Abstract
Background
Mesenchymal stromal cells (MSCs) exhibit immunosuppressive effects in vitro and in vivo. Therapeutic utility of MSC transfer for immune-mediated diseases has been long drawing attention. However, the role of endogenous MSCs in immune regulation in vivo has remained largely unclear. MSCs constitute the hematopoietic stem cell (HSC) niche within the bone marrow, perhaps leading to privilege of HSCs to evade immunity. Our recent study have demonstrated that potent niche-residential regulatory T cells (Tregs) endow HSCs with immune privilege, promoting allogeneic HSC engraftment. This Treg-mediated immune privilege depends on cell-surface ectoenzymes CD39 and CD73 on niche Tregs, which generate extracellular adenosine, a nucleotide that is known to suppress immunity and potentiate Tregs. Another niche constituents, leptin receptor-expressing (lepr+) perivascular MSCs, also highly express CD39 and CD73, prompting us to study their roles in immune privilege and allogeneic HSC engraftment.
Objective:
The goal of the study is to test whether, like niche Tregs’ CD39, lepr+ MSCs’ CD39 positively regulates immune privilege of the HSC niche and promotes allogenic HSC engraftment,.
Study Design:
We examined whether conditional deletion of CD39 in lepr+ cells in lepr-cre CD39-flox mice (B6 background) inhibits niche Tregs and decreases allo-HSC engraftment. We also compared these effects of CD39 deletion in lepr+ cells from those of CD39 deletion in Tregs in FoxP3-cre CD39-flox mice (B6). Allogenic HSC engraftment was assessed after transplantation of MHC-mismatched BALB/cJ BM cells following nonmyeloablative conditioning. Statistical significance was determined using two-tailed t-test or one-way ANOVA with Bonferroni posttest.
Results:
Unexpectedly, CD39 deletion in lepr+ cells significantly increased and potentiated effector memory-like niche Tregs, promoting allogenic HSC engraftment. CD39 deletion in Tregs also significantly activated niche Tregs, while abrogating engraftment.
Conclusion:
Our observations suggest that adenosine from both Tregs and MSCs surprisingly inhibits niche Tregs, while adenosine from Tregs, but not from MSCs, acts as an effector molecule of immune privilege. This work identifies paradoxical effects of bone marrow MSCs and adenosine to activate immunity and inhibit immune privilege of the HSC niche.
Keywords: Allogenic bone marrow transplantation, Engraftment, Hematopoietic stem cell niche, Mesenchymal stromal cell, Treg, Adenosine
Introduction
A large body of evidence indicates that MSCs have immune suppressive potentials. MSC transfer showed therapeutic effects against various autoimmune disorders and graft-versus-host diseases in preclinical and clinical settings, leading to a growing interest in their clinical use.1 MSCs express various immune suppressive molecules, TGF-beta, IL-10, indoleamine 2,3-dioxygenase, and prostaglandin E2, inhibiting immune cells and expanding or generating Tregs.2 However, the role of endogenous MSCs in immune regulation in vivo remains largely unclear.
It was recently demonstrated that MSCs participate in the formation of the tissue-committed stem cell niches,3 leading to our hypothesis that MSCs contribute to rendering the niche an immunological sanctuary for stem cells, termed an immune privileged site.4,5 Immune privilege was originally proposed over 60 years ago within testis and placenta—where immune reaction is rigorously suppressed or prevented by various mechanisms, including suppressive cytokines, downregulation of major histocompatibility complex (MHC) molecules, and blood-barrier.4,5 Transplanted allogeneic (allo-) or xenogeneic grafts can survive in testis and placenta even without immune suppressive therapy, supporting the notion that these stem cell sites were immunologically distinct.4,5 In the 1970s, the hair follicle was proposed as another immune privileged site.4,5 While other tissue-committed stem cell niches have not been