Summary:
The correlation of clinical semiology with neuronal firing in human seizures has not been well described. Similarly, the neuronal firing patterns underlying high-frequency oscillations during seizures remain controversial. Using implanted subdural electrodes and a microelectrode array in a patient with focal status epilepticus, in which 40 habitual focal motor seizures and 101 subclinical seizures were captured, the authors analyzed the association of EEG, high-frequency oscillations, and multiunit activity to facial motor semiology. The development of ictal high-frequency oscillations in subdural electrodes overlying face motor cortex was temporally associated with clonic facial movements. In representative seizures selected for multiunit analysis, synchronization of neuronal firing in the adjacent microelectrode array aligned with clinical onset and was greater in clinical seizures compared with subclinical seizures. This report demonstrates the electrophysiologic signatures of focal seizures at the level of neuronal firing, high-frequency oscillations, and EEG as they organize from microscale to macroscale, with clinical correlation.
Keywords: Focal seizures, Epilepsy semiology, Intracranial EEG, High-frequency oscillations, Microelectrode
The electrophysiologic activity underlying seizures spans across multiple scales, from single neuron (“unit”) activity to firing of small (“multiunit”) neuronal populations, to oscillations in local field potentials, and finally to power changes across broad brain regions. The associations between brain activity and seizure semiology have been worked out in reverse order, from macroscale to microscale. Since the earliest descriptions of clonic spasms implicating specific regions of motor cortex by Bravais and Jackson,1,2 case studies have demonstrated precise co-alignment of muscle contractions with intracranial EEG discharges3 and high-frequency oscillations (HFOs) tracking the “Jacksonian march” along motor cortex.4
Determining the clinical effect of unit and multiunit firing in association with seizure semiology in spontaneous human seizures has proven challenging, in part because of limited sampling of the brain with microelectrodes capable of resolving microscale activity.5 Ictal HFOs, known to correlate with synchronized multiunit burst firing,6,7 may serve as a useful clinical biomarker of neuronal population activity. Here we characterize multiunit activity and ictal HFOs in association with seizure semiology involving focal clonic facial movements.
CASE PRESENTATION
Epilepsy surgery evaluation was performed in a 30-year-old woman with childhood-onset refractory nonlesional parietal lobe epilepsy, who had habitual clinical seizures characterized by clonic movements of the left face. On implantation with intracranial electrodes, she was found to have focal nonconvulsive status epilepticus, with multifocal epileptogenic zones.
Subdural electrodes were implanted as clinically indicated to guide epilepsy surgery and recorded at 500 Hz/channel on an Xltek system (Natus, Pleasanton, CA). During 46 hours of EEG monitoring, 160 electrographic seizures were identified, and multiple doses of benzodiazepines were given to abort seizures. Overall, 141 seizures were captured on video. Of these, 40 revealed stereotyped left face clonic movements appearing a minimum of 25 seconds after electrographic onset. Two electrographic patterns were identified, with onsets at the posterior border of the subdural grid (Fig. 1A) in 117 of 160 (73%), in the anterior grid in 30 of 160 (19%), and poorly localized or diffuse in 13 of 160 (8%). Both clinical and subclinical seizures were seen with each electrographic onset pattern, and the EEG spread pattern did not reliably differentiate between seizure types. In seizures with the most common electrographic pattern, visual EEG interpretation revealed spread from onset in the parietal convexity anteriorly through the subdural grid (Fig. 1B), as well as posteriorly to occipital cortex (electrodes not shown).
FIG. 1.
