Abstract
Background:
Immune checkpoint inhibitors (ICIs) and thoracic radiotherapy are increasingly used to treat advanced cancers. Despite data indicating exaggerated radiation toxicities in patients with autoimmune disease, the safety of thoracic radiotherapy in patients with prior ICI-associated immune-related adverse events (irAEs) is undefined.
Patients and methods:
Patients treated from 2014 to 2020 with ICIs were queried for receipt of corticosteroids and radiotherapy. Patients who received thoracic radiation after symptomatic irAEs were assessed for ≥grade 2 radiation pneumonitis (RP). Characteristics predictive of RP were assessed using logistic regression and response relationships were modeled.
Results:
Among 496 assessed patients, 41 with irAE history subsequently treated with thoracic radiotherapy were analyzed. Most irAEs were grade 2 (n = 21) and 3 (n = 19). Median time from irAE onset to radiotherapy was 8.1 months. Most patients received stereotactic body radiation therapy (n = 20) or hypofractionated radiotherapy (n = 18). In total, 25 patients (61%) developed ≥grade 2 RP at a median of 4 months from radiotherapy and 11 months from onset of irAEs. Three months from RP onset, 16 of 24 (67%) assessable patients had persistent symptoms. Among patients with prior ICI pneumonitis (n = 6), five patients (83%) developed ≥grade 2 RP (grade 2, n = 3; grade ≥3, n = 2). The mean lung radiation dose (MLD) predicted for RP (odds ratio: 1.60, P = 0.00002). The relationship between MLD and RP was strong (area under the receiver-operating characteristic curve: 0.85) and showed an exaggerated dose-response. Among patients with an MLD >5 Gy (n = 26), 21 patients (81%) developed ≥grade 2 RP.
Conclusion:
This is the first study assessing the toxicity of radiotherapy among patients with prior irAEs from ICIs. Patients with prior irAEs were found to be at very high risk for clinically significant and persistent RP from thoracic radiotherapy. Careful consideration should be given to the possibility of an increased risk of RP, and close monitoring is recommended in these patients.
Keywords: checkpoint inhibitors, immune-related adverse events, pneumonitis, radiation
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have created a paradigm shift for the treatment of advanced malignancies. Many of these patients will also be exposed to thoracic radiotherapy, particularly with the increasing utilization of stereotactic body radiation therapy (SBRT) in patients with advanced disease.1 In these patients, radiation pneumonitis (RP) is a serious toxicity that results in substantial morbidity and is a result of a dysregulated inflammatory response.2 While multiple studies have established the safety of thoracic radiotherapy with concurrent or sequential ICIs,3–5 the safety of radiotherapy among patients with prior ICI-induced immune-related adverse events (irAEs) remains undescribed.
irAEs due to ICIs may develop in any organ system and are thought to be a consequence of autoimmunity.6 Prior studies have documented exaggerated radiation toxicities in patients with pre-existing autoimmune disease, attributed to a heightened pro-inflammatory state.7,8 However, it is not currently known if a history of irAEs presents a similar risk. Therefore, we sought to assess and characterize the risk of ≥grade 2 RP among patients with prior symptomatic irAEs subsequently treated with thoracic radiotherapy.
METHODS
Patients, treatment and toxicity assessments
Consecutive patients treated with atezolizumab, nivolumab, ipilimumab, pembrolizumab, durvalumab and tremelimumab between 2014 and 2020, who subsequently received prednisone or methylprednisone followed by radiotherapy were initially queried from an institutional database. Of this cohort, patients were selected for further analysis if they received corticosteroids for the treatment of irAEs and then underwent thoracic radiotherapy targeting nodes, lung parenchyma or pleura. Patients with ≤9-week post-radiation follow-up were excluded. Patients with ≥grade 2 RP were defined as those with new or progressive respiratory symptoms within 12 months of radiotherapy that warranted treatment. Chest computed tomography (CT) imaging was reviewed for radiographic changes involving the radiated field, defined as within the 50% radiation prescription-dose volume. Toxicity grading was per common terminology criteria for adverse events (CTCAE) v. 5.0. This study was completed under an institutional review board approved protocol.
