ABSTRACT
The qnrE family was designated in 2017. To date, two qnrE alleles have been discovered that are carried by plasmids. Here, we identified a new quinolone resistance gene, qnrE3, in the chromosome of Enterobacter mori clinical isolate 08-091 in China. qnrE3 conferred decreased susceptibility to fluoroquinolones, similar to qnrE1 and qnrE2. To investigate the precise origin of qnrE1, qnrE2, and qnrE3, 79 qnrE-bearing strains producing 30 qnrE variants were retrieved from the NCBI database. Phylogenetic analysis illustrated two major clusters, QnrEEmo and QnrEEas, produced mainly by the E. mori and E. asburiae strains, respectively. Comparison of the genetic context of qnrE alleles demonstrated that qnrE3 and qnrEEas2 alleles presumably were captured by ISEcp1 and mobilized from the E. mori and E. asburiae strains to the E. xiangfangensis and Escherichia coli strains, respectively. qnrEEas2 was proposed to be named qnrE4, since it has spread to another genus. All the qnrE alleles were harbored by the Enterobacter species, except those captured by ISEcp1 and mobilized into other species of Enterobacterales. E. mori is probably the source of qnrE1 to qnrE3 alleles, and E. asburiae is the reservoir of qnrE4.
KEYWORDS: qnrE3, qnrE4, Enterobacter, Enterobacter mori, quinolone resistance, Enterobacter cloacae complex, Enterobacter asburiae
INTRODUCTION
The Enterobacter species contributes increasingly to the spread of multidrug-resistant infections (1, 2). Among the Enterobacter genus, the Enterobacter cloacae complex (ECC) is common and comprises a set of closely related species, including E. asburiae, E. cloacae, E. hormaechei, E. kobei, E. ludwigii, and E. mori (1). Since it is challenging for accurate identification of ECC strains, it is common to misname other species as ECC strains or vice versa (3).
The taxonomy of ECC and Enterobacter species is complicated and continuously updated. Hoffmann and Roggenkamp first defined the ECC as 12 genetic clusters based on the hsp60 gene (4). Chavda et al. classified ECC genomes into 18 phylogenomic groups by analyzing pairwise average nucleotide identity (ANI) with a >95% cutoff (5). Most recently, Wu et al. updated the taxonomy of the Enterobacter species and reclassified Enterobacter genomes into 22 species with a stringent ≥96% ANI cutoff, which had a high correlation with the 70% in silico DNA-DNA hybridization (isDDH) cutoff (3). Within the species, E. hormaechei subsp. hormaechei and E. hormaechei subsp. hoffmannii were reassigned from subspecies to species of E. hormaechei and E. hoffmannii, while E. hormaechei subsp. oharae, E. hormaechei subsp. xiangfangensis, and E. hormaechei subsp. steigerwaltii (6–8) were reclassified from species E. hormaechei to E. xiangfangensis (3).
qnr genes mediate low-level resistance to quinolones (9). At present, seven types of qnr genes, qnrA to qnrE, qnrVC, and qnrS, have been discovered to be transferable by plasmids (9–15). qnrE1 was provisionally named qnrB88, since it presents >70% sequence identity with qnrB alleles (13, 16). The qnrB gene has its likely origin as a chromosomal gene in the Citrobacter species (17). The qnrE family was designated in 2017, when qnrE1 was found to have been mobilized via ISEcp1 from the chromosome of the Enterobacter species to plasmid pKp1130 (13). In 2018, qnrE2 was discovered in Escherichia coli (GenBank accession no. CP018961.1). In 2021, Ebmeyer et al. proposed that the mobile qnrE genes most likely originated from E. mori (18). However, the precise origin of qnrE among the various species of Enterobacter is not yet known. In this study, we identified a new quinolone-resistant gene, qnrE3, in an E. mori clinical isolate and explored the phylogeny, origin, and mobilization of qnrE.
RESULTS AND DISCUSSION
Characterization of QnrE3-producing E. mori 08-091.
E. mori 08-091 contains one chromosome (4,773 kb) and one plasmid, pHS08-091 (213 kb; see Fig. S1 in the supplemental material). A new qnrE gene (named qnrE3), together with the natural blaACT and fosA genes, was discovered in the chromosome. No known plasmid types and no resistance genes were found in pHS08-091.
