Abstract
Objectives:
To evaluate breast multiparametric ultrasound (mpUS) and its potential to reduce unnecessary breast biopsies with one, two, or three additional quantitative parameters (Doppler, elastography, and contrast-enhanced US-CEUS) to B-mode and investigate possible variations with different reader experience.
Materials and Methods:
This prospective study included 124 women (age range 18–82 years, mean 52 years), each with one new breast lesion, scheduled for US-guided biopsy between October 2015 and September 2016. Each lesion was examined with B-mode, elastography (Virtual Touch IQ-VTIQ), Doppler, and CEUS, and different quantitative parameters were recorded for each modality. 4 readers (2 experienced breast radiologists and 2 in-training) independently evaluated B-mode images of each lesion and assigned a Breast Imaging Reporting and Data System (BI-RADS) score. Using the area under the receiver operating characteristic curve (AUC), the most accurate quantitative parameter for each modality was chosen. These were then combined with the BI-RADS scores of all readers. Descriptive statistics and AUC were used to evaluate the diagnostic performance of mpUS.
Results:
65 lesions were malignant. MpUS with B-mode and two additional quantitative parameters (VTIQ and CEUS or Doppler) showed the highest diagnostic performance for all readers (averaged AUCs=0.812–0.789 respectively vs. 0.683 for B-mode, p=0.0001). Both combinations significantly reduced the number of false-positive findings up to 46.9% (p<0.0001).
Conclusions:
Quantitative mpUS with two different triple assessment modalities (B-mode, VTIQ elastography, CEUS or Doppler) shows the best diagnostic performance for breast cancer diagnosis, and leads to a significant reduction of false-positive biopsy recommendations, for both experienced and inexperienced readers.
Keywords: Breast cancer, Multiparametric ultrasound, Elastography, Contrast-enhanced ultrasound, Doppler ultrasound
Introduction
Breast cancer is a complex and heterogeneous disease. Different functional and biological capabilities acquired by tumors during their development have been studied [1] and the importance of the tumor microenvironment, as well as the interactions of tumor cells with the extracellular matrix (ECM) have been highlighted [2]. In recent years, several functional and molecular imaging modalities, such as magnetic resonance imaging (MRI) and positron emission tomography, have focused on breast cancer heterogeneity and the elucidation of the underlying oncogenic processes to enable improved diagnosis, prediction, and prognosis [3–5]. However, these imaging techniques are relatively expensive and time-consuming. There has been increasing scrutiny of the disproportionate contribution of radiology to the rising overall healthcare expenditures. Healthcare policymakers are now focusing on curbing the use of expensive imaging examinations while continuing to promote the quality and appropriateness of imaging. In this context, ultrasound (US) is an ideal alternative, as it offers both morphologic and functional information at a low cost.
Apart from morphologic imaging with standard B-mode, several functional US-based modalities have been assessed, including elastography, Doppler, and contrast-enhanced US (CEUS) [6]. These sonographic modalities provide not only functional information about breast tumors, but also offer a multitude of quantitative parameters that can be used as imaging biomarkers [7–11].
Several studies have shown promising results for each US parameter in providing valuable diagnostic, prognostic, and predictive imaging biomarkers [7, 10, 12–16]. However, the added value of combining several US modalities, termed multiparametric (mp) US [17], and the ability to assess the heterogeneity of breast cancer biology, have not yet been explored in detail, particularly the role of quantitative imaging biomarkers [18–21].
It can be assumed that assessing multiple functional information with quantitative mpUS will enable improved breast tumor characterization and a reduction of false-positive findings, since this concept allows insights into morphological changes, as well as changes at the cellular level, such as angiogenesis, cell function, and interactions with the tumor microenvironment [22–24].
Thus, the purpose of this study was to evaluate mpUS of the breast and its potential to reduce unnecessary breast biopsies with one, two, or three additional quantitative parameters (elastography, CEUS, and Doppler) to B-mode and investigate possible variations with different reader experience.
Materials and Methods
Patients
This prospective, single-center study was approved by the local institutional review board and included 124 patients (age range, 18–82 years; mean, 52 years) between October 2015 and September 2016. All participants provided written, informed consent. The study was registered at ClinicalTrials.gov (Identifier: NCT03276845). Women at least 18 years of age, who presented with a newly diagnosed (either screening-detected or symptomatic), sonographically classified Breast Imaging Reporting and Data System (BI-RADS) 4 or 5 breast lesion [25] (>3mm in diameter) were eligible for inclusion. Lactating or breastfeeding women were excluded from the study, as were women with a known allergy to US contrast media, renal, or heart disease.
If more than one lesion was detected in the same patient, the most suspicious or the largest one was chosen as a target lesion for the study. All participants underwent US-guided needle biopsy. Histopathology served as the standard of reference in all cases.
Data acquisition
A Siemens Acuson S3000 US device (Siemens Healthineers, Erlangen, DE) was used for all examinations, which were performed by one of four breast radiologists (P.K., P.A.B., M.B., K.P.), each with examination experience of >500 breast lesions with the four described US techniques.
MpUS
All patients underwent mpUS, consisting of B-mode, Doppler, Acoustic Radiation Force Impulse (ARFI) elastography, and CEUS. A detailed description of the data acquisition for each technique is provided in the supplemental material.
Briefly, lesions were initially imaged in B-mode using an 18–6 MHz linear transducer and the maximum lesion diameter was recorded.
Power Doppler of each lesion was performed using the same transducer. If vascularity was detected within or around a lesion [12], spectral Doppler of the most prominent artery was performed and pulsatility and resistive index (RI) were calculated.
Thereafter, ARFI elastography was performed using a 9–4 MHz transducer and the Virtual Touch IQ (VTIQ) algorithm. For the VTIQ examination, a region of interest (ROI) was drawn that included the whole lesion and a small amount of surrounding tissue. Four quantification, ROIs were used for shear wave velocity (SWV) measurements: one ROI on the stiffest area of the lesion (SWVmax), one at a soft lesion area, and one on an area of intermediate stiffness. The fourth ROI was placed on the surrounding fatty tissue (SWVfat), preferably at the same depth as the lesion [13]. For each tumor, a lesion-to-fat velocity ratio was calculated using the acquired SWVmax and SWVfat [8].
