ABSTRACT
The genetic regulation of Colletotrichum (Glomerella) sexual reproduction does not strictly adhere to the Ascomycota paradigm and remains poorly understood. Morphologically different but sexually compatible strain types, termed plus and minus, have been recognized, but the biological and molecular distinctions between these strain types remain elusive. In this study, we characterized the sexual behaviors of a pair of plus and minus strains of C. fructicola with the aid of live-cell nucleus-localized fluorescent protein labeling, gene expression, and gene mutation analyses. We confirmed a genetically stable plus-to-minus switching phenomenon and demonstrated the presence of both cross-fertilized and self-fertilized perithecia within the mating line (perithecia cluster at the line of colony contact) between plus and minus strains. We demonstrated that pheromone signaling genes (a-factor-like and α-factor-like pheromones and their corresponding GPCR receptors) were differently expressed between vegetative hyphae of the two strains. Moreover, deletion of pmk1 (a FUS/KSS1 mitogen-activate protein kinase) in the minus strain severely limited mating line formation, whereas deletion of a GPCR (FGSG_05239 homolog) and two histone modification factors (hos2, snt2) in the minus strain did not affect mating line development but altered the ratio between cross-fertilization and self-fertilization within the mating line. We propose a model in which mating line formation in C. fructicola involves enhanced protoperithecium differentiation and enhanced perithecium maturation of the minus strain mediated by both cross-fertilization and diffusive effectors. This study provides insights into mechanisms underlying the mysterious phenomenon of plus-minus-mediated sexual enhancement being unique to Colletotrichum fungi.
IMPORTANCE Plus-minus regulation of Colletotrichum sexual differentiation was reported in the early 1900s. Both plus and minus strains produce fertile perithecia in a homothallic but inefficient manner. However, when the two strain types encounter each other, efficient differentiation of fertile perithecia is triggered. The plus strain, by itself, can also generate minus ascospore progeny at high frequency. This nontypical mating system facilitates sexual reproduction and is Colletotrichum specific; the underlying molecular mechanisms, however, remain elusive. The current study revisits this longstanding mystery using C. fructicola as an experimental system. The presence of both cross-fertilized and self-fertilized perithecia within the mating line was directly evidenced by live-cell imaging with fluorescent markers. Based on further gene expression and gene mutation analysis, a model explaining mating line development (plus-minus-mediated sexual enhancement) is proposed. Data reported here have the potential to allow us to better understand Colletotrichum mating and filamentous ascomycete sexual regulation.
KEYWORDS: sexual reproduction, mating type, Colletotrichum, perithecium
INTRODUCTION
Fungal sexual reproduction can be classified as being either homothallic (self-fertile, capable of sexual reproduction in solo culture) or heterothallic (stringent outcrossing requiring a compatible mating partner). The sexual reproduction mode of most filamentous ascomycetes is controlled by genes located at a single mating type (MAT) locus (1). In homothallic species, individual isolates generally contain the same mating type genes, including two functionally essential transcriptional factors, the alpha domain gene mat1-1-1 and the high-mobility group (HMG) domain gene mat1-2-1. In heterothallic species, sexually compatible individuals carry one of two versions of the MAT locus, either mat1-1-1 or mat1-2-1. These two versions of the MAT locus are referred to as idiomorphs, as sequences present at the same locus are not homologous. This self/nonself recognition determined by two sequence types at one genetic locus is also described as a bipolar mating system. In certain ascomycetes, genetic manipulation of MAT locus genes is sufficient to alter the mode of mating (2).
The Colletotrichum genus encompasses a wide range of plant pathogens that collectively damage more than 3,000 plants (3). Differing from the typical ascomycete mating paradigm, all Colletotrichum (anamorph of Glomerella) species studied to date have an atypical mating recognition system (4). First, unlike other species, being either homothallic or heterothallic, the same Colletotrichum species can contain both homothallic and heterothallic individuals, and cross fertility among isolates can range continuously from completely sterile to highly fertile (5–8). Second, all Colletotrichum genomes sequenced so far, regardless of whether the isolate was self-fertile or self-sterile, contained only the mat1-2-1 gene but not the mat1-1-1 gene at the MAT locus (4, 9). Based on an extensive study of Glomerella cingulata (the sexual phase of C. gloeosporioides senso lato), Wheeler (10) proposed an unbalanced heterothallism model in which heterothallism is derived from homothallism as a result of gene mutations outside the MAT locus. Mutations located at different loci can complement each other, allowing the corresponding strains to cross-fertilize. Subsequent genetic analysis with C. graminicola supports this model and suggests that two unlinked loci outside the MAT locus regulate cross fertility (11).
The complexity of Colletotrichum sexual reproduction has been further evidenced by an intriguing plus-to-minus switch phenomenon. Edgerton first obtained Glomerella isolates showing strikingly different culture appearances, which were termed plus type and minus type, respectively (11, 12). In pure culture, the plus type isolate forms aggregated masses of fertile perithecia, whereas the minus type isolate forms scattered perithecia being infertile or containing misshaped ascospores. In addition, the plus type isolate differentiates many fewer perithecia, produces more aerial hyphae, and is less pigmented than the minus type. Plus-selfed perithecia contain asci in which plus and minus ascospores segregate in either a 4:4 or 0:8 ratio, or, in very rare cases, an 8:0 ratio (13–15), and asci within one perithecium always share the same plus-to-minus segregation pattern (15). Genetic analysis indicates a single locus (B locus) conferring plus-minus differentiation and a single locus (M locus) controlling plus-to-minus switch frequency (16, 17). In addition to the minus-type phenotype, the plus type can also produce ascospore progenies with different culture morphologies (e.g., conidial A and conidial B) but at much lower frequencies (14, 18).
Contact between plus and minus isolates efficiently triggers the formation of a mating line between the colonies. This mating line is composed of abundant and fertile perithecia with well-developed ascospores. Three types of perithecia exist within the mating line (19). The first type contains asci in which all ascospores are the plus type. These occur rarely. The second type contains asci in which plus and minus ascospores segregate in a 4:4 ratio. The third type contains asci in which all ascospores are the minus type. These second and third ascus types occur at high frequencies. With the aid of additional phenotypic markers, Chilton et al. proposed the occurrence of cross-fertilization between plus and minus isolates within the mating line (18). In addition, Driver and Wheeler (19) proposed that the high frequency of type three perithecia was due to an “induced selfing” effect, where a plus-derived sexual pheromone boosts sexual development of the minus isolate.
Colletotrichum plant pathogens cause huge economic damage, and their sexual reproduction involves complex regulation (8, 10, 20). However, our knowledge of Colletotrichum sexual reproduction remains limited. C. fructicola belongs to the C. gloeosporioides species complex and is a dominant species associated with apple bitter rot disease, from which the plus and minus strain types were originally isolated (11, 12). In this study, we characterized the sexual behaviors of a pair of plus and minus strains in C. fructicola with the aid of live-cell nucleus-localized fluorescent protein labeling, gene expression, and gene deletion analyses. We dissected the cross-fertilization and self-fertilization events with live-cell imaging and provided molecular evidence supporting the importance of FUS/KSS1 mitogen-activated protein kinase (MAPK) signaling, GPCR signaling, and epigenetic factors in regulating proper mating line development. Together, these results shed light on factors governing the atypical mating system (plus-minus-mediated sexual enhancement) being uniquely evolved in Colletotrichum fungi.
RESULTS
Genetically stable plus-to-minus switching and sexual enhancement phenomenon in Colletotrichum fructicola.
In laboratory tests with C. fructicola isolates derived from apple Glomerella leaf spot lesions, we observed that certain single-spore-purified isolates showed strikingly different colony morphologies, with the culture appearances resembling early reports of plus and minus cultures, respectively, described for Glomerella cingulata (11, 12). From one plus-like strain, termed 1104, we obtained both plus-like and minus-like ascospore progeny. Two single-ascospore-derived strains, 1104-7 (plus-like) and 1104-6 (minus-like), were randomly chosen for further experimental characterization.
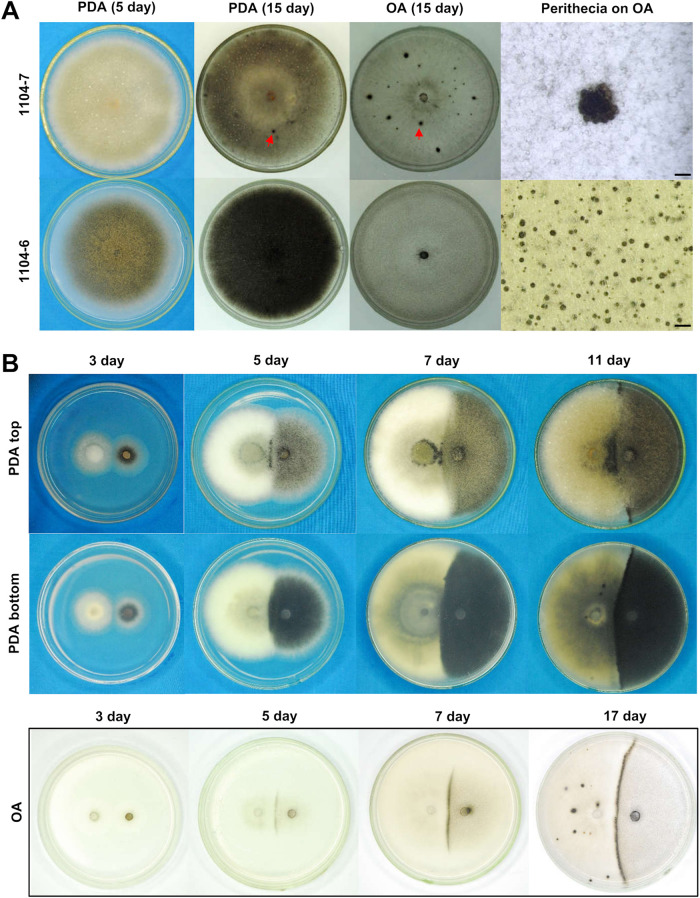
When grown on potato dextrose agar (PDA) medium, the 1104-7 colony was white to gray in color and was covered with abundant aerial hyphae, whereas the 1104-6 colony was less fluffy and more heavily melanized. On oatmeal agar (OA) medium, both colonies showed limited aerial hyphal growth (Fig. 1A). On both PDA and OA, 1104-7 formed perithecial clusters from 13 days postinoculation (dpi) onward, the frequency of which varied among plates but was, in general, higher on PDA (11.5 ± 17.6 per plate, n = 33) than on OA (8.3 ± 10.6 per plate, n = 33). Unlike 1104-7, 1104-6 differentiated scattered perithecia in abundance from 3 dpi on both media (Fig. 1A). When coculturing 1104-7 and 1104-6 on PDA and OA (mycelial plugs 2 cm apart, approximately 25°C, in darkness), a mating line made up of perithecial clusters developed at the junction of the two colonies from 5 dpi onward (Fig. 1B).
