Abstract
We previously demonstrated the safety and feasibility of mesenchymal stem cell (MSC) transplantation for bronchopulmonary dysplasia (BPD) in preterm infants in a phase I clinical trial. We thus investigated the therapeutic efficacy of MSCs for BPD in premature infants. A phase II double‐blind, randomized, placebo‐controlled clinical trial was conducted on preterm infants at 23 to 28 gestational weeks (GW) receiving mechanical ventilator support with respiratory deterioration between postnatal days 5 and 14. Infants were stratified by 23 to 24 GW and 25 to 28 GW and randomly allocated (1:1) to receive stem cells (1 × 107 cells/kg, n = 33) or placebo (n = 33). Although the inflammatory cytokines in the tracheal aspirate fluid were significantly reduced with MSCs, the primary outcome of death or severe/moderate BPD in the control group (18/33, 55%) was not significantly improved with MSC transplantation (17/33, 52%). In the subgroup analysis, the secondary outcome of severe BPD was significantly improved from 53% (8/15) to 19% (3/16) with MSC transplantation in the 23 to 24 GW group but not in the 25 to 28 GW subgroup. In summary, although MSC transplantation might be safe and feasible, this small study was underpowered to detect its therapeutic efficacy in preterm infants at 23 to 28 GW. Accordingly, we are now conducting an additional larger and controlled phase II clinical trial focusing on infants at 23 to 24 GW (NCT03392467). ClinicalTrials.gov identifier: NCT01828957.
Keywords: bronchopulmonary dysplasia, cell transplantation, mesenchymal stem cells, premature infants
1. INTRODUCTION
Bronchopulmonary dysplasia (BPD) is a chronic lung disease that develops in premature infants receiving prolonged ventilator and oxygen therapy. 1 Despite recent advances in neonatal intensive care medicine, BPD remains a major cause of mortality and long‐term respiratory and neurologic morbidities in premature infants. A few clinically effective treatment options are available. 2 , 3 New therapeutic strategies are, thus, urgently necessary to improve the prognosis of this serious intractable disorder.
Recently, our group and other research groups have reported that xenotransplantation of mesenchymal stem cells (MSCs) in immunocompetent newborn animals attenuates hyperoxic lung injuries such as impaired alveolarization, oxidative stress, inflammatory responses, increased apoptosis, and fibrosis, all of which simulate BPD in human infants. 4 , 5 , 6 , 7 , 8 , 9 These protective effects of MSCs were cell type‐dependent, showing better protection with umbilical cord blood (UCB)‐derived MSCs than with adult adipose tissue‐ or bone marrow‐derived MSCs 5 , 10 ; time‐dependent, showing better protection with early rather than late transplantation 6 ; dose‐dependent, showing better therapeutic efficacy with larger doses 7 ; and route‐dependent, showing better therapeutic efficacy with local intratracheal than systemic intraperitoneal or intravenous transplantation. 8 , 11 Furthermore, intratracheal transplantation of human UCB‐derived MSCs was proven to be safe and feasible in recently conducted phase I clinical trials 12 , 13 and was not associated with adverse respiratory, growth, or neurodevelopmental effects, as noted during the follow‐up of these infants for up to 2 years. 13 However, therapeutic efficacy of this approach has not been assessed in a clinical setting to date. Here, we report a double‐blind randomized placebo‐controlled phase II clinical trial assessing the therapeutic efficacy of human UCB‐derived MSC transplantation for BPD in extremely preterm infants.
2. MATERIALS AND METHODS
2.1. Study design and participants
This phase II clinical trial was a randomized, double‐blind, placebo‐controlled two‐center trial to assess the therapeutic efficacy of intratracheal transplantation of human UCB‐derived MSCs for BPD in preterm infants conducted between 5 March 2013 and 23 March 2015, at the neonatal intensive care units (NICUs) of Samsung Medical Center (SMC) and Asan Medical Center (AMC), Seoul, Korea. The study protocol was approved by the Korean Food and Drug Administration (no. 11276) and by the institutional review boards (IRBs) of the SMC (IRB no. 2010‐09‐092) and AMC (IRB no. 2013‐0333). External monitoring was independently performed by a contract research organization (Dream CIS Inc, Seoul, Korea). Written informed consent was obtained from both parents of each patient. A detailed study protocol was published at ClinicalTrials.gov (NCT01828957).
Preterm infants with a gestational age of 23 to 28 weeks, weighing 500 to 1250 g at birth, and who were on continuous invasive ventilator support because of respiratory deterioration were included in this study. Respiratory deterioration was defined as clinical signs of respiratory distress, including tachypnea and frequent desaturation (SaO2 < 80%), requiring an increase in ventilator settings within 24 hours before study enrollment during postnatal days 5 to 14. Patients were excluded for severe congenital anomalies, lung hypoplasia, severe septic shock, or severe (grade ≥ 3) intraventricular hemorrhage (IVH). 14
2.2. Randomization
A total of 66 infants were enrolled in the trial; blocked randomization stratified according to gestational weeks (GW) (23‐24 GW, n = 31; 25‐28 GW, n = 35; Figure 1) was used. The MSC transplantation and control groups were assigned in the same ratio (1:1) in one block, and the randomization code table was prepared using SAS (SAS Institute Inc, Cary, North Carolina). Finally, 33 infants were assigned to the control group and 33 infants were assigned to the MSC group for analysis. The parents, hospital and research staff, and medical personnel directly involved in patient care were all blinded to group allocation throughout the study. The study drug was administered by an unblinded medical professional not involved in clinical management of the patients, and the administration was monitored by the other medical staff unaffiliated with the neonatal intensive care unit.
