Abstract
In patients with localized scleroderma (LoS), facial deformity induced by subcutaneous atrophy greatly reduces life quality. Autologous fat grafting (AFG) is used for volume restoration but with low‐fat retention due to various reasons. Adipose‐derived stem cells (ADSCs) have shown potential effects in improving fat retention. We aimed to compare the feasibility and efficacy of improving fat retention in LoS patients among the ADSCs‐assisted, the stromal vascular fraction (SVF)‐assisted and conventional AFG methods. A pilot study with a 6‐month follow‐up among 18 LoS patients was conducted. Participants were randomly assigned into three AFG groups: conventional group, SVF‐assisted group, and ADSCs‐assisted group. The SVF‐assisted group received SVF‐assisted AFG at the SVF:fat ratio of 1:1. The ADSCs‐assisted group received the mixture of ADSCs‐enriched fat graft supplemented with 5 × 105 ADSCs/mL fat. Volume retention was measured by magnetic resonance imaging, and clinical photographs were taken for outcome evaluation. At sixth‐month follow‐up, the fat retention of ADSCs‐assisted group was 49.83 ± 3.61%, significantly higher than 31.75 ± 1.73% of SVF‐assisted group (P = .0004), and 21.86 ± 1.68% of the conventional group (P < .0001). A significant difference of the fat retention was also observed between the SVF‐assisted and conventional group (P = .0346). No severe adverse events occurred during the procedure and follow‐up. This pilot study suggests that ADSCs‐assisted AFG is a safe, feasible, and attractive alternative to conventional and SVF‐assisted AFG in the correction of facial atrophy of LoS patients. Future studies with large patient samples are needed for confirmation. (Chinese Clinical Trial Registry, ChiCTR1900025717).
Keywords: adipose‐derived stem cells, fat grafting, localized scleroderma, stromal vascular fraction
Adipose‐derived stem cells (ADSCs)‐assisted autologous fat grafting (AFG) significantly improved fat retention in localized scleroderma patients. This pilot study shows the potential advantage of ADSCs‐assisted AFG in the correction of facial atrophy of localized scleroderma patients. SVF, stromal vascular fraction.
Lessons learned
Adipose‐derived stem cell assisted autologous fat grafting is a safe, feasible, and attractive alternative in the correction of facial atrophy of localized scleroderma patients.
This technique significantly improved fat retention in localized scleroderma patients during a 6‐month follow‐up.
Significance statement
The present study suggests that autologous fat grafting combined with adipose‐derived stem cells is a safe, feasible, and attractive alternative to conventional fat grafting or fat grafting combined with stromal vascular fraction in the correction of facial atrophy of localized scleroderma patients.
1. INTRODUCTION
Localized scleroderma (LoS) is a rare autoimmune connective tissue disorder characterized by inflammation and fibrosis of the skin and subcutaneous fat in the affected area with an incidence of around 0.3‐3 cases per 100 000 inhabitants/year. 1 Presenting mainly as subcutaneous tissue atrophy and hyperpigmentation, this disorder seriously affects the life quality and mental health of patients. 1 , 2 With the advantages of minor invasiveness, quick recovery and natural appearance outcomes, autologous fat grafting (AFG) has replaced the method of micro‐vascular free flap transfer as the dominant surgical treatment on improving facial atrophy in LoS patients. 3 , 4 Due to the local inflammatory microenvironment, and decreased adipose‐derived stem cells (ADSCs) in adipose tissues induced by corticosteroid therapy, 5 most of the fat graft was difficult to survive in LoS patients, making them have to undergo many rounds of grafting procedures to maintain the appearance outcome and burden financial and mental pressure. Some research showed stromal vascular fraction (SVF)‐assisted fat grafting can improve the fat graft retentions by about 35% in healthy people, 6 but, it has not been proved by a similar increase in LoS patients yet. Recently, ADSCs' multiple advantages of immunomodulatory effects and the ability to promote angiogenesis and ability of differentiation into mature adipocytes have been pre‐clinically reported, showing potential effect in treatment of many autoimmune diseases 7 , 8 and improvement of fat retention. 9 , 10 This clinical study, based on our previous studies showing ADSCs have a significant effect on improving the retention of fat graft in LoS mice, 5 aimed to investigate the clinical outcomes of fat graft retention among ADSCs‐assisted, SVF‐assisted and conventional fat grafting of improving facial atrophy in LoS patients.
2. PATIENTS AND METHODS
2.1. Study design
A prospective, randomized, and controlled pilot study with three parallel comparison groups was carried out. The study followed the declaration of Helsinki guidelines, approved by the institutional ethical committee (JMLL20180902), and registered in Chinese Clinical Trial Registry (ChiCTR1900025717). The sample size of six subjects per group was determined via a power calculation. All candidates were evaluated for suitability to be enrolled in the study by physical examination and by the criteria stated in Table 1. Informed consent was obtained from each participant at study entry after an adequate explanation including the aims, methods, process, anticipated benefits, and potential risks of this study. Then participants were randomly assigned into three groups: conventional AFG group, SVF‐assisted AFG group, and ADSCs‐assisted AFG group. The allocation sequence was concealed by a statistician who was unrelated to the management group of this study. The radiologist who analyzed the magnetic resonance imaging for volume assessment, and five plastic surgery experts who evaluated the clinical outcome were blinded thorough the whole study.
TABLE 1.
The inclusion and exclusion criteria for the pilot study
| Inclusion criteria |
|
| Exclusion criteria |
|
Abbreviation: BMI, body mass index.
