Abstract
Background
Esophageal cancer is the eighth most frequent and sixth most fatal cancer worldwide. This study aimed to investigate the clinical characteristics and prognostic significance of yes related protein 1 (YAP1) and transcriptional co-activator with PDZ binding motif (TAZ) in patients with esophageal squamous cell carcinoma (ESCC).
Methods
A total of 306 ESCC pathological specimens and adjacent tissues (as control; tissues from the esophageal mucosa >5 cm from the edge of the tumor) were collected between January, 2008 and December, 2018. Immunohistochemical staining was used to assess the expression of YAP1 and TAZ proteins in the ESCC and adjacent tissues, and their relationship with clinicopathological parameters was evaluated using SPSS 21.0 software.
Results
YAP1 and TAZ proteins were highly expressed in ESCC, and their expression was closely related to TNM stage and lymph node metastasis. Expression of YAP1 was associated with tumor size (P = .029), differentiation (P = .000), depth of invasion (P = .001), and TNM stage (P = .000). Expression of TAZ was associated with tumor size (P = .034), differentiation (P = .000), depth of invasion (P = .029), lymph node metastasis (P = .006), and ethnicity (P < .001). The expression of YAP1 protein was positively correlated with the expression of TAZ protein (r = 0.257, P < .05). YAP1 and TAZ expression (P = .039 and .000, respectively), tumor size (P = .041), and lymph node metastasis (P = .001) significantly affected the overall survival of patients with ESCC, and represent independent factors for overall survival.
Conclusion
YAP1 and TAZ proteins are highly expressed in ESCC, and closely related to the clinical and pathological parameters such as the diameter of the tumor, degree of differentiation, and depth of invasion, indicating that YAP1 and TAZ may be involved in the development of ESCC. YAP1 and TAZ may be used as prognostic markers in ESCC.
Keywords: esophageal squamous cell carcinoma, immunohistochemistry, TAZ, YAP1
1. Introduction
Esophageal cancer has the eighth highest incidence and the sixth highest mortality rate worldwide. The main histological types include squamous cell carcinoma and adenocarcinoma, with esophageal squamous cell carcinoma (ESCC) accounting for >90% of the cases.[1] As the early symptoms are usually atypical, patients are often diagnosed at the later stages and have a poor prognosis. The occurrence of ESCC is related to many factors, such as abnormal cell proliferation, differentiation, and apoptosis that are complex processes involving multiple factors, genes, and multi-stage interactions.
Yes1 associated transcriptional regulator (YAP1) and WW domain-containing transcription regulator 1 (WWTR1; commonly known as transcriptional co-activator with PDZ binding motif [TAZ]) are the core factors downstream of the Hippo signaling pathway. As pivotal components of the Hippo signaling pathway, the accumulation of YAP1/TAZ in the nucleus results in many diseases including cancer.[2,3] Early studies have found that dysregulation of the Hippo signaling pathway, resulting from mutations, for instance, renders YAP1 hyperactive. Its high expression is closely related to the proliferation and metastasis of cancer cells.[4] Therefore, YAP1 is considered an oncogene that regulates the metastatic and invasive ability of cancer cells by interacting with other factors.TAZ and YAP1 are homologous and share 46% amino acid sequence identity. They function as co-transcriptional activators shuttling between the cytoplasm and nucleus. Under normal conditions, YAP1/TAZ remains highly conserved and participate in signal transduction; however, under pathological conditions, the inhibition of YAP1/TAZ by certain upstream factors is weakened, which leads to enhanced YAP1/TAZ activity and promotion of tumor proliferation, invasion, and metastasis.[5–7]
YAP1 induces matrix metalloproteinases (MMPs) and activation of matrix metalloproteinases s causes tumor invasion and increased metastatic ability.[8] Furthermore, YAP1 and TAZ may also be involved in epithelial-mesenchymal transformation, which is associated with the occurrence of ESCC.[9,10] Existing studies suggest that YAP1 and TAZ are expressed at abnormally high levels in tumor cells, indicating that these factors are closely related to the occurrence of tumors.
In this study, we aimed to investigate the expression of YAP1/TAZ in ESCC, analyze the correlation between YAP1/TAZ expression and clinicopathological parameters and prognosis of patients with ESCC, clarify the role and relationship of YAP1/TAZ in the development of ESCC, and provide a new therapeutic target for the treatment of ESCC in the clinic.
2. Materials and methods
2.1. Patients and tissue samples
Tissue samples and clinicopathological information were collected from a total of 306 patients with ESCC at the Department of Pathology of the First Affiliated Hospital of Xinjiang Medical University from 2008 to 2018. The study was approved by the medical ethics committee of the First Affiliated Hospital of Xinjiang Medical University. Clinical data including gender, age, race, depth of tumor invasion, TNM stage, lymph node metastasis were collected along with TN paraffin specimens. The inclusion criteria of the patients were as follows: 1) definitive pathologic diagnosis of ESCC; 2) the absence of preoperative radio- or chemotherapy; 3) had complete clinicopathological; and 4) the tissue samples were free from damage to the cell structure as evidenced by microscopic observation following HE staining.
2.2. Immunohistochemistry
Tissue microarrays were prepared in collaboration with the Shanghai Biochip Company (Shanghai, China). Anti-YAP1, anti-phosphorylated form of YAP1 (p-YAP1), and anti-TAZ antibodies were purchased from Abcam. Phosphate buffered saline (PBS; pH 7.4), citric acid solution for antigen retrieval (pH 6.0), and Immunohistochemical Staining kit were purchased from Bioss Reagent Co. Ltd. The anti-phosphorylated form of TAZ (p-TAZ) antibody was obtained from Santacruz. Paraffin specimens were sliced into 3 μm thick continuous sections and baked at 60°C for 2 h. The sections were then dewaxed with xylene and anhydrous ethanol gradient followed by antigen retrieval with citric acid. The sections were incubated with hydrogen peroxide in the dark to block endogenous peroxidases. Fetal bovine serum was used to block non-specific sites. Primary antibodies including anti-YAP1 (1:200), anti-p-YAP1 (1:300), anti-TAZ (1:200), and anti-p-TAZ were added and the sections were incubated overnight at 4°C. Following incubation with the corresponding secondary antibodies, the sections were stained with DAB. The sections were then evaluated by 2 senior pathologists using a double-blind method. Based on their staining intensity, the sections were categorized as unstained (colorless), weak staining (light yellow), moderate staining (light brown), and strong staining (dark brown), and scored 0, 1, 2, and 3, respectively. The criteria for positive cells were as follows: 0 (negative), 1 (1%–30%), 2 (31%–60%), and 3 (61%–90%). Based on the staining index (SI = percentage of positive cells × staining intensity), the staining results were recorded as negative (0–3 points) or positive (4–9 points), of which 4–5 points were designated as low-level expression, 6–7 points moderate expression, and 8–9 points high-level expression. Based on the above criteria, YAP1 and TAZ expression were classified as a strong positive, moderately positive, weak positive, and negative. Two independent observers evaluated the selected samples, all of which were confirmed as ESCC. Inconsistencies in scoring were reevaluated to obtain an agreement.
