ABSTRACT
We utilized the rabbit model of aortic valve infective endocarditis to examine the combined efficacy of the lysin LSVT-1701 plus daptomycin. The combination of LSVT-1701 plus daptomycin was highly effective at reducing methicillin-resistant Staphylococcus aureus (MRSA) counts in target tissue. When given for four daily doses, both lysin dose regimens in combination with daptomycin sterilized all target tissues. These findings suggest that LSVT-1701 warrants further clinical evaluation as an adjunctive therapy for the treatment of invasive MRSA infections.
KEYWORDS: MRSA, bacteremia, infective endocarditis, LSVT-1701, daptomycin
INTRODUCTION
Despite the availability of the current antibiotic armamentarium, Staphylococcus aureus infections, especially those involving the endovascular system (e.g., infective endocarditis [IE], cardiac and hemodialysis device infections, and others), are associated with high morbidity and mortality rates (1, 5). Thus, there is an urgent need for new therapeutic alternatives or adjuncts that utilize novel mechanisms of action for treating such infections.
LSVT-1701 (previously known as SAL200) is a novel, recombinantly produced, bacteriophage-encoded lysin that specifically targets staphylococci via cell wall enzymatic hydrolysis (2). LSVT-1701 has a cell wall-binding domain and two catalytic domains (an endopeptidase and an amidase) that degrade the staphylococcal cell wall peptidoglycan, eliciting rapid osmotic lysis, leading to an ultimate staphylocidal effect. LSVT-1701 is being developed as an adjunctive agent to combine with standard-of-care antibiotics against S. aureus, including both methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA). LSVT-1701 has the following important properties: (i) it is rapidly bacteriolytic, with a narrow MIC range against a wide range of S. aureus isolates, including multiresistant clinical isolates (2); (ii) it has potent antibiofilm activity (2); (iii) it is synergistic with standard-of-care antistaphylococcal antibiotics like vancomycin and daptomycin in vitro (15); (iv) it can be dosed multiple times without dose-limiting toxicities or hypersensitivity in rodents and nonhuman primates, as shown by single- and repeated-dose toxicity studies; and (v) it has a low propensity for emergence of resistance.
We therefore sought to examine the in vivo efficacy of LSVT-1701 in multiple dose regimens in combination with daptomycin, a standard-of-care anti-MRSA agent with proven efficacy against bacteremia and IE in humans (3). We employed a standard left-sided aortic valve IE model in rabbits, utilizing a prototypic MRSA strain, MW2, used in many prior experimental IE investigations (6, 12, 16). We examined microbiologic outcomes in distinct target tissues in this model.
MRSA strain MW2 (USA400; clinical complex 1 [CC1]) was used because this isolate is clinically derived, genome sequenced, represents a common hospital-acquired MRSA clonotype, is virulent in experimental IE models, and is daptomycin susceptible in vitro (7, 8). Moreover, the dose of daptomycin that yields substantial but moderate clearance of MW2 from target tissues has been well established in prior studies (6, 16). This provides a key microbiologic window to disclose the synergistic potential of adjunctive agents in this model. The daptomycin MIC of MW2, as determined by standard CLSI broth dilution methods (11), is 1 μg/ml, and that of LSVT-1701 is 0.5 μg/ml as determined by broth microdilution in cation-adjusted Mueller-Hinton broth (CA-MHB) plus 0.25 mM dithiothreitol (DTT), with an incubation time of 20 h with visual and/or spectrophotometric endpoints to assess MIC. These conditions are in line with the current CLSI M07 guidelines (11), which recommend the use of CA-MHB and incubation for 20 h for susceptibility testing of S. aureus. The addition of 0.25 mM DTT decreases assay variability.
The standard MRSA aortic valve IE rabbit model was used to compare the in vivo efficacy profiles of two dose levels of LSVT-1701 (32.5 mg/kg of body weight and 50 mg/kg) given in various dose regimens in combination with daptomycin (versus daptomycin alone). Briefly, an indwelling transcarotid artery-to-left ventricle catheter was inserted under general anesthesia with intramuscular xylazine and ketamine to induce sterile aortic vegetations. At 48 h after catheter placement, IE was induced by an intravenous (i.v.) challenge of ∼2 × 105 CFU, an inoculum that induces IE in >95% of catheterized rabbits challenged with this strain, based on extensive prior data (6, 16). One group of untreated animals (controls) were humanely sacrificed by i.v. pentobarbital at ∼24 h postinfection, and the target organs of interest were sterilely removed (vegetations, kidneys, and spleen) and quantitatively cultured to establish the baseline MRSA bioburden in these tissues. In the remaining treatment animals, similar sacrifices were done and quantitative cultures performed as described above at least 24 h after the last dose of daptomycin and/or LSVT-1701, to minimize any antimicrobial carryover impacts on cultures. In addition, baseline quantitative blood cultures were done postinfection, as well as posttherapy. Microbiologic data in the various treatment groups for each target organ were calculated as either the mean log10 CFU/ml ± standard deviation (SD) (for blood) or the mean log10 CFU/g tissue ± SD (for target tissues). The limit of detection in the organ culture assays was <10 CFU/g tissue. Experimental IE studies were approved by the IACUC Committee of The Lundquist Institute prior to performance.