Statistical analysis
Patient and treatment characteristics at the time of radiotherapy were assessed to predict for ≥grade 2 RP using logistic regression with bootstrap resampling using 1000 iterations. The dose distributions were converted into the equivalent dose in 2 Gy fractions (EQD2) assuming α/β = 3 Gy using Computational Environment for Radiotherapy Research9 and the fractionation-corrected mean lung radiation dose (MLD) for each patient was extracted for analysis. Variables that presented with a P value <0.005 (Bonferroni-corrected) were considered predictors for which the response relationship models were plotted. Model discrimination was assessed by the area under the receiver-operating characteristic curve (AUC). Kaplan–Meier estimation was used to determine the 6-month RP-free survival. For comparison, the widely-used, and validated QUANTEC RP model,10 which includes the MLD, was also assessed to predict ≥grade 2 RP in this patient population.
RESULTS
Patients and treatments
Among 496 reviewed patients, 41 patients treated with thoracic radiation after developing irAEs were analyzed. Table 1 shows patient characteristics. Most patients had prior irAEs of grade 2 (n = 21) and grade 3 (n = 19). The most common irAEs included colitis (n = 18), pneumonitis (n = 6) and hepatitis (n = 6). All irAEs were treated with systemic corticosteroids with the median initial prednisone dose of 50 mg daily [interquartile range (IQR): 40–80]; five patients (12%) received additional immunosuppressive drugs (Table 1).
Table 1.
Patient characteristics at time of radiotherapy
| No. (%) of all patients (N = 41) | |
|---|---|
| Median (range) age, years | 65 (34–83) |
| Sex | |
| Female | 21 (51) |
| Male | 20 (49) |
| Cancer type | |
| NSCLC | 24 (58) |
| Melanoma | 4 (10) |
| RCC | 3 (7) |
| SCLC | 2 (5) |
| Sarcoma | 2 (5) |
| Ovarian | 2 (5) |
| Othera | 4 (10) |
| Karnofsky Performance Status | |
| 60–70 | 4 (10) |
| 80 | 14 (34) |
| 90–100 | 23 (56) |
| Smoking history | |
| Never | 10 (24) |
| Former | 27 (66) |
| Current | 4 (10) |
| History of COPD/asthma | 6 (15) |
| Prior ICI therapy | |
| Pembrolizumab | 12 (29) |
| Ipilimumab + nivolumab | 11 (27) |
| Nivolumab | 10 (24) |
| Atezolizumab | 5 (12) |
| Durvalumab | 1 (2) |
| Tremelimumab | 1 (2) |
| Durvalumab + tremelimumab | 1 (2) |
| Prior irAE requiring systemic steroidsb | |
| Colitis | 18 (44) |
| Pneumonitis | 6 (15) |
| Hepatitis | 6 (15) |
| Arthritis | 4 (10) |
| Skin reaction | 4 (10) |
| Myositis | 3 (8) |
| Pancreatitis | 1 (2) |
| Fever | 1 (2) |
| Nephritis | 1 (2) |
| Hypophysitis | 1 (2) |
| Uveitis | 1 (2) |
| Adrenal insufficiency | 1 (2) |
| Prior irAE grade | |
| 2 | 21 (51) |
| 3 | 19 (46) |
| 4 | 1 (2) |
COPD, chronic obstructive pulmonary disease; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; NSCLC, non-small-cell lung cancer; RCC, renal cell carcinoma; SCLC, small cell lung cancer.
Other cancer diagnoses included lymphoma (n = 1), urothelial (n = 1), endometrial (n = 1) and salivary gland (n = 1).
Eleven patients had ≥1 irAE requiring systemic corticosteroids. Patients were nominally treated with systemic steroids; five patients received additional immunosuppressive therapies (n = 4 infliximab, n = 1 mycophenolate mofetil).
