Abstract
Background:
The method to evaluate the efficacy of programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors has become a big concern for researchers with its widely application. Pseudoprogressive disease (PPD) makes this process more difficult, which means that the tumor progressed at the initial evaluation, but re-evaluation after continued treatment suggested that the treatment was effective. However, PPD has not attracted enough attention of clinical doctors. This article is to systematically evaluate the incidence of PPD associated with PD-1/PD-L1 inhibitors with meta-analysis, to provide guidance for the recognition and management of PPD.
Methods:
The databases of PubMed, EMBase, Cochrane Library were retrieved from the earliest collection date of the databases until Dec 5, 2019. The search terms of “pseudoprogressive disease, anti-PD-1, anti-PD-L1, PD-1/PD-L1 inhibitor, etc” were used for logistic combination search. Published studies on PPD caused by PD-1/PD-L1 inhibitors were included. Meta-analysis was performed with Stata 15.1. Subgroup analysis was performed according to the study population, tumor type, and evaluation criteria for efficacy.
Results:
Seven researches, including 1458 patients were taken into the study. Meta-analysis showed that the overall incidence of PPD was 3.70% (95% confidence interval [CI]: 2.70%, 4.90%). Subgroup analysis showed that the incidence of PPD was 3.30% (95% CI: 1.90%, 5.90%) in non-small cell lung cancer patients and 5.10% (95% CI: 2.30%, 11.6%) in melanoma patients. There was no statistically significant difference between East and West populations and among various efficacy evaluation criteria.
Conclusion:
The incidence of PPD related to PD-1/PD-L1 inhibitors is not high, but the evaluation criteria has not yet been unified. Close monitoring, careful identification and proper application should be carried out in the clinic, and full management of the treatment with PD-1/PD-L1 inhibitors should be well done.
Keywords: incidence rate, meta-analysis, programmed cell death 1/programmed cell death ligand 1 inhibitors, pseudoprogressive disease, risk factors
1. Introduction
Immune checkpoint inhibitors (ICIs) are widely used in clinical practice, bringing new hope for patients with solid tumors. At present, the most widely used immune checkpoints are programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) and cytotoxic T lymphocyte-associated antigen 4. However, there are still great challenges to accurately evaluate the efficacy of ICIs. Pseudoprogressive disease (PPD) has attracted extensive attention from researchers as a difficult part in the evaluation of ICIs’ efficacy. PPD is defined as a transient increase in the size of an existing tumor lesion visible on imaging or the appearance of new lesions in patients receiving cancer immunotherapy. At present, there is no study systematically evaluated PPD related to PD-1/PD-L1 inhibitors. The aim of this study was to evaluate the incidence of PPD in solid tumor patients treated with PD-1/PD-L1 inhibitors, and to provide reference for clinical application of PD-1/PD-L1 inhibitors and the evaluation of efficacy.
2. Data and methods
2.1. Sources and retrieval methods
Electronic retrieval of literature databases: PubMed, EMBase, and Cochrane Library were retrieved from database construction until December 5, 2019. The search terms of “pseudoprogressive disease, anti-PD-1, anti-PD-L1, PD-1/PD-L1 inhibitor, et al” were used for logistic combination search, and the clinical studies of PPD caused by PD-1/PD-L1 inhibitor were systematically retrieved. Literature retrieval and manual search were conducted with different combinations to supply the references included in the literature.
2.2. Inclusion and exclusion criteria
Inclusion criteria: patients diagnosed with solid tumors with clear pathological diagnosis; patients treated with PD-1/PD-L1 inhibitors; reported the incidence of PPD after the application of PD-1/PD-L1 inhibitors or provided statistical incidence data; studies which was published to evaluate the efficacy and safety of PD-1/PD-L1 inhibitors, such as randomized controlled trial, controlled clinical trial, single-arm clinical trial, cohort study, case-control study or case series study; the language limited to English.