2.2. Intervention and outcomes
The study was performed in LoS patients with facial atrophy (forehead and cheek). All participants received AFG to correct facial atrophy according to the randomization sequence. The conventional group received conventional AFG, and the SVF‐assisted group received SVF‐assisted AFG according to the SVF: fat ratio of 1:1, and the ADSCs‐assisted group received fat grafting with ex vivo enriched ADSCs. The mixture of ADSCs‐enriched fat grafts was supplemented with 5 × 105 ADSCs/mL fat. This concentration was chosen based on the protocols of previous clinical studies of ADSC transplantation. 11 , 12 Magnetic resonance imaging (MRI) analysis was used to measure the facial atrophy volume of each participant preoperatively. Postoperatively and in twice follow‐ups of 6 months, the fat retention of each participant was also measured by MRI. Besides, clinical photos were taken preoperatively and at follow‐ups. An overview flowchart of this pilot study is shown in Figure 1. The primary endpoint was the fat retention at the sixth‐month follow‐up. Secondary endpoints were the assessment of safety evaluation, the feasibility, and clinical outcomes of all the AFG methods.
FIGURE 1.
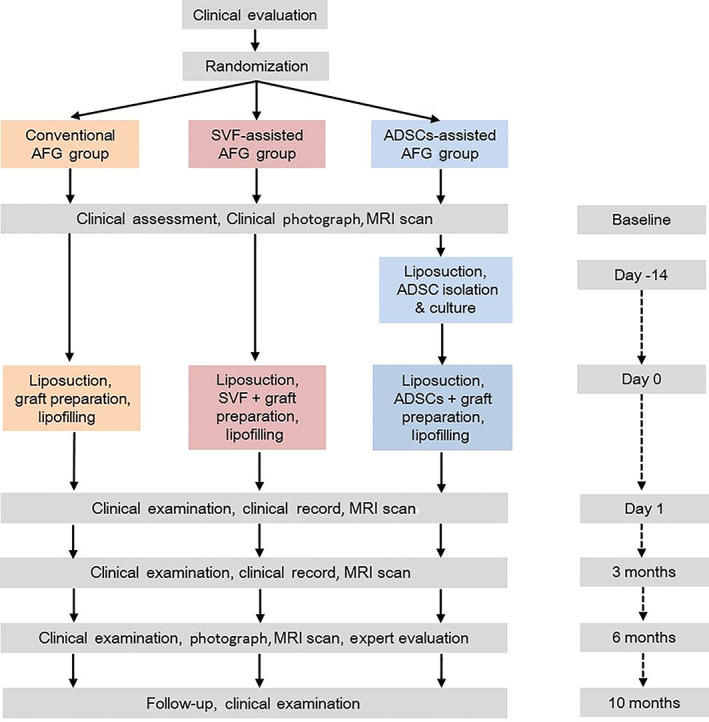
An overview description of the intervention in this pilot study. ADSCs, adipose‐derived stem cells. AFG, autologous fat grafting; MRI, magnetic resonance imaging; SVF, stromal vascular fraction
2.3. Liposuction, graft preparation, and grafting
At study entry, the facial atrophy volume of each participant was measured by MRI analysis. The assessment of facial atrophy severity and the volume of the graft was noted for each participant by comparing the volume difference of the subcutaneous adipose tissue between the target lesion site and the unaffected symmetry site preoperatively. All the participants underwent outpatient liposuction and subsequent facial fat grafting procedures under local anesthesia, and autologous SVF and ADSCs were produced and prepared at a good manufacturing practice facility (Beijing Key Laboratory of New Drug Development and Clinical Trial of Stem Cell Therapy, BZ0381). Liposuction at abdomen was performed under a low, consistent negative pressure using 50‐mL syringes to limit trauma to the adipocytes.
The SVF was obtained by means of collagenase digestion. Briefly, the lipoaspirate sample was diluted with an equal volume of PBS and centrifuge at 400g for 5 minutes, three times. Then dilute the aspirated lipid fraction with an equal volume of 0.2% collagenase I solution (Sigma Chemical Co) and incubate the mixture at 37°C for 30 minutes. The suspension was centrifuged at 600g for 5 min and washed twice and filtered through sterile 100 μm cell‐strainers to remove any residual enzyme. The cells were centrifuged at room temperature for 10 minutes at 600g. The supernatant was discarded and the SVF was suspended in sterile saline (Dazhong, China), thereby SVF solution was gained. Approximately 6 × 105 SVF cells per milliliter fat were obtained and the cell viability ranged from 85% to 95% (>70% viable) measured in Trypan blue protocols using machine (Countstar, China). Immunophenotype was analyzed by flow cytometry: positive, CD13, CD29, CD44, CD73, CD90 (>40%), CD34 (>20%); negative: CD31 (<20%), CD45 (<50%).
The isolation of ADSCs was obtained by suspending the SVF into the serum‐free medium (Lonza Group AG, Switzerland). The solution was again centrifuged at 600g for 5 minutes, resuspended in the culture medium, seeded into a culture flask, and cultured at 37°C under 5% CO2. The culture was examined the next day, and cell adherence was confirmed under a microscope. The cells were cultured to passage 2‐3 with medium replacement every 2 days. The cells were confirmed as adipose‐derived stromal cells by flow cytometry based on the criteria of the International Society for Cellular Therapy—positive: CD13, CD29, CD44, CD73, CD90, and CD105 (>80%); negative: CD31, CD45, and CD235a (<2%). 13 The assessment of ADSCs before clinical use included (a) no visible clumps in the cell suspension; (b) the absence of pathogen contamination examined by sterility testing of the medium; (c) ADSC viability measurement, which should be over 90%; (d) characteristic ADSC morphology. Each batch of 1 × 107 ADSCs was suspended into 1 mL ADSCs solution with sterile saline (Dazhong, China).
