Abstract
Introduction:
Our recent research indicated that cognitive speed of processing training (SPT) improved Useful Field of View (UFOV) among individuals with Parkinson’s disease (PD). The effects of SPT in PD have not been further examined.
Methods:
The current study investigated maintenance of training gains 3 months after post-test and examined dose effects. Indicators and predictors of continued participation in SPT were explored. Mixed effects models examined the durability of training gains among those randomized to SPT (n=44), and training dose effects among the entire sample (n=87).
Results:
The majority of participants chose to continue to use SPT. Those randomized to SPT maintained improvements in UFOV performance, and there was a significant dose effect of SPT in that more hours of training were associated with greater UFOV performance improvements.
Conclusion:
The cognitive benefits derived from SPT in PD may be maintained for up to three months. Future research should determine how long gains endure and explore if such training gains transfer.
Keywords: Mixed effect models, non-pharmacological intervention, cognitive training
In addition to typical motor dysfunction, individuals with Parkinson’s disease (PD) show deficits in various cognitive domains relative to healthy controls (e.g., 1, 2-5). The primary domains affected in PD are executive function, speed of processing, and visual cognitive abilities (1-5), with memory and attention also possibly affected (1-3). Recent interest has turned toward non-pharmacological interventions to improve cognitive performance, such as cognitive training.
Various cognitive training programs have been investigated among those with PD, each focusing on different cognitive domains such as executive functioning (6), speed of processing (7), attention (8), or sequence production (9), with others training multiple cognitive domains (10-12). Results have been promising with improvements observed in domains such as executive functioning (9, 12), memory (10, 12), speed of processing (7, 10), attention (10), visuospatial skills (10), and global cognitive function (11). However, prior training research in PD has limitations such as lack of adequate control groups (8, 9, 11) and small sample sizes (6, 8, 10, 12). Furthermore, few studies have examined longitudinal maintenance of training gains (13).
Cognitive speed of processing and visual attention, as measured by the Useful Field of View Test (UFOV; 5), is impaired in PD. The UFOV is well studied in PD (5, 7, 14-16) and poor performance is associated with reduced quality of life and independence due to driving impairments (5, 14, 15). Research with healthy older adults indicates that SPT can improve UFOV performance, and transfers to improved instrumental activities of daily living, driving mobility, and driving safety (17-20). Consequently, SPT has become of interest for persons with PD.
Our recent study examined SPT among those with PD in a randomized clinical trial (RCT) (7). Participants (n=87) with PD were randomized to either SPT or a delayed treatment group (7). Results showed a significant training group by time interaction indicating that after 3 months of training, the SPT group showed significantly greater gains in UFOV performance than the control group. Recent longitudinal data indicate that the effects of SPT in healthy older adults may last up to ten years (21). Few other types of cognitive interventions have shown such lasting effects. While our prior research has shown that SPT can improve cognitive speed of processing in those with PD (7), whether the effects of this intervention are maintained in this population has not been examined. Furthermore, the factors that affect use of the intervention among individuals with PD are unexplored. Finally, previous research has found greater training gains with larger doses of SPT (22), but dose effects have not been examined in PD.
Here we present a follow-up field study that examined if participants would continue to participate in training at home (and factors related to use), if training-related improvements observed in the prior study were maintained, and dose effects. We hypothesized that the majority of participants would continue to participate in the training program at home, that those randomized to SPT would maintain the improvements in UFOV performance previously observed at initial post-test (7), and that more hours of SPT would be associated with larger gains in UFOV performance.
Materials and Methods
Participants
Inclusion criteria for the original RCT and other study details including the CONSORT flow chart are published elsewhere (7). In all, 93 individuals were assessed for eligibility, and 6 individuals did not meet inclusion criteria, leaving 87 individuals to be randomized: 44 to the intervention (i.e., SPT) and 43 to the delayed control group. All participants randomized were included in these follow-up analyses. The USF Institutional Review Board approved the prior study and these analyses, and written informed consent was obtained from all participants.
