Abstract
The differentiation efficiency of adult stem cells undergoes a significant decline in aged animals, which is closely related to the decline in organ function and age-associated diseases. However, the underlying mechanisms that ultimately lead to this observed decline of the differentiation efficiency of stem cells remain largely unclear. This study investigated Drosophila midguts and identified an obvious upregulation of caudal (cad), which encodes a homeobox transcription factor. This factor is traditionally known as a central regulator of embryonic anterior-posterior body axis patterning. This study reports that depletion of cad in intestinal stem/progenitor cells promotes quiescent intestinal stem cells (ISCs) to become activate and produce enterocytes in the midgut under normal gut homeostasis conditions. However, overexpression of cad results in the failure of ISC differentiation and intestinal epithelial regeneration after injury. Moreover, this study suggests that cad prevents intestinal stem/progenitor cell differentiation by modulating the Janus kinase/signal transducers and activators of the transcription pathway and Sox21a-GATAe signaling cascade. Importantly, the reduction of cad expression in intestinal stem/progenitor cells restrained age-associated gut hyperplasia in Drosophila. This study identified a function of the homeobox gene cad in the modulation of adult stem cell differentiation and suggested a potential gene target for the treatment of age-related diseases induced by age-related stem cell dysfunction.
Author summary
Adult stem cells undergo an aging-related decline of differentiation efficiency in aged animals. However, the underlying mechanisms that ultimately lead to this observed decline of differentiation efficiency in stem cells still remain largely unclear. By using the Drosophila midgut as a model system, this study identified the homeobox family transcription factor gene caudal (cad), the expression of which is significantly upregulated in intestinal stem cells (ISCs) and progenitor cells of aged Drosophila. Depletion of cad promoted quiescent ISCs to become activate and produce enterocytes (ECs) in midguts under normal gut homeostasis conditions; However, overexpression of cad resulted in the failure of ISC differentiation and intestinal epithelial regeneration after injury. Moreover, cad prevents ISC-to-EC differentiation by inhibiting JAK/STAT signaling, and the expressions of Sox21a and GATAe. Reduction of cad expression in intestinal stem/progenitor cells restrained age-associated gut hyperplasia in Drosophila. These findings enable a detailed understanding of the roles of homeobox genes in the modulation of adult stem cell aging in humans. This will be beneficial for the treatment of age-associated diseases that are caused by a functional decline of stem cells.
Introduction
In metazoans, adult stem cells are responsible for the maintenance of long-term homeostasis of many organs or tissues through their ability to produce terminal differentiated cells to replace lost or damaged cells. Under homeostatic conditions, adult stem cells are largely quiescent, but will promptly be activated to proliferation and differentiation upon tissue injury [1]. However, when animals get old, both the self-renewal and differentiation efficiency of these resident stem cells exhibit significant declines [2]. Many studies have demonstrated that the functional decline of organs can be clearly delayed or even reversed when their resident stem cells are not subject to aging-related functional decline [3]. Preventing the functional decline of adult stem cells is therefore a promising area of anti-aging research. However, because of the complexities of the stem cell niche and external environment, the underlying molecular mechanisms modulating the aging-related functional decline of adult stem cells remain largely unknown.
The midgut of adult Drosophila has emerged as a powerful model system for the study of mechanisms underlying the age-related decline in stem cell function [4–6]. Because of its simple cellular components and wide array of available genetic tools for the Drosophila midgut, this system has been used to identify potential strategies to reverse or delay the dysfunction of stem cells in aged animals [7–9]. Similar to the mammalian intestine, the epithelium of the Drosophila midgut is maintained by resident intestinal stem cells (ISCs) with the ability to self-renew and differentiate into terminal function cells [5,6,10–12]. Drosophila ISCs reside in the basement membrane of the midgut epithelium and specifically express the transcription factor Escargot (Esg) and the Notch ligand Delta (Dl). The ISCs in the Drosophila midgut undergo asymmetric cell division to produce the new ISCs and progenitor cells. When asymmetric dividing of ISCs occurs, ISCs can produce either enteroendocrine mother cells (EMCs, which further differentiate into secretory enteroendocrine cells (EEs)) or non-dividing enteroblasts (EBs, which further differentiate into absorptive enterocytes (ECs)) depending on Notch activity (S1A Fig) [5,6,13–16]. A high level of Notch signaling drives ISCs to produce ECs, while a low level of Notch signaling drives ISCs to produce EEs [14,15]. Under normal homeostatic conditions, most ISCs are in a quiet state and proliferate slowly in young Drosophila. Thus, the numbers of ISCs and progenitor cells are relatively low and remain stable in the midguts of young and healthy Drosophila. However, after injury, these ISCs are rapidly activated in response to damage-induced signals, and temporarily increase their proliferation rates, thus initiating EC and/or EE production [12,17,18]. In aged Drosophila, many ISCs in the midgut are abnormally activated [19–21], and the differentiation capacity of ISCs and EBs follows a continuous decrease with aging [9,20]. This results in the accumulation of Delta positive (Dl+) expressing cells and Esg positive (Esg+) cells in the midgut of aged Drosophila [19,20,22]. Studies have shown that multiple signaling pathways, including reactive oxygen species (ROS) signaling [23,24], endoplasmic reticulum stress-induced signaling [25,26], c-Jun N-terminal kinase (JNK) signaling [20], insulin signaling [27–29], p38-MAPK signaling [22], DGF/VEGF signaling [19], and mTOR signaling [30], play roles in the modulation of aging-related ISC hyper-proliferation. Moreover, preventing the accumulation of Esg+ cells in the midgut obviously extended the lifespan of Drosophila [3]. However, the regulators and signaling pathways that regulate the aging-related decline of the differentiation capacity of ISCs and EBs remain largely unclear.
Homeobox family transcription factors, which exhibit DNA-binding transcription factor activity, are the central regulators of embryonic anterior-posterior body axis pattern formation during development [31]. Caudal (cad) homologs, which belong to the homeobox transcription factor family, have been identified in organisms ranging from Caenorhabditis elegans to humans [32–34]. These homologs have been found to play important roles in intestinal development and maintenance [35]. In the mouse gastrointestinal tract, the mammalian homologs of Cad, Caudal type homeobox 1 (Cdx1) and Caudal type homeobox 2 (Cdx2), have been reported to perform critical roles in the regulation of the developmental process of the intestine [36,37]. Moreover, dysregulations of Cdx1 and Cdx2 have been shown to be involved in the carcinogenesis of gastrointestinal cancers [38]. In addition to its function in the establishment of the anteroposterior axis during early embryogenesis, the Drosophila homeobox gene cad has been shown to regulate gut development [39,40]. It has been demonstrated that cad has very important functions in the innate immune response in the Drosophila midgut [41,42]. Recently, cad has been found to be upregulated in the posterior midgut of aged Drosophila, where it regulates gut immune homeostasis [43]. However, this study [43] did not present the cell-type specifical expression pattern of cad. The function of cad in intestinal stem/progenitor cells and gut epithelial homeostasis remains unknown.
Using the Drosophila midgut as a model system, this study found that the expression of cad increases in ISCs and EBs of aged Drosophila. Furthermore, high levels of cad expression in ISCs and EBs prevent the ISC-to-EC differentiation through modulation of a SOX21A-GATAe-mediated mechanism. Inhibition of cad expression in ISCs and EBs repress age-associated gut hyperplasia and promote healthy aging in Drosophila. This study not only elucidated a new function of the homeobox transcription factor cad in the regulation of stem/progenitor cell differentiation, but also suggests a potential approach for the promotion of healthy aging in humans.
Results
Cad expression increases in Drosophila intestinal stem and progenitor cells upon aging
To identify the potential regulators that modulate ISC aging, differentially expressed genes were analyzed in young and aged intestinal stem and progenitor cells. Analysis of published RNA-sequencing (RNA-seq) data [44] identified the homeobox transcription factor gene caudal (cad). This gene is highly expressed in ISCs and EBs in the R4 and R5 regions of the Drosophila midgut (S1B–S1D Fig). Real-time quantitative PCR (RT-qPCR) analyses using sorted esg-GFP+ cells from young and old Drosophila showed that the cad mRNA is significantly upregulated in aged esg-GFP+ cells (which indicate ISCs and their differentiating progenies) (S1E Fig).
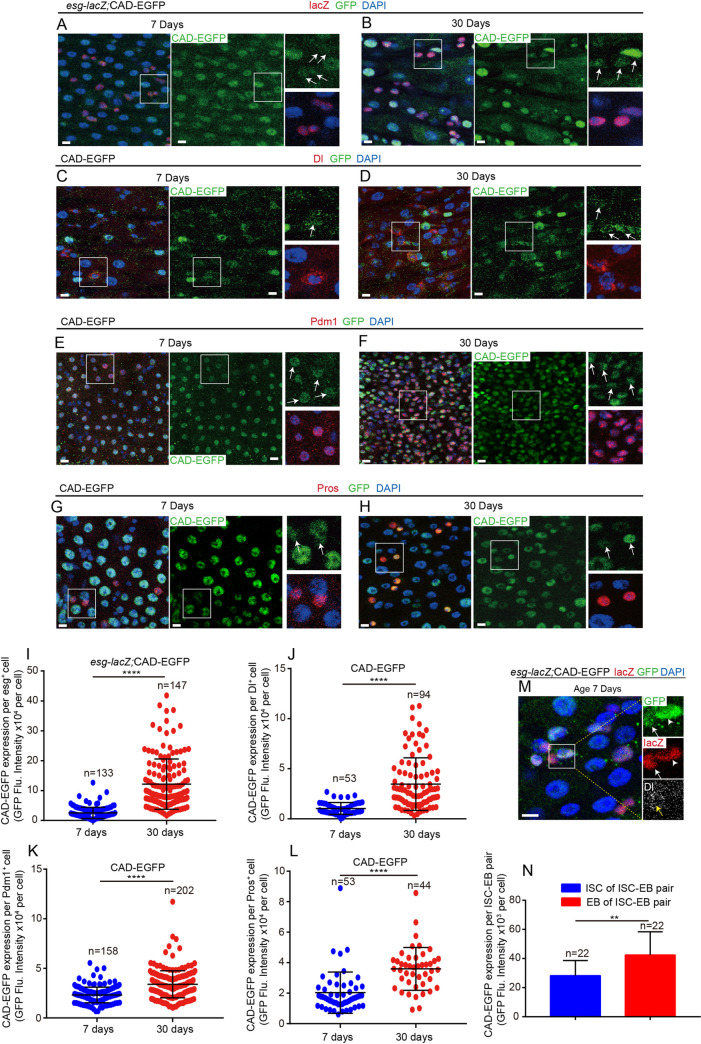
As a homeobox transcription factor, cad upregulation has been reported in the posterior midgut of aged Drosophila [43], where it regulates gut immune homeostasis [41,42]. However, these studies did not show the cell-type specifical expression pattern of cad. The function of cad in intestinal stem/progenitor cells and gut epithelial homeostasis remains unknown. To characterize the cell-type-specific expression pattern of the endogenous CAD protein (encoded by cad) upon aging, the GFP-tagged protein trap line cad-EGFPVK00033 [45] was used. Consistent with the published RNA-seq data (S1D Fig), CAD protein expressed almost in all cell types of midguts (Fig 1A–1L). Immunostaining results showed that CAD mainly expresses in the posterior (R4 and R5 regions) midgut (S1G–S1I Fig) but barely or slightly expresses in the anterior (R1 and R2 regions) midguts and CCR (R3 region) midguts (S1G–S1I Fig). Observing the CAD-EGFP reporter line showed that the protein expression of CAD had significantly increased in intestinal epithelial cells in the R4 and R5 regions of the midgut upon aging (Fig 1A–1L). In addition, the expression of CAD in some ECs and EEs seemed higher than in ISCs of young Drosophila (Fig 1C, 1E and 1G). However, the increase of CAD proteins in ISCs and EBs (Fig 1A–1L) of old Drosophila was more dramatic compared with the increase in ECs or EEs (Fig 1A–1L). The western blot analyses also showed that the expression level of CAD dramatically increased in aged midguts compared with its expression in young midguts (S1F Fig). By co-staining of esg-lacZ, Dl, and CAD-EGFP, we found that the EBs (esg-lacZ+Dl- cells) displayed a higher CAD expression level than the ISCs in the same ISC-EB pairs in young flies (Fig 1M and 1N). Moreover, paraquat (PQ)- or dextran sulphate sodium (DSS)-induced damages to Drosophila midgut could dramatically activate the expression of CAD (S1J–S1K Fig). The obtained results suggest that, except for regulating the gut immune response, cad might also function in ISCs or EBs and regulate gut homeostasis upon aging.
Fig 1. Expression of cad dramatically increases in Drosophila intestinal stem cells (ISCs) and progenitor cells upon aging.
(A-B) Expression of endogenous CAD-EGFP protein (green) in esg+ cells (ISCs and EBs; co-stained with esg-LacZ, red) in midguts from the R4 region of 7-day (A) and 30-day-old (B) Drosophila. The enlarged insets show esg-LacZ+ cells (red) with CAD-EGFP (green) staining. Arrows indicate esg-LacZ+ cells. (C-D) Expression of endogenous CAD-EGFP protein (green) in ISCs (labeled by Dl staining, red) in midguts from the R4 region of 7-day (C) and 30-day-old (D) Drosophila. The enlarged insets show ISCs (red) with CAD-EGFP (green) staining. Arrows indicate Dl+ ISCs. (E-F) Expression of endogenous CAD-EGFP protein (green) in ECs (labeled by Pdm1 staining, red) in midguts from the R4 region of 7-day (E) and 30-day-old (F) Drosophila. The enlarged insets show ECs (red) with CAD-EGFP (green) staining. Arrows indicate Pdm1+ ECs. (G-H) Expression of endogenous CAD-EGFP protein (green) in EEs (labeled by Prospero staining, red) in midguts from the R4 region of 7-day (G) and 30-day-old (H) Drosophila. The enlarged insets show EEs (red) with CAD-EGFP (green) staining. Arrows indicate Pros+ EEs. (I) Quantification of fluorescence intensity of CAD-EGFP in esg-LacZ+ cells of 7-day and 30-day-old Drosophila as shown in (A-B). (J) Quantification of fluorescence intensity of CAD-EGFP in Dl+ ISCs of 7-day and 30-day-old Drosophila as shown in (C-D). (K) Quantification of fluorescence intensity of CAD-EGFP in Pdm1+ ECs of 7-day and 30-day-old Drosophila as shown in (E-F). (L) Quantification of fluorescence intensity of CAD-GFP in Pros+ EEs of 7-day and 30-day-old Drosophila as shown in (G-H). (M) Representative images showing the expression pattern of CAD in young Drosophila midgut. The enlarged insets show esg-LacZ+ cells (red) with CAD-GFP (green) staining. Arrows indicate esg-LacZ+ Dl+ cells (ISCs). Arrowheads indicate esg-LacZ+ Dl- cells (EBs). (N) Quantification of fluorescence intensity of CAD-EGFP in ISCs or EBs from per ISC-EB pair as shown in (M). The number n is indicated. Each dot represents one cell. DAPI stained nuclei (blue). Scale bars represent 10μm (in Fig 1A-1H and 1M). Error bars represent SD. Significance was assessed via student’s t-tests: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns (non-significant) represents p > 0.05. See also S1 Fig.
Depletion of cad in intestinal stem and progenitor cells causes accumulation of premature enterocytes in the Drosophila midgut
To investigate the function of cad in Drosophila intestinal stem and progenitor cells, cad was depleted in ISCs and EBs via conditional temperature-sensitive esg-Gal4 (esgts)-driven two different RNA interference (RNAi) lines (BDSC# 57546 and BDSC# 34702). The RT-qPCR analyses showed a significant reduction of cad mRNA expression in the midguts of flies carrying either tub-Gal4>UAS-cad RNAiBL57546 or tub-Gal4>UAS-cad RNAiBL34702 compared to that in the midguts of flies carrying tub-Gal4>UAS-GFP (S2A Fig).
