Abstract
Co-infection of Hepatitis B (HBV) and Delta viruses (HDV) represent the most severe form of viral hepatitis. While treatment with pegylated Interferon alpha (PEG-IFNα) is well established, therapy with nucleoside or nucleotide analogues (NA) has been a matter of debate. We aimed to investigate the role of NA treatment in a well-defined single centre cohort.
In a retrospective approach, we observed 53 HDV RNA positive and/or anti-HDV-positive patients recruited at a German referral centre between 2000 and 2019. Patients were followed for at least 3 months (mean time of follow up: 4.6 years; range: 0.2–14.1 years). Patients who had liver transplantation or hepatocellular carcinoma at the time of presentation were excluded. 43% (n = 23) were treated with NA, 43% (n = 23) received IFNα-based therapies and 13% (n = 7) were untreated.
Liver cirrhosis was already present in 53% (28/53) of patients at first presentation. During follow-up, liver-related endpoints developed in 44% of all patients (n = 23). NA-treatment was associated with a significantly worse clinical outcome (P = .01; odds ratio [OR] = 4.92; CI = 1.51–16.01) compared to both, untreated (P = .38; OR = 0.46; CI = 0.80–2.61) and IFNα-based-treated patients (P = .04; OR = 0.29; CI = 0.89–0.94) in univariate logistic regression analysis. HBsAg levels declined by more than 50% during NA-based therapy in only 7 cases (7/23; mean time: 3.6 years; range: 0.8–8.5 years) and during IFNα-based therapy in 14 cases (14/23; mean time: 2.8 years, range 0.7–8.5 years). HDV RNA became undetectable during follow up in 30% of patients receiving NA alone (7/23; mean time: 5.0 years; range: 0.6–13.5 years), in 35% of patients receiving IFNα-based therapy (8/23; mean time: 2.9 years, range: 0.3–7.6 years).
The effect of NA in patients with HBV/HDV co-infection is limited. Treatment with NA was associated with a higher likelihood of clinical disease progression. Interferon alpha therapy was beneficial in reducing liver complications and improves long-term outcome.
Keywords: hepatitis B virus, hepatitis D (delta) virus, nucleos(t)ide analogues, pegylated interferon alpha
1. Introduction
The hepatitis D (delta) virus (HDV) was first discovered by Rizzetto et al in 1977 in the liver of individuals chronically infected with the hepatitis B virus (HBV)[1] and was accepted as a defective RNA virus that requires the presence of HBV for its viral assembly.[2] It does not produce its own envelope or capsid,[1] therefore HDV-infection can only occur in the presence of the hepatitis B surface antigen (HBsAg), either as co-infection (simultaneous infection with both HBV and HDV) or as superinfection of an individual chronically infected with HBV.[3,4] Infecting between 9 and 19 million individuals worldwide,[5] chronic hepatitis delta (CHD) represents the most severe form of viral hepatitis frequently leading to liver-related complications, such as liver cirrhosis, hepatic decompensation and hepatocellular carcinoma (HCC).[6,7] These observations have been confirmed in more recent single centre examinations published in the last decade.[8–16] Eight different genotypes of HDV have been described, which may have an impact on disease course or response to interferon therapy.[13,15,17]
Treatment options for chronic hepatitis delta are very limited and a major task for the health system. So far, the only antiviral treatment with proven efficacy against HDV is based on administration of type 1 interferons, in clinical practice pegylated interferon alpha (PEG-IFNα) for at least 48 weeks. Treatment response is observed in around 25% to 40% after 1 year of treatment.[18] Extending treatment period to 96 weeks and adding tenofovir disoproxil fumarate resulted in no significant improvement in HDV RNA response rates at the end of treatment.[19] Moreover, a large proportion of HDV infected patients cannot be treated with PEG-IFNα due to contraindications, such as advanced portal hypertension, thrombocytopenia, or autoimmune disorders.[20] Tolerability of PEG-IFNα is a particular problem in patients with advanced liver fibrosis and PEG-IFNα is even contraindicated in decompensated cirrhosis. Thus, treatment of HDV infection in patients with the most urgent clinical need is often not possible.
Still, treatment with PEG-IFNα may be useful as it has been shown in several studies that even in the absence of HBsAg loss, there is evidence that HDV RNA suppression or only reduction may be associated with an improved clinical long-term outcome.[10–12,21,22]
The standard therapy for chronic hepatitis B virus infection consists of nucleoside or nucleotide analogues (NA), which are HBV polymerase inhibitors. NA therapy leads to undetectable HBV DNA in the majority of patients,[23–25] concomitant with reducing the risk of HCC, HBV-associated mortality and morbidity and improvement in hepatic inflammation in chronic hepatitis B.[26]
Since suppression of HBV may also have indirect effects on HDV, different NAs have been tested for CHD in monotherapy and in combination with IFN. These include therapies with famiciclovir,[27] ribavirin,[28] lamivudine,[29,30] adefovir,[18] tenofovir[19] and entecavir.[31] None of them proved to be effective in reducing HDV RNA or HBsAg levels in HBV/HDV co-infected patients. However, long-term monotherapy with potent NAs in patients with human immunodeficient virus (HIV) infection has been associated with HDV RNA and even HBsAg declines in studies from Spain.[32,33] In clinical practice, cirrhotic patients infected with HDV and advanced liver disease are frequently treated with NA, to block residual HBV replication. Nevertheless, NA are not recommended in non-cirrhotic HDV patients in the absence of HIV infection.[34,35]
Thus, being unclear how NAs influence the long-term outcome of liver disease in HBV/HDV co-infected patients, we aimed to investigate in this study the role of NA treatment strategies in CHD patients. In addition, our goal was to evaluate the clinical course of HDV infection in a well-defined single centre cohort of HDV-infected patients recruited at a major liver transplant centre in Germany with a particular high frequency of immigrants from Eastern Mediterranean area.
2. Methods
2.1. Study design
In this study we screened 120 patients with an HBV/HDV co-infection, referred to the Department of Gastroenterology and Hepatology at the University Hospital Essen from 2000 to 2019. Patients with detectable HBsAg, HDV RNA and/or anti-HDV antibodies at baseline, with a minimum of 2 follow-up for at least 3 months were included. Patients which had already undergone liver transplantation or had developed hepatocellular carcinoma (HCC) before the first observation were excluded. The remaining 53 patients fulfilling the inclusions criteria were included in this analysis (Fig. 1). Retrospective clinical data of clinical routine examinations were analysed.
Figure 1.
