Abstract
Background & Aims:
Population-level familial risk estimates of inflammatory bowel diseases (IBD) are generally not available, especially in Asian countries. We aimed to quantify the familial risk of incident IBD among first-degree relatives (FDRs) of individuals with IBD according to age, sex and familial relationship.
Methods:
Using the South Korea National Health Insurance database (2002–2017), which has complete population coverage and confirmed accuracy of both FDR information and IBD diagnoses, we constructed a cohort of 21,940,795 study subjects comprising 12 million distinct families. We calculated incidence risk ratios of ulcerative colitis(UC) or Crohn’s disease(CD) in individuals of affected FDRs compared to individuals without affected FDRs.
Results:
Of 45,717 individuals with UC and 17,848 individuals with CD, 3.8% and 3.1% represented familial cases, respectively. Overall, there was a 10.2-fold (95% CI:9.39–11.1) and 22.1-fold (95% CI:20.5–24.5) significantly higher adjusted risk of UC and CD among FDRs of individuals with vs without IBD. Familial risk was highest among twins, followed by non-twin siblings, and then offspring of affected parents. Familial risk was generally higher within generation (sibling-sibling) vs between generations (parent-offspring). Familial risk also increased with increasing number of affected FDRs.
Conclusion:
According to this population-based analysis, there is a substantially increased risk of IBD among FDRs of affected individuals, with the highest risk among siblings for CD. These findings might help with earlier diagnosis and appropriate therapeutic intervention in FDRs of individuals with IBD. Dedicated studies are needed, to evaluate the contributions of shared early-in life environmental exposures and genetic factors.
Keywords: IRR, epidemiology, inherited, family
INTRODUCTION
The inflammatory bowel diseases (IBD), inclusive of ulcerative colitis (UC) and Crohn’s disease (CD), are chronic, most often progressive, inflammatory disorders of the gastrointestinal tract with or without extraintestinal involvement1,2. IBD results from the complex interaction between dysregulated immune responses to certain environmental exposures and an altered gut microbiota among genetically susceptible individuals. The timing of these critical exposures and hormonal influences are also likely relevant. The contribution of each of these elements, such as genetic predisposition, to disease pathogenesis varies based on the individual and the IBD type (UC versus CD).
A positive family history of IBD in a first-degree relative (FDR) is the strongest identifiable risk factor for incident IBD in an unaffected family member. Family history is an amalgamation of both shared genetic susceptibility and environmental exposures, especially those that occur early-in-life during decisive stages of immune development; these are further compounded by gene-environment interactions and possibly epigenetic modulation. Indeed, clinically asymptomatic family members of patients with IBD demonstrate preclinical pathology, including altered intestinal permeability, altered immune function, microbiome changes, and early biomarker positivity3–5. Studies of familial aggregation of IBD are well-represented in the literature, but these have heterogenous study designs and are vulnerable to several biases. True population-based studies quantifying precise risk estimates of familial IBD incidence are limited6–8, with none available in non-Western populations. However, there are now convincing data demonstrating differences in non-genetic and genetic disease determinants based on geography and ethnic origin, with the majority of data comparing Western versus Asian-Pacific populations9–14. Extrapolation of Western European familial IBD risk estimates to other populations might be inappropriate in light of these differences and also when considering the epidemiology of IBD in Asian-Pacific regions. The emergence of IBD in Asian-Pacific populations, including South Korea, and the remarkable rate of rise over a relatively short time span perhaps implicates shifts in environmental triggers, which might technically be modifiable, more so than shifts in genetic susceptibility. Precisely quantifying familial risk and patterns might enable more accurate risk counseling and better targeted clinical surveillance for earlier diagnosis and treatment among FDRs. Moreover, accurate definition of familial IBD risk across populations might also inform subsequent investigations untangling the various shared environmental and genetic contributions.
Accordingly, our primary objective was to conduct a population-based analysis of the South Korean population using contemporary diagnostically accurate data with complete information on blood-related FDRs in order to define the familial risk of UC and CD among FDRs of individuals with IBD.
METHODS
Data Source
South Korea has a universal health insurance system known as the, National Health Insurance (NHI), which covers the entire population of approximately 50 million residents. The NHI database distinctly contains family relationship information of all insured people and their dependents, thereby allowing identification of all blood-related family members and their relatedness, especially of FDR. The NHI is also linked to data from the National Health Screening Program (NHSP), that includes standardized biannual medical examinations, and records health behaviors including smoking status. In 2006, a Rare Intractable Diseases (RID) registration program was introduced by the NHI and includes UC and CD. For formal registry in the RID, UC and CD cases must meet the standard diagnostic criteria specified by the NHI for these conditions (Supplemental material S1).
