Abstract
The outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been rapidly spreading on a global scale and poses a great threat to human health. However, efficient indicators for disease severity have not been fully investigated. Here, we aim to investigate whether dynamic changes of lymphocyte counts can predict the deterioration of patients with COVID-19.
We collected data from 2923 patients with laboratory-confirmed COVID-19. Patients were then screened, and we focused on 145 severe cases and 60 critical cases (29 recovered cases, 31 deaths). The length of hospitalization was divided into five time points, namely admission, 25%, 50%, 75% and discharge or death, according to the principle of interquartile distance. A series of laboratory findings and clinical data were collected and analyzed during hospitalization. The results showed that there were differences in levels of leukocytes, neutrophils and lymphocytes at almost every time point in the severe cases and 60 critical cases (29 recovered cases, 31 deaths). Further analysis showed that 70.2% of the COVID-19 cases had low circulating lymphocyte count, of which 64.1% were severe cases and 85.0% were critical cases (75.9% recovered cases and 93.5% died). Moreover, the lymphocyte count in dead cases was significantly lower than that of critical cases who recovered, at almost every time point in the critical groups. We also divided critical patients into group A (<1.1 × 109/L) and group B (>1.1 × 109/L) according to number of lymphocytes. Through survival analysis, we found that there was no significant difference in survival between group A and group B at admission (P = .3065). However, the survival rate according to lymphocyte levels in group A was significantly lower than that of group B at 25% hospital stay (on average day 6.5), 50% and 75% time points (P < .001).
Lymphocyte counts that remain lower after the first week following symptom onset are highly predictive of in-hospital death of adults with COVID-19. This predictor may help clinicians identify patients with a poor prognosis and may be useful for guiding clinical decision-making at an early stage.
Keywords: COVID-19, death, lymphocytes, predict, SARS-CoV-2
1. Introduction
On March 11, 2020, coronavirus disease 2019 (COVID-19) was declared by the World Health Organization (WHO) as a global public health emergency due to its pandemicity. A lack of understanding of the severity of early COVID-19 disease, combined with the high infectivity of the virus, has caused the number of patients worldwide to dramatically increase, and the mortality rate is relatively high. As of July 15, 2020, more than 5,518,343 cases and 576,926 deaths have been recorded globally due to COVID-19. Most patients with COVID-19 have mild influenza-like symptoms, or may be asymptomatic, while a minority of patients have a clinical manifestation, including fever, cough, fatigue, muscle pain, diarrhea, and pneumonia, which can develop to severe pneumonia, acute respiratory distress syndrome (ARDS), metabolic acidosis, septic shock, coagulation dysfunction, multi-organ failure (MOF), and death.[1–3] However, efficient indicators for disease severity, therapeutic responses and disease outcome have not been fully investigated. Identifying these indicators will lead to the administration of reasonable medication and care, which can significantly reduce the mortality rate of severe and critical patients.
Currently, forecast indexes for predicting the poor prognosis of COVID-19 patients include neck circumference,[4] obesity,[5] liver injury,[6] chest CT,[7] hypoalbuminaemia,[8] alteration of taste or smell,[9] combined IL-6 and CD8+ T cell counts,[10] serum amyloid A,[11] cardiac troponin-I, homocysteine,[12] elevated N-terminal pro-brain natriuretic peptide,[13] angiopoietin-2,[14] lymphocyte-to-C-reactive protein ratio,[15] albumin,[16] peripheral lymphocyte count,[17–20] neutrophil to lymphocyte ratio.[21] However, apart from lymphocyte count, most of these indexes are limited to specific populations, with small sample sizes, and are difficult to detect or to reproduce on a large scale. Complete blood count is the most available, efficient and economic examination, as this low-cost, easily acquired biomarker is readily available even in remote areas. Laboratory biomarkers[13] to predict the mortality and severity of COVID-19 are essential in this pandemic situation.
Peripheral blood lymphocyte levels also change significantly with the progression of COVID-19 disease. SARS-CoV-2 particles spread through the respiratory mucosa and infect other cells, causing a storm of cytokines in the body,[22] producing a series of immune responses, and causing changes in peripheral leukocyte counts, lymphocytes and other immune cells. Lymphocytes are the most important immune cells in the human body, and play a significant role in regulating cellular immunity.[23] Some studies have shown that a drastic reduction in the total number of lymphocytes indicates that the coronavirus has consumed many immune cells, and a significant reduction in lymphocytes is common in critically ill patients.[24] Our primary goal was to establish whether a lower lymphocyte count could predict the severity of COVID-19. Previous studies have focused on one or several time points, but a recent research study with a small sample size showed that the dynamic changes of lymphocyte counts could predict the deterioration of COVID-19.[18] This study aims to retrospectively analyze the time courses of lymphocyte counts of cured and dead patients with COVID-19, in order to obtain a key predictor of disease outcome and to provide guidance for subsequent clinical practice.
2. Materials and methods
2.1. Patients’ involvement and data collection
All hospitalized patients (n = 2923) (admission date from February 10 to March 8, 2020) in Huoshenshan Hospital of Wuhan, diagnosed with COVID-19 based on their clinical symptoms (fever or respiratory symptoms) with typical changes in chest radiology and positive nucleic acid detection results, were involved in this study. Huoshenshan Hospital of Wuhan is a new hospital for hospitalizing patients with COVID-19 and has been entrusted to the military after completion on February 2, 2020. Pharyngeal swab specimens of these patients were collected and used for COVID-19 viral nucleic acid detection using a real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay in the designated hospitals. Patients with positive test results were admitted to hospital and included in this study. All patients involved in this study were living in Wuhan during the outbreak period of COVID-19.
