Abstract
The response of adipose progenitors to metabolic states is a crucial, but poorly understood, determinant of metabolic health. The back-to-back papers by Joffin et al. (2021) and Shao et al. (2021) in this issue of Cell Stem Cell reveal how adipose-tissue-resident PDGFRβ+ precursor cell fate is regulated by mitochondrial bio-energetic state and how such processes go wrong in obesity.
In general, obesity, as exemplified by an excess lipid accumulation in white adipose tissue (WAT), is associated with type 2 diabetes, dyslipidemia, and cardiovascular diseases. However, this is not always the case: for example, lipodystrophy (failed lipid storage in adipose tissues) frequently results in severe insulin resistance and cardiometabolic diseases. Conversely, healthy WAT expansion, e.g., less inflammatory and less fibrotic WAT, is often seen in the subcutaneous WAT of metabolically healthy obese subjects (Ghaben and Scherer, 2019). Accordingly, the field of obesity research has recognized the significance of “metabolic health” in WAT. However, what determines the healthy state remains less understood.
Under caloric excess, adipose tissues expand through two mechanisms: by increasing lipid storage (hypertrophy) in differentiated adipocytes and by increasing the number of adipocytes (hyperplasia) from adipocyte precursor cells (APCs). The process, namely adipogenesis, involves dynamic tissue remodeling in the adipose stroma that contains molecularly distinct progenitor cells. Of note, recent advances in single-cell analyses have enabled researchers to deconvolute the cellular heterogeneity of APCs. Nonetheless, molecular understanding of APC homeostasis, e.g., precursor cell proliferation, fate commitment, and maintenance, remain incomplete. The recent back-to-back papers in this issue of Cell Stem Cell by the Scherer laboratory (Joffin et al., 2021) and the Gupta laboratory (Shao et al., 2021) elegantly demonstrate how APC homeostasis is controlled in physiology and dysregulated in obesity.
The study by Joffin et al. examined how mitochondrial activity influences APCs by employing an inducible genetic mouse model of altered mitochondrial activity in a cell-type-specific manner. The authors’ group has previously demonstrated that a subpopulation of adipose perivascular cells expressing platelet-derived growth factor receptor β (PDGFRβ) in epidydimal WAT (LY6C−; CD9−; PDGFRβ+) is adipogenic (PDGFRβ+ APC), whereas LY6C+ PDGFRβ+ cells are a driver of adipose tissue inflammation, a.k.a., fibro-inflammatory progenitors (FIPs) (Hepler et al., 2018; Vishvanath et al., 2016). Notably, FIPs displayed a higher mitochondrial respiration rate than APCs, although the total content of mitochondrial DNA was the same between the two populations. To probe if the difference in mitochondrial activity affects their functional properties, the authors overexpressed mitoNEET, a mitochondrial outer-membrane protein that inhibits iron transport into the matrix, thereby limiting the mitochondrial respiration rate (Kusminski et al., 2012). The authors found that overexpression of mitoNEET promoted inflammatory responses in FIPs, but reduced adipogenic capacity in PDGFRβ+ APCs. Consistent with these results, inducible mitoNEET overexpression in PDGFRβ+ cells exacerbated the pro-inflammatory pathway in the epididymal WAT of mice. Similarly, PDGFRβ+ cell-specific overexpression of mitoNEET compromised adipogenesis while promoting inflammation in the inguinal (subcutaneous) WAT. These results suggest that mitochondrial activity is crucial for line-age commitment and adipose tissue remodeling.
It has been appreciated that decreased mitochondrial activity and increased inflammation/fibrosis in adipose tissues are closely linked to metabolic disorders (Ghaben and Scherer, 2019). Accordingly, the authors determined if mitochondrial dysfunction in PDGFRβ+ cells affects whole-body energy metabolism. Under a diet-induced obese condition, PDGFRβ+ cell-specific overexpression of mitoNEET reduced adipose tissue mass and body weight. However, it caused a partial lipodystrophy phenotype, such as increased inflammation, impaired clearance of triglycerides in the circulation, hepatic steatosis, and systemic insulin resistance. Surprisingly, the pro-inflammatory response in adipose tissues was reversed by terminating the mitoNEET overexpression within 1 week. These results suggest that mitochondrial function in PDGFRβ+ cells rapidly influences adipose tissue health and systemic energy homeostasis.
How does obesity trigger pro-inflammatory and anti-adipogenic signaling cascades in APCs? The paper from Shao et al. identified HIF1α (hypoxia-inducible factor 1α) as a critical regulator of adipose PDGFRβ+ cell function. Single-cell RNA sequencing of adipose PDGFRβ+ cells and the subsequent pathway analyses revealed that FIPs in the gonadal WAT were enriched in a gene signature associated with active HIF1α signaling. This observation is consistent with the previous studies showing that HIF1α signaling is a key driver of the pro-fibrotic gene program and adipose tissue dysfunction (Michailidou, 2019). Accordingly, the authors used a dominant-negative form of HIF1α in PDGFRβ+ cells to demonstrate that HIF1α inhibition potently attenuated adipose tissue fibrosis while promoting de novo adipogenesis on a high-fat diet. Importantly, the PDGFRβ+ cell-selective HIF1α inhibition was sufficient to reduce triglyceride levels in the liver and circulation and improve systemic glucose tolerance without altering body weight.
