Abstract
Background/Objectives:
To examine the relationship between time spent in light- (LPA) and moderate-to-vigorous intensity physical activity (MVPA) and pattern of accumulation on the risk for major mobility disability (MMD) in a large multicenter study of physical activity (PA) and aging—The Lifestyle Interventions and Independence for Elders (LIFE) study.
Design:
Data were collected from individuals randomized to a PA intervention as part of the LIFE study; an 8-center, single blind, randomized clinical trial that was conducted between February 2010 and December 2013.
Setting/Participants:
Older adult participants (78.4 years; N=507) at risk for MMD.
Intervention:
All older adults included in these analyses were randomized to structured PA intervention that included 2 center-based plus 3–4 home-based exercise sessions per week with a primary goal of walking for 150 minutes weekly. Participants attended the intervention for 2.5 years on average.
Measurements:
MMD was defined as the inability to complete a 400-meter walk within 15 minutes and without assistance. Physical function was assessed via the Short Physical Performance Battery (SPPB). Actigraph accelerometers were used to quantify amount and variability in LPA and MVPA.
Results:
In an adjusted Cox Proportional Hazards regression, we identified a significant interaction (p=0.017) between SPPB score and LPA amount and variability such that more LPA was associated with reduced risk for MMD among those with higher initial function, as was lower variability (e.g., via distributing LPA across the day). The SPPB × MVPA interaction was significant (p=0.04), such that more MVPA was associated with lower MMD risk among those with lower function. Finally, greater MVPA variability was associated with lower risk for MMD.
Conclusion:
Prescription of PA for older adults should account for key factors such as physical function and should emphasize both amount and pattern of accumulation of PA from across the intensity continuum.
Keywords: Physical activity, sedentary behavior, mobility, function
Introduction
Impaired mobility is a key marker of physical disability and is critical to the loss of functional independence as people age.1,2 More than two decades ago, reviews concluded that physical inactivity was the strongest single predictor of physical disability in aging.3,4 Conventionally, physical activity (PA) research has focused on structured exercise, or discrete bouts of purposeful moderate-to-vigorous-intensity PA (>3.0 METs; MVPA). Although the health benefits of regular MVPA are well established,5 interest in light-intensity PA (LPA) has increased in recent years. This is particularly important for older adults, as LPA may be less aversive and more attainable than more intense activity, especially for those who are frail or pre-frail.6,7 The purpose of the present study is to examine the relationship between time spent in LPA and MVPA and the pattern of accumulating these activities on the risk for major mobility disability (MMD) among older adults with compromised physical function who participated in the Lifestyle Interventions and Independence for Elders (LIFE) study.8
Physical activity is beneficial in improving mobility-related physical disability,9,10 with a number of randomized controlled clinical trials (RCT) showing that structured exercise improves mobility, even among older adults with severe chronic health conditions.11–13 As such, the health-promoting effects of MVPA are endorsed by institutions such as the American College of Sports Medicine14 and the World Health Organization.15 In 2014, Pahor and colleagues published the results of the LIFE study, a multicenter RCT comparing a PA program involving the promotion of MVPA to a health education (HE) intervention on MMD among older adults who were frail or pre-frail. They found that participants in PA had a lower 2-year incidence of MMD than those in HE.8 A subsequent publication reported that those participants classified as achieving the highest quartile for change in MVPA had the greatest reduction in risk for MMD. Interestingly, the lower bound of MVPA duration for the 4th quartile was quite modest at just 43 minutes/week.16
In recent years, there has been an increased interest in PA that falls below the threshold for MVPA. For example, whereas the first edition of the Physical Activity Guidelines for Americans recommended between 150 and 300 minutes/week of MVPA lasting at least 10 minutes per bout, the recent second edition encourages increasing both MVPA and LPA. Of note is that within the LIFE study, we observed that as many as 25% of participants were unable to reach the accelerometry-defined threshold for MVPA during center-based exercise sessions,7 and they often had to stop and rest or were unable to sustain the MVPA threshold for an extended period of time—their activity was highly variable. Moreover, the second edition of the guidelines removed the recommendation that activity persist for at least 10 minutes, reflecting a growing interest in the effect of not only the amount of PA on health, but also the pattern in which it is accumulated. For instance, does participating in LPA or MVPA frequently throughout the day have different health effects as compared with fewer, longer bouts of the behaviors?
The objective of the current study was to examine the relationships between the amount and variability in both MVPA and LPA on subsequent risk for MMD among at-risk older adults who participated in the PA intervention of the LIFE study.8 We first examine relationships between the amount and variability in MVPA with MMD. We hypothesized a dose-response effect indicating that participating in more MVPA would be associated with lower risk for MMD, though this effect would be small since all individuals participated in a program that targeted increasing MVPA. We also expected that greater variability in minutes/hour of MVPA (reflecting fewer, longer bouts of MVPA relative to lower variability) would be associated with reduced risk. Regarding LPA, we hypothesized that greater volume would be associated with lower risk for MMD and that, across individuals, a more distributed pattern of movement (i.e., lower variability) would be associated with lower risk. Lastly, we explored for potential interactions between differences in basic levels of physical functioning as assessed by the short physical performance battery (SPPB) with metrics for MVPA and LPA, as previous publications suggest that lower-functioning individuals benefitted most from the intervention,8 engage in high levels of sustained sedentary behavior,17 and are uniquely challenged in their ability to meet older adult-specific accelerometer cut points for MVPA.18
Methods
Design and Participants
Data were collected from individuals randomized to a PA intervention as part of the LIFE study; an 8-center, single blind, randomized clinical trial that was conducted between February 2010 and December 2013 (see19 for detailed procedures). LIFE examined the effect of a long-term structured exercise intervention on transition to MMD (i.e., inability to walk 400 meters) among those able to walk 400 meters at baseline. Participants were required to have an SPPB score of ≤9 out of 12 (45% had SPPB<8). Eligible individuals were 70–89 years of age, low-active (<20 min/week of structured PA; < 125 min/week MVPA over last month), able to walk 400 meters in less than 15 minutes, without major cognitive impairment, and able to safely participate in the PA intervention as determined by medical history, ECG, and physical exam. The PA intervention was well-tolerated, as supported by a median attendance rate of 71% across nearly 2 years, including medical leave. Additionally, those in the intervention condition engaged in in an additional 40 minutes per week of MVPA at month 6; which was maintained at 12 and 24 months.20
Randomization
Participants were randomized to receive PA or a health-education (HE) control group, and randomization was stratified by field center and participant sex. All participants received an orientation appointment with a health educator, at which point they reviewed their randomization assignment and clarified any outstanding questions. Because our goal in the current study was to examine how amount and variability of MVPA and LPA relate to risk for MMD, we restricted our analyses to participants randomized to the PA intervention.