2.3. Patient follow-up
Patient follow-up was conducted via telephone. The death of the patient was recorded as the end event and included the cause and time of death. The follow-up period lasted from the date of diagnosis until May, 2019. All patients who died of a cause unrelated to ESCC or due to accidents were excluded from the study. Of the 306 cases, 285 cases were followed up. The main endpoints of this study included overall survival (OS) and progression-free survival (PFS). OS of the patient was defined as the period between the initial diagnosis of ESCC until the time to death or final follow-up (May 31, 2019). PFS was defined as the period between the initial diagnosis of ESCC up to the time to tumor progression or death due to ESCC, excludes the death due to other diseases or accidents. Such as esophageal inflammatory disease, Barrett esophagus, and other malignancies, etc.
2.4. Statistical analysis
Statistical analysis was performed using SPSS 21.0 statistical package. A chi-square test was used to analyze the correlation between YAP1 and TAZ expression and the clinicopathological parameters. Survival time was estimated using OS and PFS. Survival curves of patients with ESCC with different clinical characteristics were plotted using the Kaplan–Meier method. Based on the results of the Kaplan–Meier analysis, independent factors related to the prognosis of ESCC were further analyzed using the Cox proportional risk model. Statistical significance was set at P < .05.
3. Results
3.1. Expression of YAP1 and its correlation with clinicopathological parameters in Han, Kazakh, and Uygur patients with ESCC
YAP1 expression was absent or expressed at very minimal amounts in normal esophageal tissues (Fig. 1A). p-YAP1 was only expressed in cytoplasm in both normal esophageal and ESCC tissues (Fig. 1B-C). However, YAP1 was expressed in both the cytoplasm and nucleus in ESCC tissues (Fig. 1D–F). In the paraffin-embedded ESCC tissues, weak expression of YAP1 was observed in 15 tumors (8.30%), moderate expression in 20 tumors (11.10%), and strong expression in 145 tumors (80.60%) by immunohistochemistry. Next, we analyzed the correlation between positive expression of YAP1 and clinicopathological parameters in Han, Kazakh, and Uygur patients. The result showed that high expression of YAP1 was related to tumor size (P = .004) in Han patients, and to the degree of differentiation (P = .017), depth of invasion (P = .001), TNM stage (P = .003), and vascular invasion in Kazakhs. In Uygur patients, high expression of YAP1 was closely related to the degree of differentiation (P = .016) and TNM stage (P = .012). However, there was no significant correlation between YAP1 expression and ethnic groups (P = .059). Thus, high expression of YAP1 was closely associated with tumor size (P = .029), degree of differentiation (P = .000), infiltration depth (P = .001), and TNM stage (P = .000), whereas no correlation was found with gender (P = .215), age (P = .77), lymph node metastasis (P = .051), vascular invasion (P = .055), nerve invasion (P = .686), or race (P = .059) in ESCC (Table 1).
Figure 1.
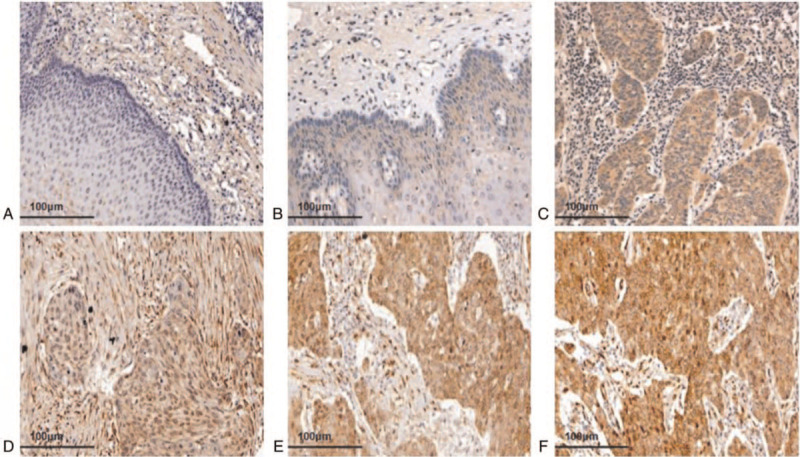
The expression of YAP1 in ESCC and normal esophageal tissues. (A) YAP1 negative expression in normal esophageal tissues (20×). (B) p-YAP1 expressed in normal esophageal tissues, mainly located in the cytoplasm (20×). (C) p-YAP1 was expressed in ESCC cytoplasm (20×). (D) YAP1 weakly positive expression in ESCC (20×). (E) YAP1 moderately positive expression in ESCC (20×). (F) YAP1 strongly positive expression in ESCC (20×). ESCC = esophageal squamous cell carcinoma, p-YAP1 = phosphorylated form of YAP1, YAP1 = yes related protein 1.
Table 1.
