SUMMARY
Sonic hedgehog (Shh), which regulates proliferation in many contexts, functions as a limb morphogen to specify a distinct pattern of digits. How Shh’s effects on cell number relate to its role in specifying digit identity is unclear. Deleting the mouse Shh gene at different times using a conditional Cre line, we find that Shh functions to control limb development in two phases: a very transient, early patterning phase regulating digit identity, and an extended growth-promoting phase during which the digit precursor mesenchyme expands and becomes recruited into condensing digit primordia. Our analysis reveals an unexpected alternating anterior-posterior sequence of normal mammalian digit formation. The progressive loss of digits upon successively earlier Shh removal mirrors this alternating sequence and highlights Shh’s role in cell expansion to produce the normal digit complement.
INTRODUCTION
Manipulation of Shh levels in both chick and mouse embryo limb buds has demonstrated a quantitative, dose-dependent requirement for Shh to regulate both digit type (specified anterior-posterior, digits 1–5 or d1–d5, thumb to pinky) and total digit number (reviewed by Zeller, 2004; Tickle, 2006). Models for digit patterning by Shh include the classic spatial morphogen gradient model and the more recent expansion-based temporal gradient model (Harfe et al., 2004; reviewed by Zeller, 2004; Tickle, 2006), which both predict that the highest cumulative levels (either spatial or temporal) of Shh specify the most posterior digit. Yet, other work indicates that the most posterior Shh-expressing cells (d5 progenitors) become refractory to Shh signals over time and are only transiently Shh dependent (Marigo et al., 1996; Platt et al., 1997; Ahn and Joyner, 2004). Prior genetic tests of models of Shh function have manipulated Shh dose by modifying expression levels and/or altering Shh lipid modification (Lewis et al., 2001; Chen et al., 2004; Li et al., 2006; Scherz et al., 2007). The temporal requirements for Shh signals during normal digit development have not been evaluated directly by genetic removal, but only with the pharmacologic agent cyclopamine (Scherz et al., 2007; Panman et al., 2006; Stopper and Wagner, 2007), which blocks signal transduction by all hedgehog ligands (reviewed by Beachy et al., 2004), conflating later effects of Shh on digit skeletal morphology with those of Ihh required for normal chondrogenesis.
We have determined the temporal dependence of digit specification on Shh using a tamoxifen-inducible Cre-deleter line to remove a conditional (floxed) Shh allele at defined times during limb development. We find that Shh is required only very early and transiently for digit patterning, but continuingly to ensure sufficient cell numbers to produce the normal complement of digits. Hence Shh plays dual and distinct roles in regulating digit identity and number that can be uncoupled temporally.
RESULTS AND DISCUSSION
Shh Activity Is Completely Removed by the Hoxb6/CreER Deleter Line
A transgenic Hoxb6/CreER mouse line expressing CreERT under the control of a Hoxb6 promoter (Schughart et al., 1991) was used to drive high-level tamoxifen-dependent Cre activity in limb bud mesenchyme, spanning the entire period of Shh expression in limb (~E9.5–E12 for forelimb, and E10–E12.5 for hindlimb) (Buscher et al., 1997; Platt et al., 1997; Lewis et al., 2001) (see also Figure S1 in the Supplemental Data available with this article online). Using the Rosa26LacZ reporter, substantial Cre-mediated recombination was evident just 8 hr following single-dose tamoxifen intraperitoneal injections ranging from E9.5 to E10.5, and was complete by 12 hr (E9.5 shown, Figure 1A). Cre recombinase was active in the posterior two-thirds of the forelimb and the entire hindlimb bud. When this Cre transgenic line was crossed with a previously described conditional knockout (floxed) Shh allele (Lewis et al., 2001), tamoxifen-induced Shh deletion prior to the onset of Shh expression in hindlimb bud (E9.5) resulted in a high frequency of Shh null phenotype in hindlimbs (~90%, 29/32; Table S1; e.g., Figure 1B), indicating that recombination of the floxed allele was efficient enough to reduce Shh activity below a functional threshold. Earlier onset of Shh expression may explain the low frequency of Shh null phenotype in forelimbs (2/14 limbs; Table S1), which more often developed two digits (e.g., Figure 1B).
Figure 1. Efficacy of Recombination and Shh Removal Using a Hoxb6CreERT Deleter Line.
(A) Cre+;LacZ reporter-positive embryos show substantial recombination at 8 hr and essentially complete recombination by 12 hr after tamoxifen (Tam) injection.
(B) Tam-induced deletion of Shh prior to the normal onset of expression in hindlimb reproduces a null mutant phenotype.
(C) Shh (exon2-deleted) and Ptc1 RNA are undetectable in limb buds at 18 hr after Tam-induced Shh removal. RNAs analyzed at both 18 hr and at 24 hr after each of the Tam injection times gave the same results. Examples shown were collected at 18 hr, except E9.5- and E10-treated Shh, and E10-treated Ptc1, which were at 24 hr. Ptc1 transcripts were occasionally weakly detected in forelimb bud after Tam at E10.5 (arrow). Controls used were Shh+/flox;Cre+ and Shh+/Δ;Cre− sibling embryos; both showed similar Shh and Ptc1 expression.
(D) Blue timelines summarize normal duration of Shh activity (Ptc1 detection; see also Buscher et al., 1997; Platt et al., 1997; Lewis et al., 2001) and duration after Tam-activated Cre removal of Shh, based on a detailed time course of Shh and Ptc1 expression (data from Figure S1) assayed at 3–5 hr intervals after different Tam injection times (marked by arrowheads).