FIGURE 1.
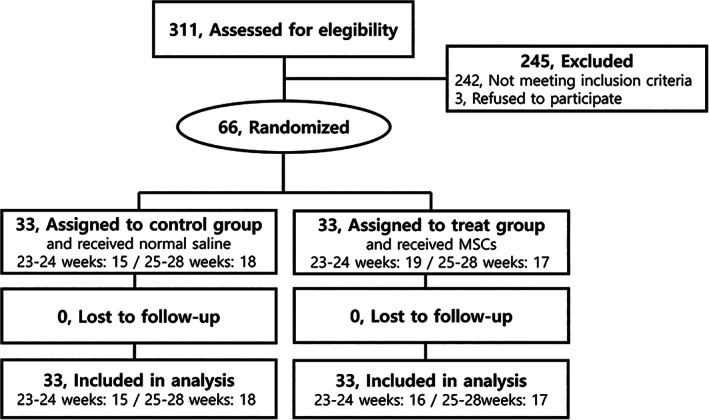
Trial profile. Flow chart of participants and visits during the trial. MSC, mesenchymal stem cell
2.3. Procedures
For the treatment arm, standardized human UCB‐derived MSCs at passage 6, prepared in strict compliance with good manufacturing practices (Pneumostem, Medipost Co, Seoul, Korea), were administered intratracheally via a gavage tube in two fractions into the left and right lungs at a total dose of 1 × 107 cells/kg in 2 cc/kg of normal saline, as previously reported. 12 An equal volume of normal saline (Medipost Co) was administered as the placebo to the control group.
2.4. Outcome measures
All patients were regularly assessed until 6 months after transplantation, and the clinical data were prospectively collected according to the schedule shown in the Supplemental Online Data. Clinical characteristics, including gestational age, birth weight, Apgar scores, sex, small size for gestational age, mode of delivery, antenatal steroid use, patent ductus arteriosus (PDA; defined as clinical signs of symptomatic PDA including heart murmur and wide pulse pressure plus ductal size >1.5 mm by echocardiography), and chorioamnionitis were analyzed. The tracheal aspirate fluid (TAF) was collected before transplantation and 7 days after MSC transplantation or placebo administration for the assessment of changes in the cytokine levels and factors known to be associated with the development or prevention of BPD. The samples were collected only when suctioning was clinically required during routine care; the details are described in the Supplemental Online Data.
Primary outcome measures included death or moderate/severe BPD, which was defined as the need for supplemental oxygen/respiratory support to maintain oxygen saturation >90% at 36 GW. 15
Secondary outcome measures included mild, moderate, and severe BPD or death, pulmonary hemorrhage, duration of assisted respiratory support, oxygen use, hospitalization, postnatal steroid given with respiratory deterioration requiring increased ventilator settings, retinopathy of prematurity (ROP) stage ≥3 16 or requiring treatment, pneumothorax requiring treatment, pulmonary hemorrhage, growth, necrotizing colitis (stage >2b), 17 blood culture proven nosocomial sepsis, and inflammatory cytokines in the TAF.
2.5. Sample size and statistical analysis
The study sample size was determined for assessing the efficacy of MSCs compared with placebo with respect to the primary endpoint in order to decrease the incidence of moderate/severe BPD or death with a two‐sided α level of 0.05% and 80% power. The target enrollment sample size of 70 and the minimum sample size of 62 necessary for assessing validation were calculated based on our previous phase I clinical trial 12 in which a 38% decrease in the incidence of moderate/severe BPD or death was noted in the MSC transplantation group (33%) compared with that in a historical control group (71%). This is compatible with our nationwide Korean Neonatal Network data of the 74% prevalence of death or severe/moderate BPD in infants at 23 to 28 GW receiving invasive ventilator care for more than 14 days in 2013, and also accounted for a potential 10% dropout rate. The analysis plan for the primary endpoint was prespecified and performed with the per‐protocol study population for testing efficacy. The data are expressed as means ± SD. The continuous variables were statistically compared between the groups using Student's t test or the Mann‐Whitney U test. The χ2 test analysis was used for comparing the other nominal variables, and repeated measures analysis of variance was used for the assessment of the temporal profile of BPD biomarkers in the TAF. A value of P < .05 was considered statistically significant. The software package SPSS version 18 (IBM SPSS, Armonk, New York) was used for the aforementioned analyses. The Bayesian method was also used. To estimate the posterior probability of MSC treatment effect, that is, the relative risk (RR), 95% credible interval, and therapeutic benefit with the probability of a posterior RR of <1, Bayesian analysis was performed using SAS software version 14.3. R version 3.6.1 was used for Bayesian calculation of absolute risk reduction using the “neutral” prior of Laptook et al. 18 The prior probability may be skeptical, neutral, or enthusiastic, as follows: (a) skeptical prior, assuming a 30% increase in the risk of death or BPD (RR, 1.30); (b) neutral prior, assuming no MSC treatment effect (RR, 1.0); and (c) enthusiastic prior, assuming a 37% decrease in the risk of death or BPD, where RR is estimated using binomial logistic regression analysis for analyzing the difference between severe BPD or death and moderate BPD and under.
