Abstract
Huntington’s disease arises from polyQ expansion within the exon-1 region of huntingtin (httex1), resulting in an aggregation prone protein that accumulates in neuronal inclusion bodies. We investigate the interaction of various httex1 constructs with the bacterial analog (GroEL) of the human chaperonin Hsp60. Using fluorescence spectroscopy and electron and atomic force microscopy we show that GroEL inhibits fibril formation. The binding kinetics of httex1 constructs with intact GroEL and a mini-chaperone comprising the apical domain is characterized by relaxation-based NMR measurements. The lifetimes of the complexes range from 100-400 μs with equilibrium dissociation constants (KD) of ~1-2 mM. The binding interface is formed by the N-terminal amphiphilic region of httex1 (which adopts a partially helical conformation) and the H and I helices of the GroEL apical domain. Sequestration of monomeric httex1 by GroEL likely increases the critical concentration required for fibrillization.
Keywords: chaperones, htt polypeptides, amyloid-chaperonin interactions, NMR spectroscopy, electron microscopy, atomic force microscopy
Graphical Abstract

Polyglutamine expansion within the region of huntingtin encoded by exon-1 of the htt gene results in an aggregation prone protein that accumulates in neuronal inclusion bodies. We show that the chaperonin GroEL, the bacterial homolog of human mitochondrial Hsp60, inhibits fibril formation of huntingtin exon-1 peptides and probe the interaction using NMR relaxation-based experiments designed to explore short-lived sparsely-populated states.
Introduction
CAG expansion resulting in abnormally long polyglutamine (polyQ) tracts, is responsible for a number of protein misfolding diseases,[1] including Huntington’s disease, a fatal, autosomal dominant, neurodegenerative condition.[1, 2] Huntington’s disease arises when the CAG (polyQ) region within exon-1 of the huntingtin (htt) gene expands beyond 36 glutamine repeats. Fragments of huntingtin exon-1 (httex1) are generated through proteolysis or incomplete mRNA splicing, yielding aggregation-prone polypeptides that form fibrillar neuronal tangles.[2] A key component in the very earlies stages of httex1 fibrillization is the N-terminal, 16-residue amphiphilic region (httNT)[3-7] which, with as few as 7 glutamines C-terminal to the NT region (httNTQ7), undergoes branched oligomerization on the micro to sub-millisecond timescale.[8] The latter involves transient, sparsely-populated species, with the productive pathway leading to helical coiled-coil tetramer via a coiled-coiled helical dimer, and the non-productive pathway to an amorphous ensemble of partially helical dimers.[8-10]
A number of chaperones, including the class II chaperonin TRiC[11, 12] and Hsp70, Hsp110 and Hsp40 either alone or in combination[13] inhibit httex1 aggregation into oligomers and fibrils. Mitochondrial deformation and dysfunction are hallmarks of Huntington’s disease [14-16]. The class I chaperonin Hsp60, as well as the chaperone Hsp70, constitute the major components of the protein quality control machinery in the mitochondrion.[17] Hsp60 comprises two heptameric rings stacked upon one another, each ring enclosing a “folding” chamber.[18-20] The bacterial Hsp60, known as GroEL,[21, 22] is ~50% sequence identical to human mitochondrial Hsp60,[23] and the isolated apical domain, known as a mini-chaperone, retains residual chaperone activity in the absence of the co-chaperone Hsp10 and nucleotides.[24-26]
Previously we showed that GroEL interacts with amyloid β preventing both fibril formation and neurotoxicity.[27] Here we make use of a variety of biophysical techniques to study the interaction of various httex1 constructs with GroEL and the GroEL mini-chaperone. We first show using electron (EM) and atomic force (AFM) microscopy and fluorescence spectroscopy that GroEL and the mini-chaperone inhibit httex1 fibril formation. We then make use of relaxation-based NMR experiments to probe the ‘dark’ invisible states of a variety of httex1 constructs bound to both intact GroEL and the GroEL mini-chaperone. We show that binding of httex1 to GroEL exclusively involves the NT region of httex1 and the apical domain of GroEL. The interaction is weak (in the mM range) with a lifetime on the sub-millisecond timescale.