Physical Activity Intervention
The focus of the PA intervention was on aerobic PA via walking, with a goal of 150 weekly minutes of MVPA paired with brief balance, flexibility, and lower body strengthening exercises. Participants aimed to engage in exercise 5–6 days per week, including 2 weekly center-based exercise sessions. For the first 2–3 weeks of training, participants progressed from exercising at a light-intensity to a rating of perceived exertion21 of 13/20 during walking, and 15–16/20 during strength training (clinicaltrials.gov Identifier: NCT01072500).19
Measures
Major Mobility Disability
Our dichotomous outcome measure is onset of MMD, defined as the inability to complete a 400-meter walk test within 15 minutes without assistance. This test was completed prior to randomization, and again at 6-month intervals.
Actigraphy
Participants wore a GT3X accelerometer (Actigraph, Pensacola, FL) on the right hip during waking hours (except while bathing or swimming) for one week at baseline, 6, 12, and 24-month visits. Data were processed in ActiLife (v4.4.1), utilizing the Choi et al22 algorithm to classify non-wear time. Specifically, periods of 90 consecutive minutes with zero counts per minute (CPM), allowing for a 1-minute spike tolerance, were classified as non-wear. Participants with at least 3 days of at least 600 minutes of wear were retained in analyses. Sedentary was defined as <100CPM, LPA was defined as 100–1041CPM, and MVPA was defined as >1040CPM.23 As low functioning older adults often achieve MVPA at intensities below conventional accelerometer cut points for MVPA, we ran an additional sensitivity analysis wherein 100–760 represented LPA, and >760 classified MVPA.18,24 Average daily minutes of LPA and MVPA were computed across valid days. Daily variance in minutes/hour of LPA and MVPA were computed and averaged across valid days before a square root transformation was applied.25 This measure provides insight into the distribution of movement during the day, as illustrated in Figure 1 wherein the profile marked with the solid line and the profile denoted by the dashed line represent different LIFE participants with similar minutes of daily LPA (solid: 7.97 min/hour; dashed: 8.01 min/hour), but relatively high (solid: 9.87) and low (dashed: 3.93) variability in LPA. Here, profile A (high variability) engages in fewer but longer bouts of LPA, with lengthy daily periods with little LPA, and profile B (low variability) frequently maintains approximately 5–20 minutes/hour of LPA during waking hours.
Figure 1.
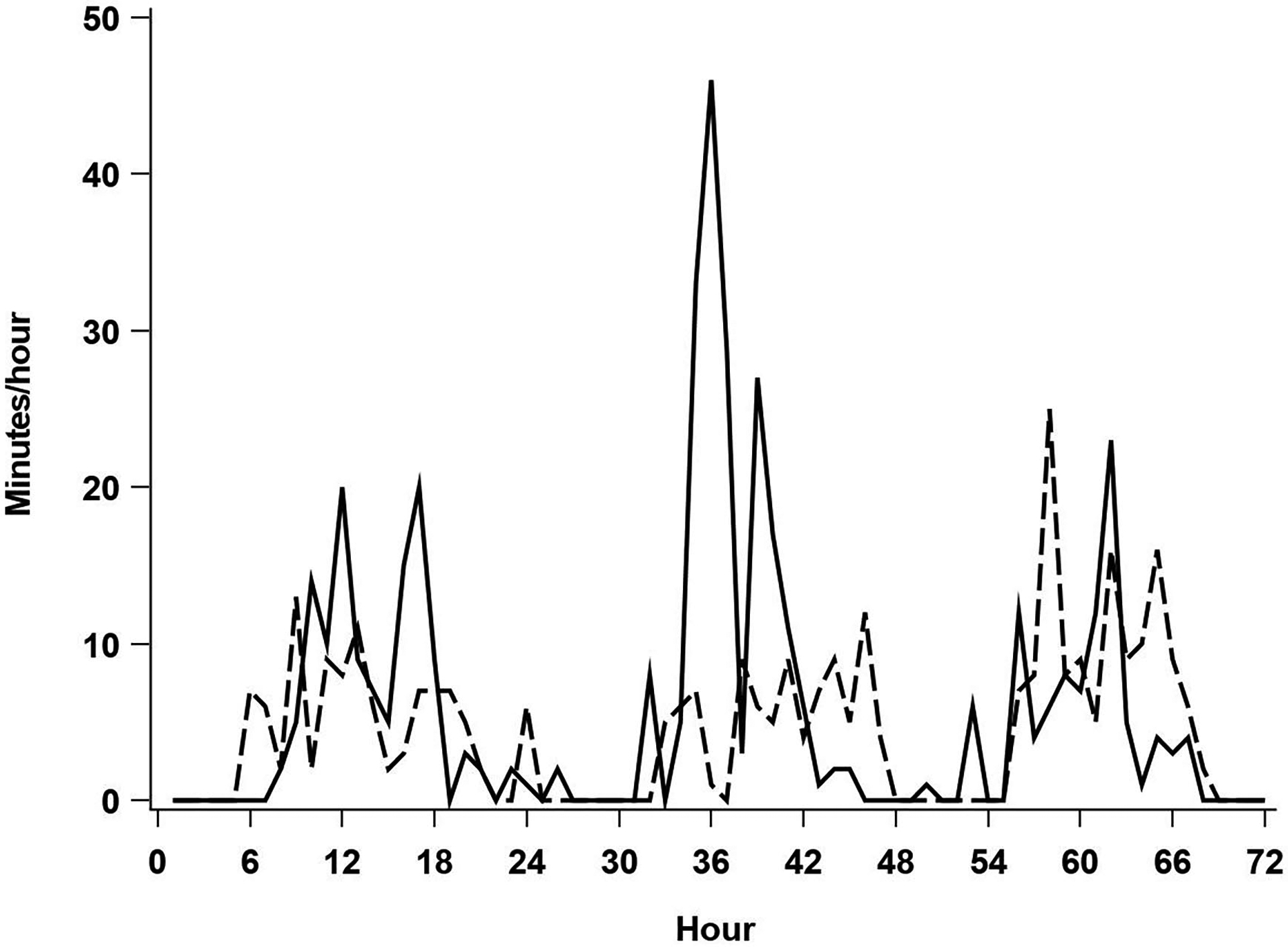
Illustration of two individuals with similar average daily minutes of LPA, and high (solid line) vs low (dashed line) LPA variability.
