Abstract
Background:
A case control study was performed to define clinical and genetic risk factors associated with osteonecrosis of the jaw in patients with metastatic cancer treated with bisphosphonates.
Methods:
Clinical data and tissues were collected from patients treated with bisphosphonates for metastatic bone disease who were diagnosed with osteonecrosis of the jaw (cases) and matched controls. Clinical data included patient, behavioral, disease, and treatment information. Genetic polymorphisms in CYP2C8 (rs1934951) and other candidate genes were genotyped. Odds ratios from conditional logistic regression models were examined to identify clinical and genetic characteristics associated with case or control status.
Results:
The study population consisted of 76 cases and 126 controls. In the final multivariable clinical model, patients with osteonecrosis of the jaw were less likely to have received pamidronate than zoledronic acid (odds ratio=0.18, 95% Confidence interval: 0.03–0.97, p=0.047) and more likely to have been exposed to bevacizumab (OR=5.15, 95% CI: 1.67–15.95, p=0.005). The exploratory genetic analyses suggested a protective effect for VEGFC rs2333496 and risk effects for VEGFC rs7664413 and PPARG rs1152003.
Conclusions:
We observed patients with ONJ were more likely to have been exposed to bevacizumab and zoledronic and identified potential genetic predictors that require validation prior to clinical translation.
Keywords: bisphosphonate, pharmacogenetics, bevacizumab, zoledronic acid, risk factors, oncology, VEGFC
Introduction:
Bone is a common site for cancer metastases. Approximately 400,000 US adults are living with evidence of bone metastases(Mundy 2002), which are associated with considerable morbidity and mortality. The common skeletal complications of malignancy designated “skeletal-related events” (SRE) include pathologic fractures, spinal cord compression, hypercalcemia of malignancy and the need for radiotherapy, or surgery, to bone. Prior to the routine use of bisphosphonate therapy, patients with bone metastases would experience an SRE every 3–12 months.(Lipton 2010). The potent osteoclast inhibitors, bisphosphonates and denosumab decrease the rate of SREs by approximately 30%-50%(Coleman 2007; Ford et al. 2013). Therefore, these agents are routinely recommended in the management of patients with metastatic bone disease(Gralow et al. 2013; Van Poznak et al. 2017).
The bisphosphonates and denosumab are associated with an oral condition known as osteonecrosis of the jaw (ONJ) also known as antiresorptive agent-induced osteonecrosis of the jaw (ARONJ), bisphosphonate related osteonecrosis of the jaw (BRONJ) and medication related osteonecrosis of the jaw (MRONJ). The American Association of Oral and Maxillofacial Surgeons (AAOMS) 2014 position paper provides the case definition of (MR)ONJ in patients if all of three of the following conditions are met: 1) current or prior treatment with anti-resorptive or anti-angiogenesis agents, 2) exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region that has persisted for more than eight weeks, and 3) no history of radiation to the jaws or obvious metastatic disease to the jaws(Ruggiero et al. 2014). An ONJ lesion typically is non-healing or slow to heal, and often is complicated by secondary infection with associated pain and swelling.
The prevalence of ONJ has not been well defined. In patients treated with zoledronic acid or denosumab for up to 36 months, the incidence appears to be 1%-2%(Saad et al. 2012); however, the rates of ONJ appear to increase over time. In patients with metastatic breast cancer or prostate cancer the rates of ONJ are between 5% and 8% with drug exposures of 5 to 5.6 years(Stopeck et al. 2016). The majority of the 400,000 patients with bone metastases in the US will be treated with an osteoclast inhibitor. If 1%-2% develop ONJ, then 4,000 to 8,000 of these patients will have ONJ at any given time. This estimate would be higher if one calculates the risk using rates reported with longer term exposure.
The etiology of ONJ is unknown. Potential mechanisms involved in the development of ONJ include infection, immune dysfunction, inflammation, vascular effect, over suppression of bone remodeling, drug interactions, and genetic predisposition(Van Poznak 2006). Identifying risk factors associated with the development of ONJ will aid in counseling patients on their individual risks, and may influence dental monitoring and early detection, as well as inform translational research investigating the mechanism of ONJ. Therefore, we performed a case-control study to define clinical and genetic risk factors associated with ONJ.
Materials and Methods:
Trial Conduct:
A multi-institutional, translational study was performed. Indiana University, MD Anderson, Memorial Sloan-Kettering and University of Michigan (UM) collaborated to identify cases of ONJ and matched controls. The retrospective study protocol (ClinicalTrials.gov Identifier: NCT01325142) was reviewed and approved by the Institutional Review Board of each of the four collaborating sites and the work was conducted in full accordance with the Declaration of Helsinki.
All study patients had cancer involving the bone, had received bisphosphonate therapy and had not received radiation to the jaw. Cases were defined as having ONJ using the American Society of Bone and Mineral Research definition of ONJ(Khosla et al. 2007), which is consistent with present definition used by AAOMS(Ruggiero et al. 2014), as outlined above although antiangiogenic therapy was not included in the ONJ definition at the time of study data acquisition. Cases were identified using medical, dental and surgical databases as well as health care provider recall. Patients were selected as controls if they had bone metastases and received bisphosphonate treatment but did not have ONJ. Control subjects were matched to the case based on age (within 5 years), primary tumor type (breast, multiple myeloma, prostate, lung, other), and gender (male, female). The medical records were reviewed to confirm eligibility as a case or control. To assess clinical and epidemiologic characteristics associated with risk of ONJ, it is critical that the case and controls not be “over” matched to key suspected risks such as duration of bisphosphonate, steroid exposure, cancer therapies, and comorbidities which are evaluated as clinical risk factors. Of note, denosumab was not FDA approved for the treatment of bone metastases at the time this study was initiated.
