ABSTRACT
Chloroquine (CQ) is the first-line treatment for Plasmodium vivax malaria in most countries where malaria is endemic. Monitoring P. vivax CQ resistance (CQR) is critical but remains challenged by the difficulty to distinguish real treatment failure from reinfection or liver relapse. The therapeutic efficacy of CQ against uncomplicated P. vivax malaria was evaluated in Gia Lai Province, Vietnam. Sixty-seven patients were enrolled and followed for 42 days using microscopy and quantitative PCR. Adequate clinical and parasitological response (ACPR) was 100% (66/66) on day 28 but 75.4% (49/65) on day 42. Eighteen recurrences (27.7%) were detected, with a median time to recurrence of 42 days (interquartile range [IQR], 35 to 42) and blood CQ concentration of <100 ng/ml. Primary infections leading to recurrence occurred in younger individuals (median age for ACPR = 25 years [IQR, 20 to 28]; recurrences = 18 [16 to 21]; P = 0.002) had a longer parasite clearance time (PCT for ACPR = 47.5 h [IQR, 36.2 to 59.8 h]; recurrences = 54.2 [48.4 to 62.0]; P = 0.035) and higher pvcrt gene expression (median relative expression ratio for ACPR = 0.09 [IQR, 0.05 to 0.22]; recurrences = 0.20 [0.15 to 0.56]; P = 0.002), but showed no differences in ex vivo CQ sensitivity. Parasite genotyping by microsatellites, single nucleotide polymorphism (SNP) barcoding, and whole-genome sequencing (WGS) identified a majority of homologous recurrences, with 80% (8/10) showing >98% identity by descent to paired day 0 samples. This study shows that CQ remained largely efficacious to treat P. vivax in Gia Lai; i.e., recurrences occurred late (>day 28) and in the presence of low blood CQ concentrations. However, the combination of both WGS and gene expression analysis (pvcrt) data with clinical data (PCT) allowed us to identify potential emergence of low-grade CQR, which should be closely monitored. (This study has been registered at ClinicalTrials.gov under identifier NCT02610686.)
KEYWORDS: Plasmodium vivax, Vietnam, antimalarial agents, chloroquine, drug resistance, malaria, pvcrt, recurrence, therapeutic efficacy, whole-genome sequencing
INTRODUCTION
Plasmodium vivax was responsible for 6.4 million malaria cases globally according to the World Health Organization (WHO) estimates for 2019, with most of them occurring in Asia (1). Historically mistaken as benign, P. vivax is becoming the predominant malaria species in countries where the incidence of Plasmodium falciparum is decreasing as they move toward malaria elimination (2). Compared to P. falciparum, the control and elimination of P. vivax present additional unique challenges due to key biological differences, such as the presence of liver hypnozoites responsible for relapses (i.e., reactivation of dormant parasite forms leading to blood-stage infection), the low parasite density in peripheral blood, and the production of gametocytes (i.e., parasite transmissible stages) before the onset of symptoms (2, 3).
Chloroquine (CQ) is the first-line drug treatment for P. vivax in most countries where it is endemic (1). Chloroquine resistance (CQR) in malaria parasites emerged first in P. falciparum shortly after the start of its widespread use as an antimalarial treatment in the 1950s (4). The first report of P. vivax CQR emerged 30 years later in Papua New Guinea, and since then, decreasing efficacy of CQ against P. vivax has been reported across areas of malaria endemicity around the world, although with different degrees of certainty (3, 4). In countries where high-level CQR has been confirmed (i.e., Papua New Guinea, Indonesia, and Malaysia), artemisinin-based combination therapies have been adopted as the universal antimalarial first-line treatment instead of CQ (1, 4).
In order to evaluate the efficacy of CQ regimens against P. vivax, the WHO recommends conducting therapeutic efficacy studies (TES) with at least 28 days of patient follow-up after treatment administration (5). Recurrences within this period with a confirmed CQ blood concentration over the minimum effective concentration (MEC) of 100 ng/ml would support the presence of CQR. However, it has also been shown that a long-acting antimalarial drug like CQ can linger in the bloodstream for approximately 35 days and delay appearance of relapses up to 6 weeks (6, 7). Extended follow-up periods of 42 or 63 days are therefore recommended to detect recurrences with parasites at early stage of CQR (5, 8). Nonetheless, a main challenge of P. vivax TES is to distinguish a parasite recrudescence caused by real treatment failure from a relapse or new infection, for which additional ex vivo and in vitro laboratory tests are required (8). On one hand, genotyping of parasites at polymorphic genome regions can show whether recurrent parasite clones differ from those in the initial infection, especially when high-resolution approaches at the whole-genome level are used (9, 10), but if applied alone, these approaches remain limited in their ability to unambiguously differentiate a recrudescence from a genetically homologous relapse. On the other hand, short-term ex vivo cultures of isolates exposed to CQ and genotyping of drug resistance markers can be combined to characterize isolates’ CQR properties.
The identification and validation of CQR markers in P. vivax has unfortunately been hampered by the inability to carry out long-term culturing of the parasite and evaluate adaptation to extended drug exposure (11). Despite this limitation, numerous studies have linked CQR to both the chloroquine resistance transporter gene (pvcrt) and multidrug resistance gene-1 (pvmdr1). Associations with single nucleotide polymorphisms (SNPs) and/or copy number variation in both pvcrt and pvmdr1 have remained weak and sometimes contradictory (12–16). At the transcriptional level, increased expression of pvcrt and pvmdr1 was found in CQR infections from the Brazilian Amazon (17), whereas pvcrt expression was upregulated in a genetic cross of P. vivax parasites differing in their CQ response in experimental nonhuman primate infections (18).
In Vietnam, P. vivax accounts for 40% of all malaria cases, which occur mainly among ethnic minorities in forested areas in central and southern provinces (19). National guidelines for the treatment of P. vivax infection follows WHO recommendation of 3 days of CQ (25 mg/kg) followed by 14 days of primaquine (PQ; 0.25 mg/kg) radical-cure treatment to eliminate liver hypnozoites. To date, only one study, conducted in Quang Nam Province (Central Highlands region) between 2009 and 2011, confirmed P. vivax CQR by reporting a 3.5% failure rate (n = 8) at day 28 and three cases with CQ blood concentrations of >100 ng/ml, although no genetic analysis of recurrences was performed (20). A few other reports of suspected P. vivax CQR have been published in the last 20 years in Binh Thuan, Binh Phuoc, and Ninh Thuan provinces (recurrence rates at day 28 or later ranging from 13% to 57%), but CQR could not be confirmed due to the lack of either blood CQ levels or parasite genotyping in the recurrent infections (21–23).
In the present study, we assessed the in vivo susceptibility of P. vivax to CQ in Gia Lai (Central Highlands), the province with the highest malaria burden in Vietnam in recent years (19, 24). We used good manufacturing practice-certified CQ and administered the currently recommended radical cure regimen of PQ at the end of the 42 days of follow-up. Importantly, we provide a thorough analysis of treatment failures by combining ex vivo drug sensitivity assays, three technical approaches to genetic analysis of recurrences, and genotyping of candidate markers of drug resistance, including gene expression analysis.
RESULTS
Patient recruitment and characteristics at baseline.
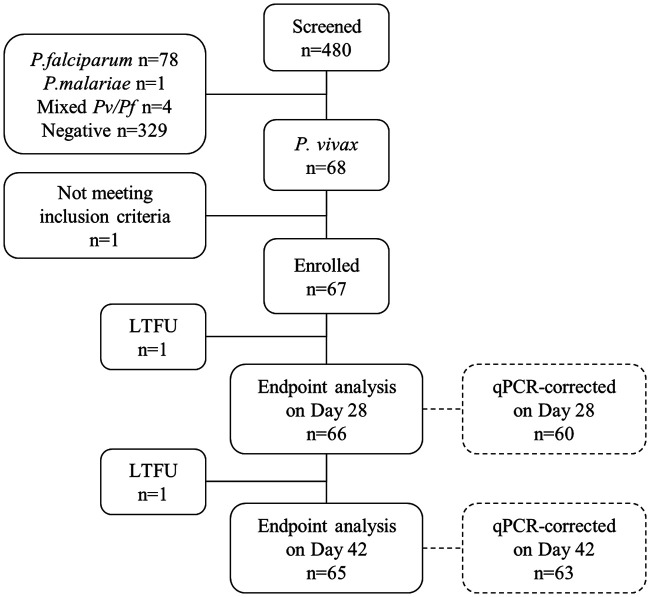
A total of 480 patients were screened between 2015 and 2017 at commune health centers (CHC) of Krong Pa district, of whom 68 (14.2%) had P. vivax infection confirmed by light microscopy (LM). Sixty-seven were enrolled and completed CQ treatment (Fig. 1). One patient vomited medication 40 min after treatment and received an extra half-dose. Twenty-seven (40.3%) patients self-presented at Chu R’Cam CHC, whereas the other 40 (59.7%) were referred from nearby communes. The age range was 8 to 45 years old, and most patients were male (91%) (Table 1). Two thirds of participants reported having had an episode of malaria in the past year (62.7%).
FIG 1.
Flow chart of patient screening, enrollment, and follow-up. LTFU, lost to follow-up.
TABLE 1.
Patient characteristics at baseline
| Variablea | Value (n = 67) |
|---|---|
| Age (yrs) [median (IQR)] | 22 (18–26) |
| No. (%) in age group (yrs) | |
| >1–15 | 6 (9.0) |
| >15–25 | 37 (55.2) |
| >25 | 24 (35.8) |
| No. (%) of males | 61 (91.0) |
| No. (%) in ethnic group | |
| J’Rai | 52 (77.6) |
| Kinh | 15 (22.4) |
| Wt (kg) [median (IQR)] | 52.5 (48, 61) |
| No. who: | |
| Had malaria in the previous yr | 42 (62.7) |
| Took antimalarials in the previous 14 days | 0 (0) |
| Had G6PD deficiency | 1/67 (1.5) |
| No. (%) with fever at enrollment (≥37.5°C) | 56 (83.6) |
| Body temp (°C) [median (IQR)] | |
| All | 38.5 (37.5, 39.5) |
| Febrile only | 39.0 (38.4, 39.5) |
| No. (%) with symptom | |
| Headache | 67 (100.0) |
| Fatigue | 66 (98.5) |
| Dizziness | 48 (71.6) |
| Chills | 37 (55.2) |
| Sweats | 21 (31.3) |
| Nausea | 17 (25.4) |
| Pain | 6 (9.0) |
| Vomiting | 6 (9.0) |
| Diarrhea | 3 (4.5) |
| Hemoglobin (g/dl) [median (IQR)] | 13.5 (12.5, 14.8) |
| No. (%) with anemia (Hb < 11 g/dl) | 7 (10.4) |
| Parasitological data by LM | |
| No. of asexual parasites/μl [geometric mean (95% CI)] | 5,992 (4,594–7,815) |
| % ring stage [median (IQR)]b | 75 (43–91) |
| Gametocyte prevalence [no. (%)] | 58 (86.6) |
| No. of gametocytes/μl [geometric mean (95% CI)] | 223 (171–290) |
Hb, hemoglobin; G6PD, glucose-6-phosphate dehydrogenase.
n = 64.
