Abstract
Corrected QT (QTc) interval prolongation has been associated with poor patient prognosis. In this study, we assessed the effects of different drugs and cardiac injury on QTc interval prolongation in patients with coronavirus disease 2019 (COVID-19).
The study cohort consisted of 395 confirmed COVID-19 cases from the Wuhan Union Hospital West Campus. All hospitalized patients were treated with chloroquine/hydroxychloroquine (CQ/HCQ), lopinavir/ritonavir (LPV/r), quinolones, interferon, Arbidol, or Qingfei Paidu decoction (QPD) and received at least 1 electrocardiogram after drug administration.
Fifty one (12.9%) patients exhibited QTc prolongation (QTc ≥ 470 ms). QTc interval prolongation was associated with COVID-19 severity and mortality (both P < .001). Administration of CQ/HCQ (odds ratio [OR], 2.759; 95% confidence interval [CI], 1.318–5.775; P = .007), LPV/r (OR, 2.342; 95% CI, 1.152–4.760; P = .019), and quinolones (OR, 2.268; 95% CI, 1.171–4.392; P = .015) increased the risk of QTc prolongation. In contrast, the administration of Arbidol, interferon, or QPD did not increase the risk of QTc prolongation. Notably, patients treated with QPD had a shorter QTc duration than those without QPD treatment (412.10 [384.39–433.77] vs 420.86 [388.19–459.58]; P = .042). The QTc interval was positively correlated with the levels of cardiac biomarkers (creatine kinase-MB fraction [rho = 0.14, P = .016], high-sensitivity troponin I [rho = .22, P < .001], and B-type natriuretic peptide [rho = 0.27, P < .001]).
In conclusion, QTc prolongation was associated with COVID-19 severity and mortality. The risk of QTc prolongation was higher in patients receiving CQ/HCQ, LPV/r, and quinolones. QPD had less significant effects on QTc prolongation than other antiviral agents.
Keywords: antiviral drugs, cardiac injury, coronavirus disease 2019, hydroxychloroquine, qingfei Paidu decoction, corrected QT prolongation
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic is continuing to impact populations around the globe. There is currently no effective treatment for patients with confirmed COVID-19. Corrected QT (QTc) prolongation is an important drug safety concern, including for drugs used for COVID-19.
Prolonged ventricular repolarization may lead to early afterdepolarizations, thereby inducing QTc prolongation that may provoke potentially fatal ventricular arrhythmias known as torsade de pointes (TdP).[1] Though usually self-limited, TdP may develop into ventricular fibrillation, causing sudden death.[2] QTc prolongation has been suggested to contribute to adverse outcomes. In a previous investigation of critically ill patients, patients with QTc prolongation were more likely to require prolonged hospitalization.[3] Gibbs et al[4] have demonstrated that QTc prolongation was a powerful predictor of short-term (30 days) and long-term (3 years) mortality in patients hospitalized for various reasons. QTc prolongation has also been associated with critical illness.[5,6] Nevertheless, there have been few investigations on the clinical implication of QTc prolongation in COVID-19 patients.
QTc prolongation can result from genetic variations or acquired factors. Clinical factors for acquired QTc prolongation include drug-related and non-drug-related risk factors.[7] Drugs are the most common cause for acquired QTc prolongation, and the role of cardiovascular factors is also of great concern.[7–9] Among drugs, chloroquine/hydroxychloroquine (CQ/HCQ), lopinavir/ritonavir (LPV/r), quinolones, and interferon have been shown to prolong QTc.[1,7,9] In patients with COVID-19, CQ/HCQ administration was associated with QTc prolongation.[10,11] However, the effects of other QTc-prolonging drugs used for the treatment of COVID-19 (LPV/r, quinolones, and interferon) on QTc interval remain unknown. Additionally, the effects of Arbidol and Qingfei Paidu decoction (QPD) on QTc interval should also be investigated as both are widely used for the treatment of COVID-19 in China. Prolonged QTc intervals in patients with cardiac injury have not been comprehensively analyzed during hospitalization for COVID-19. In the absence of safety data of drug therapies and combined effects of cardiac injury among patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), further studies are required to minimize arrhythmia during COVID-19 treatment.