long examined in the immunological context, our recent studies suggest that the HSC niche acts as an immune privileged site. Unique niche-residential Tregs with high expression of an HSC marker, CD150, endow HSCs with immune privilege, enabling allo-HSC persistence in non-irradiated immune-competent mice without immune suppression.6,7 These CD150high niche Tregs frequently show effector memory T cell-like phenotype (CD44highCD62Llow) with high expression of cell-surface ectoenzymes CD39 and CD73 that tandemly act to generate extracellular adenosine, an immunosuppressive nucleotide known to suppress T cells, NK cells, and myeloid cells, and activate or generate Treg.7,8 The following three approaches: conditional deletion of CD39 in Tregs; global CD73 knockout (KO) mice; and adenosine receptor antagonist treatment, all consistently inhibited allo-HSC engraftment, indicating that adenosine from niche Tregs plays a critical role in maintenance of immune privilege.6,7
Among BM hematopoietic cell populations, CD39highCD73high cells are predominantly CD150high niche Tregs, whereas CD39highCD73high nonhematopoietic cells are frequently found within CD140a+CD140b+CD45-mesenchymal cells9 that exclusively overlap with lepr+ stromal cells, which are putative constituents of the peri-sinusoidal HSC niche.10 Medium expression levels of CD39 and CD73 by lepr+ cells were ten times lower than those by CD150high Tregs, while being equivalent to those of CD150low Tregs and 100~1000 times higher than those by the rest of mesenchymal cells.9 In addition, our previously study indicates that BM Tregs frequently form direct contact with CXCL12high stromal cells,7 which largely overlap with lepr+ cells11 that play critical roles in Treg recruitment.7 These observations prompt us to study the role of lepr+ cells in immune privilege and allo-HSC engraftment. This work demonstrates paradoxical negative regulation of immune privilege and allo-HSC engraftment by MSC-derived adenosine, further revealing previously unreported inhibitory effects of adenosine on effector memory-like niche Tregs.
Methods
Mice.
BALB/cJ mice, CD73 KO mice, and FoxP3-YFP cre mice (Jackson Laboratory) were housed in a specific pathogen-free environment. Lepr-cre mice were kindly provided by Dr. Lei Ding. CD39-flox mice were provided by Dr. Simon C. Robson. 7-week-old mice were analyzed. The mice were sacrificed by CO2 inhalation and cervical dislocation. Studies were conducted with approval from Institutional Review Boards and Animal Care and Use Committees at Columbia University.
Nonmyeloablative BM transplantation.
Age-matched Leprcre CD39fl/wt or Leprcre recipient mice (B6) were treated with a nonmyeloablative dose of total body irradiation (TBI, 3 Gy) followed by an intravenous injection of MHC-mismatched BALB/cJ BM cells (2×107/each).12 Anti-mouse CD8 mAbs (2.43; 1.44 mg/each) was injected intraperitoneally (i.p.) on day −1. On day 0, anti-mouse CD40L mAbs (MR1; 2mg/each) was injected i.p. following TBI. Anti-CD8 mAbs and anti-mouse CD40L mAbs were purchased from BioXcell.
Flow cytometry.
BM cells were isolated by crushing bones. Flow cytometry was performed using an LSRII and LSRFortessa (BD Biosciences) followed by analysis using FlowJo. We used APC/Cy7- or PE-TxRed-NK1.1, APC/Cy7- or BV510-CD3, BV605-CD4, PE/Cy7- or PE-CD150, APC-, BV786- or PerCP/Cy5.5-CD62L, AF700- or PB-CD44, PerCP/Cy5.5-H2Kb, PE-H2Dd, PerCP/Cy5.5-GITR, PE/Cy7- or APC-CD39, APC- or PB-CD73 mAbs (Biolegend), and FITC-FoxP3 and PB-PD1 mAbs (eBioscience). Intracellular FoxP3 staining was performed according to the manufacture’s protocol (ebioscience).
Statistical analysis
Statistical analyses were performed with GraphPad Prism. Statistical significance was determined using two-tailed t-test or one-way ANOVA with Bonferroni posttest. P-values lower than 0.05 were considered to be significant. All data are presented as mean ± SD.
For original data, please contact jf2819@cumc.columbia.edu.
