Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, coronavirus disease 2019, mechanical ventilation, mortality, noninvasive ventilation, venovenous extracorporeal membrane oxygenation
Abstract
OBJECTIVES:
Implantation of venovenous extracorporeal membrane oxygenation as an alternative to invasive mechanical ventilation, an “awake approach,” may facilitate a lung- and diaphragm-protective ventilatory strategies without the associated harms of endotracheal intubation, positive pressure ventilation, and continuous sedation. This report presents the characteristics and outcomes of the patients treated with the awake venovenous extracorporeal membrane oxygenation approach.
DESIGN:
Retrospective case series.
SETTING:
Monocenter study.
PATIENTS:
Severe acute respiratory syndrome coronavirus 2 patients with acute respiratory failure treated with venovenous extracorporeal membrane oxygenation instead of invasive mechanical ventilation from March 2020 to March 2021.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Physiologic and laboratory data were collected at admission to the ICU, prior to and after venovenous extracorporeal membrane oxygenation implantation, and at decannulation. Seven patients were treated with venovenous extracorporeal membrane oxygenation instead of invasive mechanical ventilation due to hypoxemia with a median Pao2/Fio2 ratio at implantation of 76 (interquartile range, 59–92). Four patients in the awake group subsequently required invasive mechanical ventilation, and only one patient (14.3%) died. There were no significant complications attributed venovenous extracorporeal membrane oxygenation.
CONCLUSIONS:
This report demonstrates that in a selected group of patients, an “awake” venovenous extracorporeal membrane oxygenation approach is feasible and may result in favorable outcomes.
A complication of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is the acute respiratory distress syndrome (ARDS) (1). In the most extreme scenarios, where patients have either severe hypoxemia or hypercapnia that persist, despite lung-protective mechanical ventilation, neuromuscular blockade, and prone positioning, venovenous extracorporeal membrane oxygenation (VV-ECMO) can be used to improve gas exchange while maintaining lung rest (2).
Using VV-ECMO instead of invasive mechanical ventilation (IMV) in selected patients may facilitate lung- and diaphragm-protective ventilatory strategies without the harmful effects of endotracheal intubation, positive pressure ventilation, and continuous sedation (3). Whether VV-ECMO can effectively prevent the need for IMV altogether in the setting of coronavirus disease 2019 (COVID-19) ARDS remains uncertain. Identifying patients who potentially benefit from this therapeutic approach challenges contemporary indications for the use of VV-ECMO.
This report presents the clinical characteristics and outcomes of patients with SARS-CoV-2–associated respiratory failure treated with “awake” VV-ECMO in an effort to avoid IMV.
MATERIALS AND METHODS
A retrospective cohort study of COVID-19 patients admitted to the ICU at the Royal Victoria Hospital, Montreal, QC, Canada, who required support with VV-ECMO due to acute respiratory failure from March 2020 to March 2021 was performed. The McGill University Health Center research ethics board approved the study protocol (approval number p2021-7217), and informed consent was waived due to the retrospective nature of the analysis. Patients who had a diagnosis of COVID-19 and were supported with VV-ECMO instead of IMV were included.
VV-ECMO support was initiated when the patients had signs of respiratory failure that would mandate IMV such as oxygen saturation (Spo2) less than 90%, Fio2 greater than 70%, or respiratory rate (RR) greater than 35 breaths/min. These patients had clinical presentation consistent with moderate-to-severe ARDS on the basis of Pao2/Fio2 (P/F) ratio, clinical presentation, and imaging. The decision to initiate an awake ECMO approach was adjudicated on a case-by-case basis by a multidisciplinary team. Factors taken into consideration were past medical history, body habitus, duration of hospitalization and state of deconditioning, severity of radiological finding, baseline functional status, and ability to cooperate and consent to treatment. The treatment approach, risks, and benefits with either VV-ECMO or mechanical ventilation were explained to the patient or their surrogate prior to obtaining consent to treatment. Implantation was done with a bifemoral approach to avoid intubation for transesophageal echocardiography. Blood flow rate and sweep gas flow rate were adjusted to maintain a Spo2 of 85–92% and a Pco2 less than 45 mm Hg. After ECMO implantation, noninvasive positive pressure ventilation (NIPPV) was alternated with high-flow nasal oxygen (HFNO), and administration of analgo-sedation was minimized. Physiotherapy and incentive spirometry were initiated within 24–48 hours following implantation.
Demographic data, laboratory data, and mechanical ventilation variables were collected at ICU admission, prior to and after VV-ECMO implantation, and at the time of decannulation. Values associated with lowest P/F ratio within 6 hours prior to these time points were collected. Data were analyzed using the R program Version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) (4).
RESULTS
Patient Features
From March 2020 to March 2021, 55% of the 199 patients with COVID-19 admitted to the ICU required IMV. In this mechanically ventilated group, the mortality rate was 20%. Twenty-eight patients (14% of all ICU admission) were supported with VV-ECMO, of which seven patients were treated with the “awake” VV-ECMO approach (Table 1).
