Abstract
Background:
Endochondral ossification is a major bone forming mechanism in vertebrates, defects in which can result in skeletal dysplasia or craniofacial anomalies in humans. The zebrafish holds great potential to advance our understanding of endochondral growth zone development and genetics, yet several important aspects of its biology remain unexplored. Here we provide a comprehensive description of endochondral growth zones in the pharyngeal skeleton, including their developmental progression, cellular activity, and adult fates.
Results:
Postembryonic growth of the pharyngeal skeleton is supported by endochondral growth zones located either at skeletal epiphyses or synchondroses. Col2a1a and col10a1a in situ hybridization and anti-PCNA immunostaining identify resting-, hypertrophic- and proliferative zones, respectively, in pharyngeal synchondroses. Cellular hypertrophy and matrix deposition contribute little, if at all, to axial growth in most skeletal elements. Zebrafish endochondral growth zones develop during metamorphosis and arrest in adults.
Conclusions:
Two endochondral growth zone configurations in the zebrafish pharyngeal skeleton produce either unidirectional (epiphyses) or bidirectional (synchondroses) growth. Cell proliferation drives endochondral growth and its modulation, in contrast to mammalian long bones in which bone length depends more on cell enlargement during hypertrophy and intramembranous ossification is the default mechanism of bone growth in zebrafish adults.
Keywords: bone, cartilage, hypertrophy, indeterminate, metamorphosis, postembryonic
1 |. INTRODUCTION
Bone size and shape are controlled by local regions of proliferating cells along growing fronts that polarize growth in certain directions. In tetrapods, postembryonic elongation of endochondral bones takes place in cartilage growth zones (GZ), where cell proliferation, volume increase and matrix synthesis combine to drive growth along a major axis.1 These processes take place by cell progression through three spatially distinct zones. Immature, slowly dividing cartilage cells from the resting zone (RZ) increase their division rate as they transition into the proliferative zone (PZ). Subsequent entry into the hypertrophic zone (HZ) triggers cell cycle arrest, volume increase and matrix synthesis. HZ cells are ultimately replaced by bone after undergoing apoptosis at the resorption front.1,2 The vast majority of studies on GZ development and function have focused on the epiphyseal growth plates (GPs) of tetrapod long bones, yet endochondral bones that comprise the majority of the mammalian axial and appendicular skeleton come in many shapes, reflecting a largely unexplored diversity of developmental regulatory mechanisms. For example, postembryonic growth of the mammalian skull is mediated in part by GZs located within synchondroses (ie, cartilage joints) of the cranial base.3 Unlike unidirectional growth in long-bone GPs, the mirror-image organization of these cranial GZs produces bidirectional growth.4
The zebrafish (Danio rerio) has many advantages as a model for the study of GZs and their roles in skeletal development. To date, the vast majority of developmental and genetic studies of the zebrafish skeleton have focused on embryonic patterning,5 and these studies have relied on thorough descriptions of the embryonic skeleton and its development.6,7 Post-embryonic skeletal anatomy is comparatively more complex, due to the greater number of (a) skeletal elements,8 (b) tissue types and associated categories of skeletal cells9 and (c) constant remodeling through growth and resorption.2 Similar to tetrapods, teleosts have both a dermal skeleton and an endoskeleton,10,11 with the same major skeletal cell types.9 Cartilage bones develop from cartilage models. The first step in their ossification is the formation of perichondral bone within the perichondrium. Endochondral ossification may follow if hypertrophic chondrocytes are replaced by bone. The formation of trabeculae in place of the cartilage model is a patent sign of this process. However, in contrast to tetrapods, the typical absence of trabecular bone in teleosts, especially in smaller species,2 has led to a debate over the presence of true endochondral ossification. This debate was recently resolved by experimental evidence in zebrafish (a species lacking bone trabeculae) for the conversion of hypertrophic chondrocytes into osteocytes.12 In addition, although the bone marrow is fatty and does not become the site of hematopoiesis in teleosts12–14,15 blood vessels do invade the zebrafish bone marrow similar to tetrapods.12
While endochondral ossification and GZs have been reported in zebrafish,12–14 several important aspects remain unexplored. There is no comprehensive anatomical description of endochondral GZs in zebrafish, or any other teleost for that matter, and it is unclear which are unidirectional vs bidirectional. In addition, of the three basic cellular mechanisms driving endochondral growth in tetrapods (proliferation, cellular enlargement and matrix deposition), proliferation alone has been assayed in zebrafish.12 Lastly, some ray-finned fishes have indeterminate growth (ie, they grow throughout life),16 while others have determinate growth like mammals (ie, they reach an adult steady state).17 The zebrafish growth type appears to be indeterminate as growth continues beyond the onset of sexual maturity.18,19 However, the adult fate of zebrafish endochondral GZs remains undescribed.
Here we describe the locations of endochondral GZs in the zebrafish, focusing on the pharyngeal skeleton. We find that most transition regions between bone collar and bare cartilage are GZs, as indicated by localized bone matrix deposition, and include both unidirectional and bidirectional configurations. In addition, we examine cell proliferation, hypertrophy and matrix deposition in these GZs. Surprisingly, our results suggest that hypertrophy and matrix deposition contribute very little, if at all, to axial growth in most skeletal elements, and that proliferation is the major driver of endochondral growth in zebrafish. Lastly, we find that zebrafish endochondral GZs develop during metamorphosis and that they become inactive in adults. In contrast, intramembranous bone growth remains active in adults, providing a default mechanism for further growth.
2 |. RESULTS
2.1 |. Anatomical locations of endochondral growth zones in the zebrafish pharyngeal skeleton
In order to gain a better understanding of juvenile and adult bone growth in zebrafish we focus on the skull, which is composed of pharyngeal and neurocranial skeletons, each made of dermal and endochondral bones as in other bony fishes (Osteichthyes).8,20 We consider all areas of transition between bone and cartilage as putative growth zones (GZs). The pharyngeal skeleton, including the mandibular, hyoid and branchial arches, contains both distal cartilages and synchondroses (Figure 1A). In the neurocranium, synchondroses join bones associated with the ethmoid cartilage anteriorly, the occipital region ventrally and posteriorly, and the otic region dorsally and posteriorly (Figure 1B).8 Here, we restrict our description of bone growth at putative GZs to the pharyngeal skeleton of juvenile zebrafish (13.5 mm SL).18 To assay bone growth at putative endochondral GZs, we use a pulse-chase bone live stain assay in which juveniles (SL = 12 mm) are first stained with calcein green followed by alizarin red 14 days later. New bone is thus stained by alizarin red alone.
FIGURE 1.