3. RESULTS
3.1. Study population
Sixty‐six infants were enrolled from a pool of 311 preterm infants born at 23 to 28 GW, with a birth weight of 500 to 1250 g, who born and admitted to SMC/AMC NICU between 5 March 2013 and 23 March 2015. Using a blocked randomization method, the enrolled infants were stratified into subgroups based on gestational age (23‐24 GW, n = 31; 25‐28 GW, n = 35), and this study planned to recruit equal numbers of infants into each stratum. Enrolled infants were randomly allocated in a 1:1 ratio to either the MSC transplantation arm or the placebo control arm in each block (16 and 15 in the 23‐24 GW subgroup and 17 and 18 in the 25‐28 GW subgroup, respectively; Figure 1).
3.2. Clinical characteristics
The demographical and clinical characteristics of the infants in each gestational age‐stratified study group are shown in Table 1. Although the age at MSC transplantation showed a delayed tendency (P = .05) in the MSC group (12.1 ± 2.0 postnatal days) compared with the control group (10.3 ± 2.7 postnatal days) among the infants born at 23 to 24 GW, there were no significant differences in the other clinical characteristics, including gestational age, birth weight, and Apgar scores, between the study groups.
TABLE 1.
Clinical characteristics according to the gestational groups
| Characteristic | Total | 23‐24 weeks | 25‐28 weeks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 33) | MSC (n = 33) | P value | Control (n = 15) | MSC (n = 16) | P value | Control (n = 18) | MSC (n = 17) | P value | |
| Gestational age (weeks) | 25.2 ± 1.0 | 25.2 ± 1.2 | .92 | 24.4 ± 0.4 | 24.3 ± 0.4 | .93 | 25.9 ± 0.8 | 26.0 ± 1.0 | .62 |
| Birth weight (g) | 755 ± 152 | 740 ± 153 | .67 | 709 ± 88 | 690 ± 92 | .57 | 793 ± 183 | 786 ± 185 | .92 |
| Apgar score, 1 min | 4.3 ± 1.5 | 3.9 ± 1.6 | .31 | 4.1 ± 1.5 | 4.0 ± 1.5 | .90 | 4.6 ± 1.5 | 3.9 ± 1.8 | .23 |
| Apgar score, 5 min | 6.6 ± 1.5 | 6.4 ± 1.6 | .58 | 6.5 ± 1.5 | 6.3 ± 1.6 | .78 | 6.7 ± 1.5 | 6.4 ± 1.6 | .64 |
| Male | 24/33 (73%) | 17/33 (52%) | .08 | 9/15 (60%) | 6/16 (38%) | .21 | 15/18 (83%) | 11/17 (65%) | .21 |
| Cesarean section | 24/33 (73%) | 27/33 (82%) | .38 | 11/15 (73%) | 12/16 (75%) | .92 | 13/18 (72%) | 15/17 (88%) | .24 |
| SGA | 4/33 (12%) | 5/33 (15%) | .72 | 1/15 (7%) | 0/16 (0%) | .29 | 3/18 (17%) | 5/17 (29%) | .37 |
| Antenatal corticosteroids | 32/33 (97%) | 31/33 (94%) | .54 | 15/15 (100%) | 16/16 (100%) | 17/18 (94%) | 15/17 (88%) | .51 | |
| PIH | 2/33 (6%) | 1/33 (3%) | .56 | 0/15 (0%) | 0/16 (0%) | 2/18 (11%) | 1/17 (6%) | .58 | |
| Chorioamnionitis | 15/32 (47%) | 15/31 (48%) | .91 | 9/15 (60%) | 7/15 (47%) | .46 | 6/17 (35%) | 8/16 (50%) | .39 |
| Early‐onset sepsis | 1/33 (3%) | 3/33 (9%) | .30 | 1/15 (7%) | 2/16 (13%) | .58 | 0/18 (0%) | 0/1 (6%) | .30 |
| PDA | 30/33 (97%) | 28/33 (85%) | .09 | 15/15 (100%) | 15/16 (94%) | .33 | 17/18 (94%) | 13/17 (77%) | .13 |
| Age at MSC transplantation (postnatal days) | 11.4 ± 2.4 | 11.8 ± 2.0 | .51 | 10.3 ± 2.7 | 12.1 ± 2.0 | .05 | 12.3 ± 1.7 | 11.5 ± 2.1 | .22 |
Note: Data are presented as means ± SD or as number and percentage of total.
Abbreviations: MSC, mesenchymal stem cell; PDA, patent ductus arteriosus; PIH, pregnancy‐induced hypertension; SGA, small for gestational age.
3.3. Outcomes
The short‐term outcomes of the infants in each gestational age‐stratified study group are shown in Table 2. The primary outcome of death or severe/moderate BPD in the control group (18/33, 55%) was not significantly improved with MSC transplantation (17/33, 52%). In the subgroup analysis, the secondary outcome of severe BPD was significantly improved from 53% (8/15) to 19% (3/16) with MSC transplantation in the 23 to 24 GW group but not in the 25 to 28 GW subgroup. Other secondary outcomes, including duration of assisted respiratory support and hospitalization; incidence of ROP, pulmonary hemorrhage, and pneumothorax; steroid use for ventilator weaning; and incidence of other major complications associated with prematurity, were not significantly different between the study groups. No serious adverse events related to MSCs were observed until 6 months after transplantation. No newly developed abnormal mass lesions were found on chest radiography, and no significant abnormal results requiring correction were presented in laboratory tests, including complete blood count, glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, alkaline phosphatase, total protein, albumin, blood urea nitrogen, creatinine, and C‐reactive protein, which were checked at 6 months after transplantation in all infants.