Analyses
We explored relationships between amount and variability in both MVPA and LPA and risk of MMD using a series of Cox Proportional Hazards regression models that incorporated these activity measures as time-dependent covariates (i.e., updating the accelerometry variables at 6-, 12- and 24-months) using time until the initial occurrence of MMD as the dependent variable. Each model controlled for baseline sex (as a stratifying factor for the baseline hazard), age, body mass index (BMI), race, history of high blood pressure, cardiovascular disease, lung disease, and osteoarthritis. We first fit a model for MVPA that included the 3-way and lower-order interaction terms between baseline SPPB classified as frail (scores of ≤7) or pre-frail (scores of 8–9)25, amount and variability of MVPA, and all covariates. The highest order interaction terms with p<0.05 via likelihood-ratio tests, and associated lower-order interactions, were retained. Next, this model building process was repeated for LPA, log-transformed due to skewness. In our final model we included all previously identified LPA and MVPA amount and variability terms with p<0.05.
Results
Of the 14,831 individuals assessed for eligibility, 818 were randomized to PA (Figure 2; participant description provided in Table 1). Following accelerometer cleaning procedures, 507 individuals had accelerometer data at baseline (7.5 days on average) and follow-up MMD data and were included in the analyses. Of these, updated activity information was obtained at month 6 on 354 participants (6.6 days on average), 406 at month 12 (6.9 days on average) and 382 at month 24 (6.9 days on average). Baseline participant characteristics of this group have been previously reported (see Table 1 of citation25), and reveal that the average age and BMI were 78.4 years and 30.4 kg/m2, with 64% women, and 26% coming from minority racial groups. Included participants had a median follow-up time for MMD (time until censoring or an event) of 2.5 years, 291 were categorized as pre-frail, and 144 were determined to have MMD at least once during follow-up.
Figure 2.
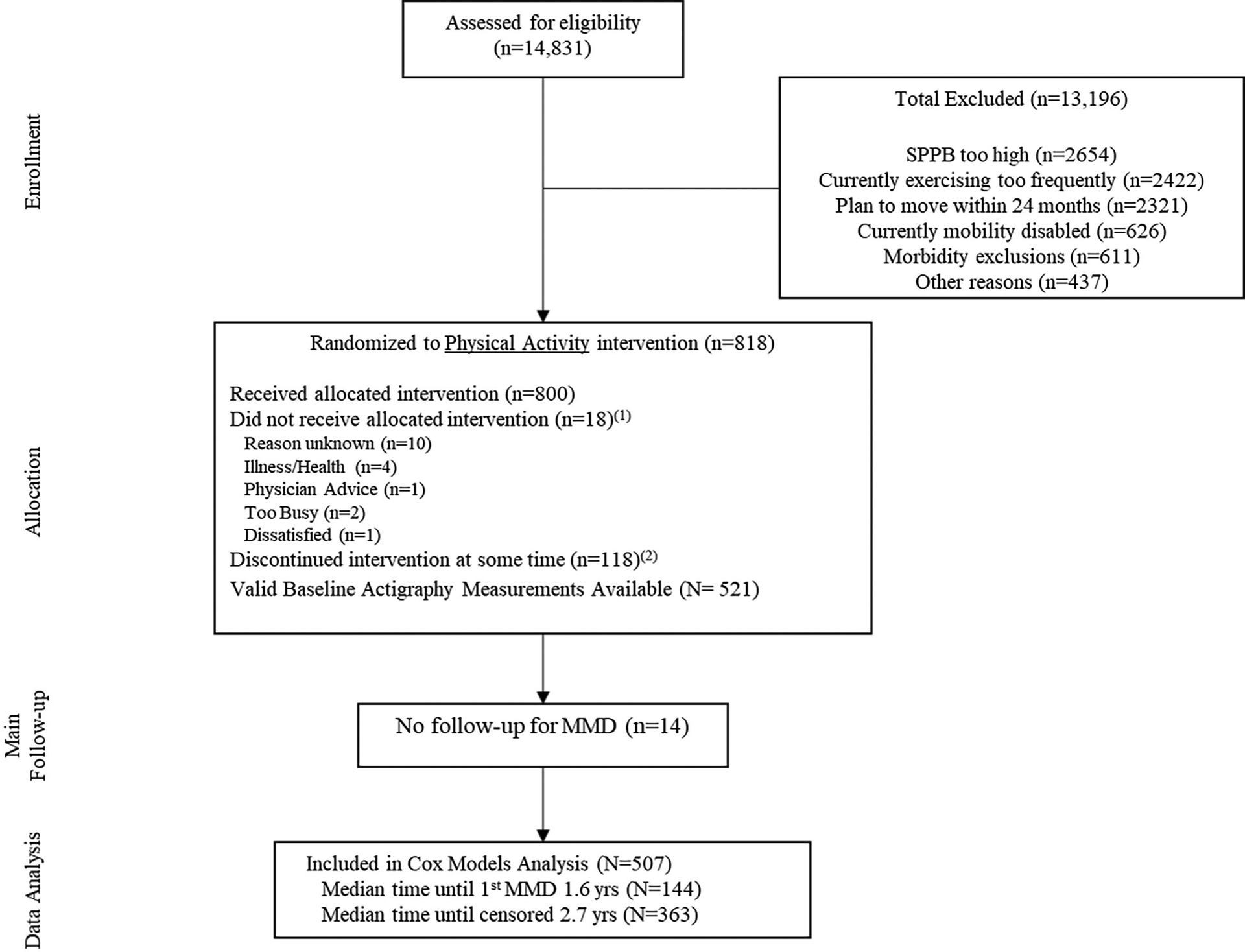
Participant Flow. Notes: 1Participants who did not receive the allocated intervention (i.e. attended no intervention sessions); 2Discontinuation of the intervention was operationalized as participants who did not attend at least one intervention session during their last 6-months of follow-up prior to the last planned follow-up visit date. Deaths and intervention withdrawals are included in these numbers.
