Abstract
BACKGROUND
Outcomes in children and adolescents with recurrent or progressive high-grade glioma are poor, with a historical median overall survival of 5.6 months. Pediatric high-grade gliomas are largely immunologically silent or “cold,” with few tumor-infiltrating lymphocytes. Preclinically, pediatric brain tumors are highly sensitive to oncolytic virotherapy with genetically engineered herpes simplex virus type 1 (HSV-1) G207, which lacks genes essential for replication in normal brain tissue.
METHODS
We conducted a phase 1 trial of G207, which used a 3+3 design with four dose cohorts of children and adolescents with biopsy-confirmed recurrent or progressive supratentorial brain tumors. Patients underwent stereotactic placement of up to four intratumoral catheters. The following day, they received G207 (107 or 108 plaque-forming units) by controlled-rate infusion over a period of 6 hours. Cohorts 3 and 4 received radiation (5 Gy) to the gross tumor volume within 24 hours after G207 administration. Viral shedding from saliva, conjunctiva, and blood was assessed by culture and polymerase-chain-reaction assay. Matched pre- and post-treatment tissue samples were examined for tumor-infiltrating lymphocytes by immunohistologic analysis.
RESULTS
Twelve patients 7 to 18 years of age with high-grade glioma received G207. No dose-limiting toxic effects or serious adverse events were attributed to G207 by the investigators. Twenty grade 1 adverse events were possibly related to G207. No virus shedding was detected. Radiographic, neuropathological, or clinical responses were seen in 11 patients. The median overall survival was 12.2 months (95% confidence interval, 8.0 to 16.4); as of June 5, 2020, a total of 4 of 11 patients were still alive 18 months after G207 treatment. G207 markedly increased the number of tumor-infiltrating lymphocytes.
CONCLUSIONS
Intratumoral G207 alone and with radiation had an acceptable adverse-event profile with evidence of responses in patients with recurrent or progressive pediatric high-grade glioma. G207 converted immunologically “cold” tumors to “hot.” (Supported by the Food and Drug Administration and others; ClinicalTrials.gov number, NCT02457845.)
MALIGNANT HIGH-GRADE GLIOMA ACcounts for 8 to 10% of pediatric brain tumors and is regularly fatal, with a rapid course. Survival rates have not improved in 30 years; the 3-year event-free survival among patients with newly diagnosed tumors who are treated with standard radiation and chemotherapy is 11 to 22%.1,2 At recurrence, the median life expectancy is only 5.6 months.2,3 For the few children cured of high-grade glioma, conventional therapies can irreversibly injure the developing brain.4 The lack of progress in developing an effective immunotherapy for pediatric high-grade glioma is attributable to several factors: low somatic mutational burden, leading to limited neoantigens and actionable alterations; intertumoral and intratumoral heterogeneity; immunologically “cold” and silent tumors with few tumor-infiltrating lymphocytes; and drug-delivery challenges due to the blood–brain barrier.4,5
We investigated genetically engineered oncolytic herpes simplex virus type 1 (HSV-1) G207, which has the potential to overcome many treatment challenges posed by pediatric high-grade glioma. G207 contains deletion of the diploid γ134.5 neurovirulence gene and has viral ribonucleotide reductase (UL39) disabled by insertion of Escherichia coli lacZ.6 These mutations prevent a productive infection of normal cells while permitting conditional replication in tumor cells (see the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org).7,8 G207 is neurotropic, making brain tumors ideal targets. It can be inoculated intratumorally to circumvent the blood–brain barrier, and it is easily detected (lacZ reporter gene) and hypersensitive to antiviral drugs owing to a retained thymidine kinase gene. In addition to infecting and lysing tumor cells directly, G207 can reverse tumor immune evasion, increase cross-presentation of tumor antigens, and promote an antitumor immune response even in the absence of virus permissivity.9–12 A single radiation dose enhances G207 efficacy in animal models by increasing viral replication and spread.13
In trials involving adults with high-grade glioma, G207 was safe when inoculated intratumorally, into brain surrounding tumor resection cavities, and combined with a single radiation dose.14–16 In preclinical studies, pediatric murine and patient-derived xenograft brain-tumor models, including cancer stem cells, were highly sensitive to oncolysis by G207.17–20 Pediatric brain tumors were 11 times as sensitive as adult glioblastoma xenografts, a finding that suggests that children are ideal candidates for G207.17 Together, these data provided a strong rationale for conducting a trial involving children and adolescents and informed our scientific hypothesis that intratumoral G207 would increase the amount of tumor-infiltrating lymphocytes and thereby convert immunologically “cold” pediatric brain tumors to “hot” and “inflamed.” Herein, we report the results of a phase 1 trial of G207 immunovirotherapy in children and adolescents with high-grade glioma.
METHODS
OBJECTIVES
The primary objective was to evaluate the safety and adverse-event profile of intratumoral G207 alone or combined with a single 5-Gy radiation dose in children and adolescents with recurrent or progressive malignant supratentorial brain tumors. The secondary objective was to assess for potential efficacy and biologic response to G207 by measuring radiographic response, progression-free and overall survival, performance score, immune response, and the occurrence of G207 viremia or shedding.
PATIENT POPULATION
Children and adolescents (3 to 18 years of age) with a pathologically proven malignant supratentorial brain tumor with a diameter of 1.0 cm or more that progressed after surgery, radiotherapy, or chemotherapy were eligible. Full recovery from acute toxic effects from previous therapy with suitable hematologic, renal, and liver function and a Karnofsky or Lansky performance score of 60 or more (on a scale from 0 to 100, with higher scores indicating better function) were required, as described in the inclusion and exclusion criteria (Table S1 in the Supplementary Appendix). Patients with tumors that would require cerebellar, brain-stem, or ventricular inoculation were excluded.
