Abstract
Aim:
We investigated potential neuron types that code sugar information and how sodium-glucose cotransporters (SGLTs) and T1Rs are involved.
Methods:
Whole-nerve recordings in the chorda tympani (CT) and the glossopharyngeal (GL) nerves and single-fibre recordings in the CT were performed in T1R3-KO and wild-type (WT) mice. Behavioural response measurements were conducted in T1R3-KO mice using phlorizin (Phl), a competitive inhibitor of SGLTs.
Results:
Results indicated that significant enhancement occurred in responses to sucrose and glucose (Glc) by adding 10 mmol/L NaCl but not in responses to KCl, monopotassium glutamate, citric acid, quinine sulphate, SC45647(SC) or polycose in both CT and GL nerves. These enhancements were abolished by lingual application of Phl. In single-fibre recording, fibres showing maximal response to sucrose could be classified according to responses to SC and Glc with or without 10 mmol/L NaCl in the CT of WT mice, namely, Phl-insensitive type, Phl-sensitive Glc-type and Mixed (Glc and SC responding)-type fibres. In T1R3-KO mice, Phl-insensitive-type fibres disappeared. Results from behavioural experiments showed that the number of licks and amount of intake for Glc with or without 10 mmol/L NaCl were significantly suppressed by Phl.
Conclusion:
We found evidence for the contribution of SGLTs in sugar sensing in taste cells of mouse tongue. Moreover, we found T1R-dependent (Phl-insensitive) type, Glc-type and Mixed (SGLTs and T1Rs)-type fibres. SGLT1 may be involved in the latter two types and may play important roles in the glucose-specific cephalic phase of digestion and palatable food intake.
Keywords: glucose, SGLTs, sugar, sweet taste, T1Rs, taste
1 |. INTRODUCTION
Several studies have indicated the existence of T1R-independent mechanisms for detecting sugars. The first observation of residual responses to sugars was reported in a study by Damak et al, showing chorda tympani (CT) and glossopharyngeal (GL) nerve responses to sugars and behavioural preference for sucrose (Suc) and glucose (Glc) in mice genetically lacking T1R3.1 In that study, T1R3-KO mice showed severely diminished responses to non-caloric sweeteners, such as SC45647 (SC) and sucralose, indicating that sensing of the sweetener requires the presence of T1R3, although there was no statistical difference between the KO and its wild-type (WT) mice in response to 500 mmol/L Glc. That research was followed by some studies showing that residual responses to sucrose and Glc increased in a temperature-dependent manner2 and that oral administration of Suc and Glc, but not fructose, mediate cephalic-phase insulin release (CPIR)3 in the same KO model.
Recently, plausible candidates of sugar sensors for those mechanisms were reportedly expressed in gustatory tissues in rats4 and mice.5,6 Considering the phenotypical similarity between intestinal cells and taste receptor cells, they found expression of glucose transporter (GLUT) 2, GLUT5 and sodium-glucose cotransporter (SGLT) 1 in rat circumvallate (CV) papillae,4 and expression of GLUT2, GLUT4, GLUT8, GLUT9 and SGLT1,5 and GLUT1 and SGLT16 in mouse papillae of the tongue. Without focusing on gustatory tissues, it was suggested that SGLT1, GLUT1, GLUT2 and GLUT3 are expressed in the epithelial cells of human oral mucosa.7 Among these reported transporters, SGLT1 is pivotal for intestinal mass absorption of D-glucose8 and triggers the upregulation of GLUT2.9 Additionally, it was found that SGLT1 serves as the intestinal Glc sensor for the Glc-induced secretion of GIP from the K-cell10 and GLP-1 from the L-cell.9 In mice lacking SGLT1, tissue retention of tracer Glc was drastically reduced throughout the entire small intestine, and blood Glc was elevated and GIP and GLP-1 secretion were considerably decreased in response to Glc.11
In this study, we investigated the function of SGLTs as a sugar taste receptor in taste tissues of the tongue by examining nerve responses to sweet compounds and short-chain Glc polymers with and without NaCl in the CT and the GL nerves of WT mice. We show a significant enhancement of responses to sugars and a Glc analogue by adding NaCl, and a significant reduction of those enhanced responses by lingual application of phlorizin (Phl), a competitive inhibitor of SGLTs. We also analysed Phl-sensitive CT neurones in WT and T1R3-KO mice to investigate potential neuron types that code sugar information. Subsequent behavioural response measurements were performed to examine the function of SGLTs in T1R3-KO mice.
2 |. RESULTS
2.1 |. Examination of concentration-response relationships in the whole nerve
Figure 1 shows sample recordings of integrated whole-nerve responses from the CT and the GL nerve of WT mice. We observed enhancement of responses between Glc or Suc and 10 mmol/L NaCl in the CT and GL. To investigate the effective concentration of NaCl and Glc for producing enhanced responses in taste tissues, we tested whole-nerve responses to concentration series of NaCl or Glc and a binary mixture of these compounds in the CT (Figure 2A,B). The magnitude of relative response to 0.5 mol/L Glc is shown as a filled circle at ordinate in Figure 2A. Responses to 1–100 mmol/L NaCl are shown as open circles with a dotted line. Responses to a series of mixture solution concentrations of NaCl with 0.5 mol/L Glc were significantly enhanced (filled circles) when compared with the sum of each response (Table 1). After lingual treatment of Phl, CT nerve responses to the mixture solutions (black triangles) were significantly reduced, and the response to mixture solutions and the sum of each response were not significantly different (Table 1). Post hoc Fisher’s test revealed that 10 mmol/L NaCl was the most effective concentration for eliciting enhancement (P = .005). Concerning whole-nerve responses to a concentration series of Glc and a binary mixture with 10 mmol/L NaCl, responses to the mixture solution were significantly enhanced compared with the sum of each response (Figure 2B, Table 1). After lingual treatment of Phl, CT nerve responses to concentration series of Glc with and without NaCl solutions were significantly reduced. Next, we recorded CT nerve responses to 0.5 mol/L Glc + 10 mmol/L NaCl with a concentration series of Phl in WT mice (Figure 2C). Phl significantly suppressed CT responses to the mixture in a dose-dependent manner (one-way ANOVA: F(1, 38) = 6.532; P < .001). Therefore, we chose 1 mmol/L Phl to obtain maximal suppression in subsequent experiments.
FIGURE 1.
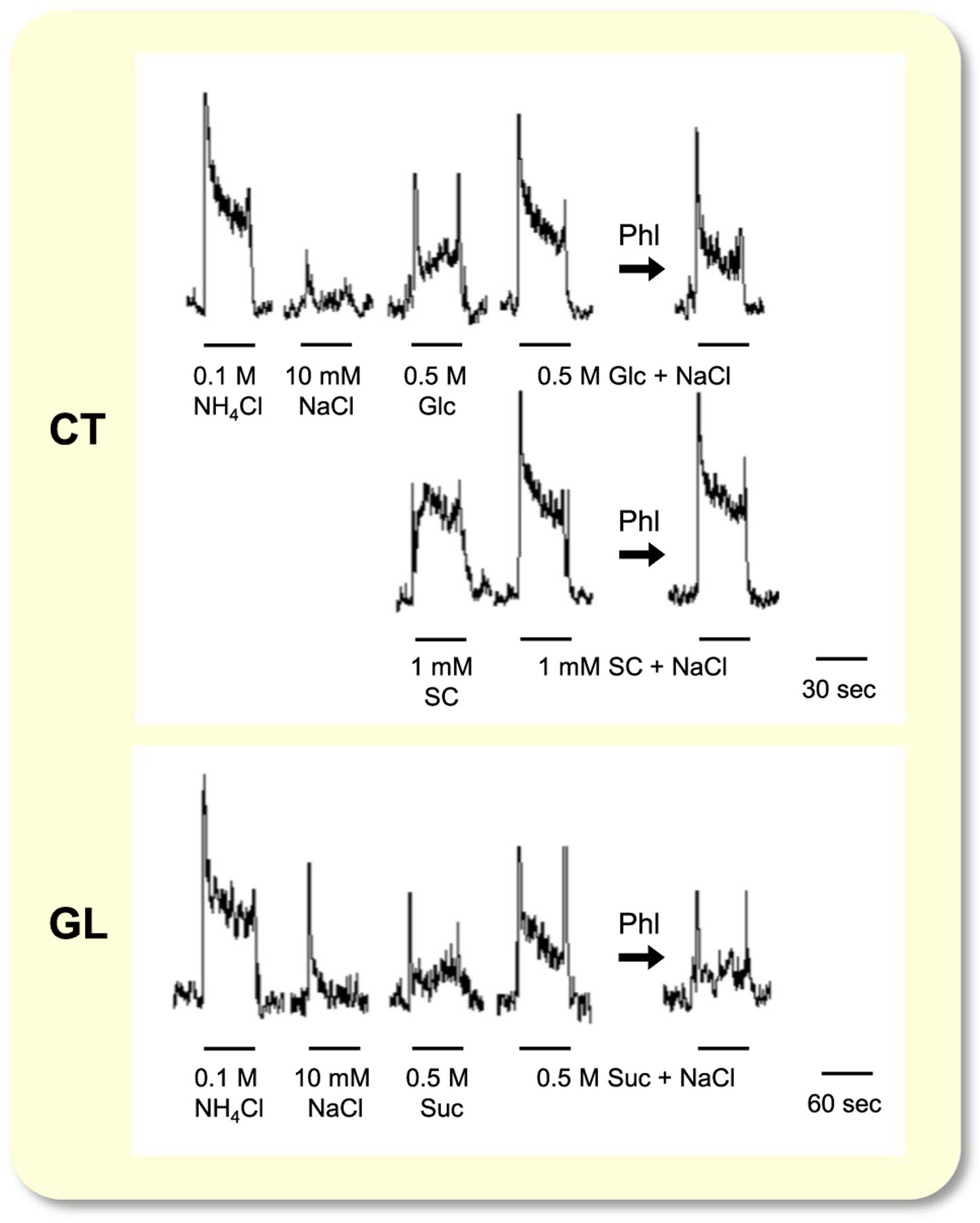
Sample recordings of integrated whole-nerve responses from the chorda tympani CT (upper and middle) and the GL nerve (bottom trace) of a WT mouse. Taste stimuli were 0.1 mol/L NH4Cl, 10 mmol/L NaCl, 0.5 mol/L glucose (Glc), 0.5 mol/L Glc + 10 mmol/L NaCl, 1 mmol/L SC45647 (SC), 1 mmol/L SC + 10 mmol/L NaCl, 0.5 mol/L sucrose (Suc), 0.5 mol/L Suc + 10 mmol/L NaCl. 1 mmol/L phlorizin (Phl) was applied to the tongue. Bars indicate application of taste stimuli. CT, chorda tympani; GL, glossopharyngeal; WT, wild-type
FIGURE 2.
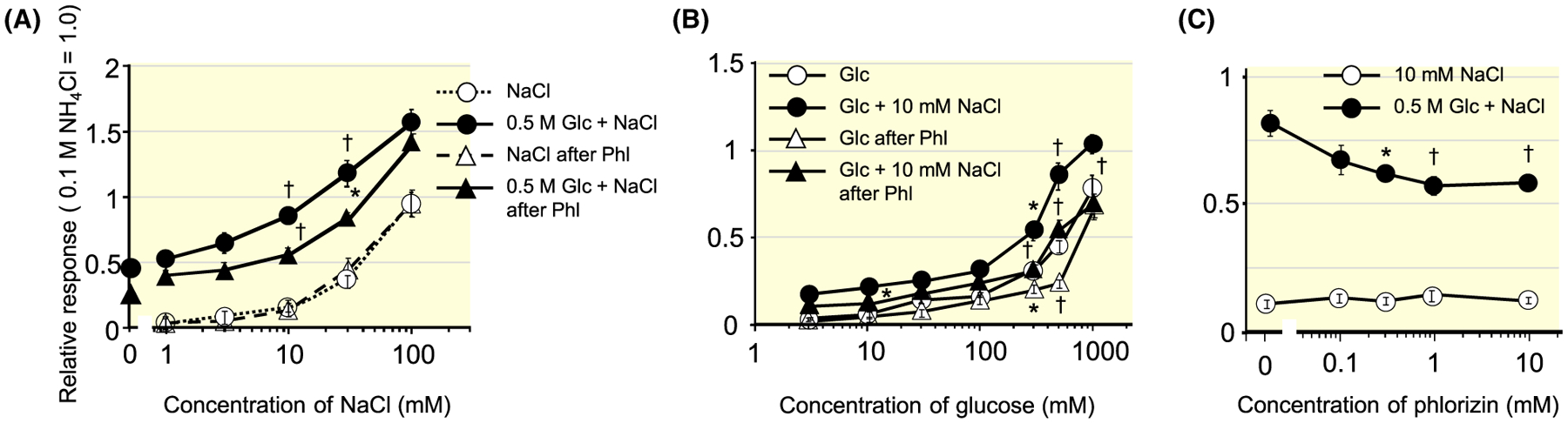
Whole-nerve responses to concentration series of NaCl or Glc and binary mixture of these compounds in the chorda tympani nerves of WT mice. Response to 0.1 mol/L NH4Cl was used as a unity (1.0). A, Responses to concentration series of NaCl with and without 0.5 mol/L Glc before and after lingual treatment of Phl. Responses to 1–100 mmol/L NaCl were shown as open circles (before Phl) or open triangles (after Phl) with black dotted line. B, Whole-nerve responses to concentration series of Glc with or without 10 mmol/L NaCl before and after lingual treatment of Phl. C, Dose-dependent effect of Phl on CT nerve response to 0.5 mol/L Glc + 10 mmol/L NaCl. Values indicated are means ± SE. Results of repeated measures ANOVA are shown in Table 1. Results of post hoc Fisher test to evaluate enhancement of the response by Glc and NaCl mixture was shown by top symbols, and that to evaluate suppression by Phl was shown by other symbols; *P < .05, †P < .01. CT, chorda tympani; WT, wild-type
TABLE 1.