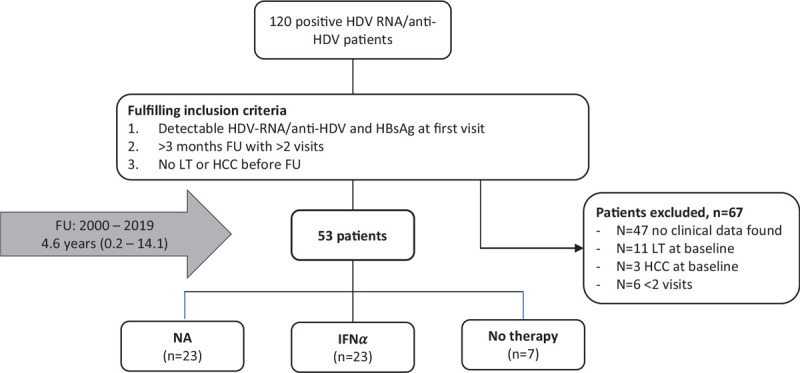
Number of patients recruited in the different treatment groups including the inclusion and exclusion criteria. FU = follow-up, HCC = hepatocellular carcinoma, HDV = hepatitis delta virus, IFNα = interferon alpha, LT = liver transplantation, NA = nucleos(t)ide analogues.
2.2. Definitions
Liver-related clinical endpoints were defined as hepatic decompensation (ascites, encephalopathy and variceal bleeding), liver transplantation, hepatocellular carcinoma or liver-related death. Presence of liver cirrhosis was diagnosed either histologically, clinically or biochemically. Patients who were liver biopsied and showed a “Metavir score F4,” or were detected by transient elastography (>145 kPa, kilopascals) were considered to have liver cirrhosis. If these data were unavailable, presence of cirrhosis was recognized if patients had already clinical evidence of hepatic decompensation in the past or if at least 2 of the following criteria were present: aspartate/alanine aminotransferase (AST/ALT) ratio > 1, platelets < 100,000/mL, international normalized ratio (INR) > 1.5, and/or splenomegaly (largest dimension > 13 cm). Abnormal biochemical parameters were characterized as AST, ALT as well as gamma-Glutamyltransferase (gGT) > 35 U/I, alkaline phosphatase > 100 U/I, platelets < 100,000/mL, albumin < 3.5 g/dL, total bilirubin > 1.2 mg/dL and INR > 15. The AST/ALT ratio > 1 indicates a more severe liver disease.
2.3. Laboratory parameters
Routine laboratory parameters were measured in the central laboratory of University Hospital Essen. Serum samples were tested for HBsAg, anti-HBs, anti-HBc, anti-HBe, and HBeAg (Architect i2000SR, Abbott, Wiesbaden, Germany). HBsAg was measured qualitatively and/or quantitatively (S/CO > 1 or > 0.05 IU/mL defined as positive, respectively). Anti-HBc, HBeAg and anti-HBe were measured qualitatively (S/CO > 1 defined as positive). Anti-HBs positivity was defined as anti-HBs ≥10 IU/L. HBV DNA was quantified by real-time PCR performed on the m2000 Abbott PCR system (Abbott, Wiesbaden, Germany), Qiagen Artus HBV LC PCR kit (Qiagen, Hilden, Germany) or Siemens bDNA HBV test system (Siemens, Munich, Germany). The detection limit for qualitative and quantitative PCR prior to 2013 was 100 IU/mL; afterwards the limit was set to 10 IU/mL. Serum HDV RNA was measured with the Robogene assay (Analytik Jena, Jena, Germany). The detection limit for qualitative and quantitative HDV RNA prior to 2013 was 400 IU/mL; afterwards the limit was set to 60 IU/mL. RNA extraction for HDV was performed using an automated method, MagNA pure (Roche, Mannheim, Germany) prior to June 2018, afterwards using a hand extraction method, INSTANT Virus DNA/RNA kit (Analytik Jena, Jena, Germany).
2.4. Statistical analysis
All statistical analyses were performed by using the SPSS software version 25 (SPSS Inc., Chicago, IL). If data were lost during follow-up, the patient data were not included in the analysis. Baseline characteristics were described as mean ± standard deviation and range. In case of non-normal distribution, the median ± standard deviation and range was used and Mann–Whitney U tests were performed for their comparison. A Chi-Squared test was calculated for the comparison of qualitative variables. Differences between groups for continuous variables were evaluated using Student t test, Mann–Whitney U test or ANOVA as applicable. P values ≤.05 were considered statistically significant. Variables found to be significant in univariate analysis, were used in multivariable logistic regression analysis. The results were expressed as odds ratio (OR), including their 95% confidence intervals (CI). Kaplan–Meier analysis was performed to estimate the cumulative event-free survival within the groups of therapy. Log-rank tests were used to show significant differences in those groups. Using Cox regression model, we calculated the association of parameters with clinical long-term outcome, as well as hazard ratios (HR). Graphics were performed by GraphPad Software (San Diego, CA).
2.5. Ethics
The data protection corresponds to that of the University Hospital Essen. The study was approved by the local ethics boards (Ethik-Kommission der Medizinischen Fakultät der Universität Duisburg-Essen; 19–8726-BO).
3. Results
3.1. Sample background and therapy
Of 53 eligible patients (enrolled from 2000 to 2019), 60% males (n = 32) and 40% females (n = 21) were studied with a mean follow-up of 4.6 years (range, 0.2–14.1 years). The average age of patients was 48 years (range, 24–69 years; Table 1).
Table 1.
Baseline characteristics.