E. mori 08-091 has a 97.17% ANI and an 81.1% isDDH value (Table S1) and belongs to E. mori in terms of the definition of the homogeneous species: ANI of ≥96% and isDDH of ≥70% (19, 20). E. mori LMG 25706 was first defined as a plant pathogen in China that can cause a severe bacterial wilt in mulberry leaves (21, 22). MLST analysis showed E. mori 08-091 belongs to ST1006.
Antimicrobial susceptibility.
E. mori 08-091 was susceptible to cefepime, imipenem, meropenem, fluoroquinolones, and fosfomycin but resistant to the first-, second-, and third-generation cephalosporins, aztreonam, and cefoxitin (Table 1). Supplemented cloxacillin could decrease the MICs of cephalosporins and cefoxitin more than 16-fold against E. mori 08-091, which was probably due to the production of chromosome-encoded ACT-type AmpC β-lactamase. When the qnrE3 gene was cloned in E. coli, the MICs of ciprofloxacin and levofloxacin were at least 16 times higher than those of the original E. coli TOP10 (Table 1), indicating that the expression of qnrE3 led to an increase of resistance to fluoroquinolones, similar to qnrE1 and qnrE2.
TABLE 1.
The MICs for clinical isolate E. mori 08-091 and qnrE3 transformanta
| Strain | MIC (μg/ml) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP | LEV | CZO | CXM | CRO | CAZ | FEP | ATM | FOX | IPM | MEM | FOS | |
| E. mori 08-091 | 0.06 | 0.12 | >128 | >128 | 32 | 32 | 0.5 | 16 | >128 | 0.5 | 0.06 | 32 |
| 2* | 8* | ≤0.5* | 0.25* | 4* | ||||||||
| E. coli TOP10(pHSG398-qnrE3) | 0.25 | 0.25 | 2 | 8 | ≤0.5 | 0.5 | ≤0.06 | ≤1 | ND | 0.125 | ≤0.03 | ND |
| E. coli TOP10 | ≤0.015 | ≤0.015 | 8 | 4 | ≤0.5 | 0.5 | ≤0.06 | ≤1 | ND | 0.125 | ≤0.03 | 0.125 |
CIP, ciprofloxacin; LEV, levofloxacin; CZO, cefazolin; CXM, cefuroxime; CRO, ceftriaxone; CAZ, ceftazidime; FEP, cefepime; ATM, aztreonam; FOX, cefoxitin; IPM, imipenem; MEM, meropenem; FOS, fosfomycin. *, the MICs decreased with supplementation of cloxacillin (200 μg/ml). ND, not determined.
Phylogenetic analysis of qnrE alleles and origin of plasmid-mediated qnrE.
Through a BLASTP and BLASTN search, 79 strains were found to carry qnrE alleles (Table S2). These qnrE alleles encoded 30 intact QnrE variants with identities of 100% to 91.1% to QnrE1 and 83.6% to 86% to QnrB1. The QnrB family is phylogenetically closest to the QnrE family among transferable Qnr proteins (13). Chromosome-encoded Qnr proteins from genera other than Enterobacter were not included, although some of them, for instance, the genera Serratia, Lelliottia, and Buttiauxella, showed relatively high amino acid identities to QnrE1 (89% to 86%).
Since misclassification or inaccurate bacterial species designation in GenBank is frequent, precise taxonomic assignation was conducted on 69 qnrE-like-bearing Enterobacter strains (NCBI species assignation) based on the ANI and isDDH results as described by Wu et al. (3). Thirty-three (47.8%) misidentified ECC strains were corrected, including two strains assigned to Lelliottia nimipressuralis and one assigned to an unclassified genus (Table S1).