FIG 1.
Colony morphologies of 1104-7 (plus strain), 1104-6 (minus strain), and the mating line response. (A) Colony morphologies on PDA and OA. Red arrows point to examples of perithecial clusters. Scale bar, 1 mm. (B) Mating line formation induced by coculturing 1104-7 (left) and 1104-6 (right) on PDA and OA. dpi, days postinoculation.
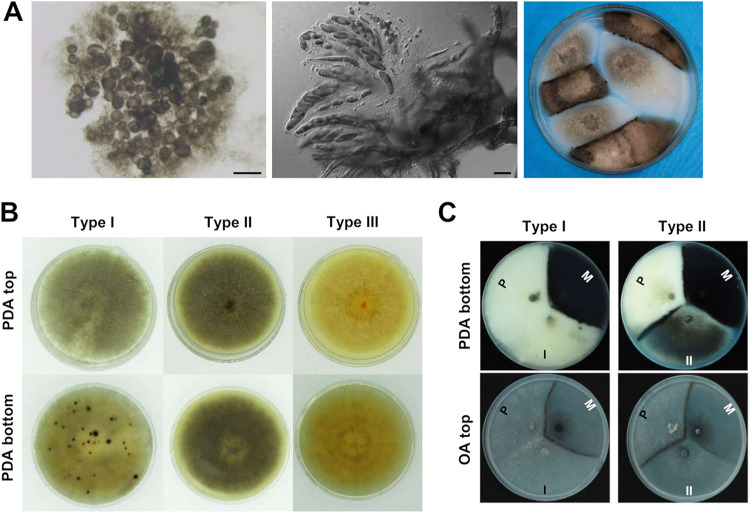
Perithecial clusters formed by 1104-7 solo cultures were highly fertile, with well-developed asci (55.8 ± 5.8 μm long; 10.5 ± 0.8 μm wide). The ascospore progeny exhibited segregation for colony morphology (Fig. 2A) and, in general, could be classified into three types (Fig. 2B). Type I cultures resembled 1104-7, with light melanization, the production of clustered perithecia, and fluffy aerial hyphae; type II cultures resembled 1104-6, with the production of scattered perithecia and darkly melanized hyphae and melanin accumulation levels; however, levels varied and were generally less than those of 1104-6; type III cultures resembled neither 1104-7 nor 1104-6, with no perithecia production. These type III cultures quickly lost viability and could not survive subculture or freeze-storage. In two independent experiments, the three culture types segregated in ratios of 66:141:39 and 21:22:16 for type I, II, and III phenotypes, respectively. In coculturing assays with 1104-7, which we designate P type, and 1104-6, which we designate M type, type I progeny cultures formed a mating line with 1104-6 but not with 1104-7, whereas type II progeny cultures exhibited the opposite pattern, forming a mating line with 1104-7 but not with 1104-6 (Fig. 2C). Thus, 1104-7 (P type) could generate non-parental-type progeny at high frequency in solo culture, and P (1104-7) and M type (1104-6) could form mating lines with type II and type I progeny, respectively.
FIG 2.
Colony morphology phenotypes of 1104-7 ascospore progeny. (A) From left to right, squashed perithecial cluster (scale bar, 200 μm), maturing asci, and ascospores (scale bar, 10 μm), representative culture types of ascospore progeny on PDA medium. (B) Representative colony photos of the three types of 1104-7 ascospore progeny, type I resembles the 1104-7 plus parent, type II resembles the minus 1104-6 strain but with reduced melanization, and type III does not produce perithecia, is light yellow in colony color, and has poor viability. (C) Mating response of type I and type II strains with 1104-7 (P) and 1104-6 (M).
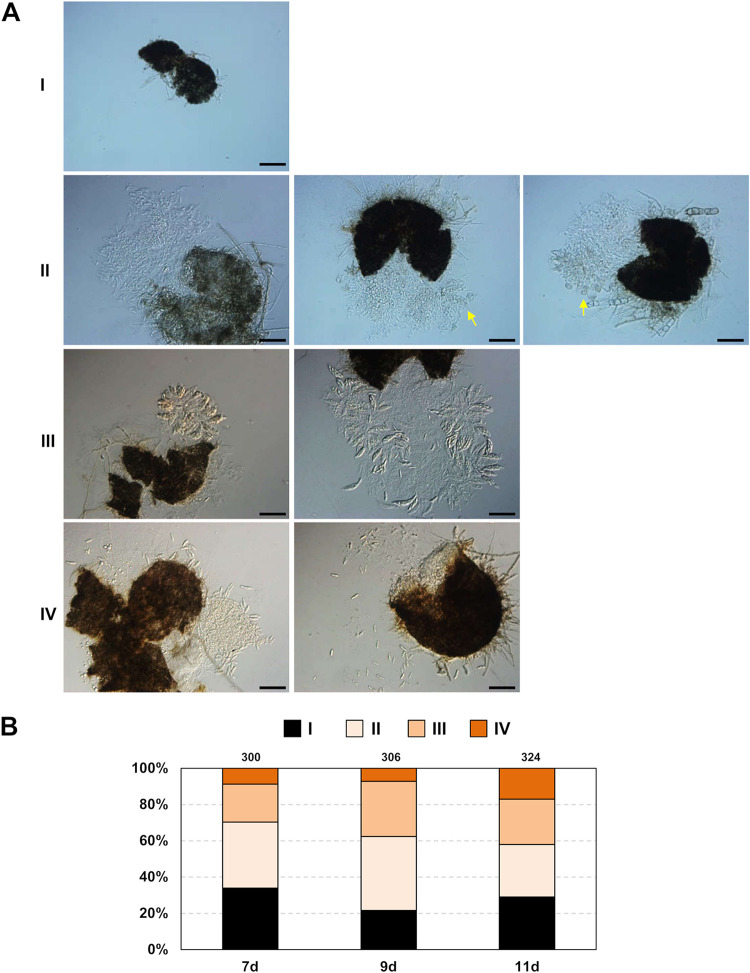
Perithecia formed by 1104-6 solo cultures were reduced in fertility compared to those of strain 1104-7. On OA, approximately 60% of perithecia were defective in ascus development, and these perithecia contained either no or undeveloped ascus initials (perithecial forms I and II in Fig. 3A); other perithecia produced asci and released viable ascospores (perithecial forms III and IV in Fig. 3A). These mature asci, however, were more compact (35.9 ± 3.3 μm long; 11.8 ± 1.3 μm wide) than those produced by 1104-7. Figure 3B depicts the relative ratios of different perithecia types at different days after OA inoculation. All ascospore progeny of 1104-6 produced colonies with the parental phenotype. No change in colony phenotype was observed with the conidial progeny of 1104-7 or 1104-6 (n > 100).
FIG 3.
Low fertility of 1104-6 selfed perithecia on OA. (A) Classification of perithecia based on ascus and ascospore development. Form I, empty perithecium, no inner tissue; form II, abundant inner tissue, ascus initials may be observed (yellow arrowhead); form III, obvious ascus rosette; form IV, ascus dissolved but abundant ascospores. Scale bar, 50 μm. (B) Relative ratios of the four perithecia types at different days (d) postinoculation.
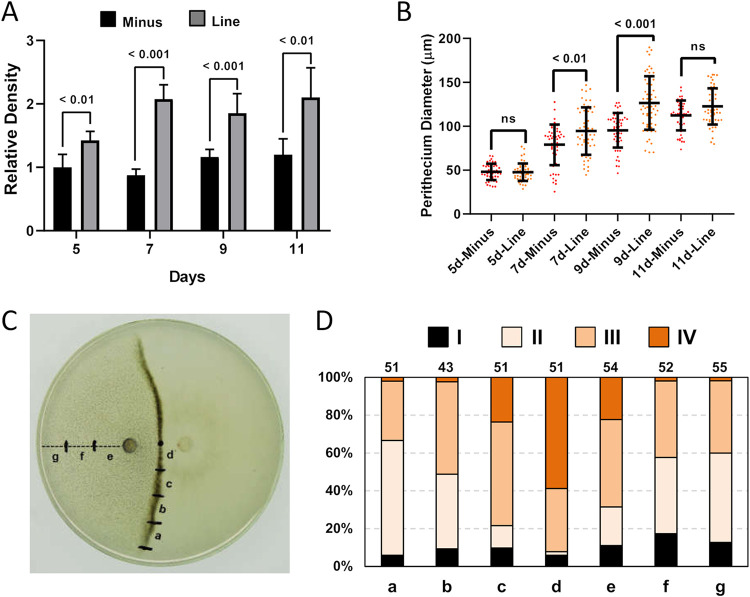
The mating line development process in cocultured 1104-7 and 1104-6 plates was also characterized (Fig. 4). At all four sampled time points (5,7,9, and 11 dpi), perithecial density within the mating line was, on average, 1.5 to 2 times higher than that found in other regions of the 1104-6 culture (Fig. 4A), supporting the hypothesis that plus-minus hyphal proximity induces perithecial differentiation. Compared with 1104-6 solo culture, a size increase for perithecia within the mating line was statistically significant (Student's t test, P < 0.05) for 7 dpi and 9 dpi but not for 5 dpi and 11 dpi (Fig. 4B). Fertility of perithecia within the mating line (perithecial forms III and IV) was 92.2% (Fig. 4D), which was over two times higher than that for 1104-6 solo culture (approximately 40%).
FIG 4.
Mating line formation between 1104-7 and 1104-6 involves increased perithecial differentiation and elevated extent of perithecial maturation. (A) Relative perithecium density for the 1104-6 (minus, corresponding to region f in panel C) and the mating line (line, corresponding to region d in panel C). The values were normalized to the minus region at 5 days. (B) Perithecium diameter in the minus region and line region over time. (C) Example of a paired cross showing the sampled regions in panels A, B, and D. (D) Fertility of different regions (shown in panel C) at 11 days postinoculation. The number of perithecia examined is shown above individual columns. Form I, empty perithecium, no inner tissue; form II, abundant inner tissue, ascus initials may be observed; form III, obvious ascus rosette; form IV, ascus dissolved but abundant ascospores. Representative photos for different perithecial types are shown in Fig. 3A.
Taken together, we confirmed a genetically stable plus-to-minus switching phenomenon in C. fructicola. Moreover, plus-minus hyphal proximity enhances sexual development by triggering the formation of a mating line. This mating line formation involves increased perithecial differentiation and elevated perithecial maturation, as evidenced by the increases in perithecial density, perithecial size, and perithecial fertility within the mating line relative to the minus solo culture. Such a plus-to-minus switch phenomenon represents a fecundity-boosting strategy specific to Colletotrichum fungi.
Perithecia within the mating line are mostly minus derived, being either cross-fertilized or self-fertilized.