To assess the efficiency of tamoxifen-regulated Cre deletion of Shh, transcript levels were checked at different times following tamoxifen treatment. Shh RNA was undetectable in both forelimb and hindlimb buds at 18–24 hr following tamoxifen treatment at either E9.5 (n = 11), E10 (n = 3), or E10.5 (n = 8) (e.g., Figure 1C). Since signaling molecules may function at very low levels, Patched1 (Ptc1), a direct target of Shh (reviewed by Ingham and McMahon, 2001), mRNA levels were assayed to report residual Shh activity. Ptc1 RNA was undetectable in most hindlimb buds within 18–24 hr following tamoxifen treatment at E9.5 (7/7), E10 (8/9), or E10.5 (8/9) (e.g., Figure 1C). In forelimb buds, Ptc1 RNA was completely undetectable in ~50% of forelimb buds 18–24 hr after tamoxifen injection at E9.5 (4/8), E10 (5/9), or E10.5 (4/9), with only trace expression in the remainder (e.g., Figure 1C, arrow). These results show that tamoxifen injection eliminates Shh function in hindlimb buds and removes or reduces it to trace levels in forelimb buds. To delimit the window of Shh activity more precisely following tamoxifen injections at each of the different times, Shh and Ptc1 transcript levels were checked over a time course of the first 3–15 hr after IP tamoxifen injection (Figure S1). These data (summarized in Figure 1D) indicate that Shh transcripts are absent by ~5–6 hr after IP injection and that Shh activity is lost (based on Ptc1 reporting) by ~12 hr after IP injection.
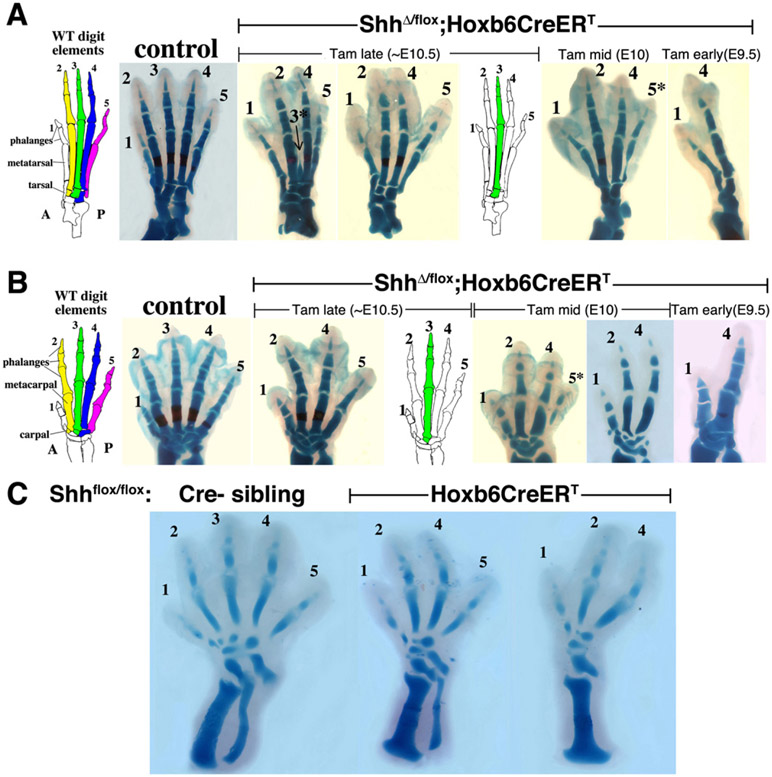
Tamoxifen-Induced Shh Removal at Successively Earlier Stages Does Not Perturb Digit Identity but Results in Progressive Digit Loss in the Sequence: d3, d5, d2, d4
We analyzed the digit skeletal phenotypes following a single tamoxifen injection at different times between E9.5 and E11 (e.g., Figure 2; summary, Table S1). As expected, progressively earlier injections elicited increasingly severe phenotypes (more digits lost). Determination of digit identity (see Experimental Procedures for criteria) was facilitated by the surprisingly normal digit morphologies in most of the embryos, even when only 2–3 digits remained; changes in number of phalanges were never observed. The order of digit loss was further confirmed by observed intermediate stages in which a clearly attenuated digit formed (asterisks in Figures 2A and 2B). Examination of 100 (each) fore- and hindlimbs revealed a highly consistent and unexpected order of digit loss: d3 first, d5 second, d2 third; leaving d4 as the last of the Shh-dependent digits (d2–d5) to be lost (e.g., Figure 2; summary, Table S2). This same sequence occurred in both fore- and hindlimbs. Assignment of the third digit lost (d2 versus d4) was more difficult than of the first two, because of fewer examples and the prior loss of adjacent skeletal landmarks. The last Shh-dependent digit was determined to be d4 based on the shape and size of the remaining carpal/tarsal bone, which is distinct from and larger than for d2, and on the proximal metatarsal shape, which has different joint morphology between d2 and d4, and was confirmed by analysis of posterior marker expression in mutant embryos at the earliest condensation stage (see below).
Figure 2. ShhΔ/flox;Hoxb6CreERT Skeletal Phenotypes after Tam-Induced Shh Removal at E10.5, E10, or E9.5, with Progressive Loss of Digits d3, d5, d2, and d4.