CEUS was performed subsequently using the same transducer at a low mechanical index (≤ 0.07). A second-generation US contrast medium (sulfur hexafluoride, SonoVue®, Bracco, Milan, Italy) was intravenously administered at a dose of 4.8 ml as a bolus and was subsequently flushed with 10 ml of saline [26]. The examination was documented with a 90 s long clip, starting at the beginning of the bolus injection [27]. The entire data acquisition lasted approximately 10 minutes per patient.
Kinetic analysis of the CEUS data was performed with dedicated software (VueBox™, Bracco Suisse SA, Geneva, Switzerland) at a separate workstation. An ROI was manually drawn on the enhancement area of each lesion, leaving out areas of evident non-enhancement. The software generated time-to-intensity curves for each ROI. With the help of these, eleven quantitative perfusion parameters were calculated: Peak Enhancement; Wash-in Area Under the Curve (AUC); Rise Time; mean Transit Time (local) (mTTl); Time-To-Peak; Wash-in Rate; Wash-in Perfusion Index; Wash-out AUC; Wash-in-Wash-out AUC; Fall Time; and Wash-out Rate.
Data evaluation
B-mode US
Four radiologists (P.C., R.W., G.J.W., M.L.), other than those who performed the examinations, independently re-evaluated the B-mode images of each lesion according to the BI-RADS lexicon [25] and assigned a BI-RADS score. Scores 2 and 3 were considered negative and scores 4a-c and 5 positive. All four readers were blinded to the clinical, mammographic, and histopathological findings of each lesion, as well as to any other US findings, except the B-mode images. Two of the readers were specialized breast radiologists [six (reader 1) and four (reader 2) years of experience respectively] and two were radiologists in-training (both in the fourth year of residency- readers 3 and 4).
Quantitative parameters
To evaluate the quantitative parameters obtained by Doppler, VTIQ, and CEUS, a receiver operating characteristic (ROC) curve analysis was performed. ROC curve analysis demonstrated that RI (cut-off >0.68) showed the highest discriminating power for Doppler, SWVmax (cut-off >3.2 m/s) for VTIQ elastography, and mTTl (cut-off >102.53 s) for CEUS (Table 1).
Table 1.
Diagnostic performance of the quantitative parameter with the highest area under the curve (AUC) for each modality.
| Parameter | Cut-off | Median | Q1 | Q3 | AUC | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|---|
| VTIQ | SWVmax | >3,2 | 3.09 | 2.4 | 5.4 | 0.873 | 0.785 – 0.962 | <0.0001 |
| Doppler | RI | >0,68 | 0.69 | 0.62 | 0.79 | 0.795 | 0.692 – 0.898 | <0.0001 |
| CEUS | mTTl | >102,53 | 85.8 | 52.99 | 145.05 | 0.643 | 0.524 – 0.762 | 0.028 |
Q1: first quartile, Q3: third quartile, CI: confidence interval, VTIQ: virtual touch IQ, SWVmax: maximum shear wave velocity, RI: resistive index, CEUS: contrast-enhanced ultrasound, mTTl: mean transit time (local).
Subsequently, all results were dichotomized for analysis. Lesions with RI ≤0.68, SWVmax ≤3.2 m/s, and mTTl ≤102.53 s were considered negative for Doppler, VTIQ elastography, and CEUS, respectively, whereas those with RI >0.68, SWVmax >3.2 m/s, and mTTl >102.53 s positive for the respective modalities.
Finally, four different parameters were used for mpUS: B-mode; RI (Doppler); SWVmax (VTIQ elastography); and mTTl (CEUS) (Figures 1–2). B-mode was always considered one of the parameters in any mpUS combination, since it is the backbone of any given US protocol and is used to identify and characterize lesions in clinical practice. Therefore, for each mpUS combination, the dichotomized BI-RADS score of each reader was combined with one or more of the dichotomized aforementioned quantitative parameters.
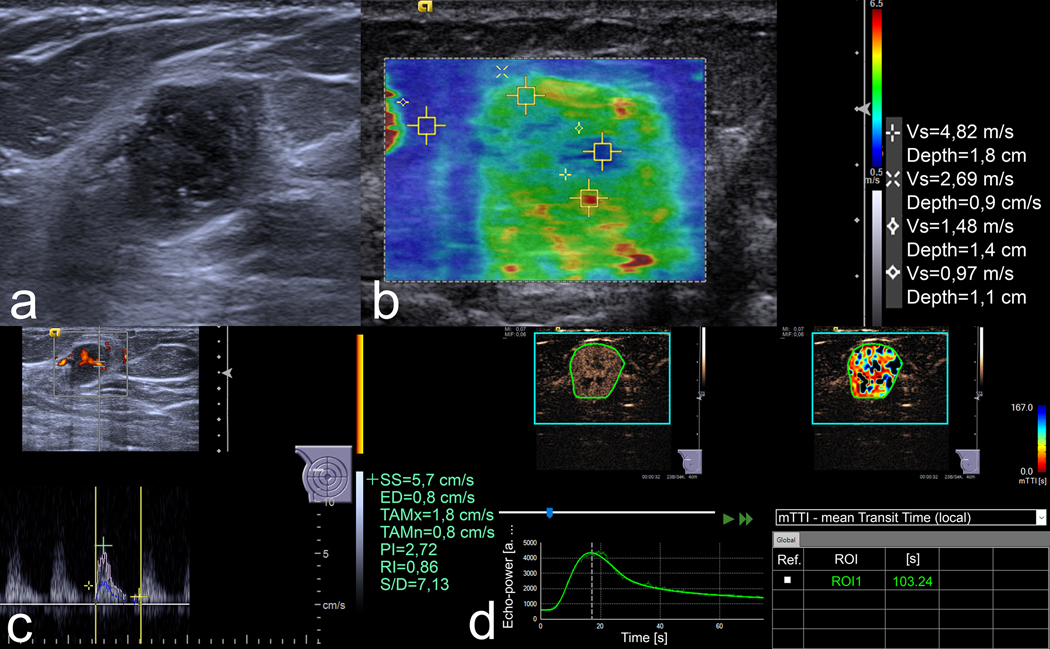
Figure 1.