Demographic information, clinical characteristics (including medical history, exposure history, comorbidities and symptoms), chest computed tomographic (CT) scan results, and laboratory findings of each patient were obtained from the electronic medical record system of Huoshenshan Hospital of Wuhan. Access was permitted by the hospital director.
The date of disease onset, hospital admission date and time of discharge or death, as well as the severity of COVID-19, were also recorded. The onset date was defined as the day when any symptoms were observed by the patients. COVID-19 severity was defined according to the diagnostic and treatment guidelines for COVID-19 issued by the Chinese National Health Committee (version 7). Severe COVID-19 was designated when the patients had one of the following criteria:
-
1.
respiratory distress with respiratory frequency ≥30/min;
-
2.
pulse oximeter oxygen saturation ≤93% at rest; and
-
3.
oxygenation index (artery partial pressure of oxygen/inspired oxygen fraction, PaO2/FiO2) ≤300 mm Hg.
Critical COVID-19 was designated when the patients had one of the following criteria:
-
1.
respiratory failure with mechanical ventilation;
-
2.
shock; and
-
3.
combination with other organ failure; requirement of ICU for monitoring and treatment.
2.2. Laboratory testing
Blood testing for all patients was performed by the clinical laboratory of Huoshenshan Hospital of Wuhan. Medical laboratory results in this study, including the numbers of leukocytes, lymphocytes, and eosinophils; and percentages of lymphocytes and eosinophils, were collected for each patient. All medical laboratory data were generated by the clinical laboratory of Huoshenshan Hospital of Wuhan. As the disease progressed, the most updated secondary results for laboratory findings (including numbers of lymphocytes, leukocytes and neutrophils) during the hospital stay were also collected.
2.3. Statistical analysis
Categorical variables were expressed as a number (%), and continuous variables were summarized using interquartile range (IQR) values. To compare continuous variables for the data of different patient groups, variance analysis and the Kruskal–Wallis H(K) test were used appropriately. The frequencies of categorical variables were compared using the χ2 test, as appropriate. Statistical analyses were processed by the statistic package deal SPSS 19.0. Variables, correlation analysis and survival analysis were processed by GraphPad Prism version 5.00 software. All graphs were generated and plotted using GraphPad Prism version 5.00 software (GraphPad Software Inc). P values of less than .05 were considered statistically significant.
2.4. Ethical approval
Clinical and laboratory information was collected approved by Huoshenshan Hospital of Wuhan. Moreover, when the information is obtained, the patient's name, ID number, work unit, home address, contact person and telephone information are hidden, and there is no patient privacy exposure. Thirdly, Huoshenshan Hospital of Wuhan has stopped running before the article is completed. Fourth, this is a descriptive retrospective study. Therefore, this study has not been reviewed by the hospital ethics committee.
3. Results
3.1. Demographics and clinical characteristics
A total of 2923 patients diagnosed with COVID-19 were included in this study, with 20 patients categorized as mild cases; 2104 moderate patients; 711 severe cases, and 88 critical cases (37 recovered cases, 51 deaths) (Fig. 1). The mean age for all patients was 58.1 years, ranging from 16 to 97 years old, and 2174 (74.9%) patients were above 50 years old. The majority of patients were non-moderate, and almost all patients who died were over 50 years old. About half (50.2%) of cases were male. In order to facilitate the study, we divided the length of hospitalization into five time points, namely admission, 25%, 50%, 75% and discharge, according to the principle of interquartile distance. We focused on the 711 severe cases and 88 critical cases. Blood was collected from patients on the above five time points. Patients with five blood tests were 145 severe cases and 60 critical cases (29 recovered cases, 31 deaths), (Figs. 1 and 2).
Figure 1.
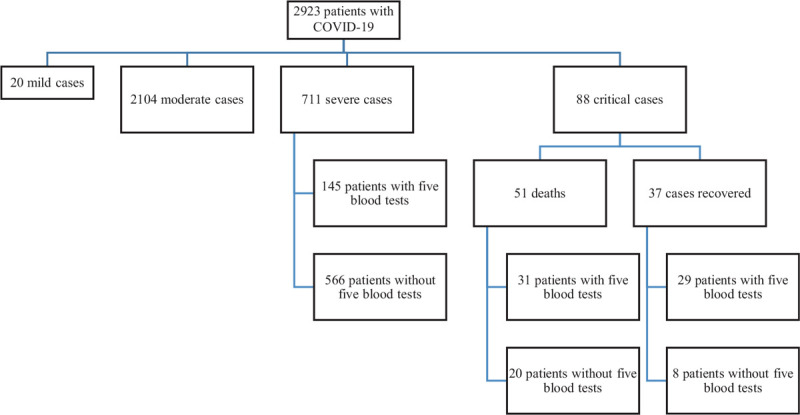
Study flow chart. A total of 2923 patients diagnosed with COVID-19 were included in this study, with 20 patients categorized as mild cases; 2104 moderate patients; 711 severe cases, and 88 critical cases (37 recovered cases, 51 deaths). Among them, patients with five blood tests were 145 severe cases and 60 critical cases (29 recovered cases, 31 deaths).
Figure 2.
Time points of blood routine collection during hospitalization. we divided the length of hospitalization into five time points, namely admission, 25%, 50%, 75% and discharge, according to the principle of interquartile distance.
