Abstract
Work over the last 40 years has described macrophages as a heterogeneous population that serve as the frontline surveyors of tissue immunity. As a class, macrophages are found in almost every tissue in the body and as distinct populations within discrete microenvironments in any given tissue. During homeostasis, macrophages protect these tissues by clearing invading foreign bodies and/or mounting immune responses. In addition to varying identities regulated by transcriptional programs shaped by their respective environments, macrophage metabolism serves as an additional regulator to temper responses to extracellular stimuli. The area of research known as “immunometabolism” has been established within the last decade, owing to an increase in studies focusing on the crosstalk between altered metabolism and the regulation of cellular immune processes. From this research, macrophages have emerged as a prime focus of immunometabolic studies, although macrophage metabolism and their immune responses have been studied for centuries. During disease, the metabolic profile of the tissue and/or systemic regulators, such as endocrine factors, become increasingly dysregulated. Owing to these changes, macrophage responses can become skewed to promote further pathophysiologic changes. For instance, during diabetes, obesity, and atherosclerosis, macrophages favor a proinflammatory phenotype; whereas in the tumor microenvironment, macrophages elicit an anti-inflammatory response to enhance tumor growth. Herein we have described how macrophages respond to extracellular cues including inflammatory stimuli, nutrient availability, and endocrine factors that occur during and further promote disease progression.
Keywords: macrophage, inflammation, immunometabolism, Krebs cycle, epigenetic modifications
Graphical Abstract
Graphical Abstract.
Essential Points.
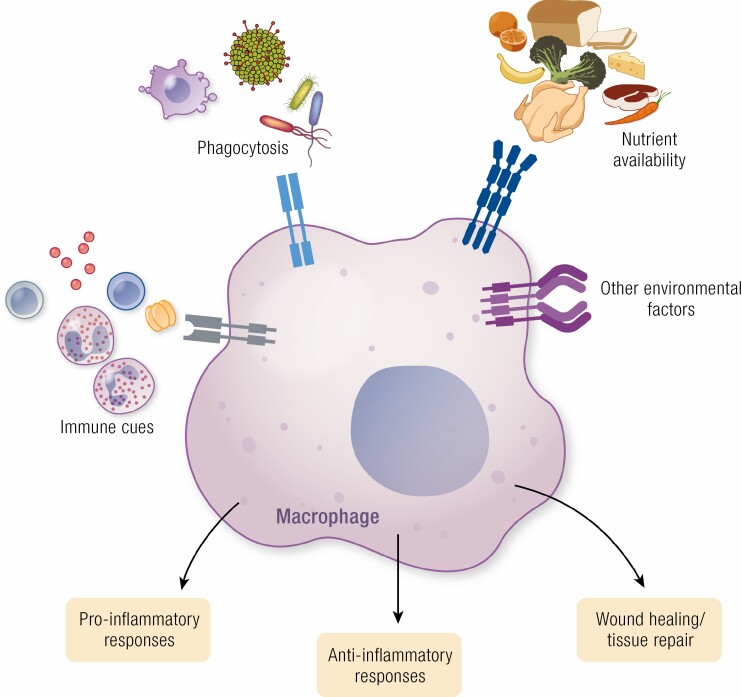
Macrophages are an integral part of the innate immune system, equipped with cell surface receptors that allow them to respond to their environment
Macrophage phenotypes can be generally classified as proinflammatory or anti-inflammatory, although macrophages exist along a spectrum rather than discrete inflammatory states
Epigenetic changes help establish macrophages identity, through the engagement of transcription factor complexes, as well as macrophage responses to future inflammatory challenges termed immune memory
Metabolic rewiring of macrophages in response to changes in environmental stimuli, such as metabolic substrates or endocrine factors, alter macrophage phenotype to drive their pathogenic contributions to a variety of diseases, such as atherosclerosis, obesity, diabetes, and cancer
Macrophages were first recognized as a cell type capable of phagocytosis in the late 19th century by Russian zoologist and Nobel laureate Élie Metchnikoff. Macrophages, as we now understand them, are an integral member of the immune system. They are found in virtually all tissues and carry out diverse physiological roles that regulate homeostasis through promoting the clearance of cellular debris and microbes, tissue repair, and mounting immune responses through cytokine production (1-3). Beyond their functions that preserve homeostasis, macrophages also play crucial roles in promoting pathogenesis, through changes in their numbers, the maintenance of their pool in tissues, and by their diverse tissue-specific functions.
Macrophages express a host of cell surface receptors that allow these cells to interact with and respond to changes in their microenvironment, which facilitate their functions as the first responders of the immune system at the tissue level. These include receptors recognize molecules indicating cellular stress, such as danger-associated molecular patterns (DAMPs), also known as alarmins, including exogenous nucleic acids and other stress proteins; or pattern-associated molecular patterns (PAMPs), namely microbial components that are detected by pattern recognition receptors such as toll-like receptors (TLRs) (4-6). In addition to recognition of inflammatory stimuli, macrophages also express receptors and other surface molecules to allow for the phagocytosis of foreign bodies, such as bacteria or apoptotic cells, that limit infection and preserve homeostatic cellular turnover (5-7). Macrophages thus act as immune surveyors that are exquisitely designed and equipped with the cellular machinery necessary to protect tissues from pathogens and accumulation of cell debris by mounting an immune response, including induction of microbicidal molecules and proinflammatory cytokines, clearance of cell debris/apoptotic cells, or contributing to tissue repair. These responses are typical during homeostasis but become dysregulated and actively contribute to pathogenesis. Therefore, macrophages represent a critical balance between inflammation as a process of pathogen resolution and tissue repair vs proinflammatory processes that potentiate disease.
Macrophages possess a variety of functions, including antigen presentation, phagocytosis/recycling of substrates in their environment, and a variety of other tissue-specific functions that have been covered in greater detail elsewhere (1, 8-10). However, one of the most widely studied functions of macrophages are as potent modulators of the inflammatory response. Simplistically, macrophages are broadly classified as polarized to either a proinflammatory (also referred to as classically activated or “M1”) or anti-inflammatory (also referred to as alternatively activated or “M2”) state (11-15). In addition to production of proinflammatory cytokines, M1 macrophages also produce microbicidal factors such as reactive oxygen species (ROS) during infection; whereas M2 macrophages produce anti-inflammatory factors that can dampen this response and participate in wound healing and the clearance of apoptotic cells/microorganisms (16). Macrophages demonstrate a great degree of plasticity, whereby their phenotypes are dictated through an interplay between changes in their transcriptional programs predominating metabolic pathways imprinted by their varying environment stimuli (Fig. 1).
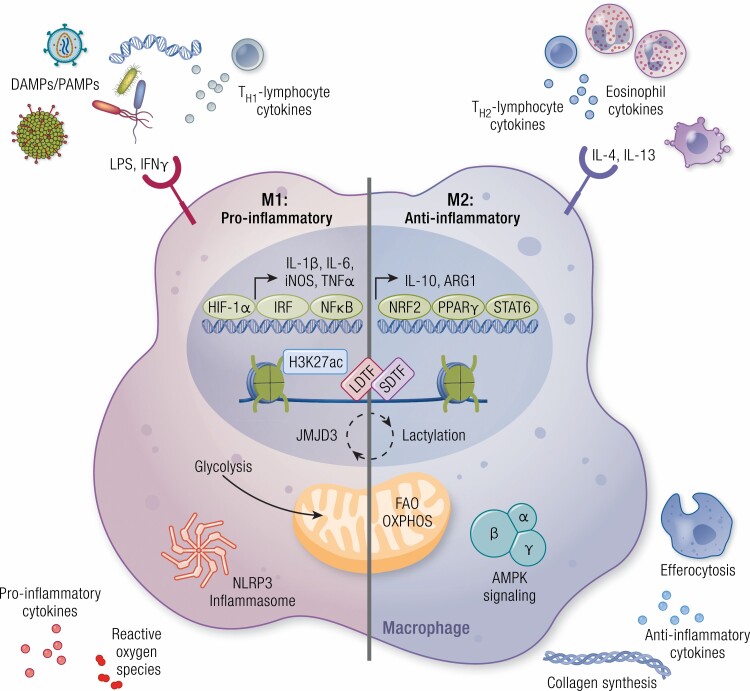
Figure 1.
Extracellular cues from the environment polarize macrophages. Macrophages express a variety of cell surface receptors that sense extracellular factors that induce macrophage polarization toward a proinflammatory (M1) or anti-inflammatory (M2) state. In cultures, proinflammatory macrophages are polarized by stimulation with lipopolysaccharide (LPS) and interferon γ (IFN-γ). The proinflammatory phenotype of macrophages can also be stimulated by detection of danger-associated molecular patterns (DAMPs)/pattern-associated molecular patterns (PAMPs), such as bacteria, viruses, and exogenous DNA, as well as by cytokines produced by TH1-lymphocytes. Polarization is in part dictated by the activation of transcription factors, such as hypoxia-inducible factor-1α (HIF-1α), IFN-regulatory factor (IRF), and nuclear factor κB (NFκB), and the induction of their proinflammatory gene networks. Furthermore, metabolic reprogramming occurs in these macrophages, primarily by the upregulation of glycolysis. Together these transcriptional and metabolic changes result in inflammasome activation and the production of proinflammatory cytokines and reactive oxygen species. Anti-inflammatory macrophages are produced by the treatment of macrophages with interleukin-4 (IL-4) or IL-13 in vitro and in vivo signals are received from eosinophils and TH2-lymphocytes. Transcription factors required for M2-like polarization include nuclear factor erythroid 2-related factor 2 (NRF2), peroxisome proliferator-activated receptor γ (PPARγ), and signal transducer and activator of transcription 6 (STAT6), leading to the production of anti-inflammatory genes such as IL-10 and arginase 1 (ARG1). In these anti-inflammatory macrophages, metabolism is shifted toward fatty acid oxidation (FAO) and oxidative phosphorylation (OXPHOS). Here, adenosine 5′-monophosphate–activated protein kinase (AMPK) signaling is promoted, and in turn leads to the production of anti-inflammatory cytokines, collagen synthesis, and efferocytosis. Epigenetic modifications also play a role in determining macrophage identity and function, including chromatin remodeling such as acetylation of lysine 27 of histone 3 (H3K27ac) accompanied by binding of transcription factors (LDTF, lineage-determining transcription factors; SDTF, signal-dependent transcription factors), an epigenetic signature that is strongly influenced by environment-derived factors. The plasticity of macrophages between its proinflammatory and anti-inflammatory polarized states can be induced by feedback pathways engaged by the metabolic programs that involve epigenetic changes, such as histone lactylation or demethylation (JMJD3).
Macrophage polarization status has been most widely studied in cultures by cytokine stimulation to promote M1 and M2 polarization (14, 17). A list of these factors has been comprehensively reviewed elsewhere (11, 14, 17). In vitro, the proinflammatory state of macrophages (herein referred to as M1 macrophages) is induced by engagement of TLR4 by PAMPs, such as lipopolysaccharide (LPS), which mimic infection with gram-negative bacteria, or other receptors by DAMPs such as interferon-γ (IFN-γ). These cell surface events initiate cell signaling cascades involving the activation of key transcription factors including nuclear factor κ-light-chain-enhancer of activated B cells (NFκB) or interferon-regulatory factor (IRF) (11, 12, 15). Promoting anti-inflammatory states (herein referred to as M2 macrophages) in cultured macrophages mainly rely on interleukin-4 or -13 (IL-4 or IL-13) that induce transcription factors including signal transducer and activator of transcription 6 (STAT6) and peroxisome proliferator-activated receptor γ (PPARγ) dependent signaling (14, 18). In vivo, the expression of a variety of other pattern recognition receptors allow macrophages to mount appropriate inflammatory responses. These include other TLRs and retinoic acid-inducible gene I (RIG-I)-like receptors that sense microorganismal-derived nucleic acids, nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) for the recognition of bacterial components, and C-type lectin receptors for the detection of carbohydrate moieties on the surface of invading microbes, along with scavenger receptors and other receptors for the detection of intracellular DNA (19, 20).
Aside from monocytes that differentiate to macrophages, other immune cell types are recruited to the tissues during disease, where they release cytokines that direct macrophages in their local tissue environment. These cytokines include proinflammatory mediators including IFNs, primarily IFNγ, ILs (ie, IL-1α and -1β), and tumor necrosis factor (TNF) superfamily members; as well as anti-inflammatory mediators including transforming growth factor β (TGFβ) and other ILs (ie, IL-4, IL-10, and IL-13). This has been most well described in the context of macrophages responding to cytokines released by T helper (TH) cell subsets. TH-1 lymphocytes produce the prototypical inflammatory cytokines such as IFN-γ and TNFα, which promote macrophage polarization toward an M1 phenotype (17). In turn, these inflammatory macrophages feed back to the lymphocytes to further mount a proinflammatory response through the release of proinflammatory cytokines, such as TNFα and IL-1β, -12, and -23 (17). Conversely, M2 polarization of macrophages can be stimulated in response to TH-2 lymphocyte-derived factors, such as IL-4 or IL-13 (21, 22). Other T-cell subsets can also promote macrophage polarization, including effector T cells to stimulate M1 polarization and regulatory T cells that inhibit this inflammatory phenotype (23, 24). In addition to lymphocytes, macrophages also receive cues from other immune cell types such as eosinophils, such as those found in the adipose tissue, where eosinophils secrete IL-4 and IL-13 in response to cold stress to direct macrophages to adopt a more M2-like phenotype to promote insulin sensitivity and browning of the adipose tissue via macrophage production of catecholamines (25-27). However, a more recent study has challenged these findings, showing that neither alternatively activated monocyte-derived macrophages nor adipose tissue resident tissue macrophages (RTMs) were capable of producing catecholamines required to stimulate adipose tissue thermogenesis (28). The authors instead postulate that macrophages may instead take up catecholamines, such as norepinephrine, in an IL-4-dependent manner that can then be released to induce thermogenesis in recipient adipocytes. Apart from inflammatory status, immune cell-derived factors have been demonstrated to maintain macrophage identity. Basophils, for example, through the release of critical inflammatory cytokines and growth factors, can contribute to the environment that imprints the identity of alveolar macrophages (29). Nevertheless, together these responses of macrophages to immune cues help shape their phenotype and inflammatory responses to promote tissue homeostasis or disease progression.
It is important to note that this classification of M1 or M2 macrophages represents extremes on a spectrum in which macrophages can exist. Factors that dictate macrophage polarization coexist in vivo and those that predominate during homeostasis vs disease shift macrophage inflammatory phenotype. Importantly, these factors do not exist in mutually exclusive states, and macrophages can coexpress prototypical markers of M1 and M2 phenotypes (15, 18, 30, 31). Moreover, as will be discussed in detail later, changes in the environment that occur during disease in which the macrophage resides influences the macrophage identity and thus tissue-specific function beyond the binary system of proinflammatory or anti-inflammatory.
Macrophages have been a major focus of the emerging field of “immunometabolism,” the intersection between metabolism and immunity, by which the cell responds to its environment by altering its metabolism to elicit an immunomodulatory effect. Beyond their inflammatory responses, M1 and M2 macrophages are characterized by their varying metabolic profiles. The metabolic rewiring of macrophages in response to external stimuli has received much attention within the last decade. Very generally, M1 macrophages prefer glycolytic metabolism, whereas M2 macrophages favor fatty acid oxidation (FAO, also referred to as β-oxidation) and oxidative phosphorylation (OXPHOS) (14, 18). This allows macrophages to fine-tune their functions rapidly in response to differing environmental stimuli. During diseases such as diabetes, obesity, and atherosclerosis, metabolic substrates such as glucose and fatty acids, along with classical immune and endocrine regulators, become dysregulated. This influences macrophage metabolism and subsequently inflammatory status to contribute to the pathophysiological states. This is also exemplified in cancer, in which within the tumor microenvironment, macrophages are subject to an environment with an altered metabolic profile. In this review we will discuss the mechanisms by which macrophages respond to environmental cues, including immune signals or the availability of metabolic substrates, and how this influences macrophage phenotype and contribution to health or disease.
