Abstract
In 2002, a transmembrane protein—now known as FNDC5—was discovered and shown to be expressed in skeletal muscle, heart, and brain. It was virtually ignored for 10 years, until a study in 2012 proposed that, in response to exercise, the ectodomain of skeletal muscle FNDC5 was cleaved, traveled to white adipose tissue, and induced browning. The wasted energy of this browning raised the possibility that this myokine, named irisin, might mediate some beneficial effects of exercise. Since then, more than 1000 papers have been published exploring the roles of irisin. A major interest has been on adipose tissue and metabolism, following up the major proposal from 2012. Many studies correlating plasma irisin levels with physiological conditions have been questioned for using flawed assays for irisin concentration. However, experiments altering irisin levels by injecting recombinant irisin or by gene knockout are more promising. Recent discoveries have suggested potential roles of irisin in bone remodeling and in the brain, with effects potentially related to Alzheimer’s disease. We discuss some discrepancies between research groups and the mechanisms that are yet to be determined. Some important questions raised in the initial discovery of irisin, such as the role of the mutant start codon of human FNDC5 and the mechanism of ectodomain cleavage, remain to be answered. Apart from these specific questions, a promising new tool has been developed—mice with a global or tissue-specific knockout of FNDC5. In this review, we critically examine the current knowledge and delineate potential solutions to resolve existing ambiguities.
Keywords: FNDC5, irisin, myokine, metabolism, bone, brain
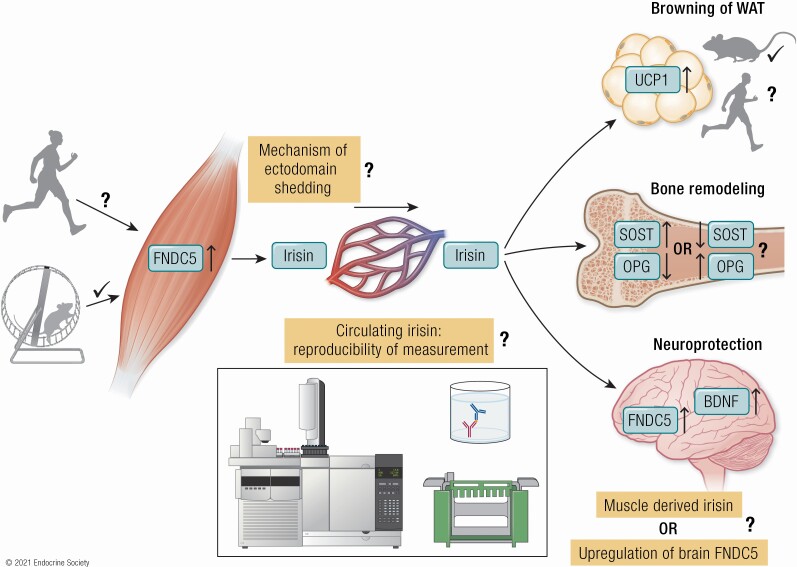
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
Irisin was discovered in 2012 as a potential myokine, cleaved from its parent FNDC5 in response to exercise, and inducing browning of white adipose tissue (WAT)
Hundreds of studies reported irisin levels in response to different physiological challenges but are compromised by flawed quantitative assays
Some studies confirmed that irisin mediates exercise-induced browning of inguinal WAT in mice, whereas others failed to confirm
In humans there is little or no data supporting an effect of exercise on browning of WAT
Effects of FNDC5/irisin in bone remodeling were reported in mice but with contradictory findings
FNDC5/irisin was linked to regulation of BDNF and neurogenesis in mice; pathways and mechanisms need to be confirmed and extended
FNDC5 knockout mice provide a new tool to study many aspects of FNDC5/irisin biology
In 2002, 2 independent groups of researchers characterized a hitherto unknown gene expressed in heart, brain, skeletal muscle, and other tissues of mice during embryonic development and in adult animals. It was named peroxisomal protein (PeP) (1) or fibronectin type III repeat containing protein 2 (FRCP2) due to its specific motif (2). Only a decade later, this gene—now annotated as fibronectin type III domain-containing protein 5 (FNDC5)—gained attention again when it was identified as one of the target genes of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α; PPARGC1A), a transcriptional coactivator that is induced in muscle by exercise (3). FNDC5 was upregulated in muscle of transgenic mice overexpressing PGC-1α, and in mice subjected to endurance exercise. Conditioned media from cultured myocytes of the PGC-1α transgenic mice caused primary mouse adipocytes to upregulate genes related to brown adipose tissues (BAT). The authors proposed that the extracellular part of FNDC5 was released from skeletal muscle into the blood stream and induced a transition of white adipose tissue (WAT) to adipose tissue with more BAT-like morphology (browning), and a thermogenic program dissipating energy as heat. They named the secreted domain irisin and concluded that irisin could be responsible for at least some of the beneficial effects of exercise on energy expenditure via an unexpected browning of adipose tissue (3). It was unexpected because browning of WAT is usually associated with cold exposure to preserve vital functions of the brain and heart (4).
Since then, more than 1000 original articles have been published investigating relationships between irisin or FNDC5 and different exercise regimens, pathological conditions, and nutritional interventions. Moreover, in vitro and in vivo effects of administration of recombinant irisin (r-irisin) on a variety of cell types and in rat and mouse models have been elucidated. However, there are many contradictions and ambiguities due to the unreliability of measurements of endogenous irisin and varying doses of r-irisin used in different studies. Furthermore, putative species differences in FNDC5 expression and the role of glycosylation for the biological activity of irisin need to be investigated further.
In this article, we review the literature regarding the purported skeletal muscle secreted myokine irisin and its precursor transmembrane protein FNDC5 in rodents and humans. We pay special attention to measurement of endogenous irisin in mice and humans as several hundreds of studies rely on poorly validated assays. Furthermore, we critically analyze the proposed physiological roles of FNDC5/irisin in health and disease, also those based on injected recombinant irisin and gene knockouts.
FNDC5: Sequence Conservation, Start Codon, Cleavage of the Ectodomain and Structure of Irisin
FNDC5 in Mice and Humans: Sequence Conservation and the Start Codon
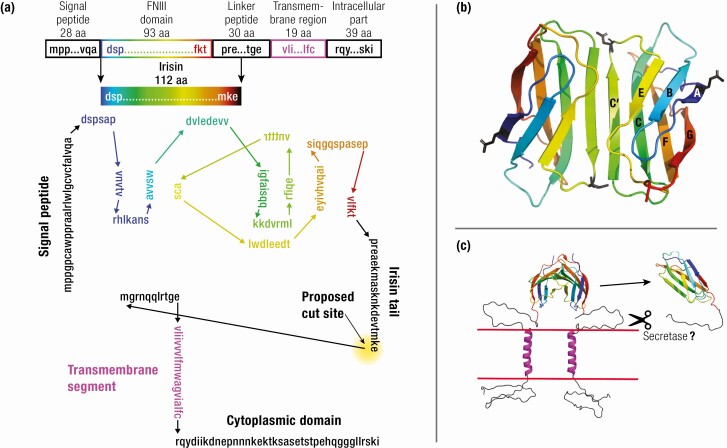
The mouse FNDC5 locus comprises 6 exons and encodes a protein of 209 amino acids (aa). FNDC5 protein, diagrammed in Fig. 1A, comprises a 28-aa signal peptide, a 93-aa fibronectin type III domain (FNIII), a 30-aa linker, a 19-aa transmembrane segment, and a 39-aa intracellular part. The N-terminal signal sequence, which provides transport of the protein across the membrane, is then cleaved (5). Without the signal peptide, the predicted molecular weight (MW) of the FNDC5 protein is 20 300. The FNIII domain plus 19 aa of the linker, comprising aa 29 to 140, was proposed to be proteolytically cleaved from this transmembrane protein (Fig. 1A), and was named irisin after the Greek messenger goddess of the rainbow (3). The 112-aa irisin peptide has a theoretical MW of 12 600 without glycosylation.
Figure 1.
Structure of FNDC5 and irisin. (A) Schematic structure of the mouse FNDC5 protein with functional units (upper part). Diagram of the FNDC5 protein. The FNIII domain is in color, with beta strands vertical and connecting loops horizontal. The mature irisin peptide runs from the signal peptide cleavage site to the proposed irisin cut site (lower part). (B) Ribbon diagram of the irisin dimer (pdb 4LSD chains A,B, (5). The C’ strands pair to form a continuous eight-strand beta sheet in the dimer. Asn36 and Asn81, the putative sites of N-linked glycosylation, are shown in black sticks. (C) A model of transmembrane FNDC5, where the linker between the FNIII domain and the transmembrane helix is shown as unstructured coil. The cleavage step should involve a secretase but this has not been explored. Alternatively, the dimeric FNDC5 could function as a transmembrane receptor, with unknown ligand. Figures B and C were constructed in PyMOl (The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC).
The irisin peptide is 100% conserved in human, mouse, rat, and cattle and there are only 3 conservative substitutions in chicken (3, 6). The peptide is more divergent in fish, and the FNDC5 gene is completely missing in amphibians. The signal peptide and segments from C-terminal to irisin are more variable, but overall, FNDC5 is a highly conserved gene. There is one striking exception: the human FNDC5 gene has a mutant start codon, ATA instead of the canonical ATG. Raschke et al (7) tested experimentally the effect of the ATA codon on FNDC5 expression efficiency by transfecting HEK293 cells with FNDC5 constructs containing either the canonical or the noncanonical codon. Constructs with an ATA codon gave a FNDC5 protein level only 1% that of constructs with ATG. They concluded that the human FNDC5 gene has substantially lost the ability to be translated into full-length FNDC5 protein (7).
Jedrychowski et al (8) suggested that genes with non-ATG codons may be fully expressed endogenously in their native environment as part of a complex translation regulation. However, Ivanov et al (9) identified only 59 human genes that were translated from a non-ATG start codon. Only 6 human genes have an ATA start codon. For 5 of these, the ATA start is present in other mammalian species (TEAD3, SP3, IFT46, VANGL2, and FAM217B), consistent with these being functional and conserved across species. The sixth—human FNDC5—is the only gene described so far with an ATA start codon that is not conserved in any other species. The ATA start of FNDC5 is also found in Denisovan and Neanderthal genomes, whereas all other great apes have ATG. This suggests that reduced expression of FNDC5 has become beneficial in the hominid lines.
Ivanov et al (9) also noted that “for initiation at non-ATG codons, the presence of a good Kozak context is crucial.” The most important bases are −3 “A/G” and +4 “G” (counted from the start of ATA). The aforementioned 5 human genes have these favorable bases, whereas the human FNDC5 gene has an unfavorable “C” at +4. This is consistent with the greatly reduced expression observed by Raschke et al (7).
We have found 3 examples of human genes where a rare mutation of the start codon resulted in essentially null expression of the protein: α2 globin, phenylalanine hydroxylase (PAH), and albumin (ALB). A mutation from ATG to ACG in the start codon of α2 globin reduced translation enough to cause clinical thalassemia, which is otherwise produced by gene deletion or severe truncation (10). The Kozak sequence had favorable A and G at −3 and +4. The mutation from ATG to ATA in PAH leads to strongly diminished expression of the gene and causes genetic phenylketonuria (11). The +4 position in its Kozak sequence is an unfavorable “A.” Caridi et al (12) reported an ATG to CTG mutation in the ALB gene promoting almost total loss of albumin in 2 homozygous patients. Again, there is an “A” at position +4 of its Kozak sequence. These cases are consistent with the original study of Kozak (13) showing that single-base changes in the canonical start codon reduced expression 20-fold or more.
Whereas these 3 examples support the idea that human FNDC5 should be a null protein, or express only a truncated peptide from the downstream ATG codon, this is contradicted by experimental evidence for the existence of a full-length FNDC5 protein in humans by different methods. Although the transcript variability of human FNDC5 obviously is higher than currently annotated, our recent data do not support expression of truncated FNDC5 from its downstream canonical start codon (14). The most important evidence for translation of full-length FNDC5 is that we and others have detected a peptide signature by mass spectrometry (MS; discussed below) that only can be detected if a transcript starting from the ATA start codon is translated (8, 14, 15). Hence, the conclusion is that the ATA is, for reasons we do not yet understand, sufficiently active to produce plasma irisin at a level that can be detected by MS.