Intervention
InSight is a self-administered version of adaptive SPT, a computerized, process-based, cognitive training technique that involves perceptual practice targeting basic fluid abilities (23). InSight training was completed at home by the participants. The program includes five exercises containing visual stimuli that progress from simple to complex in a gradual fashion as performance improves (i.e., adaptive in difficulty). See Table 1. As detailed elsewhere (7), participants were instructed to alternate between the daily recommended schedule, which included all five exercises, and choosing only the Road Tour exercise, which is most similar to a prior version of SPT that has shown substantial efficacy (18).
Table 1.
Speed of Processing Training Program Exercises
| Exercise | Targeted Ability | Description |
|---|---|---|
| Sweep Seeker | Visual processing | Identify order of visual sweeps; finer & faster sweeps |
| Bird Safari | Visual target identification | Visual discrimination of peripheral targets; degrading visual conditions & increasing speed of presentation |
| Jewel Diver | Visual tracking speed & memory | Track & remember visual targets; increasing number, speed, & background complexity |
| Road Tour | Visual attention | Discriminate center target & locate peripheral target; increasing speed & background complexity |
| Master Gardener | Visual speed & memory | Detect & remember targets; increasing speed & background complexity |
Procedure
In the original RCT, eligible participants completed a baseline testing visit and were randomized to either the SPT or delayed treatment condition. Participants in the SPT condition were given the InSight software to take home and were instructed to begin training immediately following the baseline visit with the goal of completing at least 20 hours over three months. Post-test was scheduled 3 months after baseline for participants in both conditions. Prior results of this RCT demonstrated greater gains in UFOV performance from baseline to three months among those randomized to training relative to controls (7).
At the completion of the RCT, delayed control participants were given the SPT software and were instructed to complete at least 20 hours over the next three months. Participants in the training group continued to have access to the intervention, and were not provided specific instructions.
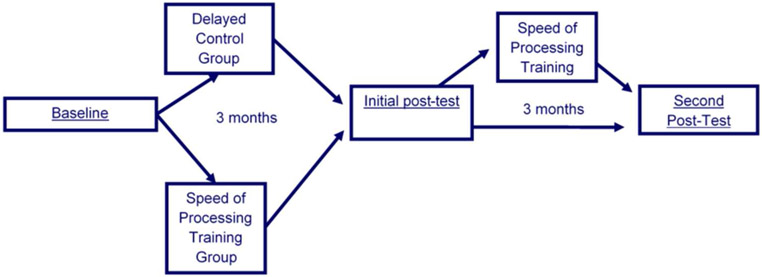
The present study is a field trial of effectiveness that examined participants three months after the RCT ended. All participants were invited to complete an additional study visit, six months after their baseline. See Figure 1. All participants had access to the training prior to this study visit. A benefit to this study design is that monitoring participants’ independent continued use of the program (outside study instruction) provides real world evidence of the likelihood of PD patients using the program outside of a research study environment. Participants completed the program at home, and hours of training completed were recorded online by the software for monitoring.
Figure 1.
Study Timeline
Measures
Demographic characteristics included sex, age, years of education and race. Mental status was assessed with the Mini Mental State Exam (MMSE). Scores of 24 or better were required for inclusion. epressive symptoms were measured using the Center for Epidemiologic Depression Scale short-form (CES-D; 24). Participants indicated the number of days from the prior week that they felt or behaved in ways indicative of depression across 20 items with ratings ranging from 0 (less than one day per week) to 3 (5 to 7 days per week). Ratings were summed with a possible range of 0 to 60 with higher scores indicating more depressive symptoms.