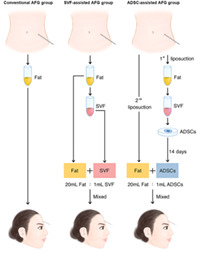
The solution of SVF or ADSCs was kept in a sterile container labeled according to current regulations and transferred to the operating room in a sealed sterile box. The target grafting volume was determined by 20% overcorrections of measured facial atrophy volume. 14 , 15 The conventional group received fat grafting directly. A graft mixture according to the fat: SVF ratio of 1:1 (20 mL fresh fat: 1 mL fresh SVF solution collected from 20 mL fat tissue) was injected into the SVF‐assisted group patients. The mixture of fresh fat and cultured ADSCs, corresponding to 5 × 105 ADSCs per milliliter of fat (20 mL fresh fat: 1 mL non‐cryopreserved ADSCs solution), was grafted into the ADSCs‐assisted group (Supporting Information Table S1). A homogenous solution of adipocytes and ADSCs solution or SVF solution was ensured by rotating and gently stirring the mixed transplant product until uniform in color and consistency. For all groups, AFG or the AFG mixture with SVF/ADSCs was transferred into the subcutaneous tissue of the target lesion by a fan‐like pattern technique using 1.4‐mm blunt cannulas.
2.4. Clinical assessment
Preoperative variables included age, sex, LoS severity index (LoSSI), and BMI. Postoperatively, participants were asked to return for follow‐up assessments at 3 and 6 months. At each follow‐up visit, surgical complications were documented, and participants were photographed.
Clinical outcomes were separately evaluated by five plastic surgery experts. Photos of each participant pre‐operation and follow‐ups were presented blinded and at an equal profile to each expert and expert satisfaction was assessed by the question: “how satisfied would you be with the retention and the aesthetic improvement of this participant?” The expert satisfaction included five grades: below average, average, above average, good, and excellent. 16
2.5. Magnetic resonance imaging analysis
MRI was performed pre‐operation, post‐operation, and each follow‐up with a 3.0 Tesla MR scanner (Discovery MR750 3T, GE Healthcare, Milwaukee, Wisconsin), using a 32‐channel head and neck coil. The MRI examination was conducted from the frontal bone to the neck base, including all six cervical lymph node levels, excluding only the supraclavicular region. Axial TSE T1‐FLAIR weighted (acquisition parameters: TR = 1725 ms, TE = 24 ms, flip angle 111°, TA 3min6s; 50 slices; FOV 22 × 20 mm) was performed with a slice thickness of 4 mm. Volumetric measurement was performed using Horos V3.3.1 (Horos Project). Preoperative and postoperative and each follow‐up imaging included the location, appearance, and volume of the adipose tissue of target lesion (Figure 2). The percentage change in the volume of adipose tissue of LoS lesion was measured as followed: Retention = (the volume of remain fat at follow‐up − the volume of initial fat tissue)/the volume of fat graft. In addition, attention was paid to the presence of complication. All imaging was measured by a radiologist with 6 years of experience.
FIGURE 2.
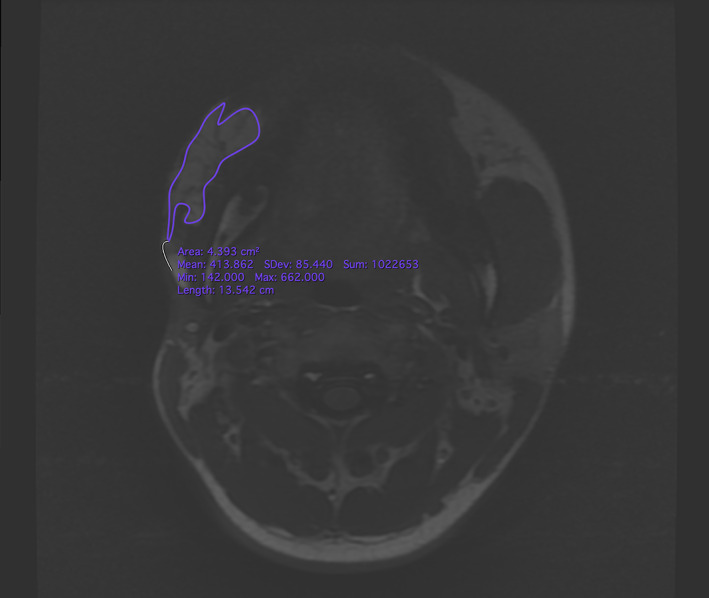
Magnetic resonance images and delineation of the subcutaneous adipose tissue. A region of interest (ROI) was precisely drawn on each axial slide by comprising pre‐ and post‐operational scans. After ensuring that the entire targeted subcutaneous adipose tissue was included in the ROIs, the volumes were calculated automatically
2.6. Statistical analysis
The sample size was calculated to be significant with 18 participants allocated equally (1:1:1) to show a significant difference in retention of 20%, with a two‐sided 5% significance level and a power of 80%. Statistical analyses were conducted using GraphPad Prism 8.0.2 (GraphPad Software). The difference significance was evaluated by one‐way ANOVA with the Tukey's multiple comparisons test. Significance was set to the level of P < .05. All data are presented as mean ± SEM. Exploratory data analysis was further performed using Python 3.8.3 (Python) to visualize the potential relationships between exposure and outcome variables.