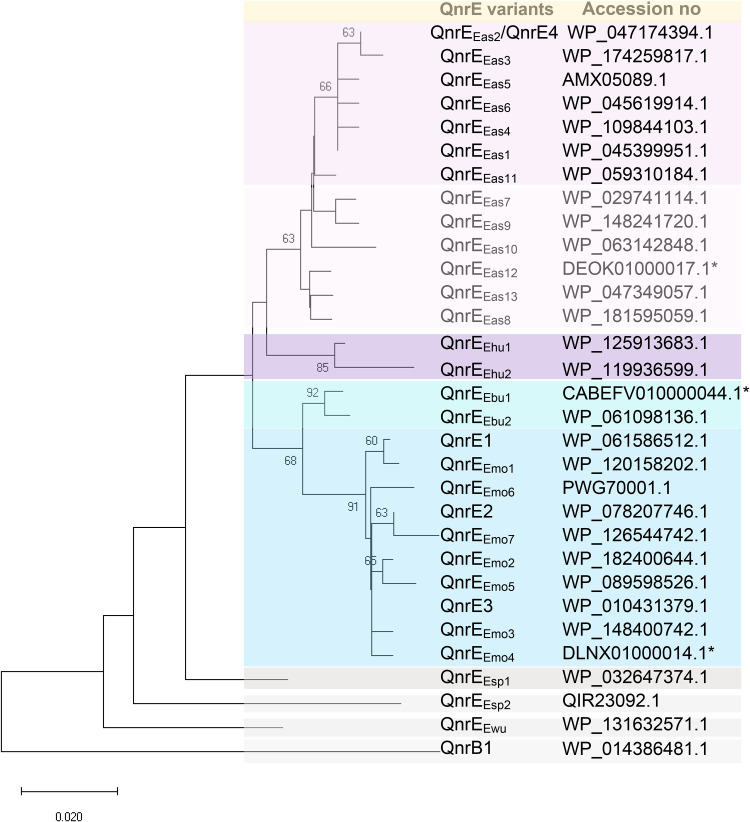
Phylogenetic analysis showed that most of the QnrE variants were classified into two major clusters (Fig. 1), QnrEEmo and QnrEEas, so named because most of them are produced by the E. mori or E. asburiae strain. The QnrEEmo cluster included QnrE1, QnrE2, QnrE3, and seven QnrEEmo variants. Compared to QnrE3, an amino acid alteration was observed in QnrE1 (V211A), QnrE2 (E49A), QnrEEmo2, QnrEEmo3, and QnrEEmo4, while there were two or three amino acid changes in the rest of the QnrEEmo members (Fig. S2, Table S3). E. mori is a rare species that is prone to misidentification. At present (7 February 2021), 12 genomes have been submitted as E. mori (taxid 539813) in GenBank (https://www.ncbi.nlm.nih.gov/genome/), among which E. mori WCHEM090044 was reconfirmed as E. quasimori (RXRX01) and E. mori HSW1412 as an unknown species (CP061801.1; Table S1). In addition, one E. cloacae and three Enterobacter strains were reassigned as E. mori (Tables S1 and S2). All the E. mori strains produced QnrE3 or QnrEEmo variants. E. quasimori WCHEM090044 produced QnrEEmo7. E. quasimori is a novel species, named by Zong et al. (3), that is phylogenetically closest to E. mori (isDDH, 67.1%; ANI, 95.28%). This and the study mentioned above (18) indicate that E. mori strains are the reservoir of QnrE3 and its derivatives. Nearly half (6/14) of the E. mori strains came from China, while the others came from the United States, United Kingdom, Japan, Austria, and India.
FIG 1.
Phylogenetic analysis of 30 QnrE variants. Evolutionary analyses were conducted in MEGA X using the neighbor-joining method in the bootstrap test (1,000 replicates) and rooted on the midpoint. Reference protein accession or sequence ID numbers are provided. For those QnrEs without protein IDs, genomic accession numbers are provided with asterisks. Branch length represents the distance between them.
The QnrEEas cluster is composed of 13 QnrEEas proteins (1 to 6 amino acid differences). Most of them (27/35) are produced by E. asburiae strains, whereas QnrEEas9 to QnrEEas13 are produced by species close to E. asburiae, which are assigned Enterobacter tentative taxon 14, taxon 11, and taxon 5 (Table S2) (3). Taxon 5 strains are mainly composed of Enterobacter genomospecies O (NCBI species assignation) (3). In addition, there are five QnrE clusters, named QnrEEbu, QnrEEhu, QnrEEwu, QnrEEsp1, and QnrEEsp2, produced by taxon 6 (close to E. bugandensis), E. huaxiensis, E. wuhouensis, and taxon 2 (close to E. quasiroggenkampii) (Fig. 1).
The phylogenetic tree of qnrE alleles from 79 Enterobacterales strains is similar to that of QnrEs (Fig. S3). Cluster qnrEEas is the largest one and has 36 members, including 15 qnrEEas1 and seven qnrEEas2 variants. The second largest cluster is qnrEEmo, which contains 29 members, including eight qnrE1, four qnrE2, 10 qnrE3, and seven qnrEEmo variants. All the qnrE alleles were from the Enterobacter species, except 12 non-Enterobacter strains carrying transferable qnrE1, qnrE2, or qnrEEas2 (Fig. 2 and 3) from Enterobacterales of Klebsiella pneumoniae, Citrobacter freundii, Salmonella spp. and E. coli. qnrE3 and qnrEEmo variants are carried by E. mori or E. quasimori except one, qnrE3, which is proposed to be mobilized into E. xiangfangensis (Fig. 2A), and one that is harbored by an E. cloacae strain that is unavailable for genome sequence and species reidentification (Table S1). The third largest cluster is qnrEEsp1, which has eight members from Enterobacter tentative taxon 2.