TRIAL DESIGN AND OVERSIGHT
The investigator-initiated trial was approved by institutional review boards at the University of Alabama at Birmingham (UAB) and Nationwide Children’s Hospital and was conducted under a Food and Drug Administration (FDA) Investigational New Drug application in accordance with International Council for Harmonization Good Clinical Practice guidelines. The trial began at the UAB in May 2016, and Nationwide Children’s Hospital was added as a trial site in 2019 to confirm the feasibility of treatment at a different institution. The open-label, nonrandomized phase 1 trial used a 3+3 design (see the Methods section in the Supplementary Appendix) with four dose cohorts: 107 plaque-forming units (PFU), 108 PFU, 107 PFU with 5 Gy of radiation, and 108 PFU with 5 Gy of radiation. The G207 dose was not escalated further, on the basis of data from the phase 1 trial involving adults, which indicated that responses were not dose-dependent and in which participants with the longest survival received 107 or 108 PFU.14 Stopping rules included establishment of the maximum tolerated or planned dose or unacceptable toxic effects at the lowest proposed dose.
The first author, trial coordinator, and UAB Clinical Trials Network and Monitoring Office Manager continuously monitored safety and data for all the patients. The UAB Clinical Trials Monitoring Committee met approximately monthly to provide oversight and to serve as the data and safety monitoring committee, as part of the UAB O’Neal Comprehensive Cancer Center Data and Safety Monitoring Plan. The first author, trial coordinator, and authors with expertise in neurosurgery, radiology, neuropathology, and immunology tabulated and analyzed the data. A biostatistician with expertise in clinical trials confirmed the accuracy of the data and analyses. The first author wrote the first draft of the manuscript, and all the authors participated in the manuscript writing, reviewing, and editing and agreed with the contents. All the authors vouch for the completeness and accuracy of the data and for adherence of the trial to the protocol, available at NEJM.org. There were no confidentiality agreements related to the data between the authors, any institutions, or Aettis/Treovir, which provided the trial drug only and participated in editing an earlier version of the manuscript but not in writing the manuscript or in analyzing the data.
Parents, guardians, or patients provided written informed consent and patients 7 to 13 years of age provided assent before screening and treatment. After confirmation of the tumor by stereotactic biopsy and of satisfactory intratumoral catheter locations by computed tomography (CT) the next morning, G207 was inoculated over a period of 6 hours in 2.4 ml total volume. In two of the dose cohorts, the gross tumor volume plus 2-mm margins received 5 Gy of radiation within 24 hours after G207 administration. Patients were monitored in the hospital for 3 days after G207 administration and in outpatient clinics while enrolled in the trial. HSV-1 serologic status was determined by immunofluorescence assay. G207 shedding from saliva, conjunctiva, and blood was assessed by culture and polymerase-chain-reaction (PCR) assay. Serial complete blood counts with quantitative lymphocyte subsets were obtained. Matched pre- and post-treatment tissue samples were examined for tumor-infiltrating lymphocytes by immunohistologic analysis. (For more on trial design and oversight, see the Methods section in the Supplementary Appendix.)
SAFETY EVALUATION
Patients were continuously monitored for toxic effects and adverse events, which were graded according to the Common Terminology Criteria for Adverse Events, versions 4.03 to 5.0. Dose-limiting toxic effects were defined as any grade 3 or 4 toxic effect possibly related to G207 within 30 days after G207 administration. Owing to the possibility of pseudoprogression (a well-known occurrence in oncolytic virotherapy, other immunotherapies, and the treatment of malignant brain tumors21,22), neurologic changes were treated with the supportive care agents bevacizumab, glucocorticoids, or both at the discretion of the investigator. A reduction in symptoms with supportive care was not considered to be dose-limiting.
RESPONSE ASSESSMENT
Magnetic resonance imaging (MRI) was performed at screening, day 3, and outpatient visits starting at day 28. Acquired imaging sequences are outlined in the protocol. No generally accepted, well-defined criteria for measuring response after intratumoral immunovirotherapy exist; apparent tumor enlargement may be seen that can be associated with new or enlarging central tumor cavities or cysts from tumor-cell death, inflammation related to an influx of immune cells (pseudoprogression), or delayed antitumor immune responses.23–25 Radiographic response was determined primarily according to immunotherapy Response Assessment in Neuro-Oncology (iRANO) criteria, despite their limitations.26
STATISTICAL ANALYSIS
Adverse events were summarized according to frequency, grade, and relation to G207. Descriptive statistics were tabulated on HSV-1 serologic status, G207 shedding, radiographic response, and performance score. Overall survival was defined as the time from G207 infusion to death. The median overall survival was calculated and Kaplan–Meier survival curves generated with the use of SigmaPlot, version 12.0 (Systat Software).
