Abstract
The ethical, legal, and social implications (ELSI) of emerging neurobiobanks and data resources are unclear in an African scientific landscape with unique cultural, linguistic, and belief systems. The overarching goal of the African Neurobiobank for Precision Stroke Medicine—ELSI Project is to identify, examine, and develop novel approaches to address ELSI issues of biobanking and stroke genomic research in sub-Saharan Africa (SSA). To accomplish the goal we will (1) explore knowledge, attitude, perceptions, barriers, and facilitators influencing ELSI issues related to biobanking and stroke genomic research; (2) use information obtained to craft a community intervention program focused on ELSI issues; and (3) build capacity and careers related to genomics and biobanking for effective client/community engagement while enhancing regulatory, governance, and implementation competences in biobanking science in SSA. A community-based participatory research and mixed-methodological approach, focused on various levels of the social ecological model, will be used to identify and examine relevant ELSI issues. Contextual intervention tools, platforms, and practices will be developed to enhance community understanding and participation in stroke biobanking and genomics research activities while facilitating enduring trust, and equitable and fair utilization of biobanking resources for genetic and trans-omics research. A concurrent capacity building program related to genetic counseling and biobanking will be implemented for early career researchers. The huge potential for neurobiobanking and genomics research in Africa to advance precision medicine applicable to stroke and other neurological disorders requires addressing ELSI challenges while building sustainable research, career, and regulatory capacities in trans-omics and biobanking science.
Keywords: biobanking, neurobiobanking, stroke, genomics, precision medicine, ethical, legal, social issues, ELSI, Africa
Background
Africa has historically been underrepresented in the biobanking revolution (Vaught, 2016a, 2016b). However, with the Human, Heredity and Health in Africa (H3Africa) Initiative biorepository science has been bolstered and biobanking-related activities are growing on the continent (Abimiku et al., 2017; Akinyemi et al., 2018; Mayne et al., 2017). Biobanking is a rapidly evolving field of biomedical science that involves the collection, processing, storage, and distribution of biospecimens along with creating necessary policies and procedures for each stage of the process. Although initially focused on collecting samples for diagnostic purposes in pathology settings, biobanks have since evolved into organized repositories and networks that are now engaged in cutting-edge translational research, which form the bedrock of personalized (or precision) medicine. Neurobiobanking derives its uniqueness in the storage of data from brain images, clinical neurological information, and neural tissue in addition to other biological materials stored by other kinds of biobanks. However, organized neurobiobanking is still burgeoning in sub-Saharan Africa (SSA) with the Stroke Investigative Research and Education Network (SIREN) Biobank (Akinyemi et al., 2018) and the IBADAN Brain Bank (Akinyemi, Salami, et al., 2019) representing pioneering efforts in the region.
In SSA, stroke persists as a leading cause of death, disability, and dementia with incidence rates rising, outcomes worse than those found in higher income countries, and relatively younger people affected compared to White populations (Ezejimofor et al., 2017; Owolabi et al., 2015). Developing trans-omics approaches to predict, prevent, diagnose, and treat stroke in the framework of current emphasis on precision/personalized medicine (Collins & Varmus, 2015; Hewitt, 2011) requires a clear understanding of the interplay of environmental and genomic risk factors (Akinyemi et al., 2016; Owolabi et al., 2017). The substantial genetic diversity of African populations offers a unique opportunity to identify novel genes and molecular pathways (Xu et al., 2017); hence, the National Institutes of Health–funded SIREN (Akpalu et al., 2015) has focused on furthering understanding of the environmental and genetic risk factors for stroke in SSA and, in the process, has developed a robust biobank of invaluable brain imaging data and human tissue resources, including blood and its fractions (serum, plasma, buffy coat, and red cell concentrates) and genetic materials (DNA) (Akinyemi et al., 2018). In addition, the IBADAN Brain Bank Project, the first organized brain bank in Africa, has emerged to accrue brain tissue for future complementary trans-omic studies (Akinyemi, Salami, et al., 2019). However, the ethical, legal, and social implications (ELSI) of the developing neurobiobank and data resources are unclear in an emerging African scientific landscape with unique cultural, linguistic, and belief systems operating in a global “open science” research community that increasingly supports the principles of trust, openness, storage, sharing, and secondary use of biological materials (Bledsoe, 2017). The huge potential for neurobiobanking in Africa to advance precision stroke medicine (Hinman et al., 2017) requires that important ELSI issues be addressed while building sustainable research and regulatory capacity, careers, and infrastructure in trans-omics and biobanking science.
Biobanking and Precision Stroke Medicine
Precision medicine (often used interchangeably with personalized medicine) is broadly described as treatments targeted to the needs of individual patients on the basis of genetic, biomarker, phenotypic, or psychosocial characteristics that distinguish a given patient from other patients with similar clinical presentation (Collins & Varmus, 2015; Hewitt, 2011). The purpose of precision medicine is to ensure that the patient gets the right treatment, at the right dose, at the right time, with minimum ill consequences and maximum efficacy. Precision stroke medicine (Hinman et al., 2017) involves the acquisition of multiple data sets including clinical phenotype, biological data (genomic, blood biomarkers), imaging data, and data integration (bioinformatic analysis, systems biology, and modeling including artificial intelligence) toward individualized diagnosis and treatment, as well as prediction of recurrence risk, medication effects, procedural outcomes, and stroke recovery. Biological samples stored in a biobank provide resources to generate biological data (omics data sets), which contribute to the process of delivering tailored interventions (Hewitt, 2011). For instance, gene expression profiling based on interrogation of RNA molecules circulating in peripheral blood has already demonstrated promise in differentiating stroke from acute nonstroke conditions and in differentiating subtypes (Hinman et al., 2017). Genomic study of stroke among Africans presents a unique opportunity for the discovery, validation, fine mapping and functional annotation, and translation of genomic determinants of stroke with implications for global populations (Owolabi et al., 2017).
Scientific Gaps in the ELSI of Neurobiobanking and Stroke Genomics in Africa
Genomic and biobanking-based research is increasingly being conducted in the context of collaborations between researchers in high-income countries and those in low- and middle-income countries (LMICs) (Munung et al., 2017). Although African researchers now have unique opportunities to contribute to cutting-edge stroke genomic research, existing ethical, legal, and regulatory frameworks have also been challenged (de Vries et al., 2016, 2017; Tindana & de Vries, 2016). Gaps exist in the key ELSI elements of biobanking, including informed consent (broad, blanket, restricted, and tiered), respect, trust building, privacy, confidentiality, sample reuse and sharing, feedback of results, community engagement (CE), governance, and capacity strengthening related to neurobiobanking and stroke genomics research in SSA. The key question is: How do we enhance sustainable, equitable, and trustworthy use of stored biological samples and pragmatically put genomics and other biomarker data therefrom to beneficial use in the best interest of the public and scientists in SSA? In particular, the ELSI surrounding emerging stroke biobank and data resources and ELSI principles that should govern return of individual results and incidental findings of stroke genomic research in SSA remain unclear. Additionally, there does not seem to be culturally appropriate, context-relevant, sustainable tools available for addressing the ELSI of neurobiobanking, and stroke genomics research in the region. Certainly, original, region-based, evidence-driven exploration and intervention into ELSI of neurobiobanking and stroke genomics in SSA is urgently required.