TABLE 2.
Short‐term outcomes
| Outcome | Total | Gestational age | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 23‐24 weeks (n = 31) | 25‐28 weeks (n = 35) | ||||||||
| Control (n = 33) | MSC (n = 33) | P value | Control (n = 15) | MSC (n = 16) | P value | Control (n = 18) | MSC (n = 17) | P value | |
| Primary outcome | |||||||||
| Moderate/severe BPD or death, n (%) | 18 (55) | 16 (49) | .62 | 11 (73) | 9 (56) | .32 | 7 (39) | 7 (41) | .89 |
| Secondary outcomes | |||||||||
| Mild BPD, n (%) | 15 (46) | 17 (52) | .62 | 4 (27) | 7 (44) | .32 | 11 (61) | 10 (59) | .89 |
| Mod BPD, n (%) | 3 (9) | 4 (12) | .69 | 2 (13) | 3 (19) | .68 | 1 (6) | 1 (6) | .97 |
| Severe BPD, n (%) | 14 (42) | 9 (27) | .20 | 8 (53) | 3 (19) | .04 | 6 (33) | 6 (35) | .90 |
| Death at discharge, n (%) | 1 (3) | 3 (9) | .30 | 1 (6) | 3 (19) | .32 | 0 (0) | 0 (0) | |
| Severe BPD or death, n (%) | 15 (46) | 12 (36) | .45 | 9 (60) | 6 (38) | .21 | 6 (33) | 6 (36) | .90 |
| Respiratory support duration, median (IQR), days | |||||||||
| Intubation | 23 ± 17 | 24 ± 12 | .75 | 27 ± 12 | 24 ± 13 | .54 | 19 ± 19 | 24 ± 12 | .39 |
| Nasal CPAP | 36 ± 20 | 31 ± 15 | .26 | 39 ± 10 | 34 ± 11 | .27 | 34 ± 25 | 28 ± 17 | .48 |
| Total ventilator | 59 ± 29 | 55 ± 19 | .55 | 66 ± 15 | 59 ± 21 | .27 | 53 ± 36 | 52 ± 18 | .97 |
| Oxygen duration | 69 ± 35 | 68 ± 28 | .88 | 77 ± 22 | 71 ± 29 | .54 | 63 ± 42 | 66 ± 29 | .82 |
| Postnatal steroid, n (%) | 23 (70) | 23 (70) | 11 (73) | 11 (69) | .78 | 12 (67) | 12 (71) | .80 | |
| ROP, n (%) | |||||||||
| Grade ≥3 | 13 (39) | 7/30 (23) | .17 | 8 (53) | 4/13 (31) | .23 | 5 (28) | 3 (18) | .48 |
| Treatment required | 11/32 (34) | 8/30 (27) | .51 | 7/14 (50) | 4/13 (31) | .31 | 4 (22) | 4 (24) | .93 |
| Pneumothorax, n (%) | 3 (9) | 1 (3) | .30 | 2 (13) | 1 (6) | .51 | 1 (6) | 0 (0) | .32 |
| Pneumonia, n (%) | 8 (24) | 12 (36) | .28 | 4 (27) | 5 (31) | .78 | 4 (22) | 7 (41) | .23 |
| Pulmonary hypertension, n (%) | 1 (3) | 0 (0) | .31 | 0 (0) | 0 (0) | 1 (6) | 0 (0) | .32 | |
| Pulmonary hemorrhage, n (%) | 2 (6) | 1 (3) | .56 | 0 (0) | 0 (0) | 2 (11) | 1 (6) | .58 | |
| Late‐onset sepsis, n (%) | 3 (9) | 6 (18) | .28 | 1 (7) | 3 (19) | .32 | 2 (11) | 3 (18) | .58 |
| NEC (stage ≥2b), n (%) | 5 (15) | 3 (9) | .45 | 5 (33) | 1 (6) | .60 | 0 (0) | 2 (12) | .13 |
| Hospitalization duration, mean (SD), days | 107 ± 24 | 108 ± 28 | .91 | 108 ± 14 | 106 ± 30 | .80 | 107 ± 31 | 110 ± 26 | .75 |
Note: Data are presented as median ± interquartile range or as number and percentage of total.
Abbreviations: BPD, bronchopulmonary dysplasia, CPAP, continuous positive airway pressure; IQR, interquartile range; MSC, mesenchymal stem cell; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity.
3.4. Cause of death
Death before discharge was observed in 7% (1/15) of the patients in the control group and 19% (3/16) of the patients in the MSC group; this was noted among the infants born at 23 to 24 GW but not for those born at 25 to 28 GW (Table 2). One infant in the control group was extubated at postnatal day 56 and died of septic shock while on continuous positive airway pressure (CPAP) support. Two infants in the MSC group also died of septic shock at postnatal days 26 and 59 while on mechanical ventilation. One infant in the MSC group was extubated at postnatal day 23 and died of intestinal perforation because of fulminant necrotizing colitis while on CPAP support.