Fever was detected in 56 patients (83.6%) at the time of enrollment, 7 patients (10.4%) presented anemia (hemoglobin [Hb] < 11 g/dl), and 1 patient was found to have G6PD deficiency. All patients reported headache and fatigue together with other clinical symptoms, most commonly dizziness and chills. Up to 87% of infections carried levels of gametocytes detectable by LM (Table 1). Asexual parasite density was significantly correlated with body temperature (ρ = 0.435; P < 0.001), density of gametocytes (n = 58; ρ = 0.525; P < 0.001), and proportion of ring stages (n = 64; ρ = 0.431, P < 0.001) but did not differ between age groups (P = 0.971).
CQ efficacy endpoints.
Overall, CQ treatment was well tolerated, with 3 patients reporting moderate adverse effects (dizziness, diarrhea, and abdominal pain). The median CQ dose received by patients was 24.5 mg/kg (range, 20.0 to 26.8). Endpoint analysis was conducted using LM data. Parasite clearance time (PCT) and parasite clearance half-life (PC1/2) were estimated at 48.3 h (maximum, 95.3 h) and 4.1 h (maximum, 13 h), respectively (Table 2). The percentage of patients that cleared infection based on LM was 62.7% (42/67) by day 2, 97.0% (64/66) by day 3, and 100% (66/66) by day 4. Sixty-six (98.5%) and 65 (97.0%) patients completed the 28-day and the 42-day follow-up, respectively (Table 2). At day 28, the rate of adequate clinical and parasitological response (ACPR) was 100% (66/66), whereas at day 42, the rate of ACPR was 75.4% (49/65). Of 16 patients with recurrences detected by LM, three presented with fever (late clinical failure [LCF], days 33 to 42), while the other 13 were afebrile (late parasitological failure [LPF], >day 35). Mean parasitemia at the day of recurrence (DRec) was 538.7 parasites/μl (95% confidence interval [CI], 227.8 to 1,274.0) and was higher in LCF (n = 3; 3,024.2 parasites/μl [95% CI, 1,946.9 to 4,697.6]) than LPF (n = 13; 361.7 [95% CI, 143.5 to 911.9]; P = 0.026). Gametocytes were observed in 6/16 (37.5%) infections at DRec.
TABLE 2.
Study endpoints
| Endpointa | Value [no./total (%) or median (IQR)] |
|---|---|
| Primary | |
| ACPR | |
| Day 28 | 66/66 (100.0) |
| Day 42 | 49/65 (75.4) |
| Treatment failure (day 42) | |
| LCF | 3/65 (4.6) |
| LPF | 13/65 (20.0) |
| Time to recurrence (days) | 42 (35–42) |
| Secondary | |
| PCT, hours (n = 66) | 48.3 (36.8–60.1) |
| PC1/2, hours (n = 63) | 4.1 (3.5–5.4) |
| Patients with asexual parasitemia at: | |
| Day 1 | 56/67 (83.6) |
| Day 2 | 25/67 (37.3) |
| Day 2, qPCR corrected | 28/63 (44.4) |
| Day 3 | 2/66 (3.0) |
| Day 3, qPCR corrected | 9/65 (13.9) |
| Patients with gametocytes at: | |
| Day 1 | 29/67 (43.3) |
| Day 2 | 8/67 (11.9) |
| Day 2, RT-qPCR corrected | 27/68 (46.6) |
| Day 3 | 0/66 (0) |
| Day 3, RT-qPCR corrected | 7/62 (11.3) |
| No. with severe anemia (Hb < 7 g/dl; day 7) | 0/34 (0) |
ACPR, adequate clinical and parasitological response; LCF, late clinical failure; LPF, late parasitological failure; PCT, parasite clearance time; PC1/2, parasite clearance half-life, i.e., time to decrease initial parasitemia by half in log-linear phase; Hb, hemoglobin.
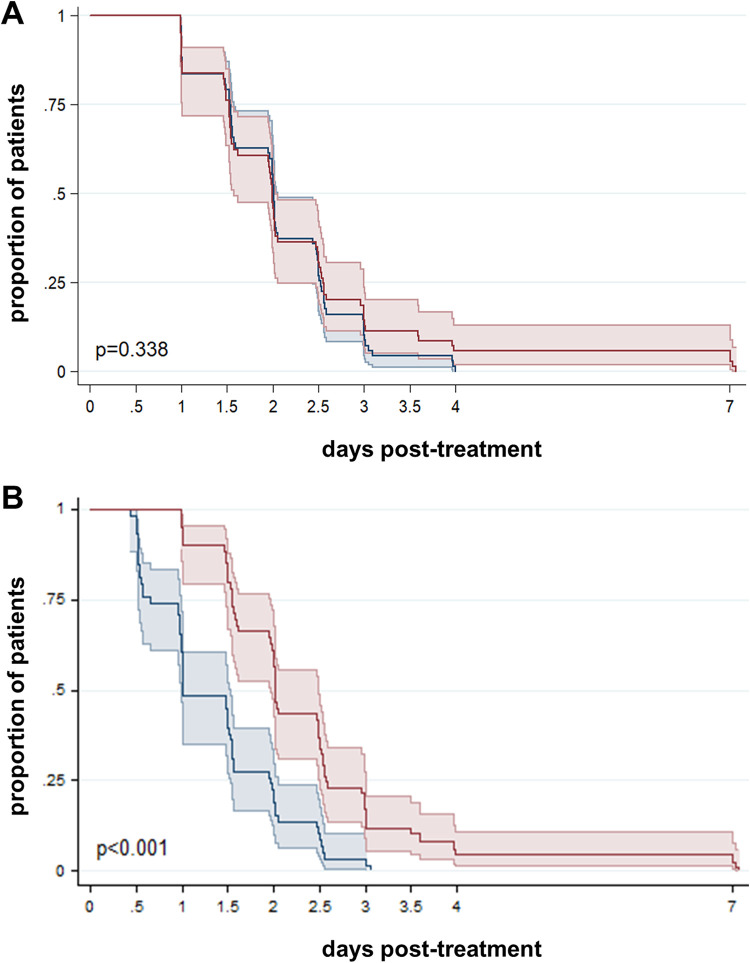
After quantitative PCR (qPCR) correction, parasite clearance rate did not differ significantly from that determined by LM (P = 0.338, log rank test) (Fig. 2A). The number of day 3 positives increased to 13.9% (9/65) by qPCR compared to 3% (2/65) by LM. On the other hand, gametocyte clearance rate was significantly lower by pvs25 reverse transcription-qPCR (RT-qPCR) than by LM (P < 0.001, log rank test) (Fig. 2B), and 11.3% (7/62) were positive by RT-qPCR on day 3. Molecular methods detected two additional recurrences on day 42 (total recurrences = 18), resulting in a qPCR-corrected ACPR of 73% (47/65) at the end of the 42-day follow-up. Kaplan-Meier plots for parasite clearance separating recurrent and nonrecurrent courses of infection did not show differences in parasite clearance rate trends (see Fig. S3 in the supplemental material).
FIG 2.
Time to parasite clearance. Kaplan-Meier survival curves for total parasite clearance (A) and gametocyte clearance (B). Results are stratified by light microscopy (n = 67; blue line) and qPCR (n = 63; red line) (A) or RT-qPCR (n = 59, red line) (B). Colored areas indicate 95% confidence intervals. P values were calculated using a log rank test. One patient with no day 7 sample tested by qPCR/RT-qPCR was excluded from this analysis.
All 18 recurrences occurred after day 33 (median 42 days [interquartile range {IQR}, 35 to 42]) and affected younger individuals (median age, 18 years old [IQR, 16 to 21]), compared to patients with ACPR (25 years [IQR, 20 to 28], P = 0.002) (Table 3). Recurrent and nonrecurrent infections did not differ in the CQ dose administered (24.6 mg/kg [IQR, 24.0 to 25.5] versus 24.5 mg/kg [IQR, 24.0 to 25.0], P = 0.787) (Fig. S4). Blood concentrations of CQ and DCQ at DRec were found to be below 100 ng/ml (median, 30.3 ng/ml [range, 1.9 to 52.3]) in the 14 patients with a valid test result (of note, all day 7 concentrations were >100 ng/ml; median, 502 ng/ml [range, 225 to 874]; n = 15). PCT was longer in infections that presented with recurrence (n = 18; 54.2 h [IQR, 48.4 to 62.0]) than those with ACPR (n = 46; 47.5 h [IQR, 36.2 to 59.8], P = 0.035; Table 3), and a similar trend was observed for PC1/2 (recurrent, 4.8 h [IQR, 4.0 to 5.5]; ACPR, 4.0 h [IQR, 3.4 to 5.2], P = 0.078). Asexual parasitemia at day 0 was not significantly different between recurrent (n = 49; mean, 6,762.3 parasites/μl [95% CI, 3,811.7 to 11,997.0]) and nonrecurrent infections (n = 16; 7,114.3 parasites/μl [95% CI, 3,726.7 to 13,581.3], P = 0.462), and nor was positivity at day 2 (P = 0.409) or day 3 (P = 0.772) (Table 3).
TABLE 3.
