Abstract
Background:
Despite increases in cannabis use generally and for pain management, data regarding cannabis use in patients undergoing surgery are lacking. This study examined the prevalence of cannabis use among patients undergoing elective surgery and explored differences in clinical characteristics and surgical outcomes between cannabis users and non-cannabis users.
Methods:
This prospective study included 1,335 adults undergoing elective surgery. Participants completed self-report questionnaires preoperative and at 3- and 6-months post-surgery to assess clinical characteristics and surgical outcomes.
Results:
Overall, 5.9% (N=79) of patients reported cannabis use (53.2% medical, 19.0% recreational, and 25.3% medical and recreational). On the day of surgery, cannabis users reported worse pain, more centralized pain symptoms, greater functional impairment, higher fatigue, greater sleep disturbances, and more symptoms of anxiety and depression versus non-cannabis users (all ps < 0.01). Additionally, a larger proportion of cannabis users reported opioid (27.9%) and benzodiazepine use (19.0%) compared to non-cannabis users (17.5% and 9.2% respectively). At 3 and 6 months, cannabis users continued to report worse clinical symptoms; however, both groups showed improvement across most domains (ps ≤ 0.05). At 6-months, the groups did not differ on surgical outcomes, including surgical site pain (p = 0.93) or treatment efficacy (p = 0.88).
Conclusions:
Cannabis use is relatively low in this surgical population, yet cannabis users have higher clinical pain, poorer scores on quality of life indicators, and higher opioid use before and after surgery. Cannabis users reported similar surgical outcomes, suggesting that cannabis use did not impede recovery.
Keywords: Perioperative cannabis use, Pain management, Total Knee Arthroplasty, Total Hip Arthroplasty, Surgical outcomes
INTRODUCTION
There are approximately 100 million procedures and surgeries performed each year in the United States.1 Substance use can complicate surgical outcomes, including post-surgical pain management. For example, a diagnosis of drug misuse is associated with poorer outcomes following total hip or knee arthroplasty including longer hospital stays, more frequent postoperative complications, and a five-fold increase in mortality rate.2 Cannabis remains one of the most prevalent substances of abuse.3 In surgical contexts, cannabis use is particularly relevant because cannabis is often used for acute and chronic pain management. Indeed, chronic pain is the most common reason for medical cannabis certification nationwide, accounting for >65% of qualifying conditions for cannabis licensure.4 The degree to which cannabis carries additional risks for surgical patients remains to be determined, as the published data regarding cannabis use among adults undergoing surgical procedures is limited.
Cannabis use in adults has been increasing in tandem with the growing number of US states that have legalized medical and recreational cannabis.3 Historically, cannabis has been considered in the context of substance misuse and addiction, but as more states legalize cannabis, its medical benefits are being reconsidered. On one hand, cannabis use is associated with a number of negative biomedical and psychosocial consequences, such as, chronic bronchitis, minor cognitive defects, psychosis, and others.5 However, patients using medical cannabis report benefits,6 and recent studies support cannabis use for some medical purposes, including chronic pain.7 Cannabis is also being investigated as a substitute for opioids in pain management contexts,8,9 given the risks of long-term use of opioid medications.10 Rates of cannabis use will likely continue to rise, and given that cannabis poses potential risks and uncertain benefits, more research is needed to better understand cannabis use in surgical settings.7 Surgeons lack a strong evidence base to make informed decisions pertaining to the potential effect of cannabis use on perioperative risk and postoperative outcomes.
Thus, we aimed to examine the prevalence and features of cannabis use in a large cohort of surgical patients receiving elective interventions. Next, we aimed to compare pre- and post-surgical clinical characteristics of cannabis users and non-cannabis users and evaluate change across time within each group. Given that prior research suggests medical cannabis users experience worse health and functioning compared to the general public,11 we hypothesized that cannabis users in this sample may have more severe symptoms than their non-cannabis using counter parts on several pain-related measures. Finally, given the limited studies on cannabis and surgical outcomes, we aimed to investigate whether cannabis use was associated with worse post-surgical outcomes (i.e., surgical site pain and perceptions of treatment efficacy).
METHODS
Participants and study design
The current study included 1,335 surgical patients enrolled in the Analgesic Outcomes Study (AOS) between December 1, 2015 and July 31, 2017 (post-legalization of medical cannabis in 2008, prior to recreational legalization in 2018). AOS is an ongoing prospective observational registry of pain- and opioid-related outcomes in patients who undergo elective surgery at the University of Michigan.12,13 On the day of surgery, potential participants are approached by a research assistant who determines eligibility and provides a brief overview of AOS. Patients are eligible to participate in AOS if they are ≥18 years old and scheduled for the following surgeries: hysterectomy, primary unilateral total knee or total hip arthroplasty, thoracic, hand, ankle, abdominal, breast, or inguinal hernia surgery. Exclusion criteria includes being a non-English speaker, inability to provide written informed consent, and current incarceration. Study participation includes completing a survey on the day of surgery (baseline) and up to four post-operative surveys (2 weeks, 1 month, 3 months and 6 months). All surveys include a comprehensive assessment of pain, functioning, mood, and opioid use, with relevant surgical outcomes assessed as part of the follow up surveys. Participants are compensated $10 for each time point for a total of up to $50.
