Abstract
Background:
Positive end-expiratory pressure (PEEP) is an important part of the lung protection strategies for one-lung ventilation (OLV). However, a fixed PEEP value is not suitable for all patients. Our objective was to determine the prevention of individualized PEEP on postoperative complications in patients undergoing one-lung ventilation.
Method:
We searched the PubMed, Embase, and Cochrane and performed a meta-analysis to compare the effect of individual PEEP vs fixed PEEP during single lung ventilation on postoperative pulmonary complications. Our primary outcome was the occurrence of postoperative pulmonary complications during follow-up. Secondary outcomes included the partial pressure of arterial oxygen and oxygenation index during one-lung ventilation.
Result:
Eight studies examining 849 patients were included in this review. The rate of postoperative pulmonary complications was reduced in the individualized PEEP group with a risk ratio of 0.52 (95% CI:0.37–0.73; P = .0001). The partial pressure of arterial oxygen during the OLV in the individualized PEEP group was higher with a mean difference 34.20 mm Hg (95% CI: 8.92–59.48; P = .0004). Similarly, the individualized PEEP group had a higher oxygenation index, MD: 49.07mmHg, (95% CI: 27.21–70.92; P < .0001).
Conclusions:
Individualized PEEP setting during one-lung ventilation in patients undergoing thoracic surgery was associated with fewer postoperative pulmonary complications and better perioperative oxygenation.
Keywords: lung-protective ventilation strategy, one-lung ventilation, positive end-expiratory pressure
1. Introduction
Postoperative pulmonary complications have a strong effect on the morbidity and mortality of patients who have suffered surgery.[1–3] In thoracic surgery, one-lung ventilation is prone to volutrauma, barotrauma, atelectrauma, and oxygen toxicity, which are important aspects of ventilator-induced lung injury.[4] Intraoperative lung-protective ventilation strategy has been recommended to reduce postoperative pulmonary complications.[5–8] The term “protective ventilation” was defined as the combination of low tidal volumes, positive end-expiratory pressure (PEEP), and recruitment maneuvers.[9,10] During mechanical ventilation, low tidal volumes are considered to reduce intrapulmonary strain and stress, while the recruitment maneuvers and PEEP are used to avoid atelectasis formation and to maintain blood oxygenation.[11] However, there is no verdict as to whether high or low levels of intraoperative PEEP are better to reduce postoperative pulmonary complications.
Several randomized controlled trials (RCTs) of intraoperative ventilation showed that reduced tidal volume combined with high levels of PEEP during intraoperative ventilation prevents postoperative pulmonary complications.[6,8,12] However, other RCTs have shown no difference in the development of postoperative pulmonary complications after intraoperative ventilation with low tidal volumes with either high or low levels of PEEP.[13,14]
Previous evidence suggests that 1 fixed PEEP value is unlikely to be appropriate for all patients and that there is considerable variability in the requirements for PEEP due to individual characteristics such as chest wall dimensions and shape, abdominal content, lung weights, and pleural pressures.[15–21] Application of individualized optimal PEEP intraoperatively not only reduces driving pressure and improves respiratory compliance and oxygenation but also reduces the incidence and severity of postoperative atelectasis.[22–25] It has not been reported for thoracic anesthesia where isolated, inflated lungs may be especially at risk. Therefore, we conducted a meta-analysis of RCTs to investigate the effect of individualized PEEP on one-lung ventilation during thoracic surgery.
2. Method
Ethical approval and patient consent are not required because this is a systematic review and meta-analysis of previously published studies. This investigation was conducted following the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” statement recommended process.[26] The protocol was registered on PROSPERO. The electronic databases PubMed, Embase, Cochrane were searched until May, 2020, and the following words were searched as keywords (individualized positive end-expiratory pressure OR individualized PEEP OR individual positive end-expiratory pressure OR individual PEEP OR personalized PEEP) AND (one-lung ventilation OR single lung ventilation OR Thoracic surgery). The results of this search strategy were limited to RCTs and humans, we excluded case reports and observational studies. No language limits were placed on the search. We screened all articles after excluding duplicates and checked the reference lists of selected articles for other relevant studies.