Structure and Biochemistry of Irisin
The crystal structure of irisin showed the typical structure of an FNIII domain, a beta sandwich with 3 beta strands on one side and 4 on the other (5). It is remarkable that 2 subunits are associated to form a tight dimer (Fig. 1B). The primary association is via the 2 C′ strands associating antiparallel to form a beta zipper, making an extended 8-strand beta sheet across the 2 domains. The association is strengthened by van der Waals and hydrophobic contacts and buries an area of 1400 Å 2, consistent with a high-affinity association.
Irisin has 2 sites for N-linked glycosylation, which increases its molecular weight. Albrecht et al (16) measured the apparent MW of 2 well-characterized r-irisins in Western blots. Nonglycosylated r-irisin produced in E. coli ran at an apparent MW of 13 000, close to the 12 300 for this 112-aa peptide. Glycosylated r-irisin ran as a fuzzy band at an apparent MW of 20 000. Incubation with Peptide:N-glycosidase F (PNGase F) reduced the glycosylated band largely to a sharp band at ~13 000. Thus, glycosylation adds 7000 to the apparent MW on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Full-length FNDC5 (without the signal peptide) consists of 181 aa and has a predicted MW of 20 300. Glycosylation, which is limited to the 2 sites in the irisin domain, should again add 7000, for a total of 28 000. Several groups have shown Western blot bands for FNDC5 and irisin at least close to the expected size ranges, with the exception for deglycosylated FNDC5 (Table 1). According to the theoretical MW, 1 nmol/L of glycosylated irisin corresponds to a circulating level of ~ 20 ng/mL.
Table 1.
Predicted and observed molecular weights for human glycosylated (glyc) and nonglycosylated (nonglyc) FNDC5 and irisin
| Product | Amino acids* | Predicted MW | Observed MW | References | ||
|---|---|---|---|---|---|---|
| nonglyc | glyc | nonglyc | glyc | |||
| FNDC5 | 32-212 | 20 300 | 28 000 | 25 000** | 23 – 28 000 | (14, 17-20) |
| Irisin | 32-143 | 12 600 | 20 000 | 13 000 | 20 000 | (16) |
* Numbers refer to entry Q8NAU1 (UniProtKB, https://www.uniprot.org).
** The only report on the apparent size of deglycosylated FNDC5 in Western blots (14).
Cleavage of the FNDC5 Ectodomain
A central hypothesis for the proposed role of FNDC5 in metabolism is that the ectodomain of FNDC5 is cleaved to produce the soluble irisin, which circulates and targets tissues. Surprisingly, the only experimental evidence for cleavage is 2 experiments in the original study of Boström et al (3). Their first experiment was a Western blot purporting to show irisin released into the medium from HEK 293 cells transfected with mouse FNDC5. However, the Western blots showed bands at a MW of ~ 32 000 before and 20 000 after deglycosylation. These bands are much too large to represent irisin species. Moreover, the antibody used in this study was raised against a C-terminal peptide of FNDC5 that is not included in the irisin domain. It was noted in a later study that this antibody should not stain irisin (21). The second experiment used MS to analyze the site of cleavage. However, the peptide they reported as showing the C-terminal cut site, KDEVTMKE, seems not to be a tryptic peptide (trypsin cuts after K and R, whereas the aa preceding this peptide is N). Wrann et al (22) repeated this experiment with cultured neurons, using an antibody that should recognize irisin. Their Western blot showed a band with a MW 13 000 in the media of cells transfected with FNDC5, which was not present in cells transfected with green fluorescent protein. This is consistent with cleavage, although it did not define the cut site. The cleavage step is key to the proposed role of irisin as a myokine.
In addition to the problems identifying the cut site, the cleavage is lacking important mechanistic details. No experiments have been described so far to identify a secretase and a signaling mechanism to activate it, which are important features of ectodomain shedding (23). If there is a secretase that operates on skeletal muscle FNDC5, one would have to propose that a related secretase is present in the HEK 293 cells used by Boström et al (3), and in the cultured neurons in the study of Wrann et al (22). Also, no one has quantitated the fraction of FNDC5 that is cleaved in the cell culture systems. It would be useful to know if the cleaved irisin was a large or small fraction of the FNDC5 on the cell surface.
Two more recent studies have considered the question of cleavage. Nie et al (24) attempted to address the maturation of FNDC5 and the cleavage of irisin by expressing different FNDC5-constructs in HEK 293T cells. However, most of their constructs lacked an N-terminal signal peptide, so they should remain in the cytoplasm. Overall, their results did not provide evidence for cleavage of irisin from FNDC5. Lourenco et al (25) analyzed hippocampal proteins in mice by MS. They reported 2 peptides that are characteristic for full-length FNDC5 but are not part of the irisin sequence. The peptides were found in 4 bands in the MW range from 29 000 to 75 000. Thus, a substantial fraction, perhaps all, of FNDC5 in brain appears to remain as an uncut transmembrane protein.
A scale model of the complete FNDC5 molecule is shown in Fig. 1C, where the link between the FNIII domain and the transmembrane helix are indicated as unstructured coil. The cytoplasmic domain is also indicated as unstructured. In the initial discovery of FNDC5 and FNDC4, Teufel et al (2) suggested that they “are likely receptors of an as yet to be identified ligand.” Until we have a better understanding of a mechanism for cleavage, the alternative hypothesis, that FNDC5 is a transmembrane receptor, should remain on the table.
Although many questions remain regarding generation and quantitation of endogenous irisin, there exists clear evidence from MS measurements that irisin is present in biological fluids of humans and mice. Moreover, there are numerous reports on biological effects of exogenous (recombinant) irisin in cells and rodent models. These are addressed later.
Detection and Quantification of FNDC5 and Irisin
FNDC5 and irisin have been detected mainly by 3 antibody-dependent methods: (i) Western blot (qualitative and semi-quantitative); (ii) enzyme-linked immunosorbent assays (ELISA, quantitative), and (iii) protein liquid chip assay (Milliplex Map Human Myokine Magnetic Bead Panel; Merck, Darmstadt, Germany, quantitative). In addition, different MS approaches have been employed for identification or quantification of irisin.
The first rough estimate of circulating irisin levels in mice was 40 nmol/L corresponding to 800 ng/mL glycosylated irisin (3). This is > 2500-fold higher than the level reported by the same group later using quantitative MS (0.3 ng/mL in mouse plasma (26)). These highly discordant data delineate the dilemma in determining circulating irisin levels. Among all the methods, MS with quantification peptides is considered the “gold standard” for measurement of protein concentrations (27). However, there are some methodological problems even with this method discussed below. Nevertheless, the only available reference values for circulating irisin: 3 to 4 ng/mL in humans (8) and 0.3 ng/mL in mice (26) were determined with this method. These “gold standard” MS values should be kept in mind when assessing the methods for irisin detection described in the following sections.
Antibody-Based Methods—Western Blot
The initial description of irisin was largely based on Western blot detection of bands at an MW of ~ 20 000 – 22 000 in mouse and human plasma and serum, after deglycosylation with PNGase F (3). However, this research group used an antibody directed against aa 149 to 178 of human FNDC5 (aa 146-175 in mice), which are not part of the irisin domain (aa 29-140; reviewed in 28). Lee et al (21) used the same inappropriate antibody and found protein bands of ~36 000 and ~24 000 before and after deglycosylation of human plasma. These MWs are all higher than those expected for glycosylated and deglycosylated r-irisin as discussed above (Table1). These authors—aware that this specific antibody (Abcam Cat. # ab93373) should not stain irisin—speculated that shivering might cause release into circulation of FNDC5 fragments containing the C-terminal epitope (21). This contradicted the active release mechanism proposed by Boström et al (3). Consequently, these Western blots apparently did not show irisin. Eventually, the antibody used in these studies was discontinued by the provider and these initial results were never questioned or clarified by the authors.
Targets at ~ 25 000 were correctly assigned as FNDC5 and not as irisin in later work on skeletal muscle and adipose samples using antibodies raised against the C-terminus of FNDC5 (14, 16, 17, 19, 29). However, Lourenco et al (25) reported bands at 29 000, 40 000, 58 000, and 75 000 in mouse hippocampus with FNDC5 peptide signatures verified by MS, and suggested these might be due to posttranslational modifications and/or the existence of multimers. Posttranslational modifications producing these large increases in size are unlikely, and multimers should be disrupted in denaturing gel electrophoresis. In conclusion, detection of FNDC5 at 25 000 – 28 000 in Western blots in different tissues is reproducible with a variety of antibodies. Higher MW species of glycosylated FNDC5 and the existence of FNDC5 multimers remain questionable.
Relative to the picture with FNDC5, there is still much more confusion concerning detection of circulating irisin in Western blots. Wrann et al (22) named a 22 000 band in deglycosylated mouse plasma as irisin although irisin was shown at ~ 15 000 in deglycosylated cell culture supernatant in another experiment of this work. Only few studies demonstrated bands close to the expected size range for deglycosylated irisin (13 000) in plasma or serum of mice and humans (8, 18, 30). However, the antibodies used are not available anymore or the results could not be reproduced independently (14). In contrast to these results identifying irisin as bands of too high MW, Chen et al (31) reported a band at MW of 13 000 as irisin in mouse serum without prior deglycosylation, which is too small for glycosylated irisin.
We have tested 7 different irisin antibodies for detection in Western blots. All of them had a comparable sensitivity against r-irisin and glycosylated r-irisin after deglycosylation (~125 pg deglycosylated irisin per lane) but failed to detect a specific band in plasma or serum of different species. Instead, we observed massive binding to unspecific proteins in Western blots of serum or plasma proteins (14, 16). To match our experimentally determined detection limit, a circulating concentration of > 10 ng/mL irisin would be required, considering the processing steps including deglycosylation and the maximum applicable protein amount in our Western blots of 160 µg per lane. Thus, the 0.3 to 4 ng/mL plasma irisin determined by quantitative MS in mice and humans would not be detectable by Western blots.
In summary, there are still no reproducible results concerning the detection of circulating irisin by Western blot in any species.
Antibody-Based Methods—ELISA
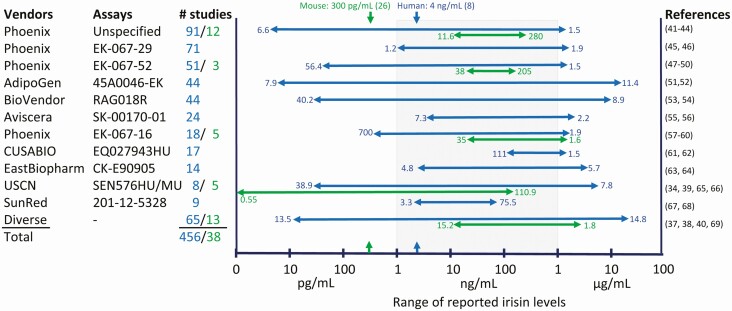
Huh et al (32) were the first to test 2 ELISAs available at that time and reported levels of 113.1 ± 20.6 ng/mL in a large cohort of middle-aged, healthy women, and of 112.7 ± 32.2 ng/mL in a small group of obese female and male individuals. Surprisingly, the basal irisin level in young, trained males was 4 times higher (473.4 ± 36.4 ng/mL). The next published study used a different ELISA and found levels between 770 ng/mL in normal weight subjects and 917 ng/mL in severely obese persons (33). This started a period with studies reporting extremely divergent levels of circulating irisin in humans that lasts until today. Numerous producers and suppliers offer irisin ELISAs mostly validated against the immunogen in artificial systems but not in biological samples. More than 100 publications rapidly appeared with irisin values varying by 6 orders of magnitude (40 pg/mL (34) vs 4.3 µg/mL (35))until we warned about the unreliability of irisin levels based on ELISAs in 2015 (16). Perakakis et al (36) predicted that the available irisin assays would consolidate and converge with time, based on past experience with several other hormones such as luteinizing hormone, growth hormone, and leptin. Unfortunately, this expectation has not been fulfilled even today. Instead, the range of reported irisin levels in humans has actually increased in recent years (13.5 pg/mL (37) vs 14.8 µg/mL (38)). The situation in rodents is similar to that of humans albeit fewer studies have been published. Reported circulating irisin levels in mice span a range from less than 1 pg/mL (39) to more than 1.5 µg/mL (40). The heterogeneous results may largely be due to nonspecific binding of the irisin antibodies to other plasma/serum proteins as observed in Western blots (14, 16). A graphical overview of measurements of circulating irisin in humans and mice is given in Fig. 2.