3.5. Bayesian analyses
Bayesian analysis using a neutral prior indicated a 59% posterior probability of reduced incidence of death or moderate/severe BPD, with an estimated posterior risk ratio (PRR) of 0.97 (95% credible interval, 0.58‐1.41) and 75% posterior probability of reduced incidence of death or severe BPD with an estimated PRR of 0.86 (95% credible interval, 0.44‐1.33; Table 3). The posterior probability of reduced death or moderate/severe BPD was 64% and 56%, and probability of reduced death or severe BPD was 79% and 72%, respectively, when using enthusiastic prior or skeptical prior. Overall, the decrease in the incidence of severe BPD or death showed a higher probability of therapeutic benefit than a decrease in the incidence of moderate/severe BPD or death. In addition, this probability of therapeutic benefit was more prominent in the 23 to 24 GW group than in the 25 to 28 GW group.
TABLE 3.
Bayesian analysis for mesenchymal stem cells benefit in reduction of death or BPD
| Outcome | Enthusiastic prior (RR, 0.74) | Neutral prior (RR, 1.0) | Skeptical prior (RR, 1.10) | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | P‐TB, % | RR (95% CI) | P‐TB, % | RR (95% CI) | P‐TB, % | |
| Moderate/severe BPD or death | ||||||
| 23‐24 weeks | 0.80 (0.42‐1.21) | 84 | 0.83 (0.44‐1.26) | 81 | 0.84 (0.46‐1.29) | 78 |
| 25‐28 weeks | 1.12 (0.49‐1.91) | 42 | 1.20 (0.49‐2.02) | 35 | 1.24 (0.52‐2.08) | 31 |
| Total | 0.94 (0.55‐1.35) | 64 | 0.97 (0.58‐1.41) | 59 | 0.98 (0.58‐1.42) | 56 |
| Severe BPD or death | ||||||
| 23‐24 weeks | 0.71 (0.29‐1.16) | 89 | 0.75 (0.33‐1.23) | 85 | 0.77 (0.34‐1.28) | 83 |
| 25‐28 weeks | 1.30 (0.35‐2.51) | 55 | 1.09 (0.39‐1.91) | 47 | 1.14 (0.42‐2.03) | 42 |
| Total | 0.82 (0.42‐1.28) | 79 | 0.86 (0.44‐1.33) | 75 | 0.88 (0.45‐1.36) | 72 |
Abbreviations: BPD, bronchopulmonary dysplasia; CI, Bayesian credible interval; P‐TB, posterior probability of treatment benefit; RR, posterior risk ratio estimate.
Expressed as an absolute risk difference, using a neutral prior, the posterior probability that death or severe BPD in MSC group was at least 1%, 2%, or 3% less than control group was 70%, 67%, and 63%, respectively. A 2% absolute risk difference was associated with a 2.4‐fold (67%/27%) higher probability of reduced, compared with increased death or severe BPD among MSC group infants relative to control group infants, assuming a range of risk differences viewed as equivalent.
3.6. Temporal profiles of cytokines and growth factors from TAF
The temporal profiles of cytokines and growth factors from the TAF are shown in Figure 2. Whereas the levels of the cytokines and growth factors at baseline showed no significant differences between the study groups, the levels of inflammatory cytokines, such as interleukin (IL)‐1β, IL‐6, IL‐8, tumor necrosis factor‐α, and matrix metalloproteinase‐9, at post‐transplantation day 7 were significantly lower in the MSC group than in the control group.
FIGURE 2.
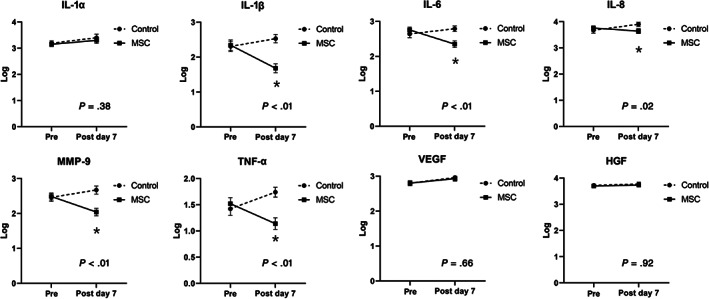
Temporal changes in levels of cytokines and growth factors from tracheal aspirate fluid assessed by enzyme‐linked immunosorbent assay in control group and MSC group. Tracheal aspirate fluid was collected before transplantation and at 7 days after transplantation and IL‐1 α/β, IL‐6, IL‐8, MMP‐9, TNF‐α, VEGF, and HGF were measured. The lines depict mean log‐transformation levels of cytokines and growth factors and error bars depict SEM for each group. At baseline, before stem cells or placebo transplantation, no difference in levels of cytokines and growth factors was observed between two groups. At 7 days after transplantation, MSC group showed significantly decreased levels of inflammatory cytokines of IL‐β, IL‐6, IL‐8, MMP‐9, and TNF‐α, when compared with control group. *Values of P for between‐group comparisons computed with the repeated measures analysis of variance were <.05. HGF, hepatocyte growth factor; IL, interleukin; MMP‐9, matrix metalloproteinase‐9; MSC, mesenchymal stem cell; TNF‐α, tumor necrosis factor‐α; VEGF, vascular endothelial growth factor
4. DISCUSSION
We performed a double‐blind randomized placebo‐controlled phase II clinical trial to assess the safety and therapeutic efficacy of MSC transplantation for BPD in extremely premature infants. We were unable to find previous randomized human studies evaluating the therapeutic efficacy of MSC transplantation for BPD in preterm infants.
