Abstract
Quorum sensing is a chemical communication process that bacteria use to coordinate group behaviors. In the global pathogen Vibrio cholerae, one quorum-sensing receptor and transcription factor, called VqmA (VqmAVc), activates expression of the vqmR gene encoding the small regulatory RNA VqmR, which represses genes involved in virulence and biofilm formation. Vibriophage VP882 encodes a VqmA homolog called VqmAPhage that activates transcription of the phage gene qtip, and Qtip launches the phage lytic program. Curiously, VqmAPhage can activate vqmR expression but VqmAVc cannot activate expression of qtip. Here, we investigate the mechanism underlying this asymmetry. We find that promoter selectivity is driven by each VqmA DNA-binding domain and key DNA sequences in the vqmR and qtip promoters are required to maintain specificity. A protein sequence-guided mutagenesis approach revealed that the residue E194 of VqmAPhage and A192, the equivalent residue in VqmAVc, in the helix-turn-helix motifs contribute to promoter-binding specificity. A genetic screen to identify VqmAPhage mutants that are incapable of binding the qtip promoter but maintain binding to the vqmR promoter delivered additional VqmAPhage residues located immediately C-terminal to the helix-turn-helix motif as required for binding the qtip promoter. Surprisingly, these residues are conserved between VqmAPhage and VqmAVc. A second, targeted genetic screen revealed a region located in the VqmAVc DNA-binding domain that is necessary to prevent VqmAVc from binding the qtip promoter, thus restricting DNA binding to the vqmR promoter. We propose that the VqmAVc helix-turn-helix motif and the C-terminal flanking residues function together to prohibit VqmAVc from binding the qtip promoter.
Author summary
Bacteria use a chemical communication process called quorum sensing (QS) to orchestrate collective behaviors. Recent studies demonstrate that bacteria-infecting viruses, called phages, also employ chemical communication to regulate collective activities. Phages can encode virus-specific QS-like systems, or they can harbor genes encoding QS components resembling those of bacteria. The latter arrangement suggests the potential for chemical communication across domains, i.e., between bacteria and phages. Ramifications stemming from such cross-domain communication are not understood. Phage VP882 infects the global pathogen Vibrio cholerae, and “eavesdrops” on V. cholerae QS to optimize the timing of its transition from existing as a parasite to killing the host, and moreover, to manipulate V. cholerae biology. To accomplish these feats, phage VP882 relies on VqmAPhage, the phage-encoded homolog of the V. cholerae VqmAVc QS receptor and transcription factor. VqmAVc, by contrast, is constrained to the control of only V. cholerae genes and is incapable of regulating phage biology. Here, we discover the molecular mechanism underpinning the asymmetric transcriptional preferences of the phage-encoded and bacteria-encoded VqmA proteins. We demonstrate how VqmA transcriptional regulation is crucial to the survival and persistence of both the pathogen V. cholerae, and the phage that preys on it.
Introduction
Quorum sensing (QS) is a cell-cell communication process that allows bacteria to coordinate collective behaviors [1]. QS relies on the production, release, and group-wide detection of extracellular signaling molecules called autoinducers (AIs). In the global pathogen Vibrio cholerae, the AI, 3,5-dimethyl-pyrazin-2-ol (DPO), together with its partner cytoplasmic QS receptor and transcription factor, VqmA (VqmAVc), comprises one of the QS circuits that controls group behaviors [2–4]. VqmAVc, following binding to DPO, activates transcription of the vqmR gene encoding the small RNA, VqmR, which, in turn, represses the expression of genes required for biofilm formation and virulence factor production [2–4].
Recently, bacteria-specific viruses, called phages, have been shown to engage in density-dependent regulation of their lysis-lysogeny decisions via chemical dialogs [5,6]. Germane to our studies are phages that encode proteins resembling bacterial QS components [5,7]. Vibriophage VP882 is one such phage: It encodes the QS receptor VqmA (VqmAPhage), a homolog of the V. cholerae QS receptor VqmAVc [5]. VqmAPhage, like VqmAVc, binds host-produced DPO. DPO-bound VqmAPhage activates transcription of the phage gene qtip. Qtip is an antirepressor that sequesters the phage VP882 repressor of lysis, leading to derepression of the phage lytic program and killing of the Vibrio host at high cell density [5,8]. Thus, the DPO AI mediates both bacterial and phage lifestyle decisions. Curiously, VqmAPhage can substitute for VqmAVc to activate the V. cholerae vqmR promoter (PvqmR) [5]. In contrast, VqmAVc cannot substitute for VqmAPhage and recognize the phage VP882 qtip promoter (Pqtip). Presumably, the ability of VqmAPhage to bind both PvqmR and Pqtip provides phage VP882 the capacity to influence host QS and simultaneously enact its own lysis-lysogeny decision.
VqmAPhage shares ~43% amino acid sequence identity with VqmAVc, and most of the key residues required for ligand and DNA binding are conserved [5,9]. Thus, how VqmAPhage can recognize two different promoters, while VqmAVc cannot, is not understood. Here, we define the mechanism underlying this asymmetry. We show that VqmA selectivity for target promoters is driven by the DNA-binding domain (DBD) of the respective protein. We identify 6 key nucleotides within PvqmR and Pqtip that contribute to VqmA promoter-binding selectivity, as exchanging these critical DNA sequences inverts the DNA-binding preferences of the two VqmA proteins. The 192nd and 194th residues in VqmAVc and VqmAPhage, respectively, within the helix-turn-helix (HTH) motifs, contribute to promoter-binding specificity. Isolation of VqmAPhage mutants capable of activating vqmR expression but incapable of activating qtip expression revealed conserved or functionally conserved residues in VqmAPhage and VqmAVc, indicating that VqmAVc likely possesses an additional feature that prevents it from binding Pqtip DNA. A mosaic VqmAVc protein containing the VqmAPhage HTH motif along with the C-terminal 25 flanking VqmAPhage residues was capable of binding Pqtip. Thus, the two corresponding regions in VqmAVc must function in concert to prevent VqmAVc from binding to Pqtip. Together, our analyses demonstrate how VqmAPhage, via its promiscuous DNA-binding activity, can control phage VP882 functions and drive host V. cholerae QS. Moreover, we discover why V. cholerae VqmAVc cannot do the reverse, as its DNA binding is strictly constrained to the host V. cholerae genome.
Results
VqmA promoter-binding selectivity is conferred by the DNA-binding domain
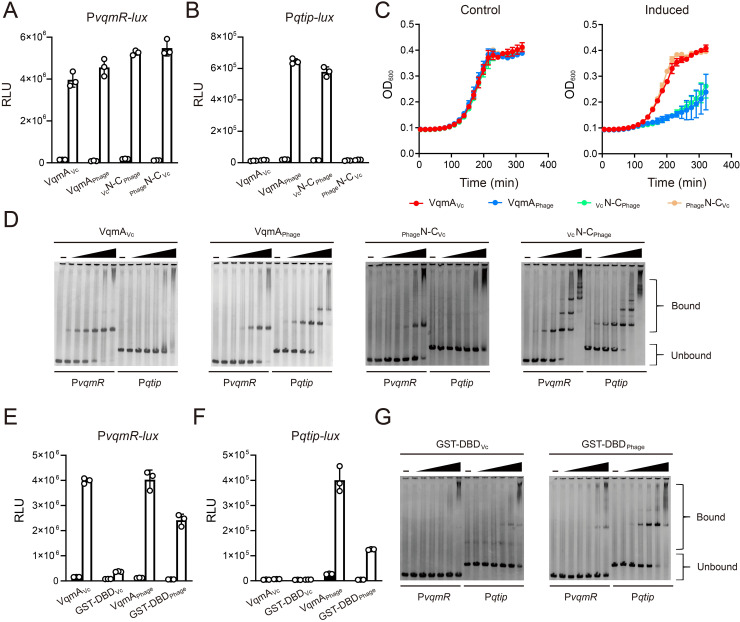
VqmA proteins are composed of N-terminal Per-Arnt-Sim (PAS) domains responsible for binding the DPO AI and C-terminal DBDs containing HTH motifs [10]. Both VqmAVc and VqmAPhage bind DPO. By contrast, with respect to DNA binding, VqmAPhage binds to Pqtip and PvqmR, whereas VqmAVc only binds to PvqmR [5]. We reasoned that this asymmetric DNA-binding pattern arises from differences in the DBDs (S1 Fig). To test this idea, we constructed chimeras in which we exchanged the VqmAVc and VqmAPhage C-terminal domains to produce VcN-CPhage and PhageN-CVc proteins. We chose to make the junction at a residue near the C-terminal end of the PAS domain immediately following an amino acid stretch (GTIF) that is identical in both VqmAVc and VqmAPhage (S1 Fig). We cloned vqmAVc, vqmAPhage, VcN-CPhage, and PhageN-CVc under an arabinose-inducible promoter and transformed each construct into recombinant Δtdh E. coli harboring a PvqmR-lux or a Pqtip-lux reporter. The Tdh enzyme is required for DPO biosynthesis, therefore a Δtdh E. coli strain makes no DPO [3]. Apo-VqmA displays basal transcriptional activity in vivo [9]. Thus, while DPO enhances VqmA DNA-binding activity, it is not an absolute requirement for binding. Using Δtdh E. coli for these studies ensured that any transcriptional activity that occurred was exclusively a consequence of the DNA-binding capabilities of the chimeras and not ligand-binding-driven transcriptional activation of the chimeras. Consistent with our hypothesis, promoter activation by each chimera was determined by the protein from which the DBD originated: All four versions of VqmA activated PvqmR-lux, whereas only VqmAPhage and VcN-CPhage activated Pqtip-lux (Fig 1A and 1B, respectively). Next, we conjugated the four versions of VqmA into Δtdh ΔvqmAVc V. cholerae lysogenized by a phage VP882 mutant in which the endogenous vqmAPhage was inactive (VP882 vqmAPhage::Tn5). Thus, the only source of VqmA protein was that made from the plasmid. As expected, following arabinose-induction, only VqmAPhage and VcN-CPhage activated qtip expression and induced host-cell lysis (Fig 1C).