The Cochrane Collaboration's Risk of Bias Tool for RCTs was employed to assess the methodological quality of each randomized trial, considering the following possible sources of bias: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessors; incomplete outcome data; selective outcome reporting; and other bias. The 2 participants independently assessed the risk of bias for the selected articles.
The primary outcome was the occurrence of postoperative pulmonary complications during follow-up. The secondary outcome was the PaO2 and oxygenation index during one-lung ventilation. We use the Review Manager software (RevMan version 5.4) to conduct the meta-analyses. The coefficient I2 was calculated to evaluate heterogeneity, with predetermined thresholds defined for low (25%–49%), moderate (50%–74%), and high (>75%) levels. In cases of moderate or high heterogeneity, a random-effects model was applied; otherwise, a fixed-effect model was employed. Whenever significant heterogeneity is present, we search for potential sources of heterogeneity via omitting 1 study in turn for the meta-analysis. Publication bias is not evaluated because of the limited number (<10) of included studies.
For dichotomous outcomes, we calculated risk ratios (RR) with 95%CI, and for continuous outcomes, we used the mean difference (MD). When the continuous outcome was reported in some studies as median, range, and interquartile range, we estimated means and standard deviations using the method described by Weir et al.[27] For all analyses, P values less than .05 were considered significant.
3. Results
In our initial electronic search, we identified 726 potential articles. No additional studies were detected through manual scrutiny of reference lists of studies. After removal of duplicates, non-RCTs, and non-full texts, we screened 125 articles by title and abstract for eligibility. From these studies, we only included 8 trials for full-text evaluation.[22,28–34] We excluded 1 cross-over trial due to an unreasonable PEEP setting[35] (Fig. 1). A total number of 849 participants were included in the 8 studies. All participants were adult patients with American Society of Anesthesiologists physical status I–III. Application of the Cochrane Collaboration Risk of Bias tool (Fig. 2) suggested that the majority of trials had a low risk of bias. Publication bias was not assessed because the number of included studies was insufficient to explore a funnel plot or use more advanced regression-based assessments appropriately.
Figure 1.
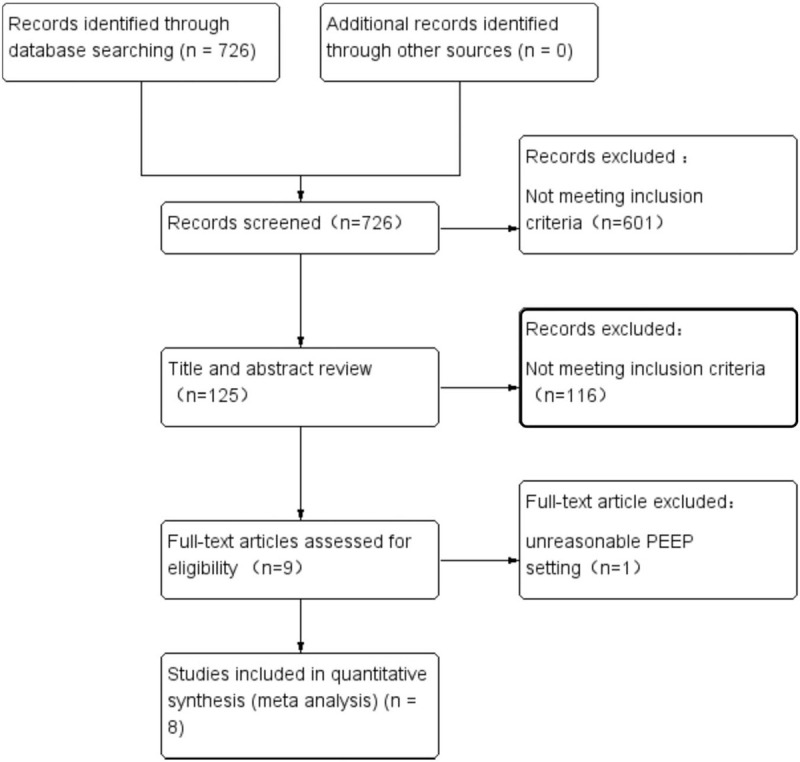
PRISMA flow diagram showing literature search results. Eight randomized controlled trials were included in the analysis. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Figure 2.