RESULTS
PATIENT CHARACTERISTICS
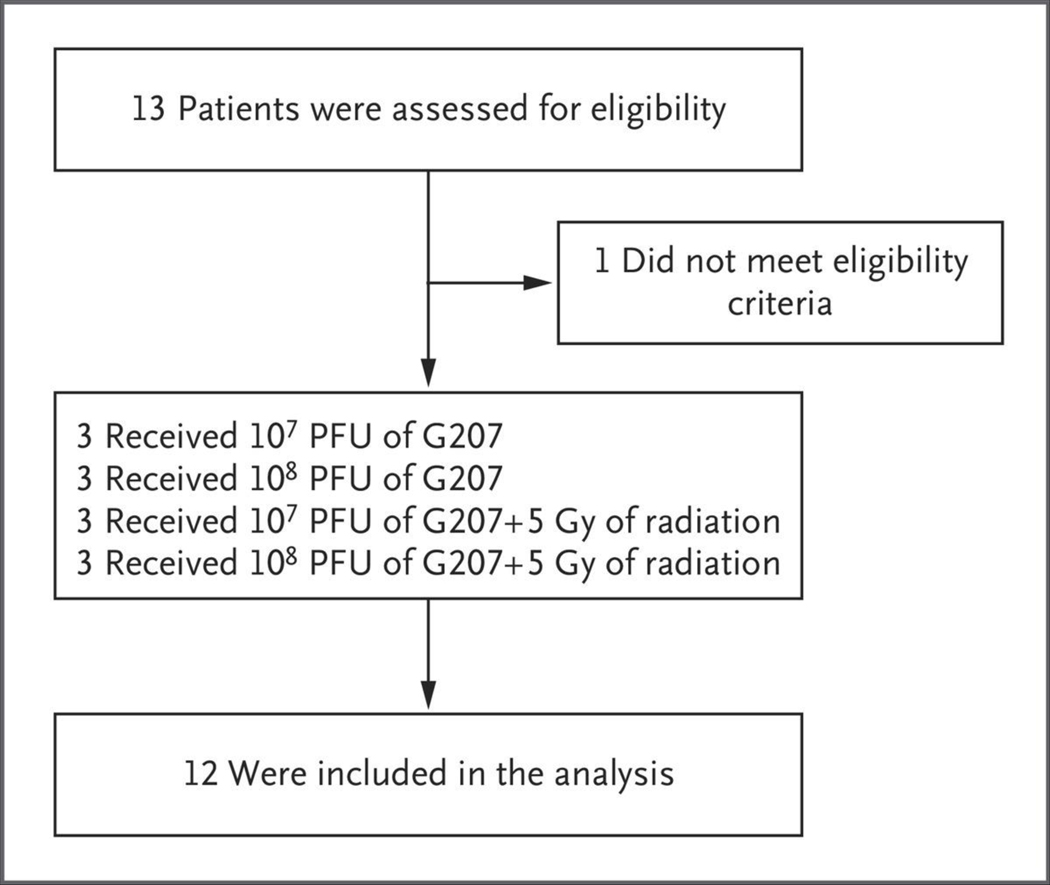
Between October 2016 and April 2020, a total of 13 patients were screened, and 12 (6 male patients and 6 female patients; age range, 7 to 18 years) (Table S2) with progressive or recurrent supratentorial high-grade glioma received G207 after biopsy specimens showed tumor presence (Fig. 1). Tumor classifications included 10 glioblastomas, 1 anaplastic astrocytoma, and 1 high-grade glioma, not otherwise specified. All tumors were IDH wild-type (Table S3). Ten tumors were large, with a bi-perpendicular sum of 5.5 cm or more at screening. Three tumors were multifocal, and 1 had leptomeningeal spread. The majority of patients were heavily pretreated; before G207 administration, 8 patients had at least two previous failed treatments and 4 had at least three previous failed treatments. Performance scores ranged from 70 to 100 before G207 administration (Table S4).
Figure 1. Enrollment, Treatment, and Analysis.
PFU denotes plaque-forming units.
SAFETY OUTCOMES
Three catheters (in 4 patients) or 4 catheters (8 patients) were safely placed throughout the cerebrum, including the thalamus, insula, corpus callosum, and the frontal, parietal, temporal, and occipital lobes. Of 44 catheters placed, 2 (5%) were retracted at the bedside into a suitable position by the neurosurgeon before G207 infusion, and one (2%) was not used because of proximity to the ventricle. No neurologic sequelae from the surgical procedures were noted. Two minor complications were attributed by the investigators to the neurosurgery: a small catheter tract hemorrhage noted on a CT scan before G207 infusion that did not warrant intervention, and a cerebrospinal fluid leak from the initial biopsy and catheter placement site that required oversewing on day 8.
G207 delivery was not associated with grade 3 or 4 toxic effects in any patients, including at the maximum planned dose (108 PFU plus 5 Gy of radiation). Eleven of 12 patients had at least one adverse event that was possibly related to G207; however, all 20 adverse events were of grade 1 (Table S5). Table 1 lists the cumulative incidence of all adverse events within 30 days after G207 administration. Tables S6 and S7 list adverse events for each patient who received G207 alone or with radiation. No dose-limiting toxic effects or serious adverse events (Table S8) were attributed to G207 by the investigators. Furthermore, no patient had evidence of peripheral G207 shedding or viremia. One patient who was HSV-1 seropositive before receiving G207 had a positive saliva HSV PCR assay at day 28; however, it was negative for lacZ and G207 sequences, and this negative result confirmed reactivation of wild-type HSV-1 (see the Methods section in the Supplementary Appendix).
Table 1.