(A) Hindlimb skeletal phenotypes at E17.5 compared to sibling controls (as in Figure 1) at left, and schematic of wild-type limb skeleton showing different digits color-highlighted with their associated distal tarsal element, which was typically lost together with the overlying digit. Sometimes attenuated digits (asterisk) were observed prior to complete loss.
(B) Forelimb skeletal phenotypes at E17.5 (or E16.5 for E10 Tam) with controls and wild-type schematic showing carpals associated with specific digits.
(C) Hindlimb skeletons of E10 Tam-treated sibling embryos collected at E14.5, showing progressive loss of d3 followed by d5 at a very early skeletal stage, with unambiguous digit assignment based on very different initial tarsal sizes (note much larger size of the posterior-most tarsal in each case), and different lengths of d4 and d5. As the fibula is reduced or lost, the position of d4 and associated tarsal shift anteriorly to overlie the tibia.
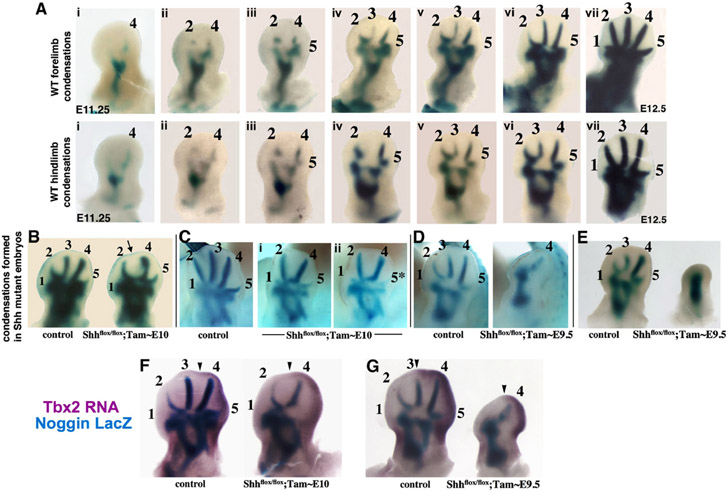
Wild-Type Digit Condensations Form in Reverse Sequence of Digit Loss upon Shh Removal
The unanticipated order of digit loss (d3, d5, d2, d4) following Shh removal is not predicted by any of the current spatial or temporal gradient models for Shh function in anterior-posterior patterning (reviewed by Zeller, 2004; Tickle, 2006). However, digit identity transformation superimposed upon digit loss could confound the simple interpretation of these results. There was no evidence at late skeletal stages (i.e., altered phalanx number or morphology) to suggest such transformations, and evaluation of very early skeletal phenotypes revealed the same sequence of digit loss as seen at later stages (Figure 2C). To further confirm that digit loss was unassociated with transformations in identity, phenotypes due to tamoxifen-induced Shh deletion were evaluated at the earliest stage that mesenchymal digit condensations first appear. Noggin is expressed very early in these condensations before cartilage differentiation markers (beginning at ~E11.25), and a Noggin-LacZ knockin allele (NogginLacZ/+) provides a very sensitive tool to detect these early condensations (Brunet et al., 1998).
Remarkably, the sequence of Shh-dependent digit condensation formation in wild-type mouse embryos, visualized with Noggin-LacZ, was d4,d2,d5,d3, exactly the reverse order of digit loss following Shh removal (Figure 3A; see also Figure S2). This order of normal mesenchymal condensation differs from that of their subsequent chondrogenic differentiation, which progresses in a more posterior-to-anterior order (Shubin and Alberch, 1986). Sox9 is essential to form proper, stable mesenchymal condensations that undergo chondrogenic differentiation (Bi et al., 1999; Barna and Niswander, 2007) and is expressed slightly before Noggin. Although Sox9 expression initiates weakly and almost synchronously in all digit condensations (making condensation order difficult to ascertain), a Sox9-3′UTR-LacZ knockin allele visualized early condensations and confirmed that digit 4 condensation appears first, then digit 2, followed by the rest in rapid succession (Figure S3). Sox9 expression onset thus corroborated Noggin expression showing that digit mesenchymal condensations appear in a different order than the posterior-anterior progression of differentiation previously observed (i.e., d4, d2, d5, d3 rather than d4, d5, d3, d2). Whether an alternating AP mesenchymal condensation order occurs in other, nonmammalian tetrapods is not known; indeed, the order of appearance of differentiated chondrogenic condensations is not invariant between different tetrapods (Shubin and Alberch, 1986).
Figure 3. Formation of Digit Condensations in Wild-Type and Shhflox/flox;Cre+ Embryos.
(A) Normal condensation sequence of d4, d2, d5, d3 in fore and hindlimb buds visualized by LacZ activity in Shh+/+;NogginLacZ/+ embryos at E11.25–E12.5. Digit 1 was not obvious in either wild-type or mutant condensations until very late (due to small size) and was not included in evaluating order.
(B–E) Condensation formation visualized by LacZ activity in Shhflox/flox;Cre+;NogginLacZ/+ and control sibling (Cre−) limbs after tamoxifen given at the times indicated ([B] and [C] show forelimb; [D] and [E] show hindlimb). Note gap for d3 (arrow in [B]) and attenuated d5 (asterisk in [C]) upon progressively earlier Shh removal in mutants. The order of condensation loss was the same as in skeletal elements (Figure 2). In severer phenotypes, narrowing of the space between long bone condensations and conversion to a single condensation were also seen frequently ([D] and not shown).