This study analyzed AOS data from the baseline and 3- and 6-month assessments. Baseline data is collected in person using pen and paper questionnaires. At 3 months, data is collected via a telephone call and responses are recorded by a research assistant. At 6 months, data is collected via a telephone call and a mailed questionnaire. Participation in AOS is completely voluntary and as part of the consent process, participants are given information about privacy protections, including the use of coded study ID numbers without HIPAA identifiers and limiting data access and sharing to authorized research personnel only. Data collection and subsequent analyses were approved by the Institutional Review Board at the University of Michigan (Ann Arbor, MI).
Measures
Demographics
Demographic variables assessed included age, gender, ethnicity, education, relationship status, and disability.
Cannabis use
The AOS survey was updated in December of 2015 and the following cannabis use questions were added to the survey. At baseline, cannabis use (i.e., tetrahydrocannabinol (THC) products, not cannabidiol (CBD) only products) was recorded using the following items: do you currently use marijuana (yes/no), do you have a medical marijuana card (yes/no), please check the reason/s you use marijuana (recreational, medical, both, other), check all of the medical reasons you use marijuana (list of medical conditions including a write-in option), and how many times per month do you typically use marijuana. At 6-months, cannabis use was reassessed with a single item in the mailed questionnaire: do you currently use marijuana (yes/no).
Opioid and benzodiazepine use
All current medications were reviewed with the patient on the day of surgery. Patients were considered “opioid users” if they confirmed the documented use of prescription opioids. Patients who reported no current opioid use at the time of surgery were considered “opioid naïve.” Patients were considered “benzodiazepine users” if, on the day of surgery, they confirmed the documented use of benzodiazepines. Opioid use was reassessed at 3-months and 6-months via a phone call. Benzodiazepine use was only assessed at baseline. Average daily opioid use was calculated by converting average dose into oral morphine equivalents (OME).
Clinical characteristics
Overall pain severity
Overall pain severity (i.e., “pain that is separate or different from pain in the surgical site”) was assessed using the average of two items from the Brief Pain Inventory (BPI).14 The average of “worst pain” and “average pain” in the last week was used as a single composite measure of overall pain severity (0 to 10-point scale, where 0 was “no pain” and 10 was “worst pain imaginable”).
Centralized pain
The Fibromyalgia Survey Criteria is a surrogate measure of centralized pain consisting of the Michigan Body Map (MBM) and the Symptom Severity Index (SSI).15,16 The MBM displays the front and back of a human form and asks participants to check boxes to indicate areas of pain. Nineteen body areas are summed to calculate an index of widespread pain. The SSI is the sum of 6 items assessing additional somatic symptoms, including fatigue, pain or cramps in the lower abdomen, and headache, with scores ranging from 0 to 12 points. The Fibromyalgia Survey Criteria score is calculated by summing the MBM and SSI, with a range of 0 to 31. Higher scores are suggestive of pain that is driven more by the central nervous system.17
Physical functioning
Physical functioning was assessed using the Patient Reported Outcomes Measurement Information System (PROMIS) 4a physical function scale, a 4-item measure of ability to do daily activities.18 Each item is measured on a 5-point scale ranging from 1 (without any difficulty) to 5 (unable to do). The scores are summed, and total raw scores range from 4 to 20.
Fatigue
Fatigue was assessed using the PROMIS 4a fatigue scale, a 4-item measure that measures fatigue during the past 7 days.18 Each item is measured on a 5-point scale ranging from 1 (not at all) to 5 (very much). The scores are summed, and total raw scores range from 4 to 20. Fatigue was reassessed at 6-months only.
Sleep impairment
The PROMIS 8a sleep disturbance scale is an 8-item measure to assess sleep difficulties in the last 7 days.18 Each item is measured on a 5-point scale ranging from 1 to 5. The scores are summed, and total raw scores range from 8 to 40. Sleep was reassessed at 6-months only.
Symptoms of anxiety and depression
The PROMIS 4a anxiety scale is a 4-item measure that assesses how much general fear and worry patients experienced in the past 7 days.18 Each item is measured on a 5-point scale ranging from 1 (never) to 5 (always). The PROMIS 4a depression scale is a 4-item measure that assesses how much depression, helplessness, and hopelessness patients experienced in the past 7 days.18 Each item is measured on a 5-point scale ranging from 1 (never) to 5 (always). For both scales, the scores are summed, and total raw scores range from 4 to 20.
Post-surgical outcomes
Surgical site pain severity
Surgical site pain severity (i.e. pain in the affected joint) was assessed using two items from the BPI.14 The average of two items assessing “worst pain” and “average pain” at surgical site in the last week was used as a single composite measure of surgical site pain severity (0 to 10-point scale, where 0 was “no pain” and 10 was “worst pain imaginable”).
Treatment efficacy
The self-report measure Patient Global Impression of Change (PGIC) reflects a patient's belief about the efficacy of treatment. The PGIC is a 7-point scale from +3 to – 3 depicting a patient's rating of overall improvement. Patients rate their change as “very much improved,” “much improved,” “minimally improved,” “no change,” “minimally worse,” “much worse,” or “very much worse.” The PGIC was rescored as a binary variable where a value of “1” indicated “very much improved” or “much improved” and a value of “0” indicated “minimally improved” to “very much worse”.
