Pilocytic astrocytoma (PA) is the most common glioma in children, with an excellent overall survival following surgical resection; however, a small subset of tumors recur, especially following incomplete resection. Since PAs contain a large number of non-neoplastic cells, immunomodulatory therapies have been proposed, particularly in light of results using experimental mouse models of low-grade glioma (LGG), where microglia and T cells were found to be critical non-neoplastic drivers of tumor formation and growth.1
To define the immune cell landscape of PAs, we leveraged tissue microarrays from 47 children (1–14 years; mean age, 7 years) with sporadic (non-NF1-associated) PA diagnosed between 1992 and 2004. Twenty-three (48.9%) were female, 21 (44.7%) were male, and 3 lacked clinical data. Tumors were located in the posterior fossa (n = 28; 59.5%), spinal cord (n = 2; 4.2%), and supratentorial (ST) compartment (n = 17; 34%), including one (2.1%) in the hypothalamus. Twenty-three patients (48.9%) underwent gross total resection, 22 (46.8%) subtotal resection, and 1 (2.1%) biopsy alone, with no surgical data available for one case.
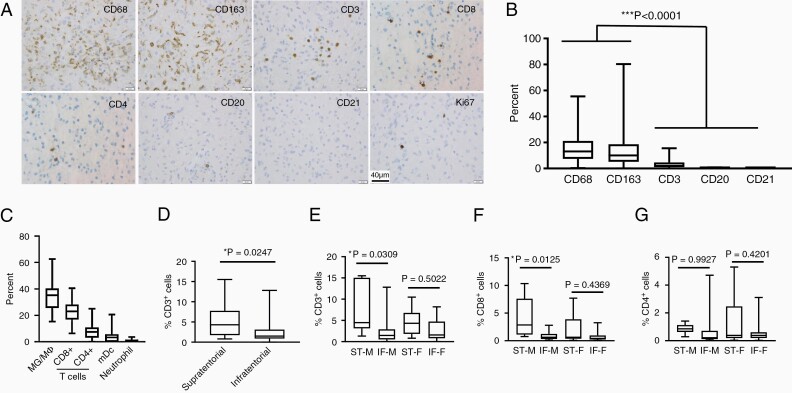
Tumor-associated microglia/macrophages were present as either scattered cells and/or perivascular aggregates (Figure 1A), with 15.3 ± 1.7 % CD68+ and 14.4 ± 2.2% CD163+ cells. CD3+ T cells (3.5 ± 0.6%) including CD8+ cells (1.6 ± 0.36%) and CD4+ cells (0.9 ± 0.19%) were less abundant, whereas CD20+ B cells (0.1 ± 0.02%) and CD21+ dendritic cells (0%) were scarce (Figure 1B). To validate these findings, we analyzed RNA expression data from 82 sporadic PAs with CIBERSORTx2 using reference file LM22.3 Similar to the immunostaining results, microglia/macrophages constituted the majority of the immune-like cells (33.5% ± 9.7% [SEM = 1.1%]), with CD8+ cells constituting the next most abundant population (Figure 1C), as recently reported for both human4 and mouse5 NF1-LGG.
Figure 1.
(A) Representative immunohistochemistry images from the PA tissue microarray. (B) Higher percentages of CD68+ and CD163+ tumor-associated microglia/macrophages were observed relative to CD3+ T cells, CD20+ B cells, and CD21+ dendritic cells (***P < .0001 by one-way analysis of variance). (C) CIBERSORTx analysis of RNA microarray data from 82 sporadic PAs (GSE44971, n = 46; GSE50161, n = 15; GSE12907, n = 21), in which macrophage and monocyte populations (MG/MΦ), as well as CD8+ cell populations (CD8+ T cells and NK cells), were combined. The means and standard deviations for these deconvoluted results are included. (D) Percentage of CD3+ T lymphocytes was higher in supratentorial relative to infratentorial PAs as a group (*P = .0247; Welch’s t-test). (E) Percentage of CD3+ T lymphocytes was significantly higher in supratentorial compared to infratentorial PAs from boys (*P = .0309), but not girls (P = .5022). ST-M: n = 6; IF-M: n = 15; ST-F: n = 10; IF-F: n = 13. (F) Percentage of CD8+ T lymphocytes was significantly higher in supratentorial compared to infratentorial PAs from boys (*P = .0125), but not girls (P = .4369). ST-M: n = 6; IF-M: n = 13; ST-F: n = 9; IF-F: n = 12. (G) There was no significant difference in percentage of CD4+ T lymphocytes in supratentorial compared to infratentorial PAs from either boys (*P = .9927) or girls (P = .4201). ST-M: n = 6; IF-M: n = 13; ST-F: n = 9; IF-F: n = 12. ST-M, supratentorial-male; IF-M, infratentorial-male; ST-F, supratentorial-female; IF-F, infratentorial-female. P values in panels (E), (F), and (G) by Tukey’s multiple comparisons test. Immunohistochemistry for microglia/macrophages (CD68; Ventana, CD163; Cell Marque), T cells (Ventana; CD3, 2GV6; CD8, SP57; CD4, SP35), B cells (CD20; Ventana), and dendritic cells (CD21; Cell Marque) was performed, and percentage of positive cells was manually counted. Ki67 immunohistochemistry (Ventana) was performed to determine the proliferation indices. Whole slide images were scanned on a Ventana iScan Coreo (Ventana Medical Systems) at a magnification of 200× and analyzed with the companion algorithm image analysis software (Roche).