Clinical and epidemiologic data were abstracted from the patient’s medical and dental health records as available at the patient’s home institution using study specific case report forms. Covariates from the clinical data contained patient demographics, including age at time of diagnosis of ONJ or for controls, age at last follow up at time of study, gender, tumor diagnosis, cancer therapies, history of bisphosphonate exposure, steroid exposure, tobacco and alcohol use. Medical records from oral health providers were reviewed to categorize patients’ dental hygiene as good, moderate, or poor and identify evidence of prior periodontal disease. Details of ONJ lesions included location and number of ONJ lesions and clinical presentation.
Genotyping
To assess for a genetic predisposition for developing ONJ, genotyping of candidate single nucleotide polymorphisms (SNPs) that had previously been reported as associating with ONJ or are associated with the angiogenesis or bisphosphonate mechanism of action were genotyped. The a priori defined primary SNP hypothesis was that CYP2C8 rs1934951 would increase ONJ risk, as this SNP has been reported as a predictor of ONJ risk in several studies(Kastritis et al. 2017; Sarasquete et al. 2008; Zhong et al. 2013). CYP2C8 rs1934951 was genotyped using Taqman Allelic Discrimination Assays, as previously described(Sikora et al. 2011). Additional SNPs of interest(Nicoletti et al. 2012; Yang et al. 2019) in CYP2C8 (rs1341162, rs1934980), PPARG (rs1152003), VEGFC (rs7664413, rs2333496, rs6838834, rs3775203) VEGFA (rs833061, rs699947, rs2010963), FDPS (rs11264359, rs17367421), IGFBP7 (rs11934877) and ABCC4 (rs1678387) were genotyped on a single multiplex Sequenom panel as previously described(Hertz et al. 2015b) for inclusion in an exploratory pharmacogenetic analysis.
DNA was isolated from archived FFPE specimens, following our previously validated technique for confirming accurate germline genetic assessment using archived specimens(Hertz et al. 2015b). DNA was extracted from FFPE specimens using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) as previously described(Sikora et al. 2011). Amplification-Quality DNA (AQ-DNA) was quantified using a standard curve set with 100bp GAPDH fragment primers and SYBR Green real-time PCR. Samples with yields below the limit of AQ-DNA thresholds were excluded from genotyping analysis. Taqman genotyping methods are compatible with levels of FFPE DNA down to 50 pg whereas Sequenom requires a higher input of DNA(Hertz et al. 2015a). After AQ-DNA quantification 56 cases and 115 controls were acceptable for use with Taqman genotyping and 46 cases and 109 controls were acceptable for use with Sequenom genotyping.
Statistical Design:
Conditional logistic regression was used to adjust for the matched nature of the data(BRESLOW et al. 1978). Odds ratios from conditional logistic regression models were examined to assess the univariate association between clinical characteristics and ONJ status. The study then created multivariable conditional logistic regression models to evaluate the adjusted associations between the clinical characteristics with ONJ status. A stepwise selection procedure was implemented to aid in the variable selection for the final multivariable model, where the significant univariate clinical characteristics were considered for the multivariable model selection. Once the multivariable model was selected, odds ratios were examined to determine which factors have a significant adjusted association with ONJ status. The analysis here was limited to use of pamidronate and zoledronic acid.
A similar conditional logistic regression analysis was used to determine the association between each SNP and ONJ status. Each SNP analysis evaluated the unadjusted linear effect from conditional logistic regression models assuming an additive genetic effect, expecting that the risk of ONJ will be greatest in the variant (A) group in the primary analysis of CYP2C8 rs1934951, as previously reported(Kastritis et al. 2017; Sarasquete et al. 2008; Zhong et al. 2013). Exploratory statistically uncorrected pharmacogenetic analyses of all other SNPs were conducted similarly, using conditional logistic regression assuming additive genetic models. All nominally significant univariate associations (p<0.05) were introduced into multivariable logistic regression models adjusting for bisphosphonate (pamidronate vs. zoledronic acid) and bevacizumab (yes vs. no).
During the course of the study, it became apparent that the target 100 cases and 200 controls would not be obtained. The availability of study materials was less than expected, and the duration of time to collect data abutted the end date of funding support. Plans were made to adjust the analysis to optimize conditions including adjustments for the matching with the analysis of the collected data with odds ratios. All analyses were performed at the University of Michigan using SAS, version 9.4 (SAS Institute Inc.).
Results:
Patients:
The study population consisted of 202 patients with cancer involving bone who received bisphosphonate therapy without radiation therapy to the jaw. There were 76 cases of ONJ and 126 controls. All cases had at least one matched control, and none had more than 2 matched controls, Figure 1. The demographics of the patients included in the analysis stratified by case or control designation are outlined in Table 1. Eight patients had exposure to oral bisphosphonates used for osteoporosis, four each in the case and control group (data not shown). Although not matched on duration of bisphosphonate use, the cases and controls had similar duration of exposure.