Statistical Analyses
Descriptive statistics including frequencies and percentages of cannabis use at day of surgery and 3- and 6-months post-surgery were calculated. Using t-tests and chi-square tests, we examined differences between cannabis users and non-cannabis users on several clinical characteristics at day of surgery and at 3- and 6-months post-surgery. Additionally, mixed effects models were used with different link functions for dichotomous and continuous outcomes using a random intercept for study participants to examine whether changes in clinical characteristics from day of surgery to 3 months and 6 months’ post-surgery differed for cannabis users and non-users. Surgical outcomes were assessed using surgical site pain and the PGIC. Within-subjects simple effects were computed without statistical correction because these were a priori hypotheses. Analyses were conducted with Stata version 15.1.
Missing Data
See Figure 1 for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) chart. Participants may have had complete missing data at 3 and 6 months due to attrition (n = 191). At 6 months, participants may have had partial missing data due to only completing the phone call (n = 264) or only completing the mailed questionnaire (n = 88). Multiple imputation with chained equations generating 25 imputed datasets was used to account for missing data.
Figure 1.
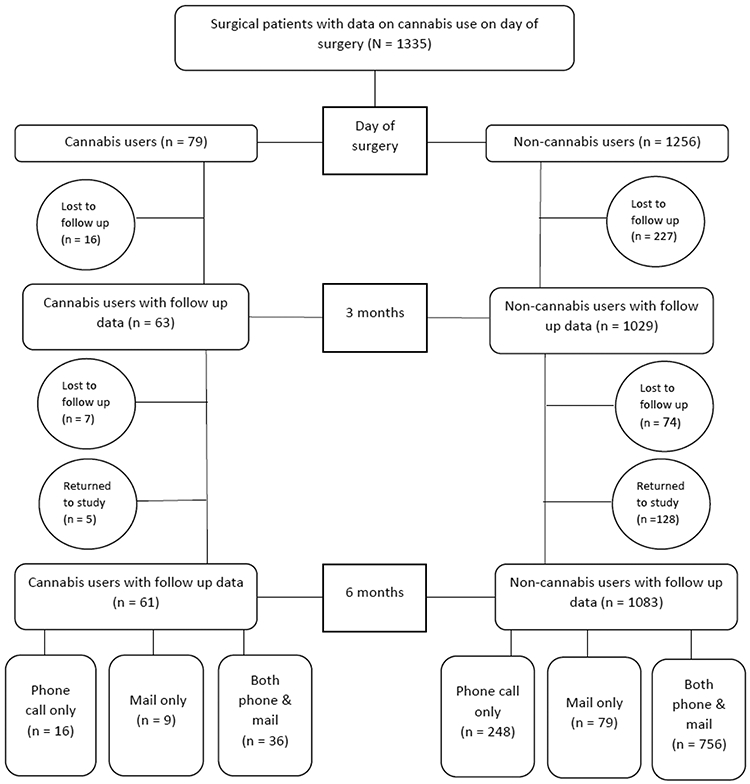
Strengthening the Reporting of Observational studies in Epidemiology (STROBE) diagram for patient participation at each time point
RESULTS
Day of surgery cannabis use
Descriptive data for cannabis use at baseline is presented in Table 1 and baseline cannabis use by surgery type is presented in Figure 2.
Table 1.
Cannabis use among 1,335 patients presenting for surgery and enrolled in the Analgesic Outcomes Study (AOS)
| n | % | 95% CI | |
|---|---|---|---|
| Use cannabis | 79 | (5.9%) | (4.8%; 7.3%) |
| Reasons for cannabis use | |||
| Medical only | 42 | (53.2%) | (42.0%; 64.0%) |
| Recreational only | 15 | (19.0%) | (11.7%; 29.3%) |
| Medical and recreational | 20 | (25.3%) | (16.8%; 36.2%) |
| Other | 2 | (2.5%) | (0.6%; 9.8%) |
| Medical reasons | |||
| Chronic pain | 57 | (91.9%) | (81.8%; 96.7%) |
| Sleep | 26 | (41.9%) | (30.2%; 54.7%) |
| Anxiety | 21 | (33.9%) | (23.0%; 46.7%) |
| Nausea | 10 | (16.1%) | (8.8%; 27.7%) |
| Migraine | 6 | (9.7%) | (4.3%; 20.2%) |
| Glaucoma | 1 | (1.6%) | (0.2%; 11.0%) |
| Other | 6 | (9.7%) | (4.3%; 20.2%) |
| Have medical cannabis card | 36 | (45.6%) | (34.8%; 56.8%) |
| Times per monthab | 23.7 | (34.1) | (16.0; 31.4) |
Note. Percentage of patients who use cannabis is calculated from full sample of 1,335 patients. Percentage of patients who have a cannabis card and reasons for cannabis use are calculated from the subsample of 79 patients who reported cannabis use. Percentages of medical reasons for cannabis use are calculated from the subsample of 62 patients who reported medical reasons for cannabis use.
mean (sd) presented for this item
One person reported using cannabis 240 times per month. Removing this person from the analysis reduces the mean number of times per month to 20.9 (23.3)
Figure 2.
