Abstract
Leptin is known to selectively suppress neural and taste cell responses to sweet compounds. The sweet suppressive effect of leptin is mediated by the leptin receptor Ob-Rb, and the ATP-gated K+ (KATP) channel expressed in some sweet-sensitive, taste receptor family 1 member 3 (T1R3)-positive taste cells. However, the intracellular transduction pathway connecting Ob-Rb to KATP channel remains unknown. Here we report that phosphoinositide 3-kinase (PI3K) mediates leptin’s suppression of sweet responses in T1R3-positive taste cells. In in situ taste cell recording, systemically administrated leptin suppressed taste cell responses to sucrose in T1R3-positive taste cells. Such leptin’s suppression of sucrose responses was impaired by co-administration of PI3K inhibitors (wortmannin or LY294002). In contrast, co-administration of signal transducer and activator of transcription 3 inhibitor (Stattic) or Src homology region 2 domain-containing phosphatase-2 inhibitor (SHP099) had no effect on leptin’s suppression of sucrose responses, although signal transducer and activator of transcription 3 and Src homology region 2 domain-containing phosphatase-2 were expressed in T1R3-positive taste cells. In peeled tongue epithelium, phosphatidylinositol (3,4,5)-trisphosphate production and phosphorylation of AKT by leptin were immunohistochemically detected in some T1R3-positive taste cells but not in glutamate decarboxylase 67-positive taste cells. Leptin-induced phosphatidylinositol (3,4,5)-trisphosphate production was suppressed by LY294002. Thus, leptin suppresses sweet responses of T1R3-positive taste cells by activation of Ob-Rb–PI3K–KATP channel pathway.
Keywords: energy homeostasis, leptin signaling, metabolic sensor, obesity, sweet receptor cell
1 |. INTRODUCTION
Leptin is an adipocyte-derived hormone that regulates food intake, energy homeostasis, and body weight (Friedman, 2004; Friedman & Halaas, 1998). Leptin exerts its function by activating the leptin receptor (Ob-R) expressed in the hypothalamus and other peripheral tissues. For example, leptin hyperpolarizes a subset of hypothalamic neurons (Irani et al., 2008; Mirshamsi et al., 2004; Spanswick et al., 1997; Williams et al., 2011) and suppresses insulin release from pancreatic β-cells (Harvey et al., 2000; Harvey, McKay, et al., 2000; Kieffer et al., 1997; Ning et al., 2009; Park et al., 2013). In line with such leptin’s functions, leptin modulates taste sensitivities. Mice homozygous for the db point mutation of the Lepr gene (db/db mice) showed increased gustatory neural responses to sweet compounds compared to wild-type (WT) mice (Ninomiya et al., 1995, 1998; Sako et al., 1996), suggesting a possibility that leptin suppresses sweet taste sensitivity. Indeed, exogenous administration of recombinant leptin selectively suppressed gustatory nerve responses to sweet compounds in WT mice (Kawai et al., 2000) and decreased sweet-induced Ca2+ responses of and transmitter secretions from isolated mouse taste buds (Meredith et al., 2015). On the other hand, some other studies reported different results; no or inverse effect of leptin on sweet responses in mice (Glendinning et al., 2015; Lu et al., 2012). Although the exogenous administration of leptin showed different results possibly because of different experimental conditions, the endogenous leptin may function to reduce the sweet taste sensitivity in healthy subjects. In mice, the administration of leptin antagonist increased sweet taste responses of WT mice (Niki et al., 2015). In humans, the polymorphisms of Lep and Lepr are associated with sweet preference (Mizuta et al., 2008). Plasma leptin levels may specifically affect sweet taste sensitivity in healthy adults (Nakamura et al., 2008), and a decrease in circulating leptin levels is associated with a decrease in sweet taste thresholds during weight loss in obese females (Umabiki et al., 2010). In addition, the association between salivary leptin concentration and sweet taste sensitivity is demonstrated in healthy adults (Han et al., 2017) and female children (Rodrigues et al., 2017). Thus, leptin would regulate sweet taste sensitivity in both mice and humans. Sweet taste is essential for the detection of calorie-rich carbohydrates, therefore, the regulation of sweet taste sensitivity by leptin may contribute to ingesting the proper amount of carbohydrate-derived calories in healthy subjects.
Some mechanistic aspects of leptin’s effect on sweet taste have been elucidated. The functional leptin receptor Ob-Rb is expressed in mouse taste cells (TCs) in fungiform (FP) and circumvallate papillae (Glendinning et al., 2015; Kawai et al., 2000; Martin et al., 2010; Shigemura et al., 2003). The majority of Ob-Rb-expressing TCs also expressed a sweet/umami receptor component taste receptor family 1 member 3 (T1R3), but not a Type I TC marker glutamate/aspartate transporter (GLAST) and a Type III TC marker glutamate decarboxylase 67 (GAD67) (Yoshida et al., 2015). In TC recordings with separate stimulation of the apical (taste-sensing) and basolateral (leptin-responding) faces, responses of T1R3-positive TCs to sweet compounds were significantly suppressed by administration of leptin, whereas leptin did not affect bitter responses of gustducin-positive TCs and sour responses of GAD67-positive TCs, suggesting that sweet-sensitive TCs are the targets for leptin (Yoshida et al., 2015). Leptin’s suppression of sweet responses of TCs was impaired by the administration of the KATP channel blocker glibenclamide, and mimicked by administration of the KATP opener diazoxide (Yoshida et al., 2015). Taken together, leptin may function to activate Ob-Rb and KATP channel expressed in T1R3-positive TCs. However, the intracellular transduction pathway connecting Ob-Rb to the KATP channel has not been elucidated. Therefore, in this study, we thought to determine how leptin affects sweet taste sensitivity of T1R3-positive TCs.
Binding of leptin to Ob-Rb activates the Janus kinase 2 (JAK2) (Baumann et al., 1996). Subsequently, multiple signal transduction pathways could be recruited (Zhou & Rui, 2013). Those include the signal transducer and activator of transcript 3 (STAT3) (White et al., 1997), the STAT5 (Gong et al., 2007), the Src homology region 2 domain of protein tyrosine phosphatase 2 (SHP2) (Bjørbaek et al., 2001) and the phosphoinositide 3-kinase (PI3K) (Niswender et al., 2001). We hypothesized that one or some of these transduction components may play a key role in leptin’s suppression of sweet responses of TCs.
Here, we examined the possible involvement of leptin signaling components in sweet suppressive effect of leptin on T1R3-positive TCs. The expression of leptin signaling components in T1R3-positive TCs were determined by single-cell reverse transcription (RT)-PCR. Then, we tested whether PI3K inhibitors (wortmannin and LY294002), STAT3 inhibitor (static) or SHP2 inhibitor (SHP099) diminished leptin’s effect on T1R3-positive TCs. In addition, we immunohistochemically examined the production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) and phosphorylation of AKT in T1R3-positive TCs in response to leptin stimulation.
2 |. METHODS
2.1 |. Animals
All experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the ARRIVE guidelines, and approved by the committee for Laboratory Animal Care and Use at Kyushu University (A29-081) and Okayama University, Japan (OKU-2018801). The experimental procedures were summarized in Figure 1. Subjects were adult (>8 weeks old) male and female transgenic mice expressing green fluorescent protein (GFP) under control of the T1R3 promoter (T1R3-GFP mice, n = 52 for taste cell recording, n = 3 for single-cell RT-PCR, n = 6 for immunostaining) (Damak et al., 2008) or the GAD67 promoter (GAD67-GFP mice, n = 3 for immunostaining) (Tamamaki et al., 2003; RRID:IMSR_APB:1601). All mice were housed under a 12:12-hr light-dark cycle (lights on 0800-2000h) and had ad libitum access to tap water and food pellets. The study was not pre-registered. No randomization was performed to allocate subjects. No blinding and no sample calculation were performed, but we used the sample sizes similar to previously published studies (n = 7–27 cells for recording, n = 3 mice for RT-PCR and immunohistochemistry: Kusuhara et al., 2013; Yoshida et al., 2015). No exclusion criteria were pre-determined.
FIGURE 1.