Figure 1:
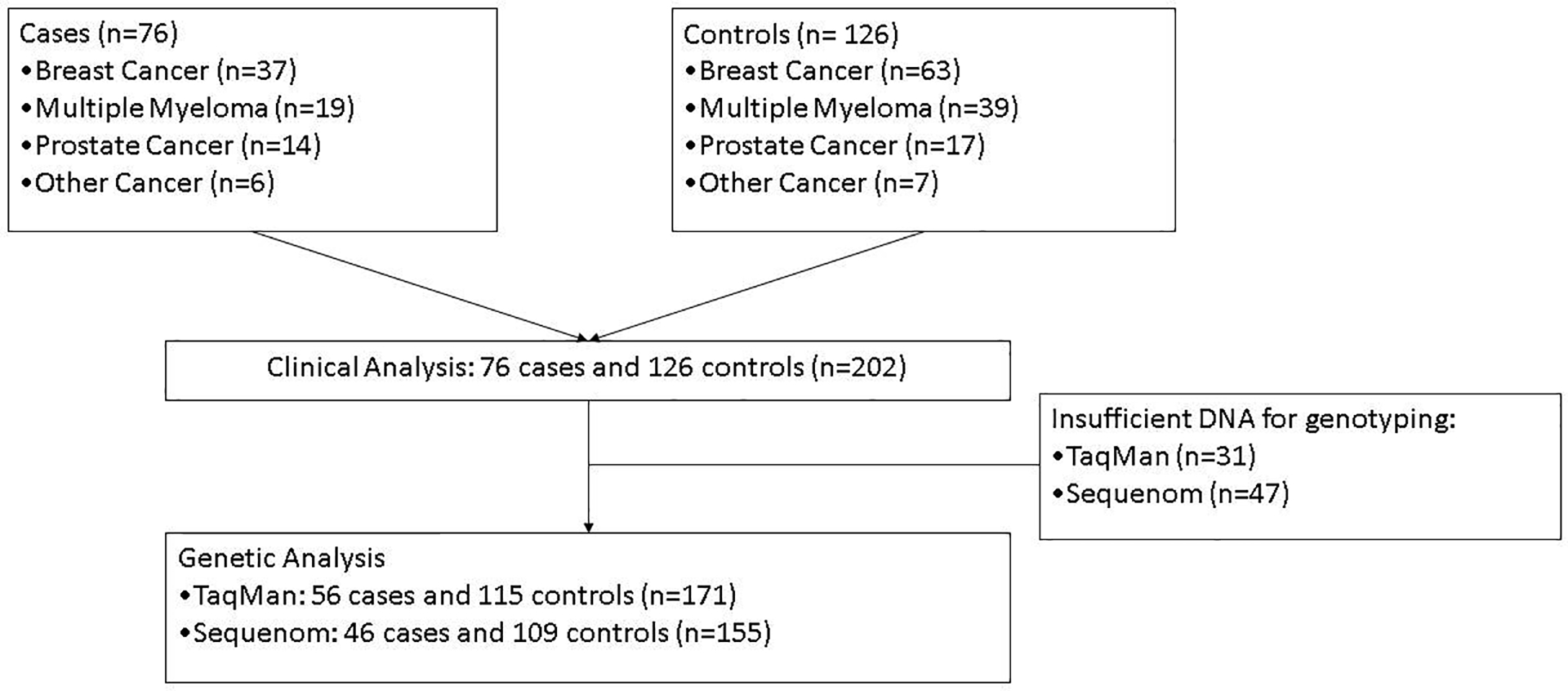
Diagram illustrating patient matriculation from the clinical through the genetic analyses.
Table 1.
Demographics of the study population
| Demographic | Case N=76 |
Control N=126 |
|
|---|---|---|---|
| Age | Years | 63.4 (±10.6) | 65.6 (±10.5) |
| Gender | Female | 32 (42.1%) | 50 (39.7%) |
| Race | White | 68 (89.5%) | 101 (80.2%) |
| Asian | 1 (1.3%) | 3 (2.4%) | |
| Black | 6 (7.9%) | 19 (15.1%) | |
| Other | 1 (1.3%) | 2 (1.6%) | |
| Not reported | 0 (0%) | 1 (0.79%) | |
| Tumor diagnosis | Breast | 37 (48.7%) | 63 (50%) |
| Multiple myeloma | 19 (25%) | 39 (31%) | |
| Prostate | 14 (18.42%) | 17 (13.5%) | |
| Other | 6 (7.9%) | 7 (5.6%) | |
| Bisphosphonate use | Pamidronate | 6 (7.9%) | 24 (19.0%) |
| Zoledronic acid | 56 (73.7%) | 75 (59.5%) | |
| Pamidronate and zoledronic acid | 14 (18.4%) | 27 (21.4%) | |
| Months of Bisphosphonate Treatment | Pamidronate | 29.0 (±12.4) | 38.6 (±40.6) |
| Zoledronic Acid | 25.7 (±121.7) | 28.8 (±24.1) | |
| Zoledronic Acid plus Pamidronate | 55.1 (±43.6) | 51.0 (±36.2) | |
| Radiation to head or neck area | Yes (%) | 9 (11.8%) | 0 (0%) |
| Tobacco use* | Prior or present | 40 (52.6%) | 24* (48.0%) |
| Alcohol use | Prior or present | 34 (44.7%) | 52 (41.3%) |
| Exposure to Bevacizumab | Yes (%) | 20 (26.3%) | 11 (8.7%) |
| Exposure to multiple classes of chemotherapy | Yes (%) | 34 (44.7%) | 65 (51.6%) |
| ONJ Characteristics at presentation | Mandible | 29 (38.2%) | X |
| Maxilla | 11 (14.5%) | X | |
| Multiple lesions | 34 (44.7%) | X | |
| Unspecified location(s) | 2 (2.6%) | X | |
| Pain | 29 (38%) | X | |
| Inflammation/Infection | 24 (31.6%) | X |
Tobacco data not available for 76 controls. All data reported as mean (± standard deviation) or n (%)
Analysis of Clinical Factors
Univariate analysis was performed to identify clinical and epidemiologic characteristics associated with an increased risk for ONJ (Table 2). Exposure to the anti-angiogenic therapy, bevacizumab was associated with increased ONJ (Odds Ratio (OR) = 5.82, 95% Confidence Interval (95% CI): 1.92–17.65, p=0.002). ONJ cases were less likely to have received pamidronate than zoledronic acid (OR=0.244, 95% CI: 0.07–0.80, p=0.04). Irritants of the aerodigestive tract (tobacco or alcohol), the status of dental hygiene or periodontal disease, and the number of months a patient had taken zoledronic acid or pamidronate were not associated with the presence of ONJ.