Results and Discussion
We examined whether CD39 deletion in lepr+ cells inhibits induction of donor tolerance and allo-HSC engraftment following nonmyeloablative conditioning, as previously observed in recipients with CD39 deletion in Tregs.7Fig.4B Nonmyeloablative conditioning was employed to assess the degree of tolerance induction based on the donor chimerism; myeloablative conditioning induces powerful immunosuppression and 100% donor chimerism, thereby leading to the difficulty to assess the degree of tolerance induction. Unexpectedly, recipients with CD39 deletion in lepr+ cells showed significantly increased allo-donor blood chimerism (Figure 1A–B), which is opposite to our previous observations of decreased engraftment in recipients with CD39 deletion in Tregs, CD73 global knockout recipients, and adenosine receptor 2A (A2AR) antagonist-treated recipients. The observation suggests that, unlike Tregs’ CD39, lepr+ cells’ CD39 negatively regulates immune privilege, promoting donor rejection. Next, flow cytometric analysis was performed to assess how CD39 deletion in lepr+ cells influences CD150high niche Tregs.7 CD39 deletion in lepr+ cells did not alter BM Treg numbers, Treg frequencies in BM mononuclear cells or Treg frequencies in BM CD4 T cells (Figure 2A), while CD39 expression levels were upregulated on host BM Tregs, suggesting compensatory increase (Figure 2B). Of note, CD39 deletion in lepr+ cells significantly increased pool size of host- and donor-derived effector memory-like niche Tregs (CD44highCD62lowCD150highFoxP3+CD4+ BM Tregs) (Figures 2C–D). In control mice, these effector memory-like niche Tregs highly expressed different suppressive or costimulatory molecules, compared with other Tregs (CD150lowCD44lowCD62low BM Tregs, or lymph node (LN) Tregs) (Figure 2E). CD39 deletion in lepr+ cells further upregulated expression levels of these suppressive or costimulatory molecules