decontamination was monitored by plating aliquots on blood agar. Results of LD assays performed without CF are referred to as MPN−CF and those with CF as MPN+CF. CFU and MPN plates were placed inside partially sealed Ziploc bags and placed inside a 37°C incubator (unhumidified and without CO2) for up to 9 weeks, with reads performed after 3 weeks and then every 2 weeks thereafter until no additional growth was observed between two consecutive reads. Positive colonies were identified visually, and any suspect positive wells were confirmed for presence of M. tuberculosis by using an SD Bioline TB AG MPT64 rapid antigen test (only for MPN−CF plates). Contamination was ruled out by plating on blood agar.
Data analysis.
Data were uploaded to REDCap (20, 21), a secure web-based software platform designed to support data capture for research studies. A second investigator used the raw data files to repeat and verify the calculations. Confidence intervals (95%) were calculated separately for assays with and without CF from the results of 10 independent dilution series per sample (22). These were compared to the average CFU values on two agar plates for a sample taken from each dilution of each LD series. DD-Mtb was recorded as present when the number of CFU calculated for the original sample was less than the lower 95% confidence interval of the number of viable M. tuberculosis bacteria in the original sample calculated by the MPN method based on growth of M. tuberculosis in the LD series. CFU and MPN-LD assays had a lower limit of detection (LLD) of 3 M. tuberculosis CFU/ml. In instances of contamination of CFU and/or MPN-LD plates, the LLD increased proportionately. If a sample showed no M. tuberculosis growth, the next whole number below the LLD was used for the purposes of MPN/CFU ratios. Samples were assigned an MPN/CFU ratio of 1 if <10 M. tuberculosis/ml were detected in all three assay formats (CFU, MPN−CF, and MPN+CF).
Numbers of viable M. tuberculosis are presented as log10 values per ml of sputum. The ratio of DD-Mtb to CFU in a sample is calculated by subtracting the log10 values for CFU assays from the log10 value for MPN-LD assays and determining the antilog of the difference, which is presented as a whole number. Results at day 0 and week 2 were compared only for subjects whose data were available at both time points. The Wilcoxon signed rank test (a nonparametric version of the paired t test) was used to compare two quantitative results at one time point and the change in one quantitative result with time. The Wilcoxon rank sum test (a nonparametric version of a two-sample t test) was used to compare distribution of continuous features between samples from subjects with DS versus DR TB. Fisher’s exact test was used to assess the association between two categorical features (presence of DD-Mtb and DS/DR status of the M. tuberculosis at the outset). All tests were performed for two-tailed hypotheses, and P values of <0.05 were considered statistically significant. Statistical analyses were performed using R v4.0.2.
ACKNOWLEDGMENTS
We thank the subjects who volunteered for this study, as well as the clinical, research, and administrative staff of GHESKIO who made this study possible, including Circee Phara Jean, Rose Laure Heriveaux, Daphie Jean-François, Marie-Marcelle Mabou, Alexandra Apollon, and Kathrine McAulay. We are grateful to the Foundation Mérieux for helping to build and maintain the biosafety level 3 facility at GHESKIO.
This work was supported by NIH grants U19AI111143 and K24AI098627 and the Milstein Program in Chemical Biology and Translational Medicine. The Department of Microbiology and Immunology is supported by the William Randolph Hearst Trust. K.S. is supported by NIH grant K08 AI139360, and K.Z. is supported by a VECD Global Health Fellowship funded by the Fogarty International Center (FIC) of the NIH (D43 TW009337).
The views expressed are solely those of the authors and do not necessary represent the views of the NIH.
Footnotes
Supplemental material is available online only.
Contributor Information
Carl Nathan, Email: cnathan@med.cornell.edu.
Daniel W. Fitzgerald, Email: dwf2001@med.cornell.edu.