Macrophage Fate and Subtypes in Health and Disease
Macrophages are found in nearly every tissue of the body. Early views of macrophages regarded them solely as mononuclear phagocytes, which are derived from circulating monocytes that infiltrate tissue and encounter growth factors/cytokines that promote their differentiation to macrophages (32, 33). However, evidence for macrophages derived in the absence of monocyte recruitment began to mount in the 1980s (34-38). In comparison to macrophages that terminally differentiate from monocytes of the hematopoietic lineage during adulthood, RTMs have the capacity for self-renewal and possess unique expression profiles and functions that have been described in detail in several other reviews (1, 8-10). Recently, the emergence of lineage tracing and fate mapping techniques have shown that while some RTM populations are maintained by the recruitment of monocytes, tissue macrophages also are self-maintained through continued proliferation of an embryonic pool of macrophages (10, 39-47). These macrophages arise from embryonic hematopoietic progenitors of the yolk sac, fetal liver, or aorta-gonad-mesonephros axis (reviewed in depth in [38]). While studies have demonstrated the proliferation of monocyte-derived macrophages, these macrophages show a limited capacity for self-renewal, in the case of infiltrating macrophages within the atherosclerotic plaque, surviving only 1 to 2 months (38, 48, 49). A prominent example of the environmental-derived factors known to support macrophage self-renewal and proliferation is macrophage colony-stimulating factor (also known as CSF1) (1, 2). This factor has been shown to be critical in the setting of atherosclerotic plaque (49, 50). Within the plaque, macrophages also depend on the expression of scavenger receptor class A, also known as macrophage scavenger receptor 1, which senses modified lipoproteins in the atherosclerotic plaque to also sustain the renewal of this population (48). Together these observations further support the role of cholesterol and inflammation in driving the pathogenesis of atherosclerosis. In other tissues, like the lung, factors such as granulocyte-macrophage colony-stimulating factor facilitate the maintenance of the pool of macrophages (10).
Stemming from these observations is the notion that in adulthood and under steady state, macrophage subtypes in tissues are maintained according to 1 of 3 dynamic scenarios: 1) self-renewal of embryonic-derived macrophages with no monocyte recruitment, 2) monocyte recruitment minimally contributes to the maintenance of the macrophage pool in the tissue, and 3) monocyte recruitment is almost exclusively responsible for the macrophage pools (38). Recently, using an elegant new fate mapping model, it was demonstrated that indeed tissue macrophages develop during waves of hematopoiesis, either embryonic or during adulthood (51). Using the Ms4a3 reporter mouse, which labels monocytes and their progenitors but not RTMs or dendritic cells, the authors were able to demonstrate these 3 dynamic paradigms for macrophage populations found in various tissues. Work in this article recapitulates the results of previous studies, which show that microglia and Kupffer cells do not rely on monocyte recruitment (scenario 1), macrophages in tissues such as the lung and spleen minimally require monocyte contributions (scenario 2), and macrophages in tissues such as the skin or gut require continual replenishment from monocyte recruitment (scenario 3). However, distinct from the scenario during steady state, inflammation alters the dynamics of macrophage pools. This has been shown to be crucial for cardiac macrophages after inflammation, where monocyte recruitment and local proliferation drive their expansion (52). Previous reports have demonstrated that on infection with Listeria monocytogenes, Kupffer cells are replenished by recruited monocytes through upregulated monocyte chemoattractant protein-1(MCP-1), whereas treatment with IL-4, which is an important cytokine during parasitic infection that can be derived from basophils, leads to enhanced resident Kupffer cell proliferation to replenish its pools (53, 54). Using the Ms4a3 reporter mouse, these authors also demonstrate differences in peritoneal macrophage dynamics, whereby intraperitoneal administration of thioglycolate significantly induced monocyte recruitment and replenishment of macrophages compared to LPS administration, which promotes RTM proliferation in the peritoneum (51).
Recent advances in our capability to survey tissues at the single-cell level have allowed us to detect genome-wide changes in macrophages, which has further allowed the dissection of the contributions of monocyte and RTM to tissue macrophage pools. Furthermore, using this technique, researchers have also been able to interrogate the macrophage subpopulations within various depots in response to environmental stimuli during homeostasis, or adaptations in macrophage phenotype that alter pathogenesis.
Heterogeneity of Macrophage Subpopulations
Whole-transcriptome sequencing at the single-cell level has revolutionized our understanding of tissue heterogeneity. Single-cell RNA sequencing (scRNA-seq) has also facilitated the study of differences that occur in macrophage subtypes under a variety of experimental conditions, including their origins, the influence of environmental factors or in diseases, and their corresponding transcriptomic signatures. Here we will highlight major breakthroughs using this technology that have increased our understanding of macrophage heterogeneity and adaptations during health and disease.
A critical aspect to macrophage maintenance is the influence of sex. For instance in male mice, maintenance of the peritoneal macrophage pool shows a greater reliance on monocyte contributions as compared to female mice (51). More recent studies using peritoneal macrophages have described the influence of sex on the microenvironment, whereby bone marrow transplantation from female donors into male recipients yielded a higher chimerism of the F4/80hiCD102+ (large) peritoneal macrophages than when transplanted into female recipients (55). In addition to their numbers, the microenvironment conferred by sex differentially regulated the transcriptome, where female large peritoneal macrophages showed an enhanced signature for genes related to embryonic or resident-derived macrophages. One of these key genes was identified as CD209b, which is a receptor involved in sensing PAMPs. Accordingly, female mice showed better clearance of bacteria compared to their male counterparts when infected by Streptococcus pneumoniae in a peritonitis model (55). The authors demonstrate that this effect of sex is likely not due to the direct stimulation of macrophages with sex-specific hormones, as estradiol treatment did not overcome the effects of ovariectomy, and macrophages from both sexes were found to have equivalent expression of receptors for other sex hormones such as androgen and progesterone. Beyond the presence of hormones, the peritoneal microenvironment is composed of other resident cell types, such as stromal cells that produce retinoic acid (56), that may themselves exhibit sex-specific traits to regulate peritoneal macrophage populations.
Interestingly, through the use of scRNA-seq, recent studies have demonstrated that the origin of brain cancers greatly influence the macrophage composition in the brain (57). For instance, cancers originating in the brain, such as gliomas, show an enrichment of microglia, whereas cancers that have metastasized to the brain are enriched in macrophages derived from infiltrating leukocytes. Further discrimination has revealed that low-grade gliomas (those possessing isocitrate dehydrogenase [IDH] mutations) show a higher abundance of microglia as compared to higher-grade gliomas (wild-type for IDH), which show greater macrophage content from monocyte recruitment in the tumor microenvironment (58). The inflammatory status of macrophages has been demonstrated to play a central role in macrophage accumulation during atherosclerosis, as well as the qualitative change in macrophages that promote disease regression. It is therefore no surprise that several groups have applied scRNA-seq technology to elucidate the macrophage subtypes in mouse and human atherosclerotic plaques (59-63). One of these studies has demonstrated drastic macrophage heterogeneity within the plaque, with macrophages characterized as highly inflammatory or high in lipid content (also known as foam cells) (63). Together, these studies demonstrated the ability of scRNA-seq to uncover macrophage subpopulations that drive disease and thus leveraging these data may therefore allow us to develop better targeted therapeutics and exploit the endogenous capabilities of the macrophage to treat or prevent disease.
The ability to translate findings from animal models to humans presents a major obstacle in the therapeutic pipeline. While it is well accepted that macrophages play critical roles in promote homeostasis both in lower-order organisms and humans, the differing heterogeneity of macrophage populations and their transcriptomic profiles may offer additional insights into the translation of results from bench to bedside. In this regard, single-cell technologies have also allowed us to evaluate species differences in macrophage phenotype and responses. scRNA-seq has demonstrated similarities between myeloid populations (ie, neutrophils and dendritic cells) in lung cancer between mice and humans (64). However, this was not the case for alveolar macrophages, which showed a great deal of species divergence, where in addition to transcriptomic changes, the number of macrophage subtypes were altered between species (9 populations in human lung tumor biopsies vs 4 populations in mouse lung tumors). Additionally, others have demonstrated a greater diversity in microglia subtypes in humans compared to other mammals (65). These highlighted studies clearly demonstrate the power of scRNA-seq to produce rich data sets that allow us to characterize macrophage subpopulations and infer phenotypic differences from transcriptomic changes during health and disease, which may better serve our ability to extrapolate macrophage influence from preclinical models to the clinical setting.
Environmental Control of Macrophage Subtypes by Epigenetic Modifications and Transcription Factor Binding
Macrophage identity in response to environmental stimuli has also been described through the concept of chromatin remodeling, which was first demonstrated by the Medzhitov group in 2007 (66). This essential role for epigenetic modifications in enhancers occurs in response to environmental cues to dictate macrophage fate and function. Primed enhancers, denoted by specific histone markers such as H3K4me1/2 (histone 3, lysine 4, monomethylation or dimethylation), act at sites distal to known promoters that regulate gene expression (67). These studies have therefore described a major contribution of the environment to shaping the transcriptome and identity of macrophages (68, 69). For example, analysis of gene expression signatures reveals a closer association between resident and monocyte-derived peritoneal macrophages as compared to macrophages derived from other environments such as microglia from the brain (69). The effects of the tissue microenvironment on shaping gene expression signatures have also been described, whereby macrophages derived from monocytes adopt transcriptomic profiles similar to those of RTMs when exposed to the same environment and how reciprocal culture conditions between macrophage populations (ie, peritoneal vs microglia) lead to the acquisition of alternative gene expression profiles (69-72).
This chromatin remodeling allows for greater accessibility and binding of key transcription factors capable of influencing macrophage identity (73, 74). These transcription factors, known as lineage-determining transcription factors (LDTFs), bind to enhancers and, along with other enhancer-binding transcription factors native to macrophages that serve “in collaboration” with the LDTFs, lead to further histone modifications that shape the responses and identity of the macrophages to environmental stimuli. In macrophages, binding of the LDTF PU.1, along with macrophage transcription factor C/EBPα/β, leads to the methylation of histone 3 (H3K4me1/2), allowing for a primed response to external stimuli, such as an inflammatory challenge from the environment including the engagement of TLR4 by LPS. This leads to a downstream signaling pathway involving the activation and binding of the NFκB subunits, which is considered a signal-dependent transcription factor (SDTF), resulting in an open or active enhancer conformation (denoted by H3K27ac) and subsequent transcriptomic changes (73, 74). The repertoire of these environmental-derived factors establish macrophage identity within their individual environmental niches (reviewed in [75]). Several studies have described a role for the nuclear receptor superfamily of ligand-activated transcription factors, such as the liver X receptors (LXRs), glucocorticoid receptors, and Rev-Erbs, to act as SDTFs (73, 74, 76, 77). Another example of an SDTF that also demonstrates the intricate communication between macrophage metabolic and immunomodulatory responses is the activation and requirement for the master regulator of cholesterol synthesis sterol regulatory element-binding factor 2 (SREBF2) to induce TNFα-dependent expression of proinflammatory genes (78). Nevertheless, the sequential binding of these LDTFs, collaborative factors, and SDTFs in macrophages elicits specific responses that occur as a result of macrophage-specific gene expression in response to environmental influences (73, 74).
The study of these epigenetic alterations has driven our understanding of how the environment imprints the identity of distinct macrophage populations from different tissues. Among these observations, enrichment of active (not poised or primed) histone markers has been identified at enhancers corresponding to retinoic acid receptor β binding in macrophage subsets isolated from the peritoneum, where retinoic acid production is known to regulate macrophage responses (13, 56). In fact during cancer, tumors also produce an abundance of retinoic acid that promotes monocyte differentiation to macrophages, but not dendritic cells, through repression of dendritic cell–dependent transcription factor IRF4 (79), providing further evidence that retinoic acid derived from the microenvironment alters macrophage fate. These findings have also been extended to RTMs of the liver (Kupffer cells), which describe the requirement for transcription factors including LXRα and SMADs for maintaining Kupffer cell identity through upstream signals derived from the environment, including hepatocyte-derived desmosterol and TGFβ signaling from sinusoidal endothelial cells (80, 81). Interestingly, during nonalcoholic steatohepatitis (NASH), disruption of these LDTFs contributes to disease progression, whereby alterations in collaborative factors with LXR decrease Kupffer cell identity, while promoting the induction of gene expression that promote NASH (82). Loss of these RTMs Kupffer cells during NASH leads to the recruitment of monocyte-derived Kupffer cells, which possess a more proinflammatory profile that further perpetuate liver injury during NASH (83).
Despite the influences of the environment in shaping the identity of macrophage subpopulations, many studies have also demonstrated that even within the same niche, macrophage subtypes still possess unique transcriptional profiles. Furthermore, despite similar origins, TGFβ signaling has been shown to play a critical role in the development of microglia vs border-associated macrophages in the brain (84). This has also been described in monocyte-derived macrophages in the lung vs resident alveolar macrophages, and alveolar vs lung-interstitial macrophages (85, 86). In line with these observations of differential responses to the same environmental stimuli, using complex genomic interrogation, it has been shown that RTMs of the lung (alveolar vs interstitial) display differences in their responses to LPS administered through independent routes (intraperitoneal vs intranasal) (87). This was attributed to the separate origins of these macrophages and the combination of LDTFs required for epigenetic modifications along the chromatin surrounding TLR4-related downstream genes.
Apart from differential responses of macrophage to the same stimuli depending on their origin (88), many tissues are heterogeneous and thus comprise multiple microenvironments. This has been demonstrated in the brain using scRNA-seq (89, 90), which has greatly enhanced our understanding of homeostatic functions of this tissue. In a distinct RTM population, PU.1 binding sites are enriched near transcription factor binding sites known to regulate microglia identity and function (69). This has best been exemplified by the distribution of macrophages throughout the red and white pulps of the spleen. Work from several groups has established the requirement for transcription factors such as LXRα and Spi-C in the development of marginal zone and red pulp macrophages, respectively (1, 91, 92). Furthermore, recent studies have shown that development of splenic red pulp macrophages is shaped by IL-33, which can be released by damaged cells in the red pulp splenic microenvironment, to stimulate their differentiation from monocytes by chromatin remodeling and transcriptional changes dictated by GATA2, which further demonstrate the ability of macrophages to respond to environmental stressors (93). These brief examples together highlight the role of transcription factor specificity and chromatin dynamics in response to microenvironmental cues to regulate macrophage fate and function. Further examples and in-depth mechanisms outlining combinations of transcription factors required to elicit these responses have been covered in greater detail elsewhere (67, 94).
Chromatin dynamics also play a role in immune memory. “Trained immunity” refers to the heightened inflammatory responses after restimulation, whereas “immune tolerance” refers to a diminished inflammatory response on subsequent stimulation (95, 96). Changes in chromatin structure leading to immune memory has been demonstrated in microglia, which influence neurologic diseases after acute vs chronic LPS administration (66, 67, 97, 98). Macrophage tolerance has also been described after the resolution of inflammation, whereby infection with pneumonia causes decreased phagocytic activity of alveolar macrophages for weeks after clearance of the infection (99). Interestingly, these epigenetic changes in the alveolar macrophages that lead to defective phagocytosis were secondary to the effects of the infection on other cell types that released tolerogenic factors such as signal-regulatory protein α to dampen macrophage phagocytic capabilities. Immune memory in macrophages can also depend on signals derived from other immune cell types. For example, in the context of respiratory viral infection, CD8+ T cells educate resident alveolar macrophages, conferring enhanced glycolytic metabolism and gene expression signatures associated with antigen presentation and defense to bacteria (100). On subsequent respiratory bacterial infection, these educated alveolar macrophages increase the release of neutrophil chemotactic factors, required for neutrophil recruitment and resolution of the bacterial infection. Apart from infection, local injury, such as myocardial infarction or stroke, results in transcriptional changes in macrophages in distal tissues leading to primed responses for subsequent challenges (101). An example of this trained immunity was increased clearance of bacteria by alveolar macrophages (lung) after myocardial infarction (local injury in the heart). In the setting of breast cancer, myocardial infarction has also been demonstrated to potentiate tumor growth. This was recently shown to occur through epigenetic changes that alter the inflammatory status of Ly6Chi monocytes, which are recruited and differentiate to macrophages found in the tumor microenvironment (102). These examples clearly highlight the importance of understanding the molecular signatures of rewired macrophages and their ability to serve as predictive markers for future disease, including autoimmune diseases. Exploring the potential to predict and manipulate macrophage responses could harness and boost the body’s natural defenses against further inflammatory insults, such as during infection, autoimmune diseases, or organ transplant rejection.