Correlation between YAP1 expression and clinicopathological parameters in ESCC.
| Han | P value | Kazakh | P value | Uygur | P value | Total | P value | |||||
| Clinicopathological parameters | YAP1 | YAP1 | YAP1 | YAP1 | ||||||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |||||
| Gender | ||||||||||||
| Male | 38 (65.5%) | 14 (82.4%) | .24 | 49 (60.5%) | 16 (66.7%) | .584 | 63 (67.7%) | 9 (75%) | .749 | 150 (64.7%) | 39 (73.6%) | .215 |
| Female | 20 (34.5%) | 3 (17.6%) | 32 (39.5%) | 8 (33.3%) | 30 (32.3%) | 3 (25%) | 82 (35.3%) | 14 (26.4%) | ||||
| Age | ||||||||||||
| <60 | 10 (17.2%) | 4 (23.5%) | .724 | 33 (40.7%) | 11 (45.8%) | .657 | 54 (58.1%) | 6 (50%) | .595 | 97 (41.8%) | 21 (39.6%) | .77 |
| ≥60 | 48 (82.8%) | 13 (76.5%) | 48 (59.3%) | 13 (54.2%) | 39 (41.9%) | 6 (50%) | 135 (58.2%) | 32 (60.4%) | ||||
| Tumor size | ||||||||||||
| <3 cm | 12 (20.7%) | 9 (52.9%) | .004 | 25 (30.9%) | 8 (33.3%) | .891 | 28 (30.1%) | 6 (50%) | .197 | 65 (28%) | 23 (43.4%) | .029 |
| ≥3 cm | 46 (79.3%) | 8 (47.1%) | 56 (69.1%) | 16 (67.7%) | 65 (69.9%) | 6 (50%) | 167 (72%) | 30 (56.6%) | ||||
| Degree of differentiation | ||||||||||||
| Poorly differentiated | 2 (3.4%) | 3 (17.6%) | .086 | 8 (9.9%) | 8 (33.3%) | .017 | 6 (6.5%) | 4 (33.3%) | .016 | 16 (6.9%) | 15 (28.3%) | .0001 |
| Moderately differentiated | 38 (65.5%) | 11 (64.8%) | 36 (44.4%) | 9 (37.5%) | 67 (72%) | 5 (41.7%) | 141 (60.8%) | 25 (47.2%) | ||||
| Well differentiated | 18 (31.1%) | 3 (17.6%) | 37 (45.7%) | 7 (29.2%) | 20 (21.5%) | 3 (25%) | 75 (32.3%) | 13 (24.5%) | ||||
| Depth of invasion | ||||||||||||
| Mucosa | 5 (8.6%) | 2 (11.8%) | .914 | 0 (0%) | 4 (16.7%) | .000 | 0 (0%) | 1 (8.3%) | .43 | 5 (2.2%) | 7 (13.2%) | .001 |
| Muscularis | 26 (43.1%) | 7 (41.2%) | 36 (44.4%) | 14 (16.7%) | 45 (48.4%) | 3 (25%) | 107 (46.1%) | 24 (45.2%) | ||||
| Full-thickness | 27 (46.5%) | 8 (47%) | 45 (55.6%) | 6 (66.6%) | 48 (51.6%) | 8 (66.7%) | 120 (51.7%) | 22 (41.5%) | ||||
| TNM stage | ||||||||||||
| IA + B | 13 (22.4%) | 6 (35.3%) | .504 | 16 (19.8%) | 4 (16.7%) | .003 | 27 (29%) | 6 (50%) | .012 | 56 (24.1%) | 16 (30.2%) | .0001 |
| IIA + B | 28 (48.3%) | 5 (29.4%) | 60 (74.1%) | 12 (50%) | 59 (63.4%) | 3 (25%) | 147 (63.4%) | 20 (37.8%) | ||||
| IIIA + B | 5 (8.6%) | 2 (11.8%) | 5 (6.1%) | 8 (33.3%) | 4 (4.3%) | 3 (25%) | 14 (6.0%) | 13 (24.5%) | ||||
| IVA + B | 12 (20.7%) | 4 (23.5%) | 0 (0%) | 0 (0%) | 3 (3.2%) | 0 (0%) | 15 (6.5%) | 4 (7.5%) | ||||
| Lymph node metastasis | ||||||||||||
| Yes | 24 (41.4%) | 4 | .181 | 22 (27.5%) | 4 (16.7%) | .282 | 34 (36.6%) | 3 (25%) | .533 | 80 (34.6%) | 11 (20.8%) | .051 |
| No | 34 (58.6%) | 13 | 58 (72.5%) | 20 (83.3%) | 59 (63.4%) | 9 (75%) | 151 (65.4%) | 42 (79.2%) | ||||
| Vascular invasion | ||||||||||||
| Yes | 8 (13.8%) | 2 (11.8%) | 1 | 6 (7.4%) | 8 (33.3%) | .003 | 18 (19.6%) | 3 (25%) | .705 | 32 (13.8%) | 13 (24.5%) | .055 |
| No | 50 (86.2%) | 15 (88.2%) | 75 (92.6%) | 16 (66.7%) | 74 (80.4%) | 9 (75%) | 199 (86.2%) | 40 (75.5%) | ||||
| Nerve invasion | ||||||||||||
| Yes | 6 (10.3%) | 2 (11.8%) | 1 | 24 (29.6%) | 4 (16.7%) | .207 | 15 (16.1%) | 3 (25%) | .428 | 45 (19.4%) | 9 (17%) | .686 |
| No | 52 (89.7%) | 15 (88.2%) | 57 (70.4%) | 20 (83.3%) | 78 (83.9%) | 9 (75%) | 187 (80.6%) | 44 (83%) | ||||
| Ethnic | ||||||||||||
| Han | 58 (25.6%) | 17 (29.3%) | ||||||||||
| Kazakh | 81 (35.7%) | 24 (41.4%) | .059 | |||||||||
| Uygur | 93 (38.7%) | 12 (29.3%) | ||||||||||
(Unorganized absence).
ESCC = esophageal squamous cell carcinoma, YAP1 = yes related protein 1.
3.2. Expression of TAZ and its correlation with clinicopathological parameters in Han, Kazakh, and Uygur patients with ESCC
Immunohistochemical results showed that TAZ was not expressed or showed very minimal expression in normal esophageal tissues (Fig. 2A). p-TAZ was only expressed in the cytoplasm in both normal esophageal and ESCC tissues (Fig. 2B and C). However, TAZ was expressed in ESCC, and was mainly localized in the nucleus and cytoplasm (Fig. 2D to F). Statistical analyses were used to analyze the correlation between TAZ expression and the clinicopathological parameters, and showed that high expression of TAZ was related to the degree of differentiation (P = .371) and lymph node metastasis (P = .002) in Han patients. In Uygur patients, it was related to the depth of tumor invasion (P = .015), and in Kazakhs it was associated with lymph node metastasis (P = .009). We also found a significant correlation between the high expression of TAZ and the ethnic groups (P < .001).
Figure 2.