Since both 1104-7 and 1104-6 strains can produce perithecia, we attempted to determine the strain origin of perithecia produced within the mating line. Hyphal entanglement prevented light microscopy-based tracing; thus, we tested the utility of melanin-deficient mutants and fluorescent protein-labeled strains. pks1 and scd1, two key genes regulating the biosynthesis of the melanin precursor 1,8 dihydroxynaphthalene (DHN), were deleted in 1104-7 or 1104-6 using gene replacement strategies (see Fig. S2 in the supplemental material). We also tagged histone H1 with fluorescent protein green fluorescent protein (GFP) or monomeric red fluorescent protein (mRFP), which allowed for fluorescent labeling of nuclei (Fig. S2). Additional derivative strains (Fig. S2) were generated by genetic retransformation.
The PDA colony appearances of Δscd1 and Δpks1 mutants were in accordance with those reported in other Colletotrichum fungi (21, 22), with the Δscd1 mutant being reddish-brown in color (due to scytalone overaccumulation) and the Δpks1 mutant being albino or light yellow. These melanin deficiency markers, however, were ineffective for dissecting perithecial origin in mixed cultures, as the mating line region would still melanize if the culture confrontation involved a WT strain on one side or when the confronted strains were genetically complementary (e.g., Δscd1 and Δpks1) (Fig. S3). PKS1 and SCD1 catalyze the sequential production of 1,3,6,8-tetrahydroxynaphthalene and scytalone, respectively, during melanin biosynthesis (23), and diffusion of these intermediate compounds within the mating line apparently complemented the mutant defect, complicating data interpretation.
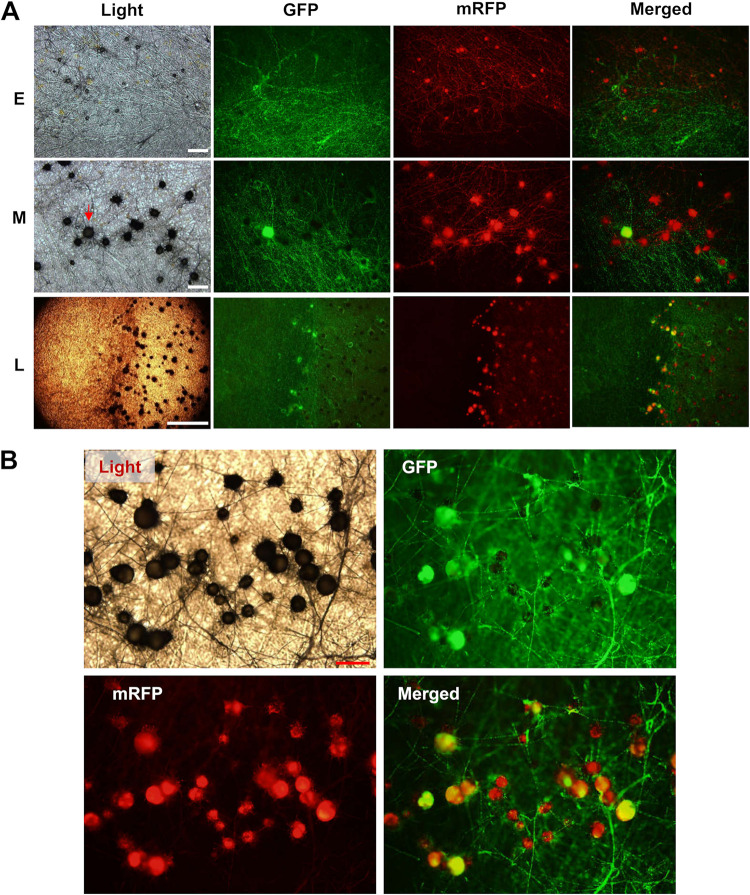
Fluorescent protein markers, on the other hand, were highly effective for dissecting mating line development (Fig. 5). As shown in Fig. S4, perithecial GFP/mRFP signals emitted by the fluorescent strains were much stronger than autofluorescence emitted by the control strains. When coculturing 1104-7-hisH1-GFP and 1104-6-hisH1-mRFP, all perithecial initials (early stage) within the mating line emitted red fluorescence (Fig. 5A), supporting their 1104-6 origin. As the line continued to develop (middle stage), maturing perithecial bodies showed two types of fluorescence patterns (Fig. 5A and B). The first type emitted red fluorescence only, whereas the second type emitted both red and green fluorescence. For the second type, most green fluorescence was emitted by the perithecial body but not the entangling superficial hyphae (Fig. 5B). Perithecia emitting both types of fluorescence were generally larger in size than ones emitting only red fluorescence. Moreover, certain minus hyphal strands produced lines of perithecia extending into the plus colony, demonstrating their high-efficiency perithecial differentiation (Fig. 5A). We postulated that red fluorescent perithecia are derived from self-fertilization of the minus strain, whereas double fluorescent perithecia are minus-derived fruiting bodies being fertilized by the plus strain. In our observations of cocultures between 1104-7-hisH1-GFP and 1104-6-hisH1-mRFP, no perithecium producing only green fluorescence was observed.
FIG 5.
Development of perithecia within the mating line between 1104-7-hisH1-GFP (P-His-GFP) and 1104-6-hisH1-mRFP (M-His-mRFP) on OA. (A) Perithecial fluorescence pattern at early (E), middle (M), and late (L) stages of mating line formation. Note that all perithecia initials emit red fluorescence, whereas from the middle stage onward, some perithecia start to emit double fluorescence (red arrowhead). Scale bars for E, M, and L represent 100 μm, 100 μm, and 1 mm, respectively. (B) A magnified view of the maturing mating line (late stage). Note the two types of perithecia differing in fluorescence pattern (red only and double fluorescence). Scale bar, 100 μm.
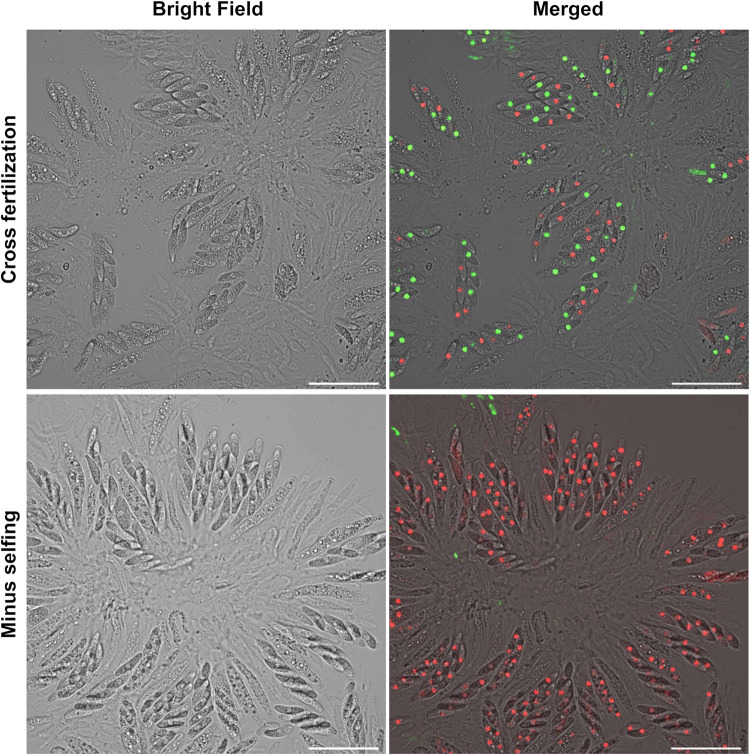
When individual perithecia within the mating line were examined, asci exhibited one of two fluorescence patterns (Fig. 6). The first pattern contained 8 ascospores emitting both green and red fluorescence, typically segregating in a 4:4 ratio. These asci are presumed to arise from plus-minus cross-fertilization. In contrast, all eight ascospores in the second pattern ascus emitted red fluorescence only, which is presumed to arise from minus (1104-6-hisH1-mRFP) selfing. Asci within the same perithecium always exhibited the same ascospore fluorescence pattern, which was in accordance with previous studies indicating that all asci from one perithecium are derived from the same fertilization event (15, 24). We examined over 90 perithecia formed within the mating line between paired cultures of 1104-7-hisH1-GFP and 1104-6-hisH1-mRFP on OA medium and did not observe any perithecia containing ascospores emitting green fluorescence only. The green and red pattern of ascospores constituted 40% and the red ascospores only pattern constituted 60% of the total perithecia produced, supporting a slightly higher frequency of minus self-fertilization over cross-fertilization within the mating line.
FIG 6.
Mating line contains both cross-fertilized and self-fertilized perithecia. 1104-7-hisH1-GFP (P-His-GFP) and 1104-6-hisH1-mRFP (M-His-mRFP) were cocultured on cellophane overlaid on OA medium. Individual perithecia were squashed to examine ascospore fluorescence patterns. (Top) 4:4 segregation of green to red fluorescence of ascospores within individual asci, indicating cross-fertilization between the two strains. (Bottom) 0:8 segregation of green to red fluorescence for ascospores within individual asci, indicating self-fertilization of the minus strain; no 8:0 green to red fluorescence pattern was observed with over 90 examined perithecia. In top and bottom panels, merged photos were generated by combining views from the bright-field green (GFP) channel and red (mRFP) channel, respectively. Scale bar, 40 μm.
Pheromone signaling genes are differently expressed between the plus and minus strains.
Sexual development in filamentous ascomycetes requires the coordinated functions of mating type genes and genes related to pheromone signaling (25). Based on the draft genome assemblies generated for 1104-7 and 1104-6 in our laboratory, mating type genes, pheromones, and pheromone receptors were manually annotated. In both strains, the MAT locus contained only the mat1-2-1 gene but no mat1-1-1 gene here or elsewhere in the genome (data not shown), as reported for other sequenced Colletotrichum genomes (4). In addition, two putative pheromone peptides (ppg1 and ppg2) and two putative pheromone receptors (pre1 and pre2) were identified in each of the two strains (Fig. S5 and S6). No DNA sequence variation was observed between plus (1104-7) and minus (1104-6) strains in the coding or flanking regions of any of these five genes (mat1-2-1, ppg1, ppg2, pre1, and pre2) (Fig. S7).
We further examined the expression of the above-mentioned genes using quantitative reverse transcription-PCR (qRT-PCR) (Fig. 7). Expression of an additional four sex-related genes (mcm1, ste12, velB, and gpcr1) was also examined. qRT-PCR tissues were sampled from 0.5 cm of the OA colony edges of 1104-7 and 1104-6 (vegetative hyphae lacking perithecial initial) and the mating line at different time points postinoculation (3, 5, 7, and 9 dpi).