Fig 1. Promoter DNA-binding selectivity is conferred by the VqmA DBD.
(A and B) Normalized reporter activity from Δtdh E. coli harboring (A) PvqmR-lux or (B) Pqtip-lux and arabinose-inducible VqmAVc, VqmAPhage, VcN-CPhage, or PhageN-CVc. Black, no arabinose; white, 0.2% arabinose. Data are represented as mean ± SD (error bars) with n = 3 biological replicates. (C) Growth curves of the Δtdh ΔvqmAVc V. cholerae harboring phage VP882 vqmAPhage::Tn5 and arabinose-inducible VqmAVc, VqmAPhage, VcN-CPhage, or PhageN-CVc in medium lacking (Control) or containing 0.2% arabinose (Induced). (D) EMSAs showing binding of VqmA proteins to PvqmR and Pqtip DNA. From left to right are, VqmAVc, VqmAPhage, PhageN-CVc, and VcN-CPhage. 25 nM PvqmR or Pqtip DNA was used in all EMSAs with no protein (designated -) or 2-fold serial dilutions of proteins. The lowest and highest protein (dimer) concentrations are 18.75 nM and 600 nM, respectively. (E and F) Normalized reporter activity from WT E. coli as in panels A and B harboring arabinose-inducible VqmAVc, GST-DBDVc, VqmAPhage, and GST-DBDPhage. (G) EMSAs showing binding of GST-DBDVc and GST-DBDPhage to PvqmR and Pqtip DNA. Probe and protein concentrations as in panel D.
We verified the above findings in vitro using electrophoretic mobility shift assays (EMSAs). Consistent with the cell-based assays, the purified VqmAVc, VqmAPhage, VcN-CPhage, and PhageN-CVc proteins shifted PvqmR DNA, whereas only the VqmAPhage and VcN-CPhage proteins shifted Pqtip DNA (Fig 1D). Assessing the ratios of bound to total DNA across varying protein concentrations allowed us to calculate the relative binding affinities (EC50) of the VqmA proteins for PvqmR and Pqtip DNA (S2A Fig). Our EMSA analyses show that PhageN-CVc, like VqmAVc, only bound PvqmR, but with an estimated ~7-fold lower affinity. Consistent with our previous findings, VqmAPhage bound Pqtip about 3-fold more strongly than it bound PvqmR [5]. By contrast, VcN-CPhage showed a modest increase in its preference for Pqtip relative to that for PvqmR, with binding to both promoters at a level similar to that with which VqmAPhage bound Pqtip. Indeed, in agreement with our EC50 measurements, when Pqtip and PvqmR DNA were supplied at equimolar concentrations in a competitive DNA-binding assay, lower amounts of VqmAPhage and VcN-CPhage were required to shift Pqtip DNA than to shift PvqmR DNA (S2B Fig). In conclusion and in agreement with our in vivo results, the respective DBD of each purified VqmA protein drives promoter selectively.
We next assayed the VqmAVc and VqmAPhage DBDs lacking their PAS domains (DBDVc and DBDPhage, respectively) for activation of PvqmR-lux and Pqtip-lux. Deletion of the PAS domains resulted in inactive proteins as neither DBD activated transcription (S3A and S3B Fig, respectively), and likewise, EMSA analyses showed that neither DBD bound either promoter (S3C Fig). Gel filtration analyses indicated that the DBD proteins purified as monomers (S3D Fig), suggesting that the DBDs were unable to dimerize in the absence of their partner PAS domains. This result is consistent with previous findings that, in addition to sensing DPO, the VqmAVc PAS domain is responsible for dimerization [9,11].
Transcriptional activity driven by HTH-containing proteins typically depends on dimer formation. Soluble glutathione S-transferase (GST) spontaneously forms a homodimer [12], and so GST can be employed as a substitute for native dimerization domains of proteins [13]. Thus, to examine the VqmA requirement for dimerization, we fused GST to the N-terminus of each VqmA DBD to yield recombinant GST-DBDVc and GST-DBDPhage and we tested whether DNA-binding function was restored. Indeed, the GST-DBD proteins purified as dimers (S3D Fig). PvqmR-lux and Pqtip-lux expression analyses revealed that the DBDs, when fused to GST, regained function, with the caveat that the GST-DBDVc exhibited 10-fold reduced activity compared to wild-type (WT) VqmAVc (Fig 1E). Importantly, the DNA-binding preferences mimicked those of the full-length proteins: GST-DBDPhage activated both PvqmR-lux and Pqtip-lux, whereas GST-DBDVc only activated PvqmR-lux (Fig 1E and 1F). Companion EMSA analyses showed that GST-DBDPhage bound Pqtip ~5-fold more strongly than it bound PvqmR, whereas GST-DBDVc showed almost no binding to PvqmR and, unexpectedly, some weak binding could be detected to the Pqtip DNA (Fig 1G). We confirmed that purified GST alone did not bind either PvqmR or Pqtip (S3E Fig). Given that the GST-DBDVc driven activation of Pqtip-lux was undetectable in vivo (Fig 1F), we presume that the observed in vitro GST-DBDVc binding to Pqtip DNA is a consequence of the simplified context in which the EMSA is performed. Likely, the DNA:VqmA ratio in the EMSA is far higher than in cells, which, in the case of GST-DBDVc, fosters modest non-specific DNA binding. Taken together, our results show that VqmA promoter-binding selectivity is conferred by the DBD, and that dimerization is necessary.
VqmA DNA-binding preferences can be inverted by exchanging key DNA sequences in PvqmR and Pqtip
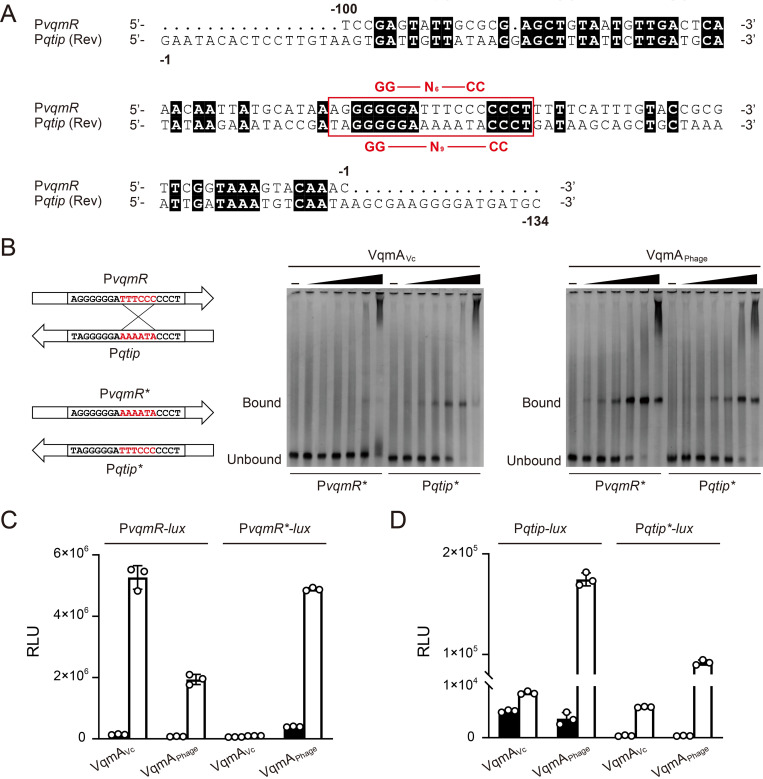
To study the VqmA promoter-binding asymmetry from the aspect of the DNA, our next goal was to identify the critical DNA sequence within Pqtip that prevents VqmAVc from binding. In the phage VP882 genome, Pqtip resides between vqmAPhage and qtip and VqmAPhage activates its own and qtip expression, suggesting that VqmAPhage binding may involve both DNA strands. Similarly, VqmAVc has been shown to interact with both strands of PvqmR [11]. Thus, in each case, both DNA strands need to be considered (Fig 2A). Previous work revealed that the critical region in PvqmR required for VqmAVc binding is -AGGGGGGATTTCCCCCCT- [2,11]. The corresponding fragment from Pqtip, but on the opposite DNA strand, -TAGGGGGAAAAATACCCT-, possesses ~56% sequence identity to this region suggesting it could be the key stretch of DNA that drives VqmAPhage promoter selection. The highest divergence in the two promoters is in the central 6 nucleotides: “-AAAATA-” in Pqtip and “-TTTCCC-” in PvqmR. We synthesized DNA probes in which we exchanged the “-AAAATA-” in Pqtip with “-TTTCCC-” from PvqmR and tested VqmAVc and VqmAPhage binding by EMSA analysis. We call these probes PvqmR* and Pqtip*, respectively. Indeed, promoter DNA-binding specificity was exchanged: VqmAVc shifted Pqtip*, whereas it only weakly shifted PvqmR* (Fig 2B). VqmAPhage bound to PvqmR* twice as strongly as it bound to Pqtip*, showing the opposite preference for the two synthetic promoters compared to the native promoters (Fig 2B). PvqmR*-lux and Pqtip*-lux transcriptional fusions mimicked the EMSA results: VqmAVc only activated expression of Pqtip*-lux, whereas VqmAPhage activated expression of PvqmR*-lux and Pqtip*-lux (Fig 2C and 2D). Thus, this 6-nucleotide stretch is the key sequence that determines the DNA-binding specificity for the two VqmA proteins. Moreover, the presence of the -AAAATA- nucleotide sequence in Pqtip is sufficient to prevent VqmAVc from activating transcription of Pqtip.