2. Methods
2.1. Study design and population
In this retrospective and observational study, we analyzed data from 395 consecutive patients hospitalized at Wuhan Union Hospital West Campus of Huazhong University of Science and Technology from February 1, 2020 to April 30, 2020, one of the earliest designated hospitals for patients with COVID-19 in China. All patients with confirmed COVID-19 as per the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7), China” were enrolled in the study. Demographic data and information on comorbidities, laboratory parameters, electrocardiograms (ECGs), and treatment were collected from the electronic medical records.
Drugs used included QPD (one package per day for 6 days), LPV/r (400 mg/100 mg, twice per day for up to 10 days), quinolones (400 mg per day for at least 3 days), interferon (5 million IU, twice per day for up to 10 days), CQ/HCQ (500 mg, twice per day for 7 days), and Arbidol (200 mg, 3 times per day for up to 10 days). We only enrolled patients who were treated with these drugs. Patients without 12-lead ECG data after drug treatment were excluded from the study. Patients with ECGs unsuitable for accurate QT interval measurements were also excluded from the study. We only analyzed ECGs from patients with physiological electrolyte levels (3.5 mmol/L < potassium < 5.3 mmol/L; 137 mmol/L < sodium < 147 mmol/L; 2.0 mmol/L < calcium < 2.5 mmol/L; Fig. 1).
Figure 1.
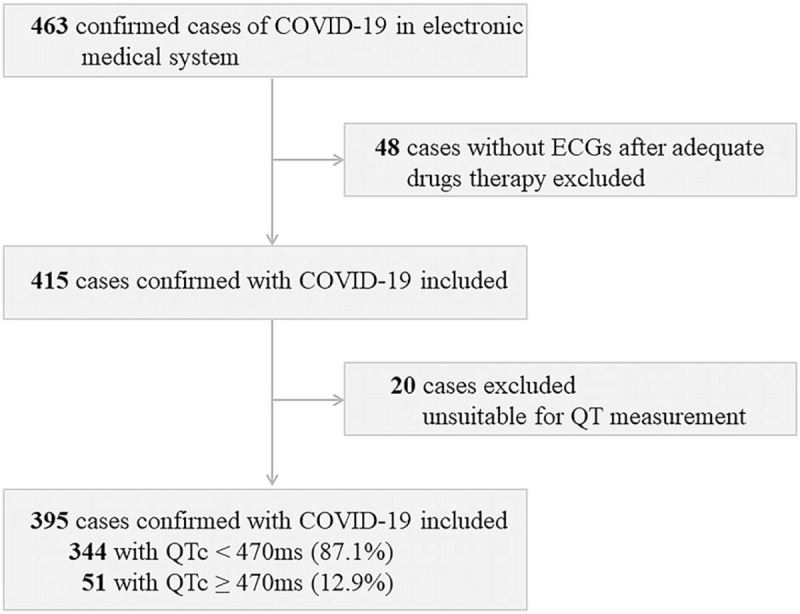
Flowchart of patient recruitment. COVID-19 = coronavirus disease 2019, ECG = electrocardiogram, QTc = corrected QT, ms = millisecond.
2.2. Definitions
The basic formula of QPD included Ephedra Herba (9 g), Glycyrrhizae Radix (6 g), Armeniacae Semen (9 g), Gypsum fibrosum (15–30 g), Cinnamomi Ramulus (9 g), Alismatis Rhizoma (9 g), Polyporus (9 g), Atractylodis macrocephalae Rhizoma (9 g), Poria (15 g), Bupleuri Radix (16 g), Scutellariae Radix (6 g), Pinellinae Rhizoma Praeparatum (9 g), Zingiberis Rhizoma recens (9 g), (Asteris Radix (9 g), Farfarae Flos (9 g), Belamcandae Rhizoma (9 g), Asari Radix et Rhizoma (6 g), Dioscoreae Rhizoma (12 g), Aurantii Fructus immaturus (6 g), Citri reticulatae Pericarpium (6 g), and Pogostemonis Herba (9 g).