| Total Cohort | NA | IFNα | P Value | |
| Total, n (%) | 53 | 23 (43) | 23 (43) | |
| Sex, n (%) | Female = 21 (40) Male = 32 (60) | Female = 8 (35) Male = 15 (65) | Female = 9 (40) Male = 14 (60) | .76 |
| Age, y, mean (range) | 48 (24–69) | 48 (28–67) | 45 (24–69) | .37 |
| BMI, kg/m2, mean (range) | 26 ± 4 (17–41) | 25 ± 4 (17–32) | 25 ± 3 (21–32) | .97 |
| Previous therapy, n (%) | 18 (34) | 9 (39) | 9 (39) | |
| AST, U/I, median ± SD (range) | 80 ± 989 (16–5968) | 83 ± 1225 (20–5968) | 80 ± 886 (25–3536) | .45 |
| ALT, U/I, median ± SD (range) | 73 ± 1210 (10–6666) | 94 ± 1370 (32–6666) | 79 ± 1241 (15–5038) | .99 |
| AST/ALT ratio, mean ± SD (range) | 1.0 ± 0.4 (0.3–2.3) | 1.1 ± 0.4 (0.4–2.0) | 1.0 ± 0.4 (0.3–1.9) | .23 |
| gGT, U/I, median ± SD (range) | 54 ± 138 (16–938) | 63 ± 194 (16–938) | 59 ± 68 (19–254) | .57 |
| AP, U/I, mean ± SD (range) | 103 ± 57 (42–309) | 111 ± 43 (45–203) | 119 ± 66 (42–309) | .91 |
| Bilirubin, mg/dL, median ± SD (range) | 1.0 ± 4.2 (0.3–19.8) | 1.1 ± 2.7 (0.4–13.4) | 0.6 ± 5.7 (0.3–19.8) | .04 |
| Albumin, g/dL, median ± SD (range) | 3.8 ± 0.7 (2.2–4.6) | 3.4 ± 0.7 (2.2–4.2) | 4.1 ± 0.4 (3.4–4.6) | .004 |
| Platelets, 1000/mL, median ± SD (range) | 115 ± 80.1 (21–359) | 102 ± 63.1 (26–248) | 180 ± 81.4 (21–319) | .03 |
| INR, mean ± SD (range) | 1.24 ± 0.25 (0.97–2.34) | 1.28 ± 0.22 (0.98–2.00) | 1.22 ± 0.30 (0.99–2.34) | .08 |
| MELD, mean ± SD (range) | 11 ± 5 (6–27) | 12 ± 4 (7–22) | 10 ± 6 (6–27) | .04 |
| Child Pugh classes, n patients (%) | A = 26/41 (63) B = 13 (32) C = 2 (5) | A = 7/18 (39) B = 10 (56) C = 1 (6) | A = 14/17 (82) B = 2 (12) C = 1 (6) | .02 |
| APRI score, median ± SD (range) | 1.5 ± 14.5 (0.1–88.4) | 3.3 ± 17.9 (0.2–88.4) | 1.2 ± 12.8 (0.3–50.5) | .14 |
| HBsAg levels, IU/mL, median ± SD (range) | 10068 ± 8295 (38–28060) | 9858 ± 6976 (1924–26136) | 12121 ± 9795 (554–28060) | .83 |
| HBeAg, n patients (%) | 7/41 (17) | 5/20 (25) | 2/14 (14) | .45 |
| Anti-HBs, n patients (%) | 0/42 (0) | 0 (0) | 0 (0) | |
| Anti-HBc, n patients (%) | 44/44 (100) | 20/20 (100) | 17/17 (100) | |
| Anti-HBe, n patients (%) | 29/41 (71) | 12/20 (60) | 12/15 (80) | .21 |
| HBV DNA viremia, n patients (%) | 26/49 (53) | 7/22 (32) | 13/20 (65) | .03 |
| HBV DNA levels, IU/mL, median ± SD (range) | 16 ± 2578473 (0–17860000) | 0 ± 217813 (0–217813) | 84 ± 4095899 (0–17860000) | .09 |
| HDV RNA levels, IU/mL, median ± SD (range) | 60800 ± 411327 (60–1983000) | 63800 ± 275451 (60–786000) | 218000 ± 554163 (60–1983000) | .85 |
| Liver cirrhosis at baseline, n patients (%) | 28 (53) | 15 (65) | 11 (48) | .23 |
| Clinical endpoints at baseline, n patients (%) | 13/52 (25) | 8 (35) | 5 (22) | .33 |
Statistical comparison between NA and IFNα-based therapy group based on ANOVA (continuous values), Chi-Squared analysis (discrete values) and Mann–Whitney U test (non-normal distributed). ALT = alanine-aminotransferase, Anti-HBc = antibody against hepatitis B core antigen, Anti-HBe = antibody against hepatitis B envelope antigen, Anti-HBs = antibody against hepatitis B surface antigen, AP = alkaline phosphatase, APRI = AST to platelet ratio index, AST = aspartate-aminotransferase, gGT = gamma-glutamyltransferase, HBeAg = hepatitis B envelope antigen, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HDV = hepatitis delta virus, IFNα = interferon alpha, INR = international normalized ratio, MELD = Model of End-Stage Liver Disease, n = number, NA = nucleos(t)ide analogues, SD = standard deviation, U/l = Units per litre.
All 53 patients were distributed into 3 treatment groups: Group 1 (n = 23, 43%) received therapies only with nucleos(t)ide analogues, group 2 (n = 23, 43%) received IFNα-based therapies (with, n = 22, or without, n = 1, concomitant or subsequent NA-therapy) and group 3 (n = 7, 13%) were untreated. Mean duration of NA-therapy was 4.2 years (range, 2.2–6.0 years) and of IFNα-based therapy was 2.6 years (range, 0.0–14.1 years). A total of 9 patients had been previously treated with IFNα and 9 with NAs. Patient characteristics of both antiviral treatment groups are found in Table 1. Detailed treatment combinations with the mean duration are shown in Table 2. Patient characteristics of untreated patients including baseline characteristics and the development of clinical endpoints during follow-up are found in Supplementary Table S1. None of the patients were co-infected with hepatitis C or human immunodeficiency virus (HIV).
Table 2.
Antiviral combinations during follow-up.
| Antiviral therapy | Number of patients (%) | Mean duration, years (range) |
| NA only, total | 23/53 (43) | 4.2 (2.2–6.0) |
| Entecavir | 10/23 (44) | 4.4 (1.0–8.2) |
| Entecavir mono | 7/10 (70) | 3.8 (1.0–7.2) |
| Entecavir + Lamivudin | 1/10 (10) | |
| Entecavir + Adefovir | 0/10 (0) | |
| Tenofovir | 15/23 (65) | 2.2 (0.3–8.2) |
| Tenofovir mono | 12/15 (80) | |
| Tenofovir + Entecavir | 2/15 (13) | |
| Tenofovir + Lamivudin | 1/15 (7) | |
| Tenofovir + Entecavir + Lamivudin | 0/15 (0) | |
| Tenofovir + Adefovir | 0/15 (0) | |
| Lamivudin | 2/23 (9) | 6.0 (5.0–7.0) |
| Lamivudin mono | 0/23 (0) | |
| IFNα-based, total (%) | 23/53 (43) | 2.6 (0.0–14.1) |
| IFNα-mono | 1/23 (4) | |
| Combination with NA | 22/23 (96) | |
| Entecavir | 5/22 (23) | |
| Entecavir + Lamivudin | 1/22 (5) | |
| Tenofovir | 11/22 (50) | |
| Tenofovir + Entecavir | 3/22 (14) | |
| Tenofovir + Lamivudin | 1/22 (5) | |
| Tenofovir + Lamivudin + Adefovir | 1/22 (5) | |
| Lamivudin | 0/22 |
IFNα = interferon alpha, mono = monotherapy, NA = nucleos(t)ide analogues.
3.2. Biochemical baseline characteristics
At baseline, AST, ALT, and gGT were elevated above the upper limit of normal in more than 85% of patients in all 3 groups (AST [85%, 45/53], ALT [89%, 47/53], gGT [85%, 45/53], Table 1, Supplementary Table S1). We observed that at baseline 38% (20/53) of patients had a platelet count below 100,000/mL, 45% (24/53) presented with an AST platelet ratio index score > 2, 36% (19/53) showed elevated bilirubin levels and 27% (9/33) showed albumin levels below the lower limit of normal (LLN). Model of End-stage Liver Disease > 10 could be confirmed in 36% (19/53).
Of note, the NA-therapy group showed at baseline significantly higher levels of bilirubin (P = .04), while albumin (P < .01), and platelet levels (P = .03) were lower compared to IFNα-treated. Additionally, the Model of End-Stage Liver Disease (P = .04) and the Child Pugh score (P = .02) were significantly higher in NA-treated patients (Table 1). Hence, NA-treated patients had the most advanced stage of liver disease at baseline. In addition, we could see that biochemical outcome during follow-up was insufficient in NA, as well as in IFNα-treated patients (Fig. 2).