Table 2.
Clinical Associations with ONJ
| Clinical Variables | Comparison vs Reference | Odds Ratio | 95% Confidence Interval | P-Value | Adjusted Odds Ratio | 95% Confidence Interval | P-Value |
|---|---|---|---|---|---|---|---|
| Race/Ethnicity | Asian vs White | 0.593 | 0.047–7.432 | 0.94 | |||
| Black vs White | 0.405 | 0.138–1.188 | 0.42 | ||||
| Other vs White | 0.720 | 0.060–8.674 | 0.91 | ||||
| Osteoporosis | Yes vs No | 1.636 | 0.639–4.192 | 0.37 | |||
| Tobacco History | Yes vs No | 1.195 | 0.482–2.965 | 0.70 | |||
| Alcohol History | Yes vs No | 1.186 | 0.652–2.158 | 0.58 | |||
| Dental Hygiene | Moderate vs Good | 0.718 | 0.288–1.792 | 0.46 | |||
| Poor vs Good | 1.070 | 0.357–3.209 | 0.68 | ||||
| Periodontal Disease History | Yes vs No | 0.575 | 0.253–1.307 | 0.19 | |||
| Steroid Exposure | Yes vs No | 1.238 | 0.633–2.422 | 0.53 | |||
| Bevacizumab Exposure | Yes vs No | 5.816 | 1.916–17.652 | 0.002 | 5.152 | 1.665–15.945 | 0.005 |
| Bisphosphate agent | Pamidronate vs Zoledronic Acid | 0.244 | 0.074–0.799 | 0.04 | 0.181 | 0.033–0.974 | 0.047 |
| Zoledronic & Pamidronate vs Zoledronic Acid | 0.653 | 0.283–1.507 | 0.54 | 0.580 | 0.217–1.552 | 0.28 | |
| Time on Bisphosphonate | Months (reported as β-coefficient) | 0.995 | 0.982–1.007 | 0.49 |
Bold indicates statistically significant associations (p<0.05)
Only bevacizumab exposure and which bisphosphonate was used were significant in the univariate analysis and considered in multivariable model building. Stepwise selection procedures selected both covariates for the final multivariable model (Table 2). In the fully adjusted model, ONJ cases were more likely to have received bevacizumab (OR=5.15, 95% CI: 1.67–15.95, p=0.005) and less likely to have received pamidronate than zoledronic acid alone (OR=0.18, 95% CI: 0.03–0.97, p=0.047).
Analysis of Genetic Factors
All SNP analyses are described as the effect of carrying the variant, relative to the wild-type allele. In the pre-specified primary SNP analysis, CYP2C8 rs1934951 was not associated with ONJ status (OR=0.96, 95% CI: 0.55–1.68, p=0.89, Table 3). Several SNPs were nominally associated with ONJ status in the statistically uncorrected secondary genetic analyses. VEGFC rs7664413 was associated with increased ONJ risk (β=3.22, 95% CI: 1.32–7.84, p=0.01) and maintained significance after adjustment for bevacizumab and bisphosphonate use (OR=4.08, 95% CI: 1.29–12.87, p=0.02). VEGFC rs2333496 had a protective effect (OR=0.52, 95% CI: 0.27–0.97, p-0.04) and PPARG rs1152003 had a risk effect (OR=1.90, 95% CI: 1.09–3.30, p=0.02) on ONJ in univariate analyses, however, neither maintained significance after covariate adjustment (both p>0.05).
Table 3.