Patients received thoracic radiation at a median of 8.1 months (IQR: 4–14) from the onset of irAEs at which time 78% (n = 32) of patients had complete resolution of irAE symptoms. Most patients were treated with SBRT (n = 20) or hypofractionated radiotherapy (n = 18) and treatments mostly targeted lesions in the lung parenchyma (n = 28) (Table 2). Most patients received radiotherapy for the management of oligo-progressive or oligometastatic disease (n = 17 and n = 10, respectively) as well as for palliative local therapy (n = 10) (Table 2).
Table 2.
Thoracic radiotherapy characteristics
| No. (%) of all patients (N = 41) | |
|---|---|
| Radiation prescription dosea | |
| Median (interquartile range) | 58 (48–84) |
| Radiation treatment type | |
| SBRT | 20 (49) |
| Hypofractionated | 18 (44) |
| Conventional | 3 (7) |
| Thoracic disease radiation target | |
| Lung parenchyma | 18 (44) |
| Parenchyma and nodes | 10 (24) |
| Thoracic lymph nodes | 9 (22) |
| Pleura | 4 (10) |
| Concurrent systemic therapyb | 11 (27) |
| Concurrent systemic corticosteroidsc | 13 (32) |
| Mean lung dosed | |
| Median (interquartile range) | 6 (4–11) |
SBRT, stereotactic body radiation therapy.
Radiation dose is shown as the biologic effective dose (BED) (α/β 10 = Gy).
Systemic anticancer therapy given during or within 14 ±days of radiotherapy, anti-programmed cell death protein 1 (PD-1) therapy (n = 5); cytotoxic chemotherapy (n = 4); tyrosine kinase inhibitor (n = 2).
Oral steroids taken during radiotherapy course.
Mean lung dose is shown as the equivalent dose in 2 Gy fractions (α/β = 3 Gy).
Incidence and characterization of RP