Figure 2.
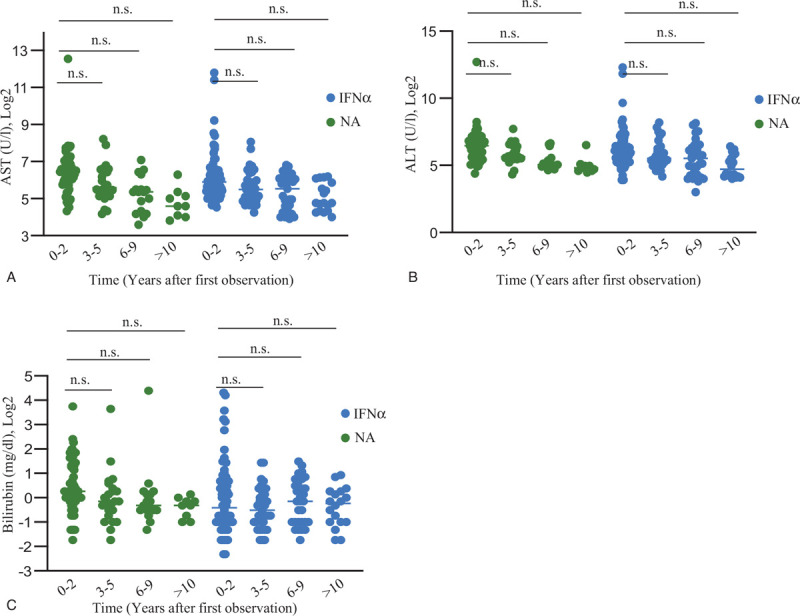
(A) Course of AST during follow-up in NA and IFNα therapy compared to baseline (B) Course of ALT during follow-up in NA and IFNα therapy compared to baseline. (C) Course of bilirubin during follow-up in NA and IFNα therapy compared to baseline. Data are presented as mean and plotted in Log2 scale. ALT = alanine-aminotransferase, AST = aspartate-aminotransferase, IFNα = interferon alpha, NS. = not significant (two-tailed Student's t-tests), NA = nucleos(t)ide analogues.
3.3. Clinical long-term outcome
Liver cirrhosis at baseline was present in more than half of the patients (53%, 28/53). Group 1 (NA therapy) showed 65% (15/23), group 2 (IFNα-based therapy) 48% (11/23) of cirrhotic patients at baseline (Table 1). From the 25 non-cirrhotic patients at baseline, 9 of them (36%) progressed to liver cirrhosis during follow-up (group 1, n = 5; group 2, n = 3, group 3, n = 1). At last available observation, it was evident that 62% (33/53) of all patients had liver cirrhosis, most of them were treated with NA-therapy. Using Chi-Squared analysis, NA-treated patients showed more often liver cirrhosis during follow-up compared to IFNα-treated group (NA vs. IFNα P = .01). In univariate regression analysis, we could confirm that NA-therapy is associated with developing liver cirrhosis (P = .01; OR = 5.43; CI = 1.49–19.82) during follow-up. Of all patients (one patient had no data about clinical endpoints), 19% (10/52) had already developed a clinical endpoint before first observation (mean follow-up of 1.4 years before first observation; range, 0.1–9.0 years), all of them evolved hepatic encephalopathy. Three patients (3/52) had a clinical endpoint (hepatic decompensation) at day of first observation. During follow-up, 44% (23/52) of all patients developed liver-related clinical endpoint with a mean duration of 3.8 years (range, 0.0–14.1 years). 31% (16/52) presented more than 1 clinical endpoint. Hepatic decompensation occurred in 42% of (22/52) patients with a mean duration of 1.0 year (range, 0.0–12.5 years), with ascites being the leading symptom in 17 cases (33%, 17/52), hepatic encephalopathy in 14 (27%, 14/52) cases and variceal bleeding in 7 (13%, 7/52) cases. HCC could be detected in this cohort only in 3 patients (6%, 3/52) after a mean follow-up of 6.3 years (range, 0.0–12.5 years). For 27% patients (14/52), a liver transplantation was necessary after 2.6 years (range, 0.3–8.3 years). Five patients (9%, 5/52) died after 3.7 years (range, 1.6–7.0 years), all of them due to liver-related causes of death. Supplementary Table S2 illustrates the clinical endpoints during follow up in both antiviral therapy groups.
3.4. Clinical complications and antiviral treatment
In all further analysis, we excluded the untreated group due to the small number of patients (n = 7). Of note, NA-treated patients developed more often clinical endpoints than IFNα-treated patients (Chi-square analysis, NA vs. IFNα, P = .01; CI = 0.56–0.71 and Cox regression model, P = .02; HR = 0.33; CI = 0.13–0.87). In addition, clinical endpoints parameter, such as liver transplantation (NA vs IFNα, P ≥ .01; CI = 0.24–0.69), hepatic decompensation (NA vs IFNα, P = .02; CI = 0.07–0.85), including hepatic encephalopathy (NA vs IFNα, P = .001; CI = 0.06–0.45) occurred more frequent in the NA-treated group. Of all 5 liver-related death cases, 4 of them were treated with NA and 1 treated with IFNα-based therapy, although this difference was not statistically significant (NA vs IFNα, P = .16; CI = 0.02–2.10; Chi-Squared analysis). Cumulative event-free survival is described by Kaplan-Meier method, where NA-therapy was also associated with a more malign clinical outcome (Fig. 3). Due to the small number of HCC-cases (6%, 3/52) it was impossible to show any association with therapy.
Figure 3.
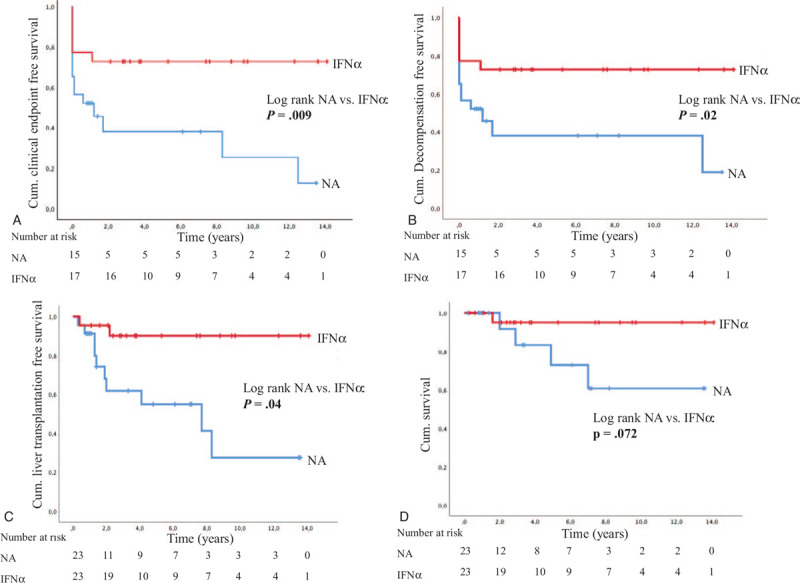
(A) Cumulative clinical endpoint-free survival of patients treated with IFNα compared to patients treated with NA. Patients treated with NA developed more often clinical endpoints compared to those treated with IFNα (P = .009). (B) Cumulative hepatic decompensation-free survival of patients treated with IFNα compared to patients treated with NA. Patients treated with NA developed more often hepatic decompensation compared to those treated with IFNα (P = .02). (C) Cumulative liver transplantation-free survival of patients treated with IFNα compared to patients treated with NA. In patients treated with NA liver transplantation was more often necessary than IFNα-treated patients (P = .004). (D) Cumulative death-free survival of patients treated with IFNα compared to patients treated with NA. There were no statistically significant differences in death rates along both treatment groups (P = .072). cum = cumulative, IFNα = interferon alpha, NA = nucleos(t)ide analogues.