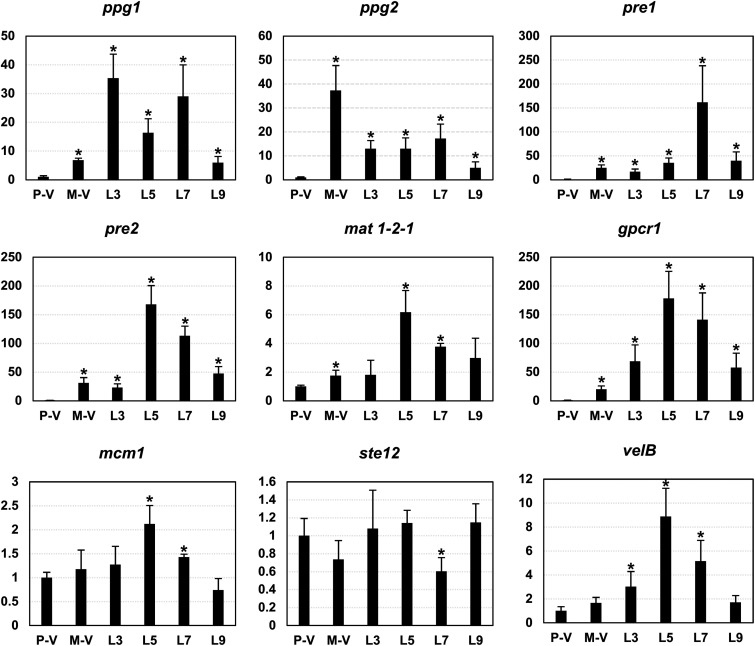
FIG 7.
qRT-PCR expression analysis of genes related to fungal sexual development. Fungal tissues were sampled from the 0.5-cm OA colony edge of 1104-7 (plus vegetative hyphae, P-V), 1104-6 (minus vegetative hyphae, M-V), and mating line (L) at different days postinoculation (days 3, 5, 7, and 9). The C. fructicola alpha-tubulin gene (XP_031882639.1) was used as the internal control, and gene expression levels were normalized to plus vegetative hyphae (P-V). Numbers on the y axes represent relative expression fold change. The error bar represents standard deviations calculated from three independent replicates. An asterisk represents significant difference compared with P-V based on two-tailed t test at a significance level of 0.05.
Compared with 1104-7, the transcript accumulation levels of gpcr1, pheromones (ppg1 and ppg2) and pheromone receptors (pre1 and pre2) were strongly and significantly upregulated in the vegetative hyphae of 1104-6 (ranging from 7- to 37-fold), whereas mat1-2-1 transcript accumulation was mildly elevated (approximately 2-fold). Except for ppg2, the expression levels of all genes were higher in the mating line tissues (5 or 7 dpi) than in 1104-6 vegetative hyphae (Fig. 7). velB, a transcription factor regulating sexual development and light signaling in ascomycetes (26), was similarly expressed between the vegetative hyphae of 1104-7 and 1104-6 but was upregulated in the mating line. No obvious change in transcript accumulation level was observed with mcm1 and ste12, two transcription factors downstream of FUS/KSS1 MAPK (27). In sum, pheromone signaling genes were differently expressed between plus and minus strains, and their expression levels were also increased during mating line development.
Proper perithecial and mating line development require GPCR/MAPK signaling and histone modification factors.
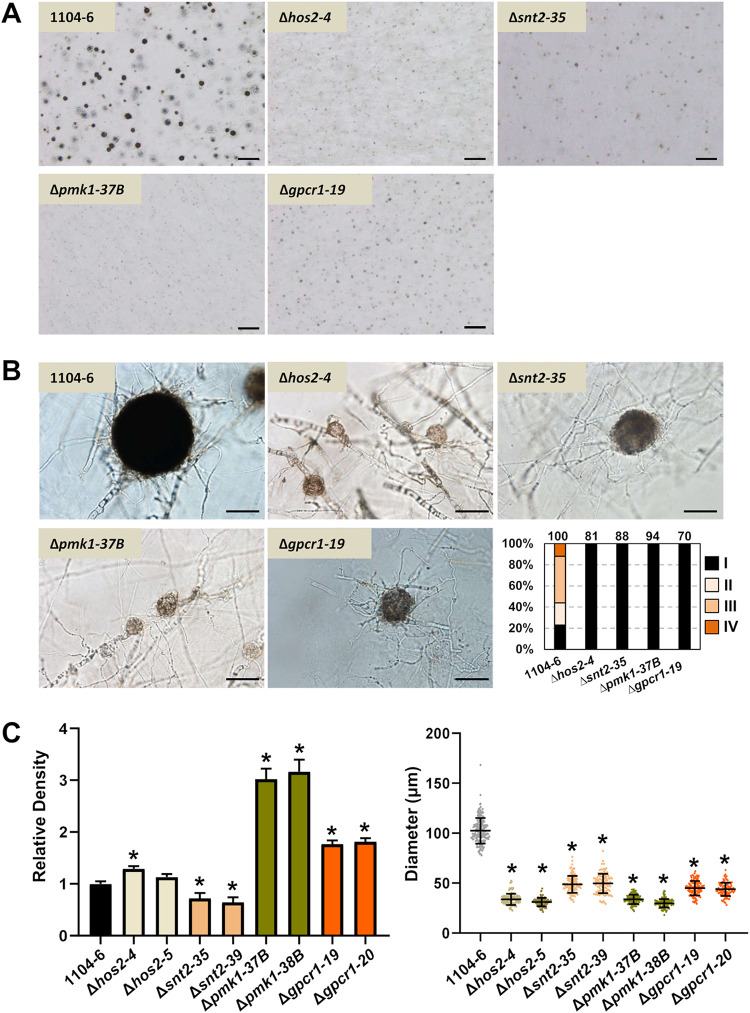
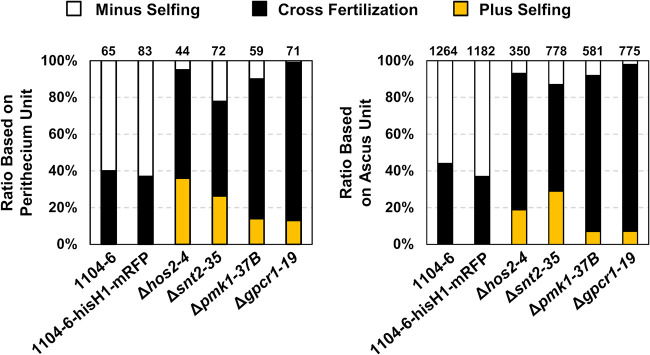
To further dissect plus-minus differentiation and mating line development in C. fructicola, we generated deletion mutants of several genes putatively regulating sexual development, which included the pheromone receptors pre1 and pre2, the class II histone deacetylase hos2, the RPD3 histone deacetylase complex component snt2, and the G protein coupled receptor gpcr1, which is orthologous to FGSG_05239 playing a sex-specific function in Fusarium graminearum (28). The previously generated pmk1 (FUS/KSS1 MAPK) deletion mutants (29) were also included for phenotype characterization. For simplicity, gene deletion mutants were only generated in 1104-6, and two independent mutants were used for phenotypic analysis. PCR identification of target gene deletion events is shown in Fig. S8. For each strain, sexual development phenotypes in the presence or absence of 1104-7-hisH1-GFP coculturing were documented. In either solo culture or coculture, no obvious phenotypic change was observed with gene deletion mutants of pre1 or pre2 (data not shown). On the other hand, perithecial development defects were observed with all other gene deletion mutants (Δhos2, Δsnt2, Δpmk1, and Δgpcr1). Compared with the 1104-6 wild type (WT), perithecia formed by these mutants were obviously smaller, less melanized, and irregular in shape (Fig. 8A to C). The perithecial densities of Δpmk1 and Δgpcr1 mutants were higher than that of the WT, whereas those of Δsnt2 mutants were slightly but significantly lower (Fig. 8C). In addition, all perithecia from these four mutants were completely sterile (Fig. 8B). When coculturing with 1104-7-hisH1-GFP, Δhos2, Δsnt2, and Δgpcr1 mutants induced obvious mating line formation, whereas mating line formation induced by the Δpmk1 mutant was almost completely abolished (Fig. 9). Based on the segregation pattern of ascospore GFP fluorescence within individual asci, perithecial fertilization patterns within the mating line were further dissected (Fig. 10). Coculture of mutants with the 1104-7-hisH1-GFP plus strain led to decreased frequencies of minus self-fertilization but increased frequencies of plus self-fertilization (Fig. 10).
FIG 8.
Effects of target gene deletion on perithecial development. All cultures were grown on OA medium. (A) Perithecial morphology observed under dissecting microscope magnification. Scale bar, 500 μm. (B) Perithecial morphology observed under light microscope magnification (scale bar, 50 μm) and quantification of perithecial fertility at 11 days postinoculation (right bottom corner; the number of examined perithecia are shown above individual columns). (C, left) Relative perithecial density normalized to the WT (11 days). Means ± standard deviations (n = 9) were plotted. An asterisk represents significant difference (adjusted P < 0.05) compared with the WT based on Dunnett’s multiple-comparison test following one-way analysis of variance (ANOVA). (Right) Perithecial diameter (11 dpi). Means ± standard deviations were plotted as horizontal and vertical lines above individual values (dots; n ≥ 100). An asterisk represents significant difference (adjusted P < 0.05) compared with the WT based on Dunnett’s multiple-comparison test following one-way ANOVA.
FIG 9.
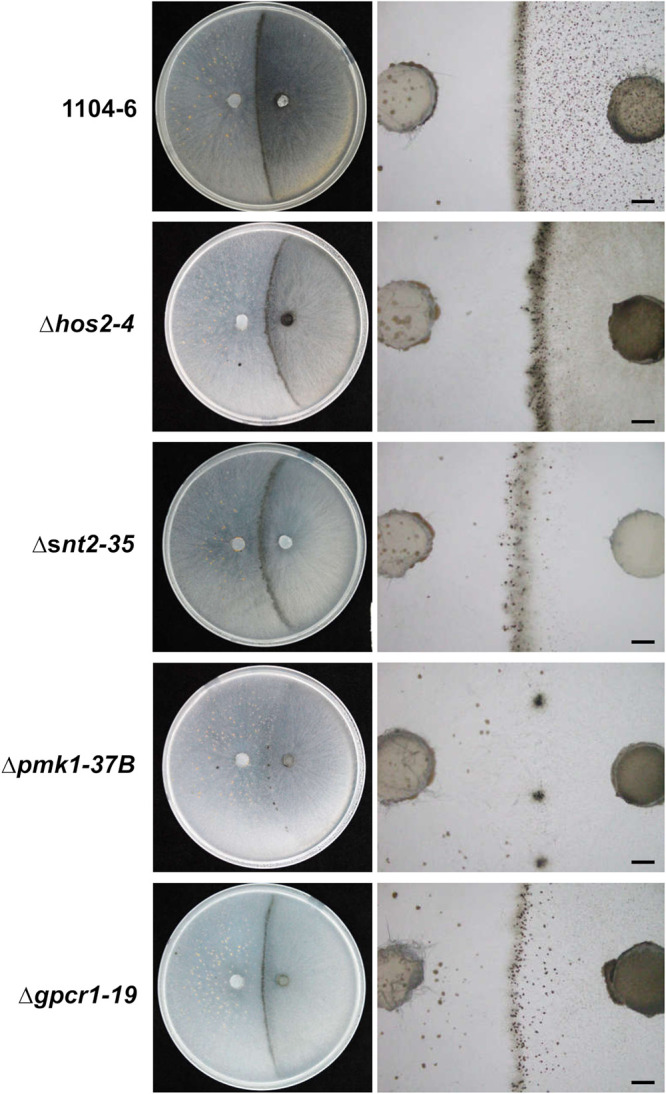
Mating line formation phenotypes of 1104-6 gene deletion mutants when cocultured with 1104-7-hisH1-GFP on OA. Mycelial plugs of 1104-7-hisH1-GFP- and 1104-6-derived strains were inoculated on the left and right sides of the OA plate, respectively. Mating line formation phenotypes were photographed at 11 days postinoculation. Scale bar, 2 mm.