Study population: Inclusion and exclusion criteria
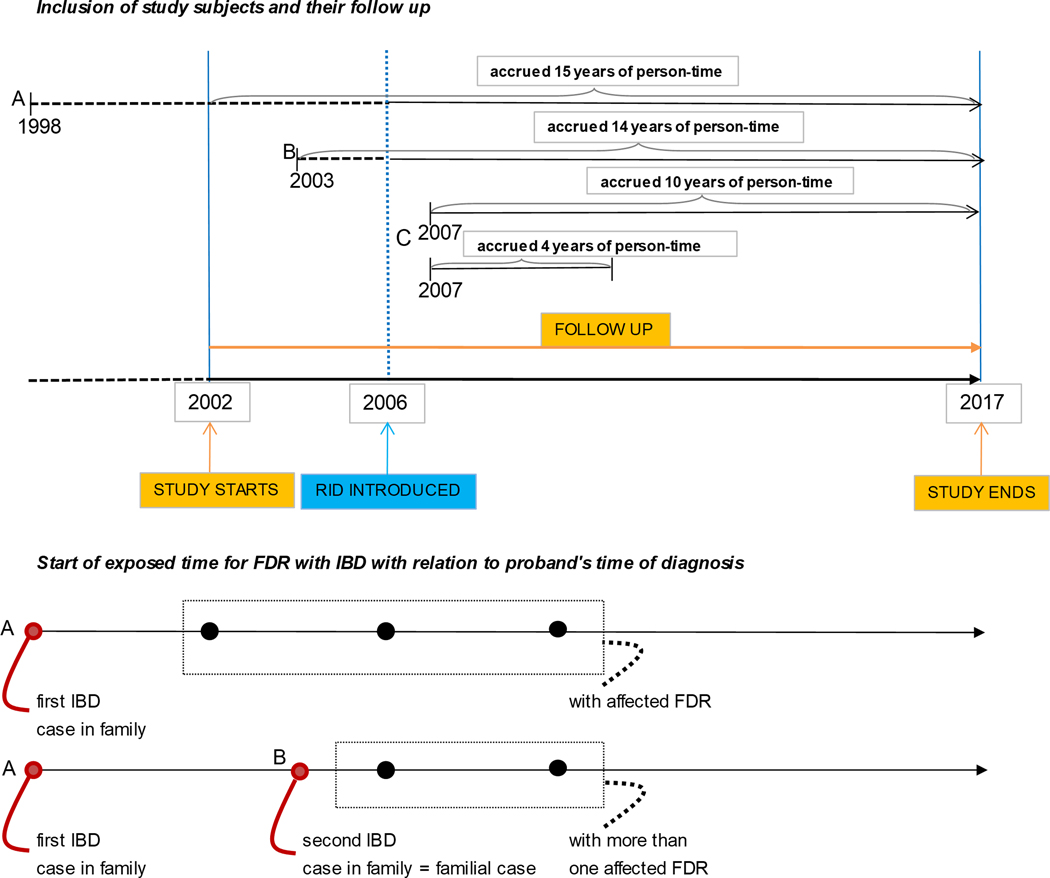
This population-based cohort study used data from the NHI-RID and linked NHSP databases during the predefined study period January 1, 2002 to December 31, 2017. From approximately 50 million individuals, we constructed a cohort of 21,940,795 study subjects with blood related FDRs, ultimately comprising 12 million distinct families. Only individuals who had an identifiable biological father and mother were included. While our defined study period is 2002–2017, we included all IBD cases (even those prior to 2002) as long as they were registered in the RID database (Supplemental material S2a). Individuals diagnosed with IBD before 2002 were included as prevalent cases, only if they were registered in the RID database. Individuals diagnosed with IBD prior to the initiation of the RID program (i.e. 2002–2006) were included as incident cases; and their date of diagnosis was considered as their initial date of diagnosis. Individuals diagnosed with IBD after 2006 were included as incident cases and their date of diagnosis was the considered as their date of registration in the RID database. Subject entry and follow up time are illustrated in Figure 1 (see Supplemental material S2b–c for details of diagnostic accuracy/verification and data abstracted from the NHI database).
Figure 1:
Study diagram to illustrate study population
A Individuals diagnosed with IBD before 2002 were included as prevalent cases as long as they were registered in the RID database.
B Individuals diagnosed with IBD between 2002 to 2006 (e.g. 2003) were included as an incidence case as long they were registered in the RID database and their date of diagnosis was the initial date of diagnosis (i.e. 2003). Though there is a low chance that it could be a prevalent case
C Individuals diagnosed with IBD after 2006 (e.g. 2007) were included as an incidence case and were registered in the RID database from their date of diagnosis (i.e. 2007).
From the date of diagnosis of the first IBD case in the family (A), all other members still unaffected were categorized as exposed and were assigned “with affected FDR”. The second family member to be diagnosed was defined as the first “familial case” (B) and from that point forward, the still unaffected family members were then considered as “exposed to two familial cases” from the date of diagnosis of the second case and were assigned “more than one affected FDR”.
IBD cases not registered in the RID database were excluded from our study. To ensure only blood-related FDRs were included and to minimize heterogeneity, we excluded individuals who had single parents, parents with changed partnership, and half-siblings (i.e. siblings with a different biological father or mother). Children who were not registered as dependent at birth were also excluded (Supplemental Figure).
Exposure
The primary “exposure” in this study was having an FDR with IBD. Additional details on assessment of familial relation and FDR definition is provided in Supplemental material S2d.