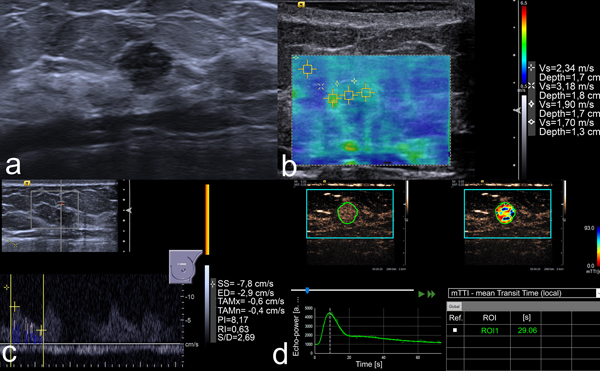
US images of an 8 mm lesion in the left breast of a 41-year-old woman. (a) B-mode image shows a round, non-circumscribed, hypoechoic lesion. This was classified as BI-RADS 4b by two readers and 4c by the other two. (b) VTIQ elastography shows an SWVmax of 3.18 m/s. (c) Spectral Doppler demonstrates an RI of 0.63. (d) Kinetic analysis of the CEUS data calculates an mTTl of 29.06 s. All quantitative parameters were below the respective cut-off values, thus mpUS was considered negative. US-guided needle biopsy demonstrated a fibroadenoma.
Figure 2.
US images of an 18 mm lesion in the right breast of a 54-year-old woman. (a) B-mode image demonstrates an irregular, non-circumscribed, heterogeneous lesion with posterior enhancement. Three readers classified it as BI-RADS 4b and the fourth one as 4c. (b) In VTIQ elastography, the SWVmax is 4.82 m/s. (c) Spectral Doppler shows an RI of 0.86. (d) In CEUS, the kinetic analysis shows an mTTl of 103.24 s. All quantitative parameters were above the respective cut-off values, rendering a positive mpUS. US-guided needle biopsy proved a Grade 3 invasive carcinoma of no special type.
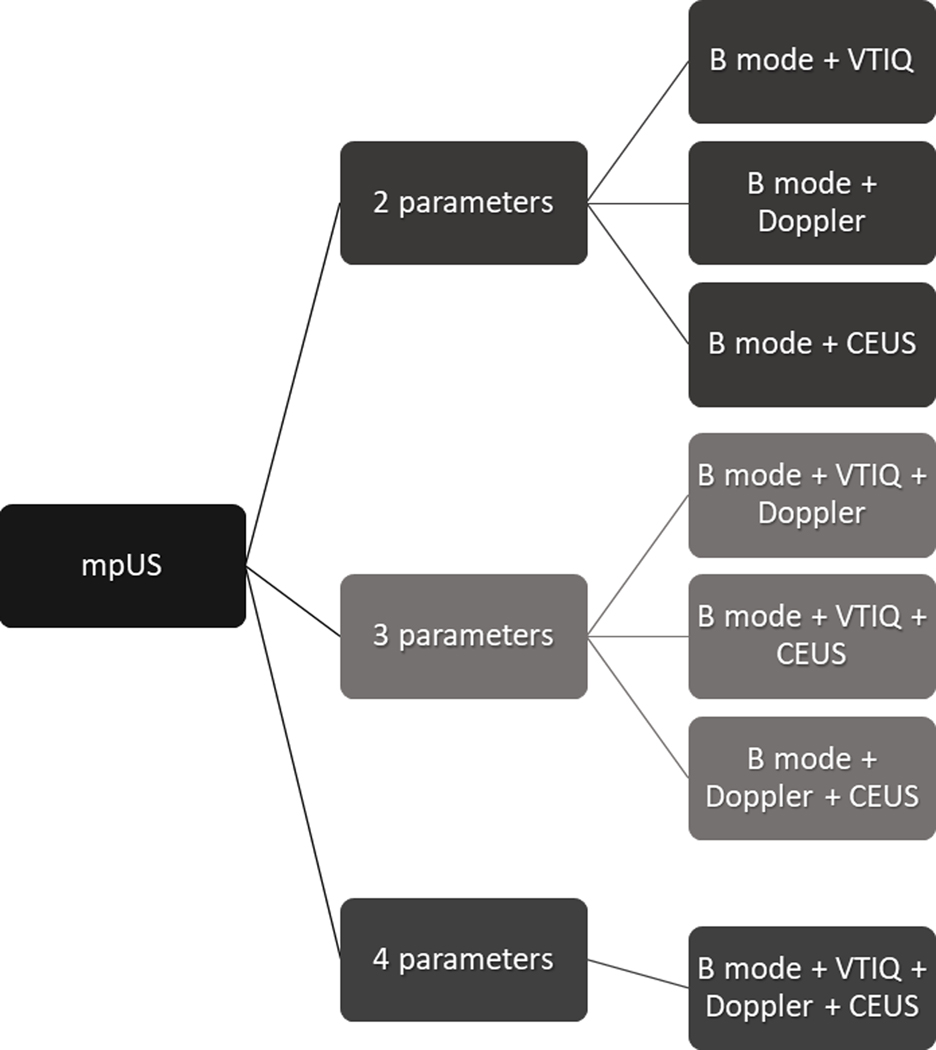
The seven possible combinations of mpUS with two, three, and four parameters are shown in Figure 3. MpUS with two parameters was regarded positive, if one was positive. MpUS with three parameters was considered positive if two or three were positive. MpUS with four parameters was regarded positive if three or four were positive. If only two of the four parameters were positive, then mpUS was considered positive if B-mode was indicative of malignancy and negative if B-mode was indicative of benignity.
Figure 3.
Graph demonstrating the seven possible combinations of multiparametric ultrasound (mpUS) of the breast, with two, three, and four parameters. VTIQ: virtual touch IQ elastography, CEUS: contrast-enhanced ultrasound.
Histopathological examination
All patients underwent US-guided needle biopsy. The pathological results of the biopsy served as the reference standard for benign lesions and for malignant lesions in patients who received neoadjuvant chemotherapy (NAC). For patients not undergoing NAC or receiving surgery because of a lesion of uncertain malignant potential, the pathological, post-surgical results served as the reference standard.