Although COVID-19 infections clustered within 26 (12.7%) patients whose family members or friends were also infected with COVID-19 in this study, 179 patients (87.3%) did not have a clear history of exposure. About 77.1% of patients had one or more underlying comorbidity, the most common of which were chronic diseases, such as hypertension, diabetes, coronary heart disease, cerebrovascular disease, stroke, malignant tumor, chronic obstructive pulmonary disease and nephropathy. In particular, many patients concomitantly suffered from a variety of underlying comorbidities (Table 1).
Table 1.
Demographics and baseline characteristics of patients with COVID-19.
| All patients | Diseases severity | |||||
| Severe | Critical (cases recovered) | Critical (Death) | ||||
| (n = 205) | (n = 145) | (n = 29) | (n = 31) | F /χ2 value | P value | |
| Age—mean (range) | 67.2 (24–91) | 66.6 (24–91) | 67.1 (37–88) | 69.7 (25–89) | 0.937 | .394 |
| Age-groups-n (%) | ||||||
| ≦30y | 2 (1.0) | 1 (0.7) | 0 | 1 (3.2) | 7.541 | .274 |
| 30–49y | 9 (4.4) | 7 (4.8) | 2 (6.9) | 0 | — | — |
| 50–69y | 111 (54.1) | 84 (57.9) | 14 (48.3) | 13 (41.9) | — | — |
| ≥70y | 83 (40.5) | 53 (36.6) | 13 (44.8) | 17 (54.9) | — | — |
| Sex-n (%) | ||||||
| Female | 87 (42.4) | 64 (44.1) | 13 (44.8) | 10 (32.3) | 1.554 | .46 |
| Male | 118 (57.6) | 81 (55.9) | 16 (55.2) | 21 (67.7) | ||
| Exposure history-n (%) | ||||||
| Yes | 26 (12.7) | 22 (15.2) | 2 (6.9) | 2 (6.5) | 2.775 | .25 |
| No | 179 (87.3) | 123 (84.8) | 27 (93.1) | 29 (93.5) | ||
| Comorbidity-n (%) | ||||||
| 0 | 47 (22.9) | 36 (24.8) | 5 (17.2) | 6 (19.4) | 2.942 | .816 |
| 1 | 70 (34.1) | 46 (31.7) | 13 (44.8) | 11 (35.5) | — | — |
| Hypertension | 31 (15.1) | 21 (14.5) | 5 (17.2) | 5 (16.1) | — | — |
| Diabetes mellitus | 7 (3.4) | 6 (4.1) | 1 (3.4) | 0 | — | — |
| Chronic bronchitis | 4 (2.0) | 4 (2.8) | 0 | 0 | — | — |
| Coronary heart disease | 1 (0.5) | 1 (0.7) | 0 | 0 | — | — |
| Respiratory failure | 8 (0.4) | 0 | 4 (13.8) | 4 (12.9) | — | — |
| Other | 20 (9.8) | 14 (9.7) | 3 (10.3) | 2 (6.5) | — | — |
| 2 | 40 (19.5) | 30 (20.7) | 5 (17.2) | 5 (16.1) | — | — |
| Hypertension+Coronary heart disease | 6 (2.9) | 6 (4.1) | 0 | 0 | — | — |
| Hypertension+Diabetes mellitus | 11 (5.4) | 6 (4.1) | 2 (6.9) | 3 (9.7) | — | — |
| Coronary heart disease+Diabetes mellitus | 2 (1.0) | 2 (1.4) | 0 | 0 | — | — |
| Other | 21 (10.2) | 16 (11.0) | 3 (10.3) | 2 (6.5) | — | — |
| ≧3 | 48 (23.5) | 33 (22.8) | 6 (20.8) | 9 (29) | — | — |
| Hypertension+Coronary heart disease+Diabetes mellitus | 3 (1.5) | 3 (2.1) | 0 | 0 | — | — |
| Hypertension+Coronary heart disease+X | 10 (4.5) | 7 (4.8) | 1 (3.4) | 2 (6.5) | — | — |
| Hypertension+Diabetes mellitus+X | 12 (5.9) | 9 (6.2) | 1 (3.4) | 2 (6.5) | — | — |
| Other | 24 (11.7) | 14 (9.7) | 5 (17.2) | 5 (16.1) | — | — |
COVID-19 = coronavirus disease 2019; X = Chronic obstructive pulmonary disease, Cerebrovascular disease, Malignant, Anemia, Hypoproteinemia, Arrhythmia, Liver cirrhosis, etc. 0,1,2, ≥3: the number of comorbidities.
Clinical symptoms of all patients at the onset of illness are shown in Table 2. The most common symptoms were cough (80.0%), followed by fever (76.6%), chest tightness/dyspnea (71.7%), fatigue (60.0%) and muscle aches (33.7%). Less common symptoms were gastrointestinal symptoms, dizziness/headache, expectoration, sore throat etc. Frequently, patients also had multiple symptoms at the same time.
Table 2.