Results and Discussion
A summary of the httex1 constructs used in the current work, together with a depiction of GroEL and the mini-chaperone, is shown in Figs. 1A and B, respectively. All httex1 constructs eventually form fibrils as can be seen from EM and AFM microscopy (Fig. S1). Details of expression and purification are provided in the Supporting Information. Assignments of the 1H-15N correlation spectra of various httex1 constructs and of the mini-chaperone are provided in the Supplementary Figs. S2 and S3, respectively; and NMR characterization of very weak (Kdimer ~ 10 mM) mini-chaperone dimerization is shown in Fig. S4.
Figure 1.
Summary of constructs used in the current work. Httex1 is composed of a N-terminal amphiphilic domain (NT), a polyQ stretch and a polyproline rich domain (PRD) comprising two polyproline sequences. The PDB codes for the GroEL and GroEL mini-chaperone are 1XCK[29, 30] and 1FYA, [30] respectively.
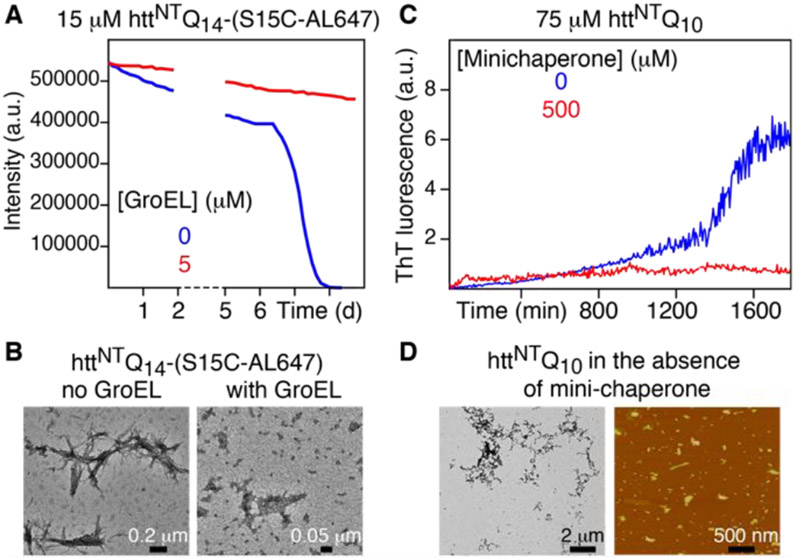
The effect of GroEL on fibril formation was studied using the httNTQ14-(S15C) construct with a cysteine residue substituted in place of a serine at position 15 to allow for the covalent attachment of the Alexa Fluor 647 (AL647) fluorophore. In the absence of GroEL, fluorescence of 15 μM httNTQ14-(S15C-AL647) is quenched within 6 days at 37 °C without shaking (Fig. 2A , blue trace), and negative stain electron microscopy (EM) clearly shows the presence of fibrils (Fig. 2B, left panel). In the presence of 5 μM (in subunits) GroEL, however, fluorescence is not quenched and no fibrils are observed by EM (Fig. 2B, right panel).
Figure 2.
Impact of GroEL and the GroEL mini-chaperone on fibril formation of httex1 constructs. (A) Time-dependence of fluorescence quenching of 15 μM httNTQ14(S15C-AL647) in the absence (blue) and presence (red) of 5 μM (in subunits) GroEL. AL647 was excited at 625 nm and emission monitored at 690 nm. (B) Negative-stain EM of samples from (A) after 8 days. In the presence of GroEL, no fibrils are observed but densely packed particles of GroEL are clearly apparent. (C) Time course of ThT fluorescence monitoring aggregation of 75 μM httNTQ10 in the absence (blue) and presence (red) of 500 μM mini-chaperone. (D) Negative stain EM (left) and atomic force microscopy (right) at the end of the ThT experiment in panel C in the absence of mini-chaperone. In the presence of the mini-chaperone, no fibrils, protofibrils or large oligomers are observed at the end of the ThT experiment by either EM or AFM. All experiments were carried out at pH 6.5 in 20 mM sodium phosphate and 50 mM NaCl, at 37 °C and not shaken.
The effect of the mini-chaperone, which contains the two helices H and I involved in substrate binding (Fig. 1B),[20] on the aggregation of httNTQ10 was monitored using a thioflavin T (ThT) fluorescence assay.[28] For 75 μM httNTQ10 an increase in ThT fluorescence emission, indicative of the formation of amyloid-like fibrils containing cross-β structure,[28] is seen after about 1 day (Fig. 2C, blue trace), with oligomers and fibrils observed by EM and AFM (Fig. 2D). When 500 μM mini-chaperone is added to the 75 μM httNTQ10 solution, however, no increase in ThT fluorescence emission is observed over the same time period (Fig. 2C, red trace), and no fibrils, protofibrils or large oligomers are observed by EM or AFM.