Table 1.
Participant characteristics.
| Baseline Characteristic | Event Classification | Comparison of Event Groups P-value |
Overall (n=507) | |
|---|---|---|---|---|
| No MMD Event (n=363) | MMD Event (n=144) | |||
| Age; mean (SD) | 78.1 (5.09) | 79.4 (5.74) | 0.01 | 78.4 (5.31) |
| Sex (Female); n, (%) | 234 (64.5) | 92 (63.9) | 0.90 | 326 (64.3) |
| Race/Ethnicity (Non-Hispanic White); n, (%) | 264 (72.7) | 109 (75.7) | 0.26 | 373 (73.6) |
| Education (Some college); n, (%) | 248 (68.3) | 82 (56.9) | 0.04 | 330 (65.1) |
| SPPB total score; mean (SD) | 7.7 (1.45) | 6.9 (1.66) | <0.001 | 7.47 (1.56) |
| SPPB ≤ 7 (Yes); n, (%) | 133 (36.6) | 83 (57.6) | <0.001 | 215 (42.6) |
| 400m walk speed (m/sec); mean, (SD) | 0.87 (0.15) | 0.74 (0.16) | <0.001 | 0.83 (0.16) |
| Body Mass Index; mean (SD) | 30.1 (5.52) | 31.0 (6.70) | 0.12 | 30.4 (5.89) |
| History of Hypertension (Yes); n, (%) | 247 (68.6) | 103 (72.5) | 0.39 | 350 (69.7) |
| History of CVD (Yes); n, (%) | 87 (24.0) | 58 (40.3) | < 0.001 | 145 (28.6) |
| History of Lung Disease; (Yes); n, (%) | 65 (18.1) | 16 (11.1) | 0.06 | 81 (16.1) |
| History of Arthritis (Yes); n, (%) | 62 (17.2) | 25 (17.4) | 0.96 | 87 (17.2) |
Notes: MMD = major mobility disability; SPPB = short physical performance battery; CVD = cardiovascular disease
Results for all Cox Proportional Hazards models are provided in Supplemental Table 1. In the preliminary model (amount and variability of MVPA, SPPB frailty classification), there was no significant 3-way interaction (p=0.64). After removal of non-significant (p>0.05) higher order interactions, there remained a 2-way interaction between the amount of MVPA and baseline SPPB (p=0.017), and a main effect for variability (p=0.058; HR=0.81, 95% CI 0.64, 1.00) of borderline statistical significance. For frail individuals, more MVPA was associated with a lower risk of MMD (HR=0.61 for a 1-min/hr difference, 95% CI 0.31, 1.08); whereas among pre-frail individuals, more MVPA was associated with an elevated risk (HR=1.12 for a 1-min/hr difference, 95% CI 0.70, 1.70). Our model which included main effects for amount and variability in LPA, SPPB classification, and all relevant interaction terms revealed a significant 3-way interaction term (p = 0.003), which we explored further in the final, full model described below.
The final model included amount and variability of MVPA plus their interaction, as well as LPA amount, variability, amount × variability, and SPPB × amount × variability. Here, conclusions from the MVPA amount × SPPB interaction and variability terms were essentially unchanged, and results for LPA were similar to the model without MVPA. Specifically, the baseline SPPB × LPA amount × LPA variability interaction retained statistical significance (p=0.02). This interaction is depicted in Figure 3, with the upper panel depicting frail individuals, and the lower panel depicting pre-frail individuals. LPA amount is depicted on the x-axis and variability on the y-axis. All plots provide HRs (obtained by back-transforming the log-transformed LPA values) for a 1-unit difference on each axis at selected values of the other axis (e.g., a 1 min/hr difference in amount at a specific level of variability). As depicted in the interaction plot, for frail participants, both amount and variability in LPA were minimally related to risk for MMD. Among pre-frail participants who were engaging in very little LPA, there was a risk reduction for greater amount and variability of LPA. At higher amounts of LPA, adding more movement had little effect on risk. However, for these higher-active, higher-functioning participants, lower variability was associated with a risk reduction. Finally, our sensitivity analyses utilizing a lower threshold for MVPA (i.e., 760 CPM) did not differ meaningfully from the results presented herein, except that findings related to variability in higher-functioning individuals appeared amplified (see Supplemental Figure 1 and Supplemental Table 2).
Figure 3.