Furthermore, NA-therapy was independently associated with a more severe clinical outcome in univariate logistic regression (P = .01; OR = 4.92; CI = 1.51–16.01) and Cox regression analysis (P = .01; HR = 3.01; CI = 1.27–7.18). In comparison, IFNα-based therapy showed a more beneficial clinical long-term outcome in univariate logistic regression (P = .04; OR = 0.29; CI = 0.89–0.94) (Table 3), although in multivariable logistic regression it was not statistically significant.
Table 3.
Parameters associated with clinical long-term outcome.
| Parameter | Univariate regression analysis (Significance) | Multivariable regression analysis† (Significance) |
| AST/ALT ratio | P = .01; OR = 1.23; CI = 1.05–1.44 | Not significant |
| INR | P = .04; OR = 1.38; CI = 1.01–1.88 | Not significant |
| IFNα-based therapy (yes vs no) | P = .04; OR = 0.29; CI = 0.89–0.94 | Not significant |
| NA-therapy (yes vs no) | P = .01; OR = 4.92; CI = 1.51–16.01 | Not significant |
| Liver cirrhosis (yes vs. no) | P = .001; OR = 7.44; CI = 2.16–25.62 | Not significant |
| HBV DNA (yes vs no) | P = .05; OR = 0.30; CI = 0.09–0.99 | Not significant |
| Albumin, g/dl | P = .04; OR = 0.04; CI = 0.00–0.34 | P = .01; OR = 0.71; CI = 0.55–0.91 |
| Platelets, 1000/nl | P = .002; OR = 0.98; CI = 0.97–0.99 | P = .05; OR = 0.98; CI = 0.96–1.00 |
All parameters with P < .05 were considered for multivariable regression analysis. Nonsignificant parameters included in analysis were bilirubin, AST, ALT and gGT. ALT = alanine-aminotransferase, AST = aspartate-aminotransferase, CI = confidence interval, HBV = hepatitis B virus, IFNα = interferon alpha, INR = international normalized ratio, NA = nucleos(t)ide analogues, OR = odds ratio.
3.5. Virological responses
During follow-up, loss of HBsAg occurred in only 9% (5/53) of all patients, 3 of them were treated with NA (mean duration of 2.4 years; range, 0.8–3.4 years; 1/3 tenofovir + lamivudin, 1/3 entecavir, 1/3 entecavir + tenofovir), one of them was treated with IFNα-based therapy (loss after 7.6 years) and one was untreated (loss after 9.4 years) (Fig. 4). HBsAg relapse was seen in 2 cases, one was with IFNα-based therapy (HBsAg stayed positive), one was untreated (HBsAg became undetectable again during follow-up). Given that loss HBsAg was rarely achieved, we further studied whether a 50% decline of HBsAg was obtained. In total, 42% (22/53) of all patients could reach this aim. IFNα-treated cohort showed more often an HBsAg response (61%, 14/23, after 2.8 years, range, 0.7–8.5 years; 1 patients’ HBsAg increased again after 17 months) compared to NA-treated patients (30%, 7/23; after 3.6 years, range, 0.8–8.5 years; 1 patients’ HBsAg increased again after 13 months) and untreated patients (14%, 1/7).
Figure 4.
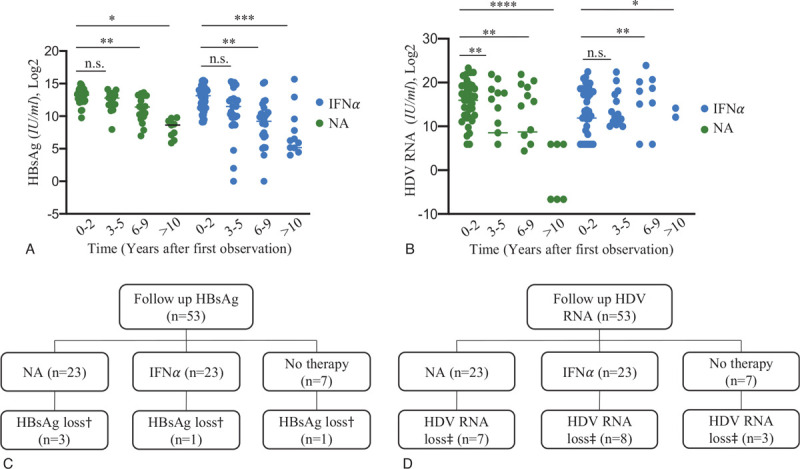
(A) Course of HBsAg during follow-up in both antiviral treatment groups. Data are presented as mean (two-tailed Student's t-test) and plotted in Log2 scale. (B) Course of HDV RNA during follow-up in both antiviral treatment groups. Data are presented as mean (F-test) and plotted in Log2 scale. (C) Loss of HBsAg during follow-up according the 3 treatment groups. Undetectability of HBsAg could not show statistical significance, mostly due to the small number of cases. (D) HDV undetectability according the 3 treatment groups along follow-up. There was no significant difference in the achievement of HDV-RNA loss observed. Statistical significance, ∗P < .05; ∗∗P < .001; ∗∗∗P < .001; ∗∗∗∗P < .0001. †, loss of HBsAg at last observation; ‡, loss of HDV RNA at last observation. IFNα = interferon alpha, NA = nucleos(t)ide analogues.