FIG 10.
Fertilization events occurring within the mating line formed between 1104-7-hisH1-GFP and various 1104-6-derived strains. Based on ascospore fluorescence segregation patterns, each ascus or perithecium could be classified as minus selfing (fluorescent/nonfluorescent ascospore ratio, 0:8), cross fertilization (fluorescent/nonfluorescent ascospore ratio, 4:4), or plus selfing (fluorescent/nonfluorescent ascospore ratio, 8:0). The relative ratios of the three types of fertilization events (based on either perithecia or asci) are plotted. The total number of observations is given above individual columns.
DISCUSSION
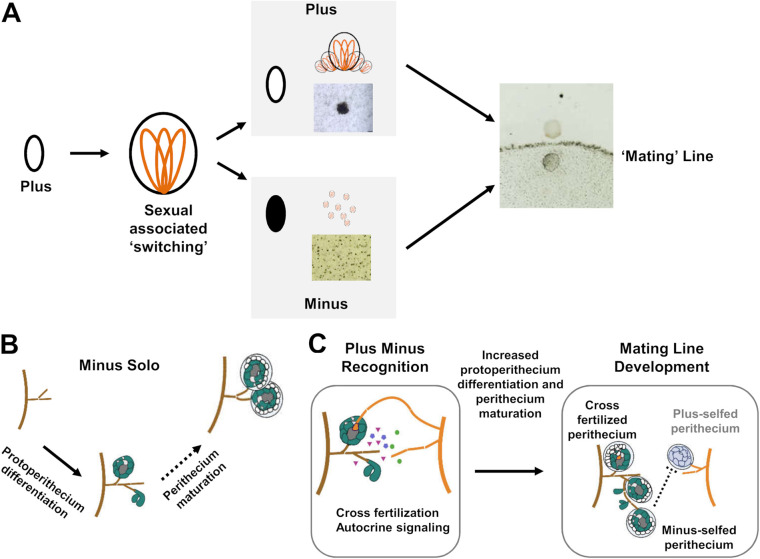
Plus-minus regulation of sexual differentiation in Colletotrichum species was reported in the early 1900s, and subsequent reports have remained restricted to Colletotrichum (Glomerella) species (5, 10–12, 30, 31). Both plus and minus strains produce fertile perithecia in a homothallic but inefficient manner. However, when the two strain types encounter each other, efficient differentiation of fertile perithecia involving both cross-fertilization and self-fertilization is triggered. Thus, plus-minus switching facilitates sexual reproduction and should be evolutionarily advantageous from the perspectives of fecundity and generation of genetic recombinants. Genetic mechanisms underlying this unique adaptive behavior, however, have been under investigation for more than half a century. By taking advantage of available molecular tools, we demonstrate that phenotypic variation between plus and minus strains involves the differential expression of pheromone signaling genes. Moreover, our data support that proper perithecial development and mating line differentiation require FUS/KSS1 MAPK and GPCR signaling as well as the epigenetic regulators HOS2 and SNT2. Based on our experimental data and previous reports, here we propose a model (Fig. 11) explaining the plus-minus differentiation and plus-minus-mediated sexual enhancement phenomenon occurring in C. fructicola and likely closely related species as well. The model is discussed below in detail.
FIG 11.
Model describing the atypical mating system of C. fructicola. (A) The plus-to-minus switching phenomenon. The plus strain, via an unknown mechanism related to sexual reproduction, produces minus ascospore offspring at high frequency. The pairing of plus and minus strains boosts sexual reproduction, leading to the formation of a mating line between colonies. (B) Minus perithecium development (minus solo). The minus wild type forms abundant perithecia but many are infertile, indicating a defect in perithecium maturation (dash arrow). Deletion mutants of pmk1, gpcr1, hos2, and snt2 still formed abundant perithecia but were smaller, aberrant, and completely sterile, indicating their importance in both protoperithecium development and perithecium maturation (not shown). (C) Mating line development. When plus (orange) and minus (dark brown) strains come into contact, the minus strain is fertilized by the plus strain, which complements or derepresses its perithecium maturation defect; moreover, local diffused effectors (e.g., pheromones) may directly boost perithecium differentiation and development via autocrine signaling, leading to the development of abundant minus-selfed perithecia and, under certain conditions (e.g., minus selfing being suppressed), the development of fertile plus-selfed perithecia as well.
Plus-to-minus switch.
So far, the plus-to-minus switch phenomenon has been studied in detail only in Glomerella cingulata, the sexual stage of Colletotrichum gloeosporioides (5, 10, 31). In the recently established Colletotrichum taxonomy, C. gloeosporioides represents a species complex (CGSC) encompassing more than 20 closely related species (32). In our laboratory study, a similar plus-to-minus switching phenomenon has been observed in C. aenigma (unpublished data), a close relative of C. fructicola. It is unclear, however, whether the plus-to-minus switch phenomenon is ubiquitous among different CGSC lineages.
Within ascomycetes, a bidirectional mating type switch has been documented in several yeast species (e.g., Saccharomyces cerevisiae, Schizosaccharomyces pombe, Hansenula polymorpha, and Pichia pastoris), where the mating type switch involves the transfer of a silent mating type cassette to an active transcriptional locus via either DNA copying or DNA inversion (33). In filamentous fungi, bidirectional mating type switching has not been observed, but unidirectional mating type switching does occur where a self-fertile strain gives rise to both self-fertile and self-sterile strains (34). Unidirectional mating type switching has been reported in Ceratocysis, Chromocrea, and Sclerotinia (34, 35). In Ceratocysis and Sclerotinia, mating type switching is related to DNA deletion or inversion at the mating type locus (35–38). The plus-to-minus switch in C. fructicola reported in this study differs from unidirectional mating type switching in several aspects: (i) both plus strains and minus strains derived from plus strains are self-fertile, although at low efficiency in the latter; (ii) plus-to-minus switching facilitates sexual reproduction by boosting both cross-fertilization and self-fertilization, whereas unidirectional mating type switching boosts outcrossing only; and (iii) plus-to-minus switching involves no DNA change at the mating type locus (discussed below), implying a novel regulatory mechanism.
Factor(s) regulating plus-minus phenotypic differentiation.
Morphologically, plus and minus strains differ dramatically in terms of cultural appearance, perithecial production, and perithecial fertility. Early genetic analysis (17) supported that such phenotypic differentiation is controlled by a single genetic locus (B locus), with the B and b alleles conferring plus and minus phenotypes, respectively. In addition, there is another genetic factor, termed mutator, or M, which confers an elevated frequency of B to b mutation (17). The molecular natures of the proposed B and M loci, however, are unclear. In ascomycetes, mating type and pheromone genes are key factors regulating cell identity and mating partner recognition (1). In heterothallic fungi, strains with opposite mating types usually harbor different mating type genes, which leads to distinct expressions of pheromones and pheromone receptors. We have generated draft genome assemblies for 1104-7 and 1104-6; however, the corresponding genes (mat1-2-1, ppg1, ppg2, pre1, and pre2) showed no DNA sequence variation. However, our qRT-PCR result did show that pheromone signaling genes in these two strain types are differently expressed, with all expression levels being higher in 1104-6. We proposed that B to b mutation or epigenetic alteration at the B locus alters the function of one or more developmental regulator(s), which impacts various phenotypes, including aerial hypha growth, perithecial differentiation, ascospore development, and pheromone gene expression. In this study, ascospore progeny of selfed 1104-7 can be classified into one of three types based on culture morphology: resembling 1104-7 (type I), resembling 1104-6 but with reduced melanization (type II), and resembling neither type I nor II and with low viability (type III). While it is known that ascospore progeny of a plus strain encompasses strain types other than minus (e.g., conidial A and conidial B) (14, 18), the death-prone type III progeny has not been reported previously. Mechanisms underlying such sex-associated phenotype diversification are also unclear. The high frequency of nonparental phenotype implies a specialized mechanism other than spontaneous DNA mutation. In-depth comparisons of genome-wide structural variations between 1104-7 and 1104-6 are ongoing.
Compared with 1104-7, 1104-6 produces scattered perithecia in abundance, but a large fraction of these perithecia are infertile. Thus, in the minus strain, protoperithecia differentiation is boosted, whereas further perithecial maturation (ascospore development) is suppressed. Ascomycete pheromones can regulate sexual structure development in addition to mate recognition (39, 40). The vegetative hyphae of 1104-6 showed higher expression levels of pheromones and pheromone receptors than 1104-7. It is possible that elevated pheromone accumulation in the minus strain directly promotes perithecial differentiation. Relevant to this idea, Fusarium oxysporum pheromones can function as density-dependent autocrine signals regulating conidial germination (41). We have generated single-gene deletion mutants for the pheromone receptor genes pre1 and pre2 in 1104-6; however, no obvious change in perithecial density was observed. In filamentous ascomycetes, PPG1/PRE2 and PPG2/PRE1 form two cognate pheromone-receptor pairs. The importance of the two signaling pairs, however, varies among homothallic ascomycetes. In Sordaria macrospora (40), single pheromone or receptor deletion mutant showed no discernible phenotype, whereas deletion of both receptor genes eliminated fruiting body formation. In Aspergillus nidulans (42), deletion of a single pheromone receptor reduced cleistothecium size and ascospore numbers, whereas deletion of both receptors eliminated fruiting body formation. In Gibberella zeae (39), the PPG1/PRE2 pair enhances but is not essential for selfing, whereas the PPG2/PRE1 pair has no discernible role. So far, the importance of pheromone signaling in Colletotrichum sexual reproduction has not been dissected. Based on results aforementioned, we hypothesize that the two pheromone-receptor pairs function redundantly in C. fructicola, and one complete pair would be sufficient to mediate proper sexual development. In the future, it would be interesting to generate and characterize double deletion mutants of pheromones and pheromone receptor genes.