Study goal.
The overarching goal of the African Neurobiobank for Precision Stroke Medicine—ELSI Project is to identify, examine, and develop novel approaches to address ELSI issues of stroke genomics and biobanking in SSA. The project will develop novel, context-relevant, and culturally appropriate interventions to enhance community understanding and participation in stroke genomic and biobanking activities in an equitable, trusting, and fair space while building sustainable careers in genetic counseling and biobanking science.
Theoretical frameworks for the African Neurobiobank for Precision Stroke Medicine ELSI Project.
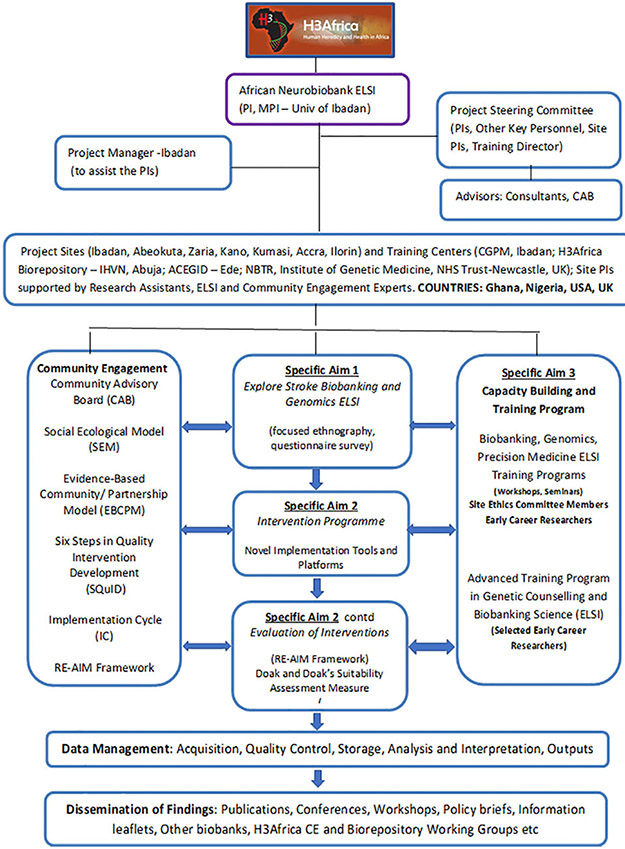
The guiding theoretical frameworks for the project are the social ecological model (SEM; Young et al., 2006), evidence-based community partnership model (EBCPM; Coughlin & Jenkins, 2017; Wells et al., 2004), the Six Steps in Quality Intervention Development (SQuID; Wight et al., 2016), implementation cycle (IC; Owolabi et al., 2016), and the RE-AIM Framework (Glasgow & Estabrooks, 2018; Harden et al., 2018). Participants in the research are viewed through the lens of the SEM and community-based participatory research (CBPR; Coughlin & Jenkins, 2017), while the processes of development and implementation of the novel interventions are based on the SQuID (Wight et al., 2016), EBCPM (Wells et al., 2004), and IC (Owolabi et al., 2016). We have also adopted the Reach, Effectiveness–Adoption, Implementation, Maintenance (REAIM) framework to enable the team to comprehensively evaluate the processes of our interventions and accomplishments (Sweet et al., 2014). Figure 1 shows the overall organizational plan of the project, while Table 1 provides further details on the guiding frameworks.
Figure 1.
Organizational plan for the African Neurobiobank for Precision Stroke Medicine ethical, legal, and social implication.
Table 1.
Theoretical and Model Underpinnings and Standardized Definitions.
| Model/Theory | Description | Application |
|---|---|---|
| Social ecological model (SEM) | SEM constructs are used to explore individual and population health related to biobanking and stroke genomics including individual factors (e.g., genetics, individual knowledge, beliefs, behaviors, consent, privacy, confidentiality, return of results, and incidental findings), interpersonal factors (e.g., family, friends, peers, benefit sharing, and return of results), institutional processes (e.g., governance and regulatory framework, biobank networks, and social support systems), and community factors (e.g., relationships among networks, biobanking governance), and public policy (e.g., local, state, and national laws/policies on biobanking). | Provides overall framework for organizing our research. Each participant is viewed through the lens of the SEM. The SEM will guide the development of the focused ethnography (FE) within Aim 1, with in-depth interviews with stroke survivors and formal and informal caregivers, researchers, public health practitioners, community leaders, faith leaders, traditional healers, and lay public. The survey research methodology will incorporate information from the FE related to the SEM and will guide Aim 2. |
| Evidence-based community/partnership model (EBCPM) | The EBCPM process is designed to implement community-based participatory research and evidence-based practice “to support health improvement goals through evidence-based strategies while building community and practice capacity to implement those strategies in a manner consistent with community priorities, culture, and values.” | The EBCPM will be operationalized within Aims 1 and 2 in parts. Step 1 will include negotiation of goals among local community stakeholders, practices, and researchers (already done in planning grant proposal). Step 2 will include active involvement of the Biobanking Community Advisory Board (CAB) to guide researchers and actively participate in engaging the community in process. This will be done by matching community needs, resources, and values with evidence-based practice strategies to address unmet needs and tailor these to the community contexts (Aim 2). |
| Six Steps in Quality Intervention Development (SQuID) | The SQuID is a six-step process-based approach used to guide researchers in developing high-quality interventions. Steps: (1) Defining and understanding the problem and its causes, (2) clarifying which causal or contextual factors are malleable and have greatest scope for change, (3) identifying how to bring about change and change mechanism, (4) identifying how to deliver change mechanism, (5) testing and refining on small scale, and (6) collecting sufficient evidence of effectiveness to justify rigorous evaluation/implementation. | The six steps are used for capturing the process for development of the proposed interventions (in combination with the EBCPM). Given the scope of this work, pretesting/testing will be limited. FE and survey will incorporate Steps 1–4 (Aims 1 and 2), and Aim 2 will focus on Steps 2–5. Application of Step 6 will be tested in future grant. |
| Implementation cycle (IC) | The IC is a seven-step process that involves identification and exploration of evidence gaps and subsequent development, contextualization, communication of evidence-based consensus interventions to combat cardiovascular diseases in LMICs. It also entails the evaluation of the processes and outcomes involved. The cycle includes a Translatability Scale to grade the ease of implementation especially in resource-limited settings. | The seven steps of the IC are adaptable to the processes of African Neurobiobank Precision Stroke Medicine ELSI Project. Aim 1 of our project is in tandem with Steps A and B of the cycle, while Aim 2 aligns with Steps C–G of the IC. The training component (Aim 3) of the project will ensure an adequate supply of expertise for effective implementation, evaluation and scaling up the interventions tools, and platforms for wider population-based impact. |
| Reach, Effectiveness-Adoption, Implementation, Maintenance (RE-AIM) framework for Evaluation | RE-AIM provides a functional framework for assessing the public health impact of health promotion strategies. Evaluative criteria include: Reach assesses the percentage, and characteristics of individuals within population who participate in a program; effectiveness explores positive and negative programmatic outcomes; adoption encompasses the number, percentage, and representativeness of program participants in relation to the overall population of interest; implementation is an indicator of the extent of program delivery, adherence, and cost; and maintenance reflects sustained adoption/adherence of the primary outcomes (>6 months) at the individual level and sustainability of delivered programs at an organizational level. | We will use RE-AIM to evaluate the overall African Neurobiobank for Precision Stroke Medicine: Ethical Legal Social Implications (ELSI) Project. Specifically, REACH will be used to capture the numbers of investigator, trainee, and community participants in the project. Community participation will include CAB participation as well as community members participating in various aspects of the program. EFFECTIVENESS will be operationalized to capture both the negative and positive effects/outcomes for investigators and trainees, and the communities. Adoption process and outcome evaluation will include measurement of number of settings, organizations, trainees, and community members that actively participate in our ELSI project. Implementation will measure the overall and specific activities implemented as planned. Maintenance will examine the changes or outcomes that are potentially “lasting outcomes” or changes as a result of ELSI activities. We will specifically examine (1) new ELSI-related knowledge regarding stroke genomics and biobanking; (2) novel interventions to address the ELSI issues so identified; (3) processes, outcomes, and impact of the interventions; (4) training early career researchers in ELSI research and practice; and (5) enhanced capacity of ethics committees to deal with ELSI issues in new research projects being developed in biobanking, genomics, and precision stroke medicine according to the various levels of SEM and RE-AIM frameworks. |
Note. LMIC = low- and middle-income countries.