(F and G) Double staining for posterior marker Tbx2 and condensations (LacZ activity) in Shhflox/flox;Cre+;NogginLacZ/+ embryos demonstrates Tbx2 RNA (arrowheads mark anterior margin) in mesenchyme surrounding the distal posterior condensation of mutant embryos with two remaining digit precursors (F), and with a single, remaining long-digit precursor (G). In control siblings (Cre−), Tbx2 RNA extends anteriorly up to d3 (arrowheads) but is completely absent from d2.
Digit Loss Following Shh Removal after the Onset of Shh Expression Is Not Associated with Transformations in Identity
Following tamoxifen-induced Shh removal, Noggin-LacZ-marked condensations revealed the same order of digit loss as observed later during skeletal stages (Figures 3B-3E), indicating that digit loss was unaccompanied by transformations in identity. The remaining single large condensation after early Shh removal (~E9.5; e.g., Figure 3D) was suspected to be d4 based on posterior swelling of the limb bud mesenchyme and the early appearance of this condensation in mutant limb buds at a time when only d4 had condensed in wild-type (data not shown). Expression analysis of the posterior marker Tbx2 confirmed that the last remaining condensation following early Shh removal was d4 (Figures 3F and 3G). Although there are no known molecular markers that uniquely determine the identity of either d2 or d4, Tbx2 expression at the condensation stage is restricted to the posterior mesenchyme surrounding the condensations of d4 and d5, whereas the mesenchyme surrounding d2 has no expression of this marker (Suzuki et al., 2004; Figure S2).
Because the normal number of condensations depends on the total available cell mass, when the mesenchymal cell population is reduced, fewer condensations form (reviewed by Mariani and Martin, 2003). Together, these results suggest that Shh deletion at all but the earliest stages (~E9.5, which gives a null phenotype in hindlimb) reduces cell number, and consequently fewer condensations form, but digit identity is still properly specified. In this case, recruitment of normal numbers of cells to the early-forming condensations yields morphologically normal digits, but cell depletion over time short-changes the latest-forming condensations. The attenuated digits observed at intermediate stages of digit loss (asterisks in Figure 2), support this view. Although it is impossible to judge whether or not absent digits were specified correctly (but simply failed to form), the persistence of d4 as the Shh-dependent digit that is least sensitive to Shh loss suggests that even very posterior digits (specified by high-level Shh activity) only require early, transient Shh function for correct specification (i.e., ~12 hr total of Shh activity, based on Ptc1 expression; see Figure 1D and Figure S1). Though Shh activity is absent after ~12 hr, the blueprint for specifying different digit types is apparently preserved, suggesting there is simply insufficient material for making a normal complement of digits.
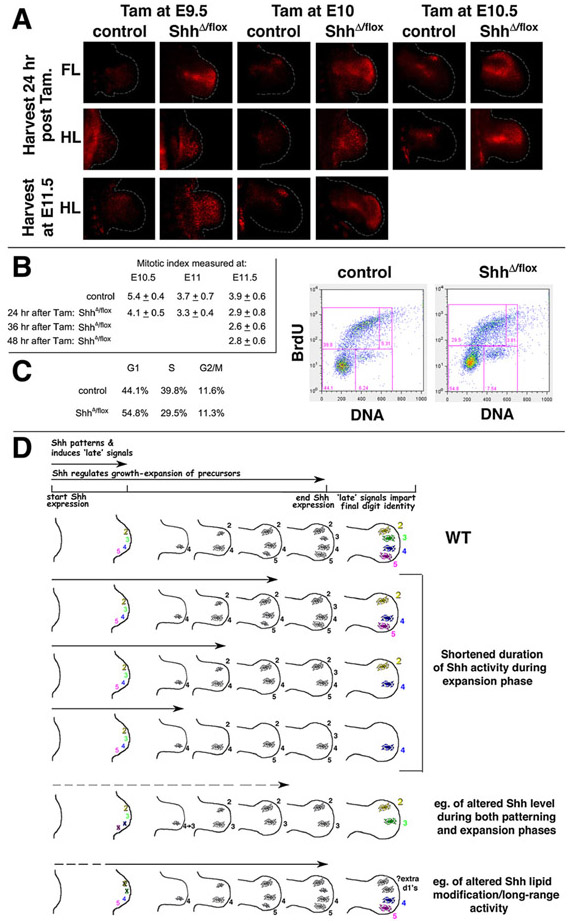
Shh Is Required Continuously to Maintain Cell Number for Limb Bud Expansion
Shh-related reduced cell number must reflect reduced proliferation and/or an increase in cell death at early stages in mutant limbs. Shh is a mitogen and a cell survival factor in many developmental systems and in cancer (reviewed by Ingham and McMahon, 2001; Beachy et al., 2004), and complete Shh removal during limb development (null mutant embryos) causes marked apoptosis of limb bud mesenchyme (Chiang et al., 2001). If changes in digit number following tamoxifen-induced Shh deletion are due to changes in cell number, then the severity of early cell reduction should parallel the severity of subsequent skeletal phenotypes. We evaluated apoptosis and proliferation levels in early limb buds following tamoxifen-induced Shh removal at different times. Shh deletion-induced apoptosis increased in spatial extent following progressively earlier tamoxifen treatment times (Figure 4A). Although slightly higher levels of apoptosis were noted in the anterior limb bud, there was no strict correlation between the spatial distribution of the apoptosis and the digit condensations lost. Mitotic cell indices were determined using anti-phospho-histone H3 to stain cells in M phase (e.g., Figure S4). There was no statistically significant difference between Shh mutant and sibling controls 24 hr following tamoxifen treatment. However the mitotic index was slightly decreased in mutant compared to control hindlimb buds 36–48 hr after tamoxifen treatment, decreasing from a mean of 3.9% in controls to 2.6% and 2.8%, respectively, in the mutant limb buds (p < 0.05; see Figure 4B and Figure S4). The effect of Shh removal on proliferation appeared to be delayed relative to the time of onset of apoptosis; however, mitotic index is an insensitive reporter of cell cycle changes. To confirm altered proliferation after Shh removal by an independent method, limb buds from mutant and control embryos were dissociated and analyzed by flow cytometry to evaluate cell cycle parameters. Relative to controls, Shh mutant limb bud mesenchyme analyzed at 24 hr after tamoxifen injection showed an increase in the proportion of cells in G1 phase (54% versus 44%) and a decreased proportion entering S phase, consistent with a G1 cell cycle arrest in mutant limb buds (Figure 4C). Hence, removal of Shh activity alters both cell survival and proliferation in limb mesenchyme, and both effects may contribute to the resulting decreased cell number in mutant limbs.