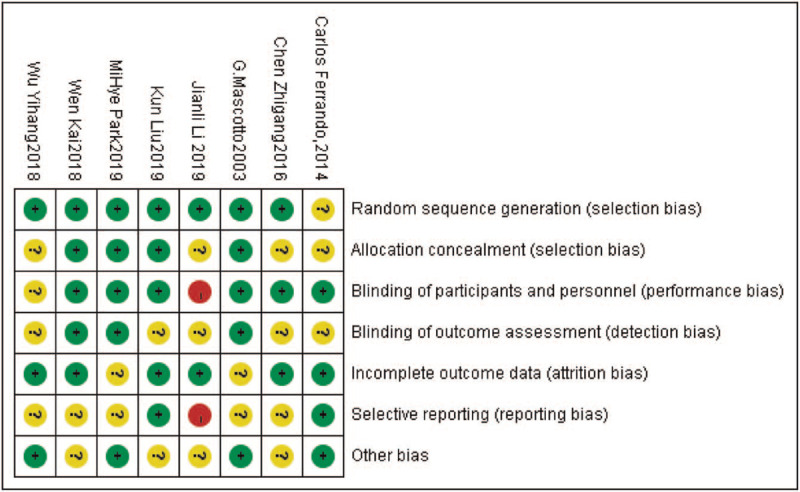
Risk of bias summary of included trials: evaluation of bias risk items for each included study. Green circle, low risk of bias; red circle, high risk of bias; yellow circle, unclear.
Table 1 presents the trial characteristics. Seven trials[22,28,29,31–34] PEEP fixed at 5 cm H2O in their control group, 1 trial[30] set zero PEEP as the control group. One trial[29] determined the individualized PEEP by electrical impedance tomography, 1 trial[31] by Pulmonary ultrasound, and 5 trials[22,30,32–34] determined PEEP by thoracopulmonary compliance measured. One[28] trial set the individualized PEEP to produce the lowest driving pressure. Regarding the ventilation patterns during one-lung ventilation, patients in 6 trials[28–33] underwent volume-controlled ventilation (VCV), while patients in 1 trial[22] experienced pressure-controlled ventilation (PCV), and in 1 study[34] PCV and VCV are reported and compared.
Table 1.
The characteristics of the included studies.
| Reference | Population (n) | Surgery | Control group | Study group | Outcomes |
| Park et al (2019) | Control: n = 145 individualized PEEP: n = 147 | Elective pulmonary resection or esophagectomy | Protective ventilation group: PEEP 5 cm H2O | The driving pressure group: individualized PEEP | Postoperative complication, pneumonia or ARDS, in-hospital deaths, durations of intensive care unit stay, hospital stay, cerebral ischemic events, atrial fibrillation |
| Ferrando et al (2014) | Control: n = 15 individualized PEEP: n = 15 | Elective lung resection | Control group: PEEP 5 cm H2O | Study group: individualized PEEP | Dynamic compliance, oxygenation during OLV, airway resistance, cardiac index |
| Liu et al (2019) | PEEP5 group: n = 50 PEEPEIT group: n = 50 | Pneumonectomy, wedge resection, lobectomy, wedge + lobectomy | Control group: PEEP 5 cm H2O | Study group: individualized PEEP by EIT | pH, PCO2, PaO2/FiO2, Cdyn, Ppeak, Pmean, and Pplat during OLV, use of vasoactive drugs, lung complications, duration of hospitalization |
| Mascott et al (2003) | Control: n = 22 individualized PEEP: n = 28 | Pneumonectomy, lobectomy, atypical lung resection | Control group: receive zero PEEP | Study group: individualized PEEP on the best thoracopulmonary compliance | Lung chest wall compliance, PaO2/FiO2, hypoxic events during OLV, postanesthesia care unit discharge |
| Wu (2018) | Control: n = 28 individualized PEEP: n = 28 | Thoracoscopic lobectomy | control group: PEEP 5 cm H2O | Study group: individualized PEEP on the maximal static pulmonary compliance | Static pulmonary compliance, PaO2/FiO2 during OLV, Length of stay days of indwelling drainage tube, postoperative pulmonary complications |