Statistical analysis
Due to the lack of literature data regarding quantitative breast mpUS at the time of study planning, calculation of the sample size was based on the hypothesis that the AUC of B-mode would be improved from 0.800 to 0.900 with a 5% type I and a 20% type II error. Since our institution is a breast imaging assessment center with a high number of breast cancer patients, we hypothesized a ratio of benign to malignant lesions of 0.75. The AUC values of 0.800 and 0.900 were determined as average values by considering several studies on breast US (B-mode, elastography, Doppler and CEUS) [7, 10, 13, 18, 28–32].
Statistical analysis was performed by two of the authors (P.K. and P.A.B.) using MedCalc 12.5.00 (MedCalc Software bvba, Ostend, Belgium-2013) and SPSS 20.0.0 (IBM Corp, Armonk NY, USA-2012) software. ROC curve analysis was used to evaluate the diagnostic accuracy of B-mode and all quantitative parameters, as well as to determine cut-off values and the corresponding AUC. Using descriptive statistics, sensitivity, specificity, positive, and negative likelihood ratios and their 95% confidence intervals were calculated for B-mode BI-RADS scores and for mpUS with two, three, and four parameters. Significant differences in the diagnostic performance of B-mode and mpUS were assessed with the two-tailed McNemar’s x2 test, both averaged separately for each reader. Proportions were compared using the Z test. P-values of ≤0.05 were considered significant.
Results
Lesion characteristics
Of the 124 lesions (median size: 13 mm; Q1: 10 mm, Q3: 22 mm), 59 were benign (median size: 13 mm; Q1: 10 mm, Q3: 22mm) and 65 were malignant (median size: 13 mm, Q1: 9 mm, Q3: 20 mm). The median sizes of benign and malignant lesions did not differ significantly (p>0.05). Table 2 summarizes all histopathological diagnoses.
Table 2.
Histopathological diagnoses of all lesions.
| Malignant lesions | n | Benign lesions | n |
|---|---|---|---|
| Invasive carcinoma NST | 53 (81.6%) | Fibroadenoma | 24 (40.7%) |
| Invasive lobular carcinoma | 6 (9.2%) | Fibroadenomatous hyperplasia | 8 (13.6%) |
| Ductal carcinoma in situ | 4 (6.2%) | Papilloma | 5 (8.5%) |
| Mucinous carcinoma | 1 (1.5%) | Fibrosis | 4 (6.7%) |
| Neuroendocrine carcinoma | 1 (1.5%) | Others | 18 (30.5%) |
NST: no special type.
Diagnostic performance of B-mode
The averaged AUC for B-mode was 0.683, with a sensitivity of 90.8% and a specificity of 45.8%. Diagnostic performance of the BI-RADS scores varied between the four readers, reflecting their different experience levels. AUCs ranged between 0.577 for the least-experienced reader to 0.759 for one of the experienced breast radiologists. The false-positive rate ranged from 39% to 69.5% (average, 54.2%). A comparison of the sensitivity and specificity of B-mode US with mpUS for each reader is shown in Figure 4. The averaged diagnostic performance of B-mode is demonstrated in Table 3. More details on the diagnostic performance of each reader’s B-mode BI-RADS scores are shown in the supplemental material.
Figure 4.
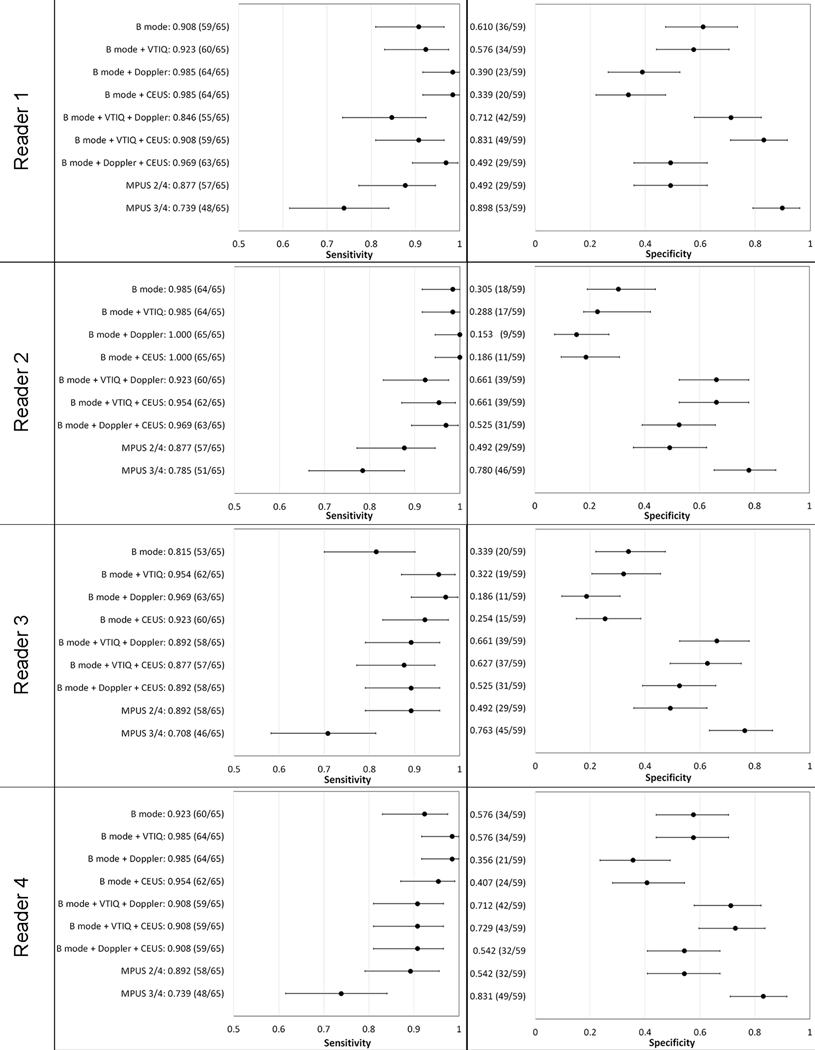
Scatter-plots summarizing the ranges of the achieved sensitivity and specificity for B-mode and all multiparametric combinations using a 95% confidence interval (error bars) for Readers 1–4. The absolute patient numbers are shown in parentheses.
Table 3.