by effector memory-like niche Treg (Figure 2F), suggesting potentiation of niche Treg. Pool of effector memory-like LN Tregs was not altered by CD39 deletion in lepr+ cells (Figure 2G). Effector memory-like niche Treg frequencies were consistently increased in non-transplanted Leprcre CD39fl/wt mice (Figure 2H). This increase and activation of effector memory-like niche Tregs is likely to explain the increase of allo-BM engraftment in Leprcre CD39fl/wt mice. While our previous studies demonstrated reductions of allo-BM engraftment in FoxP3cre CD39fl/wt mice and CD73 global deletion mice,7 these two models also showed expansion of effector memory-like niche Tregs without altering pool of effector memory-like LN Treg as in Leprcre CD39fl/wt mice (Figures 3A, 3B), suggesting that regardless of cell sources, adenosine inhibits effector memory-like niche Tregs. Flow cytometric analysis of BM Tregs further demonstrated that effector memory-like niche Tregs highly expressed adenosine 2A receptors (A2ARs) as compared to other BM Tregs and LN Tregs (Figure 3C). In vivo adenosine 2A receptor (A2AR) agonist treatment (PSB-0777, Tocris) decreased pool size of effector memory-like niche Treg (Figure 3D), in line with the increased effector memory-like niche Tregs in Leprcre CD39fl/wt mice, FoxP3cre CD39fl/wt mice and CD73 knockout mice. These results suggest that adenosine inhibits effector memory-like niche Tregs via A2ARs.
Figure 1. CD39 deletion in lepr+ cells promoted allo-HSC engraftment.
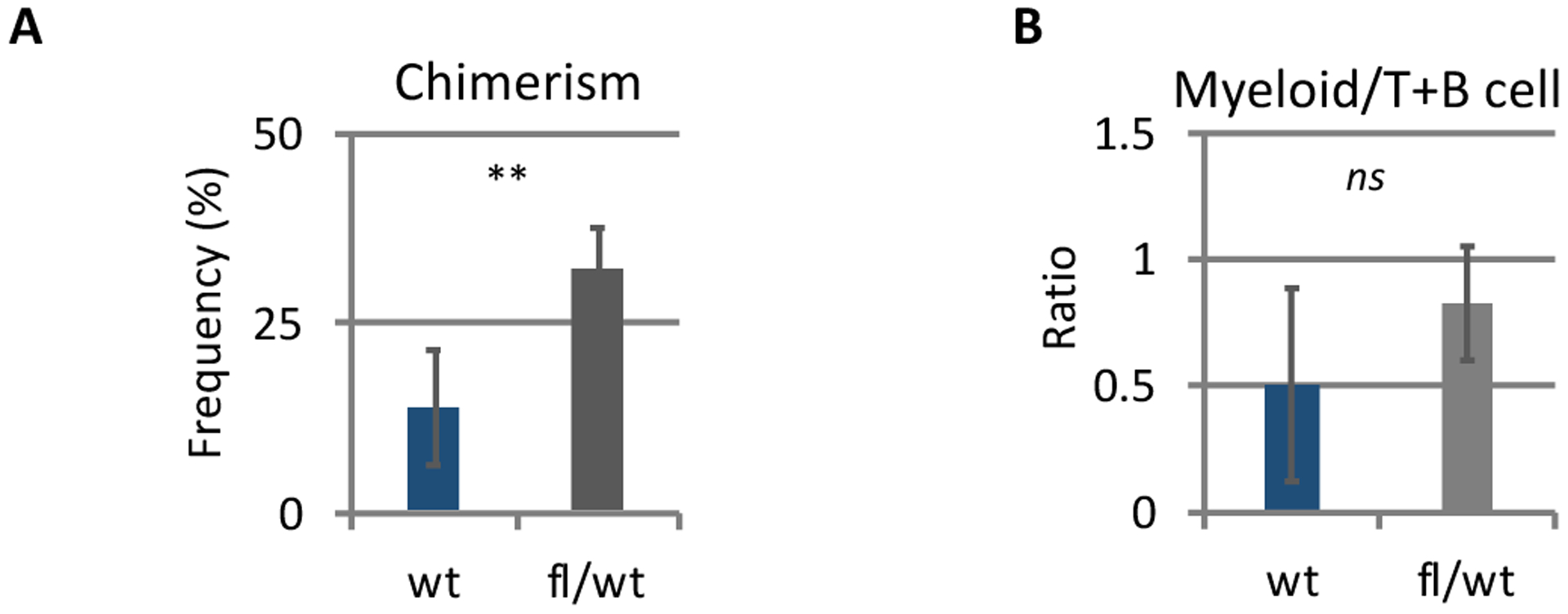
A. Allo-donor blood chimerism in Leprcre CD39fl/wt or control Leprcre mice. Recipient mice (B6) received 3-Gy irradiation followed by intravenous injection of MHC-mismatched BALB/c BM cells (2 × 107/each). Anti-CD8 mAb was intraperitoneally injected on day −1 and anti-mouse CD40L mAb on day 0. Donor chimerism frequencies in the peripheral blood 4 weeks after transplantation. Experiment included four-five recipients/group. Data were reproduced by an additional experiment (five mice/group).