QT was measured manually by at least 2 trained and experienced cardiologists; heart rate and QRS interval was automatically recorded and assessed from the ECGs. For the QT measurement, we used the “Tangent” technique. Briefly, after drawing a tangent down the steepest slope of the terminal limb of a specified T wave, the end of the T wave was defined as the intersection of this tangent with the baseline. Corrected QT was calculated using Bazett formula (heart rate <90 bpm [beat per minute]) or Fridericia formula (heart rate ≥90 bpm).[12] QTc prolongation was defined as ≥470 ms, which represents the 99th percentile of QTc distribution in the general population and has been associated with increased risk of TdP.[1,13]
2.3. Ethics
The study was conducted according to the Declaration of Helsinki, and the study protocol was approved by the institutional ethics board of the Wuhan Union Hospital of Huazhong University of Science and Technology (No. 20200254). Written informed consent was waived.
2.4. Power analysis and statistical analysis
The power analysis was conducted with G∗Power (version 3.1.9.7, Franz Faul, University of Kiel, Germany). The endpoint of interest was change in corrected QT intervals. To detect a medium effect of 0.30 with 90% power at an alpha level of 0.05, a sample size of 374 participants (325 in one group and 49 in the other group) was required. With 395 participants (344 patients with QT < 470 ms and 51 patients with QT ≥ 470 ms) included in this study, analytical power was sufficient to detect significant differences.
Categorical variables were reported as numbers and percentages. Continuous variables were expressed as medians and interquartile ranges (IQRs). Categorical variables were analyzed using the Chi-squared test or Fisher exact test, as appropriate. Continuous variables were analyzed using the Mann–Whitney U test or Student's t test depending on the distribution of the data determined by the Kolmogorov–Smirnov test; normally distributed data were analyzed using the Student t test. Kaplan–Meier survival curves and the log-rank test were used to assess differences in patient survival. All statistical analyses were performed using SPSS version 22.0 (IBM Corporation, Armonk, NY.); 2-sided P values <.05 were considered statistically significant.
3. Results
A total of 395 patients were enrolled in the study, and 51 (12.9%) patients presented with prolonged QTc intervals (QTc ≥ 470 ms). The demographic characteristics and drug therapy of the 395 patients confirmed with COVID-19 are detailed in Table 1. The median age of the patients was 61 years (IQR, 51–68 years). Patients with QTc ≥ 470 ms were older (66 [59–73] vs 60 [50–68]; P < .001) and had shorter time from symptom onset to hospitalization (35 [28–43] vs 45 [34–53]; P < .001). No comorbidities were found to be associated with a prolonged QTc interval.
Table 1.
Demographic characteristics and drugs therapy of COVID-19 patients.
| Characteristics | All patients | QTc < 470 ms | QTc ≥ 470 ms | P value |
| No. of patients | 395 (100.0%) | 344 (87.1%) | 51 (12.9%) | NA |
| Age, median (IQR), y | 61 (51–69) | 60 (50–68) | 66 (59–73) | <.001 |
| Male, n (%) | 190 (48.1%) | 164 (47.7%) | 26 (51.0%) | .659 |
| Time from symptom, median (IQR), d | 43 (33–53) | 45 (34–53) | 35 (28–43) | <.001 |
| Time from admission, median (IQR), d | 25 (17–37) | 25 (17.25–38) | 21 (16–29) | .093 |
| Comorbidities | ||||
| Hypertension, n (%) | 139 (35.2%) | 115 (33.4%) | 24 (47.1%) | .057 |
| Diabetes mellitus, n (%) | 59 (14.9%) | 49 (14.2%) | 10 (19.6%) | .316 |
| Coronary heart disease, n (%) | 40 (10.1%) | 32 (9.3%) | 8 (15.7%) | .158 |
| Heart failure, n (%) | 6 (1.5%) | 5 (1.5%) | 1 (2.0%) | .566 |
| Cardiomyopathy, n (%) | 4 (1.0%) | 3 (0.9%) | 1 (2.0%) | .426 |
| Chronic lung disease, n (%) | 14 (3.5%) | 10 (2.9%) | 4 (7.8%) | .170 |
| Drugs therapy during hospitalization | ||||
| Arbidol, n (%) | 323 (81.8%) | 277 (80.5%) | 46 (90.2%) | .095 |
| Lopinavir/Ritonavir, n (%) | 72 (18.2%) | 53 (15.4%) | 19 (37.3%) | <.001 |
| Quinolones, n (%) | 187 (47.3%) | 152 (44.2%) | 35 (68.6%) | .001 |
| Interferon, n (%) | 79 (20.0%) | 60 (17.4%) | 19 (37.3%) | .001 |
| Chloroquine/Hydroxychloroquine, n (%) | 52 (13.2%) | 38 (11.0%) | 14 (27.5%) | .001 |
| Qingfei Paidu decoction, n (%) | 294 (74.4%) | 263 (76.5%) | 31 (60.8%) | .017 |
| Clinical outcomes | ||||
| Discharged, n (%) | 348 (88.1%) | 314 (91.3%) | 34 (66.7%) | <.001 |
| Died, n (%) | 47 (11.9%) | 30 (8.7%) | 17 (33.3%) | |
| Disease severity | ||||
| Non-critical, n (%) | 300 (75.9%) | 273 (79.4%) | 27 (52.9%) | <.001 |
| Critical, n (%) | 95 (24.1%) | 71 (20.6%) | 24 (47.1%) |
Data are median (IQR) or n (%).