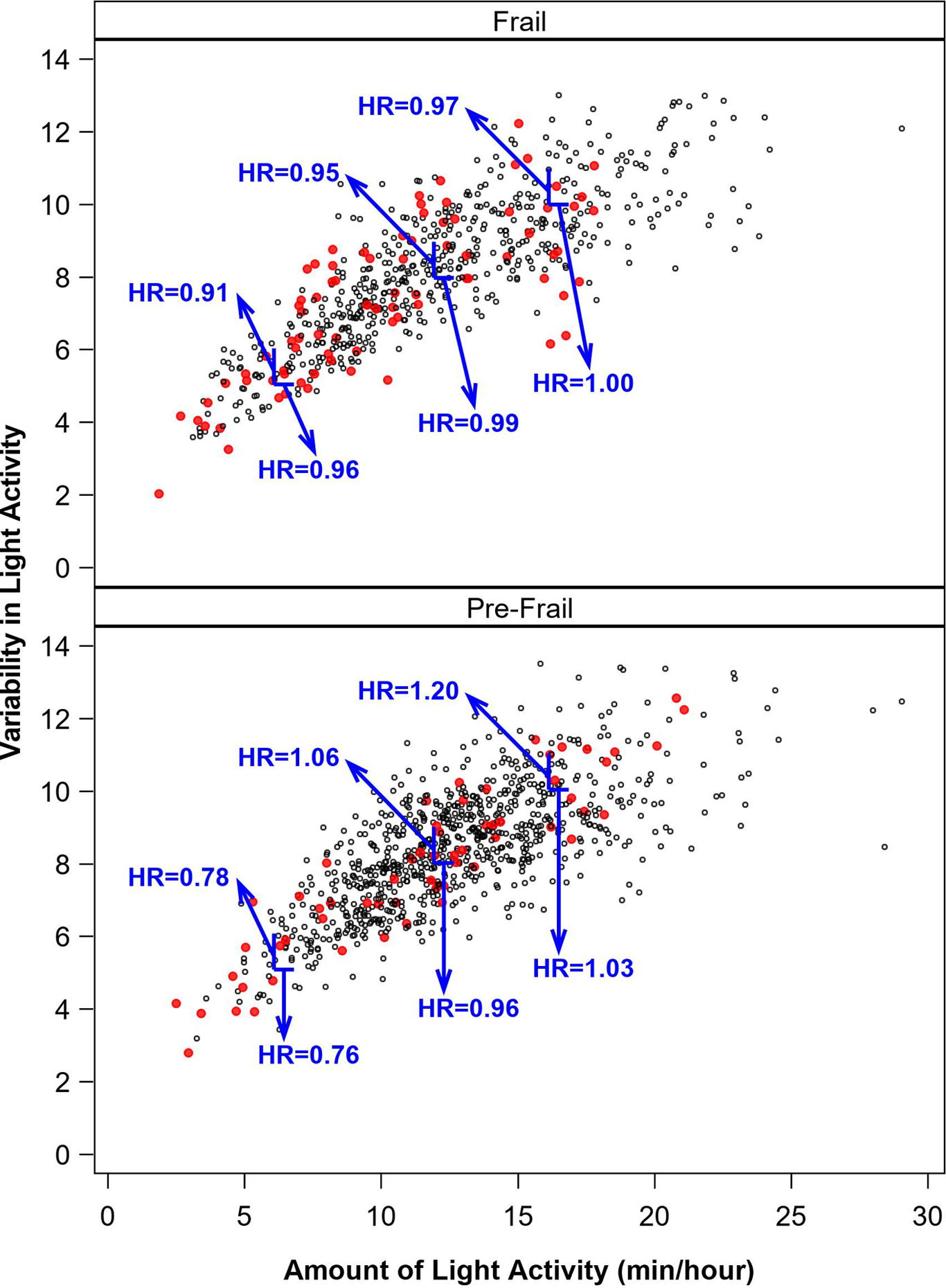
Illustration of the difference in risk for MMD that a 1-unit higher level of variability has at different amounts of LPA (vertical lines), and difference in risk associated with a 1-min/hr higher level of amount at different levels of variability in LPA (horizontal lines) among those with a short physical performance battery (SPPB) score ≤ 7 (bottom panel) and SPPB = 8–9, extracted from a model including all activity predictors. Note that we have illustrated variability hazard ratios at selected values of 6 vs. 5, 9 vs. 8, and 11 vs. 10, and amount hazard ratios at selected values of 7 vs. 6 min/hour, 13 vs. 12 min/hour, and 17 vs. 16 min/hour. Red dots represent activity measurement intervals followed by MMD events. HRs for LPA were obtained through back-transformation from models generated using log-transformed values of LPA.
Discussion
It is important to place our results in the context of the LIFE sample and PA intervention. Participants were 70–89 years of age, obese, and physically compromised (SPPB≤9, average gait speed during 400m walk of 0.83m/sec) at baseline. They had multiple comorbidities, and none had major cognitive impairment. The structured PA sessions were individualized and progressed toward a goal of 30 min of daily walking at a moderate intensity, 10 min of primarily lower extremity strength training by means of ankle weights, and 10 min of balance training/large muscle group flexibility exercises.19 Notably, only 25% of participants in the LIFE study were able to reach a common older adult-specific cut point for MVPA during the adoption phase of training.7 Moreover, many participants had to take a break during exercise.6 Thus, when older adults who are either frail or pre-frail exercise, they frequently engage in what clinicians would traditionally conceptualize as LPA or non-exercise PA (i.e., brief bouts of oftentimes slow walking).26 We would like to emphasize here that even this movement that appears low in intensity is likely to fall within the individual’s true moderate-to-vigorous range (i.e., >3.0 METs). Older adults with limited function may achieve 3.0 METs at walking speeds as slow as 1.5 mph, which falls well below what is characterized as LPA in the current PA guidelines.24,26,27 We illustrate this point in Figure 4, which shows changes in CPM the first 6 months of the intervention by SPPB category. Those with lower SPPB scores increased activity in lower-intensity bins compared to those with higher function. Finally, older adults are prone to serious health events. Within the PA group of the LIFE study, 48.4% of participants had a hospitalization that was unrelated to the intervention. A total of 58.6% went on medical leave at least once with a median(IQR) length of 49(21–140) days.8 Mobility was often limited following this leave and interventionists had to focus on strength and balance to assist the participant in returning to extended bouts of walking. Only 14.5% (5.9% due to death) of participants dropped out or were unable to continue with the intervention and complete the final assessments. In sum, these were resilient older adults challenged by compromised function and comorbidities, engaging in an intervention comprised of aerobic MVPA paired with LPA and strength and balance training as needed.
Figure 4.