Macrophage Response to and Support of the Immune System
Support of Hematopoiesis
In addition to their development from the hematopoietic system, macrophages also regulate the production of factors that play a critical role in supporting hematopoiesis (103, 104). In this setting, macrophages sense the external environment to modulate the need for hematopoiesis according to systemic cues, including inflammatory signals. Production of matrix metalloproteinases released by macrophages liberates hematopoietic stem and progenitor cells (HSPCs) from the aorta-gonad-mesonephros region of the mesoderm, which houses these HSPCs during embryogenesis, allowing for their migration to the fetal liver and establishment of early hematopoiesis (105). During adulthood and under steady-state conditions, HSPCs are maintained in a highly regulated microenvironment of cytokine/growth factors and accessory cells within the bone marrow that support HSPC quiescence or promote their differentiation to the mature immune cell types found in circulation (103). Accordingly, coordinated responses of bone marrow macrophages to circulating factors regulate hematopoiesis. As niche cells of the hematopoietic environment in the bone marrow, macrophages respond to molecules from the microbiota, including bacterial DNA (106). Through TLR signaling, CX3CR1+ macrophages produce proinflammatory cytokines that stimulate HSPC production of mature myeloid cells, allowing for further resolution of the microbes. Furthermore, increases in circulating granulocyte colony-stimulating factor (G-CSF), which occur during inflammatory diseases, can be sensed by bone marrow macrophages through their expression of the G-CSF receptor (107-111). Macrophages in turn modulate the effects of G-CSF by favoring the production of proinflammatory populations (ie, neutrophils and monocytes) at the cost of producing red blood cells by altering factors required for HSPC quiescence (ie, kit ligand, angiopoietin 1, and C-X-C motif chemokine 12/CXCL12, also known as stromal cell-derived factor 1α) (107-111). Macrophages also participate in the regulation of hematopoiesis through another prominent immune factor, IFN-γ, which in acute settings promotes the expansion of HSPCs; however, chronic exposure leads to bone marrow exhaustion (112). This effect of IFN-γ can occur directly on the HSPCs themselves, but also through bone marrow niche macrophages, whereby through signaling in macrophages, IFN-γ promotes expansion of bone marrow macrophages while reducing HSPC pools (104, 112, 113).
Macrophages in the bone marrow microenvironment also promote myelopoiesis in response to myocardial infarction (114). However, on a secondary infarct, soluble circulating factors enhance the expression of macrophage retention factors that suppress HSPC differentiation, including increases in the adhesion molecule vascular cell adhesion molecule 1 (VCAM-1) and angiopoietin 1, potentially to temper inflammatory responses on secondary infarct (114). Macrophages also support hematopoiesis in the spleen, in part through the retention of HSPCs that have homed to the spleen by the expression of VCAM-1 (115). Deletion of the macrophage colony-stimulating factor receptor (CD115), to reduce this macrophage pool, or depletion of these marginal zone macrophages blunts splenic hematopoiesis in response to LPS administration, myocardial infarction, or atherosclerosis (115).
Aside from direct effects on HSPCs, macrophages can communicate with other environmental cell types to indirectly dictate hematopoiesis. Macrophages of the bone marrow niche regulate the expression of critical factors expressed by stromal cells that promote the retention and quiescence of HSPCs, including the expression of VCAM-1, CXCL12, angiopoietin 1, and kit ligand (116). Furthermore, macrophage interaction with osteoblasts comprise a niche to retain HSPCs in the bone marrow (117, 118). Here autophagy represses NFκB signaling in osteoblasts, leading to C-C chemokine motif ligand 4 (CCL4) expression and enhanced retention of HSPCs in the bone marrow through interaction of CCL4 with C-C chemokine receptor 5 (CCR5) expressed on HSPCs (118, 119). Macrophages in the bone marrow also phagocytose senescent neutrophils from circulation (120). Through the engulfment of aged neutrophils, which occurs in a circadian manner, LXRs become activated in these macrophages, which in turn inhibit the expression of CXCL12 by CXCL12-abundant reticular cells to promote HSPC egress and differentiation, thereby replenishing the pool of circulating neutrophils.
Modulating Responses to Infection
As a critical regulator of tissue immunity, macrophages survey the environment. One of their primary functions is the detection and clearance of foreign bodies, including microbes, as well as mounting immune responses to further promote the resolution of these challenges. Many of these classical immune-derived factors that elicit macrophage responses have been covered elsewhere (reviewed in [19, 20]). Here we will highlight the roles of macrophages in response to microbes and in the maintenance of the gut. Commensal microorganisms comprising the microbiota, which are constantly present in the body but whose composition is altered during disease, provide sustained signals to macrophages to induce chronic low-grade stimuli to promote a proinflammatory phenotype, and thus provide the body with constant surveillance and protection against potential future inflammatory challenges (121, 122). Apart from the detection of microbes, such as bacteria associated with infection, macrophages can also respond to the microbiota to promote homeostatic functions. This includes the crosstalk between muscularis macrophages and enteric neurons, an interaction facilitated by macrophage responses to microbial components of the microbiota, together promoting gastric motility (123). However, during infection, muscularis macrophages support enteric neuronal viability in response to infection through the induction of β 2-adrengeric signaling, arginase-1 (ARG1), and the synthesis of polyamines (124). Macrophage support of neuronal signaling has also been demonstrated in other cell types, such as in the dermis in response to physical injury (125). However, even within a given population, macrophages do not exhibit a homogeneous response to these microbial challenges. As demonstrated by scRNA-seq, a proinflammatory signature was found in macrophages engulfing Salmonella typhimurium that display no growth (126). Conversely, those macrophages that engulfed growing S typhimurium became polarized toward a more anti-inflammatory state.
A timely observation at the time of this publication is the emerging role of macrophages in coronavirus disease 2019 (COVID-19), stemming from SARS-CoV-2 infection. This novel virus has created a global pandemic, requiring concerted efforts for vaccine development and characterization of the virus for rapid treatments after infection. Angiotensin-converting enzyme 2 (ACE2) is the receptor responsible for SARS-CoV-2 viral uptake into the cell, leading to heightened immune responses and ultimately respiratory failure (127-129). At this time, data surrounding the virus are preliminary and, given the need for rapid knowledge dissemination, some of the work described herein has not undergone peer review. A more comprehensive overview of the emerging roles of macrophages in the pathophysiologic processes that underlie COVID-19 can be found elsewhere (128, 130-133). Nevertheless, tissue analysis performed from autopsy samples of 6 patients revealed a crucial role for macrophages in promoting virus-induced pathology (127, 128). Macrophage uptake of the SARS-CoV-2 virus, among other pathologies, together are proposed to promote the observed lymphocytopenia in patients infected with the virus (127, 134). In fact, infected patients exhibit increased circulating levels of a range of proinflammatory mediators associated with macrophage activation, including IL-1β, IL-6, TNFα, IFN-γ, MCP-1, macrophage inflammatory protein-1α and -1β (MIP-1α, -1β, also known as CCL3 and CCL4 respectively), producing what is termed a “cytokine storm” (130, 131, 134, 135). Interestingly, IFN stimulation of airway epithelial cells enhances the expression of ACE2 (136), suggesting a feed-forward mechanism for macrophage-induced SARS-CoV-2 infection. In a large cohort of patients, serum levels of IL-6 and TNFα were found to be predictors of disease severity and death (137). Single-cell analysis demonstrated an enrichment of monocyte-derived macrophages in the bronchoalveolar lavage fluid from severe compared to mild cases of COVID-19 (138, 139). Interestingly, these macrophages were found to have proinflammatory, hypoxic, and oxidative signatures, consistent with an overall M1-like phenotype. Work from the Human Cell Atlas Lung Biological Network has also demonstrated the expression of ACE2 by RTMs, including those in the heart, as well as, importantly to this respiratory disorder, alveolar macrophages (129). This group also identified interactions between alveolar epithelial type II and macrophages, which enhances of the inflammatory signatures found in ACE2+ epithelial cells of the lung after infection (129, 140). These data together therefore provide preliminary evidence that macrophages may promote the rapid disease progression that occurs with SARS-CoV-2 infection and play a crucial role in the development of treatment regimens for those infected with the virus.
Nutrient Responses of Macrophages and Their Dysregulation During Disease
Aside from their response to inflammatory cues, changes in systemic nutrient availability during disease, such as during diabetes, obesity, and cancer, among others, alter macrophage metabolism and their contributions to pathophysiologic processes (Fig. 2) (141, 142). This has been best exemplified by the metabolic states induced by macrophage polarization in cultures. Stimulation of macrophages with LPS and/or IFN-γ shifts polarization toward an M1-like state, which is characterized by increased glycolysis, pentose phosphate pathway, and a disrupted Krebs cycle needed to support the proinflammatory state (14, 18). Conversely, IL-4 or IL-13 treatment induces an M2-like state, which favors FAO and OXPHOS.
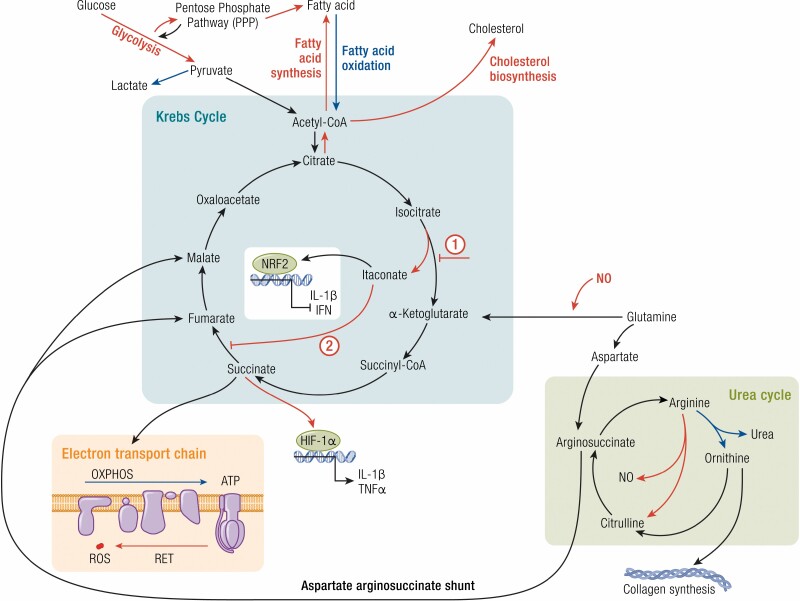
Figure 2.
Macrophage metabolism influences their function. Metabolic changes in macrophages primarily involve alterations to the Krebs cycle. Proinflammatory macrophages (red) and anti-inflammatory macrophages (blue) favor specific pathways that influence their function or inflammatory state. Proinflammatory macrophages favor glycolysis and fatty acid and cholesterol synthesis pathways, whereas anti-inflammatory macrophages prefer fatty acid oxidation. Within the Krebs cycle proinflammatory macrophages exhibit 2 key break points: (1) the inhibition of isocitrate conversion to α-ketoglutarate (by isocitrate dehydrogenase) and the production of itaconate and (2) the inhibition of succinate dehydrogenase, which catalyzes the conversion of succinate to fumarate. This accumulation of succinate allows for the stabilization of the hypoxia-inducible factor-1α (HIF-1α) and the transcription of proinflammatory genes. Succinate metabolism fuels the electron transport chain, which is important for promoting oxidative phosphorylation (OXPHOS) in anti-inflammatory macrophages. However dysfunction in the electron transport chain, including reverse electron transport (RET), produces reactive oxygen species (ROS), characteristic of proinflammatory macrophages. In the urea cycle, arginine metabolism by arginase-1, a prototypical anti-inflammatory marker, produces ornithine, which is a precursor for collagen synthesis used by these macrophages to promote tissue repair and wound healing. Proinflammatory macrophages, on the other hand, express inducible nitric oxide synthase (iNOS), which metabolizes arginine to produce citrulline and nitric oxide (NO), further enhancing the inflammatory status of these macrophages.
As would be expected with M1 and M2 representing 2 extremes along the spectrum of macrophage polarization rather than mutually exclusive populations, additional studies have demonstrated that glycolysis can be used but is not essential for M2 polarization, provided that OXPHOS is still functional (143-147). Conversely, FAO has also been shown to play a role in M1 proinflammatory responses (148-150). Additionally, further studies have challenged the notion that FAO is an absolute requirement for M2-like polarization (151-153). Together these data demonstrate that these simple associations between macrophage polarization state and metabolic processes are not steadfast and demonstrate context dependence. Nevertheless, in the following sections we will focus on the contributions of individual metabolic factors that promote macrophage responses both in health and disease.
Glucose
To support the high metabolic demand for their roles as immunomodulatory cells, macrophages favor glucose metabolism during disease, leading to the Warburg effect in macrophages, whereby glycolysis is favored over oxidative phosphorylation (14, 18). The rapid production of acetyl-CoA by glycolysis facilitates, among other metabolic changes, expansion of the Golgi and endoplasmic reticulum (ER) for the production of proinflammatory cytokines (154). The use of glucose by macrophages leading to proinflammatory responses, such as the production of IL-1β and IL-6, has been described in patients with coronary artery disease (155). Interestingly, these authors showed enhanced glucose metabolism and proinflammatory signatures in macrophages derived from coronary artery disease patients, compared to macrophages from patients with giant cell arteritis, in which macrophages exhibit lower glucose use and reduced production of these proinflammatory cytokines.
Mechanistically, many of the changes that occur in macrophage metabolism with respect to downstream glycolytic processes involve alterations in the Krebs cycle, which will be covered in more detail in following sections. As part of the interplay between metabolism and inflammation, the enhanced glycolytic response of proinflammatory macrophages is mediated by increased expression of the glucose transporter GLUT1. Increased glucose uptake mediated by this transporter has been shown to drive the production of proinflammatory cytokines and reactive oxygen species (ROS) characteristic of the M1 macrophages (156, 157). Beyond metabolic rewiring of the macrophages in response to proinflammatory stimuli, enhanced glycolytic signaling in these macrophages leads to epigenetic changes that also imprint M1-like phenotypes. Recent studies have demonstrated that the glycolytic enzyme, pyruvate kinase M2, interacts with histone deacetylases to help shape the epigenetic landscape induced by glycolysis that is required for proinflammatory responses of the macrophages (158). Furthermore lactate accumulation, which is produced by enhanced glycolytic activity of these proinflammatory macrophages, leads to histone lactylation and subsequently changes in gene expression signatures consistent with M2-like macrophages, a mechanism by which macrophage metabolic processes may feed back to temper glycolytic-induced proinflammatory responses (159). Apart from intracellular responses to changes in lactic acid accumulation, macrophages can also respond to environment-derived lactic acid. Importantly, tumors, which produce and release lactic acid because of their own enhanced glycolytic signaling, trigger an M2-like polarization of macrophages (160). This in turn induces the expression of ARG1, which promotes angiogenic signaling that increases tumor growth. Furthermore, endothelial-derived lactate also promotes M2-like macrophage polarization, which supports muscle recovery during hindlimb ischemia (161).
Fatty Acids
As macrophages adopt an anti-inflammatory or proresolving M2-like phenotype, they preferentially shift their metabolism toward FAO and OXPHOS. Entry of fatty acids into macrophages can occur as free fatty acids, or through their incorporation into lipoproteins (162). Macrophages respond to changes in fatty acid content in their surroundings by uptake through the scavenger receptor CD36 or fatty acid transport protein, which promotes M2 polarization and dampens macrophage inflammatory responses (143, 163). Like glucose, changes in fatty acid metabolism in macrophages have profound impacts on disease. Favoring FAO and OXPHOS in macrophages has been described to reduce adipose tissue inflammation and promote systemic insulin sensitivity through their actions in the adipose and liver (164-167). Furthermore, inhibition of FAO promotes macrophage accumulation that drives atherosclerotic plaque progression, consistent with a more proinflammatory phenotype (168).