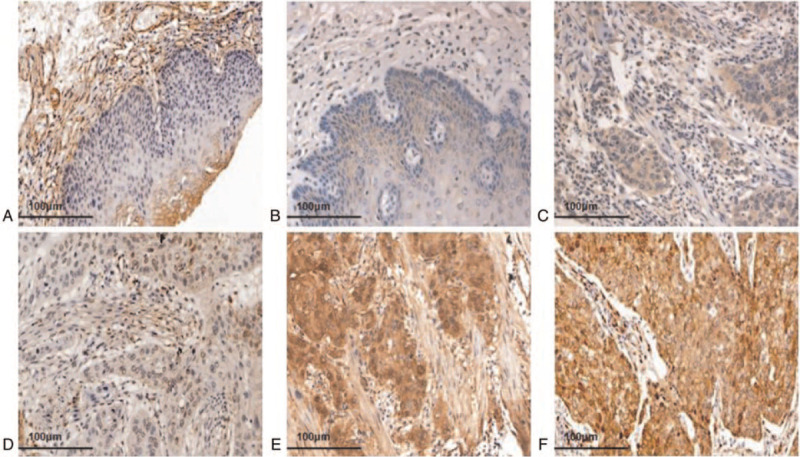
The expression of TAZ in ESCC and normal esophageal tissues. (A) TAZ negative expression in normal esophageal tissues (20×). (B) p-TAZ was low expressed in normal esophageal tissues, mainly located in the cytoplasm (20×). (C) p-TAZ was expressed in ESCC cytoplasm (20×). (D) TAZ weakly positive expression in ESCC (20×). (E) TAZ moderately positive expression in ESCC (20×). (F) TAZ strongly positive expression in ESCC (20×). ESCC = esophageal squamous cell carcinoma, p-TAZ = phosphorylated form of TAZ, TAZ = transcriptional co-activator with PDZ binding motif.
In general, high expression of TAZ was closely associated with the tumor size (P = .034), degree of differentiation (P < .001), depth of invasion (P = .029), and lymph node metastasis (P = .006). However, there is no association with gender (P = .316), age (P = .674), TNM stage (P = .083), vascular invasion (P = .676), or nerve invasion (P = . 496) in ESCC. (Table 2)
Table 2.
Correlation between TAZ expression and clinicopathological parameters in ESCC.
| Han | P value | Kazakh | P value | Uygur | P value | Total | P value | |||||
| Clinicopathological parameters | TAZ | TAZ | TAZ | TAZ | ||||||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |||||
| Gender | ||||||||||||
| Male | 38 (64.4%) | 14 (87.5%) | .125 | 45 (61.6%) | 20 (62.5%) | .934 | 65 (67.7%) | 7 (77.8%) | .716 | 148 (64.9%) | 41 (71.9%) | .316 |
| Female | 21 (35.6%) | 2 (12.5%) | 28 (38.4%) | 12 (37.5%) | 31 (32.3%) | 2 (22.2%) | 80 (35.1%) | 16 (28.1%) | ||||
| Age | ||||||||||||
| <60 | 11 (18.6%) | 3 (18.75%) | 1 | 28 (38.4%) | 16 (50%) | .266 | 54 (56.25%) | 6 (66.7%) | .729 | 93 (40.8%) | 25 (43.9%) | .674 |
| ≥60 | 48 (81.4%) | 13 (81.25%) | 45 (61.6%) | 16 (50%) | 42 (43.75%) | 3 (33.3%) | 135 (59.2%) | 32 (56.1%) | ||||
| Tumor size | ||||||||||||
| <3 cm | 16 (27.1%) | 5 (31.25%) | .76 | 28 (38.4%) | 5 (15.6%) | .021 | 33 (34.4%) | 1 (11.1%) | .266 | 77 (33.8%) | 11 (19.3%) | .034 |
| ≥3 cm | 43 (72.9%) | 11 (68.75%) | 45 (61.6%) | 27 (84.4%) | 63 (65.6%) | 8 (88.9%) | 151 (66.2%) | 46 (80.7%) | ||||
| Degree of differentiation | ||||||||||||
| Poorly differentiated | 3 (5.1%) | 2 (12.5%) | .371 | 4 (5.5%) | 12 (37.5%) | 0 | 9 (9.4%) | 1 (11.1%) | 1 | 16 (7.0%) | 15 (26.4%) | .000 |
| Moderately differentiated | 38 (64.4%) | 11 (68.75%) | 41 (56.2%) | 4 (12.5%) | 66 (68.7%) | 6 (66.7%) | 145 (63.6%) | 21 (36.8%) | ||||
| Well differentiated | 18 (30.5%) | 3 (18.75%) | 28 (38.3%) | 16 (50%) | 21 (21.9%) | 2 (22.2%) | 67 (29.4%) | 21 (36.8%) | ||||
| Depth of invasion | ||||||||||||
| Mucosa | 7 (11.9%) | 0 (0%) | .348 | 4 (5.5%) | 0 (0%) | .029 | 0 (0%) | 1 (11.1%) | .015 | 11 (4.8%) | 1 (1.8%) | .029 |
| Muscularis | 25 (42.4%) | 8 (50%) | 29 (39.7%) | 21 (65.6%) | 42 (43.75%) | 6 (66.7%) | 96 (42.1%) | 35 (61.4%) | ||||
| Full-thickness | 27 (45.7%) | 8 (50%) | 40 (54.8%) | 11 (34.4%) | 54 (56.25%) | 2 (22.2%) | 121 (53.1%) | 21 (36.8%) | ||||
| TNM stage | ||||||||||||
| IA + B | 14 (24.1%) | 5 (31.25%) | .88 | 16 (21.9%) | 4 (12.5%) | .027 | 26 (27.1%) | 7 (77.8%) | .011 | 56 (17.4%) | 16 (27.6%) | .083 |
| IIA + B | 27 (46.6%) | 6 (37.5%) | 52 (71.2%) | 20 (62.5%) | 61 (63.5%) | 1 (11.1%) | 140 (67.6%) | 27 (46.5%) | ||||
| IIIA + B | 5 (8.6%) | 1 (6.25%) | 5 (6.9%) | 8 (25%) | 7 (6.25%) | 1 (11.1%) | 17 (7.7%) | 10 (19.0%) | ||||
| IVA + B | 12 (20.7%) | 4 (25%) | 0 (0%) | 0 (0%) | 3 (3.1%) | 0 (0%) | 15 (7.3%) | 4 (6.9%) | ||||
| Lymph node metastasis | ||||||||||||
| Yes | 21 (35.6%) | 7 (43.75%) | .55 | 24 (28.9%) | 2 (6.25%) | .009 | 37 (38.5%) | 0 (0%) | .025 | 82 (34.5%) | 9 (15.8%) | .006 |
| No | 38 (64.4%) | 9 (56.25%) | 59 (71.1%) | 30 (93.75%) | 59 (61.5%) | 9 (100%) | 156 (65.5%) | 48 (84.2%) | ||||
| Vascular invasion | ||||||||||||
| Yes | 9 (15.2%) | 1 (6.25%) | .679 | 8 (11%) | 6 (18.75%) | .351 | 20 (21%) | 1 (11.1%) | .683 | 37 (16.3%) | 8 (14%) | .676 |
| No | 50 (84.8%) | 15 (93.75%) | 65 (89%) | 26 (81.25%) | 75 (78.9%) | 8 (88.9%) | 190 (83.7%) | 49 (86%) | ||||
| Nerve invasion | ||||||||||||
| Yes | 7 (11.9%) | 1 (6.25%) | 1 | 20 (27.4%) | 8 (25%) | 1 | 18 (18.75%) | 0 (0%) | .352 | 45 (19.7%) | 9 (15.8%) | .496 |
| No | 52 (88.1%) | 15 (93.75%) | 53 (72.6%) | 24 (75%) | 78 (81.25%) | 9 (100%) | 183 (80.3%) | 48 (84.2%) | ||||
| Ethnic | ||||||||||||
| Han | 59 (25.9%) | 16 (28.1%) | ||||||||||
| Kazakh | 73 (32%) | 32 (56.1%) | <.001 | |||||||||
| Uygur | 96 (42.1%) | 9 (15.8%) | ||||||||||
There were 21 missing data due to the loss of connection in some patients during the follow-up.