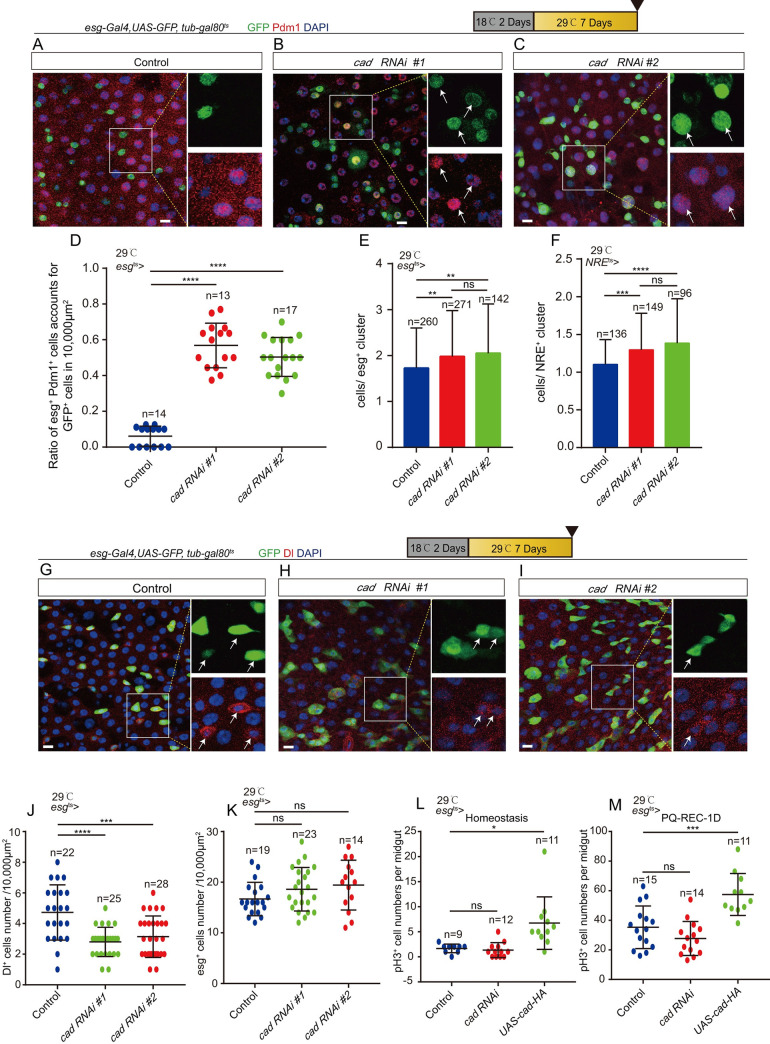
Depletion of cad in ISCs and EBs promoted the progenitors (esg+ cells) to differentiate into ECs (Pdm1 positive and polyploid cells), as indicated by the increase of both esg+ and Pdm1+ transient premature ECs (pre-ECs) in midguts (Figs 2A–2D and S2B–S2D). This phenotype is quite similar to that reported in Drosophila carrying esgts-driven Sox21a overexpression (a Drosophila Sox transcription factor that drives EB-to-EC differentiation [46–48]). Similar to the phenotype of overexpression of Sox21a in esg+ cells [48], we found that knockdown of cad in esg+ cells led to an increase in the number of cells in the esg+ clusters (Fig 2E). Besides, we found that depletion of cad in the EBs by NRE-Gal4 (NREts) also formed NRE+ clusters (Fig 2F). Moreover, depletion of cad in esg+ cells did not decrease the number of esg+ cells but decreased the number of ISCs (Delta (Dl)+ cells) (Figs 2G–2K and S2E–S2H) in the posterior region (PMG) of the midguts. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analyses were used to detect cell death in the midguts. No significant increase in cell death was found in the midguts of Drosophila carrying esgts-driven cad RNAi compared with the controls (S2M and S2N Fig). Inhibition of cell apoptosis by depletion of reaper (rpr) in esg+ cells could not rescue the phenotype of Dl+ cell loss caused by cad depletion (S2O–S2R Fig). Thus, we ruled out the possibility that the reduction of ISCs in cad-depleted flies was caused by ISC cell death. Moreover, we found that depletion of cad in esg+ cells also did not significantly affect the proliferation rate of ISCs (indicated by phosphor-histone H3 (pH3) staining) either under homeostatic conditions or after injury (Fig 2L and 2M).
Fig 2. Depletion of cad in ISCs and progenitor cells promotes ISC-to-EC differentiation.
(A-C) Immunofluorescence images of esg-GFP (green) and Pdm1 (red) staining with the midgut section from the R4 region of control Drosophila (A, esgts-Gal4>UAS-GFP) and the cad-depleted Drosophila by esgts-Gal4-driven two different cad RNAi lines (B, cad RNAi #1: BDSC #57546; C, cad RNAi #2: BDSC #34702). esg-GFP (green) represents ISCs and their differentiating cells. Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (both esg-GFP+ and Pdm1+ cells). esg-GFP+ and Pdm1- cells are ISCs or EBs. esg-GFP- and Pdm1+ cells are mature ECs. (D) Quantification of the ratio of esg-GFP+ and Pdm1+ cells account for GFP+ cells per 10,000 μm2 area of the R4 region midguts as shown in (A-C). The number n represents the ROI in midguts from each experiment. One dot corresponds to one ROI (10,000 μm2 area). (E) Quantification of the average number of cells in each esg-GFP+ cluster in midguts of control flies (esgts>UAS-GFP) and flies knocking down of cad in esg-GFP+ cells. (F) Quantification of the average number of cells in each NRE-GFP+ cluster in midguts of control flies (NREts>UAS-GFP) and flies knocking down of cad in NRE-GFP+ cells. (G-I) Immunofluorescence images of esg-GFP (green) and Dl (red) staining with the midgut section from the R4 region of control Drosophila (G, esgts-Gal4>UAS-GFP) and cad-depleted Drosophila by esgts-Gal4-driven two different cad RNAi lines (H, cad RNAi #1; I, cad RNAi #2). esg-GFP (green) represents ISCs and their differentiating cells. Dl staining (red) was used to visualize ISCs. White arrows indicate Dl+ ISCs. (J-K) Quantification of the numbers of Dl+ ISCs (J) and esg-GFP+ cells (K) in a 10,000 μm2 area of the R4 region midguts from control Drosophila and Drosophila carrying esgts-Gal4>cad RNAi as shown in (G-I). The number n represents the ROI in midguts from each experiment. One dot corresponds to one ROI (10,000 μm2 area). (L) Quantification of the pH3+ number in the whole midgut from control flies (esgts-Gal4-driven UAS-GFP), Drosophila carrying esgts-Gal4-driven cad RNAi, and Drosophila carrying esgts-Gal4-driven UAS-cad-HA in homeostasis. The number n is indicated. Each dot represents one midgut. (M) Quantification of the pH3+ number in the whole midgut from control flies (esgts-Gal4-driven UAS-GFP), Drosophila carrying esgts-Gal4-driven cad RNAi, and Drosophila carrying esgts-Gal4-driven UAS-cad-HA. Flies were treated with PQ for one day, then recovered on normal food for 1 day (hereafter referred to as PQ-REC-1D). The number n is indicated. Each dot represents one midgut. DAPI stained nuclei (blue). Scale bars represent 10 μm (Fig 2A–2C and 2G–2I). Error bars represent SD. Student’s t-tests were used to assess statistical significane as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, while ns (non-significant) represents p > 0.05. See also S2 Fig.
Overexpression of cad in enteroblasts prevents enterocyte formation
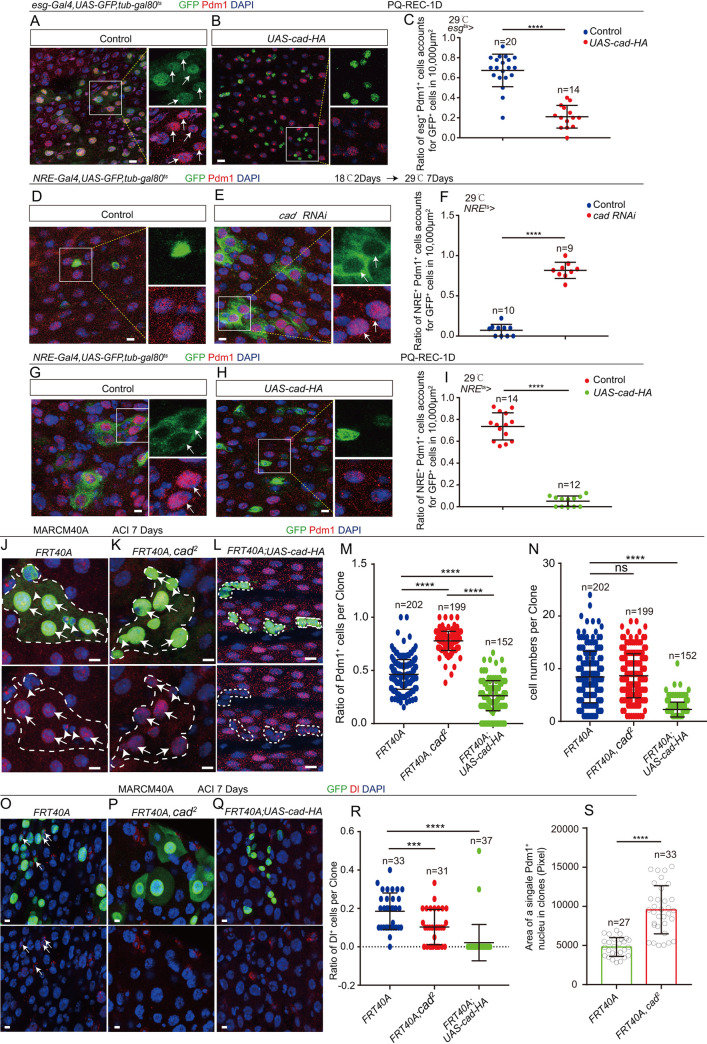
Since depletion of cad promotes ISCs to differentiate into ECs, this study hypothesized that overexpression of this protein might prevent the progenitor cells from differentiating into mature ECs. Under normal conditions, the majority of ISCs in young Drosophila midguts are in a quiescent state [10,12,49,50]. As expected, under normal conditions overexpression of cad in esg+ or in NRE+ cells did not induce dramatic proliferation or differentiation phenotype (Figs 2L, S2E–S2H, and S3A–S3F). To induce these quiescent ISCs to become active and produce ECs, paraquat (PQ) was used to induce epithelial injury in the midgut [12]. After the damage had been induced, the ISCs in the control flies were activated and began to quickly proliferate and differentiate into ECs. During gut epithelial repair (after 24 h of PQ treatment and 24 h of regeneration), many transient pre-ECs (both esg+ and Pdm1+ cells) were found in the midguts of the control Drosophila carrying UAS-GFP (Fig 3A and 3C). However, in the midguts of Drosophila carrying esgts-driven cad overexpression only diploid esg+ cells (either ISCs or EBs), but few differentiating pre-ECs (both esg+ and Pdm1+ polyploid cells), were found (Fig 3B and 3C).
Fig 3. Overexpression of cad in EBs prevents ISCs to produce differentiated ECs.
(A-B) Immunofluorescence images of esg-GFP (green) and Pdm1 (red) staining with the midgut section from the R4 region of control Drosophila (A, esgts-Gal4>UAS-GFP) and Drosophila carrying esgts-Gal4> UAS-cad-HA (B). Drosophila were treated with PQ (PQ-REC-1D). esg-GFP (green) represents ISCs and their differentiating cells. Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (esg-GFP+ and Pdm1+cells). esg-GFP+ and Pdm1- cells are ISCs or EBs. esg-GFP- and Pdm1+ cells are mature ECs. (C) Quantification of the ratio of esg-GFP+ and Pdm1+ cells in a 10,000 μm2 area of the R4 region of midguts of Drosophila as shown in (A-B). The number n represents the ROI in midguts from each experiment. One dot corresponds to one ROI (10,000 μm2 area). (D-E) Immunofluorescence images of NRE-GFP (green) and Pdm1 (red) staining with the midgut section from the R4 region of 9-day-old (2 days at 18°C, then 7 days at 29°C) control Drosophila (D, NREts-Gal4>UAS-GFP) and cad-depleted Drosophila carrying NREts-Gal4> UAS-cad RNAi (E). NRE-GFP (green) represents EBs. Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (NRE-GFP+ and Pdm1+ cells). NRE-GFP+ and Pdm1- cells are EBs. NRE-GFP- and Pdm1+ cells are mature ECs. (F) Quantification of the ratio of NRE-GFP+ and Pdm1+ cells in a 10,000 μm2 area of the R4 region of midguts as shown in (D-E). The number n represents the ROI in midguts from each experiment. One dot corresponds to one ROI (10,000 μm2 area). (G-H) Immunofluorescence images of NRE-GFP (green) and Pdm1 (red) staining with the midgut section from the R4 region of control Drosophila (G, NREts-Gal4>UAS-GFP) and Drosophila carrying NREts-Gal4>UAS-cad-HA (H). Drosophila were treated with PQ (PQ-REC-1D). NRE-GFP (green) represents EBs. Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (NRE-GFP+ and Pdm1+cells). NRE-GFP+ and Pdm1- cells are EBs. NRE-GFP- and Pdm1+ cells are mature ECs. (I) Quantification of the ratio of NRE-GFP+ and Pdm1+ cells account for GFP+ cells in a 10,000 μm2 area of the R4 region midguts as shown in (G-H). The number n represents the ROI in midguts from each experiment. One dot corresponds to one ROI (10,000 μm2 area). (J-L) Immunofluorescence analyses of control (FRT40A, J), cad2 mutant (K), and cad-HA overexpressing (L) mosaic analysis with repressible cell marker (MARCM) clones (green, outlined by white dotted lines) 7 days after clone induction (ACI) of flies. Pdm1 staining (red) was used to visualize ECs. White arrows indicate Pdm1+ polyploid ECs, and white arrowheads indicate Pdm1- diploid cells in (J). (M) Quantification of the ratio of Pdm1+ cells per clone with indicated genotypes of experiments presented in (J-L). Each dot corresponds to one clone. (N) Quantification of the GFP+ cells per clone with indicated genotypes of experiments presented in (J-L). Each dot corresponds to one clone. (O-Q) Immunofluorescence analyses of control (FRT40A, O), cad2 mutant (P), and cad-HA overexpressing (Q) mosaic analysis with repressible cell marker (MARCM) clones (green) 7 days after clone induction (ACI) of flies. Dl staining (red) was used to visualize ISCs. White arrows indicate ISCs in clones. (R) Quantification of the ratio of Dl+ cells per clone with indicated genotypes of experiments presented in (O-Q). Each dot corresponds to one clone. (S) Quantification of the average size of Pdm1+ ECs in control clones (FRT40A) and cad2 mutant clones. The number n represents Pdm1+ ECs in clones. DAPI-stained nuclei (blue). Scale bars represent 10μm (Fig 3A, 3B, 3D, 3E, 3G and 3H), or 5μm (Fig 3J–3L and 3O–3Q). Error bars represent SD. Student’s t-tests were used to assess statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns (non-significant), which represents p > 0.05. See also S3 Fig.
Moreover, we found that overexpression of cad in esg+ cells led to a slight increase of ISC proliferation (indicated by the increases of pH3+ cells, Dl+ cells, and total esg + cells) after PQ injury (Figs 2M and S2I–S2L). The slight increase of ISC proliferation in cad-overexpressed in the midguts may because that overexpression of CAD in esg+ cells caused the failure of ISC-to-EC differentiation, which in turn caused the formation of EB tumors in midguts. The EB tumors send EGF (Spi) and JAK-STAT ligands (Upd2) to neighboring ISCs to drive their proliferation [46,47].
To further delineate the role of cad in progenitor differentiation, cad was either specifically overexpressed or depleted in EBs using the temperature-sensitive NRE-Gal4 (NREts) [51] or in ISCs using the temperature-sensitive ISC-Gal4 (ISCts) [51]. Depletion of cad in EBs (but not in ISCs) induced the accumulation of pre-ECs in the midgut (Figs 3D–3F and S3G–S3I). After injury (induced by PQ treatment), overexpression of cad in EBs (but not ISCs) led to the failure of the formation of pre-ECs (Figs 3G–3I and S3J–S3L). Thus, the regulation of cad expression in EBs is critical for regulating the ISC-to-EC differentiation and maintaining gut epithelial homeostasis.