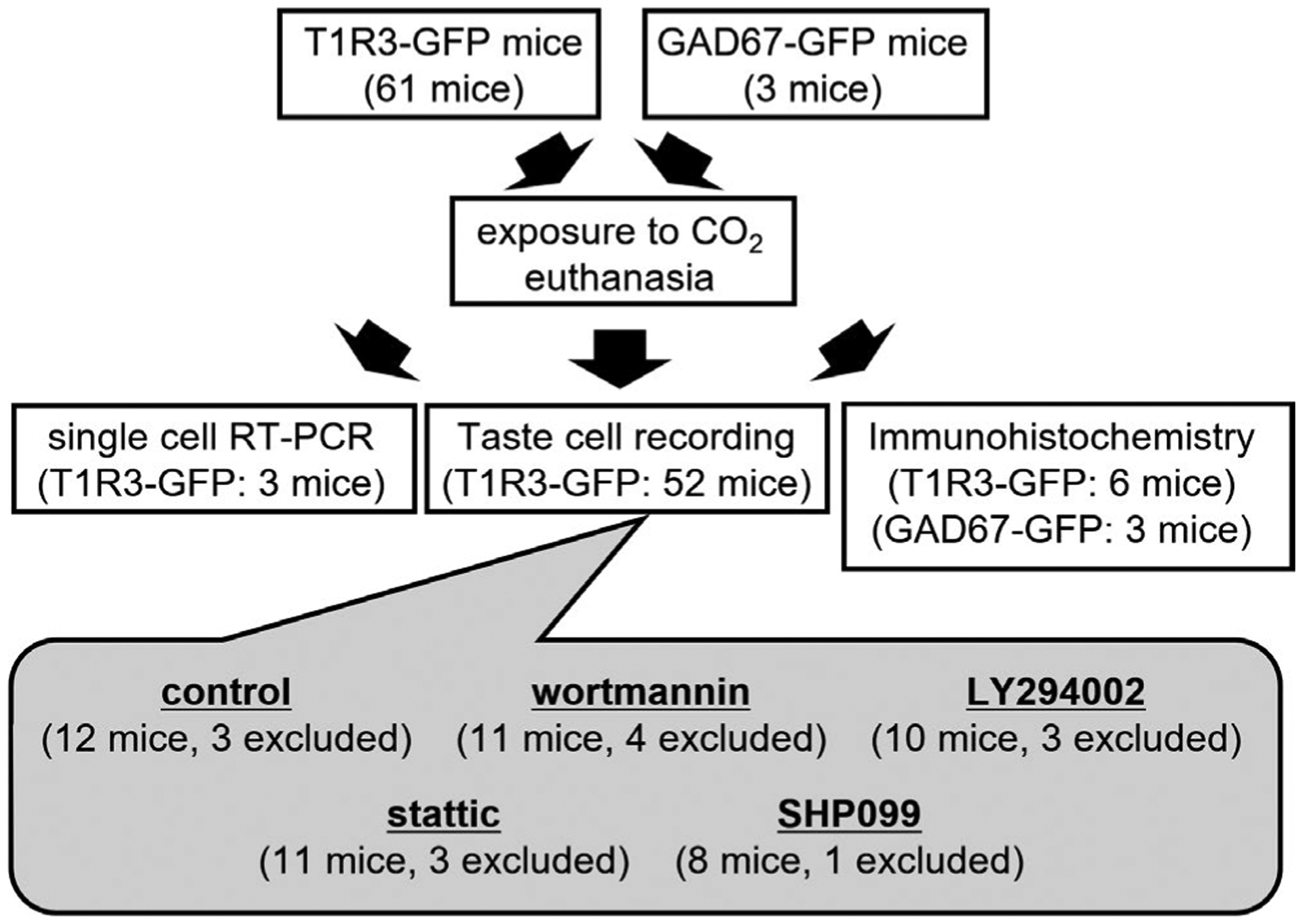
Experimental outline of animals used in this study. In total, 58 taste receptor family 1 member 3 (T1R3)-green fluorescent protein (GFP) mice and 3 glutamate decarboxylase 67 (GAD67)-GFP mice at >8 weeks of age were used for single-cell RT-PCR (3 T1R3-GFP mice), taste cell recording (52 T1R3-GFP mice) and immunohistochemistry (6 T1R3-GFP mice, 3 GAD67-GFP mice). Of 52 T1R3-GFP mice used for taste cell recording, data from 14 mice (14 cells) were excluded from analysis (3 cells/ 3 mice for control, 4 cells/ 4 mice for wortmannin, 3 cells/ 3 mice for LY294002, 3 cells / 3 mice for static, 1 cell / 1 mouse for SHP099)
2.2 |. Taste cell recordings
Recording procedures were as used previously (Yoshida, Horio, et al., 2009; Yoshida, Miyauchi, et al., 2009; Yoshida et al., 2015). Animals were sacrificed by exposure to CO2. The anterior tongue was removed and administrated with 100 μl of Tyrode solution containing 0.2–0.5 mg/ml elastase (Elastin Products, Cat# LE425) to peel the tongue epithelium. Individual taste buds of fungiform papillae with a piece of surrounding epithelium were excised from this sheet and the mucosal side containing a taste pore was drawn into the orifice of the stimulating pipette. To perfuse taste solutions and to hold the taste bud in place, a constant suction on the stimulating pipette was applied through the recording. Tyrode solution was continuously flowed into the recording chamber with a peristaltic pump at approximately 2 ml/min. The mucosal side was rinsed with distilled water at least 30 s before and after taste stimulation (15–20 s). GFP-positive TCs were identified by confocal laser scanning microscopy (FV-300; Olympus) and were approached by a recording electrode (pipette resistances 1.5–3.5 MΩ filled with Tyrode solution). Action potentials were recorded by a high-impedance patch-clamp amplifier (Axopatch 200B; Axon Instruments) interfaced to a computer by an analog-to-digital board (Digidata 1440A; Axon Instruments). Application of taste stimulus (500 mM sucrose) was restricted to the receptor membrane, while leptin and inhibitors was applied to the basolateral membrane of taste cells. To assess the effect of leptin, we recorded control response, leptin-affected response >2 min after leptin application and washout response >2 min after washout of leptin. Inhibitors (wortmannnin, LY294002, SHP099 or stattic) were applied to the basorateral membrane throughout the recordings.
2.3 |. Solutions
Tyrode solution contained (in mM): NaCl, 140; KCl, 5; CaCl2, 1; MgCl2, 1; NaHCO3, 5; HEPES, 10; Glucose, 10; sodium pyruvate, 10; pH adjusted to 7.4 with NaOH. Sucrose (500mM) was used as a sweet tastant. Recombinant murine leptin (20 ng/ml; PeproTech, Cat# 450-31), wortmannin (10 nM; Tocris Bioscience, Cat# 1,232), LY294002 (30 μM; Sigma-Aldrich, Cat# L9908), SHP099 (10 μM; Cayman Chemical, Cat# 20,000), and stattic (10 μM; Axon Medchem, Cat# 2,314) were applied from the basolateral side of TCs. Other chemicals were purchased from Wako Pure Chemical Industries or Nakarai Tesque. These concentrations of agents were selected as used in previous studies (Chen et al., 2016; Mirshamsi et al., 2004; Schust et al., 2006; Yoshida et al., 2015).
2.4 |. Single-cell RT-PCR
The protocol for the multiplex single-cell RT-PCR was as used previously described (Kusuhara et al., 2013; Yoshida, Horio, et al., 2009; Yoshida, Miyauchi, et al., 2009). Peeling of the tongue epithelium was done as described above. Peeled tongue epithelium was soaked in Ca2+, Mg2+ free solution (Ca2+ and Mg2+ in Tyrode solution were substituted by EDTA) for 5–10 min. Then, fungiform taste buds were isolated and transferred to the bottom of the culture dish containing Ca2+, Mg2+-free solution by aspiration with a transfer pipette (inner φ approximately 100 μm). Single GFP-positive TCs were identified by laser scanning microscopy, harvested by a thin glass pipette (inner φ 1–3 μm), and transferred to a PCR tube containing 1.5 μl ultra-pure distilled water (Invitrogen, Cat# 10,977,015) and 0.5 μl RNase inhibitor (Invitrogen, Cat# 10,777,019). Reverse transcription (RT) and first round PCR took place in the same tube using One Step RT-PCR kit (Qiagen, Cat# 210,212). A 50-μl reaction mixture contained 10 μl QIAGEN OneStep RT-PCR buffer (×5), 2 μl QIAGEN One Step RT-PCR enzyme mix, 0.4 mM of each dNTP, 1 μl RNase inhibitor, 0.2–0.6 mM of each outside primer (Table 1) and the sample. After the RT reaction at 50°C for 30 min, the first round PCR was subsequently performed with a 15 min pre-incubation at 95°C followed by 30 cycles (94°C for 30 s, 52°C for 60 s, 72°C for 90 s) in a thermal cycler (T-100; BioRad). Then, the first round PCR products were re-amplified for 35 cycles (94°C for 30 s, 60°C for 30 s, 72°C for 60 s) in separate reactions using the internal primer pairs (Table 1) for each template. Each 10 μl reaction mixture contained 0.25 Units of Taq DNA polymerase (TaKaRa Ex Taq HS: Takara, Cat# RR006A), 1 μl of 10 × PCR buffer containing 20 mM Mg2+, 0.2 mM of each dNTP, 0.5 mM of each primer pair, and 0.2 μl of the first round PCR products. After the second amplification, reaction solutions were subjected to 2% agarose gel electrophoresis. Positive (purified RNA from a taste bud) and negative control (without cell) reactions were run in parallel. β-actin was used as internal control. All primer sets were designed to span exon-intron boundaries to distinguish PCR products derived from genomic DNA and mRNA.
TABLE 1.
Nucleotide sequences of primers used in single-cell RT-PCR experiments
| Target | Accession no. | Forward | Reverse | Size (bp) |
|---|---|---|---|---|
| STAT3 | NM_011486 | TTCAAGCACCTGACCCTTAG | TGTCTAGCCAGACCCAGAAG | 478 |
| TGATCGTGACTGAGGAGCTG | CTTGGTGGTGGACGAGAACT | 263 | ||
| STAT5a | NM_001164062 | ACTACACCTTCTGGCAGTGG | GTTGGGTGGGTACTAGTTGT | 469 |
| GATGGAGGTGCTGAAGAAGC | CGGTCTGGGAACACGTAGAT | 288 | ||
| SHP2 | NM_001109992 | ACCTCTATGGTGGGGAGAAG | TTTCAGCAGCATTGATACGA | 486 |
| TCCATGGTCACTTGTCTGGA | AATGCTCCACCAGGTCTGTC | 252 | ||
| PI3Kr1 | NM_001024955 | AAGAAGCAGGCAGCTGAGTA | ACGAGGGAGGTGTGTTGATA | 464 |
| GGCAGAAGAAGCTGAACGAG | CCGGTGGCAGTCTTGTTAAT | 265 | ||
| GLAST | NM_148938 | GGTAAAATCGTGCAGGTCAC | CCACACCATTGTTCTCTTCC | 673 |
| ACATGTTCCCTCCCAATCTG | CAAGAAGAGGATGCCCAGAG | 362 | ||
| SNAP25 | NM_011428 | AAGGGATGGACCAAATCAAT | CAATGGGGGTGACTACTCTG | 601 |
| AAAAAGCCTGGGGCAATAAT | AGCATCTTTGTTGCACGTTG | 304 | ||
| T1R3 | NM_031872 | TGCCTGAATTTTCCCATTAT | AGGACACTGAGGCAGAAGAG | 889 |
| CTACCCTGGCAGCTCCTGGA | CAGGTGAAGTCATCTGGATGCTT | 343 | ||
| β-actin | NM_007393 | CCTGAAGTACCCCATTGAAC | GTAACAGTCCGCCTAGAAGC | 943 |
| GGTTCCGATGCCCTGAGGCTC | ACTTGCGGTGCACGATGGAGG | 370 |
Note: Upper: outside primers, Lower: inside primers.