Outcome
The primary outcome was the incident occurrence of IBD. An incident case was defined as an individual diagnosed and registered as having UC or CD in the NHI-RID database in the corresponding year (Supplemental material 2a). FDRs of the proband (defined as the individual diagnosed with IBD) were categorized as ‘exposed’ from the date of UC or CD diagnosis of the first affected case in the family and were assigned “with affected FDR”. The second family member to be diagnosed was defined as the first “familial case” and from that point forward, the still unaffected family members were then considered as “exposed to two familial cases” from the date of diagnosis of the second case and were assigned “more than one affected FDR”. A similar incremental pattern was applied in situations of three family members diagnosed with IBD, and so forth (Figure 1).
Statistical approach
Individuals meeting inclusion criteria were followed from January 1, 2002 up to the first occurrence of a diagnosis of UC or CD, death, or the study end (December 31, 2017). Individuals born after January 1, 2002 were followed starting at their date of birth. Subjects were censored at bowel resection or loss to follow up.
The number of person-years was calculated for each study subject starting from the date of diagnosis of the proband up to the subject’s date of IBD diagnosis or respective censoring point. The incidence rate (IR) was calculated as the number of incident UC or CD cases divided by the respective total person-years at risk.
We estimated familial risk of UC or CD represented as incidence rate ratios (IRRs) with 95% confidence intervals (CIs) by comparing the IR of individuals with an affected UC (or CD) FDR to the IR of individuals without a FDR with IBD (reference group). We also estimated the IRRs of UC and CD in individuals according to each type of FDR relationship (i.e. mother, father, siblings and twins) and compared these to IRRs among individuals with the same relationship of FDR, but unaffected by the same type of IBD. Similar definitions were used when assessing risk of CD in FDRs of probands with UC and for risk of UC in FDRs of probands with CD.
Cox proportional hazards method was applied without and with adjustment of covariates to derive and present both crude and adjusted IRRs. Analyses were adjusted for age, sex, calendar periods (2002–2006, 2007–2011 and 2012–2017), maternal and paternal age at birth.
To evaluate the effect of age or sex on familial risk of IBD, we conducted separate age and sex stratified familial analyses. IRRs were analyzed according to age groups having 10-year intervals (0 to 60 years) and according to sex (male, female). Age-group stratified familial risk was estimated as the incidence of UC (or CD) in individuals of a particular age group with affected FDR compared to the incidence of UC (or CD) in individuals of the same age group but without an affected FDR. Sex- stratified familial risk was calculated separately for males and females, and the incidence of UC (or CD) in males with an affected FDR was compared to the incidence of UC (or CD) in males without an affected FDR. Similarly, familial risk was estimated for females. Sex- stratified and age group- stratified familial risk was analyzed according to each category of FDR relationship. We also calculated familial risk for each age group (10–19,20–29,30–39,40–49) stratified for birth year (1980–1984,1985–1989,1990–1994,1995–1999,2000–2004, 2005–2009) to analyze possible birth cohort effect.
Using the linked NHSP data, we were also able to evaluate the familial risk adjusted for smoking status for the subset of subjects (~70% of the cohort) for whom data on smoking status were available.
RESULTS
General cohort characteristics
From the cohort of 21,940,795 study subjects meeting the detailed inclusion criteria, 45,717 (26,828 males, 18,889 females) individuals with UC and 17,848 (10,999 males, 6,849 females) individuals with CD were identified. The mean annual incidence rates of UC and CD were 4.6 and 3.2 per 10⁵ person-years, respectively (Table 1), and this was relatively stable during the study period.