To further demonstrate that cad participates in the promotion of ISC-to-EC differentiation, the lineages of cad-null (cad2) and cad-overexpressed ISCs were traced by performing mosaic analysis with repressible cell marker (MARCM) [5,6]. After clone induction (ACI) for 7 days, wild-type clones contained a few Pdm1 negative (Pdm1-) diploid cells and a cluster of Pdm1+ polyploid ECs (Fig 3J); however, cad-overexpressing clones almost exclusively consisted of Pdm1 negative (Pdm1-) diploid cells (few Pdm1+ polyploid ECs were found in cad-overexpressing clones) (Fig 3L). By calculating the ratio of Pdm1+ polyploid ECs per clone, the cad-null clones (Fig 3K) were found to show an obvious increase in EC production, while cad-overexpressing clones showed a significant failure of EC production compared with the wild-type control (FRT40A) (Fig 3J and 3M). Interestingly, cad-overexpressing clones showed a significantly decreased clone size compared with the wild-type control (FRT40A) (Fig 3N). This proliferation defect of cad-overexpressing clones was similar to the Sox21a mutant clones [46]. Moreover, we detected the composition of cell types in cad-null and cad-overexpressing clones (Figs 3O–3R and S3M–S3O). The results showed that cad-overexpressing and cad mutant clones exhibited a significant low ratio of Dl+ cells compared to genotype control clones (FRT40A) (Fig 3O and 3R). These cells in cad-overexpression clones did not express the EE marker Prospero (S3O Fig). In addition, the average size of Pdm1+ ECs in the Caudal-null clones exceeded that of the control clones (FRT40A) (Fig 3S). This suggests that cad might regulate the endopolyploidy process of ISC-to-EC differentiation.
Cad regulates intestinal stem cell to enterocyte differentiation by modulating Sox21a expression
To investigate the mechanism with which cad regulates ISC-to-EC differentiation, RNA-seq was performed on dissected midguts with cad depleted in esg+ cells (esgts> cad RNAi) as well as control midguts carrying esgts-Gal4 only (S1 Table).
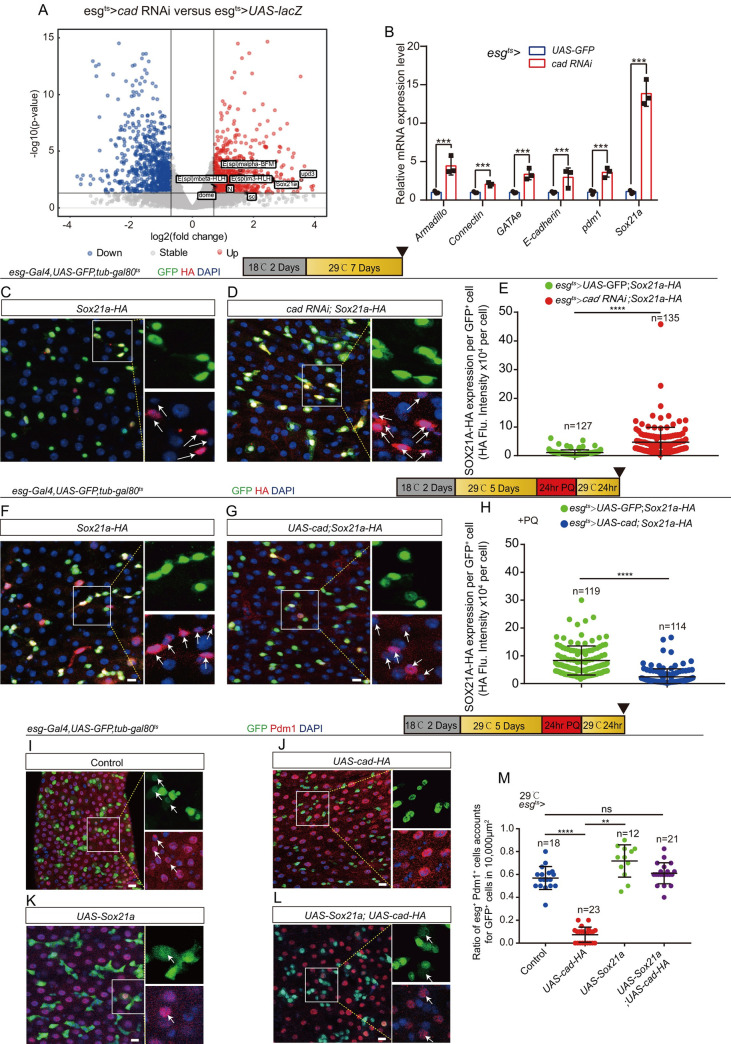
A number of genes that have been reported to regulate the differentiation of ISCs or progenitor cells in Drosophila were significantly upregulated in midguts with cad depleted in esg+ cells (Fig 4A). Among these genes, the SOX family transcription factor SOX21A, which is essential for ISC-to-EC differentiation, was found [46–48]. RT-qPCR analyses using sorted esg-GFP+ cells also showed that the mRNA expression of Sox21a and several demonstrated downstream genes of Sox21a (including pdm1, GATAe, Connectin, armadillo, and E-cadherin) significantly increased in the midgut of cad-depleted Drosophila (Fig 4B). Since cad mutant has a similar differentiation defect to SOX21A overexpression in EBs [46], it is reasonable to hypothesize that cad might regulate the ISC-to-EC differentiation through a SOX21A-mediated mechanism.
Fig 4. Cad regulates ISC-to-EC differentiation by modulating Sox21a expression.
(A) Volcano plots of differentially expressed genes in a pair-wise comparison of cad-depleted Drosophila (esgts-Gal4>cad RNAi) midguts to control Drosophila (esgts-Gal4>UAS- lacZ) midguts. (B) Relative mRNA fold changes of Armadillo, Connectin, GATAe, E-cadherin, pdm1, and Sox21a in sorted esg+ cells from of young (2 days at 18°C then transferred to 29°C for 5 days) genotype control flies’ midguts (blue lines, esgts-Gal4-driven UAS-GFP) and cad-depleted midguts (red lines, esgts-Gal4-driven cad RNAi). The changes of expressions were plotted relative to genotype controls, which was set to 1. Error bars indicate the standard deviation (SD) of three independent experiments. (C-D) Representative images showing the expression of endogenous SOX21A-HA (red) in midguts of Drosophila carrying esgts-Gal4>UAS-GFP (control; C) and midguts carrying esgts-Gal4>cad RNAi (D). The right enlarged insets from (C-D) show the single channel of esg-GFP (green) and SOX21A-HA (red). (E) Quantification of fluorescence intensity of SOX21A-HA with indicated genotypes as shown in (C-D). One dot represents one esg-GFP+ cell. (F-G) Representative images showing the expression of endogenous SOX21A-HA (red) in midguts of Drosophila carrying esgts-Gal4>UAS-GFP (control; F) and midguts carrying esgts-Gal4>UAS-cad (G). Drosophila were treated with PQ (PQ-REC-1D). The right enlarged insets from (F-G) show the single channel of esg-GFP (green) and SOX21A-HA (red). (H) Quantification of fluorescence intensity of SOX21A-HA with indicated genotypes as shown in (F-G). One dot represents one esg-GFP+ cell. (I-L) Immunofluorescence images of esg-GFP (green) and Pdm1 (red) staining with the midgut section from the R4 region of control Drosophila (I; esgts-Gal4>UAS-GFP), Drosophila carrying esgts-Gal4>UAS-cad-HA (J), Drosophila carrying esgts-Gal4>UAS-Sox21a (K), and Drosophila carrying esgts-Gal4-driven UAS-cad-HA and UAS-Sox21a (L). Drosophila were treated with PQ. esg-GFP (green) represents ISCs and their differentiating cells. Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (esg-GFP+ and Pdm1+ cells). esg-GFP+ and Pdm1- cells are ISCs or EBs. esg-GFP- and Pdm1+ cells are mature ECs. (M) Quantification of the ratio of esg-GFP+ and Pdm1+ cells per 10,000 μm2 area of the R4 region midguts as indicated in (I-L). The number n represents the ROI in midguts from each experiment. One dot corresponds to one ROI (10,000 μm2 area). DAPI stained nuclei (blue). Scale bars represent 10 μm (Fig 4C, 4D, 4F, 4G and 4I–4L). Error bars represent SD. Student’s t-tests were used to assess significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns (non-significant), which represents p > 0.05. See also S4 Fig.
To further confirm the results of the RNA-seq analyses (i.e., that cad regulates the expression of Sox21a in esg+ cells), the endogenous SOX21A reporter line Sox21a-HA [52] was used. As expected, the expression of SOX21A-HA protein in cad-depleted esg+ cells was stronger compared with its expression in the control esg+ cells (Fig 4C–4E). Moreover, the western blot analyses also showed that the expression level of SOX21A-HA dramatically increased in cad-depleted midguts compared with its expression in the control midguts (S4A–S4C Fig). Interestingly, we noticed that, while the expression of CAD in the intestinal stem/progenitor cells increased upon aging, the expression of SOX21A in these stem/progenitor cells declined (S4D, S4E, S4G, S4H and S4I Fig).
After injury, while SOX21A expression was dramatically upregulated in the control esg+ cells (Figs 4F, 4H and S4F), it still retained a relatively low level in cad-overexpressing esg+ cells (Fig 4G and 4H). More importantly, forced expression of Sox21a in esg+ cells fully restored the failure of cad-overexpressed esg+ cells to produce pre-ECs after injury as evidenced by the generation of both Pdm1+ and esg+ cells (Fig 4I–4M). These data suggest that cad regulates ISC-to-EC differentiation by inhibiting the expression of Sox21a in esg+ cells.
GATAe functions as a downstream of cad to regulate intestinal stem cell to enterocyte differentiation
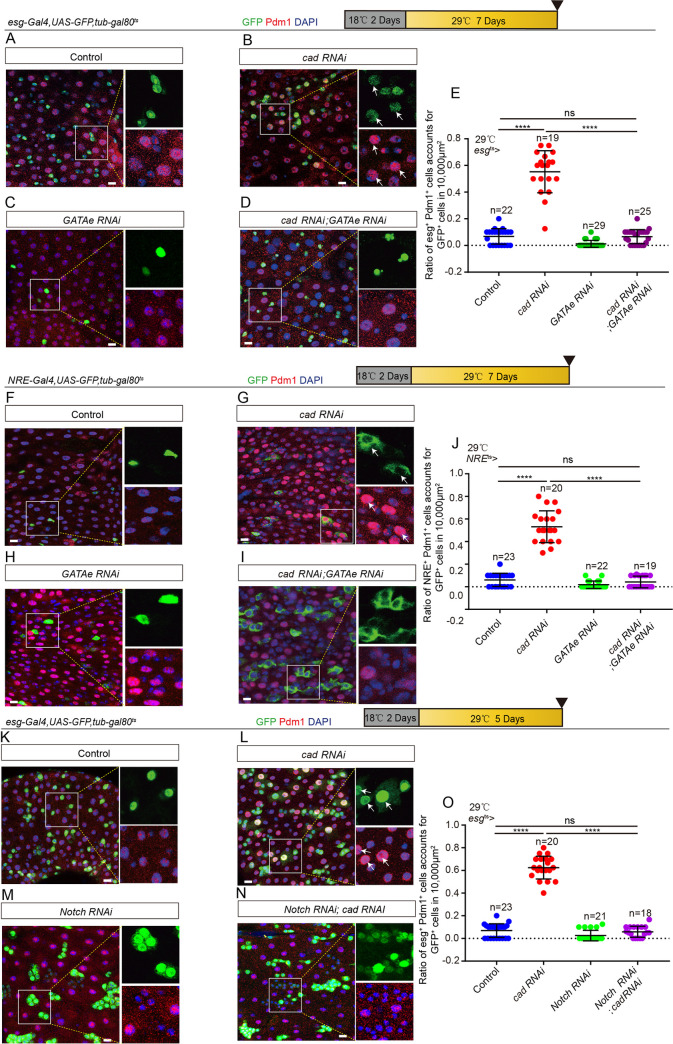
Since previous studies have shown that GATAe (a gene encoding a GATA family transcription factor, which is a downstream of Sox21a) regulates ISC-to-EC differentiation in Drosophila [48,52], the genetic interaction between cad and GATAe was tested. Depletion of GATAe in either esg+ cells (Fig 5A–5E) or EBs (Fig 5F–5J) significantly restored the differentiation defect of the accumulation of pre-ECs seen in cad-depleted midguts as evidenced by decreases of both esg+ and Pdm1+ cells. Overexpression of cad in esg+ cells did not show a noticeable rescue of the defect of the accumulation pre-ECs seen in GATAe-overexpressing midguts (S5A–S5E Fig). Thus, GATAe functioned as a downstream gene of cad to regulate ISC-to-EC differentiation in Drosophila.
Fig 5. GATAe functions downstream of cad to regulate ISC-to-EC differentiation.
(A-D) Immunofluorescence images of esg-GFP (green) and Pdm1 (red) staining with the midgut section from the R4 region of control Drosophila (A, esgts-Gal4>UAS-GFP), Drosophila carrying esgts-Gal4>cad RNAi (B), Drosophila carrying esgts-Gal4>GATAe RNAi (C), and Drosophila carrying esgts-Gal4-driven cad RNAi and GATAe RNAi (D), under normal conditions. esg-GFP (green) represents ISCs and their differentiating cells. Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (esg-GFP+ and Pdm1+cells). esg-GFP+ and Pdm1- cells are ISCs or EBs. esg-GFP- and Pdm1+ cells are mature ECs. (E) Quantification of the ratio of esg-GFP+ and Pdm1+ cells per 10,000 μm2 area of the R4 region midguts of Drosophila with genotypes as indicated in (A-D). The number n represents the ROI in midguts from each experiment. One dot corresponds to one ROI (10,000 μm2 area). (F-I) Immunofluorescence images of NRE-GFP (green) and Pdm1 (red) staining of the midgut section from the R4 region of control Drosophila (F, NREts-Gal4>UAS-GFP), Drosophila carrying NREts-Gal4>cad RNAi (G), Drosophila carrying NREts-Gal4>GATAe RNAi (H), and Drosophila carrying NREts-Gal4-driven cad RNAi and GATAe RNAi (I), under normal conditions. Pdm1 staining (red) visualizes differentiating ECs. White arrows indicate differentiating pre-ECs (NRE-GFP+ and Pdm1+ cells). NRE-GFP+ and Pdm1- cells are EBs. NRE-GFP- and Pdm1+ cells are mature ECs. (J) Quantification of the ratio of NRE-GFP+ and Pdm1+ cells per 10,000 μm2 area of the R4 region midguts as indicated in (F-I). The number n represents the ROI in midguts from each experiment. One dot corresponds to one ROI (10,000 μm2 area). (K-N) Immunofluorescence images of esg-GFP (green) and Pdm1 (red) staining with the midgut section from the R4 region of control Drosophila (K, esgts-Gal4>UAS-GFP), Drosophila carrying esgts-Gal4>cad RNAi (L), Drosophila carrying esgts-Gal4>Notch RNAi (M), and Drosophila carrying esgts-Gal4-driven cad RNAi and Notch RNAi (N), under normal conditions. esg-GFP (green) represents ISCs and their differentiating cells. Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (esg-GFP+ and Pdm1+cells). esg-GFP+ and Pdm1- cells are ISCs or EBs. esg-GFP- and Pdm1+ cells are mature ECs. (O) Quantification of the ratio of esg-GFP+ and Pdm1+ cells per 10,000 μm2 area of the R4 region midguts as indicated in (K-N). The number n represents the ROI in midguts from each experiment. One dot corresponds to one ROI (10,000 μm2 area). DAPI stained nuclei (blue). Scale bars represent 10 μm (Fig 5A–5D, 5F–5I and 5K–5N). Error bars represent SD. Student’s t-tests were used to assess statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and NS (non-significant), which represents p > 0.05. See also S5 Fig.
Since it has been shown that GATAe regulates ISC-to-EC differentiation by modulating the Notch signaling [53], the genetic interaction between cad and the Notch signaling pathway was also tested. Depletion of Notch in esg+ cells significantly restored the increase of pre-ECs production in midguts with cad-depleted esg+ cells (Fig 5K–5O).