Symptomatic and radiological characteristics of patients with COVID-19.
| All patients | Diseases severity | |||||
| Severe | Critical (cases recovered) | Critical (Death) | ||||
| (n = 205) | (n = 145) | (n = 29) | (n = 31) | χ2 value | P value | |
| Signs and symptoms- n (%) | 1.168 | .883 | ||||
| 1 | 10 (4.9) | 6 (4.1) | 2 (6.9) | 2 (6.5) | ||
| Fever | 5 (2.4) | 2 (1.4) | 1 (3.4) | 2 (6.5) | ||
| Cough | 3 (1.5) | 3 (2.1) | 0 | 0 | ||
| Chest tightness/dyspnea | 2 (1.0) | 1 (0.7) | 1 (3.4) | 0 | ||
| 2 | 40 (19.5) | 27 (18.6) | 7 (24.1) | 6 (19.4) | ||
| Fever+Fatigue | 4 (2.0) | 2 (1.4) | 1 (3.4) | 1 (3.2) | ||
| Fever+Cough | 7 (3.4) | 6 (4.1) | 0 | 1 (3.2) | ||
| Fever+Chest tightness/dyspnea | 5 (2.4) | 4 (2.8) | 0 | 1 (3.2) | ||
| Fever+Muscle aches | 1 (0.5) | 1 (0.7) | 0 | 0 | ||
| Cough+Chest tightness/dyspnea | 14 (6.8) | 10 (6.9) | 3 (10.3) | 1 (3.2) | ||
| Chest tightness/dyspnea+Fatigue | 3 (1.5) | 1 (0.7) | 1 (3.4) | 1 (3.2) | ||
| Other | 6 (2.9) | 3 (2.1) | 2 (6.9) | 1 (3.2) | ||
| ≥3 | 155 (75.6) | 112 (77.3) | 20 (69.0) | 23 (74.1) | ||
| Fever+Cough+Fatigue | 5 (2.4) | 3 (2.1) | 1 (3.4) | 1 (3.2) | ||
| Fever+Cough+Chest tightness/dyspnea | 22 (10.7) | 13 (9.0) | 4 (13.8) | 5 (16.1) | ||
| Fever+Cough+Chest tightness/dyspnea+Fatigue | 17 (8.3) | 10 (6.9) | 4 (13.8) | 3 (9.7) | ||
| Fever+Chest tightness/dyspnea+Fatigue | 6 (2.9) | 4 (2.8) | 1 (3.4) | 1 (3.2) | ||
| Fever+Cough+Fatigue+Muscle aches | 9 (4.4) | 7 (4.8) | 1 (3.4) | 1 (3.2) | ||
| Cough+Chest tightness/dyspnea+Fatigue | 7 (3.4) | 4 (2.8) | 1 (3.4) | 2 (6.5) | ||
| Fever+Cough+Chest tightness/dyspnea+Fatigue+Muscle aches | 48 (23.4) | 38 (26.2) | 4 (13.8) | 6 (19.4) | ||
| Other | 41 (20.0) | 33 (22.8) | 4 (13.8) | 4 (1.9) | ||
| Chest CT images- n (%) | 27.802 | <.001 | ||||
| Multiple patchy shadows of both lungs | 61 (29.8) | 29 (20.0) | 14 (48.3) | 18 (58.1) | ||
| Multiple ground glass of both lungs | 127 (62.0) | 103 (71.0) | 15 (51.7) | 9 (29.0) | ||
| Multiple Consolidation of both lungs | 11 (5.4) | 8 (0.6) | 0 | 3 (9.7) | ||
| Other | 6 (2.8) | 5 (8.4) | 0 | 1 (3.2) | ||
COVID-19: coronavirus disease 2019; denoted the comparison between severe and critical cases. COVID-19 = coronavirus disease 2019.0,1,2, ≥3: the number of symptoms.
3.2. Radiological and laboratory findings
Abnormalities in chest CT images were detected in all patients. Of the 205 patients, 127 (62.0%) had multiple ground glass in both lungs; 61 (29.8%) patients had multiple patchy shadows in both lungs and 11 (5.4%) patients had multiple consolidation in both lungs (Table 2). There were significant differences between the severe cases, critical recovered cases and deaths. Multiple patchy shadows in both lungs were as high as 68% in dead patients.
The blood counts of patients during their hospitalization were collected at admission, 25%, 50%, 75% and discharge. The results showed that there were differences in leukocytes, neutrophils and lymphocytes at almost every time point in the three groups (Table 3). Compared with the severe cases and critical recovered cases, the leukocytes and neutrophils of dead patients increased gradually, while their lymphocytes remained at a low level (Fig. 3: A–C). Further analysis found that 70.2% of COVID-19 cases displayed low circulating lymphocyte counts, respectively 64.1% of severe cases and 85.0% of critical cases (75.9% recovered cases, 93.5% deaths). The lymphocyte count in dead patients was significantly lower than that of critical recovered cases, at almost every time point in the critical groups (Fig. 3D).
Table 3.
Dynamic changes of blood cell count in patients with COVID-19.