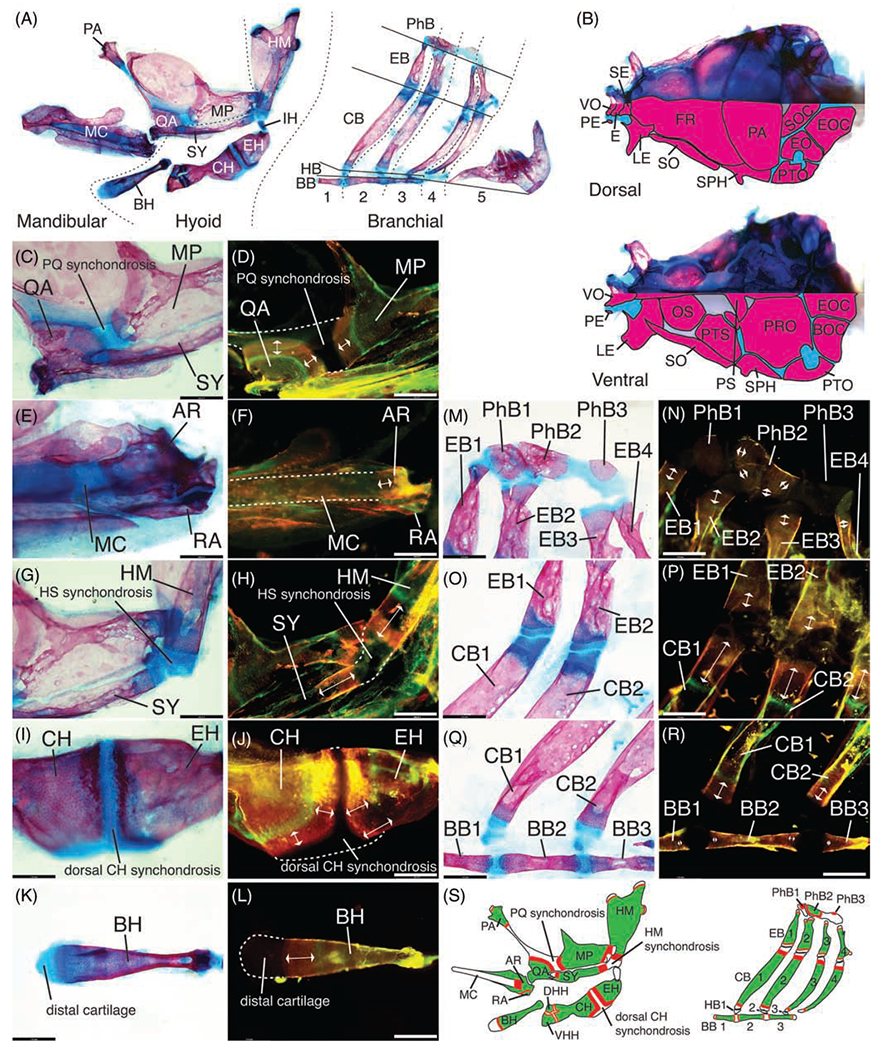
Endochondral growth zone locations in the zebrafish pharyngeal skeleton (13.5 mm SL). Bone stained with alizarin red, cartilage stained with alcian blue in panels A, B, C, E, G, I, K, M, O, Q. Old bone stained with calcein green, new bone stained with alizarin red in panels D, F, H, J, L, N, P, R. A, Anatomy of pharyngeal skeleton, including mandibular, hyoid, and branchial regions. B, Anatomy of neurocranial skeleton in dorsal and ventral views, with camera lucida outlines of bones in lower half of each image to facilitate identification. Synchondroses separating bones are colored in blue. C-F, Mandibular skeleton regions. C, QA and MP bones across PQ synchondrosis. D, QA and MP growth. E, AR and RA bones and MC. F, AR growth. G-L, Hyoid skeleton regions. G, HM and SY bones across HS synchondrosis. H, HM and SY growth. I, CH and EH bones across dorsal CH synchondrosis. J, CH and EH growth. K, BH bone and distal cartilage. L, BH growth. M-R, Branchial skeleton regions. M, PhB1-3 bones with cartilage pads and EB1-4 bones with dorsal distal cartilages. N, PhB 1and 3 ossification, PhB2 growth and EB dorsal growth. O, EB1-2 and CB1-2 bones with distal cartilages. P, EB ventral growth and CB dorsal growth. Q, CB1-2 bones with ventral distal cartilages and BB1-3 bones across BB synchondroses. R, CB ventral growth and BB growth. S, Summary of locations of new bone (red) deposition detected in the pharyngeal arch cartilage-derived skeleton. Scale bar: 140 μm. Dorsal mandibular arch: MP, metapterygoid, PA, palatine, PQ, palatoquadrate, QA, quadrate. Ventral mandibular arch: AR, articular; MC, Meckel’s cartilage; RA, retroarticular. Dorsal hyoid arch: HM, hyomandibula; HS, hyosymplectic; IH, interhyal; SY, symplectic. Ventral hyoid arch: BH, basihyal; CH, ceratohyal; DHH, dorsal hypohyal; EH, epihyal; VHH, ventral hypohyal. Branchial arches: BB, basibranchial; CB, ceratobranchial; EB, epibranchial; HB, hypobranchial; PhB, pharyngobranchial. Neurocranium: BOC, basioccipital, E, ethmoid; EO, epioccipital; EOC, exoccipital; FR, frontal; LE, lateral ethmoid; OS, orbitosphenoid; PE, preethmoid; PRO, prootic; PS, parasphenoid; PTO, pterotic; PTS, pterosphenoid; SE, supraethmoid; SO, supraorbital; SOC, supraoccipital; SPH, sphenotic; VO, vomer
The mandibular arch cartilage skeleton is composed of the palatoquadrate (PQ; dorsal) and Meckel’s cartilage (MC; ventral) separated by the jaw joint.6,20 Dorsally, three bones ossify on the PQ: the palatine (PA; anterior), quadrate (QA; ventral) and metapterygoid (MP; posterior) (Figure 1A).8 Cartilage is located at the anterior tip of the PA, posterior tip of the MP and within the synchondrosis separating the three bones (Figure 1A,C). Pulsed alizarin staining revealed bone growth in the PA, QA, and MP at all cartilage-bone transitions, with greatest amounts in the QA and MP regions flanking the PQ synchondrosis (Figure 1D,S). Ventrally, MC ossifies proximally at two centers that become the articular (AR) and retroarticular (RA) bones (Figure 1E,F).8 Bone growth was detected at the cartilage-bone transition between MC and the AR, and in small amounts across the AR-RA joint (Figure 1F,S).
The hyoid arch cartilage skeleton is composed of four cartilage elements (hyosymplectic-HS, interhyal-IH, ceratohyal-CH and basihyal-BH) that ossify into eight bones (Figure 1A).6,8 Dorsally the HS cartilage ossifies into two bones (hyomandibula, HM; symplectic, SY) separated by a synchondrosis and each flanked by cartilage at their distal ends (Figure 1A,C,G). Bone growth was detected at all cartilage-bone transitions in the HM and SY (Figure 1H,S). The IH cartilage physically links the dorsal CH to the HS synchondrosis—it was not ossified at the juvenile stage examined (Figure 1A). Ventrally, the CH cartilage ossifies into four bones (epihyal—EH, CH, and dorsal- and ventral hypohyals—DHH and VHH) with putative endochondral GZs located within two synchondroses (Figure 1A,I).8 Bone growth was detected at all cartilage-bone transitions in the EH, CH, DHH and VHH (Figure 1J,S), with greatest amounts in the CH and EH regions flanking the dorsal CH synchondrosis (Figure 1J). The unpaired basihyal (BH) cartilage ossifies at the ventral midline into a single bone with one putative GZ (Figure 1A,K), where bone growth was detected (Figure 1L).
The skeleton of the five branchial arches is composed of a repetitive dorso-ventral array of cartilage-derived skeletal elements named from dorsal to ventral: pharyngo- (PhB), epi- (EB), cerato- (CB), hypo- (HB) and basi-branchial (BB) each numbered 1 to 5 for its corresponding branchial arch (Figure 1A).6,8 Three PhBs ossify dorsally (PhB1-3) and retain cartilage pads that articulate with EB1-4 (Figure 1A,M).8 Growth was detected at the cartilage-bone transition flanking PhB2, while PhB1 and 3 started ossifying between the calcein green pulse and the alizarin red chase staining (Figure 1N,S). Growth was also detected at both ends of EB1-4 (Figure 1N,P,S). Ventrally, CBs 1 to 4 are long bones flanked by cartilage at either end (Figure 1A,O,Q). Here also, bone growth was detected at each of these putative GZs (Figure 1P,R,S). Zebrafish HBs do not ossify, while three BB elements commonly ossify along the ventral midline of the branchial skeleton (Figure 1A,Q).8 Growth was barely detected at the ends of each BB (Figure 1R,S). In branchial arch 5, the toothed CB5 loses all cartilage during post-embryonic development and there is no EB (Figure 1A).8
2.2 |. Zebrafish pharyngeal synchondroses are mirror-image endochondral growth zones
In mammals, endochondral GPs, produce axial growth at the distal ends of each long bone. In this configuration, the RZ is distal-most and closest to the articular surface, flanked proximally by the PZ, followed by the HZ.1 A different spatial arrangement is found in the neurocranial base, where synchondroses (ie, cartilage joints) house mirror-image GZs for adjacent bones. In this configuration, a central RZ is flanked by two sets of PZs and HZs, creating bidirectional bone growth in opposing directions.3,4,21–23
The anatomical sites of bone growth described above suggest that both configurations are present in the zebrafish skull (Figure 1). Examples of distal, unidirectional GZs are found in CB1-4 and EB1-4 (Figure 1K–M), while examples of mirror-image, bidirectional GZs are found in the mandibular arch between the QA and MP (Figure 1B–D), in the hyoid arch between the SY and HM dorsally (Figure 1E–G), and between the CH and EH ventrally (Figure 1H–J).