Main parasitological and drug-resistance characteristics of P. vivax infections, by treatment outcome at baselinea
| Characteristic | ACPR |
All recurrences |
IBD homologous recurrences |
|||||
|---|---|---|---|---|---|---|---|---|
| n | Median (IQR) or no. (%) | n | Median (IQR) or no. (%) | Pb | n | Median (IQR) or no. (%) | Pb | |
| Age | 49 | 25 (20–28) | 18 | 18 (16–21) | 0.002 | 8 | 19 (18–26) | 0.103 |
| CQ dose uptake (mg/kg) | 47 | 24.5 (24.0–25.0) | 18 | 24.6 (24.0–25.5) | 0.787 | 8 | 24.1 (23.0–25.2) | 0.545 |
| Parasitological indicators | ||||||||
| Parasitemia at day 0 (LM) (parasites/μl)c | 49 | 5,731.8 (4,210.8–7,802.2) | 16 | 6,762.3 (3,811.7–11,997.0) | 0.621 | 8 | 12,415 (5,792–14,031) | 0.142 |
| PCT (h) | 48 | 47.5 (36.2–59.8) | 18 | 54.2 (48.4–62.0) | 0.035 | 8 | 60.3 (48.6–73.0) | 0.019 |
| PC1/2 (h) | 46 | 4.0 (3.4–5.2) | 17 | 4.8 (4.0–5.5) | 0.078 | 8 | 4.8 (4.2–5.7) | 0.125 |
| Positive at day 2 by: | ||||||||
| LM | 49 | 16 (32.7) | 18 | 9 (50) | 0.193 | 8 | 5 (62.5) | 0.13 |
| qPCR | 46 | 19 (41.3) | 17 | 9 (52.3) | 0.409 | 8 | 5 (62.5) | 0.443 |
| Positive at day 3 by: | ||||||||
| LM | 49 | 2 (4.0) | 17 | 0 (0) | 1.000 | 8 | 0 (0) | 1.000 |
| qPCR | 48 | 7 (14.6) | 17 | 2 (11.8) | 1.000 | 8 | 1 (12.5) | 1.000 |
| Ex vivo drug sensitivity (day 0) | ||||||||
| CQ IC50 (nM)c | 13 | 41.5 (24.4–80.4) | 8 | 38.7 (10.4–108.0) | 0.856 | 5 | 51.3 (24.3–108.2) | 0.301 |
| Candidate molecular markers of CQR (day 0) | ||||||||
| pvmdr1 SNP | ||||||||
| Y976F | 47 | 34 (72.3) | 16 | 10 (62.5) | 0.459 | 6 | 3 (50.0) | 0.351 |
| F1076L | 47 | 45 (96) | 16 | 15 (93.8) | 1.000 | 6 | 6 (100) | 1.000 |
| pvcrt expression (R) | 33 | 0.09 (0.05–0.22) | 16 | 0.20 (0.15–0.56) | 0.002 | 8 | 0.17 (0.14–0.38) | 0.038 |
| pvmdr1 expression (R) | 35 | 0.11 (0.04–0.28) | 16 | 0.17 (0.06–0.47) | 0.138 | 8 | 0.09 (0.05–0.35) | 0.779 |
ACPR, adequate clinical and parasitological response; LM, light microscopy; CQR, chloroquine resistance; R, relative expression ratio.
P values correspond to results of a Wilcoxon rank sum test (for continuous variables) and a chi-square test (for proportions).
Geometric mean (95% CI).
Genotyping of recurrent infections.
Treatment failures were corrected by genotyping and classified as genetically homologous or genetically heterologous infections relative to the initial infection at day 0, using three technical approaches in increasing order of resolution. Initially, day 0/DRec sample pairs were genotyped at microsatellite (MS) markers Pvmsp1F3, MS4, MS10, and PvSal1814 (Table 4 and Table S3). Overall, 2/18 (11%) samples at day 0 and 4/15 (27%) at DRec were monoclonal. Fifteen sample pairs had data for at least 2 markers (Table 4). Median complexity of infection (COI) was 3 (IQR, 2 to 3) at day 0 compared to 2 (IQR, 1 to 3) at DRec (P = 0.080, signed-rank test). Homologous recurrences determined by MS analysis accounted for 80% (12/15) of total recurrences.
TABLE 4.
Genotyping and categorization of recurrent infectionsa
| Patient code | MS marker analysis |
SNP barcode analysis |
WGS |
|||||
|---|---|---|---|---|---|---|---|---|
| Discordant MS (no./total) | Typeb | Discordant SNP (no./total) | Typec | IBS |
IBD |
|||
| Discordant SNP (%) | Typed | % IBD | Typee | |||||
| 003 | 0/3 | Homologous | 1/38 | Heterologous | ||||
| 007 | 0/4 | Homologous | 2/38 | Heterologous | 14.3 | Heterologous | 35.2 | Heterologous |
| 008 | 0/4 | Homologous | 0/37 | Homologous | 2.2 | Homologous | 99.9 | Homologous |
| 010 | 1/38 | Heterologous | ||||||
| 018 | 0/2 | Homologous | ||||||
| 021 | 4/4 | Heterologous | ||||||
| 022 | 0/4 | Homologous | 0/37 | Homologous | 6.8 | Heterologous | 98.1 | Homologous |
| 027 | 0/4 | Homologous | 2.3 | Homologous | 99.5 | Homologous | ||
| 028 | ||||||||
| 034 | 1/4 | Heterologous | 0/38 | Homologous | 1.6 | Homologous | 99.8 | Homologous |
| 037 | 2.0 | Homologous | 99.7 | Homologous | ||||
| 043 | 0/3 | Homologous | 1/38 | Heterologous | 12.6 | Heterologous | 99.8 | Homologous |
| 044 | 0/3 | Homologous | ||||||
| 047 | 1/3 | Heterologous | 0/22 | Homologous | 8.0 | Heterologous | 32.5 | Heterologous |
| 048 | 0/3 | Homologous | 0/20 | Homologous | 1.7 | Homologous | 99.5 | Homologous |
| 050 | 0/3 | Homologous | ||||||
| 053 | 0/4 | Homologous | 0/38 | Homologous | ||||
| 054 | 0/4 | Homologous | 5/38 | Heterologous | 2.4 | Homologous | 100 | Homologous |
An extended version is provided in Table S4. MS, microsatellites (Pvmsp1F3 + MS4 + MS10 + PvSal1814); IBS, identity by state; IBD, identity by descent; WGS, whole-genome sequencing.
Rate of homologous infections, 12/15 (80%).
Rate of homologous infections, 6/11 (55%).
Rate of homologous infections, 6/10 (60%).
Rate of homologous infections, 8/10 (80%).
In a second step, recurrences were genotyped using a 38-SNP molecular barcode (25). Barcodes were successfully generated in 11 day 0/DRec sample pairs, although two of them at only 20 and 22 SNP positions, respectively (Table 4 and Data Set S1). Median COI was 1 (IQR, 1 to 1) at day 0 and 2 (IQR, 1 to 2) at DRec (P = 0.034, signed-rank test). The homologous recurrence rate was 55% (6/11). Compared to MS (n = 10 pairs), the SNP barcode identified 4 additional heterologous infections and categorized two infections heterologous by MS analysis as homologous.
Finally, samples were analyzed using whole-genome sequencing (WGS), aiming for a maximum level of resolution. Fourteen day 0/DRec DNA sample pairs generated sequencing reads in both samples. Mean sequencing coverage ranged from 1.6 to 50.1 (overall mean, 30.3 ± 14.5), and there was no significant difference in coverage between samples from day 0 and DRec (P = 0.280, paired t test) (Table S2). COI was 1 in 58% (7/12) of samples at day 0 and in 73% (8/11) of samples at DRec. WGS analysis of identity by state (IBS; i.e., number of discordant SNPs) identified 60% (6/10) of homologous recurrences (Table 4). Compared to MS (n = 9 pairs), IBS categorized three originally homologous recurrences as heterologous. WGS data analysis of identity by descent (IBD; i.e., considering inheritance from a common ancestor) identified 80% (8/10) recurrent infections as homologous, all of them with >98% of their genome identical by descent to the paired day 0 sample (median, 99.7% [IQR, 99.5 to 99.8%], n = 8). The median IBD found between day 0/DRec pairs (99.6% [IQR, 98.4 to 99.8%], n = 10) was significantly higher than the IBD found in all between-patient comparisons (0% [IQR, 0 to 0.60%]; n = 196; P < 0.001, Mann-Whitney U test), suggesting that it is unlikely that the high IBD observed was caused by independent infections with a closely related clone. Results between IBS and IBD were discordant in 2 of the 10 sample pairs (patients 022 and 043), which had a considerably high number of discordant SNPs by IBS (6.8% and 12.6%) but high IBD proportion (98.1% and 99.8%); mean sequencing coverage was >34.2 for both samples and time points. Assuming that IBD provides the highest level of resolution and most restricted definition of homology, the final rate of homologous recurrence (IBD-homologous) in the study was at least 12% (8/65) in all patients that completed the 42-day follow-up and at least 44% (8/18) of total recurrences.
Compared to patients with ACPR, patients with IBD-homologous recurrences (n = 8) were not different in CQ uptake (24.1 mg/kg [IQR, 23.0 to 25.2]; P = 0.565) or parasite density at day 0 (P = 0.142) (Table 3). However, median PCT among patients with IBD-homologous recurrences was significantly longer than in those with ACPR (P = 0.019).
Drug resistance characteristics.
Phenotypic assessment of CQ susceptibility was conducted using short-term schizont maturation assays (SMA) at day 0. A total of 41 cultures were conducted, of which 21 were valid for analysis (51% success rate). The overall CQ geometric mean half-maximal inhibitory concentration (IC50) was 40.4 nM (95% CI, 31.5 to 51.8; range, 10.4 to 108.0). The infection with the highest CQ IC50 (108.0 nM) corresponded to a patient with the longest PCT (95.1 h) and presented with an IBD-homologous recurrence, although there were no differences in overall ex vivo CQ sensitivity between recurrent and nonrecurrent infections (Table 3 and Fig. 3).
FIG 3.