The balance of fatty acid catabolic vs anabolic processes, and FAO vs fatty acid synthesis (FAS), respectively, is critical for macrophage polarization and subsequent function. As part of promoting their overall proinflammatory response, M1 macrophages shift fatty acid metabolism toward synthesis and away from oxidation (14, 18). Induction of FAS gene fatty acid synthase during M1 polarization promotes activation of the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome and alteration of membrane composition and remodeling (169-171). This subsequent production of proinflammatory mediators has been shown to perpetuate adipose tissue inflammation and insulin resistance during obesity. Additionally stearoyl-CoA desaturase 1, another enzyme involved in FAS, has been also shown to reduce the reparative functions of macrophages, specifically in repressing the proresolving ability of microglia to promote remyelination (172). FAS is directed by the transcriptional activities of the master regulator of lipogenesis SREBF1a, which is required for inflammasome activation and production of proinflammatory mediators such as IL-1β (173, 174). Interestingly M1 polarization of macrophages shows a drastic transcriptional induction of genes involved in FAS and triglyceride synthesis (175). Inhibition of triglyceride synthesis, through targeting a key enzyme in the pathway acyl-CoA:diacylglycerol acyltransferases 1 (DGAT1), significantly increases M1-polzarized production of ROS and inflammatory cytokines, while decreasing their phagocytic capabilities (175). While prostaglandin E2 (as discussed further later) synthesis is upregulated with M1 polarization, inhibition of DGAT1 also reduces the production of prostaglandin E2, which drives inflammation. DGAT1 inhibition through pharmacologic intervention during LPS endotoxemia therefore promotes an overall anti-inflammatory response.
Fatty acids, however, are present in a variety of species, including the length of the fatty acid chain, and saturated vs unsaturated bonds along the fatty acid chain, all of which have varying implications on health and disease. The effects of fatty acid species on macrophages during disease have been extensively detailed elsewhere (176); however, here we will highlight key differences as they relate to contributing to disease processes. In general, treatment of macrophages with saturated fatty acids induces an overall M1 phenotype of the macrophages, whereas polyunsaturated fatty acids decrease the production of these inflammatory cytokines and ROS (176). Saturated fatty acids, which are particularly prevalent during chronic inflammatory diseases such as obesity and atherosclerosis, have been shown to involve cell surface receptors such as TLR2 and TLR4. This occurs either through direct binding of the fatty acids to these receptors or priming receptor-dependent signaling to induce an NFκB signaling cascade and NLRP3 activation, thus promoting a proinflammatory phenotype of the macrophages, or promoting apoptosis in macrophages undergoing ER-stressed macrophages (148, 177-185). Conversion of fatty acid species also plays a role in macrophage polarization dynamics. On entry in the cell, fatty acids are esterified with acyl-CoA for subsequent metabolism. During diabetes, upregulation of one of these esterification enzymes, acyl-CoA synthetase 1 (ACSL1), significantly increases the production of proinflammatory cytokines and the extent of plaque burden in a mouse model of atherosclerosis, again highlighting the role of fatty acid anabolism in promoting M1-like processes (186). Interestingly, ACSL1 is induced by LPS treatment of macrophages (186-188), providing further evidence for FAS in promoting proinflammatory vs anti-inflammatory processes. Recent studies have also shown that inflammatory signaling through TLRs alters the macrophage lipid profile (189). This in part involves the engagement of lipid biosynthetic programs, leading to the production of monounsaturated long-chain fatty acids that dampen the inflammatory responses of these macrophages, including bacterial clearance in vivo, highlighting the intricate relationship between macrophage metabolism and inflammatory functions.
Cholesterol and Oxidized Phospholipids
Similar to fatty acids, macrophages are able to respond to cholesterol from the environment through scavenger receptor-mediated uptake of cholesterol-rich lipoproteins (162). On entry into the macrophages, cholesterol can be metabolized to oxysterols, some of which are the endogenous ligands of LXRs, leading to the transcriptional repression of NFκB and activator protein 1 to downregulate proinflammatory responses (190, 191). Cholesterol is a major component of the plasma membrane, and as such modulation of the cholesterol content of the macrophage membrane can also alter signaling pathways to dictate inflammatory status. Indeed, increasing the cholesterol content of macrophage plasma membranes has been demonstrated to increase TLR4 signaling, whereas increasing intracellular free cholesterol content within endosomes potentiates TLR3 signaling (192). Together these events converge on downstream proinflammatory mediators including the phosphorylation of p38 mitogen-activated protein kinase (MAPK). Removal of cholesterol from the membrane by high-density lipoprotein (HDL) treatment to macrophages reduced this TLR4-dependent proinflammatory profile, although extended removal of intracellular cholesterol with HDL promoted proinflammatory responses in these macrophages (193). Nevertheless, in vivo, administration of HDL was found to diminish proinflammatory signaling in peritoneal macrophages.
Intracellular concentrations of cholesterol are detected, which in turn can alter the endogenous cholesterol biosynthetic program by inhibiting the processing of the master regulator of cholesterol biosynthesis SREBP2 (162). Changes in sterol metabolism have been intricately linked with macrophage inflammatory responses. Processing of the SREBP cleavage-activating protein–SREBP2 complex, apart from driving cholesterol biosynthesis, promotes NLRP3 inflammasome activation. Along with this complex, NLRP3 is transported to the Golgi, which in turn allows for localization to mitochondrial clusters thus promoting inflammasome activation (194). Furthermore, during high-fat diet feeding, expression of the rate-limiting enzyme of cholesterol biosynthesis, β-hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase, enhances macrophage recruitment and increases proinflammatory cytokine production in the adipose tissue, leading to adipose tissue pathology and ultimately systemic insulin resistance (195). Mevalonate, an intermediate in the cholesterol biosynthetic pathway, has been shown to promote trained immunity in monocytes, where it enhances insulin-like growth factor 1 (IGF-1) receptor signaling and subsequent metabolic processes leading to epigenetic modifications (H3K27ac) that drive future inflammatory responses (196). Conversely, statin treatment, which inhibits cholesterol biosynthesis, diminishes this trained immunity response.
Because one of the key functions of macrophages is the clearance of bacteria through phagocytosis, macrophages themselves must escape infection by these pathogens. Recent work has uncovered another critical role for IFN signaling in macrophages when addressing microbial challenges. The engagement of TLR3 or TLR4 increases cholesterol uptake and decreases cholesterol biosynthesis in macrophages (197). This reduction in endogenous synthesis was shown to be crucial for macrophages to elicit a type I IFN response, which was coordinated through the stimulator of IFN genes (STING) signaling. In other studies, these authors demonstrate that macrophages, along with neutrophils, evade bacterial toxins that target plasma membrane cholesterol to induce target cell pathogenicity, by redistributing cellular cholesterol pools, without altering plasma membrane cholesterol levels (198). IFN signaling was linked to increased production of the cholesterol derivative 25-hydroxycholesterol and increased esterification of free cholesterol to mitigate membrane binding of toxins released by these bacteria. Together these studies highlight that the regulation of cholesterol metabolism is critical for macrophage immunity in response to infection.
Oxidized phospholipids also play a role in potentiating macrophage inflammation, including the oxidized phospholipids contained in the cholesterol-rich oxidized low-density lipoprotein. Studies have demonstrated NLRP3 activation and proinflammatory responses after treatment with oxidized phospholipid species (oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylcholine, OxPAPC), coupled with a decrease in phagocytosis (199-202). These oxidized phospholipid species also enhance macrophage apoptosis during ER stress (182). Furthermore, OxPAPC treatment after LPS stimulation heightens the proinflammatory responses of macrophages, in part through glutamine fueling of the Krebs cycle (discussed later), leading to oxaloacetate accumulation and HIF-1α–dependent IL-1β production (203). Importantly, inhibition of these metabolic pathways that promote IL-1β production in response to these oxidized phospholipids (including adenosine triphosphate [ATP]-citrate lyase and glutaminase) both reduce plaque inflammation and overall lesion area in atherosclerosis-prone mice.
Lipid Mediators
Apart from the direct rewiring of metabolic programs, fatty acid metabolism also produces lipid intermediates that themselves alter macrophage function. Like other cell types, macrophages also use fatty acids as the building blocks for other lipid-signaling molecules, including resolvins, eicosanoids, arachidonic acid, as well as for incorporation into the phospholipid bilayer of the plasma membrane (204). Very low-density lipoprotein (VLDL) signaling through its receptor (VLDLR) in macrophages has been shown to promote adipose tissue inflammation and insulin resistance (205). This was attributed to an increase in intracellular ceramide levels. However, other studies have demonstrated that macrophage deletion of genes involving de novo ceramide synthesis does not influence adipose tissue inflammation, suggesting that exogenous VLDL signaling through the VLDLR may occur independently of ceramide synthesis (206, 207). Interestingly, some of these lipid mediators that arise from FAS, favored by M1-like macrophages, themselves have proinflammatory properties. Desaturation of fatty acids by desaturation enzymes are required for the production of lipid mediators including arachidonic acid, eicosapentaenoic acid (EPA), and leukotriene B4 (176). In fact, inhibition of fatty acid desaturase 1 and 2 alters the balance of these proresolving lipids in the macrophage and in turn favors more proinflammatory processes (208, 209). Furthermore, inhibiting fatty acid conversion to very long-chain fatty acids revealed an overall proinflammatory phenotype associated with reductions in the production of the lipid intermediate, an omega-3 fatty acid, docosahexaenoic acid (DHA) (210, 211). Specialized proresolvin mediators such as resolvin D2 have also been demonstrated to decrease inflammasome assembly, leading to decreased production and secretion of IL-1β, as well as promoting an M2-like phenotype (212). Resolvin D1 treatment of macrophages also promotes the efferocytic capabilities of macrophages by promoting the engulfment and clearance of apoptotic and necroptotic cells, which led to an overall resolution of atherosclerotic plaque (213). Additionally, accumulation of citrate as occurs during M1 polarization (as discussed further in subsequent sections), has been demonstrated to stimulate prostaglandin E2 production (214, 215). Prostaglandin E2 itself reduces mitochondrial membrane potential, through the induction of a gene expression profile that ultimately leads to the acquisition of key M2-like genes including resistin-like molecule α (216). Indeed, the ratio of lipid mediators including the class of resolvins (D1, D2, E1) maresin 1, leukotriene B4, and prostaglandin E2 levels becomes dysregulated during atherosclerosis, which influences macrophage inflammatory properties (217-221). In the clinical setting, patients undergoing carotid endarterectomy receiving supplementation with omega-3 fatty acids demonstrated increases in plaque content of EPA and DHA, which were associated with decreases in carotid plaque inflammation (222). More recently, pharmacologic administration of an EPA derivative, as part of the REDUCE-IT clinical trial, reduced the incidence of cardiovascular events by 25% to 30% (223, 224) and in the EVAPORATE trial reduced low-attenuation plaque volume by 17% (225), demonstrating the importance of these lipid intermediates in driving macrophage inflammation during disease in the clinical setting.
Krebs Cycle and Associated Metabolic Processes
A majority of the research on the metabolic programming and rewiring of macrophages and their inflammatory functions have focused on the Krebs cycle, also referred to as the tricarboxylic acid cycle or citric acid cycle. Among the changes that occur, metabolites can feed into and enhance the Krebs cycle, a process known as anaplerosis, whereas removal of metabolites from the Krebs cycle to feed other metabolic pathways is known as cataplerosis (226). An in-depth analysis of the perturbations in the Krebs cycle during macrophage polarization has been described in a landmark paper by Jha and colleagues (227). Importantly, these data helped describe how the induction of metabolic pathways leading to metabolite accumulation coordinates the overall phenotype of macrophages during health and disease. Generally, polarization of macrophages toward an M1 phenotype by the TLR4 ligand LPS shifts overall macrophage metabolism to glycolysis over FAO or OXPHOS in part by the induction of transcriptional programs mediated by HIF-1α, as well as by inhibition of adenosine 5′-monophosphate–activated protein kinase (AMPK)-mediated signaling (228-233). Compared to LPS treatment, M2 polarization of macrophages promotes AMPK activity (234).
Macrophage polarization toward the proinflammatory M1-like population by LPS creates 2 critical break points in the Krebs cycle. The first break point is the transcriptional downregulation of IDH, the enzyme in the Krebs cycle that catalyzes the conversion of isocitrate to α-ketoglutarate (227, 228) (Fig. 2). Downregulation of IDH leads to an accumulation of isocitrate and a decrease of α-ketoglutarate. Additionally, inhibition of IDH enzymatic activity occurs in an inducible nitric oxide synthase (iNOS)-dependent manner (235, 236). iNOS, a commonly used marker of M1-polarized macrophages, produces nitric oxide, which leads to nitrosation of cysteine residues and the subsequent inactivation of IDH. The accumulation of isocitrate that occurs during M1 polarization promotes the synthesis of itaconate, a potent metabolite that has profound effects on macrophage inflammatory status, including the production of ROS and inflammasome activation (227, 237, 238). Synthesis of itaconate occurs by induction of the enzyme responsible for its conversion from aconitate, immune-responsive gene 1 (IRG1, also referred to as ACOD1), which is upregulated during inflammatory diseases such as sepsis (227, 239-241).
The second critical break point in the Krebs cycle induced by LPS polarization is the inhibition of succinate dehydrogenase (SDH). Itaconate competitively inhibits SDH, the complex II of the electron transport chain (ETC), which directly downregulates OXHPHOS (227, 238, 239, 242, 243). Furthermore, this accumulation of succinate leads to stabilization of HIF-1α, resulting in enhanced glycolysis, the production of proinflammatory cytokines such as IL-1β and TNFα, coupled with a dampening of anti-inflammatory cytokines, together which drive proinflammatory processes necessary to promote microbial resolution as in inflammatory mouse models including sepsis and colitis (228, 244-247). These associations have also been described in vivo with multiple macrophage populations. In the adipose tissue, for instance, inhibition of OXPHOS shifts macrophage polarization toward a more proinflammatory (M1-like) phenotype, and this preference for glycolytic metabolism and HIF-1α–dependent proinflammatory responses drives adipose tissue insulin resistance during obesity (181, 248).
Another key regulator of the inflammatory phenotype is the pyruvate kinase M2 (PKM2) complex. Studies have shown that treatment with LPS upregulates the expression of PKM2 (232). Interestingly PKM2 confirmation dictates inflammatory outcomes. Dimeric PKM2 promotes HIF-1α–dependent production of inflammatory cytokines, whereas small-molecule activation of PKM2, which induces its tetrameric confirmation, reduces the rate of glycolysis and succinate accumulation to favor the expression of M2-associated markers, including a significant upregulation of the anti-inflammatory cytokine IL-10 (232, 245).
Succinate can also be released by M1-polarized macrophages, which can act in the local environment in an autocrine or paracrine manner through macrophage expression of the succinate receptor 1 to potentiate succinate-dependent inflammatory signaling in macrophages (249). Interestingly, extracellular succinate is present in high levels in samples from patients with inflammatory diseases such as rheumatoid arthritis, atherothrombosis, hypertension, and diabetes (226, 249-251). Modulation of extracellular succinate levels has been exploited to temper inflammatory responses. For example, neuronal stem cells scavenge extracellular succinate to repress a proinflammatory phenotype of macrophages, which in turn prevent neuroinflammatory responses (252).