Cognitive speed of processing was measured using the UFOV (25), a computerized measure of speed of processing for visual attention tasks, which includes three subtests of progressively increasing complexity. This task has been well studied and is used in clinical practice among individuals with PD (5, 14-16). In each subtest, visual targets (cars and trucks) are shown on the computer screen at display durations ranging from 17 to 500 ms. Subtest 1 involves central target identification alone, while subtest 2 involves simultaneous identification of a central target and localization of a peripheral target. Subtest 3 is the same as subtest 2, except the peripheral target is embedded in distracters (triangles). Scores for each subtest represent the briefest display durations at which the participant performed correctly 75% of the time, with higher scores indicating worse performance. Scores from all three subtests were summed with a possible range of 51 to 1,500ms. The UFOV has high reliability ranging from r=.74-.81 (25).
Analyses
ANOVA and Chi-square analyses were conducted to determine if there were significant baseline differences between the SPT and control groups on depressive symptoms or descriptive characteristics: age, race, sex, and education.
Two mixed effects models were used to investigate the hypotheses. An intent-to-treat approach was used in which all 87 participants randomized in the original study were included in analyses regardless of adherence to the training protocol. Mixed effects models are an alternative to repeated measures ANOVA in modeling change over time(26). Similar to repeated measures ANOVAs, mixed effects models can statistically test main effects and interactions. Mixed models do not require complete data at all time points and can measure time as a continuous variable, making them commonly accepted for longitudinal data analysis (27, 28).
Training Use.
Data were collected measuring the average number of hours of training completed by each group at each time point. Regression analyses were performed to examine factors predicting training use including age, depressive symptomology, years of education, baseline MMSE score, and randomization group.
Maintenance of Training Gains.
The first mixed effects model was conducted using only participants from the SPT group to investigate if the significant training gains previously observed in UFOV performance at initial post-test (7) were maintained at 6 months, with time as a predictor of cognitive performance. A significant main effect of time would demonstrate that UFOV performance improved across the study period. Following a significant effect of time, planned contrasts were conducted using two paired sample t-tests to determine if there was a significant difference between baseline and post-test (confirming the previously observed training effect), as well as between initial post-test and follow up (to examine durability). No difference in UFOV performance between post-test and follow-up would indicate that the training gains endured.
Examination of Training Dose.
A second mixed effects model was conducted among the entire sample to investigate whether the number of hours of SPT completed was a significant predictor of improved UFOV performance (i.e., dose effect). A significant main effect of hours of SPT was expected, indicating that UFOV performance improved with increasing doses of training.
Results
Sample Characteristics
The analytic sample consisted of all 87 eligible participants and had a mean age of 68.9 years, an average of 15.4 years of education, and an average of 10.0 on the CES-D. The sample was 62.1% male and 97.7% Caucasian.
Baseline Differences
To examine potential differences across conditions, the participants in the SPT and control groups were compared at baseline on age, education, depressive symptoms, and UFOV using multivariate analysis of variance (MANOVA). Baseline differences between groups on sex and race were examined using Chi-square. Overall, there were no differences between the groups, Wilks λ=.99, F(4,82)=.30, p=.88, partial η2=.01, in terms of age, F(1,85)=.60, p=.44, partial η2=.007; education, F(1,85)=.02, p=.09, partial η2=.001; depressive symptoms, F(1,85)=.06, p=.81, partial η2=.001; or UFOV, F(1,85)=.99, p=.32, partial η2=.01. Chi-square indicated the participants in the SPT and control conditions did not differ significantly in terms of sex, X2(1)=.09, p=.76, or race, X2(1)<.001, p=.99.
Training Use
At the end of the RCT, the SPT group had completed an average of 22.59 hours of training with an average of 10.98 of those hours spent specifically on the Road Tour exercise, while the control group participants had not yet completed any training. For this field trial, at the follow-up study visit, the SPT group had completed, on average, an additional 8.14 hours of training (30.73 hours total) with an average of 2.22 of the additional hours spent specifically on the Road Tour exercise (13.2 hours total of Road Tour). At follow-up, the control group had completed on average 23.45 hours of training, with an average of 10.48 of those hours spent specifically on the Road Tour exercise. Of those in the SPT group 52% completed additional training between post-test and follow-up on their own volition, 48% did not complete additional training. Among those in the control group 84% completed training between post-test and follow-up, and 16% did not complete any training.