Adverse Events in the 12 Patients.*
| Event | Grade 1 | Grade 2 | Grade 3 | Total |
|---|---|---|---|---|
| no. | no. (%) | |||
| Cardiac disorders | ||||
| Bradycardia | 2 | 0 | 0 | 2 (17) |
| Hypertension | 1 | 0 | 0 | 1 (8) |
| Hypotension | 1 | 0 | 0 | 1 (8) |
| Tachycardia | 1 | 0 | 0 | 1 (8) |
| Ear and labyrinth disorders: external ear inflammation | 0 | 1 | 0 | 1 (8) |
| Gastrointestinal disorders | ||||
| Abdominal pain | 1 | 0 | 0 | 1 (8) |
| Constipation | 1 | 0 | 0 | 1 (8) |
| Diarrhea | 3 | 0 | 0 | 3 (25) |
| Nausea | 2 | 0 | 0 | 2 (17) |
| Vomiting | 3 | 2 | 0 | 5 (42) |
| General disorders | ||||
| Chills | 1 | 0 | 0 | 1 (8) |
| Fatigue | 3 | 0 | 0 | 3 (25) |
| Fever | 4 | 0 | 0 | 4 (33) |
| Blood and lymphatic system: anemia | 1 | 0 | 1 | 2 (17) |
| Infections and infestations: perirectal abscess | 0 | 0 | 1 | 1 (8) |
| Procedural complications: postoperative hemorrhage | 6 | 0 | 0 | 6 (50) |
| Investigations | ||||
| Increased ALT level | 0 | 1 | 1 | 2 (17) |
| Increased AST level | 0 | 2 | 0 | 2 (17) |
| Decreased lymphocyte count | 0 | 0 | 1 | 1 (8) |
| Decreased neutrophil count | 1 | 0 | 1 | 2 (17) |
| Decreased platelet count | 3 | 0 | 0 | 3 (25) |
| Weight loss | 1 | 0 | 0 | 1 (8) |
| Decreased white-cell count | 0 | 1 | 1 | 2 (17) |
| Metabolism and nutrition | ||||
| Anorexia | 2 | 0 | 0 | 2 (17) |
| Hyperphosphatemia | 1 | 0 | 0 | 1 (8) |
| Obesity | 0 | 0 | 1 | 1 (8) |
| Nervous system disorders | ||||
| Cerebrospinal fluid leak | 0 | 1 | 0 | 1 (8) |
| Dizziness | 1 | 0 | 0 | 1 (8) |
| Facial nerve disorder | 1 | 0 | 0 | 1 (8) |
| Headache | 2 | 1 | 0 | 3 (25) |
| Peritumoral vasogenic edema | 0 | 1 | 0 | 1 (8) |
| Seizure | 2 | 0 | 0 | 2 (17) |
| Skin disorders: contact dermatitis | 1 | 1 | 0 | 2 (17) |
| Total | 45 | 11 | 7 | 63 |
Shown is the cumulative incidence of adverse events according to highest grade irrespective of cause within 30 days after G207 administration. There were no events of grade 4. ALT denotes alanine aminotransferase, and AST aspartate aminotransferase.
TREATMENT OUTCOMES
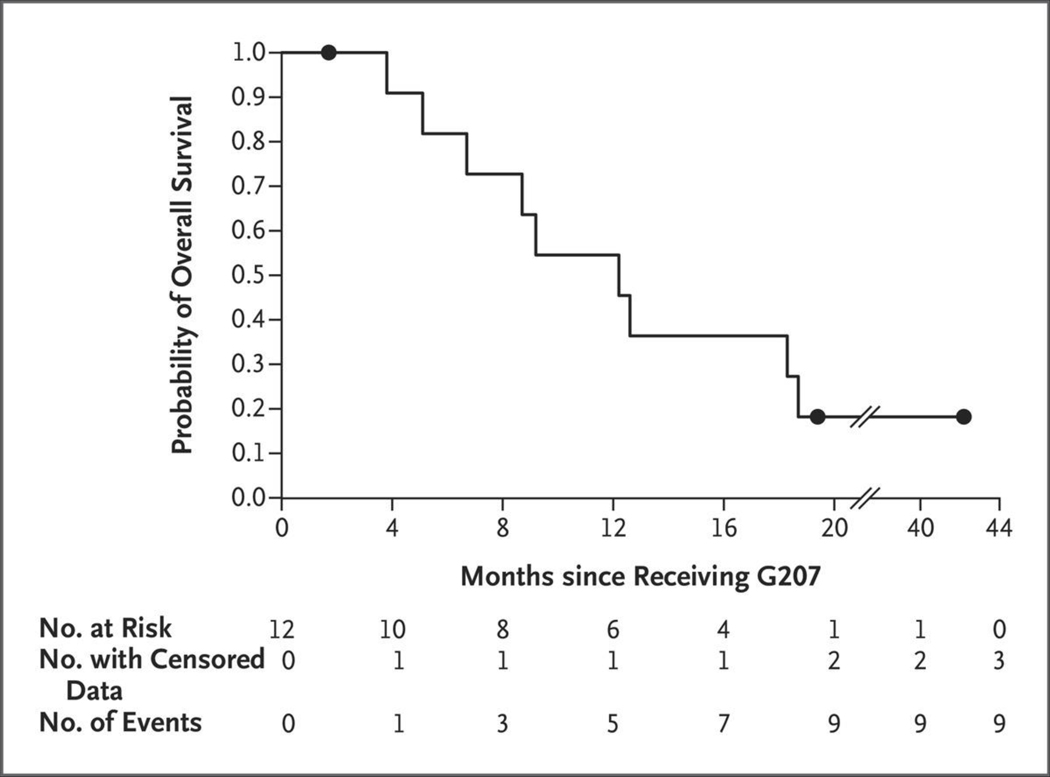
Radiographic, neuropathological, or clinical responses were seen in 11 patients. The median overall survival among patients who were treated with G207 was 12.2 months (95% confidence interval [CI], 8.0 to 16.4); as of June 5, 2020, a total of 4 of 11 patients (36%) were still alive 18 months after G207 treatment (Fig. 2). As with other immunovirotherapies,23 determination of the timing of progression was difficult owing to pseudoprogression and mixed responses except when disease was stable (see Table S4 for the time that each patient met iRANO criteria for progression). At initial evaluation, two types of imaging responses were seen: stable appearance and pseudoprogression. Stable disease was seen in 7 of 12 patients (58%) at 1 month, in 4 of 11 patients (36%) at 3 months, in 3 of 11 patients (27%) at 5 months, and in 2 of 11 patients (18%) at 12 months. Imaging from Patient 002 (Fig. 3) and Patient 005 (Fig. S1) showed stable disease. The tumor of Patient 002 was stable through 3 months; by 5 months, polycystic degradation (“Swiss cheese” appearance) was apparent. The number and size of cysts increased over time and corresponded with an increase in the performance score from 80 before treatment to 100 at 12 months after treatment. Similarly, cystic changes developed in the tumor of Patient 005, who had stable disease through 12 months after G207 treatment. In contrast, imaging from Patient 007 showed pseudoprogression (Fig. S2). The patient was asymptomatic and had an unremarkable physical examination; these clinical findings were consistent with pseudoprogression. A biopsy that was performed 3 months after G207 treatment to clarify imaging findings revealed semiliquified, friable tissue with residual tumor cells.