Genetic Associations with ONJ
| Gene | rsID | Odds Ratio | 95% Confidence Interval | P-Value | Adjusted* Odds Ratio | 95% Confidence Interval | P-Value |
|---|---|---|---|---|---|---|---|
| CYP2C8 | rs1934951** | 0.960 | 0.548–1.682 | 0.89 | |||
| rs1341162 | 0.913 | 0.505–1.651 | 0.76 | ||||
| rs1934980 | 0.911 | 0.473–1.754 | 0.78 | ||||
| PPARG | rs1152003 | 1.898 | 1.093–3.297 | 0.02 | 1.918 | 0.996–3.692 | 0.05 |
| VEGFC | rs7664413 | 3.222 | 1.324–7.844 | 0.01 | 4.078 | 1.292–12.873 | 0.02 |
| rs2333496 | 0.516 | 0.273–0.973 | 0.04 | 0.542 | 0.257–1.141 | 0.11 | |
| rs6838834 | 2.000 | 0.518–7.718 | 0.31 | ||||
| rs3775203 | 1.245 | 0.646–2.397 | 0.51 | ||||
| VEGFA | rs833061 | 1.995 | 0.974–4.087 | 0.06 | |||
| rs699947 | 1.407 | 0.767–2.583 | 0.27 | ||||
| rs2010963 | 1.347 | 0.677–2.683 | 0.40 | ||||
| FDPS | rs11264359 | 0.788 | 0.417–1.489 | 0.46 | |||
| rs17367421 | 2.253 | 0.336–15.112 | 0.40 | ||||
| IGFBP7 | rs11934877 | 1.310 | 0.642–2.672 | 0.46 | |||
| ABCC4 | rs1678387 | 0.757 | 0.212–2.701 | 0.67 |
Significant associations in the univariate analysis were adjusted for Bevacizumab and Bisphosphonate
Pre-specified primary SNP analysis
Bold indicates statistical significance (p<0.05)
Discussion
In this retrospective case control study, individuals with ONJ were more likely to have been exposed to zoledronic acid and bevacizumab. Lesions occurred more commonly in the mandible and were not affected by duration of bisphosphonate therapy or dental hygiene. The genetic analysis did not confirm an increased ONJ risk for patients carrying CYP2C8 rs1934951, though hypothesis-generating associations were detected for SNPs in VEGFC (rs7664413, rs2333496) and PPARG (rs1152003).
Our results are consistent with the literature demonstrating that ONJ occurs more commonly in the setting of exposure to zoledronic acid than with pamidronate and zoledronic acid or pamidronate alone(Estilo et al. 2008; Hoff et al. 2008). Interestingly, oral hygiene and periodontal disease, conditions that typically correlate with dental extraction(McFall 1982; Services 2000) were not associated with an increased risk for developing ONJ in this study. Epidemiologic studies have suggested poor oral health status and dental extraction increase the likelihood of ONJ(Barasch et al. 2013)Yarom et al 2019). The lack of association in this study between oral health and ONJ is likely due to the limitations of the retrospective abstraction of oral health data from clinical notes. Prospective analysis of SWOG S0702, an ONJ registry study, will likely provide insight into associations between oral health and risk of ONJ.
The findings of this study support the previously reported association between increased risk for ONJ and bevacizumab, a monoclonal antibody targeting VEGF that is known to affect vascularization(Arjaans et al. 2016; McArthur et al. 2008). Indeed, a history of antiangiogenic therapy was incorporated into the definition of ONJ in an updated American Association of Oral and Maxillofacial Surgeons(Ruggiero et al. 2014). The 2019 guideline addressing medication related ONJ produced by the American Society of Clinical Oncology, the Multinational Association of Supportive Care in Cancer and the International Society of Oral Oncology also includes exposure to anti-angiogenic therapies in the definition of medication related ONJ(Yarom et al. 2019). The strong (OR>5) association of ONJ with antiangiogenic therapies in our study may reflect alterations in vascular/wound healing that may increase risk for developing ONJ. Although bevacizumab is a monoclonal antibody targeting VEGF and zoledronic acid is a nitrogen containing bisphosphonate with different chemical structures and mechanisms of action, zoledronic acid has also been shown to impact the angiogenesis pathway in preclinical models(Wood et al. 2002)(Fournier et al. 2002; Ohba et al. 2014) and clinically(Ferretti et al. 2005; Santini et al. 2003; Vincenzi et al. 2005). In addition, a phase II study using zoledronic acid and bevacizumab together with docetaxel, thalidomide and prednisone reported an usually high incidence of ONJ of 18%(Aragon-Ching et al. 2009) suggesting a possible additive risk when multiple therapies are co-administered. It is of note that a meta-analysis of clinically relevant studies demonstrated that bisphosphonates use did not delay fracture healing time(Li et al. 2015) as might be expected if there were a clinically meaningful impact on the healing process and angiogenesis The etiology of ONJ remains undefined, and may be multifactorial(Allen and Burr 2009; Van Poznak 2006).