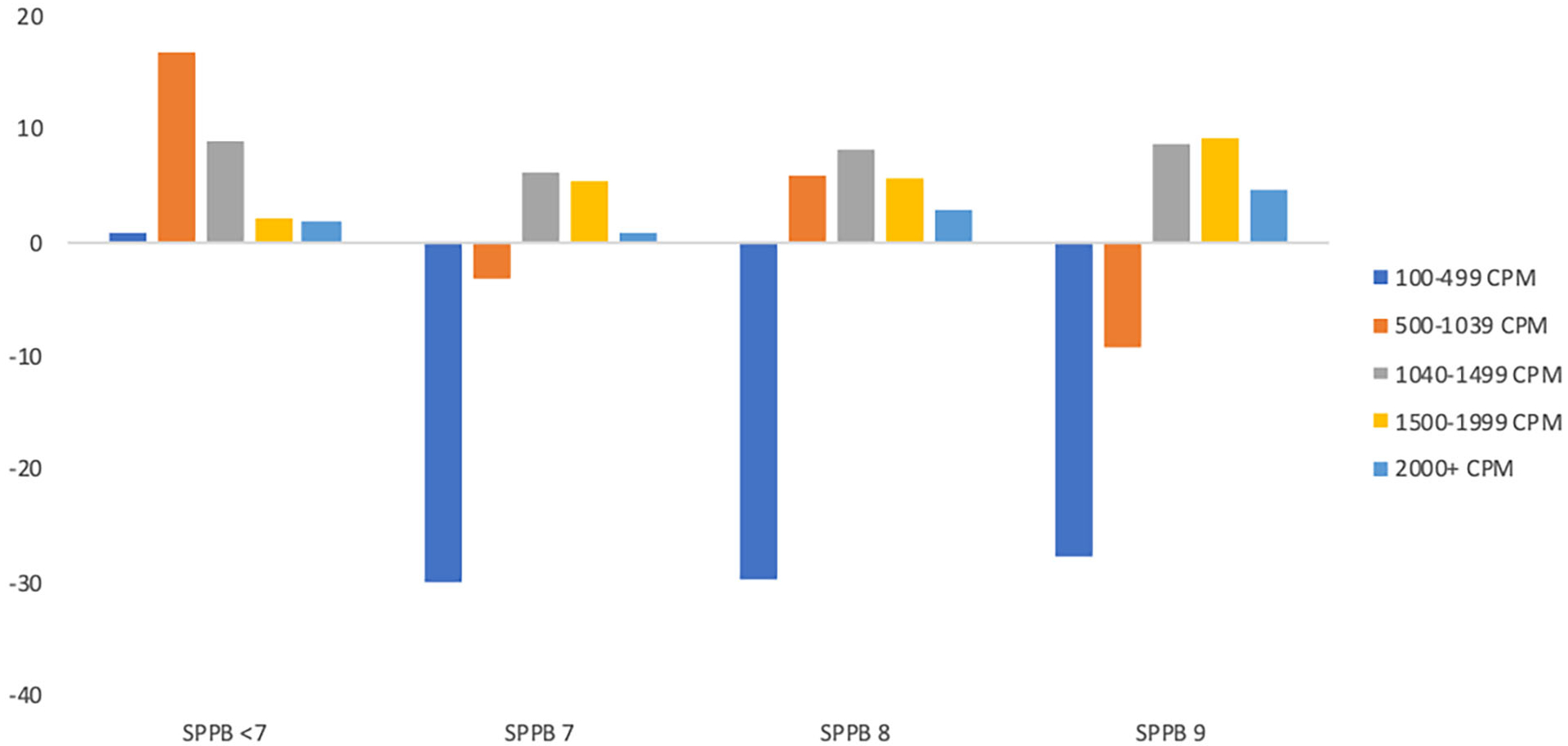
Change in accelerometer counts per minute (median difference per day) in the first six months of the intervention by short physical performance battery score.
Here we investigated the extent to which the amount and variability in both MVPA and LPA were associated with the development of MMD. Our results made clear that the relationship between PA and MMD must be viewed through the lens of function. What follows is a discussion of findings for pre-frail (i.e., SPPB=8–9) and frail (i.e., SPPB≤7) individuals.
Structuring Movement Interventions for Pre-Frail Older Adults
Recent data from the National Health and Aging Trends study (NHATS; N=4377)28 indicate that as many as 14.6% of older adults aged 65–69 are pre-frail (SPPB=8–9). Functional scores decrease with age such that 77.2% of those aged 90+ qualify as frail, and an additional 12% are pre-frail. For pre-frail individuals, “move more, more often” appears a good recommendation for reducing risk for MMD. Interestingly, counter to our hypothesis and to an analysis of the overall study sample,16 additional minutes of MVPA were associated with a small increase in risk for MMD; a phenomenon described in prior LIFE study publications and perhaps attributable to increased risk for injury.8 Both pre-frail and frail participants demonstrated benefit from more sustained bouts (i.e., higher variability) of MVPA, suggesting those most capable of adhering to the intervention had generally lower risk for MMD.
In line with our hypothesis, greater LPA was associated with lower risk for MMD among members of this group with very low levels of LPA; an effect that held when controlling for participation in MVPA. Thus, for pre-frail individuals who engage in low levels of LPA, clinical recommendations should first emphasize “moving more” to increase LPA. Recommended activities might include a variety of lifestyle behaviors such a frequent leisure walks and engaging in hobbies that challenge a variety of functional abilities. Once a greater total volume of LPA has been achieved, recommendations can be made to “move more often” by engaging in frequent bouts of LPA and MVPA throughout the day to maintain a high volume of movement with reduced variability. In sum, pre-frail older adults are likely to benefit from recommendations emphasizing greater volume in movement throughout the day paired with daily participation in MVPA.
Structuring Movement Interventions for Frail Older Adults
Our findings for frail participants highlighted unique challenges to both the assessment and promotion of health-promoting PA among this group. Nationally, less than one third of older adults under age 80 fall in this category, but this number rises quickly with increasing age.28 Poorer functional status places individuals at a greater risk for mobility disability and acts as a powerful barrier to engaging in MVPA.29 Thus, a key first step in exercise prescription for frail older adults should be the development of strength and balance to support and foster improved mobility.30 Our experience in the LIFE study following return from medical leave due to acute health events is supportive of this position. Then, as older individuals find they are more capable of MVPA, recommendations should focus on accumulating a larger daily volume of MVPA; recognizing that we are talking about a low criterion for defining MVPA. It is intriguing to note there were no MMD events observable in individuals engaging in very high levels of LPA (Figure 3). It may be that older adults with a high degree of functional impairment benefit from a high volume of this behavior.
Integrating Function-Oriented Prescriptions into a Movement Medicine Framework
Our results add to a building “movement medicine” approach to PA prescription for older adults; one that considers the individual’s specific needs and desired outcomes when crafting clinical activity recommendations. Unfortunately, there are numerous barriers to prescribing PA in the clinic, including lack of time and inadequate training on the complexities of traditional approaches to activity prescription, which has resulted in very low PA counseling rates.32 Therefore, we have provided a simple stepwise process for clinicians prescribing PA to older adults with compromised function (see Supplemental Figure 2). For low-active older adults who present with difficulty ambulating, we suggest focusing first on developing strength and balance. The next biggest return recommendation would focus on the accumulation of more daily MVPA, which can be done in brief bouts (e.g., climbing a flight of stairs) to minimize task aversion. At this point, a shift in moving more via LPA is warranted. Finally, once individuals are doing a modest amount of PA across the full intensity continuum, one may focus on moving more often, allowing the individual to accumulate movement while guarding against the ill effects of sustained sitting.