Figure 4. Effect of Timed Shh Removal on Cell Survival and Proliferation.
(A) Apoptosis (red fluorescence) in whole-mount forelimb (FL) and hindlimb (HL) buds of ShhΔ/flox;Cre+ embryos and control siblings (as in Figure 1) was analyzed at the ages indicated (left), following tamoxifen (Tam) injection at the times indicated (top). Dotted white lines indicate limb bud borders. Note the low level of increased apoptosis in Shh+/flox;Cre+ embryos over Shh+/Δ;Cre− controls (Cre+ controls are shown for all E9.5 embryos), which was negligible compared to apoptosis in Shh mutants. By E11.5, apoptosis in mutant FLs (after Tam at E9.5 or E10) was declining (data not shown), but still prominent in HLs.
(B) Mitotic cell indices (mean ± σ) determined by anti-pH3 staining of Shh mutant and sibling controls (see Experimental Procedures and Figure S4).
(C) Cell cycle analysis of limb bud cells from Shh mutant and sibling controls at 24 hr after Tam at E10. Dot plots show bivariate flow cytometry analysis of BrdU/DNA-stained cells from sibling control (7,148 cells) or mutant (13,187 cells) embryos (1 hr BrdU pulse). G1 and S phase distributions were altered, but cell size indices were unchanged in Shh mutant compared to control limb cells (data not shown).
(D) Schematic showing dual roles of Shh in relation to formation of digit condensations, and to other signals regulating digit identity downstream of Shh. Digit 1, which is Shh independent (Chiang et al., 2001), is not shown. Panels below wild-type (WT) show phenotypes resulting when only the duration of Shh function is shortened during the expansion phase (this report), compared to quantitatively reduced Shh activity or altered Shh lipid modification during both phases. Condensations shown below different Shh-duration times (arrows) indicate the number of condensations ultimately forming after limb bud expansion, not already-formed elements.
Shh regulates cell survival, at least in part, indirectly by effects on apical ectoderm ridge (AER) maintenance (Litingtung et al., 2002; te Welscher et al., 2002; Michos et al., 2004; reviewed in Tickle, 2006). AER-Fgfs have been shown to be essential cell survival factors in the limb (Sun et al., 2002), and in fact, AER morphology and Fgf8 expression are perturbed in the Shh null mutant (Chiang et al., 2001), which likely contribute to the altered cell survival observed. Indeed, after timed removal of Shh with tamoxifen, both the AER anterior extent and Fgf8 expression level were reduced in mutant limb buds relative to controls after 24–36 hr (Figure S5), indicating that modulated AER maintenance may contribute to the observed changes in cell survival. However, loss of AER-Fgf function does not appear to directly alter cell proliferation (Sun et al., 2002); the cell cycle effects of Shh are likely mediated independently, via regulation of other targets in the limb.
A Biphasic Model for Shh Function in the Limb
Altogether, the results suggest an ongoing need for Shh to ensure adequate cell numbers for subsequent condensation and digit formation, by regulating both cell survival and proliferation. Shh removal may reduce total cell number proportionate with the duration of Shh absence so that the extent of digit loss is directly related to the timing of tamoxifen-induced Shh removal. If Shh removal at later times leaves AP patterning (digit identity specification) unperturbed, then the digits that still form would remain morphologically normal despite the cell number reduction, as we observed. The progressive loss of cells and of consequent digit number, with preserved identities of the remaining digits, together with digit loss in the reverse order that normal digit primordia condense, are most parsimoniously explained by a model in which Shh acts early and transiently to specify digits properly, and thereafter is required mainly to regulate cell numbers (see Figure 4D). The results do not negate a role for Shh in specifying different digit identities and conferring AP polarity, but these critical activities must be confined to a narrow window of early limb development when Shh is first expressed. Previous genetic fate mapping showed that d3 partly, and d4–d5 completely derive from cells that previously expressed Shh (Harfe et al., 2004), leading to a model for Shh patterning by signal integration over time. Our results are more compatible with Shh acting very early as a spatial morphogen or trigger. Although d4 and d5 normally derive from Shh-expressing precursors, maintenance of this expression over time is not essential for their specification per se, suggesting that the normal order of condensation formation also does not require Shh.