In order to further determine if zebrafish pharyngeal synchondroses are bidirectional GZs, we used molecular markers to label RZs, PZs, and HZs in the synchondroses separating (a) the QA and MP (mandibular arch) and (b) the CH and EH (hyoid arch; Figure 2). col2a1a and col10a1a were used as RZ and HZ markers, respectively. Proliferative cell nuclear antigen (PCNA) was used as a PZ marker. In situ hybridization and immunostaining were conducted on cryosections of early juvenile stage individuals (7 mm SL). In both synchondroses examined, a central region of col2a1a expression (Figure 2B, E) is flanked on both sides by the partially overlapping expression of col10a1a (Figure 2C, F). Two distinct PCNA positive regions identify PZs within the col2a1a positive region of the PQ synchondrosis (Figure 2D). In the CH synchondrosis, a single PCNA-positive region is observed within the col2a1a expression domain (Figure 2G). We interpret this single region as a narrow RZ, where larger and rounded nuclei are flanked by two PZs, where labeled nuclei have the distinctly flattened shape of proliferative chondrocytes, correlating with the narrowness of this synchondrosis. These results show that zebrafish pharyngeal synchondroses are mirror-image, bidirectional GZs, where a central RZ is flanked by two sets of PZs and HZs.
FIGURE 2.
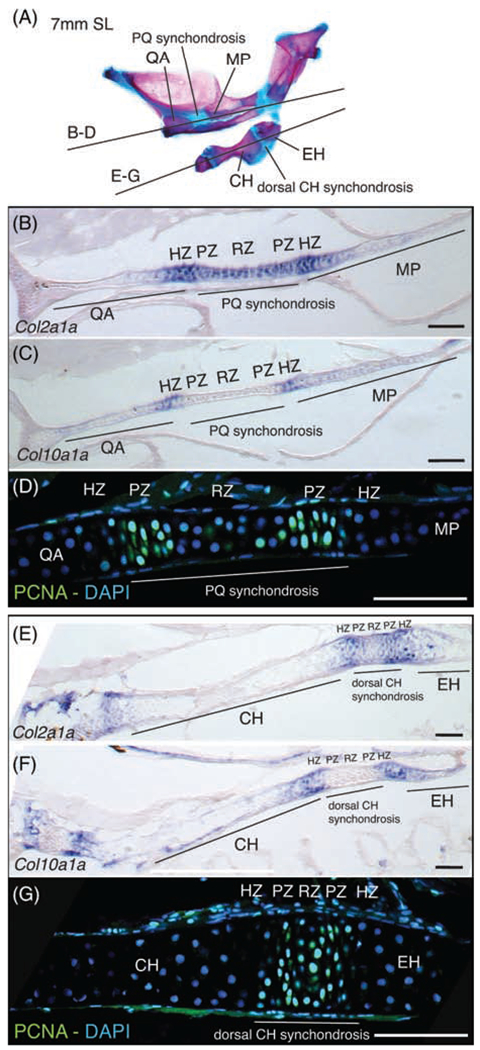
Molecular analysis of bidirectional endochondral growth zones in zebrafish. A, Section planes shown on alizarin red and alcian blue stained skeleton of 7 mm (SL) zebrafish mandibular and hyoid arches. B, Col2a1a expression in PQ synchondrosis and flanking QA and MP bones. C, Col10a1a expression in putative hypertrophic zones on either side of the PQ synchondrosis. D, anti-PCNA immunostaining of presumptive proliferative and resting zone cells in PQ synchondrosis. E, Col2a1a expression in dorsal CH synchondrosis and flanking CH and EH bones. F, Col10a1a expression in putative hypertrophic zones on either sides of the dorsal CH synchondrosis. G, anti-PCNA immunostaining of presumptive proliferative and resting zone cells in CH synchondrosis. Scale bar: 75 μm. CH, ceratohyal; EH, epihyal; HZ, hypertrophic zone; MP, metapterygoid; PZ, proliferative zone; PQ, palatoquadrate; QA, quadrate; RZ, resting zone
2.3 |. Hypertrophy and matrix deposition are negligible contributors to zebrafish endochondral growth
col10a1a expression suggests the presence of HZs in zebrafish, but do cells in these zones actually undergo hypertrophy? Hypertrophy is gradual in mammals, as hypertrophic chondrocytes may increase 4-fold in height and 10-fold in volume during maturation from prehypertrophy to terminal differentiation just prior to apoptosis and resorption at the chondro-osseous junction.24 Therefore, we examined zebrafish GZs at multiple stages (8 dpf, 6 mm SL, 10 mm SL, 13.5 mm SL, 18 mm SL and 23 mm SL) to narrow down the stage(s) when hypertrophy contributes to bone growth. We focused on 18 mm SL individuals because we found many similarities between their GZs and mammalian GZs, namely the presence at this stage of a bone collar, alcian blue-negative cartilage matrix in the HZ and a chondro-osseous junction (Figure 3A). Cell cross-sectional area and height (cell diameter parallel to the direction of growth) were used to assay enlargement within HZ chondrocytes from flat-mounted QAs. To measure cell enlargement across the HZ, cell dimensions were assayed in 2 areas: HZ cells within a 45μm radius of the PZ were designated prehypertrophic, while cells within a 45μm radius of the chondro-osseous junction were designated terminal. Surprisingly, small insignificant increases in cell cross-sectional area (8.7%; P = .3; Tukey’s HSD test) and height (3.7%; P = .04; Tukey’s HSD test) were observed between prehypertrophic (n = 85) and terminal (n = 158) HZ chondrocytes (Figure 3A–C; Table 1). In addition, HZ cells were even smaller than RZ cells as cell cross-sectional area and height were respectively 15.1% (P < .0001; Tukey’s HSD test) and 15.7% (P < .0001; Tukey’s HSD test) smaller in terminal HZs than in RZs. Lastly, extracellular matrix (ECM) synthesis was assayed by comparing the surface area of extra-cellular space in pre- vs terminal hypertrophy regions and no significant increases in ECM content were detected in the QA HZ (Figure 3D; Table 1).
FIGURE 3.

Cellular enlargement and cartilage matrix synthesis in zebrafish hypertrophic zones. A, Resting, proliferative, prehypertrophic and terminal hypertrophic zones and bone front in the QA bone and flanking PQ synchondrosis stained with alizarin red and alcian blue. B,C, Cell cross-sectional area (μm2) and height (μm), respectively, in RZ, preH and terminal HZ cells of the QA bone. C, Cell height (μm) in RZ, preH and terminal HZ cells of the QA bone. D, Percentage of surface occupied by ECM in preH and terminal HZs of the QA bone. E,F,G, PreH and terminal hypertrophic zones in the SY, CB1 and CH bone and flanking HS synchondroses, respectively, stained with alizarin red and alcian blue. H,I, Cell cross-sectional area (μm2) and height (μm), respectively, in preH and terminal HZ cells of the SY, CB1 and CH bones. J, Percentage of surface occupied by ECM in preH and terminal HZs of the SY, CB1 and CH bones. Scale bar: 50 μm. CB1, ceratobranchial 1; CH, ceratohyal; ECM, extracellular matrix; HS, hyosymplectic; PQ, palatoquadrate; preH, prehypertrophic; PZ, proliferating zone; QA, quadrate; RZ, resting zone; SY, symplectic
TABLE 1.