| Chen (2016) | Control: n = 39 individualized PEEP: n = 39 | Video-assisted right pulmonary lobectomy | Control group: PEEP 5 cm H2O | Study group: individualized PEEP | Dynamic compliance, arterial blood gas analysis during OLV |
| Wen (2018) | Control: n = 33 individualized PEEP: n = 34 | Elective lobectomy | General lung protective ventilation group (P group): PEEP 5 cm H2O | Pulmonary ultrasound (L group): individualized PEEP by lung ultrasonography | Intraoperative hypoxic events, PaO2/FiO2 value during OLV, postoperative pulmonary complications, postoperative pain scores, cough, sputum, hospital stay |
| Li et al (2020) | PCV + OLA group: n = 45 PCV group: n = 44 VCV + OLA group: n = 45 VCV group: n = 42 | Lobectomy wedge resection segmentectomy | PCV group: PEEP 5 cm H2O VCV group: PEEP 5 cm H2O | PCV + OLA group: PEEP produce the greatest dynamic compliance (Cdyn) VCV + OLA group: PEEP produce the greatest Cdyn | PaCO2, pH, PaO2/FiO2, plasma concentration of neutrophil elastase, postoperative pneumonia, atelectasis, acute respiratory failure, duration of intensive care unit stay, duration of hospital stay |
EIT = electrical impedance tomography, OLA = open-lung approach, OLV = one-lung ventilation, PCV = pressure-controlled ventilation, PEEP = positive end-expiratory pressure, VCV = volume-controlled ventilation.
Six studies[28–32,34] reported postoperative pulmonary complications. However, in 1 study, only postoperative radiological examination suggested atelectasis.[30] All reported postoperative pulmonary complications were followed up during the hospital stay. Two trials[28,34] reported the occurrence of postoperative acute respiratory distress syndrome (ARDS), in which the total number of patients who developed pneumonia or ARDS within a postoperative day were included in our analysis. Fixed effect models were chosen to reflect the heterogeneity of settings, interventions, and patient populations of the included studies. The number of patients with postoperative pulmonary complications was 42/375 (11.2%) in the individualized PEEP group and 79/366 (21.6%) in the control group, risk ratio: 0.52 (95% CI: 0.37–0.73; P = .0001) (Fig. 3).
Figure 3.
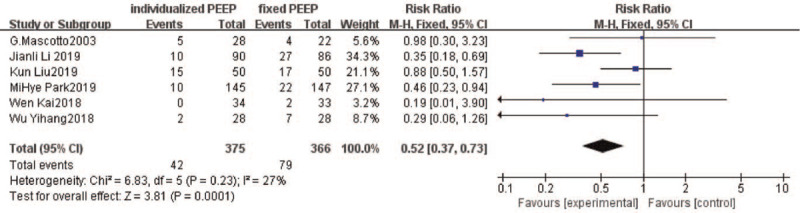
Forest plot of pooled data for the number of patients with postoperative pulmonary complications. CI = confidence interval.
Oxygenation during OLV was reported in 4 trials.[22,28,32,33] In regard to the time points, 1 trial[28] measured PaO2 15 minutes after the PEEP setting during one-lung ventilation, 1 trial[22] measured 20 minutes after PEEP setting, 1[32] measured 30 min after PEEP setting, and 1[33] after 60 minutes. Although the time points of measurement were different, no significant heterogeneity was detected in the results (Fig. 4). The MD in PaO2 during the OLV between the individualized PEEP group and control groups was 34.20 mm Hg, (95% CI: 8.92–59.48; P = .0004).
Figure 4.