IQR = interquartile range, ms = millisecond, NA = not applicable, QTc = corrected QT.
Laboratory findings are presented in Table 2. Most laboratory parameters differed significantly between patients with and without prolonged QTc intervals (all P < .05), with the exception of alanine aminotransferase (P = .213). Notably, patients with QTc ≥ 470 ms had higher levels of creatine kinase-MB fraction, high-sensitivity troponin I, and B-type natriuretic peptide (all P < .001). The relationship between QTc interval and cardiac biomarkers in COVID-19 patients was assessed by Spearman rank correlation analysis, and the results are presented in Fig. 2. Importantly, the levels of creatine kinase-MB fraction (rho = 0.14, P = .016), high-sensitivity troponin I (rho = .22, P < .001), and B-type natriuretic peptide (rho = 0.27, P < .001) were positively correlated with the QTc interval (Fig. 2).
Table 2.
Laboratory findings of COVID-19 patients.
| Laboratory findings | All patients | QTc < 470 ms | QTc ≥ 470 ms | P value |
| WBC, median (IQR), G/L | 8.15 (6.26–11.70) | 7.91 (6.16–11.15) | 10.90 (6.70–18.16) | .003 |
| Lymphocyte, median (IQR), G/L | 0.91 (0.52–1.37) | 0.97 (0.55–1.42) | 0.53 (0.27–1.08) | <.001 |
| Eosinophil, median (IQR), G/L | 0.02 (0.00–0.09) | 0.03 (0.00–0.09) | 0.00 (0.00–0.06) | .001 |
| AST, median (IQR), U/L | 40 (27–70) | 38 (26–68) | 52 (33–90) | .009 |
| ALT, median (IQR), U/L | 58 (34–103) | 56.5 (33–103) | 65 (41–101) | .213 |
| Albumin, median (IQR), g/L | 29.30 (24.50–35.40) | 29.85 (25.03–35.88) | 26.70 (22.70–30.90) | <.001 |
| Creatinine, median (IQR), μmol/L | 72.00 (59.50–91.00) | 70.15 (58.83–88.05) | 81.00 (65.00–153.20) | .004 |
| BUN, median (IQR), mmol/L | 6.38 (4.96–8.80) | 6.15 (4.85–8.43) | 7.79 (6.13–17.37) | <.001 |
| CRP, median (IQR), mg/L | 23.65 (4.21–87.73) | 18.66 (4.02–79.98) | 71.15 (9.11–151.96) | .001 |
| Procalcitonin, median (IQR), ng/L | 0.08 (0.05–0.33) | 0.07 (0.05–0.24) | 0.16 (0.06–1.70) | .009 |
| Ferritin, median (IQR), ng/mL | 410.77 (180.41–950.32) | 369.86 (161.60–896.65) | 800.46 (468.46–1419.64) | .015 |
| Cardiac biomarkers | ||||
| CKMB, median (IQR), ng/mL | 0.80 (0.50–2.10) | 0.70 (0.50–1.70) | 2.00 (0.63–7.75) | <.001 |
| hs-TNI, median (IQR), ng/L | 4.80 (1.90–24.35) | 4.25 (1.80–16.55) | 23.35 (3.73–396.55) | <.001 |
| BNP, median (IQR), pg/mL | 37.10 (10.10–230.95) | 24.05 (10.00–147.13) | 165.70 (45.53–667.28) | <.001 |
Data are median (IQR) or n (%).