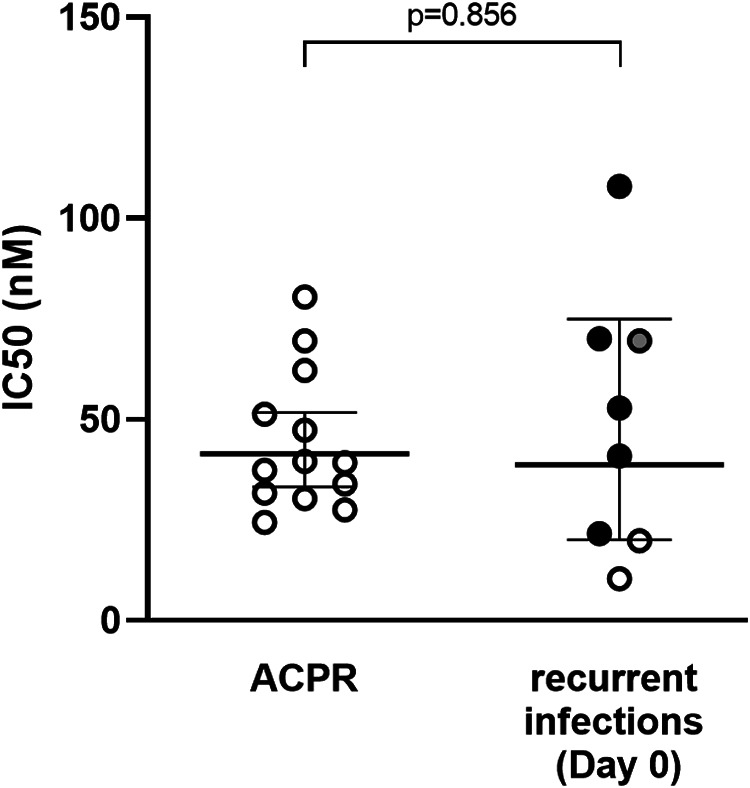
Ex vivo susceptibility to chloroquine in schizont maturation assays (SMA). Data are CQ IC50 in day 0 samples, stratified by treatment outcome. Dots indicate the type of recurrence based on identity-by-descent (IBD) analysis (black, IBD-homologous recurrence; gray, IBD-heterologous recurrence; white, undetermined by WGS). ACPR, adequate clinical and parasitological response.
At the genetic level, mutations in the pvmdr1 F976Y and F1076L codons were common (30.2% [19/63] for F976Y and 95.2% [60/63] for F1076L), but no differences were found in prevalence of mutations between treatment outcome groups at day 0 (Table 3). In samples sequenced at DRec (n = 10), F976Y and F1076L variants were found in 20% (2/10) and 100% (10/10) of the sequences, respectively.
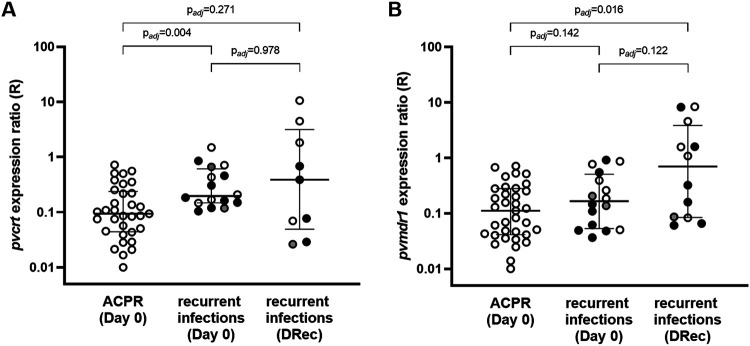
Gene expression of pvcrt and pvmdr1 was determined in all samples with sufficient RNA quantity at both day 0 (n = 51) and DRec (n = 13). At day 0, median pvcrt levels were 2.2-fold higher in infections that led to recurrence than in those with ACPR (P = 0.002; Wilcoxon rank sum test), an increase that was also observed when only IBD-homologous recurrences were considered (Table 3). No difference between treatment outcome groups at day 0 was found for pvmdr1 levels (P = 0.138) (Table 3). Comparisons at day 0 were not affected after adjustment by multiple comparisons (Fig. 4). We did not find an association between ring-stage proportion and gene expression of either pvcrt or pvmdr1 in linear regression models to suggest that there was a potential confounding effect of parasite life stage composition on transcript levels (Fig. S5). There was no correlation between parasite ex vivo CQ susceptibility in SMA (defined by IC50s) and gene expression of pvcrt (n = 13; ρ = −0.217; P = 0.476) or pvmdr1 (n = 17; ρ = −0.120; P = 0.646).
FIG 4.
Gene expression of pvcrt and pvmdr1. Expression ratios (R) for pvcrt (A) and pvmdr1 (B) relative to the reference β-tubulin gene is displayed in log scale. Samples are stratified by treatment outcome and day of collection. Horizontal lines indicate medians and IQR. Dots indicate the type of recurrence based on identity-by-descent (IBD) analysis (black, IBD-homologous recurrence; gray, IBD-heterologous recurrence; white, undetermined by WGS). Adjusted P values from a pairwise Wilcoxon rank sum test with the Benjamini-Hochberg correction for multiple testing are reported. ACPR, adequate clinical and parasitological response; DRec, day of recurrence.
Parasites collected at DRec showed a wide range of pvcrt expression levels (n = 9; R = 0.39 [IQR, 0.07 to 1.83]), which did not differ significantly from those in the ACPR group (Padj = 0.271, rank sum test) (Fig. 4) or in day 0 samples with a later recurrence, either in unpaired (Padj = 0.978) or in day 0/DRec matched-pair analysis (n = 8; ΔR[DRec − day 0] = −0.1 [IQR, −0.6 to 0.8]; P = 1.000, signed-rank test) (Fig. S6). Expression of pvmdr1 was higher at DRec (n = 12; R = 0.70 [IQR, 0.08 to 3.09]) than in day 0 samples with ACPR (Padj = 0.016) (Fig. 4) but did not differ from day 0 samples with a later recurrence, either in unpaired analysis (Padj=122) or day 0/DRec matched-pair analysis (n = 10; ΔR = 0.2 [IQR, −0.1 to 1.3]; P = 0.203) (Fig. S6).
DISCUSSION
This study evaluated CQ therapeutic efficacy for uncomplicated P. vivax malaria in Gia Lai Province, Vietnam. The success rate for P. vivax treatment was 100% by day 28 and 75.4% by day 42. All recurrences detected by qPCR (n = 18) occurred after day 33 in the presence of CQ levels below the MEC (<100 mg/ml) and were associated with longer PCT of primary infection, younger age, and higher transcript levels of pvcrt at baseline. Eighty percent of recurrences (8/10) with WGS data were homologous to primary infections using IBD analysis, leading to a homologous recurrence rate at day 42 of at least 44% (8/18).
Among previous CQ TES conducted in Vietnam that detected early recurrences (i.e., <day 28) (20, 21, 23), only one confirmed recurrent infections (n = 3) with blood CQ concentrations above the MEC in Quang Nam in 2011 (20). Although the present study was conceived as a continuation of CQR monitoring in Quang Nam Province (20), malaria cases rapidly decreased there after 2013, with mostly residual P. vivax by 2015, which was insufficient to conduct classical TES designs due to obvious time and logistic constraints (26). The primary endpoints presented in this study suggest that CQ remained largely efficacious in Gia Lai Province until 2017, with mostly late recurrences detected after day 28 in the presence of blood CQ concentrations below the MEC, similar to findings in the southern province of Binh Phuoc (22).
Recurrences occurring after day 28 of follow-up and in the presence of blood CQ concentrations below the MEC have often been assumed to be relapses (4, 27), which would be in line with the frequent relapse phenotype of P. vivax strains in the study region (i.e., mean time to relapse of 41 days [3]). Nevertheless, a thorough review of P. vivax relapses in tropical areas also showed that recrudescent infections of low-grade CQR parasites will become patent at the same time as relapsing infections, due to parasite expansion coincident with the fall in drug levels (7). Some of the findings in the present study undoubtedly suggest that low-grade P. vivax CQR should not be ruled out.
First, recurrences had a significantly longer PCT than ACPR infections. The difference in PCT was independent of parasite densities at day 0, suggesting that they were not attributable to an incomplete clearance of a higher parasite inoculum (7). The observation that recurrences occurred in younger individuals (i.e., those less frequently exposed to P. vivax infection throughout life [7, 28] and hence with weaker immune response to support drug-mediated clearance) could also have further contributed to the persistence of low-grade CQR parasites. Regardless, given that the median age in both treatment outcome groups falls within early adulthood (18 versus 25 years old), the effect of age, if any, would probably be small.
Second, genetic analysis revealed a remarkably high proportion of homologous recurrences using both common microsatellite genotyping (80% rate using 4 MS markers) but also fine-scale genomic approaches, which resulted in a minimum homologous recurrence rate of 44% (8/18) with a degree of relatedness to day 0 infections above 98% IBD. An earlier review of P. vivax clinical studies concluded that relapses in areas where it is endemic commonly occur with heterologous genotypes (as overrepeated liver inoculations lead to the accumulation of genetically diverse hypnozoites), whereas homologous relapses are more likely to occur either in patients recently treated with PQ (i.e., those that cleared preexisting hypnozoites) or in those living in areas with very little parasite genetic diversity (7). More recently, a study conducted in Cambodia investigated recurrent P. vivax infections among 20 patients reallocated to an area with no malaria transmission, in order to exclude the possibility of acquiring new infections (10). Although recurrences were comparable to those in our study in terms of recurrence time (>day 33) and blood CQ concentrations (below the MEC), they were classified as genetically heterologous based on allelic differences by WGS or high-throughput SNP genotyping (6/8), in contrast to the low heterologous rate in our study despite using the WGS-IBD approach (2/10). The authors concluded that all recurrences were thus caused by relapses (10). The possibility that IBD-homologous recurrences we identified in Vietnam were also caused by relapses (or even reinfections) with an identical parasite genotype cannot be totally excluded, as we could be underestimating the presence of new alleles due to a comparatively lower WGS coverage in our study. However, the latter seems unlikely given (i) the excess of genetically identical parasites in recurrent samples observed using IBD (low genome coverage would underestimate relatedness by IBD compared to IBS analysis, which can misclassify unrelated parasites as homologous due to sharing of alleles by chance), (ii) the fact that the number of genetically different sample pairs remained the same when only the dominant clones were considered in the Cambodian study (thus, increased depth does not explain the differences in heterologous rate between studies), and (iii) the higher rate of polyclonality among primary infections with a later recurrence (89% [16/18]; mean COI = 3 by MS analysis) compared to 50% (10/20) in Cambodia (suggesting a higher genetic diversity in Vietnam that would increase the chance of detecting heterologous recurrences if those were caused by a relapse; indeed, the very limited use of PQ as radical cure in Vietnam may further favor high hypnozoite diversity in the country [29]).