Fig 2. Promoter selectivity is reversed by exchanging key nucleotide fragments.
(A) DNA sequence alignment (ClustalW) of PvqmR and Pqtip. The reverse strand of Pqtip is shown. Numbering indicates positions relative to the transcription start sites. Identical nucleotides are designated with black shading. The reported 18-bp DNA stretch in PvqmR required for VqmAVc to bind (2,11) and the corresponding region in Pqtip are highlighted in the red box. The GG-N6-CC palindrome in PvqmR (2,11) and the recently identified GG-N9-CC palindrome in Pqtip (15) are indicated above and below the red box, respectively. (B) EMSAs showing binding of the designated VqmA proteins to PvqmR* and Pqtip* DNA. The cartoon at the left illustrates the key sequences exchanged in the probes. Probe and protein concentrations as in Fig 1D. (C) Normalized reporter activity from Δtdh E. coli harboring PvqmR-lux or PvqmR*-lux and arabinose-inducible VqmAVc or VqmAPhage. Black, no arabinose; white, 0.2% arabinose. Data are represented as mean ± SD (error bars) with n = 3 biological replicates. (D) As in C for Pqtip-lux or Pqtip*-lux.
Protein sequence-guided mutagenesis reveals that residue E194 in phage VP882 VqmAPhage and the equivalent A192 residue in V. cholerae VqmAVc contribute to specificity for Pqtip
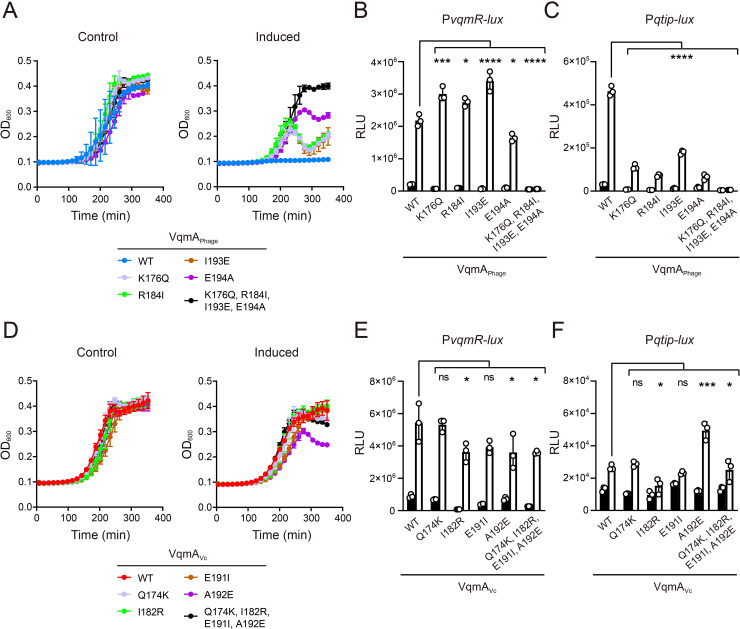
We considered two possible mechanisms that could underpin the asymmetric VqmA DNA-binding patterns: phage VP882 VqmAPhage could possess a feature that relaxes its DNA-binding specificity, and/or V. cholerae VqmAVc could possess a feature that restricts its DNA-binding ability. To distinguish between these possibilities, we first probed which residues drive VqmAPhage interactions with Pqtip but do not contribute to interactions with PvqmR. To do this, we performed site-directed mutagenesis of VqmAPhage with the goal of identifying mutants that fail to bind Pqtip but retain binding to PvqmR. Charged residues in HTH motifs typically mediate interactions between VqmA-type transcription factors and DNA, and indeed, both VqmA HTHs are enriched in positively-charged amino acids [9,11,14]. Sequence alignment of the HTHs in VqmAPhage and VqmAVc revealed four obvious differences in charged residues that could underlie the DNA-binding asymmetry between the two proteins (S1 Fig). We mutated those residues in VqmAPhage to the corresponding VqmAVc residues. The changes are: VqmAPhageK176Q, VqmAPhageR184I, VqmAPhageI193E, and VqmAPhageE194A. To test the combined effect of these mutations on VqmAPhage DNA-binding function, we also constructed the quadruple VqmAPhageK176Q, R184I, I193E, E194A mutant. VqmAPhageK176Q, VqmAPhageR184I, VqmAPhageI193E retained the ability to induce phage lysis showing that in vivo binding to Pqtip was not eliminated (Fig 3A). VqmAPhageE194A induced only low-level cell lysis suggesting that, while binding to Pqtip is not eliminated, it is compromised (Fig 3A). Analysis of PvqmR-lux and Pqtip-lux expression revealed that all four VqmAPhage single point mutants possessed levels of activity within 2-fold of that of WT PvqmR-lux. By contrast, they displayed ~2-7-fold reductions in Pqtip-lux activity, with VqmAPhageE194A being the least active (Fig 3B and 3C, respectively). The quadruple mutant was unable to induce phage lysis in a V. cholerae lysogen and it did not activate PvqmR-lux or Pqtip-lux expression showing it is defective in binding to both promoters (Fig 3A–3C). Western blot analysis demonstrated that all of the VqmAPhage variants were produced at levels similar to WT in both V. cholerae and E. coli (S4A Fig). Thus, our results indicate that, among these charged residues, only the VqmAPhage residue E194 in the HTH motif plays a role in VqmAPhage selection of Pqtip.
Fig 3. VqmAPhage residue E194 and the corresponding VqmAVc residue A192 contribute to specificity for binding to Pqtip.
(A) Growth curves of Δtdh ΔvqmAVc V. cholerae harboring phage VP882 vqmAPhage::Tn5 and the indicated 3xFLAG-VqmAPhage alleles in medium lacking (Control) or containing 0.2% arabinose (Induced). (B and C) Normalized reporter activity from Δtdh E. coli harboring (B) PvqmR-lux or (C) Pqtip-lux and the indicated arabinose-inducible 3xFLAG-VqmAPhage alleles. Black, no arabinose; white, 0.2% arabinose. Data are represented as mean ± SD (error bars) with n = 3 biological replicates. (D) Growth curves of Δtdh ΔvqmAVc V. cholerae harboring phage VP882 vqmAPhage::Tn5 and the indicated 3xFLAG-VqmAVc alleles in medium lacking (Control) or containing 0.2% arabinose (Induced). (E and F) Normalized reporter activity from Δtdh E. coli harboring (E) PvqmR-lux or (F) Pqtip-lux and the indicated arabinose-inducible 3xFLAG-VqmAVc alleles. Black, no arabinose; white, 0.2% arabinose. Data are represented as mean ± SD (error bars) with n = 3 biological replicates. ns = not significant, ****P <0.0001, ***P <0.0005, **P <0.005, *P <0.05 in one-way ANOVA compared to WT VqmA proteins.
While the residues we mutated in the phage VP882 VqmAPhage HTH motif do not dramatically perturb site-specific recognition of Pqtip, the corresponding residues in the V. cholerae VqmAVc HTH motif could nonetheless restrict its capacity to bind Pqtip. Therefore, we also mutated the analogous VqmAVc residues to the corresponding VqmAPhage residues. We made: VqmAVcQ174K, VqmAVcI182R, VqmAVcE191I, VqmAVcA192E, and VqmAVcQ174K, I182R, E191I, A192E. Here, our goal was to test whether the variants gained the ability to bind Pqtip. Only VqmAVcA192E induced a modest level of lysis in the V. cholerae lysogen, whereas all other VqmAVc variants failed to do so (Fig 3D). All of the VqmAVc variants drove the WT level of PvqmR-lux activity (Fig 3E). VqmAVcA192E generated low but detectable Pqtip-lux expression, while the other VqmAVc variants did not (Fig 3F). The VqmAVc variants were produced at similar levels to WT VqmAVc in V. cholerae and E. coli (S4B Fig). We conclude that, among the tested residues, only A192 plays a role in preventing VqmAVc from binding Pqtip.
Our mutagenesis analyses for VqmAVc are consistent with our analyses for VqmAPhage: The residue at the 192nd position in V. cholerae VqmAVc and the analogous residue at the 194th position in phage VP882 VqmAPhage contribute to selection of Pqtip. However, given that the A192E substitution in VqmAVc results in only partial activation of Pqtip expression, and the E194A substitution in VqmAPhage results in only partial loss of activation of Pqtip, the E194 residue in VqmAPhage cannot be the sole amino acid responsible for the preference VqmAPhage shows for Pqtip. Rather, additional residues in VqmAPhage must participate in conferring specificity.