MANOVA and Chi-square analyses were conducted to compare the characteristics of the 52% of participants in the SPT condition who chose to complete additional training between post-test and follow up with the 48% who did not. There were no significant differences at baseline between these two groups on age, depressive symptomology, years of education, MMSE, UFOV performance, age at diagnosis, race, or sex, p’s>.05.
A multiple linear regression was conducted with the entire sample to examine the association between hours of training completed and age, depressive symptoms, years of education, MMSE score and randomization group. This regression yielded non-significant results, F(5,74)=0.82, p=.54, R2=.06, with neither age, depressive symptoms, years of education, MMSE score or randomization group being significantly associated with hours of training completed.
Training Maintenance
To examine the durability of training effects across six months, the first mixed effects model included only those randomized to SPT. Results are presented in Table 2. An unconditional growth model showed significantly better fit than an intercept-only model, Δ-2LL X2(1)= 23.53, p<0.05. Findings indicated significant improvement in UFOV performance over time, p<.001. Fixed effects were estimated for both intercept and slope, but a random effect was estimated for intercept only, as the model failed to converge when a random effect for slope was included. Two planned contrasts were conducted using paired samples t-tests to determine if there was a significant difference between baseline and post-test, or between post-test and follow up. There was a significant difference between baseline and post-test, t(32)=4.39, p<.001, such that performance was better at post-test (M=354.91, SD=285.47) than at baseline (M=497.06, SD=288.86). However, the difference between post-test and second-post-test was not significant, t(32)=.99, p=.33, follow up (M=333.70, SD=270.08). This indicates that the training gains were maintained from post-test to follow up. See Figure 2.
Table 2.
Mixed effects models: Training durability and effect of training dose on Useful Field of View performance.
| Analysis 1: Training Durability | Analysis 2: Effect of Training Dosage | |||
|---|---|---|---|---|
| No Growth Model | Unconditional Growth Model |
No Growth Model | Conditional Growth Model |
|
| Value | Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) |
| −2LL | 1508.82 | 1485.28 | 3167.34 | 3126.16 |
| AIC | 1514.82 | 1493.28 | 3173.34 | 3134.16 |
| Parameters | 3 | 4 | 3 | 4 |
| Fixed Effects | ||||
| Intercept | 401.59 (38.80)*** | 460.48 (40.40)*** | 391.83 (27.17)*** | 427.73 (27.95)*** |
| Time | ----- | −5.26 (.99)*** | ----- | ----- |
| InSightHours | ----- | ----- | ----- | −3.63 (.64)*** |
| Random Effects | ||||
| Intercept | 57520.90 (14200.30)*** | 60762.62 (14295.61)*** | 57075.50 (9866.60)*** | 58906.38 (9914.22)*** |
| Residual | 20996.53 (3627.50)*** | 14677.90 (2544.82)*** | 19925.48 (2336.19)*** | 16352.48 (1924.85)*** |
Note.
p<.001. Analysis 1, n = 44, only those randomized to immediate training. Analysis 2, n = 87, the entire sample regardless of randomization or adherence. Those with missing data were included in the models.
Figure 2.