Methodological Approach
This project uses a CBPR (Coughlin & Jenkins, 2017) and mixed-methodological approach involving the various levels of the SEM: (a) individual/patient; (b) family/significant other caregiver; (c) healthcare/organizations, traditional/faith healers, hospital administrators, and ethics committee members; and (d) the community (Young et al., 2006). The SEM will help identify and examine relevant ELSI issues and develop novel, context-relevant, and culture-appropriate interventions. A key concept in CBPR is identifying and collaborating with community partners to meet local needs and priorities that build toward a sustainable and equitable academic and community partnership (Israel et al., 2013). Our study continues work from the existing SIREN study and CBPR by enhancing community partnerships and expanding efforts to establish ELSI-specific Community Advisory Boards (CABs). Using CBPR approaches will enable us to engage with community partners, enhance community input, and identify community-specific preferences and approaches toward ELSI neurobiobanking and stroke genomic research.
Settings, Sites, and Study Population
The project will recruit stroke survivors, caregivers, healthy controls, providers, hospital administrators, researchers, public health practitioners, ethics committee members, and laypersons from seven sites within the established SIREN from communities in southern (Abeokuta and Ibadan), central (Ilorin), and northern (Kano and Zaria) Nigeria and southern (Accra) and central (Kumasi) Ghana (Table 2). Ethical approval will be sought from the institutional review board of each site and informed consent from each participant in the focused ethnography and questionnaire survey.
Table 2.
Participating Sites.
| Site | Description |
|---|---|
| Korle Bu Teaching Hospital (KBTH), Accra, Ghana | This is a 1,500-bed tertiary hospital serving as the main referral hospital of the Southern part Ghana and the capital Accra. Stroke is the leading cause of admissions to the medical wards of the KBTH with a high case fatality rate. There is currently an ongoing collaboration to improve stroke care in the Wessex–Ghana stroke project. |
| Komfo Anokye Teaching Hospital (KATH), Kumasi, Ghana | KATH is the second largest hospital in the country (~ 1,000 beds) and the only tertiary health institution in the Ashanti Region, located in Kumasi, Ghana. It is the main referral hospital for the Ashanti, Brong Ahafo, Northern, Upper East and Upper West Regions. It is the teaching hospital affiliated to the medical school of the Kwame Nkrumah University of Science and Technology (KNUST), also located in Kumasi. Stroke also forms a major cause of admission at KNUST with mortality rates of up to 30%. |
| The University College Hospital (UCH) and Blossom Center for Neurorehabilitation, Ibadan, Nigeria | UCH has 850 bed spaces and 163 examination couches. The hospital is a tertiary institution with several affiliated community care centers where the hospital offers secondary and primary health care. Blossom Center for Neurorehabilitation was established in 2010 through the support of the World Federation for Neurorehabilitation as the first center for Neurorehabilitation in East, West, and Central Africa. |
| The Federal Medical Center (FMC), Abeokuta, Nigeria and Sacred Heart Hospital, Abeokuta | FMC is a 250-bed regional tertiary center established in April 1993. It receives patients from Ogun and neighboring states and countries and relates closely with community care clinics within and outside the Abeokuta metropolis. The Sacred Hospital Abeokuta is the oldest missionary community hospital in Nigeria focusing on underserved communities in the subregion. |
| Aminu Kano Teaching Hospital (AKTH), Kano, Nigeria | AKTH hospital is a tertiary referral health center established in 1988 for Kano state and its neighboring states like Jigawa, Katsina, Zamfara, Bauchi, Gombe, and Yobe. |
| The Ahmadu Bello University Teaching hospital, Zaria, Nigeria | It is located in Zaria, Northern Nigeria, and consist of three sites located in Kaduna and Zaria (in Kaduna State), and Malumfashi (in Katsina State), with a total bed capacity of 768. It remains a major referral center for the 19 Northern States but receives patients predominantly from the Northwestern States of Kaduna, Katsina, Zamfarad Kano, and adjoining Northcentral States of Niger, Kogi, and Nassarawa, including Abuja, the Federal Capital. |
| University of Ilorin Teaching Hospital (UITH), Ilorin, Nigeria | UITH is a 550-bed hospital with 130 beds for medical inpatients, of which 10 are dedicated to acute stroke care. This is in addition to 23 beds for medical emergencies. Between 20 and 30 stroke cases are admitted per month and most of these are managed in a dedicated acute stroke care unit. The hospital is equipped with CT and MRI scanners while carotid Doppler studies and cardiac Holter monitoring are also available for patients who require these. In addition, the hospital provides community-based primary health care services in three adjoining states, namely, Esie in Kwara, Ihima in Kogi, and Kishi in Oyo states. |
CAB and CE
A CAB (Guidance Tools for CABs, 2012; Newman et al., 2011) will be formed in each community consisting of six to nine members (minimum) at each study site, consistent with CBPR principles. The membership will be comprised of stroke survivor(s), faith-based leader(s), community leader(s), public health leader(s), ethics committee representative(s), and profession-based organizational leader(s), including the media and legal practice. Potential CAB members will be approached by the study CE coordinators at each site through personal contact from an existing pool of key community members residing in the SIREN study areas. The CABs will serve as a community liaison at each of the seven sites and will guide the investigators in explaining research to the local residents and facilitate the recruitment of participants, sustainable and responsive interactions with the community as well as facilitating feedback of research findings back to the community. Initial meetings/discussions will be held with the CAB members to ensure that they understand the goals of the study, strengthen community-academic partnerships, and facilitate stakeholders’ input into the operations and programs of CE and the research processes.