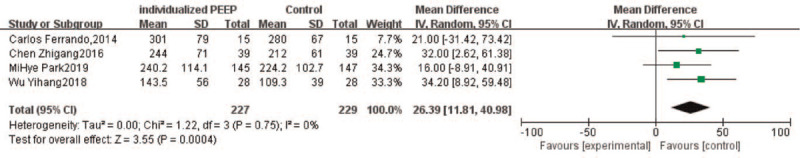
Forest plot of PaO2 during one-lung ventilation. CI = confidence interval.
Five studies[29–32,34] reported the oxygenation index (PaO2/FiO2) during one-lung ventilation, 1[30] of which was reported graphically but did not specify what the error bars represented, and for this reason, we excluded this study from our meta-analysis. One study[34] reported the PaO2/FiO2 in different ventilation patterns (VCV or PCV), we incorporated the results into the analysis separately, In all included studies, PaO2/FiO2 decreased in both groups during OLV compared with DLV. Our analysis of PaO2/FiO2 during OLV resulted in a higher level in the individualized PEEP group compared with the control group (MD: 49.07 mmHg; 95% CI: 27.21–70.92; P < .0001) (Fig. 5). However, we detected a moderate degree of heterogeneity between the studies (I2 = 57%). To further explore potential causes of this high heterogeneity, we performed a sensitivity analysis by omitting 1 study from our pooled data synthesis.[31] The results of this analysis demonstrated that setting an individualized PEEP during one-lung ventilation leads to higher oxygenation index compared with setting a fixed PEEP with reduced heterogeneity, the MD (95%CI) being 37.72 (22.53–52.9) mmHg, I2 = 13%, P < .00001.
Figure 5.
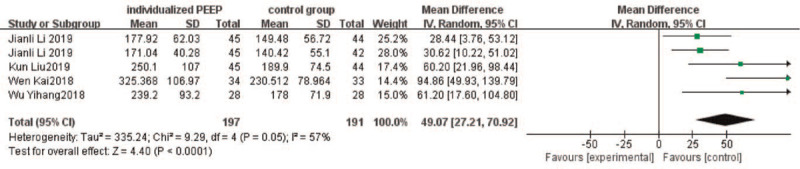
Oxygenation index (PaO2/FiO2) during one-lung ventilation. CI = confidence interval.
4. Discussion
This meta-analysis investigated the effect of individualized PEEP, compared with constant PEEP during one-lung ventilation on postoperative pulmonary complications. The setting of individualized PEEP during one-lung ventilation is of positive significance for reducing postoperative pulmonary complications. Although the included studies had different ventilation patterns (VCV or PCV) during one-lung ventilation, the resulting heterogeneity was minimal.
There are several types of PEEP titration methods to determine the individual PEEP, such as static or dynamic pulmonary compliance directed methods, electrical impedance tomography, esophageal manometry, and transpulmonary pressure directed PEEP titration procedures.[36] The optimum PEEP is the PEEP level that results in the greatest respiratory system compliance.[37,38] In the absence of previous lung injury, mechanical ventilation can destroy the fragile intercellular matrix structure of the lung.[39] General and local ischemia-reperfusion induced by hypotension, and hypoperfusion, surgical intervention, intraoperative blood loss, as well as tissue trauma itself, might lead to the release of inflammatory mediators and spread of bacteria that can prime the lungs further to the stress of mechanical ventilation.[40] Titrating PEEP to achieve individual optimal levels has a strong pathophysiological rationale with potential benefits.[37] Individualized PEEP may potentially prevent alveolar collapse in the dependent lung in the lateral posture, increase the residual volume, improve the ventilation/perfusion ratio, reduce the shear damage caused by periodic opening and closing of the alveoli.[29] Patients with thoracic surgery often have a potential difference in their respiratory compliance because of mass size or site or frequently accompanying lung disease.[28] For these reasons, fixed-setting PEEP may lead to over-distend lungs or under-ventilated lungs.[28] In addition, the use of 100% FiO2 is the first rescue therapy in the presence of hypoxemia. In this case, the use of an individualized level of PEEP would prevent reabsorptive atelectasis more than a standardized level of PEEP.[22]
The PaO2 and PaO2/FiO2 during OLV were higher in the individualized PEEP group than the control group. Although the PaO2 in the study group was higher than that in the control group, due to the management of intraoperative ventilation, PaO2 in the 2 groups was not lower than normal. These promising results were still clinically advantageous. A change in PaO2/FiO2 ratios has been shown to represent a much more sensitive endpoint for ventilatory settings.[35] However, both criteria provide information on oxygenation reduction, and within safe ranges. The main mechanism by which PEEP improves oxygenation is the reduction of the right-to-left pulmonary shunt by keeping the alveolar open.[41]
However, we detected a high degree of heterogeneity in the analysis of PaO2/FiO2 during OLV, the reason may be the difference in the time point of blood gas analysis. In 1 study,[31] blood gas analysis during one-lung ventilation was performed immediately after PEEP setting, while in other studies, PaO2/FiO2 calculations were performed 30 to 60 minutes after the start of one-lung ventilation.