Whereas the percent of CD68+ or CD163+ cells did not correlate with tumor location (P = .3814 and .1506), the percent of T cells was approximately 2-fold higher in ST PAs relative to their infratentorial (IT) counterparts (5.4 ± 1.1% vs 2.5 ± 0.5%, P = .0247, Wilcoxon rank sum test; Figure 1D). In addition, the increased CD3+ T lymphocyte content in ST PAs was only observed in males (ST-M: 7.4 ± 2.5%, IF-M: 2.4 ± 0.8%, P = .0309; ST-F: 4.7 ± 1.1%, IF-F: 2.6 ± 0.7%; P = .5022, Tukey’s multiple comparisons test; Figure 1E). Further analysis demonstrated increased CD8+ T lymphocyte content in ST PAs from males (ST-M: 4.1 ± 1.5%, IF-M: 0.9 ± 0.2%, P = .0125; ST-F: 2.2 ± 0.9%, IF-F: 0.9 ± 0.3%; P = .4369, Tukey’s multiple comparisons test; Figure 1F), but not CD4+ T lymphocyte content (ST-M: 0.9 ± 0.1%, IF-M: 0.7 ± 0.4%, P = .9927; ST-F: 1.5 ± 0.6%, IF-F: 0.6 ± 0.2%; P = .4201, Tukey’s multiple comparisons test; Figure 1G). This intriguing location-specific enrichment in boys suggests potential sexually dimorphic differences in tumor biology for these spatially distinct LGGs.
Given the T-cell differences in ST versus IT PAs, we also examined the relationship between immune cell content and patient outcome. The percentage of CD68+ and CD163+ microglia/macrophages was higher in tumors that did not recur based on postoperative surveillance imaging (16.36 ± 2.013%, 14.9 ± 2.655%, respectively) compared to those that did (11.63 ± 2.19%, 11.38 ± 3.31%, respectively); however, this difference was not statistically significant (P = .4163 and .1244, respectively). Similarly, neither the percentage of CD3+ T cells nor the Ki67 proliferation index correlated with the tumor recurrence rate (P = .7822 and .4972, respectively; unpaired t-test) or recurrence-free survival, either in the entire cohort (P = .6312 and .2850, respectively; log-rank test) or in the 22 patients who underwent subtotal tumor resection (P = .6940 and .3768, respectively; log-rank test).
Relevant to considering immune-targeted therapies for these tumors, we examined the expression of immune checkpoint proteins by immunohistochemistry. No PD-1 (Clone NAT105) or PD-L1 (Clone E1L3N) expression was detected in these PAs, consistent with previous reports demonstrating higher PD-L1 expression in malignant gliomas relative to lower-grade counterparts.6
Taken together, our findings are consistent with multiple prior studies demonstrating links between immune cell content and tumor location for PAs that are validated by the current study. We further identify an association between immune cell content and patient sex. Given the emerging role of T cells in microglia/macrophage-mediated LGG growth, these findings of sex-specific differences in T-cell content in ST versus IT PAs and the lack of tumoral PD-1/PD-L1 expression support the need for further investigations aimed at defining additional immune cell-directed targets for adjuvant LGG therapy.
Funding
D.H.G. is supported by an RPA grant from the National Institute of Neurological Disorders and Stroke (1-R35-NS097211-01).
Conflict of interest statement. The authors have no relevant interests to disclose.
References
- 1. Guo X, Pan Y, Xiong M, et al. Midkine activation of CD8+ T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth. Nat Commun. 2020;11(1):2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Newman AM, Steen CB, Liu CL, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. 2019;37(7):773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D’Angelo F, Ceccarelli M, Tala, et al. The molecular landscape of glioma in patients with neurofibromatosis 1. Nat Med. 2019;25(1):176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo X, Pan Y, Gutmann DH. Genetic and genomic alterations differentially dictate low-grade glioma growth through cancer stem cell–specific chemokine recruitment of T cells and microglia. Neuro Oncol. 2019;21(10):1250–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martin AM, Bell WR, Yuan M, et al. PD-L1 expression in pediatric low-grade gliomas is independent of BRAF V600E mutational status. J Neuropathol Exp Neurol. 2020;79(1):74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]