This study did not validate an association between CYP2C8 rs1934951 and increased ONJ risk, which has been reported in several independent cohorts(Kastritis et al. 2017; Sarasquete et al. 2008; Zhong et al. 2013). Our results (OR=0.96, p=0.89) suggest that our lack of finding was not merely due to lack of power, but formal meta-analysis approaches are needed to further explore this possibility. The secondary pharmacogenetic analysis detected an increased ONJ risk in patients carrying PPARG rs1152003, which has been previously reported to be both a risk(Kastritis et al. 2017) and protective(Di Martino et al. 2011) factor. PPARG is a biologically plausible candidate gene due to its involvement in bone homeostasis and remodeling(Wan 2010), however, the inconsistent direction of effect across studies and lack of significance after adjustment in our study necessitate additional studies to confirm this association. Similarly, the hypothesis generating finding that VEGFC rs7664413 increases ONJ risk warrants further research in independent cohorts to determine whether this SNP could be useful for predicting ONJ risk. VEGFC rs7664413 has not been previously investigated as a genetic predictor of ONJ to our knowledge; however, it has been associated with increased risk of other phenotypes related to VEGF function including preeclampsia(Srinivas et al. 2010). SNPs in VEGFC and VEGFA have been a major focus of candidate-gene ONJ pharmacogenetic studies based on the anti-angiogenic effects of bisphosphonates and role of VEGF in angiogenesis(Arduino et al. 2011; Yang et al. 2019)(Choi et al. 2015). (Srinivas et al. 2010)There are additional potential SNPs of interest that were reported to be associated with ONJ after our genetic analyses were conducted that were not included in this analysis.(Yang et al. 2019). In this study, different genotype techniques were used for different SNPs, but cases and controls were always genotyped using the same technique, thereby eliminating concern for bias
The small sample size and the retrospective study design limit confidence in our findings, particularly the lack of association for some clinical factors such as race, oral hygiene, time on bisphosphonates, and periodontal disease that have been reported to be associated with ONJ(McFall 1982; Services 2000; Yang et al. 2019). Additionally, genetic risk factors for ONJ from bisphosphonates and bevacizumab may be distinct, though a pharmacogenetic analysis in the subset of cases and controls not receiving bevacizumab did not have meaningfully different findings (data not shown). Yet, with these limitations, the data generated on risk of ONJ were consistent with the findings of most clinical and epidemiologic data demonstrating both zoledronic acid and bevacizumab as drugs associated with increased ONJ risk. Our case control study was performed prior to the introduction of denosumab into the routine clinical care of patients with bone metastases. Hence, we cannot comment on ONJ and denosumab, a drug that is in common use at this time.
With potentially hundreds of thousands of patients with metastatic bone disease at risk of ONJ, the condition remains a clinically relevant concern. This study demonstrated exposure to bevacizumab and use of zoledronic acid are associated with ONJ, as well as a suggestion that a genetic vulnerability to ONJ could exist. These data, along with other case control studies, provide a solid foundation for targeted analysis of ONJ risk factors in the prospective, observational clinical study run through SWOG “S0702: Observational study of osteonecrosis of the jaw (ONJ) in patients with metastatic bone disease starting zoledronic acid”, NCT00874211. Developing an index to use for ONJ risk assessment remains a worthy target. Patients, dentists and medical oncologists will benefit from an instrument that will provide a personalized ONJ risk assessment to guide both oral care interventions and use of systemic anticancer therapies. By identifying a population at high risk for ONJ, future studies may provide evidence-based guidelines for dental monitoring and management of early detected ONJ, as well as inform research investigating the mechanism of ONJ.
Acknowledgements
Funding for this study was provided by NIDCR (K23DE020197, CVP), Breast Cancer Research Foundation (BCRF; N003173 to JM Rae and DF Hayes), and Michigan Institute for Clinical and Health Research (MICHR, UL1RR024986, CVP). We would also like to thank the following individuals for their contribution to this project: Monika Burnett Blue, Katherine Cooney, Vicki Ellingrod, Candace Flaherty, David Flockhart, Tatiana Foroud, Jennifer Griggs, Katherine Cooney, Laurie McCauley, and Vered Stearns. The authors disclose no relevant conflicts of interest.
Footnotes
Data Availability:
Data available upon reasonable request to corresponding author.
References:
- Allen MR, Burr DB. 2009. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: So many hypotheses, so few data. J Oral Maxillofac Surg. 67(5 Suppl):61–70. doi: 10.1016/j.joms.2009.1001.1007. [DOI] [PubMed] [Google Scholar]