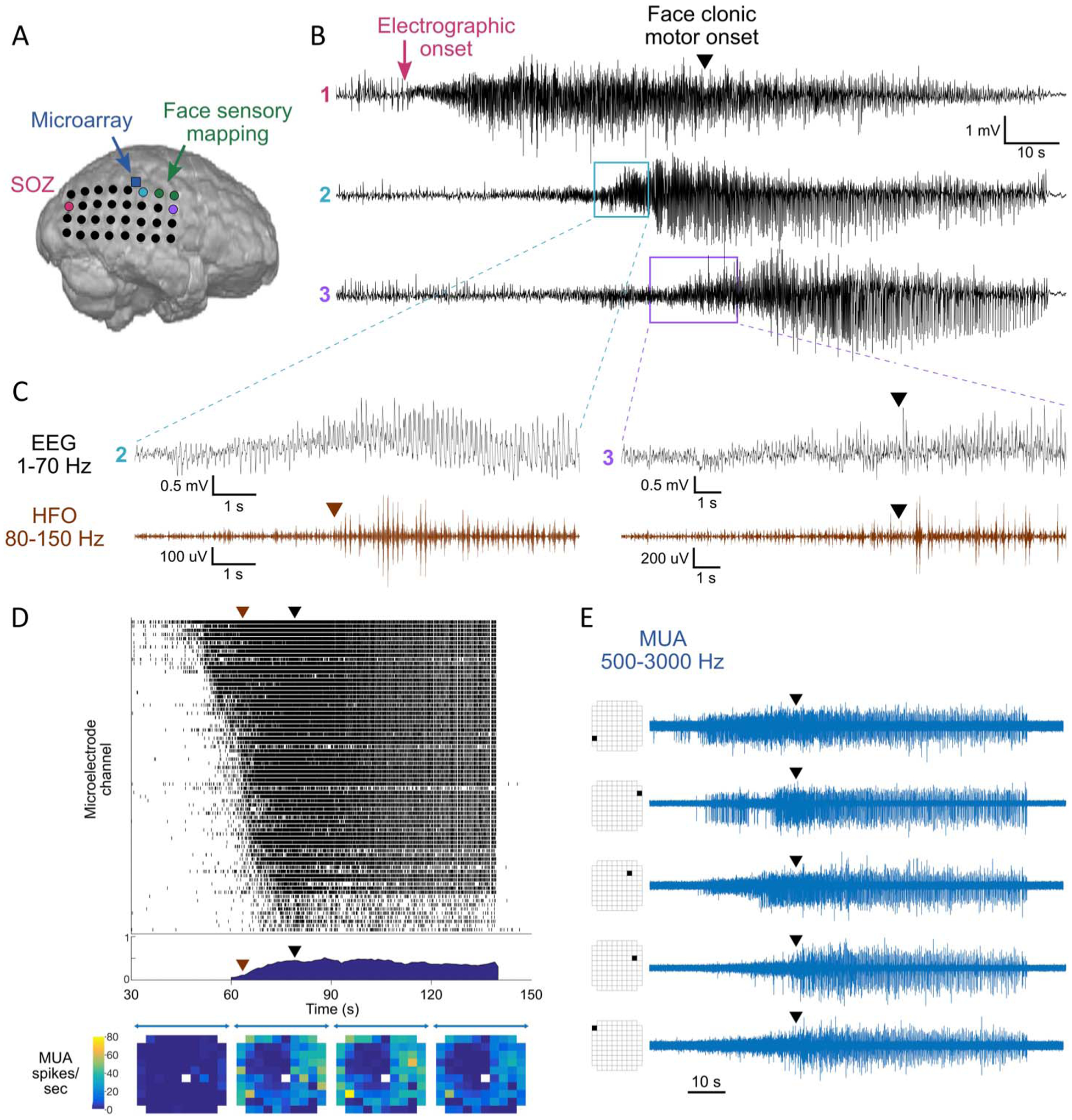
Multiscale seizure analysis. A, Layout of subdural electrode grid and location of microelectrode array. B, Standard EEG (1–70 Hz) recorded from the SOZ (#1, pink), a clinical electrode adjacent to the microarray (#2, light blue), and in the face motor region (#3, purple), showing propagation anteriorly across the grid. C, Ictal HFOs (80–150 Hz, brown) in channels 2 and 3. Black arrowhead represents clinical onset of face clonic motor activity, corresponding to buildup of ictal HFOs in channel 3. D, Raster plot of multiunit firing in microarray channels with >1 spike per second (top), cross-correlation between channels with >5 spikes per second (middle), and heatmap of mean channel firing in 30-second epochs (bottom). Brown arrowhead represents ictal HFO onset in an adjacent macroelectrode, corresponding to increasing synchronization in multiunit firing. E, Example of variability in early firing patterns between microelectrode channels, with synchronized cessation of firing at seizure termination. HFO, high-frequency oscillation; MUA, multiunit activity; SOZ, seizure onset zone.