Summary of repeated measures ANOVA results of the CT nerve responses (Glc + NaCl mixture vs sum of Glc and NaCl or before vs after phloridzin treatment, Figure 2) and behavioural responses to glucose-quinine mixture (Figure 10B)
| Stimulus | Treatment | Concentration | Treatment × Concentration |
|---|---|---|---|
| Effect of mixing of 0.5 mol/L Glc and concentration series of NaCl (Figure 2A) | |||
| Before Phl | F(1, 48) = 6.167, P = .029 | F(4, 48) = 131.39, P < .001 | F(4, 48) = 3.219, P = .020 |
| After Phl | F(1, 36) = 4.614, P = .060 | F(4, 36) = 305.69, P < .001 | F(4, 36) = 0.817, P = .523 |
| Effect of phloridzin (Figure 2A) | |||
| Glc + NaCl | F(1, 44) = 7.103, P = .023 | F(4, 44) = 174.25, P < .001 | F(4, 44) = 2.226, P = .082 |
| NaCl | F(1, 44) = 0.317, P = .585 | F(4, 44) = 158.72, P < .001 | F(4, 44) = 0.316, P = .866 |
| Effect of mixing of 10 mmol/L NaCl and concentration series of glucose (Figure 2B) | |||
| Before Phl | F(1, 56) = 12.363, P = .003 | F(4, 56) = 100.84, P < .001 | F(4, 56) = 2.794, P = .035 |
| After Phl | F(1, 52) = 0.903, P = .359 | F(4, 52) = 70.907, P < .001 | F(4, 52) = 3.409, P = .015 |
| Effect of 1 mmol/L phloridzin (Figure 2B) | |||
| Glc | F(1, 48) = 12.928, P = .004 | F(4, 48) = 70.307, P < .001 | F(4, 48) = 1.545, P = .204 |
| Glc + NaCl | F(1, 56) = 15.062, P = .002 | F(4, 56) = 147.41, P < .001 | F(4, 56) = 8.450, P < .001 |
| Effect of mixing of 10 mmol/L NaCl and glucose QHCl mixtures in behavioural experiment (Figure 10B) | |||
| Before Phl | F(1, 59) = 19.617, P = .001 | F(6, 59) = 25.366, P < .001 | F(6, 59) = 2.834, P = .017 |
| After Phl | F(1, 60) = 1.545, P = .242 | F(6, 60) = 2.962, P = .013 | F(6, 60) = 1.163, P = .338 |
| Effect of 1 mmol/L phloridzin on lick counts in behavioural experiment (Figure 10B) | |||
| Glc | F(1, 60) = 50.194, P < .001 | F(6, 60) = 12.814, P < .001 | F(6, 60) = 7.635, P < .001 |
| Glc + NaCl | F(1, 59) = 31.660, P < .001 | F(6, 59) = 13.959, P < .001 | F(6, 59) = 4.570, P < .001 |
Abbreviations: CT, chorda tympani; QHCl, quinine HCl.
2.2 |. Survey of stimuli showing synergistic responses by the addition of NaCl
To survey taste compounds showing synergistic responses when mixed with 10 mmol/L NaCl, we recorded whole-nerve responses to 100 mmol/L KCl, 5 mmol/L citric acid, 0.3 mmol/L (for the GL) and 1 mmol/L (for the CT) quinine sulphate (QSO4), 100 mmol/L monopotassium glutamate (MPG), 500 mmol/L Glc, 500 mmol/L Suc, 500 mmol/L galactose, 1 mmol/L SC, 10 mmol/L saccharin and 16% polycose in the CT and/or GL (Figure 3). In the CT nerve, significant enhancement occurred in responses to mixture solutions of Suc, Glc or galactose with 10 mmol/L NaCl but not in responses to citric acid, KCl, QSO4, MPG, SC, polycose or saccharin with 10 mmol/L NaCl (Table 2). In the GL nerve, a significant enhancement of responses occurred in responses to mixture solutions of Suc or Glc with 10 mmol/L NaCl but not in responses to citric acid, KCl, QSO4, MPG, SC or polycose with 10 mmol/L NaCl (Table 2). These enhanced responses significantly decreased after Phl treatment of the tongue in both CT and GL nerves (Table 3). As in the case of the response to Glc, the response to Suc without NaCl significantly decreased after Phl treatment in the CT.
FIGURE 3.
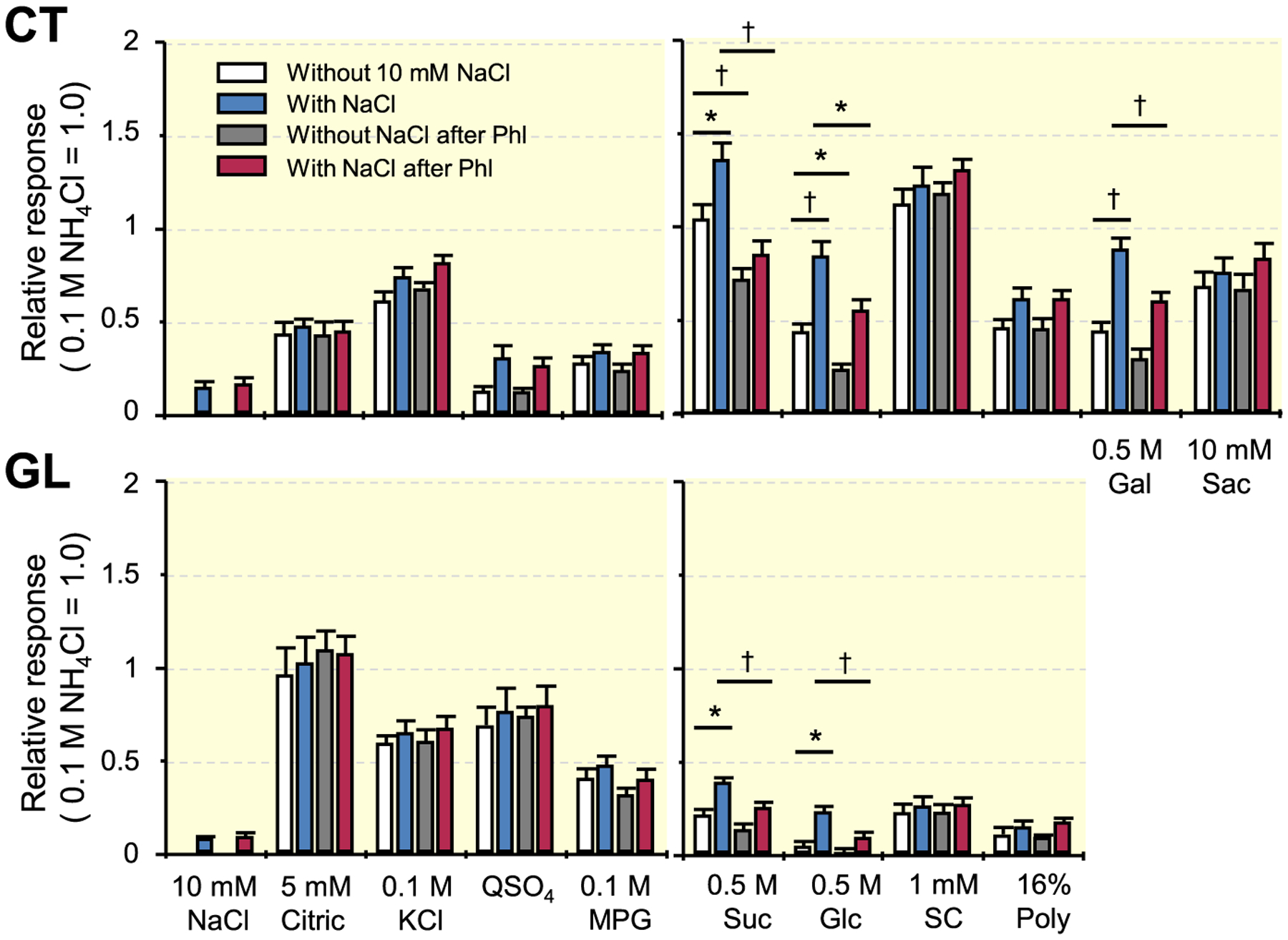
Whole-nerve responses to 100 mmol/L KCl, 5 mmol/L citric acid, 0.3 mmol/L (for the GL) and 1 mmol/L (for the CT) QSO4, 100 mmol/L MPG, 500 mmol/L Glc, 500 mmol/L Suc, 500 mmol/L galactose, 1 mmol/L SC, 10 mmol/L saccharin and 16% polycose in the CT and/or GL of WT mice. Response to 0.1 mol/L NH4Cl was used as a unity (1.0). Values indicated are means ± SE. Results of unpaired t test for the effect of the addition of 10 mmol/L NaCl and the effect of 1 mmol/L phlorizin (Phl) on responses to taste stimuli are shown in Table 2 and Table 3 respectively; *P < .05, †P < .01. CT, chorda tympani; GL, glossopharyngeal; WT, wild-type
TABLE 2.
Results of unpaired t test for the effect of the addition of 10 mmol/L NaCl on responses to taste stimuli in the CT and GL of WT mice (Figure 3)
| Stimulus | Before phloridzin | After phloridzin |
|---|---|---|
| CT nerve | ||
| Citric acid | t(8) = 1.258, P = .244 | t(10) = 1.275, P = .231 |
| KCl | t(10) = 0.568, P = .582 | t(12) = 0.561, P = .585 |
| QSO4 | t(10) = 0.601, P = .561 | t(12) = 0.548, P = .594 |
| MPG | t(10) = 1.940, P = .081 | t(12) = 1.187, P = .258 |
| Sucrose | t(10) = 2.306, P = .044 | t(9) = 0.253, P = .806 |
| Glucose | t(10) = 3.376, P = .007 | t(9) = 2.229, P = .053 |
| SC45647 | t(10) = 0.067, P = .948 | t(12) = 0.109, P = .915 |
| Polycose | t(11) = 0.025, P = .980 | t(11) = 0.122, P = .905 |
| Galactose | t(12) = 3.804, P = .003 | t(11) = 1.663, P = .125 |
| Saccharin | t(12) = 0.293, P = .775 | t(12) = 0.292, P = .775 |
| GL nerve | ||
| Citric acid | t(12) = 0.099, P = .923 | t(10) = 0.844, P = .418 |
| KCl | t(14) = 0.499, P = .696 | t(10) = 0.298, P = .772 |
| QSO4 | t(12) = 0.067, P = .947 | t(10) = 0.336, P = .744 |
| MPG | t(14) = 0.254, P = .803 | t(10) = 0.221, P = .830 |
| Sucrose | t(12) = 2.570, P = .025 | t(10) = 0.429, P = .677 |
| Glucose | t(12) = 2.344, P = .037 | t(10) = 0.534, P = .605 |
| SC45647 | t(14) = 0.882, P = .393 | t(12) = 1.020, P = .328 |
| Polycose | t(14) = 0.813, P = .430 | t(10) = 0.426, P = .679 |
Note: Differences between mixture of stimulus with NaCl and the sum of each component were assessed by unpaired t test in the CT (upper) and GL (lower).
Abbreviations: CT, chorda tympani; GL, glossopharyngeal; WT, wild-type.
TABLE 3.