Out of 41 patients, 25 (61%) developed ≥grade 2 RP. The rate of ≥grade 3 RP was 15% (n = 6). The 6-months RP-free survival estimate was 49% (95% CI: 33% to 65%) (Figure 1A). The onset of RP symptoms was at a median of 4 months (IQR: 3–6 months) from the start of radiation, 11 months (IQR: 9–20) from the onset of irAEs and 19 months (IQR: 12–27) from starting ICI therapy. Patients with RP presented with symptoms of new or progressive non-productive cough (n = 22), dyspnea (n = 21), wheezing (n = 5) and/or chest tightness (n = 3) and most, 96% (n = 24), were treated with systemic steroids. Chest CT imaging showed new patchy or dense consolidations (n = 15), and ground-glass changes (n = 11) most pronounced in the radiation field. Figure 2 shows representative diagnostic CT images. At 3 and 6 months from the onset of RP symptoms, respectively, 16 of 24 (67%) and 9 of 15 (60%) assessable patients had persistent RP symptoms including dyspnea and/or cough.
Figure 1.
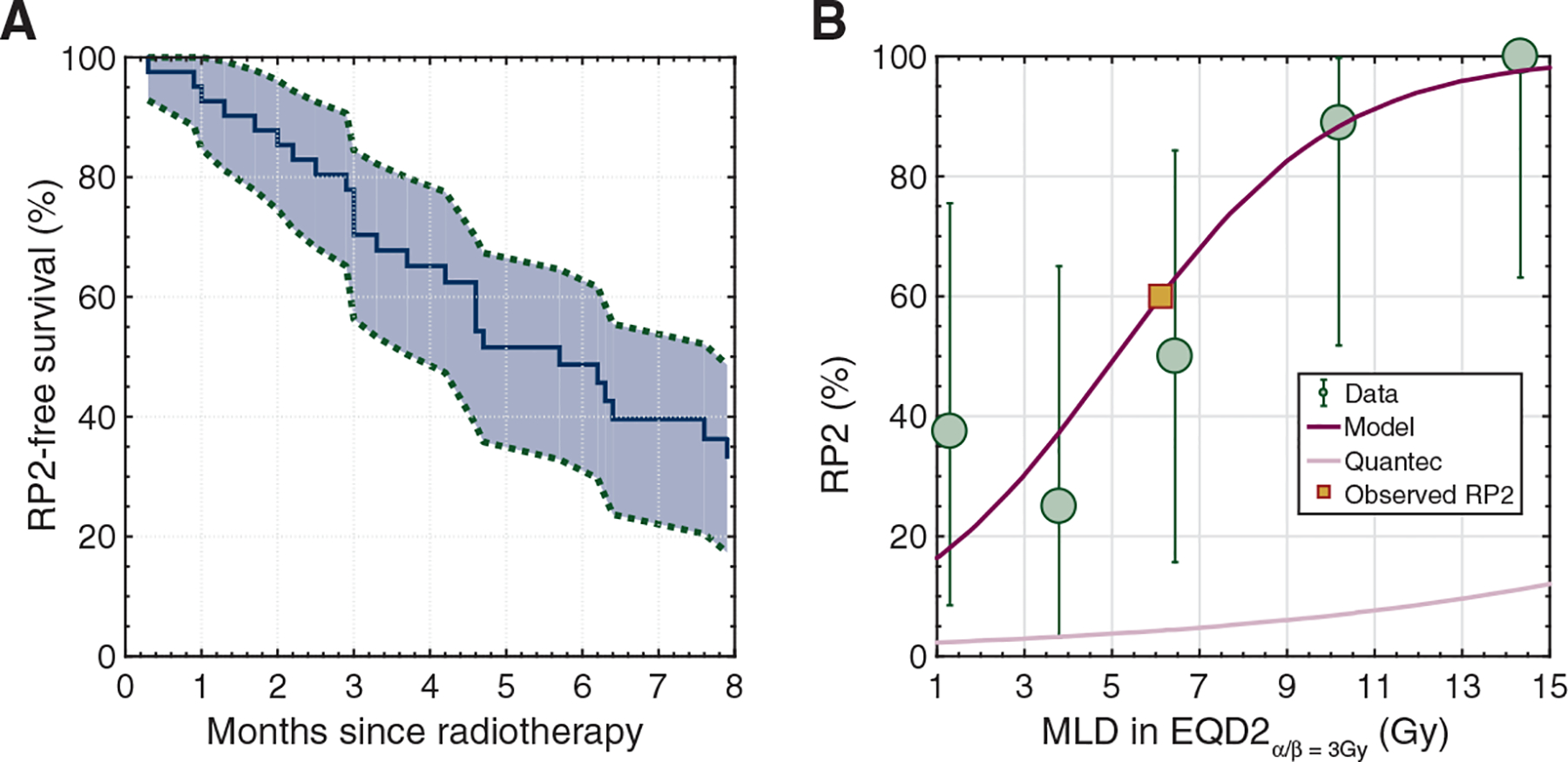
(A) Kaplan–Meier estimation (and 95% confidence bounds) of ≥grade 2 radiation pneumonitis-free survival. (B) Dose-response curve (magenta) of the mean lung dose (MLD) and ≥grade 2 radiation pneumonitis (RP). The observed RP rate is denoted by the yellow square. Also, the QUANTEC model10 (light pink) is located far below the observed RP data (green dots) indicating the inability of this widely used model to capture the observed RP distribution in this patient population. Note: error bars denote the exact binomial 95% confidence interval in each observed data quintile.
Figure 2. Radiation planning imaging showing the 100% prescription-dose volume (red) and 50% dose volume (blue) in patients treated with (A) conventional definitive, (B) hypofractionated palliative, (C) stereotactic body radiation therapy (SBRT) for oligo-progression, and (D) hypofractionated definitive radiotherapy (top row).