The finding that loss of Shh during the “expansion” phase reduces digit number without altering identities of remaining digits supports the view that digit identity is in fact determined at a very early stage, in so far as Shh requirement is concerned. However, there is also evidence that Shh may act in part through secondary signals (Yang et al., 1997; Drossopoulou et al., 2000) and that interdigit signals downstream of Shh function to regulate digit identity at late stages (Dahn and Fallon, 2000), thus relaying the patterning function of Shh beyond the time period of direct Shh activity. Our data do not contradict a contribution of late signals acting downstream of Shh, but rather suggest that the signal relay is set in motion during the earliest phases of Shh action (Figure 4D). Indeed, the action of late signals regulating identity would provide one mechanism for maintaining normal digit identities when reduced cell numbers become allocated into fewer condensations following later Shh removal. Alternatively, normal digit identities could be maintained in the face of reduced cell number if early digit condensation precursors were expanded at different times. However, there is currently no evidence for differential spatial proliferation zones in the limb bud as required by this model (Hornbruch and Wolpert, 1970; this report).
In previous studies where genetic manipulation of Shh lipid modifications and/or expression levels in mouse embryos produced partial digit loss (Lewis et al., 2001; Chen et al., 2004; Li et al., 2006; Scherz et al., 2007), the engineered alterations commenced immediately upon Shh expression onset. Thus, both early (digit identity specification) and late (control of digit number) Shh functions were simultaneously altered, resulting in different patterns of digit loss than we observed (Figure 4D). The same argument can be made for Wnt7a null mutant phenotypes, in which abnormal regulation of Shh results in highly attenuated Shh expression early (Parr and McMahon, 1995). In the only prior analysis of genetic Shh removal after expression-onset, Shh loss was evaluated at a single embryonic time point using a PrxCre line. PrxCre expression begins very early in prelimb mesenchyme (~E9; Hasson et al., 2007), yet Shh transcripts were not completely lost until much later (E11; Scherz et al., 2007), making it likely that Shh activity was reduced during both early patterning and later expansion phases. The resulting phenotype, with three digits usually remaining, has been variously interpreted as either d1, d2, d3 (Lewis et al., 2001; Scherz et al., 2007) or as d1, d2, d4 (Lewis et al., 2001). However, when the Shh expression level was reduced after onset (apparently without altered duration) using ShhCre, a single digit was lost, reported as d2 (Scherz et al., 2007). Distinction between d2 and d3 loss (in the absence of a phenotypic series with attenuated intermediates, as we have generated) is difficult. Notably, the late attenuation of Shh expression by genetic removal of Fgfr1 also resulted in the consistent loss of just a single digit, reported as d3 (Verheyden et al., 2005).
The inhibitory drug cyclopamine has been used to evaluate the temporal requirements for Shh function, but results from such studies may be complicated by potential off-target effects and pharmacokinetic issues. For example, Ihh loss has striking effects on digit morphology (see Pacifici et al., 2005). In the chick, digit loss following cyclopamine was invariably associated with changes in digit identity (number of phalanges altered) as well as digit number, whereas we never observed phalangeal changes following genetic removal of Shh (Scherz et al., 2007). Whether this difference reflects the approach used or an inherent difference between birds and mammals remains to be seen. In mouse limb buds, cyclopamine’s effects on gene expression were analyzed in short-term organ culture and showed that mid-phase 5′Hoxd expression recovered only slowly over time following drug-induced Shh inhibition (Panman et al., 2006), suggesting a prolonged requirement for Shh in regulating digit identity. 5′Hoxd genes play a role in regulating digit pattern downstream of Shh, but the timing of their functional requirement is still not established, and they may be required only very late following activation by other (as yet unknown) intermediaries downstream of Shh (reviewed by Mariani and Martin, 2003). Using timed removal of a floxed Hoxd11-d13 allele (Zakany and Duboule, 1996), we have found that 5′Hoxd function is in fact essential after E11.5 for correct digit patterning; the phenotype resulting from tamoxifen-induced deletion at E11.5 is similar to the complete null phenotype (unpublished data). Furthermore, in the nonconditional Shh null mutant, although mid-phase Hoxd expression is absent in the limb, late-phase Hoxd13 expression recovers completely in the hindlimb bud (and is clearly expressed by E11.5), indicating that late-phase Hoxd expression is Shh independent (Chiang et al., 2001).
Altogether, the data in this report provide strong support for the model that Shh, whether acting as a morphogen or trigger, specifies digit identity very early and that later, in the undifferentiated limb bud, Shh acts mainly to ensure sufficient cell numbers to form a normal five-digit limb. Mutant phenotypes attest to uncoupled growth in the face of absent or perturbed patterning (e.g., Litingtung et al., 2002; te Welscher et al., 2002); it is less clear whether the reverse, preserved patterning in the context of compromised growth, also holds. Our results suggest that patterning can in fact be uncoupled from growth. Such uncoupling of AP pattern from growth regulation may serve as one avenue for evolution to diversify and adapt digit number without disturbing normal digit morphology, as previously suggested (Shapiro et al., 2003; Stopper and Wagner, 2007).