Figure 2. Kaplan–Meier Curve of Overall Survival Probability among Patients Treated with G207.
The median survival was 12.2 months (95% confidence interval, 8.0 to 16.4). Circles represent patients with censored data.
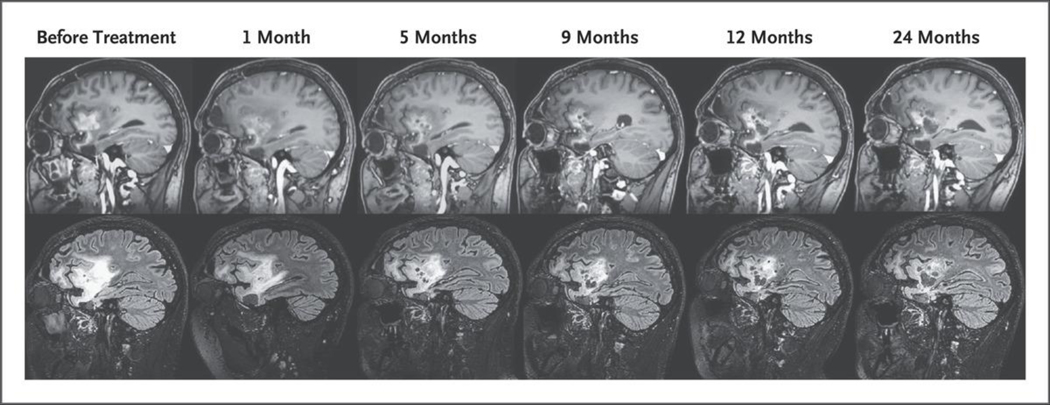
Figure 3. Response in Patient 002 Treated with G207.
In the top row, post-gadolinium sagittal T1-weighted images show the decreased size of the enhancing component of the tumor with interval development of numerous enlarging cystic spaces where G207 was inoculated. In the bottom row, sagittal fluid-attenuated inversion recovery (FLAIR) images show an interval decrease in the size of the FLAIR hyperintense component of the tumor in the frontal and temporal lobes after G207 treatment. These changes corresponded with an increase in the performance score from 80 before treatment to 100 by 12 months after treatment (on a scale from 0 to 100, with higher scores indicating better function). The patient had a continuous response 42 months after G207 treatment without any additional therapy
In four patients who had undergone a post-treatment biopsy or resection owing to indeterminate MRI findings (three patients) or new-onset neurologic symptoms (one patient), we assessed matched pre- and post-treatment tissues for T lymphocytes by immunohistochemical analysis (see the Methods section in the Supplementary Appendix). Pretreatment tissue had few tumor-infiltrating lymphocytes, as expected for pediatric high-grade glioma. In contrast, tissue 2 to 9 months post-treatment from patients in each dose cohort revealed substantive increases in CD3+, CD4+, and CD8+ tumor-infiltrating lymphocytes. (Fig. S3 shows quantification and Table S9 shows differences in median positive cells between the pretreatment and post-treatment tissue samples.) Clusters of CD8+ (Fig. 4) and CD4+ (Fig. S4) tumor-infiltrating lymphocytes were observed after G207 treatment. In Patient 009, T cells infiltrated throughout the resected tumor in areas adjacent to and distant from where G207 was inoculated 5 months earlier (Fig. S5). In Patient 010, tumor-infiltrating lymphocytes increased between 2 and 3 months post-treatment (Fig. S6). No evidence of HSV-1 staining was seen in any post-treatment tissue, which indicated that G207 was no longer replicating or present (Fig. S7). Assessment of CD20+ B lymphocytes (Fig. S8) and CD138+ plasma cells (Fig. S9) in Patients 006, 009, and 010 by immunohistochemical analysis revealed few pretreatment CD20+ or CD138+ cells (Fig. S3). However, post-treatment, CD20+ cells were increased in Patient 009, and CD138+ cells were increased in Patients 009 and 010 (Table S9).