ESCC = esophageal squamous cell carcinoma, TAZ = transcriptional co-activator with PDZ binding motif.
3.3. Relationship between YAP1 and TAZ expression in ESCC
The positive expression rates of YAP1 and TAZ in ESCC were 81.4% (232/285) and 80.0% (228/285), respectively, and were significantly higher compared to those in normal esophageal tissues (P < .05). Positive expression of both YAP1 and TAZ was detected in 197 cases. Spearman correlation analysis revealed that the expression of YAP1 was positively correlated with that of TAZ (r = 0.257, P < .001) (Table 3).
Table 3.
Relationship between YAP1 and TAZ expression.
| TAZ | ||||||
| Protein | Positive | Negative | sum | r | P value | |
| YAP1 | Positive | 197 (69.1%) | 35 (12.3%) | 232 (81.4%) | ||
| Negative | 31 (10.9%) | 22 (7.7%) | 53 (18.6%) | 0.257 | <.001 | |
| sum | 228 (80.0%) | 57 (20%) | 285 (100%) | |||
TAZ = transcriptional co-activator with PDZ binding motif, YAP1 = yes related protein 1.
3.4. Correlation between YAP1 expression and prognosis of patients with ESCC
Kaplan–Meier survival curve indicated that the median OS of patients with ESCC positive for YAP1 expression was 21 months, whereas that of patients negative for YAP1 expression was 33 months. The difference in OS was found to be statistically significant (P = .0047). When the tumor diameter was ≥3 cm, patients with lymph node metastasis had lower OS values (P < .005) (Fig. 3A–C). Moreover, the PFS in patients with ESCC negative for YAP1 expression was longer compared to those positive for YAP1 expression (P = .0129) (Fig. 3D). Further analysis showed that when the tumor diameter ≥3 cm and YAP1 expression level was high, patients had a shorter OS (P = .0013) (Fig. 3E). Similarly, OS values were lower in patients with lymph node metastasis and full-thickness invasion (Fig. 3F and G). More importantly, positive expression of YAP1 has a significant effect on PFS in patients with lymph node metastasis (P < .000) (Fig. 3H). At TNM stages I and II, high expression of YAP1 resulted in poor OS in patients with ESCC (P = .035) (Fig. 3I).
Figure 3.
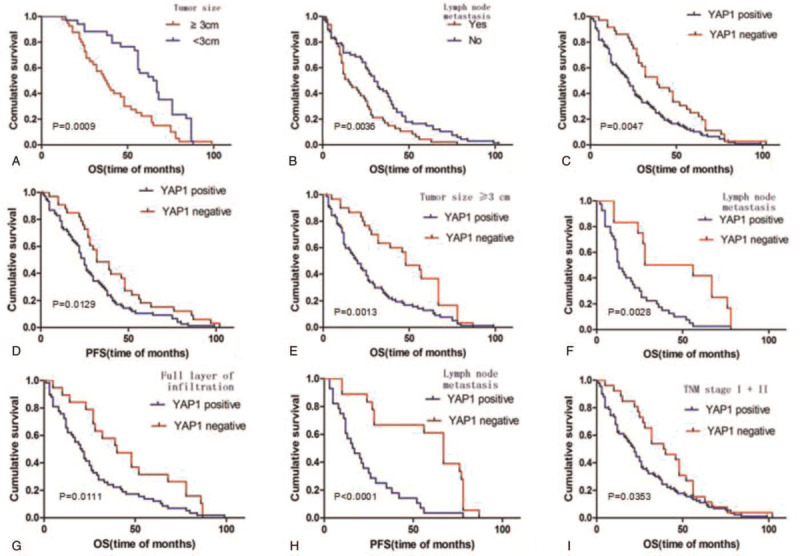
Kaplan–Meier survival analysis in negative and positive YAP1 group. YAP1 = yes related protein 1.
3.5. Correlation between TAZ expression and prognosis of patient with ESCC
Kaplan–Meier survival curve indicated that the OS value of TAZ high expression group was lower than that of the TAZ low expression group (P = .025) (Fig. 4A). When the tumor diameter was >3 cm and TAZ was highly expressed, the OS of patients was lower (P = .018) (Fig. 4B), and the difference was statistically significant (P < .05). High TAZ expression accompanied by tumor infiltration and lymph node metastasis, resulted in lower OS values (P < .05) (Fig. 4C and D). Further analysis showed that positive expression of TAZ had a greater impact on PFS in patients with lymph node metastasis (P = .034) (Fig. 4E). At TNM stages I and II, high expression of TAZ showed poor OS in patients with ESCC (P = .035) (Fig. 4F).
Figure 4.