Specific Aim 1: To identify and examine ethical, legal, and social issues relating to stroke biobanking in the context of African research landscape, culture, and beliefs. We will explore knowledge, attitude, perceptions, barriers, and facilitators influencing ELSI-related to the use of blood and blood fractions in the context of stroke genomic research. The research questions will be explored using a mixed qualitative (focused ethnography; Higginbottom et al., 2013) and quantitative (questionnaire survey) approach and focus on the key issues of the ELSI of donating blood for research, storage, reuse of stored blood fractions, and return of individual research results and incidental findings.
Plan to Accomplish Specific Aim 1
Qualitative approach.
Focus group discussions (FGDs) and key informant interviews (KIIs) will be conducted following the guidance of Higginbottom et al. (2013). To develop the interview guidance notes, we reviewed the peer-reviewed literature related to the ELSI of neurobiobanking and genomic research as well as lay literature available to the communities. The research team further reviewed and refined the interview guides to ensure the aforementioned research questions are appropriately captured. Interview prompts include a social ecological perspective (i.e., patient, family/caregiver, health systems [including traditional healers], and community factors; Green et al., 1996; Young et al., 2006). The FGDs will explore the perspectives of the ELSI CABs members, stroke patients, stroke caregivers, and stroke-free laypersons, while the KIIs will explore the views of sampled researchers, health care providers, public health practitioners, hospital administrators, and religious and community leaders on key ELSI elements of neurobiobanking. These ELSI elements include informed consent (broad, blanket, restricted, or tiered), trust, privacy and confidentiality, ownership, sample storage and secondary use, feedback of research results (general and individual) and incidental findings, governance, and regulatory issues (Bledsoe, 2017) within the context of stroke genomic research. Similar approaches have been used in previous studies exploring perspectives of laypersons about biobanking (Igbe & Adebamowo, 2012) and of research participants about use of biological samples (Moodley et al., 2014).
FGDs.
Trained interviewers will conduct FGDs at SIREN participating site settings described in Table 2. Each FGD will include six to eight participants to sustain a meaningful but guided discussion and four FGDs will be conducted per site. Respective FGDs will be comprised of (1) site CAB members, (2) stroke survivors previously enrolled in SIREN, (3) their primary caregivers, and (4) stroke-free laypersons who have not been previously enrolled in SIREN. Participants will be selected by purposive sampling (maintaining careful balance for age, gender, socioeconomic status, and types/levels of disabilities and caregiver support distribution, as appropriate) until no new data related to biobanking ELSI are identified (saturation). Participants will be 18 years and older and have no condition that could limit FGD participation, such as cognitive impairment/dementia (Brief Community Screening Interview for Dementia (CSID) score < 6; Prince et al., 2011) and severe global disability (modified Rankin score ≥ 3; Owolabi, Akinyemi, Gebregziabher, et al., 2014; Owolabi, Akinyemi, Hurst, et al., 2014). We will conduct the sessions with a member of the ELSI team taking field notes (video and/or audio). Written consent will be obtained from participants prior to the start of any session. The interviews will occur at a location convenient to participants. Methods developed by Tremblay (2002) will guide interview processes. All interviews, along with field notes and audio/video (as appropriate), will be recorded and audio transcribed verbatim for qualitative analysis.
KIIs.
Interviews will be carried out with health care providers (doctors, nurses, pharmacists, medical laboratory scientists, medical records personnel, and other allied health workers), health administrators, religious and community leaders, and traditional healers using semistructured qualitative inquiry. Approximately 10 participants from each SIREN participating site will be recruited. A KII protocol and specific questions will be developed to guide the discussion regarding key ELSI elements of neurobiobanking focusing on the key research questions. Participants in the KIIs will also be “purposively sampled” from the various stakeholder groups. However, such interviewees will be with persons with demonstrable interest and/or work experience with patients with stroke. Regarding religious leaders, we will liaise with the CAB of the SIREN participating sites to identify relevant religious leaders representing the major faith blocs (Christianity, Islam, and Traditional Religion).
Qualitative data management, analyses, and reporting.
All data will be uploaded and stored on REDCap (Harris et al., 2009), a secure data storage system. Transcripts of the audio recordings and field notes will be used for data analysis. Transcriptions will be compared with recordings to confirm accuracy and authenticity. The transcripts will be imported into the text analysis software (i.e., NVivo, version 9.0) for data management and analyses. For the initial analysis of the data, we will review the transcripts and undertake the process of (a) coding for descriptive labels, (b) sorting for patterns, (c) identification of outliers or negative cases, (d) generalizing the constructs and theories, and (e) conduct memoing, including reflective remarks (Miles et al., 2013). Saturation of data will be determined when no new descriptive labels are identified. The “framework analysis” approach (Srivastava & Thomson, 2009) will be used and it includes five key stages: familiarization, identifying a thematic framework, indexing, charting, mapping, and interpretation to meet the processes of Steps 1 and 2 and partially address Step 3 of the 6 SQuID (Wight et al., 2016). To maintain and evaluate rigor, reliability and validity issues in qualitative studies are best addressed as trustworthiness, which are dependent on credibility, confirmability, transferability, and dependability (Shenton, 2004).
Quantitative approach (questionnaire survey)
Design.
Drawing lessons from the approaches of Jallinoja and colleagues (Jallinoja & Aro, 1999; Jallinoja & Aro, 2000), Moodley et al. (2014), and Yamamoto et al. (2017), as well as key highlights of the pilot phase of the project, we will develop a survey questionnaire to elicit demographic information and perspectives on elements of the key study questions: ELSI of donating blood for research, storage and secondary use of stored blood and blood fractions, and preference for return of individual research results and incidental findings within the context of stroke genomics research. We will explore the association of factors that might influence respondents’ perspectives: “demographic data,” “genetic knowledge,” “cultural and religious beliefs,” “health-conscious behaviors,” and “communication with health professionals” (Yamamoto et al., 2017). For “genetic knowledge,” we will utilize the genetic knowledge questionnaire developed by Jallinoja et al. (2000).