Exclusion criteria: animal experiments and mechanism studies; interventions were not only PD-1/PD-L1 inhibitors; case reports; literature reviews, commons, expert opinions, clinical guidelines, etc; no cases of PPD reported or the incidence of PPD could not be calculated; repeated published studies.
2.3. Literature information extraction
SPSS 20.0 was used to design the data extraction table. The extracted information includes: literature information: first author, year of publication, location of research institute, sample size, type of study; research object information: male proportion, tumor type, intervention measures; treatment program: type of PD-1/PD-L1 inhibitors, efficacy evaluation criteria, clinical efficacy; number of cases of PPD.
2.4. Literature quality evaluation
For randomized controlled trial and controlled clinical trial, the quality assessment was performed by the Cochrane Reviewer's Handbook 5.2.0 evaluation criteria,[1] with a full score of 7 and a total score of <4 for high-quality literature; for single-arm clinical studies, cohort studies, and case control studies, the Newcastle-Ottawa scale was used for quality assessment,[2] with a full score of 9 and a total score of <5 for high-quality studies. Case series studies were evaluated by the recommended criteria of the National Institute for Clinical Excellence,[3] with a full score of 8 and a score of <4 for high-quality studies. Literature retrieval, literature screening, information extraction, and quality assessment were performed independently by 2 researchers, disagreements were resolved by discussion or solicitation of a third researcher when they arise.
2.5. Statistical methods
Meta-analysis of rates was performed using Stata 15.1 software. P-value was used to analyze the heterogeneity among the studies, and I2 value was used to evaluate the heterogeneity. If P ≥ .10 and I2 ≤ 35%, it indicated that there was no statistical heterogeneity among the studies or the heterogeneity was small, then the fixed-effect model was used for meta-analysis. If P < .10 or I2 > 35%, indicating statistical heterogeneity among studies, a random effect model was used for meta-analysis to calculate the combined results of PPD incidence and its 95% confidence interval (95% CI). Subgroup analysis was performed according to the population, tumor type, and efficacy evaluation criteria. SPSS20.0 was used to measure P-value between groups. Egger regression was used to evaluate the risk of publication bias. The difference was statistically significant with P < .05.
3. Results
3.1. Literature search and screening results
A total of 332 records were obtained in the initial examination, and 51 duplicates were excluded. The remaining literatures were screened out by reading titles and abstracts and 20 literatures were selected. Finally, the full text was read and 7 literatures were included.[4–10] The flow chart of literature retrieval and screening is shown in Fig. 1.
Figure 1.
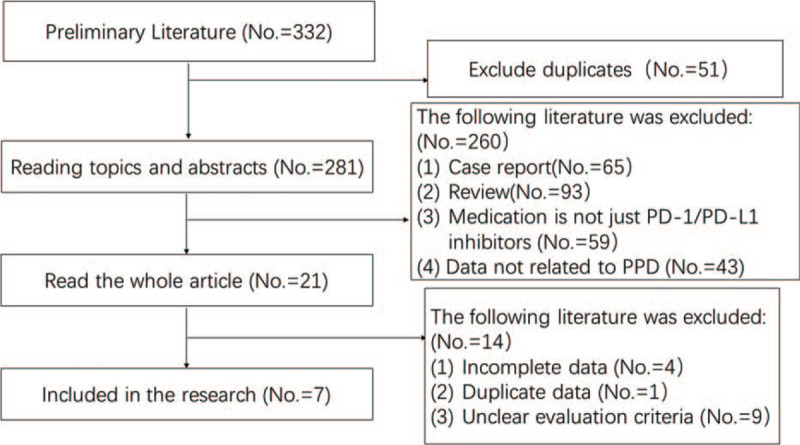
Flow diagram of literature retrieval and screening.
3.2. Basic characteristics and quality evaluation of literature
A total of 7 clinical studies were included in this study, with 1458 patients available for inclusion. The basic characteristics and quality evaluation of the literature are shown in Table 1.
Table 1.