Figure 2.
Plasma concentrations of irisin measured with ELISA methodologies in human (blue) and mice (green). The numbers given on the arrowheads represent the lowest and highest irisin levels (units on the x-axis) reported for the specific assay in both species. All values are means or medians of control groups. Concentrations measured by quantitative MS for humans and mice are marked by arrows above the graph and on the x-axis.
Some studies measured irisin in subsets or in their total samples with 2 different ELISA/radioimmunoassays and noted differences in the values by factors of 2 to 25 (70, 71), or correlations as low as r = 0.03 between measurements using different assays (16). Additional problems arose from missing lot-to-lot reproducibility even within a single assay. Montes-Nieto et al (72) observed a correlation of only r = 0.22 comparing 2 lots of an ELISA from the same manufacturer. Moreover, inadequate description of reagents in the literature (missing lot and/or catalog numbers) hinders reproducibility and contributes to the current confusion about circulating levels of irisin.
In summary, all published data on irisin levels based on ELISAs are compromised by major methodical problems even 9 years after its discovery.
Antibody-Based Methods—Protein Liquid Chip Assay
A single study presented serum irisin levels of 1.1 ± 0.2 ng/mL in healthy, newborn infants measured with a Luminex bead-based multiplex detection system (Merck Millipore; (31)). This system detects 15 myokines, including irisin, simultaneously. Because this system is also antibody-based, further verification is required.
Label-Free Methods—Qualitative Mass Spectrometry
MS was employed for characterization of the putatively secreted FNDC5 fragment in the initial study (3). Untargeted and targeted MS was subsequently used to identify specific FNDC5/irisin peptide signatures in different tissues of humans and mice. Besides the fact that antibodies often are used to visualize the region of interest on gels, the analysis itself requires no label. After excision of the bands of interest from unmarked gels, in-gel digestion of the proteins and subsequent chromatographic separation, specific peptides can be detected. Lee et al (21) detected a peptide (FIQEVNTTTR) within bands at a MW of 32 000 and 24 000 among human serum proteins before and after deglycosylation (see comments on the antibody from this study above under “Antibody-Based Methods—Western Blot”). In contrast, Albrecht et al (16) found the same signature in the MW region 20 000 to 25 000 in native human serum samples, in spite of this region showing no bands stained by an irisin antibody. This corresponded to the MW of glycosylated irisin. Accordingly, Peng et al (73) identified the peptide in the MW fraction of mouse serum from 10 000 to 50 000. It must be noted that the detected fragment (FIQEVNTTTR) is part of FNDC5 as well as of irisin, making the apparent MW of the excised bands important for interpretation. Thus, the results of Chen et al (31) were surprising, because they observed the signature in a band at a MW of 13 000 of mouse serum without prior deglycosylation although irisin should have been present at a MW of 20 000 under these conditions. In contrast, Barja-Fernandez et al (74) did not report any signatures of FNDC5 or irisin in antibody-stained bands of rat plasma or gastric mucosa homogenates. Lourenco et al (25) analyzed mouse hippocampus by Western blot and identified 4 bands with 2 different FNDC5 peptides (but not FIQEVNTTTR) by MS. As discussed above, these bands are much too large to be FNDC5. Overall, it seems that MS can detect the FNDC5 peptides, especially FIQEVNTTTR, with very high sensitivity. Surprisingly, this and further FNDC5-specific peptide signatures were also found in SDS-PAGE bands that are too large or too small to be FNDC5 or irisin. Thus, identification of a band as FNDC5 based on detection of a peptide may be questionable, although we cannot propose a specific mechanism.
Label-Free Methods—Quantitative Mass Spectrometry
Three different laboratories have employed quantitative MS for measuring circulating irisin (8, 14, 15, 26). A targeted search for irisin-specific peptides was combined with the addition of defined amounts of labeled peptides to the sample. The amount of labeled irisin peptides is used to calculate the amounts of (“natural,” unlabeled) irisin in the sample by direct comparison of the peak heights or areas. Jedrychowski et al (8) were the first to use 2 labeled irisin peptides for quantification. Besides the well-known tryptic fragment, FIQEVNTTTR, they labeled DSPSAPVNTVR. This fragment, the N-terminus of FNDC5 following cleavage of the signal peptide, can only be detected when FNDC5 is translated from the ATA start codon. Both fragments were detected in largely similar amounts in human plasma proteins, giving a circulating irisin concentration of ~ 4 ng/mL. Jedrychowski et al (8) measured a slightly higher value (4.3 ng/mL) in a small group undergoing aerobic training as compared to the controls (3.6 ng/mL). This small increase was later named a “burst of circulating irisin” in response to acute exercise (75). Albrecht et al (14) questioned the biological meaning of this minor increase because similar differences were also observed between the 2 detected irisin fragments in the exercise group of that study. In contrast, another group employing the same method failed to detect irisin in human plasma at all, although they successfully measured irisin levels of 0.3 to 1.9 ng/mL in paired samples of cerebrospinial fluid (15).
We have used the labeled peptide FIQEVNTTTR to measure irisin in human serum and initially failed to detect endogenous irisin peptides in 2 human samples (14). After a new preparation, an irisin level of 0.09 ng/mL was monitored in 1 of 2 samples. A repeated measurement of this sample yielded 0.15 ng/mL, which is still much lower than the ~ 4 ng/mL reported previously (8). In a rat serum sample, we determined irisin concentrations between 0.6 and 0.9 ng/mL in 2 technical replicates and 4 measurements. This is somewhat higher than the 0.3 ng/mL reported in mice (26). Notably, all concentrations reported by MS are markedly below the detection limit of antibodies in Western blots. This suggests that the bands stained in Western blots are not irisin.
Calibrated MS was expected to be the “gold standard” for determining the existence of plasma irisin and its concentration (27). However, the differences even between repeated measurements of the same sample and more pronounced differences between subsequent sample preparations from the same donor indicates a rather high methodological variability. This casts serious doubts on a meaningful interpretation of small differences between groups of individuals based on single measurements for each individual. In addition, preparation of plasma or serum samples for MS requires removal of highly abundant albumins and immunoglobulins and subsequent concentration, which leads to varying amounts of retained proteins for analysis (14). This hinders an exact and reproducible quantification.
Current Situation in Determination of Circulating Irisin
Nine years after its discovery, no confirmed reference values for irisin are available for rodents and humans. There are several reasons for that. First, the initial study suggested rather high amounts of irisin in circulation of mice and humans even detectable by Western blots (3). Consequently, early ELISAs measuring levels in the range between 50 and 900 ng/mL were considered valid. Second, uncritical use of poorly validated ELISAs as well as publication of values beyond the linear range and even below the detection limit, led to a fast expansion of the reported levels over a wide range from pg/mL to µg/mL. Third, the group who discovered irisin later reported levels of ~ 4 ng/mL in humans and 0.3 ng/mL in mice measured by quantitative MS (8, 26). These MS values are far lower than their own initial estimate of roughly 40 nmol/L circulating irisin (~800 ng/mL) in mice (3). Importantly, the very low MS values question the validity of a large proportion of all reported irisin levels measured with ELISA, which are closer to the originally proposed levels.
Although calibrated MS has the potential to resolve these issues, there is still no independent confirmation for irisin levels in mice, and attempts to reproduce values in humans have failed or revealed inconsistent results (14, 15). Considering missing or low correlations between irisin levels measured with different ELISAs and rather low correlations between values determined by ELISA and MS (r = 0.4 (76)), we conclude that a reliable, reproducible method for measurement of circulating irisin is still not available for any species. Consequently, we will only briefly review studies relying on ELISA measurement of endogenous irisin but put more focus on experiments applying r-irisin to cultured cells and rodent models.
Physiological Roles of Irisin
Irisin and Metabolic Conditions or Diseases
Hundreds of studies have been conducted measuring irisin and relating it to diseases or physiological conditions. All these data have to be considered with caution due to the problems with ELISA methodologies described above. Consequently, only selected meta-analyses are reviewed here. An early meta-analysis of 7 studies on type 2 diabetes revealed lower irisin levels in patients compared to controls. The authors noted the extreme heterogeneity of the ELISA-based irisin levels compromising their analysis (77). Similarly, lower irisin levels were found in an analysis of 22 studies of gestational diabetes (78). In contrast, a weak but significant positive relation of irisin levels with insulin resistance was found in nondiabetic individuals from 17 studies (79). Analysis of 18 studies on obesity resulted in a weak positive correlation between circulating irisin and increasing overweight or obesity, which is in contrast to results in diabetes (80). Further meta-analyses reported increased irisin concentrations in patients with polycystic ovary disease, lower irisin levels in coronary artery disease, and inconclusive results for associations with cancer and C-reactive protein (81-84). Irisin levels were weakly correlated with bone mineral density (BMD) in subjects with osteoporosis (85). All meta-analyses have struggled with extremely varying irisin levels reported within a condition—often ranging from pg to µg/mL—and thus have limited validity.
FNDC5/Irisin in Cellular Signaling Pathways—Cell Culture Assays
Numerous in vitro experiments, mostly applying r-irisin to different cell types, have been published. In these studies, authors proposed a multitude of signaling pathways affected by irisin or regulating irisin. In a recent review, Rabiee et al (86) summarized evidence that irisin exerts biological effects mainly via mitogen-activated protein kinases (MAPKs) and the authors list additional pathways that may be affected by irisin. In addition to the proposed effects of irisin on adipose tissue, bone, and brain described in more detail below, irisin had anti-inflammatory and antimetastatic effects, improved glucose uptake in muscle cells, facilitated endothelial cell proliferation, lowered blood pressure, and improved cardiac hypertrophy (86). The reviewed studies all applied r-irisin to cells in a range corresponding to 50 to 2500 ng/mL, which is 10- to 8000-fold more than the assumed physiological concentrations in humans and mice. Based on very early ELISA measurements, Moon et al (87) considered 65 to 130 ng/mL r-irisin physiological and 650 to 1300 ng/mL pharmacological concentrations. Even under this assumption, which later proved 20 to 50 times too high, the apparent physiological concentration promoted no effects on mouse hippocampal neuronal cells.
There are only a few exceptions of cellular response at assumed physiological concentrations. Recent studies on murine osteocytes, a murine adipocyte subpopulation, and HEK293T cells, revealed induction of integrin/focal adhesion kinase (FAK) signaling by glycosylated r-irisin at concentrations as low as 1 to 10 pmol/L corresponding to 20 to 200 pg/mL (26, 88). Oguri et al (88) reported a response of adipocyte precursors to 1 ng/mL r-irisin but the response was reduced at 5 ng/mL.
Zhang et al (89) found greater effects of glycosylated compared to nonglycosylated r-irisin (500 ng/mL) in 3T3-L1–derived adipocytes, whereas Liu et al (90) did not observe any differences in the biological activity of the 2 r-irisin forms (application range 250-2500 ng/mL) on pancreatic cancer cells. Shan et al (91) incubated primary mouse adipocytes with a recombinant C-terminal FNDC5 peptide (20 nmol/L) and observed browning effects. They attributed the effect to irisin, although the applied peptide did not share any sequence with irisin and does not circulate under physiological conditions (reviewed in (92)). The results of many of the in vitro studies raise the question whether effects of r-irisin in supraphysiological concentrations are specific and meaningful with respect to in vivo conditions.
Irisin, Exercise, and Browning of WAT
In contrast to classic unilocular (one big lipid droplet per cell) WAT, BAT is rich in mitochondria and contains many small (multilocular) lipid droplets. BAT specifically expresses uncoupling protein 1 (UCP1) allowing mitochondrial uncoupling and dissipation of energy as heat during respiration instead of ATP production. The term “browning” within WAT typically refers to a more BAT-like morphology with increased expression of UCP1 (93). Boström et al (3) presented the first suggestion and one of the major reasons for the focus on browning of WAT in response to exercise. Several groups have confirmed their findings of browning of inguinal subcutaneous WAT in rodents after long-term exercise (94-96). However, exercise-induced browning of subcutaneous WAT might be specific for inguinal WAT in rodents. Browning of subcutaneous WAT after 3 weeks of physical training was only observed in inguinal WAT but not in other subcutaneous WAT depots (anterior and inter-scapular adipose tissue (96)). In contrast, cold exposure induces browning of all murine adipose tissue depots (97).