- Aragon-Ching JB, Ning YM, Chen CC, Latham L, Guadagnini JP, Gulley JL, Arlen PM, Wright JJ, Parnes H, Figg WD et al. 2009. Higher incidence of osteonecrosis of the jaw (onj) in patients with metastatic castration resistant prostate cancer treated with anti-angiogenic agents. Cancer Invest. 27(2):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduino PG, Menegatti E, Scoletta M, Battaglio C, Mozzati M, Chiecchio A, Berardi D, Vandone AM, Donadio M, Gandolfo S et al. 2011. Vascular endothelial growth factor genetic polymorphisms and haplotypes in female patients with bisphosphonate-related osteonecrosis of the jaws. Journal of Oral Pathology & Medicine. 40(6):510–515. [DOI] [PubMed] [Google Scholar]
- Arjaans M, Schroder CP, Oosting SF, Dafni U, Kleibeuker JE, de Vries EG. 2016. Vegf pathway targeting agents, vessel normalization and tumor drug uptake: From bench to bedside. Oncotarget. 7(16):21247–21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasch A, Cunha-Cruz J, Curro FA, Hujoel P, Sung AH, Vena D, Voinea-Griffin AE, Beadnell S, Craig RG, DeRouen T et al. 2013. Risk factors for osteonecrosis of the jaws: A case-control study from the condor dental pbrn. Texas dental journal. 130(4):299–307. [PubMed] [Google Scholar]
- BRESLOW NE, DAY NE, HALVORSEN KT, PRENTICE RL, SABAI C. 1978. Estimation of multiple relative risk functions in matched case-control studies. American Journal of Epidemiology. 108(4):299–307. [DOI] [PubMed] [Google Scholar]
- Choi H, Lee JH, Kim HJ, Park W, Lee JH, Kim JH. 2015. Genetic association between vegf polymorphisms and bronj in the korean population. Oral diseases. 21(7):866–871. [DOI] [PubMed] [Google Scholar]
- Coleman RE. 2007. The benefits and costs of bisphosphonates. J Support Oncol. 5(10):483–484. [PubMed] [Google Scholar]
- Di Martino MT, Arbitrio M, Guzzi PH, Leone E, Baudi F, Piro E, Prantera T, Cucinotto I, Calimeri T, Rossi M et al. 2011. A peroxisome proliferator-activated receptor gamma (pparg) polymorphism is associated with zoledronic acid-related osteonecrosis of the jaw in multiple myeloma patients: Analysis by dmet microarray profiling. British journal of haematology. 154(4):529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estilo CL, Van Poznak CH, Wiliams T, Bohle GC, Lwin PT, Zhou Q, Riedel ER, Carlson DL, Schoder H, Farooki A et al. 2008. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 13(8):911–920. [DOI] [PubMed] [Google Scholar]
- Ferretti G, Fabi A, Carlini P, Papaldo P, Cordiali Fei P, Di Cosimo S, Salesi N, Giannarelli D, Alimonti A, Di Cocco B et al. 2005. Zoledronic-acid-induced circulating level modifications of angiogenic factors, metalloproteinases and proinflammatory cytokines in metastatic breast cancer patients. Oncology. 69(1):35–43. doi: 10.1159/000087286. Epub 000082005 Aug 000087282. [DOI] [PubMed] [Google Scholar]
- Ford JA, Jones R, Elders A, Mulatero C, Royle P, Sharma P, Stewart F, Todd R, Mowatt G. 2013. Denosumab for treatment of bone metastases secondary to solid tumours: Systematic review and network meta-analysis. Eur J Cancer. 49(2):416–430. [DOI] [PubMed] [Google Scholar]
- Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M, Clézardin P. 2002. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 62(22):6538–6544. [PubMed] [Google Scholar]
- Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar R, Litsas G, McKay R, Podoloff DA, Srinivas S et al. 2013. Nccn task force report: Bone health in cancer care. Journal of the National Comprehensive Cancer Network : JNCCN. 11 Suppl 3:S1–50; quiz S51. [DOI] [PubMed] [Google Scholar]
- Hertz DL, Kidwell KM, Thibert JN, Gersch C, Regan MM, Skaar TC, Henry NL, Hayes DF, Van Poznak CH, Rae JM. 2015a. Genotyping concordance in dna extracted from formalin-fixed paraffin embedded (ffpe) breast tumor and whole blood for pharmacogenetic analyses. Molecular oncology. 9(9):1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz DL, Kidwell KM, Thibert JN, Gersch C, Regan MM, Skaar TC, Henry NL, Hayes DF, Van Poznak CH, Rae JM. 2015b. Genotyping concordance in dna extracted from formalin-fixed paraffin embedded (ffpe) breast tumor and whole blood for pharmacogenetic analyses. Mol Oncol. 9(9):1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, Nooka A, Sayegh G, Guarneri V, Desrouleaux K et al. 2008. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 23(6):826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastritis E, Melea P, Bagratuni T, Melakopoulos I, Gavriatopoulou M, Roussou M, Migkou M, Eleutherakis-Papaiakovou E, Terpos E, Dimopoulos MA. 2017. Genetic factors related with early onset of osteonecrosis of the jaw in patients with multiple myeloma under zoledronic acid therapy. Leuk Lymphoma. 58(10):2304–2309. [DOI] [PubMed] [Google Scholar]
- Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S et al. 2007. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the american society for bone and mineral research. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 22(10):1479–1491. [DOI] [PubMed] [Google Scholar]
- Li YT, Cai HF, Zhang ZL. 2015. Timing of the initiation of bisphosphonates after surgery for fracture healing: A systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 26(2):431–441. [DOI] [PubMed] [Google Scholar]
- Lipton A 2010. Implications of bone metastases and the benefits of bone-targeted therapy. Semin Oncol. 37 Suppl 2:S15–29. [DOI] [PubMed] [Google Scholar]