Our study is substantially different from the previous analysis of individualized PEEP in several respects. We focused exclusively on non-critically ill patients with uninjured lungs undergoing short-term ventilation for surgery. Thus, our results extend knowledge about protective ventilation and the potential role of the individualized PEEP. The protective role of intraoperative PEEP has been a matter of intense debate. This should be confirmed in future RCTs, in which the benefits of intraoperative ventilation strategies aiming at individualized PEEP for one-lung ventilation are determined.
Our meta-analysis had several limitations. First, postoperative pulmonary complications included postoperative lung injury, atelectasis, pulmonary infection, or barotrauma. Since only 2 of the trials included in the analysis provided subgroup data, we did not conduct a subgroup analysis. Second, the agreement of definitions of postoperative pulmonary complications and timeframe of diagnosis was heterogeneous among the included studies. Third, in 1 study,[30] the postoperative radiological examination showed atelectasis, but the pulmonary complications were not followed up. We still include corresponding data, for a fair majority of studies suggests that postoperation atelectasis is harmful. It can last for several days after surgery, increasing pulmonary complications, impairing respiratory function, and ultimately delaying patient discharge.[25,42,43] Finally, our study neglected thoracic surgery in children, so further research is needed for pediatric patients.
In conclusion, in patients undergoing one-lung ventilation, individualized PEEP is associated with less postoperative pulmonary complications and better perioperative oxygenation. However, to confirm these findings, some large randomized clinical trials are necessary.
Author contributions
All authors conceived and designed the study. Pule Li and Xia Kang conducted the literature review. Pule Li and Xia Kang abstracted the data. Pule Li conducted the analysis and drafted the manuscript. All authors revised the manuscript for important intellectual content.
Conceptualization: Pule Li, Mengrong Miao.
Data curation: Xia Kang.
Formal analysis: Pule Li, Xia Kang, Mengrong Miao, Jiaqiang Zhang.
Investigation: Pule Li.
Methodology: Pule Li.
Resources: Pule Li.
Software: Pule Li.
Supervision: Jiaqiang Zhang.
Visualization: Pule Li.
Writing – original draft: Pule Li.
Writing – review & editing: Pule Li, Mengrong Miao, Jiaqiang Zhang.
Footnotes
Abbreviations: ARDS = acute respiratory distress syndrome, CI = confidence interval, EIT = electrical impedance tomography, OLV = one-lung ventilation, PCV = pressure-controlled ventilation, PEEP = positive end-expiratory pressure, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCTs = randomized controlled trial, VCV = volume-controlled ventilation.
How to cite this article: Li P, Kang X, Miao M, Zhang J. Individualized positive end-expiratory pressure (PEEP) during one-lung ventilation for prevention of postoperative pulmonary complications in patients undergoing thoracic surgery: a meta-analysis. Medicine. 2021;100:28(e26638).