Random mutagenesis of the VqmAPhage DBD reveals that residues G201, A202, E207, and M211 are required for VqmAPhage to bind Pqtip but are dispensable for binding PvqmR
Our protein sequence-guided approach did not reveal the primary mechanism underlying promoter-binding specificity for either of the VqmA proteins. We therefore performed a genetic screen to forward our goal of identifying phage VP882 VqmAPhage mutants that fail to bind Pqtip but retain the ability to bind PvqmR. We constructed a library of random mutations in the region of vqmAPhage encoding the DBD in the context of the full-length gene, cloned them into a plasmid under an arabinose-inducible promoter, and introduced them into Δtdh ΔvqmAVc V. cholerae harboring PvqmR-lux on the chromosome and lysogenized by phage VP882 harboring inactive vqmAPhage (vqmAPhage::Tn5). The logic of the screen is as follows: When propagated on agar plates supplemented with arabinose, V. cholerae exconjugants harboring vqmAPhage alleles possessing reasonable Pqtip-binding activity will lyse because those VqmAPhage proteins will bind Pqtip on the phage VP882 genome and launch the phage lytic cascade (S5 Fig). Such exconjugants will die and thus be eliminated from the screen. Exconjugants that survive but carry vqmAPhage null alleles will produce no light because those VqmAPhage proteins will fail to bind PvqmR-lux, so they also can be eliminated from the screen. The vqmAPhage alleles of interest to us are those that are maintained in surviving exconjugants (because they encode proteins that cannot bind Pqtip) and produce light (because they encode proteins that can bind PvqmR-lux).
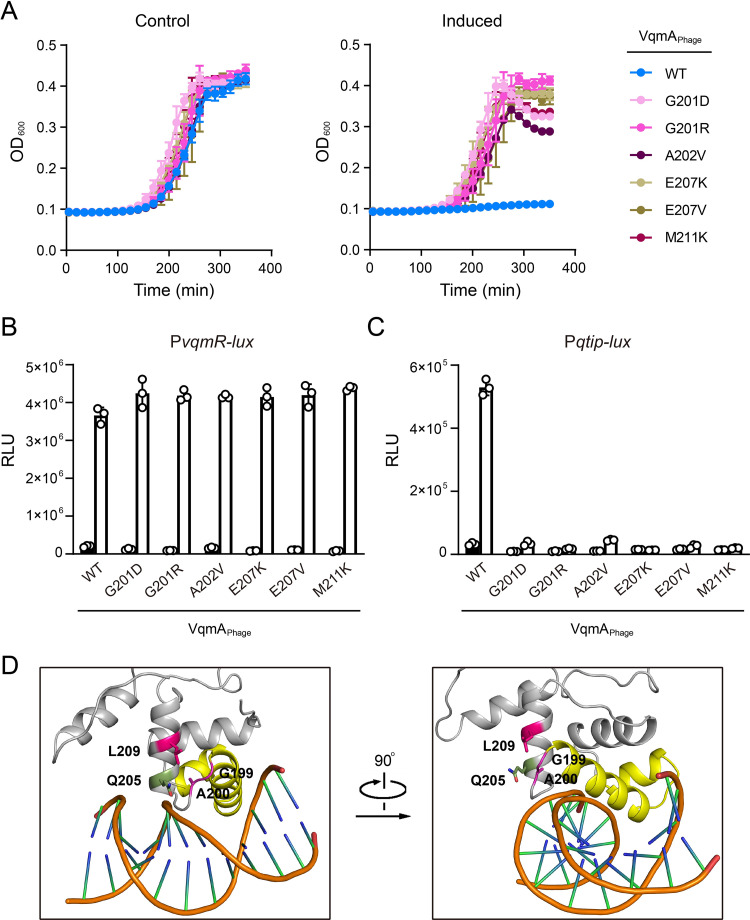
Our screen yielded the following mutants: VqmAPhageG201D, VqmAPhageG201R, VqmAPhageA202V, VqmAPhageE207K, VqmAPhageE207V, and VqmAPhageM211K (Fig 4A). To verify that these VqmAPhage mutants were indeed defective in binding Pqtip, we individually transformed them into Δtdh E. coli carrying the Pqtip-lux reporter or the PvqmR-lux reporter and measured light production. All variants retained WT capability to activate PvqmR-lux, but they did not harbor WT capability to activate Pqtip-lux expression (>10-fold reductions in activity) (Fig 4B and 4C, respectively). Thus, any residual Pqtip binding by these mutant VqmAPhage proteins is insufficient to induce host-cell lysis in the phage VP882 lysogen (Fig 4A). We verified that the VqmAPhage variants are produced at the same level as WT VqmAPhage in V. cholerae and E. coli (S4C Fig). According to the protein sequence alignment, VqmAPhage residues (175–200) corresponding to positions 173–198 in VqmAVc comprise the VqmAPhage HTH motif (S1 Fig). Thus, the residues identified in the mutagenesis (G201, A202, E207, and M211) are located C-terminal to the VqmAPhage HTH motif. Mapping the analogous V. cholerae VqmAVc residues (G199, A200, Q205, and L209) to the DPO-VqmAVc-PvqmR structure (there is no DPO-VqmAPhage-Pqtip structure) also shows that all of these residues cluster in a flexible loop region and helix adjacent to, but distinct from the HTH motif that directly contacts DNA (Figs 4D and S1). Surprisingly, the residues identified in the VqmAPhage mutagenesis are either identical (VqmAPhage G201 and A202 versus VqmAVc G199 and A200) or similar (VqmAPhage E207 and M211 versus VqmAVc Q205 and L209) between VqmAPhage and VqmAVc. To test whether possession of the similar residues is sufficient to confer DNA-binding specificity for Pqtip, we constructed VqmAVcQ205E and VqmAVcL209M and tested their DNA-binding functions as above. VqmAVcQ205E and VqmAVcL209M, like WT VqmAVc, activated PvqmR-lux but failed to activate Pqtip-lux (S6A and S6B Fig, respectively). We make the following four conclusions from these findings: 1) There are at least four residues (G201, A202, E207, and M211) required for VqmAPhage to recognize Pqtip DNA. 2) Because the VqmAPhage G201D, G201R, A202V, E207K, E207V, and M211K variants exhibit WT binding to PvqmR, the substitutions at these four residues must not significantly affect PvqmR recognition. 3) Because these residues are conserved or similar between VqmAPhage and VqmAVc, one would expect VqmAVc to have the capacity to bind Pqtip. 4) However, because VqmAVc in fact does not bind Pqtip, VqmAVc likely possesses an additional feature that resides elsewhere in the protein that prevents Pqtip binding from occurring.
Fig 4. VqmAPhage residues G201, A202, E207, and M211 are required for binding to Pqtip.
(A) Growth curves of Δtdh ΔvqmAVc V. cholerae harboring phage VP882 vqmAPhage::Tn5 and the indicated 3xFLAG-VqmAPhage alleles in medium lacking (Control) or containing 0.2% arabinose (Induced). (B and C) Normalized reporter activity from Δtdh E. coli harboring (B) PvqmR-lux or (C) Pqtip-lux and the indicated arabinose-inducible 3xFLAG-VqmAPhage alleles. Black, no arabinose; white 0.2% arabinose. Data are represented as mean ± SD (error bars) with n = 3 biological replicates. (D) Close up views of the DBD from the crystal structure of DPO-VqmAVc bound to PvqmR (PDB: 6ide, protein in gray with the HTH motif in yellow, and the DNA in orange). The color scheme for VqmAVc residues G199, A200, Q205, and L209 mirrors that used in panel A.
The restrictive element that prevents VqmAVc from binding Pqtip is located in its HTH motif and the adjacent C-terminal region of 25 residues
To test the hypothesis that a feature in the VqmAVc DBD restricts its DNA-binding capacity to PvqmR, we performed a genetic screen aimed at identifying VqmAVc mutants capable of activating Pqtip-lux expression. To do this, we constructed a library of random vqmAVc DBD alleles containing, on average, 1–2 substitutions, and we cloned them into a plasmid under an arabinose-inducible promoter. The library was transformed into the Δtdh E. coli strain harboring the Pqtip-lux reporter and transformants were propagated on plates containing arabinose. We screened ~10,000 transformants for colonies that produced light indicating that they contained VqmAVc proteins that activated Pqtip-lux. This strategy yielded no such transformants. Several possibilities could explain our result: We did not screen sufficient numbers of mutants, the mutagenesis did not yield the crucial change, or no alteration of a single residue can enable VqmAVc binding to Pqtip.