3. RESULTS
Eighteen participants (11 female and 7 male) were enrolled into the study and retained throughout the 6 months to study completion, whose average age was 26.68 ± 1.28 years, average BMI was 21.22 ± 0.34 kg/m2 and average LoSSI was 3.46 ± 0.11 scores. None of age, BMI, scleroderma severity (LoSSI) or facial atrophy severity (fat graft volume) was found significantly different among the three groups (Table 2, Figure 3). No participants experienced infection, hematoma, seroma, or oil cysts. No perioperative antibiotics were used. No serious adverse events or unanticipated events occurred.
TABLE 2.
Description of baseline
| Conventional group (n = 6) | SVF‐assisted group (n = 6) | ADSCs‐assisted group (n = 6) | P value1 | P value2 | P value3 | |
|---|---|---|---|---|---|---|
| Age (yr) | 24.43 ± 2.67 | 28.14 ± 2.12 | 27.38 ± 1.93 | 0.6222 | 0.9675 | 0.4976 |
| Gender | ||||||
| Female | 4 | 3 | 4 | |||
| Male | 2 | 3 | 2 | |||
| Site | ||||||
| Forehead | 3 | 3 | 3 | |||
| Cheek | 3 | 3 | 3 | |||
| BMI (kg/m2) | 21.04 ± 0.62 | 21.88 ± 0.72 | 20.82 ± 0.42 | 0.9616 | 0.4134 | 0.5868 |
| LoSSI | 3.29 ± 0.18 | 3.43 ± 0.20 | 3.63 ± 0.18 | 0.4255 | 0.7440 | 0.8625 |
| ISFV (cm3) | 8.90 ± 1.24 | 8.46 ± 1.46 | 9.04 ± 1.01 | 0.9969 | 0.9424 | 0.9654 |
Notes: P value1, the difference between ADSCs‐assisted group and conventional group. P value2, ADSCs‐assisted group to SVF‐assisted group. P value3, conventional and SVF‐assisted group.
Abbreviations: BMI, body mass index; ISFV, initial subcutaneous fat volume; LoSSI, localized scleroderma severity index.
FIGURE 3.
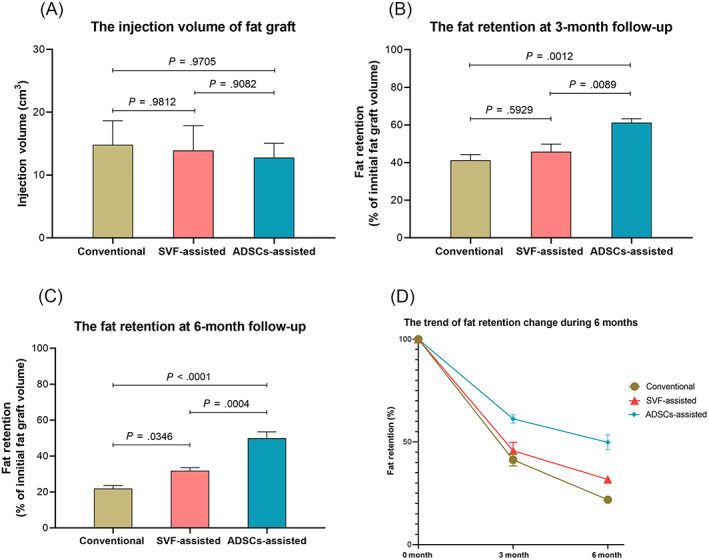
Statistical outcomes. A, The injection volumes of the fat graft of three groups. B, The fat retention at the 3‐month follow‐up. C, The fat retention at 6‐month follow‐up. D, The trend of fat retention changes during the 6 months period
At the 3‐month follow‐up, the fat retention of ADSCs‐assisted group was 61.25 ± 2.03%, significantly higher than 45.69 ± 4.11% of SVF‐assisted group (P = .0089), and 41.26 ± 3.00% of the conventional group (P = .0012). But there was no significant difference between the conventional and SVF‐assisted group (P = .5929). At sixth‐month follow‐up, the fat retention of ADSCs‐assisted group was 49.83 ± 3.61%, significantly higher than 31.75 ± 1.73% of SVF‐assisted group (P = .0004), and 21.86 ± 1.68% of the conventional group (P < .0001). A significant difference of the fat retention was also observed between the SVF‐assisted and conventional group (P = .0346) (Figure 3). Furthermore, the AFG method was confirmed by exploratory data analysis as the only exposure variable affecting the outcome variable (fat retention) (Supporting Information Figure S1). The expert satisfaction of the ADSCs‐assisted group was 3.98 ± 0.11, significantly higher than 3.07 ± 0.18 of the SVF‐assisted group (P = .0092) and 2.20 ± 0.24 of the conventional fat grafting (P < .0001) (Figures 4 and 5).
FIGURE 4.
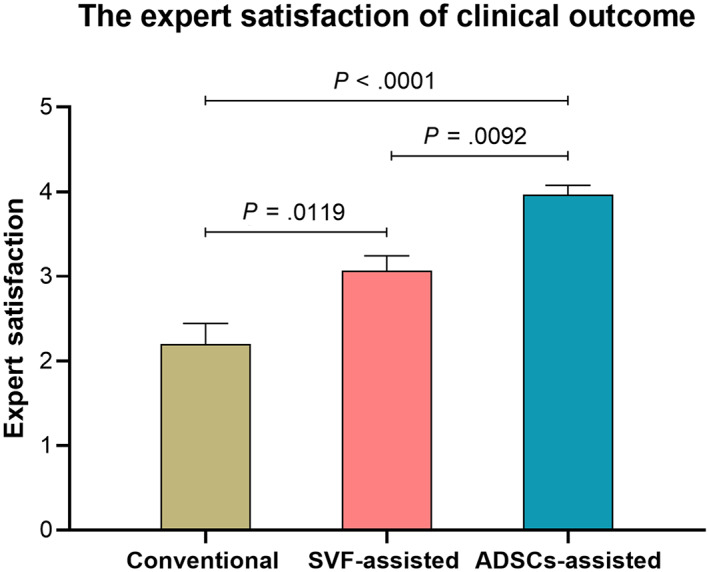
The expert satisfaction on clinical outcome of the three groups
FIGURE 5.