Finally, a third finding that supports some degree of CQR is the higher baseline transcript levels of pvcrt in recurrent infections compared to patients with ACPR, independent of parasite stage composition (28, 30). This suggests that P. vivax parasites expressing higher levels of pvcrt may have reduced CQ susceptibility. Data on pvcrt expression in response to CQ treatment are limited. In line with our results, a previous study in Brazil found increased pvcrt (and pvmdr1) levels in P. vivax parasites with CQR phenotype both at day 0 and at the time of recurrence (17). A recent study in nonhuman primates infected with a genetic cross of P. vivax parasites with different CQ responses identified a strong selection of the chromosome 1 region that includes pvcrt, linked to the CQR phenotype and upregulation of pvcrt expression (18), supporting a role of pvcrt in the molecular mechanism of CQR. In contrast, pvcrt expression did not correlate with the ex vivo response to CQ in isolates from Papua, Indonesia (28). As for pvmdr1, increased expression found at DRec (although not confirmed in the matched-pairs analysis) could hypothetically reflect a mechanism of drug resistance or be a consequence of an altered membrane transport after drug exposure, but current knowledge of the physiological function of pvmdr1 is still very limited (31). There is a clear need to further investigate the patterns of pvcrt and pvmdr1 expression in a higher number of samples from infections with ACPR and recurrent infections (both homologous and heterologous) to elucidate their role in CQ susceptibility.
Considering a genetically homologous recurrence a true recrudescence would imply that parasites observed at day 0 remain in the human host at levels below the qPCR detection limit for several weeks while CQ blood concentrations are over the MEC. It has been proposed that P. vivax parasites seen in the bloodstream represent only a portion of the total parasite biomass and that a fraction of parasites may hide in tissues such as the bone marrow or the spleen (32–34). Several published studies strongly support the role of bone marrow as a parasite reservoir, in both humans and nonhuman primates (35, 36). An hypothetical scenario could be one in which a subpopulation of parasites with higher pvcrt expression persists in the tissue parenchyma, where CQ levels are lower, leading to homologous recurrences as soon as drug concentrations decrease significantly below the MEC threshold, furthermore coinciding with the time when hypnozoite stages are reactivated, producing relapse (7).
A limitation of this study was the impossibility of characterizing the phenotype of isolates at the time of recurrence to provide further evidence of low-grade CQR recrudescence and support findings at the genomic level. Additionally, the number of samples eligible for ex vivo assays or genomic analysis was lower than the total number of patients recruited, a common challenge of P. vivax field studies (i.e., low parasite density infections and/or poor adaptation to ex vivo conditions) and may be the reason why some comparisons did not reach statistical significance. Another difficulty is the intrinsic logistical constraints linked to TES in settings where transmission is being drastically reduced, which questions the feasibility of classical in vivo studies under these epidemiological conditions. Such limitation could potentially be overcome once a CQR marker is validated and can be implemented into molecular surveillance, similarly to the ongoing surveillance of P. falciparum artemisinin resistance in Greater Mekong Subregion based on the pfkelch-13 gene (37).
In conclusion, despite the full efficacy of P. vivax CQ treatment at day 28, we found clinical (longer PCT), genomic (high rate of IBD homology), and transcriptional (increased pvcrt expression) evidence suggesting that recurrences after day 28 can be attributed to low-grade CQR recrudescence rather than genetically homologous relapses of liver hypnozoites. Emergence of CQR parasites should be closely monitored. The conclusive identification of a molecular marker is of utmost importance to unequivocally define P. vivax CQR and detect early warning signs of CQR in molecular surveillance studies conducted in areas where malaria is endemic. The finding that pvcrt (and even pvmdr1) may play a role in in vivo CQ susceptibility deserves further attention.
MATERIALS AND METHODS
Study site.
The study was conducted between May 2015 and February 2017 in Chu R’Cam commune’s health center (CHC), Krong Pa district (Gia Lai Province, Central Highlands, Vietnam), where a small field laboratory was set up. In addition, CHC in the nearby communes of Ia R’Sai, Ia R’Suom, and Uar were asked to refer all potentially eligible patients to the reference CHC. Chu R’Cam is located 120 km southeast of the provincial capital Pleiku, next to the Ba river in a large valley surrounded by hilly and partially forested areas (38). The local population is mainly from the J’Rai ethnic minority, which has in general a low socioeconomic status and education level. Local housing structures consist of government-supported brick or wooden houses built on concrete floors. The main occupation is slash-and-burn agriculture in forest fields, which often requires sleeping in the forest. The climate is tropical, with the dry season from November to April and the rainy season from May to October. Malaria transmission usually begins in May and peaks from September to December, with the lowest incidence between February and April. At the time of study initiation in 2015, Gia Lai province had reported an incidence of 3.2 malaria cases per 1,000 individuals and 4,367 microscopically confirmed cases—the highest burden in the country—of which 2,175 (49.8%) occurred in Krong Pa district (1,051 P. falciparum and 1,124 P. vivax) (19, 24). Malaria control by the provincial malaria station relies on early diagnosis by light microscopy (LM) or rapid diagnostic tests, annual distribution of long-lasting insecticide-treated nets, promotion of community-based prevention behavior, and monitoring of drug resistance through regular TES. The presence of P. falciparum artemisinin resistance was confirmed in the province in 2017 (38).
Study design and trial procedures.
The study was designed as a 42-day drug efficacy study to evaluate clinical and parasitological responses after treatment of P. vivax uncomplicated malaria infections. Patients aged >1 year old presenting to the CHC with fever (≥37.5°C) and/or history of fever during the previous 48 h were screened for malaria infection by blood smears from finger pricks, and hemoglobin (Hb) concentration was measured using the Hb201+ system (Hemocue). Those with LM-confirmed P. vivax monoinfection and an asexual parasite density of >250 parasites/μl were invited to enroll and provide written informed consent. Individuals diagnosed with P. falciparum or mixed infections, signs of severe malaria, febrile diseases other than malaria, or any chronic medical condition, as well as pregnant and breastfeeding women, were excluded. Prior to treatment, 5 ml of whole blood was collected by venipuncture for nucleic acid purification and ex vivo drug assays; 200 μl was transferred into an EDTA BD Microtainer tube (Becton, Dickinson and Company), 100 μl was transferred into tubes prefilled with RNAprotect cell reagent (Qiagen) and stored at −20°C, and the remaining blood was processed as described below. After treatment initiation, patients remained in the health center, where they were monitored by LM every 12 h for 72 h (day 3) or until parasite clearance was confirmed by two consecutive LM-negative slides. On days 7, 14, 21, 28, 35, and 42, patients were asked to return for follow-up visits or were visited at home by health center staff. At each visit, a finger prick was conducted to prepare thick and thin smears, 200 μl of blood was transferred into EDTA tubes (for DNA extraction), and another 100 μl was transferred into an RNAprotect-containing tube (for RNA extraction). On day 7, 100 μl of blood was collected onto chromatography paper for drug concentration measurements (31ETCHR; Whatman). Hb levels were measured on days 14, 28, and 42. In cases of confirmed recurrence, a 5-ml venous blood sample and a blood spot on chromatography paper were collected prior to rescue treatment administration. A system of passive detection of fever cases was maintained throughout the study period.
Treatment.
All patients received a full treatment with CQ (Nivaquine; Sanofi) tablets at a total dose of 25 mg of CQ base/kg of body weight, given over 3 days and under direct observation (10 mg the first 2 days and 5 mg on the third) (39). The number of tablets and exact dosage given per patient were registered on case report forms. Individuals who vomited their medication within the first 30 min after treatment received another full dose, whereas those vomiting between 30 and 60 min after treatment received another half dose. The time when patients received the first dose was defined as day 0. PQ (0.25 mg/kg/day for 14 days; Sanofi/Valeant Pharmaceuticals) was given at the end of follow-up on day 42 to all patients testing negative for glucose-6-phosphate dehydrogenase deficiency (G6PD; CareStart, Access Bio) under the supervision of health workers. Patients with recurrences at day 42 or earlier were administered radical cure treatment as per national guidelines, consisting of CQ (25 mg/kg, 3 days) together with PQ (0.25 mg/kg/day for 14 days) (39).
Microscopy.
Malaria diagnosis by LM was conducted by examining thick and thin smears stained with freshly prepared 10% Giemsa for 15 min under ×1,000 magnification. Parasite density was calculated by determining the number of asexual parasites per 200 white blood cells (WBC) with a hand tally counter and expressed as parasites per microliter of blood, assuming a WBC density of 8,000 cells per μl. Blood smears were considered negative when no asexual parasites were found after counting of 1,000 WBC. All smears were read in duplicate by two trained microscopists, and the final density was expressed as the average result. A third reading was conducted by another technician in case of discrepancy in positivity or species diagnosis or if difference in parasite count was >25%. Randomly selected blood smears (15% of the total) were sent to ITM (Antwerp, Belgium) for external quality control.
P. vivax quantification by qPCR and RT-qPCR.
DNA was extracted from 200 μl whole blood in EDTA using a Favorprep 96-well genomic DNA kit (Favorgen) and eluted in 200 μl of water. Duplex qPCR targeting P. vivax and P. falciparum 18S rRNA genes was performed in samples from day 0 using TaqMan Universal Master Mix II and a StepOne Plus real-time PCR system (Applied Biosystems) (40). The same method was adapted as a P. vivax monoplex qPCR and used for P. vivax quantification in follow-up samples. RNA was extracted from day 0 samples and from all follow-up samples positive in the pv18S rRNA qPCR, using an RNeasy Plus 96 kit (Qiagen) (38). Final RNA elution was conducted in 100 μl of RNase-free water, and the presence of quality RNA was confirmed in a random selection of 80 samples by one-step reverse transcription-qPCR (RT-qPCR) targeting pv18S rRNA transcripts, using LightCycler multiplex RNA virus master mix (Roche) in a LightCycler 480 thermocycler (Roche). The presence of P. vivax stage V gametocytes was determined by RT-qPCR amplification of pvs25 RNAs (41). A standard curve (106 to 1 copy/μl) of plasmids containing the pvs25 PCR fragment was included in each plate for copy number quantification (41).
Ex vivo SMA.
