ABSTRACT
To test the hypothesis that the addition of an aminoglycoside to a β-lactam antibiotic could provide better outcomes than β-lactam monotherapy for the initial empirical treatment of hematological neutropenic patients with subsequently documented Gram-negative bacillus (GNB) bloodstream infection (BSI), a multinational, retrospective, cohort study of GNB BSI episodes in hematological neutropenic patients in six centers (2010 to 2017) was conducted. Combination therapy (β-lactam plus aminoglycoside) was compared to β-lactam monotherapy. The primary endpoint was the case fatality rate, assessed at 7 and 30 days from BSI onset. Secondary endpoints were nephrotoxicity and persistent BSI. Propensity score (PS) matching was performed. Among 542 GNB BSI episodes, 304 (56%) were initially treated with combination therapy, with cefepime plus amikacin being most common (158/304 [52%]). Overall, Escherichia coli (273/304 [50.4%]) was the main etiological agent, followed by Pseudomonas aeruginosa, which predominated in the combination group (76/304 [25%] versus 28/238 [11.8%]; P < 0.001). Multidrug resistance rates were similar between groups (83/294 [28.2%] versus 63/233 [27%]; P = 0.95). In the multivariate analysis, combination therapy was associated with a lower 7-day case fatality rate (odds ratio [OR], 0.37; 95% CI, 0.14 to 0.91; P = 0.035) with a tendency toward lower mortality at 30 days (OR, 0.56; 95% CI, 0.29 to 1.08; P = 0.084). After PS matching, these differences remained for the 7-day case fatality rate (OR, 0.33; 95% CI, 0.13 to 0.82; P = 0.017). In addition, aminoglycoside use was not significantly associated with renal function impairment (OR, 1.12; 95% CI, 0.26 to 4.87; P = 0.9). The addition of an aminoglycoside to the initial empirical therapy regimen for febrile neutropenic hematological patients should be considered.
KEYWORDS: febrile neutropenia, hematological patients, aminoglycosides, combination empirical treatment, Gram-negative bloodstream infection
INTRODUCTION
Patients with hematological malignancies are at high risk of Gram-negative bacillus bloodstream infections (GNB BSI), especially those with severe and prolonged neutropenia. Multidrug-resistant (MDR) GNB BSI, including those caused by extended-spectrum β-lactamase (ESBL)-producing Enterobacterales and carbapenem-resistant (CR) GNB, are increasing worldwide in cancer patients (1–3). This is of special concern in hematological neutropenic patients, in whom a delay in initiating adequate empirical antibiotic therapy can negatively affect outcomes (4–6). Currently, the optimal initial empirical therapy for high-risk hematological patients with febrile neutropenia (FN) remains a matter of intense debate.
A prospective study including patients with severe GNB BSI found reduced mortality in the subgroup of neutropenic patients who received empirical combination therapy with a β-lactam plus an aminoglycoside (7). However, a later meta-analysis by the same group failed to confirm the benefit of combination therapy over β-lactam monotherapy in neutropenic cancer patients (8). Of note, whereas the 2011 Infectious Diseases Society of America (IDSA) guidelines advocate monotherapy with an antipseudomonal β-lactam for FN treatment (9), the most recent European Conference on Infections in Leukaemia (ECIL) guidelines recommend escalation or de-escalation strategies based on the clinical presentation and individual risk of MDR infections (10). The administration of a β-lactam plus an aminoglycoside facilitates using noncarbapenem β-lactams while ensuring broad empirical antibiotic coverage that can later be narrowed according to microbiological results. In fact, reducing carbapenem use is a cornerstone of limiting the development of antibiotic resistance.
In the current era of widespread antimicrobial resistance, it can be hypothesized that adding an aminoglycoside to a β-lactam may broaden the antibiotic spectrum, improve empirical treatment adequacy, and thereby reduce mortality (11). Moreover, aminoglycosides have pharmacological properties that offer additional advantages, such as their rapid bactericidal activity, postantibiotic effect, and potential synergy with β-lactams (12). The main limitation of this strategy is the potential for aminoglycoside-associated nephrotoxicity (13). However, it is difficult to determine aminoglycosides’ net contribution to nephrotoxicity because relevant confounders, such as septic shock, prolonged treatment courses, and the use of other nephrotoxic agents, may overestimate this adverse event (14). Therefore, a short-course aminoglycoside treatment could reliably improve outcomes without significantly affecting renal function.
We aimed to test the hypothesis that the addition of a short-course aminoglycoside regimen to a broad-spectrum β-lactam could provide better outcomes than β-lactam monotherapy, without significant renal function impairment, for the empirical treatment of hematological neutropenic patients with GNB BSI.
RESULTS
Clinical characteristics and BSI etiology.