ALT = alanine aminotransferase, AST = aspartate aminotransferase, BNP = B-type natriuretic peptide, BUN = blood urea nitrogen, CK-MB = creatine kinase-MB fraction, CRP = C-reactive protein, hs-TNI = high-sensitivity troponin I, IQR = interquartile range, ms = millisecond, NA = not applicable, QTc = corrected QT, WBC = white blood cell.
Figure 2.

Correlation between cardiac biomarkers and corrected QT interval. Correlation between cardiac biomarkers and corrected QT interval. (A) CK-MB, (B) hs-TNI, (C) BNP. BNP = B-type natriuretic peptide, CK-MB = creatine kinase-MB fraction, hs-TNI = high-sensitivity troponin I.
Most of the patients (323 [81.8%]) received Arbidol. QPD, LPV/r, quinolones, interferon, and CQ/HCQ were administered to 294 (74.4%), 72 (18.2%), 187 (47.3%), 79 (20.0%), and 52 (13.2%) patients, respectively. Prolonged QTc intervals were more frequent in patients treated with LPV/r, quinolones, interferon, and CQ/HCQ (all P < .05). However, prolonged QTc intervals were less frequent in patients treated with QPD than those not receiving QPD, (31 [60.8%] vs 263 [76.5%]; P = .017; Table 1). We also assessed the effects of QPD on the characteristics of the ECG. Heart rate was lower in patients treated with QPD than those without QPD treatment (80 [71–88] vs 86 [75–100]; P < .001). Furthermore, QTc intervals were shorter in patients treated with QPD than those without QPD treatment (412.10 [384.39–433.77] vs 420.86 [388.19–459.58]; P = .042; Table 3). Multivariable logistic regression analyses revealed that compared with patients not receiving drugs, the risk of prolonged QTc intervals was higher in patients receiving drugs, including quinolones (odds ratios [OR], 2.268; 95% confidence interval [CI], 1.171–4.392; P = .015), LPV/r (OR, 2.342 95% CI, 1.152–4.760; P = .019), and CQ/HCQ (OR, 2.759; 95% CI, 1.318–5.775; P = .007). However, treatment with Arbidol (OR, 1.792; 95% CI, 0.658–4.878; P = .254), interferon (OR, 1.889; 95% CI, 0.938–3.804; P = .075), and QPD (OR, 0.731; 95% CI, 0.370–1.447; P = .369) did not affect the QTc interval (Fig. 3).
Table 3.
ECG characteristics of COVID-19 patients with and without QPD.
| ECG characteristics | All patients | Without QPD | With QPD | P value |
| No. of patients, n (%) | 395 (100.0%) | 101 (25.6%) | 294 (74.4%) | NA |
| ECG Characteristics | ||||
| Heart rate, median (IQR), bpm | 82 (72–91) | 86 (75–100) | 80 (71–88) | <.001 |
| QT, median (IQR), ms | 360 (340–400) | 360 (330–400) | 360 (340–400) | .550 |
| QTc, median (IQR), ms∗ | 413.00 (385.00–438.00) | 420.86 (388.19–459.58) | 412.10 (384.39–433.77) | .042 |
| QRS, median (IQR), ms | 84 (78–92) | 84 (76–97) | 84 (78–92) | .683 |
| Clinical outcomes | ||||
| Discharged, n (%) | 348 (88.1%) | 60 (59.4%) | 288 (98.0%) | <.001 |
| Died, n (%) | 47 (11.9%) | 41 (40.6%) | 6 (2.0%) | |
| Disease severity | ||||
| Non-critical, n (%) | 300 (76.0%) | 40 (39.6%) | 260 (88.4%) | <.001 |
| Critical, n (%) | 95 (24.0%) | 61 (60.4%) | 34 (11.6%) |
Data are median (IQR) or n (%).
bpm = beat per minute, COVID-19 = coronavirus disease 2019, ECG = electrocardiogram, IQR = interquartile range, ms = millisecond, NA = not applicable, QPD = Qingfei Paidu decoction, QTc = corrected QT.