Table 1.
Demographics of people with IBD-affected first degree relatives compared to IBD-unaffected first-degree relatives
| Individuals with UC affected FDR | Individuals with CD affected FDR | Total individuals | Total population | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| with affected FDR | with unaffected FDR | |||||||||
|
| ||||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
|
| ||||||||||
| Sex | ||||||||||
| Male | 39,440 | (54) | 14,585 | (52) | 54,100 | (54) | 12,067,365 | (55) | 12,121,465 | (55) |
| Female | 33,112 | (46) | 13,717 | (48) | 46,895 | (46) | 9,772,435 | (45) | 9,819,330 | (45) |
| Period of birth | ||||||||||
| ~1971 | 4,655 | (6) | 1,126 | (4) | 5,781 | (6) | 1,394,947 | (6) | 1,400,733 | (6) |
| 1972~1981 | 15,948 | (22) | 4,778 | (17) | 20,726 | (21) | 3,653,394 | (17) | 3,674,152 | (17) |
| 1982~1991 | 20,411 | (28) | 7,901 | (28) | 28,312 | (28) | 5,275,359 | (24) | 5,303,716 | (24) |
| 1992~2001 | 17,386 | (24) | 8,307 | (29) | 25,693 | (26) | 5,794,872 | (27) | 5,820,614 | (27) |
| 2002~ | 14,152 | (19) | 6,190 | (22) | 20,342 | (20) | 5,721,228 | (26) | 5,741,580 | (26) |
| Total | 72,552 | 28,302 | 100,995 | 21,839,800 | 21,940,795 | |||||
N – number of individuals, IBD – inflammatory bowel disease, UC - ulcerative colitis, CD - Crohn’s disease, FDR – first degree relatives
Familial risk of IBD
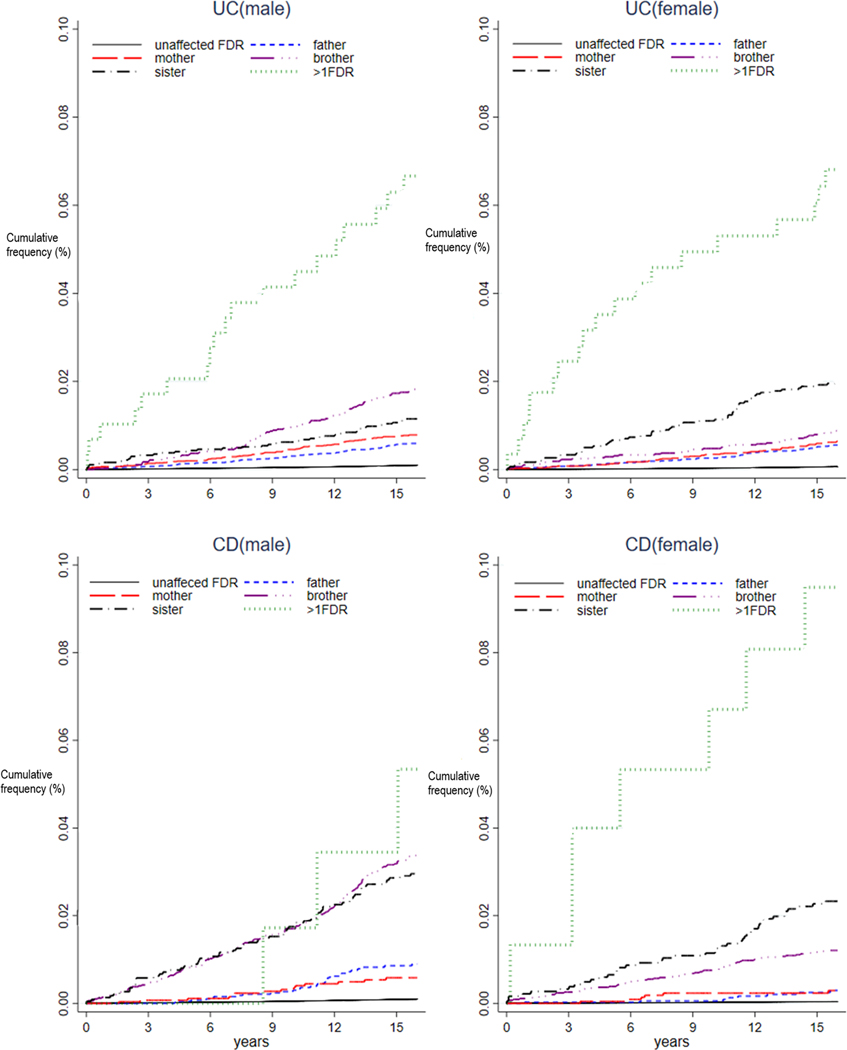
Familial cases of incident IBD accounted for 3.8% of all UC diagnoses and 3.1% of all CD diagnoses. Of the 100,854 individuals who had an FDR with IBD, 72,552 individuals (71.9%; 39,440 males and 33,112 females) had an FDR with UC, while 28,302 (28.1%; 14,585 males and 13,717 females) had an FDR with CD. Of these, 576 (0.79%) developed UC and 400 (1.4%) developed CD. Among those with an FDR with IBD, the incidence of UC and CD was 54.5 and 99.2 per 105 person-years, respectively. Contrastingly, among 21,839,800 people with FDRs without a history of IBD, 14,988 (0.07 %) developed UC and 12,983 (0.06 %) developed CD, which corresponded to a respective incidence rates of 4.9 and 4.2 per 105 person-years for UC and CD (Table 2, Figure 2)
Table 2.
Familial risk of incident UC and CD with affected FDR of the same subtype according to type of family relationship
| With affected father* | With affected mother* | With affected sibling* | With affected twin* | With more than one affected FDR* | With positive family history* | No family history of IBD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
|
| ||||||||||||||
| UC | ||||||||||||||
| Individuals at risk | 32,800 | 23,093 | 15,949 | 138 | 572 | 72,552 | 21,839,800 | |||||||
| Male | 18,045 | (55) | 12,811 | (55) | 8,212 | (52) | 83 | (60) | 289 | (51) | 39,440 | (54) | 12,067,365 | (55) |
| Female | 14,755 | (45) | 10,282 | (45) | 7,737 | (48) | 55 | (38) | 283 | (49) | 33,112 | (46) | 9,772,435 | (45) |
| Patients | 165 | 147 | 206 | 20 | 38 | 576 | 14,988 | |||||||
| Person-year | 465,748 | 327,633 | 250,507 | 2,062 | 8,583 | 1,056,686 | 307,000,000 | |||||||
| Incidence rate per 10 5 person-years (95% CI) | 35.4 (30.4–41.3) | 44.9 (38.2–52.7) | 82.2 (71.7–94.3) | 969.8 (620–1500) | 442.8 (322.2–608.5) | 54.5(50.2–59.1) | 4.9(4.8–5.0) | |||||||
| Crude IRR (95% CI) | 7.26 (6.19–8.46) | 9.19 (7.76–10.81) | 16.84 (14.61–19.33) | 198.64 (121–306) | 90.68(64.15–124.54) | 11.17 (10.26–12.14) | 1.00 | |||||||
| Adjusted ** IRR (95%CI) | 7.11 (6.10–8.29) | 8.77 (7.46–10.32) | 13.09 (11.41–15.02) | 163.73 (105–253) | 72.36 (52.63–99.49) | 10.20(9.39–11.09) | 1.00 | |||||||
| CD | ||||||||||||||
| Individuals at risk | 8,460 | 5,267 | 14,312 | 130 | 133 | 28,302 | 21,839,800 | |||||||
| Male | 4,490 | (55) | 2,837 | (55) | 7,109 | (52) | 91 | (70) | 58 | (51) | 14,585 | (54) | 12,067,365 | (55) |
| Female | 3,970 | (45) | 2,430 | (45) | 7,203 | (48) | 39 | (30) | 75 | (49) | 13,717 | (46) | 9,772,435 | (45) |