REFERENCES
- 1.World Health Organization. 2019. World tuberculosis report 2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Burki TK. 2018. The global cost of tuberculosis. Lancet Respir Med 6:13. doi: 10.1016/S2213-2600(17)30468-X. [DOI] [PubMed] [Google Scholar]
- 3.Dhillon J, Fourie PB, Mitchison DA. 2014. Persister populations of Mycobacterium tuberculosis in sputum that grow in liquid but not on solid culture media. J Antimicrob Chemother 69:437–440. doi: 10.1093/jac/dkt357. [DOI] [PubMed] [Google Scholar]
- 4.Huang W, Qi Y, Diao Y, Yang F, Zha X, Ren C, Huang D, Franken KLMC, Ottenhoff THM, Wu Q, Shen J. 2014. Use of resuscitation-promoting factor proteins improves the sensitivity of culture-based tuberculosis testing in special samples. Am J Respir Crit Care Med 189:612–614. doi: 10.1164/rccm.201310-1899LE. [DOI] [PubMed] [Google Scholar]
- 5.Almeida Júnior PS, Schmidt Castellani LG, Peres RL, Combadao J, Tristão TC, Dietze R, Hadad DJ, Palaci M. 2020. Differentially culturable tubercle bacteria dynamics during standard anti-tuberculosis treatment: a prospective cohort study. Tuberculosis (Edinb) 124:101945. doi: 10.1016/j.tube.2020.101945. [DOI] [PubMed] [Google Scholar]
- 6.Beltran CGG, Heunis T, Gallant J, Venter R, Du Plessis N, Loxton AG, Trost M, Winter J, Malherbe ST, Kana BD, Walzl G. 2020. Investigating non-sterilizing cure in TB patients at the end of successful anti-TB therapy. Front Cell Infect Microbiol 10:443. doi: 10.3389/fcimb.2020.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dusthackeer A, Balasubramanian M, Shanmugam G, Priya S, Nirmal CR, Sam Ebenezer R, Balasubramanian A, Mondal RK, Thiruvenkadam K, Hemanth Kumar AK, Ramachandran G, Subbian S. 2019. Differential culturability of Mycobacterium tuberculosis in culture-negative sputum of patients with pulmonary tuberculosis and in a simulated model of dormancy. Front Microbiol 10:2381. doi: 10.3389/fmicb.2019.02381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito K, Warrier T, Somersan-Karakaya S, Kaminski L, Mi J, Jiang X, Park S, Shigyo K, Gold B, Roberts J, Weber E, Jacobs WR, Nathan CF. 2017. Rifamycin action on RNA polymerase in antibiotic tolerant Mycobacterium tuberculosis results in differentially detectable populations. Proc Natl Acad Sci U S A 114:E4832–E4840. doi: 10.1073/pnas.1705385114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukamolova GV, Turapov O, Malkin J, Woltmann G, Barer MR. 2010. Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am J Respir Crit Care Med 181:174–180. doi: 10.1164/rccm.200905-0661OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chengalroyen MD, Beukes GM, Gordhan BG, Streicher EM, Churchyard G, Hafner R, Warren R, Otwombe K, Martinson N, Kana BD. 2016. Detection and quantification of differentially culturable tubercle bacteria in sputum from patients with tuberculosis. Am J Respir Crit Care Med 194:1532–1540. doi: 10.1164/rccm.201604-0769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAulay K, Saito K, Warrier T, Walsh KF, Mathurin LD, Royal-Mardi G, Lee MH, Ocheretina O, Pape JW, Fitzgerald DW, Nathan CF. 2018. Differentially detectable Mycobacterium tuberculosis cells in sputum from treatment-naive subjects in Haiti and their proportionate increase after initiation of treatment. mBio 9:e02192-18. doi: 10.1128/mBio.02192-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordhan BG, Peters JS, McIvor A, Machowski EE, Ealand C, Waja Z, Martinson N, Kana BD. 2021. Detection of differentially culturable tubercle bacteria in sputum using mycobacterial culture filtrates. Sci Rep 11:6493. doi: 10.1038/s41598-021-86054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colwell RR, Brayton PR, Grimes DJ, Roszak DB, Huq SA, Palmer LM. 1985. Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Nat Biotechnol 3:817–820. doi: 10.1038/nbt0985-817. [DOI] [Google Scholar]
- 14.Turapov O, O’Connor BD, Sarybaeva AA, Williams C, Patel H, Kadyrov AS, Sarybaev AS, Woltmann G, Barer MR, Mukamolova GV. 2016. Phenotypically adapted Mycobacterium tuberculosis populations from sputum are tolerant to first-line drugs. Antimicrob Agents Chemother 60:2476–2483. doi: 10.1128/AAC.01380-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan C. 2012. Fresh approaches to anti-infective therapies. Sci Transl Med 4:140sr2. doi: 10.1126/scitranslmed.3003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opie E, Aronson J. 1927. Tubercle bacilli in latent tuberculous lesions and in lung tissue without tuberculous lesion. Arch Pathol Lab Med 4:1–21. [Google Scholar]
- 17.Dartois V, Saito K, Warrier T, Nathan C. 2016. New evidence for the complexity of the population structure of Mycobacterium tuberculosis increases the diagnostic and biologic challenges. Am J Respir Crit Care Med 194:1448–1451. doi: 10.1164/rccm.201607-1431ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant SS, Kaufmann BB, Chand NS, Haseley N, Hung DT. 2012. Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc Natl Acad Sci U S A 109:12147–12152. doi: 10.1073/pnas.1203735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NIAID. 2014. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events. National Institutes of Health. Bethesda, MD. [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, REDCap Consortium. 2019. The REDCap consortium: building an international community of software partners. J Biomed Inform 95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvis B, Wilrich C, Wilrich PT. 2010. Reconsideration of the derivation of most probable numbers, their standard deviations, confidence bounds and rarity values. J Appl Microbiol 109:1660–1667. doi: 10.1111/j.1365-2672.2010.04792.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1 to S3 and S5 to S8. Download AAC00608-21_Supp_1_seq9.pdf, PDF file, 0.8 MB (845.4KB, pdf)
Supplemental Table S4. Download AAC00608-21_Supp_2_seq3.xlsx, XLSX file, 0.02 MB (19.2KB, xlsx)