Health conscious behavior type questions will deal with regular physical activity, eating a healthy diet, and intentionally reducing alcohol intake and/or smoking. Questions concerning communication with health professionals seek to assess whether participants have a health care provider for consultation regarding their health checkup results. Participant’s preferences on receiving individual research results and incidental findings will be explored in relation to the following types of conditions: lifestyle diseases, pharmacogenetics, adult-onset clinically actionable diseases, and adult-onset nonclinically actionable diseases (multiple answers possible; Yamamoto et al., 2017). The questionnaire will be pretested in a non-SIREN hospital site and further refined in a FGD. FGD participants will be asked about readability, comprehension, and cultural appropriateness of questionnaire items. Data from FGDs will allow further questionnaire refinement. We will invite sampled SIREN participants via telephone (cases and controls) to complete the survey questionnaire, while non-SIREN laypersons will be recruited through in-person CE activities at their respective sites. Questionnaires will be administered by trained interviewers and written informed consent will be obtained prior to completion of the survey.
Questionnaire survey (sample size, power calculation, and statistical analysis).
The survey will be conducted in SIREN participating sites (Table 2) and involve 1,000 SIREN-enrolled stroke survivors, 1,000 SIREN-enrolled stroke-free controls, and 1,000 non-SIREN stroke-free laypersons. Including diverse perspectives will allow the examination of the influence of previous contact with the SIREN study as well as stroke disease status on the key ELSI outcome measures being explored. Survey sample numbers are estimated based on preliminary data, which showed 48.7% of stroke patients and 57.0% of stroke-free individuals have knowledge of stroke heritability (Akinyemi, Sarfo, et al., 2019). Using these as a premise and with a 3% degree of precision and 95% confidence level, 829 stroke-free controls would be required. Adjusting for 85% anticipated response rate, the effective sample size is estimated to be 975. The survey is targeted at capturing the knowledge, attitude, behavior, and practice (KABP) of the three distinct groups (SIREN stroke survivors, SIREN-enrolled stroke-free controls, and non-SIREN stroke-free laypersons) with similar sample size estimations for these three groups, yielding an average of 1,000 per group across all sites. The number of participants to be surveyed per site will be proportionate to the size of SIREN recruitment from each site. Respondents will be selected by stratified random sampling using the recruitment list as sampling frame.
Data analysis.
Descriptive statistics will be used to summarize the demographic and other variables. For hypothesis testing, χ2 or Fisher’s exact test will be used to investigate factors associated with KABP regarding genetic testing. p Values of .05 will be considered statistically significant. The Mann–Whitney U test will be used to analyze ranked responses including participants’ preferences for receiving genetic test results. To identify the independent factors associated with the outcomes, logistic regression models with robust (clustered) standard error will be fitted to adjust for clustering or withinsite correlations. Data analysis results from the survey will be triangulated with the results of analyses of the qualitative data sets to provide input into the process of designing interventions in Specific Aim 2.
Specific Aim 2: To use information obtained from Aim 1 to craft a novel intervention program to address the ELSI issues related to stroke genomic and biobanking research in SSA using the CBPR and a multimodal approach (to target different components of the SEM).
Plan to Accomplish Specific Aim 2
The key goal of this specific aim is to develop contextual interventions to enhance community understanding and participation in neurobiobanking activities in an environment where there is enduring trust and equitable and fair utilization of biobanking resources for genetic and trans-omics research to enhance precision medicine using stroke as a model. Intervention development and pretesting will focus on working with the CAB and a purposively selected group of researchers, poststroke patients, families, community members, health care providers, faith-based organizations, and regulatory bodies. The overarching purpose of this group will be to integrate findings from Aim 1 to develop and refine culturally and scientifically relevant intervention strategies and incorporate potential variations across regions/sites.
Development of intervention tools.
Our team has prior experience producing a documentary on stroke suitable for the lay public (Akinyemi et al., 2015; Owolabi, Akinyemi, Gebregziabher, et al., 2014). We will employ our knowledge from this experience and work with communication experts, as well as technical partners, to produce high-quality materials. These materials will be reviewed by the CAB and research team members for input and further refinement. Using themes and content details synthesized from the outputs of Specific Aim 1, we will develop short, novel, and informative training videos, comics, and case vignettes on neurobiobanking, genomics, and precision medicine with an emphasis on the associated ethical, legal, and societal issues (Table 3). During the developmental stages of the intervention tools, we will ask SIREN stroke patients, stroke caregivers, SIREN stroke-free control subjects, and stroke-free laypersons (four Nigerians and four Ghanaians for each category) to view the videos and provide feedback via semistructured interviews and narratives. We plan to adapt Doak and Doak’s Suitability Assessment Measure (Doak et al., 1994; Weintraub et al., 2004) to help us evaluate the tools along with the semistructured interview prompts based on Willis’s cognitive interviewing techniques (Willis, 2004; Willis & Miller, 2011). This feedback will inform further refinements of the tools to ensure they are contextually relevant, culturally sensitive, and can be clearly understood by those viewing the materials.
Table 3.
Novel Intervention Tools and Platforms.
| Novel Tool/Platform | Goal | Target |
|---|---|---|
| Biobanking and Personalized Medicine Community Advisory Board (CAB) | To bridge the academic-community gap an enhance participant and public education regarding ELSI related to biobanking and stroke genomics. | Stroke community and general public |
| Neurobiobank Expert Consensus Guideline | Government and professional association sanctioned expert consensus ELSI stroke biobanking and genomics policy guideline document to enhance best practices among professionals involved in biobanking and genomics research | Stroke community, stroke genomic and neurobiobank researchers |
| Biobanking training programs (workshops, seminars) | To enhance biobanking awareness and understanding of related ELSI issues | Researchers, ethics committee members, policy makers, public health practitioners, health administrators, and CAB members |
| Biobanking and Precision Medicine Community Awareness Programs | To promote public understanding regarding biobanking and stroke genomics ELSI | Faith-based organizations, lay public, print and electronic media, mHealth technologies, social media platforms, and film industry such as Nollywood |
| Stimulate the establishment of the African Neurobiobank Network | To provide a platform for regular engagement of biobank researchers, development of harmonized guidelines for neurobiobanking in SSA based on international best practices | Stroke community, stroke genomic, and neurobiobank researchers |
| Development of informative video on neurobiobanking, genomics and precision medicine | To enhance biobanking, stroke genomics, and precision medicine literacy and related ELSI | Stroke community (patients and persons at risk, caregivers, advocates) and lay public |
| Development of training video on neurobiobanking and precision medicine | To enhance biobanking, stroke genomics and precision medicine literacy and related ELSI | Researchers and academics (stroke, genomics, biobanking) and ethics committee members |
| Development of comics and case vignettes relating to neurobiobanking, genomics, and precision medicine | To use comics and case vignettes to illustrate key ELSI-related scenarios and how to deal with them | Stroke community, lay public, and especially young people |
| Capacity training program in genetic counseling and biobanking science | To develop a crop of professionals with relevant competences in neurobiobanking and genetic counseling. | Biomedical scientists, public health professionals, postgraduate students with relevant qualifications |
Note. ELSI = ethical, legal, and social implications; SSA = sub-Saharan Africa.
CE, dissemination, and implementation.