Chondrocyte cross-sectional area and height in 18 mm (SL) zebrafish
| Element | Cell type | cross-sectional area (μm2) ± SD | vs preH P-value | Height (μm) ± SD | vs preH P-value | % ECM ± SD |
|---|---|---|---|---|---|---|
| QA | preH | 60.1 ± 11.3 | 6.1 ± 1.1 | 15.4 ± 2.2 | ||
| terminal | 65.4 ± 13.0 | .324 | 6.3 ± 1.0 | .038 | 15.2 ± 0.8 | |
| RZ | 75.2 ± 20.0 | 0 | 7.3 ± 1.3 | 0 | ||
| SY | preH | 73.7 ± 12.8 | 6.8 ± 0.9 | 14.1 ± 1.3 | ||
| terminal | 74.9 ± 16.0 | .999 | 6.4 ± 1.0 | .650 | 13.8 ± 2.0 | |
| CH | preH | 73.3 ± 14.4 | 7.3 ± 1.0 | 12.7 ± 0.7 | ||
| terminal | 106.2 ± 18.0 | 0 | 13.1 ± 1.7 | 0 | 14.8 ± 0.7 | |
| CB1 | preH | 74.5 ± 14.8 | 6.7 ± 0.9 | 10.9 ± 1.0 | ||
| terminal | 73.3 ± 12.1 | .999 | 6.6 ± 0.8 | 1.0 | 11.3 ± 2.1 |
To determine if cell enlargement is similarly negligible in other parts of the zebrafish pharyngeal skeleton at this stage (18 mm SL), we compared the size and height of prehypertrophic vs terminal HZ chondrocytes at two other bidirectional GZs: the dorsal SY (Figure 3E) and dorsal CH (Figure 3G), and one monodirectional GZ, the ventral CB1 (Figure 3F). No significant enlargement (Figure 3H,I) or increase in ECM content (Figure 3J) were observed in SY and CB1 GZs (Table 1). However, significant increases in cross-sectional area (44.9%; P-value < .0001; Tukey’s HSD test) and height (80.6%; P-value < .0001; Tukey’s HSD test) were observed between prehypertrophic and terminal HZ chondrocytes in the CH (Figure 3G–I), where maturation distance between PZ and chondro-osseous junction is over 2 times greater than in QA, SY and CB1. A 16.4% increase in ECM content was also observed in the CH (Figure 3J; Table 1). Overall these results show that cell enlargement and ECM synthesis are negligible contributors to endochondral bone growth in much of the zebrafish pharyngeal skeleton, except in the CH, where cell height of terminal HZ chondrocytes (13.1um) is nearly double that of prehypertrophic chondrocytes (7.3 μm).
2.4 |. Presumptive hypertrophic chondrocytes transiently enlarge at the onset of growth zone development
Based on the near absence of hypertrophy in mature zebrafish GZs (Figure 3), we asked if hypertrophy occurs at earlier stages of development and when zebrafish GZs first develop.
Three stages were examined: 8 dpf, 6 mm (SL) and 10 mm (SL). 8 dpf larva have fully developed cartilages without detectable GZs (Figure 4A–D). 6 mm (SL) individuals undergo the early stage of metamorphosis, as defined by the appearance of the first melanophores and rays in dorsal and anal fins and of the first joints between caudal fin ray segments .18 At this stage, flattening of chondrocytes at presumptive PZs and the previously reported appearance of a bone collar25 indicate early GZ development (Figure 4E–H). 10 mm (SL) individuals were found to be in a more advanced stage of GZ development as the cartilage matrix of their HZ chondrocytes changed in composition as demonstrated by lack of alcian staining (Figure 4I–L). Enlargement of cell cross-sectional area ranging from 14% (SY; P = .21; Tukey’s HSD test) to 95% (CH; P < .0001; Tukey’s HSD test) was observed between 8 dpf and 6 mm (SL) in the presumptive hypertrophic chondrocytes of examined elements (Figure 4M–P; Table 2). However, this cell cross-sectional area increase was not typical of hypertrophy as observed in tetrapods: it was not concomitant with a cell elongation along the axis of skeletal growth. In addition, this enlargement was transient: cell size at 10 mm (SL) decreased below 8 dpf values in all elements except for the QA, where cell cross-sectional area decreased to 33% above 8 dpf-size (P < .0001; Tukey’s HSD test; Figure 4M–P; Table 2). We conclude that while the initiation of chondrocyte hypertrophy and bone collar formation coincide in zebrafish GZs, similar to mammals, hypertrophy appears to regress later.
FIGURE 4.

Endochondral growth zone development in zebrafish. A-D, Alcian blue stained cartilages of 8 dpf zebrafish with presumptive A, QA; B, SY; C, CH; and D, CB1 labeled (dashed line). E-H, Alcian blue stained cartilages of 6 mm (SL) zebrafish with labeled presumptive resting (RZ), proliferative (PZ) and hypertrophic (HZ) zones associated with the, E, QA; F, SY; G, CH; and H, CB1 (dashed lines). I-L, Alcian blue stained cartilages of 10 mm (SL) zebrafish with labeled presumptive RZ, PZ and HZ associated with the, I, QA; J, SY; K, CH; and L, CB1 (dashed lines). M-P, Cell cross-sectional area (μm2) in 8 dpf, 6 mm (SL) and 10 mm (SL) presumptive HZ cells of the, M, QA; N, SY; O, CH; and P, CB1. Scale bar: 50 μm. CB1, ceratobranchial 1; CH, ceratohyal; HZ, hypertrophic zone; PZ, proliferative zone; QA, quadrate; RZ, resting zone; SY, symplectic, *, presumptive
TABLE 2.
Chondrocyte cross sectional area in 8 dpf, 6 mm (SL), and 12 mm (SL) zebrafish
| Element | Stage | Cross-sectional area (um2) ± SD | vs 8 dpf P-value |
|---|---|---|---|
| QA | 8 dpf | 50.3 ± 6.2 | |
| 6 mm | 85.4 ± 17.9 | 0 | |
| 12 mm | 66.4 ± 11.5 | 0 | |
| SY | 8 dpf | 88.1 ± 19.4 | |
| 6 mm | 99.7 ± 23.6 | .217 | |
| 12 mm | 78.4 ± 13.4 | .247 | |
| CH | 8 dpf | 81.7 ± 16.4 | |
| 6 mm | 159.8 ± 28.5 | 0 | |
| 12 mm | 69.6 ± 13.2 | .001 | |
| CB1 | 8 dpf | 81.9 ± 12.4 | |
| 6 mm | 126.7 ± 36.7 | 0 | |
| 12 mm | 66.5 ± 10.9 | .021 |
2.5 |. Endochondral growth zones shut down in zebrafish adults
A potentially significant difference between zebrafish and mammalian or bird endochondral GZs is their activity lifespan. Determinate growth is the general condition in mammals and birds, as individuals stop growing when they reach a predictable size and this results largely from the arrest of cartilage cell proliferation, hypertrophy, matrix deposition and its replacement by bone.1 In contrast, growth in ray-finned fishes is either indeterminate, generally in long-lived species of colder regions, or determinate in short-lived species of warmer regions,16,17,26 with numerous exceptions including zebrafish, which growth appears to be indeterminate.18,19
To determine if zebrafish endochondral growth is maintained in adults, we fixed and stained 1 year-old adults (2.3-2.5 cm SL) with alcian blue and alizarin red to visualize the cellular structure of their GZs (Figure 5A). Similar to earlier time points examined above, alcian blue-positive cartilage was detected at the synchondroses separating the QA and MP (Figure 5B), the MC (Figure 5D), the SY and HM (Figure 5F), the CH and EH (Figure 5H), and at the ventral extremity of the CB1 (Figure 5J). However, alcian-negative HZ chondrocytes had completely disappeared and there was no overlap between bone and cartilage in any of the GZs examined.