EXPERIMENTAL PROCEDURES
Mouse Lines and Embryo Analyses
The Shh-floxed (Lewis et al., 2001), Shh-deleted (Chiang et al., 2001), and Noggin/lacZ knockin lines (Brunet et al., 1998) were described previously. The generation and characterization of the Hoxb6/CreERT line will be described elsewhere (M.T.N. et al., unpublished data). All alleles were maintained on a predominantly FVB/n background, and expression profiles (in Figure 1D) are typical for this background. Somite numbers are also given for all intervals in detailed time course analyses (Figure S1). Noon on the date of the vaginal plug was defined as E0.5. Tamoxifen treatment (single IP dose of 3 mg), embryo collection, and analyses were described previously (Nakamura et al., 2006). A 246 bp Shh exon2 probe detecting only the floxed region was used to evaluate Shh transcript loss (generated with PCR primers 5′-GTGCAAAGACAAGTTAAATGCCT-3′ and 5′-CAGTGGATGTGAGCTTTGGA-3′). Lysotracker (Molecular Probes) and anti-phospho-Histone H3 staining (Upstate Biotechnology) were used as recommended. Shhflox/flox;Cre+ mice were mated with Shh+/Δ or Shhflox/flox to generate mutant embryos with comparable results. Control siblings, including Shh+/flox;Cre+ and ShhΔ/flox;Cre− genotypes (and in a few experiments, Shhflox/flox;Cre−), and wild-type embryos had very similar gene expression, proliferation, and skeletal morphologies. Shh+/flox;Cre+ embryos had slightly more apoptosis than Cre− controls. Mitotic indices were determined for control and mutant sibling embryos limb buds (Figure 4B) by counting pH3+/DAPI nuclei in the distal limb bud area (400–600 cells) on 3–4 stained sections from each of 1–3 independent limb buds of each genotype. Statistical significance of differences in average mitotic indices were determined using a two-tailed t test. For flow cytometry, 1 mg of BrdU was injected IP 60 min prior to embryo harvest; limb buds were dissected and dissociated (with 0.1% trypsin-EDTA, 1 mg/ml collagenase), and pooled forelimb or hindlimb pairs from an embryo were analyzed using a FITC-BrdU Flow kit (BD PharMingen) and FlowJo software.
Skeletal Analysis
E15.5–E17.5 embryos were processed and stained with alizarin red and alcian blue for skeletal analysis. Criteria used for assigning digit identity were: (1) importantly, loss of associated carpals or tarsals: for digits 2, 3, and 4, the shape and size of articulating wrist/ankle bones are distinctive (see diagrams in Figure 2) and were always lost together with the metapodial and phalangeal digit elements; (2) digit attenuation: often observed as an “intermediate” state (e.g., asterisks in Figure 2) that anticipated the next digit to be lost; (3) metapodial shape, particularly the proximal articular end; and (4) element length and timing of ossification, which were somewhat variable (even in wild-type and control siblings) and used mainly for secondary confirmation. Phalanx number is distinctive only for digit 1 and was not useful; all other digits normally have three phalanges, and loss of phalangeal elements was not observed in mutant embryos.
Supplementary Material
ACKNOWLEDGMENTS
We thank Cliff Tabin for stimulating discussions and comments on the manuscript, Richard Behringer and Rolf Zeller for critical reading of the manuscript, Mike Lenardo for insightful advice on key experiments, Seth Finger for assistance in assay development, Heiner Westphal and Chin Chiang for providing the Shh null allele mouse line, and Heinz Arnheiter for Ptc1, Benoit Bruneau for Tbx2 and Tbx3, and Gail Martin for Fgf8 probes. We also thank Andy McMahon for his generosity and service to the entire scientific community in making genetically engineered lines generated in his laboratory readily available to everyone. This research was supported by the Center for Cancer Research, National Cancer Institute, NIH.
Footnotes
SUPPLEMENTAL DATA
Supplemental Data include five figures and two tables (detailed kinetic analyses of Shh and Ptc1 expression decay following tamoxifen; Tbx2 and Tbx3 expression at normal two-digit condensation stage; Sox9-lacZ visualization of normal digit condensation order; mitotic levels and AER-Fgf8 expression in mutant embryos; mutant phenotype frequencies and characteristics) and are available at http://www.developmentalcell.com/cgi/content/full/14/4/624/DC1/.
REFERENCES
- Ahn S, and Joyner AL (2004). Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118, 505–516. [DOI] [PubMed] [Google Scholar]
- Barna M, and Niswander L (2007). Visualization of cartilage formation: insight into cellular properties of skeletal progenitors and chondrodysplasia syndromes. Dev. Cell 12, 931–941. [DOI] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, and Berman DM (2004). Tissue repair and stem cell renewal in carcinogenesis. Nature 432, 324–331. [DOI] [PubMed] [Google Scholar]
- Bi W, Deng JM, Zhang Z, Behringer RR, and de Crombrugghe B (1999). Sox9 is required for cartilage formation. Nat. Genet 22, 85–89. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, and Harland RM (1998). Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science 280, 1455–1457. [DOI] [PubMed] [Google Scholar]
- Buscher D, Bosse B, Heymer J, and Ruther U (1997). Evidence for genetic control of Sonic hedgehog by Gli3 in mouse limb development. Mech. Dev 62, 175–182. [DOI] [PubMed] [Google Scholar]
- Chen MH, Li YJ, Kawakami T, Xu SM, and Chuang PT (2004). Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev. 18, 641–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, and Fallon JF (2001). Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev. Biol 236, 421–435. [DOI] [PubMed] [Google Scholar]
- Dahn RD, and Fallon JF (2000). Interdigital regulation of digit identity and homeotic transformation by modulated BMP signaling. Science 289, 438–441. [DOI] [PubMed] [Google Scholar]