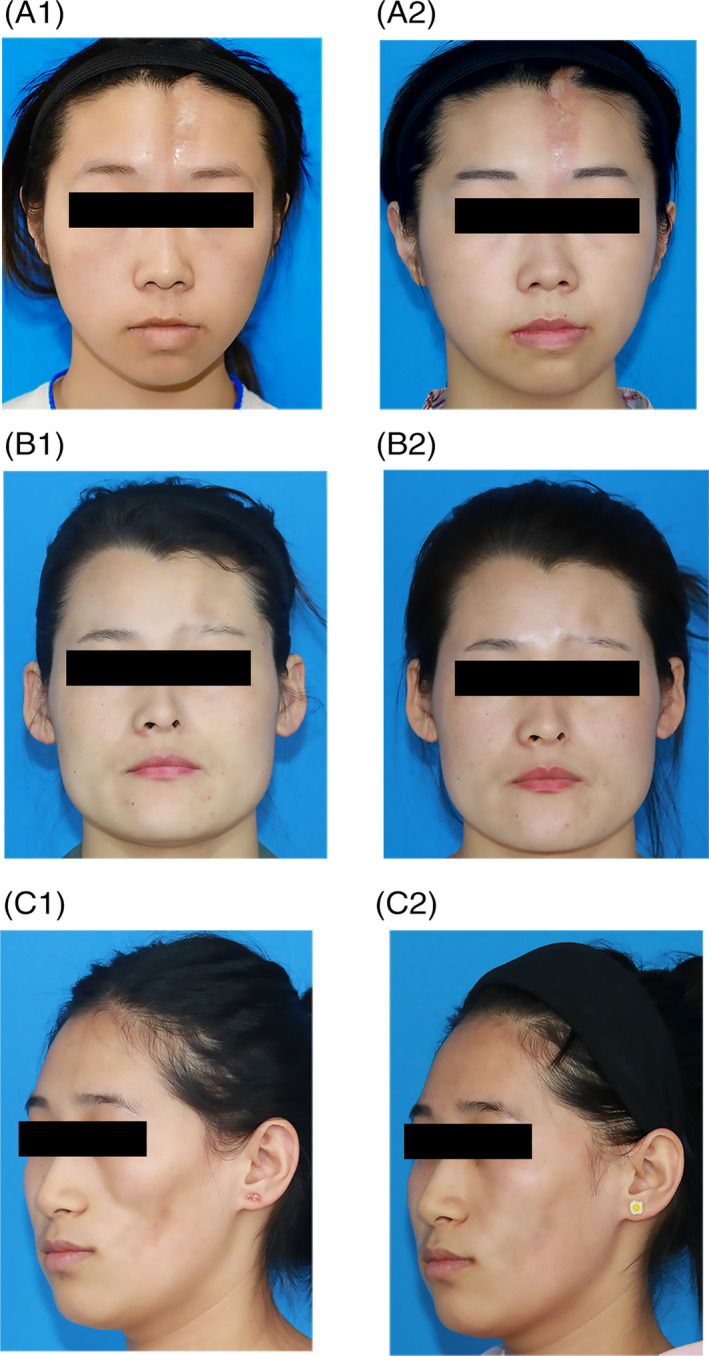
A, A 24‐year‐old female LoS patient underwent ADSCs‐assisted AFG in the forehead (A1, pre‐operation; A2, after 6 months). B, A 25‐year‐old female LoS patient underwent ADSCs‐assisted AFG in the forehead (B1, pre‐operation; B2, after 6 months). C, A 22‐year‐old female LoS patient underwent ADSCs‐assisted AFG in the cheek (C1, pre‐operation; C2, after 6 months)
4. DISCUSSION
LoS is a chronic inflammatory connective tissue disease whose specific etiological factors remain unknown. LoS manifests as various plaques of different shapes and sizes with signs of skin sclerosis and subcutaneous atrophy, whose disorder classically presents benign and self‐limited evolution. 17 Medical treatment can only provide partial clinical improvement (about 60% reported) with side effects such as systemic toxicity, chronic immune suppression, and bleeding, and fails to correct the facial aesthetic impairment that has occurred. 4 , 18 AFG has been widely used to correct the scleroderma‐induced facial atrophy. 4 , 19 , 20 In addition, basic research and clinical practice also showed that AFG has a certain therapeutic effect on scleroderma, including the improvement of skin fibrosis and the reduction of pigmentation. 4 , 21 , 22 , 23 However, what has been found in clinical practice is that the fat retention in scleroderma patients was significantly lower than that in normal people (approximately 25% in scleroderma patients VS 60% in normal people). 4 , 19 The reason for the occurrence of low‐fat retention was thought to be related to the local inflammatory microenvironment, lack of local blood supply induced by vascular injuries, and increased apoptosis of adipocytes. 5 , 15 , 23 Therefore, improving the fat retention in scleroderma patients has become an urgent problem to be solved in clinical research. 19
Our study was the first clinical study using ADSCs‐assisted fat grafting to treat facial atrophy of LoS lesion and comparing the differences in fat graft retentions between ADSCs‐assisted, SVF‐assisted and conventional fat grafting. The results showed that the fat graft retention of ADSCs‐assisted group was significantly higher than that of SVF‐assisted and conventional fat grafting groups. In our study, the ADSC‐assisted group showed notable graft retention over the rest groups at an early stage and exhibited the highest level of graft retention among the three groups in the relatively long run. In comparison, the SVF‐assisted group did not show a statistically significant advantage of graft retention over the conventional group after 3 months. Still, it also presented a relatively long‐term efficacy that the SVF‐assisted group had a higher level of fat retention than the conventional group after 6 months.