Mechanistically, the authors found that HIF1α promoted the phosphorylation of PPARγ Serine 112 (S112)—a post-translational modification of PPARγ that represses its transcriptional activity (Hu et al., 1996)—leading to an impaired adipogenic capacity of PDGFRβ+ cells. The HIF1α-induced phosphorylation of PPARγ S112 was mediated by autocrine/paracrine actions of PDGF-C and PDGF-D and the subsequent activation of ERK signaling. Significantly, Imatinib (known as GLEEVEC), a tyrosine kinase inhibitor for PDGFRs, powerfully suppressed PPARγ S112 phosphorylation and promoted adipogenesis in the WAT of diet-induced obese mice. These results identified a novel mechanism through which the HIF1α-ERK signaling cascade triggers the pro-inflammatory/fibrotic program while inhibiting adipogenesis in PDGFRβ+ precursor cells.
Together, the back-to-back papers (Joffin et al., 2021; Shao et al., 2021) provide compelling evidence that adipose PDGFRβ+ precursor cells play a central role in the regulation of adipose tissue health (Figure 1). Since tissue hypoxia can occur in obese WAT and inhibit mitochondrial activity, tissue oxygen tension is one of the central determinants of adipose tissue health. Consistent with that notion, HIF1α inhibition in adipose tissues protected mice against diet-induced metabolic disorders, such as glucose intolerance; however, it should be noted that targeting different components of the HIF pathway (e.g., HIF1α, HIF2α, and HIF-prolyl hydroxylases) leads to conflicting metabolic outcomes (Michailidou, 2019). Hence, the specificity and compensatory responses of HIF1 blockade need future investigation.
Figure 1. The mechanisms through which obesity leads to adipose tissue dysfunction.
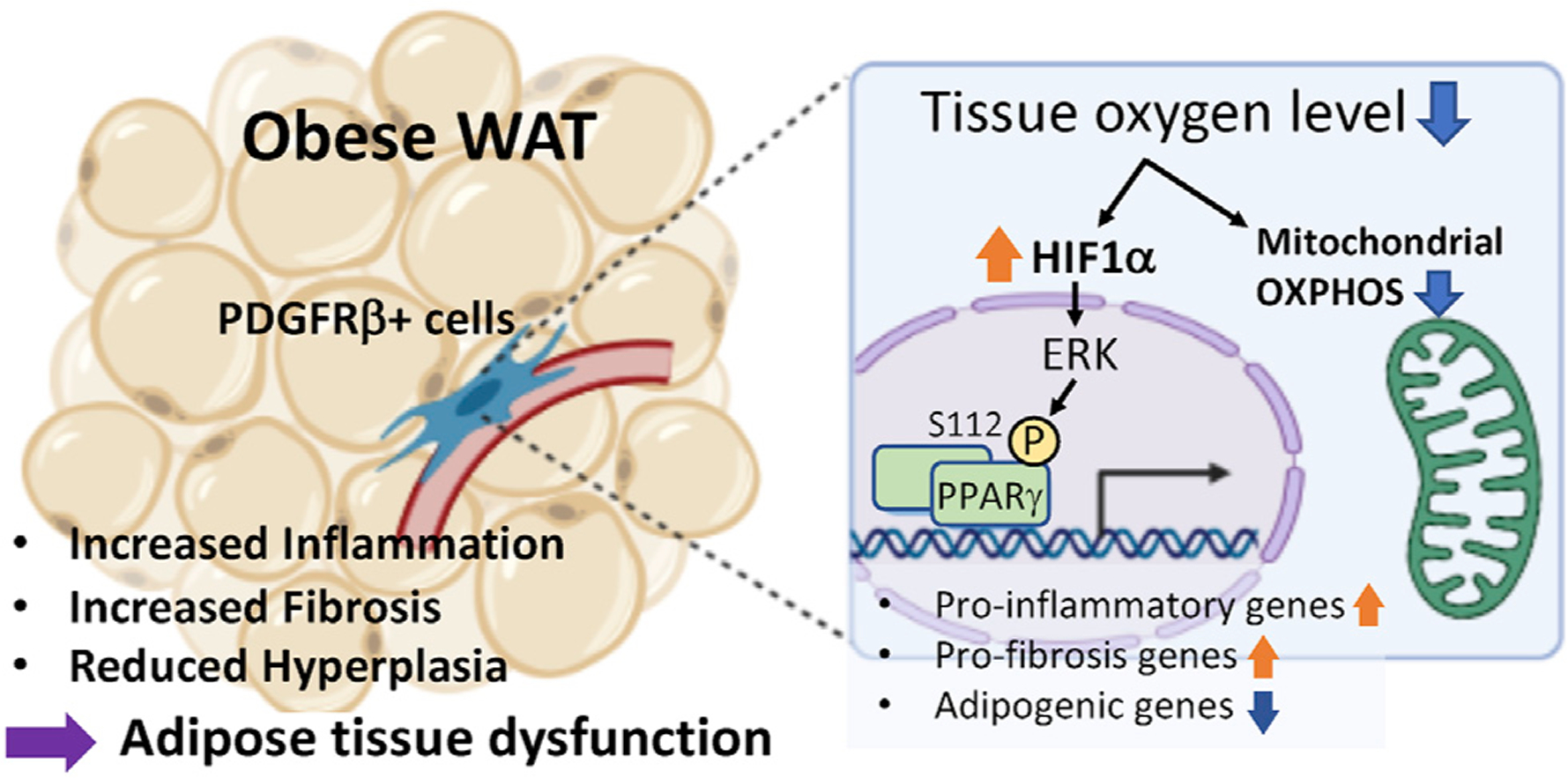
Under an obese condition, reduced oxygen level (hypoxia) in adipose tissues trigger the HIF1α-induced phosphorylation of PPARγ S112 via ERK signaling in PDGFRβ+ progenitor cells. This signaling cascade leads to the activation of pro-fibrotic genes while suppressing the pro-adipogenic program. Reduced mitochondrial OXPHOS activity also leads to the activation of pro-inflammatory genes and repression of adipogenic potential in adipose PDGFRβ+ cells.