Using our previously developed IC framework (Owolabi et al., 2016), we will implement a multilevel CE program for deployment of each intervention tool and platform and collect measurable outcome data. Furthermore, as part of our CBPR approach, we will engage with relevant professional organizations and groups to develop and refine current biobanking guidelines, as applicable to neurobiobanking. Workshops and seminars will be organized targeting early career researchers (ECRs), members of the research community, ethics committee members, policy makers, public health practitioners, and health administrators to promote awareness and understanding of ELSI related to neurobiobanking, genomics, and precision medicine. Given our prior experience with this type of deployment, we anticipate a robust impact.
Specific Aim 3: To build capacity and careers related to ELSI of genomic (genetic counselors) and biobanking (biobank scientists), to enhance regulatory (ethics committees) and implementation competencies in biobanking science in SSA.
Plan to Accomplish Specific Aim 3
A prime goal of the H3Africa program is capacity building for the next generation of African researchers and professionals to use genomic approaches to advance health research. In line with this vision, our project is designed to facilitate the growth of ECRs and ethics committee members drawn from the study sites in ELSI related to neurobiobanking, genomics, and precision medicine through training workshops and seminars. From the cohort of ECRs, four talented and highly motivated ECRs will be competitively selected for advanced training. The focus on genetics and genomics (two genetic counselors) and biobanking science (two biobank scientists/managers) will ensure that advanced competencies are acquired by these emerging leaders to handle ELSI-related issues including governance, sample use and storage, genomic and biobanking education, effective client/CE, international sample and data sharing, and other emerging ELSI issues. The program is designed with sufficient exposure to training in ELSI related to biobanking, genomics, and precision medicine. Specific components of the training include (i) responsible conduct of research; (ii) informed consent; (iii) biosample reuse; (iv) biorights; (v) data sharing; (vi) CBPR focused on building the capacity of the researchers to work effectively with our communities; (vii) qualitative and quantitative research processes and analyses; (viii) genetic, genomic, and phenomic research; (ix) statistical analysis; (x) genetic epidemiology and phenotyping; (xi) bioinformatics; and (xii) mentoring. After the training, the project will facilitate continued support and institutionalized mentoring of the ECRs through the Centre for Genomic and Precision Medicine at the College of Medicine, University of Ibadan, Nigeria. Group, peer, and one-on-one mentoring models will be facilitated with the local and international faculty as well as consultants on the project. The training program will also enhance regulatory and governance competencies of health research ethics committee members across study sites in dealing with ELSI-related matters of genetics/genomics and biobanking science in SSA. By this, the project will greatly enhance capacity for ELSI research in Africa and initiate a favorable trajectory of successive generations of highly trained African personnel within this important field.
Evaluation of the Interventions
To assess the processes and outcomes of the intervention, we will adapt the RE-AIM model (Glasgow & Estabrooks, 2018; Harden et al., 2018; Sweet et al., 2014) to evaluate the reach, effectiveness, adoption, implementation, and maintenance of the novel ELSI tools, platforms, and interventional activities we develop (Table 3). The use of RE-AIM will enhance the quality of the evaluation and will be used to evaluate the overall and specific activities of the ELSI Project. We will use the “hybrid approach” that is guided by pragmatic decisions regarding prioritization of strategies that deserve a more thorough RE-AIM evaluation and which are better evaluated using one or two RE-AIM dimensions described by Glasgow and colleagues (Glasgow & Estabrooks, 2018; Harden et al., 2018).
Dissemination of Products
Products from this project (videos, vignettes, comics, scholarly publications, newsletters, policy briefs, etc.) will be disseminated widely and made available across Africa and globally through peer-reviewed publications, scientific conferences, including the biannual H3Africa Consortium meetings, other biobanks and CE programs/strategies, regular meetings with CAB members, electronic, print, and social media, as well as presenting results at community-based activities. Electronic versions of all products will be available on the project website. Translation into local languages will be encouraged for wider dissemination.
Conclusion
This study will provide currently nonexistent but much-needed practical tools and interventions for addressing the ELSI of neurobiobanking and neurogenomics in Africa at several levels, with stroke genomics as a model. It will explore the utilization of technology-driven tools that facilitate ready integration across different tiers of health care facilities and the community. Additionally, the approach developed in this project may serve as a model for addressing neurobiobanking ELSI in LMICs around the world, and even underresourced areas within high-income countries, as well as for addressing biobanking and genomics ELSI of other chronic diseases. To our knowledge, this will be the first study to (1) establish a culturally sensitive, multilevel, systematic effort to explore and document ELSI issues relating to neurobiobanking in Africa; (2) evaluate the impact/influence of stroke genomics ELSI on precision stroke medicine; (3) develop the first-ever community-based intervention for neurobiobanking by engaging relevant stakeholders; (4) implement a neurobiobanking study in Africa using focused ethnography; (5) develop a capacity building program for training genetic counselors and biorepository scientists focused on brain disorders; and (6) strengthen the development of the first organized brain bank in Africa.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The African Neurobiobank for Precision Stroke Medicine ELSI Project, the SIREN Study, and the Systematic Investigation of Blacks with Stroke using Genomics (SIBS Genomics) Study are supported by Grants U01HG010273, U54HG007479, and R01NS107900, respectively, from the National Institutes of Health as part of the H3Africa Consortium. Rufus O. Akinyemi is further supported by the FLAIR fellowship funded by the UK Royal Society and the African Academy of Sciences.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abimiku A, Mayne ES, Joloba M, Beiswanger CM, Troyer J, & Wideroff L (2017). H3Africa Biorepository Program: Supporting genomics research on African populations by sharing high-quality biospecimens. Biopreservation and Biobanking, 15(2), 99–102. 10.1089/bio.2017.0005 [DOI] [Google Scholar]