Abbreviations: GLAST, glutamate/aspartate transporter; PI3K, phosphoinositide 3-kinase; SHP2, Src homology region 2 domain-containing phosphatase-2; STAT3, signal transducer and activator of transcription 3; T1R3, taste receptor family 1 member 3.
2.5 |. Immunohistochemistry
The procedures were modified from those used previously (Niki et al., 2015; Yoshida et al., 2015). Peeling of the tongue epithelium was done as described above. The peeled tongue epithelium was cut into thirds and each epithelium was pinned out in a Sylgard-coated culture dish. The peeled lingual epithelia were pretreated with Tyrode solution for 10 min, then treated with Tyrode solution (−Lep) or that containing 20 ng/ml leptin (+Lep) for 10 min at room temperature (20–25°C). For LY294002 treatment (+Lep + LY), 30 μM LY294002 was added to these solutions. Immediately after these treatments, the epithelia were fixed in phosphate buffer solution (PBS) with 4% paraformaldehyde. Subsequently, preparations were treated with 0.3% H2O2 + 0.5% saponin in PBS, 1% blocking reagent (Roche, Mannheim, Germany, Cat# 11,096,176,001), and the purified anti-PIP3 monoclonal antibody conjugated with horseradish peroxidase (1:100, Z-H345, RRID:AB_427220; Echelon Bioscience) or anti-phospho-AKT rabbit antibody (1:100, Cat# 4,060, RRID:AB_2315049; Cell Signaling Technology) in tris-buffered saline. After that, tissues were incubated with Amplibuffer containing tyramide-Alexa 647 substrate (TSA Kit; Molecular Probes, Cat# T20925) or donkey anti-rabbit IgG antibody conjugated with Alexa Fluor 568 (1:200, Cat# A10042, RRID:AB_2534017; Thermo Fisher Scientific). The fluorescent labeled TCs were observed with a laser scanning microscope (FV-1000 or FV-300; Olympus) and analyzed with FLUOVIEW software (Olympus).
2.6 |. Data analysis
Action potential waveform analyses were as described previously (Yoshida et al., 2006). The number of spikes per unit time was counted throughout the recording. The mean spontaneous impulse discharge was calculated by averaging the number of spikes over the 10-s period in which distilled water flowed over the taste pore prior to each stimulation. Response magnitude was obtained by counting the total number of impulses for the first 10 s after taste stimulation and subtracting the spontaneous impulse discharge. Data were excluded if the spontaneous impulse discharge rate after washout of agents (i.e., at the end of experiment) was significantly different from that before treatment of agents (i.e., at the start of experiment). Potential baseline differences indicative of washout were noted by comparing six 10-s-periods by t-test for baseline levels. Data were also excluded if taste responses after washout did not recover to ≥70% of the control response. In total, 14 cell/14 mice were excluded from data analysis (3 cells/3 mice for control, 4 cells/4 mice for wortmannin, 3 cells/3 mice for LY294002, 3 cells/3 mice for stattic, 1 cell/1 mouse for SHP099). These samples were replaced with additional samples until sample size exceed at least seven in each condition. The data were not assessed for normality. No test for outliers was conducted.
Statistically significant differences were evaluated by one-way repeated measures analysis of variance (ANOVA) followed by post hoc t test with Holm correction or one-way ANOVA followed by post hoc Tukey HSD test. Statistical calculations were performed using the EZR program on Windows 10 (Kanda, 2013), and differences were considered significant at values of p < .05.
3 |. RESULTS
3.1 |. Expression of leptin signaling components in T1R3-positive TCs
T1R3 is a sweet receptor component and T1R3-positive TCs respond to multiple sweeteners and have leptin sensitivity (Yoshida et al., 2015). It is known that various molecules are involved in leptin signaling. Leptin binds to Ob-Rb and activates the JAK2/STAT3, JAK2/STAT5, PI3K/AKT, and SHP2/extracellular signal-regulated kinase (ERK) pathways (Zhou & Rui, 2013). To investigate which signaling pathway is involved in sweet suppressive effect of leptin in TCs, we first thought to determine the expression of leptin signaling components in T1R3-positive TCs by single-cell RT-PCR.
Among 20 T1R3-positive TCs confirmed the expression of T1R3 but not type I TC marker GLAST and type III TC marker SNAP25, 19 (95%) cells expressed PI3K regulatory subunit 1 (Figure 2, Table 2). STAT3 and SHP2 were expressed in 18 (90%) and 7 (35%) cells, respectively, whereas only 2 (10%) cells expressed STAT5a. Although we did not exclude a possibility of false-negative results for STAT5a, we focused on the role of PI3K, STAT3 and SHP2 on leptin’s suppression of sweet responses.
FIGURE 2.
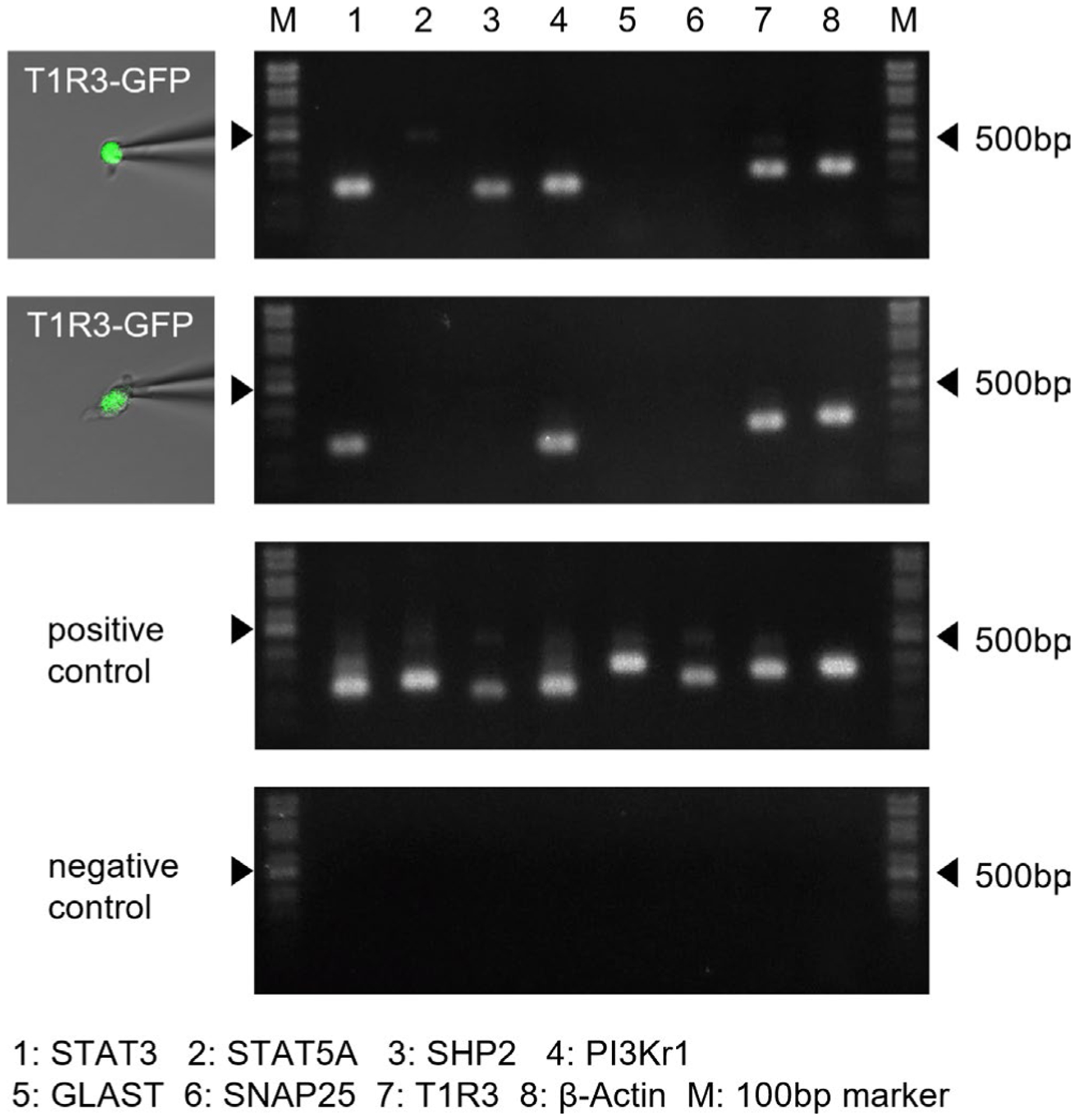
Gene expression analysis of taste receptor family 1 member 3 (T1R3)-positive taste cells (TCs) by single-cell RT-PCR. Examples of single-cell RT-PCR from typical profiled cells. After harvesting single T1R3-green fluorescent protein (GFP) TCs (left pictures), gene expression (signal transducer and activator of transcription 3 [STAT3], STAT5a, Src homology region 2 domain-containing phosphatase-2 [SHP2], phosphoinositide 3-kinase [PI3Kr1], glutamate/aspartate transporter [GLAST], SNAP25, T1R3, β-actin) was analyzed by multiplex single-cell RT-PCR (right pictures showing second PCR reaction products). Positive and negative control reactions were always run in parallel with reactions with samples. Arrowheads indicate marker bands of 500 bp. Summarized data are shown in Table 2
TABLE 2.