Cad promotes intestinal stem cell to enterocyte differentiation through manipulation of the JAK/STAT signaling pathway
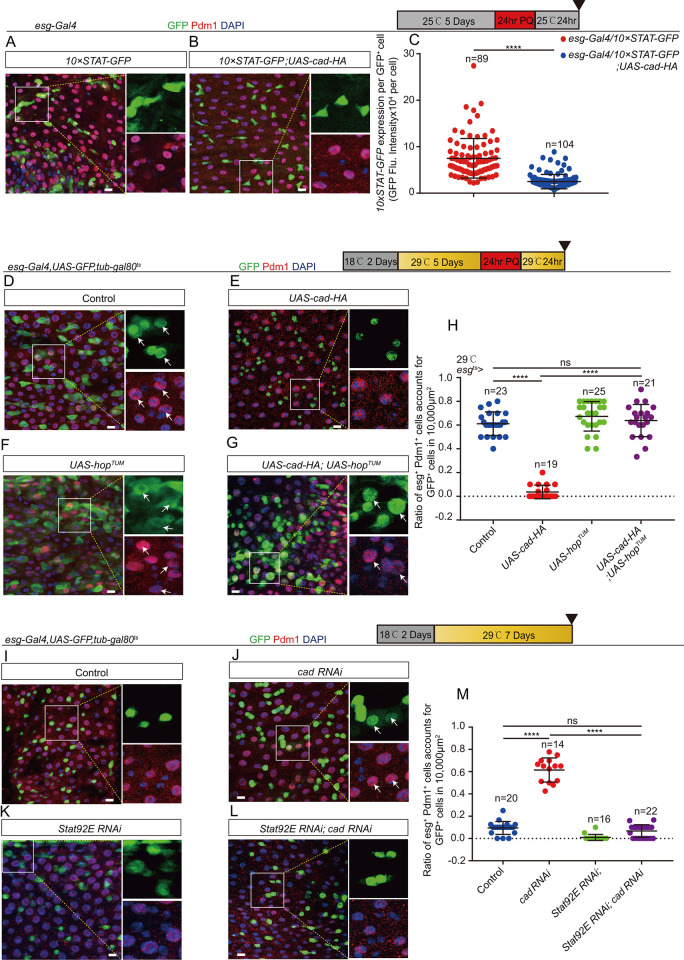
Previous studies have shown that the Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway functions as a upstream of Sox21a in EBs and promotes the maturation of ECs [46,48]. Thus, the genetic interaction between cad and the JAK/STAT signaling pathway was explored. After injury (induced by PQ treatment), while the activity of JAK/STAT signaling was significantly upregulated in control midguts, as indicated by the 10XSTAT-GFP transcriptional reporter (Fig 6A and 6C), it still retained a relatively low level in cad-overexpressing midguts (Fig 6B and 6C).
Fig 6. Cad promotes ISC-to-EC differentiation by manipulating the JAK/STAT signaling pathway.
(A-B) Representative images showing the expression of 10xSTAT-GFP (green) in midguts of Drosophila carrying esg-Gal4 only (control; A) and Drosophila carrying esg-Gal4>UAS-cad-HA (B) treated with PQ. Pdm1 (red) staining identifies pre-ECs and matured ECs. (C) Quantification of fluorescence intensity of 10xSTAT-GFP per esg+ cells in midguts with genotypes as indicated in (A-B). The number n represents counted cells. (D-G) Immunofluorescence images of esg-GFP (green) and Pdm1 (red) staining with the midgut section from the R4 region of control Drosophila (D, esgts-Gal4>UAS-GFP), Drosophila carrying esgts-Gal4>UAS-cad-HA (E), Drosophila carrying esgts-Gal4>UAS-hopTUM (F), and Drosophila carrying esgts-Gal4-driven UAS-cad-HA and UAS-hopTUM (G). Drosophila were treated with PQ. esg-GFP (green) identifies ISCs and their differentiating cells. Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (esg-GFP+ and Pdm1+cells). esg-GFP+ and Pdm1- cells are ISCs or EBs. esg-GFP- and Pdm1+ cells are matured ECs. (H) Quantification of the ratio of esg-GFP+ and Pdm1+ cells per 10,000 μm2 area of the R4 region midguts as indicated in (D-G). The number n represents the ROI in midguts from each experiment. One dot corresponds to one ROI (10,000 μm2 area). (I-L) Immunofluorescence images of esg-GFP (green) and Pdm1(red) staining with the midgut under normal condition. The midgut section from the R4 region of control flies (I, esgts-Gal4-driven UAS-GFP), Drosophila carrying esgts-Gal4-driven cad RNAi (J), Drosophila carrying esgts-Gal4-driven Stat92E RNAi (K), and Drosophila carrying esgts-Gal4-driven cad RNAi and Stat92E RNAi (L). esg-GFP (green) identifies ISCs and their differentiating cells. Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (esg-GFP+ and Pdm1+cells). esg-GFP+ and Pdm1- cells are ISCs or EBs. esg-GFP- and Pdm1+ cells are mature ECs. (M) Quantification of the ratio of esg-GFP+ and Pdm1+ cells per 10,000 μm2 area of the R4 region midguts as indicated in (I-L). The number n represents the region of interest in midguts from each experiment. One dot corresponds to one region of interest (ROI = 10,000 μm2 area). DAPI stained nuclei are shown in blue. Scale bars represent 10 μm (Fig 6A, 6B, 6D–6G and 6I–6L). Error bars represent SD. Student’s t-tests were used to assess statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and NS (non-significant), which represents p > 0.05.
More importantly, after gut injury, forced expression of a constitutively active version of JAK (i.e., hopscotch (hop)) (UAS-hopTUM) in ISCs and EBs by esgts-Gal4, or only in EBs by NREts-Gal4, significantly restored the ISC-to-EC differentiation defect of cad-overexpressing midguts. This was evidenced by the generation of both esg+ and Pdm1+ cells (Figs 6D–6H and S6A–S6E). In addition, depletion of Stat92E in ISCs and EBs by esgts-Gal4 significantly rescued the defect of pre-EC accumulation of cad-depleted midguts (Fig 6I–6M). In summary, these findings show that cad participates in the regulation of ISC-to-EC differentiation through the JAK/STAT-SOX21A-GATAe cascade in intestinal progenitor cells.
Reduction of cad expression in intestinal stem and progenitor cells represses age-associated gut hyperplasia in Drosophila
The ISCs and progenitor cells in aged Drosophila midguts undergo malignant proliferation and show decreased differentiation efficiency [19,20]. These results in the continuous accumulation of ISCs and their differentiating progenies (esg+ polyploid cells) in aged midguts [19,20]. Previous studies have shown that the failure of ISC-to-EC differentiation causes the undifferentiated EBs sending EGF (Spi) and JAK/STAT ligands (Upd2) to neighboring ISCs to drive their proliferation [46,47]. In addition, the accumulation of undifferentiated EBs caused niche EC detachment [54] and up-regulating paracrine EGF (Vn) and JAK/STAT ligands (Upd3) expression further accelerated ISC proliferation. Thus, the age-associated gut hyperplasia of aged Drosophila might be increased by the age-associated decrease of the differentiation efficiency of ISCs. This study showed that cad is upregulated in ISCs and EBs in aged Drosophila, while a high level of cad expression in EBs repressed the differentiation of intestinal progenitor cells. Based on these findings, the following hypothesis was proposed: the age-associated upregulation of cad in ISCs and EBs contributes to the decrease of differentiation efficiency and gut hyperplasia in aged Drosophila.
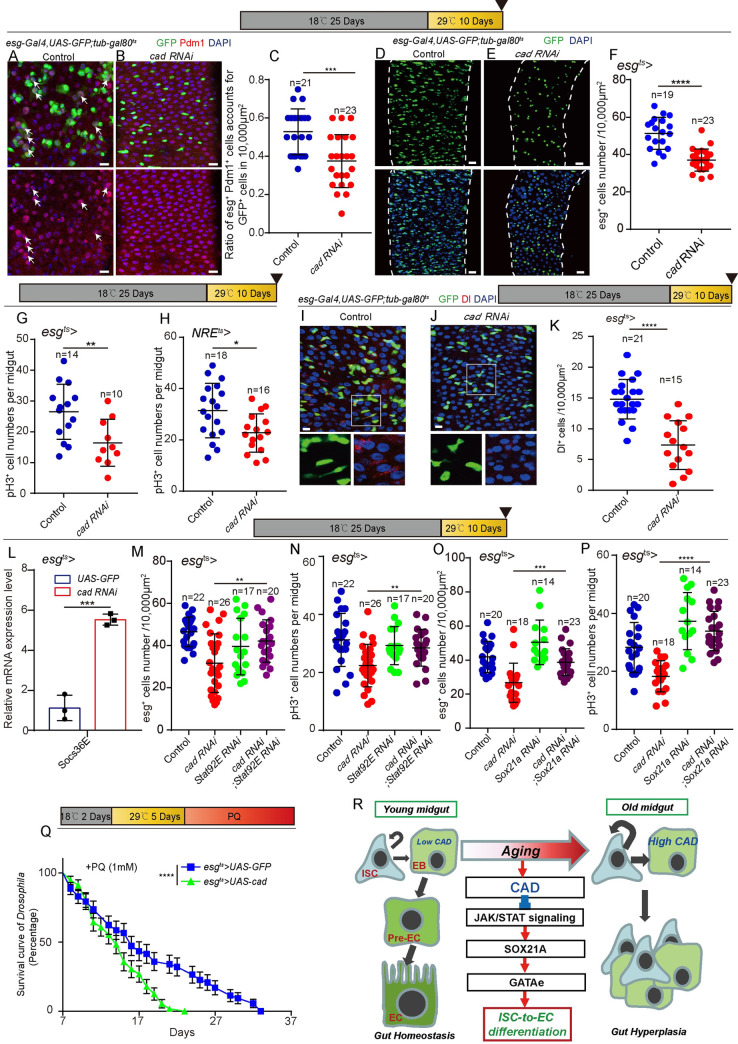
To test this hypothesis, the expression of cad in ISCs and EBs was lowered by using esgts-driven cad RNAi. The differentiation capacities of ISCs and EBs appear to decrease with age, which leads to the accumulation of Esg and Dl expressing cells in the aged midgut [55]. Therefore, this study tested whether the reduction of CAD expression could repress aging-related esg+ cells, Dl+ cells and esg+Pdm1+ cells in the midguts of aged Drosophila. The midguts of 35-day-old Drosophila (Drosophila were cultured at 18°C for 25 days and transferred to 29°C for another 10 days to induce RNAi), which induced esgts-driven cad RNAi at middle age (i.e., the 25th day after eclosion), indeed showed a significantly lower level of hyperplasia compared with the midguts of control flies aged 35 days as indicated by less accumulation of esg+Pdm1+ cells (Fig 7A–7C), esg+ cells (Figs 7D–7F and S7A and S7B) and Dl+ cells (Fig 7I–7K). TUNEL analyses indicated that depletion of cad in aged Drosophila did not cause more cell death compared to the control (S7E and S7F Fig). We found that knockdown of CAD either in esg+ cells or EBs significantly reduced the ISC hyper-proliferation defect of aged flies as indicated by pH3+ staining (Fig 7G and 7H). Since overexpression of cad in esg+ cells of injured young Drosophila prevents ISC-to-EC differentiation, which in turn led to an increase in ISC proliferation and progenitor cell accumulation (Figs 2M and S2I–S2L), it is reasonable to predict that knockdown of cad in esg+ cells of aged Drosophila prevents gut hyperplasia by promoting intestinal progenitor cell differentiation.
Fig 7. Reduction of cad expression in ISCs and progenitor cells represses age-associated gut hyperplasia in Drosophila.
(A-B) Immunofluorescence images of esg-GFP (green) and Pdm1 (red) staining with midgut from the PMG in 35-day-old (25 days at 18°C, then 10 days at 29°C) control Drosophila (A, esgts-Gal4>UAS-GFP) and 35-day-old Drosophila carrying esgts-Gal4>cad RNAi (B). esg-GFP (green) represents ISCs and their differentiating cells. Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (esg-GFP+ and Pdm1+ cells). esg-GFP+ and Pdm1- cells are ISCs or EBs. esg-GFP- and Pdm1+ cells are mature ECs. (C) Quantification of the ratio of esg-GFP+ Pdm1+ cells per 10,000 μm2 area from PMG of control Drosophila with genotypes as indicated in (A-B). The number n is indicated. Each dot represents one midgut. Each dot corresponds to one ROI (10,000 μm2 area). (D-E) Immunofluorescence images of midgut sections from the R4 region in 35-day-old (25 days at 18°C, then 10 days at 29°C) control Drosophila (D, esgts-Gal4>UAS-GFP) and Drosophila carrying esgts-Gal4>cad RNAi (E). esg-GFP (green) indicates ISCs and their differentiating cells. (F) Quantification of esg-GFP+ cell numbers in experiments (D-E). The number n represents the ROI in midguts from each experiment. One dot corresponds to one ROI (10,000 μm2 area). (G-H) Quantification of pH3+ cell numbers in midguts from 35-day-old (25 days at 18°C, then 10 days at 29°C) control Drosophila (esgts-Gal4>UAS-GFP in G and NREts-Gal4>UAS-GFP in H), Drosophila carrying esgts-Gal4>cad RNAi (G), and NREts-Gal4>cad RNAi (H). The number n represents the whole midguts. One dot corresponds to one midgut. (I-J) Immunofluorescence images of midgut sections from the R4 region in 35-day-old (25 days at 18°C, then 10 days at 29°C) control Drosophila (I, esgts-Gal4>UAS-GFP) and Drosophila carrying esgts-Gal4>cad RNAi (J). esg-GFP (green) indicates ISCs and their differentiating cells. Dl (red) staining was used to visualize ISCs. (K) Quantification of Dl+ cell numbers in experiments (I-J). The number n represents the ROI in midguts from each experiment. One dot corresponds to one ROI (10,000 μm2 area). (L) Relative mRNA fold changes of Socs36E in sorted esg+ cells from midguts of aged (25 days at 18°C add 10 days at 29°C) control Drosophila (blue lines, esgts-Gal4>UAS-GFP) and aged Drosophila with cad depleted (red lines, esgts-Gal4>cad RNAi). The changes of expressions were plotted relative to the control Drosophila, which was set to 1. (M-N) Quantification of esg+ (M) or pH3+ (N) cell numbers in midguts from 35-day-old (25 days at 18°C, then 10 days at 29°C) control Drosophila (esgts-Gal4>UAS-GFP), Drosophila carrying esgts-Gal4> cad RNAi, and Drosophila carrying esgts-Gal4> cad RNAi; Stat92E RNAi. The number n represents the whole midguts (N). One dot corresponds to one midgut in N. The number n represents the ROI in midguts from each experiment in M. One dot corresponds to one ROI (10,000 μm2 area) in M. (O-P) Quantification of esg+ (O) or pH3+ (P) cell numbers in midguts from 35-day-old (25 days at 18°C, then 10 days at 29°C) control Drosophila (esgts-Gal4>UAS-GFP), Drosophila carrying esgts-Gal4> cad RNAi, and Drosophila carrying esgts-Gal4-driven cad RNAi and Sox21a RNAi. The number n represents the whole midguts in P. One dot corresponds to one midgut in P. The number n represents the ROI in midguts from each experiment in O. One dot corresponds to one ROI (10,000 μm2 area) in O. (Q) Survival rate of control Drosophila (esgts-Gal4-driven UAS-GFP) and Drosophila carrying esgts-Gal4> UAS-cad under PQ treatment (see methods). (R) Schematic model of the underlying mechanism. In young Drosophila, CAD expresses at a basal level in ISCs and EBs. The basal expressed cad prevents the overexpression of SOX21A and GATAe transcription factors in ISCs and EBs, which prevents the ISCs from producing ECs unless the intestinal epithelia are damaged. CAD represses SOX21A and GATAe expression in ISCs and EBs by inhibiting the activation of JAK/STAT signaling. The expression of CAD increases in ISCs and EBs of Drosophila during aging. Ultimately, this leads to a decrease of differentiation efficiency of ISCs and EBs with age, which, in turn, results in gut hyperplasia as indicated by the continuous accumulation of ISCs and their differentiating progenies (i.e., esg+ polyploid cells) in the midguts of aged Drosophila. DAPI stained nuclei are shown in blue. Scale bars represent 10 μm (Fig 7A, 7B, 7I and 7J) or 20 μm (Fig 7D and 7E). Error bars represent SD. Student’s t-tests were used to assess statistical significance. For the survival test, the log-rank test was used to analyze the statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and NS (non-significant), which represents p > 0.05. See also S7 Fig.
As expected, knockdown of cad in esg+ cells of aged Drosophila promoted the activate of JAK/STAT signaling pathway as indicated by the upregulation of Socs36E (a demonstrated downstream of JAK/STAT pathway [56]) transcription (Fig 7L). Moreover, knockdown of either Stat92E or Sox21a restored the effect of cad RNAi-mediated reduction of gut hyperplasia of aged Drosophila (Fig 7M–7P).
In addition, overexpression of CAD in esg+ cells caused more severe gut hyperplasia defect in aged midgut indicated by pH3+ staining (S7C Fig) and esg-GFP staining (S7D Fig), and an increase of mortality of flies under low dose PQ-induced injury compared to the control flies (Fig 7Q). These data suggest that the upregulation of cad expression in ISCs and EBs in aged Drosophila might be an important cause of the functional decline of ISCs and reduces the overall fitness of Drosophila upon aging.