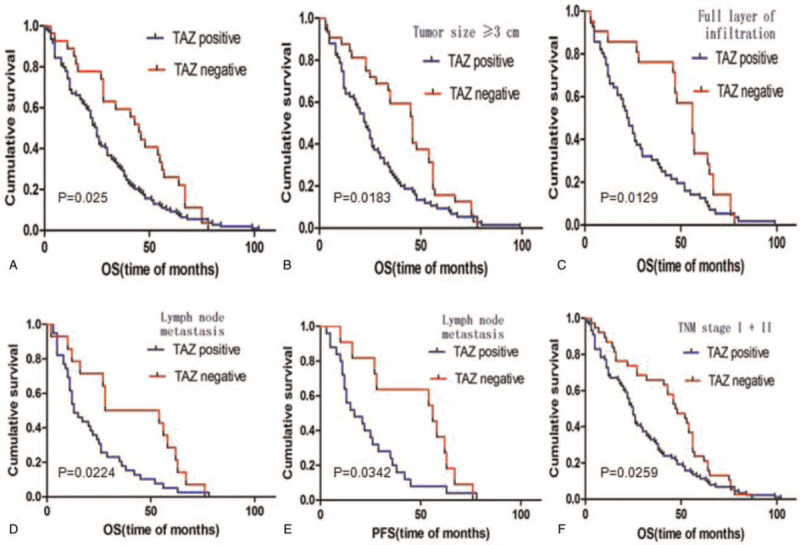
Kaplan–Meier survival analysis in negative and positive TAZ group. TAZ = transcriptional co-activator with PDZ binding motif.
3.6. Correlation between the expression of YAP1 and TAZ in ESCC tissues with patient prognosis
Survival analysis showed that the OS of the YAP1+/TAZ+ group was significantly shorter than that of the YAP1−/TAZ− group (P = .0211, Fig. 5). The OS of YAP1−/TAZ− group was significantly longer compared to that of YAP1+/TAZ+, YAP1+/TAZ−, and YAP1−/TAZ+ groups (P < .05, Fig. 5). There was no significant difference in OS among YAP1+/TAZ+, YAP1+/TAZ− and YAP1−/TAZ+ groups (P > .05). This data indicates that the expression level of the YAP1 and TAZ is closely related to the prognosis of patients with ESCC (Fig. 5A). The survival rate of patients with tumor diameter ≥3 cm, was lower than that of patients with tumor diameter <3 cm. Thus, the larger the tumor diameter, the lower the survival rate, and the worse the prognosis of the patient (Fig. 5B). These results also showed that positive expression of either YAP1 or TAZ, or both proteins, resulted in poorer OS (23 months) compared to cases that were negative for YAP1 and TAZ expression (OS = 31 months). Also, these figures also indicated that among the YAP1 and TAZ positive cases, the OS was worse in cases that showed lymph node metastasis (Fig. 5C and D). Cox multivariate regression analysis showed that YAP1 expression, TAZ expression, tumor size, and lymph node metastasis were associated with poor survival outcomes in patients with ESCC, and represent independent factors for OS (P = .039, .000, .041, and .001, respectively) (Table 4). In summary, our results showed that the absence of YAP1 and TAZ expression resulted in longer OS in ESCC patients. Therefore, YAP1 and TAZ may serve as potential biomarkers for poor prognosis in ESCC.
Figure 5.
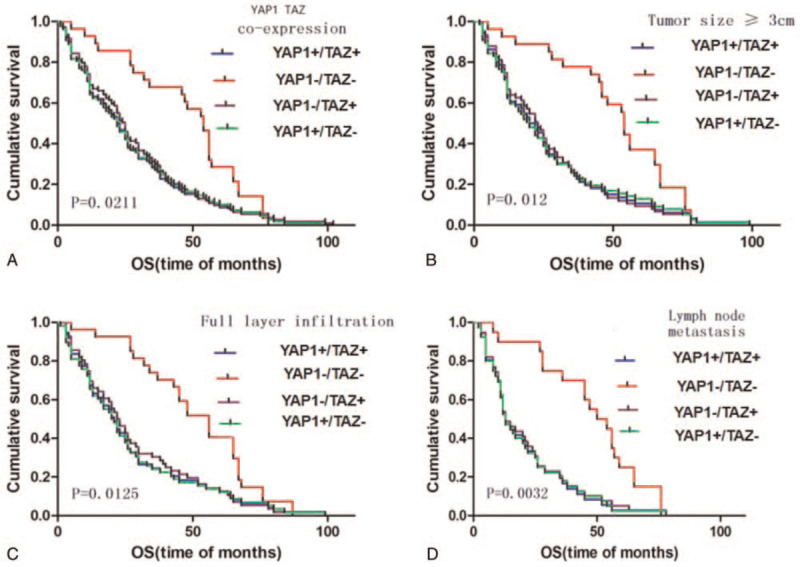
Kaplan–Meier survival analysis in YAP1+/TAZ+ group, YAP1−/TAZ− group, YAP1+/TAZ− group, and YAP1−/TAZ+ group. TAZ = transcriptional co-activator with PDZ binding motif, YAP1 = yes related protein 1.
Table 4.
Multivariate analysis of prognostic factors in relation to patient survival.
| Variable | B | SE | Wald | df | Sig. | Exp(B) | 95% Confidence interval |
| YAP1 expression | −0.446 | 0.215 | 4.280 | 1 | 0.039 | 0.640 | 0.420–0.977 |
| TAZ expression | −0.706 | 0.199 | 12.559 | 1 | 0.000 | 0.493 | 0.334–0.729 |
| Gender | 0.261 | 0.161 | 2.612 | 1 | 0.106 | 1.298 | 0.946–1.781 |
| Age | −0.211 | 0.155 | 1.842 | 1 | 0.175 | 0.810 | 0.597–1.098 |
| Tumor size | 0.345 | 0.169 | 4.180 | 1 | 0.041 | 1.413 | 1.014–1.967 |
| Degree of differentiation | 0.103 | 0.122 | 0.706 | 1 | 0.401 | 1.108 | 0.872–1.408 |
| Depth of invasion | −0.089 | 0.177 | 0.257 | 1 | 0.612 | 0.914 | 0.647–1.292 |
| TNM stage | 0.056 | 0.182 | 0.094 | 1 | 0.759 | 1.058 | 0.740–1.512 |
| Lymph node metastasis | 0.626 | 0.193 | 10.512 | 1 | 0.001 | 1.871 | 1.281–2.732 |
| Vascular invasion | 0.218 | 0.207 | 1.111 | 1 | 0.292 | 1.244 | 0.829–1.867 |
| Nerve invasion | 0.224 | 0.193 | 1.346 | 1 | 0.246 | 1.251 | 0.857–1.825 |
| Ethnic | −0.019 | 0.096 | 0.040 | 1 | 0.841 | 0.981 | 0.813–1.183 |
4. Discussion
Esophageal carcinoma is 1 of the top 10 malignant tumors in the world, with high morbidity and mortality rates. In China, the incidence of esophageal cancer is increasing gradually. ESCC constitutes the major pathological subtype of esophageal cancer. Currently, the treatment modalities for ESCC mainly include surgery, chemotherapy, and radiotherapy. However, most of the patients are diagnosed at later stages, resulting in a poor prognosis. Therefore, the pathogenesis of ESCC needs to be studied further to optimize diagnosis and treatment options.