| Patients | 37 | 19 | 304 | 30 | 10 | 400 | 12,983 | |||||||
| Person-year | 105,367 | 71,279 | 222,649 | 1,788 | 1,971 | 403,054 | 307,000,000 | |||||||
| Incidence rate per 10 5 person-years (95% CI) | 35.1(25.4–48.5) | 26.7(17.0–41.8) | 136.5(122.0–152.8) | 1678.3(1170–2400) | 507.4(273.0–942.9) | 99.2(90.0–109.5) | 4.2(4.2–4.3) | |||||||
| Crude IRR (95% CI) | 8.30(5.84–11.45) | 6.30(3.79–9.85) | 32.29(28.72–36.18) | 396.86(267–566) | 119.97(57.52–220.7) | 23.47(21.19–25.92) | 1.00 | |||||||
| Adjusted ** IRR (95%CI) | 9.40(6.81–12.98) | 6.54(4.17–10.25) | 27.56(24.59–30.88) | 336.21(234–481) | 107.52(57.84–199.8) | 22.15(20.05–24.47) | 1.00 | |||||||
Familial risk of incident IBD with same subtype of affected FDR (e.g. risk of UC with UC affected FDR)
adjusted for age, sex, calendar periods, maternal and paternal age at birth
N – number of individuals, CD - Crohn’s disease, CI – confidence interval, FDR – first degree relatives, IRR – incidence risk ratio, IBD – inflammatory bowel disease, UC - ulcerative colitis.
Figure 2:
Time related incidence of ulcerative colitis or Crohn’s disease according to type to affected first degree relatives in males and females.
The familial risk for CD was significantly higher than for UC. Compared to individuals without an FDR with IBD (reference group), individuals with an FDR with CD had a 22.2-fold higher risk of CD (IRR 22.2, 95% CI 20.5–24.5) and individuals with an FDR with UC had a 10.2-fold higher risk of UC (IRR 10.2, 95% CI 9.39–11.1), respectively. We also analyzed the risk of CD in individuals with an FDR with UC and the risk of UC in individuals with an FDR with CD (Supplemental Table 1). Compared to individuals without a FDR with IBD, individuals with an FDR with CD had 3.5-fold higher risk of UC (IRR 3.54, 95% CI 2.80–4.47) and individuals with an FDR with UC had a 2.8-fold increased risk of CD (IRR 2.81, 95% CI 2.36–3.34).
Compared to individuals without an affected FDR, the familial risk of UC and CD after adjusting for smoking status was 7.9-fold (IRR 7.94, 95% CI 6.98–9.03) and 19-fold (IRR 19.03, 95% CI 15.58–23.25) respectively (Supplemental Tables 2, 3).
There was no significant difference in familial risk of UC and CD when adjusted for calendar periods, nor was there an apparent birth cohort effect (Supplemental tables 4 and 5). For example, for age groups 20–29 and 30–39 years, the IRRs for UC ranged from 9- fold to 11-fold and 6- fold to 13-fold respectively for the birth years 1975–1989. A similar pattern was observed for CD.
Familial risk of IBD according to type of familial relationship and number of affected FDRs
The reference group for these analyses was individuals with the same type of FDR but without IBD (Table 2). The risk of incident CD among an FDR was greatest for a twin sibling (IRR 336.2, 95% CI 235.0–481.1), followed by a non-twin sibling (IRR 27.6, 95% CI 24.6–30.9).
There was a 6.5- to 9.4-fold significantly higher risk of CD among the offspring of affected parents. For individuals with an affected father and mother the IRRs for CD were 9.40, 95% CI 6.81–13.0) and 6.54, 95% CI 4.17–10.3) respectively. Overall, the familial risk of CD when there were at least 2 affected FDRs was 107.5-fold higher (IRR 107.5, 95% CI 57.8–200) compared to individuals who had no FDRs with IBD.
Although the magnitude of familial risk for UC was lower compared to CD, the pattern of familial risk was similar in that the risk was highest when the affected FDR was a twin sibling (IRR 163.7, 95% CI 105.6–253.9), followed by a non-twin sibling (IRR 13.1, 95% CI 11.4–15.0), and then parents. The risk of UC among offspring when the parent was an affected mother and affected father was IRR 8.77, 95% CI 7.46–10.3) and 7.11, 95% CI 6.10–8.29) respectively. Overall, the familial risk of UC when there were at least 2 affected DRs was 72.4-fold (IRR 72.4, 95% CI 52.6–99.5) higher compared to individuals who had no FDRs with IBD.