Averaged diagnostic performance of B-mode ultrasound (US) and all possible combinations of multiparametric US with two, three, and four parameters.
| Sensitivity (%) | 95% CI | p-values | Specificity (%) | 95% CI | p-values | +LR | -LR | AUC | p-values | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Parameter | B-mode | 90.8 (236/260) | 86.6 – 94.0 | 45.8 (108/236) | 39.3 – 52.3 | 1.67 | 0.2 | 0.683 | |||
| 2 Parameters | B-mode + VTIQ | 96.2 (250/260) | 93.0 – 98.1 | 0.0005 | 44.1 (104/236) | 37.6 – 50.7 | 0.1336 | 1.72 | 0.087 | 0.701 | 0.0241 |
| B-mode + Doppler | 96.5 (251/260) | 93.5 – 98.4 | 0.0035 | 39.8 (94/236) | 33.5 – 46.4 | 0.0824 | 1.6 | 0.087 | 0.682 | 0.9643 | |
| B-mode + CEUS | 96.5 (251/260) | 93.5 – 98.4 | 0.0003 | 29.7 (70/236) | 23.9 – 35.9 | < 0.0001 | 1.37 | 0.12 | 0.631 | 0.0002 | |
| 3 Parameters | B-mode + VTIQ + Doppler | 89.2 (232/260) | 84.8 – 92.7 | 0.5563 | 68.6 (162/236) | 62.3 – 74.5 | < 0.0001 | 2.85 | 0.16 | 0.789 | 0.0001 |
| B-mode + VTIQ + CEUS | 91.2 (237/260) | 87.0 – 94.3 | 1 | 71.2 (168/236) | 65.0 – 76.9 | < 0.0001 | 3.16 | 0.12 | 0.812 | 0.0001 | |
| B-mode + Doppler + CEUS | 93.5 (243/260) | 89.7 – 96.1 | 0.7103 | 52.1 (123/236) | 45.5 – 58.6 | 0.0872 | 1.95 | 0.13 | 0.728 | 0.0183 | |
| 4 Parameters | B-mode + VTIQ + Doppler + CEUS | 81.5 (212/260) | 76.3 – 86.1 | < 0.0001 | 62.3 (147/236) | 55.8 – 68.5 | < 0.0001 | 2.16 | 0.3 | 0.719 | 0.023 |
P-values refer to B-mode US. CI: confidence interval, LR: likelihood ratio, AUC: area under the curve, VTIQ: virtual touch IQ elastography, CEUS: contrast-enhanced US.
Diagnostic performance of mpUS
The averaged results of all different combinations of mpUS are summarized in Table 3. Detailed results for each reader are shown in the supplemental material.
Of all combinations, including B-mode as a single modality, a triple assessment by B-mode, VTIQ elastography, and CEUS performed best (averaged AUC 0.812). The triple combination of B-mode, VTIQ elastography, and Doppler was the second-best at an averaged AUC of 0.789. The AUCs of these two triple assessments did not differ significantly (p=0.1967), while both demonstrated a significantly higher averaged AUC than all other approaches (p<0.0001 in all cases).
Combinations with four or two parameters demonstrated AUCs of 0.719 and 0.631–0.701, respectively.
MpUS and the effect on the reduction of false-positives
Of all combinations, the triple assessment using mpUS with B-mode, VTIQ elastography, and CEUS led to the highest significant reduction of false-positives (averaged reduction 46.9%, p<0.0001), followed by the triple combination of B-mode, VTIQ elastography, and Doppler (averaged reduction 42.2%, p<0.0001).
However, mpUS with the two aforementioned combinations did not significantly influence the recommendations for follow-up on malignant lesions (p=0.3721–0.8234). Table 4 summarizes the averaged effect of mpUS on false-positive biopsy and false-negative follow-up recommendations. Reader-specific details are shown in the supplemental material.
Table 4.
Averaged comparison of false-positive biopsy and false-negative follow-up recommendations with B-mode ultrasound (US) alone and multiparametric US with three parameters (B-mode US, virtual touch IQ [VTIQ] elastography], and contrast-enhanced ultrasound [CEUS] and B-mode US, VTIQ elastography, and Doppler).
| Averaged | |||
|---|---|---|---|
| B-mode | B-mode + VTIQ + CEUS | B-mode + VTIQ + Doppler | |
| False-positive biopsies | 128 | 68 | 74 |
| Difference | −60 (−46.9%) | −54 (−42.2%) | |
| p-value | < 0.0001 | < 0.0001 | |
| 95% CI | 23.1 – 35 | 25.5 – 37.7 | |
| False-negative follow-up | 24 | 23 | 28 |
| Difference | −1 (−4.2%) | +4 (+16.7%) | |
| p-value | 0.8234 | 0.3721 | |
| 95% CI | 5.7 – 12.9 | 7.3 – 15.2 | |
Percentage reduction is given in parentheses. P-values refer to B-mode US. CI: confidence interval.
Effect of reader experience
The triple assessment of B-mode, VTIQ elastography, and CEUS showed the best diagnostic performance (AUC 0.807–0.869 vs. 0.719–0.747 for B-mode, p-values <0.0001–0.0115) and the highest reduction of false-positives (36–56.5%, p-values <0.0001–0.0174) for three of the four readers (two experienced and one in-training; readers 1, 2 and 4), followed by the triple combination of B-mode, VTIQ elastography, and Doppler. The latter showed the highest diagnostic performance (AUC 0.752 vs. 0.672 for B-mode, p-value <0.0001) and reduction of false positives (48.7%, p-value <0.0001) for the fourth reader (one radiologist in-training; reader 3), while the triple assessment using B-mode, VTIQ elastography, and CEUS was the second-best. In all cases, the sensitivity was not significantly affected by the implementation of mpUS with three parameters. More details on the diagnostic performance of each reader can be found in the supplemental material.
Discussion
Our study shows that quantitative mpUS with two different triple assessment modalities (B-mode, VTIQ elastography, CEUS or Doppler) showed the best diagnostic performance in characterizing breast lesions compared to several other mpUS combinations and B-mode as a single parameter. Quantitative mpUS would have significantly reduced unnecessary breast biopsies in up to 47%, for both experienced and inexperienced readers.