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Neto AS, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012;58:1651–9. [DOI] [PubMed] [Google Scholar]
- [2].Hemmes SNT, Neto AS, Schultz MJ. Intraoperative ventilatory strategies to prevent postoperative pulmonary complications: a meta-analysis. Curr Opin Anaesthesiol 2013;26:126–33. [DOI] [PubMed] [Google Scholar]
- [3].Serpa Neto A, Hemmes SNT, Barbas CSV, et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med 2014;1007–15. [DOI] [PubMed] [Google Scholar]
- [4].Lohser J. Evidence-based management of one-lung ventilation. Anesthesiol Clin 2008;26:241–72. [DOI] [PubMed] [Google Scholar]
- [5].Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery? Chest 2011;139:530–7. [DOI] [PubMed] [Google Scholar]
- [6].Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 2013;118:1307–21. [DOI] [PubMed] [Google Scholar]
- [7].Güldner A, Kiss T, Neto AS, et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications. Anesthesiology 2015;123:692–713. [DOI] [PubMed] [Google Scholar]
- [8].Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013;369:428–37. [DOI] [PubMed] [Google Scholar]
- [9].Neto AS, Simonis FD, Barbas CSV, et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome. Crit Care Med 2015;28:2155–63. [DOI] [PubMed] [Google Scholar]
- [10].Futier E, Constantin JM, Jaber S. Protective lung ventilation in operating room: a systematic review. Minerva Anestesiol 2013;80:726–35. [PubMed] [Google Scholar]
- [11].Stberg E, Thorisson A, Enlund M, Zetterstrm H, Hedenstierna G, Edmark L. Positive end-expiratory pressure alone minimizes atelectasis formation in nonabdominal surgery: a randomized controlled trial. Anesthesiology 2018;128:01. [DOI] [PubMed] [Google Scholar]
- [12].Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Eng J Med 2013;369:2126–36. [DOI] [PubMed] [Google Scholar]
- [13].Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ. PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014;384:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Treschan TA, Schaefer M, Kemper J, et al. Ventilation with high versus low peep levels during general anaesthesia for open abdominal surgery does not affect postoperative spirometry: a randomised clinical trial. Eur J Anaesthesiol 2017;34:534–43. [DOI] [PubMed] [Google Scholar]
- [15].Fumagalli J, Berra L, Zhang C, et al. Transpulmonary pressure describes lung morphology during decremental positive end-expiratory pressure trials in obesity. Crit Care Med 2017;45:1374–81. [DOI] [PubMed] [Google Scholar]
- [16].Karsten J, Grusnick C, Paarmann H, Heringlake M, Heinze H. Positive end-expiratory pressure titration at bedside using electrical impedance tomography in post-operative cardiac surgery patients. Acta Anaesthesiol Scand 2015;59:723–32. [DOI] [PubMed] [Google Scholar]
- [17].Maisch S, Reissmann H, Fuellekrug B, et al. Compliance and dead space fraction indicate an optimal level of positive end-expiratory pressure after recruitment in anesthetized patients. Anesth Analg 2008;106:175–81. [DOI] [PubMed] [Google Scholar]
- [18].Ferrando C, Soro M, Canet J, et al. Rationale and study design for an individualized perioperative open lung ventilatory strategy (iPROVE): study protocol for a randomized controlled trial. Trials 2015;16:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pirrone M, Fisher D, Chipman D, et al. Recruitment maneuvers and positive end-expiratory pressure titration in morbidly obese ICU patients. Crit Care Med 2016;44:300–7. [DOI] [PubMed] [Google Scholar]
- [20].Nestler C, Simon P, Petroff D, et al. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: a randomized controlled clinical trial using electrical impedance tomography. Br J Anaesth 2017;119:1194–205. [DOI] [PubMed] [Google Scholar]
- [21].Ferrando C, Tusman G, Suarez-Sipmann F, et al. Individualized lung recruitment maneuver guided by pulse-oximetry in anesthetized patients undergoing laparoscopy: a feasibility study. Acta Anaesthesiol Scand 2018;62:608–19. [DOI] [PubMed] [Google Scholar]
- [22].Ferrando C, Mugarra A, Gutierrez A, et al. Setting individualized positive end-expiratory pressure level with a positive end-expiratory pressure decrement trial after a recruitment maneuver improves oxygenation and lung mechanics during one-lung ventilation. Anesth Analg 2014;118:657–65. [DOI] [PubMed] [Google Scholar]