At 24 h after induction of IE, 10 animals each were randomized into one of the following seven treatment groups: (i) untreated controls (sacrificed at 24 h postinfection); (ii) daptomycin at 4 mg/kg i.v. once daily (QD) by bolus for 4 days; (iii) LSVT-1701 at 32.5 mg/kg i.v. given as a single dose plus daptomycin 4 mg/kg i.v. QD for 4 days; (iv) LSVT-1701 at 50 mg i.v. single dose plus daptomycin 4 mg/kg i.v. QD for 4 days; (v) LSVT-1701 at 32.5 mg/kg QD for 2 days plus daptomycin 4 mg/kg i.v. QD for 4 days; (vi) LSVT-1701 at 32.5 mg/kg QD for 4 days plus daptomycin 4 mg/kg i.v. QD for 4 days; and (vii) LSVT-1701 at 50 mg/kg i.v. QD for 4 days plus daptomycin 4 mg/kg i.v. QD for 4 days. The LSVT-1701 i.v. dose was given by an i.v. infusion of 1-h duration administered within 30 min following the a.m. daptomycin i.v. bolus dose. We did not include an LSVT-1701-alone group, as pilot IE studies found no microbiologic impacts with this lysin at either of two dose levels (at 25 mg/kg, the mean log10 CFU/g ± SD in vegetations was 8.24 ± 0.82 [n = 5], and at 32.5 mg/kg, the mean log10 CFU/g ± SD in vegetations was 9.06 ± 0.23 [n = 5]).
These data (primary endpoints) were statistically compared by a mixed-effects model with treatment groups as fixed effects and rabbits nested within each treatment as a random effect (using SAS version 9.4 or later). The P values were adjusted for multiple comparisons using the Tukey-Kramer method. P values of <0.05 were considered statistically significant.
Baseline (pretherapy) MRSA blood culture counts were similar in all groups, including untreated controls (between 3.18 and 3.50 log10 CFU/ml). All of the LSVT-1701 and daptomycin combinations, as well as the daptomycin-alone regimen, rendered blood cultures negative at the time of sacrifice (24 h after the last dose of both study drugs).
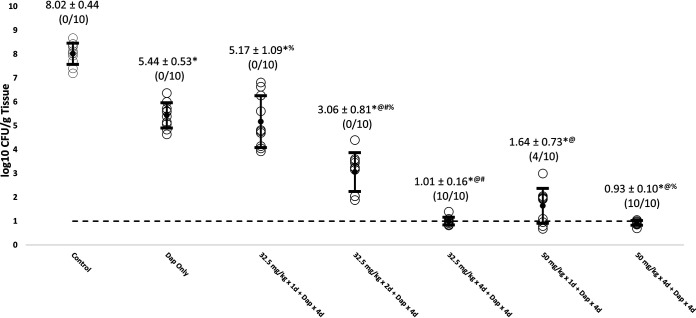
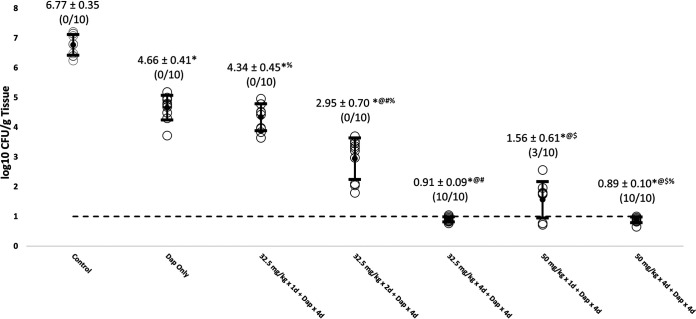
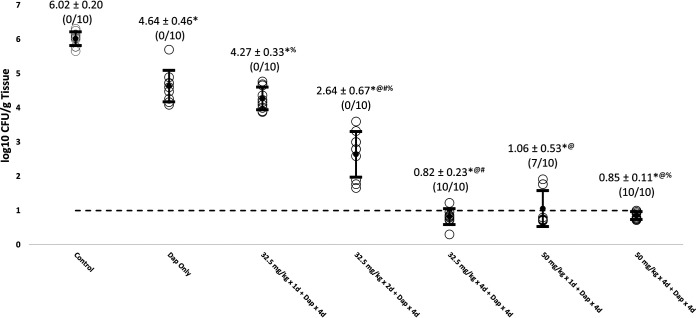
All of the LSVT-1701 regimens in combination with daptomycin significantly reduced MRSA burdens in all target tissue compared to untreated controls (Table 1; Fig. 1, 2, and 3). The reductions in MRSA counts were statistically significant for both increasing LSVT-1701 doses (i.e., single doses of 50 mg/kg versus 32.5 mg/kg i.v.) and an increased number of lysin doses (i.e., four daily doses versus a single dose or two daily doses) in combination