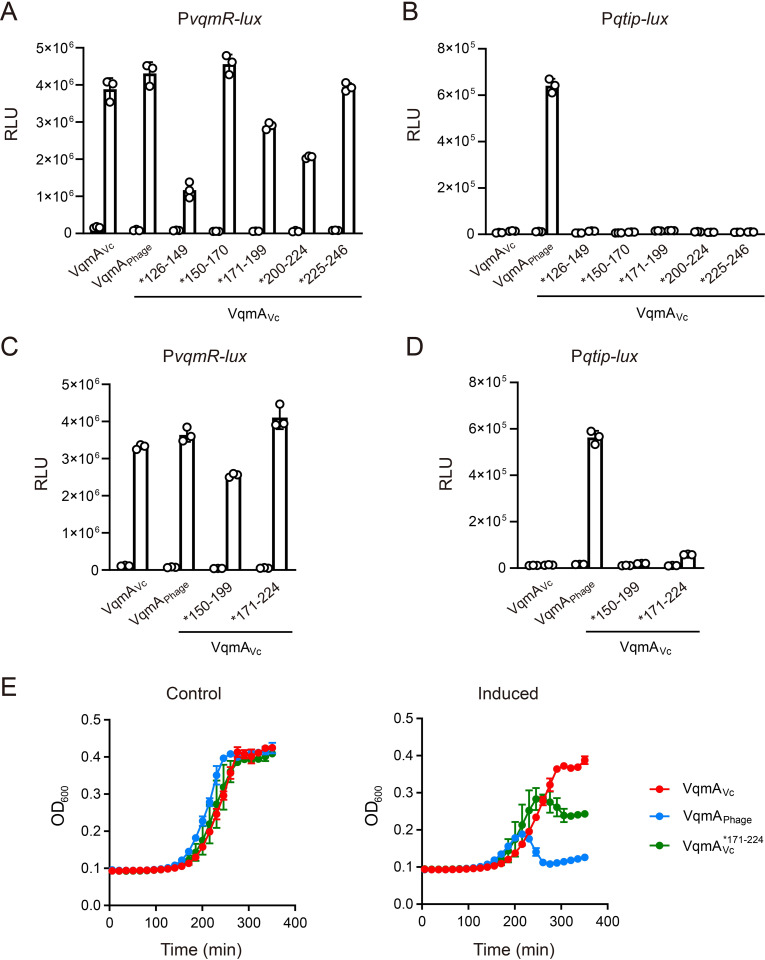
We expanded our search for the DNA-binding restrictive element present in VqmAVc by assessing whether a particular region in the VqmAVc DBD constrains promoter binding to PvqmR. To do this, we constructed five VqmAVc mosaic proteins by replacing ~20–30 residues in the V. cholerae VqmAVc DBD with the corresponding residues from the phage VP882 VqmAPhage DBD. We call these proteins VqmAVc*126–149, VqmAVc*150–170, VqmAVc*171–199, VqmAVc*200–224, and VqmAVc*225–246 (see S1 Fig for relevant protein segments). Each superscript denotes the VqmAVc amino acid residues that have been replaced by the corresponding residues from VqmAPhage. In all the mosaics, either the intact VqmAVc HTH or the intact VqmAPhage HTH was present. For reference, the VqmAVc HTH motif consists of residues 173 to 198 and the VqmAPhage HTH spans residues 175 to 200. We tested the mosaic VqmAVc proteins for activation of the PvqmR-lux and Pqtip-lux reporters. The DNA specificity of all the VqmAVc mosaics mimicked WT VqmAVc as PvqmR-lux was expressed but Pqtip-lux was not (Fig 5A and 5B, respectively). We confirmed that the mosaic VqmAVc proteins are expressed at levels similar to WT VqmAVc (S7 Fig). Our results suggest that the feature that prevents V. cholerae VqmAVc from binding to Pqtip is larger than the regions delineated by any of the VqmAVc mosaics, or it could be that multiple patches in the VqmAVc DBD that are not contiguous in amino acid sequence are responsible.
Fig 5. The VqmAVc HTH motif and the immediate C-terminal 25 residues, together, constrain binding to PvqmR.
(A-D) Normalized reporter activity from Δtdh E. coli harboring (A and C) PvqmR-lux or (B and D) Pqtip-lux and arabinose-inducible VqmAVc, VqmAPhage, or the indicated VqmAVc allele. Data are represented as mean ± SD (error bars) with n = 3 biological replicates. Black, no arabinose; white, 0.2% arabinose. (E) Growth curves of Δtdh ΔvqmAVc V. cholerae harboring phage VP882 vqmAPhage::Tn5 and VqmAVc, VqmAPhage, or VqmAVc*171–224 in medium lacking (Control) or containing 0.2% arabinose (Induced).
Pinpointing non-contiguous regions that could, together, contain the VqmAVc restrictive element is challenging. However, testing for a larger contiguous expanse that could contain the putative restrictive element is straightforward. Thus, we constructed two additional V. cholerae VqmAVc mosaic proteins. In one construct, called VqmAVc*150–199, we introduced the VqmAPhage HTH along with the immediate N-terminal 25 amino acids in place of the corresponding VqmAVc region. Second, in a construct called VqmAVc*171–224, we introduced the VqmAPhage HTH together with the immediate C-terminal 25 amino acid stretch in place of that VqmAVc region. VqmAVc*150–199 and VqmAVc*171–224 activated PvqmR-lux to approximately WT levels, whereas only VqmAVc*171–224 activated Pqtip-lux, albeit weakly (Fig 5C and 5D, respectively). Consistent with this result, VqmAVc*171–224 induced partial lysis in the V. cholerae phage VP882 lysogen (Fig 5E). VqmAVc*171–224 was produced at levels similar to WT VqmAVc, eliminating the possibility that the observed binding to Pqtip was a consequence of overexpression (S7 Fig). We conclude that the region encompassing both the HTH motif and the C-terminal 25 residues are required to restrict the VqmAVc DBD from binding Pqtip.
Discussion
The DPO-VqmA QS AI-receptor pair controls lifestyle transitions in the pathogen V. cholerae and in the vibriophage VP882. Here, we studied the DNA-binding function of VqmA. VqmA proteins are cytoplasmic transcription factors composed of N-terminal PAS domains responsible for binding the DPO ligand and C-terminal DBDs containing HTH motifs. Most of the key residues required for binding the DPO ligand and for binding to PvqmR DNA are conserved between the two VqmA proteins. Indeed, both VqmAVc and VqmAPhage bind DPO and activate transcription of vqmR. By contrast, only VqmAPhage activates the phage gene qtip. Here, we investigated this asymmetric DNA-binding pattern. Our work shows that, in both proteins, the DBD determines promoter recognition. We have previously shown that DPO binding enhances VqmA transcriptional activity [9]. This earlier work, together with our present results, suggest a model in which the PAS domain specifies DNA-binding affinity (between the apo- and holo- states), and the DBD specifies DNA-binding selectivity.
The main goal of the present work was to discover features of the VqmA proteins that confer specificity in transcriptional activity. We propose that phage VP882 VqmAPhage possesses a feature that relaxes its DNA-binding specificity and V. cholerae VqmAVc possesses a feature that restricts its DNA-binding capability. Regarding VqmAVc, our genetic analyses support the hypothesis that the VqmAVc DBD harbors elements that prevent it from binding Pqtip. This hypothesis stems from our finding that residues G201, A202, E207, and M211 are crucial for VqmAPhage recognition of Pqtip. These residues are conserved between VqmAVc and VqmAPhage. Specifically, in VqmAVc they are: G199, A200, Q205, and L209, respectively. More broadly, sequence alignments of VqmA proteins among Vibrios reveal that the residue at the 207th position in VqmAPhage (205th position in VqmAVc) is most frequently either a Glu or a Gln [5]. Similarly, the residue at the 211th position in VqmAPhage (209th position in VqmAVc) is commonly a hydrophobic residue, like Met, Leu, Ile, or Val. Thus, E207 and M211 are not unique to VqmAPhage, but rather occur in most VqmA proteins. We propose that because the key residues for Pqtip binding are conserved in VqmAPhage, VqmAVc, and other Vibrio VqmA proteins, VqmAVc is likely restricted from binding Pqtip by additional features elsewhere in its DBD. Regarding VqmAPhage, the DPO-VqmAPhage structure was reported during review of this manuscript [15]. Superimposition of this new structure (7DWM) onto the DPO-VqmAVc and DPO-VqmAVc-PvqmR structures (6KJU and 6IDE, respectively, and [9,11,14]) reveals two insights (S8 Fig). First, the conformations of the three PAS domains are similar except for the orientations of the first 20 N-terminal residues in each protein, indicating that the PAS domains do not confer the differences in promoter DNA specificity. Second, the DPO-VqmAPhage DBDs adopt a conformation that is intermediate between that of the more open DBDs in the DPO-VqmAVc structure and the closed DBDs in the DPO-VqmAVc-PvqmR structure. Additionally, the interaction interface between the VqmAPhage DBDs is less extensive, and thus more relaxed than that of the VqmAVc DBDs [15]. Likely, the more relaxed conformation exhibited by the VqmAPhage DBDs underpins its promiscuity for promoter binding with respect to PvqmR and Pqtip.
In the case of VqmAPhage, the residues G201, A202, E207, and M211 identified in our mutagenesis screen as necessary for Pqtip binding are, surprisingly, not in the HTH motif, nor do the corresponding VqmAVc residues make direct contacts with DNA in the DPO-VqmAVc-PvqmR crystal structure (Fig 4D). Thus, we wonder how the G201, A202, E207, and M211 residues could govern recognition of Pqtip. Our in vivo analyses showed that substitutions in VqmAPhage at these residues enable activation of vqmR expression to WT levels, whereas only residual activation of qtip expression occurs (Fig 4A–4C). Surprisingly, the purified VqmAPhage mutant proteins maintained some capability to bind Pqtip in vitro. A representative experiment using the VqmAPhageG201D protein is shown in S9A Fig.