HDV RNA became undetectable in only 34% (18/53) of all patients during follow-up after 3.6 years (range, 0.3–13.5 years; Fig. 4). Seven patients were treated with NA (loss after 5.0 years, range, 0.6–13.5 years; 2/7 received entecavir [entecavir + lamivudin, entecavir monotherapy], 4/7 tenofovir [3/4 tenofovir monotherapy, 1/4 tenofovir + lamivudin], 1/7 entecavir + tenofovir; all NA-patients lost HDV RNA during therapy). Eight patients had IFNα-based therapy (mean duration of 2.9 years, range 0.3–7.6 years). Three patients were untreated (mean duration of 2.2 years, range, 0.6–4.5 years; Fig. 4). Furthermore, HDV RNA relapse was seen in 3 patients, 1 patient received NA-based therapy, 2 patients received IFNα-based therapy. There was 1 patient who developed clinical endpoints (hepatic encephalopathy and HCC) 6.4 years after loss of HDV RNA, during NA-therapy (entecavir + lamivudin). Negativity of both HDV RNA and HBsAg during follow-up was observed in only 3 cases (6%), 2 receiving NA-therapies, one was untreated. The course of HBsAg and HDV RNA can be found in Figure 4.
3.6. Virological response and clinical long-term outcome
Neither HBsAg nor HDV RNA loss could be significantly linked to a beneficial clinical long-term outcome independent of treatment (Chi-square analysis: HBsAg, P = .39; OR = 2.25; CI = 0.34–14.83; HDV RNA, P = .59; OR = 0.73; CI = 0.22–2.39; Kaplan-Meier analysis: HBsAg, P = .50; HDV RNA, P = .32; univariate Cox-regression analysis HBsAg, P = .54; HR = 1.47; CI = 0.43–5.03; HDV RNA, P = .36; HR = 0.64; CI = 0.24–1.68). By testing the virological parameters in univariate regression analysis, loss of HBsAg (P = .44; OR = 1.91; CI = 0.37–9.87) was not associated with a favorable clinical long-term outcome.
3.7. Factors associated with clinical long-term outcome
In univariate logistic regression the AST/ALT ratio (P = .01; OR = 1.23; CI = 1.05–1.44), INR (P = .04; OR = 1.38; CI = 1.01–1.88), presence of liver cirrhosis at baseline (P = .001; OR = 7.44; CI = 2.16–25.62) and NA-therapy (P = .01; OR = 4.92; CI = 1.51–16.01) were associated with a more severe clinical outcome. Furthermore, higher albumin levels (P = .04; OR = 0.04; CI = 0.00–0.34), higher platelet counts (P = .002; OR = 0.98; CI = 0.97–0.99) and IFNα-based therapy (P = .04; OR = 0.29; CI = 0.89–0.94) indicated a more beneficial clinical long-term outcome. A multivariate logistic regression analysis was performed containing all above-mentioned explanatory variables. Only albumin levels (P = .001; OR = 0.71; CI = 0.55–0.91) and platelet count (P = .05; OR = 0.98; CI = 0.96–1.00) remained significantly associated with a favorable outcome. All factors are summarized in Table 3.
4. Discussion
In this retrospective study, we analyzed the clinical and virological outcome of nucleos(t)ide analogue therapy and IFNα-based therapy in HBV/HDV co-infected patients, who were observed for a median period of 4.6 years at a single center. Our study confirms that CHD represents the most serious form of viral hepatitis with high rates of liver-related complications. We could show that (I) NA therapy alone was not associated with any obvious clinical and virological benefits and may even be associated with a worse clinical outcome with high risks of developing clinical endpoints, (II) IFNα-based treated patients showed a better clinical outcome and furthermore, (III) liver cirrhosis, low levels of albumin and platelets at baseline are predictors of a progressive liver disease.
The role of NA therapy in CHD is a matter of debate, since administration of NAs seems to be ineffective in treating CHD. However, studies from Spain and Switzerland, evaluating cohorts of HIV/HBV/HDV triple-infected patients who have been exposed to an extended treatment of tenofovir for 54 months, suggested that long-term treatment of NAs might be beneficial.[32,33,36] Possible explanations of this benefit could be due to immunomodulatory effects, as tenofovir was found to induce interferon lambda.[37,38] Another factor might be extended period of tenofovir treatment. In contrast, our study could not show a significant better long-term clinical outcome, even with long-term therapy of NAs (4.2 years).
We observed that patients treated with NA therapies only, either in monotherapy or with concomitant or subsequent NAs, developed a progressive liver disease during follow-up but it has to be mentioned that 65% of the patients being treated with NA had already liver cirrhosis at baseline. Almost 2 third of NA-treated patients developed clinical endpoints: 52% decompensated during follow-up, 43% needed a liver transplantation and 17% died due to liver-related complications. Importantly, NA therapy was significantly associated with a worse clinical long-term outcome with a rather high OR of 4.92. In a report by Brancaccio et al with 56 patients with CHD not eligible for PEG-IFNα therapy, it was observed that even after prolonged suppression of HBV DNA, which should be a main goal of NA-treatment, HDV patients maintained an increased risk of severe clinical events as compared to HBV mono-infected patients.[39] These studies do not answer the question if long-term NA therapy could have been even harmful effects in some individuals with advanced liver disease. Prospective long-term clinical studies with NA treatment would be needed to address this question.
The number of HCC cases was rather low in our cohort, as only 3 patients developed HCC during follow-up. This observation may support the hypothesis that HDV has only minor direct oncogenic effects[10] and that HCC development is mainly due to more rapid progression to liver cirrhosis.[40] Still, a recent meta-analysis described an increased HCC risk for patients with HDV infection in more decent studies (published after 2010) and, in particular, in Asian cohorts.[41] Our data indicate that hepatic decompensation, and not HCC, is the dominant complication of cirrhosis which is in line with several previous reports.[8,10,16,40,42] HDV viremia at study inclusion has been associated with the development of liver-related complications in different cohorts,[12,43] which supports the high number of clinical events in our study as all of our patients were HDV RNA positive at inclusion.
Given that suppression of HDV RNA and HBsAg are the main goals in treatment of CHD, none of the antiviral therapies applied showed satisfactory effects. Less than 10% of our patients experienced a loss of HBsAg and only one third reached undetectable HDV RNA during follow-up (Fig. 4). Still, a 50% decline in quantitative HBsAg levels was achieved in 42% of patients, with more frequent declines in IFNα-treated patients. Overall, our findings on low rates of HBsAg declines during NA therapy are in line with a report from Switzerland analyzing HIV-positive patients with HBV/HDV coinfection.[36]
In contrast to other reports, both, loss HBsAg and HDV RNA could not correlate with a favorable clinical outcome, neither in univariable nor in multivariate analysis. However, the number of patients achieving the virological endpoints was very small and a larger sample size would be required to address this issue in more detail. Moreover, on might suggest that, once an individual has already entered an advanced and severe stage of CHD, the suppression of HDV RNA or loss of HBsAg could not prevent the development of clinical endpoints.