with daptomycin. Of note, LSVT-1701 at both 50 mg/kg daily and 32.5 mg/kg daily given for 4 days in combination with daptomycin sterilized all target tissues (i.e., quantitative cultures were at or below the lower limit of detection of 1 log10 CFU/g tissue). Also, LSVT-1701 given as a 50-mg/kg single dose in combination with daptomycin sterilized 4/10 vegetations, 3/10 kidney abscesses, and 7/10 splenic abscesses. In comparison, daptomycin alone or in combination with the lower LSVT-1701 dose of 32.5 mg/kg as a single dose or two daily doses failed to sterilize a single target tissue sample.
TABLE 1.
MRSA bioburdens in blood, vegetations, kidney, and spleen in rabbit IE model
| Treatment group (n = 10 animals/group)a | Mean log10 CFU/g tissue ± SD (no. of sterile cultures/total no. of cultures)b |
Mean log10 CFU/ml blood ± SD (pretherapy baseline) | ||
|---|---|---|---|---|
| Vegetations | Kidney | Spleen | ||
| Untreated control | 8.02 ± 0.44 (0/10) | 6.77 ± 0.35 (0/10) | 6.02 ± 0.20 (0/10) | 3.18 ± 0.45 |
| Dap 4 mg/kg i.v. QD for 4 days | 5.44 ± 0.53* (0/10) | 4.66 ± 0.41* (0/10) | 4.64 ± 0.46* (0/10) | 3.33 ± 0.40 |
| LSVT-1701 32.5 mg/kg i.v. single dose + Dap 4 mg/kg i.v. QD for 4 days | 5.17 ± 1.09*% (0/10) | 4.34 ± 0.45*% (0/10) | 4.27 ± 0.33*% (0/10) | 3.22 ± 0.49 |
| LSVT-1701 32.5 mg/kg i.v. QD for 2 days + Dap 4 mg/kg i.v. QD for 4 days | 3.06 ± 0.81*@#% (0/10) | 2.95 ± 0.70*@#% (0/10) | 2.64 ± 0.67*@#% (0/10) | 3.37 ± 0.27 |
| LSVT-1701 32.5 mg/kg i.v. QD for 4 days + Dap 4 mg/kg i.v. QD for 4 days | 1.01 ± 0.16*@# (10/10) | 0.91 ± 0.09*@# (10/10) | 0.82 ± 0.23*@# (10/10) | 3.30 ± 0.22 |
| LSVT-1701 50 mg/kg i.v. single dose + Dap 4 mg/kg i.v. QD for 4 days | 1.64 ± 0.73*@ (4/10) | 1.56 ± 0.61*@$ (3/10) | 1.06 ± 0.53*@ (7/10) | 3.50 ± 0.39 |
| LSVT-1701 50 mg/kg i.v. QD for 4 days + Dap 4 mg/kg i.v. QD for 4 days | 0.93 ± 0.10*@% (10/10) | 0.89 ± 0.10*@$% (10/10) | 0.85 ± 0.11*@% (10/10) | 3.42 ± 0.33 |
Dap, daptomycin.
*, P < 0.05 for LSVT-1701 treatment groups compared to untreated control; @, P < 0.05 for LSVT-1701 treatment groups compared to daptomycin alone; #, P < 0.05 for LSVT-1701 32.5 mg/kg i.v. QD for 4 days compared to i.v. QD for 2 days and single dose; $, P < 0.05 for LSVT-1701 50 mg/kg i.v. QD for 4 days compared to single dose; %, P < 0.05 for LSVT-1701 50 mg/kg i.v. QD for 4 days compared to 32.5 mg/kg i.v. QD for 2 days and single dose.
FIG 1.