Complex fertilization events within the mating line.
Differing from a typical mating line formed between opposite mating types, where only cross-fertilization event takes place, three perithecial types, termed plus selfing, minus selfing, and cross-fertilization, were previously suggested to occur within the mating line (19). In the current study, we generated nuclear-fluorescent protein-labeled strains, which enabled the direct dissection of different fertilization events with live-cell fluorescence imaging. In our coculture combinations involving 1104-7-hisH1-GFP/1104-6-hisH1-mRFP or 1104-7-hisH1-GFP/1104-6, around 60% of total perithecia were derived from minus selfing, with the rest being derived from cross-fertilization. No perithecia derived from plus selfing were observed. We postulated that most, if not all, protoperithecial initials within the mating line are minus derived, these maternal initials could be fertilized by either the plus or the minus type. Whereas efforts to directly observe fertilization events failed due to intense hyphal entanglement, dynamics of perithecial fluorescence pattern support this postulation. All early perithecial initials emitted red fluorescence (corresponding to minus strain origin), whereas maturing perithecia emitted either red fluorescence alone (corresponding to minus selfing) or emitted both red and green fluorescence (corresponding to cross-fertilization).
Differing from minus solo culture bearing perithecia with low fertility, minus selfing perithecia within the mating line were highly fertile. Driver and Wheeler proposed the term induced selfing and showed that an active substance derived from the plus strain could boost minus self-fertilization (19). The nature of this diffusive substance, its specificity to the plus strain, and its relationship with known peptide mating pheromones (a-factor and α-factor) remain to be determined.
The common occurrence of induced selfing perithecia on the mating line implies that care should be taken when performing segregation analysis in C. fructicola or related CGSC species, as the self-fertilization events would significantly distort the expected Mendelian ratio in progeny. A fluorescence marker strain, in this scenario, would be advantageous in aiding the identification of cross-fertilized perithecia.
Genetic regulation of plus-minus-mediated sexual enhancement.
Plus and minus strains are inefficient in producing perithecia when cultured separately. However, when the two strain types are cocultured, a strong mating line develops at the contact zone between the two cultures. Apparently, along with oriented hyphal growth, plus-minus recognition triggers multiple downstream events, including increased perithecial differentiation, cross- and self-fertilization, and elevated perithecia maturation. These events culminate in the formation of a dense mating line, which we termed here the plus-minus-mediated sexual enhancement (PMSE) phenomenon. So far, no genetic factor regulating this intriguing sexual phenomenon has been reported. In the current study, we showed that the proper function of FUS3/KSS1 MAPK (PMK1) in the minus strain is required for both normal perithecial development and for normal mating line formation. On the other hand, one GPCR (GPCR1; FGSG_05239 homolog) and two histone modification enzymes (HOS2 and SNT2) were required for normal perithecial development in minus solo culture but not for mating line formation. Deletion of any one of these genes in the minus strain, however, altered the ratio of cross-fertilized and self-fertilized perithecia within the mating line.
In ascomycetes, FUS3/KSS1 is a central component of pheromone signaling and a key regulator of both mating partner recognition and fruiting body development, corresponding gene deletion mutants are generally defective in hyphal fusion-mediated heterokaryon formation, female fertility, and sexual fruiting body development (26, 43, 44). Deletion of pmk1 in the minus strain of C. fructicola (1104-6) nearly abolished mating line development when cocultured with a plus WT. We hypothesize that PMK1 is critical for mate recognition at the early perithecial development phase, the mating line formation defect of the Δpmk1 mutant could be explained by the combined effects of perithecial minus origin and their fertilization defect. Differing from pmk1, gene deletion of gpcr1, hos2, and snt2 obviously affected perithecial development in minus solo culture but not perithecia development within the mating line. GPCR1, HOS2, and SNT2 likely mainly regulate perithecial development at the postplasmogamy phase. The fertilization of the protoperithecia of these gene deletion mutants by a plus WT appears to rescue the mutant developmental defect. In line with such postulation, the mutant mating lines involved significantly higher ratios of cross-fertilization and a significantly lower ratio of minus-selfing compared with the WT.
In contrast with the mating line formed between plus and minus WT strains, where only cross-fertilization and minus-selfing perithecia were observed, the mating lines involving all gene deletion mutants also contained plus-selfing perithecia. The occurrences of these plus-selfing perithecia were concomitant with a significant reduction of minus-selfing perithecia. We propose that, within the mating line, both plus and minus hyphae are responsive to perithecium boosting signals, but minus perithecial development may somehow inhibit plus differentiation via an unknown mechanism.
Conclusions.
Genetic regulation of Colletotrichum sexual reproduction is complex and has been poorly understood. Molecular dissection of plus-minus differentiation and perithecial mating line development in C. fructicola should provide important insights into the evolution of Colletotrichum sexual regulation and development. Since the 1950s, however, few investigations into this plus-minus system have been performed. The current study revisits this mystery, and we propose that (i) the minus strain serves as the female counterpart and differentiates abundant protoperithecia, which both plus and minus strains can fertilize; (ii) the minus-selfed perithecium by itself exhibits poor efficiency in structural maturation (ascospore development), which can be complemented by either cross-fertilization or by diffusive signals released within the contact zone; (iii) the development of minus-selfed perithecia somehow inhibit plus differentiation; and (iv) perithecial development and mating line formation require proper G protein and MAPK signaling and proper epigenetic regulation. Data reported in this study lays a foundation for future studies addressing factors shaping plus-minus differentiation and plus-minus-mediated sexual enhancement, for which the establishment of additional cellular and genetic tools, and the development of relevant genomic and transcriptomic resources will be important.
MATERIALS AND METHODS
Fungal strains, culture conditions, and nucleic acid manipulations.
The C. fructicola strain 1104 was obtained from an apple Glomerella leaf spot lesion in a private orchard in Hebei province, China. The derived strains (1104-7, 1104-6, gene knockout mutants, and fluorescent protein-labeled strains) were maintained on potato dextrose agar (PDA) and preserved as 15% glycerol conidial stocks at −80°C in the Fungal Laboratory, College of Plant Protection, Northwest A&F University. Genomic DNAs were extracted from aerial hyphae scraped from PDA colonies with a modified cetyl trimethylammonium bromide (CTAB) procedure (45). Total RNAs were extracted with a Tiangen RNAsimple kit (Tiangen, Beijing, China). Reverse transcription and qRT-PCR were performed in the same way as previously described (46). PCR primers and qRT-PCR primers used in this study are listed in Table 1.
TABLE 1.
Primers used in this study
| Name | Purpose | Sequence (5′–3′) |
|---|---|---|
| SNT2-LF | snt2 gene deletion | TTGTCATTGTCCTGGTCCCTG |
| SNT2-LFNest | snt2 gene deletion | GGCAATGGAAGGCAAGGTAAG |
| SNT2-LR | snt2 gene deletion | CGTCAGATCGATGGTAGTTGTCGTCGACTGGCAGAAGGCAGGTTGAAGAT |
| SNT2-RF | snt2 gene deletion | TGACAGTTCTGGTTAGCCGTCACCAGTGTTTTGCTTTCTTAGGCGACGAT |
| SNT2-RRNest | snt2 gene deletion | TCCACCATGTAAGTCGAAACCT |
| SNT2-RR | snt2 gene deletion | ACAATGAACGACGCAGAGGGT |
| SNT2-DF | snt2 gene deletion | GCATGTCACCTGCGCTGTCTGG |
| SNT2-DR | snt2 gene deletion | GAGGCTGACATCAATACCGCAAGT |
| GPCR1-LF | gpcr1 gene deletion | ATTGGTTCTCCTGCGTGACTG |
| GPCR1-LFNest | gpcr1 gene deletion | TATTCGCACCCAGCCTTACC |
| GPCR1-LR | gpcr1 gene deletion | CGTCAGATCGATGGTAGTTGTCGTCGACTAGGACGCATAATGAGCAGAGC |
| GPCR1-RF | gpcr1 gene deletion | TGACAGTTCTGGTTAGCCGTCACCAGTGTTCGGCCATTATTGAGGAGTC |