In addition, we have also analyzed the lifespan of Drosophila carrying esgts>UAS cad RNAi (flies were cultured at 18°C for 25 days, then transferred to growth at 29°C to induce cad RNAi). However, we didn’t find a significant increase (but a minor reduction) in the longevity of flies with cad depletion compared to the control flies (esgts>UAS-GFP) (S7G Fig). The possible reason that knockdown cad does not increase fly lifespan may be because that depletion of cad causes over-reduction of ISCs under normal conditions or cad has other unknown functions in ISCs/progenitor cells.
Discussion
Over the past decades, studies on the homeobox gene cad mainly focused on exploring its roles in antero-posterior (A-P) patterning, organ morphogenesis during development, tumorigenesis, and the innate immune response [38,39,41,57–61]. It has been reported that the transcriptional expression of cad increases in the midguts of both young Drosophila after injury and aged Drosophila [43,44]. However, the function of cad in the context of ISCs and aging remains largely unexplored. This study showed that the expressions of cad in ISCs and EBs of aged Drosophila midguts were significantly upregulated compared with the expressions in ISCs and EBs of young Drosophila midguts. Importantly, the aging-related CAD increase in ISCs and EBs contributes to gut hyperplasia in the midguts of aged Drosophila (Fig 7R).
In addition to the regulation of the formation of body sections during Drosophila embryogenesis [39,57,58], cad has also been shown to repress the expressions of antimicrobial peptides (AMPs) in Drosophila midguts [41] and to function in the regulation of innate immune homeostasis [41,62–66]. Although the immune function of cad in intestinal epithelia has been well studied [41,62–66], the roles of cad in ISCs and progenitor cells and how it regulates gut epithelial homeostasis were largely ignored. This study showed that deletion of cad in EBs induced EB-to-EC differentiation, while overexpression of cad inhibited EC formation. Using the Drosophila genetic strategy, this study demonstrated that CAD functions in EBs to regulate ISC-to-EC differentiation by modulating the JAK/STAT-SOX21A-GATAe cascade (Fig 7R).
In this study, we showed that the expression of cad was significantly upregulated in ISCs and EBs of aged Drosophila. The failure of ISC-to-EC differentiation, and thus inducing gut hyperplasia during aging may attribute to excessive cad expression. But how the expression of cad in aged animals is regulated remains unclear. Cad functions as an effector of induction of AMPs in immune homeostasis have been reported[61,66]. Besides its roles in the immune system, oxidative stress has also been shown to regulate the expression of cad [43,67]. It is conceivable that these dynamic changes of cad expression during aging could be influenced by these external physiological factors since the levels of reactive oxygen species and oxidative damage increase with age, and the innate immune response tends to become hyperactive in older organisms [68,69]. On the other hand, the cad-related homeobox gene has been reported to be regulated by Relish and NF-κB pathway, while the activation of NF-κB has been firmly linked to the age-related inflammatory processes under increased oxidative stress [43,70–72]. DRE/DREF is another molecular effector that has been reported to regulate the transcription of cad [73]. Previous studies have indicated that DREF activity is modulated by the intracellular redox state [74]. These intriguing correlations imply to us that the age-associated changes of expression of cad may probably be the result of alters of these upstream molecular effectors and related signaling pathways caused by the accumulation of immune damage and oxidative stress during aging. Understanding the dynamic changes of cad expression under young and aging conditions may be useful for us to clarify the mechanisms through which external stresses result in the differentiation disorder of ISC in the aging process, and then lead to intestinal function degeneration and tumorigenesis.
In this study, we showed that high levels of cad expression in ISCs and EBs results in the failure of ISC-to-EC differentiation during aging. This defect leads to the continuous accumulation of differentiation defective EBs and immature ECs, and consequently tumorigenesis. There are some cytokines/peptides, like Hh, Upd1, Upd2 produced by ISCs/EBs to maintain local tissue homeostasis [75–77]. Elevated Hh and JAK-STAT signaling pathways are essential for the regenerative proliferation of ISCs. However, excessive secretion and ectopic activation of Hh signals may be a stimulator in many types of cancers, including lung, prostate, pancreas, liver, bladder, and skin [78–83]. Moreover, the over-release of insulin-like factor Ilp6 and TNF ligand Eiger derived from stem/progenitors may also induce colorectal cancer, diabetic enteropathy, and commonly triggers chronic systemic inflammation [84–86]. Aside from the uncontrolled retention of transient undifferentiated cells, another consequence of ISC-to-EC differentiation defect is the ablation of mature ECs in the midguts. As one of the differentiated cells in Drosophila intestine, ECs are responsible for the absorption of nutrients, and the secretion of digestive enzymes. Unable to produce mature ECs may directly lead to the interruption of normal digestion, absorption, and transport of a variety of nutrients, which results in malnutrition, diarrhea, steatorrhea, and weight loss, named malabsorption syndrome clinically [87,88]. Poor intestinal absorption and secretion also have been associated with a variety of different human diseases, such as coeliac disease, lactase deficiency, or Whipple’s disease [88]. In addtion, lack of ECs also contributes to the disruption of the gut barrier, which increases intestinal permeability. This dysfunctional intestinal barrier in turn facilitates the translocation of harmful substances and pathogens to the bloodstream, and thus impacts on other distal organs and gives rises to pathology and physiology of several systemic disorders, such as inflammation, allergic diseases, chronic kidney disease, hyperglycemia, and Alzheimer’s disease [89–94].
In aged Drosophila, midguts showed dysplasia, caused by both ISC hyper-proliferation [19–21] and aberrant differentiation [20,28,55]. Several signaling pathways, such as JNK [20], insulin receptor [27–30], p38-MAPK [19,22], ROS [23], and unfolded protein response (UPR) [25,26], have been identified to regulate aging-associated ISC hyper-proliferation. However, the causes of the aberrant differentiation of ISCs and progenitor cells upon aging remain largely unexplored. This study demonstrated that the upregulation of the homeobox gene cad resulted in a decline of ISC-to-EC differentiation in Drosophila midguts upon aging. These findings identify a novel regulator that specifically modulates ISC-to-EC differentiation during gut epithelial regeneration and aging.
Materials and methods
Drosophila breeding and maintenance
The stocks of Drosophila were fed on standard yeast food (cornmeal 50 g, yeast 18.75 g, sucrose 80 g, glucose 20 g, agar 5 g, and propionic acid 30 ml combined in 1 L water) at 25°C, 65% humidity, and on a 12-h light/dark daylight cycle unless otherwise stated, unless specifically mentioned.
The temporal and regional gene expression targeting (TARGET) method [95] was used to manipulate the Gal4-UAS-mediated RNAi and overexpression experiments in this study. To repress the GAL4 system, the crosses were maintained at 18°C when driving temperature-sensitive GAL4-mediated RNAi or gene overexpression. When flies eclosion or at a certain age after flies eclosion, the adults were shifted to 29°C to turn on the GAL4 system, which induces RNAi or gene overexpression. Eclosed flies were incubated at 29°C for indicated days followed by dissection of midguts for immunostaining or western blot. In this study we used the mated females for experiments on the Drosophila midguts.
For the aging-associated experiments, the tub-Gal80ts transgenic Drosophila combined with esg-Gal4 or NRE-Gal4 were grown at a permissive temperature (18°C) to limit Gal4 activity. Adult flies continue to raise in 18°C for 25 days after eclosion then transferred to a non-permissive temperature (29°C) for 10 days until dissection midguts for immunostaining or western blot analyses.
Drosophila lines in this study
The following Drosophila lines were obtained from the TsingHua Fly Center (THU, Beijing, China): FRT40A (Thu0389), 10×Stat-GFP (Thu0274), and GATAe RNAi (Thu1490). The following Drosophila line was obtained from the Zurich ORFeome Project (FlyORF): UAS-caudal-3×HA (F000471). The following Drosophila lines were obtained from the Bloomington Drosophila stock center (BDSC): cad RNAi (BDSC# 57546 and BDSC# 34702), w1118 (BDSC# 3605), UAS-Sox21a (BDSC# 68155), cad-EGFP (BDSC# 30875), Notch RNAi (BDSC# 7078), and cad2 FRT40A (BDSC# 7091). The following Drosophila line was obtained from the Vienna Drosophila Resource Center (VDRC): Stat92E RNAi (v106980), rpr RNAi (v101234).
The following Drosophila lines were kindly provided as indicated in the following: tub-GAL4 line, esg-GFP, esg-Gal4 and UAS-lacZ line, by Dr. Allan Spradling; esgts-Gal4 line [5], MyolAts-Gal4 line [18], ISCts-Gal4 line [51], NREts-Gal4 line [51], and UAS-hopTUM line by Dr. Benjamin Ohlstein [52]; esg-lacZ line by Dr. Mark Van Doren [96]. The following Drosophila lines were available in our laboratory: Sox21a-HA lines [52] and UAS-GATAe-HA line [52]. All Drosophila lines used in this study are listed in S3 Table.
The transgenic Drosophila line UAS-cad-HA was constructed in our laboratory. In brief, cDNA of cad sub-cloned was used into the pEntry-3xHA vector [52] through the pEASY-Uni seamless cloning and assembly kit (TransGen Biotech, CU101-02). Then, the previous product was sub-cloned into the pTW vector (obtained from Allan Spradling Lab) through LR recombination reaction. Finally, the vectors were cloned and tested through sequencing, and sent to UniHuaii Corporation (Zhuhai, China) for Drosophila egg injection. The cad cDNA used the following primers: Cad L: 5’ ATGGTTTCGCACTACTACAA 3’; Cad R: 5’ TCACATCGAGAGCGTGCCCA 3’.
To obtain the Drosophila line UAS-cad. The transgenic Drosophila line UAS-cad was constructed in our laboratory. In brief, cDNA of cad sub-cloned was used into the pEntry vector [52] through the pEASY-Uni seamless cloning and assembly kit (TransGen Biotech, CU101-02). Then, the previous product was sub-cloned into the pTW vector (obtained from Allan Spradling Lab) through LR recombination reaction. Finally, the vectors were cloned and tested through sequencing, and sent to UniHuaii Corporation (Zhuhai, China) for Drosophila egg injection. The cad cDNA used the following primers: Cad L: 5’ ATGGTTTCGCACTACTACAA 3’; Cad R: 5’ TCACATCGAGAGCGTGCCCA 3’.
Mosaic analysis with repressible cell marker clone induction
To generate cad2 [97] mutant MARCM clones, the cad2 mutant carrying the FRT40A [98]line was obtained from the BDSC (BDSC# 7091). To generate UAS-cad MARCM clones, FRT40A was crossed to UAS-cad to produce the following Drosophila: FRT40A/cyo; UAS-cad /TM6B. Then, these Drosophila were crossed to yw hsFLP::tub-Gal4::UAS-nls GFP/FM7; tubG80 FRT40A/CyO (gifted from Allan Spradling) was used to obtain yw hsFLP::tub-Gal4::UAS-nls GFP/+; tubG80 FRT40A/ FRT40A::cad2, and yw hsFLP::tub-Gal4::UAS-nls GFP/+; tubG80 FRT40A/ FRT40A; UAS-cad/+ flies. The crosses were kept at 25°C. Three-day-old adult flies were heat-shocked for 1 h twice at 37°C to induce clones and then kept at 29°C for 7 days until dissection. UAS-cad was only expressed in the clone which presented GFP.
For MARCM clone production, adult Drosophila were fed at 25°C for 2–3 days and heat-shocked at 37°C for 1 h twice. After heat-shock treatment, Drosophila were kept at 25°C, and then, these flies were dissected and observed at 7 days ACI [52].
Paraquat and DSS treatment
For the Paraquat (PQ) or DSS treatment, Drosophila were starved in empty vials for 2 h and were exposed in 5% (wt/vol) sucrose with 10 mM PQ (Aladdin, China) or 5% (wt/vol) DSS (MP Biomedicals, CAS: 9011-18-1, 36~50KD) for 24 h, followed by recovery for 24 h. Then, Drosophila were dissected in PBS. Drosophila were transferred from medium to empty bottles for 2 h. Filter paper was cut into 3.5 × 6.0 cm pieces, and infiltrated in 5% (wt/vol) sucrose with 10 mM PQ or with 5% (wt/vol) DSS. Then, the moist paper was added to the empty bottles for 24 h, and Drosophila were transferred into new standard medium without PQ or DSS. Identical Drosophila fed with 5% sucrose were used as control [52]. The PQ- or DSS-induced damages experiments were performed in the temperature incubator at 25°C, unless specifically mentioned.
For manipulation of the PQ-treatment RNAi or overexpression experiments, the GAL4ts lines and UAS lines were maintained at 18°C to repress the GAL4 system. One day after eclosion, the adults were shifted to 29°C to activate the GAL4 system, which induces RNAi or overexpression. Eclosed flies were incubated at 29°C for 5 days. Then Drosophila were starved in empty vials for 2 h and were exposed in 5% (wt/vol) sucrose with 10 mM PQ for 24 h, followed by recovery for 24 h. The controls were treated in the same way in parallel. The process of PQ treatment was manipulated at 29°C.
Immunostaining and microscopy
Guts were dissected in PBS, and were immersed in PBS with 4% EM-paraformaldehyde (PBS formula: 100 mM glutamic acid, 26 mM KCl, 20 mM MgSO4, 4.5 mM Na2HPO4, 1 mM MgCl2, pH 7.4) at room temperature (25°C) for 45 min. Guts were washed in PBST (PBS, 0.1% Triton X-100) 3 times, 15 min each, followed by incubation with primary and second antibodies in PBST buffer after soaking in BSA buffer (PBS, 0.5% BSA, 0.1%Triton X-100) for 30 mins.
The following primary antibodies were used: Chicken polyclonal anti-GFP (Abcam, AB_300798, 1:1000), rabbit polyclonal anti-GFP (Proteintech, AB_11042881, 1:1000), rabbit anti-HA (Cell Signaling Technology, AB_1549585, 1:1000), mouse anti-Delta (anti-Dl) (Developmental Studies Hybridoma Bank, AB_528194, 1:100), rabbit anti-Pdm1 (from Dr. XiaoHang Yang, 1:500), and mouse anti-Prospero (Developmental Studies Hybridoma Bank, RRID: AB_528440). The method of gut immunostaining with Dl used methanol-heptane and methanol to replace the PFA and PBST before BSA incubation. The secondary antibodies (Alexa 488, Alexa 568, and Alexa 647, purchased from MolecularProbes/Invitrogen) were diluted and used at 1:2000. The final concentration of 4,6-diamidino-2-phenylindole (DAPI; Sigma) was 1 μg/ml.
Confocal images were acquired by a Leica TCS-SP8 confocal microscope. Images for each set of experiments were acquired as confocal stacks under the same settings. These settings including the distinguish at 1024x1024 and using bi-direction scanning. The gain value up reaches 800 and off-set arrange from -0.1%~-0.2% in each image. To illuminate the Z-axis detail staining and get clear Images, they were acquired as a confocal z-stacks number usually range from 5 to 10 and finally projected to one image. Adobe Photoshop and Adobe Illustrator CS5 were used to assemble the images. The number of midgut cells in all quantifications was counted using a Leica DM6-B microscope.
Guts lysate and Western blotting analyses
Dissected Drosophila midguts, mixed in RIPA Lysis Buffer (P0013B, Beyotime Biotechnology) with appropriate protease inhibitors, then transferred to liquid nitrogen. Put the tissues on ice 30 minutes after used grinding rod for homogenization. Collected lysate supernatant transferred to total proteins through BCA kit (PierceRapid Gold BCA Protein Assay Kit, Thermo, A53226). Each sample added 5× loading buffer to boiled 12 min. Transferred the protein gel to polyvinylidene difluoride (PVDF) membranes and used 5% TBST with defatted milk to blocked 1 h. After blocking, incubated in primary antibody for whole night at 4°C and used TBST to wash 3 times at room temperature 25°C, each time 10 min. Shaking PVDF membranes with horseradish peroxidase-labelled secondary antibody at room temperature 25°C for incubation.
The Western blotting antibodies were shown in the Table of Antibodies List. The secondary antibodies were horseradish peroxidase-conjugated Goat anti-mouse (Beyotime Biotechnology, #A0216, 1:1000), Goat anti-rabbit (Thermo, #A0208, 1:1000).