The accumulation of succinate also leads to an increase in its oxidation by SDH, leading to ROS production from complex I and complex III of the mitochondrial ETC (228, 247, 253-256). However, recent studies have provided evidence to suggest that ROS production by M1 macrophages is instead produced by reverse electron transport, which also occurs because of succinate accumulation (247). The generation of these ROS and the previously mentioned nitric oxide production by M1 macrophages are crucial for their microbicidal properties. This mitochondrial-derived ROS also contributes to activation of the NLRP3 inflammasome and production of proinflammatory factors (257). M1 macrophages also upregulate the pentose phosphate pathway, which shifts metabolism away from OXPHOS and promotes FAS and NADPH production necessary for the microbiocidal properties of M1 macrophages (228, 258, 259). It has been well established that the production of these oxidative species has a profound impact on contributing diseases. In addition to the processes already described, oxidative stress from macrophages in the brain plays a prominent role in promoting neurodegenerative diseases. Recent work has used multiple sequencing modalities to characterize macrophages, among other myeloid populations, in the brain producing these oxidative species and their contribution to these neurological disorders, including chronic experimental autoimmune encephalomyelitis (260). Here the authors describe the contribution both of monocyte-derived macrophages and tissue-resident microglia producing ROS. Among these observations, these ROS-producing macrophages showed an enrichment for a glutathione-degrading enzyme, gamma-glutamyl transferase. Using transcriptional profiling, these authors identify the metabolism of glutathione, an endogenous antioxidant, as a druggable target for the treatment of neurodegenerative diseases.
Despite its production during M1 polarization, itaconate has also displayed potent anti-inflammatory actions. Key among these functions is the stabilization of the transcription factor nuclear factor erythroid 2-related factor 2 (NRF2), which promotes an overall anti-inflammatory response through promoting the transcription of anti-inflammatory mediators while repressing proinflammatory gene expression (239, 261). NRF2 is also well known for its prominent role as a critical regulator of the antioxidant response (262). The discrepancy between the aforementioned proinflammatory effects on itaconate through the inhibition of SDH may be related to the separate roles of SDH in promoting succinate oxidation vs its role as complex II of the mitochondrial ETC (238, 239, 243, 247). Furthermore, itaconate has been demonstrated to be an antimicrobial compound (263), which may also explain the duality of the role of this metabolite in eliciting macrophage inflammatory responses (264). Further studies have shed light on these apparent discrepancies in the actions of itaconate. These opposing functions of itaconate were shown to be a function of individual derivatives of itaconate used for experiments, which elicit proinflammatory or immunosuppressive functions in treated macrophages (265).
Interestingly, the metabolic rewiring of macrophages in response to the proinflammatory modulators LPS and IFN-γ occurs in 2 phases (266). During the first stage (within 6-24 hours), early responses show accumulation of key metabolic intermediates such as itaconate and succinate, as described during the proinflammatory activation of these macrophages. However, this response is tempered in the second phase (48-72 hours post stimulation), where there is dampened accumulation of these intermediates, an effect that is dictated by the lipoylation of the E2 subunit of the pyruvate and oxoglutarate dehydrogenase complexes, along with the phosphorylation of the E1 subunit of pyruvate dehydrogenase complex. Furthermore, recent studies have identified further targets of itaconate, which reveal its potential to inhibit aerobic glycolytic enzymes such as aldolase A, glyceraldehyde 3-phosphate dehydrogenase, and lactate dehydrogenase A (267). The regulation of itaconic acid in macrophages has also has profound effects during cancer, whereby tumors induce OXPHOS and the accumulation of itaconic acid in peritoneal macrophages, which in turn may promote tumor growth through M2 polarization (268). Inhibition of IRG1 in these macrophages displayed a profound capacity to reduce peritoneal tumors, clearly indicating the capacity of itaconate to promote anti-inflammatory or proresolving responses of macrophages.
Apart from increased glycolysis that feeds into the Krebs cycle, during M1 polarization there is concomitant upregulated expression of ATP-citrate lyase, which catalyzes the conversion of citrate produced from the Krebs cycle to acetyl-CoA (214). Both glycolytic and Krebs cycle production of acetyl-CoA provide the substrate for synthesis of fatty acids and other lipid intermediates, which, as previously described, promote proinflammatory processes of these cells. This accumulation of acetyl-CoA can also be used for histone acetylation, which leads to epigenetic changes that facilitate the expression of proinflammatory cytokines (269). Bempedoic acid, a pharmacologic ATP-citrate lyase inhibitor, has been introduced as a new line of cholesterol-lowering therapeutics. Treatment of macrophages with bempedoic acid (ETC-1002) activated AMPK and reduced the production of inflammatory mediators through a macrophage liver kinase B1–dependent mechanism, and in vivo reduced macrophage infiltration and adipose tissue inflammation (270).
Amino Acids
Amino acids play a major role in modulating Krebs cycle signaling to influence macrophage inflammatory processes. For example, catabolism of branched-chain amino acids through the regulation of the branched-chain aminotransferase 1 enzyme promotes an overall proinflammatory response of the macrophages, in part by inducing the production of the metabolite itaconate (271). However, a majority of the interaction between amino acid metabolism and macrophage responses have focused on 2 particular amino acids: glutamine and arginine.
Glutamine participates in anaplerosis, through its metabolism into glutamate by glutaminolysis and then to α-ketoglutarate, which feeds the Krebs cycle. Interestingly, glutamine uptake by macrophages can promote either M1 or M2 responses depending on the route of glutamine metabolism. Glutamine anaplerosis promotes succinate synthesis through the Krebs cycle, and this accumulation of succinate has been demonstrated to stabilize HIF-1α–dependent proinflammatory responses including the production of IL-1β (228, 272). On the other hand, glutamine is also responsible for glycosylation pathways, specifically UDP-GlcNAc, that overall promote an anti-inflammatory phenotype (227). This glycosylation pattern is a posttranslational modification of cell surface molecules, such as lectin and the mannose receptor among other molecules, which are required for the resolution of inflammation by phagocytosis, a prototypical M2 function (14). Furthermore, glutamine anaplerosis induces epigenetic changes that facilitate trained immunity in monocytes (273). Glutamine production of α-ketoglutarate also promotes FAO through the acquisition of M2-related genes, which occur in response to epigenetic changes in part dictated by the histone demethylase JMJD3 (274).
Another key amino acid crucial for dictating macrophage function is arginine. Apart from extracellular sources, arginine can also be produced by the aspartate-arginosuccinate shunt pathway from the Krebs cycle, tying metabolic processes of the macrophage to arginine production. Arginine participates in M1-associated proinflammatory processes, as arginine conversion by iNOS enzyme, in addition to citrulline, produces nitric oxide, a potent oxidative species used by macrophages as part of their microbicidal response (15). Nitric oxide also plays other roles that promote macrophage inflammatory responses, such as to promote or sustain an M1-polarization state by shifting endogenous metabolism, including glutamine feeding into the Krebs cycle (275). Furthermore, nitric oxide prolongs the proinflammatory responses by disrupting mitochondrial respiration and thus macrophage plasticity, which attenuates the subsequent polarization to an M2-like state after an initial M1-associated stimulus (152). The citrulline produced as part of this iNOS-dependent production of nitric oxide can cycle back through the aspartate-arginosuccinate shunt pathway to regenerate arginine and provide a positive feedback loop for the production of nitric oxide, thus potentiating this prolonged proinflammatory response (276). However, M2 polarization of macrophages protects against this proinflammatory function loop by expression of ARG1, which also serves as a marker of M2 macrophages, which metabolizes arginine into ornithine and urea, and effectively scavenges arginine metabolism away from iNOS and the production of nitric oxide (277). Ornithine is subsequently metabolized to polyamines, which serve as intermediates for the synthesis of collagen, to promote wound healing, another M2-associated macrophage phenotype (278). Interestingly aminooxyacetic acid, an endogenous inhibitor of the aspartate-arginosuccinate shunt, attenuates macrophage glycolysis and promotes an overall M2 response, which in turn improves cardiac function post myocardial infarction (279).
Phagocytosis
One of the first described functions of macrophages, one that continually occurs to promote homeostasis and development, is the capacity for macrophages to promote the resolution of inflammation through the engulfment of foreign bodies or apoptotic cells/debris (2, 3). Engulfment of these microbes or cell remnants therefore provides macrophages with exogenous nutrient sources that alter their metabolism, and therefore immune responses. These engulfed particles are subject to lysosomal degradation, which subsequently alters the metabolism of the macrophages (280). During steady-state conditions, engulfment of apoptotic cells provides the macrophages with metabolites, such as fatty acids and oxysterols, that activate the nuclear receptors PPARs and LXRs, which as described earlier promote an overall anti-inflammatory, M2-like phenotype (281-283). Clearance of apoptotic cells also provides an exogenous source of glucose for these efferocytes. Uptake of these apoptotic cells by efferocytic macrophages induces the solute carrier family genes that enhance aerobic glycolysis and lactate release, while downregulating oxidative phosphorylation (284). This process in turn further enhances efferocytosis, whereby i) glycolytic metabolism also facilitates cytoskeletal changes that promote the continued uptake of apoptotic cells, and ii) lactate produced from aerobic glycolysis is released, which, as previously mentioned, can promote macrophage polarization toward an anti-inflammatory status. The engulfment of apoptotic cells also leads to alterations in the AKT and extracellularly regulated kinase 1/2 signaling cascades to inhibit macrophage proliferation, while promoting survival (285). Interestingly, in the elderly population, inflammatory pathways such as p38 MAPK signaling predominate in macrophages. This is associated with an overall proinflammatory phenotype with reduced efferocytotic potential. Recent evidence has shown that oral administration of a p38 inhibitor to elderly individuals, losmapimod, promoted macrophage efferocytosis and the resolution of dermal inflammation to levels comparable to younger participants, in part through enhancing the expression of the efferocytosis receptor TIM-4 (286). Furthermore, efferocytosis of apoptotic cells provides an exogenous source of arginine and ornithine, which produce putrescine, leading to a signaling cascade that promotes cytoskeletal rearrangement and subsequent engulfment of apoptotic cells (283, 287). In vivo this feed-forward loop of clearing apoptotic cells has been demonstrated to promote plaque regression during atherosclerosis (287).
Insulin and Endocrine Regulation of Macrophage Function
In addition to their contributions to insulin resistance in the adipose tissue and liver, macrophages themselves can also develop defects in insulin signaling that promote overall pathogenesis. This has been described in patients with metabolic syndrome, who display defects in macrophage insulin signaling and decreased phagocytosis, along with increased ROS production (288-292). Furthermore, macrophages derived from diabetic or obese mice demonstrate an increase in scavenger receptors, responsible for the entry of fatty acids and modified lipoproteins, thereby augmenting nutrient use by macrophages and altering their metabolic processes, favoring FAS and promoting a proinflammatory phenotype (288, 293-295). Indeed, activation of the insulin signaling pathway participates in intracellular processes on phagocytosis and protects the macrophage from apoptosis (285, 296, 297).
Another mediator of insulin signaling, IGF-1, whose levels have been inversely correlated with metabolic syndrome, plays an important role in influencing macrophage immunomodulatory responses (298). IGF-1, in addition to being secreted by M2-like macrophages, stimulates macrophage polarization toward an M2-like phenotype, which in the adipose tissue leads to improved insulin sensitivity and pathogen clearance (299). Furthermore, IGF-1 promotes plaque stability during atherosclerosis, whereby deletion of the IGF-1 receptor in macrophages resulted in an overall proinflammatory profile of the macrophages, which in vivo promoted increases in total plaque area and markers of plaque instability (300).
Another prominent endocrine target is the glucagon-like peptide-1 (GLP-1), which promotes insulin secretion and thus improves hyperglycemia during metabolic disease (301). GLP-1, through the GLP-1 receptor expressed in macrophages, shifts macrophage polarization toward an M2-like phenotype by induction of STAT3, which reduces macrophage-induced adipose tissue inflammation during obesity (302-304). Specifically, use of a GLP-1 analogue, lixisenatide, decreased atherosclerosis, in part by promoting an M2-like phenotype of macrophages in atherosclerotic plaque (305). These results were recapitulated when using the dipeptidyl-peptidase 4 (DPP4) inhibitor sitagliptin, which prevented the enzymatic cleavage of GLP-1 by DPP4 in mouse models of diet-induced obesity and atherosclerosis (306, 307).
Several other endocrine factors have been widely investigated as systemic metabolic regulators, particularly in diseases of metabolic syndrome. One such class of these factors is the growth differentiation factor (GDF) class belonging to the TGFβ superfamily. Recent studies have demonstrated that exogenous GDF3 shifts the inflammatory balance of macrophages, favoring an M2-like phenotype, through a downregulation of the NLRP3 inflammasome and proinflammatory mediators to preserve cardiac function in response to sepsis (308). Similarly, GDF15 promotes OXPHOS in macrophages to promote an M2-like response (248). Beyond metabolic syndrome, tumor production of GDF15 dampens the proinflammatory response of macrophages that promote tumor growth (309). Fibroblast growth factor 21 (FGF21) has also been of interest as a target in the regulation of metabolic diseases. FGF21 promotes insulin sensitivity, in part through M2 polarization of adipose tissue macrophages, which can be in part attributed to attenuating NFκB signaling (310, 311).
Interestingly, different dietary regimens have demonstrated profound effects on macrophage immune responses. Tissue-specific macrophage populations have been shown to respond differently on challenge with varying dietary challenges. Among these changes, adipose tissue macrophages, and their upregulation of proinflammatory mediators, have been shown to be among the greatest changes in fasting-refeeding experiments (312). Recent studies using scRNA-seq on adipose tissue from mice undergoing caloric restriction revealed an alteration in adipose tissue leukocyte content, along with an adipose tissue macrophage population with enhanced transcriptomic signatures for phagocytic activity, suggesting a proresolving phenotype of these macrophages with caloric restriction (313). Additionally, high-protein diets have been demonstrated to activate mechanistic target of rapamycin signaling in macrophages, which decrease mitophagy, leading to mitochondrial dysfunction and subsequently atherosclerotic plaque development (314). Finally, the so-called “keto diet” has received much attention as of late. This diet, rich in fats and low in carbohydrates, has been investigated for promoting systemic use and metabolism of fatty acids. Recent data show that short-term feeding of the ketogenic diet (1 week) decreased macrophage content and Il-1β expression in adipose tissue compared to a chow diet (315). However, prolonged feeding (3-4 months) promoted glucose intolerance and weight gain, which was associated with an accumulation of adipose tissue macrophages and an increase in overall proinflammatory gene expression (ie, MCP1, TNFα, and IL-1β). Although weight gain was improved in mice lacking γδ T cells, this did not completely rescue the glucose intolerance, suggesting that ketogenic diets induced M1-associated gene expression signatures in the adipose to promote systemic insulin resistance. Fasting has also been described to mediate inflammatory responses in part by regulating hepatic energy regulation, through the repression of CCL2, reducing the number inflammatory monocytes found in the circulation both of mice and humans (316), which can then be recruited to tissues to promote the accumulation of proinflammatory macrophages. Interestingly, fasting did not affect the emergency monocyte mobilization out of the bone marrow during acute challenges, indicating that fasting regulation of monocyte mobilization attenuates chronic inflammation, while preserving the ability of these monocyte/macrophages to respond to promote tissue homeostasis during acute stressors. Although the precise components and their effects on macrophages, for instance primary vs incidental pathologies, these studies nonetheless highlight the influence of diet on macrophage pathogenicity.
Concluding Remarks
Characterized by their common capacity for promoting inflammation and phagocytosis of extracellular debris, macrophages are a heterogeneous population of individual tissue-specific subtypes. These cells contribute to tissue homeostasis through responses to molecules in the microenvironment via their expression of cell surface receptors. Recent observations have exquisitely linked macrophage metabolic processes to their inflammatory phenotypes and highlight how macrophages switch from tissue protection to contribution to pathophysiologic processes during disease. Our current understanding of the molecular mechanisms of macrophage responses and contribution to disease, along with further investigation, will continue to open a variety of avenues for therapeutic intervention.