FIGURE 5.
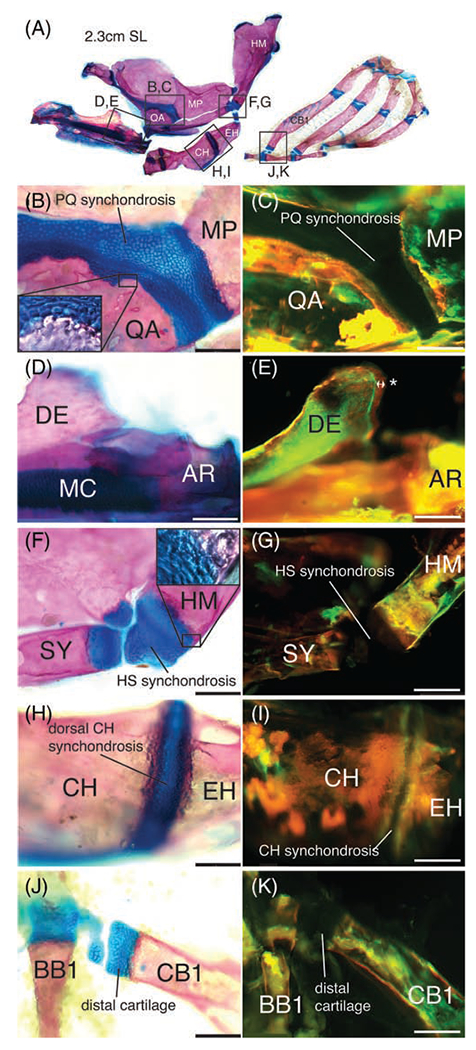
Endochondral growth arrest in adult zebrafish. A, Anatomy of one-year old adult zebrafish cartilage-derived pharyngeal skeleton with alizarin red stained bone and alcian blue stained cartilage. Frames indicate regions highlighted in panels B to K. B, Alcian blue stained PQ synchondrosis flanked by alizarin red stained QA and MP bones. Chondrocytes are absent from the QA and MP, as shown in higher magnification inset. C, Bone matrix of the QA and MP flanking the PQ synchondrosis is stained by both alizarin red and calcein green. D, Alcian blue stained MC flanked posteriorly by alizarin red stained AR bone. E, Bone matrix of the AR flanking MC is stained by both alizarin red and calcein green. New bone growth on DE is stained by alizarin red alone (double-arrow and *). F, Alcian blue stained HS synchondrosis flanked by alizarin red stained SY and HM bones. Chondrocytes are absent from the SY and HM, as shown in higher magnification inset. G, Bone matrix of the SY and HM flanking the HS synchondrosis is stained with both alizarin red and calcein green. H, Alcian blue stained dorsal CH synchondrosis flanked by alizarin red stained CH and EH bones. Chondrocytes are absent from the CH and EH. I, Bone matrix of the CH and EH flanking the dorsal CH synchondrosis is stained by both alizarin red and calcein green. J, Alcian blue stained CB1 distal cartilage flanked by alizarin red stained CB1 bone. Chondrocytes are absent from the CB1. K, Bone matrix of the CB1 flanking the distal cartilage is stained by both alizarin red and calcein green. Scale bar: 640 μm. AR, articular; BB1, basibranchial 1; CB1, ceratobranchial 1; CH, ceratohyal; DE, dentary; EH, epihyal; HM, hyomandibula; HS, hyosymplectic; MC, Meckel’s cartilage; MP, metapterygoid; PQ, palatoquadrate; QA, quadrate; SY, symplectic
In order to detect if actual bone growth was deposited at adult GZs, adults were live-stained with calcein green, followed by live alizarin red stain 14 days later. In these conditions, new bone is stained red alone, while old bone is stained red and green. An overlap of green and red stain was observed at all the bone fronts flanking the examined growth zones: QA and MP (Figure 5C), MC and AR (Figure 5E), SY and HM (Figure 5G), CH and EH (Figure 5I), and CB1 (Figure 5K), indicative of lack of bone growth. New bone growth was however detected at the dorsal posterior edge of the DE (Figure 5E), indicating that intramembranous ossification was still active in adults. We conclude that endochondral GZs shut down in zebrafish adults, similar to mammals and birds, although the effects of fish density and tank size on this process remain to be determined since low density and large tank size promote growth in many fish species.27–29 Unlike in tetrapods the cartilage areas derived from earlier functioning RZs and PZs are not replaced by bone.
3 |. DISCUSSION
In this study, we show that endochondral GZs located either at skeletal epiphyses or synchondroses support postembryonic growth of the zebrafish pharyngeal skeleton. The spatial organization of epiphyseal GZs resembles that of mammalian GPs and produces growth in one direction. In contrast, synchondral GZs have a mirror-image organization that produces bidirectional growth. Surprisingly, cell proliferation drives endochondral growth in this skeleton, while cellular enlargement and matrix production contribute little to growth, unlike mammalian GPs. In addition, zebrafish endochondral GZs develop during metamorphosis and shut down in adults under standard raising conditions, while intramembranous ossification remains active.
3.1 |. Diversity of endochondral growth strategies
Strikingly, we find negligible contributions of cellular enlargement (hypertrophy) to axial growth in zebrafish endochondral GZs, which contrasts starkly with the major role of hypertrophy in endochondral growth and growth rate modulation in mammals. The vast majority of studies of mechanisms regulating endochondral bone growth have focused on GPs of rodent long bones.1 In these GPs, a chondrocyte differentiation cascade with first proliferation, succeeded by enlargement and matrix synthesis in the HZ, mediates postnatal elongation of endochondral bone. However, these three cellular mechanisms do not contribute equally to bone elongation, as shown in the rat proximal tibia where enlargement contributes 59% of axial growth, matrix synthesis 32% and proliferation 9%.30 In addition, periodic accelerated growth almost exclusively involves cellular enlargement in the HZ.31 Differential growth rates in GZs also occur within an individual, notably accounting for length differences between tibia and radius in rats,30 metacarpal elongation in bat wings32 and metatarsal elongation in jerboa hindlimbs vs forelimbs.33 In all three cases, dramatic cellular enlargement in the HZ drives differential growth. In contrast, modulation of endochondral elongation in birds primarily results from cell proliferation, although enlargement and matrix synthesis are also detected.34
Our results in zebrafish suggest that proliferation drives endochondral growth and changes in growth rate between different skeletal GZs. We detect some cell enlargement and matrix deposition, but they are moderate and either transient (6 mm SL) or localized to single skeletal elements (CH 18 mm SL). The largest average cell cross-sectional area observed in this study (160 μm2; CH 6 mm SL) is 24.8 times smaller than for a 21 day rat HZ cell (3971 μm2) and 6.4 times smaller than a rat PZ cell (1030 μm2).31 Why would mammals use cartilage hypertrophy for growth more than fishes? Speed might be the answer, as suggested by the dominant role of hypertrophy in supporting accelerated growth in mammals.1,30,32 Additional studies in fishes from other taxa and with different growth rates are necessary to determine if the zebrafish condition is representative of other ray-finned fishes.