Results of unpaired t test for the effect of 1 mmol/L phloridzin on responses to taste stimuli with or without 10 mmol/L NaCl in the CT and GL of WT mice (Figure 3)
| Stimulus | Without NaCl | With NaCl |
|---|---|---|
| CT nerve | ||
| Citric acid | t(9) = 0.139, P = .892 | t(9) = 0.420, P = .685 |
| KCl | t(11) = 1.089, P = .300 | t(11) = 1.246, P = .239 |
| QSO4 | t(11) = 0.002, P = .999 | t(11) = 1.047, P = .318 |
| MPG | t(11) = 0.535, P = .604 | t(11) = 0.146, P = .887 |
| Sucrose | t(11) = 3.149, P = .009 | t(10) = 4.274, P = .002 |
| Glucose | t(10) = 2.804, P = .019 | t(9) = 2.968, P = .016 |
| SC45647 | t(11) = 0.926, P = .375 | t(11) = 0.818, P = .431 |
| Polycose | t(12) = 0.126, P = .902 | t(10) = 0.014, P = .989 |
| Galactose | t(11) = 1.456, P = .173 | t(12) = 3.892, P = .002 |
| Saccharin | t(12) = 0.121, P = .906 | t(12) = 0.706, P = .494 |
| GL nerve | ||
| Citric acid | t(11) = 0.825, P = .427 | t(11) = 0.307, P = .764 |
| KCl | t(12) = 0.315, P = .758 | t(12) = 0.266, P = .795 |
| QSO4 | t(11) = 0.542, P = .599 | t(11) = 0.203, P = .843 |
| MPG | t(12) = 1.315, P = .213 | t(12) = 1.062, P = .309 |
| Sucrose | t(11) = 1.687, P = .120 | t(11) = 3.829, P = .003 |
| Glucose | t(11) = 0.828, P = .425 | t(11) = 3.942, P = .002 |
| SC45647 | t(13) = 0.251, P = .806 | t(13) = 0.155, P = .879 |
| Polycose | t(12) = 0.077, P = .940 | t(12) = 0.708, P = .493 |
Abbreviations: CT, chorda tympani; GL, glossopharyngeal; WT, wild-type.
2.3 |. Whole-nerve responses in T1R3-KO mice
If SGLT1 contributed to the above-mentioned responses to the mixtures, T1R3-KO mice would show an enhancement of the responses to the same mixtures and suppression of the responses by Phl. Therefore, we recorded responses to Suc, Glc, saccharin and SC in the CT nerve of T1R3-KO mice. As shown in Figure 4, significant enhancement occurred in the responses to binary mixtures of Suc or Glc with 10 mmol/L NaCl but not in responses to binary mixtures of saccharin or SC with 10 mmol/L NaCl (Table 4). The responses to Suc, Glc and the mixtures of these sugars with NaCl were significantly decreased after Phl treatment of the tongue (Table 5).
FIGURE 4.
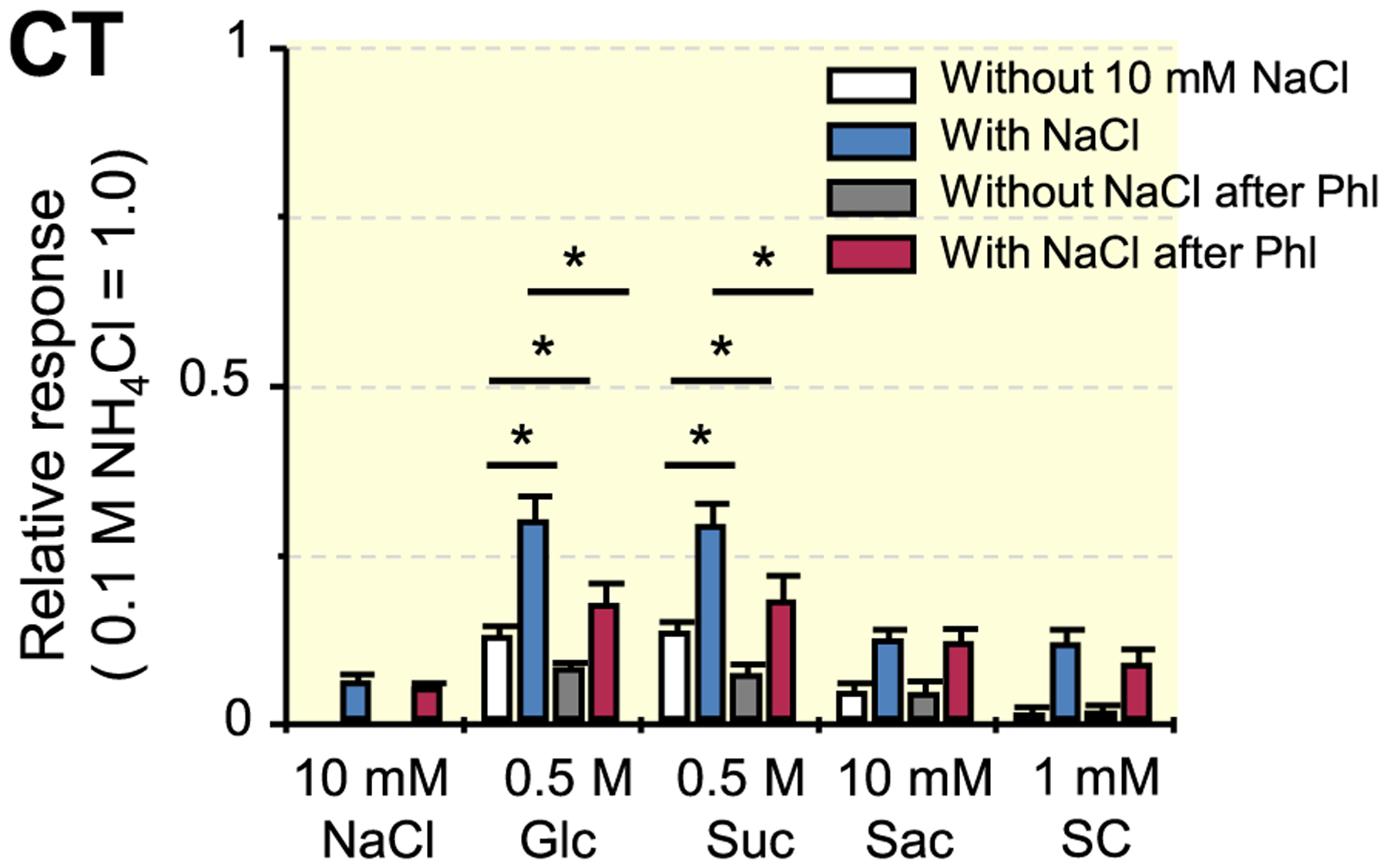
Whole-nerve responses to Suc, Glc, saccharin and SC in the chorda tympani nerves of T1R3-KO mice. Response to 0.1 mol/L NH4Cl was used as a unity (1.0). Values indicated are means ± SE. Results of unpaired t test for the effect of the addition of 10 mmol/L NaCl and the effect of 1 mmol/L phlorizin (Phl) on responses to taste stimuli are shown in Table 4 and Table 5 respectively; *P < .05
TABLE 4.
Results of unpaired t test for the effect of the addition of 10 mmol/L NaCl on responses to taste stimuli in the CT of T1R3-KO mice (Figure 4)
| CT nerve | ||
|---|---|---|
| Stimulus | Before phloridzin | After phloridzin |
| Glucose | t(10) = 2.895, P = .016 | t(8) = 1.278, P = .237 |
| Sucrose | t(10) = 2.500, P = .031 | t(8) = 1.298, P = .230 |
| Saccharin | t(10) = 0.034, P = .974 | t(10) = 0.494, P = .632 |
| SC45647 | t(10) = 1.859, P = .093 | t(10) = 0.745, P = .474 |
Note: Differences between mixture of stimulus with NaCl and the sum of each component were assessed by unpaired t test in the CT.
Abbreviation: CT, chorda tympani.
TABLE 5.
Results of unpaired t test for the effect of 1 mmol/L phloridzin on responses to taste stimuli with or without 0.1 mol/L NaCl in the CT of T1R3-KO mice (Figure 4)
| CT nerve | ||
|---|---|---|
| Stimulus | Without NaCl | With NaCl |
| Glucose | t(9) = 3.133, P = .012 | t(9) = 2.642, P = .027 |
| Sucrose | t(9) = 3.220, P = .010 | t(9) = 2.355, P = .043 |
| Saccharin | t(10) = 0.746, P = .473 | t(10) = 0.710, P = .868 |
| SC45647 | t(10) = 0.706, P = .496 | t(10) = 1.202, P = .257 |
Abbreviation: CT, chorda tympani.
2.4 |. 2-NBDG uptake by taste cells through GLUTs
2-NBDG enters the cell through glucose transporters such as GLUTs and SGLTs.12 To test whether functional glucose transporters exist on the apical side of taste cells, we observed 2-NBDG uptake by taste cells in the intact tongue of WT mice (Figure 5). After apical 2-NBDG treatment on the tongue, some taste cells in the fungiform taste buds clearly showed 2-NBDG fluorescence (Figure 5A). 2-NBDG uptake by taste cells was concentration dependent (Figure 5B). To confirm whether 2-NBDG uptake by taste cells occurred through glucose transporters, Phl or phloretin (an inhibitor of sodium-glucose co-transporters) was applied before and during 2-NBDG treatment. 2-NBDG uptake by taste cells was significantly reduced by application of Phl or phroretin (Figure 5B, P < .001, Tukey’s HDS test). The inhibitory effect of Phl was greater than that of phloretin. In addition, 2-NBDG uptake was significantly greater in the condition with NaCl than without NaCl (Figure 5B, P < .001, Tukey’s HSD test). These results suggest that a subset of taste cells was able to uptake glucose through SGLTs and GLUTs expressed on the apical membrane.
FIGURE 5.
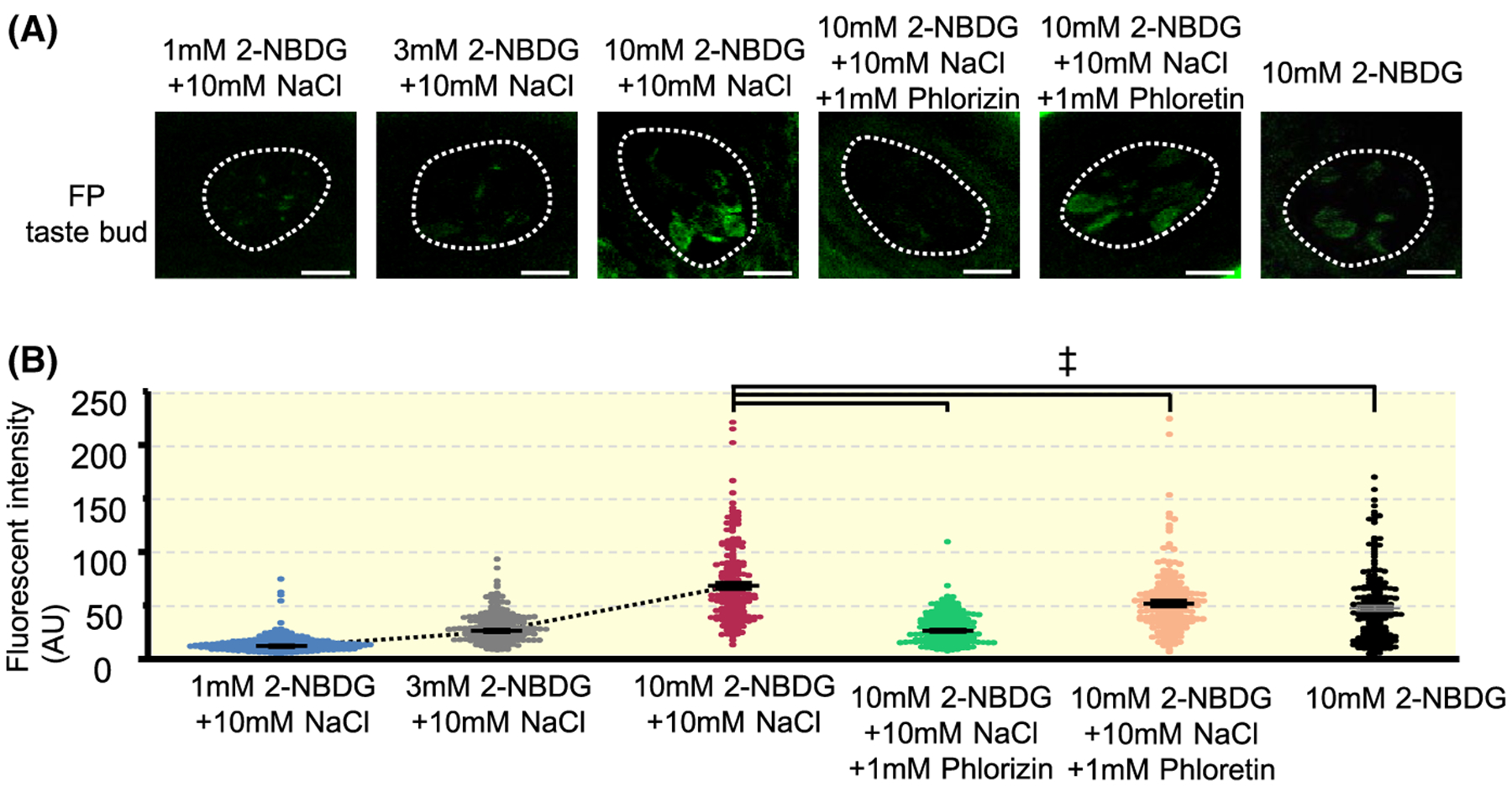
2-NBDG uptake by taste cells in the intact mouse tongue. A, Representative images of fungiform taste buds after apical treatment of 2-NBDG with different conditions. Dotted lines indicate the outlines of taste buds. Scale bar, 10 μm. B, Fluorescent intensities (arbitrary unit: AU) of taste buds after apical treatment of 2-NBDG with different conditions (1 mmol/L 2-NBDG + 10 mmol/L NaCl, 3 mmol/L 2-NBDG + 10 mmol/L NaCl, 10 mmol/L 2-NBDG + 10 mmol/L NaCl, 10 mmol/L 2-NBDG + 10 mmol/L NaCl + 1 mmol/L Phlorizin, 10 mmol/L 2-NBDG + 10 mmol/L NaCl + 1 mmol/L Phloretin and 10 mmol/L 2-NBDG). Each dot represents fluorescent intensity of each taste bud (10th optical section from the apical side). Data were obtained from three animals for each condition (58–60 taste buds for each animal). Summarized data are shown in means ± SE. F = 55.65, P < .001, one-way ANOVA test. ‡P < .001, Tukey HSD test
2.5 |. Identification for enhanced taste quality
To investigate which taste quality (sweet or salty) was enhanced in the responses to sugars with NaCl mixture solutions, we recorded single-fibre responses to the mixture in fibres that showed responses to Glc and/or NaCl of WT mice (Figure 6). In an initial survey of single fibres of the CT, we measured the number of impulses as the response when various taste stimuli were applied to the tongue. Among the Glc- and/or NaCl-responsive fibres, we segregated S-type, E-type and N-type fibres according to the maximal net response (ie the best stimulus for the response) to Suc, HCl and NaCl among five compounds, NaCl, Suc, HCl, quinine HCl (QHCl) and MPG. Next we discriminated between N-type and E-type fibres according to their response characteristics to electrolytes and the susceptibility of their NaCl responses to amiloride (Ami), as reported previously (Ninomiya, 1998).13 That is, N-type fibres are selectively responsive to NaCl and their responses to NaCl is largely inhibited by Ami, whereas E-type fibres are broadly sensitive to various electrolytes, including acids and salts, and Ami did not inhibit their responses to NaCl. Figure 6 shows response profiles of three types of fibres that responded to Glc and/or NaCl. Although E-type fibres showed robust responses to NaCl, the number of impulses evoked by lingual stimulation of a 10-mmol/L NaCl + 0.5-mol/L Glc mixture was not statistically different from the arithmetic sum of the NaCl response and the Glc response. Similarly, the number of impulses evoked by stimulation with the mixture were not different from the sum of each number of impulse of those evoked by NaCl and Glc in N-type fibres. Contrary to these NaCl-responsive fibres, S-type fibres showed significantly larger number of impulses by mixing these compounds than what was shown for the sum of each impulse of those evoked by each compound, indicating sweet taste enhancement by adding NaCl (paired t test, t(13) = 2.878, P = .013).