FIG 2.
Comparison of genetic environments of qnrE3 and qnrE4 (qnrEEas2) carried by chromosomes with those carried by putative plasmids. (A) Genetic context comparison among qnrE3-bearing E. mori strains (08-091, WP8-W19-CRE-02, CCUG 72520 [CP071063, AP022274, and QZDQ02000002.1]), E. xiangfangensis strain C2-37 (SWFV01000049), qnrE1-bearing K. pneumoniae plasmid pKp1130 (KY073238), and S. enterica strain PNUSAS069606 (AADHEF010000020). (B) Genetic context comparison of qnrE4 (qnrEEas2) from E. asburiae MNCRE20 (JYMM01000029) with those from E. coli strain PNUSAE000281 (AATIWT010000137 and AATIWT010000024) and those from the IncHI2 plasmid pKST313 (LN794248.1). Different genes are annotated and denoted by colored arrows. Sequence identities are denoted between two broken lines. Filled gray circles and black squares indicate target site duplications (TSD). The putative target site of ISEcp1-qnrE4/qnrEEas2 transposition unit is indicated in blue with TSDs (TATTA).
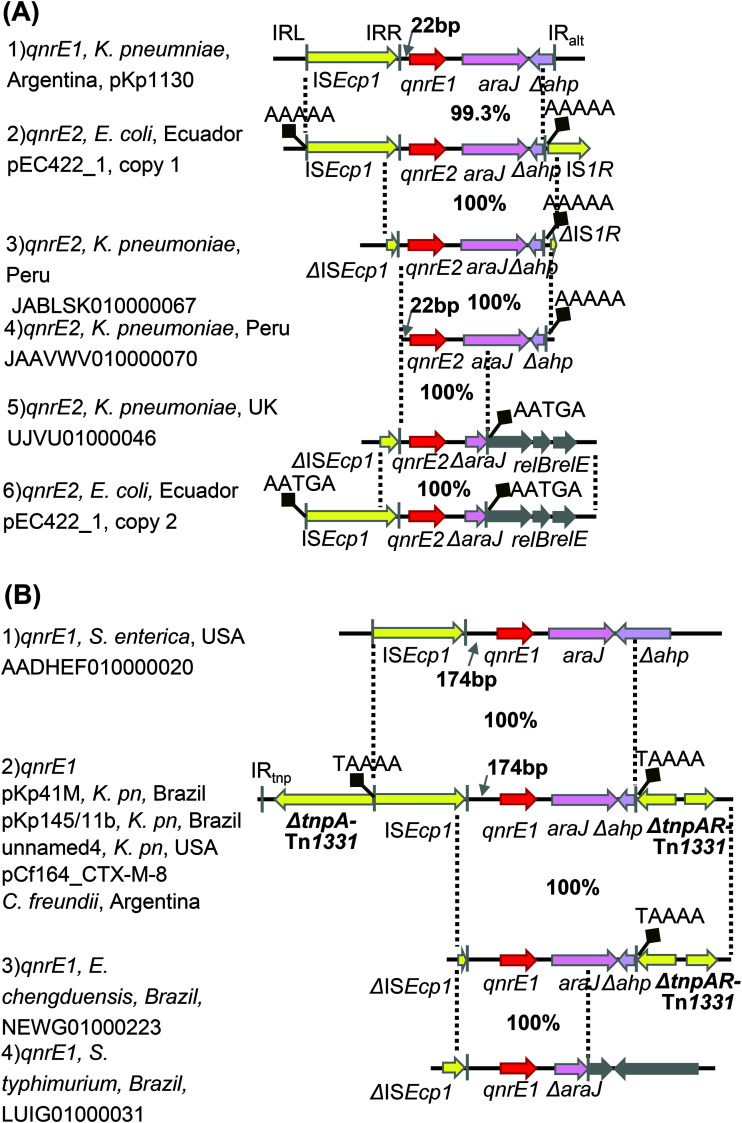
FIG 3.