In the present study, although intratracheal transplantation of MSCs in preterm infants was safe and feasible, this small study was underpowered to detect its statistically significant therapeutic efficacy for improving the primary outcome of death or severe/moderate BPD in preterm infants at 23 to 28 GW. The underpower of this study might be primarily attributable to the much lower 32% rate of the primary outcome (especially in infants at 25‐28 GW) than the 71% expected rate of the baseline primary outcome in the control group, and to the implausibly large 38% treatment effect size used for calculating the sample size of this trial. Nonetheless, considering the paucity of available clinical data on the therapeutic efficacy of MSC transplantation, the reduced tendency of the primary outcome from 73% to 56% with MSC transplantation observed in the 23 to 24 GW subgroup may provide valuable information for future clinical trials with a larger sample size.
In this study, the gestational age cutoffs of 23 to 24 GW and 25 to 28 GW were used for the strata based on our previous institutional and nationwide Korean Neonatal Network data showing significant differences in mortality and the prevalence of at least moderate BPD especially between the 23 to 24 GW and 25 to 28 GW subgroups. 19 , 20 The effect size of Pearson's correlation was −0.331, indicating medium strength of the relationship between gestational age and the primary outcome of moderate/severe BPD or death. In subgroup analysis, the secondary outcome of severe BPD was significantly improved from 53% (8/15) to 19% (3/16) with MSC transplantation in the 23 to 24 GW group but not in the 25 to 28 GW subgroup. The Bayesian analysis using a neutral prior indicated an 81% posterior probability of reduced primary outcomes with stem cells relative to the placebo in the 23 to 24 GW subgroup. Accordingly, we are now conducting an additional larger controlled phase II clinical trial (NCT03392467) focusing on the most immature infants born at 23 to 24 GW who are at the highest risk of BPD or death.
Although male sex showed a tendency to be higher in the control group than in the MSC group, it was not associated with increased risk of BPD on multivariate analysis. The higher death rate in the MSC group than in the control group occurred only in the 23 to 24 and not in the 25 to 28 GW subgroup. This rate is much lower than the death rate of our institution and nationwide data, 21 , 22 suggesting that mortality might be associated with gestational age rather than MSC transplantation. Although a physiologic test of BPD was not used to define its severity in this study, we plan to use this test in a future clinical trial as it could improve the precision definition of BPD by minimizing variations in clinical oxygen use.
As the antibacterial effects of MSCs were observed in our previous animal studies, 23 , 24 sepsis itself was not a contraindication for enrollment in this study. For the timing of sepsis diagnosis, two cases in the MSC transplantation 23 to 24 GW subgroup were culture‐positive on routine blood culture performed 1 day after transplantation. For etiology, one infant grew Acinetobacter baumannii, the same bacteria grown on skin and tracheal aspirate culture performed before transplantation. Another infant who developed hyperglycemia and aggravation of respiratory distress 1 day before transplantation grew Staphylococcus aureus. Considering the much lower incidence of sepsis than that observed in our previous study, 25 the trend for more sepsis in the MSC transplantation 23 to 24 GW subgroup might be attributable to lower gestational age rather than MSC transplantation.
Death or BPD are difficult to predict in the first week of an infant's life 26 ; hence, biomarkers might serve to aid early prediction of adverse outcomes beyond the rather crude tools of gestational age and the need for ventilation. 20 Previously, using an animal model, we demonstrated that inflammation mediated by proinflammatory cytokines played a seminal role in the development of BPD 4 , 6 , 7 , 8 and that the protection offered by MSCs against neonatal hyperoxic lung injury was primarily mediated by their paracrine anti‐inflammatory, antioxidative, and antifibrotic effects rather than by the regenerative capacity of the MSCs. 4 , 6 , 8 Furthermore, in concordance with our phase I clinical trial, 12 the levels of inflammatory cytokines, such as IL‐1β, IL‐6, and IL‐8, tumor necrosis factor‐α, and matrix metalloproteinase‐9, in the TAF were significantly reduced on post‐MSC transplantation day 7 compared with those in the control group in this study. However, owing to our small sample size and lack of correlation with the clinical outcomes, our results pertaining to the biomarkers should be interpreted cautiously. Further studies are warranted to clarify whether any biomarker in the TAF could be used to predict death, severe BPD, and therapeutic efficacy of the MSCs.