ADSCs in the adipose tissue have been considered playing a crucial role in regulating the balance between apoptosis and proliferation of adipocytes in the dynamic remodeling process of the adipose tissue after lipotransfering. 24 Previous studies have revealed that ADSCs can secrete multiple cytokines to prompt vascularization, suppress the apoptosis of adipocytes, and increase fat survival and regeneration. 24 , 25 ADSCs also have the advantage of immunomodulatory effects to limit inflammation and reduce fibrosis in scleroderma model. 26 , 27
SVF obtained from collagenase‐digested adipose tissue comprises multiple heterogeneous cell populations including endothelial cells, hematopoietic cells, pericyte origin cells, and a minor fraction of ADSCs (less than 3%). 28 It is reported that a concentration of approximately 1 × 10 4 ADSCs per mL is achieved with the use of an SVF: fat ratio of 1:1 in healthy people samples, which equals a doubling in the physiological number of ADSCs in adipose tissue of healthy people. 28 , 29 As a method of cell‐assisted lipotransfer, SVF‐assisted fat grafting is regarded a timesaving technique to obtain ADSCs supplementation by collagenase isolation without extra culture. 14 , 29 However, to date, no existing data have convincingly demonstrated that the SVF‐assisted method could stably produce comparable or increased fat graft retention compared with what can be achieved in two sessions of conventional fat grafting. 14 In our study, the fat retention in the SVF‐assisted group increased 45% than that in the conventional group after 6 months. However, a method could not be considered having clinical application value until it can stably improve retention by over 1.5 times. 28
Due to the composition of the SVF, the number and the activity of ADSCs vary greatly among patients, contributing to the unstable outcome of the SVF‐assisted method. Though our previous study compared the biological activity of ADSCs between LoS patients and healthy people, finding no differences in morphology or adipogenic differentiation potential among the isolated ADSC lines, the number of ADSCs in scleroderma patients decreased by 50% of that in healthy people, meaning the number of ADSCs in the scleroderma SVF is also lower than that in healthy people SVF samples. 5 These above factors limit the potential clinical application of the SVF enriched method in the field of treating scleroderma patients.
Ex vivo expanded ADSC‐assisted method not only can obtain enough stem cells but also can identify the biological activity of these cells before transplantation. 30 This advantage makes it more superior in the unique field of correcting facial atrophy of LoS patients. Compared with the fat retention of around 50% from the case series studies in LoS patients, 31 our study's fat retention in conventional fat grafting group of about 20% was low. Because the previous cases studies failed to require the enrolled patients having no history of fat grafting, which would increase the fat retention. Receiving additional rounds of fat grafting would soften the skin, provide improved skin laxity, and extend subcutaneous space, allowing for increased fat retention in the next round. 20 , 32 Though ADSCs‐assisted and SVF‐assisted methods improved the fat retentions in our study, fat retention rates in LoS patients were still lower than that of healthy people, 14 , 22 which means that LoS patients still need multiple rounds of grafting to maintain a relatively ideal appearance. Further modification of the transplantation protocols, such as the increased concentration of ADSCs in fat graft, could be carried to improve fat retention in scleroderma patients.
Due to insufficient understanding of the physiological mechanism of stem cell in human bodies, some clinicians were concerned about the potential complications of stem cell therapy. Our preclinical vivo study has presented that mesenchymal stem cell transplantation had low tumorigenicity risk. 33 We also performed MRI scans 6 months post‐operation, revealing no complications or hazards associated with ADSCs use in LoS patients. As shown in abundant clinical and basic studies on ADSCs with no report of cell mutation or tumor formation, the expansion of passaged ADSC does not affect the differentiation capacity of stem cells and does not confer a cancerous state or capacity in vitro to the cells. 12 , 30 Moreover, it would be beneficial to understand the potential benefits and complications of cell therapy by using novel monitoring methods, such as imaging, to track labeled stem cell products, determining the distribution of infused cells in the body as well as the tendency for migration and homing of transplanted cells. There are good reasons to consider the use of such technology in future clinical trials.
Considering the future clinical application, regulatory‐wise and cost‐wise shall also produce major consequences depending on the choice of method. In the United States and the European Union, the SVF is regarded as a pharmacological product requiring manufacturing authorization which may hamper its easy applicability. The ADSC‐assisted route also faces the same regulatory issues, in addition, costs will increase substantially due to the necessity of expansion in highly specialized GMP facilities. These aspects may eventually largely determine the feasibility and success rate of SVF/ADSC‐assisted AFG optimizations in the long run.
Although this pilot study was with a limited number of participants and relatively brief follow‐up, it showed that the ADSCs‐assisted fat grating was not only safe and well‐tolerated in patients with LoS, but also may be feasible and superior to conventional fat grafting or SVF‐assisted fat grafting in improving facial atrophy of LoS patients. Longer‐term, larger, and controlled clinical trials will be important to confirm the long‐term efficacy of this novel cell therapy of improving fat retention in LoS patients.