FACS and qRT-PCR
200 midguts were dissected from young or old female flies, respectively. Flies were dissected in 4°C diethyl pyrocarbonate (DEPC)-treated water-PBS, then incubated in 1 mg/ml Elastase (Sigma, cat. no. E0258) combined DEPC-PBS mixture at room temperature, mixed the sample softly 5 times every 15 min during incubation. After dissociation, the samples were centrifuged at 400×g for 20 min at 4°C, then re-suspended in 4°Cdiethyl pyrocarbonate (DEPC)-treated water-PBS, filtered with 70 mm filters (Biologix) and sorted by FACS Aria II sorter (BD Biosciences). GFP+ cells in the esg-GFP Drosophila midguts were sorted out, the midguts of w1118 flies were set as fluorescence gate. We used three biological replicates to detect, each replicate contains 50,000 esg-GFP+ cells, the total RNA was analyzed in the Arcturus PicoPure RNA isolation kit (Applied Biosystems) follow the protocol. We used the PrimeScript RT reagent Kit (TaKaRa) to synthesize cDNA, RNA was reverse-transcript by oligo dT, the first-strand cDNA was diluted with sterile water 50 times and transfer to real-time PCR. The Real-time PCR was accomplished in Quant-Studio 5 System (Thermo Fisher Scientific), each sample employs SYBRGreen (Genestar). The reference standard group is Rp49, method of expression value calculation is 2-△△CT. The expression of the standard sample was normalized to 1, All of the primer sequences of qPCR are available upon reasonable request.
RNA-Seq and data analysis
The Drosophila crosses were cultured on normal food at 18°C. esgts>UAS-lacZ Drosophila were used as the control for the cad-depleted Drosophila (esgts>cad RNAi). To induce RNAi, Drosophila were maintained at 29°C for 7 days. About 100 female flies for each biological replicate were dissected in PBS on ice. Midguts were frozen on drikold and isothiocyanate-alcoholphenyl-chloroform was used to collect total RNA after dissection. Then, the total RNA was sent to Berry Genomics Corporation (China) for sequencing on a novaseq 6000 platform (Illumina, San Diego, CA, USA), and quality control was performed by FastQC (version 0.11.8).
The raw data of RNA-seq read lengths of 150 bp were aligned to the Drosophila reference genome (Ensembl BDGP6 release-89). The aligned reads (sam files) were transferred to bam files and sorted by SAMtools. Gene expressions in different samples were determined by DESeq2 (version 1.22.2). The differentiation of expression was verified if p ≤ 0.05 following a Benjamini and Hochberg correction for multiple hypothesis testing.
Fluorescence intensity statistics
Immunofluorescence images were analyzed via Confocal microscopy. The fluorescence intensity statistics in the region of interest (ROI) was calculated using ImageJ. The detailed process of the fluorescence intensity statistics was previously described [52]. The steps in brief:
Open image: File -> Open.
Split Channels: Image-> Color -> Split Channels. Select the channel which needs to calculate and strike out redundant channels.
Scale Setting: Analyze-> Set scale-> click to remove scale. Set scale to determine the unit of length is Pixel.
Measurements Setting: Analyze-> Set Measurements. Choose the boxes named "Area", "Integrated Density", and "limit to threshold". Click on the "OK" box after chosen.
Choose the ROI or Cell by different kinds of drawing/selection tools and select another smaller region around the ROI or cell as background.
Choose different types of ROI, Cell and background regions through using boxes named"ROI Manager".
Select the boxes named"Measure", calculate the integrated density.
Integrated Density = Integrated Density of ROI or cell—Integrated Density of background region/Area of background region × Area of ROI or cell.
Band Gray Value analysis of Western blotting
Band Gray Value analysis of Western Blotting was used the software ImageJ. The specific methods are shown:
File -> Open. Choose the selected image.
Edit -> Invert.
Analyze -> Set Scale -> Choose the image scale to remove. Set scale to determine the unit of length is Pixel.
Analyze -> Set Measurements. Choose the bottom of “Area” ->“Mean Gray Value”, then limited the number to attain threshold, Choose the botton of “OK”.
Pick up the region of band and choose a smaller area as the background.
Choose the ROI Manager to select the different regions of band and background region.
Click the box of “Measure” to compute the average value of Gray Value.
(Band Gray Value = (Mean Gray Value of band region × Area of Band Region)-(Background Mean Gray Value × Area of background Region))
Lifespan assays under normal and stressful conditions
For survival tests under normal conditions, 100 female flies (2 days old) with the same phenotype were collected and fed on the temperature incubator at 18°C to repress the GAL4 system. Two days after fly eclosion, the adults were transferred to 29°C temperature turn on GAL4 activity for survival tests analysis. Living flies were counted and transported to new food tubes every 2 days. The survival tests were repeated 3 times as 3 independent experiments.
For survival tests under chronic oxidative damage conditions, 100 female flies (2 days old) with the same phenotype were collected and fed on the temperature incubator at 18°C to repress the GAL4 system. Two days after fly eclosion, the adults were transferred to 29°C temperature turn on GAL4 activity for 5 days. In order to finish the viability test, 100 female flies (2 days old) have same genetic background were collected and divided into five tubes with food medium. Transferred flies into empty tubes for starvation at 29°C before Viability Tests. Then put the filter paper (3.7×5.8 cm) saturated with 1 mM PQ in 5% sucrose solution at 29°C. The number of flies alive still counted, the filter paper saturated with 1 mM PQ in 5% sucrose solution exchanged continually every day.
Quantification of statistical analysis
GraphPad Prism v7.0 was used to evaluate the statistical significance after verifying normality and equivalence of variances. Data in this study are presented as the means ± standard deviation (SD) from at least three independent biological replicates unless otherwise specified. Statistical significance was determined using two-tailed Student’s t-tests unless otherwise specified. Significance is stated in the text or figure legends. For all tests, a p < 0.05 was considered statistically significant.
Software availability
The software package R (version 3.5.3, download from https://www.r-project.org/) was used for downstream analysis of RNA-seq results. The custom ImageJ used for immunofluorescent staining and Western blotting quantification (download from https:https://imagej.nih.gov/ij/). Prism 7.0 (GraphPad) was also used in this study (download from https://www.graphpad.com/).
Supporting information
(A) Cartoon model of Drosophila ISC lineages. An ISC (Dl+ and esg+) undergoes asymmetric division once to produce a new ISC and a diploid precursor enteroblast (EB; esg+ and NRE+) or a diploid precursor enteroendocrine mother cell (EMC; esg+ and Pros+). The post-mitotic EB further differentiates into premature enterocytes (pre-ECs) (esg+ and Pdm1+), which continue to differentiate into octoploid mature ECs (Pdm1+). The EMC divides once to produce a pair of diploid enteroendocrine cells (EEs; Pros+). (B-C) The cad gene reads per kilobase per million mapped reads (RPKM) values in ISCs (B) and EBs (C) of five regions from the midguts of Drosophila. Expression was determined using RNAseq in experiments described in [44]. (D) The cad gene RPKM values in ISC, EB, EC, and EE from R1-R5 of midguts. Expression was determined using RNAseq in experiments described in [44]. (E) Relative mRNA fold change of cad in sorted esg-GFP+ cells of young (green bar) and aged (red bar) Drosophila (esg-GFP/CyO). The cad expressions in esg-GFP+ cells of Drosophila with different ages are plotted relative to 5-day Drosophila, which was set to 1. Error bars indicate the standard deviation (SD) of three independent experiments. (F) Western blot result of CAD expression in midguts from young (age 5 days) and old (age 30 days). (G) The whole posterior midgut immunofluorescence images of CAD-EGFP (green) staining from young (age 7 days) and old (age 30 days) Drosophila carrying CAD-EGFP (green). (H-I) Immunofluorescence images of CAD-EGFP (green) staining with the midgut section from R1-R5 region (except R4 which were showed in Fig 1) of young (7 days; H) and old (30 days; I) Drosophila carrying CAD-EGFP midgut. (J) Immunofluorescence images of CAD-EGFP (green) staining with the midgut section from the PMG (the posterior midgut) of young cad-EGFP Drosophila which were treated with 5% sucrose (used as control), PQ, or DSS, respectively. (K) Quantifications of fluorescence intensity of CAD-EGFP in per region of interest (ROI) from the PMG as shown in (J). The number n is indicated. Each dot represents one ROI (10,000 μm2). DAPI stained nuclei are shown in blue. Scale bars represent 50 μm (S1G Fig), 25 μm (S1H, S1I, and S1J Fig), 10μm (S1J Fig). Error bars represent SD. Student’s t-tests were used to assess significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and NS (non-significant), which represents p > 0.05.
(TIF)
(A) Relative mRNA fold change of cad-depleted Drosophila by tub-Gal4-driven two different cad RNAi lines. The cad expressions in Drosophila with different RNAi are plotted relative to control flies (tub-Gal4), which was set to 1. Error bars indicate the standard deviation (SD) of three independent experiments. (B-C) Immunofluorescence images of esg-GFP (green) and Pdm1 (red) staining with the midgut under normal conditions. The midgut section from AMG of control flies (B, esgts-Gal4-driven UAS-GFP) and AMG of Drosophila carrying esgts-Gal4-driven cad RNAi (C). esg-GFP+ and Pdm1- cells are ISCs or EBs. esg-GFP- and Pdm1+ cells are matured ECs. (D) Quantification of the ratio of esg-GFP+ and Pdm1+ cells per 10,000 μm2 area of the AMG as indicated in (B-C). The number n represents counted ROI in midguts from each experiment. Each dot corresponds to one ROI (10,000 μm2 area). (E-H) Quantification of the esg+ cells (E-F) and the Dl+ cells (G-H) per 10,000 μm2 area of the anterior midgut (AMG; E and G) and the posterior midgut (PMG; F and H) from control Drosophila (esgts-Gal4-driven UAS-GFP), Drosophila carrying esgts-Gal4>cad RNAi, and Drosophila carrying esgts-Gal4>UAS-cad-HA, under homeostatic conditions. The number n is indicated. Each dot corresponds to one ROI (10,000 μm2 area). (I-L) Quantification of the esg+ cells (I-J) and the Dl+ cells (K-L) per 10,000 μm2 area of the AMGs (I and K) and the PMGs (J and L) from control Drosophila (esgts-Gal4-driven UAS-GFP), Drosophila carrying esgts-Gal4>cad RNAi, and Drosophila carrying esgts-Gal4>UAS-cad-HA, under PQ-treated conditions (PQ-REC-1D). The number n is indicated. Each dot corresponds to one ROI (10,000 μm2 area). (M-N) Immunofluorescence images of esg-GFP (green) and TUNEL (red) staining with the midgut under normal conditions. The midgut section from PMG of control flies (M, esgts-Gal4-driven UAS-GFP) and Drosophila carrying esgts-Gal4-driven cad RNAi (N). esg-GFP (green) identifies ISCs and their differentiating cells. White arrowhead indicates cells in apoptosis. (O-Q) Immunofluorescence images of esg-GFP (green) and Dl (red) staining with the midgut section from the R4 region of Drosophila carrying esgts-Gal4>UAS-GFP (O), Drosophila carrying esgts-Gal4> cad RNAi (P), and Drosophila carrying esgts-Gal4>cad RNAi and rpr RNAi (Q). esg-GFP (green) represents ISCs and their differentiating cells. Dl staining (red) was used to visualize ISCs. White arrows indicate Dl+ ISCs. (R) Quantification of the Dl+ cell numbers per 10,000 μm2 area of midgut as indicated in (O-Q). The number n represents counted ROIs in midguts from each experiment. Each dot corresponds to one ROI (10,000 μm2 area). DAPI stained nuclei are shown in blue. Scale bars represent 10 μm (S2B, S2C, S2M, S2N and S2O–S2Q Fig). Error bars represent SD. Student’s t-tests were used to assess significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and NS (non-significant), which represents p > 0.05.
(TIF)
(A-B) Immunofluorescence images of esg-GFP (green) and Pdm1(red) staining with the midgut section from the R4 region of control Drosophila (A, esgts-Gal4>UAS-GFP) and Drosophila carrying esgts-Gal4>UAS-cad (B), under normal conditions. esg-GFP (green) represents ISCs and their differentiating cells. Pdm1 staining (red) was used to visualize matured ECs. (C) Quantification of the ratio of esg-GFP+ and Pdm1+ cells per 10,000 μm2 area of the midgut as indicated in (A-B). The number n represents counted ROIs in midguts from each experiment. Each dot corresponds to one ROI (10,000 μm2 area). (D-E) Immunofluorescence images of NRE-GFP (green) and Pdm1(red) staining with the midgut section from the R4 region of control Drosophila (D, NREts-Gal4>UAS-GFP) and Drosophila carrying NREts-Gal4>UAS-cad (B), under normal conditions. NRE-GFP (green) represents EBs. Pdm1 staining (red) was used to visualize matured ECs. (F) Quantification of the ratio of NRE-GFP+ and Pdm1+ cells per 10,000 μm2 area of the midgut as indicated in (D-E). The number n represents counted ROIs in midguts from each experiment. Each dot corresponds to one ROI (10,000 μm2 area). (G-H) Immunofluorescence images of ISC-GFP (ISCts-Gal4-driven UAS-GFP; green) and Pdm1 (red) staining with the midgut section from the R4 region of control flies (G, ISCts-Gal4-driven UAS-GFP) and cad-depleted Drosophila by ISCts-Gal4-driven cad RNAi (H). ISC-GFP (green) indicates ISCs. Pdm1 staining (red) was used to visualize differentiating ECs. ISC-GFP+ and Pdm1- cells are ISCs. ISC-GFP- and Pdm1+ cells are mature ECs. (I) Quantification of the ratio of ISC-GFP+ and Pdm1+ cells per 10,000 μm2 area of the R4 region of midguts as shown in (G-H). The number n represents counted regions of interest in midguts from each experiment. Each dot corresponds to one region of interest (ROI = 10,000 μm2 area). (J-K) Immunofluorescence images of ISC-GFP (green) and Pdm1 (red) staining with the midgut treated with PQ-REC-1D. The midgut section from the R4 region of control flies (J, ISCts-Gal4-driven UAS-GFP) and cad-overexpressing Drosophila by ISCts-Gal4-driven UAS-cad-HA (K). ISC-GFP (green) represents ISCs. Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (ISC-GFP+ and Pdm1+cells). ISC-GFP+ and Pdm1- cells are ISCs. ISC-GFP- and Pdm1+ cells are mature ECs. (L) Quantification of the ratio of ISC-GFP+ and Pdm1+ cells per 10,000 μm2 area of R4 region midguts from control Drosophila and cad-overexpressing Drosophila carrying ISCts-Gal4-driven UAS-cad-HA as shown in (J-K). The number n represents counted ROIs in midguts from each experiment. Each dot corresponds to one ROI (10,000 μm2 area). (M-O) Immunofluorescence analyses of control (FRT40A, M), cad2 mutant (N), and cad-HA overexpressing (O) mosaic analysis with repressible cell marker (MARCM) clones (green) 7 days after clone induction (ACI) of flies. Prospero staining (red) was used to visualize EEs. White arrows indicate EEs in clones. DAPI stained nuclei are shown in blue. Scale bars represent 10 μm (S3A, S3B, S3D, S3E, S3G, S3H and S3J–S3K Fig), 5μm (S3M–S3O Fig). Error bars represent SD. Student’s t-tests were used to assess statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and NS (non-significant), which represents p > 0.05.
(TIF)
(A-B) Western blotting of SOX21A-HA of whole midguts from young (7 days; A) and old (35 days; B) control Drosophila (esgts-Gal4>UAS-GFP; Sox21a-HA) and Drosophila carrying esgts-Gal4>cad RNAi; Sox21a-HA. Loading controls, esg-GFP (GFP) and a-tubulin (a-Tub). (C) Quantification of the relative band intensity of SOX21A-HA to esg-GFP as shown in experiments (A-B). (D) Western blotting of SOX21A-HA of whole midguts from young (7 days) and old (35 days) control Drosophila (esgts-Gal4>UAS-GFP; Sox21a-HA). Loading controls, esg-GFP (GFP) and a-tubulin (a-Tub). (E) Quantification of the relative band intensity of SOX21A-HA to esg-GFP as shown in experiments (D). (F) Western blotting of SOX21A-HA of whole midguts carrying Sox21a-HA were treated with or without PQ (PQ-REC-1D). Loading controls, a-tubulin (a-Tub). (G) Relative mRNA fold changes of Sox21a in sorted esg+ cells from midguts of young (10 days) and old (50 days) Drosophila carrying esg-GFP and Sox21a-HA. The changes of expressions were plotted relative to the young Drosophila, which was set to 1. Error bars indicate the standard deviation (SD) of three independent experiments. (H) Immunofluorescence images of CAD-EGFP (green) and SOX21A-HA (red) staining with the midgut section from PMG of young (5 days) and old (30 days) Drosophila carrying cad-EGFP and Sox21a-HA. The separated channels of GFP and HA were indicated in the lower panel. The enlarged insets show Sox21a-HA+ cells (red) with CAD-GFP (green) staining. (I) Quantification of fluorescence intensity of CAD-EGFP and SOX21A-HA per HA+ cell as shown in (H). The number n is indicated. Each dot represents one SOX21A-HA+ cell. DAPI stained nuclei are shown in blue. Scale bars represent 10μm (S4H Fig). Error bars represent SD. Student’s t-tests were used to assess significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and NS (non-significant), which represents p > 0.05.