In humans, exercise seems to have little or no effect of browning of abdominal subcutaneous WAT (70, 98-100) or activation of classic BAT (101). Only one study showed a small increase in UCP1 gene expression in abdominal subcutaneous WAT after long-term exercise in obese participants (102). Importantly, human abdominal subcutaneous WAT expresses only minor amounts of UCP1 (70, 103, 104).
Activation of PGC-1α in response to exercise was proposed to increase the level and cleavage of FNDC5 from skeletal muscle (3). The secreted domain of FNDC5, irisin, was furthermore suggested to signal browning to WAT. Whereas intravenous injection into mice of FNDC5-expressing adenoviral particles induced Ucp1 expression in inguinal WAT (3), FNDC5 knockout mice exhibited attenuated exercise-induced browning of inguinal WAT (95). Although these mechanistic studies show that FNDC5 has the potential to induce browning of WAT in mice, whether and how much FNDC5/irisin might increase by exercise is controversial. Shortly after the initial publication of Boström et al (3), Timmons et al (105) reported that FNDC5 expression in skeletal muscle was only increased in a minority of ~200 human subjects in response to long-term exercise. A systematic review published a few years later analyzing the link between PGC-1α and FNDC5 in muscle, and circulating irisin and UCP1 of white adipocytes, summarized that the existing evidence does not allow for conclusions regarding irisin responses to physical activity (106). A recent meta-analysis on the responses of the skeletal muscle transcriptome to exercise (MetaMEx) provides an online interface to interrogate 66 published datasets (107). Whereas PGC-1α expression is significantly increased by acute exercise (aerobic and strength) and decreased by inactivity, FNDC5 expression in skeletal muscle is regulated neither by acute nor by long-term exercise, or by inactivity (107). In contrast, expression of skeletal muscle FNDC5 (mRNA and/or protein) in mice has been shown to increase in response to both acute and long-term exercise in some (3, 108-111), but not in all studies (30, 112, 113).
As discussed above, studies using ELISA or Western blots to measure plasma irisin in response to exercise are compromised by the unreliability of the available antibodies. There is one study that used quantitative MS to measure irisin levels in response to exercise. Six young individuals who underwent 12 weeks high-intensity aerobic exercise were compared with 4 individuals without exercise (8). The exercised individuals showed ~19 % higher levels of plasma irisin than the controls. This study still awaits independent confirmation with a larger pool of human subjects.
In summary, experiments in rodents suggest that exercise induces significant browning of inguinal WAT. However, there is very little evidence that this extends to humans. This might be explained by the comparison of different subcutaneous WAT depots between the species, or that subcutaneous WAT plays different roles in humans and mice.
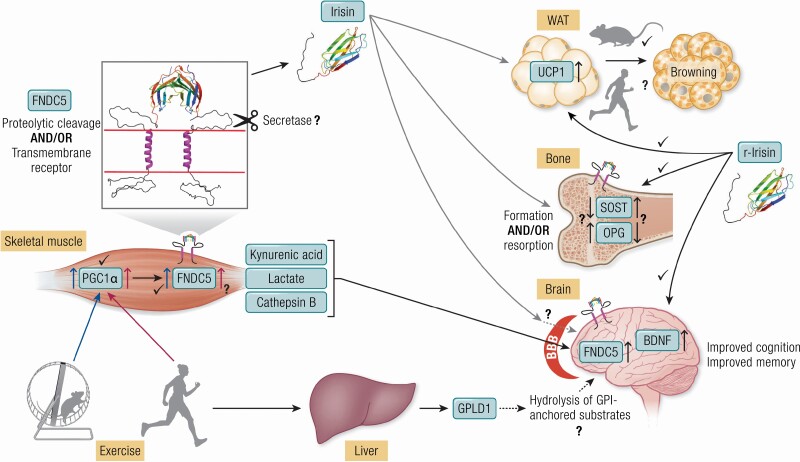
Furthermore, existing evidence points to only minor effects of all modes of exercise on skeletal muscle FNDC5 mRNA expression in humans (Fig. 3).
Figure 3.
Exercise increases PGC1α in skeletal muscle of mice and humans. The subsequent increase in FNDC5 is established in mice but not in humans. FNDC5 may reside as dimeric transmembrane receptor, for example, in skeletal muscle, bone, and brain. The putative cleavage mechanism releasing the extracellular part as irisin is still unclear. Irisin acts on WAT and induces browning, is involved in bone remodeling and improves cognition and memory. Many of these effects have been shown by application of r-irisin to cells or by injections into mice. Browning of WAT was induced by application of high doses of r-irisin or by forced ectopic expression of FNDC5 in mice but was not shown in humans so far. Further open questions are: Does irisin enhance bone formation or resorption or both in a dose dependent manner? Does irisin cross the BBB to increase FNDC5 and BDNF levels as was demonstrated for exercise kynurenic acid, lactate, and cathepsin B? In a recent report (155) a liver-to-brain axis was established with a central role of exercise-induced, liver-derived GPLD1. The figure was partly created with biorender.org. Abbreviations: BDNF, brain-derived neurotrophic factor; BBB, blood-brain barrier; FNDC5, Fibronectin type III domain-containing protein 5; GPI, glycosylphosphatidyl inositol; GPLD1, GPI–specific phospholipase D1; OPG, osteoprotegerin; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1 alpha; r-Irisin, recombinant irisin; SOST, sclerostin; UCP1, uncoupling protein 1; WAT, white adipose tissue.
Effects of Irisin on Bone Metabolism
Some of the more intriguing studies of irisin reported enhanced bone production when injecting r-irisin into mice. Colaianni et al (114) began with an analysis of tibia bone following 4 weekly injections of 100 µg/kg r-irisin. This is a low dose for testing a protein in vivo and far below the daily 500 µg/kg injections used in other irisin studies (115, 116). Considering that r-irisin is cleared from plasma in less than an hour (26), it is difficult to see how these 4 small injections a week apart could have such a strong physiological effect. Nevertheless, following the 4-week treatment Colaianni et al (114) observed a significant 7% increase in cortical BMD, but no change in trabecular bone. The authors noted that no effect on trabecular bone is “in stark contrast to any other known therapy.” The cortical bone showed an increase in mRNA for osteopontin, associated with osteoblastic bone formation, and a reduction of sclerostin (SOST), an inhibitor of bone formation. In a separate experiment, bone marrow stromal cells incubated with 100 ng/mL r-irisin showed increase in several markers of osteoblast differentiation. This suggested that irisin might interact directly with bone cells to enhance bone formation. A number of subsequent studies applying irisin to cell cultures also supported a direct interaction with bone cells (117-119).
A related study by Narayanan et al (120) investigated the bone loss induced by a model of inflammatory bowel disease (IBD). Rats induced to have IBD suffered substantial bone loss, but when injected with r-irisin, the IBD rats showed an increase in osteoblast and a reduction of osteoclast activities specifically on trabecular bone. The authors did not look at cortical bone, but the effect on trabecular bone is in contrast to Colaianni et al (114). A remarkable aspect of this study was that the r-irisin injection caused a 100% cure of this particular induced model of IBD. In a follow-up study, Metzger et al (121) used a different model of IBD, where r-irisin had less effect on the primary inflammation and on associated bone loss. These studies show that the immune system and inflammation can cause bone loss, probably via released cytokines, and that injected r-irisin can moderate the inflammation. We suggest that effects of irisin on bone may be mediated by this inflammatory response. This could be in addition to a direct effect of r-irisin on bone cells indicated in other studies (114, 117, 119).
In a later study, Colaianni et al (122) subjected mice to hindlimb unloading, which caused loss of BMD. Following 4 weeks of suspension, femurs lost ~4% of cortical BMD and ~40% of trabecular BMD. The similar weekly injections of 100 µg/kg r-irisin used previously prevented all the cortical and half the trabecular BMD loss. The inactivity-induced BMD in control mice was accompanied by an increase in SOST and reduction in osteoprotegerin mRNA in femurs. Irisin injection decreased SOST and increased osteoprotegerin about halfway back to control levels. Overall, these studies painted a consistent picture of irisin enhancing bone formation by lowering the inhibitory SOST and raising the protective osteoprotegerin. A recent study has extended these results by sending bone cell cultures into space. Consistent with previous studies, 14 days of microgravity aboard the SpaceX Dragon caused a substantial downregulation of genes favoring bone deposition and upregulation of genes favoring bone erosion. Incubation with 100 ng/mL r-irisin largely reversed these effects of microgravity (123).
Luo et al (124) reported a similar protection of bone loss by injecting r-irisin into female mice subjected to ovariectomy. The control ovariectomized mice suffered a significant loss of bone mass over the following weeks. Biweekly injections of 100 µg/kg r-irisin blocked the bone loss and enhanced most bone parameters beyond the control levels, similar to the weekly injections of Colaianni et al (122). Unfortunately, Luo et al (124) did not reference or discuss the apparently contradictory study of Kim et al (26).
Kim et al (26) observed that glycosylated r-irisin increased SOST both in mice and in cultured osteocytes, the opposite of Colaianni et al (114). The effect in mice was seen after 6 daily injections of 1 mg/kg, each dose 10 times higher than the weekly doses used by Colaianni et al (114). An important development in this study was the generation of FNDC5-knockout (KO) mice (26). Remarkably, the KO caused no change in bone structure in male mice. In contrast, female KO mice showed significantly enhanced trabecular bone volume, BMD, and connectivity. Female mice were then subjected to ovariectomy, promoting a significant loss of bone in the control littermates. In the FNDC5-KO mice the ovariectomy-induced bone loss was completely blocked.
In a recent follow-up study, Estell et al (75) looked more closely at the effect of irisin on osteoclasts. Incubation of bone marrow cells from male mice with 2 to 10 ng/mL r-irisin caused enhanced differentiation of active osteoclasts. They then created transgenic mice in which the FNDC5 transgene was expressed from a muscle-specific promoter. FNDC5 mRNA expression was also increased in bone tissue, which raises the question of whether effects on bone are due to the extra bone FNDC5 or to circulating irisin released from muscle. Male mice, exhibiting no change in bone in the FNDC5 KO (26), showed a significant reduction in trabecular bone in the FNDC5 transgenic mice. However, they did not report on female mice, which had improved bone parameters in the FNDC5 KO (26). Both groups (26, 75) note that their results—that irisin inhibits bone formation—are the opposite of Colaianni et al (114, 122). They did not reference the results of other groups who also found irisin enhanced bone formation (120, 124, 125). Estell et al (75) suggested that the opposite effects of irisin (inhibition vs enhancement of bone formation) might be due to the concentration of irisin and the duration or frequency of application.
In another recent study, Zhu et al (125) performed a selective KO of FNDC5 in bone cells using an osterix-Cre driver. Osterix-Cre has been used to knock out genes specifically in osteoblasts and osteocytes. Expression analyses showed major reduction of FNDC5 in bone tissue but normal expression in WAT and muscle. These KO mice showed a significant reduction in trabecular bone mass and delayed bone development and mineralization from early postnatal stage to adulthood. This is the opposite of the report of Kim et al (26) and consistent with the conclusions of Colaianni et al (114, 122) that irisin enhances bone parameters.
These intriguing studies leave a number of questions: Does FNDC5 or irisin enhance or inhibit bone formation (Fig. 3)? Does irisin in vivo operate primarily directly on bone cells, or is the effect on bone partially or largely a secondary response of other cells responding to irisin? If the FNDC5 knockout does alter bone, which tissue KO has the major effect, bone or muscle?
An Irisin Receptor?