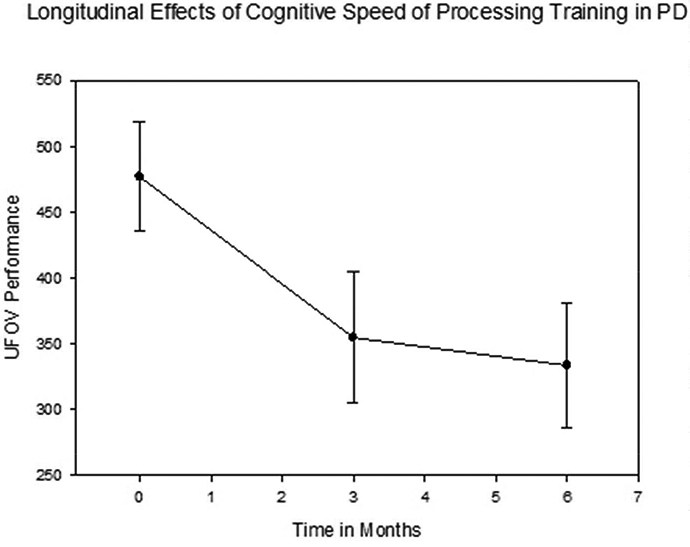
Longitudinal effects of speed of processing training among those with Parkinson’s disease in the immediate speed of processing training group. UFOV = Useful Field of View. PD = Parkinson’s Disease. Lower UFOV scores indicate better performance.
Training Dose
Next, to examine the dose effect of the training, a mixed effects model was conducted that included all participants (N=87) from both SPT and control groups, as the control group completed training between initial post-test and follow up. Fixed effects were estimated for both intercept and slope, but a random effect was estimated for intercept only, as the model failed to converge when a random effect for slope was included. A growth model using total number of hours trained as a predictor showed a significantly better fit than an intercept-only model, Δ-2LL X2(1)=53.11, p<0.001, which indicated significant improvement in UFOV performance with greater hours of training, p<.001 (i.e., a dose-response effect). Among the entire study sample, more hours of SPT was associated with lower UFOV scores (i.e., better performance). Results are presented in Table 2.
Discussion
We examined use, maintenance, and dose effects of SPT among individuals with PD. The majority of participants continued to use SPT at home after the RCT. Our hypothesis that those randomized to SPT would maintain the improvements in UFOV performance observed at initial post-test was supported. Our hypothesis that more hours of SPT would be associated with better UFOV performance was also supported. Our results showed that older adults with PD who were randomized to SPT longitudinally maintained the training gains observed at initial post-test. Further, larger doses of training were associated with greater performance gains.
It is encouraging that 52% of participants in the SPT group and 84% of participants in the control groups continued to use the program after the RCT ended. This provides real world evidence for the feasibility of and adherence to this program among PD patients outside of a research study environment. However, there were no significant baseline differences between those who chose to continue using the program and those who did not. Further, none of our baseline demographics predicted number of hours completed. Future research should explore who is and is not likely to use these programs and why.
These findings extend our previous research (7) by showing that the previously observed benefits from SPT are maintained 3 months after post-test. This is important because it suggests that the benefits of SPT do not immediately dissipate. The results are similar to other research with SPT in healthy and cognitively impaired older adults, which suggests SPT training gains have lasting effects. Research from healthy older adults and those with mild cognitive impairment indicate that SPT gains may endure 5-10 years (21, 29).
While there is a plethora of cognitive training literature, there is a relative dearth of research regarding the necessary dose or amount of training needed to see a benefit. There is even less information regarding necessary dose of cognitive training among those with PD. Our results show that increasing amounts of SPT were associated with better performance on the UFOV. Each hour of training was associated with 3.63 ms faster performance on the UFOV. If participants completed the instructed 20 hours of training, this would lead to an average 72.6 ms faster performance on the UFOV task, compared to the annualized declines of 15.6 ms slower performance on the UFOV task seen among healthy older adults (30). Future research should investigate if there is an optimal dose of cognitive training to see benefits.
Strengths and Limitations
A strength of the current study is its relatively large sample size. The original RCT to which this is a follow-up is the largest cognitive training trial in PD to date (31). The RCT provided sufficient evidence for the feasibility and efficacy of computerized cognitive training among individuals with PD. Further, our results demonstrate the durability of training gains after immediate post-test. Thus, cognitive training gains in PD do not immediately dissipate. Another strength of this study is its examination of training dose, an area which is understudied. Results show stronger effects with larger doses..