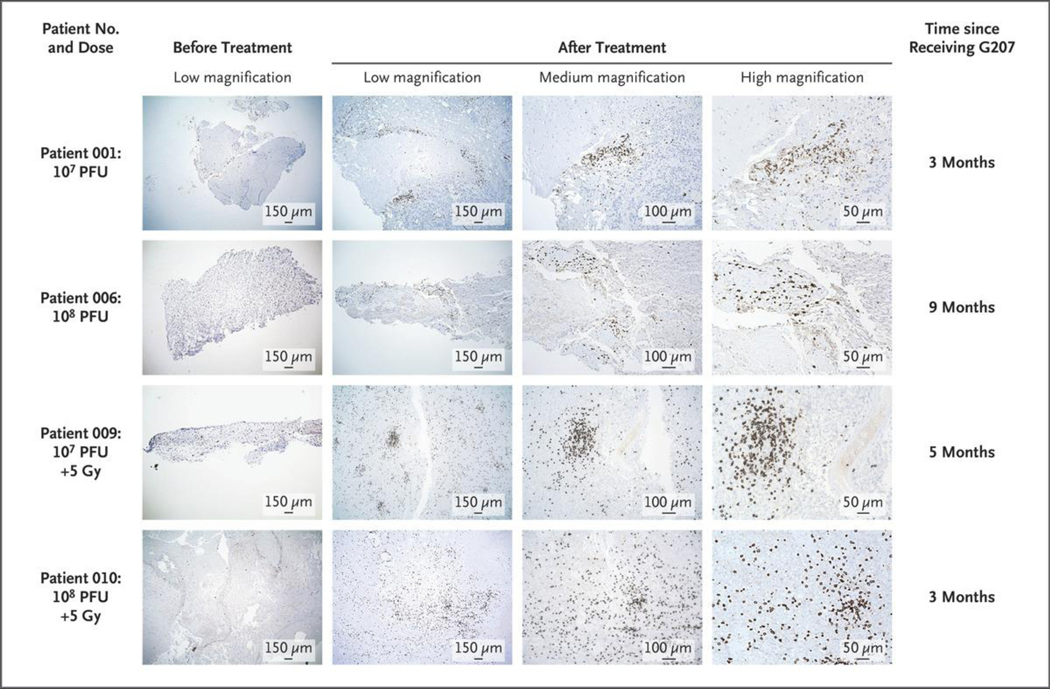
Figure 4. Immunohistologic Staining for CD8+ Cytotoxic T Lymphocytes in Matched Pre- and Post-treatment Tissue from Four Patients.
The left column shows the initial core biopsies before G207 administration; there were few immune-related cells, a finding consistent with immunologically silent or “cold” tumors. The other three columns show tumor tissues between 3 and 9 months after G207 administration from these same four patients. Post-G207 tissue revealed a brisk infiltration of CD8+ cells, which indicates an immune response to G207 and a shift to immunologically “hot” tumors. Photomicrographs were taken at low, medium, and high magnifications. Doses are in plaque-forming units (PFU) of G207 and in grays (Gy) of radiation.
IMMUNE RESPONSES IN BLOOD, POST-G207 SUPPORTIVE CARE, AND OTHER TREATMENTS
We considered that previous infection with HSV-1 might interfere with the efficiency of G207 infection owing to neutralizing antibodies, and we deemed seroconversion as measurable confirmation of an immune response to G207 infection.27 The median overall survival among patients with baseline HSV-1 IgG antibodies (three patients) was 5.1 months (95% CI, 3.0 to 7.2), as compared with 18.3 months (95% CI, 9.2 to 27.4) among patients who had seroconversion after G207 (three patients) (Table S10). No patients had seroconversion after treatment with 107 PFU. Table S11 summarizes peripheral white-cell counts and absolute neutrophil, lymphocyte, natural killer (NK) cell, and T-lymphocyte counts at baseline as well as activated T-cell counts and CD4:CD8 ratios at baseline and on days 7, 14, and 28. Six patients had lymphopenia at baseline. Despite having severe lymphopenia before G207 treatment, Patient 010 had a sizeable infiltration of tumor with lymphocytes after G207 treatment (Fig. S6). NK cells are important in controlling herpesvirus infections in humans and may contribute to antitumor immunity.28 When patients who were seropositive before treatment were excluded, baseline levels of absolute NK cells correlated positively with survival (Spearman’s rank correlation coefficient, 0.81 for all patients and 0.88 for patients who had died) (Fig. S10). Table S4 summarizes supportive care with glucocorticoids (three patients) and bevacizumab (six patients) and subsequent treatments received after G207 treatment (eight patients).
DISCUSSION
Pediatric high-grade glioma is a devastating disease that lacks effective, targeted therapies and results in high morbidity and mortality. Several promising cancer-selective immunovirotherapy approaches are in various stages of clinical development to target high-grade glioma in both adults and children. Replication-deficient viruses are being tested to deliver enzymes (e.g., cytosine deaminase and thymidine kinase) to tumor cells to convert prodrugs to active cytotoxic agents and induce gene-mediated cytotoxic immunotherapy29 or to produce cytokines (e.g., interleukin-12) locally when in the presence of a small-molecule activator to overcome the immunosuppressive microenvironment of high-grade glioma.30 Several replication-competent viruses, both unmodified (e.g., reovirus) and genetically engineered for tumor selectivity (e.g., herpesvirus, poliovirus, adenovirus, and measles virus),31 are being investigated in malignant pediatric brain tumors.