Boström et al (3) speculated that irisin effects might be mediated by a cell surface receptor. Although binding of recombinant irisin to 3T3L1 cells and of synthetic irisin to a subpopulation of mouse stromavascular cells was demonstrated (89, 126), no receptor was identified until 2018. Then, Kim et al (26) reasoned that if cultured osteocytes respond to r-irisin by upregulating SOST, they must have a cell surface receptor for irisin. They initially screened for a receptor using crosslinking and MS, and identified integrin β1 as a candidate. No integrin α was identified by this crosslinking. Cultured osteocytes incubated with irisin showed signs of integrin signaling. The authors then transfected HEK 293T cells with different integrins and tested the effect of r-irisin. The best response was observed with αVβ5 integrin, which they identified as a likely irisin receptor. A major problem with this receptor identification is lack of specificity: the only integrin identified in the initial MS screen, β1 has been abandoned in all subsequent experiments. The favored receptor is now αVβ5, which gave the best response in the screen of integrins. Several subsequent studies have supported αVβ5 as an irisin receptor. Bi et al (127, 128) demonstrated co-immunoprecipitation of integrin αVβ5 with irisin as well as co-localization of this integrin complex with irisin in human colon carcinoma or microvascular endothelial cells thus indirectly supporting a receptor-ligand binding. A recent study reported that CD81 is a partner in the integrin signaling (88). The signaling required 2 different integrin complexes, αVβ1 and αVβ5, in addition to CD81. The authors concluded that CD81 sensitized the HEK293T cells to irisin (88). Estell et al (75) showed that a commercial antibody inhibiting αVβ5 blocked osteoclast response to irisin, apparently confirming this receptor. However, the major integrin on osteoclasts is αVβ3; αVβ5 integrin has not previously been identified on osteoclasts (129, 130).
A problem with an integrin receptor is that it would not be able to utilize the dimeric structure of irisin. The irisin dimer is about 40 Å wide, compared with 47 Å for a single integrin head. Thus, an irisin dimer could bind only a single integrin. Of course, this would be a moot point if irisin were monomeric at the nanomolar concentrations in circulation. The crystal structure is consistent with a high-affinity dimer, but the KD has not been measured.
The Muscle-Brain Axis
Exercise has beneficial effects on life expectancy and prevention of coronary heart disease, diabetes mellitus, osteoporosis, and frailty (131-134). Furthermore, exercise improves several conditions related to the brain, such as cognitive function, depression, dementia, and sleep (135-139). The effects of exercise on promoting learning and memory formation may be mediated by inducing brain-derived neurotrophic factor (BDNF) expression and signaling in the hippocampus (140).
A twist to the story of the interaction between the brain and exercise was provided by the discovery of a new family of myokines including cathepsins that were increased in response to 11 weeks of resistance training among sedentary men (141). Exercise in mice increased the plasma concentration of cathepsin B and its capacity to pass the blood-brain barrier. Cathepsin B induced the production of BDNF in certain parts of the hippocampus, thereby promoting neurogenesis and enhanced learning and memory (142). Another study suggests that cathepsin B and BDNF are affected by long-term exercise in men (143).
Additional metabolites linking exercise to BDNF expression include beta-hydroxybutyrate (144, 145), and the tryptophane metabolites kynurenine and kyrunenic acid (146-149). Only recently, Contrepois et al (150) observed an increase of circulating kynurenic acid in response to acute exercise in humans in line with previous results. Recently, FNDC5/irisin has joined this field of exercise-induced brain modulators. FNDC5 was found to be upregulated in muscle when the translational coactivator PGC1α was enhanced transgenically or in response to exercise (3). PGC-1α and FNDC5 are both highly expressed in the brain (1, 2, 22, 151). Previous studies have shown that exercise improves several brain functions, and these benefits may be mediated by upregulation of BDNF. Wrann et al (22) reported that in response to 30 days of endurance exercise, mice showed elevated expression of PGC-1α, FNDC5, and BDNF in the hippocampus but not in the rest of the brain. As in muscle, increasing or decreasing expression of PGC-1α in the hippocampus increased or decreased expression of FNDC5. In several experiments, BDNF increased or decreased in synchrony with expression of FNDC5, independent of PGC1α. All of this was consistent with BDNF expression being regulated by expression of FNDC5 within each neuron. Wrann et al (22) then obtained indirect evidence that peripheral overexpression of irisin, from an injected adenovirus vector presumably expressing FNDC5 in liver, could stimulate hippocampal BDNF.
Ruan et al (15) used quantitative MS with labeled peptides to assay for irisin in cerebrospinal fluid (CSF). They detected irisin in all 6 samples from elderly men, with a wide range of concentrations: 0.26 to 1.86 ng/mL. They intended to compare CSF irisin with paired samples of plasma, but they failed to detect irisin in any of the 6 plasma samples. Thus, the CSF irisin may be derived from FNDC5 in the brain.
Lourenco et al (25) investigated a potential relation of FNDC5/irisin to Alzheimer's disease. They found that small hairpin-FNDC5 RNA injected into mouse brains caused loss of hippocampal long-term potential (LTP). A similar loss of LTP was caused by injecting amyloid-β oligomers (AβOs), a treatment which has been used to model Alzheimer's disease. AβO injection also caused defects on 2 tests of memory and behavior. However, when glycosylated r-irisin was injected along with AβOs, the LTP loss was reversed, as were the behavioral defects. In a related experiment, they injected adenovirus expressing FNDC5 into the brain, and 6 days later injected AβOs. The FNDC5 expression again rescued the behavioral defects. Exercise also reversed the behavioral defects of AβO injection, consistent with the result from Wrann et al (22) that exercise increased expression of hippocampal FNDC5. Lourenco et al (25) then turned to a strain of mice considered a model for Alzheimer's disease. The Alzheimer's disease mice showed loss of LTP in hippocampal neurons and behavioral defects similar to the AβO-injected mice. All effects were reversed by injection of adenovirus expressing FNDC5 into the brain. They then tested the effect of peripheral FNDC5 by injecting the adenovirus intravenously, similar to Wrann et al (22). This presumed peripheral expression rescued the AβO-induced loss of LTP and behavioral defects, similar to the injection into the brain. This suggests that the peripheral FNDC5/irisin might enter the brain and mimic the effect of the brain FNDC5 in improving function. In a more recent human study of patients with Alzheimer's disease and control subjects, Lourenco et al (152) found a positive correlation of irisin levels in CSF with BDNF, Aβ42, and memory. A cautionary note is that irisin was measured with a commercial ELISA, which has not been validated for measurement in plasma or body fluids.
Neither Wrann et al (22) nor Lourenco et al (25) addressed how peripheral irisin might cross the blood-brain barrier (BBB). The BBB efficiently blocks the transfer of large molecules, including proteins, from plasma to CSF. The block is not complete, and small amounts of some proteins enter the central nervous system by passive diffusion: eg, albumin is present in CSF at 0.5% of its concentration in plasma. Some cytokines have selective transporters; for example, leptin is present in CSF at 4% to 7% of its 10 ng/mL plasma concentration (153). If peripheral irisin were to play a role in brain physiology, it would need a selective BBB transporter and a very-high-affinity receptor on brain cells. As outlined above, FNDC5/irisin is only one among several exercise-induced substances that have been reported to improve various brain functions in a BDNF-dependent manner. Cathepsin B and lactate might both cross the BBB and convey hippocampal-dependent learning and memory in a BDNF-dependent manner (142, 154). El Hayek et al (154) proposed an alternative mechanism linking exercise to improved learning and memory in mice. Voluntary exercise promoted increased circulating lactate levels. Lactate, in turn, entered hippocampal neurons via the monocarboxylate transporter (MCT) 2 and induced a signaling cascade (SIRT1 to PGC-1α to FNDC5 to BDNF), leading to increased BDNF levels in exercised mice. This mechanism would circumvent the necessity of irisin passing the BBB, and it is consistent with previously reported increased FNDC5 levels in the brain of exercised animals. Only recently, liver-derived circulating glycosylphosphatidylinositol–specific phospholipase D1 (GPLD1) was identified linking exercise to improved cognitive functions in old mice (155).
Overall, the studies of Wrann et al (22) and Lourenco et al (25) present intriguing preliminary results implicating hippocampal FNDC5 in normal brain physiology and in Alzheimer's disease. FNDC5, cathepsin B, and lactate may all increase with exercise and in turn upregulate expression of hippocampal BDNF. The possible role of peripheral irisin is more problematic (Fig. 3). However, FNDC5-KO mouse models would be a tool to explore these preliminary leads and crossing the FNDC5-KO with the AD mice should be instructive to reveal potential interactions.
Fndc5 Knockout Mice
Most early studies of physiological functions of FNDC5/irisin are based on gain-of-function experiments, adding r-irisin to cultured cells or injecting it into mice. These are indirect approaches, especially for injections into mice, where it would simply increase the level of endogenous irisin by an unknown amount.
More recently, several research groups have created FNDC5 KO mice. Most groups reported that mice with global KO of FNDC5 are viable, fertile, and have no obvious phenotypic abnormalities under standard conditions (26, 94, 156, 157). In particular, they have normal weight and growth. However, there are exceptions: Luo et al (158) described female FNDC5-KO mice with significantly lower body weight than wild-type (WT) mice, and Kim et al (26) reported that FNDC5-KO mice suffer less than WT from ovariectomy. The research group of GQ Zhou investigated the effects of several specific challenges. They found that fasting FNDC5 KO mice promoted severe hepatic steatosis, which did not occur in WT mice (156). In contrast, when mice were fed a high fat diet for 6 months, they developed liver fibrosis, which was aggravated in the FNDC5 KO mice (159). The effects on liver are somewhat surprising because hepatic expression of FNDC5 is low. In another study, using a separately derived FNDC5 KO, Xiong et al (95) observed that the high fat diet also induced obesity and insulin resistance, which were exacerbated in the KO mice. Zhou et al (160) also induced vascular oxidative stress and inflammation by injecting angiotensin II. The effects were aggravated by the absence of FNDC5. Overall, these experiments suggest that whereas FNDC5 KO mice appear normal under standard husbandry conditions, they suffer more than WT mice during several specific physiological challenges. Li et al (157) created FNDC5 KO mice, as well as transgenic mice overexpressing FNDC5. Human FNDC5 was overexpressed in skeletal muscle of the latter mice under control of the chicken β-actin promoter and cytomegalovirus enhancer. Circulating irisin was more than 2-fold increased in transgenic mice, but the reported levels were far above the reference values determined by MS, even in the controls (26). They then subjected mice to transverse aortic constriction for 4 weeks, which caused cardiac hypertrophy. The hypertrophy was enhanced in the FNDC5 KO mice, whereas the transgenic FNDC5 overexpression reduced the hypertrophy.
A major interest in the FNDC5/irisin story is its potential role in metabolism and in adipose tissue. Xiong et al (95) reported that skeletal muscle in their FNDC5 KO mice was not different from WT in size, histology, and fiber type. KO and WT mice showed similar levels of glucose and serum lipids, and similar recovery from an intraperitoneal glucose injection. However, they found a difference when mice were subjected to 8 weeks of treadmill exercise. Exercised WT mice showed glucose recovery identical to unexercised mice, whereas exercised KO mice showed ~50 % higher blood glucose concentrations 30 to 60 minutes after injection. They also had significantly reduced insulin sensitivity and lower maximum oxygen uptake. BAT was similar in exercised WT and KO mice, but inguinal WAT showed “formation of many smaller multilocular beige adipocytes” in WT but not in KO mice. They also noted differences in glucose tolerance. Interestingly, the metabolic changes could affect bone indirectly. These results need to be confirmed and hopefully tied to a common mechanism.
Conclusions and Further Perspectives
Unsolved Issues
There are still many open questions after 9 years of intensive research on irisin. Following 10 years of a Sleeping Beauty–like fate as a disregarded transmembrane protein, FNDC5 was kissed awake in 2012 as the supposed precursor of a circulating, hormone-like peptide, irisin. Since then, irisin evolved rapidly to a factor apparently involved in an amazing variety of diseases and metabolic conditions. This elaboration of pathways is seriously compromised by flawed methods for detection and quantification, and the high doses of r-irisin applied to cells or injected to animals. Thus, validation of available methods and confirmation of major results by independent groups is a most urgent task.
Moreover, the mutant start codon of FNDC5 exclusively in the hominid lineage still awaits exploration. How can significant amounts of a protein be translated from the noncanonical ATA start codon located in an unfavorable Kozak sequence?
Furthermore, the proposed cleavage mechanism of the irisin peptide from FNDC5 needs confirmation and exploration of mechanisms. Is exercise required for cleavage—this might be opposed by reasons given below—but if yes, how is this mediated?
Finally, an important drawback about the suggested browning of adipose tissue induced by irisin is that the carefully controlled intervention studies in humans find no sign of uncoupling and enhanced energy expenditure that could be explained by irisin.