We consider several possibilities to explain our findings:
First, the VqmAPhage G201, A202, E207, and M211 residues could mediate interactions with an additional bacterial factor involved in transcription. Importantly, the failure of these VqmAPhage variants to activate Pqtip expression in V. cholerae lysogens also occurred in E. coli, eliminating the possibility that these residues interact with a phage-specific or Vibrio-specific factor. Rather, these residues could be important for coordinating interactions with a conserved factor, such as RNA polymerase. If so, these mutant VqmAPhage proteins, while capable of binding promoter DNA, are incapable of activating transcription. This situation would be analogous to the positive control mutants of the lambda phage cI repressor (cIlambda). So called pc mutants bind DNA and exhibit repressor activity, but are deficient in positive transcriptional regulation due to the inability of the mutant cIlambda proteins to productively interact with RNA polymerase [16,17]. In our case, the VqmAPhage mutants maintain the capacity to activate vqmR expression so they must successfully interact with RNA polymerase at least at PvqmR. For this reason, we consider it unlikely that these VqmAPhage mutants are analogous to lambda pc mutants.
Second, a global transcriptional regulator could be involved that is present in both V. cholerae and E. coli. One candidate is the histone-like nucleoid structuring protein (H-NS) that functions as a universal repressor of transcription [18]. In Vibrio harveyi, the QS master regulator, LuxR, displaces H-NS at promoter DNA to activate expression of QS-controlled genes [19]. Perhaps, the VqmAPhage G201, A202, E207, and M211 mutants cannot successfully compete with H-NS for binding at Pqtip in vivo, whereas in an EMSA assay, since H-NS is not present, binding to Pqtip DNA occurs. To address this possibility, we examined whether WT VqmAPhage and VqmAPhageG201D competed with H-NS for binding to Pqtip using EMSA assays. There was no difference between WT VqmAPhage and VqmAPhageG201D binding to Pqtip DNA in the presence of purified H-NS (S9C and S9D Fig). These experiments suggest that it is unlikely that H-NS competition underlies our findings.
Third, the binding of the VqmAPhage G201, A202, E207, and M211 mutants to Pqtip in vitro, while demonstrating loss of activity in vivo, could be a consequence of the unnaturally high DNA: VqmAPhage stoichiometry in the EMSA, similar to what we observed for the GST-DBDVc construct (Fig 1G). Thus, the EMSA is not sufficiently sensitive to distinguish between the strength of DNA binding of WT VqmAPhage and the residual binding by the VqmAPhage G201, A202, E207, and M211 mutants. If this is the case, we propose that VqmAPhage G201, A202, E207, and M211 could play allosteric roles in correctly positioning the VqmAPhage HTH for proper contact with particular DNA nucleotides. Here, we compare this possibility to how site-specific recognition is accomplished by cIlambda. Genetic and biochemical studies revealed that residues outside of the cIlambda HTH motif are crucial for site-specific DNA recognition [20–24]. The crystal structure of the cIlambda repressor bound to DNA shows that charged residues adjacent to those in the HTH interact with the DNA sugar phosphate backbone [25]. Additionally, the N-terminal arm of cIlambda wraps around the DNA and makes contacts on the backside of the helix [25]. It is presumed that the backbone contacts function to position the HTH residues to contact specific DNA nucleotides. Thus, while the VqmAPhage residues that we identified as important for Pqtip recognition (G201, A202, E207, and M211) do not function perfectly analogously to those in cIlambda because they do not make contact with the DNA backbone, their role in site-specific recognition could be similar. A caveat of our interpretation is that, as noted, we do not have a structure of VqmAPhage bound to Pqtip and we mapped the residues identified in our VqmAPhage mutagenesis to the DPO-VqmAVc-PvqmR crystal structure. Therefore, it remains possible that the residues we identified here do indeed make contacts with DNA. A further possibility is that the residues we identified foster increased plasticity to the VqmAPhage DBDs, perhaps, allowing VqmAPhage to bind the longer palindrome that exists in Pqtip, which we discuss below. The recently reported DPO-VqmAPhage crystal structure [15], together with the existing DPO-VqmAVc structures, could enable modeling to predict the roles played by particular residues in conferring a relaxed conformation to the VqmAPhage DBDs. To our knowledge, no region analogous to the one we discovered in VqmAPhage has been shown to confer promoter specificity to a transcription factor. Going forward, determining the structure of VqmAPhage bound to Pqtip DNA should reveal the mechanism enabling recognition of Pqtip and the role that these residues play, individually and collectively, in determining DNA-binding specificity.
Previous work demonstrated that VqmAVc recognizes a key GG-N6-CC palindrome in PvqmR [2,11]. Our sequence alignment of PvqmR and Pqtip showed that Pqtip does not possess this palindrome. Rather, the corresponding sequence in Pqtip is GG-N6-TA (Fig 2A). The most obvious divergence between the two sequences is in the central six nucleotides: “-AAAATA-” in Pqtip and “-TTTCCC-” in PvqmR (Fig 2A). We hypothesized that this nucleotide stretch could be responsible for conferring the asymmetric DNA-binding patterns to the two VqmA proteins. Indeed, exchanging these nucleotides in Pqtip and PvqmR reversed the promoter binding preferences of the VqmA proteins. We verified our conclusion that this core 6 nucleotide stretch drives VqmA DNA-binding preference using our VqmA chimeric proteins (VcN-CPhage and PhageN-CVc), a representative mosaic protein (VqmAVc*171–224), and a representative protein containing a point mutation (VqmAPhageG201D) (S9A, S9B, and S10 Figs). While the present manuscript was under review, Gu et al. reported that a GG-N9-CC palindrome in Pqtip is the key sequence for VqmAPhage recognition [15]. According to our DNA sequence alignment, the GG-N6-CC palindrome required for VqmAVc binding is only present in PvqmR, while the key GG-N9-CC palindrome required for VqmAPhage binding exists in both Pqtip and PvqmR (Fig 2A). Together, our results and those of Gu et. al. [15] explain, at the level of the promoter DNA, why VqmAPhage binds both Pqtip and PvqmR while VqmAVc recognizes only PvqmR.
Genomic sequencing data have revealed the presence of many QS receptor-transcription factors encoded in phage genomes [26]. In general, however, their transcriptional outputs are uncharacterized, with the exception of VqmAPhage, which is promiscuous with respect to binding to PvqmR and Pqtip, the only two promoters tested to our knowledge. It remains possible that VqmAPhage regulates additional genes specifying bacterial and or/phage functions. Given that VqmAPhage can regulate biofilm formation through its control of V. cholerae vqmR, probing the host regulon controlled by VqmAPhage under various growth conditions could reveal unanticipated roles of QS in phage-Vibrio interactions.
Finally, we found that the VqmAVcA192E variant exhibited modest, but detectable binding to Pqtip, whereas the VqmAVc quadruple mutant, and the VqmAVc*171–199 mosaic protein did not. Western blot and PvqmR-lux assays eliminated the possibility that any of the mutant proteins were not expressed or were misfolded. Rather, we infer that a particular regional conformation in the VqmA proteins is required for this key residue to function properly. Our results also show that exchanging both the VqmAVc HTH motif and C-terminal 25 residues with the corresponding residues from VqmAPhage enables some but not WT-level binding to Pqtip. This finding supports the notion that a set of non-contiguous amino acids or a particular conformation of the VqmAVc DBD prevents binding to Pqtip. This arrangement is perhaps not surprising given that V. cholerae would pay a significant penalty if VqmAVc bound the phage VP882 qtip promoter, as the consequence would be the launch of the phage lytic program and death of the host cell. To our knowledge, VqmAVc binds to only one promoter, PvqmR [3]. Thus, even in the context of the V. cholerae genome, VqmAVc transcriptional activity is tightly constrained. It is possible that other negative ramifications stem from non-specific VqmAVc binding in the V. cholerae genome. Distinct mechanisms are employed to restrict other QS receptor/transcription factors from promiscuously binding to DNA. For example, LuxR-type QS receptors can typically bind >100 promoters, but their solubilization, stability, and DNA-binding capabilities strictly rely on being bound to an AI whose availability is, in turn, highly regulated [27–31]. Therefore, precise control of gene expression is maintained in many QS circuits by confining QS receptor activity to the ligand-bound form coupled with discrete affinities of the ligand-receptor complexes for target promoters. By contrast, VqmAVc is expressed constitutively, and its DNA-binding capabilities are not limited by the presence of an AI. Thus, exquisitely tight control over promoter DNA-binding specificity by VqmAVc—restricting it to one and only one promoter—is apparently crucial for proper regulation of gene expression and survival.
Materials and methods
Bacterial strains, plasmids, primers, and reagents
Strains, plasmids, primers, and gBlocks used in this study are listed in S1–S4 Tables, respectively. In all experiments, Δtdh V. cholerae and Δtdh E. coli strains were used except in the experiment assaying expression of PvqmR-lux and Pqtip-lux in response to the DBDVc, DBDPhage, GST-DBDVc, and GST-DBDPhage proteins. In that case, the E. coli strain contained the WT tdh gene. V. cholerae and E. coli were grown aerobically in lysogeny broth (LB) at 37°C. Antibiotics and inducers were used at the following concentrations: 50 units mL-1 polymyxin B, 200 μg mL-1 ampicillin, 5 μg mL-1 chloramphenicol, 100 μg mL-1 kanamycin, 0.2% arabinose, and 1 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG).