Percentage of pre-surgical cannabis users for each surgical type
Note. A Fisher’s exact test to examine differences in cannabis use by surgery type found no statistically significant differences (p = 0.571).
Pre-and post-surgical clinical characteristics of cannabis users compared to non-cannabis users
Pre- and post-surgical clinical characteristics of cannabis users and non-cannabis users are presented in Table 2. Relative to non-cannabis users, cannabis users were more likely to be younger, male, less educated, and to receive disability benefits (all ps<0.01). On the day of surgery, cannabis users reported worse overall pain severity, more centralized pain symptoms, greater functional impairment, more fatigue, greater sleep disturbances, and more symptoms of anxiety and depression (all ps<0.01). Additionally, a larger proportion of cannabis users reported opioid (27.9%) and benzodiazepine use (19.0%) compared to non-cannabis users (17.5%, p=0.02 and 9.2%, p<.01, respectively). However, there was not a significant difference in average daily OME use between cannabis users and non-users at baseline (p=0.72).
Table 2.
Descriptive statistics of demographics, clinical characteristics, and surgical outcomes of cannabis users compared to non-cannabis users pre-and post-surgery
| Cannabis users (n = 79) |
Non-cannabis users (n = 1256) |
|||||
|---|---|---|---|---|---|---|
| Baseline | Month 3 | Month 6 | Baseline | Month 3 | Month 6 | |
| Mean age in years (SD) | 52.4 (13.2)a | -- | -- | 59.1 (13.4) | -- | -- |
| No. of males (%) | 42 (53.2)a | -- | -- | 420 (33.4) | -- | -- |
| No. white (%) | 65 (87.8) | -- | -- | 1095 (90.0) | -- | -- |
| No. with a disability (%) | 18 (24.7)a | -- | -- | 147 (12.0) | -- | -- |
| No. with a college degree (%) | 14 (18.7)a | -- | -- | 598 (48.9) | -- | -- |
| No. living with a partner (%) | 50 (66.7) | -- | -- | 928 (76.0) | -- | -- |
| Mean BPI pain severity score (SD) | 5.0 (2.8)a | 3.7 (3.0)b | 3.6 (2.7)c | 3.6 (2.9) | 2.6 (2.5) | 2.7 (2.6) |
| Mean Centralized pain score (SD) | 7.9 (5.4)a | 5.9 (6.0)b | 5.5 (6.0)c | 5.3 (4.1) | 3.5 (3.7) | 3.7 (4.0) |
| Mean Functional impairment score (SD) | 12.0 (4.8)a | 9.1 (4.6)b | 9.0 (4.8)c | 10.0 (4.9) | 7.6 (4.3) | 7.3 (4.1) |
| Mean PROMIS Fatigue score (SD) | 11.0 (4.5)a | -- | 9.3 (4.4)c | 9.3 (4.3) | -- | 7.9 (3.8) |
| Mean PROMIS Sleep Disturbance score (SD) | 27.0 (6.9)a | -- | 22.0 (8.2)c | 23.0 (7.4) | -- | 20.0 (7.0) |
| Mean PROMIS Anxiety score (SD) | 8.2 (3.8)a | 6.5 (3.1)b | 6.4 (3.2)c | 6.4 (3.0) | 5.3 (2.4) | 5.2 (2.3) |
| Mean PROMIS Depression score (SD) | 7.3 (4.1)a | 5.9 (3.2)b | 6.0 (3.6)c | 5.7 (2.7) | 5.0 (2.2) | 4.9 (2.2) |
| No. using opioids (%) | 22 (27.9)a | 15 (21.4)b | 9 (17.7)c | 220 (17.5) | 115 (10.1) | 100 (10.0) |
| Mean average daily OME value (SD) | 17.7 (22.8) | 18.2 (19.4) | 16.6 (11.7) | 21.1 (35.2) | 20.4 (23.9) | 17.5 (20.5) |
| No. using benzodiazepines (%) | 15 (19.0)a | -- | -- | 115 (9.2) | -- | -- |
| No. with PGIC score of 2 or 3 (%) | -- | 50 (80.7) | 44 (84.6) | -- | 834 (81.5) | 800 (80.1) |
| Mean BPI surgical site pain score (SD) | 5.5 (3.0)a | 1.5 (2.3)b | 0.64 (1.6) | 4.2 (3.3) | 0.94 (1.7) | 0.73 (1.6) |
Note. Independent samples t-tests were used to test the between group differences for continuous measures. Opioid use, benzodiazepine use, and PGIC differences were assessed with chi-square tests. Observed means (standard deviations) and frequencies (percentages) are presented. P-values calculated from tests using multiple imputation to handle missing data. Superscripts denote statistical significance at p < 0.05 or lower for the following tests:
day of surgery differences between cannabis users and non-users
Month 3 differences between cannabis users and non-users
Month 6 differences between cannabis users and non-users.