QTc was calculated by Bazett's formula (heart rate < 90 bpm) or Fridericia's formula (heart rate ≥ 90 bpm).
Figure 3.

Effects of drug treatments on the risk of QTc prolongation. CI = confidence interval, CQ/HCQ = chloroquine/hydroxychloroquine, LPV/r = lopinavir/ritonavir, OR = odds ratio, QPD = Qingfei Paidu decoction.
QTc prolongation was associated with the clinical outcomes and disease severity in COVID-19 patients. Importantly, patients with QTc ≥ 470 ms had a higher risk of death (17 [33.3%] vs 30 [8.7%]; P < .001) and critical condition (24 [47.1%] vs 71 [20.6%]; P < .001), compared with patients with QTc < 470 ms. Kaplan–Meier survival analyses revealed that patients with QTc ≥ 470 ms had a higher risk of death than those with QTc < 470 ms, both during the time from symptom onset and from admission (all P < .001, Fig. 4).
Figure 4.

Kaplan–Meier survival curves for COVID-19 patients. Survival curves analyses for patients with QTc < 470 ms and QTc ≥ 470 ms during symptom onset (A) and admission (B).ms = milliseconds, QTc = corrected QT.
4. Discussion
In this study, we explored the influence of different parameters on QTc prolongation in patients infected with SARS-CoV-2. QTc prolongation (QTc ≥ 470 ms) was observed in 12.9% of hospitalized COVID-19 patients after drug therapy. Consistently, Hooks et al[14] reported that 8.2% of patients with COVID-19 had QTc ≥ 470 ms. Importantly, we found that QTc prolongation was associated with disease severity and increased mortality in COVID-19 patients; a prolonged QTc interval was associated with drug administration (quinolones, LPV/r, and CQ/HCQ); QPD, commonly used in Chinese medicine, had a lower effect on QTc interval.
Recent studies have shown that COVID-19 patients with prolonged QTc intervals had a higher risk of death and disease exacerbation during intensive care.[15,16] In this study, we found QTc prolongation (>470 ms) to be an aggravating factor associated with increased mortality. Previous studies have also reported higher levels of inflammatory markers (e.g., C-reactive protein and procalcitonin) in critically ill COVID-19 patients.[17] Here, we found that levels of C-reactive protein and procalcitonin were higher in patients with QTc ≥ 470 ms. Inflammation has also been implicated in QTc prolongation in patients with rheumatoid rrthritis,[18,19] suggesting that inflammatory responses may contribute to QTc prolongation associated with COVID-19 severity and mortality.
Our findings suggest that the administration of drugs during hospitalization could prolong the QTc interval in COVID-19 patients. Drugs previously linked to QTc prolongation (LPV/r, quinolones, interferon, CQ/HCQ), as well as those without known effects on the QTc interval (Arbidol, QPD) were included in our analyses. Patients treated with CQ/HCQ had the highest risk of QTc prolongation, followed by those treated with LPV/r and quinolones. CQ/HCQ, LPV/r, and quinolones have been demonstrated to prolong the QTc interval by blocking voltage-dependent potassium channels in HEK293 cells.[20–22] The strong correlation between CQ/HCQ administration and QTc prolongation could be due to the stronger blocking effects of CQ/HCQ on potassium channels compared with LPV/r and quinolones. The cardiovascular side effects of CQ/HCQ, LPV/r, and quinolones are well-demonstrated; these side effects include QTc interval prolongation and arrhythmias. Here, we found that QTc interval prolongation was significantly associated with the administration of CQ/HCQ, LPV/r, and quinolones. However, QPD treatment was not associated with QTc interval prolongation. There are currently no reports suggesting adverse cardiovascular effects of QPD. Hence, compared with other antiviral agents, QPD may be a better choice for COVID-19 patients with a history of cardiovascular diseases.