Genetic context of qnrE1 and qnrE2. ISEcp1 is present at two different locations, 22 bp upstream of qnrE1 and qnrE2 (A) and 174 bp upstream of seven qnrE1 genes (B). qnrE1 genes are carried by K. pneumoniae plasmids pKp1130, pKp41M, pKp145/11b, and unnamed4, C. freundii plasmid pCf164_CTX-M-8 (KY073238, KY781949.1, KX118608.1, CP027699.1, and MN187903.1), E. chengduensis strain EkBL-II-14(1), and two S. Typhimurium strains, CFSAN033917 and PNUSAS069606 (NEWG01000223, LUIG01000031, and AADHEF010000020). E. coli plasmid pEC422_1 harbored two copies of qnrE2 (CP018961.1). qnrE2 genes are also carried by three K. pneumoniae strains (JABLSK010000067, JAAVWV010000070, and UJVU01000046). Sequence identities are denoted between two broken lines. Filled black squares indicate target site duplications (TSD).
Among 79 Enterobacterales strains, 66 are Enterobacter strains with whole-genome sequencing (WGS) data. Phylogenomic analyses based on the Genome BLAST Distance Phylogeny (GBDP) method showed that QnrEEas-producing E. asburiae strains belonged to four subspecies, and qnrEEas1-bearing ST252 strains were predominant (Fig. S4). QnrE-producing E. mori strains belonged to two subspecies with various sequence types (STs). No prevalent STs were observed.
Comparison of genetic contexts of transferable qnrE genes.
The genetic context of qnrE3 in E. mori 08-091 is closely related to that of the chromosome of E. mori WP8-W19-CRE-02 (AP022274; identity, 99.7%; Fig. 2A). Unlike the common genetic surroundings of qnrE in E. mori strains (ppk2-qnrE-araJ-ahp-luciferase gene), E. mori 08-091 has an extra unknown sequence (3,651 bp) incorporated downstream of qnrE3 (Fig. 2A).
The prevalent qnrE1 genes carried by many plasmids are highly conserved, with intact or remnant ISEcp1 insertion sequences upstream and varied lengths of fragments araJ-ahp downstream of qnrE1 (Fig. 3B). A similar scenario is observed in qnrE2 (Fig. 3A). The gene context of qnrE3 (ΔISEcp1-qnrE3-araJ-Δahp; SWFV01000049.1) in an E. xiangfangensis strain C2-37 (submitted as E. hormaechei) has high identity (99.4%) to that of qnrE1 in pKP1130 (Fig. 2A), indicating that they share the same origin. The segment qnrE1-araJ-Δahp has identities of 99.6% with that of the E. mori strain CCUG 72520 (QZDQ02000002.1; Fig. 2A). Since qnrE1, qnrE2, and qnrE3, together with their flanking sequences, are genetically closely related, it is suggested that E. mori is the origin of qnrE1 to qnrE3, which have been disseminated among Enterobacteriaceae via ISEcp1. ISEcp1 is present at two different locations, 22 bp upstream of qnrE1, qnrE2, and qnrE3 or 174 bp upstream of qnrE1 (Fig. 3 and 2A), indicating that different transposition events happened.
The ISEcp1-qnrE1 transposition unit was discovered in four CTX-M-8-bearing IncM1 plasmids from South America (pKp41M, pKp145/11b, and pCf164_CTX-M-8) (23, 24) and the United States (plasmid unnamed4), disrupting tnpA of Tn1331 with the same duplications (TAAAA; Fig. 3B). A similar transposition event was observed in an E. chengduensis strain [submitted as E. kobei EkBL-II-14(1)] from Brazil, leaving an identical TSD. Genomic comparison of strain EkBL-II-14 with plasmid pKp145/11b revealed 96.6% DNA coverage (Fig. S5), indicating the existence of a CTX-M-8-bearing IncM1 plasmid in this strain and the circulation of this kind of plasmid in South America.