YAP1 and TAZ are the core factors in the Hippo signaling pathway and have been implicated in tumorigenesis and development. They are localized in the cytoplasm, and translocate into the nucleus following activation to bind to TEA domain DNA-binding family of transcription factors (TEADs), and have dual functions of signal transduction and transcriptional regulation.[11] Under normal conditions, the Hippo signaling pathway mediates tumor inhibition, and YAP1 and TAZ mainly exist in the cytoplasm in their phosphorylated forms. Under the pathological condition, the upstream factors of the Hippo pathway, such as Mst1/2 and LATS1/2 become activated, relieving the inhibition of YAP1 and TAZ. Once YAP1 and TAZ are released, MST1/2 and LATS1/2 become deactivated, YAP1 and TAZ are dephosphorylated, and then they translocate into the nucleus to bind to TEADs to promote tumor development.[12,13]
Studies have shown that YAP1 and TAZ are abnormally expressed in various tumor tissues and cell lines such as head and neck squamous cell carcinoma,[14] oral squamous cell carcinoma,[15] esophageal adenocarcinoma cells,[16] non-small cell lung cancer,[17] gastric cancer,[18] colorectal cancer,[19] breast cancer,[20] and other cancers. YAP1 and TAZ were highly expressed in these tumors, which is consistent with our experimental results.
Previous studies have revealed that YAP plays an important role in ESCC proliferation and progression. In the normal esophageal developmental process, low levels of nuclear YAP are maintained via interaction with TEADs, thereby inhibiting YAP signaling in normal esophageal epithelium tissues. However, in ESCC tissues, abnormal overactivation of YAP promotes ESCC development. Knockdown of YAP expression resulted in a decrease in the number of tumor spheres and tumor size. YAP is closely related to the acquisition of cancer stem cell-like characteristics in ESCC. Our study found that patients with ESCC who were YAP1 negative tended to have a better prognosis, which is consistent with previous studies to a certain extent.[21]
Analysis of our data revealed distinctly different expression patterns of YAP1 and TAZ in ESCC and normal esophageal tissues. In normal esophageal epithelium tissues, YAP1 and TAZ were not expressed or showed very minimal expression. However, both proteins were highly expressed in the nucleus and cytoplasm of ESCC tissues. p-YAP1 and p-TAZ were only expressed in the cytoplasm of normal esophageal and ESCC tissues that indicates that YAP1 and TAZ are translocated between nucleus and cytoplasm during tumorigenesis, which may provide an explanation for their role in promoting tumorigenesis and development. The expression level of YAP1 was significantly related to the tumor size, differentiation, depth of invasion, and TNM stage. High expression of TAZ was closely related to the size, differentiation, depth of invasion, lymph node metastasis, and ethnic. Furthermore, analysis of the correlation between YAP1 and TAZ using Spearman correlation analysis revealed that the expression of YAP1 and TAZ were positively correlated with each other.
In recent years, more and more novel markers have provided a better reference to the diagnosis and treatment of tumors, identification of new molecular biomarkers may help in identifying tumor origin, differentiating precancerous lesions from cancer, defining tumor malignancy, and it is conducive to the early diagnosis and treatment of tumors. For example, in esophageal cancer, gastric cancer, liver cancer,[22] colorectal cancer, breast cancer,[23] chronic myeloid leukemia,[24] and other tumors, the discovery of many new tumor markers provides great help to the diagnosis and treatment of most tumors. Such as octamer-binding transcription factor 4 (Oct4) is a transcription factor that plays a valuable role in the progression and prognosis of esophageal cancer and gastric cancer.[25,26] Serum metadherin mRNA expression can be considered a useful non-invasive biomarker screening for early diagnosis and prognosis in patients with colorectal cancer.[27] Similarly, YAP1 and TAZ are expected to be novel diagnostic and therapeutic targets for ESCC. In this study, the OS and PFS in YAP1−/TAZ− group were markedly better than that in YAP1+/TAZ+ group. Cox regression analysis revealed that the YAP1/TAZ expression, tumor size, and lymph node metastasis were associated with poor survival outcomes in patients with ESCC, and represent independent factors for OS. Our data also indicated that the prognosis of patients with ESCC can be assessed more accurately by detecting the expression of YAP1 and TAZ. This work provides a theoretical basis and a strategy for the targeted treatment of ESCC. However, the study has some limitations: the study was mainly based on immunohistochemical analysis, and due to its retrospective nature, may be prone to lead time and ascertainment biases. Thus, further studies are needed to clarify the role of YAP1 and TAZ in the occurrence, development, invasion, and metastasis of ESCC based on functional experiments at a molecular level, so as to generate more comprehensive and effective therapeutic options for the clinical treatment of ESCC.
5. Conclusions
We demonstrated that YAP1 and TAZ are highly expressed in ESCC and closely associated with each other, suggesting that they may be used as prognostic indicators in ESCC patients.
Author contributions
Conceptualization: Liping Su, Hongwei Pu.
Data curation: Li Liu, Ziyang Lu, Liping Su, Hongwei Pu.
Formal analysis: Li Liu, Ziyang Lu, Xiayun Hu, Tianyuan Su.
Funding acquisition: Liping Su, Hongwei Pu.
Investigation: Li Liu.
Methodology: Ziyang Lu, Xiayun Hu, Tianyuan Su, Liping Su, Hongwei Pu.
Resources: Hongwei Pu.
Software: Ziyang Lu, Xiayun Hu.
Supervision: Hongwei Pu.
Writing – original draft: Li Liu, Ziyang Lu, Hongwei Pu.