- Akinyemi RO, Akinwande K, Diala S, Adeleye O, Ajose A, Issa K, Owusu D, Boamah I, Yahaya IS, Jimoh AO, Imoh L, Fakunle G, Akpalu A, Sarfo F, Wahab K, Sanya E, Owolabi L, Obiako R, Osaigbovo G, … Owolabi M (2018). Biobanking in a challenging African environment: Unique experience from the SIREN project. Biopreservation and Biobanking, 16(3), 217–232. 10.1089/bio.2017.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemi RO, Owolabi MO, Adebayo PB, Akinyemi JO, Otubogun FM, Uvere E, Adjenijio O, Adeleye O, Aridegbe O , Taiwo FT, Ogun SA, & Ogunniyi A (2015). Task-shifting training improves stroke knowledge among Nigerian non-neurologist health workers. Journal of the Neurological Sciences, 359(1–2), 112–116. 10.1016/j.jns.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemi RO, Owolabi MO, Oyeniyi T, Ovbiagele B, Arnett DK, Tiwari HK, Walker R, Ogunniyi A, Kalaria RN, & the SIREN group of the H3 Africa Consortium. (2016). Neurogenomics in Africa: Perspectives, progress, possibilities and priorities. Journal of the Neurological Sciences, 366, 213–223. 10.1016/j.jns.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemi RO, Salami A, Akinyemi J, Ojagbemi A, Olopade F, Coker M, Farombi T, Nweke M, Arulogun O, Jegede A, Owolabi M, Kalaria RN, & Ogunniyi A (2019). Brain banking in low and middle-income countries: Raison D’etre for the Ibadan brain ageing, dementia and neurodegeneration (IBADAN) brain bank project. Brain Research Bulletin, 145, 136–141. 10.1016/j.brainresbull.2018.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemi RO, Sarfo FS, Akinyemi J, Singh A, Onoja Akpa M, Akpalu A, Owolabi L, Adeoye AM, Obiako R, Wahab K, Sanya E, Komolafe M, Ogbole G, Fawale M, Adebayo P , Osaigbovo G, Sunmonu T, Olowoyo P, Chukwuonye I, Ovbiagele B, … for the SIREN Investigators. (2019). Knowledge, attitudes and practices of West Africans on genetic studies of stroke: Evidence from the SIREN study. International Journal of Stroke, 14(1), 69–79. 10.1177/1747493018790059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpalu A, Sarfo FS, Ovbiagele B, Akinyemi R, Gebregziabher M, Obiako R, Owolabi L, Sagoe K, Jenkins C, Arulogun O, Adamu S, Appiah LT, Adadey MA, Quansah JA, Mensah YB, Adeoye AM, Singh A, Tosin AO, Ohifemen O, Owolabi M, … on behalf of SIREN as part of the H3Africa Consortium. (2015). Phenotyping stroke in Sub-Saharan Africa: Stroke investigative research and education network (SIREN) phenomics protocol. Neuroepidemiology, 45(2), 73–82. 10.1159/000437372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe MJ (2017). Ethical legal and social issues of biobanking: Past, present, and future. Biopreservation and Biobanking, 15(2), 142–147. 10.1089/bio.2017.0030 [DOI] [PubMed] [Google Scholar]
- Collins FS, & Varmus H (2015). A new initiative on precision medicine. New England Journal of Medicine, 372(9), 793–795. 10.1056/NEJMp1500523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S, & Jenkins C (2017). Engaging communities in translational research. In Coughlin SS, Smith SA, & Fernandez ME (Eds.), Handbook of community-based participatory research. Oxford University Press. [Google Scholar]
- de Vries J, Munung SN, Matimba A, McCurdy S, Ouwe Missi Oukem-Boyer O, Staunton C, Yakubu A, & Tindana P, & the H3 Africa Consortium. (2017). Regulation of genomic and biobanking research in Africa: A content analysis of ethics guidelines, policies and procedures from 22 African countries. BMC Medical Ethics, 18(1), 8. 10.1186/s12910-016-0165-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J, Munung SN, & Tindana P (2016). Deliberation to promote shared sovereignty in health research: Four questions to clarify goals, methods, and scope. American Journal of Bioethics, 16(10), 50–52. 10.1080/15265161.2016.1214326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doak C, Doak L, Miller K, & Wilder L (1994). Suitability assessment of materials (SAM). American Public Health Association. [Google Scholar]
- Ezejimofor MC, Uthman OA, Maduka O, Ezeabasili AC, Onwuchekwa AC, Ezejimofor BC, Asuquo E, Chen YF, Stranges S, & Kandala NB (2017). Stroke survivors in Nigeria: A door-to-door prevalence survey from the Niger Delta region. Journal of the Neurological Sciences, 372, 262–269. 10.1016/j.jns.2016.11.059 [DOI] [PubMed] [Google Scholar]
- Glasgow RE, & Estabrooks PE (2018). Pragmatic applications of RE-AIM for health care initiatives in community and clinical settings. Preventing Chronic Disease, 15, E02. 10.5888/pcd15.170271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LW, Richard L, & Potvin L (1996). Ecological foundations of health promotion. American Journal of Health Promotion, 10(4), 270–281. 10.4278/0890-1171-10.4.270 [DOI] [PubMed] [Google Scholar]
- Guidance Tools for Community Advisory Boards. (2012). International AIDS vaccine initiative. http://www.iavi.org
- Harden SM, Smith ML, Ory MG, Smith-Ray RL, Estabrooks PA, & Glasgow RE (2018). RE-AIM in clinical, community, and corporate settings: Perspectives, strategies, and recommendations to enhance public health impact. Frontiers in Public Health, 6, 71. 10.3389/fpubh.2018.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (RED-Cap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt RE (2011). Biobanking: The foundation of personalized medicine. Current Opinion in Oncology, 23(1), 112–119. 10.1097/CCO.0b013e32834161b8 [DOI] [PubMed] [Google Scholar]
- Higginbottom GM, Pillay JJ, & Boadu NY (2013). Guidance on performing focused ethnographies with an emphasis on healthcare research. The Qualitative Report, 18, 1–6. [Google Scholar]
- Hinman JD, Rost NS, Leung TW, Montaner J, Muir KW, Brown S, Arenillas JF, Feldman E, & Liebeskind DS (2017). Principles of precision medicine in stroke. Journal of Neurology, Neurosurgery, and Psychiatry, 88(1), 54–61. 10.1136/jnnp-2016-314587 [DOI] [PubMed] [Google Scholar]
- Igbe MA, & Adebamowo CA (2012). Qualitative study of knowledge and attitudes to biobanking among lay persons in Nigeria. BMC Medical Ethics, 13, 27. 10.1186/1472-6939-13-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel BA, Eng E, Schulz AJ, & Parker EA (2013). Methods for community-based participatory research for health (2nd ed.). Jossey-Bass. [Google Scholar]
- Jallinoja P, & Aro AR (1999). Knowledge about genes and heredity among Finns. New Genetics & Society, 18(101–110). 10.1080/14636779908656892 [DOI] [Google Scholar]
- Jallinoja P, & Aro AR (2000). Does knowledge make a difference? The association between knowledge about genes and attitudes toward gene tests. Journal of Health Communication, 5(1), 29–39. 10.1080/10810730050019546 [DOI] [PubMed] [Google Scholar]
- Mayne ES, Croxton T, & Abimiku A (2017). Genes for life: Biobanking for genetic research in Africa. Biopreservation and Biobanking, 15(2), 93–94. 10.1089/bio.2017.0007 [DOI] [Google Scholar]