(TIF)
(A-D) Immunofluorescence images of esg-GFP (green) and Pdm1 (red) staining with the midgut in homeostasis. The midgut section from PMG of control Drosophila (A, esgts-Gal4>UAS-GFP), Drosophila carrying esgts-Gal4>UAS-cad-HA (B), Drosophila carrying esgts-Gal4>UAS-GATAe (C), and Drosophila carrying esgts-Gal4>UAS-cad-HA and UAS-GATAe (D). esg-GFP (green) represents ISCs and their differentiating cells. Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (esg-GFP+ and Pdm1+ cells). esg-GFP+ and Pdm1- cells are ISCs or EBs. esg-GFP- and Pdm1+ cells are mature ECs. (E) Quantification of the ratio of esg-GFP+ and Pdm1+ cells per 10,000 μm2 area of R4 region midguts of control Drosophila with genotypes as indicated in A-D. The number n represents counted ROI in midguts from each experiment. Each dot corresponds to one ROI (10,000 μm2 area). DAPI stained nuclei are shown in blue. Scale bars represent 10μm (S5A–S5D Fig). Error bars represent SD. Student’s t-tests were used to assess significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and NS (non-significant), which represents p > 0.05.
(TIF)
(A-D) Immunofluorescence images of NRE-GFP (green) and Pdm1 (red) staining with the midgut fed with PQ (PQ-REC-1D). The midgut section from the R4 region of control flies (A, NREts-Gal4-driven UAS-GFP), Drosophila carrying NREts-Gal4-driven UAS-cad-HA (B), Drosophila carrying NREts-Gal4-driven UAS-hopTUM (C), and Drosophila carrying NREts-Gal4-driven UAS-cad-HA and UAS-hopTUM (D). Pdm1 staining (red) was used to visualize differentiating ECs. White arrows indicate differentiating pre-ECs (NRE-GFP+ and Pdm1+ cells). NRE-GFP+ and Pdm1- cells are EBs. NRE-GFP- and Pdm1+ cells are matured ECs. (E) Quantification of the ratio of NRE-GFP+ and Pdm1+ cells Per 10,000 μm2 area of the R4 region midguts as shown in (A-D). The number n represents counted regions of interest in midguts from each experiment. Each dot corresponds to one region of interest (ROI = 10,000 μm2 area). DAPI stained nuclei are shown in blue. Scale bars represent 10μm (S6A–S6D Fig). Error bars represent SD. Student’s t-tests were used to assess significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and NS (non-significant), which represents p > 0.05.
(TIF)
(A-B) Quantification of the esg+ cell number in AMG (B) or PMG (A) from 35-day-old (25 days at 18°C, then 10 days at 29°C) control Drosophila (esgts-Gal4>UAS-GFP) and Drosophila carrying esgts-Gal4>cad RNAi. The number n is indicated. Each dot corresponds to one ROI (10,000 μm2 area). (C-D) Quantification of pH3+ (C) or esg+ (D) cell numbers in midguts from 35-day-old (25 days at 18°C, then 10 days at 29°C) control Drosophila (esgts-Gal4>UAS-GFP) and Drosophila carrying esgts-Gal4>UAS-cad-HA. The number n represents the whole midguts in C. One dot corresponds to one midgut in C. The number n represents the ROI in midguts from each experiment in D. One dot corresponds to one ROI (10,000 μm2 area) in D. (E-F) Immunofluorescence images of esg-GFP (green) and TUNEL (red) staining with the midgut section from PMG of 35-day-old (25 days at 18°C, then 10 days at 29°C) control Drosophila (E, esgts-Gal4>UAS-GFP) and Drosophila carrying esgts-Gal4>cad RNAi (F). esg-GFP (green) identifies ISCs and their differentiating cells. TUNEL staining (red) was used to visualize the apoptotic cells. White arrowheads indicate apoptotic cells. (G) Survival rate of control Drosophila (esgts-Gal4-driven UAS-GFP) and Drosophila carrying esgts-Gal4-driven cad RNAi. Adult flies were cultured at 18°C for 25 days and shifted to grow at 29°C to turn on the UAS-Gal4 system. The survival rates of these flies were recorded on the 25th day. DAPI stained nuclei are shown in blue. Scale bars represent 10 μm (S7A, S7B, S7K and S7L Fig). Error bars represent SD. Student’s t-tests were used to assess significance. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and NS (non-significant), which represents p > 0.05.
(TIF)
Gene symbols and gene locations are indicated.
(XLSX)
Gene symbols, gene locations, fold changes (i.e., logFC, log2 of the fold change), and P values (obtained by hypergeometric test) are indicated.
(XLSX)
(XLSX)
Acknowledgments
We thank the BDSC, the VDRC, and the THU for fly strains, and the DSHB for antibodies.
Data Availability
The RNA-seq data that support the findings of this study have been deposited in the Sequence Read Archive (SRA) under BioProject accession PRJNA686687. Source data of Fig 4A have been provided as S1 Table and S2 Table.
Funding Statement
This work was supported by the National Key R&D Program of China (2020YFA0803602 and 2018YFA0108301), the National Natural Science Foundation of China (31622031, 31671254, and 91749110) (H.C.), the Guangdong Natural Science Funds for Distinguished Young Scholars (2016A030306037) (H.C.), the National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2020201006) (H.C.), and the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (H.C.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Otsuki L, Brand AH. Cell cycle heterogeneity directs the timing of neural stem cell activation from quiescence. Science. 2018;360(6384):99–102. Epub 2018/04/07. doi: 10.1126/science.aan8795 ; PubMed Central PMCID: PMC6538531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell. 2017;20(6):771–84 e6. Epub 2017/03/24. doi: 10.1016/j.stem.2017.02.009 ; PubMed Central PMCID: PMC5459794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gervais L, Bardin AJ. Tissue homeostasis and aging: new insight from the fly intestine. Curr Opin Cell Biol. 2017;48:97–105. Epub 2017/07/19. doi: 10.1016/j.ceb.2017.06.005 . [DOI] [PubMed] [Google Scholar]
- 4.Stainier DY. No organ left behind: tales of gut development and evolution. Science. 2005;307(5717):1902–4. Epub 2005/03/26. doi: 10.1126/science.1108709 . [DOI] [PubMed] [Google Scholar]
- 5.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439(7075):475–9. Epub 2005/12/13. doi: 10.1038/nature04371 . [DOI] [PubMed] [Google Scholar]
- 6.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439(7075):470–4. Epub 2005/12/13. doi: 10.1038/nature04333 . [DOI] [PubMed] [Google Scholar]
- 7.Du G, Qiao Y, Zhuo Z, Zhou J, Li X, Liu Z, et al. Lipoic acid rejuvenates aged intestinal stem cells by preventing age-associated endosome reduction. EMBO Rep. 2020;21(8):e49583. doi: 10.15252/embr.201949583 ; PubMed Central PMCID: PMC7403706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Fernandez IA, Tauc HM, Jasper H. Hallmarks of aging Drosophila intestinal stem cells. Mech Ageing Dev. 2020;190:111285. Epub 2020/06/17. doi: 10.1016/j.mad.2020.111285 . [DOI] [PubMed] [Google Scholar]
- 9.Jasper H. Intestinal Stem Cell Aging: Origins and Interventions. Annu Rev Physiol. 2020;82:203–26. Epub 2019/10/15. doi: 10.1146/annurev-physiol-021119-034359 . [DOI] [PubMed] [Google Scholar]
- 10.Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell. 2011;9(5):402–11. Epub 2011/11/08. doi: 10.1016/j.stem.2011.10.004 ; PubMed Central PMCID: PMC3212030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, Edgar BA. Intestinal stem cell function in Drosophila and mice. Curr Opin Genet Dev. 2012;22(4):354–60. Epub 2012/05/23. doi: 10.1016/j.gde.2012.04.002 ; PubMed Central PMCID: PMC3426656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4(1):49–61. Epub 2009/01/09. doi: 10.1016/j.stem.2008.10.016 ; PubMed Central PMCID: PMC2659574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Z, Lucchetta E, Rafel N, Ohlstein B. Maintenance of the adult Drosophila intestine: all roads lead to homeostasis. Curr Opin Genet Dev. 2016;40:81–6. Epub 2016/07/09. doi: 10.1016/j.gde.2016.06.009 ; PubMed Central PMCID: PMC5135564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Xu N, Wang C, Huang P, Huang H, Jin Z, et al. Transient Scute activation via a self-stimulatory loop directs enteroendocrine cell pair specification from self-renewing intestinal stem cells. Nat Cell Biol. 2018;20(2):152–61. Epub 2018/01/18. doi: 10.1038/s41556-017-0020-0 . [DOI] [PubMed] [Google Scholar]
- 15.Guo Z, Ohlstein B. Stem cell regulation. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science. 2015;350(6263). Epub 2015/11/21. doi: 10.1126/science.aab0988 ; PubMed Central PMCID: PMC5431284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He L, Si G, Huang J, Samuel ADT, Perrimon N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature. 2018;555(7694):103–6. Epub 2018/02/08. doi: 10.1038/nature25744 ; PubMed Central PMCID: PMC6101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136(3):483–93. Epub 2009/01/15. doi: 10.1242/dev.026955 ; PubMed Central PMCID: PMC2687592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137(7):1343–55. Epub 2009/07/01. doi: 10.1016/j.cell.2009.05.014 ; PubMed Central PMCID: PMC2753793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7(3):318–34. Epub 2008/02/21. doi: 10.1111/j.1474-9726.2008.00380.x ; PubMed Central PMCID: PMC2408640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3(4):442–55. Epub 2008/10/23. doi: 10.1016/j.stem.2008.07.024 ; PubMed Central PMCID: PMC3225008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultz MB, Sinclair DA. When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development. 2016;143(1):3–14. Epub 2016/01/07. doi: 10.1242/dev.130633 ; PubMed Central PMCID: PMC4725211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JS, Kim YS, Yoo MA. The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging (Albany NY). 2009;1(7):637–51. Epub 2010/02/17. doi: 10.18632/aging.100054 PubMed Central PMCID: PMC2806044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen F, Liu Y, Wong NK, Xiao J, So KF. Oxidative Stress in Stem Cell Aging. Cell Transplant. 2017;26(9):1483–95. Epub 2017/11/09. doi: 10.1177/0963689717735407 ; PubMed Central PMCID: PMC5680960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8(2):188–99. Epub 2011/02/08. doi: 10.1016/j.stem.2010.12.006 ; PubMed Central PMCID: PMC3035938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Zeng X, Ryoo HD, Jasper H. Integration of UPRER and oxidative stress signaling in the control of intestinal stem cell proliferation. PLoS Genet. 2014;10(8):e1004568. Epub 2014/08/29. doi: 10.1371/journal.pgen.1004568 ; PubMed Central PMCID: PMC4148219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Ryoo HD, Qi Y, Jasper H. PERK Limits Drosophila Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress. PLoS Genet. 2015;11(5):e1005220. Epub 2015/05/07. doi: 10.1371/journal.pgen.1005220 ; PubMed Central PMCID: PMC4422665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14(6):810–23. Epub 2014/06/07. doi: 10.1016/j.stem.2014.04.014 ; PubMed Central PMCID: PMC4102383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6(10):e1001159. Epub 2010/10/27. doi: 10.1371/journal.pgen.1001159 ; PubMed Central PMCID: PMC2954830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao SH, Tseng CY, Wan CL, Su YH, Hsieh CC, Pi H, et al. Aging and insulin signaling differentially control normal and tumorous germline stem cells. Aging Cell. 2015;14(1):25–34. Epub 2014/12/04. doi: 10.1111/acel.12288 ; PubMed Central PMCID: PMC4326914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493(7432):338–45. Epub 2013/01/18. doi: 10.1038/nature11861 ; PubMed Central PMCID: PMC3687363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young T, Deschamps J. Chapter 8 Hox, Cdx, and Anteroposterior Patterning in the Mouse Embryo. Hox Genes. Current Topics in Developmental Biology 2009. p. 235–55. doi: 10.1016/S0070-2153(09)88008-3 [DOI] [PubMed] [Google Scholar]
- 32.Bürglin TR, Finney M, Coulson A, Ruvkun G. Caenorhabditis elegans has scores of homoeobox-containing genes. Nature. 1989;341(6239):239–43. Epub 1989/09/21. doi: 10.1038/341239a0 . [DOI] [PubMed] [Google Scholar]
- 33.Gamer LW, Wright CV. Murine Cdx-4 bears striking similarities to the Drosophila caudal gene in its homeodomain sequence and early expression pattern. Mech Dev. 1993;43(1):71–81. Epub 1993/09/01. doi: 10.1016/0925-4773(93)90024-r . [DOI] [PubMed] [Google Scholar]
- 34.Drummond F, Putt W, Fox M, Edwards YH. Cloning and chromosome assignment of the human CDX2 gene. Ann Hum Genet. 1997;61(Pt 5):393–400. Epub 1998/02/12. doi: 10.1046/j.1469-1809.1997.6150393.x . [DOI] [PubMed] [Google Scholar]
- 35.Beck F, Stringer EJ. The role of Cdx genes in the gut and in axial development. Biochem Soc Trans. 2010;38(2):353–7. Epub 2010/03/20. doi: 10.1042/BST0380353 . [DOI] [PubMed] [Google Scholar]
- 36.Beck F. Homeobox genes in gut development. Gut. 2002;51(3):450–4. Epub 2002/08/13. doi: 10.1136/gut.51.3.450 ; PubMed Central PMCID: PMC1773336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and cdx2 expression during intestinal development. Gastroenterology. 2000;119(4):961–71. Epub 2000/10/21. doi: 10.1053/gast.2000.18142 . [DOI] [PubMed] [Google Scholar]
- 38.Guo RJ, Suh ER, Lynch JP. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther. 2004;3(7):593–601. Epub 2004/05/12. doi: 10.4161/cbt.3.7.913 . [DOI] [PubMed] [Google Scholar]
- 39.Macdonald PM, Struhl G. A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature. 1986;324(6097):537–45. Epub 1986/12/11. doi: 10.1038/324537a0 . [DOI] [PubMed] [Google Scholar]
- 40.Wu LH, Lengyel JA. Role of caudal in hindgut specification and gastrulation suggests homology between Drosophila amnioproctodeal invagination and vertebrate blastopore. Development. 1998;125(13):2433–42. Epub 1998/06/04. . [DOI] [PubMed] [Google Scholar]
- 41.Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319(5864):777–82. Epub 2008/01/26. doi: 10.1126/science.1149357 . [DOI] [PubMed] [Google Scholar]
- 42.Zhai Z, Huang X, Yin Y. Beyond immunity: The Imd pathway as a coordinator of host defense, organismal physiology and behavior. Dev Comp Immunol. 2018;83:51–9. Epub 2017/11/18. doi: 10.1016/j.dci.2017.11.008 . [DOI] [PubMed] [Google Scholar]
- 43.Choi YJ, Hwang MS, Park JS, Bae SK, Kim YS, Yoo MA. Age-related upregulation of Drosophila caudal gene via NF-kappaB in the adult posterior midgut. Biochim Biophys Acta. 2008;1780(10):1093–100. Epub 2008/07/29. doi: 10.1016/j.bbagen.2008.06.008 . [DOI] [PubMed] [Google Scholar]
- 44.Dutta D, Dobson AJ, Houtz PL, Glasser C, Revah J, Korzelius J, et al. Regional Cell-Specific Transcriptome Mapping Reveals Regulatory Complexity in the Adult Drosophila Midgut. Cell Rep. 2015;12(2):346–58. Epub 2015/07/07. doi: 10.1016/j.celrep.2015.06.009 . [DOI] [PubMed] [Google Scholar]