Basic characteristics and quality evaluation.
| No. | Author | Year | Country | Disease | Eligible | PPD | Criteria | Quality |
| 1 | Fujimoto D. | 2019 | Japan | NSCLC | 542 | 14 | RECIST | High |
| 2 | Ferreira G. | 2019 | Portugal | melanoma | 30 | 2 | PERCIST∗ | High |
| 3 | Han J. | 2019 | China | NSCLC | 51 | 3 | RECIST1.1 | High |
| 4 | Dumoulin D. | 2019 | Netherlands | mesothelioma | 91 | 2 | RECIST | High |
| 5 | Ferrara R. | 2018 | France | NSCLC | 406 | 19 | RECIST1.1 | High |
| 6 | Nishino M. | 2017 | U.S.A | melanoma | 96 | 4 | irRECIST | High |
| 7 | Ferrara R. | 2017 | France | NSCLC | 242 | 3 | RECIST1.1 | High |
NSCLC = non-small cell lung cancer, PPD = pseudoprogressive disease.
PERCIST = PET response criteria in solid tumors.
3.3. Meta-analysis results
A total of 1458 subjects were included in the 7 studies, and 47 patients had PPD events. The incidence of PPD reported in various studies ranged from 1.30% to 7.10%. There was less heterogeneity in the incidence of PPD among the 7 studies (P = .198, I2 = 30.2%), thus a fixed-effect model was used for meta-analysis and forest plotting (Table 2, Fig. 2). The overall incidence of PPD in solid cancer patients treated with PD-1/PD-L1 inhibitors was 3.70% (95% CI: 2.70%, 4.90%).
Table 2.
Meta-analysis results.
| No. | Study | ES | [95% confidence interval] | % Weight | |
| 1 | Fujimoto D. | 0.027 | 0.016 | 0.045 | 30.18 |
| 2 | Ferreira G. | 0.071 | 0.017 | 0.300 | 4.13 |
| 3 | Han J. | 0.063 | 0.019 | 0.201 | 6.25 |
| 4 | Dumoulin D. | 0.022 | 0.006 | 0.091 | 4.33 |
| 5 | Ferrara R. | 0.049 | 0.031 | 0.078 | 40.08 |
| 6 | Nishino M. | 0.043 | 0.016 | 0.118 | 8.48 |
| 7 | Ferrara R. | 0.013 | 0.004 | 0.039 | 6.56 |
| D+L pooled ES | 0.037 | 0.027 | 0.049 | 100.00 | |
Figure 2.
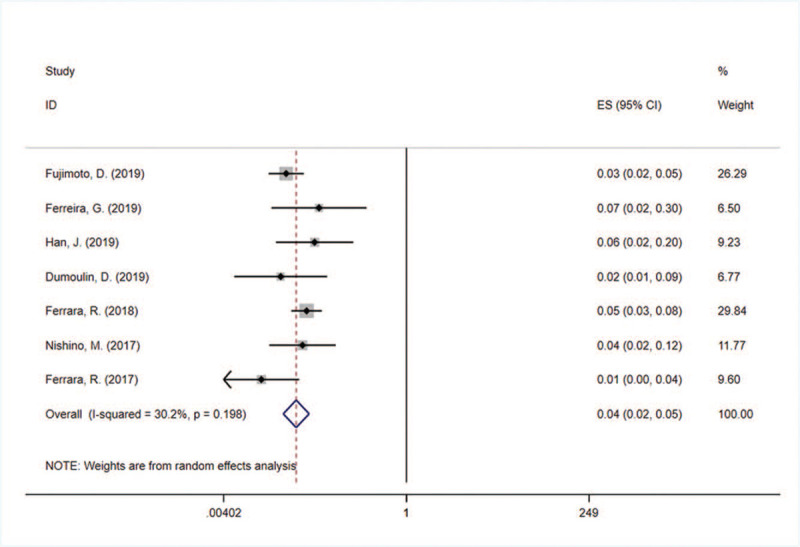
Meta-analysis forest map of PPD incidence in cancer patients treated with PD-1/PD-L1 inhibitors. PD-1 = programmed cell death 1, PD-L1 = programmed cell death ligand 1, PPD = pseudoprogressive disease.