A Problem for Evolution of an Exercise Hormone
The original hypothesis and much of the later research posits that irisin is released from muscle in response to exercise, and acts on WAT, where it generates browning and an accompanying thermogenic response. It is claimed that this places irisin as a prime mediator of the beneficial effects of exercise. This would make sense if irisin evolved in modern humans, where one of the major benefits of exercise is weight loss. However, it is difficult to rationalize this for evolution in wild animals engaged in the struggle for life and survival of the fittest. Exercise in this context would involve chasing down another animal for food or escaping a predator. It is hard to imagine any value in following these exertions by a physiological response that continues to burn energy. On the contrary, survival would seem to require a rapid shutdown of any extra energy consumption and restoration of stores of fat and glycogen for the next encounter.
A Search for Other Functions for FNDC5.
We should still look for alternative functions for FNDC5 that may have nothing to do with browning of WAT or metabolism. An important lead may be found in birds, which have FNDC5 but lack BAT and its canonical UCP1. Li et al (161) cloned the FNDC5 gene in chicken and determined the tissue distribution of its expression. It is remarkably similar to the distribution in mammals, with high expression in brain, heart, and skeletal muscle. This would suggest looking for functions in these tissues, independent of the still-hypothetical cleavage and browning of WAT.
FNDC5 Knockout Mice—High Potential for Future Research
FNDC5 KO mice might provide an excellent tool to test and validate assays for plasma irisin. The plasma of these mice should preserve all nonspecific components but be completely lacking irisin. Glycosylated r-irisin could be titrated into KO plasma to determine the nonspecific background and the sensitivity of the ELISA to real irisin. Plasma of amphibians, which are lacking the FNDC5 locus, would provide an alternative background test.
FNDC5 KO mice might be a model to demonstrate the functions of FNDC5. The absence of obvious phenotypes under normal husbandry frustrates easy answers, but specific physiological challenges have revealed defects that need to be followed up. One important test seems possible for any observed phenotype: does injection of glycosylated r-irisin reverse the phenotypes caused by the KO? If major functions of FNDC5 are mediated by cleavage and release of irisin, the r-irisin should reverse the defect. If FNDC5 actually functions as a transmembrane protein on its own cell, the injected r-irisin should have no effect.
The recently described mouse line with floxed FNDC5 should be especially useful (125). This line could be used to generate a conditional KO in any tissue for which a Cre driver is available. Of particular interest would be skeletal muscle, heart, and brain, the tissues of highest FNDC5 expression, and bone, where responses and expression have been found. For any phenotype already observed in a global FNDC5 KO, the tissue-specific KO should provide confirmation and be informative of the pathway.
Perhaps most important is whether FNDC5 operates primarily as soluble irisin released from muscle and acting on receptors in an autocrine, paracrine, or endocrine manner, or as a transmembrane receptor in the tissue where it is expressed. For example, if the true agent is soluble irisin, a conditional KO in skeletal muscle should eliminate phenotypes in WAT, bone, and brain. If the true agent is FNDC5 receptors, or if irisin is acting as a paracrine/autocrine regulator, KO in the target tissues should produce results, whereas the muscle KO should have no effect.
In summary, more than 1000 published studies suggest intriguing roles for FNDC5/irisin, originally in muscle and adipose tissue, and now expanding into bone and brain. Many of these studies are compromised by use of questionable antibody-based assays; valid assays to quantitate circulating irisin are sorely needed. However, several physiological roles have been elucidated by exposing cells and rodent models to exogenous r-irisin, thus proving it a potent biological agent. The combined use of up- and downregulation by injecting r-irisin or FNDC5 KO may have the potential to resolve controversies and to rigorously establish physiological pathways.
Acknowledgments
Financial Support: The publication of this article was funded by the Open Access Fund of the Leibniz Institute for Farm Animal Biology (FBN).
Glossary
Abbreviations
- aa
amino acid(s)
- AβO
amyloid-β oligomer
- BAT
brown adipose tissue
- BBB
blood-brain barrier
- BDNF
brain-derived neurotrophic factor
- BMD
bone mineral density
- CSF
cerebrospinal fluid
- ELISA
enzyme-linked immunosorbent assay
- FNDC5
fibronectin type III domain-containing protein 5
- FNIII
fibronectin type III domain
- IBD
inflammatory bowel disease
- KO
knockout
- LTP
long-term potential
- MS
mass spectrometry
- MW
molecular weight
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator 1α
- PNGase F
Peptide:N-glycosidase F
- r-irisin
recombinant irisin
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SOST
sclerostin
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
- WT
wild-type
Additional Information
Disclosures: We have nothing to disclose.
References
- 1. Ferrer-Martínez A, Ruiz-Lozano P, Chien KR. Mouse PeP: a novel peroxisomal protein linked to myoblast differentiation and development. Dev Dyn. 2002;224(2):154-167. [DOI] [PubMed] [Google Scholar]
- 2. Teufel A, Malik N, Mukhopadhyay M, Westphal H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene. 2002;297(1-2):79-83. [DOI] [PubMed] [Google Scholar]
- 3. Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277-359. [DOI] [PubMed] [Google Scholar]
- 5. Schumacher MA, Chinnam N, Ohashi T, Shah RS, Erickson HP. The structure of irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer: implications for receptor activation. J Biol Chem. 2013;288(47):33738-33744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Komolka K, Albrecht E, Schering L, Brenmoehl J, Hoeflich A, Maak S. Locus characterization and gene expression of bovine FNDC5: is the myokine irisin relevant in cattle? Plos One. 2014;9(1):e88060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raschke S, Elsen M, Gassenhuber H, et al. Evidence against a beneficial effect of irisin in humans. Plos One. 2013;8(9):e73680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jedrychowski MP, Wrann CD, Paulo JA, et al. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015;22(4):734-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ivanov IP, Firth AE, Michel AM, Atkins JF, Baranov PV. Identification of evolutionarily conserved non-AUG-initiated N-terminal extensions in human coding sequences. Nucleic Acids Res. 2011;39(10):4220-4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pirastu M, Saglio G, Chang JC, Cao A, Kan YW. Initiation codon mutation as a cause of alpha thalassemia. J Biol Chem. 1984;259(20):12315-12317. [PubMed] [Google Scholar]
- 11. Eiken HG, Knappskog PM, Apold J, Skjelkvåle L, Boman H. A de novo phenylketonuria mutation: ATG (Met) to ATA (Ile) in the start codon of the phenylalanine hydroxylase gene. Hum Mutat. 1992;1(5):388-391. [DOI] [PubMed] [Google Scholar]
- 12. Caridi G, Dagnino M, Lugani F, et al. A novel mutation in the albumin gene (c.1A > C) resulting in analbuminemia. Eur J Clin Invest. 2013;43(1):72-78. [DOI] [PubMed] [Google Scholar]
- 13. Kozak M. Context effects and inefficient initiation at non-AUG codons in eucaryotic cell-free translation systems. Mol Cell Biol. 1989;9(11):5073-5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Albrecht E, Schering L, Buck F, et al. Irisin: still chasing shadows. Mol Metab. 2020;34:124-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruan Q, Zhang L, Ruan J, et al. Detection and quantitation of irisin in human cerebrospinal fluid by tandem mass spectrometry. Peptides. 2018;103:60-64. [DOI] [PubMed] [Google Scholar]
- 16. Albrecht E, Norheim F, Thiede B, et al. Irisin - a myth rather than an exercise-inducible myokine. Sci Rep. 2015;5:8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roca-Rivada A, Castelao C, Senin LL, et al. FNDC5/irisin is not only a myokine but also an adipokine. Plos One. 2013;8(4):e60563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Löffler D, Müller U, Scheuermann K, et al. Serum irisin levels are regulated by acute strenuous exercise. J Clin Endocrinol Metab. 2015;100(4):1289-1299. [DOI] [PubMed] [Google Scholar]
- 19. Matsuo Y, Gleitsmann K, Mangner N, et al. Fibronectin type III domain containing 5 expression in skeletal muscle in chronic heart failure-relevance of inflammatory cytokines. J Cachexia Sarcopenia Muscle. 2015;6(1):62-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deng J, Zhang N, Wang Y, et al. FNDC5/irisin improves the therapeutic efficacy of bone marrow-derived mesenchymal stem cells for myocardial infarction. Stem Cell Res Ther. 2020;11(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee P, Linderman JD, Smith S, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19(2):302-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wrann CD, White JP, Salogiannnis J, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18(5):649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayashida K, Bartlett AH, Chen Y, Park PW. Molecular and cellular mechanisms of ectodomain shedding. Anat Rec (Hoboken). 2010;293(6):925-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nie Y, Dai B, Guo X, Liu D. Cleavage of FNDC5 and insights into its maturation process. Mol Cell Endocrinol. 2020;510:110840. [DOI] [PubMed] [Google Scholar]
- 25. Lourenco MV, Frozza RL, de Freitas GB, et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med. 2019;25(1):165-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim H, Wrann CD, Jedrychowski M, et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell. 2018;175(7):1756-1768.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polyzos SA, Mathew H, Mantzoros CS. Irisin: a true, circulating hormone. Metabolism. 2015;64(12):1611-1618. [DOI] [PubMed] [Google Scholar]
- 28. Erickson HP. Irisin and FNDC5 in retrospect: an exercise hormone or a transmembrane receptor? Adipocyte. 2013;2(4):289-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gul-Kahraman K, Yilmaz-Bozoglan M, Sahna E. Physiological and pharmacological effects of melatonin on remote ischemic perconditioning after myocardial ischemia-reperfusion injury in rats: role of Cybb, Fas, NfκB, Irisin signaling pathway. J Pineal Res. 2019;67(2):e12589. [DOI] [PubMed] [Google Scholar]
- 30. Brenmoehl J, Albrecht E, Komolka K, et al. Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int J Biol Sci. 2014;10(3):338-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen K, Xu Z, Liu Y, et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci Transl Med. 2017;9(418):eaao6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huh JY, Panagiotou G, Mougios V, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61(12):1725-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stengel A, Hofmann T, Goebel-Stengel M, Elbelt U, Kobelt P, Klapp BF. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity–correlation with body mass index. Peptides. 2013;39:125-130. [DOI] [PubMed] [Google Scholar]
- 34. Choi YK, Kim MK, Bae KH, et al. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract. 2013;100(1):96-101. [DOI] [PubMed] [Google Scholar]
- 35. Lawson EA, Ackerman KE, Slattery M, Marengi DA, Clarke H, Misra M. Oxytocin secretion is related to measures of energy homeostasis in young amenorrheic athletes. J Clin Endocrinol Metab. 2014;99(5):E881-E885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perakakis N, Triantafyllou GA, Fernández-Real JM, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13(6):324-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aslan R, Alp HH, Eryılmaz R, et al. Can the Irisin be a Biomarker for Prostate Cancer? A Case Control Study. Asian Pac J Cancer Prev. 2020;21(2):505-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang L, Tong Y, Zhang F, et al. The association of circulating irisin with metabolic risk factors in Chinese adults: a cross-sectional community-based study. BMC Endocr Disord. 2019;19(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bi J, Zhang J, Ren Y, et al. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 2019;20:296-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Natalicchio A, Marrano N, Biondi G, et al. The Myokine Irisin Is Released in Response to Saturated Fatty Acids and Promotes Pancreatic β-Cell Survival and Insulin Secretion. Diabetes. 2017;66(11):2849-2856. [DOI] [PubMed] [Google Scholar]