TABLE 1.
Patient Characteristics and Outcomes in the Awake Group
| Patient Characteristics | Awake Group, N = 7 |
|---|---|
| Male, n (%) | 4 (57.1) |
| Age, yr, median (IQR) | 55 (50.5–63) |
| Duration of symptoms prior to ICU admission, d, median (IQR) | 7 (5.5–8) |
| SOFA score at ICU admission, median (IQR)a | 4 (3–4) |
| SOFA score at ECMO cannulation, median (IQR)a | 4 (4–4) |
| Pao2/Fio2 at ICU admission, median (IQR) | 108 (97.5–288.5) |
| Pao2/Fio2 prior to ECMO cannulation, median (IQR) | 76 (59–92) |
| Paco2 at ICU admission, mm Hg, median (IQR) | 40.3 (4.2) |
| Paco2 prior to ECMO cannulation, mm Hg, median (IQR) | 41.8 (36.6–42.8) |
| Respiratory rate prior to ECMO cannulation, breaths/min, median (IQR) | 30 (28.5–41) |
| Adjunctive treatment prior to venovenous-ECMO cannulation | |
| Awake prone > 1 hr, n (%) | 5 (71.4) |
| IV corticosteroids, n (%) | 7 (100.0) |
| Tocilizumab, n (%) | 3 (42.9) |
| Duration from ICU admission to cannulation, d, median (IQR) | 2 (1.40–4.16) |
| ECMO complications, n (%) | |
| Bleeding | 2 (28.6) |
| Ventilator-acquired pneumonia | 3 (42.9) |
| Septic shock | 3 (42.9) |
| Acute renal failure requiring renal replacement therapy | 1 (14.3) |
| Outcomes | |
| Duration of ECMO support, d, median (IQR) | 14.8 (9.2–28.3) |
| Duration of mechanical ventilation, d, median (IQR) | 8 (0–34.5) |
| Duration of ICU stay, d, median (IQR) | 27 (14–44.5) |
| Ventilator-free days at 28 d, median (IQR) | 20 (0–28) |
| ECMO decannulation, n (%) | 6 (85.7) |
| ICU mortality, n (%) | 1 (4.3) |
ECMO = extracorporeal membrane oxygenation, IQR = interquartile range, SOFA = Sequential organ failure assessment score.
aPoints for mechanical ventilation were not added to the respiratory component of the SOFA score.
Prior to ECMO implantation, all patients were asked to self-prone and received corticosteroids and oxygenation support with HFNO or NIPPV. All patients selected for this approach were cooperative and did not exhibit signs of encephalopathy. Two patients had neuromuscular disease (myasthenia gravis and myotonic dystrophy type I), where endotracheal intubation would have resulted in prolonged ICU stay. One patient refused endotracheal intubation. Four patients were selected due to anticipation of poor outcome if invasively ventilated due to high body mass index or extent of pulmonary disease (Supplemental Table 1, http://links.lww.com/CCX/A722).
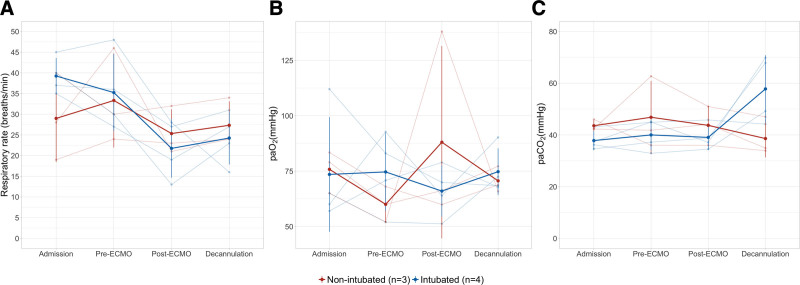
Oxygenation was maintained, and arterial Co2 was normalized upon initiation of VV-ECMO with a reduction of RR (Fig. 1). The median duration of ECMO support was 15 days (interquartile range [IQR], 9–28 d). Six patients received passive range of motion exercise and chest physiotherapy on days 2–4 after VV-ECMO implantation.
Figure 1.
Respiratory variables at admission, extracorporeal membrane oxygenation (ECMO) cannulation, after ECMO cannulation and prior to ECMO decannulation (A) respiratory rate (breaths/min), (B) arterial oxygen (mm Hg), (C) arterial Co2 (mm Hg).