Reduction of MRSA bioburden in cardiac vegetations with LSVT-1701 in combination with daptomycin (Dap). Open circles, individual bioburdens; filled circles, mean bioburdens; error bars, standard deviations; dashed line, limit of experimental sterility. Values are mean log10 CFU/g tissue ± standard deviations (sterilized tissues/total tissues). *, P < 0.05 for LSVT-1701 treatment groups compared to untreated control; @, P < 0.05 for LSVT-1701 treatment groups compared to daptomycin alone; #, P < 0.05 for LSVT-1701 32.5 mg/kg i.v. QD for 4 days compared to i.v. QD for 2 days and single dose; $, P < 0.05 for LSVT-1701 50 mg/kg i.v. QD for 4 days compared to single dose; %, P < 0.05 for LSVT-1701 50 mg/kg i.v. QD for 4 days compared to 32.5 mg/kg i.v. QD for 2 days and single dose.
FIG 2.
Reduction of MRSA bioburden in kidney with LSVT-1701 in combination with daptomycin (Dap). Open circles, individual bioburdens; filled circles, mean bioburdens; error bars, standard deviations; dashed line, limit of experimental sterility. Values are mean log10 CFU/g tissue ± standard deviations (sterilized tissues/total tissues). *, P < 0.05 for LSVT-1701 treatment groups compared to untreated control; @, P < 0.05 for LSVT-1701 treatment groups compared to daptomycin alone; #, P < 0.05 for LSVT-1701 32.5 mg/kg i.v. QD for 4 days compared to i.v. QD for 2 days and single dose; $, P < 0.05 for LSVT-1701 50 mg/kg i.v. QD for 4 days compared to single dose; %, P < 0.05 for LSVT-1701 50 mg/kg i.v. QD for 4 days compared to 32.5 mg/kg i.v. QD for 2 days and single dose.
FIG 3.
Reduction of MRSA bioburden in spleen with LSVT-1701 in combination with daptomycin (Dap). Open circles, individual bioburdens; filled circles, mean bioburdens; error bars, standard deviations; dashed line, limit of experimental sterility. Values are mean log10 CFU/g tissue ± standard deviations (sterilized tissues/total tissues). *, P < 0.05 for LSVT-1701 treatment groups compared to untreated control; @, P < 0.05 for LSVT-1701 treatment groups compared to daptomycin alone; #, P < 0.05 for LSVT-1701 32.5 mg/kg i.v. QD for 4 days compared to i.v. QD for 2 days and single dose; $, P < 0.05 for LSVT-1701 50 mg/kg i.v. QD for 4 days compared to single dose; %, P < 0.05 for LSVT-1701 50 mg/kg i.v. QD for 4 days compared to 32.5 mg/kg i.v. QD for 2 days and single dose.
In this MRSA IE model, both LSVT-1701 doses tested, when administered daily as a 1-h i.v. infusion for 4 days in combination with daptomycin, microbiologically sterilized all target organs tested. This is particularly impressive given the high bioburdens in such target tissues at the time of therapy initiation (i.e., >8 log10 CFU/g in vegetations and >6 log10 CFU/g in kidneys and spleen).
Of note, the same rabbit IE model caused by the same MRSA strain (MW2) as used in the present investigation was recently employed to study another antistaphylococcal phage lysin, CF-301 (Exebacase) (6); this lysin is currently in a phase 3 clinical trial for the treatment of MRSA bacteremia including right-sided IE (ClinicalTrials.gov registration number NCT04160468). Despite utilizing the identical daptomycin dose regimen as in the current study (4 mg/kg i.v. QD for 4 days), the same high rates of target tissue sterilization were not observed in the CF-301 studies (6). Although both CF-301 and LSVT-1701 are recombinant antistaphylococcal lysins, there are distinct structural differences. CF-301 is 26 kDa, has only one catalytic domain (an endopeptidase), and is limited to a single-dose regimen based on preclinical toxicology studies (dose-limiting toxicity leading to vasculitis and hypersensitivity) (14). In contrast, LSVT-1701 is 54 kDa and has two catalytic domains (an endopeptidase and an amidase) that cleave at two sites in the cell wall; it can also be administered as multiple doses, with no similar dose-limiting toxicities observed in a 4-week good laboratory practices (GLP) repeated-dose study in rats and nonhuman primates (9, 10). Because of these differences, LSVT-1701 will be evaluated in an upcoming phase 2b study of S. aureus bacteremia, including both right-sided and left-sided IE.