- 41. Boeselt T, Nell C, Lütteken L, et al. Benefits of High-Intensity Exercise Training to Patients with Chronic Obstructive Pulmonary Disease: A Controlled Study. Respiration. 2017;93(5):301-310. [DOI] [PubMed] [Google Scholar]
- 42. Bang HS, Seo DY, Chung YM, et al. Ursolic Acid-induced elevation of serum irisin augments muscle strength during resistance training in men. Korean J Physiol Pharmacol. 2014;18(5):441-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li DJ, Huang F, Lu WJ, Jiang GJ, Deng YP, Shen FM. Metformin promotes irisin release from murine skeletal muscle independently of AMP-activated protein kinase activation. Acta Physiol (Oxf). 2015;213(3):711-721. [DOI] [PubMed] [Google Scholar]
- 44. Zhou X, Li R, Liu X, et al. ROCK1 reduces mitochondrial content and irisin production in muscle suppressing adipocyte browning and impairing insulin sensitivity. Sci Rep. 2016;6:29669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fukushima Y, Kurose S, Shinno H, et al. Effects of Body Weight Reduction on Serum Irisin and Metabolic Parameters in Obese Subjects. Diabetes Metab J. 2016;40(5):386-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuzmicki M, Telejko B, Lipinska D, et al. Serum irisin concentration in women with gestational diabetes. Gynecol Endocrinol. 2014;30(9):636-639. [DOI] [PubMed] [Google Scholar]
- 47. Küster OC, Laptinskaya D, Fissler P, et al. Novel Blood-Based Biomarkers of Cognition, Stress, and Physical or Cognitive Training in Older Adults at Risk of Dementia: Preliminary Evidence for a Role of BDNF, Irisin, and the Kynurenine Pathway. J Alzheimers Dis. 2017;59(3):1097-1111. [DOI] [PubMed] [Google Scholar]
- 48. Huh JH, Ahn SV, Choi JH, Koh SB, Chung CH. High Serum Irisin Level as an Independent Predictor of Diabetes Mellitus: A Longitudinal Population-Based Study. Medicine (Baltimore). 2016;95(23):e3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Amengual J, García-Carrizo FJ, Arreguín A, et al. Retinoic Acid Increases Fatty Acid Oxidation and Irisin Expression in Skeletal Muscle Cells and Impacts Irisin In Vivo. Cell Physiol Biochem. 2018;46(1):187-202. [DOI] [PubMed] [Google Scholar]
- 50. Quiñones M, Folgueira C, Sánchez-Rebordelo E, Al-Massadi O. Circulating Irisin Levels Are Not Regulated by Nutritional Status, Obesity, or Leptin Levels in Rodents. Mediators Inflamm. 2015;2015:620919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kałużna M, Hoppe K, Schwermer K, Ibrahim AY, Pawlaczyk K, Ziemnicka K. Adropin and irisin levels in relation to nutrition, body composition, and insulin resistance in patients with end-stage renal disease on chronic hemodialysis and peritoneal dialysis. Pol Arch Med Wewn. 2016;126(7-8):474-482. [DOI] [PubMed] [Google Scholar]
- 52. Palermo A, Sanesi L, Colaianni G, et al. A Novel Interplay Between Irisin and PTH: From Basic Studies to Clinical Evidence in Hyperparathyroidism. J Clin Endocrinol Metab. 2019;104(8):3088-3096. [DOI] [PubMed] [Google Scholar]
- 53. Zhang M, Chen P, Chen S, et al. The association of new inflammatory markers with type 2 diabetes mellitus and macrovascular complications: a preliminary study. Eur Rev Med Pharmacol Sci. 2014;18(11):1567-1572. [PubMed] [Google Scholar]
- 54. Bubak MP, Heesch MWS, Shute RJ, et al. Irisin and Fibronectin Type III Domain-Containing 5 Responses to Exercise in Different Environmental Conditions. Int J Exerc Sci. 2017;10(5):666-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang S, Xiao F, Pan L, et al. Association of serum irisin and body composition with chronic kidney disease in obese Chinese adults: a cross-sectional study. BMC Nephrol. 2015;16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moreno-Navarrete JM, Ortega F, Serrano M, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98(4):E769-E778. [DOI] [PubMed] [Google Scholar]
- 57. Wiecek M, Szymura J, Sproull J, Szygula Z. Whole-Body Cryotherapy Is an Effective Method of Reducing Abdominal Obesity in Menopausal Women with Metabolic Syndrome. J Clin Med. 2020;9(9):E2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hofmann T, Elbelt U, Ahnis A, et al. The exercise-induced myokine irisin does not show an association with depressiveness, anxiety and perceived stress in obese women. J Physiol Pharmacol. 2016;67(2):195-203. [PubMed] [Google Scholar]
- 59. Xiong XQ, Chen D, Sun HJ, et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta. 2015;1852(9):1867-1875. [DOI] [PubMed] [Google Scholar]
- 60. Ge X, Sathiakumar D, Lua BJ, Kukreti H, Lee M, McFarlane C. Myostatin signals through miR-34a to regulate Fndc5 expression and browning of white adipocytes. Int J Obes (Lond). 2017;41(1):137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ghanbari-Niaki A, Saeidi A, Ahmadian M, et al. The combination of exercise training and Zataria multiflora supplementation increase serum irisin levels in postmenopausal women. Integr Med Res. 2018;7(1):44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang W, Guo Y, Zhang X, Zheng J. Abnormal irisin level in serum and endometrium is associated with metabolic dysfunction in polycystic ovary syndrome patients. Clin Endocrinol (Oxf). 2018;89(4):474-480. [DOI] [PubMed] [Google Scholar]
- 63. Ates I, Arikan MF, Erdogan K, et al. Factors associated with increased irisin levels in the type 1 diabetes mellitus. Endocr Regul. 2017;51(1):1-7. [DOI] [PubMed] [Google Scholar]
- 64. Yeniocak S, Karcıoğlu Ö, Kalkan A, et al. The diagnostic value of irisin in patients with acute abdominal pain: a preliminary study. Ulus Travma Acil Cerrahi Derg. 2018;24(6):539-544. [DOI] [PubMed] [Google Scholar]
- 65. Shi G, Tang N, Qiu J, et al. Irisin stimulates cell proliferation and invasion by targeting the PI3K/AKT pathway in human hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;493(1):585-591. [DOI] [PubMed] [Google Scholar]
- 66. Hou N, Du G, Han F, Zhang J, Jiao X, Sun X. Irisin Regulates Heme Oxygenase-1/Adiponectin Axis in Perivascular Adipose Tissue and Improves Endothelial Dysfunction in Diet-Induced Obese Mice. Cell Physiol Biochem. 2017;42(2):603-614. [DOI] [PubMed] [Google Scholar]
- 67. Mustafa AI, El-Shimi OS. Serum irisin: a prognostic marker for severe acne vulgaris. J Cosmet Dermatol. 2018;17(5):931-934. [DOI] [PubMed] [Google Scholar]
- 68. Calan M, Demirpence M. Increased circulating levels of irisin are associated with cardiovascular risk factors in subjects with acromegaly. Hormones (Athens). 2019;18(4):435-442. [DOI] [PubMed] [Google Scholar]
- 69. Pang M, Yang J, Rao J, et al. Time-Dependent Changes in Increased Levels of Plasma Irisin and Muscle PGC-1α and FNDC5 after Exercise in Mice. Tohoku J Exp Med. 2018;244(2):93-103. [DOI] [PubMed] [Google Scholar]
- 70. Norheim F, Langleite TM, Hjorth M, et al. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. Febs J. 2014;281(3):739-749. [DOI] [PubMed] [Google Scholar]
- 71. Kurdiova T, Balaz M, Vician M, et al. Are Skeletal Muscle & Adipose Tissue Fndc5 Gene Expression and Irisin Release Affected by Obesity, Diabetes and Exercise? In vivo & in vitro studies. J Physiol. 2014;592(5):1091–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Montes-Nieto R, Martínez-García MÁ, Luque-Ramírez M, Escobar-Morreale HF. Differences in analytical and biological results between older and newer lots of a widely used irisin immunoassay question the validity of previous studies. Clin Chem Lab Med. 2016;54(7):e199-e201. [DOI] [PubMed] [Google Scholar]
- 73. Peng H, Wang Q, Lou T, et al. Myokine mediated muscle-kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidneys. Nat Commun. 2017;8(1):1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Barja-Fernández S, Folgueira C, Castelao C, et al. FNDC5 is produced in the stomach and associated to body composition. Sci Rep. 2016;6:23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Estell EG, Le PT, Vegting Y, et al. Irisin directly stimulates osteoclastogenesis and bone resorption in vitro and in vivo. Elife. 2020;9:e58172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ruan Q, Huang Y, Yang L, et al. The effects of both age and sex on irisin levels in paired plasma and cerebrospinal fluid in healthy humans. Peptides. 2019;113:41-51. [DOI] [PubMed] [Google Scholar]
- 77. Zhang C, Ding Z, Lv G, Li J, Zhou P, Zhang J. Lower irisin level in patients with type 2 diabetes mellitus: a case-control study and meta-analysis. J Diabetes. 2016;8(1):56-62. [DOI] [PubMed] [Google Scholar]
- 78. Cui L, Qiao T, Xu F, et al. Circulating irisin levels of prenatal and postnatal patients with gestational diabetes mellitus: a systematic review and meta-analysis. Cytokine. 2020;126:154924. [DOI] [PubMed] [Google Scholar]
- 79. Qiu S, Cai X, Yin H, et al. Association between circulating irisin and insulin resistance in non-diabetic adults: a meta-analysis. Metabolism. 2016;65(6):825-834. [DOI] [PubMed] [Google Scholar]
- 80. Jia J, Yu F, Wei WP, et al. Relationship between circulating irisin levels and overweight/obesity: a meta-analysis. World J Clin Cases. 2019;7(12):1444-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang C, Zhang XY, Sun Y, Hou XG, Chen L. Higher circulating irisin levels in patients with polycystic ovary syndrome: a meta-analysis. Gynecol Endocrinol. 2018;34(4):290-293. [DOI] [PubMed] [Google Scholar]
- 82. Guo W, Zhang B, Wang X. Lower irisin levels in coronary artery disease: a meta-analysis. Minerva Endocrinol. 2020;45(1):61-69. [DOI] [PubMed] [Google Scholar]
- 83. Maalouf GE, El Khoury D. Exercise-Induced Irisin, the Fat Browning Myokine, as a Potential Anticancer Agent. J Obes. 2019;2019:6561726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Eslampour E, Ebrahimzadeh F, Abbasnezhad A, Khosroshahi MZ, Choghakhori R, Asbaghi O. Association between Circulating Irisin and C-Reactive Protein Levels: A Systematic Review and Meta-Analysis. Endocrinol Metab (Seoul). 2019;34(2):140-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhou K, Qiao X, Cai Y, Li A, Shan D. Lower circulating irisin in middle-aged and older adults with osteoporosis: a systematic review and meta-analysis. Menopause. 2019;26(11):1302-1310. [DOI] [PubMed] [Google Scholar]
- 86. Rabiee F, Lachinani L, Ghaedi S, Nasr-Esfahani MH, Megraw TL, Ghaedi K. New insights into the cellular activities of Fndc5/Irisin and its signaling pathways. Cell Biosci. 2020;10:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Moon HS, Dincer F, Mantzoros CS. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism. 2013;62(8):1131-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Oguri Y, Shinoda K, Kim H, et al. CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling. Cell. 2020;182(3):563-577.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang Y, Li R, Meng Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63(2):514-525. [DOI] [PubMed] [Google Scholar]
- 90. Liu J, Song N, Huang Y, Chen Y. Irisin inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway. Sci Rep. 2018;8(1):15247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. Faseb J. 2013;27(5):1981-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Elsen M, Raschke S, Eckel J. Browning of white fat: does irisin play a role in humans? J Endocrinol. 2014;222(1):R25-R38. [DOI] [PubMed] [Google Scholar]
- 93. Abdullahi A, Jeschke MG. White Adipose Tissue Browning: A Double-edged Sword. Trends Endocrinol Metab. 2016;27(8):542-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Trevellin E, Scorzeto M, Olivieri M, et al. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes. 2014;63(8):2800-2811. [DOI] [PubMed] [Google Scholar]
- 95. Xiong Y, Wu Z, Zhang B, et al. Fndc5 loss-of-function attenuates exercise-induced browning of white adipose tissue in mice. Faseb J. 2019;33(5):5876-5886. [DOI] [PubMed] [Google Scholar]
- 96. Lehnig AC, Dewal RS, Baer LA, et al. Exercise Training Induces Depot-Specific Adaptations to White and Brown Adipose Tissue. Iscience. 2019;11:425-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53(4):619-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Camera DM, Anderson MJ, Hawley JA, Carey AL. Short-term endurance training does not alter the oxidative capacity of human subcutaneous adipose tissue. Eur J Appl Physiol. 2010;109(2):307-316. [DOI] [PubMed] [Google Scholar]