The current study does have limitations. The sample included primarily highly educated Caucasians in mild to moderate stages of PD; therefore, the results may not generalize to minorities, those with lower education levels, or those in later stages of the disease. However, prior research with healthy older adults suggests that race and education do not significantly impact SPT gains (19). The study was a field trial of effectiveness, so participants’ access to the intervention was not prohibited. A RCT is needed to quantify maintenance effects. Although, it is not clear if the UFOV gains would have been maintained without continued access to the software, it is encouraging that more than 50% chose to continue use of the intervention on their own. Future work should examine the sustainability of training effects in a RCT with and without booster training. Further, an active control group would be ideal for future research in PD. Other studies have found large effects of SPT relative to active control groups (e.g., 32). One final limitation is that participants and experimenters were not blinded as to condition.
Implications and Future Research
Our prior work (7) showed that SPT is beneficial in those with PD. The current study extends this research and indicates that UFOV training gains may endure across 3 months. SPT is a particularly unique cognitive training program in that it has shown transfer to everyday functional outcomes, such as driving (20). Future research should examine if SPT also transfers to everyday functional outcomes in PD and also include longer intervals of follow up to examine the long term durability of training gains in this population. SPT may be a viable non-pharmacological intervention option that can be self-administered by individuals with PD to improve their cognitive performance with potentially lasting effects.
Acknowledgement:
We would like to acknowledge financial support from the National Institutes of Health National Institute on Aging, grant 1R21AG033332 (Edwards, PI). We would like to express our gratitude to the study participants as well as to the many other contributors to this study including, but not limited to, Holly Delgado, Eden Feldman, Christine Haley, Elizabeth Hudak, Dr. Israt Jahan, Theresa McClain, Chelsea McNee, Adina Mears, Amber Miller, Carol Peronto, Judy Smolk, Kelly Sullivan, and Dr. Theresa Zesiewicz, who helped to recruit, test, and coordinate study participants. Thank you also to Neveen Nawaway and Hope Smith for clerical assistance, as well as the undergraduate student Research Assistants at the USF Cognitive Aging Lab for data entry assistance. We would also like to thank Drs. Aryn Harrison, Jennifer Lister, and Alyssa Gamaldo as they served as members on the first author’s dissertation committee, of which a previous version of this manuscript was submitted in partial fulfilment.
Funding sources for the study:
National Institutes of Health National Institute on Aging, grant 1R21AG033332. E. Valdes was supported by the National Institutes of Health, National Institute on Aging, 1R36AG049889-01. E. Uc was additionally supported by R01 NS044930, Department of Veterans Affairs, Rehabilitation R&D Branch Merit Review Award B6261R, NIH/NIA R21 AG033332, VA Merit Review 1 I01 RX000170-01
Dr. Edwards served on Data Safety and Monitoring Boards for a National Institutes of Health grants to Posit Science, Inc., and worked as a consultant to Wilson, Sonsini, Goodrich & Rosati. Dr. Hauser served as a consultant for Auspex Pharmaceuticals, Cowan Therapeutics, Gerson Lehrman Group, Allergan, Abbvie, Eli Lilly and Company, Impax Laboratories, Lundbeck Pharmaceuticals, Michael J. Fox Foundation, Teva Pharmaceuticals, UCB BioSciences, Inc. He has served on advisory boards for Acorda Therapeutics, Acadia Pharmaceuticals, AstraZeneca, Lundbeck Pharmaceuticals, Neurocrine Biosciences, Pfizer, Inc., and Cynapsus Therapeutics. He has served on the Steering Committee for Chelsea Therapeutics, and the Speaker’s Bureau for Biotic Therapies, Novartis, and Teva Pharmaceuticals. Drs. Andel and O’Connor report no disclosures. Dr. Uc has served as a consultant to Rhythm Pharmaceuticals, Inc, Biotie Therapies, and the Michael J. Fox Foundation.
Footnotes
Disclosures of Interest:
J. Edwards worked as a limited consultant to Posit Science, Inc., the company that marketed InSight, from June to August 2008, and currently serves on the data safety and monitoring board of NIH grants awarded to employees of Posit Science.