Morphologic imaging with B-mode US as a single parameter is a widely used technique for breast lesion characterization. An important drawback of B-mode is its low specificity, which results in a high number of false-positive results [18, 29]. This was confirmed by our study, where B-mode alone led to 54.2% of unnecessary, false-positive biopsy recommendations. Apart from B-mode, several functional US-based modalities have been developed in order to improve the diagnostic performance of breast US. Seven mpUS combinations were assessed in our study, with two of them consistently raising the diagnostic performance for both experienced and inexperienced readers and leading to a significant reduction of false-positives by maintaining sensitivity levels similar to that of B-mode as a single modality.
The reduction of false-positive biopsy recommendations has a significant clinical impact, as our data could positively influence the ongoing discussion of the disadvantages associated with breast screening programs, including false-positives and overtreatment [33]. Although no formal cost-effectiveness analysis was performed, the results of this study suggest a possible reduction of costs by reducing unnecessary US-guided biopsies. Although US-guided breast biopsy is considered safe and accurate, it poses a considerable financial and psychological burden to the healthcare system and to patients [34] and should be avoided whenever possible. This is even more pronounced considering the shift in healthcare to a value-based paradigm, with value corresponding to quality or outcomes divided by cost and the radiologist’s contribution to cost reduction and outcome improvement now monitored with the use of newly developed metrics [35]. In this context, the importance of a relatively low-cost modality, which provides a plethora of morphological and functional tissue information, such as mpUS, is evident.
One might argue that elastography is primarily a morphological or structural imaging modality, since it displays tissue mechanical properties, which are based on tissue structure. However, previous basic research has demonstrated that cancerous tissue changes both induce and are promoted by substantial changes in the tumor ECM [2, 36]. The changes in the biomechanical properties of the ECM (like its tumor-associated stiffening) are caused by changes in its chemical composition and spatial arrangement of its components and in turn (de)regulate different cell behaviors, playing a causative role in cancer pathogenesis and progression [37]. These changes in the ECM properties represent dynamic processes [22]. Only recently, a study [38] has proven that the sonoelastographic estimation of tumor stiffness is correlated with collagen and fibroblast content and changes of the ECM. In this sense, since elastography depicts the results of the dynamic changes in stiffness of the tumor and its microenvironment, which are based on the one hand on functional changes and on the other on the increased tumor cellularity and possibly also on changes in tumor perfusion [39] we regard elastography as a “functional” imaging modality, in broad analogy to diffusion weighted imaging in MRI.
Our study is the first to evaluate exclusively quantitative, sonographic imaging biomarkers combined with morphologic information of breast lesions in a multiparametric setting. Imaging biomarkers have drawn substantial attention in recent years, since they provide an unbiased, quantitative estimation of biological processes and play an increasing role in the era of evidence-based medicine [40]. Imaging biomarkers offer deeper insights into intratumoral heterogeneity and aid in the decrease of variability in imaging by removing the subjective interpretation bias of qualitative evaluations. Moreover, since the combination of different imaging parameters performs better than single features [41], it appears reasonable to test the performance of different potential US quantitative biomarkers in a multiparametric setting. Regarding US imaging, the Quantitative Imaging Biomarkers Alliance® organized by the Radiological Society of North America identified CEUS, shear-wave elastography and calculations of blood flow as techniques offering possible quantitative imaging biomarkers [42]. In our study, the combined quantitative assessment of tissue elasticity (SWVmax), vascularity (preferably with mTTl derived from CEUS, alternatively with the Doppler-acquired RI which showed the second-best results), and morphologic information allowed the most accurate characterization and differentiation of benign and malignant breast lesions. It can be expected that, with further development and standardization, quantitative biomarkers will steadily gain in importance and application. In this context, our study contributes to the increasing body of evidence on the value of quantitative breast imaging.
A further potential of quantitative imaging lies in the recent advance of radiomics and artificial intelligence applications in medical imaging. The extraction of large amounts of features from medical images offers substantial information- not only diagnostic, but also possibly predictive and prognostic [43]. Deep learning algorithms make use of data acquired from medical images to predict the differential diagnosis of a lesion [44]. To-date, such research on US is relatively limited as compared to other cross-sectional imaging modalities [45], mainly due to standardization issues [46]. However, radiomics analysis has been shown to be feasible and accurate, not only on B-mode breast US images but also on data acquired with strain elastography, Doppler and CEUS [46–50]. It is possible that the input of mpUS data to deep learning algorithms may lead to more accurate diagnoses, however, this is a matter of future research.
In our study, CEUS was proven to be a valuable tool in the mpUS evaluation of breast lesions. Due to the necessary intravenous contrast application, it is considered a minimally invasive procedure and prolongs the examination time. In addition, the use of specific software for the kinetic evaluation is required. However, the consequently improved diagnostic accuracy is not only favorable for the patient, but may also be cost-effective, due to the reduction of unnecessary biopsies. In addition, several vendors offer quantification packages integrated into the US device, making CEUS quantification easily applicable in the clinical routine. If CEUS cannot be performed, vascularity can be alternatively evaluated with Doppler, taking into account that this combination still leads to a decrease in false-positives, albeit to a lesser degree.
Different groups have previously evaluated breast mpUS, mainly focusing on qualitative parameters of elastography, Doppler, or CEUS [18–21]. The results of these studies are partially comparable to ours, demonstrating an overall increase in specificity compared to B-mode US alone. Cho et al demonstrated a higher accuracy and an improved specificity leading to decrease of breast biopsy recommendation by using qualitative strain elastography and Doppler combined with B-mode [18]. Similar results have been shown on a screening population by Lee et al. [19]. Choi et al. investigated the added value of quantitative shear wave elastography and qualitative Doppler to B-mode for the evaluation of non-mass breast lesions and also demonstrated an improved diagnostic performance and increased specificity as compared to B-mode US alone [20]. On the other hand, Xiao et al. combined B-mode with strain elastography and qualitative CEUS for the characterization of sub-centimeter breast lesions [21]. In this study, the highest AUC was observed for the combination of B-mode with CEUS, followed by the combination of all three parameters, which appears in contrast to our results. However, this discrepancy may be explained by the use of different elastography technologies (strain vs. quantitative VTIQ) as well as the sole qualitative interpretation of CEUS, whereas we performed a kinetic analysis. Nevertheless, qualitative mpUS includes the inherent limitations of a subjective evaluation, such as a high degree of operator-dependence and variability, which is almost eliminated with our quantitative approach.