| Laboratory parameters | time | All patients (n = 205) | Severe cases (n = 145) | Critical cases (recovered) (n = 29) | Critical cases (deaths) (n = 31) | χ2 value | P value |
| Leukocytes (×10E9/L; normal range 3.5–9.5) | 1 | 7.00 (5.10–9.45) | 6.80 (4.80–8.55) | 9.30 (7.05–12.90) | 8.40 (6.00–10.60) | 19.642 | ≦.001 |
| 2 | 7.10 (5.30–10.25) | 6.60 (5.15–8.95) | 8.90 (6.30–11.90) | 10.90 (5.80–16.20) | 17.109 | ≦.001 | |
| 3 | 6.80 (5.20–8.95) | 6.30 (5.10–7.65) | 7.30 (5.10–9.30) | 10.50 (8.40–18.30) | 30.108 | ≦.001 | |
| 4 | 6.50 (5.10–8.80) | 6.10 (4.90–7.50) | 7.10 (5.50–9.20) | 11.00 (8.10–17.90) | 32.078 | ≦.001 | |
| 5 | 6.40 (5.20–8.65) | 6.00 (5.00–7.55) | 6.30 (5.15–9.55) | 13.60 (7.70–24.70) | 42.167 | ≦.001 | |
| χ2 value | 5.926 | 6.617 | 13.062 | 8.012 | — | — | |
| P value | .205 | .158 | .011 | .091 | — | — | |
| Neutrophils (×10E9/L; normal range 1.8–6.3) | 1 | 4.33 (3.09–6.45) | 3.68 (2.80–4.80) | 8.05 (6.14–10.20) | 6.34 (5.27–9.86) | 61.805 | ≦.001 |
| 2 | 5.24 (3.47–8.28) | 4.32 (3.23–6.62) | 6.66 (4.84–10.14) | 9.34 (5.10–15.23) | 25.648 | ≦.001 | |
| 3 | 4.44 (3.38–7.04) | 4.02 (2.85–5.46) | 4.89 (3.71–7.05) | 9.18 (7.51–16.58) | 43.05 | ≦.001 | |
| 4 | 4.25 (2.95–6.13) | 3.61 (2.72–5.28) | 5.01 (3.41–6.51) | 9.82 (6.23–17.33) | 49.825 | ≦.001 | |
| 5 | 3.95 (3.00–6.37) | 3.68 (2.80–4.80) | 3.93 (3.06–6.35) | 11.24 (6.74–23.79) | 55.885 | ≦.001 | |
| χ2 value | 23.809 | 27.368 | 23.674 | 8.33 | — | — | |
| P value | ≦.001 | ≦.001 | ≦.001 | .08 | — | — | |
| Lymphocyte (×10E9/L; normal range 1.1–3.2) | 1 | 0.85 (0.55–1.22) | 0.97 (0.65–1.31) | 0.75 (0.48–1.12) | 0.57 (0.34–0.84) | 19.582 | ≦.001 |
| 2 | 0.14 (0.06–0.41) | 1.17 (0.76–1.60) | 0.97 (0.65–1.42) | 0.54 (0.34–0.92) | 64.19 | ≦.001 | |
| 3 | 1.17 (0.72–1.71) | 1.31 (0.90–1.83) | 1.13 (0.71–1.51) | 0.50 (0.38–0.71) | 3.465 | .063 | |
| 4 | 1.32 (0.82–1.76) | 1.47 (1.09–1.83) | 1.34 (0.95–1.76) | 0.49 (0.32–0.73) | 1.011 | .315 | |
| 5 | 1.44 (0.94–1.92) | 1.57 (1.14–1.97) | 1.46 (1.00–2.12) | 0.42 (0.27–0.84) | 41.14 | ≦.001 | |
| χ2 value | 9.33 | 26.53 | 7.578 | 3.998 | — | — | |
| P value | .002 | ≦.001 | .023 | .406 | — | — |
COVID-19 = coronavirus disease 2019; P values denoted the comparison between moderate and severe, critical and death cases. Data are shown as median (IQR); IQR = interquartile range; 1, 2, 3, 4, and 5: blood cell collection time point of admissions, 25%, 50%, 75% and discharge during their hospitalization.
Figure 3.
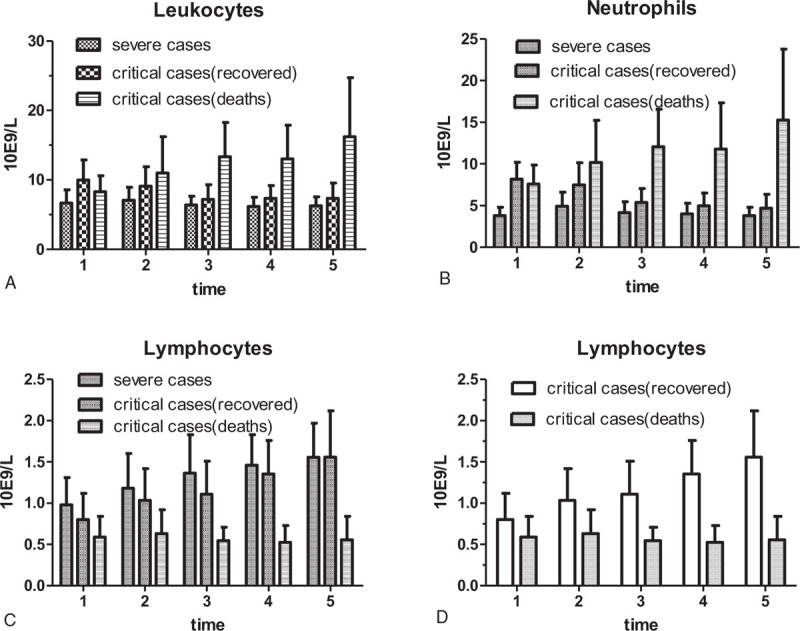
Dynamic changes of blood cell count in patients with COVID-19. Compared with the severe cases and critical recovered cases, the leukocytes and neutrophils of dead patients increased gradually, while lymphocytes from dead patients remained at a low level (A, B and C). The lymphocyte count in dead patients was significantly lower than that of critical recovered cases, at almost every time point in the critical groups (D).
3.3. Correlation analysis of lymphocytes and leukocytes or neutrophils in critical cases (recovered) and critical cases (deaths)
There was no correlation of lymphocytes and leukocytes in critical cases (recovered) (r = -0.1102, P = .1871). However, there was a positive correlation of lymphocytes and leukocytes or neutrophils in critical cases (deaths) (r = 0.3075 and P < .0001, r = 0.2651and P = .0009, respectively). Furthermore, there was a negative correlation of lymphocytes and neutrophils in critical cases (recovered) (r = -0.2885, P = .0004) (Fig. 4).
Figure 4.