Venous-blood Vacutainers were centrifuged, and plasma and buffy coat were stored at −20°C. Infected red blood cells (iRBC) were diluted to 25% hematocrit in sterile phosphate-buffered saline (PBS) and passed through an autoclaved 10-ml syringe (Terumo; SS10LE1) prefilled with a 2.5-ml layer of cellulose powder (Sigma-Aldrich; C6288) on top of a lens-cleaning paper to remove WBC. In all samples with a ring-stage proportion of ≥65%, 150 μl iRBC were separated for an ex vivo drug sensitivity schizont maturation assay (SMA), and the remaining iRBC pellet was cryopreserved in liquid nitrogen or stored at −20°C. SMA was performed by adapting the WHO microtest, as previously described (38, 42). A stock solution of 640 μM CQ in water (Sigma-Aldrich; C6628) was predosed in seven 2-fold serial dilutions in water (800 to 12.5 nM), and 25 μl/well was distributed in a 96-well plate, air-dried overnight inside a laminar flow cabinet, and stored at 4°C (43). The iRBC (150 μl) were resuspended to 2% hematocrit in prewarmed McCoy’s 5A medium (Gibco) supplemented with 20% human serum (from nonexposed Vietnamese individuals), and 200 μl/well of the iRBC-medium mixture was added to predosed plates, including drug-free wells. Plates were cultured at 37°C, and drug-free wells were monitored by LM at 34 h and every 2 to 4 h thereafter until the number of schizonts reached 40% (or until 42 h). The percentage of schizonts per each drug concentration was determined by double reading of blood smears, and results were expressed as percent schizonts relative to the drug-free well. Half-maximal inhibitory concentrations (IC50) were calculated using WWARN’s online in vitro analysis and reporting tool (44).
Drug concentration in blood.
Concentrations of CQ and desethyl-chloroquine (DCQ, i.e., the main chloroquine metabolite) were measured in paired samples on day 7 and day of recurrence (DRec). Blood expelled onto chromatography papers was air dried and stored in separate plastic bags with silica gel. Drug measurements were conducted by liquid chromatography-mass spectrometry at the Department of Clinical Pharmacology, Mahidol-Oxford Tropical Medicine Research Unit (Bangkok, Thailand). The minimum effective concentration (MEC) was considered a [CQ]+[DCQ] value of 100 ng/ml (8).
Microsatellite genotyping.
Paired day 0 and DRec samples were genotyped at polymorphic markers Pvmsp1F3, MS4, MS10, and PvSal1814 (selected based on the high degree of heterozygosity found in central Vietnam) by adapting previously published protocols (Table S1) (45–47). All amplification products were run on a 2% agarose gel, and 20-μl pools of Pvmsp1F3 (5 μl), MS4 (7.5 μl), and MS10 (7.5 μl) PCR products and PvSal1814 products were sent off for capillary electrophoresis at Genoscreen (Lille, France). Allele calling was performed using GeneTools (Syngene) and GeneMarker v2.4.0 (Softgenetics). All samples were double-checked manually, and the predominant allele (i.e., the highest peak) and minor alleles within at least two-thirds of the height of the predominant allele were scored. Alleles were considered identical if the size difference was ≤2 bp. Complexity of infection (COI; i.e., the estimated number of genetically distinct clones within an infection) was defined as the highest number of alleles found in any of the four markers. Genotypes at day 0 and DRec were classified as homologous (when all or some of the alleles present in day 0 samples were found in DRec samples for all markers), heterologous (when none of the alleles present in day 0 sample were found at DRec for at least one marker), or indeterminate (when no amplification was achieved for any marker).
SNP barcodes.
A molecular barcode consisting of 38 SNPs across the P. vivax genome was used to genotype samples in the context of MalariaGEN SpotMalaria Project (Welcome Sanger Institute, Hinxton, UK), using MALDI-TOF mass spectrometry of PCR amplicons in a MassARRAY system (Agena BioScience) (25, 38). Nucleotide sequence results were provided in the form of a genetic report card (see Data Set S1). Samples from recurrences were classified as homologous to those from day 0 if there were no nucleotide differences in any nonheterozygous position of the barcode, irrespective of the total number of successfully genotyped SNPs.
Whole-genome sequencing.
DNA was initially subjected to selective whole-genome amplification (sWGA) with phi29 DNA polymerase (New England Biolabs) according to a previously published method (30 μl of DNA in a 50-μl reaction volume) (48). Product was purified with AMPure XP beads (Beckman-Coulter), and 15 μl was used in a second sWGA round (50-μl reaction volume). The final concentration of the sWGA-purified product was determined in a Qubit 2.0 fluorometer (Life Technologies), and a minimum of 1 mg was sent to BGI (Hong Kong) for library preparation and 150-bp paired-end WGS on a HiSeq X Ten instrument (Illumina). FASTQ files were trimmed using Trimmomatic to remove adapters and low-quality reads and aligned to the reference genome PvP01 using the BWA-MEM algorithm v0.7.17 (49). Variants were called using HaplotypeCaller in GVCF mode followed by Joint-Call Cohort (GATK 4.1.2.0; Broad Institute) and filtered to include only biallelic SNPs in the core genome (MQ > 50, QUAL > 30, and combined DP ≥100), resulting in 191,849 high-quality SNPs (50). Samples with at least 10× coverage were kept for subsequent analysis (Table S2). Within-host infection complexity was assessed using within-sample F statistic (FWS), and an FWS value of >0.95 was considered a proxy for a monoclonal infection (51, 52). Pairwise comparisons between all samples were analyzed as identity by state (IBS) and identity by descent (IBD). For the IBS approach, the proportion of discordant SNPs was determined by calculating the Prevosti distance (i.e., number of allelic differences/number of possible differences). Distance was fitted as the sum of four Gaussian distributions, and the mean of the lowest distribution was set as the threshold for IBS-homologous recurrence (Fig. S1). For the IBD analysis, PED and MAP file formats were created by using VCFtools, and the proportion of the genome IBD between pairs of samples was calculated using the isoRelate R package (53). Genetic distance was calculated using an estimated mean map unit size from Plasmodium chabaudi chabaudi of 13.7 kb/centimorgan (cM) (54). We set thresholds of IBD on the minimum number of SNPs (n = 20) and length of IBD segments (50.000 bp) reported to reduce false-positive calls. Pairwise comparisons with ≥95% of the genome being identical by descent were considered homologous, and all pairwise comparisons with shared IBD of <95% were considered heterologous.
Molecular markers of drug resistance.
Gene expression of pvcrt and pvmdr1 were determined directly on RNA samples by one-step RT-qPCR. The β-tubulin gene was used as the reference gene. Each 10-μl reaction volume contained 2 μl of LightCycler EvoScript RNA SYBR green I master mix (Roche), 0.4 μM forward and reverse primers (from reference 17), and 2.5 μl of RNA template. LightCycler480 (Roche) thermal cycling conditions were 60°C for 15 min, 95°C for 10 min, and 45 cycles of 95°C for 10 s and 58°C for 30 s, followed by a melting curve step to ensure amplification specificity. Samples were run in triplicate and excluded from calculations if the final standard deviation (SD) of duplicates after removal of outliers was >0.4. Gene expression was estimated using the efficiency-adapted Pfaffl relative quantification model (55), in which the relative expression ratio (R) is calculated as the efficiency of the target genes (Fig. S2) raised to the power of the cycle threshold (CT) difference between a given sample and a control (i.e., mean CT + 2 × SD of all day 0 samples with ACPR) and divided by the same formula applied to the reference β-tubulin gene.
Mutations in pvmdr1 codons 976 (Y→F) and 1076 (F→L) were genotyped by nested PCR amplification using primers from Golassa et al. (56) (primary) and Lin et al. (57) (nested) (Table S1). The 646-bp amplification products were sequenced at Genoscreen (Lille, France). Nucleotide sequences were entered in BioEdit 7.0 and aligned using ClustalW with default parameters.
Clinical trial endpoints.
The primary endpoint was adequate clinical and parasitological response (ACPR) at 28 and 42 days of follow-up. Treatment failures were classified as early treatment failure, late clinical failure (LCF; i.e., parasitemia with fever or signs of severe malaria between day 4 and day 42), or late parasitological failure (LPF, i.e., parasitemia between day 7 and day 42), based on WHO guidelines (5). Patients who discontinued due to reappearance of parasites and subsequent rescue treatment were considered evaluable for day 42 endpoint analysis. Secondary endpoints were parasite clearance estimates obtained from the loge parasitemia-time LM data entered into WWARN Parasite Clearance Estimator (58), carriage of asexual and sexual parasites on day 0 to 3 and during follow-up (including submicroscopic), and severe anemia (Hb < 7 g/dl) on day 7.
Statistical analysis.
For sample size calculation, the initial estimation of 5% treatment failure rate (based on the Quang Nam study [20]) was amended to 20% upon completion of the first year of the study, after observing treatment outcome results together with an extremely low malaria incidence in the district. To detect a treatment failure rate of 20%, a minimum sample size of 61 patients was necessary (10% precision and 95% confidence level), which was extended to 67 to allow 10% loss to follow-up. All clinical data were double entered into a Microsoft Access database and transferred to Stata v11.0. Primary study outcomes were analyzed using a per-protocol patient population. Comparisons between groups for continuous variables were performed using the Wilcoxon rank sum test, and proportions were compared using chi-square or Fisher’s exact tests, when appropriate. Correlation between variables was assessed by Spearman’s test. Clearance time for total parasites and for gametocytes was estimated using Kaplan-Meier survival curves, and comparisons between subgroups were done using log rank test. Time was computed in hours and since treatment administration. Gene expression data were log transformed, and comparisons between groups were performed by a pairwise Wilcoxon rank sum test, with a Benjamini-Hochberg correction in cases of multiple testing. A linear model adjusted for the proportion of ring-stage parasites was applied to investigate the potential effect of stage composition on gene expression (28). Paired analyses between day 0 and DRec were done using the Wilcoxon matched-pairs signed-rank test. Statistical analysis and graphing were performed in Stata v11.0, R v4.0 or GraphPad Prism (v9.0). P values <0.05 were considered indicative of statistical significance.
Ethics approval and consent to participate.
The study received approval from ethics committee of the NIMPE-Ministry of Health, Hanoi, Vietnam (351/QD-VSR and QD2211/QD-BYT); the Institutional Review Board of the Institute of Tropical Medicine, Antwerp, Belgium (937/14); and the ethics committee of Antwerp University Hospital (UZA), Antwerp, Belgium (14/15/183). Written informed consent was obtained from all participants or their respective parents/guardians in the case of minors. The trial was registered on ClinicalTrials.gov under identifier NCT02610686.
Data availability.
The nucleotide sequences determined here have been deposited in GenBank under accession numbers MW245736 to MW245808.