In our study, different reader experience levels resulted in a varying diagnostic performance. However, a significant increase in the diagnostic performance through the simultaneous assessment of morphology, elasticity, and vascularity was consistent for all four readers, regardless of their degree of experience. This finding confirms the complementary information acquired by the different modalities of mpUS. A significant increase in accuracy through the addition of elastography was observed for less-experienced readers. All other mpUS combinations showed no experience-dependent variability.
Our study has some limitations. The study population included a high number of malignancies (52.4% of all patients). This study was conducted at a tertiary institution, potentially introducing some degree of spectrum bias and making it difficult to extrapolate to community practice. However, all lesions underwent pathologic proof, which should be seen as a considerable strength of our study. Furthermore, different molecular subtypes of breast cancer were not considered in our analysis. Previous research has implied differences in the US appearance of tumors of different molecular subtypes; however, the study population did not allow for an in-depth subgroup analysis in this direction.
In conclusion, quantitative mpUS with two different triple assessment modalities (B-mode, VTIQ elastography, CEUS, or Doppler) shows the best diagnostic performance for breast cancer diagnosis, and leads to a significant reduction of false-positive biopsy recommendations, for both experienced and inexperienced readers.
Supplementary Material
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 2.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glunde K, Jacobs MA, Pathak AP, et al. Molecular and functional imaging of breast cancer. NMR Biomed. 2009;22:92–103. [DOI] [PubMed] [Google Scholar]
- 4.Pinker K, Bogner W, Baltzer P, et al. Improved differentiation of benign and malignant breast tumors with multiparametric 18fluorodeoxyglucose positron emission tomography magnetic resonance imaging: a feasibility study. Clin Cancer Res. 2014;20:3540–9. [DOI] [PubMed] [Google Scholar]
- 5.Pinker K, Bogner W, Baltzer P, et al. Improved diagnostic accuracy with multiparametric magnetic resonance imaging of the breast using dynamic contrast-enhanced magnetic resonance imaging, diffusion-weighted imaging, and 3-dimensional proton magnetic resonance spectroscopic imaging. Invest Radiol. 2014;49:421–30. [DOI] [PubMed] [Google Scholar]
- 6.Kuhl CK. The Changing World of Breast Cancer: A Radiologist’s Perspective. Invest Radiol. 2015;50:615–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saracco A, Szabo BK, Aspelin P, et al. Differentiation between benign and malignant breast tumors using kinetic features of real-time harmonic contrast-enhanced ultrasound. Acta Radiol. 2012;53:382–8. [DOI] [PubMed] [Google Scholar]
- 8.Kapetas P, Pinker-Domenig K, Woitek R, et al. Clinical application of Acoustic Radiation Force Impulse Imaging with Virtual Touch IQ in breast ultrasound: diagnostic performance and reproducibility of a new technique. Acta Radiol. 2017;58:140–7. [DOI] [PubMed] [Google Scholar]
- 9.Sirous M, Sirous R, Nejad FK, et al. Evaluation of different aspects of power Doppler sonography in differentiating and prognostication of breast masses. J Res Med Sci. 2015;20:133–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Golatta M, Schweitzer-Martin M, Harcos A, et al. Evaluation of virtual touch tissue imaging quantification, a new shear wave velocity imaging method, for breast lesion assessment by ultrasound. Biomed Res Int. 2014;2014:960262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kettenbach J, Helbich TH, Huber S, et al. Computer-assisted quantitative assessment of power Doppler US: effects of microbubble contrast agent in the differentiation of breast tumors. Eur J Radiol. 2005;53:238–44. [DOI] [PubMed] [Google Scholar]
- 12.Ozdemir A, Ozdemir H, Maral I, et al. Differential diagnosis of solid breast lesions: contribution of Doppler studies to mammography and gray scale imaging. J Ultrasound Med. 2001;20:1091–101; quiz 102. [DOI] [PubMed] [Google Scholar]
- 13.Li DD, Xu HX, Guo LH, et al. Combination of two-dimensional shear wave elastography with ultrasound breast imaging reporting and data system in the diagnosis of breast lesions: a new method to increase the diagnostic performance. Eur Radiol. 2016;26:3290–300. [DOI] [PubMed] [Google Scholar]
- 14.Wubulihasimu M, Maimaitusun M, Xu XL, et al. The added value of contrast-enhanced ultrasound to conventional ultrasound in differentiating benign and malignant solid breast lesions: a systematic review and meta-analysis. Clin Radiol. 2018;73:936–43. [DOI] [PubMed] [Google Scholar]
- 15.Xiao X, Dong L, Jiang Q, et al. Incorporating Contrast-Enhanced Ultrasound into the BI-RADS Scoring System Improves Accuracy in Breast Tumor Diagnosis: A Preliminary Study in China. Ultrasound Med Biol. 2016;42:2630–8. [DOI] [PubMed] [Google Scholar]
- 16.Wan J, Wu R, Yao M, et al. Acoustic radiation force impulse elastography in evaluation of triple-negative breast cancer: A preliminary experience. Clin Hemorheol Microcirc. 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Sidhu PS. Multiparametric Ultrasound (MPUS) Imaging: Terminology Describing the Many Aspects of Ultrasonography. Ultraschall Med. 2015;36:315–7. [DOI] [PubMed] [Google Scholar]
- 18.Cho N, Jang M, Lyou CY, et al. Distinguishing benign from malignant masses at breast US: combined US elastography and color doppler US--influence on radiologist accuracy. Radiology. 2012;262:80–90. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Chung J, Choi HY, et al. Evaluation of Screening US-detected Breast Masses by Combined Use of Elastography and Color Doppler US with B-Mode US in Women with Dense Breasts: A Multicenter Prospective Study. Radiology. 2017;285:660–9. [DOI] [PubMed] [Google Scholar]