Ictal HFOs were visually analyzed (Persyst; Persyst Development Corp, Solana Beach, CA) after filtering (FIR order 500, 80–150 Hz) and defined by delayed-onset repetitive bursting lasting at least 10 seconds, standing out from baseline, and distinct from artifactual signals.8 Ictal HFOs displayed a similar propagation pattern as the standard EEG, from posterior parietal to posterior frontal cortex. The start of contralateral clonic face movements aligned with the buildup of HFOs at the anterior border of the array overlying the precentral gyrus, adjacent to electrodes mapped to face sensory function (Fig. 1C). Ictal HFOs at this site were observed in 40 of 40 (100%) clinical seizures compared with 34 of 101 (34%) subclinical seizures (Fisher exact test, P < 0.001).
A NeuroPort microelectrode array (Blackrock Microsystems LLC, Salt Lake City, UT) was implanted in the right superior parietal lobe as previously described9 and recorded at 30 kHz/channel. Six seizures were randomly selected for multiunit analysis, including three clinical seizures with habitual semiology and three subclinical seizures, all sharing the same electrographic onset in the posterior parietal region, occurring >24 hours after administration of anesthesia, and free of visible electrode artifact. Multiunit action potentials were identified from 500 to 3000 Hz filtered data (MATLAB; Mathworks, Natick, MA) as negative peaks at least five standard deviations below a baseline 30-second pre-ictal segment.10 Firing rates were calculated for each channel across 30-second ictal segments, excluding channels with <1 spike per second. Synchronization was measured by averaging Pearson rank correlations between all unique channel pairs in 10-ms bins across 30-second ictal segments, excluding channels with <5 spikes per second. Multiunit visual analysis was performed in each channel to approximate the time of transition from baseline to sustained spiking of >1 spike per second.
The microelectrode array, positioned approximately 5 cm from the posterior seizure onset and 3 cm from face motor region, demonstrated synchronization of multiunit firing corresponding to development of HFOs in an adjacent clinical electrode (Fig. 1D). Synchronized bursting was higher in representative clinical seizures (n = 3) during clonic movements than in corresponding epochs of subclinical seizures (n = 3) with similar electrographic onset patterns {mean correlation coefficient 0.44 (95% confidence interval [CI], 0.39–0.47) versus 0.31 (95% CI, 0.28–0.32), asymptotic general independence test, Z = 2.00, P = 0.046}. Additionally, peak firing rates per channel were greater in clinical (n = 3) compared with subclinical (n = 3) seizures (mean 24.7 [95% CI, 22.5–26.9] versus 20.0 [95% CI, 18.0–22.1] spikes per second across 30-second ictal segments, asymptotic general independence test, Z = −3.02, P = 0.002). Individual microelectrode channels showed variable early activity patterns (Fig. 1E), followed by synchronous cessation of activity at seizure termination (Figs. 1D and 1E). Among the 75 microelectrode channels with >1 spike per second in one clinical seizure, a minority (n = 9) demonstrated maximal firing delayed until the time of clinical onset.
A subtotal right parietal resection was performed targeting the primary seizure onset zone in the posterior parietal region and encompassing the microelectrode recording area, with incomplete resection of the secondary seizure onset zone involving eloquent peri-rolandic cortex anteriorly. Postoperatively, prolonged seizure improvement ensued with occasional nondisabling seizures and impaired awareness seizures largely associated with medication withdrawal or missed doses, although ultimately focal status epilepticus recurred and was controlled with medications (Engel Class 3A outcome with 162-month follow-up). Seizures recorded by scalp EEG postoperatively appeared to originate in the right hemisphere from the anterior and posterior resection margins.
DISCUSSION
We describe a correlation of clinical semiology with stereotyped neuronal firing patterns. In this case, the co-occurrence of habitual cyclic focal motor seizures and subclinical seizures, in the context of focal status epilepticus, allowed for the measurement of neuronal activity associated with clinical semiology. Using an implanted microelectrode array, we demonstrate development of ictal HFOs and synchronization of multiunit activity associated with clonic motor semiology. Clinical and subclinical seizures could not be distinguished on the macroscale by standard EEG but demonstrated distinct local microscale activity measured by ictal HFOs and multiunit activity.