| GPCR1-RRNest | gpcr1 gene deletion | CATCTGTCCACTCCGCCTAT |
| GPCR1-RR | gpcr1 gene deletion | AGCCCATCTTTCAAGGTCAC |
| GPCR1-DF | gpcr1 gene deletion | GTTGTCATTGGTCGGCGTCAC |
| GPCR1-DR | gpcr1 gene deletion | CGGAGTTGGTTGCGTTGATGG |
| HOS2-LF | hos2 gene deletion | CAACAGTAAGGTAGGCAAGTGGGAATA |
| HOS2-LFNest | hos2 gene deletion | CGACAACGGAGTGCTTAATGACTG |
| HOS2-LR | hos2 gene deletion | CGTCAGATCGATGGTAGTTGTCGTCGACTGATGAAGGTGAAGGTGACGGCTAT |
| HOS2-RF | hos2 gene deletion | TGACAGTTCTGGTTAGCCGTCACCAGTGTACAGGCAGTGCTATGCTTGAGGT |
| HOS2-RRNest | hos2 gene deletion | GTGGAGGATGATCTGGAACGACA |
| HOS2-RR | hos2 gene deletion | CAAGCATCAATGTTGTCGGGATC |
| HOS2-DF | hos2 gene deletion | TTCGAGGGTCTGTACGACTACTGCT |
| HOS2-DR | hos2 gene deletion | CATTCTCAAAGAGCCACTTGTACTGCT |
| PKS1-LF | pks1 gene deletion | TCTCGCCTTCACCAAACATTACC |
| PKS1-LFNest | pks1 gene deletion | GAAACTCTTCTTGTTAGGTAGCTCGTCGT |
| PKS1-LR | pks1 gene deletion | CGTCAGATCGATGGTAGTTGTCGTCGACTGGTTGTCGACATAGATTTCCTAGGTGA |
| PKS1-RF | pks1 gene deletion | TGACAGTTCTGGTTAGCCGTCACCAGTGTTGAGCACACGCATTGCAGG |
| PKS1-RRNest | pks1 gene deletion | CTGGCTTTCGACTTGGATGTTGTTTGT |
| PKS1-RR | pks1 gene deletion | GCGGTTATGTGACCCGAGATC |
| PKS1-DF | pks1 gene deletion | TATCCCCTTCGGCCCTACAC |
| PKS1-DR | pks1 gene deletion | CCTTGTCCGCAATAGCATCCTC |
| SCD1-LF | scd1 gene deletion | TGTTTTTTTGCGGCTGAGGTTAGA |
| SCD1-LFNest | scd1 gene deletion | AAAACGGCCAGGGGAAATCTTATCG |
| SCD1-LR | scd1 gene deletion | CGTCAGATCGATGGTAGTTGTCGTCGACTTTGTCGAAATTACTCTTCTAAATTCGGGTA |
| SCD1-RF | scd1 gene deletion | TGACAGTTCTGGTTAGCCGTCACCAGTGTGTATGTAATCTGGGCGTGGAATGG |
| SCD1-RRNest | scd1 gene deletion | TGACTCCCATCCCATAAGAGAGGTTGA |
| SCD1-RR | scd1 gene deletion | GAGCTCCGTCTATCTGTATAAATGAAGTG |
| SCD1-DF | scd1 gene deletion | GATATGGACTACCAGCAATACAGAAACTG |
| SCD1-DR | scd1 gene deletion | GAGCCCGGCAAACTTCCAC |
| PPG1-qF | qRT-PCR | ATGCCTGCTCCTAACCCTGTCG |
| PPG1-qR | qRT-PCR | CTGACGCTTCTCCATAGCCTCG |
| PPG2-qF | qRT-PCR | ACTGTCCGTAGAACATTACCAACCA |
| PPG2-qR | qRT-PCR | GAGAAGTGATATGCCTCCTCTGTGT |
| PRE1-qF | qRT-PCR | CAAGCAGATTGAGAGCACAACTCG |
| PRE1-qR | qRT-PCR | GTAGACCGCATAGACGCAGGAAAT |
| PRE2-qF | qRT-PCR | GCTACCAAAGCACCATCATCGG |
| PRE2-qR | qRT-PCR | CATTCGTTATCACCAACACCTCCAT |
| MAT1-2-1-qF | qRT-PCR | TGGACGGCAACGCAGACAGA |
| MAT1-2-1-qR | qRT-PCR | CTCAAGGAAGTGGCTTGGTGGC |
| MCM1-qF | qRT-PCR | CCGCTGACGATGAAGATGATGATGA |
| MCM1-qR | qRT-PCR | AGAAGGTGATGTGACGACGAGACT |
| STE12-qF | qRT-PCR | CTTCCATCCCGCCGTCAATGTC |
| STE12-qR | qRT-PCR | GTCTGTGCTGCTGCTGCTGTT |
| VELB-qF | qRT-PCR | CGTGTTCCAGGTCTACTCTGCCA |
| VELB-qR | qRT-PCR | CTCTTCGTTGTTCTTCTTCCCGTCC |
| GPCR1-qF | qRT-PCR | ACAACCTCATCAAGCCTAACGACAT |
| GPCR1-qR | qRT-PCR | TGACAGCATTCCACACGCCTT |
| TUBULIN-qF | qRT-PCR | CCACTTCCCTTTGGTCGCTTAC |
| TUBULIN-qR | qRT-PCR | CATGGTCATCTCCTGGACAGAGT |
| hisH1-1F | gfp/mrfp fusion | CGTCAATCTTCTTCGCAATCCGATC |
| hisH1-2R | gfp/mrfp fusion | CCTCGAGTGTGCAGAAGCTGCAGAGGCTCAGCCTTGGCCTCGTCCTTCTTG |
| hisH1-3F | gfp/mrfp fusion | ACGCGTTTCGGGTTTACCTCTTCAGCGTTGTTCTTCGT CGTCGTCTTC |
| hisH1-4R | gfp/mrfp fusion | GGTTCAAGTCTCATCTGACGCCAGG |
| GFP-HPH-5F | gfp/mrfp fusion | CCTGCTGCAGCTTCTGCACACTCGAGG |
| GFP-HPH-6R | gfp/mrfp fusion | GAAGAGGTAAACCCGAAACGCGT |
| hisH1-1FNest | gfp/mrfp fusion | CAATCGCCCTTTCGATTGCATCCGC |
| HP-R | gfp/mrfp fusion | GTATTGACCGATTCCTTGCGGTCCGAA |
| hisH1-4RNest | gfp/mrfp fusion | CAGCCCACATCCAACGAGCCATACC |
| PT-F | gfp/mrfp fusion | GATGTAGGAGGGCGTGGATATGTCCT |
| GFP-APH-F | gfp fusion, G418 selection | CAAGAAGGACGAGGCCAAGGCTGAGGTGAGCAAGGGCGAGGAGCTGTTCA |
| GFP-APH-R | gfp fusion, G418 selection | GAAGACGACGACGAAGAACAACGCTGGCTGGTGACGGAATTTTCATAGTC |
| APH-F | gfp fusion, G418 selection | GTGGAGAGGCTATTCGGCTATGACT |
| APH-R | gfp fusion, G418 selection | TGATGTTCTTCGTCCAGATCATCCT |
Genome sequencing and comparison.
The 1104-7 genome was previously sequenced with both Illumina technology (47) and Nanopore long-read sequencing technology (48). The corresponding assemblies have been deposited at GenBank under the accession number MVNS00000000. Illumina sequencing and assembly of the 1104-6 genome was performed as previously described for 1104-7 (47). The 1104-6 assembly was deposited at GenBank under the accession number JAFJZV000000000. A local BLASTN search was used to detect potential sequence variation between 1104-7 and 1104-6 at the MAT, pheromone, and pheromone receptor loci.
Genetic manipulations.
Gene deletion mutants were generated by protoplast transformation with split-marker constructs generated by overlap PCR. Protoplast preparation and genetic transformation were performed in the same way as previously described (29), and gene deletion mutants were identified by PCR screening of hygromycin-resistant transformants. For each gene, at least two independent deletion mutants were used for phenotypic analysis. To minimize interference from genetic transformation, only mutant phenotypes shared by both deletion strains were considered to have arisen from the targeted gene deletion. To achieve nuclear fluorescent protein labeling, a knock-in strategy was applied to insert a reporter gene (gfp or mrfp) fused with a hygromycin or G418 resistance selection marker at the C-terminal region of the histone H1 gene (see Fig. S1 in the supplemental material). Knock-in constructs were generated by overlap PCR. P-His-GFP-ΔSCD1 strain and M-His-GFP-ΔPKS1 strain were further generated by independent genetic transformations of the corresponding plus and minus-derived His-GFP strains, respectively. Genetic strains generated and used in this study are listed in Table 2.
TABLE 2.
Fungal strains used in this study
| Strain | Brief description | Reference or source |
|---|---|---|
| 1104-7 (P) | Plus wild type | 47 |
| 1104-6 (M) | Minus wild type | 29 |
| P-His-GFP (1104-7-hisH1-GFP) | Plus, histone H1 C-terminal GFP-hygR fusion | This study |
| M-His-mRFP (1104-6-hisH1-mRFP) | Minus, histone H1 C-terminal mRFP-hygR fusion | This study |
| P-His-GFP-ΔSCD1 | Plus, histone H1 C-terminal GFP-G418R fusion, scd1 gene knockout | This study |
| M-His-GFP-ΔPKS1 | Minus, histone H1 C-terminal GFP-G418R fusion, pks1 gene knockout | This study |
| Δhos2-4 | Minus, hos2 gene deletion mutant | This study |
| Δhos2-5 | Minus, hos2 gene deletion mutant | This study |
| Δgpcr-19 | Minus, gpcr1 (FGSG_05239 homolog) gene deletion mutant | This study |
| Δgpcr-20 | Minus, gpcr1 (FGSG_05239 homolog) gene deletion mutant | This study |
| Δsnt2-35 | Minus, snt2 gene deletion mutant | This study |
| Δsnt2-39 | Minus, snt2 gene deletion mutant | This study |
| Δpmk1-37 | Minus, pmk1 gene deletion mutant | 29 |
| Δpmk1-38 | Minus, pmk1 gene deletion mutant | 29 |
Phenotypic characterization.
Strains were cultured on PDA and oatmeal agar (OA) at room temperature (approximately 25°C) to observe colony morphology. To examine ascospore development, individual perithecia were picked up with the aid of a dissecting microscope, squashed, mounted in water, and observed under light, epifluorescent, or laser confocal microscope illumination. Quantification of perithecium density and perithecium size was performed in the same way as previously described (29). To induce mating line formation, mycelial plugs derived from plus and minus strains were inoculated on the same OA or PDA culture plate 2 cm apart and incubated at room temperature (approximately 25°C) in constant darkness. To observe the microscopic dynamics of mating line development, mycelial plugs derived from plus and minus strains were inoculated on cellophane overlaid on PDA or OA medium. After sufficient oriented fungal growth, the cellophane layer bearing fungal tissues along the mating line were peeled off and examined microscopically. To analyze the phenotypic stability of 1104-7 and 1104-6 during sexual reproduction, single-ascospore progenies were obtained with a dilution culture approach, these cultures were grown on PDA, and their morphological phenotypes were directly observed.
Data availability.
The 1104-7 and 1104-6 genome assemblies have been deposited at GenBank under the accession number MVNS00000000 and JAFJZV000000000, respectively.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (2016YFD0201132), National Natural Science Foundation of China (32072374 and 31772113), National Natural Science Foundation of Shaanxi (2020JQ-255), the China Postdoctoral Science Foundation (2016M592844), the Shaanxi Postdoctoral Science Foundation (2016BSHEDZZ115), and the China Agriculture Research System (CARS-27).
Footnotes
Supplemental material is available online only.
Contributor Information
Rong Zhang, Email: rongzh@nwsuaf.edu.cn.
Guangyu Sun, Email: sgy@nwsuaf.edu.cn.