It is known, that suppression of viral replication by interferon-based therapies in patients with hepatitis B or hepatitis C virus infection is associated with a reduction of fibrosis progression[44,45] and an improved course of liver disease. Our data suggests similar effects in HDV-patients treated with IFNα. We detected that the rates of hepatic decompensation, the need for liver transplantation and deaths were significantly lower compared to NA-treated patients. Univariable analysis confirms these effects with a beneficial clinical outcome in IFNα-treated with an OR of 0.29, a ratio similarly to the one reported in the study of Wranke et al (OR of 0.25).[10] In line with other studies, treatment with IFNα might be useful as it has been shown that HDV RNA suppression or only reduction might be associated with an improved clinical long-term outcome.[10–12,21,22,46,47]
In our hepatitis delta cohort, liver cirrhosis, low albumin and platelet levels at baseline were associated with the development of clinical endpoints by univariable, and additionally in multivariate analysis, for platelet and albumin levels. These data are comparable reported in previous studies.[10,14,21,40] Furthermore, we have used the baseline anticipation score (BEA) score[14] to distinguish patients with low, moderate and severe risk of clinical disease progression. Our results showed that 24% of patients from low risk group (BEA-A), 33% from the moderate risk group (BEA-B) and 92% from the severe risk group (BEA-C) developed clinical events, percentages which are higher than shown in the original publication[14] or other cohorts,[47] indicating a more severe course of liver disease in our patients.
The analysis was performed retrospectively, which yet entailed the advantage on being based on real-life practice. Moreover, we applied strict and well-defined inclusion criteria. Nevertheless, the current study had obvious limitations. The overall number of patients and in the analyzed subgroups was rather low. Of importance, our study was therefore not able to show differences between the different NA combinations, for example tenofovir and entecavir, as the number of the analyzed subgroups were limited. More than half of the patients had already liver cirrhosis at baseline and were in an advanced stage of liver disease, which could also explain why the rate of clinical events in our HDV cohort was higher than in other studies. IFNα-based therapy was started mainly in patients with compensated liver disease, whereas NAs were administered to patients with advanced liver disease without other treatment options. The findings of an impaired outcome in NA-treated patients may therefore be biased by the fact that NA treatment was administered to especially those with an advanced liver disease. Hence, further studies with a larger number of patients are necessary to rule out this selection bias. Moreover, virological and biochemical values were not completely available for all patients at each timepoint during the observation period.
It has been noted that this cohort of patients differed from the one's in other recent retrospective cohort studies. The median age in our cohort was 48 years, thereby the patients were older than in previous trials,[10,12,13] which in fact could also support the thesis that our cohort had a more severe course of CHD. Unfortunately, we could neither trail the region of origin in our patients nor the HDV genotype, therefore important information has been lost, as the HDV genome itself may be responsible for different outcomes among the chronically HDV-infected population. Nevertheless, studies have showed that the prominent genotype in Germany is in over 90% genotype 1.[48] Also, factors, including comorbidities and demographics were not taken into consideration.
In conclusion, our study demonstrates the high morbidity of HBV/HDV-co-infected patients in a German cohort with high rates of liver cirrhosis and clinical events. Moreover, we show that antiviral treatment with NA alone is not sufficient to improve biochemical parameters and is, additionally associated with a higher likelihood of clinical disease progression compared to IFNα-treated patients. This highlights the limitation of NA therapy in advanced liver disease. As the known treatments fail to cure HBV and HDV, targeted treatment is highly warranted.
Acknowledgments
We thank Dr. rer. nat. Nils Lehmann, Institute for Medical Informatics, Biometry and Epidemiology, University Hospital Essen, for statistical consultation and attendance.
Author contributions
Conceptualization: Heiner Wedemeyer, Katja Deterding.
Data curation: Laura Scheller, Gudrun Hilgard, Olympia Anastasiou, Ulf Dittmer, Alisan Kahraman, Katja Deterding.
Formal analysis: Laura Scheller.
Investigation: Olympia Anastasiou, Ulf Dittmer, Alisan Kahraman, Katja Deterding.
Methodology: Laura Scheller, Katja Deterding.
Project administration: Heiner Wedemeyer, Katja Deterding.
Resources: Ulf Dittmer, Katja Deterding.
Supervision: Heiner Wedemeyer, Katja Deterding.
Validation: Heiner Wedemeyer, Katja Deterding.
Visualization: Laura Scheller, Katja Deterding.
Writing – original draft: Laura Scheller, Heiner Wedemeyer, Katja Deterding.
Writing – review & editing: Heiner Wedemeyer, Katja Deterding.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ALT = alanine-aminotransferase, AST = aspartate-aminotransferase, BEA = baseline anticipation score, CHD = chronic hepatitis D (delta), CI = confidence interval, gGT = gamma-glutamyltransferase, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HDV = hepatitis D (delta) virus, HIV = human immunodeficient virus, HR = hazard ratios, IFNα = interferon alpha, INR = international normalized ratio, NA = nucleos(t)ide analogues, OR = odds ratio, PEG-IFNα = pegylated interferon alpha.
How to cite this article: Scheller L, Hilgard G, Anastasiou O, Dittmer U, Kahraman A, Wedemeyer H, Deterding K. Poor clinical and virological outcome of nucleos(t)ide analogue monotherapy in HBV/HDV co-infected patients. Medicine. 2021;100:28(e26571).
Lecture fees from AbbVie, Biotest, BMS, Intercept, Janssen, MSD/Merck; research grants from Abbvie, Biotest, BMS, Gilead, Hexal, Merck/MSD, Novartis Roche; consulting from Abbott, Abbvie, Altimmune, Biotest, BMS, BTG, Dicerna, Gilead, Janssen, MYR GmbH, Novartis, Roche, Siemens; all unrelated to the present work.