- [23].Marret E, Cinotti R, Berard L, et al. Protective ventilation during anaesthesia reduces major postoperative complications after lung cancer surgery. Eur J Anaesthesiol 2018;35:727–35. [DOI] [PubMed] [Google Scholar]
- [24].Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg 2016;152:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pereira SM, Tucci MR, Morais CCA, et al. Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology 2018;129:1070–81. [DOI] [PubMed] [Google Scholar]
- [26].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [27].Weir CJ, Butcher I, Assi V, et al. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: a systematic review. BMC Med Res Methodol 2018;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Park MH, Ahn HJ, Kim JA, et al. Driving pressure during thoracic surgery: a randomized clinical trial. Anesthesiology 2019;130:385–93. [DOI] [PubMed] [Google Scholar]
- [29].Liu K, Huang C, Xu M, et al. PEEP guided by electrical impedance tomography during one-lung ventilation in elderly patients undergoing thoracoscopic surgery. Ann Transl Med 2019;7:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mascotto G, Bizzarri M, Messina M, et al. Prospective, randomized, controlled evaluation of the preventive effects of positive end-expiratory pressure on patient oxygenation during one-lung ventilation. Eur J Anaesthesiol 2003;20:704–10. [DOI] [PubMed] [Google Scholar]
- [31].Dalian Medical University, Wen K. Lung ultrasound score to investigate thoracic surgery during single lung ventilation comparison of best PEEP with traditional lung protection ventilation. 2018. [Google Scholar]
- [32].Fujian Medical University, Wu Y. Effect of compliance guided an optimal Positive End-Expiratory Pressure on arterial oxygenation and intrapulmonary shunt during one-lung ventilation. 2018. [Google Scholar]
- [33].Second Military Medical University, Chen Z. The clinical application of lung protective ventilation strategies in the video assisted thoracic lobectomy. 2016. [Google Scholar]
- [34].Li J, Cai B, Yu D, Liu M, Wu X, Rong J. Pressure-controlled ventilation-volume guaranteed mode combined with an open-lung approach improves lung mechanics, oxygenation parameters, and the inflammatory response during one-lung ventilation: a randomized controlled trial. BioMed Res Int 2020;2020:01–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rozé H, Lafargue M, Perez P, et al. Reducing tidal volume and increasing positive end-expiratory pressure with constant plateau pressure during one-lung ventilation: effect on oxygenation. Br J Anaesth 2012;108:1022–7. [DOI] [PubMed] [Google Scholar]
- [36].Wanderer JP, Rathmell JP. Personalized PEEP: options for getting it just right. Anesthesiology 2018;129:A21. [Google Scholar]
- [37].Spadaro S, Karbing DS, Mauri T, et al. Effect of positive end-expiratory pressure on pulmonary shunt and dynamic compliance during abdominal surgery. Br J Anaesth 2016;6:855–61. [DOI] [PubMed] [Google Scholar]
- [38].Ruszkai Z, Kiss E, László I, et al. Effects of intraoperative positive end-expiratory pressure optimization on respiratory mechanics and the inflammatory response: a randomized controlled trial. J Clin Monit Comput 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Neto AS, Hemmes SNT, Barbas CSV, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med 2016;4:272–80. [DOI] [PubMed] [Google Scholar]
- [40].Fernandez-Bustamante A, Wood CL, Tran ZV, Moine P. Intraoperative ventilation: incidence and risk factors for receiving large tidal volumes during general anesthesia. BMC Anesthesiol 2011;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gattinoni L, Collino F, Maiolo G, et al. Positive end-expiratory pressure: how to set it at the individual level. Ann Transl Med 2017;5:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hedenstierna GR, Edmark L. Protective ventilation during anesthesia: is it meaningful? Anesthesiology 2016;125:1079–82. [DOI] [PubMed] [Google Scholar]
- [43].Leme AC, Hajjar LA, Volpe MS, et al. Effect of intensive vs moderate alveolar recruitment strategies added to lung-protective ventilation on postoperative pulmonary complications: a randomized clinical trial. JAMA 2017;317:1422–32. [DOI] [PubMed] [Google Scholar]