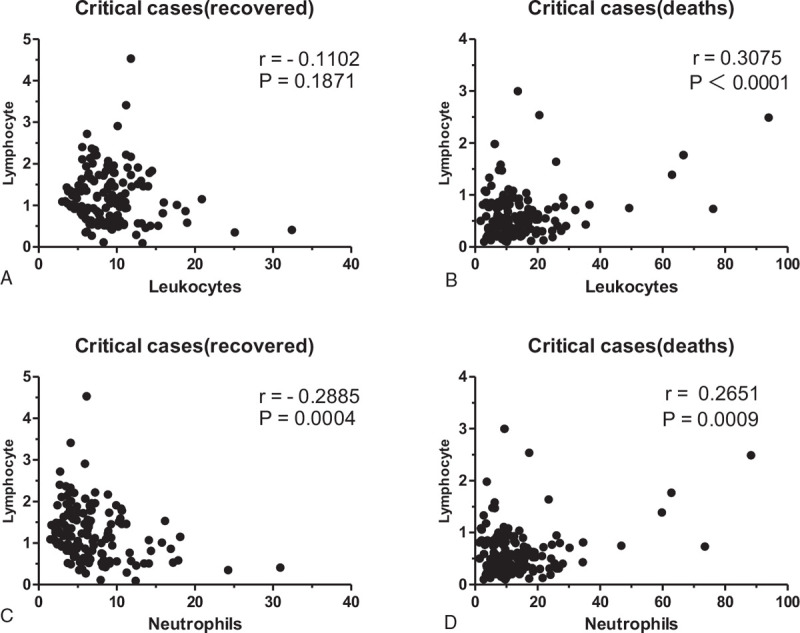
Correlation analysis of critical cases (recovered) and critical cases (deaths). There was no correlation of lymphocytes and leukocytes in critical cases (recovered) (r = -0.1102, P = .1871). There was a positive correlation of lymphocytes and leukocytes or neutrophils in critical cases (deaths) (r = 0.3075 and P < .0001, r = 0.2651and P = .0009, respectively). Furthermore, there was a negative correlation of lymphocytes and neutrophils in critical cases (recovered) (r = -0.2885, P = .0004).
3.4. Survival analysis of critical patients with COVID-19
Critical patients with COVID-19 were divided into a critical recovered cases group and a dead group according to the outcome. Of course, we can also divide them into critical patients of group A (<1.1 × 10E9/L) and group B (>1.1 × 10E9/L) according to number of lymphocytes. A total of 80 critical patients were enrolled according to the lymphocyte count at admission, with 66 in group A (27 recovered, 39 dead) and 14 in group B (9 recovered, 5 dead). Analyzing survival, we found that there was no significant difference between group A and group B (P = .3067). We then analyzed the survival rate according to lymphocytes at the 25% hospital stay (on average day 6.5). A total of 65 critical patients were enrolled, with 48 patients in group A (18 recovered, 30 dead) and 17 patients in group B (15 recovered, 2 dead). The results showed that the survival rate of group A was significantly lower than that of group B (P = .0003). The same results were also found at the 50% and 75% time points (P = .003, P < .0001, respectively) (Fig. 5).
Figure 5.
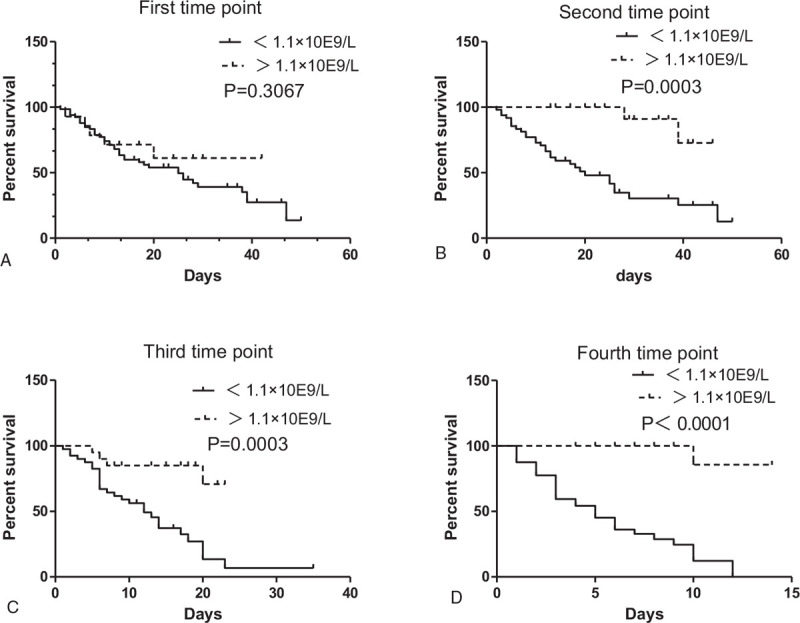
Prognosis of lymphocytes of severe and critical patients with COVID-19 on 1–4time point. We can divide critical patients with COVID-19 into the group A (<1.1 × 10E9/L) and group B (>1.1 × 10E9/L) according to their number of lymphocytes. Analyzing survival, we found that the survival rate of group A (<1.1 × 10E9/L) was significantly lower than that of group B (>1.1 × 10E9/L) at the second time point (P = .0003). The same results were also found at the third and fourth time points (P = .003, P < .0001, respectively).
4. Discussion
This was a retrospective cohort study. We reviewed clinical records, nursing records, laboratory findings, and CT scans from the data of 205 patients with laboratory-confirmed COVID-19. We have presented the important changes in blood cell count of patients with SARS-CoV-2 infection of different clinical types and at different follow-up time points in China. To study the characteristics of peripheral blood lymphocytes in critically ill patients is of great significance for timely diagnosis, accurate typing, accurate treatment, delaying or delaying the development of the disease and reducing the mortality.