At the 3- and 6-month follow ups, participants using cannabis at the time of surgery continued to report worse clinical symptoms across all clinical domains (all ps < 0.05,) compared to patients not using cannabis the day of surgery (Table 2). Cannabis users also had higher likelihood of opioid use at 3 months (p=0.01) and 6-months (p =.04). However, there was not a significant difference in average daily OME use between cannabis users and non-users at 3 months (p=0.39) or 6 months (p=0.73).
3- and 6-month surgical outcomes of cannabis users compared to non-cannabis users
On the day of surgery, cannabis users reported higher surgical site pain compared to non-cannabis users (p < 0.01) (See Table 2). At 3-months, cannabis users continued to report higher surgical site pain compared to non-cannabis users (p = 0.01); however, at 3-months the groups did not differ on perceived post-surgical improvement (PGIC) with 80.7% of cannabis users and 81.5% of non-cannabis users reporting they were “much improved” or “very much improved” since surgery (p=0.99) (See Table 2). At 6 months, there was no statistically significant difference between cannabis users and non-cannabis users on surgical site pain (p=0.93) or the PGIC (p=0.88).
Post-surgical changes in clinical characteristics for cannabis users and non-cannabis users
We first examined post-surgical changes within each group. At 3 -and 6- months, cannabis users had significant improvement in overall body pain, surgical site pain, centralized pain, functional impairment, anxiety, and depression (all ps < 0.05). At 6-months, sleep disturbance was also improved (p < 0.05). Although opioid use decreased over time for cannabis users, there was not a statistically significant change in opioid use or daily OME (ps > 0.05) among cannabis users at 3-or 6-months. Similarly, at 3- and 6- months, non-cannabis users had significant improvement in overall body pain, surgical site pain, centralized pain, functional impairment, anxiety, depression, a reduction in opioid use, and a reduction in average daily OME (all ps < 0.001). At 6-months, fatigue and sleep disturbance were improved (ps < 0.05).
Next, examining differences in change between groups, at 3 months, a significant interaction was observed for functional impairment (Coefficient = −1.12; 95% CI −2.21, −0.01; p < 0.05) and depression (Coefficient = −0.65; 95% CI −1.27 to −0.03; p = 0.04) indicating that pre-surgical cannabis users report greater improvement compared to non-cannabis users (See Table 2). At 6 months, a significant interaction was observed for centralized pain (Coefficient = −0.96; 95% CI −1.95 to −0.02; p < 0.05) and surgical site pain (Coefficient = −1.31; 95% CI −2.11 to −0.48; p < 0.01), indicating that pre-surgical cannabis users report greater improvement in both areas compared to non-cannabis users.
Cannabis use at 6-months
Of the 79 participants who reported cannabis use on the day of surgery, 61 (77.2%) completed some 6-month follow-up data (phone call, mailed survey, or both). A total of 45 (57%) completed the mailed questionnaire, which included cannabis use items. Among day of surgery cannabis users with available cannabis data at 6 months, thirty-eight (84.4%) continued cannabis use, three (6.7%) no longer used cannabis, and cannabis use was missing for four (8.9%). Of the 1,256 participants who reported no cannabis use on the day of surgery, 1,083 (86.2%) completed some 6-month follow-up data. A total of 835 (66.5%) completed the mailed questionnaire, which included cannabis use items. Among non-cannabis users with available cannabis use data at 6 months, 809 (96.9%) were still not using cannabis, 13 (1.6%) reported new cannabis use, and cannabis use was missing for 13 (1.6%).
DISCUSSION
In this large cohort of patients undergoing elective surgery, 5.9% of patients reported current cannabis use, which is consistent with the national prevalence (5.6%) of past-month cannabis use among adults ages 35 and older.19 The majority reported using cannabis medically, mostly for pain (92%), although less than half of patients (45.6%) reported having a medical card. Although rates of cannabis use among surgical patients are relatively low, given that cannabis availability is increasing as legal restrictions loosen nationwide, rates will likely rise as access expands. Therefore, it is critical for surgeons to better understand the potential consequences and benefits of cannabis use in surgical settings.
Cannabis users report worse clinical characteristics before and after surgery
On the day of surgery and at the 3- and 6-month follow-ups, cannabis users reported worse clinical characteristics across all domains relative to non-users. This is consistent with other studies in which patients using cannabis report increased pain and greater sleep interruption following surgery,20,21 as well as with studies of individuals using medical cannabis generally. For example, using data from the National Survey on Drug Use and Health, Lin et al. showed that adults who use cannabis medically report poorer health than those who use it recreationally.22 Perron et al. found that compared to the general population, medical cannabis users had higher rates of alcohol and non-cannabis drug use, and Ilgen et al. note that patients using cannabis for pain have significantly worse health and functioning than the general population.11,23 It is conceivable that cannabis contributes to these health problems, given that cannabis is a common drug of abuse and is associated with negative effects. Alternatively, it is possible that the findings from this study reflect a subset of patients who were clinically worse off prior to trying cannabis and are using cannabis in an attempt to treat their distressing symptoms. Differentiating between these two hypotheses is beyond the scope of the current study but illustrates the need for future research to better understand the associations of cannabis use, pain, and functioning in order to counsel patients appropriately. Further, while cannabis may be useful for managing pain, benefits must be considered in light of associated health risks (e.g., impaired driving, cardiovascular effects, etc.) as well as the availability of alternative options (e.g., non-pharmacological)24 for pain management.