Outcomes
Four patients were subsequently intubated at a median of 6 days (IQR, 2.5–9 d) after ECMO implantation due to worsening hypoxemia and delirium leading to poor secretion clearance. The median P/F ratio, Paco2, and respiratory system compliance at intubation were 69 (IQR, 65.4–87.4), 45.1 (IQR, 43.1–49.8), and 8 mL/cm H2O (IQR, 6.75–11 mL/cm H2O). In this group, one patient died from ventilator-associated pneumonia and septic shock after 32 days on VV-ECMO support. Three patients had acute kidney injury, and only one patient required dialysis. The only VV-ECMO–related complication was bleeding at the cannula insertion site in two patients. Six patients were discharged home after a median of 33 days of hospital stay (IQR, 18–39 d).
DISCUSSION
Main Findings
This cohort of seven patients with respiratory failure due to SARS-CoV-2 infection who were treated with VV-ECMO instead of mechanical ventilation resulted in six (85.7%) survivors. Despite subsequent IMV in four patients, the mortality rate in this awake ECMO cohort is lower than the conventional IMV group in our institution.
Rationale for Awake VV-ECMO
The rationale for the awake approach is derived from reduction of the risks associated with endotracheal intubation and preservation of safe spontaneous breathing. By avoiding IMV, there is a lower risk of ventilator-associated pneumonia, ventilator-induced lung injury, less sedation is required, the patient can participate more actively in physiotherapy, maintain a cough, and interact with family members (3). This approach has shown improved outcome in patients who require VV-ECMO in the setting of chronic lung disease as a bridge to lung transplant (5); however, there are less data in the ARDS population. A case series of 10 postoperative ARDS patients showed that intubation was avoided in seven patients with this approach (6). Another case series of six patients with ARDS from pneumonia resulted in an intubation rate of 50% (7). To date, there is only one case report of SARS-CoV-2 ARDS that was managed with the upfront awake approach (8).
Challenges of Awake VV-ECMO
Although improvement of oxygenation, normalization of Pco2, and reduction of RR were achieved after VV-ECMO implantation, inability to monitor and effectively control transpulmonary pressure to prevent further patient self-inflicted lung injury is the main limitation of this approach. Reduction of respiratory drive and effort in ARDS patients despite normalization of Pco2 with VV-ECMO has proven to be challenging due to other factors that increase respiratory drive such as lung and systemic inflammation (9, 10). Increased breathing efforts and coughing caused large swings in pleural pressure that resulted in interruption of blood flow from the femoral ECMO cannulas and frequent hypoxemia (3). In an attempt to reduce respiratory drive and effort, opioids and benzodiazepine administration resulted in delirium and derecruitment which was the main reason for intubation in this cohort. One additional challenge to this approach is case selection which is biased toward younger patients who had rapid progression of symptoms during the daytime where a multidisciplinary team could be gathered. The team’s assessment of their overall state of deconditioning, their risk of worsening on mechanical ventilation, and potential for rehabilitation played a role in case selection.
The strength of this study is that it is currently the largest published cohort of upfront VV-ECMO prior to IMV in SARS-CoV-2 respiratory failure. Being a retrospective case series, no comparison to demonstrate the benefit of this approach over conventional IMV for SARS-CoV-2 ARDS can be made.
CONCLUSIONS
This report demonstrates that an awake VV-ECMO approach is feasible and safe in a selected group of patients. Although IMV cannot be entirely avoided, good outcome is possible in almost all of the patients.
ACKNOWLEDGMENTS
We acknowledge the multidisciplinary ICU team at the Royal Victoria Hospital for their dedication during this unprecedented pandemic.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any potential conflicts of interest.
This work was performed at McGill University Health Centre at the Royal Victoria Hospital, Montreal, QC, Canada.
REFERENCES
- 1.Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med. 2021; 203:54–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: A review. JAMA. 2019; 322:557–568 [DOI] [PubMed] [Google Scholar]
- 3.Langer T, Santini A, Bottino N, et al. “Awake” extracorporeal membrane oxygenation (ECMO): Pathophysiology, technical considerations, and clinical pioneering. Crit Care. 2016; 20:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.R Core Team: R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing [computer; program; ], Vienna, Austria, 2020 [Google Scholar]
- 5.Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med. 2012; 185:763–768 [DOI] [PubMed] [Google Scholar]
- 6.Yeo HJ, Cho WH, Kim D. Awake extracorporeal membrane oxygenation in patients with severe postoperative acute respiratory distress syndrome. J Thorac Dis. 2016; 8:37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoeper MM, Wiesner O, Hadem J, et al. Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in patients with acute respiratory distress syndrome. Intensive Care Med. 2013; 39:2056–2057 [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Chambrun MPd, Lebreton G, et al. Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in a patient with severe COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021; 203:1571–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crotti S, Bottino N, Ruggeri GM, et al. Spontaneous breathing during extracorporeal membrane oxygenation in acute respiratory failure. Anesthesiology. 2017; 126:678–687 [DOI] [PubMed] [Google Scholar]
- 10.Spinelli E, Mauri T, Beitler JR, et al. Respiratory drive in the acute respiratory distress syndrome: Pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020; 46:606–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.