References
- 1.McKinlay A, Grace RC, Dalrymple-Alford JC, Roger D. Characterisitics of executive function impairment in Parkinson's disease patients without dementia. Journal of the International Neuropsychological Society. 2010;16:268–77. doi: 10.1017/S1355617709991299. [DOI] [PubMed] [Google Scholar]
- 2.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson's disease. Neurology. 2005;65:1239–45. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 3.Zgaljardic DJ, Borod JC, Foldi NS, Mattia P. A review of the cognitive and behavioral sequelae of Parkinson's disease: Relationship to frontalstriatal circuitry. Cognitive and Behavioral Neurology. 2003;16(4):193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Grossman M, Zurif E, Lee C, Prather P, Kalmanson J, Stern MB, et al. Information processing speed and sentence comprehension in Parkinson's disease. Neuropsychology. 2002;16(2):174–81. doi: 10.1037//0894-4105.16.2.174. [DOI] [PubMed] [Google Scholar]
- 5.Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson's disease without dementia. Neurology. 2005;65:1907–13. doi: 10.1212/01.wnl.0000191565.11065.11. [DOI] [PubMed] [Google Scholar]
- 6.Sammer G, Reuter I, Hullmann K, Kaps M, Vaitl D. Training of executive functions in Parkinson's disease. Journal of the Neurological Sciences. 2006;248:115–9. doi: 10.1016/j.jns.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Edwards JD, Hauser RA, O'Connor ML, Valdés E, Zesiewicz TA, Uc EY. Randomized controlled trial of cognitive speed of processing training in Parkinson's disease. Neurology. 2013;81:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohlman J, Chazin D, Georgescu B. Feasibility and acceptance of a nonpharmacological cognitive remediation intervention for patients with Parkinson's disease. Journal of Geriatric Psychiatry and Neurology. 2011;24(2):91–7. doi: 10.1177/0891988711402350. [DOI] [PubMed] [Google Scholar]
- 9.Disbrow EA, Russo KA, Higginson CI, Yund EW, Ventura MI, Zhang L, et al. Efficacy of tailored computer-based neurorehabilitation for improvement of movement initiation in Parkinson's disease. Brain Research. 2012;1452:151–64. doi: 10.1016/j.brainres.2012.02.073. [DOI] [PubMed] [Google Scholar]
- 10.Paris AP, Saleta HG, Maraver MCC, Silverstre E, Freixe MG, Torrellas CP, et al. Blind randomized controlled study of the efficacy of cognitive training in Parkinson's disease. Movement Disorders. 2011;26(7):1251–8. doi: 10.1002/mds.23688. [DOI] [PubMed] [Google Scholar]
- 11.Reuter I, Mehnert S, Sammer G, Oechsner M, Engelhardt M. Efficacy of a multimodal cognitive rehabilitation including psychomotor and endurance training in Parkinson's disease. Journal of Aging Research. 2012;2012:1–15. doi: 10.1155/2012/235765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinforiani E, Banchieri L, Zucchella C, Pacchetti C, Sandrini G. Cognitive rehabilitation in Parkinson's disease. Archives of Gerontology and Geriatrics. 2004;9:387–91. doi: 10.1016/j.archger.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 13.Goldman JG, Weintraub D. Advances in the treatment of cognitive impairment in Parkinson's disease. Movement Disorders. 2015. doi: 10.1002/mds.26352. [DOI] [PubMed] [Google Scholar]
- 14.Uc EY, Rizzo M, Johnson AM, Emerson JL, Liu D, Mills ED, et al. Real-life driving outcomes in Parkinson's disease. Neurology. 2011;76(22):1894–902. doi: 10.1212/WNL.0b013e31821d74fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Classen S, McCarthy DP, Schechtman O, Awadzi KD, Lanford DN, Okun MS, et al. Useful Field of View as a erliable screening measure of driving performance in people Parkinson's disease: Results of a pilot study. Traffic Injury Prevention. 2009;10(6):593–8. doi: 10.1080/15389580903179901. [DOI] [PubMed] [Google Scholar]