Choosing the optimal MSCs for transplantation is a critical issue for successful clinical translation of stem cell therapy in preterm infants. UCB‐derived MSCs exhibit several advantages over adult tissue‐derived MSCs, including lower immunogenicity, 10 , 27 higher proliferation capacity, paracrine potency, and therapeutic efficacy both in vitro 5 , 28 , 29 and in vivo. 5 Moreover, allogenic transplantation of MSCs might have a logistic advantage as they are ready to use “off the shelf” in the clinical setting. In the present study, we accordingly used the same clinical grade, standardized allogenic human UCB‐derived MSCs, manufactured in strict compliance with the criteria for good manufacturing practice, from passage 6, as those used in previous phase I clinical trials for BPD 12 and severe IVH. 30
Determining the optimal route for MSC transplantation is another important consideration. We previously demonstrated that the efficacy of local intratracheal transplantation of the MSCs is fourfold higher than that of systemic intravenous or intraperitoneal administration for protecting against hyperoxic lung injury in newborn rats, although the two routes are not mutually exclusive. 8 , 11 In the present and previous studies, 12 the MSCs were transplanted intratracheally in two fractions using the same method as that used for administering the exogenous surfactant, without any clinical instability or complications associated with MSC transplantation, suggesting that intratracheal administration of MSCs is safe and feasible. For intubated preterm infants receiving mechanical ventilation, additional procedures are not required for local administration. However, in a meta‐analysis of MSCs in BPD, Augustine et al suggested that the intravenous route might be more efficacious than intratracheal administration of MSCs. 31 Further studies are necessary to clarify this issue. Moreover, considering the recent increase in the use of noninvasive ventilation and less invasive techniques for surfactant administration, 32 the feasibility of a new mode of MSC administration without intubation is another important issue that needs to be addressed in future clinical trials.
The major limitations of the present study include the small sample size, which was inadequate to attain statistically significant results. This was primarily due to the recently reduced incidence of BPD or death, even in the 25 to 28 GW subgroup of the control group, owing to improvements in neonatal intensive care medicine. These improvements limited the power of the study to detect small but important differences in the therapeutic efficacy of MSC transplantation according to the stratified GW subgroups, although the sample size was calculated appropriately. Accordingly, we are now conducting an additional larger and controlled phase II clinical trial (NCT03392467) focusing instead on the most immature infants born at 23 to 24 GW, who are at the highest risk of developing BPD or of death. Nevertheless, the prospective double‐blind randomized placebo‐controlled design of the trial may be a strength of this phase II clinical trial. Another limitation might be the ability to detect safety concerns when fewer than 40 infants received the intervention. Other limitations include the lack of long‐term respiratory and developmental outcome data. As we have just completed the 5‐year long‐term follow‐up of these infants, we hope to publish these data in the near future.
5. CONCLUSION
Although intratracheal transplantation of MSCs might be safe and feasible, it did not significantly improve the primary outcome of death or severe/moderate BPD in preterm infants at 23 to 28 GW. In subgroup analysis, the secondary outcome of severe BPD was significantly improved with MSC transplantation in the 23 to 24 GW group but not in the 25 to 28 GW subgroup. Accordingly, we are now conducting an additional larger and controlled phase II clinical trial, focusing on the most immature infants at 23 to 24 GW, who are at the highest risk of BPD or death (NCT03392467).
CONFLICT OF INTEREST
Samsung Medical Center and Medipost Co, Ltd, have issued or filed patents for “Method of treating lung diseases using cells separated or proliferated from umbilical cord blood” under the names of Y.S.C. and W.S.P. The relevant application number is PCT/KR2007/000535. The other authors indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
S.Y.A., Y.S.C., A.‐R.K., W.S.P.: conception/design, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript; M.H.L., S.I.S., B.S.L., K.S.K.: administrative support, collection and/or assembly of data, data analysis interpretation, manuscript writing, final approval of manuscript.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
We are grateful to Myoung Eun Lee, Clinical Research Coordinator, for her sincere assistance in administrative support, data collection, and management. This work was funded by a grant from the Korean Health and Medical Technology R&D Program, Ministry for Health, Welfare, and Family Affairs, Republic of Korea (HI12C1821). Human umbilical cord blood‐derived mesenchymal stem cells were supplied by Medipost Co, Ltd. The sponsor had no involvement in study design, the collection, analysis, or interpretation of data, writing of the report, or the decision to submit the manuscript for publication.
Ahn SY, Chang YS, Lee MH, et al. Stem cells for bronchopulmonary dysplasia in preterm infants: A randomized controlled phase II trial. STEM CELLS Transl Med. 2021;10:1129–1137. 10.1002/sctm.20-0330
So Yoon Ahn and Yun Sil Chang contributed equally as co‐first authors.
Funding information Korean Health and Medical Technology R&D Program, Ministry for Health, Welfare, and Family Affairs, Republic of Korea, Grant/Award Number: HI12C1821
Contributor Information
Ai‐Rhan Kim, Email: arkim@amc.seoul.kr.