3.4. Subgroup analysis
The results of subgroup analysis showed that there was no significant difference in the incidence of PPD between the Eastern and Western populations, tumor types or therapeutic evaluation criteria. The results are shown in Table 3.
Table 3.
Results of subgroup analysis.
| [95% confidence interval] | Heterogeneity test | |||||||
| Subgroup analysis | Study number | D+L pooled ES | P∗ | P | I2 | Tau2 | ||
| Population | ||||||||
| Eastern | 2 | 0.035 | 0.016 | 0.075 | .536 | .190 | 41.9% | 0.1539 |
| Western | 5 | 0.037 | 0.021 | 0.063 | .197 | 33.7% | 0.1252 | |
| Disease | ||||||||
| NSCLC | 4 | 0.033 | 0.019 | 0.059 | 1.198 | .067 | 58.1% | 0.1781 |
| Melanoma | 2 | 0.051 | 0.023 | 0.116 | .578 | 0.00% | 0.0000 | |
| Mesothelioma | 1 | 0.022 | 0.006 | 0.091 | – | – | 0.0000 | |
| Criteria | ||||||||
| RECIST | 2 | 0.026 | 0.016 | 0.043 | 2.477 | .829 | 0.0% | 0.0000 |
| PET-RECIST | 1 | 0.071 | 0.017 | 0.300 | – | – | 0.0000 | |
| RECIST1.1 | 3 | 0.036 | 0.016 | 0.084 | .074 | 61.6% | 0.3383 | |
| irRECIST | 1 | 0.043 | 0.016 | 0.118 | – | – | 0.0000 | |
| Overall | 7 | 0.036 | 0.024 | 0.053 | .198 | 30.2% | 0.0787 | |
Chi-square test for comparison of combined incidence.
NSCLC = non-small cell lung cancer.
3.5. Publication bias risk assessment
The results of Egger regression showed that there was no statistical significance in the bias between the included studies (Egger test: P = .801), as shown in Fig. 3.
Figure 3.
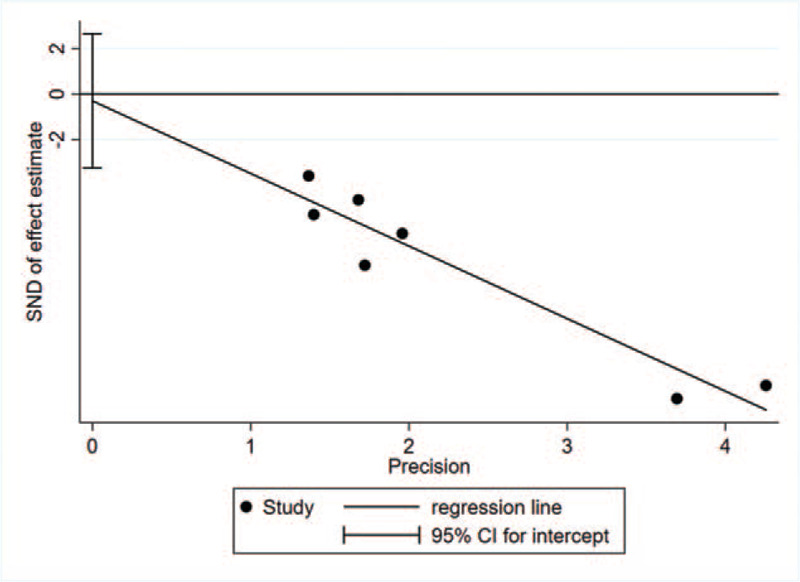
Egger detection.