ACKNOWLEDGMENTS
We sincerely thank all individuals that agreed to enroll in the trial, all staff from Chu R’Cam CHC, and local authorities in Krong Pa district. We are especially grateful to Tran Tuyet Mai, Nguyen Thi Thuy Duong, and Do Manh Ha for their support of field work and to Ric Price and Kamala Thriemer (Menzies School of Health Research) for clinical trial protocol guidelines and helpful discussion.
The study was funded by Belgium Development Cooperation (DGD) under the Framework Agreement Program between DGD and ITM (FA3-III, 2014 to 2016, FA42017 to 2021). This publication uses data from the MalariaGEN SpotMalaria Project, coordinated by the MalariaGEN Resource Centre supported by Wellcome (098051, 090770). Both chloroquine and primaquine were provided at no cost by Sanofi.
Conceived and designed the study: E.R.-V., N.V.H., A.E., A.R.-U.; performed the experiments: E.R.-V., N.V.H., J.H.K., N.T.T.H., V.T.S., N.T.H.N., P.G., E.S.; analyzed the data: E.R.-V., N.V.H., J.H.K., A.R.-U.; contributed data collection/logistics/materials/analysis tools: R.M.H., N.T.H.B., N.L.H., N.D.L., T.T.D., N.X.X., A.E.; wrote the paper: E.R.-V., J.H.K., A.R.-U. All authors read and approved the final version of the manuscript.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Eduard Rovira-Vallbona, Email: edu.rovira@outlook.com.
Anna Rosanas-Urgell, Email: arosanas@itg.be.
REFERENCES
- 1.World Health Organization. 2020. World malaria report 2020. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020
- 2.Price RN, Commons RJ, Battle KE, Thriemer K, Mendis K. 2020. Plasmodium vivax in the era of the shrinking P falciparum map. Trends Parasitol 36:560–570. 10.1016/j.pt.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howes RE, Battle KE, Mendis KN, Smith DL, Cibulskis RE, Baird JK, Hay SI. 2016. Global epidemiology of Plasmodium vivax. Am J Trop Med Hyg 95:15–34. 10.4269/ajtmh.16-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price RN, von Seidlein L, Valecha N, Nosten F, Baird JK, White NJ. 2014. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis 14:982–991. 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. 2009. Methods for surveillance of antimalarial drug efficacy. https://www.who.int/malaria/publications/atoz/9789241597531/en/
- 6.Baird JK. 2004. Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother 48:4075–4083. 10.1128/AAC.48.11.4075-4083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White NJ. 2011. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J 10:297. 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price RN, Auburn S, Marfurt J, Cheng Q. 2012. Phenotypic and genotypic characterisation of drug-resistant Plasmodium vivax. Trends Parasitol 28:522–529. 10.1016/j.pt.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popovici J, Pierce-Friedrich L, Kim S, Bin S, Run V, Lek D, Hee KHD, Soon-U LL, Cannon MV, Serre D, Menard D. 2019. Recrudescence, reinfection, or relapse? A more rigorous framework to assess chloroquine efficacy for Plasmodium vivax malaria. J Infect Dis 219:315–322. 10.1093/infdis/jiy484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popovici J, Friedrich LR, Kim S, Bin S, Run V, Lek D, Cannon MV, Menard D, Serre D. 2018. Genomic analyses reveal the common occurrence and complexity of plasmodium vivax relapses in cambodia. mBio 9:e01888-17. 10.1128/mBio.01888-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noulin F, Borlon C, Van Den Abbeele J, D’Alessandro U, Erhart A. 2013. 1912–2012: a century of research on Plasmodium vivax in vitro culture. Trends Parasitol 29:286–294. 10.1016/j.pt.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Brega S, Meslin B, de Monbrison F, Severini C, Gradoni L, Udomsangpetch R, Sutanto I, Peyron F, Picot S. 2005. Identification of the Plasmodium vivax mdr‐like gene (pvmdr1) and analysis of single‐nucleotide polymorphisms among isolates from different areas of endemicity. J Infect Dis 191:272–277. 10.1086/426830. [DOI] [PubMed] [Google Scholar]
- 13.Suwanarusk R, Russell B, Chavchich M, Chalfein F, Kenangalem E, Kosaisavee V, Prasetyorini B, Piera KA, Barends M, Brockman A, Lek-Uthai U, Anstey NM, Tjitra E, Nosten F, Cheng Q, Price RN. 2007. Chloroquine resistant Plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS One 2:e1089. 10.1371/journal.pone.0001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suwanarusk R, Chavchich M, Russell B, Jaidee A, Chalfein F, Barends M, Prasetyorini B, Kenangalem E, Piera KA, Lek‐Uthai U, Anstey NM, Tjitra E, Nosten F, Cheng Q, Price RN. 2008. Amplification of pvmdr1 associated with multidrug‐resistant Plasmodium vivax. J Infect Dis 198:1558–1564. 10.1086/592451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva SR, Almeida ACG, da Silva GAV, Ramasawmy R, Lopes SCP, Siqueira AM, Costa GL, Sousa TN, Vieira JLF, Lacerda MVG, Monteiro WM, de Melo GC. 2018. Chloroquine resistance is associated to multi-copy pvcrt-o gene in Plasmodium vivax malaria in the Brazilian Amazon. Malar J 17:267. 10.1186/s12936-018-2411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnadas C, Ratsimbasoa A, Tichit M, Bouchier C, Jahevitra M, Picot S, Ménard D. 2008. Plasmodium vivax resistance to chloroquine in Madagascar: clinical efficacy and polymorphisms in pvmdr1 and pvcrt-o genes. Antimicrob Agents Chemother 52:4233–4240. 10.1128/AAC.00578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melo GC, Monteiro WM, Siqueira AM, Silva SR, Magalhães BML, Alencar ACC, Kuehn A, Portillo H.Ad, Fernandez-Becerra C, Lacerda MVG. 2014. Expression levels of pvcrt-o and pvmdr-1 are associated with chloroquine resistance and severe Plasmodium vivax malaria in patients of the Brazilian Amazon. PLoS One 9:e105922. 10.1371/journal.pone.0105922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sá JM, Kaslow SR, Moraes Barros RR, Brazeau NF, Parobek CM, Tao D, Salzman RE, Gibson TJ, Velmurugan S, Krause MA, Melendez-Muniz V, Kite WA, Han PK, Eastman RT, Kim A, Kessler EG, Abebe Y, James ER, Chakravarty S, Orr-Gonzalez S, Lambert LE, Engels T, Thomas ML, Fasinu PS, Serre D, Gwadz RW, Walker L, DeConti DK, Mu J, Bailey JA, Sim BKL, Hoffman SL, Fay MP, Dinglasan RR, Juliano JJ, Wellems TE. 2019. Plasmodium vivax chloroquine resistance links to pvcrt transcription in a genetic cross. Nat Commun 10:4300. 10.1038/s41467-019-12256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldlust SM, Thuan PD, Giang DDH, Thang ND, Thwaites GE, Farrar J, Thanh NV, Nguyen TD, Grenfell BT, Boni MF, Hien TT. 2018. The decline of malaria in Vietnam, 1991–2014. Malar J 17:226. 10.1186/s12936-018-2372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thanh PV, Van Hong N, Van Van N, Louisa M, Baird K, Xa NX, Peeters Grietens K, Hung LX, Duong TT, Rosanas-Urgell A, Speybroeck N, D’Alessandro U, Erhart A. 2015. Confirmed Plasmodium vivax resistance to chloroquine in central Vietnam. Antimicrob Agents Chemother 59:7411–7419. 10.1128/AAC.00791-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phan GT, De Vries PJ, Tran BQ, Le HQ, Nguyen NV, Nguyen TV, Heisterkamp SH, Kager PA. 2002. Artemisinin or chloroquine for blood stage Plasmodium vivax malaria in Vietnam. Trop Med Int Heal 7:858–864. 10.1046/j.1365-3156.2002.00948.x. [DOI] [PubMed] [Google Scholar]
- 22.Thuan PD, Ca NTN, Van Toi P, Nhien NTT, Thanh NV, Anh ND, Phu NH, Thai CQ, Thai LH, Hoa NT, Dong LT, Loi MA, Son DH, Khanh TTN, Dolecek C, Nhan HT, Wolbers M, Thwaites G, Farrar J, White NJ, Hien TT. 2016. A randomized comparison of chloroquine versus dihydroartemisinin-piperaquine for the treatment of plasmodium vivax infection in Vietnam. Am J Trop Med Hyg 94:879–885. 10.4269/ajtmh.15-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phong NC, Chavchich M, Quang HH, San NN, Birrell GW, Chuang I, Martin NJ, Manh ND, Edstein MD. 2019. Susceptibility of Plasmodium falciparum to artemisinins and Plasmodium vivax to chloroquine in Phuoc Chien Commune, Ninh Thuan Province, south-central Vietnam. Malar J 18:10. 10.1186/s12936-019-2640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Malaria Control and Elimination Program in Vietnam. 2015. Malaria report 2014 [internal report]. NIMPE, Hanoi, Vietnam. [Google Scholar]
- 25.Baniecki ML, Faust AL, Schaffner SF, Park DJ, Galinsky K, Daniels RF, Hamilton E, Ferreira MU, Karunaweera ND, Serre D, Zimmerman PA, Sá JM, Wellems TE, Musset L, Legrand E, Melnikov A, Neafsey DE, Volkman SK, Wirth DF, Sabeti PC. 2015. Development of a Single nucleotide polymorphism barcode to genotype Plasmodium vivax infections. PLoS Negl Trop Dis 9:e0003539. 10.1371/journal.pntd.0003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kattenberg JH, Erhart A, Truong MH, Rovira-Vallbona E, Vu KAD, Nguyen THN, Nguyen VH, Nguyen VV, Bannister-Tyrrell M, Theisen M, Bennet A, Lover AA, Tran TD, Nguyen XX, Rosanas-Urgell A. 2018. Characterization of Plasmodium falciparum and Plasmodium vivax recent exposure in an area of significantly decreased transmission intensity in Central Vietnam. Malar J 17:180. 10.1186/s12936-018-2326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor AR, Watson JA, Chu CS, Puaprasert K, Duanguppama J, day NPJ, Nosten F, Neafsey DE, Buckee CO, Imwong M, White NJ. 2019. Resolving the cause of recurrent Plasmodium vivax malaria probabilistically. Nat Commun 10:5595. 10.1038/s41467-019-13412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pava Z, Handayuni I, Wirjanata G, To S, Trianty L, Noviyanti R, Poespoprodjo JR, Auburn S, Price RN, Marfurt J. 2016. Expression of Plasmodium vivax crt-o is related to parasite stage but not ex vivo chloroquine susceptibility. Antimicrob Agents Chemother 60:361–367. 10.1128/AAC.02207-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tinh TT, Mai DTT, Thieu NQ. 2017. Quality of malaria diagnosis and treatment in some hospitals in malaria endemic areas (2015-2016). J Malar Parasite Dis Control (Vietnam) 2:3–9. [Google Scholar]