Another notable observation from the present studies is the rapid reversibility of adipose tissue health: reactivating mitochondrial oxidative phosphorylation (OXPHOS) by turning off mitoNEET or pharmacologically antagonizing PDGFR by Imatinib in PDGFRβ+ cells restored adipose tissue health and systemic glucose tolerance in diet-induced obese mice. Hence, the present studies illuminate the potential for PDGFRβ+ cells to be a therapeutically attractive target. This idea is particularly appealing given the high accessibility of perivascular cells (PDGFRβ+ cells) through the blood-stream. An exciting area of future research would be to develop approaches to target adipose PDGFRβ+ progenitors in a cell-type-specific manner pharmacologically.
Lastly, emerging evidence suggests the role of mitochondria in the regulation of cell fate determination and maintenance. In general, stem cells depend on glycolytic metabolism for self-renewal and maintenance; nonetheless, recent studies demonstrated that active mitochondrial OXPHOS is required for the activation of certain stem cells, including intestinal Lgr5+ stem cells and tumor-initiating stem-like cells (Zhang et al., 2018). Of note, mitochondrial clearance via mitophagy contributes to the maintenance of beige adipocytes that contain high levels of mitochondria (Altshuler-Keylin and Kajimura, 2017). These studies imply the existence of mitochondrial-derived retrograde signals that control the transcriptional and epigenetic machinery of adipocyte fate and differentiation. In this regard, the present studies open a new research field to explore the role of mitochondria metabolism in regulating APC fate and maintenance.
ACKNOWLEDGMENTS
The author thanks the support of the NIH (DK097441, DK125281, DK127575, and DK126160) and the Edward Mallinckrodt, Jr. Foundation.
REFERENCES
- Altshuler-Keylin S, and Kajimura S (2017). Mitochondrial homeostasis in adipose tissue remodeling. Sci. Signal 10, eaai9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaben AL, and Scherer PE (2019). Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol 20, 242–258. [DOI] [PubMed] [Google Scholar]
- Hepler C, Shan B, Zhang Q, Henry GH, Shao M, Vishvanath L, Ghaben AL, Mobley AB, Strand D, Hon GC, and Gupta RK (2018). Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 7, e39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E, Kim JB, Sarraf P, and Spiegelman BM (1996). Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 274, 2100–2103. [DOI] [PubMed] [Google Scholar]
- Joffin N, Paschoal VA, Gliniak CM, Crewe C, Elnwasany A, Szweda LI, Zhang Q, Hepler C, Kusminski CM, Gordillo R, et al. (2021). Mitochondrial metabolism is a key regulator of the fibro-inflammatory and adipogenic stromal subpopulations in white adipose tissue. Cell Stem Cell 28, this issue, 702–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, and Scherer PE (2012). MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med 18, 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidou Z (2019). Fundamental roles for hypoxia signalling in adipose tissue metabolism and inflammation in obesity. Current Opinion in Physiology 12, 39–43. [Google Scholar]
- Shao M, Hepler C, Zhang Q, Shan B, Vishvanath L, Henry GH, Zhao S, An YA, Wu Y, Strand DW, and Gupta RK (2021). Pathologic HIF1α signaling drives adipose progenitor dysfunction in obesity. Cell Stem Cell 28, this issue, 685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, and Gupta RK (2016). PDGFRβ+ Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell Metab. 23, 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Menzies KJ, and Auwerx J (2018). The role of mitochondria in stem cell fate and aging. Development 145, dev143420. [DOI] [PMC free article] [PubMed] [Google Scholar]