Glossary
Abbreviations:
- ACE2
angiotensin-converting enzyme 2
- ACSL1
acyl-CoA synthetase 1
- AMPK
adenosine 5′-monophosphate–activated protein kinase
- ARG1
arginase-1
- ATP
adenosine triphosphate
- CCL2, 4
C-C chemokine motif ligand 2, 4
- CCR5
C-C chemokine receptor 5;
- COVID-19
coronavirus disease 2019;
- CXCL12
C-X-C motif chemokine 12
- DAMP
danger-associated molecular pattern
- DHA
docosahexaenoic acid
- DGAT1
acyl-CoA:diacylglycerol acyltransferases 1
- DPP4
dipeptidyl-peptidase 4
- EPA
eicosapentaenoic acid
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FAO
fatty acid oxidation
- FAS
fatty acid synthesis
- FGF21
fibroblast growth factor 21
- G-CSF
granulocyte colony-stimulating factor
- GDF3, 5
growth differentiation factor 3, 5
- GLP-1
glucagon-like peptide-1;
- HDL
high-density lipoprotein;
- HIF-1α
hypoxia-inducible factor-1α
- HMG-CoA
β-hydroxy β-methylglutaryl-CoA
- HSPC
hematopoietic stem and progenitor cell
- IDH
isocitrate dehydrogenase
- IFN-γ
interferon-γ
- IGF-1
insulin-like growth factor-1
- IL-1β, 4, 6, 8 10, 12, 13, 23, 33
interleukin-1β, 4, 6, 8 10, 12, 13, 23, 33
- iNOS
inducible nitric oxide synthase
- IRF4
interferon-regulatory factor 4
- IRG1
immune-responsive gene 1
- LDTF
lineage-determining transcription factor
- LPS
lipopolysaccharide
- LXRα
liver X receptor α
- M-CSF
macrophage colony-stimulating factor
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein-1
- MIP-1α, 1β
macrophage inflammatory protein-1α, 1β
- NASH
nonalcoholic steatohepatitis
- NFκB
nuclear factor κ-light-chain-enhancer of activated B cells
- NLR
NOD-like receptors
- NLRP3
NOD-, LRR- and pyrin domain-containing protein 3
- NO
nitric oxide
- NOD
nucleotide-binding oligomerization domain
- NRF2
nuclear factor erythroid 2-related factor 2
- OxPAPC
oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphatidylcholine
- OXPHOS
oxidative phosphorylation
- PAMP
pattern-associated molecular pattern
- PKM2
pyruvate kinase M2
- PPARδ, γ
peroxisome proliferator-activated receptor δ, γ
- PPR
pattern recognition receptor
- RAGE
receptor for advanced glycation end products
- RIG-I
retinoic acid–inducible gene I
- ROS
reactive oxygen species
- RTM
resident tissue macrophage
- scRNA-seq
single-cell RNA sequencing
- SDH
succinate dehydrogenase
- SDTF
signal-dependent transcription factor
- SREBF(P)1a, 2
sterol regulatory element binding factor (protein) 1a, 2
- STAT3, 6
signal transducer and activator of transcription 3, 6
- STING
stimulator of interferon genes
- TGFβ
transforming growth factor β;
- TH
T helper;
- TNFα
tumor necrosis factor α
- TLR2, 4
toll-like receptor 2, 4
- VCAM-1
vascular cell adhesion molecule-1
- VLDL
very low-density lipoprotein
- VLDLR
very low-density lipoprotein receptor
Acknowledgments
Financial Support: This work was supported by the University of Ottawa Cardiac Endowment Fund (A.R.) and the Canadian Institutes of Health Research (grant No. FND148448 to K.J.R.).
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability:
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446): 445-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643-675. [DOI] [PubMed] [Google Scholar]
- 4. Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197-216. [DOI] [PubMed] [Google Scholar]
- 5. Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901-944. [DOI] [PubMed] [Google Scholar]
- 6. Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214(2):161-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin CJ, Peters KN, Behar SM. Macrophages clean up: efferocytosis and microbial control. Curr Opin Microbiol. 2014;17:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okabe Y. Molecular control of the identity of tissue-resident macrophages. Int Immunol. 2018;30(11):485-491. [DOI] [PubMed] [Google Scholar]
- 10. Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34(5):216-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750-761. [DOI] [PubMed] [Google Scholar]
- 13. Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157(4):832-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ley K. M1 means kill; M2 means heal. J Immunol. 2017;199(7): 2191-2193. [DOI] [PubMed] [Google Scholar]
- 16. Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541-566. [DOI] [PubMed] [Google Scholar]
- 17. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805-820. [DOI] [PubMed] [Google Scholar]
- 20. Ley K, Pramod AB, Croft M, Ravichandran KS, Ting JP. How mouse macrophages sense what is going on. Front Immunol. 2016;7:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doyle AG, Herbein G, Montaner LJ, et al. Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-γ. Eur J Immunol. 1994;24(6):1441-1445. [DOI] [PubMed] [Google Scholar]
- 22. Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176(1):287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914-920. [DOI] [PubMed] [Google Scholar]
- 24. Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qiu Y, Nguyen KD, Odegaard JI, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480(7375):104-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fischer K, Ruiz HH, Jhun K, et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med. 2017;23(5): 623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen M, Giladi A, Gorki AD, et al. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell. 2018;175(4):1031-1044.e18. [DOI] [PubMed] [Google Scholar]
- 30. Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40(2):274-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giladi A, Wagner LK, Li H, et al. Cxcl10+ monocytes define a pathogenic subset in the central nervous system during autoimmune neuroinflammation. Nat Immunol. 2020;21(5): 525-534. [DOI] [PubMed] [Google Scholar]
- 32. Guilliams M, Thierry GR, Bonnardel J, Bajenoff M. Establishment and maintenance of the macrophage niche. Immunity. 2020;52(3):434-451. [DOI] [PubMed] [Google Scholar]
- 33. van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845-852. [PMC free article] [PubMed] [Google Scholar]
- 34. Sawyer RT, Strausbauch PH, Volkman A. Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab Invest. 1982;46(2):165-170. [PubMed] [Google Scholar]
- 35. Parwaresch MR, Wacker HH. Origin and kinetics of resident tissue macrophages. Parabiosis studies with radiolabelled leucocytes. Cell Tissue Kinet. 1984;17(1):25-39. [DOI] [PubMed] [Google Scholar]
- 36. Czernielewski JM, Demarchez M. Further evidence for the self-reproducing capacity of Langerhans cells in human skin. J Invest Dermatol. 1987;88(1):17-20. [DOI] [PubMed] [Google Scholar]
- 37. Melnicoff MJ, Horan PK, Breslin EW, Morahan PS. Maintenance of peritoneal macrophages in the steady state. J Leukoc Biol. 1988;44(5):367-375. [DOI] [PubMed] [Google Scholar]
- 38. Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44(3):439-449. [DOI] [PubMed] [Google Scholar]
- 39. Ginhoux F, Tacke F, Angeli V, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7(3):265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Molawi K, Wolf Y, Kandalla PK, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med. 2014;211(11):2151-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoeffel G, Wang Y, Greter M, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209(6):1167-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86-90. [DOI] [PubMed] [Google Scholar]
- 44. Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bain CC, Bravo-Blas A, Scott CL, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15(10):929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ensan S, Li A, Besla R, et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1+ precursors and circulating monocytes immediately after birth. Nat Immunol. 2016;17(2):159-168. [DOI] [PubMed] [Google Scholar]
- 47. Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178(4):2000-2007. [DOI] [PubMed] [Google Scholar]
- 48. Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19(9):1166-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Williams JW, Zaitsev K, Kim KW, et al. Limited proliferation capacity of aortic intima resident macrophages requires monocyte recruitment for atherosclerotic plaque progression. Nat Immunol. 2020;21(10):1194-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sinha SK, Miikeda A, Fouladian Z, et al. Local M-CSF (macrophage colony-stimulating factor) expression regulates macrophage proliferation and apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2021;41(1):220-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Z, Gu Y, Chakarov S, et al. Fate mapping via Ms4a3-expression history traces monocyte-derived cells. Cell. 2019;178(6): 1509-1525.e19. [DOI] [PubMed] [Google Scholar]
- 52. Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Blériot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42(1):145-158. [DOI] [PubMed] [Google Scholar]
- 54. Milner JD, Orekov T, Ward JM, et al. Sustained IL-4 exposure leads to a novel pathway for hemophagocytosis, inflammation, and tissue macrophage accumulation. Blood. 2010;116(14):2476-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bain CC, Gibson DA, Steers NJ, et al. Rate of replenishment and microenvironment contribute to the sexually dimorphic phenotype and function of peritoneal macrophages. Sci Immunol. 2020;5(48):eabc4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buechler MB, Kim KW, Onufer EJ, et al. A stromal niche defined by expression of the transcription factor WT1 mediates programming and homeostasis of cavity-resident macrophages. Immunity. 2019;51(1):119-130.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Friebel E, Kapolou K, Unger S, et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell. 2020;181(7):1626-1642.e20. [DOI] [PubMed] [Google Scholar]
- 58. Klemm F, Maas RR, Bowman RL, et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 2020;181(7):1643-1660.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Winkels H, Ehinger E, Vassallo M, et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res. 2018;122(12):1675-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cochain C, Vafadarnejad E, Arampatzi P, et al. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018;122(12):1661-1674. [DOI] [PubMed] [Google Scholar]
- 61. Lin JD, Nishi H, Poles J, et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4(4):e124574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fernandez DM, Rahman AH, Fernandez NF, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25(10):1576-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim K, Shim D, Lee JS, et al. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ Res. 2018;123(10):1127-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zilionis R, Engblom C, Pfirschke C, et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50(5):1317-1334.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Geirsdottir L, David E, Keren-Shaul H, et al. Cross-species single-cell analysis reveals divergence of the primate microglia program. Cell. 2019;179(7):1609-1622.e16. [DOI] [PubMed] [Google Scholar]
- 66. Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972-978. [DOI] [PubMed] [Google Scholar]
- 67. Fonseca GJ, Seidman JS, Glass CK. Genome-wide approaches to defining macrophage identity and function. Microbiol Spectr. 2016;4(5):10.1128/microbiolspec.MCHD-0039-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gautier EL, Shay T, Miller J, et al. ; Immunological Genome Consortium . Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13(11): 1118-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gosselin D, Link VM, Romanoski CE, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159(6):1327-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lavin Y, Winter D, Blecher-Gonen R, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Suzuki T, Arumugam P, Sakagami T, et al. Pulmonary macrophage transplantation therapy. Nature. 2014;514(7523):450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gosselin D, Skola D, Coufal NG, et al. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017;356(6344):eaal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Heinz S, Benner C, Spann N, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kaikkonen MU, Spann NJ, Heinz S, et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell. 2013;51(3):310-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Blériot C, Chakarov S, Ginhoux F. Determinants of resident tissue macrophage identity and function. Immunity. 2020;52(6):957-970. [DOI] [PubMed] [Google Scholar]
- 76. Uhlenhaut NH, Barish GD, Yu RT, et al. Insights into negative regulation by the glucocorticoid receptor from genome-wide profiling of inflammatory cistromes. Mol Cell. 2013;49(1):158-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lam MT, Cho H, Lesch HP, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498(7455):511-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kusnadi A, Park SH, Yuan R, et al. The cytokine TNF promotes transcription factor SREBP activity and binding to inflammatory genes to activate macrophages and limit tissue repair. Immunity. 2019;51(2):241-257.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Devalaraja S, To TKJ, Folkert IW, et al. Tumor-derived retinoic acid regulates intratumoral monocyte differentiation to promote immune suppression. Cell. 2020;180(6):1098-1114.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bonnardel J, T’Jonck W, Gaublomme D, et al. Stellate cells, hepatocytes, and endothelial cells imprint the Kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity. 2019;51(4):638-654.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sakai M, Troutman TD, Seidman JS, et al. Liver-derived signals sequentially reprogram myeloid enhancers to initiate and maintain kupffer cell identity. Immunity. 2019;51(4):655-670.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Seidman JS, Troutman TD, Sakai M, et al. Niche-specific reprogramming of epigenetic landscapes drives myeloid cell diversity in nonalcoholic steatohepatitis. Immunity. 2020;52(6):1057-1074.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tran S, Baba I, Poupel L, et al. Impaired Kupffer cell self-renewal alters the liver response to lipid overload during non-alcoholic steatohepatitis. Immunity. 2020;53(3):627-640.e5. [DOI] [PubMed] [Google Scholar]
- 84. Utz SG, See P, Mildenberger W, et al. Early fate defines microglia and non-parenchymal brain macrophage development. Cell. 2020;181(3):557-573.e18. [DOI] [PubMed] [Google Scholar]
- 85. Aegerter H, Kulikauskaite J, Crotta S, et al. Influenza-induced monocyte-derived alveolar macrophages confer prolonged antibacterial protection. Nat Immunol. 2020;21(2):145-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ural BB, Yeung ST, Damani-Yokota P, et al. Identification of a nerve-associated, lung-resident interstitial macrophage subset with distinct localization and immunoregulatory properties. Sci Immunol. 2020;5(45):eaax8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sajti E, Link VM, Ouyang Z, et al. Transcriptomic and epigenetic mechanisms underlying myeloid diversity in the lung. Nat Immunol. 2020;21(2):221-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gundra UM, Girgis NM, Ruckerl D, et al. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood. 2014;123(20):e110-e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Van Hove H, Martens L, Scheyltjens I, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22(6):1021-1035. [DOI] [PubMed] [Google Scholar]
- 90. Masuda T, Sankowski R, Staszewski O, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566(7744):388-392. [DOI] [PubMed] [Google Scholar]
- 91. A-Gonzalez N, Guillen JA, Gallardo G, et al. The nuclear receptor LXRα controls the functional specialization of splenic macrophages. Nat Immunol. 2013;14(8):831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kohyama M, Ise W, Edelson BT, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457(7227):318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lu Y, Basatemur G, Scott IC, et al. Interleukin-33 signaling controls the development of iron-recycling macrophages. Immunity. 2020;52(5):782-793.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Glass CK. Genetic and genomic approaches to understanding macrophage identity and function. Arterioscler Thromb Vasc Biol. 2015;35(4):755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Netea MG, Domínguez-Andrés J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rodriguez RM, Suarez-Alvarez B, Lopez-Larrea C. Therapeutic epigenetic reprogramming of trained immunity in myeloid cells. Trends Immunol. 2019;40(1):66-80. [DOI] [PubMed] [Google Scholar]
- 97. Ostuni R, Piccolo V, Barozzi I, et al. Latent enhancers activated by stimulation in differentiated cells. Cell. 2013;152(1-2):157-171. [DOI] [PubMed] [Google Scholar]
- 98. Wendeln AC, Degenhardt K, Kaurani L, et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature. 2018;556(7701):332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Roquilly A, Jacqueline C, Davieau M, et al. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat Immunol. 2020;21(6): 636-648. [DOI] [PubMed] [Google Scholar]
- 100. Yao Y, Jeyanathan M, Haddadi S, et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell. 2018;175(6):1634-1650.e17. [DOI] [PubMed] [Google Scholar]
- 101. Hoyer FF, Naxerova K, Schloss MJ, et al. Tissue-specific macrophage responses to remote injury impact the outcome of subsequent local immune challenge. Immunity. 2019;51(5):899-914.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Koelwyn GJ, Newman AAC, Afonso MS, et al. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat Med. 2020;26(9):1452-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. McCabe A, MacNamara KC. Macrophages: key regulators of steady-state and demand-adapted hematopoiesis. Exp Hematol. 2016;44(4):213-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Travnickova J, Tran Chau V, Julien E, et al. Primitive macrophages control HSPC mobilization and definitive haematopoiesis. Nat Commun. 2015;6:6227. [DOI] [PubMed] [Google Scholar]
- 106. Lee S, Kim H, You G, et al. Bone marrow CX3CR1+ mononuclear cells relay a systemic microbiota signal to control hematopoietic progenitors in mice. Blood. 2019;134(16):1312-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tay J, Bisht K, McGirr C, et al. Imaging flow cytometry reveals that granulocyte colony-stimulating factor treatment causes loss of erythroblastic islands in the mouse bone marrow. Exp Hematol. 2020;32(2):107881. [DOI] [PubMed] [Google Scholar]