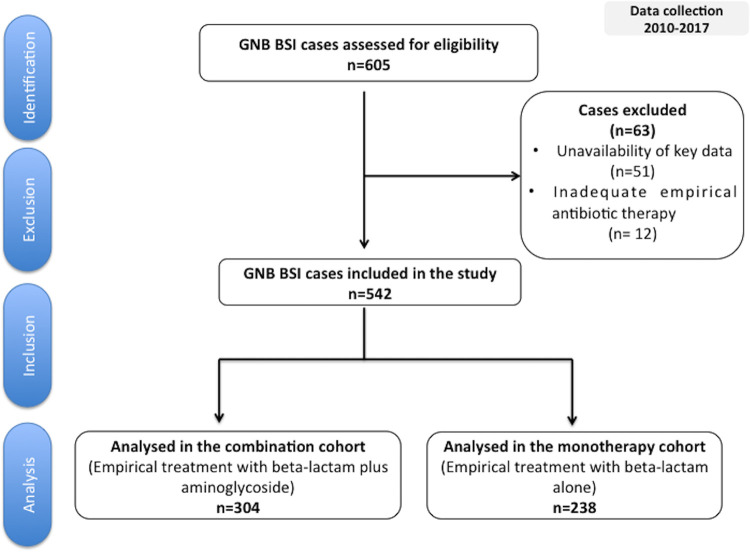
Among the 605 eligible episodes, 63 met at least one of the exclusion criteria and were excluded from analysis, leaving 542 included episodes (Fig. 1). Table 1 shows the episodes’ baseline characteristics. Patients in the monotherapy group had more comorbidities, including diabetes mellitus, and were more likely to have received previous corticosteroids. Conversely, more patients in the combination group presented with severe neutropenia and septic shock when compared to the monotherapy group. Nevertheless, all the above-mentioned variables were balanced between groups in the propensity score (PS)-matched cohort (see Table S4 in the supplemental material).
FIG 1.
Selection flowchart for episodes of bloodstream infection caused by Gram-negative bacilli. Abbreviations: GNB, Gram-negative bacilli; BSI, bloodstream infection.
TABLE 1.
Baseline characteristics of patients with bloodstream infections due to Gram-negative bacilli by treatment group
| Parametera | Value for study populationb |
Pc | ||
|---|---|---|---|---|
| Total (n = 542) | Combination therapy (n = 304 [56%]) |
Monotherapy (n = 238 [44%]) | ||
| No. (%) at center | ||||
| Bellvitge University Hospital | 216 (39.8) | 168 (77.8) | 48 (22.2) | <0.001 |
| Hospital Clínic I Provincial de Barcelona | 128 (23.6) | 73 (57) | 55 (43) | 0.885 |
| Hacettepe University School of Medicine | 82 (15.1) | 1 (1.2) | 81 (98.8) | <0.001 |
| Hospital General Universitario Gregorio Marañón | 53 (9.8) | 39 (73.6) | 14 (26.4) | 0.011 |
| Centro de Educación Médica e Investigaciones Clínicas | 32 (5.9) | 9 (28.1) | 23 (71.9) | 0.002 |
| Ramón y Cajal Hospital | 31 (5.7) | 14 (45.2) | 17 (54.8) | 0.282 |
| Age (yr) (mean ± SD) | 53.7 ± 16.3 | 54.2 ± 15.5 | 53.1 ± 17.3 | 0.48 |
| No. (%) of males | 311 (57.4) | 179 (58.9) | 132 (55.5) | 0.48 |
| No. (%) with: | ||||
| Comorbiditiesd | 121 (22.4) | 42 (13.8) | 79 (33.3) | <0.001 |
| Diabetes mellitus | 41 (7.6) | 15 (4.9) | 26 (10.9) | 0.01 |
| Acute leukemia | 251 (46.3) | 133 (43.8) | 118 (49.6) | 0.20 |
| HSCT | 160 (29.5) | 87 (28.6) | 73 (30.7) | 0.67 |
| Previous corticosteroid treatment (1 mo) | 252 (46.7) | 127 (41.9) | 125 (52.7) | 0.02 |
| Duration of previous neutropenia (days) (median [IQR]) | 4 [2–10] | 4 [2–9] | 5 [3–11.5] | 0.001 |
| No. (%) with: | ||||
| Profound neutropenia at presentation (<100 neutrophils/mm3) | 429 (80.3) | 261 (85.9) | 168 (73) | <0.001 |
| Previous antibiotic treatment (1 mo) | 268 (49.6) | 145 (47.7) | 123 (52.1) | 0.35 |
| Quinolone prophylaxis | 165 (30.5) | 74 (24.3) | 91 (38.4) | 0.001 |
| Previous BSI episode | 39 (7.2) | 18 (5.9) | 21 (8.8) | 0.3 |
| Previous hospital admission (1 mo) | 384 (71.6) | 230 (76.2) | 154 (65.8) | 0.01 |
| Kidney failure at presentation (GFR < 60 ml/min/1.73 m2) | 88 (16.3) | 46 (15.2) | 42 (17.7) | 0.49 |
| Concomitant nephrotoxic treatment | 90 (16.6) | 50 (16.4) | 40 (16.8) | 1 |
| Nosocomial acquisition | 353 (65.4) | 196 (64.5) | 157 (66.5) | 0.68 |
| High-risk BSI | 145 (26.9) | 86 (28.3) | 59 (25.0) | 0.682 |
| High-risk MASCC index score (<21) | 289 (53.3) | 155 (51) | 134 (56.3) | 0.25 |
| Septic shock at presentation | 104 (19.3) | 71 (23.4) | 33 (14) | 0.008 |
| Source of BSI | ||||
| Endogenous | 236 (43.7) | 139 (45.7) | 97 (41.1) | 0.32 |
| Unknown origin | 75 (13.8) | 41 (13.5) | 34 (14.3) | 0.89 |
| Catheter infection | 53 (9.8) | 21 (6.9) | 32 (13.6) | 0.015 |
| Urinary tract infection | 33 (6.11) | 17 (5.6) | 16 (6.8) | 0.7 |
| Respiratory tract infection | 32 (5.9) | 18 (5.9) | 14 (5.9) | 1 |
| Neutropenic enterocolitis | 28 (5.2) | 20 (6.6) | 8 (3.4) | 0.14 |
| Perianal infection | 24 (4.4) | 15 (4.9) | 9 (3.8) | 0.67 |
| Skin and soft tissue infection | 17 (3.1) | 8 (2.6) | 9 (3.8) | 0.59 |
| Mucositis | 12 (2.2) | 8 (2.6) | 4 (1.7) | 0.66 |
| Other sources | 32 (5.9) | 17 (5.6)e | 15 (6.3)f | 0.87 |
Abbreviations: BSI, bloodstream infection; HSCT, hematopoietic stem cell transplant; GFR, glomerular filtration rate; MASCC, Multinational Association for Supportive Care in Cancer.