Strengths and Limitations
This study leveraged a relatively large sample of older adults who had varied levels of compromised function recruited across a wide geographic area. We utilized a statistical approach that allowed for the modeling of the relationship between PA and MMD over time and included a lengthy follow-up period. A key limitation to this study was that the PA intervention was not designed to affect lifestyle LPA. As such, although we controlled for several baseline co-morbidities, it may be that our analyses highlighted healthy individuals who were capable of engaging in more lifestyle movement. Future interventions attempting to directly manipulate both the amount and patterning of PA are warranted. Additionally, our model utilized the most recent accelerometer value to predict subsequent MMD risk. Due to having only three activity assessments, some predictions may be based on an individual’s baseline physical activity levels alone. As a sensitivity analyses, we reran our models with only individuals with baseline and at least one follow-up assessment, which reduced our sample size by 17 and had no meaningful effect on MMD risk. Fortunately, accelerometers are becoming easier to wear, and future research should consider replicating our analyses using more robust PA datasets.
Conclusion
PA is a vital health-promoting behavior for maintaining physical functioning and an independent lifestyle. For older adults at high risk for MMD, our findings underscore the importance of a tailored approach to PA promotion. At present, the necessary behavior-change techniques that can effectively bring about a distributed pattern of movement are unknown, and we believe this represents an important path forward for research on promoting quality of life as individuals age.
Supplementary Material
Supplemental Figure 1.Utilizing 760 as the cut point for MVPA, this figure illustrates the difference in risk for MMD that a 1-unit higher level of variability has at different amounts of LPA (vertical lines), and difference in risk associated with a 1-min/hr higher level of amount at different levels of variability in LPA (horizontal lines) among those with a short physical performance battery (SPPB) score ≤ 7 (bottom panel) and SPPB = 8–9, extracted from a model including all activity predictors. Note that we have illustrated variability hazard ratios at selected values of 6 vs. 5, 9 vs. 8, and 11 vs. 10, and amount hazard ratios at selected values of 7 vs. 6 min/hour, 13 vs. 12 min/hour, and 17 vs. 16 min/hour. Red dots represent activity measurement intervals followed by MMD events. HRs for LPA were obtained through back-transformation from model generated using log-transformed values of LPA.
Supplemental Figure 2. Stepwise Activity Recommendation Guide. Notes: Recommendations build from left to right, such that fostering basic physical function is necessary for building a greater volume of higher-intensity movement. Once higher intensity movement has been built into the day, and function is stable, a focus on lower-intensity movement is warranted, followed by a focus on distribution of lower-intensity movement. *It is vital to bear in mind that intensity is relevant, and as such, many basic activities of daily living (e.g., walking for transport) should be seen as higher-intensity for some individuals with very low levels of basic functioning.
Supplemental Table 1: Development of Cox Proportional Hazards models using an accelerometer cut point of 1040 to define moderate-to-vigorous physical activity (MVPA). Each column represents a step in the development of the model for light physical activity (LPA) or MVPA, culminating in the final full model, which retained all significant predictors identified in the model building steps, plus covariates. Note: HR = hazard ratio; SPPB = short physical performance battery; BP = blood pressure, CVD = cardiovascular disease.
Supplemental Table 2: Development of Cox Proportional Hazards models using an accelerometer cut point of 760 to define moderate-to-vigorous physical activity (MVPA). Each column represents a step in the development of the model for light physical activity (LPA) or MVPA, culminating in the final full model, which retained all significant predictors identified in the model building steps, plus covariates. Note: HR = hazard ratio; SPPB = short physical performance battery; BP = blood pressure, CVD = cardiovascular disease.
Acknowledgements
Sponsor’s Role
This work is supported by the National Institutes of Health and National Institute on Aging (UO1 AG22376), the National Heart, Lung and Blood Institute (3U01AG022376-05A2S), and the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332). The funding agencies did not participate in the design or conduct of the study, nor did they participate in the preparation of the manuscript at any stage.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest.
Financial Disclosure: None Reported
Trial Registration clinicaltrials.gov Identifier: NCT01072500
References
- 1.Guralnik JM, Lacroix AZ, Abbott RD, et al. Maintaining mobility in late life. I. Demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137(8):845–857. doi: 10.1093/oxfordjournals.aje.a116746 [DOI] [PubMed] [Google Scholar]
- 2.Lonergan ET, Krevans JR. A national agenda for research on aging. N Engl J Med. 1991;324(25):1825–1828. doi: 10.1056/NEJM199106203242527 [DOI] [PubMed] [Google Scholar]
- 3.Buchner DM, Beresford SAA, Larson EB, LaCroix AZ, Wagner EH. Effects of physical activity on health status in older adults II: intervention studies. Annu Rev Public Health. 1992;13(1):469–488. doi: 10.1146/annurev.pu.13.050192.002345 [DOI] [PubMed] [Google Scholar]
- 4.Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: A systematic literature review. Soc Sci Med. 1999;48(4):445–469. doi: 10.1016/S0277-9536(98)00370-0 [DOI] [PubMed] [Google Scholar]
- 5.Warburton DER, Bredin SSD. Reflections on physical activity and health: what should we recommend? Can J Cardiol. 2016;32(12):1–10. doi: 10.1016/j.cjca.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 6.Rejeski WJ, Axtell R, Fielding R, et al. Promoting physical activity for elders with compromised function: the lifestyle interventions and independence for elders (LIFE) study physical activity intervention. Clin Interv Aging. 2013;8:1119–1131. doi: 10.2147/CIA.S49737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rejeski WJ, Marsh AP, Brubaker PH, et al. Analysis and interpretation of accelerometry data in older adults: The LIFE study. J Gerontol A Biol Sci Med Sci. 2016:71(4):521–8. doi: 10.1093/gerona/glv204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg DE, Bombardier CH, Hoffman JM, Belza B. Physical activity among persons aging with mobility disabilities: shaping a research agenda. J Aging Res. 2011;2011. doi: 10.4061/2011/708510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McPhee JS, French DP, Jackson D, Nazroo J, Pendleton N, Degens H. Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology. 2016;17(3):567–580. doi: 10.1007/s10522-016-9641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messier SP, Loeser RF, Mitchell MN, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatr Soc. 2000;48(9):1062–1072. doi: 10.1111/j.1532-5415.2000.tb04781.x [DOI] [PubMed] [Google Scholar]
- 12.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease. JAMA. 2013;310(1):57. doi: 10.1001/jama.2013.7231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rejeski WJ, Brubaker PH, Goff DC, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171(10):880–886. doi: 10.1001/archinternmed.2010.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell E Exercise is medicine. CMAJ. 2013;185(11):E526. doi: 10.1503/cmaj.109-4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. New physical activity recommendations for reducing disease and prevent deaths. http://www.who.int/chp/media/news/releases/2011_2_physicalactivity/en/. Accessed May 3, 2015.