Cannabis users and non-cannabis users report similar improvements in surgical outcomes
By 6 months, cannabis users reported similar improvement as non-cannabis users on surgical site pain and perceived treatment efficacy, suggesting cannabis use did not impede recovery from surgery. This finding adds to the limited literature on cannabis and surgical outcomes. We identified one observational cross-sectional study that surveyed patients between one and six months after musculoskeletal trauma (69% of cases involved surgery).9 In this study, 14% of patients used cannabis during recovery, with 90% of those using cannabis reporting that it helped with pain management, and 81% reported that it allowed them to use fewer opioids. While national trends have reported mixed results on the effects of cannabis on surgical outcomes,2,25 a systematic review of 7 clinical trials reported that cannabinoids did not outperform placebo for acute post-operative pain.26 However, cannabinoid administration in these trials occurred pre-surgery or the day after surgery for acute pain management and did not consider cannabis use over the entire recovery period.7
Cannabis use was associated with opioid and benzodiazepine use
On the day of surgery, cannabis users were more likely to report opioid and benzodiazepine use, which is perhaps not surprising given their higher pain and anxiety. Although opioid use decreased following surgery for cannabis and non-cannabis users, patients using cannabis continued to report higher rates of opioid use at 6-months, suggesting cannabis was not substituted for opioids among these patients. Of note, while cannabis users were more likely to be using opioids at each time point, they were not using significantly different dose amounts compared to non-cannabis users. These findings add to a literature which is characterized by inconsistent results of cannabinoids on postoperative opioid use. Indeed, one retrospective study showed higher post-operative analgesic requirements for individuals who smoke cannabis,27 while a retrospective case-control study showed decreased post-operative opioid consumption among individuals given dronabinol compared to those who were not.28
These mixed results highlight some challenges associated with determining what role, if any, cannabis should play in post-surgical pain management, especially in the context of using cannabis as an opioid-sparing alternative. There is data to suggest that patients report less opioid use after obtaining a medical cannabis licenses.8 However, data are mixed, with some studies suggesting decreased opioid use following initiation of cannabis use,29 while others find no such effect.30 Although we did not assess whether patients were using cannabis as a substitute for prescription medications per se, the higher rates of opioid and benzodiazepine use highlights a different concern. Polysubstance use may be present in some cannabis users undergoing surgery and using cannabis along with other prescription medications represents a distinct clinical issue compared to use of medical cannabis alone. For example, adding cannabis to the combination of opioids and benzodiazepines (which have considerable abuse liability) could be a function of a substance use disorder, rather than solely symptom management. Further, given the lack of established guidelines on using cannabis for acute and chronic pain, cannabis is often incorporated into existing drug or treatment regimens based on advice from non-medically trained friends or budtenders.31 Such practices are concerning, given that cannabis dispensaries typically sell products that are THC-dominant and quite potent, potentially leading to more adverse effects.32 Future research is needed to better understand how and why cannabis is used in combination with these high risk medications.
Strengths and Limitations
The longitudinal study design and large sample size are strengths of this study. The sample size, in combination with the similarity in rates of cannabis use in this sample to the national prevalence rates, suggest confidence in the basic characteristics of cannabis use in this population. However, findings from this study also should be considered in light of its limitations. First, although we were given the opportunity to add cannabis items to the AOS survey, we were limited to a small number of items. Therefore, we are not able to describe several important features of cannabis use including duration of cannabis use, type of cannabis (THC-dominant, CBD-dominant), or primary administration route (e.g., smoking, edible), and our dosing question was limited to frequency of use in the past month. A more granular assessment could potentially reveal relationships between use patterns and the amount of harm or benefit that individuals derive from use. Additionally, we did not assess for presence of cannabis use disorder or other substance use disorders, leaving us unable to identify whether differences in surgical outcomes may be moderated by an underlying addictive disorder. Second, the wording for our cannabis use item was “Do you currently use marijuana”, which is a term commonly used to describe cannabis products that result in a THC-related high, and not typically CBD-only products. Thus, participants who responded “yes” to cannabis use were likely using at least one THC dominant product; however, because we did not ask about CBD use specifically (as noted above), it is unknown what % of current cannabis users also use CBD products. Similarly, because it is unlikely a CBD only user would select “yes” to cannabis use, we do not have data on how many “non cannabis users” used CBD only products at the time of the survey. A third limitation is that because we did not explicitly define current use as “in the past month”, a participant who quit using marijuana recently, perhaps at the recommendation of their surgeon, may have said “no” to the item used to define current marijuana use. If the current use item included “in the past month”, it is possible more participants would have said “yes” to marijuana use; however because current cannabis use in this sample was similar to the national prevalence of past 30-day use in adults, even if some people under reported current use, it is unlikely to have impacted our outcomes of interest significantly. Fourth, data were obtained by self-report which could be subject to concerns about under-reporting due to the illegal nature of cannabis (at the federal level) despite the state-level legalization of medical cannabis. These concerns are tempered by our use of standard procedures to encourage accurate self-report33 (e.g., confidentiality assurances) and because 45.6% of cannabis users in our sample reported they did not have a medical cannabis card and were therefore using illegally (recreational cannabis was not legal in the state of MI at the time). Note also that self-report is a standard measurement practice in the substance abuse field. Additionally, because the total number of cannabis users was small, caution is warranted in generalizing the associations found here between surgical outcomes and cannabis use to other populations. That said, although we have relatively few cannabis users in this sample, the number of users is comparable to the numbers examined in other studies of surgical outcomes.27,28 Finally, as data were conducted at a single study site between 2015 and 2017 in the state of Michigan, findings may not generalize to current cannabis use in Michigan post recreational legalization, or to other locations with different medical/recreational cannabis policies and more diverse patient populations.