Given the above results showing that httex1 fibril formation is inhibited by both GroEL and the mini-chaperone, we proceeded to investigate the interaction of various httex1 constructs with GroEL and the mini-chaperone by NMR spectroscopy. 15N lifetime line-broadening (ΔR2), predominantly within the NT region of httNTQ7, httNTQ10, httNTQ7P11 and httNTQ14P11 is observed in the presence of GroEL (Figs 3A and B), as expected for exchange between the low molecular weight, NMR visible monomeric species of the httex1 constructs and an NMR-invisible ‘dark’ state bound to a slowly tumbling large macromolecule such as GroEL (~800 kDa).[31] Addition of acid-denatured Rubisco, an unfolded protein substrate that binds with nanomolar affinity to GroEL,[32] abolishes lifetime line-broadening of httNTQ7, indicating the Rubisco and httNTQ7 compete for the same binding site(s) on GroEL. In addition, no lifetime line-broadening is observed for Met7O-httNTQ7 in which Met7 is selectively oxidized to a sulfoxide by treatment with hydrogen peroxide, indicating that hydrophobic contacts involving the methyl group of Met7 play an important role in binding.
Figure 3.
15N lifetime line broadening (ΔR2) of httex1 constructs in the presence of GroEL. 15N-ΔR2 values are given as the difference in 15N-R2 values measured for the httex1 constructs in the presence and absence of 25 μM (in subunits) GroEL at a spectrometer frequency of 600 MHz. Experiments were carried out at pH 6.5 and 10 °C in 20 mM sodium phosphate and 50 mM NaCl.
1HN/15N chemical shift perturbation mapping was further used to delineate the interaction sites between httex1 constructs and the GroEL mini-chaperone. Titration of unlabelled mini-chaperone into various 15N-labeled httex1 constructs with 7 (httNTQ7; Fig. 4A and C), 14 ( httNTQ14P11; Fig. 4B and C) and 21 (GB1-httex1-Q21 fusion; Fig. S5) glutamine repeats shows that the largest 1HN/15N chemical shift perturbations occur between Leu3 and Ser15 with minimal or no perturbations beyond Gln19. These data confirm that binding occurs via the NT region of httex1 and does not involve the polyQ repeat beyond the first few glutamines. The converse experiments with 15N-labeled mini-chaperone and unlabeled httNTQ7 (Fig. 5A) or httNTQ14P11 (Fig. 5B) indicate that the predominant interaction surface on the mini-chaperone comprises helices H and I, as well as a small helical turn region close to the N-terminus. This is further confirmed from intermolecular paramagnetic relaxation enhancement (PRE) measurements[33] in which a nitroxide label (R1) is covalently attached to cysteine in httNTQ7-(S15C) resulting in an increase in 15N transverse relaxation for the same regions on the mini-chaperone that exhibit 1HN/15N chemical shift perturbations (Fig. 5C).
Figure 4.
1HN/15N chemical shift perturbation observed for 300 μM 15N/13C-labeled (A) httNTQ7 and (B) httNTQ14P11 upon addition of unlabeled GroEL mini-chaperone. (C) Corresponding ΔδNH (in ppm) perturbation profiles for httNTQ7 (top) and httNTQ14P11 (bottom) given by {[0.125ΔδN2 + ΔδH2]/2}1/2 where ΔδN and ΔδH are the differences in 15N and 1HN chemical shifts, respectively, between 300 μM samples in the presence and absence of 1 mM mini-chaperone.[34] The 1H-15N single quantum coherence correlation (HSQC) spectra were recorded at 600 MHz and 10 °C in 20 mM sodium phosphate, pH 6.5 and 50 mM NaCl.
Figure 5.