Qualitative data are expressed as number (percent), unless otherwise indicated. Quantitative data are expressed as means ± standard deviation (SD) or median and interquartile range (IQR, 25th to 75th percentiles), as appropriate.
Qualitative data were tested by the chi-square test. Quantitative data expressed as means ± SD were tested by the t test, and quantitative data expressed as medians and IQR were tested by the Kruskal-Wallis test.
Comorbidities are defined as the presence of one or more of the following diseases: chronic obstructive pulmonary disease, heart or hepatic disease, diabetes mellitus, renal failure, and cerebrovascular disease.
Includes peritonitis (n = 1), cholangitis (n = 1), other abdominal infections (n = 11), and sinusitis (n = 4).
Includes peritonitis (n = 2), cholangitis (n = 1), other abdominal infections (n = 11), and odontogenic infection (n = 1).
Microbiological results are detailed in Table 2. We observed no differences regarding BSI etiology between groups with the exception of Pseudomonas aeruginosa isolates, which were more frequently identified in the combination group.
TABLE 2.
Isolated microorganisms in patients with bloodstream infections due to Gram-negative bacilli by treatment group
| Organism or infectiona | No. (%) in study population |
Pb | ||
|---|---|---|---|---|
| Total (n = 542) | Combination therapy (n = 304) |
Monotherapy (n = 238) |
||
| Escherichia coli | 273 (50.4) | 147 (48.4) | 126 (52.9) | 0.33 |
| Pseudomonas aeruginosa | 104 (19.2) | 76 (25) | 28 (11.8) | <0.001 |
| Klebsiella pneumoniae | 98 (18.1) | 52 (17.1) | 46 (19.3) | 0.58 |
| Enterobacter cloacae | 26 (4.8) | 12 (3.95) | 14 (5.88) | 0.4 |
| Other | 41 (7.6) | 17 (5.6) | 24 (10) | 0.072 |
| MDR BSIc | 146 (27.7) | 83 (28.2) | 63 (27) | 0.95 |
| E. coli | 78 (14.8) | 40 (13.6) | 38 (16.3) | 0.25 |
| K. pneumoniae | 29 (5.5) | 13 (4.4) | 16 (6.7) | 0.26 |
| P. aeruginosa | 29 (5.5) | 25 (8.5) | 4 (1.7) | 0.001 |
| Other | 10 (1.9) | 5 (1.7) | 5 (2.1) | 0.7 |
Abbreviations: BSI, bloodstream infection; MDR, multidrug resistant.
Qualitative data were tested by the chi-square test.
Percentages are calculated based on the available data regarding MDR BSI (total study population, n = 527; monotherapy population, n = 233; combination therapy population, n = 294).
Overall, 146 episodes were caused by MDR strains, with ESBL production being present in 66 (45.2%) of them. When the BSI episodes caused by Escherichia coli and Klebsiella pneumoniae were analyzed, 41/273 isolates (15%) of the former and 21/98 isolates (21.4%) of the latter were ESBL producers. Carbapenemase expression was identified in 12/146 isolates (8.2%), particularly in K. pneumoniae (8/12).
Focusing on P. aeruginosa, 29/104 isolates (27.8%) were MDR, and among these, 25/29 (86.2%) were CR. However, only 4/29 isolates (13.8%) were carbapenemase producers. Among the 25 remaining non-carbapenemase-producing P. aeruginosa isolates, 8/25 (32%) were MDR and 17/25 (68%) were extensively drug resistant. All MDR P. aeruginosa isolates tested were susceptible to amikacin. Of note, the rate of MDR P. aeruginosa BSI was significantly higher in the combination group.
Empirical antibiotic treatment.
The empirical antibiotic therapy is detailed in Table 3. Carbapenems were predominantly used in the monotherapy group, whereas cefepime and piperacillin-tazobactam were more frequently administered in the combination group. In the combination group, 56/304 episodes (18.4%) received only one active antibiotic, with the aminoglycoside being the active drug in 47/56 (83.9%) of them. Detailed information regarding the 47 episodes in which patients received an aminoglycoside as the only active antibiotic in the combination group is provided in Table S10.
TABLE 3.
Treatment regimens of patients with bloodstream infections due to Gram-negative bacilli by treatment group
| Treatment | No. (%) in study population |
Pa | ||
|---|---|---|---|---|
| Total (n = 542) | Combination therapy (n = 304) |
Monotherapy (n = 238) |
||
| β-Lactam antibiotic | ||||
| Meropenem | 173 (31.9) | 78 (25.7) | 95 (39.9) | 0.001 |
| Cefepime | 164 (30.3) | 158 (52) | 6 (2.5) | <0.001 |
| Piperacillin-tazobactam | 120 (22.1) | 46 (15.1) | 74 (31.1) | <0.001 |
| Imipenem-cilastatin | 67 (12.4) | 15 (4.9) | 52 (21.8) | <0.001 |
| Other β-lactams | 18 (3.3) | 7 (2.3)b | 11 (4.6)c | 0.210 |
| Aminoglycosidesd | 304 (56) | 304 (100) | 0 | |
Qualitative data were tested by the chi-square test. Quantitative data were tested by the Kruskal-Wallis test.