Gene expression analysis of T1R3-positiove taste cells by single-cell RT-PCR
| Cell | STAT3 | STAT5a | SHP2 | PI3Kr1 | GLAST | SNAP25 | T1R3 | β- actin |
|---|---|---|---|---|---|---|---|---|
| FP01 | + | + | + | + | − | − | + | + |
| FP02 | + | + | + | + | − | − | + | + |
| FP03 | + | − | + | + | − | − | + | + |
| FP04 | + | − | + | + | − | − | + | + |
| FP05 | + | − | + | + | − | − | + | + |
| FP06 | + | − | + | − | − | − | + | + |
| FP07 | − | − | + | + | − | − | + | + |
| FP08 | + | − | − | + | − | − | + | + |
| FP09 | + | − | − | + | − | − | + | + |
| FP10 | + | − | − | + | − | − | + | + |
| FP11 | + | − | − | + | − | − | + | + |
| FP12 | + | − | − | + | − | − | + | + |
| FP13 | + | − | − | + | − | − | + | + |
| FP14 | + | − | − | + | − | − | + | + |
| FP15 | + | − | − | + | − | − | + | + |
| FP16 | + | − | − | + | − | − | + | + |
| FP17 | + | − | − | + | − | − | + | + |
| FP18 | + | − | − | + | − | − | + | + |
| FP19 | + | − | − | + | − | − | + | + |
| FP20 | − | − | − | + | − | − | + | + |
| Total | 18/20 | 2/20 | 7/20 | 19/20 | 0/20 | 0/20 | 20/20 | 20/20 |
Note: +: positive, −: negative.
Abbreviations: GLAST, glutamate/aspartate transporter; PI3K, phosphoinositide 3-kinase; SHP2, Src homology region 2 domain-containing phosphatase-2; STAT3, signal transducer and activator of transcription 3; T1R3, taste receptor family 1 member 3.
3.2 |. Leptin suppresses sucrose responses of T1R3-positive TCs
To confirm the leptin’s effect on sweet responses, we observed sucrose responses of individual T1R3-GFP TCs before, during, and after bath application of 20 ng/ml leptin (Figure 3a–c). Sucrose responses in some T1R3-GFP TCs were decreased >2 min after application of leptin and recovered to the control level >2 min after washout of leptin (Figure 3a,b). Leptin’s suppression of responses of T1R3-GFP TCs to sucrose was statistically significant (Figure 3c), indicating that leptin suppressed taste responses of sweet-sensitive TCs.
FIGURE 3.
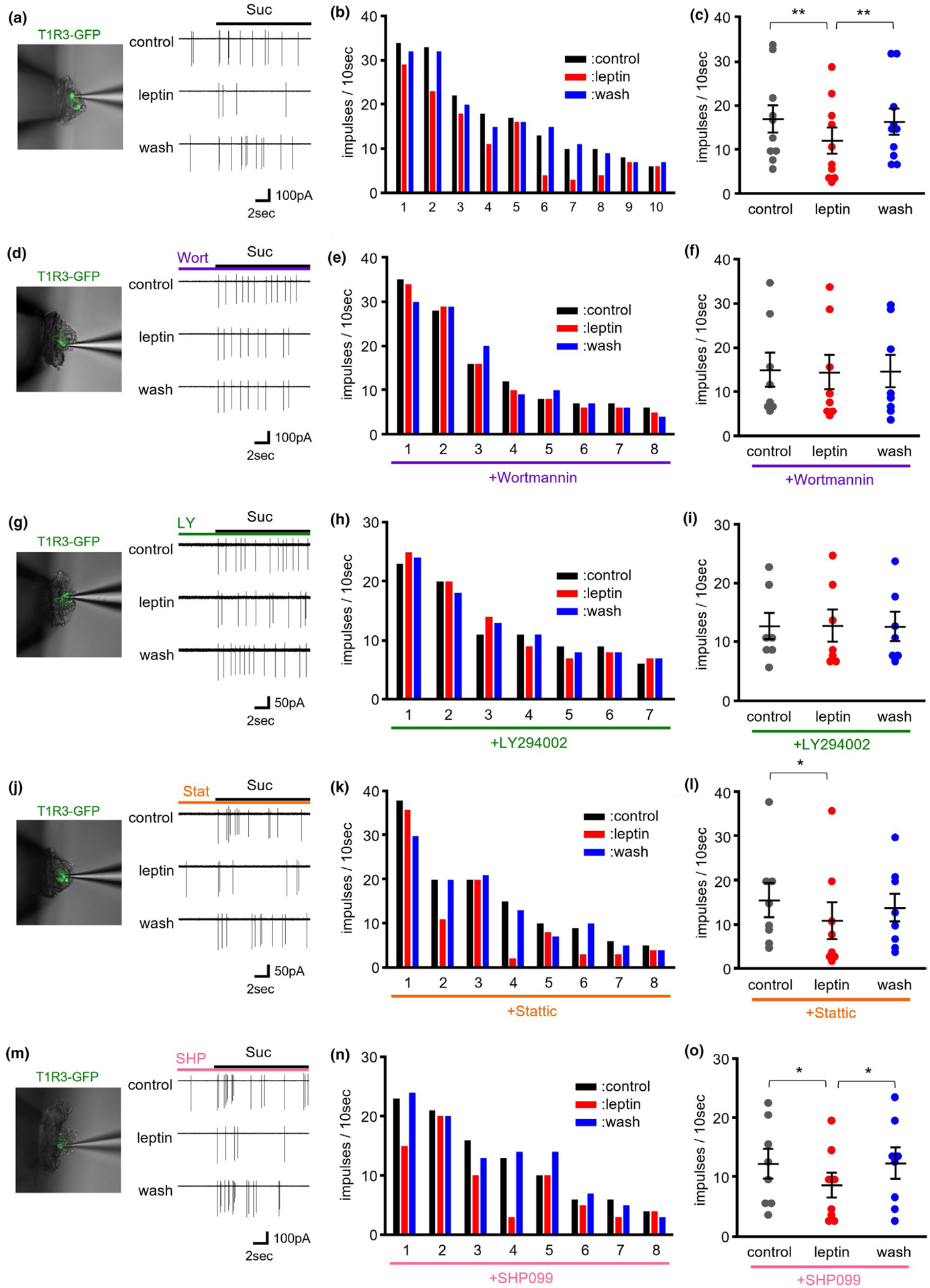
Effect of bath applied leptin on sucrose responses of taste receptor family 1 member 3 (T1R3)-green fluorescent protein (GFP) taste cells (TCs) from mouse fungiform papillae. (a) Sample recordings of TC responses to 500 mM sucrose (Suc) before (cont), during (leptin) and after (wash) treatment with 20 ng/ml leptin. The picture shows a T1R3-GFP TC from which taste responses were recorded. (b). Responses of 10 individual T1R3-GFP TCs to 500 mM sucrose before (left), during (middle) and after (right) treatment of 20 ng/ml leptin. (c) Effects of leptin on summated responses of T1R3-GFP TCs to 500 mM sucrose (n = 10 cells/ 9 mice, F = 14.869, p < .001, repeated ANOVA). Values are means ± SE. (d–i) Phosphoinositide 3-kinase (PI3K) inhibitors suppressed leptin’s suppression of TC responses to sucrose. (d, g) Sample recordings of TC responses to 500 mM sucrose (Suc) before (cont), during (leptin) and after (wash) treatment with 20 ng/ml leptin. PI3K inhibitor wortmannin (30 nM, d) or LY294002 (30 μM, g) was added to bath solution throughout the experiment. The picture shows a T1R3-GFP TC from which taste responses were recorded. (e, h) Responses of 8 and 7 individual T1R3-GFP TCs to 500 mM sucrose before (left), during (middle) and after (right) treatment of 20 ng/ml leptin under the existence of 30 nM wortmannin (e) or 30 μM LY294002 (h). (f, i) The effects of leptin on summated responses of T1R3-GFP TCs to 500 mM sucrose under the existence of 30 nM wortmannin (n = 8 cells/ 7 mice, F = 0.3577, p > .1, repeated ANOVA, f) or 30 μM LY294002 (n = 7 cells/ 7 mice, F = 0.0375, p > .1, repeated ANOVA, i). Values are means ± SE. (j–o) Signal transducer and activator of transcription 3 (STAT3) inhibitor and Src homology region 2 domain-containing phosphatase-2 (SHP2) inhibitor did not affect leptin’s suppression of TC responses to sucrose. (j, m) Sample recordings of TC responses to 500 mM sucrose (Suc) before (cont), during (leptin) and after (wash) treatment with 20 ng/ml leptin. STAT3 inhibitor stattic (10 μM, i) or SHP2 inhibitor SHP099 (10 μM, m) was added to bath solution throughout the experiment. The picture shows a T1R3-GFP TC from which taste responses were recorded. (k, n) Responses of 8 individual T1R3-GFP TCs to 500 mM sucrose before (left), during (middle) and after (right) treatment of 20 ng/ml leptin under the existence of 10 μM stattic (k) or 10 μM SHP099 (n). (l, o) Effects of leptin on summated responses of T1R3-GFP TCs to 500 mM sucrose under the existence of 10 μM stattic (n = 8 cells/ 8 mice, F = 4.0694, p < .05, repeated ANOVA, l) or 10 μM SHP099 (n = 8 cells/ 7 mice, F = 5.8569, p < .05, repeated ANOVA, o). Values are means ± SE. **p < .01, *p < .05, post hoc paired t-test with Holm correction
3.3 |. Effect of pharmacological blockers of leptin signaling in T1R3-GFP TCs
Next, we investigated the effect of pharmacological blockers of these signaling components on leptin signaling in T1R3-positive TCs. We tested PI3K inhibitors wortmannin and LY294002 (Mirshamsi et al., 2004), STAT3 inhibitor stattic (Schust et al., 2006) and SHP2 inhibitor SHP099 (Chen et al., 2016).