Delineation of mini-chaperone binding surface for httex1 constructs. tHN/15N (ΔδNH) chemical shift perturbation observed for 150 μM 15N-labeled GroEL mini-chaperone upon addition of unlabeled (A) httNTQ7 (1.5 mM) and (B) httNTQ14P11 (600 μM) measured from 1H-15N HSQC spectra obtained at 800 MHz. ΔδNH (in ppm) is given by {[0.125ΔδN2 + ΔδH2]/2}1/2 where ΔδN and ΔδH are the differences in 15N and 1HN chemical shifts, respectively, between samples in the presence and absence of httex1 polypeptides.[34] (C) PRE 1HN-Γ2 rates measured on 300 μM 15N-labeled GroEL mini-chaperone in the presence of 150 μM nitroxide spin-labeled httNT-Q7-(S15C-R1) (blue circles). 1HN-Γ2 rates are expressed as the difference in 15HN-R2 rates (measured at 600 MHz) obtained in the presence of paramagnetic (nitroxide-R1 labeled) and diamagnetic (reduced nitroxide label) species.[33] No intermolecular PREs are observed in the presence of 150 μM nitroxide-labeled maltose binding protein (E38C-R1 MBP)[39] (red circles) indicating that the intermolecular PREs observed with httNT-Q7-(S15C-R1) do not arise from non-specific interactions between the R1 nitroxide label and the mini-chaperone. Experiments were carried out at 10 °C in 20 mM sodium phosphate, pH 6.5, 50 mM NaCl. The sites of interaction with the mini-chaperone are indicated by the grey bars in (A)-(C) and in (D) are color coded in red on a ribbon diagram of the structure of the mini-chaperone (PDB code 1FYA[30]).
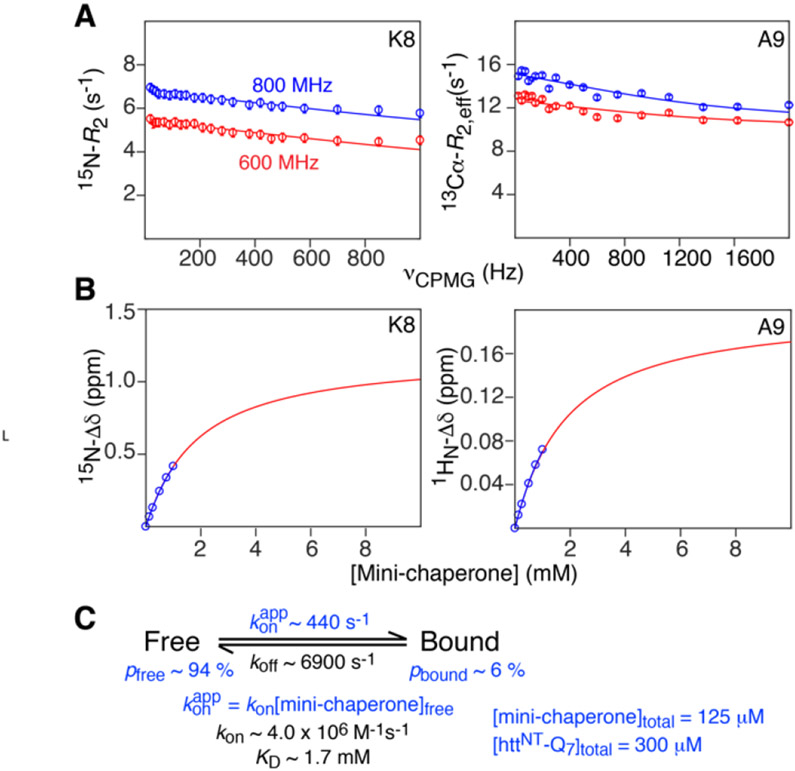
To characterize the kinetics of the interaction of httNTQ7 with the GroEL mini-chaperone, we simultaneously fit 15N[35] and 13Cα[36] Carr-Purcell-Meiboom-Gill (CPMG)[31, 37, 38] relaxation dispersion data recorded on15N/13Cα-labeled httNTQ7 in the presence of the mini-chaperone, combined with 1HN and 15N chemical shift titration data (Fig. 6). The complete relaxation dispersion (Figs. S6 and S7) and chemical shift titration (Figs. S8 and S9) data used in the fits are provided in the Supplementary Information. At the concentration of 300 μM httNTQ7 employed, no relaxation dispersion is observed as a result of pre-nucleation transient oligomerization, and hence the data in the presence of the mini-chaperone can be fully accounted for by a two-state exchange model with a lifetime for the bound state of ~140 μs, association (kon) and dissociation (koff) rate constants of ~4 x 106 M−1s−1 and 6900 s−1, respectively, and an equilibrium dissociation constant, KD, of ~1.7 mM.
Figure 6.