B. Ratios of donor myeloid (CD11b+) to lymphoid (TCRβ+ and B220+) cells in the peripheral blood.
Figure 2. CD39 deletion in lepr+ cells increased and potentiated effector memory-like niche Tregs.
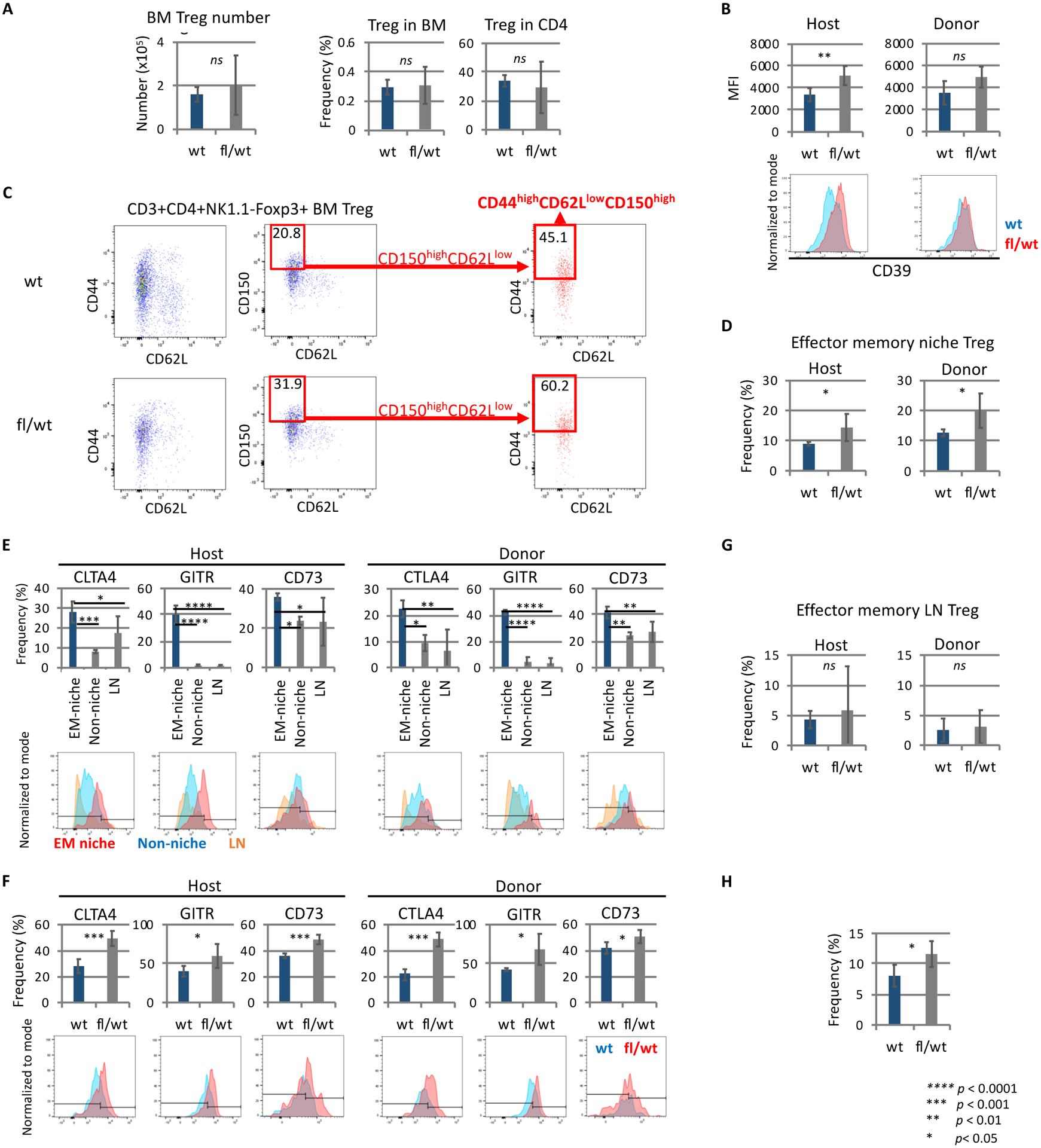
A. Treg numbers or frequencies in BM mononuclear cells or CD4 T cells. Experiment included four-five recipients/group. Analysis was performed ten weeks after transplant (B-H).
B. CD39 expression levels by BM Tregs. Experiment included four-five recipients/group. Four weeks after transplant.
C. Gating strategy of host-derived effector memory-like niche Tregs (H-2Kb+) CD44highCD62lowCD150highFoxP3+CD4+CD3+NK1.1-) in transplanted Leprcre CD39fl/wt or Leprcre mice. Experiment included four-five recipients/group.
D. Frequencies of effector memory-like niche Tregs within host- or donor-derived BM Tregs four weeks after transplantation. Experiment included four-five recipients/group.
E. Expression levels of PD1, GITR, and CD73 by host- or donor-derived effector memory-like niche Tregs (EM niche), CD150lowCD44lowCD62low BM Tregs (non-niche), and LN Tregs in transplanted Leprcre mice. Experiment included four-five recipients/group.
F. Expression levels of PD1, GITR, and CD73 by host- or donor-derived effector memory-like niche Tregs in Leprcre CD39fl/wt or Leprcre mice. Experiment included four-five recipients/group.
G. Frequencies of effector memory-like LN Tregs. Experiment included four-five recipients/group.
H. Frequencies of effector memory-like niche Tregs within Tregs in non-transplanted Leprcre CD39fl/wt or Leprcre mice. Experiment included four-five mice/group.
Figure 3. CD39 deletion in Tregs and CD73 global deletion both increased effector memory-like niche Tregs, while in vivo A2AR agonist treatment decreased effector memory-like niche Tregs.