- 16.Devo H, Vandenberghe W, Nieuwboer A, Tant M, Baten G, De Weerdt W. Predictors of fitness to drive in people with Parkinson's disease. Neurology. 2007;69:1434–41. [DOI] [PubMed] [Google Scholar]
- 17.Edwards JD, Myers C, Ross LA, Roenker DL, Cissell GM, McLaughlin AM, et al. The longitudinal impact of speed of processing training on driving mobility The Gerontologist. 2009;49(4):485–94. doi: 10.1093/geront/gnp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ball KK, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288(18):2271–81. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ball KK, Edwards JD, Ross LA. The impact of speed of processing training on cognitive and everyday functions. Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2007;62B(Special Issue 1):19–31. doi: 10.1093/geronb/62.special_issue_1.19. [DOI] [PubMed] [Google Scholar]
- 20.Ball KK, Edwards JD, Ross LA, McGwin GJ. Cognitive training decreases motor vehicle collision involvement of older drivers. Journal of the American Geriatrics Society. 2010;58(11):2107–13. doi: 10.1111/j.1532-5415.2010.03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebok GW, Ball KK, Guey LT, Jones RN, Kim H-Y, King JW, et al. Ten-year effects of the Advanced Cognitive Training for Independent and Vital Elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society. 2014;62(1):16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ball KK, Ross LA, Roth DL, Edwards JD. Speed of processing training in the ACTIVE study: Who benefits and how much? Journal of Aging and Health. 2013;25(8S):65S–84S. doi: 10.1177/0898264312470167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lustig C, Shah P, Seidler R, Reuter-Lorenz PA. Aging, training, and the brain: A review and future directions. Neuropsychological Reviews. 2009;19:504–22. doi: 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Despression) depression symptoms index. Journal of Aging and Health. 1993;5:179–93. [DOI] [PubMed] [Google Scholar]
- 25.Edwards JD, Vance DE, Wadley VG, Cissell GM, Roenker DL, Ball KK. The reliability and validity of the Useful Field of View Test as administered by personal computer. Journal of Clinical and Experimental Neuropsychology. 2005;27:529–43. doi: 10.1080/13803390490515432. [DOI] [PubMed] [Google Scholar]
- 26.Hertzog C, Nesselroade JR. Assessing psychological change in adulthood: An overview of methodological issues. Psychology and Aging. 2003;18(4):639–57. doi: 10.1037/0882-7974.18.4.639. [DOI] [PubMed] [Google Scholar]
- 27.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 28.Gueorguiena R, Krystal JH. Move over ANOVA: Progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Archives of General Psychiatry. 2004;61:310–7. [DOI] [PubMed] [Google Scholar]
- 29.Valdés EG, O'Connor ML, Edwards JD. The effects of cognitive speed of processing training among older adults with psychometrically defined mild cognitive impairment. Current Alzheimer Research. 2012;9(8). doi: 10.2174/156720512803568984 [DOI] [PubMed] [Google Scholar]
- 30.Edwards JD, Ross LA, Wadley VG, Clay OJ, Crowe M, Roenker DL, et al. The Useful Field of View Test: Normative data for older adults. Archives of Clinical Neuropsychology. 2006;21(4):275–86. doi: 10.1016/j.acn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Leung IHK, Walton CC, Hallock H, Lewis SJG, Valenzuela M, Lampit A. Cognitive training in Parinson disase: A systematic review and meta-analysis. Neurology. 2015;85(21):1843–51. doi: 10.1212/WNL.0000000000002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards JD, Wadley VG, Vance DE, Wood KM, Roenker DL, Ball KK. The impact of speed of processing training on cognitive and everyday performance. Aging and Mental Health. 2005;9(3):262–71. doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]