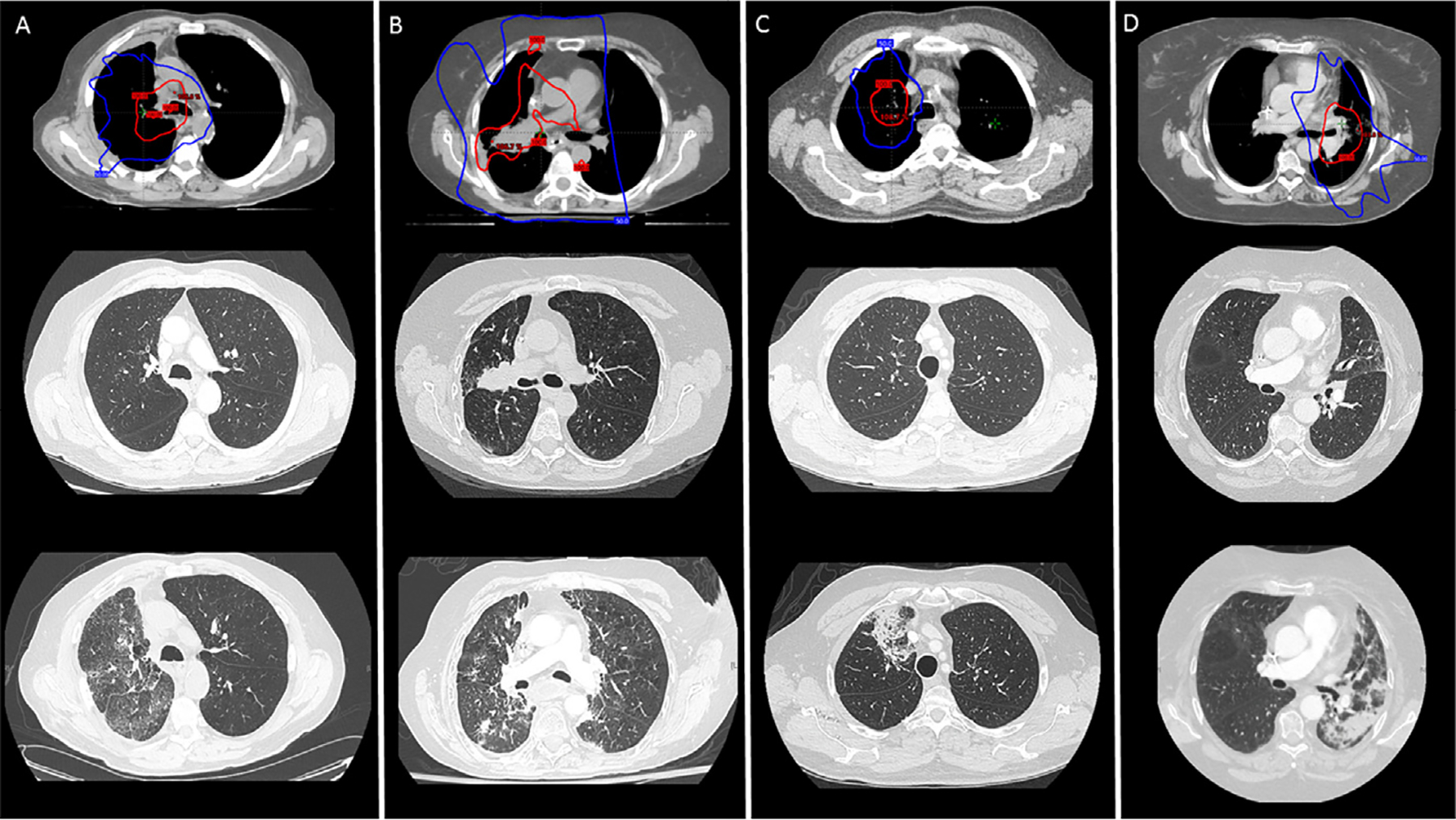
Pre-radiation diagnostic chest computed tomography (CT) imaging at the approximate axial slice (middle row). Diagnostic chest CT imaging at the time of pulmonary symptoms consistent with ≥grade 2 radiation pneumonitis (RP), imaging shows characteristic changes within the radiated lungs (bottom row).
Among patients with prior ICI pneumonitis (n = 6), all had resolution of prior respiratory symptoms at the time of radiotherapy, and five (83%) developed RP (n = 3, grade 2; n = 1, grade 3; n = 1, grade 4). The onset of RP among these patients was a median of 4 months (IQR: 3–6) from the start of radiation and 18 months (IQR: 10–46) from the time of ICI pneumonitis.