3.2 |. Development of endochondral growth zones
How can zebrafish and other teleosts advance our understanding of GZ development? Unlike GPs in tetrapod limbs, which are invariably unidirectional at the epiphyseal ends of long bones, synchondroses are bidirectional and variable in position and orientation. In mouse GPs two signals, Indian Hedgehog (Ihh) and Parathyroid Hormone-Like Hormone (Pthlh), play key roles in growth.35–38 Ihh expressed in prehypertrophic chondrocytes activates Pthlh expression at a distance in periarticular chondrocytes, which in turn negatively regulates Ihh expression. This feedback loop maintains Ihh and Pthlh expression domains at a constant distance, effectively regulating GP patterning and activity. High Pthlh/low Ihh in RZ maintains cells in an undifferentiated, slow cycling state, while high Ihh/low Pthlh in PZ promotes an undifferentiated, high proliferation state, and high Ihh/no Pthlh promotes hypertrophy in HZ. This feedback loop is largely conserved in mouse skull synchondroses, although Pthlh expression expands into both the RZ and PZ.22,23 However, several standing questions remain including how these skull GZs are patterned in the embryo? In Ihh−/− mice, both limb GP position and polarity are much more severely disrupted than GZs in skull synchondroses.22,36 Both are regulated by planar cell polarity (PCP) pathways, such as non-canonical Wnt signaling in mouse digits39 and Fat-Dachsous signaling in the zebrafish pharyngeal skeleton.40 The diverse array of GZ positions, configurations and orientations of the teleost pharyngeal skeleton combined with the molecular genetic tools available in zebrafish hold great potential to elucidate GZ patterning mechanisms.
3.3 |. Endochondral growth zone evolution
Furthermore, which configuration of skeletal growth is ancestral, unidirectional or bidirectional? Studies of the fossil record support a time of appearance of endochondral bone predating the split between ray-finned (Actinopterygians) and lobe-finned fishes (Sarcoptery gians), leading to tetrapods,11 with tenuous records in Osteostracans,41 Placoderms,42 and Acanthodians.43 However, the location and configuration of endochondral GZs in extinct gnathostomes remains unknown. Here, we report the presence of both configurations in the zebrafish pharyngeal skeleton, supporting a time of appearance of both prior to the split between ray-finned and lobe-finned fishes. In what anatomical location did endochondral GZs first evolve? The fossil record suggests that the pharyngeal skeleton came first in the vertebrate skeleton, where it supported feeding and respiration in early agnathans over 500 million years ago,11 but was composed of noncollagenous cartilage.44 The fossil appearance of perichondral bone is reported simultaneously in the pharyngeal, axial and appendicular skeletons of at least some placoderms.11 It is therefore unknown if endochondral GZs first evolved in a particular skeletal region, or in all of them simultaneously. Can the development of zebrafish cranial endochondral GZs inform our understanding of GZ evolution? Unlike in mammals, most ray-finned fish epiphyses lack secondary ossification centers and are entirely cartilaginous.45 In this context, a simple developmental change: the fusion of two skeletal elements such as the CB1 and EB1 across a normally free joint will unite their RZs into a single shared one, resulting into a mirror-image configuration. The opposite transformation actually takes place in the ventral branchial basket: BBs 1 to 3 ossify on a single cartilage rod (copula 1) in juveniles, which regresses into cartilage pads that form epiphyses in adults.8 Molecular evidence supports the close evolutionary and developmental relationship between uni- and bidirectional GZs: pthlh is expressed in both the epiphyseal cartilage of unidirectional GZs and the shared RZ of bidirectional GZs,4 as would result from epiphyseal fusion.
3.4 |. Importance of endochondral growth zones in the diversification of teleost skull morphology
More than half of all vertebrate species are ray-finned fishes and while previous studies have shown the presence of endochondral GZs in this lineage,46,47 their number and anatomical locations have not been described. Over 400 million years, ray-finned fishes have diversified into over 30 000 extant species and evolved countless morphological adaptations, many of which relate to the trophic apparatus of the head. What was the importance of endochondral GZs in enabling the evolution of new morphologies in this lineage? Recent adaptive radiations provide numerous opportunities to determine if changes in endochondral GZ development, activity and/or lifespan underlie morphological evolution in ray-finned fishes. Interestingly, studies conducted in closely related yet morphologically divergent species of East African cichlids and damselfishes (Pomacentridae family) have shown that differences in morphology largely develop during postembryonic development,48–50 leading to the idea that changes in bone remodeling may be responsible for the morphological diversification of young radiations. A large portion of the ray-finned fish head skeleton is composed of endochondral bone, and we show here that postembryonic growth of this skeleton is fueled at least in part by endochondral growth zones. The examination of GZ performance in closely related cichlid or damselfish species with different adult morphologies will help evaluate the importance of endochondral growth zones in producing new morphologies in ray-finned fishes.
3.5 |. Life-history of endochondral growth zones in bony fishes
We have shown that zebrafish endochondral GZ activity starts during metamorphosis and ends at adulthood. This transient activity parallels the mammalian condition, where endochondral GZ activity starts during fetal development and ends at sexual maturity, both of which are regulated by endocrine signaling. Thyroid hormone (TH) mediates mammalian endochondral GZ maturation,51–53 while estrogen triggers the end of GZ activity and the replacement of GZ cartilage with bone.54 Interestingly, TH controls metamorphosis in teleosts,55 suggesting conserved regulation of endochondral GZ maturation by TH in bony fishes (Osteichthyes). Does estrogen trigger endochondral GZ arrest in zebrafish? The GZ arrest of sexually mature zebrafish we observe supports this possibility, although further evidence is required, including effects of growth conditions on endochondral GZ arrest. Fish growth rate is determined in part by stocking density.27–29 The regulation of endochondral GZ maturation and arrest by TH and estrogen, respectively, would make zebrafish an appropriate model to study these aspects of mammalian GZ biology.
Zebrafish GZ arrest is interesting given the animal’s otherwise indeterminate growth.18,19 Determinate growth in mammals is intrinsically linked to the transient activity of their endochondral GZs, suggesting that indeterminate growth is supported by life-long endochondral growth. However, our results in zebrafish would argue against this hypothesis, since intramembranous ossification alone supports adult bone growth after endochondral growth arrests. Addressing this issue and the evolutionary history of endochondral GZs awaits further exploration in other ray-finned fishes and beyond.
4 |. EXPERIMENTAL PROCEDURES
4.1 |. Animal care
Adult zebrafish of the AB strain were maintained in 20 L tanks and staged as described.18,19,56 All animal work was approved by the University of North Carolina, Wilmington Institutional Animal Care and Use Committee.
4.2 |. Skeletal preparations
All animals were euthanized by immersion in a 168 mg/L tricaine-S solution (MP Biomedicals) prepared in tank water prior to fixation. For staining on fixed tissues, larvae (8 dpf, 6 mm SL and 10 mm SL) were fixed in 4% PFA and stained with alizarin red and alcian blue or alcian alone as described57 with 60 mM MgCl2. Fixed adults were stained as described58 with modifications57 for acid free stain. Live bone staining by calcein green and alizarin red was conducted as described previously59 with modifications.60 Fish were stained for 24 hours in calcein green, returned to tanks for 14 days, and then chased with alizarin red for 24 hours. Fish were then returned to tanks for 24 hours before fixation overnight in 10% normal buffered formalin at 4° C and stored in 100% ethanol in the dark at 4°C until trypsin digestion as previously described.60 Preparations were phenotyped on either a Leica M165FC dissecting microscope or a Zeiss Axioskop 2 FS Plus compound microscope. The dissecting microscope was equipped with a Planapo 1.6X M-series objective, a Prior L200 fluorescent light source and a Leica DC7000T camera controlled by LAS X software. The compound microscope was equipped with a Qimaging Micropublisher 6 camera controlled by Ocular software.