Includes ceftazidime (n = 4), aztreonam (n = 1), ceftolozane-tazobactam (n = 1), and ertapenem (n = 1).
Includes cefoperazone-sulbactam (n = 3), ceftazidime (n = 1), ceftriaxone (n = 3), doripenem (n = 1), ertapenem (n = 2), and amoxicillin-clavulanate (n = 1).
All episodes in the combination group received amikacin at a dose of 15 mg/kg once daily or equivalent dosing if renal failure was present. The median number of days of aminoglycoside treatment was 2 (IQR 1–3).
Study endpoints.
Table 4 details the study endpoints. In the multivariate analysis, empirical combination therapy was associated with a lower 7-day case fatality rate (adjusted odds ratio [aOR], 0.37; 95% confidence interval [CI], 0.14 to 0.91; P = 0.035) and showed a tendency toward lower mortality at 30 days (aOR, 0.56; 95% CI, 0.29 to 1.08; P = 0.084). Moreover, there was no significant renal function impairment (aOR, 1.77; 95% CI, 0.56 to 5.63; P = 0.3) after adjusting for baseline renal insufficiency and concomitant nephrotoxic agents. In addition, combination therapy was associated with a lower risk of persistent GNB BSI than monotherapy (aOR, 0.35; 95% CI, 0.12 to 0.89; P = 0.033). The intraclass correlation index, which measures the amount of variability explained by the hospital cluster effect, was 0.07 in the 7-day-mortality model and 0.20 in the 30-day-mortality and nephrotoxicity model. These figures suggest a low cluster effect in the 7-day-mortality model and a moderate effect in the 30-day-mortality and nephrotoxicity model. More detailed information regarding case fatality rates according to the active empirical antibiotic therapy is provided in Table S11.
TABLE 4.
Case fatality and nephrotoxicity rates at different assessment points
| Treatment | No. (%) in study population |
Pa | ||
|---|---|---|---|---|
| Total (n = 542) | Combination therapy (n = 304) | Monotherapy (n = 238) | ||
| 7-day case fatality rate | 49 (9) | 18 (5.92) | 31 (13) | 0.007 |
| 30-day case fatality rate | 115 (21.2) | 48 (15.8) | 67 (28.1) | 0.001 |
| Persistent BSIb | 24 (4.4) | 7 (2.4) | 17 (7.20) | 0.014 |
| Incidence of nephrotoxicity at end of antibiotic treatment | 40 (7.4) | 18 (5.9) | 22 (9.2) | 0.2 |
Qualitative data were tested by the chi-square test.
BSI: bloodstream infection. Percentages are calculated based on the available data regarding persistent BSI (total study population, n = 533; monotherapy population, n = 236; combination therapy population, n = 297).
For the PS analysis, we were able to match 147 pairs of episodes treated empirically with combination therapy or monotherapy. Baseline characteristics of the PS-matched cohort are detailed in Table S4. In this cohort, combination therapy was still associated with a lower 7-day case fatality rate (aOR, 0.33; 95% CI, 0.13 to 0.82; P = 0.017). In contrast, there was no association when the 30-day case fatality rate was assessed (aOR, 0.79; 95% CI, 0.41 to 1.51; P = 0.5). Persistent BSI could not be assessed due to the low number of events in the PS-matched cohort. Regarding nephrotoxicity, combination therapy continued to have no association with significant renal function impairment (aOR, 1.12; 95% CI, 0.26 to 4.87; P = 0.9).
The results obtained after performing the sensitivity analysis and an inverse probability of treatment weighting (IPTW)-based approach did not show significant differences compared to those observed with the PS analysis (Table S12 and Fig. S1).
DISCUSSION
In this multicenter, international observational study of a large cohort of neutropenic hematological patients with GNB BSI, adding a short-course aminoglycoside to the empirical therapy with a β-lactam significantly improved the 7-day case fatality rate, without significantly impairing renal function.
Our findings differ from those of other studies that failed to demonstrate a benefit from combination therapy on survival, including an important meta-analysis (8). Nevertheless, most of the randomized clinical trials (RCT) included in that meta-analysis were performed between 1983 and 2012, when the burden of bacterial resistance had not yet reached the levels we currently face (15). Thus, their results may not represent the true impact of combined treatment in the current era of multidrug resistance. Interestingly, our findings do agree with those reported in a later study of neutropenic cancer patients with BSI, where combination therapy was associated with lower mortality in hematological patients (16).
We observed relevant differences regarding the β-lactam used between treatment groups. These findings may be explained because the use of a combination therapy, based on the addition of an aminoglycoside, allows using a carbapenem-sparing β-lactam while ensuring a wide antibiotic coverage. This is of paramount importance, since a reduction of carbapenem consumption is a cornerstone in the fight against antimicrobial resistance. However, when monotherapy is used, carbapenems seem to be preferred, probably due to their activity against ESBL producers, which are currently the most prevalent multidrug-resistant microorganisms in our setting.