FIGURE 6.
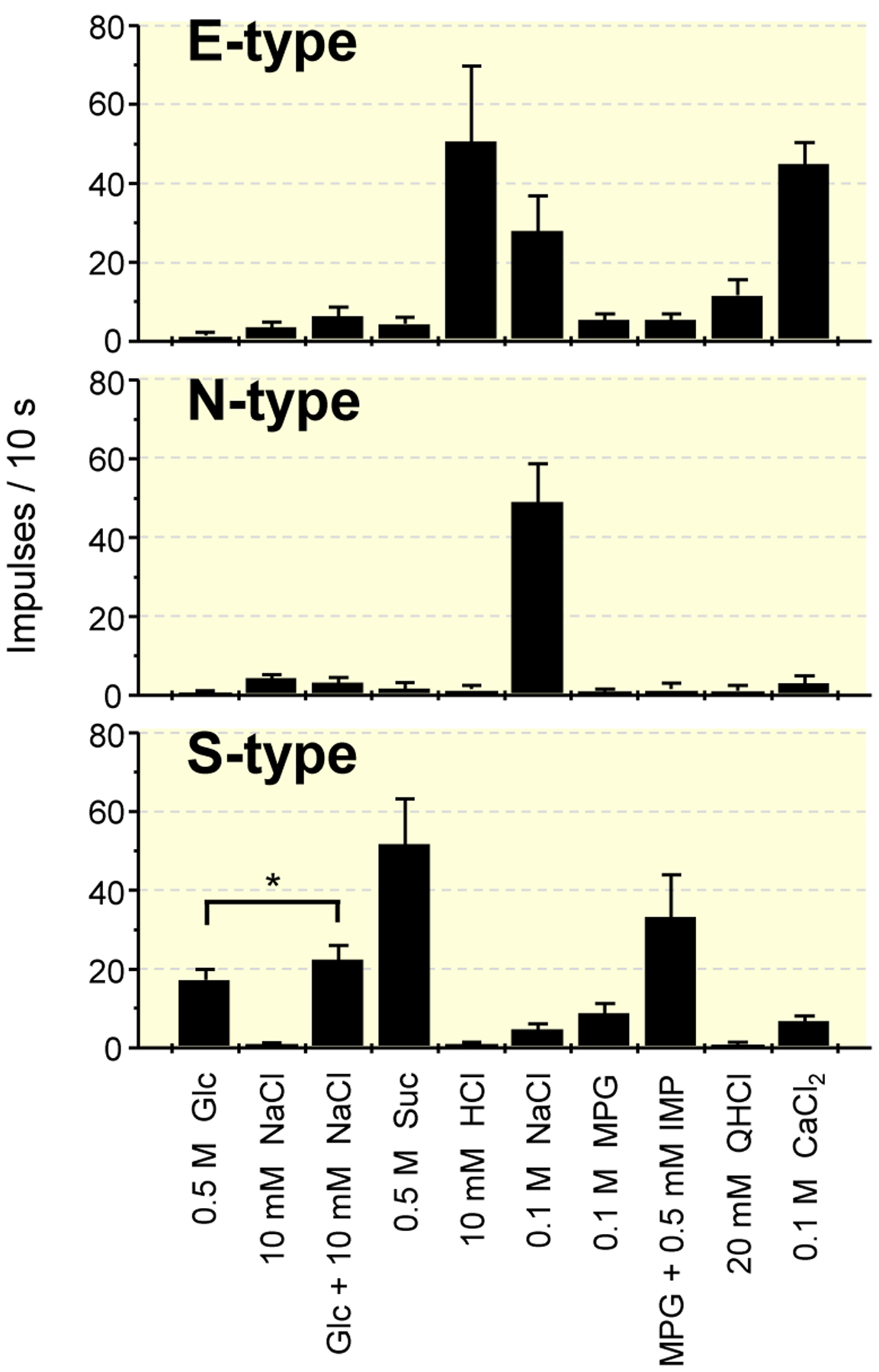
Response profiles of three types of single fibres that responded to Glc and/or NaCl in the chorda tympani nerves of WT mice. Stimuli are 0.5 mol/L glucose (Glc), 10 mmol/L NaCl, 0.5 mol/L Glc + 10 mmol/L NaCl, 0.5 mol/L sucrose (Suc), 10 mmol/L HCl, 0.1 mol/L NaCl, 0.1 mol/L monopotassium glutamate (MPG), MPG + 0.5 mmol/L inosine monophosphate (IMP), 20 mmol/L quinine HCl (QHCl) and 0.1 mol/L CaCl2. Values indicated are mean impulse frequencies ± SE. paired t test; *P < .05. WT, wild-type
2.6 |. Groupings of S-type fibres in WT mice
To investigate whether fibres that show the enhancement and Phl sensitivity independently exist, we examined Phl sensitivity of the responses to Glc and SC with or without 10 mmol/L NaCl in all S-type fibres in the CT of WT mice (Figures 7 and 8). As shown in Figure 7, we observed fibres showing a maximal response to 0.5 mol/L Glc when compared with 0.5 mol/L Suc, 0.5 mol/L Glc, 1 mmol/L SC, 0.01 mol/L HCl, 0.1 mol/L NaCl, 0.1 mol/L MPG and 20 mmol/L QHCl (left, Glc-best, 28.6%). The responses of these fibres to Glc was enhanced by the addition of 10 mmol/L NaCl (Table 6), and that to SC was not enhanced. In contrast, a group of fibres that showed maximal responses to 0.5 mol/L Suc or 1 mmol/L SC, did not show enhancement between 0.5 mol/L Glc and 10 mmol/L NaCl (middle, Suc SC-best, Table 6, 28.6%). Furthermore, we observed a group of fibres showing responses to Glc, Suc and SC, and an enhanced response to Glc by the addition of 10 mmol/L NaCl (right, Mixed-type, Table 6, 42.9%). Figure 8A shows the response profiles of PI- (Phl-insensitive, Suc SC-best), Mixed- and Glc-type fibres from CT nerves of WT mice. Each column indicates the mean number of net impulses per 10 seconds (mean response) for each fibre type responding to taste stimuli. Responses to Glc and Glc + 10 mmol/L NaCl in Mixed-, and Glc-type fibres were significantly suppressed after lingual treatment of 1 mmol/L Phl (Table 7). In addition to the classification above, we also classified these fibres using hierarchical cluster analysis according to responses to taste stimuli tested (Figure 8B). The letters Su, Sc and Gn indicate the stimulus producing the maximal response among Suc (Su), SC (Sc) and Glc + NaCl (Gn). From this analysis, fibres were classified into two clusters. The majority of PI fibres are located at the top of the figure, and the majority of Glc-type fibres are located at the bottom of the figure. Although Mixed-type fibres are in middle of the figure, half of Mixed-type fibres were in top group, and the other half were in bottom group in this analysis. Additionally, we examined fibre distribution by means of Phl sensitivity of the fibres (Figure 8C). The results showed bimodal distribution of fibres. Namely, Phl-sensitive (PS)-type fibres (a fibre group on the left in Figure 8C) and PI-type fibres (a fibre group on the right in Figure 8C). All Glc-type and Mixed-type fibres were among PS-types. From these three figures, it was suggested that T1Rs and SGLTs co-express in taste cells that connect with Mixed-type fibres, and that other sweet taste cells express either T1Rs or SGLTs.
FIGURE 7.
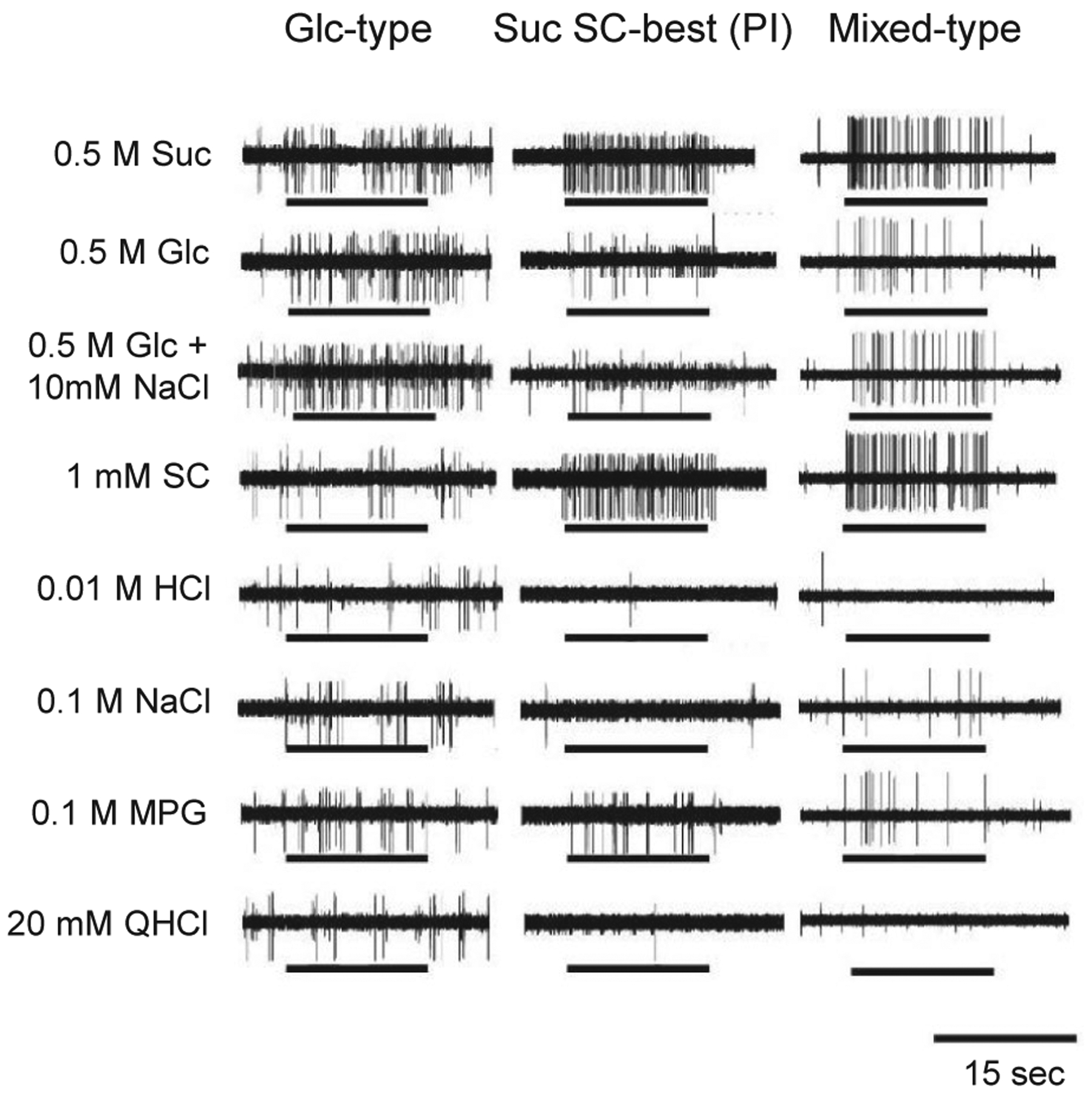
Sample recordings of Glc-, Suc SC best (PI)- and Mix-type single fibres from the chorda tympani nerves of WT mice. Stimuli were 0.5 mol/L Suc, 0.5 mol/L Glc, 0.5 mol/L Glc + 10 mmol/L NaCl, 1 mmol/L SC, 0.01 mol/L HCl, 0.1 mol/L NaCl, 0.1 mol/L MPG and 20 mmol/L QHCl. Lines indicate application of stimuli to the tongue. QHCl, quinine HCl; WT, wild-type
FIGURE 8.