In this phase 1 immunovirotherapy trial involving children and adolescents with high-grade glioma, genetically engineered herpesvirus G207 alone and with radiation elicited only low-grade toxic effects. Side effects that were attributed to G207 were mild and manageable with observation or supportive care. As in previous trials of G207 involving adults with high-grade glioma,14–16 no evidence of peripheral viral shedding was found. Most patients had a strong response to G207 despite many being heavily pretreated and having large tumors. Studies in melanoma that used FDA-approved oncolytic HSV-1 talimogene laherparepvec showed that the virus initiates delayed regional and systemic antitumor immune responses ranging from 18.4 to 29.1 weeks.25 Thus, unlike traditional therapies, patients may benefit from G207 months after receiving it even after another therapy is initiated, resulting in improved overall survival. The median overall survival of 12.2 months (95% CI, 8.0 to 16.4) among patients who received G207 is very promising. Although our phase 1 trial involving 12 patients cannot be compared directly with previous studies, the median overall survival among patients with pediatric high-grade glioma at initial progression after front-line therapy in the Children’s Oncology Group ACNS0423 study was only 5.6 months (95% CI, 4.3 to 7.2).2 This median value was confirmed in a recent meta-analysis of 17 studies involving pediatric patients with recurrent high-grade glioma (overall survival, 5.6 months; 95% CI, 3.9 to 7.3).3 In addition, 4 patients in the current trial who received G207 have lived past 18 months thus far, which exceeds the median overall survival among pediatric patients with newly diagnosed hemispheric high-grade glioma.5 These encouraging results will need to be confirmed in an upcoming phase 2 trial.
We found that seroconversion after G207 treatment occurred only in patients who received the 108-PFU dose, which indicates that seroconversion is dose-dependent when virus is inoculated in the brain. Preexisting antibodies reduced median overall survival by nearly half in studies of oncolytic adenovirus involving adults with solid tumors, whereas seropositivity did not reduce the efficacy of oncolytic HSV-1 talimogene laherparepvec in patients with melanoma.22,27 However, patients received an initial 106-PFU dose of talimogene laherparepvec followed by subsequent 108-PFU doses, which probably diminished the ability to detect any effect of preexisting neutralizing antibodies on response.22 Although 70 to 90% of adults have preexisting HSV-1 antibodies, most children are seronegative.32 Further assessment of the usefulness of baseline HSV-1 seropositivity and seroconversion as a biomarker of treatment response in children is needed.
On the basis of the neuropathological data, we surmise that responses seen in this trial were immune-mediated. G207 results in a dramatic increase in tumor-infiltrating lymphocytes. It is uncertain whether tumor-infiltrating lymphocytes seen 2 to 9 months after treatment were tumor-specific. However, they may have had an antitumor role. Active HSV infection is self-limited in immunocompetent hosts.33 In animals, wild-type HSV replication peaks at approximately 5 days, followed by abrogation by CD8+ T cells within approximately 10 days.33,34 Likewise, preclinical studies indicate that G207 infection is eliminated within a similar period.9,10 G207 has greatly diminished capacity to establish latency because a portion of latency-activated transcripts is affected by γ134.5 gene deletions.34 Although a small amount of G207 DNA may persist several months after G207 treatment,14 antiviral T cells respond to virus-derived peptides and not DNA. In preclinical studies, G207 induced cytotoxic T-lymphocyte antitumor responses within 20 days and resulted in antitumor immunity.9,10
The matched pre- and post-treatment tumor tissues are suggestive of antitumor T-cell responses. Clusters of T cells were seen up to 9 months after G207 treatment without evidence of HSV-1 staining. The tissue showed an ongoing T-cell infiltration that increased over time and was detected in distant areas several centimeters from G207 inoculation. T-cell receptor sequencing was not performed. Although rare subtypes of pediatric high-grade glioma may respond to immunotherapy (e.g., hypermutated),35 pediatric high-grade gliomas are largely immunologically silent. The informative difference in this trial is the large number of tumor-infiltrating lymphocytes induced by intratumoral G207 inoculation, although it remains unclear whether the infiltrates reflect a general inflammatory response or the response is indeed related to virus antigen or tumor antigen recognition.
We found that G207 alone and with radiation was associated with mainly low-grade toxic effects, with evidence of responses in many of the children. G207 converted immunologically “cold” tumors to “hot,” with high numbers of infiltrating T cells and other inflammatory cells. To extend and confirm the findings in this phase 1 trial, a multi-institutional phase 2 clinical trial of G207 in pediatric high-grade glioma (ClinicalTrials.gov number, NCT04482933) is forthcoming.
Supplementary Material
Acknowledgments
Supported by grants from the Food and Drug Administration (R01FD005379), the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) (UL1TR003096), Cannonball Kids’ Cancer Foundation, the Rally Foundation for Childhood Cancer Research, Hyundai Hope on Wheels, St. Baldrick’s Foundation, the Department of Defense (W81XWH-15–1-0108), Andrew McDonough B+ Foundation, Kaul Pediatric Research Institute, and NIH/National Cancer Institute Cancer Center Support Grants to the University of Alabama at Birmingham (P30CA013148) and to Memorial Sloan Kettering Cancer Center (P30CA008748), and by Kelsie’s Crew, Eli’s Block Party Childhood Cancer Foundation, the Eli Jackson Foundation, Jaxon’s F.R.O.G. Foundation, Battle for a Cure Foundation, and Sandcastle Kids.
Disclosure forms provided by the authors are available at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank the brave participant–pioneers and their families for entrusting us with their care; Shannon Ross and Tony McGrath for serving as medical monitors; Emily Waite, Erin Blodgett, and Clay Tynes for providing pharmacy support; Jan Robertson for supporting the families and patients and arranging for their travel and stay; Pamela Hardwick for monitoring the data, and Raymond Watts, Thomas Howard, Roger Berkow, Smita Bhatia, Mike Warren, Dawn Walton, Crayton (Tony) Fargason, Bridget Tate, Jess Eddington, Joshua Pavlovec, Nadine Bradley, Sasha Ramini, Marla Thompson, Juliette Hukin, Michael Rytting, David Kelly, Gerald Schatten, Timothy Cripe, David Munn, Katie Metrock, Elizabeth Alva, Girish Dhall, and countless others whose support and assistance made conducting the trial possible.