Conclusions and Future Directions
In conclusion, we show that in a surgical cohort, current cannabis use prevalence is similar to the general population and patients who use cannabis have higher clinical pain, poorer scores on quality of life indicators, and are more likely to use opioids and/or benzodiazepines, compared to individuals who do not. Despite persistent differences on most clinical measures at 3 and 6 months, cannabis users reported equally high levels of improvement following surgery, suggesting cannabis use did not impede recovery. Future studies should continue to examine the role of cannabinoids during the surgical recovery process, with a focus on identifying moderating risk factors (e.g., dose) for surgical success/failure. Generally speaking, in studies that examine cannabis use, it can be challenging to determine if associations between cannabis use and other factors (e.g., higher pain, depression) are causal in nature, reciprocally influence each other over time, or are both influenced by other variables. To resolve this, we recommend experimental and longitudinal study designs with more frequent and rigorous assessments. Additionally, future work would be improved by including more fine-grained assessments of cannabis use among surgical patients, including measures of cannabis use disorder, detail regarding relative THC and CBD levels of their cannabis (if known), route of administration, and more information regarding quantity and frequency of use. Further, given that cannabis was legalized for recreational use in Michigan in late 2018 and CBD products have become widely available since the passage of the 2018 Farm Bill, additional work is needed to examine whether prevalence of use among surgical patients increases as a result of increased access.
ACKNOWLEDGEMENTS
The authors thank the physicians, nurses and staff of the University of Michigan Health System and the research team for their assistance in this study.
Funding:
The study was funded by the following NIH grants: NIAMS R01AR060392 (MPI Clauw and Brummett), NIDA R01DA038261 (MPI Clauw and Brummett) and NIDA R01DA042859 (MPI Waljee and Brummett). Additional funding was provided by the Department of Anesthesiology, the Medical School Dean’s Office, and the Michigan Genomics Initiative of the University of Michigan (Ann Arbor, MI).
Abbreviations:
- TKA
Total Knee Arthroplasty
- THA
Total Hip Arthroplasty
- AOS
(Analgesic Outcome Study)
- THC
Tetrahydrocannabinol
- CBD
Cannabidiol
- OME
Oral Morphine Equivalents
- BPI
Brief Pain Inventory
- MBM
Michigan Body Map
- SSI
Symptom Severity Index
- PROMIS
Patient Reported Outcomes Measurement Information System
- PGIC
Patient Global Impression of Change
Footnotes
COMPETING INTERESTS:
Dr. Brummett is a consultant for Heron Therapeutics (San Diego, CA), not related to this work. Dr. Boehnke sits on a data safety and monitoring committee (unpaid) for an ongoing study with Vireo Health (New York, NY). The authors with no disclosures: Jenna McAfee, Stephanie Moser, Jennifer Waljee, and Erin Bonar.
REFERENCES
- 1.Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet 2011;377(9784):2215–25. doi: 10.1016/S0140-6736(11)60245-6 [DOI] [PubMed] [Google Scholar]
- 2.Best MJ, Buller LT, Klika AK, et al. Outcomes Following Primary Total Hip or Knee Arthroplasty in Substance Misusers. J Arthroplasty 2015;30(7):1137–41. doi: 10.1016/j.arth.2015.01.052 [DOI] [PubMed] [Google Scholar]
- 3.2019 NSDUH Annual National Report 2018. [cited Substance Abuse and Mental Health Services Administration. Available from: https://www.samhsa.gov/data/report/2018-nsduh-annual-national-report.