Characterization of binding kinetics and equilibrium for the interaction of httNTQ7 with the GroEL mini-chaperone. Examples of (A) 15N (left) and 13Cα (right) CPMG relaxation dispersion profiles and (B) 15N (left) and 1HN (right) chemical shift titration data obtained at 10°C (in 20 mM sodium phosphate, pH 6.5, 50 mM NaCl). The experimental data are shown as circles and the best-fit curves to the two-state exchange model shown in (C) are displayed as continuous lines. Data in (A) were acquired at 600 and 800 MHz on 300 μM 15N/13Cα-labeled httNTQ7 in the presence of 125 μM unlabeled GroEL; data in (B) were acquired at 600 MHz on 300 μM 15N-labeled httNTQ7 with the concentration of unlabeled mini-chaperone ranging from 0 to 1 mM. The full data sets used in the global fit are shown in Figs. S6-S9. The values of the rate constants and KD are given in (C). The populations of free and bound httNTQ7, as well as the value of konapp, shown in (C) relate to the conditions of the CPMG relaxation dispersion experiments (right-side). The relative uncertainties in the values of the fitted parameters range from 5-10%. The fitted Δω values are given in Table S2.
To confirm that httNTQ7 binds to the GroEL mini-chaperone as a monomer we carried out intermolecular 1HN-PRE measurements[33] (Fig. S10). In the absence of the GroEL mini-chaperone, the intermolecular 1HN-PRE profile observed on 15N-labeled httNTQ7 in the presence of a small amount of amino-terminal nitroxide-labeled R1-httNTQ7 (see Supplementary) is characteristic of transient, sparsely-populated, pre-nucleation helical coiled-coil dimers and tetramers, as described previously.[8] The 1HN-PRE profile remains unaltered upon addition of GroEL mini-chaperone, allowing one to conclude that the bound state of httNTQ7 is a monomer.
CPMG relaxation dispersion and chemical shift titration experiments were also performed with httNTQ14P11 and the GroEL mini-chaperone (Fig. 7, and Figs. S11-S16). Analysis is a little bit more complex since relaxation dispersion, owing to sub-millisecond pre-nucleation oligomerization,[8] is observed and therefore has to be taken into account. Although pre-nucleation oligomerization involves three sparsely populated states (two dimers and a tetramer) and a branched pathway,[8, 9] for the purposes of fitting the relaxation dispersion data at a single httNTQ14P11 concentration, these multiple events can be represented by a ‘virtual’ two-state exchange process. Thus, in the presence of mini-chaperone, we consider two states that interconvert with the NMR observable monomeric species: a mini-chaperone bound state and a ‘virtual’ state representing the pre-nucleation oligomer ensemble. Both the CPMG relaxation dispersion data in the absence and presence of the mini-chaperone are included in the global fits. The resulting kinetic and equilibrium parameters are comparable to those obtained with httNTQ7. The lifetime of httNTQ14P11 bound to the mini-chaperone is ~170 μs, with kon ~2.7 x 106 M−1s−1, koff ~ 5040 s−1 and KD ~1.9 mM (Table S1). Thus, the length of the polyQ repeat (beyond the first few glutamines) and the presence or absence of a polyproline stretch has little impact on the binding of httex1 constructs to GroEL.
Figure 7.
Characterization of binding kinetics and equilibrium for the interaction of httNTQ14P11 with the GroEL mini-chaperone. Examples of (A) 15N and 13Cα CPMG relaxation dispersion profiles in the presence (left) and absence (right) of the mini-chaperone, and (B) 1HN (left) and 15N (right) chemical shift titration data obtained at 10°C (in 20 mM sodium phosphate, pH 6.5, 50 mM NaCl). The experimental data are shown as circles and the best-fit curves to the three-state exchange model shown in (C) are displayed as continuous lines. Data in (A) were acquired at 600 and 800 MHz on 300 μM 15N/13Cα-labeled httNTQ11P14 in the presence of 300 μM unlabeled GroEL; data in (B) were acquired at 600 MHz on 300 μM 15N-labeled httNTQ11P14 with the concentration of unlabeled mini-chaperone ranging from 0 to 1 mM. The full data sets used in the global fit are shown in Figs. S11-S16. The values of the rate constants and KD are given in (C). The populations of free and bound httNTQ11P14, as well as the value of konapp, shown in (C) relate to the conditions of the CPMG relaxation dispersion experiments (right-side). The conformation of httNTQ11P14 bound to the mini-chaperone is shown as a transparent blue helix to indicate that the bound state samples an ensemble of partially helical conformations; note also that the depiction is purely a cartoon as we have no information on the orientation of bound httNTQ11P14 on the surface of the mini-chaperone. The pre-nucleation oligomers that exchange with free monomeric httNTQ11P14 represent an ensemble of helical dimers and tetramers,[8, 9] that are represented in this instance by a single virtual species with apparent interconversion rate constants k1* and k−1*. The relative uncertainties in the values of the fitted parameters range from 5-10%. The fitted Δω values are given in Tables S3 and S4.