5. CONCLUSION
This pilot study suggests that ADSCs‐assisted AFG is a safe, feasible, and attractive alternative to conventional and SVF‐assisted AFG in the correction of facial atrophy of LoS patients. Future studies with large patient samples are needed for confirmation.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
C.W. and X.L.: writing the manuscript, analyzed and interpreted the patient data; L.S.: analyzed and interpreted the patient data; B.C.: design of the work; Y.Z. and T.S.: acquisition of the data; X.Z., R.C.Z., X.W.: conception and design of the work, revised the manuscript.
ETHICS STATEMENT
The study followed the declaration of Helsinki guidelines, approved by the institutional ethical committee (JMLL20180902), and registered in Chinese Clinical Trial Registry (ChiCTR1900025717). Informed consent was obtained from each participant at study entry.
Supporting information
Table S1 Overview of the volume of fat grafted and total number of injected ADSCs in the ADSCs‐assisted AFG group
Figure S1 The exploratory data analysis confirmed that among all the exposure variables, the AFG method was the only variable that affected the outcome variable (fat retention). (red square)
Figure S2 The changes of the subcutaneous adipose tissue of a patient who received ADSCs‐assisted AFG in the cheek lesion (red square) presented by MRI scans. (A, pre‐operation; B, immediate post‐operation; C, after 3 months; D, after 6 months)
ACKNOWLEDGMENTS
We thank Mr. Nan Lin (University of Chinese Academy of Sciences) for the help with the statistical analysis. We thank Prof. Fadi Issa (University of Oxford) and Prof. Qin Han (Chinese Academy of Medical Sciences and Peking Union Medical College) for providing valuable suggestions to improve this manuscript. We thank Dr. Ruijia Dong (Chinese Academy of Medical Sciences and Peking Union Medical College) for drawing the graphical abstract.
Wang C, Long X, Si L, et al. A pilot study on ex vivo expanded autologous adipose‐derived stem cells of improving fat retention in localized scleroderma patients. STEM CELLS Transl Med. 2021;10:1148–1156. 10.1002/sctm.20-0419
Chenyu Wang and Xiao Long contributed equally as co‐first authors.
Xiuqin Zhang, Robert Chunhua Zhao, and Xiaojun Wang contributed equally as senior authors.
Contributor Information
Xiuqin Zhang, Email: zhangxiuqin2712@163.com.
Robert Chunhua Zhao, Email: zhaochunhua@vip.163.com.
Xiaojun Wang, Email: pumchwxj@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90(1):62‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lis‐Swiety A, Skrzypek‐Salamon A, Ranosz‐Janicka I, Brzezinska‐Wcislo L. Associations between disease activity/severity and damage and health‐related quality of life in adult patients with localized scleroderma—a comparison of LoSCAT and visual analogue scales. J Clin Med. 2020;9(3):756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee JH, Lim SY, Lee JH, Ahn HC. Surgical management of localized scleroderma. Arch Craniofac Surg. 2017;18(3):166‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strong AL, Rubin JP, Kozlow JH, Cederna PS. Fat grafting for the treatment of scleroderma. Plast Reconstr Surg. 2019;144(6):1498‐1507. [DOI] [PubMed] [Google Scholar]
- 5. Chen B, Wang X, Long X, et al. Supportive use of adipose‐derived stem cells in cell‐assisted lipotransfer for localized scleroderma. Plast Reconstr Surg. 2018;141(6):1395‐1407. [DOI] [PubMed] [Google Scholar]
- 6. Matsumoto D, Sato K, Gonda K, et al. Cell‐assisted lipotransfer: supportive use of human adipose‐derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12(12):3375‐3382. [DOI] [PubMed] [Google Scholar]
- 7. Maria AT, Maumus M, Le Quellec A, Jorgensen C, Noel D, Guilpain P. Adipose‐derived mesenchymal stem cells in autoimmune disorders: state of the art and perspectives for systemic sclerosis. Clin Rev Allergy Immunol. 2017;52(2):234‐259. [DOI] [PubMed] [Google Scholar]
- 8. Kotani T, Masutani R, Suzuka T, Oda K, Makino S, Ii M. Anti‐inflammatory and anti‐fibrotic effects of intravenous adipose‐derived stem cell transplantation in a mouse model of bleomycin‐induced interstitial pneumonia. Sci Rep. 2017;7(1):14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen B, Cai J, Wei Y, et al. Exosomes are comparable to source adipose stem cells in fat graft retention with up‐regulating early inflammation and angiogenesis. Plast Reconstr Surg. 2019;144(5):816e‐827e. [DOI] [PubMed] [Google Scholar]
- 10. Rasmussen BS, Sorensen CL, Kurbegovic S, et al. Cell‐enriched fat grafting improves graft retention in a porcine model: a dose‐response study of adipose‐derived stem cells versus stromal vascular fraction. Plast Reconstr Surg. 2019;144(3):397e‐408e. [DOI] [PubMed] [Google Scholar]
- 11. Onesti MG, Fioramonti P, Carella S, Fino P, Marchese C, Scuderi N. Improvement of mouth functional disability in systemic sclerosis patients over one year in a trial of fat transplantation versus adipose‐derived stromal cells. Stem Cells Int. 2016;2016:2416192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koh KS, Oh TS, Kim H, et al. Clinical application of human adipose tissue‐derived mesenchymal stem cells in progressive hemifacial atrophy (Parry‐Romberg disease) with microfat grafting techniques using 3‐dimensional computed tomography and 3‐dimensional camera. Ann Plast Surg. 2012;69(3):331‐337. [DOI] [PubMed] [Google Scholar]