Predictors of RP
The fractionation-corrected mean radiation dose delivered to the lungs (MLD) significantly predicted for ≥grade 2 RP (odds ratio: 1.49, range: 1.11–8.11E165, P = 0.00002). No other explored variable including smoking history, concurrent systemic therapy, grade of prior irAEs, presence of irAE symptoms at the time of radiotherapy or the time between irAE or last ICI treatment and radiotherapy predicted for RP (Table 3). Additionally, among the 24 patients with RP, the majority had radiotherapy as the only new therapy introduced before the onset of pneumonitis symptoms. Only four patients had a change in their systemic therapy between the start of radiotherapy and the onset of RP symptoms.
Table 3.
Univariate analysis results
| ≥Grade 2 radiation pneumonitis OR (range) | P value | |
|---|---|---|
| Age at RTa | 1.04 (0.94–1.28) | 0.14 |
| Sex | ||
| Female (ref.) | 2.16 (0.28–5.54E44) | 0.24 |
| Male | ||
| Smoking history | ||
| Never (ref.) | 3.27 (0.00–4.48E40) | 0.12 |
| Former | ||
| Current | ||
| Concurrent systemic therapy | ||
| Yes | 1.17 (0.09–5.11E44) | 0.51 |
| No | ||
| Concurrent systemic corticosteroids | ||
| Yes | 1.30 (0.08–2.06E44) | 0.55 |
| No | ||
| Resolution of irAE symptoms | ||
| Yes | 1.30 (5E-113–2.88E44) | 0.55 |
| No | ||
| Grade of irAE | ||
| Grade 2 | 1.29 (0.04–7.56) | 0.56 |
| Grade 3–4 | ||
| Time between irAE and RTb | 1.00 (1.00–1.01) | 0.48 |
| Time between last ICI treatment and RTc | 1.00 (1.00–1.01) | 0.50 |
| Mean lung dosed | 1.49 (1.11–8.11E165) | 0.00002 |
ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; OR, odds ratio; ref, reference; RT, radiotherapy.
Age was assessed as a continuous variable.
Time between onset of ICI-associated irAE and RT was assessed as a continuous variable.
Time between last ICI treatment and RT was assessed as a continuous variable.
Mean lung dose was assessed as a continuous variable as the equivalent dose in 2 Gy fractions (EQD2) assuming α/β = 3 Gy, which take into consideration dose and fraction size (see Methods).
The relationship between MLD and RP was strong (AUC = 0.85) and the predicted RP rates agreed with observed RP events (Figure 1B.). According to this model, an MLD of only 5 Gy would predict for a 50% risk of ≥grade 2 RP. For comparison, the QUANTEC RP model suggested the RP risk to be between 2% and 12%, considerably underestimating the observed RP event rate in the quintiles ranging from 25% to 100%. Among the 26 patients with an MLD of >5 Gy, 81% (n = 21) developed ≥grade 2 RP.
DISCUSSION
To our knowledge, this is the first study assessing the toxicity of radiotherapy among patients with prior irAEs due to ICIs. We found patients with prior irAEs to be at exquisitely high-risk for developing >grade 2 RP, with 51% developing clinically significant and persistent RP at 6 months. A strong and exaggerated dose-response between MLD and RP was also identified, suggesting an increased sensitivity to RP that is potentially modifiable.
Pneumonitis is one of the most serious toxicities of thoracic radiation. The risk of ≥grade 2 RP is estimated to be between 10% and 25% with fractionated radiotherapy concurrent with platinum-based chemotherapy and/or with sequential ICI therapy.11,12 However, highly conformal radiotherapy, such as SBRT, is associated with a 10%−15% risk of RP, even with concurrent ICI therapy.13,14 Therefore, our finding that 61% of patients developed ≥grade 2 RP, despite highly conformal radiotherapy, suggests that patients with prior irAEs are at a unique and increased risk for toxicity from radiotherapy.