The CPMG relaxation dispersion and chemical shift titration data also yield the changes in 13Cα, 15N and 1HN shifts of httNTQ7 (Table S2) and httNTQ14P11 (Table S3) upon binding the mini-chaperone. In both cases, the shift changes within the NT region are positive for 13Cα, negative for 15N, and negative for 1HN with the exception of Leu3 and Glu4. These shifts are largely impacted by the backbone ϕ/ψ angles,[40] and the magnitude of the 13Cα shifts (ranging from ~0.4 to 1 ppm) is suggestive of an ensemble of partially helical conformations in the bound state. It is also worth noting that for httNTQ14P11, the 13Cα shift differences between the monomer and the sparsely-populated (0.4%) virtual oligomeric state (Table S4) are indicative of helix formation within the NT region, consistent with the detailed kinetic and structural characterization of pre-nucleation olgomerization presented earlier on both httNTQ7 and the entire httex1 with 7 glutamine repeats.[8, 9]
The kinetics of httNTQ7 binding to intact GroEL were investigated by a combination of 15N Dark state Exchange Saturation Transfer (DEST), lifetime line broadening (ΔR2) and CPMG relaxation dispersion.[41, 42] Examples of these data are shown in Fig. 8 and the full data set used for fitting to a two-state exchange model are provided in Figs. S17 and S18. The lifetime of the complex is ~370 μs, with kon ~2.3 x 106 M−1s−1, koff ~2650 s−1, and KD ~ 1.3 mM (Table S1). (Note that kon and KD are calculated under the assumption that the apical domain of all 14 subunits (7 per ring) can potentially bind httNTQ7). Under the conditions of the experiment comprising 200 μM httNTQ7 and 25 μM GroEL (in subunits), one can conclude that each GroEL cavity is occupied by a single httNTQ7 molecule (i.e. the bound population of httNTQ7 is ~1.8%, corresponding to a concentration of ~3.6 μM bound httNTQ7, which is equal to the total concentration of GroEL cavities. The best-fit 15N-R2bound profile for httNTQ7 bound to GroEL indicates that the region most immobilized upon binding extends from residues 3-10 (Fig. 8E). The average <15N-R2bound> value for residues 3-10 is ~800 s−1 at 900 MHz, consistent with the molecular weight of GroEL.
Figure 8.
Characterization of binding kinetics and equilibrium for the interaction of 15N-labeled httNTQ7 with unlabeled intact GroEL. (A) 15N-ΔR2 lifetime line broadening at 600 and 900 MHz; (B) Examples of 600 MHz 15N DEST profiles acquired at two radiofrequency saturation fields (250 and 500 Hz); (C) Examples of 15N-CPMG relaxation dispersion curves at 600 MHz. The full 15N-DEST and 15N-CPMG relaxation dispersion data sets used in the fitting are shown in Figs. S17 and S18, respectively. The experimental data is shown as circles, and the best fit curves to the two-state exchange model shown in (C) are displayed as continuous lines. Note, the inclusion of the CPMG relaxation dispersion data in the analysis of the DEST and ΔR2 data serves to decorrelate pbound from R2bound in the exchange regime where (konapp + koff)/R2bound > 1.[42, 43] The depiction of httNTQ7 bound (as a transparent blue helix to represent an ensemble of partially helical states) to GroEL in (D) is purely a cartoon as we have no information on the orientation of bound httNTQ11P14 on the surface of the GroEL apical domain. The experiments were carried out with 200 μM 15N-labeled httNTQ7 and 25 μM (in subunits) unlabeled GroEL at 10 °C in 20 mM sodium phosphate, pH 6.5, 50 mM NaCl. The relative uncertainties in the values of the fitted parameters range from 5-10%. The Δω values required to fit the 15N-CPMG relaxation dispersion data were fixed to the values obtained from the data with the GroEL mini-chaperone (Table S1). (D) Best-fit 15N-R2bound values for httNTQ7 bound to intact GroEL.