- 99. Stinkens R, Brouwers B, Jocken JW, et al. Exercise training-induced effects on the abdominal subcutaneous adipose tissue phenotype in humans with obesity. J Appl Physiol (1985). 2018;125(5):1585-1593. [DOI] [PubMed] [Google Scholar]
- 100. Tsiloulis T, Carey AL, Bayliss J, Canny B, Meex RCR, Watt MJ. No evidence of white adipocyte browning after endurance exercise training in obese men. Int J Obes (Lond). 2018;42(4):721-727. [DOI] [PubMed] [Google Scholar]
- 101. Vosselman MJ, Hoeks J, Brans B, et al. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes (Lond). 2015;39(12):1696-1702. [DOI] [PubMed] [Google Scholar]
- 102. Otero-Díaz B, Rodríguez-Flores M, Sánchez-Muñoz V, et al. Exercise Induces White Adipose Tissue Browning Across the Weight Spectrum in Humans. Front Physiol. 2018;9:1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bettini S, Favaretto F, Compagnin C, et al. Resting Energy Expenditure, Insulin Resistance and UCP1 Expression in Human Subcutaneous and Visceral Adipose Tissue of Patients With Obesity. Front Endocrinol (Lausanne). 2019;10:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lim J, Park HS, Kim J, et al. Depot-specific UCP1 expression in human white adipose tissue and its association with obesity-related markers. Int J Obes (Lond). 2020;44(3):697-706. [DOI] [PubMed] [Google Scholar]
- 105. Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature. 2012;488(7413):E9-10; discussion E10. [DOI] [PubMed] [Google Scholar]
- 106. Dinas PC, Lahart IM, Timmons JA, et al. Effects of physical activity on the link between PGC-1a and FNDC5 in muscle, circulating Ιrisin and UCP1 of white adipocytes in humans: a systematic review. F1000Res. 2017;6:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Pillon NJ, Gabriel BM, Dollet L, et al. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat Commun. 2020;11(1):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dehghani M, Kargarfard M, Rabiee F, Nasr-Esfahani MH, Ghaedi K. A comparative study on the effects of acute and chronic downhill running vs uphill running exercise on the RNA levels of the skeletal muscles PGC1-α, FNDC5 and the adipose UCP1 in BALB/c mice. Gene. 2018;679:369-376. [DOI] [PubMed] [Google Scholar]
- 109. Tiano JP, Springer DA, Rane SG. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) during exercise. J Biol Chem. 2015;290(12):7671-7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pang M, Yang J, Rao J, et al. Time-Dependent Changes in Increased Levels of Plasma Irisin and Muscle PGC-1α and FNDC5 after Exercise in Mice. Tohoku J Exp Med. 2018;244(2):93-103. [DOI] [PubMed] [Google Scholar]
- 111. Abedpoor N, Taghian F, Ghaedi K, et al. PPARγ/Pgc-1α-Fndc5 pathway up-regulation in gastrocnemius and heart muscle of exercised, branched chain amino acid diet fed mice. Nutr Metab (Lond). 2018;15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Quinn LS, Anderson BG, Conner JD, Wolden-Hanson T. Circulating irisin levels and muscle FNDC5 mRNA expression are independent of IL-15 levels in mice. Endocrine. 2015;50(2):368-377. [DOI] [PubMed] [Google Scholar]
- 113. Lally JS, Ford RJ, Johar J, Crane JD, Kemp BE, Steinberg GR. Skeletal muscle AMPK is essential for the maintenance of FNDC5 expression. Physiol Rep. 2015;3(5):e12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Colaianni G, Cuscito C, Mongelli T, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A. 2015;112(39):12157-12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang Y, Li R, Meng Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63(2):514-525. [DOI] [PubMed] [Google Scholar]
- 116. Zhu G, Wang J, Song M, et al. Irisin Increased the Number and Improved the Function of Endothelial Progenitor Cells in Diabetes Mellitus Mice. J Cardiovasc Pharmacol. 2016;68(1):67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Qiao X, Yong Qiao X, Nie Y, et al. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep. 2016;6:18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Xin X, Wu J, Zheng A, et al. Delivery vehicle of muscle-derived irisin based on silk/calcium silicate/sodium alginate composite scaffold for bone regeneration. Int J Nanomedicine. 2019;14:1451-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Storlino G, Colaianni G, Sanesi L, et al. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J Bone Miner Res. 2020;35(4):766-775. [DOI] [PubMed] [Google Scholar]
- 120. Narayanan SA, Metzger CE, Bloomfield SA, Zawieja DC. Inflammation-induced lymphatic architecture and bone turnover changes are ameliorated by irisin treatment in chronic inflammatory bowel disease. Faseb J. 2018;32(9):4848-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Metzger CE, Narayanan SA, Elizondo JP, et al. DSS-induced colitis produces inflammation-induced bone loss while irisin treatment mitigates the inflammatory state in both gut and bone. Sci Rep. 2019;9(1):15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Colaianni G, Mongelli T, Cuscito C, et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep. 2017;7(1):2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Colucci S, Colaianni G, Brunetti G, et al. Irisin prevents microgravity-induced impairment of osteoblast differentiation in vitro during the space flight CRS-14 mission. Faseb J. 2020;34(8):10096-10106. [DOI] [PubMed] [Google Scholar]
- 124. Luo Y, Ma Y, Qiao X, et al. Irisin ameliorates bone loss in ovariectomized mice. Climacteric. 2020;23(5):496-504. [DOI] [PubMed] [Google Scholar]
- 125. Zhu X, Li X, Wang X, et al. Irisin deficiency disturbs bone metabolism. J Cell Physiol. 2021;236(1):664-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wucherpfennig TG, Müller S, Wolfrum C, Bode JW. Chemical Synthesis of the 12 kDa Human Myokine Irisin by α-Ketoacid-Hydroxylamine (KAHA) Ligation. Helv Chim Acta. 2016;99(12):897–907. [Google Scholar]
- 127. Bi J, Zhang J, Ren Y, et al. Irisin reverses intestinal epithelial barrier dysfunction during intestinal injury via binding to the integrin αVβ5 receptor. J Cell Mol Med. 2020;24(1):996-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bi J, Zhang J, Ren Y, et al. Exercise hormone irisin mitigates endothelial barrier dysfunction and microvascular leakage related diseases. JCI Insight. 2020b;5(13):e136277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Duong LT, Lakkakorpi P, Nakamura I, Rodan GA. Integrins and signaling in osteoclast function. Matrix Biol. 2000;19(2):97-105. [DOI] [PubMed] [Google Scholar]
- 130. Yavropoulou MP, Yovos JG. Osteoclastogenesis–current knowledge and future perspectives. J Musculoskelet Neuronal Interact. 2008;8(3):204-216. [PubMed] [Google Scholar]
- 131. Franco OH, de Laet C, Peeters A, Jonker J, Mackenbach J, Nusselder W. Effects of physical activity on life expectancy with cardiovascular disease. Arch Intern Med. 2005;165(20):2355-2360. [DOI] [PubMed] [Google Scholar]
- 132. Ozemek C, Laddu DR, Lavie CJ, et al. An Update on the Role of Cardiorespiratory Fitness, Structured Exercise and Lifestyle Physical Activity in Preventing Cardiovascular Disease and Health Risk. Prog Cardiovasc Dis. 2018;61(5-6):484-490. [DOI] [PubMed] [Google Scholar]
- 133. Görgens SW, Eckardt K, Jensen J, Drevon CA, Eckel J. Exercise and Regulation of Adipokine and Myokine Production. Prog Mol Biol Transl Sci. 2015;135:313-336. [DOI] [PubMed] [Google Scholar]
- 134. Pérez-Martínez P, Mikhailidis DP, Athyros VG, et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: an international panel recommendation. Nutr Rev. 2017;75(5):307-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. [DOI] [PubMed] [Google Scholar]
- 137. Pascoe MC, Parker AG. Physical activity and exercise as a universal depression prevention in young people: a narrative review. Early Interv Psychiatry. 2019;13(4):733-739. [DOI] [PubMed] [Google Scholar]
- 138. Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. 2019;15(7):383-392. [DOI] [PubMed] [Google Scholar]
- 139. Vanderlinden J, Boen F, van Uffelen JGZ. Effects of physical activity programs on sleep outcomes in older adults: a systematic review. Int J Behav Nutr Phys Act. 2020;17(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472. [DOI] [PubMed] [Google Scholar]
- 141. Norheim F, Raastad T, Thiede B, Rustan AC, Drevon CA, Haugen F. Proteomic identification of secreted proteins from human skeletal muscle cells and expression in response to strength training. Am J Physiol Endocrinol Metab. 2011;301(5):E1013-E1021. [DOI] [PubMed] [Google Scholar]
- 142. Moon HY, Becke A, Berron D, et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016;24(2):332-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. De la Rosa A, Solana E, Corpas R, et al. Long-term exercise training improves memory in middle-aged men and modulates peripheral levels of BDNF and Cathepsin B. Sci Rep. 2019;9:3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sleiman SF, Henry J, Al-Haddad R, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife. 2016;5:e15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Norwitz NG, Hu MT, Clarke K. The Mechanisms by Which the Ketone Body D-β-Hydroxybutyrate May Improve the Multiple Cellular Pathologies of Parkinson’s Disease. Front Nutr. 2019;6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10(9):1103-1109. [DOI] [PubMed] [Google Scholar]
- 147. Dantzer R. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression: Preclinical Approaches. Curr Top Behav Neurosci. 2017;31:117-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Schlittler M, Goiny M, Agudelo LZ, et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol. 2016;310(10):C836-C840. [DOI] [PubMed] [Google Scholar]
- 150. Contrepois K, Wu S, Moneghetti KJ, et al. Molecular Choreography of Acute Exercise. Cell. 2020;181(5):1112-1130.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ma D, Li S, Lucas EK, Cowell RM, Lin JD. Neuronal inactivation of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) protects mice from diet-induced obesity and leads to degenerative lesions. J Biol Chem. 2010;285(50):39087-39095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lourenco MV, Ribeiro FC, Sudo FK, et al. Cerebrospinal fluid irisin correlates with amyloid-β, BDNF, and cognition in Alzheimer’s disease. Alzheimers Dement (Amst). 2020;12(1):e12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Banks WA. Leptin transport across the blood-brain barrier: implications for the cause and treatment of obesity. Curr Pharm Des. 2001;7(2):125-133. [DOI] [PubMed] [Google Scholar]
- 154. El Hayek L, Khalifeh M, Zibara V, et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J Neurosci. 2019;39(13):2369-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Horowitz AM, Fan X, Bieri G, et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369(6500):167-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Liu TY, Xiong XQ, Ren XS, et al. FNDC5 Alleviates Hepatosteatosis by Restoring AMPK/mTOR-Mediated Autophagy, Fatty Acid Oxidation, and Lipogenesis in Mice. Diabetes. 2016;65(11):3262-3275. [DOI] [PubMed] [Google Scholar]
- 157. Li RL, Wu SS, Wu Y, et al. Irisin alleviates pressure overload-induced cardiac hypertrophy by inducing protective autophagy via mTOR-independent activation of the AMPK-ULK1 pathway. J Mol Cell Cardiol. 2018;121:242-255. [DOI] [PubMed] [Google Scholar]
- 158. Luo Y, Qiao X, Ma Y, Deng H, Xu CC, Xu L. Disordered metabolism in mice lacking irisin. Sci Rep. 2020;10(1):17368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhou B, Ling L, Zhang F, et al. Fibronectin Type III Domain-Containing 5 Attenuates Liver Fibrosis Via Inhibition of Hepatic Stellate Cell Activation. Cell Physiol Biochem. 2018;48(1):227-236. [DOI] [PubMed] [Google Scholar]
- 160. Zhou B, Qiu Y, Wu N, et al. FNDC5 Attenuates Oxidative Stress and NLRP3 Inflammasome Activation in Vascular Smooth Muscle Cells via Activating the AMPK-SIRT1 Signal Pathway. Oxid Med Cell Longev. 2020;2020:6384803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Li X, Fang W, Hu Y, Wang Y, Li J. Characterization of fibronectin type III domain-containing protein 5 (FNDC5) gene in chickens: cloning, tissue expression, and regulation of its expression in the muscle by fasting and cold exposure. Gene. 2015;570(2):221-229. [DOI] [PubMed] [Google Scholar]