Low-level heterogenous neuronal firing patterns during seizures have been reported in multiple human microelectrode recordings11,12; however, in certain instances, a band of intense tonic firing (i.e., the ictal wavefront) followed by synchronized burst firing is observed.10 In adjacent clinical electrodes, delayed-onset ictal HFOs correspond to synchronized multiunit activity,6 and therefore ictal HFOs may serve as a biomarker of intense synchronized neuronal firing. This report replicates these findings and additionally demonstrates that the clinical effect of seizure recruitment at a specific site, as well as the onset of ictal HFOs, is spatiotemporally associated with synchronized neuronal population firing. Multiunit synchronization was observed as ictal HFOs tracked seizure recruitment across cortex, however without evidence of an ictal wavefront on seizure invasion, suggesting multiple potential mechanisms by which brain territory can be recruited.
Variability in early firing patterns between individual microelectrode channels is expected as a result of recording from different neuronal cell types and population sizes at each contact, as well as from long-range synaptic effects from distant recruited territory, which are dynamic over the course of the seizure.5,7 This variability is unlikely to result from technical or external factors (e.g., electrode malfunction or subdural hematoma) because the majority of channels became fully recruited as the seizures evolved, and individual channels showed similar firing patterns from seizure to seizure across recording days. Per protocol, the research microelectrode array was implanted in non-eloquent cortex, and therefore, we measured seizure recruitment in territory closely associated with motor cortex but not directly overlying it. Persistent focal motor seizures were attributed to the unresected portion of the epileptogenic zone in the peri-rolandic region. However, we cannot exclude the possibility of a remote unsampled seizure focus contributing to multiple seizure patterns.
This report demonstrates electrophysiologic signatures of focal seizures as they organize across scales. Multiscale analysis reveals a seizure structure that is invisible by standard EEG interpretation. Further research into the dynamics and structure of seizures across scales may yield insight into how seizures spread across large brain areas and improve identification of sites critical to seizure generation.
ACKNOWLEDGMENTS
The authors thank the patient and family, whose contribution and participation are deeply appreciated.
Supported by NIH/NINDS R01-NS084142 (C. A. Schevon) and R01-NS095368 (C. A. Schevon).
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Bravais LF. Recherches sur les symptômes et le traitement de l’épilepsie hémiplégique. Paris: Didot, 1827;1–46. [Google Scholar]
- 2.Jackson JH. Lectures on the diagnosis of epilepsy. Br Med J 1879;1:33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamer HM, Lüders HO, Knake S, Fritsch B, Oertel WH, Rosenow F. Electrophysiology of focal clonic seizures in humans: a study using subdural and depth electrodes. Brain 2003;126:547–555. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama T, Chan DW, Go CY, et al. Topographic movie of intracranial ictal high-frequency oscillations with seizure semiology: epileptic network in Jacksonian seizures. Epilepsia 2011;52:75–83. [DOI] [PubMed] [Google Scholar]
- 5.Schevon CA, Tobochnik S, Eissa T, et al. Multiscale recordings reveal the dynamic spatial structure of human seizures. Neurobiol Dis 2019;127:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss SA, Banks GP, Mckhann GM, et al. Ictal high frequency oscillations distinguish two types of seizure territories in humans. Brain 2013;136:3796–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eissa TL, Tryba AK, Marcuccilli CJ, et al. Multiscale aspects of generation of high-gamma activity during seizures in human neocortex. eNeuro 2016;3:ENEURO.0141–15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jirsch JD, Urrestarazu E, Levan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain 2006;129:1593–1608. [DOI] [PubMed] [Google Scholar]
- 9.Waziri A, Schevon CA, Cappell J, Emerson RG, Mckhann GM, Goodman RR. Initial surgical experience with a dense cortical microarray in epileptic patients undergoing craniotomy for subdural electrode implantation. Neurosurgery 2009;64:540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schevon CA, Weiss SA, Mckhann G, et al. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun 2012;3:1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truccolo W, Donoghue JA, Hochberg LR, et al. Single-neuron dynamics in human focal epilepsy. Nat Neurosci 2011;14:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bower MR, Stead M, Meyer FB, Marsh WR, Worrell GA. Spatiotemporal neuronal correlates of seizure generation in focal epilepsy. Epilepsia 2012;53:807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]