Although the KD values for the binding of httNTQ7 to GroEL and the mini-chaperone are very similar (~1.3 and ~1.7 mM, respectively; Table S1), the approximately two-fold lower values of kon and koff obtained with intact GroEL (Fig. 8) compared to the mini-chaperone (see Fig. 6) may be due to two potential factors: (a) multiple trials required for httNTQ7 to penetrate the GroEL cavity and bind to an apical domain; and (b) geminate rebinding of httNTQ7 to the same or another apical domain within the cavity prior to exiting the cavity into bulk solution.
Conclusions
In summary we have shown that bacterial Hsp60 (GroEL) inhibits fibril formation of a variety of httex1 constructs comprising differing lengths of the polyQ repeat with and without the polyproline-rich region. Binding predominantly involves residues 3-13 within the NT region of httex1 and a contiguous surface on the apical domain of GroEL formed by helices H and I and a small helical turn. The affinity for both GroEL and the mini-chaperone is in the low millimolar range with complex lifetimes in the 100-400 μs range. Despite the low affinity, the avidity of httex1 for intact GroEL is high on account of the presence of multiple binding sites (7 per ring). Since GroEL binds monomeric httex1, the population of pre-nucleation, transient oligomers (dimers and tetramer) formed by the self-association of the NT region will necessarily be reduced, thereby effectively increasing the critical concentration required for nucleation and consequent fibril formation.
Supplementary Material
Acknowledgements
We thank Dr. V. Tugarinov for useful discussions, and Drs. J. Baber, D. Garrett and J. Ying for technical support. This work was supported by the Intramural Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (DK-029023 to G.M.C.).
References
- [1].Orr HT, Zoghbi HY, Ann. Rev. Neuroscience 2007, 30, 575–621. [DOI] [PubMed] [Google Scholar]
- [2].Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, Nance M, Ross CA, Scahill RI, Wetzel R, Wild EJ, Tabrizi SJ, Nature Rev. Dis. Primers 2015, 1, 15005. [DOI] [PubMed] [Google Scholar]
- [3].Fiumara F, Fioriti L, Kandel ER, Hendrickson WA, Cell 2010, 143, 1121–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sivanandam VN, Jayaraman M, Hoop CL, Kodali R, Wetzel R, van der Wel PC, J. Am. Chem. Soc 2011, 133, 4558–4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Crick SL, Ruff KM, Garai K, Frieden C, Pappu RV, Proc. Natl. Acad. Sci. U. S. A 2013, 110, 20075–20080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen M, Wolynes PG, Proc. Natl. Acad. Sci. U. S. A 2017, 114, 4406–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Urbanek A, Popovic M, Morato A, Estana A, Elena-Real CA, Mier P, Fournet A, Allemand F, Delbecq S, Andrade-Navarro MA, Cortes J, Sibille N, Bernado P, Structure 2020, 28, 733–746. [DOI] [PubMed] [Google Scholar]
- [8].Kotler SA, Tugarinov V, Schmidt T, Ceccon A, Libich DS, Ghirlando R, Schwieters CD, Clore GM, Proc. Natl. Acad. Sci. U. S. A 2019, 116, 3562–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ceccon A, Tugarinov V, Ghirlando R, Clore GM, Proc. Natl. Acad. Sci. U. S. A 2020, 117, 5844–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ceccon A, Tugarinov V, Clore GM, J. Phys. Chem. Lett 2020, 11, 5643–5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tam S, Geller R, Spiess C, Frydman J, Nat. Cell Biol 2006, 8, 1155–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shahmoradian SH, Galaz-Montoya JG, Schmid MF, Cong Y, Ma B, Spiess C, Frydman J, Ludtke SJ, Chiu W, Elife 2013, 2, e00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Scior A, Buntru A, Arnsburg K, Ast A, Iburg M, Juenemann K, Pigazzini ML, Mlody B, Puchkov D, Priller J, Wanker EE, Prigione A, Kirstein J, EMBO J. 2018, 37, 