- 45.Venken KJ, Carlson JW, Schulze KL, Pan H, He Y, Spokony R, et al. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nat Methods. 2009;6(6):431–4. Epub 2009/05/26. doi: 10.1038/nmeth.1331 ; PubMed Central PMCID: PMC2784134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhai Z, Kondo S, Ha N, Boquete JP, Brunner M, Ueda R, et al. Accumulation of differentiating intestinal stem cell progenies drives tumorigenesis. Nat Commun. 2015;6:10219. Epub 2015/12/23. doi: 10.1038/ncomms10219 ; PubMed Central PMCID: PMC4703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Xu N, Huang H, Cai T, Xi R. A feedback amplification loop between stem cells and their progeny promotes tissue regeneration and tumorigenesis. eLife. 2016;5. Epub 2016/05/18. doi: 10.7554/eLife.14330 ; PubMed Central PMCID: PMC4905741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhai Z, Boquete JP, Lemaitre B. A genetic framework controlling the differentiation of intestinal stem cells during regeneration in Drosophila. PLoS Genet. 2017;13(6):e1006854. Epub 2017/07/01. doi: 10.1371/journal.pgen.1006854 ; PubMed Central PMCID: PMC5510897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ayyaz A, Li H, Jasper H. Haemocytes control stem cell activity in the Drosophila intestine. Nat Cell Biol. 2015;17(6):736–48. Epub 2015/05/26. doi: 10.1038/ncb3174 ; PubMed Central PMCID: PMC4449816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohlmaier A, Fassnacht C, Jin Y, Reuter H, Begum J, Dutta D, et al. Src kinase function controls progenitor cell pools during regeneration and tumor onset in the Drosophila intestine. Oncogene. 2015;34(18):2371–84. Epub 2014/07/01. doi: 10.1038/onc.2014.163 . [DOI] [PubMed] [Google Scholar]
- 51.Zeng X, Chauhan C, Hou SX. Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in drosophila. Genesis (New York, NY: 2000). 2010;48(10):607–11. Epub 2010/08/04. doi: 10.1002/dvg.20661 ; PubMed Central PMCID: PMC2958251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Du G, Xiong L, Li X, Zhuo Z, Zhuang X, Yu Z, et al. Peroxisome Elevation Induces Stem Cell Differentiation and Intestinal Epithelial Repair. Dev Cell. 2020;53(2):169–84 e11. doi: 10.1016/j.devcel.2020.03.002 . [DOI] [PubMed] [Google Scholar]
- 53.Okumura T, Takeda K, Kuchiki M, Akaishi M, Taniguchi K, Adachi-Yamada T. GATAe regulates intestinal stem cell maintenance and differentiation in Drosophila adult midgut. Developmental biology. 2016;410(1):24–35. Epub 2016/01/01. doi: 10.1016/j.ydbio.2015.12.017 . [DOI] [PubMed] [Google Scholar]
- 54.Patel PH, Dutta D, Edgar BA. Niche appropriation by Drosophila intestinal stem cell tumours. Nat Cell Biol. 2015;17(9):1182–92. Epub 2015/08/04. doi: 10.1038/ncb3214 ; PubMed Central PMCID: PMC4709566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci U S A. 2012;109(52):21528–33. Epub 2012/12/14. doi: 10.1073/pnas.1215849110 ; PubMed Central PMCID: PMC3535647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karsten P, Häder S, Zeidler MP. Cloning and expression of Drosophila SOCS36E and its potential regulation by the JAK/STAT pathway. Mechanisms of development. 2002;117(1–2):343–6. Epub 2002/09/03. doi: 10.1016/s0925-4773(02)00216-2 . [DOI] [PubMed] [Google Scholar]
- 57.Mlodzik M, Fjose A, Gehring WJ. Isolation of caudal, a Drosophila homeo box-containing gene with maternal expression, whose transcripts form a concentration gradient at the pre-blastoderm stage. The EMBO journal. 1985;4(11):2961–9. Epub 1985/11/01. ; PubMed Central PMCID: PMC554605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mlodzik M, Gehring WJ. Expression of the caudal gene in the germ line of Drosophila: formation of an RNA and protein gradient during early embryogenesis. Cell. 1987;48(3):465–78. Epub 1987/02/13. doi: 10.1016/0092-8674(87)90197-8 . [DOI] [PubMed] [Google Scholar]
- 59.van den Akker E, Forlani S, Chawengsaksophak K, de Graaff W, Beck F, Meyer BI, et al. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development. 2002;129(9):2181–93. Epub 2002/04/18. . [DOI] [PubMed] [Google Scholar]
- 60.Joo MK, Park JJ, Chun HJ. Impact of homeobox genes in gastrointestinal cancer. World J Gastroenterol. 2016;22(37):8247–56. Epub 2016/10/13. doi: 10.3748/wjg.v22.i37.8247 ; PubMed Central PMCID: PMC5055856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryu JH, Nam KB, Oh CT, Nam HJ, Kim SH, Yoon JH, et al. The homeobox gene Caudal regulates constitutive local expression of antimicrobial peptide genes in Drosophila epithelia. Mol Cell Biol. 2004;24(1):172–85. Epub 2003/12/16. doi: 10.1128/MCB.24.1.172-185.2004 ; PubMed Central PMCID: PMC303351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muyskens JB, Guillemin K. Bugs inside Bugs: what the fruit fly can teach us about immune and microbial balance in the gut. Cell Host Microbe. 2008;3(3):117–8. Epub 2008/03/11. doi: 10.1016/j.chom.2008.02.011 . [DOI] [PubMed] [Google Scholar]
- 63.Buchon N, Silverman N, Cherry S. Immunity in Drosophila melanogaster—from microbial recognition to whole-organism physiology. Nat Rev Immunol. 2014;14(12):796–810. Epub 2014/11/26. doi: 10.1038/nri3763 ; PubMed Central PMCID: PMC6190593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Sloan MA, Ligoxygakis P. Intestinal NF-kappaB and STAT signalling is important for uptake and clearance in a Drosophila-Herpetomonas interaction model. PLoS Genet. 2019;15(3):e1007931. Epub 2019/03/02. doi: 10.1371/journal.pgen.1007931 ; PubMed Central PMCID: PMC6415867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanson MA, Lemaitre B. New insights on Drosophila antimicrobial peptide function in host defense and beyond. Curr Opin Immunol. 2020;62:22–30. Epub 2019/12/14. doi: 10.1016/j.coi.2019.11.008 . [DOI] [PubMed] [Google Scholar]
- 66.Sarvari M, Mikani A, Mehrabadi M. The innate immune gene Relish and Caudal jointly contribute to the gut immune homeostasis by regulating antimicrobial peptides in Galleria mellonella. Dev Comp Immunol. 2020;110:103732. Epub 2020/05/20. doi: 10.1016/j.dci.2020.103732 . [DOI] [PubMed] [Google Scholar]
- 67.Zhang R, Kang KA, Kim KC, Na SY, Chang WY, Kim GY, et al. Oxidative stress causes epigenetic alteration of CDX1 expression in colorectal cancer cells. Gene. 2013;524(2):214–9. Epub 2013/04/27. doi: 10.1016/j.gene.2013.04.024 . [DOI] [PubMed] [Google Scholar]
- 68.Stadtman ER. Protein oxidation and aging. Science (New York, NY). 1992;257(5074):1220–4. Epub 1992/08/28. doi: 10.1126/science.1355616 . [DOI] [PubMed] [Google Scholar]
- 69.Badinloo M, Nguyen E, Suh W, Alzahrani F, Castellanos J, Klichko VI, et al. Overexpression of antimicrobial peptides contributes to aging through cytotoxic effects in Drosophila tissues. Archives of insect biochemistry and physiology. 2018;98(4):e21464. Epub 2018/04/11. doi: 10.1002/arch.21464 ; PubMed Central PMCID: PMC6039247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong NA, Wilding J, Bartlett S, Liu Y, Warren BF, Piris J, et al. CDX1 is an important molecular mediator of Barrett’s metaplasia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(21):7565–70. Epub 2005/05/17. doi: 10.1073/pnas.0502031102 ; PubMed Central PMCID: PMC1140438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim S, Domon-Dell C, Wang Q, Chung DH, Di Cristofano A, Pandolfi PP, et al. PTEN and TNF-alpha regulation of the intestinal-specific Cdx-2 homeobox gene through a PI3K, PKB/Akt, and NF-kappaB-dependent pathway. Gastroenterology. 2002;123(4):1163–78. Epub 2002/10/03. doi: 10.1053/gast.2002.36043 . [DOI] [PubMed] [Google Scholar]
- 72.Kim HJ, Kim KW, Yu BP, Chung HY. The effect of age on cyclooxygenase-2 gene expression: NF-kappaB activation and IkappaBalpha degradation. Free radical biology & medicine. 2000;28(5):683–92. Epub 2000/04/08. doi: 10.1016/s0891-5849(99)00274-9 . [DOI] [PubMed] [Google Scholar]
- 73.Choi YJ, Choi TY, Yamaguchi M, Matsukage A, Kim YS, Yoo MA. Transcriptional regulation of the Drosophila caudal homeobox gene by DRE/DREF. Nucleic acids research. 2004;32(12):3734–42. Epub 2004/07/16. doi: 10.1093/nar/gkh688 ; PubMed Central PMCID: PMC484175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park SY, Kim YS, Yang DJ, Yoo MA. Transcriptional regulation of the Drosophila catalase gene by the DRE/DREF system. Nucleic acids research. 2004;32(4):1318–24. Epub 2004/02/26. doi: 10.1093/nar/gkh302 ; PubMed Central PMCID: PMC390290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Osman D, Buchon N, Chakrabarti S, Huang YT, Su WC, Poidevin M, et al. Autocrine and paracrine unpaired signaling regulate intestinal stem cell maintenance and division. Journal of cell science. 2012;125(Pt 24):5944–9. Epub 2012/10/06. doi: 10.1242/jcs.113100 . [DOI] [PubMed] [Google Scholar]
- 76.Tian A, Shi Q, Jiang A, Li S, Wang B, Jiang J. Injury-stimulated Hedgehog signaling promotes regenerative proliferation of Drosophila intestinal stem cells. The Journal of cell biology. 2015;208(6):807–19. Epub 2015/03/11. doi: 10.1083/jcb.201409025 ; PubMed Central PMCID: PMC4362464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou X, Ding G, Li J, Xiang X, Rushworth E, Song W. Physiological and Pathological Regulation of Peripheral Metabolism by Gut-Peptide Hormones in Drosophila. Frontiers in physiology. 2020;11:577717. Epub 2020/10/30. doi: 10.3389/fphys.2020.577717 ; PubMed Central PMCID: PMC7552570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432(7015):324–31. Epub 2004/11/19. doi: 10.1038/nature03100 . [DOI] [PubMed] [Google Scholar]
- 79.Peng YC, Levine CM, Zahid S, Wilson EL, Joyner AL. Sonic hedgehog signals to multiple prostate stromal stem cells that replenish distinct stromal subtypes during regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(51):20611–6. Epub 2013/11/13. doi: 10.1073/pnas.1315729110 ; PubMed Central PMCID: PMC3870668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fendrich V, Esni F, Garay MV, Feldmann G, Habbe N, Jensen JN, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135(2):621–31. Epub 2008/06/03. doi: 10.1053/j.gastro.2008.04.011 ; PubMed Central PMCID: PMC2666349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ochoa B, Syn WK, Delgado I, Karaca GF, Jung Y, Wang J, et al. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology (Baltimore, Md). 2010;51(5):1712–23. Epub 2010/05/01. doi: 10.1002/hep.23525 ; PubMed Central PMCID: PMC2920129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472(7341):110–4. Epub 2011/03/11. doi: 10.1038/nature09851 ; PubMed Central PMCID: PMC3676169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cellular and molecular life sciences: CMLS. 2013;70(12):2059–81. Epub 2012/10/12. doi: 10.1007/s00018-012-1152-9 ; PubMed Central PMCID: PMC3663196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doupé DP, Marshall OJ, Dayton H, Brand AH, Perrimon N. Drosophila intestinal stem and progenitor cells are major sources and regulators of homeostatic niche signals. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(48):12218–23. Epub 2018/11/09. doi: 10.1073/pnas.1719169115 ; PubMed Central PMCID: PMC6275525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.D’Addio F, La Rosa S, Maestroni A, Jung P, Orsenigo E, Ben Nasr M, et al. Circulating IGF-I and IGFBP3 Levels Control Human Colonic Stem Cell Function and Are Disrupted in Diabetic Enteropathy. Cell stem cell. 2015;17(4):486–98. Epub 2015/10/03. doi: 10.1016/j.stem.2015.07.010 ; PubMed Central PMCID: PMC4826279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mizoguchi E, Mizoguchi A, Takedatsu H, Cario E, de Jong YP, Ooi CJ, et al. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology. 2002;122(1):134–44. Epub 2002/01/10. doi: 10.1053/gast.2002.30347 . [DOI] [PubMed] [Google Scholar]
- 87.Tabrez S, Roberts IM. Malabsorption and malnutrition. Primary care. 2001;28(3):505–22, v. Epub 2001/08/03. doi: 10.1016/s0095-4543(05)70050-6. [DOI] [PubMed] [Google Scholar]
- 88.Schulzke JD, Tröger H, Amasheh M. Disorders of intestinal secretion and absorption. Best practice & research Clinical gastroenterology. 2009;23(3):395–406. Epub 2009/06/10. doi: 10.1016/j.bpg.2009.04.005 . [DOI] [PubMed] [Google Scholar]
- 89.König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, et al. Human Intestinal Barrier Function in Health and Disease. Clinical and translational gastroenterology. 2016;7(10):e196. Epub 2016/10/21. doi: 10.1038/ctg.2016.54 ; PubMed Central PMCID: PMC5288588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weström B, Arévalo Sureda E, Pierzynowska K, Pierzynowski SG, Pérez-Cano FJ. The Immature Gut Barrier and Its Importance in Establishing Immunity in Newborn Mammals. Frontiers in immunology. 2020;11:1153. Epub 2020/06/26. doi: 10.3389/fimmu.2020.01153 ; PubMed Central PMCID: PMC7296122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McKenzie C, Tan J, Macia L, Mackay CR. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunological reviews. 2017;278(1):277–95. Epub 2017/06/29. doi: 10.1111/imr.12556 . [DOI] [PubMed] [Google Scholar]
- 92.Shinozaki Y, Furuichi K, Toyama T, Kitajima S, Hara A, Iwata Y, et al. Impairment of the carnitine/organic cation transporter 1-ergothioneine axis is mediated by intestinal transporter dysfunction in chronic kidney disease. Kidney international. 2017;92(6):1356–69. Epub 2017/07/30. doi: 10.1016/j.kint.2017.04.032 . [DOI] [PubMed] [Google Scholar]
- 93.Song W, Cheng D, Hong S, Sappe B, Hu Y, Wei N, et al. Midgut-Derived Activin Regulates Glucagon-like Action in the Fat Body and Glycemic Control. Cell metabolism. 2017;25(2):386–99. Epub 2017/02/09. doi: 10.1016/j.cmet.2017.01.002 ; PubMed Central PMCID: PMC5373560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Honarpisheh P, Reynolds CR, Blasco Conesa MP, Moruno Manchon JF, Putluri N, Bhattacharjee MB, et al. Dysregulated Gut Homeostasis Observed Prior to the Accumulation of the Brain Amyloid-β in Tg2576 Mice. International journal of molecular sciences. 2020;21(5). Epub 2020/03/07. doi: 10.3390/ijms21051711 ; PubMed Central PMCID: PMC7084806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302(5651):1765–8. Epub 2003/12/06. doi: 10.1126/science.1089035 . [DOI] [PubMed] [Google Scholar]
- 96.Ashburner M, Misra S, Roote J, Lewis SE, Blazej R, Davis T, et al. An exploration of the sequence of a 2.9-Mb region of the genome of Drosophila melanogaster: the Adh region. Genetics. 1999;153(1):179–219. Epub 1999/09/03. ; PubMed Central PMCID: PMC1460734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moreno E, Morata G. Caudal is the Hox gene that specifies the most posterior Drosophile segment. Nature. 1999;400(6747):873–7. Epub 1999/09/07. doi: 10.1038/23709 . [DOI] [PubMed] [Google Scholar]
- 98.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development (Cambridge, England). 1993;117(4):1223–37. Epub 1993/04/01. . [DOI] [PubMed] [Google Scholar]