Writing – review & editing: Li Liu, Hongwei Pu.
Footnotes
Abbreviations: ESCC = esophageal squamous cell carcinoma, MMPs = matrix metalloproteinases, OS = overall survival, PFS = progression-free survival, p-TAZ = phosphorylated form of TAZ, p-YAP1 = phosphorylated form of YAP1, TAZ = transcriptional co-activator with PDZ binding motif, TEADs = TEA domain DNA-binding family of transcription factors, YAP1 = yes related protein 1.
How to cite this article: Liu L, Lu Z, Hu X, Su T, Su L, Pu H. Clinical significance of YAP1 and TAZ in esophageal squamous cell carcinoma. Medicine. 2021;100:28(e26597).
LL and ZL contributed equally to this work.
This work was supported by the Natural Science Foundation of Xinjiang Uyghur Autonomous Region (2020D01C257); State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia Fund(SKL-HIDCA-2018-28), and 13th Five-Year Plan of Xinjiang key discipline (2016-07).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Malhotra GK, Yanala U, Ravipati A, Follet M, Vijayakumar M, Are C. Global trends in esophageal cancer. J Surg Oncol 2017;115:564–79. [DOI] [PubMed] [Google Scholar]
- [2].Raj N, Bam R. Reciprocal crosstalk between YAP1/Hippo pathway and the p53 family proteins: mechanisms and outcomes in cancer. Front Cell Dev Biol 2019;7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell 2016;29:783–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xiao Y, Zhang H, Ma Q, et al. YAP1-mediated pancreatic stellate cell activation inhibits pancreatic cancer cell proliferation. Cancer Lett 2019;462:51–60. [DOI] [PubMed] [Google Scholar]
- [5].Zanconato F, Cordenonsi M, Piccolo S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nat Rev Cancer 2019;19:454–64. [DOI] [PubMed] [Google Scholar]
- [6].Liu H, Du S, Lei T, et al. Multifaceted regulation and functions of YAP/TAZ in tumors (review). Oncol Rep 2018;40:16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sudol M. YAP1 oncogene and its eight isoforms. Oncogene 2013;32:3922–13922. [DOI] [PubMed] [Google Scholar]
- [8].Chen J, Mei Z, Huang B, et al. IL-6/YAP1/β-catenin signaling is involved in intervertebral disc degeneration. J Cell Physiol 2019;234:5964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shao DD, Xue W, Krall EB, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 2014;158:171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tiffon C, Giraud J, Molina-Castro SE, et al. TAZ controls Helicobacter pylori-induced epithelial-mesenchymal transition and cancer stem cell-like invasive and tumorigenic properties. Cells 2020;9:1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Crawford JJ, Bronner SM, Zbieg JR. Hippo pathway inhibition by blocking the YAP/TAZ-TEAD interface: a patent review. Expert Opin Ther Pat 2018;28:867–73. [DOI] [PubMed] [Google Scholar]
- [12].Song S, Li Y, Xu Y, et al. Targeting Hippo coactivator YAP1 through BET bromodomain inhibition in esophageal adenocarcinoma. Mol Oncol 2020;14:1410–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yu L, Gao C, Feng B, et al. The role of hippo pathway in cancer stem cell biology. Mol Cells 2018;41:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Verduci L, Ferraiuolo M, Sacconi A, et al. The oncogenic role of circPVT1 in head and neck squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD transcription-competent complex. Genome Biol 2017;18:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tu MS, Li YS, Zhang X. Expression and significance of YAP and TAZ proteins in oral squamous cell carcinoma. Shanghai Kou Qiang Yi Xue 2018;27:415–8. [PubMed] [Google Scholar]
- [16].Song S, Honjo S, Jin J, et al. The Hippo coactivator YAP1 mediates EGFR overexpression and confers chemoresistance in esophageal cancer. Clin Cancer Res 2015;21:2580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lo Sardo F, Forcato M, Sacconi A, et al. MCM7 and its hosted miR-25, 93 and 106b cluster elicit YAP/TAZ oncogenic activity in lung cancer. Carcinogenesis 2017;38:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huang S, Zhu L, Cao Y, et al. Significant association of YAP1 and HSPC111 proteins with poor prognosis in Chinese gastric cancer patients. Oncotarget 2017;8:80303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu R, Huang S, Lei Y, et al. FGF8 promotes colorectal cancer growth and metastasis by activating YAP1. Oncotarget 2015;6:935–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Andrade D, Mehta M, Griffith J, et al. YAP1 inhibition radiosensitizes triple negative breast cancer cells by targeting the DNA damage response and cell survival pathways. Oncotarget 2017;8:98495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang L, Zhang Z, Yu X, et al. Unbalanced YAP-SOX9 circuit drives stemness and malignant progression in esophageal squamous cell carcinoma. Oncogene 2019;38:2042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abdel Ghafar MT, Elkhouly RA, Elnaggar MH, et al. Utility of serum neuropilin-1 and angiopoietin-2 as markers of hepatocellular carcinoma. J Investig Med 2021;jim-2020-001744. [DOI] [PubMed] [Google Scholar]
- [23].Abdel Ghafar MT, Gharib F, Al-Ashmawy GM, et al. Serum high-temperature-required protein A2: a potential biomarker for the diagnosis of breast cancer. Gene Rep 2020;20:100706. [Google Scholar]
- [24].Habib EM, Nosiar NA, Eid MA, et al. Circulating miR-146a expression predicts early treatment response to imatinib in adult chronic myeloid leukemia. J Investig Med 2021;69:333–7. [DOI] [PubMed] [Google Scholar]
- [25].Li C, Zhu M, Lou X, et al. Transcriptional factor OCT4 promotes esophageal cancer metastasis by inducing epithelial-mesenchymal transition through VEGF-C/VEGFR-3 signaling pathway. Oncotarget 2017;8:71933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].El-Guindy DM, Wasfy RE, Abdel Ghafar MT, et al. Oct4 expression in gastric carcinoma: association with tumor proliferation, angiogenesis and survival. J Egypt Natl Canc Inst 2019;31:03. [DOI] [PubMed] [Google Scholar]
- [27].Abdel Ghafar MT, Gharib F, Abdel-Salam S, et al. Role of serum Metadherin mRNA expression in the diagnosis and prediction of survival in patients with colorectal cancer. Mol Biol Rep 2020;47:2509–19. [DOI] [PubMed] [Google Scholar]