Interestingly, we found that higher levels of cardiac biomarkers were associated with QTc ≥ 470 ms, suggesting that cardiac injury may also have contributed to QTc prolongation. The QT interval is a summation of the duration of ventricular depolarization and repolarization; hence, any electrical disturbance of cardiomyocytes caused by cardiac injury may results in prolongation of the QT interval.[23] Multiple studies have shown that QTc prolongation is a predominant feature of cardiac injury.[24–26] It has also been demonstrated that cardiac injury was common in hospitalized patients with COVID-19.[27,28] Cardiac injury may prolong the QTc interval by promoting myocardial electrical instability in patients infected with SARS-CoV-2. The length of the QT interval is clinically important because QT prolongation can potentially predispose to life-threatening ventricular arrhythmias. The administration of certain drugs (CQ/HCQ, LPV/r, and quinolones) in our patients could have increased the risk of QTc prolongation. Our findings provide evidence that QTc prolongation can occur in association with cardiac injury. The possible reason for the QTc prolongation in patients with SARS-CoV-2 infection may be a combined effect of medication and cardiac injury. Hence, therapies or conditions associated with QT prolongation should be taken into consideration for the treatment of COVID-19 to avoid arrhythmic risks.
Our study had several limitations. First, this was a retrospective and observational study. Recall bias could not be eliminated and may have affected our evaluation. Second, because all patients needed to have ECGs suitable for QT measurement, the cohort size was relatively small. Nevertheless, the G∗power analysis demonstrated that the sample size, albeit small, provided sufficient power to detect statistical differences. Third, we did not include patients treated with each drug alone. Future clinical trials are required to further assess the effects of various drugs on QTc prolongation.
5. Conclusions
In this retrospective study, we found that QTc prolongation was associated with COVID-19 severity and mortality. Treatment with CQ/HCQ, LPV/r, and quinolones increased risk of QTc prolongation. Notably, QPD had a weaker effect on QTc prolongation than conventional antiviral agents, and it may be a better treatment choice for COVID-19 patients with a history of cardiovascular diseases. The QTc intervals of hospitalized COVID-19 patients should be closely monitored.
Author contributions
Conceptualization: Jiaxing Ding, Hongquan Guan, Zhijian Chen.
Data curation: Jiaxing Ding, Wei Liu, Hongquan Guan.
Formal analysis: Jiaxing Ding, Wei Liu.
Funding acquisition: Zhijian Chen.
Investigation: Yu Feng, Yintu Bao, Huili Li, Xuehua Wang, Hongquan Guan.
Methodology: Jiaxing Ding, Wei Liu.
Project administration: Hongquan Guan, Zhijian Chen.
Supervision: Zihua Zhou, Zhijian Chen.
Writing – original draft: Jiaxing Ding, Wei Liu.
Writing – review & editing: Hongquan Guan, Zhijian Chen.
Footnotes
Abbreviations: CI = confidence interval, COVID-19 = coronavirus disease 2019, CQ/HCQ = chloroquine/hydroxychloroquine, ECG = electrocardiogram, hs-TNI = high-sensitivity troponin I, IQR = interquartile range, LPV/r = lopinavir/ritonavir, NA = not applicable; OR = odds ratio, QPD = Qingfei Paidu decoction, QTc = corrected QT, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
How to cite this article: Ding J, Liu W, Guan H, Feng Y, Bao Y, Li H, Wang X, Zhou Z, Chen Z. Corrected QT interval in hospitalized patients with coronavirus disease 2019: Focus on drugs therapy. Medicine. 2021;100:28(e26538).
JD, WL and HG contributed equally to this work.