Importantly, the benefit of combined therapy for the 7-day case fatality rate remained statistically significant after PS-matching analysis. This is particularly relevant because the impact of an empirical antibiotic regimen on outcomes is probably better assessed by taking early mortality into consideration. These results may be explained by different factors. First, in the combination group, 47/304 episodes (15.5%) showed an improvement empirical treatment adequacy with the aminoglycoside addition. Of note, 26 of these 47 episodes (55.3%) were caused by P. aeruginosa, with the majority of them being CR strains. This is of special concern, since 11/26 (42.3%) of these episodes were empirically treated with carbapenems, and the 7-day case fatality rate was particularly high among them (9/10). An expected finding was that case fatality rates of patients who received aminoglycoside monotherapy were higher than those of patients treated with β-lactam monotherapy. However, if the aminoglycoside had not been added to the empirical treatment, an inadequate empirical antibiotic therapy would have been administered, leading to a clinical scenario associated with very high mortality rates. (4, 17). Second, despite the similar rates of multidrug resistance between groups, persistent BSI occurred less often in the combination group, suggesting an enhanced bactericidal effect that may facilitate microbiological and clinical cure (12, 18). In addition, the lowest mortality rate was observed in the combination group when both antibiotic agents were active against the causative microorganism.
It remains controversial whether a short-course aminoglycoside regimen can benefit severely ill patients. Notably, we found improved early mortality among patients receiving combination therapy, who presented more frequently with septic shock, consistent with other reports (19, 20). However, an important meta-analysis (21) and a recent prospective observational cohort study of 648 ICU patients (22) failed to show this association. In the latter, there was no association with a faster reversal of shock or an improved 14-day survival. However, that study mainly included general nonimmunocompromised patients, and only 4% received inadequate empirical antibiotic therapy, probably due to low local levels of antibiotic resistance (22). The potential nephrotoxicity of aminoglycosides is a major concern that limits their clinical use. However, its nephrotoxicity is usually mild when it is administered in a nephroprotective once-daily dosing regimen and in a short course (13, 23). As reported in the above-mentioned meta-analysis, the mean duration of aminoglycoside treatment in most of the included trials was >7 days (8), which could explain the resulting increased nephrotoxicity. In contrast, the median duration of aminoglycoside treatment in our study was only 2 days, which might explain the lack of renal impairment.
Finally, in the current era of emerging resistance, choosing the optimal initial empirical β-lactam according to individual risk of resistance is crucial. In this regard, the new available β-lactam antibiotics play a very important role.
This study benefitted from including a large cohort and having a multicenter design, facilitating generalization. In addition, to account for possible bias, logistic regression models were adjusted by PS analysis. Nevertheless, this study has some limitations that should be acknowledged. First, this was not an RCT; thus, the choice of therapy could have been influenced by several patient-related variables and the clinical presentation. In this regard, patients who received monotherapy had more comorbidities, including diabetes mellitus, which could lead to bias if clinicians considered these patients more susceptible to developing nephrotoxicity and, consequently, avoided the use of aminoglycosides. However, the presence of renal function impairment at baseline is a well-known factor associated with increased nephrotoxicity, and in our cohort, the rate of kidney failure at baseline was not significantly different between treatment groups, which may reduce the potential treatment bias.
Second, the planned sample size was not achieved in the monotherapy group, because the final number of episodes included in the combination group was higher than initially expected. However, the difference in 30-day case fatality between treatment groups was slightly higher than expected, so the study was not underpowered to detect clinically relevant differences. Third, we included only patients with a documented BSI episode, so the results may not be applicable to all febrile neutropenic patients. Fourth, some information may have been lost due to the retrospective design, and we may not have adequately controlled for certain confounders. Fifth, the commonly used antimicrobial susceptibility testing methods may have not been able to detect piperacillin-tazobactam resistance. Finally, despite the differences among centers regarding the number of included episodes and empirical treatments, these intervariability differences had only a moderate influence on the 30-day case fatality and nephrotoxicity rates at the end of treatment.
In conclusion, we found that therapy with a broad-spectrum β-lactam plus a short-course aminoglycoside regimen significantly improved the 7-day case fatality rate for the empirical treatment of GNB BSI in hematological neutropenic patients, with no significant renal function impairment. In addition to choosing the optimal empirical β-lactam according to individual risk of resistance, our results support reconsidering the inclusion of short-course aminoglycoside therapy in combination with the β-lactam for the treatment of febrile hematological neutropenic patients. Further RCTs are warranted to assess the impact of such combination therapy in these high-risk patients.
MATERIALS AND METHODS
Study design and setting.
This was a multicenter, retrospective cohort study of prospectively collected data, conducted from January 2010 to August 2017 in six university hospitals in Spain (4 centers), Argentina (1 center), and Turkey (1 center).
Ethics.
The study was approved by the Institutional Review Board at Bellvitge University Hospital (reference number EPA052/17) and by the local research ethics committees of participating centers. It was conducted according to the Declaration of Helsinki guidelines. The need for informed consent was waived by the Clinical Research Ethics Committee due to the retrospective design. The study results are reported following the STROBE recommendations (24).
Participants.
All adult (≥18 years old) hematological neutropenic patients, including hematopoietic stem cell transplant (HSCT) recipients, were eligible for the study if they were diagnosed with at least one episode of GNB BSI for which they received adequate initial empirical antibiotic therapy for at least 48 h. Empirical antibiotic therapy was classified into a combination therapy group (β-lactam plus aminoglycoside) and a monotherapy group (β-lactam). The exclusion criteria were unavailability of key data (related to empirical therapy and death), receipt of inadequate empirical antibiotic therapy, use of a combination other than a β-lactam plus aminoglycoside, and death within the first 48 h from BSI onset. The follow-up period was 30 days from BSI onset.
Variables.