- 108. Jacobsen RN, Forristal CE, Raggatt LJ, et al. Mobilization with granulocyte colony-stimulating factor blocks medullar erythropoiesis by depleting F4/80+VCAM1+CD169+ER-HR3+Ly6G+ erythroid island macrophages in the mouse. Exp Hematol. 2014;42(7):547-561.e4. [DOI] [PubMed] [Google Scholar]
- 109. Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116(23): 4815-4828. [DOI] [PubMed] [Google Scholar]
- 110. Winkler IG, Wiercinska E, Barbier V, Nowlan B, Bonig H, Levesque JP. Mobilization of hematopoietic stem cells with highest self-renewal by G-CSF precedes clonogenic cell mobilization peak. Exp Hematol. 2016;44(4):303-314.e1. [DOI] [PubMed] [Google Scholar]
- 111. Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208(2):251-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature. 2010;465(7299):793-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. McCabe A, Zhang Y, Thai V, Jones M, Jordan MB, MacNamara KC. Macrophage-lineage cells negatively regulate the hematopoietic stem cell pool in response to interferon gamma at steady state and during infection. Stem Cells. 2015;33(7):2294-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cremer S, Schloss MJ, Vinegoni C, et al. Diminished reactive hematopoiesis and cardiac inflammation in a mouse model of recurrent myocardial infarction. J Am Coll Cardiol. 2020;75(8):901-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dutta P, Hoyer FF, Grigoryeva LS, et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med. 2015;212(4):497-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chang MK, Raggatt LJ, Alexander KA, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181(2):1232-1244. [DOI] [PubMed] [Google Scholar]
- 118. Chang KH, Sengupta A, Nayak RC, et al. p62 is required for stem cell/progenitor retention through inhibition of IKK/NF-κB/Ccl4 signaling at the bone marrow macrophage-osteoblast niche. Cell Rep. 2014;9(6):2084-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Basu S, Broxmeyer HE. CCR5 ligands modulate CXCL12-induced chemotaxis, adhesion, and Akt phosphorylation of human cord blood CD34+ cells. J Immunol. 2009;183(11):7478-7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Casanova-Acebes M, Pitaval C, Weiss LA, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153(5):1025-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Abt MC, Osborne LC, Monticelli LA, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37(1):158-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ganal SC, Sanos SL, Kallfass C, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37(1):171-186. [DOI] [PubMed] [Google Scholar]
- 123. Muller PA, Koscsó B, Rajani GM, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158(2):300-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Matheis F, Muller PA, Graves CL, et al. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell. 2020;180(1):64-78.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kolter J, Feuerstein R, Zeis P, et al. A subset of skin macrophages contributes to the surveillance and regeneration of local nerves. Immunity. 2019;50(6):1482-1497.e7. [DOI] [PubMed] [Google Scholar]
- 126. Saliba AE, Li L, Westermann AJ, et al. Single-cell RNA-seq ties macrophage polarization to growth rate of intracellular Salmonella. Nat Microbiol. 2016;2:16206. [DOI] [PubMed] [Google Scholar]
- 127. Chen Y, Feng Z, Diao B, et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv. 2020:2020.2003. 2027.20045427. [Google Scholar]
- 128. Park MD. Macrophages: a Trojan horse in COVID-19? Nat Rev Immunol. 2020;20(6):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Muus C, Luecken MD, Eraslan G, et al. ; The NHLBI LungMAP Consortium; The Human Cell Atlas Lung Biological Network. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. bioRxiv. 2020:2020.2004.2019.049254. [Google Scholar]
- 130. Zhu H, Rhee JW, Cheng P, et al. Cardiovascular complications in patients with COVID-19: consequences of viral toxicities and host immune response. Curr Cardiol Rep. 2020;22(5):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Vabret N, Britton GJ, Gruber C, et al. ; Sinai Immunology Review Project . Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473-474. [DOI] [PubMed] [Google Scholar]
- 136. Ziegler CGK, Allon SJ, Nyquist SK, et al. ; HCA Lung Biological Network . SARS-CoV-2 receptor ACE2 Is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016-1035.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Bost P, Giladi A, Liu Y, et al. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell. 2020;181(7):1475-1488.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842-844. [DOI] [PubMed] [Google Scholar]
- 140. Sungnak W, Huang N, Bécavin C, et al. ; HCA Lung Biological Network . SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148(6):1132-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Park S, Sadanala KC, Kim EK. A metabolomic approach to understanding the metabolic link between obesity and diabetes. Mol Cells. 2015;38(7):587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Huang SC, Smith AM, Everts B, et al. Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity. 2016;45(4):817-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wang F, Zhang S, Vuckovic I, et al. Glycolytic stimulation is not a requirement for M2 macrophage differentiation. Cell Metab. 2018;28(3):463-475.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Németh B, Doczi J, Csete D, et al. Abolition of mitochondrial substrate-level phosphorylation by itaconic acid produced by LPS-induced Irg1 expression in cells of murine macrophage lineage. FASEB J. 2016;30(1):286-300. [DOI] [PubMed] [Google Scholar]
- 146. Lachmandas E, Boutens L, Ratter JM, et al. Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat Microbiol. 2016;2:16246. [DOI] [PubMed] [Google Scholar]
- 147. Vijayan V, Pradhan P, Braud L, et al. Human and murine macrophages exhibit differential metabolic responses to lipopolysaccharide—a divergent role for glycolysis. Redox Biol. 2019;22:101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12(5):408-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hall CJ, Boyle RH, Astin JW, et al. Immunoresponsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating β-oxidation-dependent mitochondrial ROS production. Cell Metab. 2013;18(2):265-278. [DOI] [PubMed] [Google Scholar]
- 150. Moon JS, Nakahira K, Chung KP, et al. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat Med. 2016;22(9):1002-1012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 151. Namgaladze D, Brüne B. Fatty acid oxidation is dispensable for human macrophage IL-4-induced polarization. Biochim Biophys Acta. 2014;1841(9):1329-1335. [DOI] [PubMed] [Google Scholar]
- 152. Van den Bossche J, Baardman J, Otto NA, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17(3):684-696. [DOI] [PubMed] [Google Scholar]
- 153. Nomura M, Liu J, Rovira II, et al. Fatty acid oxidation in macrophage polarization. Nat Immunol. 2016;17(3):216-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Everts B, Amiel E, Huang SC, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15(4):323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Watanabe R, Hilhorst M, Zhang H, et al. Glucose metabolism controls disease-specific signatures of macrophage effector functions. JCI Insight. 2018;3(20):e123047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Fukuzumi M, Shinomiya H, Shimizu Y, Ohishi K, Utsumi S. Endotoxin-induced enhancement of glucose influx into murine peritoneal macrophages via GLUT1. Infect Immun. 1996;64(1):108-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Freemerman AJ, Johnson AR, Sacks GN, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289(11):7884-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Das Gupta K, Shakespear MR, Curson JEB, et al. Class IIa histone deacetylases drive toll-like receptor-inducible glycolysis and macrophage inflammatory responses via pyruvate kinase M2. Cell Rep. 2020;30(8):2712-2728.e8. [DOI] [PubMed] [Google Scholar]
- 159. Zhang D, Tang Z, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zhang J, Muri J, Fitzgerald G, et al. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab. 2020;31(6):1136-1153.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Remmerie A, Scott CL. Macrophages and lipid metabolism. Cell Immunol. 2018;330:27-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Johnson AR, Qin Y, Cozzo AJ, et al. Metabolic reprogramming through fatty acid transport protein 1 (FATP1) regulates macrophage inflammatory potential and adipose inflammation. Mol Metab. 2016;5(7):506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kang K, Reilly SM, Karabacak V, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7(6):485-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, et al. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7(6):496-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Nomura M, Liu J, Yu ZX, et al. Macrophage fatty acid oxidation inhibits atherosclerosis progression. J Mol Cell Cardiol. 2019;127:270-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Moon JS, Lee S, Park MA, et al. UCP2-induced fatty acid synthase promotes NLRP3 inflammasome activation during sepsis. J Clin Invest. 2015;125(2):665-680. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 170. Carroll RG, Zasłona Z, Galván-Peña S, et al. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J Biol Chem. 2018;293(15):5509-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wei X, Song H, Yin L, et al. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016;539(7628):294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bogie JFJ, Grajchen E, Wouters E, et al. Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain. J Exp Med. 2020;217(5):e20191660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Im SS, Yousef L, Blaschitz C, et al. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 2011;13(5):540-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Dennis EA, Deems RA, Harkewicz R, et al. A mouse macrophage lipidome. J Biol Chem. 2010;285(51):39976-39985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Castoldi A, Monteiro LB, van Teijlingen Bakker N, et al. Triacylglycerol synthesis enhances macrophage inflammatory function. Nat Commun. 2020;11(1):4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Davanso MR, Crisma AR, Murata G, Newsholme P, Curi R. Impact of dietary fatty acids on macrophage lipid metabolism, signaling and function. Immunometabolism. 2020;2(1):e200008. [Google Scholar]
- 177. Gianfrancesco MA, Dehairs J, L’homme L, et al. Saturated fatty acids induce NLRP3 activation in human macrophages through K+ efflux resulting from phospholipid saturation and Na, K-ATPase disruption. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(7):1017-1030. [DOI] [PubMed] [Google Scholar]
- 178. Nguyen MT, Favelyukis S, Nguyen AK, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279-35292. [DOI] [PubMed] [Google Scholar]
- 179. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27(1):84-91. [DOI] [PubMed] [Google Scholar]
- 181. Sharma M, Boytard L, Hadi T, et al. Enhanced glycolysis and HIF-1α activation in adipose tissue macrophages sustains local and systemic interleukin-1β production in obesity. Sci Rep. 2020;10(1):5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Seimon TA, Nadolski MJ, Liao X, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12(5):467-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Feng R, Luo C, Li C, et al. Free fatty acids profile among lean, overweight and obese non-alcoholic fatty liver disease patients: a case-control study. Lipids Health Dis. 2017;16(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lancaster GI, Langley KG, Berglund NA, et al. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab. 2018;27(5):1096-1110.e5. [DOI] [PubMed] [Google Scholar]
- 185. Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29(11):1944-1949. [DOI] [PubMed] [Google Scholar]
- 186. Kanter JE, Kramer F, Barnhart S, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci U S A. 2012;109(12):E715-E724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Kanter JE, Tang C, Oram JF, Bornfeldt KE. Acyl-CoA synthetase 1 is required for oleate and linoleate mediated inhibition of cholesterol efflux through ATP-binding cassette transporter A1 in macrophages. Biochim Biophys Acta. 2012;1821(3):358-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Rubinow KB, Wall VZ, Nelson J, et al. Acyl-CoA synthetase 1 is induced by Gram-negative bacteria and lipopolysaccharide and is required for phospholipid turnover in stimulated macrophages. J Biol Chem. 2013;288(14):9957-9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hsieh WY, Zhou QD, York AG, et al. Toll-like receptors induce signal-specific reprogramming of the macrophage lipidome. Cell Metab. 2020;32(1):128-143.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9(2):213-219. [DOI] [PubMed] [Google Scholar]
- 191. Steffensen KR, Jakobsson T, Gustafsson JÅ. Targeting liver X receptors in inflammation. Expert Opin Ther Targets. 2013;17(8):977-990. [DOI] [PubMed] [Google Scholar]
- 192. Sun Y, Ishibashi M, Seimon T, et al. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ Res. 2009;104(4):455-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Fotakis P, Kothari V, Thomas DG, et al. Anti-inflammatory effects of HDL (high-density lipoprotein) in macrophages predominate over proinflammatory effects in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2019;39(12):e253-e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Guo C, Chi Z, Jiang D, et al. Cholesterol homeostatic regulator SCAP-SREBP2 integrates NLRP3 inflammasome activation and cholesterol biosynthetic signaling in macrophages. Immunity. 2018;49(5):842-856.e7. [DOI] [PubMed] [Google Scholar]
- 195. Takei A, Nagashima S, Takei S, et al. Myeloid HMG-CoA reductase determines adipose tissue inflammation, insulin resistance, and hepatic steatosis in diet-induced obese mice. Diabetes. 2020;69(2):158-164. [DOI] [PubMed] [Google Scholar]
- 196. Bekkering S, Arts RJW, Novakovic B, et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell. 2018;172(1-2):135-146.e9. [DOI] [PubMed] [Google Scholar]
- 197. York AG, Williams KJ, Argus JP, et al. Limiting cholesterol biosynthetic flux spontaneously engages type I IFN signaling. Cell. 2015;163(7):1716-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zhou QD, Chi X, Lee MS, et al. Interferon-mediated reprogramming of membrane cholesterol to evade bacterial toxins. Nat Immunol. 2020;21(7):746-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Orozco LD, Bennett BJ, Farber CR, et al. Unraveling inflammatory responses using systems genetics and gene-environment interactions in macrophages. Cell. 2012;151(3):658-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Serbulea V, DeWeese D, Leitinger N. The effect of oxidized phospholipids on phenotypic polarization and function of macrophages. Free Radic Biol Med. 2017;111:156-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107(6):737-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Yeon SH, Yang G, Lee HE, Lee JY. Oxidized phosphatidylcholine induces the activation of NLRP3 inflammasome in macrophages. J Leukoc Biol. 2017;101(1):205-215. [DOI] [PubMed] [Google Scholar]
- 203. Di Gioia M, Spreafico R, Springstead JR, et al. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat Immunol. 2020;21(1):42-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Shin KC, Hwang I, Choe SS, et al. Macrophage VLDLR mediates obesity-induced insulin resistance with adipose tissue inflammation. Nat Commun. 2017;8(1):1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Camell CD, Nguyen KY, Jurczak MJ, et al. Macrophage-specific de novo synthesis of ceramide is dispensable for inflammasome-driven inflammation and insulin resistance in obesity. J Biol Chem. 2015;290(49):29402-29413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Chaurasia B, Tippetts TS, Mayoral Monibas R, et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019;365(6451):386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Stoffel W, Holz B, Jenke B, et al. Δ6-desaturase (FADS2) deficiency unveils the role of ω3- and ω6-polyunsaturated fatty acids. EMBO J. 2008;27(17):2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Gromovsky AD, Schugar RC, Brown AL, et al. Δ-5 fatty acid desaturase FADS1 impacts metabolic disease by balancing proinflammatory and proresolving lipid mediators. Arterioscler Thromb Vasc Biol. 2018;38(1):218-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Pauter AM, Olsson P, Asadi A, et al. Elovl2 ablation demonstrates that systemic DHA is endogenously produced and is essential for lipid homeostasis in mice. J Lipid Res. 2014;55(4):718-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Talamonti E, Pauter AM, Asadi A, Fischer AW, Chiurchiù V, Jacobsson A. Impairment of systemic DHA synthesis affects macrophage plasticity and polarization: implications for DHA supplementation during inflammation. Cell Mol Life Sci. 2017;74(15):2815-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Lopategi A, Flores-Costa R, Rius B, et al. Frontline science: specialized proresolving lipid mediators inhibit the priming and activation of the macrophage NLRP3 inflammasome. J Leukoc Biol. 2019;105(1):25-36. [DOI] [PubMed] [Google Scholar]
- 213. Gerlach BD, Marinello M, Heinz J, et al. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ. 2020;27(2):525-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Infantino V, Iacobazzi V, Palmieri F, Menga A. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem Biophys Res Commun. 2013;440(1):105-111. [DOI] [PubMed] [Google Scholar]
- 215. Infantino V, Iacobazzi V, Menga A, Avantaggiati ML, Palmieri F. A key role of the mitochondrial citrate carrier (SLC25A1) in TNFα- and IFNγ-triggered inflammation. Biochim Biophys Acta. 2014;1839(11):1217-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Sanin DE, Matsushita M, Klein Geltink RI, et al. Mitochondrial membrane potential regulates nuclear gene expression in macrophages exposed to prostaglandin E2. Immunity. 2018;49(6):1021-1033.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Hasturk H, Abdallah R, Kantarci A, et al. Resolvin E1 (RvE1) attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35(5):1123-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Salic K, Morrison MC, Verschuren L, et al. Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis. 2016;250:158-165. [DOI] [PubMed] [Google Scholar]