4. Discussion
After treatment with ICIs, tumors may present with transient volume gain or an increase in the number of lesions followed by a reduction in size and number of lesions, which was known as PPD that usually occurs when ICIs are first used.[11]
It is important to accurately identify true progression or PPD after the application of PD-1/PD-L1 inhibitors. The gold standard for the diagnosis of PPD is pathological findings. PPD can be diagnosed by biopsy of enlarged lesions following the application of PD-1/PD-L1 inhibitors and pathological findings confirming necrosis or inflammatory cell infiltration.[12] When a biopsy cannot be performed, it can also be judged by imaging criteria. However, Response Evaluation Criteria in Solid Tumors (RECIST), which is traditionally used to evaluate the efficacy of solid tumors, obviously cannot meet such needs. Therefore, some criteria for evaluating the efficacy of immunotherapy have emerged, such as immune-related RECIST (irRECIST), immune RECIST (iRECIST), etc. Katz et al[13] found that irRECIST and iRECIST criteria were more accurate in diagnosing imaging PPD as progression-free, with higher accuracy than traditional RECIST 1.1 criteria.
Researchers have also attempted to find serological indicators to differentiate PPD from true progression. Such as circulating tumor cell DNA level[14]; serum interleukin-8 level,[15] and plasma CXCL2 and MMP2 levels.[16]
However, the time of PPD occurrence, the predisposing population of PPD, and the time of tumor re-shrinkage after PPD occurrence are still up to know, and the identification and diagnosis of PPD and the first evaluation after treatment with PD-1/PD-L1 inhibitors pose difficulties for the next treatment choice of patients who have progressed.
In this study, a meta-analysis of 1458 patients with solid tumors who were treated with PD-1/PD-L1 inhibitors was conducted. The results showed that the overall incidence of PPD in tumor patients treated with PD-1/PD-L1 inhibitors was 3.70% (95% CI: 2.70%, 4.90%). This suggested that the incidence of PPD is not high. Subgroup analysis showed that the occurrence of PPD was not related to the population, to the type of solid tumor, or to the selection of evaluation criteria for evaluating efficacy.
4.1. Limitation
There were still some shortcomings in this study. Included studies were mainly case series studies with low level of evidence; there were some differences in the number of samples among the studies, and the data obtained were biased; the researches were not included in this study which did not report the occurrence of PPD, may led to bias. All included studies used only one method to evaluate PPD, but different conclusions might be drawn if different criteria were used to evaluate the same patients.
5. Conclusion
In summary, PD-1/PD-L1 inhibitors may lead to the occurrence of PPD. Although the incidence is not high, there is still unclear of the occurrence time, regression time, and accurate identification of PPD. Therefore, patients treated with PD-1/PD-L1 inhibitors should be paid more attention in the clinic, so as to make an accurate evaluation of the therapeutic efficacy of patients as far as possible, to avoid erroneous evaluation of therapeutic efficacy. Further detail studies are needed on PPD to avoid affecting patients’ treatment due to wrong efficacy evaluation and causing serious consequences.
Author contributions
Conceptualization: Jingyi Zhang, Huijuan Cui.
Data curation: Jingyi Zhang, Kexin Tan, Xuejiao Jiang, Jia Li.
Formal analysis: Xuejiao Jiang, Chongxiang Xue, Xu Zhang.
Funding acquisition: Huijuan Cui.
Methodology: Shuyue Zheng, Chongxiang Xue.
Software: Kexin Tan, Xuejiao Jiang, Shuyue Zheng, Xu Zhang.
Validation: Shuyue Zheng, Jia Li, Chongxiang Xue, Huijuan Cui.
Visualization: Jia Li.
Writing – original draft: Jingyi Zhang, Kexin Tan.
Writing – review & editing: Jingyi Zhang, Xu Zhang, Huijuan Cui.
Footnotes
Abbreviations: CI = confidence interval, ICIs = immune checkpoint inhibitors, NSCLC = non-small cell lung cancer, PD-1 = programmed cell death 1, PD-L1 = programmed cell death ligand 1, PPD = pseudoprogressive disease, RECIST = response evaluation criteria in solid tumors.
How to cite this article: Zhang J, Tan K, Jiang X, Zheng S, Li J, Xue C, Zhang X, Cui H. The incidence of pseudoprogressive disease associated with programmed cell death 1/programmed cell death ligand 1 inhibitors: a meta-analysis. Medicine. 2021;100:28(e26649).