Data regarding baseline characteristics, clinical and microbiological features, and endpoints were collected. Creatinine and glomerular filtration rate were recorded at the moment of initiation and at the end of antibiotic treatment. Empirical antibiotic therapy was defined as antibiotic therapy administered before reception of definitive susceptibility results. Adequate empirical antibiotic therapy was defined as treatment of BSI episodes with at least one in vitro active antibiotic against the causative pathogen for at least 48 h. In the monotherapy group, all episodes were treated exclusively with an empirical β-lactam, which was active against the infecting microorganism. In the combination group, adequate empirical combination therapy was considered to have occurred when the β-lactam and/or the aminoglycoside was active against the causative microorganism and was administered for at least 48 h. In this group, receiving monotherapy with an active aminoglycoside was considered an adequate combination treatment. Inadequate treatment was considered when the empirical treatment did not include any antibiotic with in vitro activity. The antipseudomonal β-lactams were uniformly administered at the doses recommended by the international guidelines for the management of febrile neutropenia (9, 10): cefepime, 2 g/8 h; piperacillin-tazobactam, 4.5 g/6 h; meropenem, 1 g/8 h; imipenem-cilastatin, 500 mg/6 h; and ceftazidime, 2 g/8 h. In the case of renal impairment, the dosing was adjusted accordingly.
Endpoints.
The primary endpoint was the case fatality rate, assessed at 7 and 30 days from BSI onset. Secondary endpoints included the incidence of nephrotoxicity and persistent BSI. Nephrotoxicity incidence was considered in patients developing acute kidney injury, grade ≥2 (25), defined as an increase in serum creatinine value of ≥2.0 times the baseline, and was recorded at the termination of antibiotic treatment in both treatment groups. Persistent BSI was considered if blood cultures were positive after the first 48 h of adequate antibiotic therapy.
Microbiological studies.
Clinical samples were processed at the microbiology laboratories of each participating center in accordance with standard operating procedures. GNB were identified using standard microbiological techniques, and in vitro susceptibility was determined according to EUCAST recommendations, except at the center in Argentina, where CLSI cutoffs were used (26). MDR GNB were defined as described elsewhere (27). ESBL production was studied in isolates with reduced susceptibility to third-generation cephalosporins and was confirmed by phenotypic methods, such as disk diffusion or Etest, or molecular characterization by PCR, depending on the participating center. Isolates were considered carbapenemase producers if a carbapenemase gene was detected by a molecular method. Due to the retrospective nature of the study, the analysis of isolates was not able to be performed in a central microbiology laboratory, and it was performed in each participating center.
Definitions.
Patients diagnosed with acute myeloid or acute lymphoblastic leukemia were classified as having acute leukemia. Neutropenia was defined as an absolute neutrophil count of <0.5 × 109 cells/mm3. The Multinational Association for Supportive Care in Cancer (MASCC) score was calculated as described elsewhere (28). Previous corticosteroid treatment was defined as the administration of ≥20 mg of prednisone, or equivalent dosing, for at least 4 weeks within the last 30 days from BSI onset.
BSI sources were established using standard U.S. Centers for Disease Control and Prevention criteria for secondary BSI (29). In addition, we defined an endogenous source of BSI in neutropenic patients with absent or mild gastrointestinal symptoms, in whom gut translocation was suspected. Neutropenic enterocolitis was defined in patients with severe (grade III or IV) extensive mucositis, involving the upper and lower gastrointestinal tract. Mucositis was considered present in patients with ulcerative lesions involving only the oral cavity. An unknown source was considered when no clear source of BSI was identified due to mixed clinical presentations and/or lack of adequate complementary diagnostic procedures. BSIs were considered low risk when originating from the urinary tract, a catheter, or endogenous sources but high risk when originating from other sources.
Comorbidities were defined as the presence of one or more of the following diseases: chronic obstructive pulmonary disease, heart disease, hepatic disease, diabetes mellitus, renal failure, and cerebrovascular disease. Baseline renal failure was defined as a glomerular filtration rate of <60 ml/min/1.73 m2, measured by the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation. Vancomycin and colistin were considered concomitant nephrotoxic drugs if used during the study period.
Sample size.
For the whole cohort, we expected a similar number of episodes to be treated with both regimens. The 30-day case fatality rates in the monotherapy and combination groups were expected to be 25% and 15%, respectively. Thus, 278 episodes was the ideal minimum sample size required to perform an unadjusted test to reject the null hypothesis of equal case fatality rates between monotherapy and combination groups, with a power of 80% and a type I error of 5%. A 10% dropout rate was assumed for the sample size estimation.
Statistical analysis.
Continuous quantitative variables were compared using the Mann–Whitney U test or the t test, as appropriate. Categorical variables were compared using Fisher’s exact test or Pearson’s χ2 test as appropriate. Incidence rates and 95% confidence intervals (CIs) were reported.
To assess potential risk factors for mortality, logistic regression was used to calculate crude and multivariable adjusted ORs with their 95% CIs. Four logistic regression models were developed, with 7- and 30-day mortality, persistent BSI, and nephrotoxicity as dependent variables. To account for the clustered data within each hospital, a mixed-model approximation was assessed. The cluster effect was found to be relevant only for 30-day mortality and nephrotoxicity; thus, a logistic mixed-effects model for both outcomes was estimated. Missing imputation was not performed because missing key data was an exclusion criterion. Predefined independent variables were tested based on clinical plausibility, as were interactions considered clinically significant. The final multivariate model included age, sex, HSCT, acute leukemia (AL), comorbidities, neutrophil count (<100 or >100/mm3), MASCC index, MDR GNB BSI, corticosteroids, BSI risk, and the interaction between combination therapy and MDR GNB BSI. The Akaike information criterion was used to assess model goodness of fit.