First, we examined the effect of PI3K inhibitors wortmannin (Figure 3d–f) and LY294002 (Figure 3g–i) on leptin’s suppression of responses of T1R3-GFP TCs to sucrose. Under the existence of 10 nM wortmannin, sucrose responses of individual T1R3-GFP TCs were not suppressed by bath application of 20 ng/ml leptin (Figure 3d,e). In total, leptin’s suppression of sucrose responses was blocked significantly by the addition of 10 nM wortmannin (Figure 3f). Similarly, suppression of sucrose responses of T1R3-GFP TCs by leptin was impaired significantly by another PI3K inhibitor LY294002 (30 μM, Figure 3g–i). These results suggest that leptin’s suppression of sweet responses in T1R3-positive TCs is mediated by PI3K.
Next, we tested the effect of other inhibitors on leptin’s suppression of sweet responses. In case of the STAT3 inhibitor stattic (10 μM), some T1R3-GFP TCs showed leptin’s suppression of sucrose responses under the existence of stattic (Figure 3j,k). Leptin significantly suppressed sucrose responses of T1R3-GFP TCs under the existence of 10 μM stattic (Figure 3l), suggesting that STAT3 is not involved in leptin’s effect on sweet responses. Similarly, treatment of SHP2 inhibitor SHP099 (10 μM) did not impair leptin’s suppression of sucrose responses in some T1R3-GFP TCs (Figure 3m,n). Leptin significantly suppressed sucrose responses of T1R3-GFP TCs under the existence of 10 μM SHP099 (Figure 3o), suggesting that SHP2 also do not contribute to leptin’s effect on sweet responses.
3.4 |. Leptin-induced PIP3 production and phosphorylation of AKT in TCs
Activation of PI3K induces PIP3 production (Whitman et al., 1988). If leptin activates PI3K in sweet-sensitive TCs, PIP3 may be produced in these cells in response to leptin stimulation. To test this hypothesis, we immunohistochemically determined the PIP3 production in TCs (Plum et al., 2006) (Figure 4).
FIGURE 4.
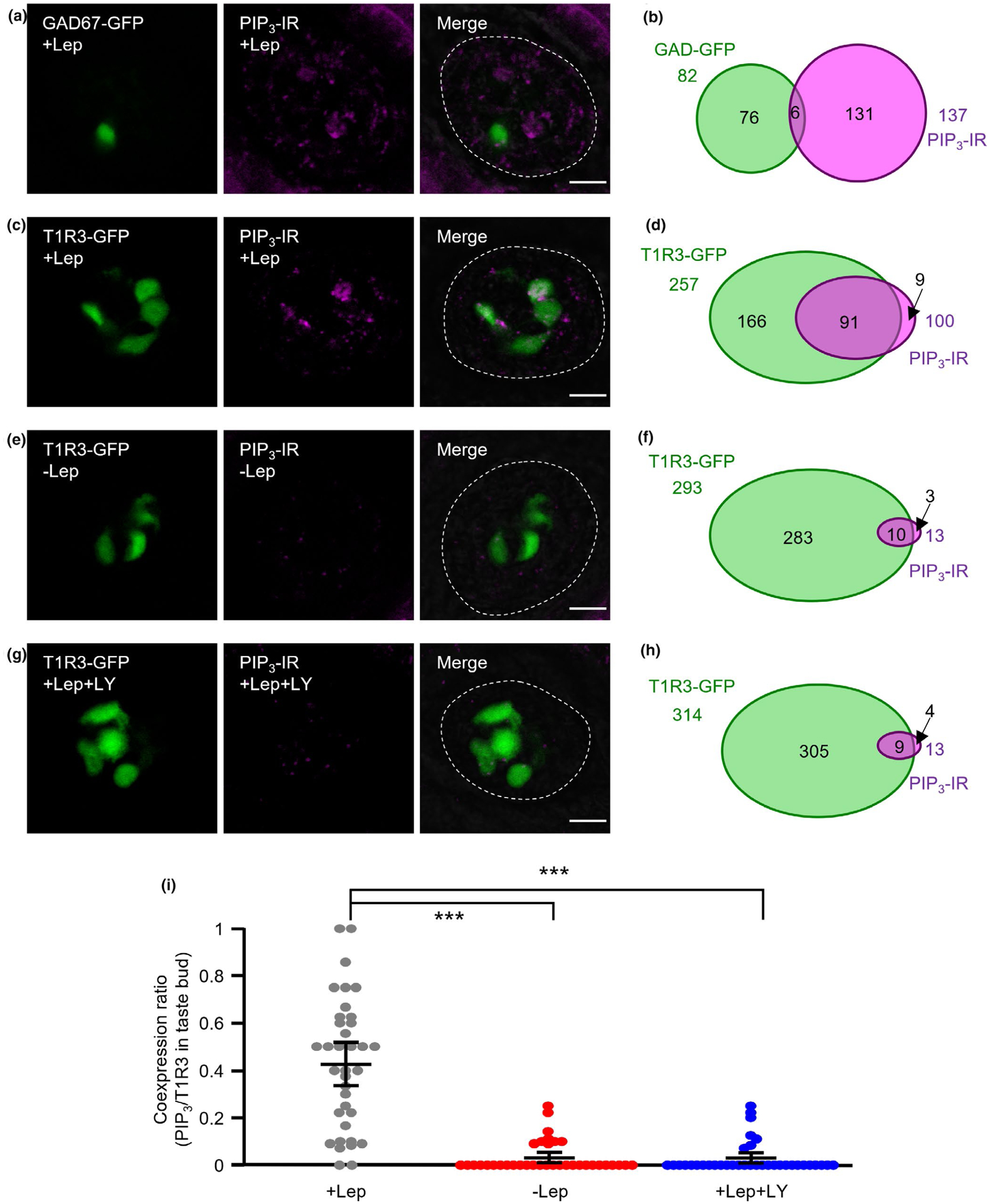
Production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) in taste cells (TCs) by leptin treatment. (a, b) Immunostaining of PIP3 in fungiform taste buds of glutamate decarboxylase 67 (GAD67)-green fluorescent protein (GFP) mice after treatment with 20 ng/ml leptin (+Lep). GAD67-GFP and immunostaining of PIP3 are shown in green and magenta, respectively (a). Schematic diagram of the pattern of coexpression of GAD67-GFP and PIP3 in mouse fungiform TCs (50 taste buds/ 3 mice, b). (c, d) Immunostaining of PIP3 in fungiform taste buds of taste receptor family 1 member 3 (T1R3)-GFP mice after treatment with 20 ng/ml leptin (+Lep). T1R3-GFP and immunostaining of PIP3 are shown in green and magenta, respectively (c). Schematic diagram of the pattern of coexpression of T1R3-GFP and PIP3 in mouse fungiform TCs (36 taste buds/ 3 mice, d). (e, f) Immunostaining of PIP3 in fungiform taste buds of T1R3-GFP mice without leptin treatment (−Lep). T1R3-GFP and immunostaining of PIP3 are shown in green and magenta, respectively (e). Schematic diagram of the pattern of coexpression of T1R3-GFP and PIP3 in mouse fungiform TCs (36 taste buds/3 mice, f). (g, h) Immunostaining of PIP3 in fungiform taste buds of T1R3-GFP mice after treatment with 20 ng/ml leptin + 30 μM LY294002 (+Lep + LY). T1R3-GFP and immunostaining of PIP3 are shown in green and magenta, respectively (g). Schematic diagram of the pattern of coexpression of T1R3-GFP and PIP3 in mouse fungiform TCs (36 taste buds/3 mice, h). Dotted lines indicate the outline of taste buds. Scale bars = 10 μm. i. Coexpression ratio of T1R3 and PIP3 in taste buds (36 taste buds/3 mice, F = 66.4, p < .001). Values are means ± 95% CI. ***p < .001, post hoc Tukey HSD test
In pealed epithelium of the mouse tongue, some taste bud cells showed immunoreactivity for PIP3 after the treatment of 20 ng/ml leptin (Figure 4a,c, magenta). The majority of these PIP3 immunopositive TCs coexpressed with T1R3-GFP (91/100, 91%) but not GAD67-GFP (6/137, 4%), which is expressed in Type III TCs (Figure 4a–d). About 35% (91/257) of T1R3-GFP TCs showed immunoreactivity for PIP3. This proportion is similar to previous data on leptin-sensitive T1R3-GFP TCs and LepRb-expressing T1R3-GFP TCs (about 30~40% of T1R3-GFP TCs) (Yoshida et al., 2015). Only a small number of taste bud cells showed PIP3 immunoreactivity if the tongue epithelium was not stimulated with leptin (Figure 4e,f). If PI3K inhibitor LY294002 (30 μM) was co-treated with leptin, the number of PIP3 positive TCs was almost similar to those without leptin stimulation (Figure 4g,h). Statistical analysis by ANOVA and post hoc Tukey HSD test demonstrated that the coexpression ratio of T1R3 and PIP3 immunoreactivity in taste buds was significantly greater in the + Lep condition than in −Lep and +Lep+LY conditions (Figure 4i). These results indicate that PI3K mediates leptin-induced PIP3 production in TCs.