A similar pattern of familial risk was observed for incident UC when the FDR had CD or for incident CD when the FDR had UC, with the risk being highest among twins, followed by siblings and then parents.
The risk for developing UC among offspring was nearly 13 times greater when both parents had UC compared to one parent having UC. There were no individuals with CD in the database who had both parents diagnosed with CD (Supplemental Table 6)
Age-group stratified familial risk of IBD
‘Age group’ refers to the ‘age of the population in the decade’ who are at risk of developing IBD in both familial and non-familial groups. The risk for both UC and CD by age-group is provided in Table 3. IRRs were generally higher in younger age groups (0–20 years). Age group-stratified analyses for UC (or CD) when the FDR had CD (or UC) are provided in Supplemental Table 7.
Table 3.
Age-group stratified familial risk of incident UC and CD with affected FDR of the same subtype according to type of family relationship
| Age group (years) | With affected father* | With affected mother* | With affected sibling* | With affected twin* | With more than oneaffected FDR* | With positive family history* | No family history of IBD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| N (%) | IRRs (95% CI) | N (%) | IRRs (95% CI) | N (%) | IRRs (95% CI) | N (%) | IRRs (95% CI) | N (%) | IRRs (95% CI) | Person-years | N (%) | IRRs (95% CI) | Person-years | N (%) | |
|
| |||||||||||||||
| CD | |||||||||||||||
| 0 to <10 | 0 | - | 1(5) | 15.93(0.4–89.53) | 5(1) | 47.37(15.2–111.9) | 0 | - | 0 | - | 83,891 | 6(1) | 19.63 (7.14–43.32) | 70,542,448 | 257(2) |
| 10 to <20 | 29(78) | 15.12(10.1–21.7) | 10(50) | 8.27(3.96–15.23) | 165(54) | 34.06(29–39.77) | 18(60) | 379.4(224.69–600.17) | 6(60) | 138.23(50.71–301.05) | 125,009 | 228(57) | 28.28(24.67–32.29) | 87,510,480 | 5643(19) |
| 20 to <30 | 7(19) | 5.09(2.05–10.50) | 6(35) | 5.36(1.97–11.67) | 113(38) | 24.04(19.7–28.9) | 12(40) | 352.4(181.97–616.11) | 3(30) | 80.14(16.52–234.33) | 116,182 | 141(36) | 19.41(16.30–22.94) | 80,635,807 | 5043(42) |
| 30 to <40 | 1(3) | 2.32(0.06–12.93) | 2(10) | 4.91(0.59–17.77) | 19(6) | 15.34(9.2–24.0) | 0 | - | 3(10) | 102.41(2.59–571.37) | 61,269 | 23(6) | 10.99(6.94–16.54) | 48,287,779 | 1650(26) |
| 40 to <50 | 0 | - | 0 | - | 2(1) | 14.88(1.7–54.1) | 0 | - | 0 | - | 14,274 | 2(0) | 6.83(0.82–24.88) | 15,605,220 | 320(8) |
| 50 + | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - | 2,429 | 0 | - | 4,422,586 | 70(3) |
| Total | 37 | 9.40(6.81–12.98) | 19 | 6.54(4.17–10.25) | 304 | 27.56(24.5–30.8) | 30 | 336.21(234.96–481.08) | 10 | 107.52(57.84–199.89) | 403,054 | 400 | 22.15(20.05–24.47) | 307,000,000 | 12983 |
| UC | |||||||||||||||
| 0 to <10 | 1(1) | 2.60(0.07–14.60) | 3(2) | 11.42(2.3–33.7) | 5(2) | 74.84(24.1–176.8) | 0 | - | 0 | - | 190,012 | 9(2) | 12.51(5.6–24.1) | 70,542,448 | 267(2) |
| 10 to<20 | 46(28) | 10.75(7.8–14.3) | 39(27) | 13.66(9.6–18.7) | 60(29) | 30.33(23.08–39.15) | 6(30) | 284.63(104.36–620.2) | 8(21) | 120.35(51.9–237.4) | 280,616 | 159(28) | 17.23(14.6–20.2) | 87,510,480 | 2877(19) |
| 20 to <30 | 71(43) | 7.07(5.51–8.93) | 67(46) | 9.67(7.48–12.92) | 84(42) | 11.73(9.34–14.54) | 11(55) | 182.45(91.03–326.68) | 25(66) | 104.23(67.4–154) | 315,036 | 258(45) | 10.54(9.2–11.9) | 80,635,807 | 6268(42) |
| 30 to <40 | 35(21) | 5.51(3.83–7.67) | 27(18) | 5.77(3.8–8.41) | 45(21) | 8.84(6.44–11.85) | 2(10) | 67.02(8.11–242.28) | 5(13) | 29.68(9.63–69.33) | 202,375 | 114(20) | 6.97(5.7–8.4) | 48,287,779 | 3902(26) |
| 40 to <50 | 11(6) | 6.37(3.17–11.43) | 11(7) | 7.19(3.58–12.92) | 11(5) | 9.18(4.57–16.49) | 1(5) | 114.76(2.9–640.55) | 0 | - | 56,474 | 34(6) | 7.54(5.2–10.5) | 15,605,220 | 1246(8) |
| 50 + | 1(1) | 2.38(0.06–13.33) | 0 | - | 1(1) | 4.09(0.1–22.91) | 0 | - | 0 | - | 12,173 | 2(1) | 1.70(0.2–6.1) | 4,422,586 | 428(3) |
| Total | 165 | 7.11(6.10–8.29) | 147 | 8.77(7.46–10.32) | 206 | 13.09(11.41–15.02) | 20 | 163.73(105.60–253.86) | 38 | 72.36(52.63–99.49) | 1,056,686 | 576 | 10.2(9.3–11.0) | 307,000,000 | 14,988 |
Familial risk of incident IBD with same subtype of affected FDR (e.g. risk of UC with UC affected FDR)
N – number of individuals, IBD – inflammatory bowel disease, UC - ulcerative colitis, CD - Crohn’s disease, FDR – first degree relatives, IRR – incidence risk ratio,
The age of diagnosis among familial cases was younger as compared to non-familial cases in both CD (58% vs 21%) and UC (30% vs 21%) (Supplemental table 8).