- 30.Kim A, Popovici J, Menard D, Serre D. 2019. Plasmodium vivax transcriptomes reveal stage-specific chloroquine response and differential regulation of male and female gametocytes. Nat Commun 10:371. 10.1038/s41467-019-08312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dean P, Major P, Nakjang S, Hirt RP, Martin ET. 2014. Transport proteins of parasitic protists and their role in nutrient salvage. Front Plant Sci 5:153. 10.3389/fpls.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markus MB. 2018. Biological concepts in recurrent Plasmodium vivax malaria. Parasitology 145:1765–1771. 10.1017/S003118201800032X. [DOI] [PubMed] [Google Scholar]
- 33.Mayor A, Alano P. 2015. Bone marrow reticulocytes: a Plasmodium vivax affair? Blood 125:1203–1205. 10.1182/blood-2014-12-614123. [DOI] [PubMed] [Google Scholar]
- 34.Silva-Filho JL, Lacerda MVG, Recker M, Wassmer SC, Marti M, Costa FTM. 2020. Plasmodium vivax in hematopoietic niches: hidden and dangerous. Trends Parasitol 36:447–458. 10.1016/j.pt.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Baro B, Deroost K, Raiol T, Brito M, Almeida ACG, de Menezes-Neto A, Figueiredo EFG, Alencar A, Leitão R, Val F, Monteiro W, Oliveira A, Armengol MDP, Fernández-Becerra C, Lacerda MV, del Portillo HA. 2017. Plasmodium vivax gametocytes in the bone marrow of an acute malaria patient and changes in the erythroid miRNA profile. PLoS Negl Trop Dis 11:e0005365. 10.1371/journal.pntd.0005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obaldia N, Meibalan E, Sa JM, Ma S, Clark MA, Mejia P, Moraes Barros RR, Otero W, Ferreira MU, Mitchell JR, Milner DA, Huttenhower C, Wirth DF, Duraisingh MT, Wellems TE, Marti M. 2018. Bone marrow is a major parasite reservoir in plasmodium vivax infection. mBio 9:e00625-18. 10.1128/mBio.00625-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noviyanti R, Miotto O, Barry A, Marfurt J, Siegel S, Thuy-Nhien N, Quang HH, Anggraeni ND, Laihad F, Liu Y, Sumiwi ME, Trimarsanto H, Coutrier F, Fadila N, Ghanchi N, Johora FT, Puspitasari AM, Tavul L, Trianty L, Utami RAS, Wang D, Wangchuck K, Price RN, Auburn S. 2020. Implementing parasite genotyping into national surveillance frameworks: feedback from control programmes and researchers in the Asia-Pacific region. Malar J 19:271. 10.1186/s12936-020-03330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rovira-Vallbona E, Van Hong N, Kattenberg JH, Huan RM, Hien NTT, Ngoc NTH, Guetens P, Hieu NL, Mai TT, Duong NTT, Duong TT, Phuc BQ, Xa NX, Erhart A, Rosanas-Urgell A. 2020. Efficacy of dihydroartemisinin/piperaquine and artesunate monotherapy for the treatment of uncomplicated Plasmodium falciparum malaria in Central Vietnam. J Antimicrob Chemother 75:2272–2281. 10.1093/jac/dkaa172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ministry of Health Vietnam. 2016. Guidelines of diagnosis, treatment and prevention of malaria disease. https://thuvienphapluat.vn/van-ban/The-thao-Y-te/Quyet-dinh-4845-QD-BYT-Huong-dan-chan-doan-dieu-tri-benh-Sot-ret-2016-321533.aspx
- 40.Rosanas-Urgell A, Mueller D, Betuela I, Barnadas C, Iga J, Zimmerman PA, del Portillo HA, Siba P, Mueller I, Felger I. 2010. Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malar J 9:361. 10.1186/1475-2875-9-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, Beck H-P, Mueller I, Felger I. 2013. Strategies for detection of Plasmodium species gametocytes. PLoS One 8:e76316. 10.1371/journal.pone.0076316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basco LK. 2007. Field application of in vitro assays sensitivity of human malaria parasites antimalarial drugs. World Health Organization, Geneva, Switzerland. https://www.who.int/malaria/publications/atoz/9789241595155/en/ [Google Scholar]
- 43.WWARN In Vitro Module. 2011. Preparation of predosed plates. WWARN procedures. https://www.wwarn.org/sites/default/files/INV03_PreparationOfPredosedPlates.pdf
- 44.Woodrow CJ, Dahlström S, Cooksey R, Flegg JA, Le Nagard H, Mentré F, Murillo C, Ménard D, Nosten F, Sriprawat K, Musset L, Quashie NB, Lim P, Fairhurst RM, Nsobya SL, Sinou V, Noedl H, Pradines B, Johnson JD, Guerin PJ, Sibley CH, Le Bras J. 2013. High-throughput analysis of antimalarial susceptibility data by the WorldWide Antimalarial Resistance Network (WWARN) in vitro analysis and reporting tool. Antimicrob Agents Chemother 57:3121–3130. 10.1128/AAC.02350-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koepfli C, Mueller I, Marfurt J, Goroti M, Sie A, Oa O, Genton B, Beck H-P, Felger I. 2009. Evaluation of Plasmodium vivax genotyping markers for molecular monitoring in clinical trials. J Infect Dis 199:1074–1080. 10.1086/597303. [DOI] [PubMed] [Google Scholar]
- 46.Hong NV, Delgado-Ratto C, Thanh PV, Van den Eede P, Guetens P, Binh NTH, Phuc BQ, Duong TT, Van Geertruyden JP, D’Alessandro U, Erhart A, Rosanas-Urgell A. 2016. Population genetics of Plasmodium vivax in four rural communities in Central Vietnam. PLoS Negl Trop Dis 10:e0004434. 10.1371/journal.pntd.0004434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van den Eede P, Erhart A, Van der Auwera G, Van Overmeir C, Thang ND, Hung LX, Anné J, D’Alessandro U. 2010. High complexity of Plasmodium vivax infections in symptomatic patients from a rural community in central Vietnam detected by microsatellite genotyping. Am J Trop Med Hyg 82:223–227. 10.4269/ajtmh.2010.09-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cowell AN, Loy DE, Sundararaman SA, Valdivia H, Fisch K, Lescano AG, Baldeviano GC, Durand S, Gerbasi V, Sutherland CJ, Nolder D, Vinetz JM, Hahn BH, Winzeler EA. 2017. Selective whole-genome amplification is a robust method that enables scalable whole-genome sequencing of Plasmodium vivax from unprocessed clinical samples. mBio 8:e02257-16. 10.1128/mBio.02257-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearson RD, Amato R, Auburn S, Miotto O, Almagro-Garcia J, Amaratunga C, Suon S, Mao S, Noviyanti R, Trimarsanto H, Marfurt J, Anstey N, William T, Boni MF, Colecek C, Tran HT, White NJ, Michon P, Siba P, Tavul L, Harrison G, Barry A, Mueller I, Ferreira MU, et al. 2016. Genomic analysis of local variation and recent evolution in Plasmodium vivax. Nat Genet 48:959–964. 10.1038/ng.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Auburn S, Campino S, Miotto O, Djimde AA, Zongo I, Manske M, Maslen G, Mangano V, Alcock D, MacInnis B, Rockett KA, Clark TG, Doumbo OK, Ouédraogo JB, Kwiatkowski DP. 2012. Characterization of within-host Plasmodium falciparum diversity using next-generation sequence data. PLoS One 7:e32891. 10.1371/journal.pone.0032891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Auburn S, Getachew S, Pearson RD, Amato R, Miotto O, Trimarsanto H, Zhu SJ, Rumaseb A, Marfurt J, Noviyanti R, Grigg MJ, Barber B, William T, Goncalves SM, Druy E, Sriprawat K, Anstey NM, Nosten F, Petros B, Aseffa A, McVean G, Kwiatkowski DP, Price RN. 2019. Genomic analysis of Plasmodium vivax in southern Ethiopia reveals selective pressures in multiple parasite mechanisms. J Infect Dis 220:1738–1749. 10.1093/infdis/jiz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henden L. 2016. isoRelate. 10.5281/zenodo.197857#. [DOI]
- 54.Martinelli A, Hunt P, Fawcett R, Cravo PVL, Walliker D, Carter R. 2005. An AFLP-based genetic linkage map of Plasmodium chabaudi chabaudi. Malar J 4:11. 10.1186/1475-2875-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golassa L, Kamugisha E, Ishengoma DS, Baraka V, Shayo A, Baliraine FN, Enweji N, Erko B, Aseffa A, Choy A, Swedberg G. 2015. Identification of large variation in pfcrt, pfmdr-1 and pfubp-1 markers in Plasmodium falciparum isolates from Ethiopia and Tanzania. Malar J 14:1–9. 10.1186/s12936-015-0783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin JT, Patel JC, Kharabora O, Sattabongkot J, Muth S, Ubalee R, Schuster AL, Rogers WO, Wongsrichanalai C, Juliano JJ. 2013. Plasmodium vivax isolates from Cambodia and Thailand show high genetic complexity and distinct patterns of P vivax multidrug resistance gene 1 (pvmdr1) polymorphisms. Am J Trop Med Hyg 88:1116–1123. 10.4269/ajtmh.12-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flegg JA, Guerin PJ, White NJ, Stepniewska K. 2011. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J 10:339. 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental tables and figures. Download AAC00095-21_Supp_1_seq5.pdf, PDF file, 0.6 MB (661.4KB, pdf)
Supplemental Data Set S1. Download AAC00095-21_Supp_2_seq6.xlsx, XLSX file, 0.03 MB (33.1KB, xlsx)
Data Availability Statement
The nucleotide sequences determined here have been deposited in GenBank under accession numbers MW245736 to MW245808.