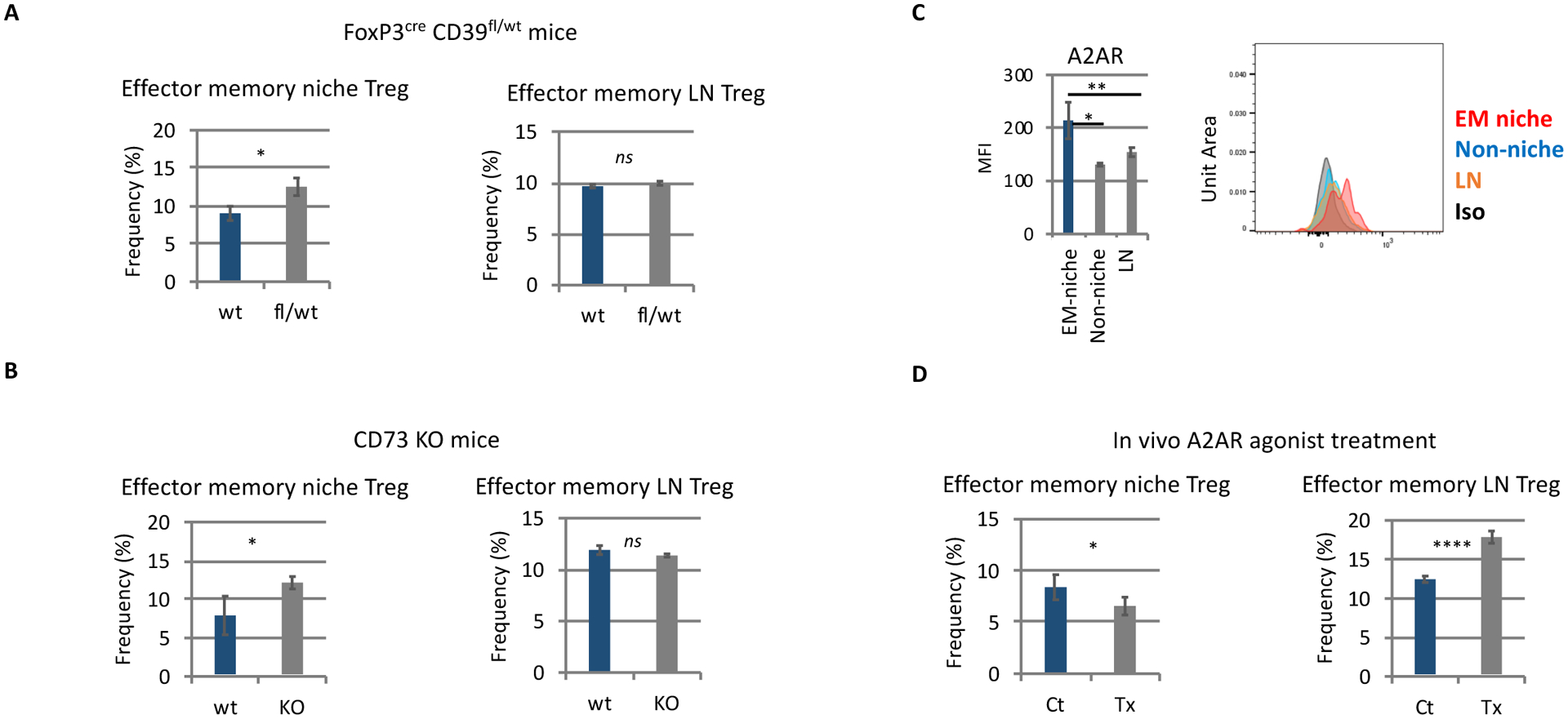
A-B, D. Frequencies of effector memory-like niche Tregs and LN Tregs within BM Tregs and LN Tregs, respectively, in FoxP3cre CD39fl/wt mice (A), CD73 KO mice (B), and A2AR agonist (PSB-0777) treated mice (D). PSB-0777 (Tocris; 1mg/kg i.p.) was given for seven consecutive days. Flow cytometric analysis was performed four hours after the final injection. Experiment included three-four mice/group.
C. Flow cytometric analysis of A2AR expression levels on effector memory-like niche Tregs. N=3. EM-niche: Effector memory-like niche Tregs. Non-niche: CD150low BM Tregs. LN: lymph node Tregs. Iso: isotype control. ****: p < 0.0001. ***: p < 0.001. **: p < 0.01. *: p< 0.05.