- 219. Fredman G, Hellmann J, Proto JD, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 2016;7:12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Viola JR, Lemnitzer P, Jansen Y, et al. Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ Res. 2016;119(9):1030-1038. [DOI] [PubMed] [Google Scholar]
- 221. Laguna-Fernandez A, Checa A, Carracedo M, et al. ERV1/ChemR23 signaling protects against atherosclerosis by modifying oxidized low-density lipoprotein uptake and phagocytosis in macrophages. Circulation. 2018;138(16):1693-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Thies F, Garry JM, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361(9356):477-485. [DOI] [PubMed] [Google Scholar]
- 223. Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. [DOI] [PubMed] [Google Scholar]
- 224. Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators . Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73(22):2791-2802. [DOI] [PubMed] [Google Scholar]
- 225. Budoff MJ, Bhatt DL, Kinninger A, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020;41(40):3925-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Ryan DG, O’Neill LAJ. Krebs cycle reborn in macrophage immunometabolism. Annu Rev Immunol. 2020;38:289-313. [DOI] [PubMed] [Google Scholar]
- 227. Jha AK, Huang SC, Sergushichev A, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419-430. [DOI] [PubMed] [Google Scholar]
- 228. Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Krawczyk CM, Holowka T, Sun J, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115(23):4742-4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Everts B, Amiel E, van der Windt GJ, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120(7):1422-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Amiel E, Everts B, Fritz D, et al. Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function. J Immunol. 2014;193(6):2821-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Palsson-McDermott EM, Curtis AM, Goel G, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the Warburg effect in LPS-activated macrophages. Cell Metab. 2015;21(1):65-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181(12):8633-8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Bailey JD, Diotallevi M, Nicol T, et al. Nitric oxide modulates metabolic remodeling in inflammatory macrophages through TCA cycle regulation and itaconate accumulation. Cell Rep. 2019;28(1):218-230.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. De Souza DP, Achuthan A, Lee MK, et al. Autocrine IFN-I inhibits isocitrate dehydrogenase in the TCA cycle of LPS-stimulated macrophages. J Clin Invest. 2019;129(10):4239-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Strelko CL, Lu W, Dufort FJ, et al. Itaconic acid is a mammalian metabolite induced during macrophage activation. J Am Chem Soc. 2011;133(41):16386-16389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Lampropoulou V, Sergushichev A, Bambouskova M, et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016;24(1):158-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Mills EL, Ryan DG, Prag HA, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Michelucci A, Cordes T, Ghelfi J, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci U S A. 2013;110(19):7820-7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Li Y, Zhang P, Wang C, et al. Immune responsive gene 1 (IRG1) promotes endotoxin tolerance by increasing A20 expression in macrophages through reactive oxygen species. J Biol Chem. 2013;288(23):16225-16234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Cordes T, Wallace M, Michelucci A, et al. Immunoresponsive gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J Biol Chem. 2016;291(27):14274-14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Bambouskova M, Gorvel L, Lampropoulou V, et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature. 2018;556(7702):501-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Liu L, Lu Y, Martinez J, et al. Proinflammatory signal suppresses proliferation and shifts macrophage metabolism from Myc-dependent to HIF1α-dependent. Proc Natl Acad Sci U S A. 2016;113(6):1564-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Wang F, Wang K, Xu W, et al. SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1β production and to prevent DSS-induced colitis in mice. Cell Rep. 2017;19(11):2331-2344. [DOI] [PubMed] [Google Scholar]
- 246. Rius J, Guma M, Schachtrup C, et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. 2008;453(7196):807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Mills EL, Kelly B, Logan A, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167(2):457-470.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Jung SB, Choi MJ, Ryu D, et al. Reduced oxidative capacity in macrophages results in systemic insulin resistance. Nat Commun. 2018;9(1):1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Littlewood-Evans A, Sarret S, Apfel V, et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016;213(9):1655-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. He W, Miao FJ, Lin DC, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429(6988):188-193. [DOI] [PubMed] [Google Scholar]
- 251. Macaulay IC, Tijssen MR, Thijssen-Timmer DC, et al. Comparative gene expression profiling of in vitro differentiated megakaryocytes and erythroblasts identifies novel activatory and inhibitory platelet membrane proteins. Blood. 2007;109(8):3260-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Peruzzotti-Jametti L, Bernstock JD, Vicario N, et al. Macrophage-derived extracellular succinate licenses neural stem cells to suppress chronic neuroinflammation. Cell Stem Cell. 2018;22(3):355-368.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. West AP, Brodsky IE, Rahner C, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472(7344):476-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Cameron AM, Castoldi A, Sanin DE, et al. Inflammatory macrophage dependence on NAD+ salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat Immunol. 2019;20(4):420-432. [DOI] [PubMed] [Google Scholar]
- 255. Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191(2):421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Sugioka K, Nakano M, Totsune-Nakano H, Minakami H, Tero-Kubota S, Ikegami Y. Mechanism of O2- generation in reduction and oxidation cycle of ubiquinones in a model of mitochondrial electron transport systems. Biochim Biophys Acta. 1988;936(3):377-385. [DOI] [PubMed] [Google Scholar]
- 257. Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221-225. [DOI] [PubMed] [Google Scholar]
- 258. Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182(3):751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Yi L, Liu Q, Orandle MS, et al. p47phox directs murine macrophage cell fate decisions. Am J Pathol. 2012;180(3):1049-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Mendiola AS, Ryu JK, Bardehle S, et al. Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat Immunol. 2020;21(5):513-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Kobayashi EH, Suzuki T, Funayama R, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Vomund S, Schäfer A, Parnham MJ, Brüne B, von Knethen A. Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci. 2017;18(12):2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Hammerer F, Chang JH, Duncan D, Castañeda Ruiz A, Auclair K. Small molecule restores itaconate sensitivity in salmonella enterica: a potential new approach to treating bacterial infections. Chembiochem. 2016;17(16):1513-1517. [DOI] [PubMed] [Google Scholar]
- 264. Artyomov MN, Sergushichev A, Schilling JD. Integrating immunometabolism and macrophage diversity. Semin Immunol. 2016;28(5):417-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Swain A, Bambouskova M, Kim H, et al. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat Metab. 2020;2(7):594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Seim GL, Britt EC, John SV, et al. Two-stage metabolic remodelling in macrophages in response to lipopolysaccharide and interferon-γ stimulation. Nat Metab. 2019;1(7):731-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Qin W, Qin K, Zhang Y, et al. S-glycosylation-based cysteine profiling reveals regulation of glycolysis by itaconate. Nat Chem Biol. 2019;15(10):983-991. [DOI] [PubMed] [Google Scholar]
- 268. Weiss JM, Davies LC, Karwan M, et al. Itaconic acid mediates crosstalk between macrophage metabolism and peritoneal tumors. J Clin Invest. 2018;128(9):3794-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Langston PK, Nambu A, Jung J, et al. Glycerol phosphate shuttle enzyme GPD2 regulates macrophage inflammatory responses. Nat Immunol. 2019;20(9):1186-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Filippov S, Pinkosky SL, Lister RJ, et al. ETC-1002 regulates immune response, leukocyte homing, and adipose tissue inflammation via LKB1-dependent activation of macrophage AMPK. J Lipid Res. 2013;54(8):2095-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Papathanassiu AE, Ko JH, Imprialou M, et al. BCAT1 controls metabolic reprogramming in activated human macrophages and is associated with inflammatory diseases. Nat Commun. 2017;8:16040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Meiser J, Krämer L, Sapcariu SC, et al. Pro-inflammatory macrophages sustain pyruvate oxidation through pyruvate dehydrogenase for the synthesis of itaconate and to enable cytokine expression. J Biol Chem. 2016;291(8):3932-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Arts RJ, Novakovic B, Ter Horst R, et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016;24(6):807-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Liu PS, Wang H, Li X, et al. α-Ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18(9):985-994. [DOI] [PubMed] [Google Scholar]
- 275. Palmieri EM, Gonzalez-Cotto M, Baseler WA, et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat Commun. 2020;11(1):698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Qualls JE, Subramanian C, Rafi W, et al. Sustained generation of nitric oxide and control of mycobacterial infection requires argininosuccinate synthase 1. Cell Host Microbe. 2012;12(3):313-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Shearer JD, Richards JR, Mills CD, Caldwell MD. Differential regulation of macrophage arginine metabolism: a proposed role in wound healing. Am J Physiol. 1997;272(2 Pt 1):E181-E190. [DOI] [PubMed] [Google Scholar]
- 278. Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017;61:3-11. [DOI] [PubMed] [Google Scholar]
- 279. Zhao P, Zhou W, Zhang Y, et al. Aminooxyacetic acid attenuates post-infarct cardiac dysfunction by balancing macrophage polarization through modulating macrophage metabolism in mice. J Cell Mol Med. 2020;24(4):2593-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Huang SC, Everts B, Ivanova Y, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15(9):846-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Han CZ, Ravichandran KS. Metabolic connections during apoptotic cell engulfment. Cell. 2011;147(7):1442-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Zhang S, Weinberg S, DeBerge M, et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 2019;29(2):443-456.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Medina CB, Mehrotra P, Arandjelovic S, et al. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020;580(7801):130-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Morioka S, Perry JSA, Raymond MH, et al. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature. 2018;563(7733):714-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Reddy SM, Hsiao KH, Abernethy VE, et al. Phagocytosis of apoptotic cells by macrophages induces novel signaling events leading to cytokine-independent survival and inhibition of proliferation: activation of Akt and inhibition of extracellular signal-regulated kinases 1 and 2. J Immunol. 2002;169(2):702-713. [DOI] [PubMed] [Google Scholar]
- 286. De Maeyer RPH, van de Merwe RC, Louie R, et al. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat Immunol. 2020;21(6):615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Yurdagul A Jr, Subramanian M, Wang X, et al. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab. 2020;31(3):518-533.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Liang CP, Han S, Okamoto H, et al. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113(5):764-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Hartman ME, O’Connor JC, Godbout JP, Minor KD, Mazzocco VR, Freund GG. Insulin receptor substrate-2-dependent interleukin-4 signaling in macrophages is impaired in two models of type 2 diabetes mellitus. J Biol Chem. 2004;279(27):28045-28050. [DOI] [PubMed] [Google Scholar]
- 290. Lee FY, Li Y, Yang EK, et al. Phenotypic abnormalities in macrophages from leptin-deficient, obese mice. Am J Physiol. 1999;276(2):C386-C394. [DOI] [PubMed] [Google Scholar]
- 291. Li SL, Reddy MA, Cai Q, et al. Enhanced proatherogenic responses in macrophages and vascular smooth muscle cells derived from diabetic db/db mice. Diabetes. 2006;55(9):2611-2619. [DOI] [PubMed] [Google Scholar]
- 292. Plotkin BJ, Paulson D, Chelich A, et al. Immune responsiveness in a rat model for type II diabetes (Zucker rat, fa/fa): susceptibility to Candida albicans infection and leucocyte function. J Med Microbiol. 1996;44(4):277-283. [DOI] [PubMed] [Google Scholar]
- 293. Mauldin JP, Srinivasan S, Mulya A, et al. Reduction in ABCG1 in type 2 diabetic mice increases macrophage foam cell formation. J Biol Chem. 2006;281(30):21216-21224. [DOI] [PubMed] [Google Scholar]
- 294. Fukuhara-Takaki K, Sakai M, Sakamoto Y, Takeya M, Horiuchi S. Expression of class A scavenger receptor is enhanced by high glucose in vitro and under diabetic conditions in vivo: one mechanism for an increased rate of atherosclerosis in diabetes. J Biol Chem. 2005;280(5):3355-3364. [DOI] [PubMed] [Google Scholar]
- 295. Griffin E, Re A, Hamel N, et al. A link between diabetes and atherosclerosis: glucose regulates expression of CD36 at the level of translation. Nat Med. 2001;7(7):840-846. [DOI] [PubMed] [Google Scholar]
- 296. Iida KT, Suzuki H, Sone H, et al. Insulin inhibits apoptosis of macrophage cell line, THP-1 cells, via phosphatidylinositol-3-kinase-dependent pathway. Arterioscler Thromb Vasc Biol. 2002;22(3):380-386. [DOI] [PubMed] [Google Scholar]
- 297. Han S, Liang CP, DeVries-Seimon T, et al. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3(4):257-266. [DOI] [PubMed] [Google Scholar]
- 298. Aguirre GA, De Ita JR, de la Garza RG, Castilla-Cortazar I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J Transl Med. 2016;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Spadaro O, Camell CD, Bosurgi L, et al. IGF1 shapes macrophage activation in response to immunometabolic challenge. Cell Rep. 2017;19(2):225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Higashi Y, Sukhanov S, Shai SY, et al. Insulin-like growth factor-1 receptor deficiency in macrophages accelerates atherosclerosis and induces an unstable plaque phenotype in apolipoprotein E-deficient mice. Circulation. 2016;133(23):2263-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Trzaskalski NA, Fadzeyeva E, Mulvihill EE. Dipeptidyl peptidase-4 at the interface between inflammation and metabolism. Clin Med Insights Endocrinol Diabetes. 2020;13:1179551420912972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Shiraishi D, Fujiwara Y, Komohara Y, Mizuta H, Takeya M. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun. 2012;425(2):304-308. [DOI] [PubMed] [Google Scholar]
- 303. Wan S, Sun H. Glucagon-like peptide-1 modulates RAW264.7 macrophage polarization by interfering with the JNK/STAT3 signaling pathway. Exp Ther Med. 2019;17(5):3573-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Lee YS, Park MS, Choung JS, et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012;55(9):2456-2468. [DOI] [PubMed] [Google Scholar]
- 305. Vinué Á, Navarro J, Herrero-Cervera A, et al. The GLP-1 analogue lixisenatide decreases atherosclerosis in insulin-resistant mice by modulating macrophage phenotype. Diabetologia. 2017;60(9):1801-1812. [DOI] [PubMed] [Google Scholar]
- 306. Dobrian AD, Ma Q, Lindsay JW, et al. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab. 2011;300(2):E410-E421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Matsubara J, Sugiyama S, Sugamura K, et al. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2012;59(3):265-276. [DOI] [PubMed] [Google Scholar]
- 308. Wang L, Li Y, Wang X, et al. GDF3 protects mice against sepsis-induced cardiac dysfunction and mortality by suppression of macrophage pro-inflammatory phenotype. Cells. 2020;9(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Ratnam NM, Peterson JM, Talbert EE, et al. NF-κB regulates GDF-15 to suppress macrophage surveillance during early tumor development. J Clin Invest. 2017;127(10):3796-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Li H, Wu G, Fang Q, et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat Commun. 2018;9(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Yu Y, He J, Li S, et al. Fibroblast growth factor 21 (FGF21) inhibits macrophage-mediated inflammation by activating Nrf2 and suppressing the NF-κB signaling pathway. Int Immunopharmacol. 2016;38:144-152. [DOI] [PubMed] [Google Scholar]
- 312. Brykczynska U, Geigges M, Wiedemann SJ, et al. Distinct transcriptional responses across tissue-resident macrophages to short-term and long-term metabolic challenge. Cell Rep. 2020;30(5):1627-1643.e7. [DOI] [PubMed] [Google Scholar]
- 313. Weinstock A, Brown EJ, Garabedian ML, et al. Single-cell RNA sequencing of visceral adipose tissue leukocytes reveals that caloric restriction following obesity promotes the accumulation of a distinct macrophage population with features of phagocytic cells. Immunometabolism. 2019;1:e190008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Zhang X, Sergin I, Evans TD, et al. High-protein diets increase cardiovascular risk by activating macrophage mTOR to suppress mitophagy. Nat Metab. 2020;2(1):110-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Goldberg EL, Shchukina I, Asher JL, Sidorov S, Artyomov MN, Dixit VD. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat Metab. 2020;2(1):50-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Jordan S, Tung N, Casanova-Acebes M, et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell. 2019;178(5):1102-1114.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.