This work was funded by National Natural Science Foundation of China (Grant No. 81770330).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Trinkley KE, Page RN, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin 2013;29:1719–26. [DOI] [PubMed] [Google Scholar]
- [2].Moss AJ, Schwartz PJ, Crampton RS, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation 1991;84:1136–44. [DOI] [PubMed] [Google Scholar]
- [3].Pickham D, Helfenbein E, Shinn JA, et al. High prevalence of corrected QT interval prolongation in acutely ill patients is associated with mortality: results of the QT in practice (QTIP) study. Crit Care Med 2012;40:394–9. [DOI] [PubMed] [Google Scholar]
- [4].Gibbs C, Thalamus J, Kristoffersen DT, et al. QT prolongation predicts short-term mortality independent of comorbidity. Europace 2019;21:1254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ding Y, Jeon R, Ran L, et al. New-onset QT prolongation is a novel predictor of mortality in critically ill patients. Crit Care 2019;23:229.doi: 10.1186/s13054-019-2514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Russell H, Churilov L, Toh L, Eastwood GM, Bellomo R. The incidence, predictors and outcomes of QTc prolongation in critically ill patients. J Crit Care 2019;54:244–9. [DOI] [PubMed] [Google Scholar]
- [7].van Noord C, Eijgelsheim M, Stricker BH. Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol 2010;70:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jayasinghe R, Kovoor P. Drugs and the QTc interval. Aust Prescr 2002;25:63–5. [Google Scholar]
- [9].Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharm 2017;39:16–25. [DOI] [PubMed] [Google Scholar]
- [10].Chorin E, Wadhwani L, Magnani S, et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm 2020;17:1425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Naksuk N, Lazar S, Peeraphatdit TB. Cardiac safety of off-label COVID-19 drug therapy: a review and proposed monitoring protocol. Eur Heart J Acute Cardiovasc Care 2020;9:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bun SS, Taghji P, Courjon J, et al. QT interval prolongation under hydroxychloroquine/azithromycin association for inpatients with SARS-CoV-2 lower respiratory tract infection. Clin Pharmacol Ther 2020;108:1090–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mason JW, Ramseth DJ, Chanter DO, et al. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol 2007;40:228–34. [DOI] [PubMed] [Google Scholar]
- [14].Hooks M, Bart B, Vardeny O, Westanmo A, Adabag S. Effects of hydroxychloroquine treatment on QT interval. Heart Rhythm 2020;17:1930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Farre N, Mojon D, Llagostera M, et al. Prolonged QT interval in SARS-CoV-2 infection: prevalence and prognosis. J Clin Med 2020;9:2712.doi: 10.3390/jcm9092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bessiere F, Roccia H, Deliniere A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol 2020;5:1067–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barry M, Almohaya A, Alhijji A, et al. Clinical characteristics and outcome of hospitalized COVID-19 patients in a MERS-CoV endemic area. J Epidemiol Glob Health 2020;10:214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chauhan K, Ackerman MJ, Crowson CS, et al. Population-based study of QT interval prolongation in patients with rheumatoid arthritis. Clin Exp Rheumatol 2015;33:84–9. [PMC free article] [PubMed] [Google Scholar]
- [19].Lazzerini PE, Acampa M, Capecchi PL, et al. Association between high sensitivity c-reactive protein, heart rate variability and corrected QT interval in patients with chronic inflammatory arthritis. Eur J Intern Med 2013;24:368–74. [DOI] [PubMed] [Google Scholar]
- [20].Traebert M, Dumotier B, Meister L, et al. Inhibition of herg K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol 2004;484:41–8. [DOI] [PubMed] [Google Scholar]
- [21].Singh M, Arora R, Jawad E. HIV protease inhibitors induced prolongation of the QT interval: electrophysiology and clinical implications. Am J Ther 2010;17:e193–201. [DOI] [PubMed] [Google Scholar]
- [22].Alexandrou AJ, Duncan RS, Sullivan A, et al. Mechanism of herg K+ channel blockade by the fluoroquinolone antibiotic moxifloxacin. Br J Pharmacol 2006;147:905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lester RM, Paglialunga S, Johnson IA. QT assessment in early drug development: the long and the short of it. Int J Mol Sci 2019;20:1324.doi: 10.3390/ijms20061324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schwartz CL, Hobbie WL, Truesdell S, et al. Corrected QT interval prolongation in anthracycline-treated survivors of childhood cancer. J Clin Oncol 1993;11:1906–10. [DOI] [PubMed] [Google Scholar]
- [25].Sultan F, Kaur R, Mir AH, et al. Rosuvastatin and retinoic acid may act as ’pleiotropic agents’ against β-adrenergic agonist-induced acute myocardial injury through modulation of multiple signalling pathways. Chem Biol Interact 2020;318:108970.doi: 10.1016/j.cbi.2020.108970. [DOI] [PubMed] [Google Scholar]
- [26].Yang P, Han P, Hou J, et al. Electrocardiographic characterization of rhesus monkey model of ischemic myocardial infarction induced by left anterior descending artery ligation. Cardiovasc Toxicol 2011;11:365–72. [DOI] [PubMed] [Google Scholar]
- [27].Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Si D, Du B, Ni L, et al. Death, discharge and arrhythmias among patients with COVID-19 and cardiac injury. CMAJ 2020;192:E791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