- 16.Fielding RA, Guralnik JM, King AC, et al. Dose of physical activity, physical functioning and disability risk in mobility-limited older adults: results from the LIFE study randomized trial. PLoS One. 2017;12(8):e0182155. doi: 10.1371/journal.pone.0182155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis MG, Fox KR, Stathi A, Trayers T, Thompson JL, Cooper AR. Objectively measured sedentary time and its association with physical function in older adults. J Aging Phys Act. 2014. doi: 10.1123/JAPA.2013-0042 [DOI] [PubMed] [Google Scholar]
- 18.Rejeski WJ, Marsh AP, Brubaker PH, et al. Analysis and interpretation of accelerometry data in older adults: The LIFE study. J Gerontol A Biol Sci Med Sci. 2016:71(4):521–8. doi: 10.1093/gerona/glv204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fielding RA, Rejeski WJ, Blair S, et al. The lifestyle interventions and independence for elders study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66A(11):1226–1237. doi: 10.1093/gerona/glr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borg GA. Perceived exertion: a note on “history” and methods. Med Sci Sports. 1973;5(2):90–93. doi:ET0016 [PubMed] [Google Scholar]
- 22.Choi N Relationship between health service use and health information technology use among older adults: analysis of the US National Health Interview Survey. J Med Internet Res. 2011;13(2):e33. doi: 10.2196/jmir.1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Copeland JL, Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Act. 2009;17(1):17–30. http://europepmc.org/abstract/med/19299836. Accessed February 18, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Barnett A, Van Den Hoek D, Barnett D, Cerin E. Measuring moderate-intensity walking in older adults using the ActiGraph accelerometer. BMC Geriatr. 2016;16(1). doi: 10.1186/s12877-016-0380-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanning J, Rejeski WJ, Chen S-H, et al. A case for promoting movement medicine: preventing disability in the LIFE randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2019; pii: glz05. doi: 10.1093/gerona/glz050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018. doi: 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall KS, Howe CA, Rana SR, Martin CL, Morey MC. METs and accelerometry of walking in older adults: standard versus measured energy cost. Med Sci Sports Exerc. 2013;45(3):574–582. doi: 10.1249/MSS.0b013e318276c73c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NHATS Public Use Data (Round 7). National Institute on Aging, Johns Hopkins Bloomberg School of Public Health. 2010. Accessed September 24, 2019. [Google Scholar]
- 29.Wanigatunga AA, Ferrucci L, Schrack JA. Physical activity fragmentation as a potential phenotype of accelerated aging. Oncotarget. 2019;10(8):807–809. doi: 10.18632/oncotarget.26631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsh AP, Rejeski WJ, Espeland MA, et al. Muscle strength and BMI as predictors of major mobility disability in the lifestyle interventions and independence for elders pilot (LIFE-P). J Gerontol A Biol Sci Med Sci. 2011:66(12):1376–83. doi: 10.1093/gerona/glr158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rejeski WJ, Walkup MP, Fielding RA, et al. Evaluating accelerometry thresholds for detecting changes in levels of moderate physical activity and resulting major mobility disability. J Gerontol A Biol Sci Med Sci. 2017: 73(5):660–667. doi: 10.1093/gerona/glx132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanWormer JJ, Pronk NP, Kroeninger GJ. Clinical counseling for physical activity: translation of a systematic review into care recommendations. Diabetes Spectr. 2009;22(1):48–55. doi: 10.2337/diaspect.22.1.48 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1.Utilizing 760 as the cut point for MVPA, this figure illustrates the difference in risk for MMD that a 1-unit higher level of variability has at different amounts of LPA (vertical lines), and difference in risk associated with a 1-min/hr higher level of amount at different levels of variability in LPA (horizontal lines) among those with a short physical performance battery (SPPB) score ≤ 7 (bottom panel) and SPPB = 8–9, extracted from a model including all activity predictors. Note that we have illustrated variability hazard ratios at selected values of 6 vs. 5, 9 vs. 8, and 11 vs. 10, and amount hazard ratios at selected values of 7 vs. 6 min/hour, 13 vs. 12 min/hour, and 17 vs. 16 min/hour. Red dots represent activity measurement intervals followed by MMD events. HRs for LPA were obtained through back-transformation from model generated using log-transformed values of LPA.
Supplemental Figure 2. Stepwise Activity Recommendation Guide. Notes: Recommendations build from left to right, such that fostering basic physical function is necessary for building a greater volume of higher-intensity movement. Once higher intensity movement has been built into the day, and function is stable, a focus on lower-intensity movement is warranted, followed by a focus on distribution of lower-intensity movement. *It is vital to bear in mind that intensity is relevant, and as such, many basic activities of daily living (e.g., walking for transport) should be seen as higher-intensity for some individuals with very low levels of basic functioning.
Supplemental Table 1: Development of Cox Proportional Hazards models using an accelerometer cut point of 1040 to define moderate-to-vigorous physical activity (MVPA). Each column represents a step in the development of the model for light physical activity (LPA) or MVPA, culminating in the final full model, which retained all significant predictors identified in the model building steps, plus covariates. Note: HR = hazard ratio; SPPB = short physical performance battery; BP = blood pressure, CVD = cardiovascular disease.
Supplemental Table 2: Development of Cox Proportional Hazards models using an accelerometer cut point of 760 to define moderate-to-vigorous physical activity (MVPA). Each column represents a step in the development of the model for light physical activity (LPA) or MVPA, culminating in the final full model, which retained all significant predictors identified in the model building steps, plus covariates. Note: HR = hazard ratio; SPPB = short physical performance battery; BP = blood pressure, CVD = cardiovascular disease.