Of note, a qnrEEas2 variant was discovered from E. coli strain PNUSAE000281 with a gene context of qnrEEas2-araJ-Δahp (AATIWT010000137.1), identical to that of E. asburiae strain MNCRE20 (JYMM01000029.1; Fig. 2B). The flanking sequences (orf3 to orf6) downstream of Δahp, together with the sequences (orf1-orf2) upstream of ISEcp1 in another contig (AATIWT010000024.1), are identical to those in IncHI2 plasmids, such as pKST313 (LN794248.1; Fig. 2B). Genomic comparison of the E. coli strain PNUSAE000281 to pKST313 revealed a coverage of 79% (238 kb versus 300 kb, identity of >99.4%), including the IncHI2 conjugative transfer system and replication region (Fig. S6A). Although the completed circular sequence of IncHI2 plasmid in PNUSAE000281 is unavailable, it is likely that ISEcp1 has transposed the context of qnrEEas2 from E. asburiae into an IncHI2 plasmid at the target site between orf2 and orf3 based on the 5-bp duplications (TATTA) flanking the left and alternative inverted repeats (IRL and IRalt) of the proposed ISEcp1-qnrEEas2 transposition unit (Fig. 2B). Thus, qnrEEas2 should be named qnrE4, since it is a transferable qnr gene.
Similarly, genomic comparison of the qnrE3-bearing E. xiangfangensis strain C2-37 showed high identity and coverage to IncHI2 plasmid pMCR-SCNJ07 (MK933279.1) from the same species (Fig. S6B). The ISEcp1-qnrE3 transposition unit integrated into the site adjacent to the pcoE gene (copper resistance gene). An mcr-9 gene together with replicative and conjugative genes were also discovered in this putative IncHI2 plasmid.
In conclusion, qnrEEmo and qnrEEas are the two largest subgroups of the qnrE family. In addition to qnrE1 and qnrE2, qnrE3 and qnrE4 alleles have been captured by ISEcp1 and mobilized from the chromosomes of E. mori and E. asburiae, respectively, to plasmids of different species of Enterobacterales. E. mori is likely the source of qnrE1, qnrE2, and qnrE3 alleles.
MATERIALS AND METHODS
Bacterial strains and WGS.
E. mori 08-091 was isolated in 2008 from the ascites specimen of a 65-year-old male patient from Shanghai, China. It was subjected to WGS based on the Illumina NovaSeq sequencing platform and the Oxford Nanopore ONT sequencing platform (25–27).
Cloning of qnrE3 and antimicrobial susceptibility testing.
The 645-bp qnrE gene was amplified from E. mori 08-091 using primers qnrE-EcoRI-F (5′-AACAGCTATGACCATGATTACGAATTCATGGCATTGATTTTTGAAGGC-3′) and qnrE-SalI-R (5′-CCAAGCTTGCATGCCTGCAGGTCGACTTAGCCTAAAACAACGATGCCA-3′) and cloned into the vector pHSG398 (TaKaRa) via the homologous recombination technique. The recombinant plasmid pHSG398-qnrE3 was transformed into E. coli TOP10 and confirmed by sequencing. An antimicrobial susceptibility test was determined by the broth microdilution method and interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (28). An inhibition test was performed on the MICs of cephalosporins and cefoxitin with supplementation of cloxacillin (200 μg/ml).
S1-PFGE.
S1-pulsed-field gel electrophoresis (PFGE) was performed as previously described to demonstrate the plasmid profile of E. mori 08-091 with the CHEF Mapper system (Bio-Rad) (29). The total DNA of Salmonella Braenderup standard strain H9812 digested with XbaI served as the molecular marker.
Bioinformatics analysis.
Annotation was conducted in silico using RAST 2.0, combined with BLASTP/BLASTN, ResFinder, and PlasmidFinder (https://cge.cbs.dtu.dk/services). A BLASTP analysis was performed with QnrE3 in the Nonredundant Protein Database (nr). BLASTN analysis of the qnrE3 gene sequence was carried out in the Nucleotide Database (nt) and the Whole-Genome Shotgun Contigs Database of E. mori (taxid 539813) and Enterobacter (taxid 547) (accessed 5 November 2020). Molecular identification for bacterial species was performed by determining the pairwise ANI with a ≥96% cutoff and isDDH with a ≥70% cutoff using the JSpecies program (http://jspecies.ribohost.com/jspeciesws/) and Genome-to-Genome Distance Calculator (GGDC; formula 2; https://ggdc.dsmz.de/ggdc.php) (30, 31). The neighbor-joining trees for QnrE or qnrE sequences was established with MEGA 10.0.5 (32). The GBDP method was used for phylogenomic analyses and genome-based taxonomy (https://tygs.dsmz.de/) (33).
Accession numbers.