- Drossopoulou G, Lewis KE, Sanz-Ezquerro JJ, Nikbakht N, McMahon AP, Hofmann C, and Tickle C (2000). A model for anteroposterior patterning of the vertebrate limb based on sequential long- and short-range Shh signaling and Bmp signaling. Development 127, 1337–1348. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, and Tabin CJ (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517–528. [DOI] [PubMed] [Google Scholar]
- Hasson P, Del Buono J, and Logan MPO (2007). Tbx5 is dispensable for forelimb outgrowth. Development 134, 85–92. [DOI] [PubMed] [Google Scholar]
- Hornbruch A, and Wolpert L (1970). Cell division in the early growth and morphogenesis of the chick limb. Nature 226, 764–766. [DOI] [PubMed] [Google Scholar]
- Ingham PW, and McMahon AP (2001). Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087. [DOI] [PubMed] [Google Scholar]
- Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, and McMahon AP (2001). Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell 105, 599–612. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang HM, Litingtung Y, and Chiang C (2006). Cholesterol modification restricts the spread of Shh gradient in the limb bud. Proc. Natl. Acad. Sci. USA 103, 6548–6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litingtung Y, Dahn RD, Li YN, Fallon JF, and Chiang C (2002). Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 418, 979–983. [DOI] [PubMed] [Google Scholar]
- Mariani FV, and Martin GR (2003). Deciphering skeletal patterning: clues from the limb. Nature 423, 319–325. [DOI] [PubMed] [Google Scholar]
- Marigo V, Johnson RL, Vortkamp A, and Tabin CJ (1996). Sonic hedgehog differentially regulates expression of GLI and GLI3 during limb development. Dev. Biol 180, 273–283. [DOI] [PubMed] [Google Scholar]
- Michos O, Panman L, Vintersten K, Beier K, Zeller R, and Zuniga A (2004). Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development 131, 3401–3410. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Nguyen MT, and Mackem S (2006). Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev. Dyn 235, 2603–2612. [DOI] [PubMed] [Google Scholar]
- Pacifici M, Koyama E, and Iwamoto M (2005). Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res. C Embryo Today 75, 237–248. [DOI] [PubMed] [Google Scholar]
- Panman L, Galli A, Lagarde N, Michos O, Soete G, Zuniga A, and Zeller R (2006). Differential regulation of gene expression in the digit forming area of the mouse limb bud by SHH and gremlin 1/FGF-mediated epithelial-mesenchymal signalling. Development 133, 3419–3428. [DOI] [PubMed] [Google Scholar]
- Parr BA, and McMahon AP (1995). Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature 374, 350–353. [DOI] [PubMed] [Google Scholar]
- Platt KA, Michaud J, and Joyner AL (1997). Expression of the mouse Gli and Ptc genes is adjacent to embryonic sources of hedgehog signals suggesting a conservation of pathways between flies and mice. Mech. Dev 62, 121–135. [DOI] [PubMed] [Google Scholar]
- Scherz PJ, McGlinn E, Nissim S, and Tabin CJ (2007). Extended exposure to Sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev. Biol 308, 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schughart K, Bieberich CJ, Eid R, and Ruddle FH (1991). A regulatory region from the mouse Hox-2.2 promoter directs gene-expression into developing limbs. Development 112, 807–811. [DOI] [PubMed] [Google Scholar]
- Shapiro MD, Hanken J, and Rosenthal N (2003). Developmental basis of evolutionary digit loss in the Australian lizard Hemiergis. J. Exp. Zoolog. B Mol. Dev. Evol 297, 48–56. [DOI] [PubMed] [Google Scholar]
- Shubin NH, and Alberch P (1986). A morphogenetic approach to the origin and basic organization of the tetrapod limb. In Evolutionary Biology, Volume 20, Hecht MK, Wallace B, and Prance GI, eds. (New York: Plenum Press; ), pp.319–387. [Google Scholar]
- Stopper GF, and Wagner GP (2007). Inhibition of Sonic hedgehog signaling leads to posterior digit loss in Ambystoma mexicanum: parallels to natural digit reduction in urodeles. Dev. Dyn 236, 321–331. [DOI] [PubMed] [Google Scholar]
- Sun X, Mariani FV, and Martin GR (2002). Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature 418, 501–508. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Takeuchi J, Koshiba-Takeuchi K, Koshiba-Takeuchi K, and Ogura T (2004). Tbx genes specify posterior digit identity through Shh and BMP signaling. Dev. Cell 6, 43–53. [DOI] [PubMed] [Google Scholar]
- te Welscher P, Zuniga A, Kuijper S, Drenth T, Goedemans HJ, Meijlink F, and Zeller R (2002). Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science 298, 827–830. [DOI] [PubMed] [Google Scholar]
- Tickle C (2006). Making digit patterns in the vertebrate limb. Nat. Rev. Mol. Cell Biol 7, 45–53. [DOI] [PubMed] [Google Scholar]
- Verheyden JM, Lewandoski M, Deng C, Harfe BD, and Sun X (2005). Conditional inactivation of Fgfr1 in mouse defines its role in limb bud establishment, outgrowth and digit patterning. Development 132, 4235–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Drossopoulou G, Chuang PT, Duprez D, Marti E, Bumcrot D, Vargesson N, Clarke J, Niswander L, McMahon A, et al. (1997). Relationship between dose, distance and time in Sonic Hedgehog-mediated regulation of anteroposterior polarity in the chick limb. Development 124, 4393–4404. [DOI] [PubMed] [Google Scholar]
- Zakany J, and Duboule D (1996). Synpolydactyly in mice with a targeted deficiency in the HoxD complex. Nature 384, 69–71. [DOI] [PubMed] [Google Scholar]
- Zeller R (2004). It takes time to make a pinky: unexpected insights into how SHH patterns vertebrate digits. Sci. STKE 259, pe53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.