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Rizzetto M. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 1977;18:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Farci P. Delta hepatitis: an update. J Hepatol 2003;39:212–9. [DOI] [PubMed] [Google Scholar]
- [3].Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet 2011;378:73–85. [DOI] [PubMed] [Google Scholar]
- [4].Zhang Z, Urban S. New insights into hepatitis D virus persistence: the role of interferon response and implications for upcoming novel therapies. J Hepatol 2020;74:686–99. [DOI] [PubMed] [Google Scholar]
- [5].Stockdale AJ. The global prevalence of hepatitis D virus infection: systematic review and meta-analysis. J Hepatol 2020;73:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fattovich G. Influence of hepatitis delta virus infection on progression to cirrhosis in chronic hepatitis type B. J Infect Dis 1987;155:931–5. [DOI] [PubMed] [Google Scholar]
- [7].Fattovich G. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut 2000;46:420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Niro GA. Outcome of chronic delta hepatitis in Italy: a long-term cohort study. J Hepatol 2010;53:834–40. [DOI] [PubMed] [Google Scholar]
- [9].Buti M. Clinical outcome of acute and chronic hepatitis delta over time: a long-term follow-up study. J Viral Hepat 2011;18:434–42. [DOI] [PubMed] [Google Scholar]
- [10].Wranke A. Antiviral treatment and liver-related complications in hepatitis delta. Hepatology 2017;65:414–25. [DOI] [PubMed] [Google Scholar]
- [11].Yurdaydin C. Interferon treatment duration in patients with chronic delta hepatitis and its effect on the natural course of the disease. J Infect Dis 2018;217:1184–92. [DOI] [PubMed] [Google Scholar]
- [12].Kamal H. Long-term study of hepatitis D infection at secondary care centers: the impact of viremia on liver-related outcomes. Hepatology 2020;72:1177–90. [DOI] [PubMed] [Google Scholar]
- [13].Spaan M. Hepatitis delta genotype 5 is associated with favourable disease outcome and better response to treatment compared to genotype 1. J Hepatol 2020;72:1097–104. [DOI] [PubMed] [Google Scholar]
- [14].Calle Serrano B. Development and evaluation of a baseline-event-anticipation score for hepatitis delta. J Viral Hepat 2014;21:e154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roulot D. Origin, HDV genotype and persistent viremia determine outcome and treatment response in patients with chronic hepatitis delta. J Hepatol 2020;73:1046–62. [DOI] [PubMed] [Google Scholar]
- [16].Romeo R. A 28-year study of the course of hepatitis (infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 2009;136:1629–38. [DOI] [PubMed] [Google Scholar]
- [17].Le Gal F. Genetic diversity and worldwide distribution of the deltavirus genus: a study of 2,152 clinical strains. Hepatology 2017;66:1826–41. [DOI] [PubMed] [Google Scholar]
- [18].Wedemeyer H. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med 2011;364:322–31. [DOI] [PubMed] [Google Scholar]
- [19].Wedemeyer H. Peginterferon alfa-2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT-II): a randomised, placebo controlled, phase 2 trial. Lancet Infect Dis 2019;19:275–86. [DOI] [PubMed] [Google Scholar]
- [20].Heidrich B, Manns MP, Wedemeyer H. Treatment options for hepatitis delta virus infection. Curr Infect Dis Rep 2013;15:31–8. [DOI] [PubMed] [Google Scholar]
- [21].Wranke A. Ten-year follow-up of a randomized controlled clinical trial in chronic hepatitis delta. J Viral Hepat 2020;27:1359–68. [DOI] [PubMed] [Google Scholar]
- [22].Heidrich B. HBeAg-positive hepatitis delta: virological patterns and clinical long-term outcome. Liver Int 2012;32:1415–25. [DOI] [PubMed] [Google Scholar]
- [23].Lok AS. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology 2016;63:284–306. [DOI] [PubMed] [Google Scholar]
- [24].Schweitzer A. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386:1546–55. [DOI] [PubMed] [Google Scholar]
- [25].Terrault NA. AASLD guidelines for treatment of chronic hepatitis B. Hepatology (Baltimore, Md) 2016;63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wong DK-H. Reduction of hepatitis B surface antigen and covalently closed circular DNA by nucleos (t) ide analogues of different potency. Clin Gastroenterol Hepatol 2013;11:1004–10. e1. [DOI] [PubMed] [Google Scholar]
- [27].Yurdaydin C. Famciclovir treatment of chronic delta hepatitis. J Hepatol 2002;37:266–71. [DOI] [PubMed] [Google Scholar]
- [28].Gunsar F. Two-year interferon therapy with or without ribavirin in chronic delta hepatitis. Antivir Ther 2005;10:721–6. [PubMed] [Google Scholar]
- [29].Yurdaydin C. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + interferon vs interferon. J Viral Hepat 2008;15:314–21. [DOI] [PubMed] [Google Scholar]
- [30].Niro GA. Lamivudine therapy in chronic delta hepatitis: a multicentre randomized-controlled pilot study. Aliment Pharmacol Ther 2005;22:227–32. [DOI] [PubMed] [Google Scholar]
- [31].Kabaçam G. Entecavir treatment of chronic hepatitis D. Clin Infect Dis 2012;55:645–50. [DOI] [PubMed] [Google Scholar]
- [32].Soriano V. Efficacy of prolonged tenofovir therapy on hepatitis delta in HIV-infected patients. AIDS 2014;28:2389–94. [DOI] [PubMed] [Google Scholar]
- [33].Sheldon J. Does treatment of hepatitis B virus (HBV) infection reduce hepatitis delta virus (HDV) replication in HIV-HBV-HDV-co-infected patients? Antivir Ther 2008;13:97–102. [PubMed] [Google Scholar]
- [34].European Association for the Study of the Liver. Electronic address, e.e.e. and L. European Association for the Study of the, EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. [DOI] [PubMed] [Google Scholar]
- [35].Cornberg M. Aktualisierung der S 3-leitlinie zur prophylaxe, diagnostik und therapie der hepatitis-B-virusinfektion. Zeitschrift für Gastroenterologie 2011;49:871–930. [DOI] [PubMed] [Google Scholar]
- [36].Beguelin C. Impact of tenofovir on hepatitis delta virus replication in the swiss human immunodeficiency virus cohort study. Clin Infect Dis 2017;64:1275–8. [DOI] [PubMed] [Google Scholar]
- [37].Murata K. Induction of IFN-lambda3 as an additional effect of nucleotide, not nucleoside, analogues: a new potential target for HBV infection. Gut 2018;67:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Murata K. Immunomodulatory mechanism of acyclic nucleoside phosphates in treatment of hepatitis B virus infection. Hepatology 2020;71:1533–45. [DOI] [PubMed] [Google Scholar]
- [39].Brancaccio G. Clinical outcomes in patients with hepatitis D, cirrhosis and persistent hepatitis B virus replication, and receiving long-term tenofovir or entecavir. Aliment Pharmacol Ther 2019;49:1071–6. [DOI] [PubMed] [Google Scholar]
- [40].Bockmann JH. High rates of cirrhosis and severe clinical events in patients with HBV/HDV co-infection: longitudinal analysis of a German cohort. BMC Gastroenterol 2020;20:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Alfaiate D. Chronic hepatitis D and hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. J Hepatol 2020;73:533–9. [DOI] [PubMed] [Google Scholar]
- [42].Farci P. Long-term benefit of interferon α therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology 2004;126:1740–9. [DOI] [PubMed] [Google Scholar]
- [43].Romeo R. High serum levels of HDV RNA are predictors of cirrhosis and liver cancer in patients with chronic hepatitis delta. PLoS One 2014;9:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].van der Meer AJ. Life expectancy in patients with chronic HCV infection and cirrhosis compared with a general population. JAMA 2014;312:1927–8. [DOI] [PubMed] [Google Scholar]
- [45].Viganò M, Lampertico P. Hepatitis B virus treatment: which patients should be treated with nucleos (t) ide analogue? Clinical Liver Disease 2013;2:21–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Manesis EK. Prevalence and clinical course of hepatitis delta infection in Greece: a 13-year prospective study. J Hepatol 2013;59:949–56. [DOI] [PubMed] [Google Scholar]
- [47].Palom A. Long-term clinical outcomes in patients with chronic hepatitis delta: the role of persistent viraemia. Aliment Pharmacol Ther 2020;51:158–66. [DOI] [PubMed] [Google Scholar]
- [48].Wranke A. Clinical and virological heterogeneity of hepatitis delta in different regions world-wide: the Hepatitis Delta International Network (HDIN). Liver Int 2018;38:842–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