The complete sequences of E. mori 08-091 have been deposited in GenBank under accession numbers CP071063.1 and CP071064.1 for the chromosome and the plasmid pHS08-091, respectively. WP_010431379 and WP_047174394 are reference sequences (Refseq) for QnrE3 and QnrE4, respectively.
ACKNOWLEDGMENTS
We are grateful to Jan Meier-Kolthoff for assistance in the phylogenomic analyses using the GBDP method.
This work was supported by grants from the National Natural Science Foundation of China (grant numbers 81872909, 81991531, and 81673479).
We have no conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Davin-Regli A, Lavigne JP, Pages JM. 2019. Enterobacter spp.: update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin Microbiol Rev 32:e00002-19. 10.1128/CMR.00002-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tommasi R, Brown DG, Walkup GK, Manchester JI, Miller AA. 2015. ESKAPEing the labyrinth of antibacterial discovery. Nat Rev Drug Discov 14:529–542. 10.1038/nrd4572. [DOI] [PubMed] [Google Scholar]
- 3.Wu W, Feng Y, Zong Z. 2020. Precise species identification for Enterobacter: a genome sequence-based study with reporting of two novel species, Enterobacter quasiroggenkampii sp. nov. and Enterobacter quasimori sp. nov. mSystems 5:e00527-20. 10.1128/mSystems.00527-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann H, Roggenkamp A. 2003. Population genetics of the nomenspecies Enterobacter cloacae. Appl Environ Microbiol 69:5306–5318. 10.1128/AEM.69.9.5306-5318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavda K, Chen L, Fouts D, Sutton G, Brinkac L, Jenkins S, Bonomo R, Adams M, Kreiswirth B. 2016. Comprehensive genome analysis of carbapenemase-producing Enterobacter spp.: new insights into phylogeny, population structure, and resistance mechanisms. mBio 7:e02093-16. 10.1128/mBio.02093-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann H, Stindl S, Ludwig W, Stumpf A, Mehlen A, Monget D, Pierard D, Ziesing S, Heesemann J, Roggenkamp A, Schleifer KH. 2005. Enterobacter hormaechei subsp. oharae subsp. nov., E. hormaechei subsp. hormaechei comb. nov., and E. hormaechei subsp. steigerwaltii subsp. nov., three new subspecies of clinical importance. J Clin Microbiol 43:3297–3303. 10.1128/JCM.43.7.3297-3303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu CT, Li CY, Yang LJ, Huo GC. 2014. Enterobacter xiangfangensis sp. nov., isolated from Chinese traditional sourdough, and reclassification of Enterobacter sacchari Zhu et al. 2013 as Kosakonia sacchari comb. nov. Int J Syst Evol Microbiol 64:2650–2656. 10.1099/ijs.0.064709-0. [DOI] [PubMed] [Google Scholar]
- 8.Sutton GG, Brinkac LM, Clarke TH, Fouts DE. 2018. Enterobacter hormaechei subsp. hoffmannii subsp. nov., Enterobacter hormaechei subsp. xiangfangensis comb. nov., Enterobacter roggenkampii sp. nov., and Enterobacter muelleri is a later heterotypic synonym of Enterobacter asburiae based on computational analysis of sequenced Enterobacter genomes. F1000Res 7:521. 10.12688/f1000research.14566.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez-Martínez L, Pascual A, Jacoby GA. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797–799. 10.1016/S0140-6736(97)07322-4. [DOI] [PubMed] [Google Scholar]
- 10.Jacoby GA, Walsh KE, Mills DM, Walker VJ, Oh H, Robicsek A, Hooper DC. 2006. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob Agents Chemother 50:1178–1182. 10.1128/AAC.50.4.1178-1182.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Guo Q, Xu X, Wang X, Ye X, Wu S, Hooper DC, Wang M. 2009. New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob Agents Chemother 53:1892–1897. 10.1128/AAC.01400-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavaco LM, Hasman H, Xia S, Aarestrup FM. 2009. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans strains of human origin. Antimicrob Agents Chemother 53:603–608. 10.1128/AAC.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albornoz E, Tijet N, De BD, Gomez S, Martino F, Corso A, Melano RG, Petroni A. 2017. qnrE1, a member of a new family of plasmid-located quinolone resistance genes, originated from the chromosome of Enterobacter species. Antimicrob Agents Chemother 61:e02555-16. 10.1128/AAC.02555-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseca EL, Dos Santos Freitas F, Vieira VV, Vicente AC. 2008. New qnr gene cassettes associated with superintegron repeats in Vibrio cholerae O1. Emerg Infect Dis 14:1129–1131. 