A major source of bias in this study was the choice of empirical therapy. To address this and to limit the differences between treatment groups, we conducted PS matching based on the estimated probability that a patient would be in the combination group based on the clinical profile. We matched episodes from each treatment group with the nearest-neighbor algorithm, using a maximum tolerance distance between matched subjects of 0.2 standard deviation (30). The PS-confounding variables were age, gender, AL, HSCT, MASCC index, diabetes mellitus, comorbidities (yes/no), length of previous neutropenia (<7 days versus ≥7 days), BSI risk, BSI acquisition, quinolone prophylaxis, prior antibiotics, urinary catheter, corticosteroids, intensive care unit (ICU) admission (previous or during hospitalization), prior BSI, severe mucositis, parenteral nutrition, hypotension, septic shock, and renal failure at presentation. We identified the imbalanced variables between groups after PS matching by calculating the standardized mean difference and included these as covariates in subsequent logistic regression models. Analyses were performed with R software version 3.4.1. (31). All models used for the multivariate and PS analysis are detailed in Tables S1 to S8. In addition, and in order to further address the potential treatment bias, we performed an inverse probability of treatment weighting (IPTW) analysis and a sensitivity analysis by center, which are detailed in Tables S9 and Fig. S1.
ACKNOWLEDGMENTS
We thank the ESCMID Study Group for Bloodstream Infections, Endocarditis and Sepsis (ESGBIES) and the ESCMID Study Group for Immunocompromised Hosts (ESGICH) for supporting the study. We thank Centers de Recerca de Catalunya (CERCA) Program and Generalitat de Catalunya for institutional support.
F.H. received MSD and Pfizer speaker’s honoraria and Pfizer’s Research and Educational grants.
This study was supported by the Spanish Plan Nacional de I+D+i2013‐2016 and the Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI-RD16/0016/0001), cofinanced by European Development Regional Fund “A way to achieve Europe.” Operative Program Intelligent Growth 2014–2020.
A.A.-P., C.G., and J.C. were responsible for the study conception and design. Data collection was performed by A.A.-P., C.G., P.P.-A., M.C.A., M.M., F.H., P.M.-D., J.L.-A., C.C., M.A., A.A.-U., D.T., J.F., C.G.-V., P.M., A.B., H.P., S.M., and X.D.-M. N.P. and E.G.-L. were responsible for the statistical analysis. A.A.-P., C.G., and J.C. drafted and revised the manuscript. All authors approved the final version of the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Gudiol C, Bodro M, Simonetti A, Tubau F, González-Barca E, Cisnal M, Domingo-Domenech E, Jiménez L, Carratalà J. 2013. Changing aetiology, clinical features, antimicrobial resistance, and outcomes of bloodstream infection in neutropenic cancer patients. Clin Microbiol Infect 19:474–479. 10.1111/j.1469-0691.2012.03879.x. [DOI] [PubMed] [Google Scholar]
- 2.Baker TM, Satlin MJ. 2016. The growing threat of multidrug-resistant Gram-negative infections in patients with hematologic malignancies. Leuk Lymphoma 57:2245–2258. 10.1080/10428194.2016.1193859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satlin MJ, Cohen N, Ma KC, Gedrimaite Z, Soave R, Askin G, Chen L, Kreiswirth BN, Walsh TJ, Seo SK. 2016. Bacteremia due to carbapenem-resistant Enterobacteriaceae in neutropenic patients with hematologic malignancies. J Infect 73:336–345. 10.1016/j.jinf.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Nadal G, Puerta-Alcalde P, Gudiol C, Cardozo C, Albasanz-Puig A, Marco F, Laporte-Amargós J, Moreno-García E, Domingo-Domenech E, Chumbita M, Martinez JA, Soriano A, Carratalà J, García-Vidal C. 2020. Inappropriate empirical antibiotic treatment in high-risk neutropenic patients with bacteremia in the era of multidrug resistance. Clin Infect Dis 70:1068–1074. [DOI] [PubMed] [Google Scholar]
- 5.Trecarichi EM, Tumbarello M, Spanu T, Caira M, Fianchi L, Chiusolo P, Fadda G, Leone G, Cauda R, Pagano L. 2009. Incidence and clinical impact of extended-spectrum-β-lactamase (ESBL) production and fluoroquinolone resistance in bloodstream infections caused by Escherichia coli in patients with hematological malignancies. J Infect 58:299–307. 10.1016/j.jinf.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Lin MY, Weinstein RA, Hota B. 2008. Delay of active antimicrobial therapy and mortality among patients with bacteremia: impact of severe neutropenia. Antimicrob Agents Chemother 52:3188–3194. 10.1128/AAC.01553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leibovici L, Paul M, Poznanski O, Drucker M, Samra Z, Konigsberger H, Pitlik SD. 1997. Monotherapy versus β-lactam-aminoglycoside combination treatment for gram-negative bacteremia: a prospective, observational study. Antimicrob Agents Chemother 41:1127–1133. 10.1128/AAC.41.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul M, Dickstein Y, Schlesinger A, Grozinsky-Glasberg S, Soares-Weiser K, Leibovici L. 2013. Beta-lactam versus beta-lactam-aminoglycoside combination therapy in cancer patients with neutropenia. Cochrane Database Syst Rev 2013:CD003038. 10.1002/14651858.CD003038.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JH, Wingard JR. 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis 52:e56–e93. 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 10.Averbuch D, Orasch C, Cordonnier C, Livermore DM, Viscoli C, Gyssens IC, Kern WV, Klyasova G, Marchetti O, Engelhard D, Akova M. 2013. European guidelines for emperical antibacterial therapy for febrile neutropenic patients in the era of growing resistance. Haematologica 98:1826–1835. 10.3324/haematol.2013.091025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat S, Fujitani S, Potoski BA, Capitano B, Linden PK, Shutt K, Paterson DL. 2007. Pseudomonas aeruginosa infections in the intensive care unit: can the adequacy of empirical beta-lactam antibiotic therapy be improved? Int J Antimicrob Agents 30:458–462. 10.1016/j.ijantimicag.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Giamarellou H. 1986. Aminoglycosides plus beta-lactams against gram-negative organisms: evaluation of in vitro synergy and chemical interactions. Am J Med 80:126–137. 10.1016/0002-9343(86)90490-0. [DOI] [PubMed] [Google Scholar]