- Miles MB, Huberman AM, & Saldana J (2013). Qualitative data analysis: A methods sourcebook (3rd ed.). SAGE Publications. [Google Scholar]
- Moodley K, Sibanda N, February K, & Rossouw T (2014). “It’s my blood”: Ethical complexities in the use, storage and export of biological samples: Perspectives from South African research participants. BMC Medical Ethics, 15, 4. 10.1186/1472-6939-15-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munung NS, Mayosi BM, & de Vries J (2017). Equity in international health research collaborations in Africa: Perceptions and expectations of African researchers. PLoS One, 12(10), e0186237. 10.1371/journal.pone.0186237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SD, Andrews JO, Magwood GS, Jenkins C, Cox MJ, & Williamson DC (2011). Community advisory boards in community-based participatory research: A synthesis of best processes. Preventing Chronic Disease, 8(3), A70. [PMC free article] [PubMed] [Google Scholar]
- Owolabi M, Miranda JJ, Yaria J, & Ovbiagele B (2016). Controlling cardiovascular diseases in low and middle income countries by placing proof in pragmatism. BMJ Global Health, 1(3). 10.1136/bmjgh-2016-000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owolabi MO, Akarolo-Anthony S, Akinyemi R, Arnett D, Gebregziabher M, Jenkins C, Tiwari H, Arulogun O, Akpalu A, Sarfo FS, Obiako R, Owolabi L, Sagoe K, Melikam S, Adeoye AM, Lackland D, Ovbiagele B,… as Members of the H3Africa Consortium. (2015). The burden of stroke in Africa: A glance at the present and a glimpse into the future. Cardiovascular Journal of Africa, 26(2 Suppl. 1), S27–38. 10.5830/CVJA-2015-038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owolabi MO, Akinyemi RO, Gebregziabher M, Olaniyan O, Salako BL, Arulogun O, & Ovbiagele B (2014). Randomized controlled trial of a multipronged intervention to improve blood pressure control among stroke survivors in Nigeria. International Journal of Stroke, 9(8), 1109–1116. 10.1111/ijs.12331 [DOI] [PubMed] [Google Scholar]
- Owolabi MO, Akinyemi RO, Hurst S, Arulogun O, Olaniyan O, Gebregziabher M, Salako BL, & Ovbiagele B (2014). Tailored hospital-based risk reduction to impede vascular events after stroke (THRIVES) study: Qualitative phase protocol. Critical Pathways in Cardiology, 13(1), 29–35. 10.1097/HPC.0000000000000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owolabi MO, Peprah E, Xu H, Akinyemi R, Tiwari HK, Irvin MR, Wahab KW, Arnett DK, & Ovbiagele B (2017). Advancing stroke genomic research in the age of trans-omics big data science: Emerging priorities and opportunities. Journal of the Neurological Sciences, 382, 18–28. 10.1016/j.jns.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Acosta D, Ferri CP, Guerra M, Huang Y, Jacob KS, Rodriguex JJL, Salas A, Sosas AL, Williams JD, & Hall KS (2011). A brief dementia screener suitable for use by non-specialists in resource poor settings—The cross-cultural derivation and validation of the brief community screening instrument for dementia. International Journal of Geriatric Psychiatry, 26(9), 899–907. 10.1002/gps.2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotimi C, Abayomi A, Abimiku A, Adabayeri VM, Adebamowo C, Ademola AD, Adeyemo A, Adu D, Affolabi D, Agongo G, Ajayi S, Akarolo-Anthony S, Akinyemi R, Akpalu A, Alberts M, Alonso Betancourt O, Alzohairy AM, Ameni G, Amodu O, Zar H,… on behalf of the H3Africa Consortium. (2014). Research capacity. Enabling the genomic revolution in Africa. Science, 344(6190), 1346–1348. 10.1126/science.1251546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton AK (2004). Strategies for ensuring trustworthiness in qualitative research projects. Education for information, 22(2), 63–75. 10.3233/EFI-2004-22201 [DOI] [Google Scholar]
- Srivastava A, & Thomson SB (2009). Framework analysis: A qualitative methodology for applied policy research. Journal of Administration and Governance, 4(2), 72–79. [Google Scholar]
- Sweet SN, Ginis KA, Estabrooks PA, & Latimer-Cheung AE (2014). Operationalizing the RE-AIM framework to evaluate the impact of multi-sector partnerships. Implementation Science, 9, 74. 10.1186/1748-5908-9-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindana P, & de Vries J (2016). Broad consent for genomic research and biobanking: Perspectives from low- and middle-income countries. Annual Review of Genomics and Human Genetics, 17, 375–393. 10.1146/annurev-genom-083115-022456 [DOI] [PubMed] [Google Scholar]
- Tremblay PR (2002). Interviewing and counseling across cultures: Heuristics and biases. Clinical Law Review, 9(1), 373–416. [Google Scholar]
- Vaught J (2016a). Biobanking and biosecurity initiatives in Africa. Biopreservation and Biobanking, 14(5), 355–356. 10.1089/bio.2016.29009.jjv [DOI] [PubMed] [Google Scholar]
- Vaught J (2016b). Biobanking comes of age: The transition to biospecimen science. Annual Review of Pharmacology and Toxicology, 56, 211–228. 10.1146/annurev-pharmtox-010715-103246 [DOI] [PubMed] [Google Scholar]
- Weintraub D, Maliski SL, Fink A, Choe S, & Litwin MS (2004). Suitability of prostate cancer education materials: Applying a standardized assessment tool to currently available materials. Patient Education and Counseling, 55, 275–280. 10.1016/j.pec.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Wells K, Miranda J, Bruce ML, Alegria M, & Wallerstein N (2004). Bridging community intervention and mental health services research. American Journal of Psychiatry, 161(6), 955–963. 10.1176/appi.ajp.161.6.955 [DOI] [PubMed] [Google Scholar]
- Wight D, Wimbush E, Jepson R, & Doi L (2016). Six steps in quality intervention development (6SQuID). Journal of Epidemiology and Community Health, 70(5), 520–525. 10.1136/jech-2015-205952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis GB (2004). Cognitive interviewing revisited: A useful technique, in theory? In Presser S, Rothgeb JM, Couper MP, Lessler JT, Martin E, Martin J, & Singer E (Eds.), Methods for testing and evaluating survey questionnaires (pp. 23–43). John Wiley & Sons. [Google Scholar]
- Willis GB, & Miller K (2011). Cross-cultural cognitive interviewing: Seeking comparability and enhancing understanding. Field Methods, 23(4), 331–341. 10.1177/1525822X11416092 [DOI] [Google Scholar]
- Xu H, Mitchell BD, Peprah E, Kittner SJ, & Cole JW (2017). The importance of conducting stroke genomics research in African ancestry populations. Global Heart, 12(2), 163–168. 10.1016/j.gheart.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Hachiya T, Fukushima A, Nakaya N, Okayama A, Tanno K, Aizawa F, Tokutomi T, Hozawa A, & Shimizu A (2017). Population-based biobank participants’ preferences for receiving genetic test results. Journal of Human Geneticist, 62(12), 1037–1048. 10.1038/jhg.2017.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young OR, Berkhout F, Gallopin GC, Janssen MA, Ostrom E, & van der Leeuw S (2006). The globalization of socio-ecological systems: An agenda for scientific research. Global Environmental Change, 16, 304–316. 10.1016/j.gloenvcha.2006.03.004 [DOI] [Google Scholar]