Published RNA-seq data (SRP094673, Sukumaran et al., 2017) shows that AKT is abundantly and ubiquitously expressed in taste bud cells, including T1R3-GFP taste cells. We also examined leptin-induced phosphorylation of AKT by immunohistochemistry (Figure 5). After leptin treatment, about 35% of T1R3-GFP TCs showed immunoreactivity for phospho-AKT (pAKT) (Figure 5a,b). A small number of taste bud cells showed pAKT immunoreactivity if the tongue epithelium was not stimulated with leptin (Figure 5c,d) or co-treated with 30 μM LY294002 (Figure 5e,f). Statistical analysis by ANOVA and post hoc Tukey HSD test demonstrated that the coexpression ratio of T1R3 and pAKT in taste buds was significantly greater in the + Lep condition than in −Lep and +Lep+LY conditions (Figure 5g). These results indicate that leptin induces activation of PI3K/AKT pathway in T1R3-positive TCs.
FIGURE 5.
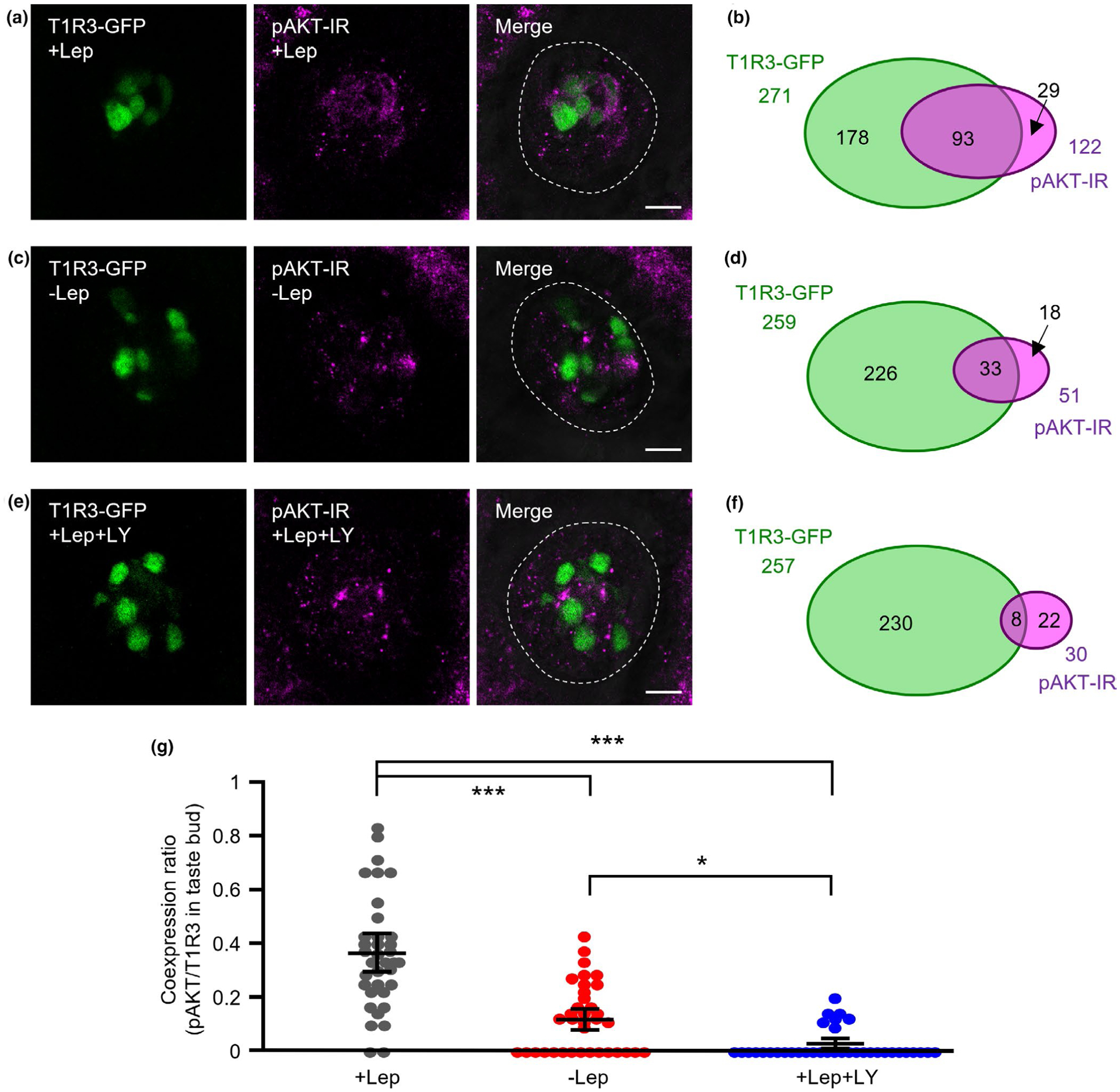
Phosphorylation of AKT in taste cells (TCs) by leptin treatment. (a, b) Immunostaining of phospho-AKT (pAKT) in fungiform taste buds of taste receptor family 1 member 3 (T1R3)-green fluorescent protein (GFP) mice after treatment with 20 ng/ml leptin (+Lep). T1R3-GFP and immunostaining of pAKT are shown in green and magenta, respectively (a). Schematic diagram of the pattern of coexpression of T1R3-GFP and pAKT in mouse fungiform TCs (36 taste buds/ 3 mice, b). (c, d) Immunostaining of pAKT in fungiform taste buds of T1R3-GFP mice without leptin treatment (−Lep). T1R3-GFP and immunostaining of pAKT are shown in green and magenta, respectively (c). Schematic diagram of the pattern of coexpression of T1R3-GFP and pAKT in mouse fungiform TCs (36 taste buds/ 3 mice, d). (e, f) Immunostaining of pAKT in fungiform taste buds of T1R3-GFP mice after treatment with 20 ng/ml leptin + 30 μM LY294002 (+Lep + LY). T1R3-GFP and immunostaining of pAKT are shown in green and magenta, respectively (e). Schematic diagram of the pattern of coexpression of T1R3-GFP and pAKT in mouse fungiform TCs (36 taste buds/ 3 mice, f). Dotted lines indicate the outline of taste buds. Scale bars = 10 μm. (g) Coexpression ratio of T1R3 and pAKT in taste buds (36 taste buds/3 mice, F = 52.8, p < .001). Values are means ± 95% CI. ***p < .001, *p < .05, post hoc Tukey HSD test
4 |. DISCUSSION
Our results suggest that PI3K is likely to be involved in leptin signaling in sweet-sensitive, T1R3-positive TCs. The contribution of PI3K to leptin signaling has been demonstrated in previous studies of hypothalamic neurons. Leptin increased the activity of KATP channels of isolated arcuate neurons in a PI3K dependent manner (Mirshamsi et al., 2004). In hypothalamic neurons located within the ventral premammillary nucleus, leptin-induced depolarization and hyperpolarization were blocked by PI3K inhibitors (Williams et al., 2011). The hyperpolarization of these neurons required the activation of a putative KATP channel. Thus leptin’s suppression of neural activities is mediated by PI3K and the KATP channel and similar mechanisms may be recruited in T1R3-positive TCs. Leptin-stimulated PI3K activation leads to production of PIP3 and phosphorylation of AKT in T1R3-positive TCs (Figures 4,5). This increase in the PIP3 level may be an important step for activation of the KATP channel. In general, the activity of the KATP channel is regulated by metabolically generated adenosine triphosphate (ATP) and adenosine diphosphate (McTaggart et al., 2010). But some phospholipids also control the activity of the KATP channel. Previous in vitro study demonstrated that PIP3 activates KATP channels directly (MacGregor et al., 2002). Therefore, leptin-induced PIP3 production in T1R3-positive TCs may lead to direct activation of KATP channels, which reduces electrical activity and responsiveness of TCs.
Another possible pathway for the PI3K-mediated activation of the KATP channel by leptin stimulation may involve actin remodeling. Leptin hyperpolarizes rat CRI-G1 insulin-secreting cells by activation of KATP channels, which is dependent upon PI3K activity and reorganization of the actin cytoskeleton (Harvey, Hardy, et al., 2000; Harvey, McKay, et al., 2000). Leptin suppresses insulin secretion by activation of KATP channels in pancreatic β-cells (Kieffer et al., 1997). This effect is mediated by the phosphatase and tensin homolog (Ning et al., 2009). Leptin inhibits phosphatase and tensin homolog by increasing PI3K-mediated phosphorylation of the phosphatase, leading to an increase in PIP3 and actin reorganization. Leptin also induces AMP-activated protein kinase-dependent KATP channel trafficking, which is a key mechanism for regulating β-cell membrane potentials (Park et al., 2013). In this case, PI3K linked Ob-Rb to AMP-activated protein kinase activation. Actin reorganization mediated by leptin-induced PI3K activation is also reported in hypothalamic cell line GT1–7 (Mirshamsi et al., 2004). Thus, actin reorganization may also contribute to leptin-induced activation of the KATP channel in T1R3-positive TCs.