To the best of our knowledge, this work for the first time identifies the role of MSCs in tolerance induction of transplanted allo-HSCs, revealing unexpected negative regulation of engraftment and immune privilege by MSC-derived adenosine. Our work further demonstrates previously undescribed roles of adenosine to inhibit effector memory-like niche Tregs via A2ARs. These observations contradicts a large amount of previous studies showing effects of MSCs and adenosine to suppress immunity and expand or generate Tregs.8,13 Adenosine did not inhibit effector memory-like LN Treg pool, suggesting that adenosine inhibits only tissue-residential effector memory-like Tregs. Adenosine-mediated inhibition of niche Tregs may prevent excessive immunosuppression within the niche, while this inhibitory effect on immune privilege is likely to be counterbalanced by the effect from lepr+ cells’ CXCL12, a chemokine ligand which was demonstrated to recruit Tregs to the BM.7
Differential effects of CD39 deletion in Tregs and lepr+ cells on allo-HSC engraftment indicate that adenosine from Tregs, but not from MSCs, acts as an effector molecule of immune privilege, suppressing immunity. Our previous study using in vivo microscopy revealed active movement of BM Tregs over time, which is likely to enable more frequent exposure of Treg-derived adenosine to other immune cells as compared to adenosine from stationary stromal cells, explaining immunosuppressive effects of adenosine only from Tregs.7 While this study was performed by using hemizygous deletion of CD39 in lepr+ cells or in Tregs, the expansion of effector memory-like niche Tregs was also confirmed in mice with homozygous deletion of CD39 in Tregs (not shown), suggesting that regardless of hemizygous or homozygous deletion, the phenotype was reproducible. It would be warranted to study roles of MSC-derived adenosine in regulation of tumor niche Tregs, given that adenosine receptor antagonist treatment is drawing attention as a promising approach of tumor immunotherapy.8
Highlights.
Although a large body of evidence indicates immunosuppressive effects of adenosine and mesenchymal stromal cells (MSCs), this work demonstrates a paradoxical effect of MSC-derived adenosine to activate immunity and decrease allogeneic bone marrow engraftment.
While it was previously demonstrated that hematopoietic stem cell (HSC) niche-residential Tregs maintains immune privilege of the HSC niche through adenosine and promotes allogeneic bone marrow (BM) engraftment, this work shows that adenosine from leptin receptor-expressing MSCs, another niche constituents, unexpectedly inhibits allogeneic BM engraftment.
While adenosine from Tregs acts as an effector molecule of immune privilege in the HSC niche, adenosine from Tregs and MSCs possesses an effect to inhibit niche Tregs, perhaps preventing excessive immunosuppression within the niche.
Acknowledgments
This work was supported by ASH Junior Faculty Basic Science Scholar Award (ASOH CU15-2897; JF), NIH NHLBI 1R01HL129506-01A1(JF), NIH NHLBI R01HL145154-01 (JF), and NIH NIDDK R01DK121889 (JF).
Footnotes
Disclosure of Conflict of Interest We have no conflicting interest to declare.
References
- 1.Galipeau J & Sensebe L Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018; 22(6): 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardo ME & Fibbe WE Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann N Y Acad Sci 2012; 1266(107–117. [DOI] [PubMed] [Google Scholar]
- 3.Kfoury Y & Scadden DT Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 2015; 16(3): 239–253. [DOI] [PubMed] [Google Scholar]
- 4.Niederkorn JY See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol 2006; 7(4): 354–359. [DOI] [PubMed] [Google Scholar]
- 5.Li N, Wang T & Han D Structural, cellular and molecular aspects of immune privilege in the testis. Front Immunol 2012; 3(152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujisaki J, Wu J, Carlson AL et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 2011; 474(7350): 216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirata Y, Furuhashi K, Ishii H et al. CD150(high) Bone Marrow Tregs Maintain Hematopoietic Stem Cell Quiescence and Immune Privilege via Adenosine. Cell Stem Cell 2018; 22(3): 445–453 e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohta A A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front Immunol 2016; 7(109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirata Y, Kakiuchi M, Robson SC & Fujisaki J CD150high CD4 T cells and CD150high Tregs regulate hematopoietic stem cell quiescence via CD73. Haematologica 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding L, Saunders TL, Enikolopov G & Morrison SJ Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012; 481(7382): 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenbaum A, Hsu YM, Day RB et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013; 495(7440): 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito H, Kurtz J, Shaffer J & Sykes M CD4 T cell-mediated alloresistance to fully MHC-mismatched allogeneic bone marrow engraftment is dependent on CD40-CD40 ligand interactions, and lasting T cell tolerance is induced by bone marrow transplantation with initial blockade of this pathway. J Immunol 2001; 166(5): 2970–2981. [DOI] [PubMed] [Google Scholar]
- 13.Deaglio S, Dwyer KM, Gao W et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007; 204(6): 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]