The safety of thoracic radiation in patients with prior ICI-induced irAEs has not been previously described. However, multiple studies have addressed the risk of re-challenge with ICIs in patients who developed irAEs. With ICI re-challenge, both the recurrence of the same irAE as well as the development of a different irAE have been described.15,16 Additionally, there are reports of other at-risk populations after ICI therapy. For example, exposure to the tyrosine kinase inhibitor osimertinib following ICI therapy has been associated with a higher rate of pneumonitis.17 Further research is necessary, but the development of irAEs may represent immune system dysregulation predisposing these patients to RP, similar to the amplified radiation toxicities seen in patients with autoimmune disease.7,8,18
We found the MLD to predict the development of RP. The exaggerated dose-response between RP and MLD (even across the fairly-low MLDs) among patients with prior irAE supports the use of strategies to reduce the MLD far below current guidelines. Deep inspiratory breath hold, margin reduction and/or proton therapy should be considered as potential technical adjustments to mitigate the risk of RP and the associated morbidity and risk of even mortality in these patients.19,20 Additionally, in appropriately selected patients, surgical therapy should also be considered. Future strategies exploring the preventative use of biologic agents to improve the safety profile of thoracic radiotherapy in high-risk patients are warranted.21,22 Overall, careful consideration should be given to the possibility of an increased risk of RP when weighing the benefits of radiotherapy and close monitoring for RP is recommended.
Limitations of this study include those inherent to retrospective analyses and, therefore, confirmatory studies are necessary. This study is limited by its sample size, but this is a unique patient population poised to grow with the increased use of thoracic radiation in patients with advanced pretreated disease. Additionally, determining the diagnosis of RP was a challenge even before the widespread use of ICIs and is now, undoubtedly, more complex.23 Prior studies have found variable imaging characteristics in patients with RP and the patients within this series have had multiple exposures.24,25 However, we present a thorough analysis on a cohort with mostly classic symptoms and imaging evidence for RP.
In conclusion, our data suggest that patients with prior ICI irAEs are a unique subgroup at very high risk for developing clinically significant and persistent RP from radiotherapy. Careful consideration of the increased risk of RP and efforts to minimize the MLD are warranted to mitigate the risk of substantial morbidity.
ACKNOWLEDGEMENTS
We are grateful to our patients and their families.
FUNDING
This work was supported by the National Institutes of Health (grant number P30 CA008748).
DISCLOSURE
MO: Reports advisory role for PharMar, Novartis and Targeted Oncology. Reports honoraria from Bristol Myers Squibb and Merck Sharp & Dohme. AFS: Reports honoraria from ASCO. AJW: Reports research support from CivaTech Oncology, Inc., non-financial support from AlphaTau Medical, personal fees from MoreHealth and personal fees from AstraZeneca. CBS II: Reports honoraria from Varian Medical Systems. MDH: Reports personal fees from Genentech, grants, personal fees and non-financial support from Bristol Myers Squibb, personal fees from Merck, personal fees and non-financial support from AstraZeneca, personal fees from Mirati, personal fees from Syndax, personal fees and equity from Shattuck Labs, personal fees and equity from Immunai, personal fees from Nektar, personal fees from Blueprint Medicines. In addition, MDH has a pending patent Determinants of Cancer Response to Immunotherapy by PD-1 Blockade (PCT/US2015/062208) licensed to PGDx. JEC: Reports both research funding and consulting roles with Bristol Myers Squibb, Merck, Genentech and AstraZeneca. AR: Reports grants from Varian Medical Systems, grants from Boehringer Ingelheim, grants from Pfizer, grants and personal fees from AstraZeneca, grants and personal fees from Merck, personal fees from Research to Practice, personal fees from Cybrexa, non-financial support from Philips/Elekta, personal fees from MoreHealth. DRG: Reports honoraria from Merck, Bristol Myers Squibb, AstraZeneca, Reflexion, Medscape, Vindico, US Oncology, and Varian. Reports research support from Merck, Bristol Myers Squibb, AstraZeneca and Varian. Serves on advisory board for AstraZeneca. All remaining authors have declared no conflicts of interest.