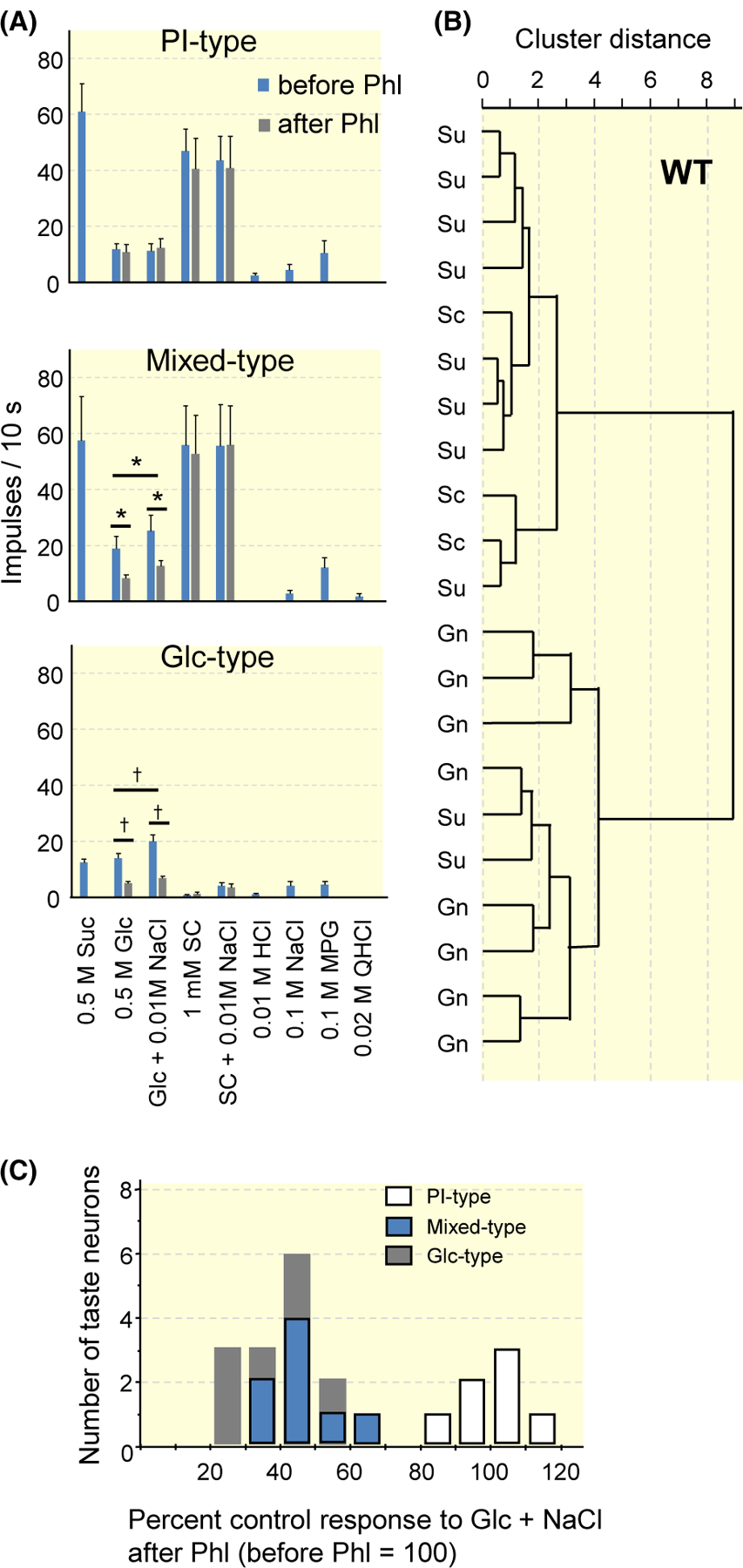
Results of single-fibre recordings from the chorda tympani nerves of WT mice. A, Response profiles of PI- (Suc SC-best), Mix- and Glc-type fibres. Stimuli are 0.5 mol/L sucrose (Suc), 0.5 mol/L glucose (Glc), 0.5 mol/L Glc + 10 mmol/L NaCl, 1 mmol/L SC45647 (SC), SC + 10 mmol/L NaCl, 10 mmol/L HCl, 0.1 mol/L NaCl, 0.1 mol/L monopotassium glutamate (MPG), 20 mmol/L quinine HCl (QHCl). Each column indicates the mean number of net impulses per 10 seconds (mean response) ± SE. Results of paired t test for the effect of the addition of 10 mmol/L NaCl and the effect of 1 mmol/L phlorizin (Phl) on response to Glc are shown in Table 6 and Table 7 respectively; *P < .05, †P < .01. B, Results from hierarchical cluster analysis according to responses to taste stimuli tested. Su, Sc and Gn indicate the stimulus producing the maximal response among Suc, SC and Glc + NaCl in the fibre. C, Fibre distribution by means of Phl sensitivity of the fibres in response to 0.5 mol/L Glc + 10 mmol/L NaCl. WT, wild-type
TABLE 6.
Results of paired t test for the effect of the addition of 10 mmol/L NaCl on single-fibre responses to Glc in the CT of WT mice (Figure 8)
| Before phloridzin | After phloridzin | |
|---|---|---|
| PI-type | t(5) = 0.775, P = .474 | t(5) = 0.914, P = .402 |
| Mixed-type | t(8) = 2.777, P = .024 | t(8) = 2.086, P = .070 |
| Glc-type | t(5) = 2.982, P = .031 | t(5) = 0.434, P = .683 |
Note: Differences between mixture of stimulus with NaCl and the sum of each component were assessed by paired t test.
Abbreviations: CT, chorda tympani; WT, wild-type.
TABLE 7.
Results of paired t test for the effect of 1 mmol/L phloridzin on responses to Glc with or without 0.1 mol/L NaCl in the CT of WT mice (Figure 8)
| Without NaCl | With NaCl | |
|---|---|---|
| PI-type | t(5) = 0.794, P = .463 | t(5) = 0.087, P = .934 |
| Mixed-type | t(8) = 2.780, P = .024 | t(8) = 3.042, P = .016 |
| Glc-type | t(5) = 4.106, P = .009 | t(5) = 4.398, P = .007 |
Abbreviation: CT, chorda tympani; WT, wild-type.
2.7 |. Groupings of S-type fibres in T1R3-KO mice
Given that T1R-expressing cells connect with PI-type fibres, those fibres were expected to be absent and Glc-type fibres were expected to exist in T1R3-KO mice. To test this hypothesis, we recorded single-fibre responses in the CT of T1R3-KO mice. As expected, the responses to Glc or Glc + NaCl were suppressed by Phl in all fibres examined (Figure 9A). Among them, we found a subset of fibres significantly responding to SC, although the response to SC was smaller than that in WT mice. We classified these fibres as Mixed-type (73.3%). Another subset of fibres was classified as Glc-type (26.7%), which had a response profile similar to those in WT mice (Tables 8 and 9). The response to 0.5 mol/L Glc was larger than that to the non-metabolizable SGLT-specific sugar αMDG in the CT of T1R3-KO mice (paired t test, t5 = 4.106, P = .009 for Glc-type, t16 = 3.238, P = .005 for Mixed-type, Figure 9A). We also observed the same tendency in responses to the mixture solutions with 10 mM NaCl (paired t test, t5 = 2.236, P = .076 for Glc-type, t5 = 1.62, P = .126 for Mixed-type). In hierarchical cluster analysis according to responses to the taste stimuli tested (Figure 9B), fibres were classified into two clusters. Glc-type fibres (four fibres in this analysis) are located at the top of the figure, and the others were Mixed-type fibres. In fibre distribution by means of Phl sensitivity of the fibres (Figure 9C), the results showed unimodal distribution of fibres. All Glc-type and Mixed-type fibres were in the PS-type group. From these three figures, it was suggested that SGLTs are the main receptors in T1R3-KO mice, and that the residual response to SC reveals the function of T1R1 or T1R2 in taste cells connecting with Mixed-type fibres.
FIGURE 9.
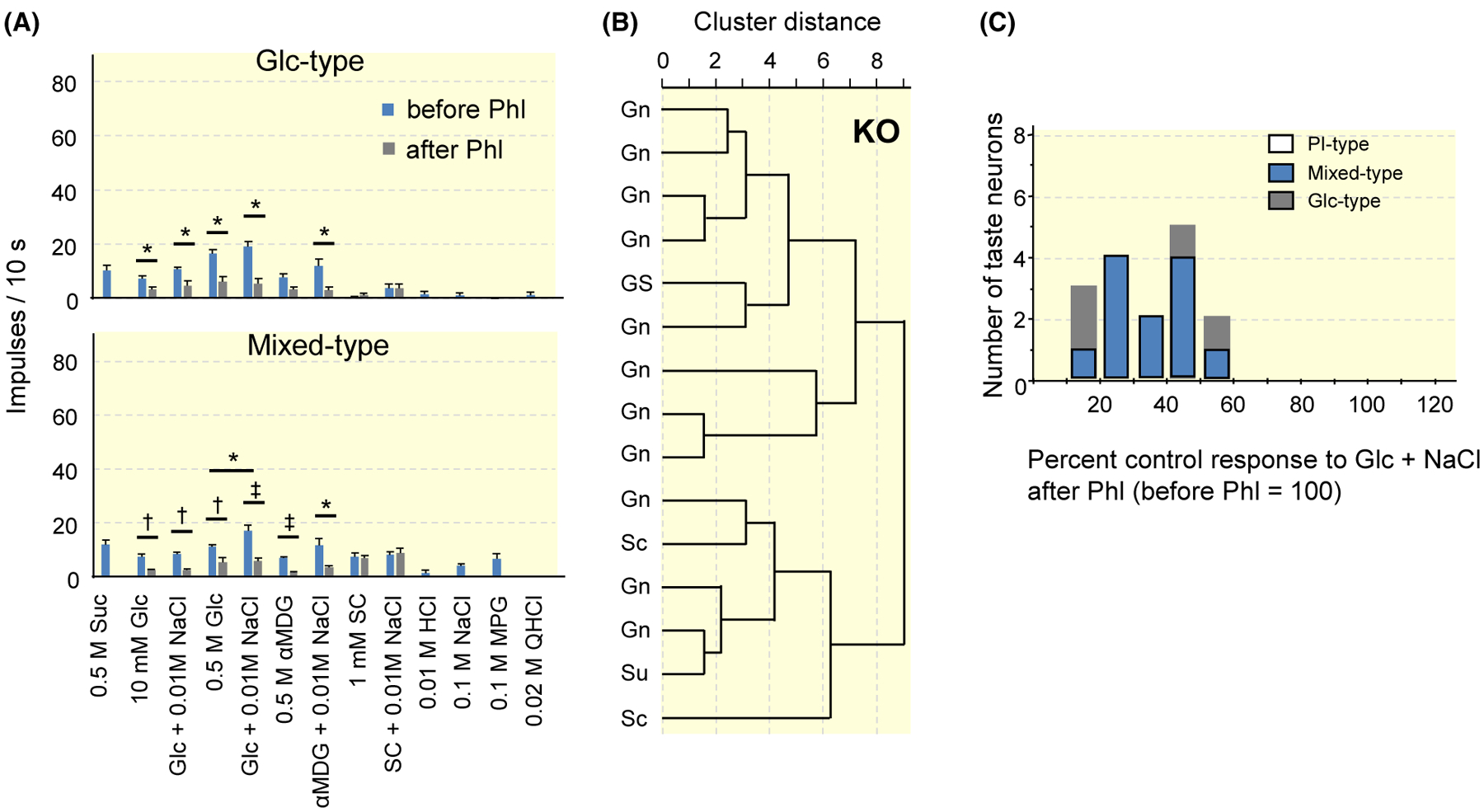
Results of single-fibre recordings from the chorda tympani nerves of T1R3-KO mice. A, Response profiles of Glc- and Mix-type fibres. Stimuli are 0.5 mol/L sucrose (Suc), 10 mmol/L glucose (Glc), 10 mmol/L Glc + 10 mmol/L NaCl, 0.5 mol/L Glc, 0.5 mol/L Glc + 10 mmol/L NaCl, 0.5 mol/L αMDG, 0.5 mol/L αMDG + 10 mmol/L NaCl, 1 mmol/L SC45647 (SC), SC + 10 mmol/L NaCl, 10 mmol/L HCl, 0.1 mol/L NaCl, 0.1 mol/L monopotassium glutamate (MPG), 20 mmol/L quinine HCl (QHCl). Each column indicates the mean number of net impulses per 10 seconds (mean response) ± SE. Results of paired t test for the effect of the addition of 10 mmol/L NaCl and the effect of 1 mmol/L phlorizin (Phl) on responses to Glc or αMDG are shown in Table 8 and Table 9 respectively; *P < .05, †P < .01, ‡P < .001. B, Results from hierarchical cluster analysis according to responses to taste stimuli tested. Su, Sc, Gn indicate the stimulus producing the maximal response among Suc, SC and Glc + NaCl in the fibre. GS indicate same impulse frequencies in responses to Glc + NaCl and Suc. C, Fibre distribution by means of Phl sensitivity of the fibres in response to 0.5 mol/L Glc + 10 mmol/L NaCl
TABLE 8.