4.3 |. In situ hybridization and immunostaining
For cryosections, larvae were processed as described.61 Larvae were cut as 16 μm sections on a Leica CSM1816 cryostat. For in situ hybridization, sections were hybridized as described62 with published antisense probes against collagen 2a1a63 or collagen 10a1a.64 Immunohistochemistry was performed as described62 without the proteinase K step, with mouse anti-PCNA (Proliferating Cell Nuclear Antigen; 1/500; Santa Cruz Biotech; PC10), Alexa Fluor 488 conjugated Donkey anti-mouse secondary antibody (1/100 Jackson Immunoresearch 715-545-150) and DAPI (1:2000 Life Technologies D1306). Fluorescence imaging was conducted on a Leica SP8 confocal microscope with a 63X oil immersion objective.
4.4 |. Data collection and analysis
Cell cross-sectional area and height measurements were collected in FIJI from images captured under Nomarski illumination with a 63x water-immersion objective on a Zeiss Axioskop 2 FS plus microscope. Cell contours in regions of interest were manually drawn using the free-hand selection tool to obtain individual cell cross-sectional areas and cell dimensions parallel to the direction of endochondral growth. Cell cross-sectional area and height averages and standard deviations were calculated in Microsoft Excel, and graphed in R using the ggplot2 package. Statistical significance was determined using Tukey’s test of Honest Significant Difference in R. To determine ECM percentage of total skeletal area for each image, cell contours were drawn on the same regions of interest as above and filled with black in Adobe Illustrator. This filled contour was saved without the underlying image as TIFF and analyzed in FIJI. The Analyze particles tool was used to measure the surface area occupied by ECM. Statistical significance was determined using Tukey’s test of Honest Significant Difference in R.
ACKNOWLEDGMENTS
Financial support provided by NIH NIDCR R01DE13828 to P.L. and T.F.S.; UNCW SURCA and CSURF awards to B.P.H.; NIH NIAMS R01AR67797 and NICHD R01HD073182 to T.F.S.
Funding information
National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant/Award Number: R01AR67797; National Institute of Child Health and Human Development, Grant/Award Number: R01HD073182; National Institute of Dental and Craniofacial Research, Grant/Award Number: R01DE13828
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
REFERENCES
- 1.Postnatal Growth of Fins and Limbs through Endochondral Ossification. Chicago: University of Chicago Press; 2007:118–151. [Google Scholar]
- 2.Witten PE, Huysseune A. A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biol Rev Camb Philos Soc. 2009;84(2):315–346. 10.1111/j.1469-185X.2009.00077.x. [DOI] [PubMed] [Google Scholar]
- 3.McBratney-Owen B, Iseki S, Bamforth SD, Olsen BR, Morriss-Kay GM. Development and tissue origins of the mammalian cranial base. Dev Biol. 2008;322(1):121–132. 10.1016/j.ydbio.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei X, Hu M, Mishina Y, Liu F. Developmental regulation of the growth plate and cranial synchondrosis. J Dent Res. 2016;95(11):1221–1229. 10.1177/0022034516651823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schilling TF, Le Pabic P. Neural crest cells in craniofacial skeletal development. In: Trainor P, ed. Neural Crest Cells: Evolution, Development and Disease. New York: Elsevier; 2014:127–151. [Google Scholar]
- 6.Schilling TF, Kimmel CB. Musculoskeletal patterning in the pharyngeal segments of the zebrafish embryo. Development. 1997;124(15):2945–2960. [DOI] [PubMed] [Google Scholar]
- 7.Kimmel CB, Miller CT, Moens CB. Specification and morphogenesis of the zebrafish larval head skeleton. Dev Biol. 2001;233 (2):239–257. 10.1006/dbio.2001.0201. [DOI] [PubMed] [Google Scholar]
- 8.Cubbage CC, Mabee PM. Development of the cranium and paired fins in the zebrafish Danio rerio (Ostariophysi, cyprinidae). J Morphol. 1996;229(2):121–160. . [DOI] [PubMed] [Google Scholar]
- 9.Huysseune A Skeletal system. London: Academic Press; 2000:307–317. [Google Scholar]
- 10.Smith MM, Hall BK. Development and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biol Rev. 1990;65(3):277–373. 10.1111/j.1469-185X.1990.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 11.Donoghue PCJ, Sansom IJ. Origin and early evolution of vertebrate skeletonization. Microsc Res Tech. 2002;59(5):352–372. 10.1002/jemt.10217. [DOI] [PubMed] [Google Scholar]
- 12.Giovannone D, Paul S, Schindler S, et al. Programmed conversion of hypertrophic chondrocytes into osteoblasts and marrow adipocytes within zebrafish bones. Elife. 2019;8:1–12. 10.7554/eLife.42736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witten PE, Hansen A, Hall BK. Features of mono- and multinucleated bone resorbing cells of the zebrafish Danio rerio and their contribution to skeletal development, remodeling, and growth. J Morphol. 2001;250(3):197–207. 10.1002/jmor.1065.abs. [DOI] [PubMed] [Google Scholar]
- 14.Weigele J, Franz-Odendaal TA. Functional bone histology of zebrafish reveals two types of endochondral ossification, different types of osteoblast clusters and a new bone type. J Anat. 2016;229(1):92–103. 10.1111/joa.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zapata A, Amemiya CT. Phylogeny of lower vertebrates and their immunological structures. Origin Evol Vertebr Immun Syst. 2000;248:67–107. [DOI] [PubMed] [Google Scholar]
- 16.Woodhead AD. Fish in studies of aging. Exp Gerontol. 1978;13 (3–4):125–140. 10.1016/0531-5565(78)90005-0. [DOI] [PubMed] [Google Scholar]
- 17.Brown ME. Experimental studies on growth. The Physiology of Fishes. London: Elsevier; 1957:361–400. [Google Scholar]
- 18.Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of postembryonic Zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn. 2009;238(12):2975–3015. 10.1002/dvdy.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schilling TF. The morphology of larval and adult zebrafish. In: Nusslein-Volhard C, Dahm R, eds. Zebrafish: A Practical Approach. New York: Oxford University Press; 2002. [Google Scholar]
- 20.de Beer GR. The Development of the Vertebrate Skull. London: Oxford University Press; 1937. [Google Scholar]
- 21.Romereim SM, Conoan NH, Chen BJ, Dudley AT. A dynamic cell adhesion surface regulates tissue architecture in growth plate cartilage. Development. 2014;141(10):2085–2095. 10.1242/dev.105452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young B, Minugh-Purvis N, Shimo T, et al. Indian and sonic hedgehogs regulate synchondrosis growth plate and cranial base development and function. Dev Biol. 2006;299(1):272–282. 10.1016/j.ydbio.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 23.Nagayama M, Iwamoto M, Hargett A, et al. Wnt/beta-catenin signaling regulates cranial base development and growth. J Dent Res. 2008;87(3):244–249. 10.1177/154405910808700309. [DOI] [PubMed] [Google Scholar]
- 24.Hunziker EB, Schenk RK, Cruzorive LM. Quantification of chondrocyte performance in growth-plate cartilage during longitudinal bone-growth. J Bone Joint Surg Am Vol. 1987;69A(2):162–173. 10.2106/00004623-198769020-00002. [DOI] [PubMed] [Google Scholar]
- 25.LeClair EE, Mui SR, Huang A, Topczewska JM, Topczewski J. Craniofacial skeletal defects of adult Zebrafish glypican 4 (knypek) mutants [Article]. Dev Dyn. 2009;238(10):2550–2563. 10.1002/dvdy.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutta H Growth in fishes. Gerontology. 1994;40(2–4):97–112. 10.1159/000213581. [DOI] [PubMed] [Google Scholar]