REFERENCES
- 1.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: longterm results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graves PR, Siddiqui F, Anscher MS, Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20(3):201–207. [DOI] [PubMed] [Google Scholar]
- 3.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Reibnitz D, Chaft JE, Wu AJ, et al. Safety of combining thoracic radiation therapy with concurrent versus sequential immune checkpoint inhibition. Adv Radiat Oncol. 2018;3(3):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non–small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young A, Quandt Z, Bluestone JA. The balancing act between cancer immunity and autoimmunity in response to immunotherapy. Cancer Immunol Res. 2018;6(12):1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga J, Haustein UF, Creech RH, Dwyer JP, Jimenez SA. Exaggerated radiation-induced fibrosis in patients with systemic sclerosis. JAMA. 1991;265(24):3292–3295. [PubMed] [Google Scholar]
- 8.Lin D, Lehrer EJ, Rosenberg J, Trifiletti DM, Zaorsky NG. Toxicity after radiotherapy in patients with historically accepted contraindications to treatment (CONTRAD): an international systematic review and meta-analysis. Radiother Oncol. 2019;135:147–152. [DOI] [PubMed] [Google Scholar]
- 9.Deasy JO, Blanco AI, Clark VH. CERR: a computational environment for radiotherapy research. Med Phys. 2003;30(5):979–985. [DOI] [PubMed] [Google Scholar]
- 10.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S70–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85(2):444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaverdian N, Thor M, Shepherd AF, et al. Radiation pneumonitis in lung cancer patients treated with chemoradiation plus durvalumab. Cancer Med. 2020;9(13):4622–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang JY, Lin SH, Yao L, et al. I-SABR phase II randomized study of nivolumab immunotherapy and stereotactic ablative radiotherapy in early stage NSCLC: interim analysis adverse effects. J Clin Oncol. 2020;38(15_suppl):9035. [Google Scholar]
- 14.Zhao J, Yorke ED, Li L, et al. Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: a pooled analysis of 88 studies. Int J Radiat Oncol Biol Phys. 2016;95(5):1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolladille C, Ederhy S, Sassier M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6(6):865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and efficacy of retreating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 2018;6(9):1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4(8):1112–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diao K, Chen YH, Catalano PJ, et al. Radiation toxicity in patients with collagen vascular disease and intrathoracic malignancy treated with modern radiation techniques. Radiother Oncol. 2017;125(2):301–309. [DOI] [PubMed] [Google Scholar]
- 19.Josipovic M, Aznar M, Rydhög J, et al. MA05.06 Locally advanced lung cancer radiotherapy in deep inspiration breath hold: dosimetric benefits from a prospective trial. J Thorac Oncol. 2018;13(10):S372–S373. [Google Scholar]
- 20.Wink KCJ, Roelofs E, Simone CB 2nd, et al. Photons, protons or carbon ions for stage I non-small cell lung cancer - results of the multicentric ROCOCO in silico study. Radiother Oncol. 2018;128(1):139–146. [DOI] [PubMed] [Google Scholar]
- 21.Liu G-Y, Li Q, Yang X-J, Xiong Z-H. The correlation between serum contents of TGF-β1 and IL-6 and acute radiation pneumonitis in patients with lung cancer. J Acute Disease. 2016;5(4):333–336. [Google Scholar]
- 22.Chen Y, Rubin P,Williams J, et al. Circulating IL-6 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2001;49(3):641–648. [DOI] [PubMed] [Google Scholar]
- 23.Kocak Z, Evans ES, Zhou SM, et al. Challenges in defining radiation pneumonitis in patients with lung cancer. Int J Radiat Oncol Biol Phys. 2005;62(3):635–638. [DOI] [PubMed] [Google Scholar]
- 24.Arbetter KR, Prakash UB, Tazelaar HD, Douglas WW. Radiation-induced pneumonitis in the “nonirradiated” lung. Mayo Clin Proc. 1999;74(1): 27–36. [DOI] [PubMed] [Google Scholar]
- 25.Thomas R, Chen Y-H, Hatabu H, Mak RH, Nishino M. Radiographic patterns of symptomatic radiation pneumonitis in lung cancer patients: imaging predictors for clinical severity and outcome. Lung Cancer. 2020;145:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]