282–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Orr AL, Li SH, Wang CE, Li H, Wang JJ, Rong J, Xu XS, Mastroberardino PG, Greenamyre JT, Li XJ, J. Neurosci 2008, 28, 2783–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Intihar TA, Martinez EA, Gomez-Pastor R, Front. Cell. Neurosci 2019, 13, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Costa V, Scorrano L, EMBO J. 2012, 31, 1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jebara F, Weiss C, Azem A, eLS. 1–9 John Wiley & Sons, Ltd: Chichester: 2017, doi: 10.1002/9780470015902.a0027152. [DOI] [Google Scholar]
- [18].Todd MJ, Lorimer GH, Thirumalai D, Proc. Natl. Acad. Sci. U. S. A 1996, 93, 4030–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thirumalai D, Lorimer GH, Annu. Rev. Biophys. Biomol. Struct 2001, 30, 245–269. [DOI] [PubMed] [Google Scholar]
- [20].Horwich AL, Fenton WA, Q. Rev. Biophys 2009, 42, 83–116. [DOI] [PubMed] [Google Scholar]
- [21].Goloubinoff P, Gatenby AA, Lorimer GH, Nature 1989, 337, 44–47. [DOI] [PubMed] [Google Scholar]
- [22].Goloubinoff P, Christeller JT, Gatenby AA, Lorimer GH, Nature 1989, 342, 884–889. [DOI] [PubMed] [Google Scholar]
- [23].Nisemblat S, Yaniv O, Parnas A, Frolow F, Azem A, Proc. Natl. Acad. Sci. U. S. A 2015, 112, 6044–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chatellier J, Hill F, Lund PA, Fersht AR, Proc. Natl. Acad. Sci. U. S. A 1998, 95, 9861–9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kobayashi N, Freund SMV, Chatellier J, Zahn R, Fersht AR, J. Mol. Biol 1999, 292, 181–190. [DOI] [PubMed] [Google Scholar]
- [26].Jain N, Knowles TJ, Lund PA, Chaudhuri TK, Biochim. Biophys. Acta - Proteins and Proteomics 2018, 1866, 941–951. [DOI] [PubMed] [Google Scholar]
- [27].Wälti MA, Steiner J, Meng FJ, Chung HS, Louis JM, Ghirlando R, Tugarinov V, Nath A, Clore GM, Proc. Natl. Acad. Sci. U. S. A 2018, 115, E11924–E11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Michaels TCT, Saric A, Habchi J, Chia S, Meisl G, Vendruscolo M, Dobson CM, Knowles TPJ, Annu. Rev. Phys. Chem 2018, 69, 273–298. [DOI] [PubMed] [Google Scholar]
- [29].Bartolucci C, Lamba D, Grazulis S, Manakova E, Heumann H, J. Mol. Biol 2005, 354, 940–951. [DOI] [PubMed] [Google Scholar]
- [30].Wang QH, Buckle AM, Fersht AR, J. Miol. Biol 2000, 298, 917–926. [DOI] [PubMed] [Google Scholar]
- [31].Anthis NJ, Clore GM, Q. Rev. Biophys 2015, 48, 35–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Van der Vies SM, Viitanen PV, Gatenby AA, Lorimer GH, Jaenicke R, Biochemistry 1992, 31, 3635–3644. [DOI] [PubMed] [Google Scholar]
- [33].Clore GM, Iwahara J, Chem. Rev 2009, 109, 4108–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Williamson MP, Prog. Nucl. Magn. Reson. Spectrosc 2013, 73, 1–16. [DOI] [PubMed] [Google Scholar]
- [35].Hansen DF, Vallurupalli P, Kay LE, J. Phys. Chem. B 2008, 112, 5898–5904. [DOI] [PubMed] [Google Scholar]
- [36].Hansen DF, Vallurupalli P, Lundstrom P, Neudecker P, Kay LE, J. Am. Chem. Soc 2008, 130, 2667–2675. [DOI] [PubMed] [Google Scholar]
- [37].Korzhnev DM, Kay LE, Acc. Chem. Res 2008, 41, 442–451. [DOI] [PubMed] [Google Scholar]
- [38].Palmer AG 3rd, J. Magn. Reson 2014, 241, 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tang C, Ghirlando R, Clore GM, J. Am. Chem. Soc 2008, 130, 4048–4056. [DOI] [PubMed] [Google Scholar]
- [40].Shen Y, Delaglio F, Cornilescu G, Bax A, J. Biomol. NMR 2009, 44, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fawzi NL, Ying J, Ghirlando R, Torchia DA, Clore GM, Nature 2011, 480, 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Libich DS, Fawzi NL, Ying J, Clore GM, Proc. Natl. Acad. Sci. U. S. A 2013, 110, 11361–11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tugarinov V, Clore GM, J. Biomol. NMR 2019, 73, 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.