Sex-stratified familial risk of IBD
Overall, sex did not appear to be an effect modifier, except perhaps in affected female siblings (sister) with UC or CD. Among females compared to males, there was a significantly higher risk of UC or CD if they had an affected female sibling (sister) with UC or CD, respectively (p-interaction: <0.05). The magnitude of risk of UC or CD among females versus males was greatest for females with an affected twin, an affected female sibling, or if there were at least two affected FDRs (Supplemental Table 9). Familial risk analyses stratified by sex for incident UC when the FDR had CD or for incident CD when the FDR had UC are provided in Supplemental Table 10.
Among twins, same sex twins had significantly higher risk compared to opposite sex twins for both UC and CD. While we do not have the breakdown of monozygotic to dizygotic twins, based on estimation for zygosity15, we can estimate that these same sex twins had a higher ratio of monozygotic twins (see Supplemental material S3 and Supplemental table 11).
DISCUSSION
This study represents the largest population-based cohort study of familial IBD risk, and the first in an Asian-Pacific population. Among approximately 22 million South Koreans comprising 12 million families, we demonstrated significantly elevated familial risk of incident IBD among FDRs of probands with IBD, ranging from 10.2- fold to 22.2-fold overall. We additionally estimated familial risk estimates according to FDR relationship type, number of affected FDRs, IBD type, sex and age. These patterns demonstrate some similarities to those observed in Western population-based analyses6, but with an overall larger magnitude of familial risk and more pronounced effect, especially within generation (sibling) versus across generation (parent-offspring).
When compared to Western populations, among whom IBD is significantly more prevalent and has been established for multiple generations, some differences in familial risk estimates are to be expected since IBD is an emerging disease over far fewer generations among Asian-Pacific populations. Moller and colleagues published the largest population-based study of familial IBD risk among a Western population (N = 8.3 million persons)6. The authors reported an overall 7.8-fold higher risk of incident CD and 4.1-fold higher risk of UC among FDRs of probands with either CD or UC, respectively. The twin siblings of probands with CD or UC were at highest risk of incident CD and the risk of IBD if the affected FDR was a non-twin sibling, mother, or father was overall not different compared to the overall FDR risk estimates. By contrast, we reported significantly higher risk estimates within generation (i.e. siblings) compared to between generation (i.e. parent to offspring) for both UC and CD, although more pronounced for CD.
Of the 45,717 individuals diagnosed with UC and 17,848 with CD, a respective 3.8% and 3.1% represented familial cases among the South Korean population between 2002–2017. While the pattern of slight predominance of familial UC versus CD is similar to prior studies from the Asia-Pacific region16,17, including South Korea18, the magnitude is slightly higher. This could reflect key differences in our study design, including our 1) complete population coverage, 2) use of direct, as opposed to indirect, calculations, 3) adjusting analyses for age and sex, 4) confirmed high diagnostic accuracy for cases and familial relationships, and 5) the more contemporary time period.
The predominant driver of familial aggregation in IBD is generally accepted to be genetic susceptibility, compounded by shared environmental risk factors and gene-environment interactions or epigenetic modifications. Our study was not designed to determine the contribution of genetic vs. non-genetic determinants to familial IBD risk, and future well-designed dedicated investigations are needed to provide this clarity. That said, on the background of shared genetic susceptibility, differences in prevalence of environmental exposures vertically between generations might at least in part explain not only the differences in familial IBD risk within versus across generations observed in the South Korean population, and plausibly other Asian-Pacific populations, but also the differences in the familial IBD risk patterns compared to Western populations. As an illustrative example, diet and cultural practices are more commonly shared within families and might impact familial risk of IBD.
Our study has several key strengths in addition to those already mentioned. Because we had complete data on all blood-related FDRs by relationship type we were able to unmask important generational differences, which are not observed in Western populations. A few non-population-based studies describe the epidemiology of familial IBD in Asian-Pacific countries in a limited manner, but confidence in some estimates might be dampened given the variable level of scrutiny of IBD diagnoses with many relying on questionnaires or patient-reported family history of IBD.14,17–19. Limitations of the study included relatively shorter follow up period which may not be sufficient to capture all familial occurrences. While there was no minimum follow-up period, IBD is a chronic disease and affected individuals tend to have long health care utilization periods, which would be captured in the universal health care system. We notably accounted for IBD diagnoses prior to 2002 to extend the captured follow-up time and adjusting for calendar period did not adversely affect our study results. There was no minimum follow-up period, however if employed, the number of excluded cases would be relatively low compared to the total population so as to impact our study results.