A very important characteristic is that lymphocytes were reduced in patients with COVID-19, especially in critically ill patients. Our research found that 70.2% of COVID-19 cases displayed low circulating lymphocyte counts, of which 64.1% were severe cases and 85.0% were critical cases (75.9% recovered cases and 93.5% died). According to new research, about 82.1% of COVID-19 cases displayed low lymphocyte counts,[1,2,24,25] which is consistent with our research. However, earlier studies have been unclear regarding the numbers and function of lymphocytes in COVID-19 patients, although a reduction in lymphocyte count is suggested.[2,25] Cytokine storm induced by virus invasion[25] is a phenomenon of an excessive inflammatory reaction that rapidly produces many cytokines, especially IL-6, in response to microbial infection. This phenomenon has been considered an important contributor to COVID-19. Persistent stimulation by the virus may lead to a reduction in lymphocytes.[26–28] The pathogenesis of lymphocyte reduction in COVID-19 may be caused by the following mechanisms:
-
1.
translocation of lymphocytes from peripheral blood to the lungs,[3,29–30] gastrointestinal tracts, and/or lymphoid tissues[20,31];
-
2.
lymphocytes express the ACE2 receptor and may be a direct target of SARS-CoV-2 infection.[32]
Another very important feature is that lymphocyte count of the severe cases and critical recovered cases of COVID-19 increased gradually, while lymphocytes in dead cases remained at low levels, during the period of disease. In addition, the lymphocyte count of dead cases was significantly lower than that of critical recovered cases at almost every time point in the critical groups. This was a novel finding which, as far as we know, has not been previously discussed in the literature. Some studies have only shown that a significant reduction in lymphocytes is common in critically ill patients.[24] There are few studies on dynamic monitoring of lymphocyte changes. Changes in the number of lymphocytes are considered a hallmark of severe immune injury.[3,33] Recent studies have found that immunosuppression is more pronounced in critically ill patients with COVID-19, and low levels of CD4+ T and CD8+ T cells are common.[24] This may result in lymphocytes not being recovered in dead cases.
The third feature is that there is a very weak correlation of lymphocytes and leukocytes or neutrophils in critical cases. This is different from the significant negative correlation that we previously believed. In addition, several studies have addressed an increased level of neutrophils along with a decrease in lymphocyte numbers in patients with COVID-19.[3,21,34] Qin et al[35] found that severe cases were likely to have a higher neutrophil count but a lower lymphocyte count compared with non-severe patients; the neutrophil-lymphocyte ratio (NLR) thus tended to be higher in the severe group.[34] A rising neutrophil count and a falling lymphocyte count indicate the intensity of the inflammatory response and damage to the immune system, respectively. These findings indicated that neutrophils or lymphocytes could be a potential marker for predicting progression of SARS-CoV-2-infected patients. However, few studies have described the relationship between lymphocytes and leukocytes or neutrophils.
Interestingly, we also found that lymphopenia can be used as a marker for poor prognosis in COVID-19, in particular in patients with a low remaining lymphocyte count. Lymphocytes in dead cases with COVID-19 remained at a low level (Fig. 3 D). Through survival analysis, we found that although there was no significant difference in survival between group A (lymphocyte count < 1.1 × 109/L) and group B (lymphocyte count > 1.1 × 109/L) in critical cases at symptom onset, group A had a significantly lower survival than that of group B after the second time point (after an average of 6.5 days) (Fig. 5). Some studies also showed that a lower lymphocyte count could be a critical factor associated with disease severity and increased mortality, ARDS, need for ICU care, critical COVID-19, and severe COVID-19.[2,20] During hospitalization, most patients have significant lymphopenia, and over time, patients with a poor prognosis develop more significant lymphopenia. Patients with a remaining low lymphocyte count, in particular, were more likely to die. A lower remaining lymphocyte count was considered to be associated with the pathologic mechanisms in the death of patients with COVID-19, which may indicate poor clinical prognosis, which is helpful in the early screening of critically ill patients with COVID-19.[35,36]
Our study has several limitations. Firstly, the lack of a randomized control group means that we cannot draw definitive conclusions. In addition, the different time points in this analysis were used for the lymphocytopenia-related survival analysis, which have strengthened our findings; however, the sample size available was not of a sufficient size to conduct such an analysis. Despite this, the present results are in line with the previously demonstrated prognostic ability of a low lymphocyte count in COVID-19, and in a previously unstudied continuous dynamic monitoring of severe and critical patients. Therefore, we believe that the predictors demonstrated here are meaningful. In addition, more detailed patient information, particularly regarding clinical outcomes, was unavailable at the time of analysis. Greater effort should be made to tackle these issues in future studies.
5. Conclusions
Taken together, a lower remaining lymphocyte count after the first week following symptom onset is highly predictive of in-hospital death in adults with COVID-19. This predictor may help clinicians identify patients with a poor prognosis and may be useful for guiding clinical decision-making at an early stage. This study revealed a feasible quantitative tool as a prognostic indicator for COVID-19. The use of such tool could potentially reduce mortality of critical COVID-19 at early stages.
Author contributions
Conceptualization: Hong-Jun Zhang.
Data curation: Hong-Jun Zhang, Gang-Qiang Qi, Xing Gu, Xiao-Yan Zhang, Yan-Feng Fang, Hong Jiang, Yan-Jun Zhao.
Writing – original draft: Hong-Jun Zhang.
Writing – review & editing: Yan-Jun Zhao.