Primers were obtained from Integrated DNA Technologies. Gibson assembly, intramolecular reclosure, and traditional cloning methods were employed for all cloning. PCR with Q5 High Fidelity Polymerase (NEB) was used to generate insert and backbone DNA. Gibson assembly relied on HiFi DNA assembly mix (NEB). All enzymes used in cloning were obtained from NEB. Mutageneses of the VqmAPhage and VqmAVc DBDs were accomplished using the GeneMorph II EZClone Domain Mutagenesis Kit (Agilent) according to the manufacturer’s instructions. Transfer of plasmids carrying vqmA genes into the V. cholerae phage VP882 lysogen employed conjugation followed by selective plating on polymyxin B, chloramphenicol, and kanamycin, based on previously described protocols [32].
Genetic screens for VqmAPhage and VqmAVc DNA-binding mutants
E. coli carrying a library of plasmid-borne vqmAPhage mutants was mated with V. cholerae harboring a phage VP882 mutant (vqmAPhage::Tn5) and the PvqmR-lux reporter integrated at the lacZ locus. Exconjugant V. cholerae colonies were collected and streaked onto LB agar plates supplemented with polymyxin B, chloramphenicol, kanamycin, and arabinose. PvqmR-lux activity of surviving exconjugants was assayed using an ImageQuant LAS4000 imager (GE). V. cholerae colonies that produced light were harvested for plasmid DNA preparation. Isolated plasmid DNA was subsequently transformed into E. coli strains carrying Pqtip-lux or PvqmR-lux to validate activity.
A library of plasmid-borne vqmAVc mutants was transformed into E. coli carrying the Pqtip-lux reporter. Transformants were plated on LB agar supplemented with ampicillin, kanamycin, and arabinose. Pqtip-lux activity was assayed using an ImageQuant LAS4000 imager.
Growth, lysis, and bioluminescence assays
To measure growth of V. cholerae phage VP882 lysogens or activation of the PvqmR-lux and Pqtip-lux reporters in bacterial strains, overnight cultures of V. cholerae or E. coli were back-diluted 1:1000 into LB medium supplemented with appropriate antibiotics prior to being dispensed (200 μL) into 96-well plates (Corning Costar 3904). Arabinose was added as specified. The plates were shaken at 37°C and a Biotek Synergy Neo2 Multi-Mode reader was used to measure OD600 and bioluminescence. For bioluminescence assays, relative light units (RLU) were calculated by dividing bioluminescence by the OD600 after 5 h.
Protein expression, purification, and electrophoretic mobility shift assay (EMSA)
Protein expression and purification were performed as described [9,19]. EMSAs were performed as described [8] with the following modifications: Following electrophoresis, 6% DNA retardation gels were stained with SYBR Green (Thermo) and visualized using an ImageQuant LAS 4000 imager with the SYBR Green settings. Unless specified otherwise, the highest concentration of VqmA assessed was 600 nM. 25 nM PvqmR or Pqtip DNA was used in all EMSAs. The percentage of promoter DNA bound was calculated using the gel analyzer tool in ImageJ and the estimated EC50 values were derived from EC50 analyses in Prism.
Western blot analysis
Western blot analyses probing for abundances of 3xFLAG-tagged proteins were performed as reported [3] with the following modifications: E. coli and V. cholerae carrying N-terminal 3xFLAG-tagged VqmAVc and N-terminal 3xFLAG-tagged VqmAPhage alleles were back-diluted 1:1000 in LB supplemented with appropriate antibiotics and harvested after 6 h and 4 h of growth at 37°C, respectively. Cells were resuspended in Laemmli sample buffer at a final concentration of 0.006 OD/μL. Following denaturation for 15 min at 95°C, 5 μL of each sample was subjected to SDS-PAGE gel electrophoresis. RpoA was used as the loading control (Biolegend Inc.). Signals were visualized using an ImageQuant LAS 4000 imager.
Sequence alignments
Protein and DNA sequences in FASTA format were aligned in the BioEdit Sequence Alignment Editor using the default setting under the ClustalW mode. Figs 2A and S1 were prepared via the ESPript 3.0 online server [33].
Statistical methods
All statistical analyses were performed using GraphPad Prism software. Error bars correspond to standard deviations of the means of three biological replicates.
Supporting information
Protein sequence alignment (ClustalW) showing VqmAVc and VqmAPhage. Black and white boxes designate identical and conserved residues, respectively. The PAS domain and HTH motif are indicated. The site used to fuse domains for chimera constructions is indicated by the red box. Key residues required for DPO binding are designated with black triangles. Conserved HTH residues are designated by black circles and open circles show residues with different charges in the HTH motifs of the two proteins. The residue in each HTH motif that contributes to Pqtip specificity is designated by the striped circle. The residues identified in the VqmAPhage screen and the equivalent residues altered by site-directed mutagenesis in VqmAVc are designated by asterisks.
(TIF)
(A) EC50 analysis of the designated VqmA proteins for binding to PvqmR and Pqtip. Data are representative of two independent experiments. The percentage of DNA bound was calculated using the gel analyzer tool in ImageJ and the estimated EC50 values were derived from Prism. (B) Competitive VqmAPhage and VcN-CPhage EMSA analysis. 25 nM PvqmR and Pqtip DNA were used and no protein (designated -) or 2-fold serially-diluted protein was added to the lanes. The lowest and highest protein (dimer) concentrations are 4.7 nM and 1200 nM, respectively.
(TIF)
(A and B) Normalized reporter activity from WT E. coli harboring (A) PvqmR-lux or (B) Pqtip-lux and arabinose-inducible VqmAVc, DBDVc, VqmAPhage, and DBDPhage. Black, no arabinose; white, 0.2% arabinose. Data are represented as mean ± SD (error bars) with n = 3 biological replicates. (C) EMSAs of DBDVc and DBDPhage proteins binding to PvqmR and Pqtip. 25 nM PvqmR or Pqtip DNA was used in all EMSAs with no protein (designated -) or 2-fold serial dilutions of proteins. The lowest and highest protein (dimer) concentrations are 18.75 nM and 600 nM, respectively. (D) Gel filtration chromatogram showing UV280 traces for the purification of (left) VqmAVc, DBDVc, and GST-DBDVc and (right) VqmAPhage, DBDPhage, and GST-DBDPhage proteins. (E) EMSA of GST protein binding to PvqmR and Pqtip DNA as in panel C.
(TIF)
Western blot showing the designated (A and C) 3xFLAG-VqmAPhage and (B) 3xFLAG-VqmAVc proteins produced by Δtdh E. coli and Δtdh ΔvqmAVc V. cholerae. A contaminating band below VqmAPhage and VqmAVc is present in all Δtdh E. coli samples. The RNAPα subunit (RpoA) was used as the loading control. Data are representative of two independent experiments.
(TIF)
Shown is growth of Δtdh ΔvqmAVc V. cholerae harboring phage VP882 vqmAPhage::Tn5 as a lysogen and arabinose-inducible 3xFLAG-VqmAPhage streaked onto agar plates with no arabinose (Control) or 0.2% arabinose (Induced).
(TIF)
(A and B) Normalized reporter activity from Δtdh E. coli harboring (A) PvqmR-lux or (B) Pqtip-lux and arabinose-inducible 3xFLAG-VqmAVc, 3xFLAG-VqmAPhage, or the indicated 3xFLAG-VqmAVc allele. Black, no arabinose; white, 0.2% arabinose. Data are represented as mean ± SD (error bars) with n = 3 biological replicates.
(TIF)
Western blot showing the designated 3xFLAG-VqmAVc mosaic proteins produced by Δtdh E. coli and Δtdh ΔvqmAVc V. cholerae. RpoA was used as the loading control. Data are representative of two independent experiments.
(TIF)
Previously reported crystal structures of DPO-VqmAVc-PvqmR (blue, PDB: 6IDE) and DPO-VqmAVc (green, PDB: 6KJU) superimposed onto the recently published crystal structure of DPO-VqmAPhage (yellow, PDB: 7DWM) based on the orientations of the PAS domains. DNA in the DPO-VqmAVc-PvqmR structure was omitted for simplicity.
(TIF)
(A) EMSA showing binding of VqmAPhageG201D to PvqmR and Pqtip DNA. 25 nM DNA was used in all EMSAs with no protein (designated -) or 2-fold serial dilutions of proteins. The lowest and highest protein (dimer) concentrations are 18.75 nM and 600 nM, respectively. (B) As in panel A for PvqmR* and Pqtip* DNA. (C) EMSA showing WT VqmAPhage and VqmAPhageG201D binding to Pqtip DNA in the presence of H-NS (300 nM). (D) EMSA showing H-NS binding to Pqtip DNA in the presence of WT VqmAPhage or VqmAPhageG201D (each protein at 300 nM).