Results of paired t test for the effect of the addition of 10 mmol/L NaCl on single-fibre responses to stimuli in the CT of WT mice (Figure 9)
| Before phloridzin | After phloridzin | |
|---|---|---|
| Glc-type | ||
| 10 mmol/L Glc | t(2) = 2.464, P = .118 | t(2) = 0.378, P = .742 |
| 0.5 mol/L Glc | t(3) = 3.000, P = .058 | t(3) = 1.567, P = .215 |
| 0.5 mol/L αMDG | t(2) = 1.429, P = .289 | t(2) = 0.718, P = .547 |
| Mixed-type | ||
| 10 mmol/L Glc | t(5) = 1.291, P = .253 | t(4) = 0.784, P = .477 |
| 0.5 mol/L Glc | t(10) = 2.824, P = .018 | t(10) = 1.064, P = .310 |
| 0.5 mol/L αMDG | t(5) = 1.056, P = .339 | t(5) = 0.395, P = .709 |
Note: Differences between mixture of stimulus with NaCl and the sum of each component were assessed by paired t test.
Abbreviations: CT, chorda tympani; WT, wild-type.
TABLE 9.
Results of paired t test for the effect of 1 mmol/L phloridzin on responses to stimuli with or without 0.1 mol/L NaCl in the CT of WT mice (Figure 9)
| Without NaCl | With NaCl | |
|---|---|---|
| Glc-type | ||
| 10 mmol/L Glc | t(2) = 6.928, P = .020 | t(2) = 6.000, P = .027 |
| 0.5 mol/L Glc | t(3) = 5.308, P = .013 | t(3) = 5.329, P = .013 |
| 0.5 mol/L αMDG | t(2) = 1.982, P = .186 | t(2) = 5.196, P = .035 |
| Mixed-type 10 | ||
| 10 mmol/L Glc | t(5) = 4.443, P = .007 | t(4) = 4.670, P = .010 |
| 0.5 mol/L Glc | t(10) = 3.480, P = .006 | t(10) = 9.114, P < .001 |
| 0.5 mol/L αMDG | t(5) = 7.348, P < .001 | t(5) = 2.870, P = .035 |
Abbreviations: CT, chorda tympani; WT, wild-type.
2.8 |. Behavioural role of SGLTs in T1R3-KO mice
Figure 10A shows the mean number of licks for 3–800 mmol/L Glc (open circles) and Glc + 10 mmol/L NaCl (filled circles) mixed with 1 mmol/L QHCl in T1R3-KO mice. The number of licks for these solutions shows a Glc concentration dependence. When Glc solutions were presented without QHCl, the number of licks for the solutions were essentially the same as those for distilled water (DW) (70–80) due to water deprivation. In addition, the number of licks for 1 mmol/L QHCl before and after Phl was ~2. The number of licks for the Glc–QHCl mixture were significantly enhanced by the addition of 10 mmol/L NaCl. (Table 1). To investigate the involvement of SGLTs in these behavioural responses to Glc or Glc + NaCl mixed with QHCl, we applied 1 mmol/L Phl to the tongue and measured the number of licks. After Phl treatment, the number of licks for the Glc (white triangles)—or Glc + NaCl (black triangles)—QHCl mixtures were significantly smaller than those for Glc or the Glc + NaCl–QHCl mixture before Phl (Table 1).
FIGURE 10.
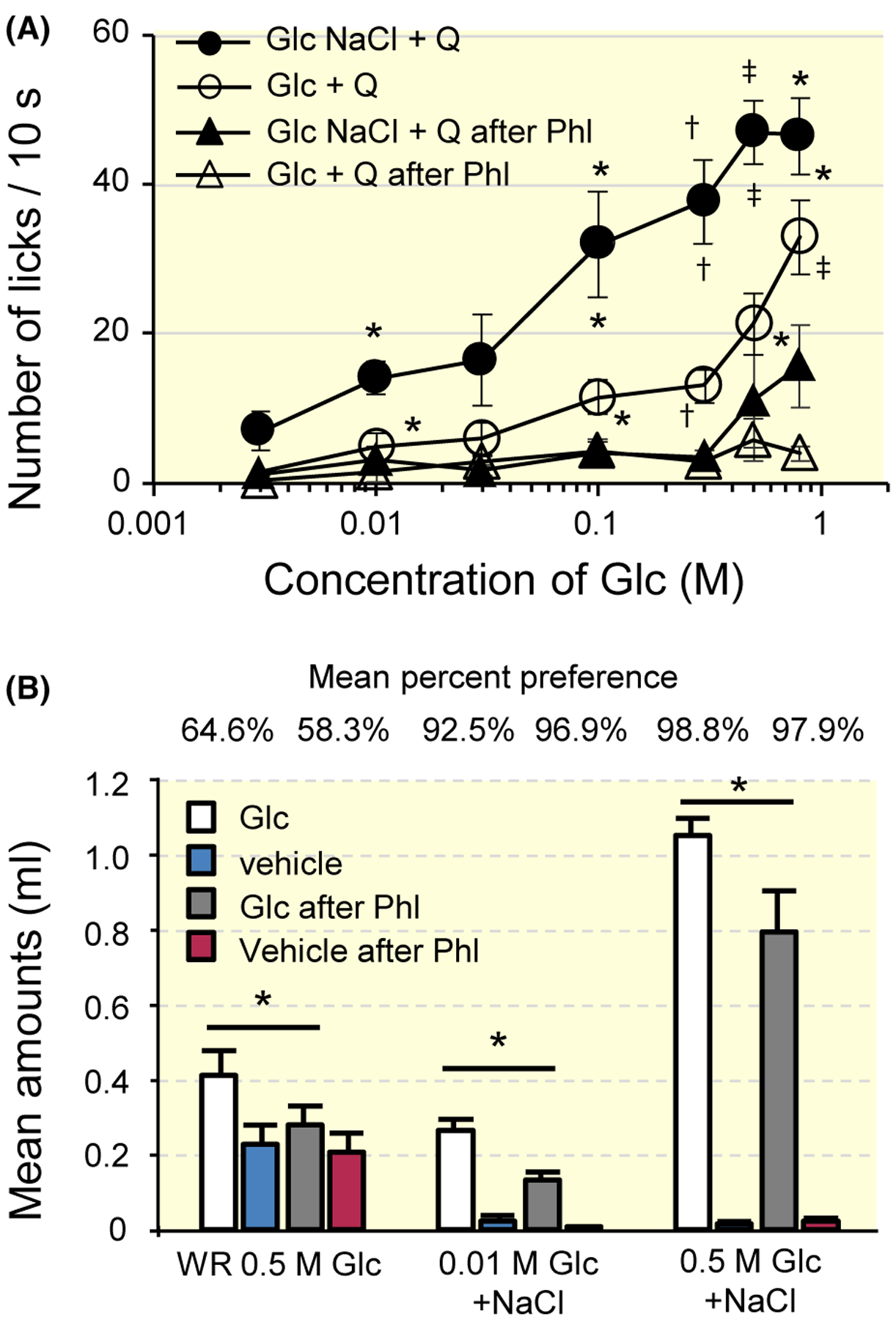
Results from behavioural experiments from T1R3-KO mice. A, Mean numbers of licks for 3–800 mmol/L Glc (open circles) and Glc + 10 mmol/L NaCl (filled circles) mixed with 1 mmol/L QHCl and those after phlorizin (Phl) treatment (triangles) in T1R3-KO mice. Values indicated are means ± SE. Results of repeated measures ANOVA are shown in Table 1. Results of post hoc Fisher test to evaluate enhancement of the response by Glc and NaCl mixture was shown by top symbols, and that to evaluate suppression by Phl was shown by other symbols; *P < .05, †P < .01, ‡P < .001. B, Short-term preference test in T1R3-KO mice. Amounts of intake for Glc + 10 mmol/L NaCl solutions were measured under water restricted (WR) or food restricted conditions. Mean percent preferences were indicated on the top. Values indicated are means ± SE. Paired t test; *P < .05. QHCl, quinine HCl
In a short-term preference test (Figure 10B), Phl suppressed the amount of intake for 0.5 mol/L Glc of water restricted T1R3-KO mice (paired t test, t5 = 2.671, P = .044), for 0.01 mol/L Glc + 10 mmol/L NaCl (paired t test, t5 = 3.515, P = .017), and for 0.5 M Glc + 10 mM NaCl (paired t test, t5 = 3.189, P = .024) of food restricted T1R3-KO mice. These results were consistent with those for the behavioural response to the Glc–QHCl mixture mentioned above (Figure 10A).
3 |. DISCUSSION
3.1 |. Functional evidence for SGLTs as a sugar taste sensor
This study demonstrated that SGLTs mediate T1R-independent responses to sugars in taste tissues of the anterior and posterior of mouse tongue. Among the six members of SGLT1-6, the Phl-sensitive galactose response was observed in the CT of WT mice, suggesting the function of SGLT1 in sugar sensing. However, we did not elucidate the function of each SGLT in this study because of the low selectivity of Phl’s effect on SGLT1, SGLT2 and SGLT4.14 Thus, we use ‘SGLTs’ for discussion. As ligands, we used sugars, including a disaccharide, Suc. Recently, mouse taste cells were reported to express maltase-glucoamylase, sucrase-isomaltase, lactase, trehalase and α-glucosidase C, which can convert oligosaccharides and disaccharides into monosaccharides.15 By these disaccharidases at the taste pores, oligosaccharides and disaccharides, including Suc, starch hydrolysis products16 can activate SGLTs after conversion into monosaccharides.
It has been reported that the responses to sugars were greatly enhanced by the presence of Na+ in the canine CT. The magnitude of the sucrose response is greatly increased with an increase in NaCl concentration up to 100 mmol/L, and the enhanced response is decreased with a further increase in NaCI concentration.17 In addition, the canine lingual epithelium showed that both monosaccharides and disaccharides in the mucosa stimulate a dose-dependent increase in the short-circuit current over the concentration range coincident with mammalian sugar taste responses in the presence of 30 mmol/L NaCl.18 These studies using canine are consistent with the present study showing the function of SGLTs in mice. The enhancing effect on responses to Glc were prominent when 10 or 30 mmol/L NaCl were added in the present study. We further observed that a fluorescent D-glucose derivative 2-NBDG uptake by taste cells occurred through Phl-sensitive glucose transporters. The fluorescent intensity of 2-NBDG in the presence of 10 mmol/L NaCl was significantly higher than that in the absence of NaCl. hSGLT1 is a 72-kDa protein with 14 transmembrane helices that transports two Na+ ions and one Glc molecule in one cycle, which takes 20 ms.19 In vitro, SGLT1 is reportedly critical for the increase in action potentials that occur via the elevation of extracellular glucose, with associated inward Na+ currents depolarizing the membrane potential of L cells.9,20,21 The influx of Na+ ions simultaneously open voltage-sensitive calcium channels and the exocytose of GLP-1-containing secretory vesicles.20 In addition, KATP channel closure is reportedly required to stimulate a full-blown glucose-induced response in rat small intestine.22 When glucose rises in the enteroendocrine cells or pancreatic β-cells, metabolism is stimulated, raising the ratio of ATP to ADP. This closes KATP channels, which depolarizes the cell, opening Ca2+ channels and triggering peptide or insulin secretion.23,24 However, a recent study concluded that, in healthy mice, the KATP channels are mostly closed and are therefore unlikely to play a major role in glucose-stimulated hormone secretion.25 Furthermore, GLP-1 secretion is reportedly triggered by the non-metabolizable monosaccharide indicating that the role of KATP channels in intestinal L-cells is minor.20,21 Based on these reports, the observed nerve responses of this study might be mainly the results of taste cell depolarization, which is generated by Na+ currents via SGLTs. However, the response to 0.5 mol/L Glc was larger than that to the non-metabolizable SGLT-specific sugar αMDG in the CT of T1R3-KO mice, suggesting involvement of other mechanisms, such as GLUTs and/or KATP channel closure, in the analysed responses. It is well known that ATP play a crucial role in taste signalling pathways.26 GLP-1 is also suggested to be involved in sweet taste signal transmission to taste nerves.27 Future work will be necessary to determine precise transduction mechanisms and transmitters of taste cells connecting to Glc-, PI- and Mixed-type fibres.