We demonstrated that STAT3 inhibitor stattic did not affect leptin’s suppression of sweet responses of T1R3-positive TCs (Figure 3j–l), suggesting that STAT3 does not contribute to leptin’s suppression of sweet responses in T1R3-positive TCs. The expression of STAT3 in taste tissues was demonstrated by RT-PCR and in situ hybridization (Shigemura et al., 2003). We also demonstrated STAT3 expression in T1R3-positive TCs by single-cell RT-PCR (Figure 2, Table 2). However, a previous study demonstrated that stimulation of leptin did not induce phosphorylated STAT3 immunoreactivity in taste tissues (Glendinning et al., 2015), indicating that leptin may not phosphorylate STAT3 in T1R3-positive TCs. STAT3 is a transcription factor, which translocates to the nucleus after phosphorylation, and then regulates gene expressions. The suppressor of cytokine signaling-3 is one of the targets of STAT3, which serves as a negative regulator of leptin signaling (Banks et al., 2000). STAT3 plays an important role in the control of feeding and body weight as a component of leptin signaling, because the disruption of the STAT3 binding site in Ob-Rb results in hyperphagia and obesity to a similar degree as that in db/db mice (Bates et al., 2003; Jiang et al., 2008) and selective deletion of STAT3 in Ob-Rb expressing neurons leads to profound obesity in mice (Piper et al., 2008). These functions of STAT3 may be exerted by the regulation of gene expression. Leptin’s suppression of sweet responses in TCs occurs within several minutes by activation of the KATP channel. Therefore, the time course of STAT3 function of gene expression may not fit with such acute effect of leptin in TCs even if it would be phosphorylated by activation of Ob-Rb. Thus STAT3 may not be involved in leptin-stimulated KATP channel activation in T1R3-positive TCs.
We also demonstrated that SHP2 inhibitor SHP099 had no effect on leptin’s suppression of sweet responses of T1R3-positive TCs (Figure 3m–o). SHP2 is a SH2-containing tyrosine-specific protein phosphatase, which mediates leptin-stimulated activation of the ERK pathway (Bjørbaek et al., 2001). Leptin-induced ERK phosphorylation in the hypothalamus was decreased in the neuron-specific SHP2-KO mice compared with the control mice (Zhang et al., 2004). In addition, the pharmacological inhibition of the ERK pathway attenuated leptin’s effect of suppression on food intake (Rahmouni et al., 2009). These previous studies indicate that SHP2-ERK pathway is involved in leptin signaling. Regarding the relationship between ERK and the KATP channel, the activation of KATP channels by ERK has demonstrated in single channel recordings of Kir6.2/SUR1 expressed in HEK cells (Lin & Chai, 2008), suggesting that leptin-induced SHP2/ERK activity could lead to activation of KATP channel. However, our results do not support this possibility. The ERK is involved in various signaling pathways and its targets are diverse including transcription factors, RNA-binding proteins and signaling proteins (Ünal et al., 2017). Leptin-stimulated SHP2/ERK pathway may possibly contribute to activation or inhibition of signaling components other than the KATP channel in T1R3-positive TCs.
In conclusion, we demonstrated that PI3K is a key component for leptin signaling in T1R3-positive TCs. Leptin suppresses sweet taste responses of T1R3-positive TCs via Ob-Rb – PI3K – KATP channel axis. Similar mechanism may function in various organs including the hypothalamus (Irani et al., 2008; Mirshamsi et al., 2004; Spanswick et al., 1997; Williams et al., 2011), pancreatic β-cells (Harvey, Hardy, et al., 2000; Harvey, McKay, et al., 2000; Kieffer et al., 1997; Ning et al., 2009; Park et al., 2013) and enteroendocrine cells (Jyotaki et al., 2016), all of which play important roles in maintaining central and peripheral homeostasis of energy balance. The existence of common signaling pathway may imply co-evolved complementary strategies to modulate behavior. Disruption or inhibition of this pathway in TCs may lead to the impairment of leptin’s effect on sweet sensitivity, which was shown in diet-induced obesity mice and db/db mice (Niki et al., 2015; Yoshida et al., 2015). Dysfunction of leptin in sweet-sensitive TCs would alter food intake and glucose homeostasis in animals and humans, which may lead to the progression of obesity. Pharmacological regulation of these components present in TCs may be one of the potions to maintain normal food intake and glucose homeostasis under the state of leptin dysfunction, such as leptin resistance.
ACKNOWLEDGEMENTS
The authors thank Dr. Yuchio Yanagawa (Gunma University, Gunma, Japan) for providing GAD67-GFP mice. This work was supported by JSPS KAKENHI JP18K09507 (R.Y.), JP18H02968, JP18K19653 (Y.N.) for Scientific Research from Japan Society for the Promotion of Science and Grant from the Naito Foundation, 2018 (Y.N.).
Funding information
Japan Society for the Promotion of Science, Grant/Award Number: JP18K09507, JP18H02968 and JP18K19653; Naito Foundation
Abbreviations:
- ADP
adenosine diphosphate
- AMPK
AMP-activated protein kinase
- ATP
adenosine triphosphate
- CV
circumvallate papillae
- DW
distilled water
- ERK
extracellular signal-regulated kinase
- FP
fungiform papillae
- GAD67
glutamate decarboxylase 67
- GFP
green fluorescent protein
- GLAST
glutamate /aspartate transporter
- JAK2
Janus kinase 2
- KATP channel
ATP gated K+ channel
- Ob-R
leptin receptor
- PI3K
phosphoinositide 3-kinase
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate
- PTEN
phosphatase and tensin homolog
- RT
reverse transcription
- SHP2
Src homology region 2 domain-containing phosphatase-2
- SOCS3
suppressor of cytokine signaling-3
- STAT3 (or 5)
signal transducer and activator of transcription 3 (or 5)
- T1R3
taste receptor family 1 member 3
- TC
taste cell
- WT
wild-type
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interests.
DATA AVAILABILITY STATEMENT
All data supporting the findings of this study are available from the corresponding author upon request. Custom-made materials will be shared upon reasonable request.
REFERENCES
- Banks AS, Davis SM, Bates SH, & Myers MG Jr. (2000). Activation of downstream signals by the long form of the leptin receptor. Journal of Biological Chemistry, 275, 14563–14572. 10.1074/jbc.275.19.14563 [DOI] [PubMed] [Google Scholar]
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, & Tartaglia LA (1996). The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proceedings of the National Academy of Sciences of the United States of America, 93(16), 8374–8378. 10.1073/pnas.93.16.8374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AWK, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, & Myers MG Jr. (2003). STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature, 421, 856–859. 10.1038/nature01388 [DOI] [PubMed] [Google Scholar]
- Bjørbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG Jr.& Flier JS (2001). Divergent roles of SHP-2 in ERK activation by leptin receptors. Journal of Biological Chemistry, 276, 4747–4755. 10.1074/jbc.M007439200 [DOI] [PubMed] [Google Scholar]
- Chen YN, LaMarche MJ, Chan HM, Fekkes P, Garcia-Fortanet J, Acker MG, Antonakos B, Chen CH, Chen Z, Cooke VG, Dobson JR, Deng Z, Fei F, Firestone B, Fodor M, Fridrich C, Gao H, Grunenfelder D, Hao HX, … Fortin PD (2016). Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature, 535, 148–152. 10.1038/nature18621 [DOI] [PubMed] [Google Scholar]
- Damak S, Mosinger B, & Margolskee RF (2008). Transsynaptic transport of wheat germ agglutinin expressed in a subset of type II taste cells of transgenic mice. BMC Neuroscience, 9, 96. 10.1186/1471-2202-9-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM (2004). Modern science versus the stigma of obesity. Nature Medicine, 10, 563–569. 10.1038/nm0604-563 [DOI] [PubMed] [Google Scholar]
- Friedman JM, & Halaas JL (1998). Leptin and the regulation of body weight in mammals. Nature, 395, 763–770. 10.1038/27376 [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Elson AE, Kalik S, Sosa Y, Patterson CM, Myers MG Jr., & Munger SD (2015). Taste responsiveness to sweeteners is resistant to elevations in plasma leptin. Chemical Senses, 40, 223–231. 10.1093/chemse/bju075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Münzberg H, & Myers MG Jr. (2007). The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. Journal of Biological Chemistry, 282, 31019–31027. 10.1074/jbc.M702838200 [DOI] [PubMed] [Google Scholar]
- Han P, Keast RSJ, & Roura E (2017). Salivary leptin and TAS1R2/TAS1R3 polymorphisms are related to sweet taste sensitivity and carbohydrate intake from a buffet meal in healthy young adults. British Journal of Nutrition, 118, 763–770. [DOI] [PubMed] [Google Scholar]
- Harvey J, Hardy SC, Irving AJ, & Ashford ML (2000). Leptin activation of ATP-sensitive K+ (KATP) channels in rat CRI-G1 insulinoma cells involves disruption of the actin cytoskeleton. Journal of Physiology, 527, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, McKay NG, Walker KS, Van der Kaay J, Downes CP, & Ashford ML (2000). Essential role of phosphoinositide 3-kinase in leptin-induced KATP channel activation in the rat CRI-G1 insulinoma cell line. Journal of Biological Chemistry, 275, 4660–4669. [DOI] [PubMed] [Google Scholar]
- Irani BG, Le Foll C, Dunn-Meynell A, & Levin BE (2008). Effects of leptin on rat ventromedial hypothalamic neurons. Endocrinology, 149, 5146–5154. 10.1210/en.2008-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, You J, Yu X, Gonzalez L, Yu Y, Wang Q, Yang G, Li W, Li C, & Liu Y (2008). Tyrosine-dependent and-independent actions of leptin receptor in control of energy balance and glucose homeostasis. Proceedings of the National Academy of Sciences of the United States of America, 105, 18619–18624. 10.1073/pnas.0804589105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyotaki M, Sanematsu K, Shigemura N, Yoshida R, & Ninomiya Y (2016). Leptin suppresses sweet taste responses of enteroendocrine STC-1 cells. Neuroscience, 332, 76–87. 10.1016/j.neuroscience.2016.06.036 [DOI] [PubMed] [Google Scholar]