- 20.Choi JS, Han BK, Ko EY, et al. Additional diagnostic value of shear-wave elastography and color Doppler US for evaluation of breast non-mass lesions detected at B-mode US. Eur Radiol. 2016;26:3542–9. [DOI] [PubMed] [Google Scholar]
- 21.Xiao X, Jiang Q, Wu H, et al. Diagnosis of sub-centimetre breast lesions: combining BI-RADS-US with strain elastography and contrast-enhanced ultrasound-a preliminary study in China. Eur Radiol. 2017;27:2443–50. [DOI] [PubMed] [Google Scholar]
- 22.Giussani M, Merlino G, Cappelletti V, et al. Tumor-extracellular matrix interactions: Identification of tools associated with breast cancer progression. Semin Cancer Biol. 2015;35:3–10. [DOI] [PubMed] [Google Scholar]
- 23.Moon WK, Im JG, Noh DY, et al. Nonpalpable breast lesions: evaluation with power Doppler US and a microbubble contrast agent-initial experience. Radiology. 2000;217:240–6. [DOI] [PubMed] [Google Scholar]
- 24.Mori N, Mugikura S, Takahashi S, et al. Quantitative Analysis of Contrast-Enhanced Ultrasound Imaging in Invasive Breast Cancer: A Novel Technique to Obtain Histopathologic Information of Microvessel Density. Ultrasound Med Biol. 2017;43:607–14. [DOI] [PubMed] [Google Scholar]
- 25.Mendelson EB, Böhm-Vélez M, Berg WA, et al. ACR BI-RADS® Ultrasound. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al., editors. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013:216–355. [Google Scholar]
- 26.Saracco A, Szabo BK, Aspelin P, et al. Contrast-enhanced ultrasound using real-time contrast harmonic imaging in invasive breast cancer: comparison of enhancement dynamics with three different doses of contrast agent. Acta Radiol. 2015;56:34–41. [DOI] [PubMed] [Google Scholar]
- 27.Wan CF, Du J, Fang H, et al. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: correlation with prognostic factors. Radiology. 2012;262:450–9. [DOI] [PubMed] [Google Scholar]
- 28.Berg WA, Cosgrove DO, Dore CJ, et al. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology. 2012;262:435–49. [DOI] [PubMed] [Google Scholar]
- 29.Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du J, Wang L, Wan CF, et al. Differentiating benign from malignant solid breast lesions: combined utility of conventional ultrasound and contrast-enhanced ultrasound in comparison with magnetic resonance imaging. Eur J Radiol. 2012;81:3890–9. [DOI] [PubMed] [Google Scholar]
- 31.Athanasiou A, Tardivon A, Tanter M, et al. Breast lesions: quantitative elastography with supersonic shear imaging--preliminary results. Radiology. 2010;256:297–303. [DOI] [PubMed] [Google Scholar]
- 32.Liu B, Zheng Y, Huang G, et al. Breast Lesions: Quantitative Diagnosis Using Ultrasound Shear Wave Elastography-A Systematic Review and Meta--Analysis. Ultrasound Med Biol. 2016;42:835–47. [DOI] [PubMed] [Google Scholar]
- 33.Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11:106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schueller G, Jaromi S, Ponhold L, et al. US-guided 14-gauge core-needle breast biopsy: results of a validation study in 1352 cases. Radiology. 2008;248:406–13. [DOI] [PubMed] [Google Scholar]
- 35.Sarwar A, Boland G, Monks A, et al. Metrics for Radiologists in the Era of Value-based Health Care Delivery. Radiographics. 2015;35:866–76. [DOI] [PubMed] [Google Scholar]
- 36.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Provenzano PP, Inman DR, Eliceiri KW, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riegler J, Labyed Y, Rosenzweig S, et al. Tumor elastography and its association with collagen and the tumor microenvironment. Clin Cancer Res. 2018;24:4455–67. [DOI] [PubMed] [Google Scholar]
- 39.Lee SH, Moon WK, Cho N, et al. Shear-Wave Elastographic Features of Breast Cancers: Comparison With Mechanical Elasticity and Histopathologic Characteristics. Invest Radiol. 2014;49:147–55. [DOI] [PubMed] [Google Scholar]
- 40.Trattnig S. The Shift in Paradigm to Precision Medicine in Imaging: International Initiatives for the Promotion of Imaging Biomarkers. In: Martí-Bonmatí L, Alberich-Bayarri A, editors. Imaging biomarkers: development and clinical integration. 1st ed. Cham, Switzerland: Springer International Publishing; 2016:1–7. [Google Scholar]
- 41.Weaver O, Leung JWT. Biomarkers and Imaging of Breast Cancer. AJR Am J Roentgenol. 2018;210:271–8. [DOI] [PubMed] [Google Scholar]
- 42.Radiological Society of North America. Quantitative Imaging Biomarkers Alliance® (QIBA®). Available at: https://www.rsna.org/QIBA/. Accessed September 16, 2018.
- 43.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi JY. Radiomics and Deep Learning in Clinical Imaging: What Should We Do? Nucl Med Mol Imaging. 2018;52:89–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tahmassebi A, Wengert GJ, Helbich TH, et al. Impact of Machine Learning With Multiparametric Magnetic Resonance Imaging of the Breast for Early Prediction of Response to Neoadjuvant Chemotherapy and Survival Outcomes in Breast Cancer Patients. Invest Radiol. 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SE, Han K, Kwak JY, et al. Radiomics of US texture features in differential diagnosis between triple-negative breast cancer and fibroadenoma. Sci Rep. 2018;8:13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Q, Xiao Y, Suo J, et al. Sonoelastomics for Breast Tumor Classification: A Radiomics Approach with Clustering-Based Feature Selection on Sonoelastography. Ultrasound Med Biol. 2017;43:1058–69. [DOI] [PubMed] [Google Scholar]
- 48.Theek B, Opacic T, Magnuska Z, et al. Radiomic analysis of contrast-enhanced ultrasound data. Sci Rep. 2018;8:11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo Y, Hu Y, Qiao M, et al. Radiomics Analysis on Ultrasound for Prediction of Biologic Behavior in Breast Invasive Ductal Carcinoma. Clin Breast Cancer. 2018;18:e335–e44. [DOI] [PubMed] [Google Scholar]
- 50.Wu T, Sultan LR, Tian J, et al. Machine learning for diagnostic ultrasound of triple-negative breast cancer. Breast Cancer Res Treat. 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.