In summary, using a population-based cohort analysis with complete coverage of the South Korean population, we demonstrated substantially increased risk of IBD among FDRs of affected individuals, with the highest risk estimates among siblings, especially for CD. Studies conducted among other populations, particularly other Asian-Pacific populations will be informative for better understanding patterns of IBD risk that are shared vs divergent between populations, which might provide clues for predisposing or risk attenuating determinants. In tandem, studies specifically designed to identify the etiologies for these observations, which may plausibly relate to shared environmental exposures on the background of shared genetic susceptibility, are direly needed in the face of the expanding global burden of IBD.
Supplementary Material
Acknowledgments
Grant and Funding Support: None.
Abbreviations used:
- IBD
Inflammatory bowel diseases
- UC
ulcerative colitis
- CD
Crohn’s disease
- FDR
first degree relatives
- NHI
national health insurance database
- ICD 10
international classification of disease 10th revision
- RID
rare intractable diseases
- IRR
incidence risk ratio
- CI
confidence interval
Footnotes
Writing Assistance: None.
Disclosures: All authors declare no conflict of interest
REFERENCES
- 1.Torres J, Mehandru S, Colombel F, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 2.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J-F. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeters M, Geypens B, Claus D, et al. Clustering of increased small intestinal permeability in families with Crohn’s disease. Gastroenterology 1997;113:802–7. [DOI] [PubMed] [Google Scholar]
- 4.Hedin CR, McCarthy NE, Louis P, et al. Altered intestinal microbiota and blood T cell phenotype are shared by patients with Crohn’s disease and their unaffected siblings. Gut 2014;63:1578–86. [DOI] [PubMed] [Google Scholar]
- 5.Török HP, Glas J, Hollay HC, et al. Serum antibodies in first-degree relatives of patients with IBD: a marker of disease susceptibility? A follow-up pilot-study after 7 years. Digestion 2005;72:119–23. [DOI] [PubMed] [Google Scholar]
- 6.Moller FT, Andersen V, Wohlfahrt J, Jess T. Familial risk of inflammatory bowel disease: a population-based cohort study 1977–2011. Am J Gastroenterol 2015;110:564–71. [DOI] [PubMed] [Google Scholar]
- 7.Orholm M, Fonager K, Sorensen HT. Risk of ulcerative colitis and Crohn’s disease among offspring of patients with chronic inflammatory bowel disease. Am J Gastroenterol 1999;94:3236–3238. [DOI] [PubMed] [Google Scholar]
- 8.Hemminki K, Li X, Sundquist K, Sundquist J. Familial association of inflammatory bowel diseases with other autoimmune and related diseases. Am J Gastroenterol 2010;105:139–147. [DOI] [PubMed] [Google Scholar]
- 9.Ng SC, Bernstein CN, Vatn MH, et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013;62:630–49. [DOI] [PubMed] [Google Scholar]
- 10.Ng SC, Tang W, Leong RW, et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut 2015;64:1063–71. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand H, Malmborg P, Askling J, Ekbom A, Montgomery SM. Early-life exposures associated with antibiotic use and risk of subsequent Crohn’s disease. Scand J Gastroenterol 2008;43:961–6. [DOI] [PubMed] [Google Scholar]
- 12.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015;12:205–17. [DOI] [PubMed] [Google Scholar]
- 13.Thompson NP, Montgomery SM, Wadsworth ME, Pounder RE, Wakefield AJ. Early determinants of inflammatory bowel disease: use of two national longitudinal birth cohorts. Eur J Gastroenterol Hepatol 2000;12:25–30. [DOI] [PubMed] [Google Scholar]
- 14.Cui G, Yuan A. A systematic review of epidemiology and risk factors associated with chinese inflammatory bowel disease. Front Med (Lausanne) 2018;5:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SH, & Lim DO (2019). Trends of Twin Birth in Korea, 1991–2018. Journal of Health Informatics and Statistics, 44(4), 427–431. doi: 10.21032/jhis.2019.44.4.427 [DOI] [Google Scholar]
- 16.Ishige T, Tomomasa T, Takebayashi T, et al. Inflammatory bowel disease in children: epidemiological analysis of the nationwide IBD registry in Japan. J Gastroenterol 2010;45:911–7. [DOI] [PubMed] [Google Scholar]
- 17.Childers RE, Eluri S, Vazquez C, Weise RM, Bayless TM, Hutfless S. Family history of inflammatory bowel disease among patients with ulcerative colitis: a systematic review and meta-analysis. J Crohns Colitis 2014;8:1480–97. [DOI] [PubMed] [Google Scholar]
- 18.Park JB, Yang S-K, Byeon J-S, et al. Familial occurrence of inflammatory bowel disease in Korea. Inflamm Bowel Dis 2006;12:1146–51. [DOI] [PubMed] [Google Scholar]
- 19.Chung SH, Park SJ, Lee HS, et al. Similar clinical characteristics of familial and sporadic inflammatory bowel disease in South Korea. World J Gastroenterol 2014;20:17120–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.