Footnotes
Abbreviations: ACE2 = Angiotensin converting enzyme II, ARDS = respiratory distress syndrome, CD = Cluster of Differentiation, COVID-19 = coronavirus disease-2019, CT = computed tomography, ICU = Intensive Care Unit, IL-6 = interleukin 6, IQR = interquartile range, MOF = multi-organ failure, NLR = the neutrophil-lymphocyte ratio, PaO2/FiO2 = artery partial pressure of oxygen/inspired oxygen fraction, RT-PCR = real-time reverse transcriptase-polymerase chain reaction, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, WHO = World Health Organization.
How to cite this article: Zhang HJ, Qi GQ, Gu X, Zhang XY, Fang YF, Jiang H, Zhao YJ. Lymphocyte blood levels that remain low can predict the death of patients with COVID-19. Medicine. 2021;100:28(e26503).
The authors have no funding to disclose.
The authors have no conflicts of interest to disclose.
Transparency declarations: None to declare.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Di Bella S, Cesareo R, De Cristofaro P, et al. Neck circumference as reliable predictor of mechanical ventilation support in adult inpatients with COVID-19: a multicentric prospective evaluation. Diabetes Metab Res Rev 2020;e3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Deng M, Qi Y, Deng L, et al. Obesity as a potential predictor of disease severity in young COVID-19 patients: a retrospective study. Obesity (Silver Spring) 2020;28:1815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fu T, Deng M, Wang X, et al. Predictor of poor prognosis of COVID-19 patients—liver injury. Expert Rev Gastroenterol Hepatol 2020;14:873–6. [DOI] [PubMed] [Google Scholar]
- [7].Gregory J, Raynaud L, Galy A, et al. Extension of COVID-19 pulmonary parenchyma lesions based on real-life visual assessment on initial chest CT is an independent predictor of poor patient outcome. Infect Dis (Lond) 2020;52:828–40. [DOI] [PubMed] [Google Scholar]
- [8].Herlekar R, Sur Roy A, Matson M. Hypoalbuminaemia in COVID-19 infection: a predictor of severity or a potential therapeutic target? J Med Virol 2020;https://www.ncbi.nlm.nih.gov/pubmed/32519791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liou JM, Chen MJ, Hong TC, Wu MS. Alteration of taste or smell as a predictor of COVID-19. Gut 2020;https://www.ncbi.nlm.nih.gov/pubmed/32620550 [DOI] [PubMed] [Google Scholar]
- [10].Luo M, Liu J, Jiang W, et al. IL-6 and CD8+ T cell counts combined are an early predictor of in-hospital mortality of patients with COVID-19. JCI Insight 2020;5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mo XN, Su ZQ, Lei CL, et al. Serum amyloid A is a predictor for prognosis of COVID-19. Respirology 2020;25:764–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ponti G, Ruini C, Tomasi A. Homocysteine as a potential predictor of cardiovascular risk in patients with COVID-19. Med Hypotheses 2020;143:109859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pranata R, Huang I, Lukito AA, Raharjo SB. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19: systematic review and meta-analysis. Postgrad Med J 2020;96:387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Smadja DM, Guerin CL, Chocron R, et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis 2020;23:611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ullah W, Basyal B, Tariq S, et al. Lymphocyte-to-C-reactive protein ratio: a novel predictor of adverse outcomes in COVID-19. J Clin Med Res 2020;12:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Violi F, Cangemi R, Romiti GF, et al. Is albumin predictor of mortality in COVID-19? Antioxid Redox Signal 2020;https://www.ncbi.nlm.nih.gov/pubmed/32524832 [DOI] [PubMed] [Google Scholar]
- [17].Yamasaki Y, Ooka S, Tsuchida T, et al. The peripheral lymphocyte count as a predictor of severe COVID-19 and the effect of treatment with ciclesonide. Virus Res 2020;198089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhao Q, Meng M, Kumar R, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis 2020;96:131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther 2020;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huang D, Lian X, Song F, et al. Clinical features of severe patients infected with 2019 novel coronavirus: a systematic review and meta-analysis. Ann Transl Med 2020;8:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020;117:10970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Feng X, Li S, Sun Q, et al. Immune-inflammatory parameters in COVID-19 cases: a systematic review and meta-analysis. Front Med (Lausanne) 2020;7:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ng CT, Snell LM, Brooks DG, Oldstone MB. Networking at the level of host immunity: immune cell interactions during persistent viral infections. Cell Host Microbe 2013;13:652–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fenwick C, Joo V, Jacquier P, Noto A, Banga R, Perreau M. T-cell exhaustion in HIV infection. Immunol Rev 2019;292:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lin L, Lu L, Cao W, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020;9:727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li J, He X, Yuanyuan, et al. Meta-analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. Am J Infect Control 2020;https://www.ncbi.nlm.nih.gov/pubmed/32540370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xu YH, Dong JH, An WM, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect 2020;80:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care 2020;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020;12:08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lin Y, Kim J, Metter EJ, et al. Changes in blood lymphocyte numbers with age in vivo and their association with the levels of cytokines/cytokine receptors. Immun Ageing 2016;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tatum D, Taghavi S, Houghton A, Stover J, Toraih E, Duchesne J. Neutrophil-to-lymphocyte ratio and outcomes in Louisiana Covid-19 patients. Shock 2020;54:652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 2020;71:762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li K, Chen D, Chen S, et al. Predictors of fatality including radiographic findings in adults with COVID-19. Respir Res 2020;21:146. [DOI] [PMC free article] [PubMed] [Google Scholar]