- 13. Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue‐derived stromal vascular fraction and culture expanded adipose tissue‐derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiong S, Yi C, Pu LLQ. An overview of principles and new techniques for facial fat grafting. Clin Plast Surg. 2020;47(1):7‐17. [DOI] [PubMed] [Google Scholar]
- 15. Slack GC, Tabit CJ, Allam KA, Kawamoto HK, Bradley JP. Parry‐Romberg reconstruction: beneficial results despite poorer fat take. Ann Plast Surg. 2014;73(3):307‐310. [DOI] [PubMed] [Google Scholar]
- 16. Kolle ST, Duscher D, Taudorf M, et al. Ex vivo‐expanded autologous adipose tissue‐derived stromal cells ensure enhanced fat graft retention in breast augmentation: a randomized controlled clinical trial. Stem Cells Translational Medicine. 2020;9(11):1277‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kreuter A, Krieg T, Worm M, et al. AWMF guideline no. 013/066. Diagnosis and therapy of circumscribed scleroderma. J Dtsch Dermatol Ges. 2009;7(suppl 6):S1‐S14. [DOI] [PubMed] [Google Scholar]
- 18. Noh JW, Kim J, Kim JW. Localized scleroderma: a clinical study at a single center in Korea. Int J Rheum Dis. 2013;16(4):437‐441. [DOI] [PubMed] [Google Scholar]
- 19. Griffin MF, Almadori A, Butler PE. Use of lipotransfer in scleroderma. Aesthet Surg J. 2017;37(suppl 3):S33‐S37. [DOI] [PubMed] [Google Scholar]
- 20. Zanelato TP, Marquesini G, Colpas PT, Magalhaes RF, Moraes AM. Implantation of autologous fat globules in localized scleroderma and idiopathic lipoatrophy—report of five patients. An Bras Dermatol. 2013;88(6 suppl 1):120‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kappos EA, Temp M, Schaefer DJ, Haug M, Kalbermatten DF, Toth BA. Validating facial aesthetic surgery results with the FACE‐Q. Plast Reconstr Surg. 2017;139(4):839‐845. [DOI] [PubMed] [Google Scholar]
- 22. Laloze J, Varin A, Gilhodes J, et al. Cell‐assisted lipotransfer: friend or foe in fat grafting? Systematic review and meta‐analysis. J Tissue Eng Regen Med. 2018;12(2):e1237‐e1250. [DOI] [PubMed] [Google Scholar]
- 23. Chen W, Xia ZK, Zhang MH, et al. Adipose tissue‐derived stem cells ameliorates dermal fibrosis in a mouse model of scleroderma. Asian Pac J Trop Med. 2017;10(1):52‐56. [DOI] [PubMed] [Google Scholar]
- 24. Li Y, Zhang W, Gao J, et al. Adipose tissue‐derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res Ther. 2016;7(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kato H, Mineda K, Eto H, et al. Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg. 2014;133(3):303e‐313e. [DOI] [PubMed] [Google Scholar]
- 26. Mert T, Kurt AH, Arslan M, Celik A, Tugtag B, Akkurt A. Anti‐inflammatory and anti‐nociceptive actions of systemically or locally treated adipose‐derived Mesenchymal stem cells in experimental inflammatory model. Inflammation. 2015;38(3):1302‐1310. [DOI] [PubMed] [Google Scholar]
- 27. Djouad F, Bouffi C, Ghannam S, Noel D, Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol. 2009;5(7):392‐399. [DOI] [PubMed] [Google Scholar]
- 28. Rasmussen BS, Lykke Sorensen C, Vester‐Glowinski PV, et al. Effect, feasibility, and clinical relevance of cell enrichment in large volume fat grafting: a systematic review. Aesthet Surg J. 2017;37(suppl_3):S46‐S58. [DOI] [PubMed] [Google Scholar]
- 29. Olenczak JB, Seaman SA, Lin KY, et al. Effects of collagenase digestion and stromal vascular fraction supplementation on volume retention of fat grafts. Ann Plast Surg. 2017;78(6S suppl 5):S335‐S342. [DOI] [PubMed] [Google Scholar]
- 30. El Atat O, Antonios D, Hilal G, et al. An evaluation of the stemness, paracrine, and tumorigenic characteristics of highly expanded, minimally passaged adipose‐derived stem cells. PLoS One. 2016;11(9):e0162332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roh MR, Jung JY, Chung KY. Autologous fat transplantation for depressed linear scleroderma‐induced facial atrophic scars. Dermatol Surg. 2008;34(12):1659‐1665. [DOI] [PubMed] [Google Scholar]
- 32. Scuderi N, Ceccarelli S, Onesti MG, et al. Human adipose‐derived stromal cells for cell‐based therapies in the treatment of systemic sclerosis. Cell Transplant. 2013;22(5):779‐795. [DOI] [PubMed] [Google Scholar]
- 33. Li HL, Wei JF, Fan LY, et al. miR‐302 regulates pluripotency, teratoma formation and differentiation in stem cells via an AKT1/OCT4‐dependent manner. Cell Death Dis. 2016;7:e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Overview of the volume of fat grafted and total number of injected ADSCs in the ADSCs‐assisted AFG group
Figure S1 The exploratory data analysis confirmed that among all the exposure variables, the AFG method was the only variable that affected the outcome variable (fat retention). (red square)
Figure S2 The changes of the subcutaneous adipose tissue of a patient who received ADSCs‐assisted AFG in the cheek lesion (red square) presented by MRI scans. (A, pre‐operation; B, immediate post‐operation; C, after 3 months; D, after 6 months)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