3.2 |. Concentration of NaCl in the oral cavity
Although the tongue adapted to DW in this study, taste receptors usually adapt to the salivary environment. The concentration of Na+ in human mixed saliva from all salivary grands is in the range of 5–100 mEq/L.28 This concentration range is consistent with results from whole-nerve responses to the concentration series of NaCl in this study (Figure 2A) Particularly, the concentration of Na+ increases according to the increase in the flow rate of saliva during meals. Therefore, SGLTs may function as a sugar taste receptor every time carbohydrates are ingested. In this study, we observed Phl-sensitive responses to Suc, Glc or Glc analogues even when salts were not added or saliva was washed out. There also is a possible effect of microenvironments. The responses might occur through co-transport of Na+ from nearby cells. The taste cells which respond to salts or sour stimuli may excrete Na+ after influx into those cells. It is also well known that type I taste cells are secretory cells. However, these possibilities have not been investigated so far.
3.3 |. Effective concentration of Glc
We used 3 mmol/L and higher concentrations of Glc as stimulants and observed significant suppression of the responses to 10 mmol/L Glc with or without NaCl by Phl in whole-nerve responses in WT mice and in single-fibre and behavioural responses in T1R3-KO mice. Regarding luminal Glc absorption in the small intestine, Km values for SGLT1-mediated transport are in the range 6–23 mmol/L for in vivo and 2–6 mmol/L for in vitro studies.29 That in vivo concentration range is consistent with what was demonstrated in this study (~10 mmol/L). The driving force of sugar transport via SGLT1 is considered to be a downward gradient of Na+ from extracellular to the intracellular fluid, which is maintained by Na+ K+-ATPase.30 Using abundant Na+ in saliva, SGLT1 may be able to transport Glc even when the extracellular concentration is smaller than the intracellular concentration. In this study, we also used a higher concentration range of Glc than that in SGLT1-mediated transport, and detected significant suppression of Phl in the responses to 0.3–1 mol/L sugars in nerve recordings. Kellett and colleagues suggested that GLUT2 is trafficked to the apical membrane after activation of SGLT1 and/or T1R2 + T1R3, which is accompanied by an increase in PLC, PKC and intracellular calcium in rat enterocytes.31,32 The involvement of tongue-expressing GLUTs will be examined in future.
3.4 |. Independence of sugar taste
In our previous study, qualitative similarities and differences among various taste stimuli were examined by comparing the generalization patterns of a conditioned aversion from single chemicals to other compounds in mice.33 In the study, one group each, among 15 groups, was conditioned to avoid a different stimulus among 15 stimuli. A hierarchical cluster analysis was performed and the results showed that generalization patterns of Glc and maltose were not significantly correlated with those of Suc, Sac, D-tryptophan, D-phenylalanine and fructose in C57BL/6 mice. This study employed SC instead of Sac in single-fibre recordings. Because these sweet compounds activate T1R3,1 and Glc and maltose (after being hydrolysed to glucose) activate SGLTs, this study provided evidence of neural mechanisms that underlie the results from our previous behavioural study. The difference in receptors or receptor sites has also been reported in human by means of cross adaptation among many sweeteners.34 It was observed that non-caloric sweeteners are fully cross-adapted by sugars, but sugars are only partially cross-adapted by non-caloric sweeteners, indicating the existence of a T1R-independent receptor site (or sites) that mediates the perception of sugar sweetness. Concerning taste intensity, although non-caloric sweeteners evoke a sweet taste at lower concentrations than sugars, indicating a higher affinity of non-caloric sweeteners for T1Rs than that of sugars for T1Rs or SGLTs, artificial sweeteners reportedly have a tendency to elicit a lower maximal sweet taste intensity than sugars in human,35,36 and to elicit a lower rate of licking in rodents.37
This study explains these differences between sugars and non-caloric (artificial) sweeteners described in previous studies. It is likely that we can discriminate sweet compounds in foods or beverages thorough the activities of T1R3-dependent or SGLT-dependent neurons. Furthermore, oral administration of sucrose (Suc) and Glc mediates CPIR in T1R3-KO mice, indicating the important role of a T1R-independent sugar receptor on CPIR.3 Glc has reportedly evoked a most significant CPIR among sugars, sugar alcohols and a non-nutritive sweeteners, suggesting the existence of Glc-specific taste receptors and neurones.38 However, there is no direct evidence of involvement of SGLTs in this CPIR. Because the SGLT1-KO and WT mice were maintained on a low-carbohydrate diet from weaning, the insulin level of the WT mice was lower than that of other WT controls.39 Future work is necessary to examine the function of tongue-expressed SGLTs by tissue-specific blocks.
3.5 |. Coexpression of sweet receptors
Among the CT fibres responding to sweet compounds, 42.9% of fibres (Mixed-type) showed responses to Glc, Suc and SC, and enhanced response to Glc by the addition of 10 mmol/L NaCl in WT mice. Previous immunohistochemistry data demonstrated that the expression of SGLT1 and members of GLUTs are preferentially expressed in T1R3-positive taste cells.5 A total of 80%–85% of SUR1, a KATP subunit, expressing taste cells coexpress T1r3 in the CV and foliate papillae. For mouse fungiform taste cells, sulphonylurea, an inhibitor of the KATP channel, reportedly inhibits outward (K+) currents, indicating the depolarization of the cell after the absorption of glucose via the transporter(s).5 At submaximal levels, the combined activation of T1Rs and transporters by sugars likely provide an enhanced perception of sweet taste over that achieved by either receptor alone. The perceptive intensity of sweetness of sugars may be due to this mechanism in human. Our previous data showed that a subset of S-type fibres also respond to glutamate with IMP40 and to long-chain fatty acids, linoleic acid and oleic acid.41 That is, we have found a group of fibres that respond to three major nutrients. We propose that taste cells connecting to this subset of S-type fibres be designated as ‘calorie sensing cells’, which may function to find energy sources. While eating meals, the gustatory system is usually activated by taste mixtures from ingredients in foods and seasonings. If the mixtures are composed of sugars, proteins and fats, the food is more palatable and stimulates appetite and consumption via activation of the calorie sensing cells. However, the link between such energy-dense foods and metabolic syndrome, including obesity, is an important issue in food-rich environments.
3.6 |. Conclusion
This study provided functional evidence for the contribution of SGLTs in sugar sensing in taste cells of both the anterior and posterior mouse tongue. Moreover, we found three types of S-type fibres, T1R-dependent (Phl-insensitive) type, Glc-type and Mixed-type fibres, which connect taste cells expressing both transporters and T1Rs. SGLT1 may be involved in the latter two types and may play an important role in sugar sensing, which is associated with palatability and the cephalic phase of digestion. Taste information of T1R-dependent and Glc-type fibres may contribute to distinguishing between artificial sweeteners and sugars, and Mixed-type fibres may contribute to finding and ingesting energy-dense foods.
4 |. METHODS
4.1 |. Ethical approval
All experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the committee for Laboratory Animal Care and Use and the local ethics committee at Kyushu University, Tokyo Dental College and Okayama University Japan.
4.2 |. Animals
Subjects were adult male C57BL/6JCrj (WT) mice (Charles River Japan, Tokyo, Japan) and T1R3- KO mice, originally developed at Mount Sinai Medical School from the C57BL/6J strain and produced by homologous recombination in C57BL/6 embryonic stem cells and maintained in this background.1 All mice were maintained on a 12/12-h light/dark cycle and fed standard rodent chow. Animals were 8–20 weeks of age, ranging in weight from 20 to 35 g.
4.3 |. Gustatory nerve recording
Whole-nerve responses to lingual application of tastants were recorded from the CT or the GL nerves and single-fibre responses were recorded from the CT as described previously.1,13,42,43 Mice were anesthetized with an injection of sodium pentobarbital (40–50 mg/kg ip) and maintained at a surgical level of anaesthesia with additional injections of sodium pentobarbital (8–10 mg/kg ip every hour). Under anaesthesia, the mouse was then fixed in the supine position with a head holder and the trachea of each mouse was cannulated. The right CT nerve was dissected free from surrounding tissues after removal of the pterygoid muscle and cut at the point of its entry to the tympanic bulla. The right GL nerve from a different animal was exposed, dissected free from underlying tissues and cut near its entrance to the posterior lacerated foramen. In the case of whole-nerve recording, the entire nerve was placed on the Ag-AgCl electrode. In the case of single-fibre recording, a single or a few fibres of the nerve were teased apart with a pair of needles and lifted onto an Ag-AgCl electrode. An indifferent electrode was placed in nearby tissue. Neural activities were amplified (K-1; Iyodenshikagaku, Nagoya, Japan), and monitored on an oscilloscope and audio monitor. Integrated whole-nerve responses with a time constant of 1.0 seconds and impulse discharges during single-fibre recordings recorded on a computer for later analysis using a PowerLab system (PowerLab/sp4; AD Instruments, Bella Vista, NSW, Australia).
For taste stimulation of fungiform papillae (FP), the anterior half of the tongue was enclosed in a flow chamber made of silicone rubber.44 For taste stimulation of the CV and foliate papillae, an incision was made on each side of the animal’s face from the corner of the mouth to just above the angle of the jaw, and the papillae were exposed and their trenches opened by slight tension applied through a small suture sewn in the tip of the tongue. All chemicals were used at ~24°C. Taste solutions were delivered to each part of the tongue by gravity flow for ~10 (single fibre), 30 (CT) and 60 seconds (GL) at the same flow rate as the DW used for rinse (~0.1 mL s−1). We used longer stimulation time for the GL nerve response recording which is thought to be, unlike stimulation of fungiform taste buds located on the tongue surface, necessary for delivered test solutions to reach CV and foliate taste buds located on basal side walls of deep and narrow trench formed by the taste papillae and surrounding tissues. The tongue was washed with DW for an interval of 1 minutes between successive stimulation. Only responses from stable recordings were used for data analysis. At the end of the experiment, animals were killed by the administration of an overdose of the anaesthetic.
4.4 |. Uptake of a fluorescent D-glucose derivative into taste bud cells
Subjects were adult (>8 weeks old) male and female WT mice (n = 18, n = 3 for each condition). After euthanasia by cervical dislocation, the anterior part of the tongue was removed and washed with Tyrode solution. Apical side of the removed tongue was wiped with KimWipe (Nippon Paper Crecia, Tokyo, Japan) to remove adhering solution then treated with 1–10 mmol/L 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl) Amino)-2-Deoxyglucose (2-NBDG) + 10 mmol/L NaCl, 10 mmol/L 2-NBDG + 10 mmol/L NaCl + 1 mmol/L Phl or 1 mmol/L phroletin, or 10 mmol/L 2-NBDG for 15 min at room temperature in the dark chamber. If Phl or phloretin mixture was used, the tongue was pre-treated with 1 mmol/L Phl or phloretin 10 minutes before 2-NBDG treatment. The treated tongue was washed with Tyrode solution and wiped with a cotton swab. Then, a part of tongue muscles was removed by forceps and pinned out in a Sylgard coated culture dish with the apical side up. Pinned tongue tissue was incubated in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 5 minutes at 4°C and washed several times with Tyrode solution. Optical sections of taste buds in the FP were obtained by laser scanning confocal microscope (FV-1000 and Fluoview; Olympus, Tokyo, Japan) with water immersion objective lens (LUMPlanFl x40, Olympus) using following conditions; excitation 488 nm, emission 500–600 nm, 1.5 μm/section, 15 sections. Fluorescent images of 10th optical sections of taste buds (15μm below the apical side) were analysed by NIH image-J software (Maryland, USA). 58–60 taste buds were analysed in each animal.
4.5 |. Behavioural responses to glucose-quinine mixture
All training and testing sessions occurred during the light phase of the light/dark cycle. Experimental protocols of this experiment were modified from those in our published papers.27,45 Because the number of licks for sweet compounds alone were nearly maximal at any concentration owing to water deprivation for 23 hours, we employed a previously used procedure in which QHCl-sweet mixtures are used to obtain concentration-dependent changes in licks for Suc.45 On the first day of training, T1R3-KO mice were water deprived for 23 hours, and then each one placed in a test box and given free access to DW during a One h-session from a polyethylene tube via a circular window (5 mm in diameter). The tip of a tube (1.5 mm in inner diameter) was located 2 mm outside the window. This arrangement prevented contact of the tip of the tube with the animal’s lips. The number of licks per 10 seconds was measured by a lickometer with a laser beam sensor (Yutaka Electronics Company, Gifu, Japan) and recorded on a pen recorder. From day 2 to day 5, training session time was reduced from 1 hour to 30 minutes. During this period, the animal was trained to drink DW on an interval schedule, consisting of 10 seconds periods of presentation of DW alternated with 20 seconds inter-trial intervals, resulting in 30–50 trials. On days 6–9, lick rates for each of the test stimuli and DW were measured during the first 10 seconds after the animal’s first lick. To examine the effect of Phl, licks for 3–800 mmol/L Glc or Glc + 10 mmol/L NaCl as mixtures with 1 mmol/L QHCl (same concentration used in Takai et al, 2015)27 were measured before and after licking 1 mmol/L Phl in T1R3-KO mice. All solutions were presented to mice at room temperature (~24°C).