10.3201/eid1407.080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata M, Suzuki M, Matsumoto M, Takahashi M, Sato K, Ibe S, Sakae K. 2005. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob Agents Chemother 49:801–803. 10.1128/AAC.49.2.801-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacoby G, Cattoir V, Hooper D, Martinez-Martinez L, Nordmann P, Pascual A, Poirel L, Wang M. 2008. qnr gene nomenclature. Antimicrob Agents Chemother 52:2297–2299. 10.1128/AAC.00147-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacoby GA, Griffin CM, Hooper DC. 2011. Citrobacter spp. as a source of qnrB alleles. Antimicrob Agents Chemother 55:4979–4984. 10.1128/AAC.05187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebmeyer S, Kristiansson E, Larsson DGJ. 2021. A framework for identifying the recent origins of mobile antibiotic resistance genes. Commun Biol 4:8. 10.1038/s42003-020-01545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richter M, Rosselló-Móra R. 2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A 106:19126–19131. 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson C, Chimetto L, Edwards R, Swings J, Stackebrandt E, Thompson F. 2013. Microbial genomic taxonomy. BMC Genomics 14:913. 10.1186/1471-2164-14-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu B, Zhang GQ, Lou MM, Tian WX, Li B, Zhou XP, Wang GF, Liu H, Xie GL, Jin GL. 2011. Genome sequence of the Enterobacter mori type strain, LMG 25706, a pathogenic bacterium of Morus alba L. J Bacteriol 193:3670–3671. 10.1128/JB.05200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu B, Lou MM, Xie GL, Wang GF, Zhou Q, Wang F, Fang Y, Su T, Li B, Duan YP. 2011. Enterobacter mori sp. nov., associated with bacterial wilt on Morus alba L. Int J Syst Evol Microbiol 61:2769–2774. 10.1099/ijs.0.028613-0. [DOI] [PubMed] [Google Scholar]
- 23.Cunha MPV, Davies YM, Cerdeira L, Dropa M, Lincopan N, Knöbl T. 2017. Complete DNA sequence of an IncM1 plasmid bearing the novel qnrE1 plasmid-mediated quinolone resistance variant and blaCTX-M-8 from Klebsiella pneumoniae sequence type 147. Antimicrob Agents Chemother 61:e00592-17. 10.1128/AAC.00592-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dabos L, Rodriguez CH, Nastro M, Dortet L, Bonnin RA, Famiglietti A, Iorga BI, Vay C, Naas T. 2020. LMB-1 producing Citrobacter freundii from Argentina, a novel player in the field of MBLs. Int J Antimicrob Agents 55:105857. 10.1016/j.ijantimicag.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Chin C-S, Peluso P, Sedlazeck FJ, Nattestad M, Concepcion GT, Clum A, Dunn C, O'Malley R, Figueroa-Balderas R, Morales-Cruz A, Cramer GR, Delledonne M, Luo C, Ecker JR, Cantu D, Rank DR, Schatz MC. 2016. Phased diploid genome assembly with single-molecule real-time sequencing. Nat Methods 13:1050–1054. 10.1038/nmeth.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu H, Giordano F, Ning Z. 2016. Oxford Nanopore MinION sequencing and genome assembly. Genomics Proteomics Bioinformatics 14:265–279. 10.1016/j.gpb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. 2017. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res 27:722–736. 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing, 30th Informational Supplement M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Wong MH, Kan B, Chan EW, Yan M, Chen S. 2016. IncI1 plasmids carrying various blaCTX-M genes contribute to ceftriaxone resistance in Salmonella enterica serovar Enteritidis in China. Antimicrob Agents Chemother 60:982–989. 10.1128/AAC.02746-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosselló-Móra R, Amann R. 2015. Past and future species definitions for bacteria and archaea. Syst Appl Microbiol 38:209–216. 10.1016/j.syapm.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Wu W, Wei L, Feng Y, Xie Y, Zong Z. 2021. Precise species identification by whole-genome sequencing of Enterobacter bloodstream infection, China. Emerg Infect Dis 27:161–169. 10.3201/eid2701.190154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier-Kolthoff JP, Goker M. 2019. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun 10:2182. 10.1038/s41467-019-10210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download AAC00456-21_Supp_S1_seq5.pdf, PDF file, 1.1 MB (1.1MB, pdf)