REFERENCES
- 1.Cohen KJ, Pollack IF, Zhou T, et al. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro Oncol 2011; 13:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jakacki RI, Cohen KJ, Buxton A, et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children’s Oncology Group ACNS0423 study. Neuro Oncol 2016; 18: 1442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kline C, Felton E, Allen IE, Tahir P, Mueller S. Survival outcomes in pediatric recurrent high-grade glioma: results of a 20-year systematic review and meta-analysis. J Neurooncol 2018; 137: 103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones C, Karajannis MA, Jones DTW, et al. Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol 2017; 19:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 2017; 32(4):520–537.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med 1995; 1: 938–43. [DOI] [PubMed] [Google Scholar]
- 7.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A 1997; 94:843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein DJ, Weller SK. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol 1988; 62: 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toda M, Rabkin SD, Kojima H, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther 1999;10: 385–93. [DOI] [PubMed] [Google Scholar]
- 10.Todo T, Rabkin SD, Sundaresan P, et al. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum Gene Ther 1999; 10: 2741–55. [DOI] [PubMed] [Google Scholar]
- 11.Benencia F, Courrèges MC, Fraser NW, Coukos G. Herpes virus oncolytic therapy reverses tumor immune dysfunction and facilitates tumor antigen presentation. Cancer Biol Ther 2008; 7: 1194–205. [DOI] [PubMed] [Google Scholar]
- 12.Leddon JL, Chen CY, Currier MA, et al. Oncolytic HSV virotherapy in murine sarcomas differentially triggers an antitumor T-cell response in the absence of virus permissivity. Mol Ther Oncolytics 2015; 1: 14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Advani SJ, Markert JM, Sood RF, et al. Increased oncolytic efficacy for high-grade gliomas by optimal integration of ionizing radiation into the replicative cycle of HSV-1. Gene Ther 2011; 18: 1098–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther 2000; 7: 867–74. [DOI] [PubMed] [Google Scholar]
- 15.Markert JM, Liechty PG, Wang W, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther 2009; 17: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markert JM, Razdan SN, Kuo H-C, et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther 2014; 22:1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman GK, Bernstock JD, Chen D, et al. Enhanced sensitivity of patient-derived pediatric high-grade brain tumor xenografts to oncolytic HSV-1 virotherapy correlates with nectin-1 expression. Sci Rep 2018;8: 13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman GK, Langford CP, Coleman JM, et al. Engineered herpes simplex viruses efficiently infect and kill CD133+ human glioma xenograft cells that express CD111. J Neurooncol 2009; 95: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman GK, Moore BP, Nan L, et al. Pediatric medulloblastoma xenografts including molecular subgroup 3 and CD133+ and CD15+ cells are sensitive to killing by oncolytic herpes simplex viruses. Neuro Oncol 2016; 18: 227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstock JD, Vicario N, Li R, et al. Safety and efficacy of oncolytic HSV-1 G207 inoculated into the cerebellum of mice. Cancer Gene Ther 2020; 27: 246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carceller F, Fowkes LA, Khabra K, et al. Pseudoprogression in children, adolescents and young adults with non-brainstem high grade glioma and diffuse intrinsic pontine glioma. J Neurooncol 2016; 129: 109–21. [DOI] [PubMed] [Google Scholar]
- 22.Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015;33: 2780–8. [DOI] [PubMed] [Google Scholar]
- 23.Desjardins A, Gromeier M, Herndon JE II, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med 2018; 379: 150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang FF, Conrad C, Gomez-Manzano C, et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol 2018; 36:1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman HL, Amatruda T, Reid T, et al. Systemic versus local responses in melanoma patients treated with talimogene laherparepvec from a multi-institutional phase II study. J Immunother Cancer 2016; 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 2015; 16(15): e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taipale K, Liikanen I, Koski A, et al. Predictive and prognostic clinical variables in cancer patients treated with adenoviral oncolytic immunotherapy. Mol Ther 2016;24: 1323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marotel M, Hasim MS, Hagerman A, Ardolino M. The two-faces of NK cells in oncolytic virotherapy. Cytokine Growth Factor Rev 2020; 56: 59–68. [DOI] [PubMed] [Google Scholar]
- 29.Kieran MW, Goumnerova L, Manley P, et al. Phase I study of gene-mediated cytotoxic immunotherapy with AdV-tk as adjuvant to surgery and radiation for pediatric malignant glioma and recurrent ependymoma. Neuro Oncol 2019; 21: 537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiocca EA, Yu JS, Lukas RV, et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci Transl Med 2019;11(505): eaaw5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foreman PM, Friedman GK, Cassady KA, Markert JM. Oncolytic virotherapy for the treatment of malignant glioma. Neurotherapeutics 2017;14: 333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu F, Lee FK, Morrow RA, et al. Seroprevalence of herpes simplex virus type 1 in children in the United States. J Pediatr 2007;151: 374–7. [DOI] [PubMed] [Google Scholar]
- 33.Lang A, Nikolich-Zugich J. Development and migration of protective CD8+ T cells into the nervous system following ocular herpes simplex virus-1 infection. J Immunol 2005; 174: 2919–25. [DOI] [PubMed] [Google Scholar]
- 34.Whitley RJ, Kern ER, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin Invest 1993; 91: 2837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackay A, Burford A, Molinari V, et al. Molecular, pathological, radiological, and immune profiling of non-brainstem pediatric high-grade glioma from the HERBY phase II randomized trial. Cancer Cell 2018; 33(5): 829–842.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.