- 4.Boehnke KF, Gangopadhyay S, Clauw DJ, et al. Qualifying Conditions Of Medical Cannabis License Holders In The United States. Health Aff (Millwood) 2019;38(2):295–302. doi: 10.1377/hlthaff.2018.05266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med 2014;371(9):879. doi: 10.1056/NEJMc1407928 [DOI] [PubMed] [Google Scholar]
- 6.Webb CW, Webb SM. Therapeutic benefits of cannabis: a patient survey. Hawaii J Med Public Health 2014;73(4):109–11. [PMC free article] [PubMed] [Google Scholar]
- 7.The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington D.C.: The National Academies Collection: Reports funded by National Institutes of Health, 2017. [PubMed] [Google Scholar]
- 8.Boehnke KF, Litinas E, Clauw DJ. Medical Cannabis Use Is Associated With Decreased Opiate Medication Use in a Retrospective Cross-Sectional Survey of Patients With Chronic Pain. J Pain 2016;17(6):739–44. doi: 10.1016/j.jpain.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 9.Heng M, McTague MF, Lucas RC, et al. Patient Perceptions of the Use of Medical Marijuana in the Treatment of Pain After Musculoskeletal Trauma: A Survey of Patients at 2 Trauma Centers in Massachusetts. J Orthop Trauma 2018;32(1):e25–e30. doi: 10.1097/BOT.0000000000001002 [DOI] [PubMed] [Google Scholar]
- 10.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 11.Ilgen MA, Bohnert K, Kleinberg F, et al. Characteristics of adults seeking medical marijuana certification. Drug Alcohol Depend 2013;132(3):654–9. doi: 10.1016/j.drugalcdep.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 12.Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol 2015;67(5):1386–94. doi: 10.1002/art.39051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain 2016;157(6):1259–65. doi: 10.1097/j.pain.0000000000000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23(2):129–38. [PubMed] [Google Scholar]
- 15.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011;38(6):1113–22. doi: 10.3899/jrheum.100594 [DOI] [PubMed] [Google Scholar]
- 16.Brummett CM, Bakshi RR, Goesling J, et al. Preliminary validation of the Michigan Body Map. Pain 2016;157(6):1205–12. doi: 10.1097/j.pain.0000000000000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311(15):1547–55. doi: 10.1001/jama.2014.3266 [DOI] [PubMed] [Google Scholar]
- 18.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Results from the 2016 National Survey on Drug Use and Health: Detailed Tables, SAmHSA, CBHSQ [Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.htm#tab1-12B accessed June 26, 2018. [Google Scholar]
- 20.Jefferson DA, Harding HE, Cawich SO, et al. Postoperative analgesia in the Jamaican cannabis user. J Psychoactive Drugs 2013;45(3):227–32. doi: 10.1080/02791072.2013.803644 [DOI] [PubMed] [Google Scholar]
- 21.Liu CW, Bhatia A, Buzon-Tan A, et al. Weeding Out the Problem: The Impact of Preoperative Cannabinoid Use on Pain in the Perioperative Period. Anesth Analg 2019;129(3):874–81. doi: 10.1213/ANE.0000000000003963 [DOI] [PubMed] [Google Scholar]
- 22.Lin LA, Ilgen MA, Jannausch M, et al. Comparing adults who use cannabis medically with those who use recreationally: Results from a national sample. Addict Behav 2016;61:99–103. doi: 10.1016/j.addbeh.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perron BE, Bohnert K, Perone AK, et al. Use of prescription pain medications among medical cannabis patients: comparisons of pain levels, functioning, and patterns of alcohol and other drug use. J Stud Alcohol Drugs 2015;76(3):406–13. doi: 10.15288/jsad.2015.76.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneiderhan J, Clauw D, Schwenk TL. Primary Care of Patients With Chronic Pain. JAMA 2017;317(23):2367–68. doi: 10.1001/jama.2017.5787 [DOI] [PubMed] [Google Scholar]
- 25.Moon AS, Smith W, Mullen S, et al. Marijuana use and mortality following orthopedic surgical procedures. Subst Abus 2019;40(3):378–82. doi: 10.1080/08897077.2018.1449054 [DOI] [PubMed] [Google Scholar]
- 26.Stevens AJ, Higgins MD. A systematic review of the analgesic efficacy of cannabinoid medications in the management of acute pain. Acta Anaesthesiol Scand 2017;61(3):268–80. doi: 10.1111/aas.12851 [DOI] [PubMed] [Google Scholar]
- 27.Jamal N, Korman J, Musing M, et al. Effects of pre-operative recreational smoked cannabis use on opioid consumption following inflammatory bowel disease surgery: A historical cohort study. Eur J Anaesthesiol 2019;36(9):705–06. doi: 10.1097/EJA.0000000000001044 [DOI] [PubMed] [Google Scholar]
- 28.Schneider-Smith E, Salottolo K, Swartwood C, et al. Matched pilot study examining cannabis-based dronabinol for acute pain following traumatic injury. Trauma Surg Acute Care Open 2020;5(1):e000391. doi: 10.1136/tsaco-2019-000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corroon JM Jr., Mischley LK, Sexton M Cannabis as a substitute for prescription drugs - a cross-sectional study. J Pain Res 2017;10:989–98. doi: 10.2147/JPR.S134330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell G, Hall WD, Peacock A, et al. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: findings from a 4-year prospective cohort study. Lancet Public Health 2018;3(7):e341–e50. doi: 10.1016/S2468-2667(18)30110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin R Medical Marijuana Is Legal in Most States, but Physicians Have Little Evidence to Guide Them. JAMA 2017;317(16):1611–13. doi: 10.1001/jama.2017.0813 [DOI] [PubMed] [Google Scholar]
- 32.Russo EB. Current Therapeutic Cannabis Controversies and Clinical Trial Design Issues. Front Pharmacol 2016;7:309. doi: 10.3389/fphar.2016.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone AAT, Jaylan S; Bachrach Christine A; Jobe Jared B; Kurtzman Howard S; Cain Virginia S, editor. The science of self-report: Implications for research and practice. : Psychology Press, 1999. [Google Scholar]