Irina S. Druzhinina, Nanjing Agricultural University
REFERENCES
- 1.Ni M, Feretzaki M, Sun S, Wang X, Heitman J. 2011. Sex in fungi. Annu Rev Genet 45:405–430. 10.1146/annurev-genet-110410-132536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang S, Staben C. 1994. Directed replacement of mt A by mt a-1 effects a mating type switch in Neurospora crassa. Genetics 138:75–81. 10.1093/genetics/138.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon PF, Damm U, Johnston PR, Weir BS. 2012. Colletotrichum–current status and future directions. Stud Mycol 73:181–213. 10.3114/sim0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crouch J, O’Connell R, Gan P, Buiate E, Torres MF, Beirn L, Shirasu K, Vaillancourt L. 2014. The genomics of Colletotrichum, p 69–102. In Dean AR, Lichens-Park A, Kole C (ed), Genomics of plant-associated fungi: monocot pathogens. Springer Press, Berlin, Germany. [Google Scholar]
- 5.Cisar CR, TeBeest DO. 1999. Mating system of the filamentous ascomycete, Glomerella cingulata. Curr Genet 35:127–133. 10.1007/s002940050441. [DOI] [PubMed] [Google Scholar]
- 6.Menat J, Cabral AL, Vijayan P, Wei Y, Banniza S. 2012. Glomerella truncata: another Glomerella species with an atypical mating system. Mycologia 104:641–649. 10.3852/10-265. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Guerra R, Ramírez-Rueda MT, Cabral-Enciso M, García-Serrano M, Lira-Maldonado Z, Guevara-González RG, González-Chavira M, Simpson J. 2005. Heterothallic mating observed between Mexican isolates of Glomerella lindemuthiana. Mycologia 97:793–803. 10.3852/mycologia.97.4.793. [DOI] [PubMed] [Google Scholar]
- 8.Vaillancourt L, Du M, Wang J, Rollins J, Hanau R. 2000. Genetic analysis of cross fertility between two self-sterile strains of Glomerella graminicola. Mycologia 92:430–435. 10.2307/3761501. [DOI] [Google Scholar]
- 9.García-Serrano M, Laguna EA, Simpson J, Rodríguez-Guerra R. 2008. Analysis of the MAT1-2-1 gene of Colletotrichum lindemuthianum. Mycoscience 49:312–317. 10.1007/S10267-008-0424-6. [DOI] [Google Scholar]
- 10.Wheeler HE. 1954. Genetics and evolution of heterothallism in Glomerella. Phytopathology 44:342–345. [Google Scholar]
- 11.Edgerton CW. 1908. The physiology and development of some anthracnoses. Bot Gaz 45:367–408. 10.1086/329594. [DOI] [Google Scholar]
- 12.Edgerton CW. 1914. Plus and minus strains in the genus Glomerella. Am J Bot 1:244–254. 10.2307/2435256. [DOI] [Google Scholar]
- 13.Andes JO. 1941. Experiments on the inheritance of the “plus” and “minus” characters in Glomerella cingulata. Bull Torrey Bot Club 68:609–614. 10.2307/2481749. [DOI] [Google Scholar]
- 14.Edgerton CW, Chilton SJP, Lucas GB. 1945. Genetics of Glomerella. II. Fertilization between strains. Am J Bot 32:115–118. 10.2307/2437529. [DOI] [Google Scholar]
- 15.Lucas GB, Chilton SJP, Edgerton CW. 1944. Genetics of Glomerella. I. Studies on the behavior of certain strains. Am J Bot 31:233–239. 10.2307/2437611. [DOI] [Google Scholar]
- 16.Chilton SJP, Wheeler HE. 1949. Genetics of Glomerella. VII. Mutation and segregation in plus cultures. Am J Bot 36:717–721. 10.2307/2438224. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler HE. 1950. Genetics of Glomerella. VIII. A genetic basis for the occurrence of minus mutants. Am J Bot 37:304–312. 10.2307/2437851. [DOI] [Google Scholar]
- 18.Chilton SJP, Lucas GB, Edgerton CW. 1945. Genetics of Glomerella. III. Crosses with a conidial strain. Am J Bot 32:549–554. 10.2307/2437685. [DOI] [Google Scholar]
- 19.Driver CH, Wheeler HE. 1955. A sexual hormone in Glomerella. Mycologia 47:311–316. 10.2307/3755453. [DOI] [Google Scholar]
- 20.Wheeler HE. 1956. Linkage groups in Glomerella. Am J Bot 43:1–6. 10.2307/2438406. [DOI] [Google Scholar]
- 21.Kubo Y, Takano Y, Endo N, Yasuda N, Tajima S, Furusawa I. 1996. Cloning and structural analysis of the melanin biosynthesis gene SCD1 encoding scytalone dehydratase in Colletotrichum lagenarium. Appl Environ Microbiol 62:4340–4344. 10.1128/AEM.62.12.4340-4344.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perpetua NS, Kubo Y, Yasuda N, Takano Y, Furusawa I. 1996. Cloning and characterization of a melanin biosynthetic THR1 reductase gene essential for appressorial penetration of Colletotrichum lagenarium. Mol Plant Microbe Interact 9:323–329. 10.1094/mpmi-9-0323. [DOI] [PubMed] [Google Scholar]
- 23.Tsuji G, Kenmochi Y, Takano Y, Sweigard J, Farrall L, Furusawa I, Horino O, Kubo Y. 2000. Novel fungal transcriptional activators, Cmr1p of Colletotrichum lagenarium and pig1p of Magnaporthe grisea, contain Cys2His2 zinc finger and Zn(II)2Cys6 binuclear cluster DNA-binding motifs and regulate transcription of melanin biosynthesis genes in a developmentally specific manner. Mol Microbiol 38:940–954. 10.1046/j.1365-2958.2000.02181.x. [DOI] [PubMed] [Google Scholar]
- 24.McGahen JW, Wheeler HE. 1951. Genetics of Glomerella. IX. Perithecial development and plasmogamy. Am J Bot 38:610–617. 10.2307/2437771. [DOI] [Google Scholar]
- 25.Wilson AM, Wilken PM, van der Nest MA, Wingfield MJ, Wingfield BD. 2019. It's all in the genes: the regulatory pathways of sexual reproduction in filamentous ascomycetes. Genes 10:330. 10.3390/genes10050330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayram Ö, Bayram ÖS, Ahmed YL, Maruyama J, Valerius O, Rizzoli SO, Ficner R, Irniger S, Braus GH. 2012. The Aspergillus nidulans MAPK module AnSte11-Ste50-Ste7-Fus3 controls development and secondary metabolism. PLoS Genet 8:e1002816. 10.1371/journal.pgen.1002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolting N, Pöggeler S. 2006. A STE12 homologue of the homothallic ascomycete Sordaria macrospora interacts with the MADS box protein MCM1 and is required for ascosporogenesis. Mol Microbiol 62:853–868. 10.1111/j.1365-2958.2006.05415.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim HK, Jo SM, Kim GY, Kim DW, Kim YK, Yun SH. 2015. A large-scale functional analysis of putative target genes of mating-type loci provides insight into the regulation of sexual development of the cereal pathogen Fusarium graminearum. PLoS Genet 11:e1005486. 10.1371/journal.pgen.1005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang X, Wei T, Cao M, Zhang X, Liu W, Kong Y, Zhang R, Sun G. 2019. The MAP kinase CfPMK1 is a key regulator of pathogenesis, development, and stress tolerance of Colletotrichum fructicola. Front Microbiol 10:1070. 10.3389/fmicb.2019.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barcelos QL, Pinto JMA, Vaillancourt LJ, Souza EA. 2014. Characterization of Glomerella strains recovered from anthracnose lesions on common bean plants in Brazil. PLoS One 9:e90910. 10.1371/journal.pone.0090910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheeler HE, Driver CH, Campa C. 1959. Cross- and self-fertilization in Glomerella. Am J Bot 46:361–365. 10.2307/2439196. [DOI] [Google Scholar]
- 32.Weir BS, Johnston PR, Damm U. 2012. The Colletotrichum gloeosporioides species complex. Stud Mycol 73:115–180. 10.3114/sim0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett RJ, Turgeon BG. 2016. Fungal sex: the Ascomycota. Microbiol Spectr 4:10.1128/microbiolspec.FUNK-0005-2016. 10.1128/microbiolspec.FUNK-0005-2016. [DOI] [PubMed] [Google Scholar]
- 34.Perkins DD. 1987. Mating-type switching in filamentous ascomycetes. Genetics 115:215–216. 10.1093/genetics/115.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu L, Jardini TM, Chen W. 2016. Direct repeat-mediated DNA deletion of the mating type MAT1-2 genes results in unidirectional mating type switching in Sclerotinia trifoliorum. Sci Rep 6:27083. 10.1038/srep27083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chitrampalam P, Inderbitzin P, Maruthachalam K, Wu BM, Subbarao KV. 2013. The Sclerotinia sclerotiorum mating type locus (MAT) contains a 3.6-kb region that is inverted in every meiotic generation. PLoS One 8:e56895. 10.1371/journal.pone.0056895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilken PM, Steenkamp ET, Wingfield MJ, de Beer ZW, Wingfield BD. 2014. DNA loss at the Ceratocystis fimbriata mating locus results in self-sterility. PLoS One 9:e92180. 10.1371/journal.pone.0092180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witthuhn RC, Harrington TC, Wingfield BD, Steimel JP, Wingfield MJ. 2000. Deletion of the MAT-2 mating-type gene during uni-directional mating-type switching in Ceratocystis. Curr Genet 38:48–52. 10.1007/s002940000131. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Leslie JF, Bowden RL. 2008. Expression and function of sex pheromones and receptors in the homothallic ascomycete Gibberella zeae. Eukaryot Cell 7:1211–1221. 10.1128/EC.00272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayrhofer S, Weber JM, Pöggeler S. 2006. Pheromones and pheromone receptors are required for proper sexual development in the homothallic ascomycete Sordaria macrospora. Genetics 172:1521–1533. 10.1534/genetics.105.047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vitale S, Di Pietro A, Turrà D. 2019. Autocrine pheromone signalling regulates community behaviour in the fungal pathogen Fusarium oxysporum. Nat Microbiol 4:1443–1449. 10.1038/s41564-019-0456-z. [DOI] [PubMed] [Google Scholar]
- 42.Seo JA, Han KH, Yu JH. 2004. The gprA and gprB genes encode putative G protein-coupled receptors required for self-fertilization in Aspergillus nidulans. Mol Microbiol 53:1611–1623. 10.1111/j.1365-2958.2004.04232.x. [DOI] [PubMed] [Google Scholar]
- 43.Hou Z, Xue C, Peng Y, Katan T, Kistler HC, Xu JR. 2002. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol Plant Microbe Interact 15:1119–1127. 10.1094/MPMI.2002.15.11.1119. [DOI] [PubMed] [Google Scholar]
- 44.Li D, Bobrowicz P, Wilkinson HH, Ebbole DJ. 2005. A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 170:1091–1104. 10.1534/genetics.104.036772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen GC, Flores-Vergara MA, Krasynanski S, Kumar S, Thompson WF. 2006. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc 1:2320–2325. 10.1038/nprot.2006.384. [DOI] [PubMed] [Google Scholar]
- 46.Liang X, Shang S, Dong Q, Wang B, Zhang R, Gleason ML, Sun G. 2018. Transcriptomic analysis reveals candidate genes regulating development and host interactions of Colletotrichum fructicola. BMC Genomics 19:557–557. 10.1186/s12864-018-4934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang X, Wang B, Dong Q, Li L, Rollins JA, Zhang R, Sun G. 2018. Pathogenic adaptations of Colletotrichum fungi revealed by genome wide gene family evolutionary analyses. PLoS One 13:e0196303. 10.1371/journal.pone.0196303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang X, Cao M, Li S, Kong Y, Rollins JA, Zhang R, Sun G. 2020. Highly contiguous genome resource of Colletotrichum fructicola generated using long-read sequencing. Mol Plant Microbe Interact 33:790–793. 10.1094/MPMI-11-19-0316-A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aem.00474-21-s0001.pdf, PDF file, 1.1 MB (981.3KB, pdf)
Data Availability Statement
The 1104-7 and 1104-6 genome assemblies have been deposited at GenBank under the accession number MVNS00000000 and JAFJZV000000000, respectively.