- 27.Hecht T, Oellermann L, Verheust L. Perspectives on clariid catfish culture in Africa. Aquat Living Resour. 1996;9:197–206. 10.1051/alr:1996054. [DOI] [Google Scholar]
- 28.Khatune-Jannat M, Rahman MM, Bashar MA, Hasan MN, Ahamed F, Hossain MY. Effects of stocking density on survival, growth and production of Thai climbing perch (Anabas testudineus) under fed ponds. Sains Malaysiana. 2012;41(10):1205–1210. [Google Scholar]
- 29.Zhu YJ, Yang DG, Chen JW, Yi JF, Liu WC, Zhao JH. An evaluation of stocking density in the cage culture efficiency of Amur sturgeon Acipenser schrenckii. J Appl Ichthyol. 2011;27(2):545–549. 10.1111/j.1439-0426.2011.01675.x. [DOI] [Google Scholar]
- 30.Wilsman NJ, Farnum CE, Leiferman EM, Fry M, Barreto C. Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J Orthop Res. 1996;14(6):927–936. 10.1002/jor.1100140613. [DOI] [PubMed] [Google Scholar]
- 31.Hunziker EB, Schenk RK. Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone-growth in rats. J Physiol Lond. 1989;414:55–71. 10.1113/jphysiol.1989.sp017676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farnum CE, Tinsley M, Hermanson JW. Forelimb versus hindlimb skeletal development in the big brown bat, Eptesicus fuscus: functional divergence is reflected in chondrocytic performance in autopodial growth plates. Cells Tissues Organs. 2008;187(1):35–47. 10.1159/000109962. [DOI] [PubMed] [Google Scholar]
- 33.Cooper KL, Oh S, Sung Y, Dasari RR, Kirschner MW, Tabin CJ. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature. 2013;495(7441):375–378. 10.1038/nature11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barreto C, Wilsman NJ. Hypertrophic chondrocyte volume and growth-rates in avian growth plates. Res Vet Sci. 1994;56(1):53–61. 10.1016/0034-5288(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 35.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273 (5275):613–622. 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 36.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation (vol 13, pg 2072, 1999). Genes Dev. 1999;13(19):2617–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung UI, Lanske B, Lee KC, Li E, Kronenberg H. The parathyroid hormone parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci U S A. 1998;95(22):13030–13035. 10.1073/pnas.95.22.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung UI, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Investig. 2001;107(3):295–304. 10.1172/jci11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao B, Song H, Bishop K, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20(2):163–176. 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Pabic P, Ng C, Schilling TF. Fat-Dachsous signaling coordinates cartilage differentiation and polarity during craniofacial development. PLoS Genet. 2014;10(10):e1004726. 10.1371/journal.pgen.1004726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janvier P Les Céphalaspides du Spitsberg: Anatomie, Phylogénie et Systématique des Ostéostracés Siluro-Devoniens; révisions des Ostéostracés de la Formation du Wood Bay (Dévonien inférieur du Spitsberg). Paris: CNRS édition; 1985:244. [Google Scholar]
- 42.Denison RH. Placodermi. Stuttgart: Gustav Fischer Verlag; 1978. [Google Scholar]
- 43.Denison RH. Acanthodii. Stuttgart: Gustav Fischer Verlag; 1979. [Google Scholar]
- 44.Wright GM, Keeley FW, Robson P. The unusual cartilaginous tissues of jawless craniates, cephalochordates and invertebrates. Cell Tissue Res. 2001;304(2):165–174. 10.1007/s004410100374. [DOI] [PubMed] [Google Scholar]
- 45.Nelson GJ. Gill arches and the phylogeny of fishes, with notes on the classification of vertebrates. Bull Am Museum Nat Hist. 1969;141(4):475–552. [Google Scholar]
- 46.Haines RW. Epiphysial growth in the branchial skeleton of fishes. Q J Microsc Sci. 1934;77:77–97. [Google Scholar]
- 47.Haines RW. The evolution of epiphyses and of endochondral bone. Biol Rev. 1942;17:267–292. [Google Scholar]
- 48.Powder KE, Milch K, Asselin G, Albertson RC. Constraint and diversification of developmental trajectories in cichlid facial morphologies. Evodevo. 2015;6:25. 10.1186/s13227-015-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Pabic P, Cooper WJ, Schilling TF. Developmental basis of phenotypic integration in two Lake Malawi cichlids. Evodevo. 2016;7:3. 10.1186/s13227-016-0040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper WJ, VanHall R, Sweet E, et al. Functional morphogenesis from embryos to adults: late development shapes trophic niche in coral reef damselfishes. Evol Dev. 2020;22(3):221–240. 10.1111/ede.12321. [DOI] [PubMed] [Google Scholar]
- 51.Shao YY, Wang L, Ballock RT. Thyroid hormone and the growth plate. Rev Endocr Metab Disord. 2006;7(4):265–271. 10.1007/s11154-006-9012-2. [DOI] [PubMed] [Google Scholar]
- 52.Kozhemyakina E, Lassar AB, Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation [Review]. Development. 2015;142(5):817–831. 10.1242/dev.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindsey RC, Aghajanian P, Mohan S. Thyroid hormone signaling in the development of the endochondral skeleton. Thyroid Hormone. 2018;106:351–381. 10.1016/bs.vh.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borjesson AE, Lagerquist MK, Windahl SH, Ohlsson C. The role of estrogen receptor alpha in the regulation of bone and growth plate cartilage. Cell Mol Life Sci. 2013;70(21):4023–4037. 10.1007/s00018-013-1317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McMenamin SK, Parichy DM. Metamorphosis in Teleosts. Anim Metamorp. 2013;103:127–165. 10.1016/b978-0-12-385979-2.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic-development of the zebrafish. Dev Dyn. 1995;203(3):253–310. [DOI] [PubMed] [Google Scholar]
- 57.Walker MB, Kimmel CB. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech Histochem. 2007;82(1):23–28. 10.1080/10520290701333558. [DOI] [PubMed] [Google Scholar]
- 58.van Eeden FJM, Granato M, Schach U, et al. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development. 1996;123:153–164. [DOI] [PubMed] [Google Scholar]
- 59.Kimmel CB, DeLaurier A, Ullmann B, Dowd J, McFadden M. Modes of developmental outgrowth and shaping of a craniofacial bone in Zebrafish. PLoS One. 2010;5(3):12. 10.1371/journal.pone.0009475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellis NA, Glazer AM, Donde NN, Cleves PA, Agoglia RM, Miller CT. Distinct developmental genetic mechanisms underlie convergently evolved tooth gain in sticklebacks. Development. 2015;142(14):2442–2451. 10.1242/dev.124248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Albertson RC, Yan YL, Titus TA, et al. Molecular pedomorphism underlies craniofacial skeletal evolution in Antarctic notothenioid fishes. BMC Evol Biol. 2010;10:1–12. 10.1186/1471-2148-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith A, Zhang J, Guay D, Quint E, Johnson A, Akimenko MA. Gene expression analysis on sections of zebrafish regenerating fins reveals limitations in the whole-mount in situ hybridization method. Dev Dyn. 2008;237(2):417–425. 10.1002/dvdy.21417. [DOI] [PubMed] [Google Scholar]
- 63.Yan YL, Hatta K, Riggleman B, Postlethwait JH. Expression of a type-II collagen gene in the Zebrafish embryonic axis. Dev Dyn. 1995;203(3):363–376. [DOI] [PubMed] [Google Scholar]
- 64.Avaron F, Hoffman L, Guay D, Akimenko MA. Characterization of two new zebrafish members of the hedgehog family: atypical expression of a zebrafish indian hedgehog gene in skeletal elements of both endochondral and dermal origins. Dev Dyn. 2006;235(2):478–489. 10.1002/dvdy.20619. [DOI] [PubMed] [Google Scholar]