- McArthur HL, Estilo C, Huryn J, Williams T, Fornier M, Traina TA, Howard J, Hudis CA, Dickler MN. 2008. Osteonecrosis of the jaw (onj) among intravenous (iv) bisphosphonate- and/or bevacizumab-treated patients (pts) at memorial sloan-kettering cancer center (mskcc). 26(15_suppl):9588–9588. [Google Scholar]
- McFall WT Jr., 1982. Tooth loss in 100 treated patients with periodontal disease. A long-term study. Journal of periodontology. 53(9):539–549. [DOI] [PubMed] [Google Scholar]
- Mundy GR. 2002. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nature reviews Cancer. 2(8):584–593. [DOI] [PubMed] [Google Scholar]
- Nicoletti P, Cartsos VM, Palaska PK, Shen Y, Floratos A, Zavras AI. 2012. Genomewide pharmacogenetics of bisphosphonate-induced osteonecrosis of the jaw: The role of rbms3. Oncologist. 17(2):279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba T, Cates JM, Cole HA, Slosky DA, Haro H, Ichikawa J, Ando T, Schwartz HS, Schoenecker JG. 2014. Pleiotropic effects of bisphosphonates on osteosarcoma. Bone. 63:110–20.(doi): 10.1016/j.bone.2014.1003.1005. Epub 2014 Mar 1014. [DOI] [PubMed] [Google Scholar]
- Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F. 2014. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 72(10):1938–1956. [DOI] [PubMed] [Google Scholar]
- Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, Diel IJ, Takahashi S, Shore N, Henry DH et al. 2012. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: Integrated analysis from three blinded active-controlled phase iii trials in cancer patients with bone metastases. Ann Oncol. 23(5):1341–1347. [DOI] [PubMed] [Google Scholar]
- Santini D, Vincenzi B, Dicuonzo G, Avvisati G, Massacesi C, Battistoni F, Gavasci M, Rocci L, Tirindelli MC, Altomare V et al. 2003. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin Cancer Res. 9(8):2893–2897. [PubMed] [Google Scholar]
- Sarasquete ME, Garcia-Sanz R, Marin L, Alcoceba M, Chillon MC, Balanzategui A, Santamaria C, Rosinol L, de la Rubia J, Hernandez MT et al. 2008. Bisphosphonate-related osteonecrosis of the jaw is associated with polymorphisms of the cytochrome p450 cyp2c8 in multiple myeloma: A genome-wide single nucleotide polymorphism analysis. Blood. 112(7):2709–2712. [DOI] [PubMed] [Google Scholar]
- Services USDoHaH. 2000. Oral health in america: A report of the surgeon general. NIH publication.155–188. [Google Scholar]
- Sikora MJ, Thibert JN, Salter J, Dowsett M, Johnson MD, Rae JM. 2011. High-efficiency genotype analysis from formalin-fixed, paraffin-embedded tumor tissues. Pharmacogenomics J. 11(5):348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas SK, Morrison AC, Andrela CM, Elovitz MA. 2010. Allelic variations in angiogenic pathway genes are associated with preeclampsia. Am J Obstet Gynecol. 202(5):445.e441–411. doi: 410.1016/j.ajog.2010.1001.1040. Epub 2010 Mar 1012. [DOI] [PubMed] [Google Scholar]
- Stopeck AT, Fizazi K, Body JJ, Brown JE, Carducci M, Diel I, Fujiwara Y, Martin M, Paterson A, Tonkin K et al. 2016. Safety of long-term denosumab therapy: Results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support Care Cancer. 24(1):447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Poznak C 2006. The phenomenon of osteonecrosis of the jaw in patients with metastatic breast cancer. Cancer Invest. 24(1):110–112. [DOI] [PubMed] [Google Scholar]
- Van Poznak C, Somerfield MR, Barlow WE, Biermann JS, Bosserman LD, Clemons MJ, Dhesy-Thind SK, Dillmon MS, Eisen A, Frank ES et al. 2017. Role of bone-modifying agents in metastatic breast cancer: An american society of clinical oncology-cancer care ontario focused guideline update. J Clin Oncol. 35(35):3978–3986. [DOI] [PubMed] [Google Scholar]
- Vincenzi B, Santini D, Dicuonzo G, Battistoni F, Gavasci M, La Cesa A, Grilli C, Virzì V, Gasparro S, Rocci L et al. 2005. Zoledronic acid-related angiogenesis modifications and survival in advanced breast cancer patients. J Interferon Cytokine Res. 25(3):144–151. doi: 110.1089/jir.2005.1025.1144. [DOI] [PubMed] [Google Scholar]
- Wan Y 2010. Pparγ in bone homeostasis. Trends in Endocrinology & Metabolism. 21(12):722–728. [DOI] [PubMed] [Google Scholar]
- Wood J, Bonjean K, Ruetz S, Bellahcene A, Devy L, Foidart JM, Castronovo V, Green JR. 2002. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 302(3):1055–1061. [DOI] [PubMed] [Google Scholar]
- Yang G, Singh S, Chen Y, Hamadeh IS, Langaee T, McDonough CW, Holliday LS, Lamba JK, Moreb JS, Katz J et al. 2019. Pharmacogenomics of osteonecrosis of the jaw. Bone. 124:75–82.(doi): 10.1016/j.bone.2019.1004.1010. Epub 2019 Apr 1022. [DOI] [PubMed] [Google Scholar]
- Yarom N, Shapiro CL, Peterson DE, Van Poznak CH, Bohlke K, Ruggiero SL, Migliorati CA, Khan A, Morrison A, Anderson H et al. 2019. Medication-related osteonecrosis of the jaw: Mascc/isoo/asco clinical practice guideline. J Clin Oncol. 37(25):2270–2290. [DOI] [PubMed] [Google Scholar]
- Zhong DN, Wu JZ, Li GJ. 2013. Association between cyp2c8 (rs1934951) polymorphism and bisphosphonate-related osteonecrosis of the jaws in patients on bisphosphonate therapy: A meta-analysis. Acta Haematol. 129(2):90–95. [DOI] [PubMed] [Google Scholar]