Won Soon Park, Email: wonspark@skku.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
REFERENCES
- 1. Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946‐1955. [DOI] [PubMed] [Google Scholar]
- 2. Horbar JD, Badger GJ, Carpenter JH, et al. Trends in mortality and morbidity for very low birth weight infants, 1991‐1999. Pediatrics. 2002;110:143‐151. [DOI] [PubMed] [Google Scholar]
- 3. Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahn SY, Chang YS, Kim SY, et al. Long‐term (postnatal day 70) outcome and safety of intratracheal transplantation of human umbilical cord blood‐derived mesenchymal stem cells in neonatal hyperoxic lung injury. Yonsei Med J. 2013;54:416‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahn SY, Chang YS, Sung DK, et al. Cell type‐dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury. Cytotherapy. 2015;17:1025‐1035. [DOI] [PubMed] [Google Scholar]
- 6. Chang YS, Choi SJ, Ahn SY, et al. Timing of umbilical cord blood derived mesenchymal stem cells transplantation determines therapeutic efficacy in the neonatal hyperoxic lung injury. PLoS One. 2013;8:e52419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang YS, Choi SJ, Sung DK, et al. Intratracheal transplantation of human umbilical cord blood‐derived mesenchymal stem cells dose‐dependently attenuates hyperoxia‐induced lung injury in neonatal rats. Cell Transplant. 2011;20:1843‐1854. [DOI] [PubMed] [Google Scholar]
- 8. Chang YS, Oh W, Choi SJ, et al. Human umbilical cord blood‐derived mesenchymal stem cells attenuate hyperoxia‐induced lung injury in neonatal rats. Cell Transplant. 2009;18:869‐886. [DOI] [PubMed] [Google Scholar]
- 9. Sutsko RP, Young KC, Ribeiro A, et al. Long‐term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia‐induced lung injury. Pediatr Res. 2013;73:46‐53. [DOI] [PubMed] [Google Scholar]
- 10. Jin HJ, Bae YK, Kim M, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14:17986‐18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sung DK, Chang YS, Ahn SY, et al. Optimal route for human umbilical cord blood‐derived mesenchymal stem cell transplantation to protect against neonatal hyperoxic lung injury: gene expression profiles and histopathology. PLoS One. 2015;10:e0135574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose‐escalation clinical trial. J Pediatr. 2014;164:966‐972.e966. [DOI] [PubMed] [Google Scholar]
- 13. Ahn SY, Chang YS, Kim JH, et al. Two‐year follow‐up outcomes of premature infants enrolled in the phase I trial of mesenchymal stem cells transplantation for bronchopulmonary dysplasia. J Pediatr. 2017;185:49‐54.e2. [DOI] [PubMed] [Google Scholar]
- 14. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529‐534. [DOI] [PubMed] [Google Scholar]
- 15. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723‐1729. [DOI] [PubMed] [Google Scholar]
- 16. An international classification of retinopathy of prematurity. II. The classification of retinal detachment. The International Committee for the Classification of the Late Stages of Retinopathy of Prematurity. Arch Ophthalmol. 1987;105:906‐912. [PubMed] [Google Scholar]
- 17. Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laptook AR, Shankaran S, Tyson JE, et al. Effect of therapeutic hypothermia initiated after 6 hours of age on death or disability among newborns with hypoxic‐ischemic encephalopathy: a randomized clinical trial. JAMA. 2017;318:1550‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim JK, Chang YS, Sung S, Park WS. Mortality rate‐dependent variations in the survival without major morbidities rate of extremely preterm infants. Sci Rep. 2019;9:7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim JK, Chang YS, Sung S, Ahn SY, Yoo HS, Park WS. Trends in survival and incidence of bronchopulmonary dysplasia in extremely preterm infants at 23‐26 weeks gestation. J Korean Med Sci. 2016;31:423‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park JH, Chang YS, Sung S, Ahn SY, Park WS. Trends in overall mortality, and timing and cause of death among extremely preterm infants near the limit of viability. PLoS One. 2017;12:e0170220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JK, Hwang JH, Lee MH, Chang YS, Park WS. Mortality rate‐dependent variations in antenatal corticosteroid‐associated outcomes in very low birth weight infants with 23‐34 weeks of gestation: a nationwide cohort study. PLoS One. 2020;15:e0240168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sung DK, Chang YS, Sung SI, Yoo HS, Ahn SY, Park WS. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta‐defensin‐2 via toll‐like receptor 4 signalling. Cell Microbiol. 2016;18:424‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahn SY, Chang YS, Kim YE, Sung SI, Sung DK, Park WS. Mesenchymal stem cells transplantation attenuates brain injury and enhances bacterial clearance in Escherichia coli meningitis in newborn rats. Pediatr Res. 2018;84:778‐785. [DOI] [PubMed] [Google Scholar]
- 25. Kim JK, Chang YS, Sung S, Ahn SY, Park WS. Trends in the incidence and associated factors of late‐onset sepsis associated with improved survival in extremely preterm infants born at 23‐26 weeks' gestation: a retrospective study. BMC Pediatr. 2018;18:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laughon MM, Langer JC, Bose CL, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715‐1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rocha V, Wagner JE Jr, Sobocinski KA, et al. Graft‐versus‐host disease in children who have received a cord‐blood or bone marrow transplant from an HLA‐identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342:1846‐1854. [DOI] [PubMed] [Google Scholar]
- 28. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294‐1301. [DOI] [PubMed] [Google Scholar]
- 29. Yang SE, Ha CW, Jung M, et al. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004;6:476‐486. [DOI] [PubMed] [Google Scholar]
- 30. Ahn SY, Chang YS, Sung SI, Park WS. Mesenchymal stem cells for severe intraventricular hemorrhage in preterm infants: phase I dose‐escalation clinical trial. Stem Cells Translational Medicine. 2018;7:847‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Augustine S, Avey MT, Harrison B, et al. Mesenchymal stromal cell therapy in bronchopulmonary dysplasia: systematic review and meta‐analysis of preclinical studies. Stem Cells Translational Medicine. 2017;6:2079‐2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klebermass‐Schrehof K, Wald M, Schwindt J, et al. Less invasive surfactant administration in extremely preterm infants: impact on mortality and morbidity. Neonatology. 2013;103:252‐258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.