There are several limitations to the current study. First, only one MRSA strain was assessed. Future studies will require multiple strains of both MRSA and MSSA to be evaluated in this same model. Second, although not the objective of this study, the rates and extents of microbiologic target tissue relapse following the 4-day daptomycin–plus–LSVT-1701 regimens were not evaluated. Such investigations of relapse are planned for future studies. Third, no MIC testing for LSVT-1701 in MRSA isolates recovered in target tissues following therapy was performed. Fourth, neither pharmacokinetic and pharmacodynamic drivers nor target attainment values most predictive of treatment outcomes were provided in this report. These latter metrics will be the topic of a separate report.
In conclusion, LSVT-1701 administered at 32.5 or 50 mg/kg daily in a 4-day regimen in combination with daptomycin resulted in microbiologic sterilization of all target organs in this rigorous MRSA IE model. These data support further clinical development of LSVT-1701 for the treatment of MRSA endovascular infections, including IE.
ACKNOWLEDGMENTS
We are grateful for the help of Surya Chitra, a consultant for Lysovant, for statistical support.
These studies were supported by a research grant to A.S.B. from Lysovant.
REFERENCES
- 1.Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler VG, Jr.. 2016. Infective endocarditis. Nat Rev Dis Primers 2:16059. doi: 10.1038/nrdp.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jun SY, Jung GM, Yoon SJ, Oh M-D, Choi Y-J, Lee WJ, Kong J-C, Seol JG, Kang SH. 2013. Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int J Antimicrob Agents 41:156–161. doi: 10.1016/j.ijantimicag.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Fowler VG, Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, Cabell CH, Link AS, DeMeyer I, Filler SG, Zervos M, Cook P, Parsonnet J, Bernstein JM, Price CS, Forrest GN, Fätkenheuer G, Gareca M, Rehm SJ, Brodt HR, Tice A, Cosgrove SE, S. aureus Endocarditis and Bacteremia Study Group. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 355:653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG, Jr.. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah SU, Xiong YQ, Abdelhady W, Iwaz J, Pak Y, Schuch R, Cassino C, Lehoux D, Bayer AS. 2020. Effect of the lysin Exebacase on cardiac vegetation progression in a rabbit model of methicillin-resistant Staphylococcus aureus endocarditis as determined by echocardiography. Antimicrob Agents Chemother 64:e00482-20. doi: 10.1128/AAC.00482-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra NN, Rubio A, Nast CC, Bayer AS. 2012. Differential adaptations of methicillin-resistant Staphylococcus aureus to serial in vitro passage in daptomycin: evolution of daptomycin resistance and role of membrane carotenoid content and fluidity. Int J Microbiol 2012:683450. doi: 10.1155/2012/683450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra NN, Yang SJ, Sawa A, Rubio A, Nast CC, Yeaman MR, Bayer AS. 2009. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 53:2312–2318. doi: 10.1128/AAC.01682-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jun SY, Jung GM, Yoon SJ, Choi YJ, Koh WS, Moon KS, Kang SH. 2014. Preclinical safety evaluation of intravenously administered SAL200 containing the recombinant phage endolysin SAL-1 as a pharmaceutical ingredient. Antimicrob Agents Chemother 58:2084–2088. doi: 10.1128/AAC.02232-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jun SY, Jung GM, Yoon SJ, Youm SY, Han HY, Lee JH, Kang SH. 2016. Pharmacokinetics of the phage endolysin-based candidate drug SAL200 in monkeys and its appropriate intravenous dosing period. Clin Exp Pharmacol Physiol 43:1013–1016. doi: 10.1111/1440-1681.12613. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed. Approved standard M07. CLSI, Wayne, PA. [Google Scholar]
- 12.Reference deleted.
- 13.Trotonda MP, Xiong YQ, Memmi G, Bayer AS, Cheung AL. 2009. Role of mgrA and sarA in methicillin-resistant Staphylococcus aureus autolysis and resistance to cell wall-active antibiotics. J Infect Dis 199:209–218. doi: 10.1086/595740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Congo K. 2018. U.S. Food and Drug Administration meeting, 22 August 2018: immunomodulator/enhancers product development. Capital Reporting Company, Washington, DC. https://www.fda.gov/media/115771/download.
- 15.Kim NH, Park WB, Cho JE, Choi YJ, Choi SJ, Jun SY, Kang CK, Song KH, Choe PG, Bang JH, Kim ES, Park SW, Kim NJ, Oh MD, Kim HB. 2018. Effects of phage endolysin SAL200 combined with antibiotics on Staphylococcus aureus infection. Antimicrob Agents Chemother 62:e00731-18. doi: 10.1128/AAC.00731-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayer AS, Lam K, Bayer AS. 1985. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J Infect Dis 151:157–165. doi: 10.1093/infdis/151.1.157. [DOI] [PubMed] [Google Scholar]