4.6 |. Short-term preference test
We next conducted measurement of amount of intake for 3 min (modified from 3-min lick test by Sclafani & Ackroff, 2018)46 to determine if T1R3-KO mice display preferences for 10 mmol/L or 500 mmol/L Glc with 10 mmol/L NaCl. T1R3-KO and WT mice were housed as in prior experiments. The animals were familiarized with test cages and two bottles by housing them in the cages with ad libitum chow and water. Next, they were water deprived overnight and allowed to drink from two water bottles in the test cages for 5 minutes. On the following 2 days the mice were given two-bottle access to Glc or Glc after Phl vs water for 3 minutes. One h after the session, a second 3-min two-bottle test was conducted with the left-right positions of the Glc and water bottles reversed. On the first two water-restriction days the mice were given 1-h access to water in the home cage after training to maintain hydration. On the third day and thereafter, they were given ad libitum access to water, but restricted chow rations (1–3 g) to maintain them at 90% of their ad libitum body weight. While food restricted, they were tested for 4 days with 10 mmol/L Glc + NaCl vs water, 10 mmol/L Glc + NaCl after Phl vs water, 500 mmol/L Glc + NaCl vs water and 500 mmol/L Glc + NaCl after Phl vs water.
4.7 |. Solutions
Test stimuli were 100 mmol/L NH4Cl, 100 mmol/L KCl, 5 mmol/L citric acid, 10 mmol/L HCl, 0.3 mmol/L (for GL) and 1 mmol/L (for CT), QSO4, 1 mmol/L (for a behavioural experiment) or 20 mmol/L (for single-fibre recordings) QHCl, 100 mmol/L mol/LPG, 0.5 mmol/L inosine monophosphate (IMP), 0.1 mol/L CaCl2, 3–1000 mmol/L Glc, 500 mmol/L Suc, 500 mmol/L galactose, 1 mmol/L SC, 10 mmol/L saccharin, 16% polycose and 0.5 mol/L methyl-α-D-glucopyranoside (αMDG). To make binary mixture solutions, 1–100 mmol/L NaCl was used. All these chemicals were dissolved in DW. Phl, a competitive inhibitor of SGLTs, was dissolved in DW (with KOH to adjust their pH to 7.0). Regents were purchased from Sigma (St Louis, MO, USA; MPG and phlorizin), and Wako Pure Chemical Industries (Osaka, Japan; others). SC was a gift from Dr. Margolskee.
4.8 |. Data analysis
In the analysis of whole-nerve recordings, integrated whole-nerve response magnitudes were measured from 5 to 25 seconds (for the CT) and, from 10 to 40 seconds (for the GL) after stimulus onset. These values were averaged and normalized to responses to 100 mmol/L NH4Cl to account for mouse-to-mouse variations in absolute responses. This relative response was used for statistical analysis (two-way ANOVA with post hoc Fisher test and Student’s unpaired t test or paired t test). Two-way ANOVA and Student’s t test were used to statistically evaluate the treatments (with and without NaCl, Phl) or concentration. The effect of Phl, phloretin and NaCl on 2-NBDG uptake were analysed by one-way ANOVA with post hoc Tukey HSD test. In the analysis of single-fibre responses, single fibres were identified with the help of spike wave form analysis (PowerLab/sp4; AD Instruments). We used waveform shape parameters (width, height, peak amplitude, antipeak amplitude, interspike interval) to segregate each single unit.13,42 For data analysis, we used the net average frequency for the 10 seconds after the stimulus onset, which was obtained by subtracting the spontaneous frequency for the 10 seconds period before stimulation. The occurrence of a responses was determined according to the criteria that the number of spikes was larger than the mean plus 2 SDs of the spontaneous discharge for three 10 seconds periods before stimulation. Hierarchical cluster analysis was used to know the similarity of response profiles, and consequently classification of fibre types was obtained. The clustering program (Excel ad-in soft for statistical analysis, SSRI, Tokyo, Japan) processed the fibre profiles based on a matrix of the Pearson correlation coefficients between all possible pairs of profiles and amalgamated the fibre sequentially into the cluster solution using the Ward method. Repeated-measures ANOVA and post hoc Fisher test or Student’s t test were used to statistically evaluate the effects of the addition of NaCl and the effect of Phl on CT fibre responses. For the analysis of behavioural experiments, paired t test and repeated-measures ANOVA followed by post hoc Fisher test were used to statistically evaluate the effects of treatment. Calculations were performed using the statistical software packages IBM SPSS Statistics (IBM, NY, USA).
Funding information
This work was supported by JSPS KAKENHI JP 26670810, JP 15H02571, JP 18H02968, JP 18K19653 (YN), JP 15K11043, JP 20H03855 (KY), JP 18K09507 (RY) for Scientific Research from the Japan Society for the Promotion of Science.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- 1.Damak S, Rong M, Yasumatsu K, et al. Detection of sweet and umami taste in the absence of taste receptor T1R3. Science. 2003;301:850–853. [DOI] [PubMed] [Google Scholar]
- 2.Ohkuri T, Yasumatsu K, Horio N, et al. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R960–R971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glendinning JI, Stano S, Holter M, et al. Sugar-induced cephalic-phase insulin release is mediated by a T1r2+T1r3-independent taste transduction pathway in mice. Am J Physiol Regul Integr Comp Physiol. 2015;309(5):R552–R660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merigo F, Benati D, Cristofoletti M, Osculati F, Sbarbati A. Glucose transporters are expressed in taste receptor cells. J Anat. 2011;219(2):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci USA. 2011;108(13):5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toyono T, Seta Y, Kataoka S, Oda M, Toyoshima K. Differential expression of the glucose transporters in mouse gustatory papillae. Cell Tissue Res. 2011;345(2):243–252. [DOI] [PubMed] [Google Scholar]
- 7.Oyama Y, Yamano H, Ohkuma A, Ogawara K-I, Higaki K, Kimura T. Carrier-mediated transport systems for glucose in mucosal cells of the human oral cavity. J Pharm Sci. 1999;88(8):830–834. [DOI] [PubMed] [Google Scholar]
- 8.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91(2):733–794. [DOI] [PubMed] [Google Scholar]
- 9.Gorboulev V, Schürmann A, Vallon V, et al. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61(1):187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab. 2009;297(6):E1358–E1365. [DOI] [PubMed] [Google Scholar]
- 11.Röder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One. 2014;9(2):e89977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada K, Saito M, Matsuoka H, Inagaki N. A real-time method of imaging glucose uptake in single, living mammalian cells. Nat Protoc. 2007;2:753–762. [DOI] [PubMed] [Google Scholar]
- 13.Ninomiya Y Reinnervation of cross-regenerated gustatory nerve fibres into amiloride-sensitive and amiloride-insensitive taste receptor cells. Proc Natl Acad Sci USA. 1998;95:5347–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raja M, Kinne RK. Identification of phlorizin binding domains in sodium-glucose cotransporter family: SGLT1 as a unique model system. Biochimie. 2015;115:187–193. [DOI] [PubMed] [Google Scholar]
- 15.Sukumaran SK, Yee KK, Iwata S, et al. Taste cell-expressed α-glu-cosidase enzymes contribute to gustatory responses to disaccharides. Proc Natl Acad Sci USA. 2016;113(21):6035–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robyt JF, French D. Multiple attach hypothesis of alpha-amylase action: action of porcine pancreatic, human salivary, and Aspergillus oryzae alpha-amylases. Arch Biochem Biophys. 1967;122(1): 8–16. [DOI] [PubMed] [Google Scholar]
- 17.Kumazawa T, Kurihara K. Large enhancement of canine taste responses to sugars by salts. J Gen Physiol. 1990;95(5):1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mierson S, DeSimone SK, Heck GL, DeSimone JA. Sugar-activated ion transport in canine lingual epithelium. Implications for sugar taste transduction. J Gen Physiol. 1988;92(1):87–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright EM, Ghezzi C, Loo DDF. Novel and unexpected functions of SGLTs. Physiology (Bethesda). 2017;32(6):435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52(5):1147–1154. [DOI] [PubMed] [Google Scholar]
- 21.Parker HE, Adriaenssens A, Rogers G, et al. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia. 2012;55(9):2445–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhre RE, Frost CR, Svendsen B, Holst JJ. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes. 2015;64(2):370–382. [DOI] [PubMed] [Google Scholar]
- 23.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440(7083):470–476. [DOI] [PubMed] [Google Scholar]
- 24.Goldspink DA, Reimann F, Gribble FM. Models and tools for studying enteroendocrine cells. Endocrinology. 2018;159(12):3874–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogata H, Seino Y, Harada N, et al. KATP channel as well as SGLT1 participates in GIP secretion in the diabetic state. J Endocrinol. 2014;222(2):191–200. [DOI] [PubMed] [Google Scholar]
- 26.Finger TE, Danilova V, Barrows J, et al. ATP signalling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. [DOI] [PubMed] [Google Scholar]
- 27.Takai S, Yasumatsu K, Inoue M, et al. Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB J. 2015;29(6):2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuo R Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med. 2000;11(2):216–229. [DOI] [PubMed] [Google Scholar]
- 29.Ferraris RP, Yasharpour S, Lloyd KC, Mirzayan R, Diamond JM. Luminal glucose concentrations in the gut under normal conditions. Am J Physiol. 1990;259(5 Pt 1):G822–G837. [DOI] [PubMed] [Google Scholar]
- 30.Crane RK. Hypothesis for mechanism of intestinal active transport of sugars. Fed Proc. 1962;21:891–895. [PubMed] [Google Scholar]
- 31.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582(Pt 1):379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr. 2008;28:35–54. [DOI] [PubMed] [Google Scholar]
- 33.Ninomiya Y, Higashi T, Katsukawa H, Mizukoshi T, Funakoshi M. Qualitative discrimination of gustatory stimuli in three different strains of mice. Brain Res. 1984;322(1):83–92. [DOI] [PubMed] [Google Scholar]
- 34.Schiffman SS, Cahn H, Lindley MG. Multiple receptor sites mediate sweetness: evidence from cross adaptation. Pharmacol Biochem Behav. 1981;15(3):377–388. [DOI] [PubMed] [Google Scholar]
- 35.Schiffman SS, Booth BJ, Losee ML, Pecore SD, Warwick ZS. Bitterness of sweeteners as a function of concentration. Brain Res Bull. 1995;36(5):505–513. [DOI] [PubMed] [Google Scholar]
- 36.Glendinning JI. Oral Post-Oral Actions of Low-calorie sweeteners: A tale of contradictions and controversies. Obesity. 2018;26(Suppl 3):S9–S17. [DOI] [PubMed] [Google Scholar]
- 37.Smith JC, Sclafani A. Saccharin as a sugar surrogate revisited. Appetite. 2002;38(2):155–160. [DOI] [PubMed] [Google Scholar]
- 38.Grill HJ, Berridge KC, Ganster DJ. Oral glucose is the prime elicitor of preabsorptive insulin secretion. Am J Physiol. 1984;246(1 Pt 2):R88–95. [DOI] [PubMed] [Google Scholar]
- 39.Glendinning JI, Frim YG, Hochman A, Lubitz GS, Basile AJ, Sclafani A. Glucose elicits cephalic-phase insulin release in mice by activating KATP channels in taste cells. Am J Physiol Regul Integr Comp Physiol. 2017;312(4):R597–R610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasumatsu K, Ogiwara Y, Takai S, et al. Umami taste in mice uses multiple receptors and transduction pathways. J Physiol. 2012;590:1155–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasumatsu K, Iwata S, Inoue M, Ninomiya Y. Fatty acid taste quality information via GPR120 in the anterior tongue of mice. Acta Physiol. 2019;226(1):e13215. [DOI] [PubMed] [Google Scholar]
- 42.Yasumatsu K, Katsukawa H, Sasamoto K, Ninomiya Y. Recovery of amiloride-sensitive neural coding during regeneration of the gustatory nerve: behavioural/neural correlation of salt discrimination. J Neurosci. 2003;23:4362–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kusuhara Y, Yoshida R, Ohkuri T, et al. Taste responses in mice lacking taste receptor subunit T1R1. J Physiol. 2013;591: 1967–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ninomiya Y, Funakoshi M. Responses of rat chorda tympani fibres to electrical stimulation of the tongue. Jpn J Physiol. 1981;31:559–570. [DOI] [PubMed] [Google Scholar]
- 45.Murata Y, Nakashima K, Yamada A, Shigemura N, Sasamoto K, Ninomiya Y. Gurmarin suppression of licking responses to sweetener-quinine mixtures in C57BL mice. Chem Senses. 2003;28(3):237–243. [DOI] [PubMed] [Google Scholar]
- 46.Sclafani A, Ackroff K. Greater reductions in fat preferences in CALHM1 than CD36 knockout mice. Am J Physiol Regul Integr Comp Physiol. 2018;315(3):R576–R585. [DOI] [PMC free article] [PubMed] [Google Scholar]