(TIF)
(A) EMSA showing binding of VqmAVc*171–224 to PvqmR and Pqtip DNA. 25 nM DNA was used in all EMSAs with no protein (designated -) or 2-fold serial dilutions of proteins. The lowest and highest protein (dimer) concentrations are 18.75 nM and 600 nM, respectively. (B) As in panel A for PvqmR* and Pqtip* DNA. (C) As in panel A for VcN-CPhage binding to PvqmR* and Pqtip* DNA. (D) As in panel C for PhageN-CVc.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Acknowledgments
We thank members of the Bassler laboratory for insightful discussions.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Howard Hughes Medical Institute, National Institutes of Health Grant R37GM065859, and National Science Foundation Grant MCB-1713731 (BLB), NIGMS T32GM007388 (OPD), a Charlotte Elizabeth Procter Fellowship provided by Princeton University, and a National Defense Science and Engineering Graduate Fellowship supported by the Department of Defense (JES). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Papenfort K, Bassler BL. Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol. 2016. Sep 1;14(9):576–88. doi: 10.1038/nrmicro.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papenfort K, Förstner KU, Cong JP, Sharma CM, Bassler BL. Differential RNA-seq of Vibrio cholerae identifies the VqmR small RNA as a regulator of biofilm formation. Proc Natl Acad Sci U S A. 2015. Feb 17;112(7):E766–75. doi: 10.1073/pnas.1500203112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papenfort K, Silpe JE, Schramma KR, Cong JP, Seyedsayamdost MR, Bassler BL. A Vibrio cholerae autoinducer-receptor pair that controls biofilm formation. Nat Chem Biol. 2017. May 1;13(5):551–7. doi: 10.1038/nchembio.2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herzog R, Peschek N, Fröhlich KS, Schumacher K, Papenfort K. Three autoinducer molecules act in concert to control virulence gene expression in Vibrio cholerae. Nucleic Acids Res. 2019;47(6):3171–83. doi: 10.1093/nar/gky1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silpe JE, Bassler BL. A host-produced quorum-sensing autoinducer controls a phage lysis-lysogeny decision. Cell. 2019. Jan 10;176(1–2):268–280.e13. doi: 10.1016/j.cell.2018.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erez Z, Steinberger-Levy I, Shamir M, Doron S, Stokar-Avihail A, Peleg Y, et al. Communication between viruses guides lysis-lysogeny decisions. Nature. 2017. Jan 26;541(7638):488–93. doi: 10.1038/nature21049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silpe JE, Bassler BL. Phage-encoded LuxR-type receptors responsive to host-produced bacterial quorum-sensing autoinducers. MBio. 2019. Mar 1;10(2):e00638–19. doi: 10.1128/mBio.00638-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silpe JE, Bridges AA, Huang X, Coronado DR, Duddy OP, Bassler BL. Separating functions of the phage-encoded quorum-sensing-activated antirepressor Qtip. Cell Host Microbe. 2020. Feb 19;27:629–641.e4. doi: 10.1016/j.chom.2020.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Duddy OP, Silpe JE, Paczkowski JE, Cong J, Henke BR, et al. Mechanism underlying autoinducer recognition in the Vibrio cholerae DPO-VqmA quorum-sensing pathway. J Biol Chem. 2020. Mar 6;295(10):2916–31. doi: 10.1074/jbc.RA119.012104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Hsiao A, Joelsson A, Zhu J. The transcriptional regulator VqmA increases expression of the quorum-sensing activator HapR in Vibrio cholerae. J Bacteriol. 2006. Apr 1;188(7):2446–53. doi: 10.1128/JB.188.7.2446-2453.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H, Li M, Guo H, Zhou H, Li B, Xu Q, et al. Crystal structure of the Vibrio cholerae VqmA–ligand–DNA complex provides insight into ligand-binding mechanisms relevant for drug design. J Biol Chem. 2019. Feb 22;294(8):2580–92. doi: 10.1074/jbc.RA118.006082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes JD, Pulford DJ. The glutathione s-transferase supergene family: Regulation of GST and the contribution of the lsoenzymes to cancer chemoprotection and drug resistance part i. Crit Rev Biochem Mol Biol. 1995;30(6):445–520. doi: 10.3109/10409239509083491 [DOI] [PubMed] [Google Scholar]
- 13.Tudyka T, Skerra A. Glutathione S-transferase can be used as a C-terminal, enzymatically active dimerization module for a recombinant protease inhibitor, and functionally secreted into the periplasm of Escherichia coli. Protein Sci. 2008. Dec 31;6(10):2180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Li M, Peng C, Yin Y, Guo H, Wang W, et al. Large conformation shifts of Vibrio cholerae VqmA dimer in the absence of target DNA provide insight into DNA-binding mechanisms of LuxR-type receptors. Biochem Biophys Res Commun. 2019. Dec 3;520(2):399–405. doi: 10.1016/j.bbrc.2019.10.063 [DOI] [PubMed] [Google Scholar]
- 15.Gu Y, Zhi SX, Yang N, Yang WS. Understanding the mechanism of asymmetric gene regulation determined by the VqmA of vibriophage. Biochem Biophys Res Commun. 2021;558:51–6. doi: 10.1016/j.bbrc.2021.04.036 [DOI] [PubMed] [Google Scholar]
- 16.Hochschild A, Irwin N, Ptashne M. Repressor structure and the mechanism of positive control. Cell. 1983. Feb 1;32(2):319–25. doi: 10.1016/0092-8674(83)90451-8 [DOI] [PubMed] [Google Scholar]
- 17.Hawley DK, McClure WR. The effect of a lambda repressor mutation on the activation of transcription initiation from the lambda PRM promoter. Cell. 1983. Feb 1;32(2):327–33. doi: 10.1016/0092-8674(83)90452-x [DOI] [PubMed] [Google Scholar]
- 18.Dorman CJ. H-NS: A universal regulator for a dynamic genome. Nat Rev Microbiol. 2004. May;2(5):391–400. doi: 10.1038/nrmicro883 [DOI] [PubMed] [Google Scholar]
- 19.Chaparian RR, Tran MLN, Miller Conrad LC, Rusch DB, Van Kessel JC. Global H-NS counter-silencing by LuxR activates quorum sensing gene expression. Nucleic Acids Res. 2020. Jan 10;48(1):171–83. doi: 10.1093/nar/gkz1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pabo CO, Lewis M. The operator-binding domain of λ repressor: Structure and DNA recognition. Nature. 1982;298(5873):443–7. doi: 10.1038/298443a0 [DOI] [PubMed] [Google Scholar]
- 21.Hecht MH, Sauer RT. Phage lambda repressor revertants. Amino acid substitutions that restore activity to mutant proteins. J Mol Biol. 1985. Nov 5;186(1):53–63. doi: 10.1016/0022-2836(85)90256-6 [DOI] [PubMed] [Google Scholar]
- 22.Lewis M, Jeffrey A, Wang J, Ladner R, Ptashne M, Pabo CO. Structure of the operator-binding domain of bacteriophage λ repressor: Implications for DNA recognition and gene regulation. Cold Spring Harb Symp Quant Biol. 1982;47(1):435–40. [DOI] [PubMed] [Google Scholar]
- 23.Eliason JL, Weiss MA, Ptashne M. NH2-terminal arm of phage λ repressor contributes energy and specificity to repressor binding and determines the effects of operator mutations. Proc Natl Acad Sci U S A. 1985. Apr 1;82(8):2339–43. doi: 10.1073/pnas.82.8.2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson HCM, Sauer RT. Lambda repressor mutations that increase the affinity and specificity of operator binding. Cell. 1985. Sep 1;42(2):549–58. doi: 10.1016/0092-8674(85)90112-6 [DOI] [PubMed] [Google Scholar]
- 25.Jordan SR, Pabo CO. Structure of the lambda complex at 2.5 Å resolution: Details of the repressor-operator interactions. Science. 1988;242(4880):893–9. doi: 10.1126/science.3187530 [DOI] [PubMed] [Google Scholar]
- 26.Duddy OP, Bassler BL. Quorum sensing across bacterial and viral domains. PLOS Pathog. 2021. Jan 7;17(1):e1009074. doi: 10.1371/journal.ppat.1009074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Kessel JC, Ulrich LE, Zhulin IB, Bassler BL. Analysis of activator and repressor functions reveals the requirements for transcriptional control by LuxR, the master regulator of quorum sensing in Vibrio harveyi. MBio. 2013. Jul 9;4(4). doi: 10.1128/mBio.00378-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J, Winans SC. Autoinducer binding by the quorum-sensing regulator TraR increases affinity for target promoters in vitro and decreases TraR turnover rates in whole cells. Proc Natl Acad Sci U S A. 1999. Apr 27;96(9):4832–7. doi: 10.1073/pnas.96.9.4832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swem LR, Swem DL, O’Loughlin CT, Gatmaitan R, Zhao B, Ulrich SM, et al. A quorum-sensing antagonist targets both membrane-bound and cytoplasmic receptors and controls bacterial pathogenicity. Mol Cell. 2009. Jul 31;35(2):143–53. doi: 10.1016/j.molcel.2009.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuster M, Urbanowski ML, Greenberg EP. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc Natl Acad Sci U S A. 2004. Nov 9;101(45):15833–9. doi: 10.1073/pnas.0407229101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Winans SC. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc Natl Acad Sci U S A. 2001. Feb 13;98(4):1507–12. doi: 10.1073/pnas.98.4.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9(4):773–86. doi: 10.1111/j.1365-2958.1993.tb01737.x [DOI] [PubMed] [Google Scholar]
- 33.Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014. Jul 1;42(W1):W320–4. doi: 10.1093/nar/gku316 [DOI] [PMC free article] [PubMed] [Google Scholar]