This work was supported by National Natural Science Foundation of China (No.81873396).
Ethical approval: This article is a meta-analysis, so it does not involve Human Participants and/or Animal.
Patient Consent: Not applicable.
Data availability: The data supporting this meta-analysis are from previously reported studies and datasets, which have been cited.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1]. The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions version 5.1.0[EB/OL]. (updated March 2011) [2019-12-5]. Available at: http://handbook-5-1.cochrane.org/. Accessed December 5, 2019. [Google Scholar]
- [2]. Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses [EB/OL]. July 2015 [2019-12-5]. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 5, 2019. [Google Scholar]
- [3]. National Institute for Health and Care Excellence (NICE). Appendix 4 quality of case series form [EB/OL]. July 2015 [2019-12-5]. Available at: https://www.nice.org.uk/guidance/index. Accessed December 5, 2019. [Google Scholar]
- [4].Fujimoto D, Yoshioka H, Kataoka Y, et al. Pseudoprogression in previously treated patients with non-small cell lung cancer who received nivolumab monotherapy. J Thorac Oncol 2019;14:468–74. [DOI] [PubMed] [Google Scholar]
- [5].Ferreira G, Castro SF, Sampaio IL, Violante LS, Teixeira JP, Duarte H. Response assessment to immunotherapy with pd1 inhibitors using metabolic tumor volume on 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging 2019;46: 1 suppl 1: S225–6. [Google Scholar]
- [6].Han J, Wang Z, Wang Y, et al. Dynamic clonality of T cell receptor differentiate atypical progression in NSCLC patients treated with PD-1/PD-L1inhibitors. Cancer Res 2019;79: 13 suppl: 3247. [Google Scholar]
- [7].Dumoulin D, Cantini L, Belderbos R, Mercieca D, Cornelissen R, Aerts J. MA05.09 Real-World Data of nivolumab and pembrolizumab in chemotherapy pre-treated mesothelioma patients. J Thorac Oncol 2019;14:S267. [Google Scholar]
- [8].Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol 2018;4:1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nishino M, Giobbie-Hurder A, Manos MP, et al. Immune-related tumor response dynamics in melanoma patients treated with pembrolizumab: identifying markers for clinical outcome and treatment decisions. Clin Cancer Res 2017;23:4671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ferrara R, Caramella C, Texier M, et al. Hyperprogressive disease (HPD) is frequent in non-small cell lung cancer (NSCLC) patients (pts) treated with anti PD1/PD-L1 monoclonal antibodies (IO). Ann Oncol 2017;28: suppl 5: v464–5. [Google Scholar]
- [11].Imafuku K, Hata H, Kitamura S, Yanagi T, Shimizu H. Ultrasonographic findings can identify ’pseudoprogression’ under nivolumab therapy. Br J Dermatol 2017;177:1726–31. [DOI] [PubMed] [Google Scholar]
- [12].Wang Q, Gao J, Wu X. Pseudoprogression and hyperprogression after checkpoint blockade. Int Immunopharmacol 2018;58:125–35. [DOI] [PubMed] [Google Scholar]
- [13].Katz SI, Hammer M, Bagley SJ, et al. Radiologic pseudoprogression during anti-PD-1 therapy for advanced non-small cell lung cancer. J Thorac Oncol 2018;13:978–86. [DOI] [PubMed] [Google Scholar]
- [14].Jensen TJ, Goodman AM, Kato S, et al. Genome-wide sequencing of cell-free dna identifies copy-number alterations that can be used for monitoring response to immunotherapy in cancer patients. Mol Cancer Ther 2019;18:448–58. [DOI] [PubMed] [Google Scholar]
- [15].Sanmamed MF, Perez-Gracia JL, Schalper KA, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol 2017;28:1988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Matsuo N, Azuma K, Hattori S, et al. Association between soluble immune mediators and tumor responses in patients with nonsmall cell lung cancer treated with anti-PD-1 inhibitor. Int J Cancer 2019;144:1170–9. [DOI] [PubMed] [Google Scholar]