- 13.Bertino JS, Booker LA, Franck PA, Jenkins PL, Franck KR, Nafziger AN. 1993. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis 167:173–179. 10.1093/infdis/167.1.173. [DOI] [PubMed] [Google Scholar]
- 14.Boyer A, Gruson D, Bouchet S, Clouzeau B, Hoang-Nam B, Vargas F, Gilles H, Molimard M, Rogues AM, Moore N. 2013. Aminoglycosides in septic shock: an overview, with specific consideration given to their nephrotoxic risk. Drug Saf 36:217–230. 10.1007/s40264-013-0031-0. [DOI] [PubMed] [Google Scholar]
- 15.Trecarichi EM, Tumbarello M. 2014. Antimicrobial-resistant Gram-negative bacteria in febrile neutropenic patients with cancer: current epidemiology and clinical impact. Curr Opin Infect Dis 27:200–210. 10.1097/QCO.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 16.Marín M, Gudiol C, Ardanuy C, Garcia-Vidal C, Jimenez L, Domingo-Domenech E, Pérez FJ, Carratalà J. 2015. Factors influencing mortality in neutropenic patients with haematologic malignancies or solid tumours with bloodstream infection. Clin Microbiol Infect 21:583–590. 10.1016/j.cmi.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Park BK, Koo Kim S, Han SB, Lee JW, Lee DG, Chung NG, Cho B, Jeong DC, Kang J. 2017. Clinical characteristics and outcomes of Pseudomonas aeruginosa bacteremia in febrile neutropenic children and adolescents with the impact of antibiotic resistance: a retrospective study. BMC Infect Dis 17:1–10. 10.1186/s12879-017-2597-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtz TO, Winston DJ, Bruckner DA, Martin WJ. 1981. Comparative in vitro synergistic activity of new betalactam antimicrobial agents and amikacin against Pseudomonas aeruginosa and Serratia marcescens. Antimicrob Agents Chemother 20:239–243. 10.1128/aac.20.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez JA, Cobos-Trigueros N, Soriano A, Almela M, Ortega M, Marco F, Pitart C, Sterzik H, Lopez J, Mensa J. 2010. Influence of empiric therapy with a β-lactam alone or combined with an aminoglycoside on prognosis of bacteremia due to gram-negative microorganisms. Antimicrob Agents Chemother 54:3590–3596. 10.1128/AAC.00115-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Zarychanski R, Light B, Parrillo J, Maki D, Simon D, Laporta D, Lapinsky S, Ellis P, Mirzanejad Y, Martinka G, Keenan S, Wood G, Arabi Y, Feinstein D, Kumar A, Dodek P, Kravetsky L, Doucette S. 2010. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med 38:1773–1785. 10.1097/CCM.0b013e3181eb3ccd. [DOI] [PubMed] [Google Scholar]
- 21.Paul M, Lador A, Grozinsky-Glasberg S, Leibovici L. 2014. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 2014:CD003344. 10.1002/14651858.CD003344.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong DSY, Frencken JF, Klein Klouwenberg PMC, Juffermans N, Van Der Poll T, Bonten MJM, Cremer OL. 2017. Short-course adjunctive gentamicin as empirical therapy in patients with severe sepsis and septic shock: a prospective observational cohort study. Clin Infect Dis 64:1731–1736. 10.1093/cid/cix186. [DOI] [PubMed] [Google Scholar]
- 23.Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. 1995. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 39:650–655. 10.1128/aac.39.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. 2007. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806–808. 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, Uchino S. 2012. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138. [Google Scholar]
- 26.Brown D, Cantón R, Dubreuil L, Gatermann S, Giske C, MacGowan A, Martínez-Martínez L, Mouton J, Skov R, Steinbakk M, Walton C, Heuer O, Struelens MJ, Diaz Högberg L, Kahlmeter G. 2015. Widespread implementation of EUCAST breakpoints for antibacterial susceptibility testing in Europe. Eurosurveillance 20:21008. 10.2807/1560-7917.ES2015.20.2.21008. [DOI] [PubMed] [Google Scholar]
- 27.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson D, Rice L, Stelling J, Struelens M, Vatopoulos A, Weber J, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 28.Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, Gallagher J, Herrstedt J, Rapoport B, Rolston K, Talcott J. 2000. The Multinational Association for Supportive Care in Cancer Risk Index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol 18:3038–3051. 10.1200/JCO.2000.18.16.3038. [DOI] [PubMed] [Google Scholar]
- 29.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. 1988. CDC definitions for nosocomial infections. Am J Infect Control 16:128–140. 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 30.Patorno E, Grotta A, Bellocco R, Schneeweiss S. 2013. Propensity score methodology for confounding control in health care utilization databases. Epidemiol Biostat Public Heal 10:e8040-16. [Google Scholar]
- 31.Bunn A, Korpela M. 2008. An introduction to dplR. Ind Commer Train 10:11–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download AAC00045-21_Supp_1_seq7.pdf, PDF file, 0.6 MB (627.1KB, pdf)