- Kanda Y (2013). Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplantation, 48, 452–458. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K, Sugimoto K, Nakashima K, Miura H, & Ninomiya Y (2000). Leptin as a modulator of sweet taste sensitivities in mice. Proceedings of the National Academy of Sciences of the United States of America, 97, 11044–11049. 10.1073/pnas.190066697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TJ, Heller RS, Leech CA, Holz GG, & Habener JF (1997). Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes, 46, 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuhara Y, Yoshida R, Ohkuri T, Yasumatsu K, Voigt A, Hübner S, Maeda K, Boehm U, Meyerhof W, & Ninomiya Y (2013). Taste responses in mice lacking taste receptor subunit T1R1. Journal of Physiology, 591, 1967–1985. 10.1113/jphysiol.2012.236604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YF, & Chai Y (2008). Functional modulation of the ATP-sensitive potassium channel by extracellular signal-regulated kinase-mediated phosphorylation. Neuroscience, 152, 371–380. 10.1016/j.neuroscience.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Lu B, Breza JM, Nikonov AA, Paedae AB, & Contreras RJ (2012). Leptin increases temperature-dependent chorda tympani nerve responses to sucrose in mice. Physiology & Behavior, 107, 533–539. 10.1016/j.physbeh.2012.04.018 [DOI] [PubMed] [Google Scholar]
- MacGregor GG, Dong K, Vanoye CG, Tang L, Giebisch G, & Hebert SC (2002). Nucleotides and phospholipids compete for binding to the C terminus of KATP channels. Proceedings of the National Academy of Sciences of the United States of America, 99, 2726–2731. 10.1073/pnas.042688899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Shin YK, White CM, Ji S, Kim W, Carlson OD, Napora JK, Chadwick W, Chapter M, Waschek JA, Mattson MP, Maudsley S, & Egan JM (2010). Vasoactive intestinal peptide-null mice demonstrate enhanced sweet taste preference, dysglycemia, and reduced taste bud leptin receptor expression. Diabetes, 59, 1143–1152. 10.2337/db09-0807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTaggart JS, Clark RH, & Ashcroft FM (2010). The role of the KATP channel in glucose homeostasis in health and disease: More than meets the islet. Journal of Physiology, 588, 3201–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith TL, Corcoran A, & Roper SD (2015). Leptin’s effect on taste bud calcium responses and transmitter secretion. Chemical Senses, 40, 217–222. 10.1093/chemse/bju066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, Sutherland C, & Ashford ML (2004). Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neuroscience, 5, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta E, Kokubo Y, Yamanaka I, Miyamoto Y, Okayama A, Yoshimasa Y, Tomoike H, Morisaki H, & Morisaki T (2008). Leptin gene and leptin receptor gene polymorphisms are associated with sweet preference and obesity. Hypertension Research, 31, 1069–1077. 10.1291/hypres.31.1069 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Sanematsu K, Ohta R, Shirosaki S, Koyano K, Nonaka K, Shigemura N, & Ninomiya Y (2008). Diurnal variation of human sweet taste recognition thresholds is correlated with plasma leptin levels. Diabetes, 57, 2661–2665. 10.2337/db07-1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki M, Jyotaki M, Yoshida R, Yasumatsu K, Shigemura N, DiPatrizio NV, Piomelli D, & Ninomiya Y (2015). Modulation of sweet taste sensitivities by endogenous leptin and endocannabinoids in mice. Journal of Physiology, 593, 2527–2545. 10.1113/JP270295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning K, Miller LC, Laidlaw HA, Watterson KR, Gallagher J, Sutherland C, & Ashford ML (2009). Leptin-dependent phosphorylation of PTEN mediates actin restructuring and activation of ATP-sensitive K+ channels. Journal of Biological Chemistry, 284, 9331–9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Imoto T, Yatabe A, Kawamura S, Nakashima K, & Katsukawa H (1998). Enhanced responses of the chorda tympani nerve to nonsugar sweeteners in the diabetic db/db mouse. American Journal of Physiology, 274, R1324–1330. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Sako N, & Imai Y (1995). Enhanced gustatory neural responses to sugars in the diabetic db/db mouse. American Journal of Physiology, 269, R930–937. 10.1152/ajpregu.1995.269.4.R930 [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG&Schwartz MW Jr. (2001). Intracellular signalling. Key enzyme in leptin induced anorexia. Nature, 413, 794–795. 10.1038/35101657 [DOI] [PubMed] [Google Scholar]
- Park SH, Ryu SY, Yu WJ, Han YE, Ji YS, Oh K, Sohn JW, Lim A, Jeon JP, Lee H, Lee KH, Lee SH, Berggren PO, Jeon JH, & Ho WK (2013). Leptin promotes KATP channel trafficking by AMPK signaling in pancreatic β-cells. Proceedings of the National Academy of Sciences of the United States of America, 110, 12673–12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ML, Unger EK, Myers MG Jr., & Xu AW (2008). Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Molecular Endocrinology, 22, 751–759. 10.1210/me.2007-0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Münzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, … Brüning JC (2006). Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. Journal of Clinical Investigation, 116, 1886–1901. 10.1172/JCI27123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Sigmund CD, Haynes WG, & Mark AL (2009). Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes, 58, 536–542. 10.2337/db08-0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues L, Espanca R, Costa AR, Antunes CM, Pomar C, Capela-Silva F, Pinheiro CC, Amado F, & Lamy E (2017). Association between salivary leptin levels and taste perception in children. Journal of Nutrition and Metabolism, 2017, 7260169. 10.1155/2017/7260169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako N, Ninomiya Y, & Fukami Y (1996). Analysis of concentration-response relationship for enhanced sugar responses of the chorda tympani nerve in the diabetic db/db mice. Chemical Senses, 21, 59–63. [DOI] [PubMed] [Google Scholar]
- Schust J, Sperl B, Hollis A, Mayer TU, & Berg T (2006). Stattic: A small-molecule inhibitor of STAT3 activation and dimerization. Chemistry & Biology, 13, 1235–1242. 10.1016/j.chembiol.2006.09.018 [DOI] [PubMed] [Google Scholar]
- Shigemura N, Miura H, Kusakabe Y, Hino A, & Ninomiya Y (2003). Expression of leptin receptor (Ob-R) isoforms and signal transducers and activators of transcription (STATs) mRNAs in the mouse taste buds. Archives of Histology and Cytology, 66, 253–260. 10.1679/aohc.66.253 [DOI] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan SD, & Ashford ML (1997). Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature, 390, 521–525. 10.1038/37379 [DOI] [PubMed] [Google Scholar]
- Sukumaran SK, Lewandowski BC, Qin Y, Kotha R, Bachmanov AA, & Margolskee RF (2017). Whole transcriptome profiling of taste bud cells. Scientific Reports, 7, 7595. 10.1038/s41598-017-07746-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, & Kaneko T (2003). Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. The Journal of Comparative Neurology, 467, 60–79. 10.1002/cne.10905 [DOI] [PubMed] [Google Scholar]
- Umabiki M, Tsuzaki K, Kotani K, Nagai N, Sano Y, Matsuoka Y, Kitaoka K, Okami Y, Sakane N, & Higashi A (2010). The improvement of sweet taste sensitivity with decrease in serum leptin levels during weight loss in obese females. Tohoku Journal of Experimental Medicine, 220, 267–271. 10.1620/tjem.220.267 [DOI] [PubMed] [Google Scholar]
- Ünal EB, Uhlitz F, & Blüthgen N (2017). A compendium of ERK targets. FEBS Letters, 591, 2607–2615. [DOI] [PubMed] [Google Scholar]
- White DW, Kuropatwinski KK, Devos R, Baumann H, & Tartaglia LA (1997). Leptin receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. Journal of Biological Chemistry, 272, 4065–4071. 10.1074/jbc.272.7.4065 [DOI] [PubMed] [Google Scholar]
- Whitman M, Downes CP, Keeler M, Keller T, & Cantley L (1988). Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature, 332, 644–646. 10.1038/332644a0 [DOI] [PubMed] [Google Scholar]
- Williams KW, Sohn JW, Donato J Lee CE, Zhao JJ, Elmquist JK, & Elias CF (2011). The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. Journal of Neuroscience, 31, 13147–13156. 10.1523/JNEUROSCI.2602-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Horio N, Murata Y, Yasumatsu K, Shigemura N, & Ninomiya Y (2009). NaCl responsive taste cells in the mouse fungiform taste buds. Neuroscience, 159, 795–803. 10.1016/j.neuroscience.2008.12.052 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Miyauchi A, Yasuo T, Jyotaki M, Murata Y, Yasumatsu K, Shigemura N, Yanagawa Y, Obata K, Ueno H, Margolskee RF, & Ninomiya Y (2009). Discrimination of taste qualities among mouse fungiform taste bud cells. Journal of Physiology, 587, 4425–4439. 10.1113/jphysiol.2009.175075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Noguchi K, Shigemura N, Jyotaki M, Takahashi I, Margolskee RF, & Ninomiya Y (2015). Leptin suppresses mouse taste cell responses to sweet compounds. Diabetes, 64, 3751–3762. 10.2337/db14-1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Shigemura N, Sanematsu K, Yasumatsu K, Ishizuka S, & Ninomiya Y (2006). Taste responsiveness of fungiform taste cells with action potentials. Journal of Neurophysiology, 96, 3088–3095. 10.1152/jn.00409.2006 [DOI] [PubMed] [Google Scholar]
- Zhang EE, Chapeau E, Hagihara K, & Feng GS (2004). Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proceedings of the National Academy of Sciences of the United States of America, 101, 16064–16069. 10.1073/pnas.0405041101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, & Rui L (2013). Leptin signaling and leptin resistance. Frontiers of Medicine, 7, 207–222. 10.1007/s11684-013-0263-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


