Abstract
We propose a mechanism for myelin plasticity that would complement synaptic plasticity by adjusting conduction velocity for optimal spike-time arrival. In the proposed treadmilling model, myelin sheath thickness is a dynamic balance between the rates of new myelin deposited adjacent to the axon and removal of the outermost layer.
The proper speed of impulse conduction is critical for information processing, spike-time dependent synaptic plasticity, and sustaining brainwave properties [1]. How the appropriate conduction velocity for optimal synchrony of spike-time arrival is achieved in individual axons is unknown. Synchronous arrival of impulses from axons converging onto the same postsynaptic neuron is critical for temporal summation of postsynaptic potentials to initiate postsynaptic action potential firing, and a few milliseconds deviation from synchronous arrival of the presynaptic action potential with respect to postsynaptic firing will initiate long-term changes that either increase or decrease synaptic strength [2]. Action potentials will not arrive synchronously if the path lengths or conduction velocities differ sufficiently between two converging inputs.
Myelin, the electrical insulation on axons, greatly speeds information transmission, because electrical impulses jump from electrogenic relay points, called nodes of Ranvier, separated by segments of axon that are wrapped by electrically inert myelin (Figure 1). By reducing electrical capacitance, thicker myelin increases the speed of action potential propagation, as does narrowing the gap of exposed axon membrane at the nodes of Ranvier [3], but how the proper thickness of myelin is achieved for optimal conduction velocity, and maintained as neural circuits develop and are modified, are long-standing questions.
Figure 1. Myelin Formation and Structure.
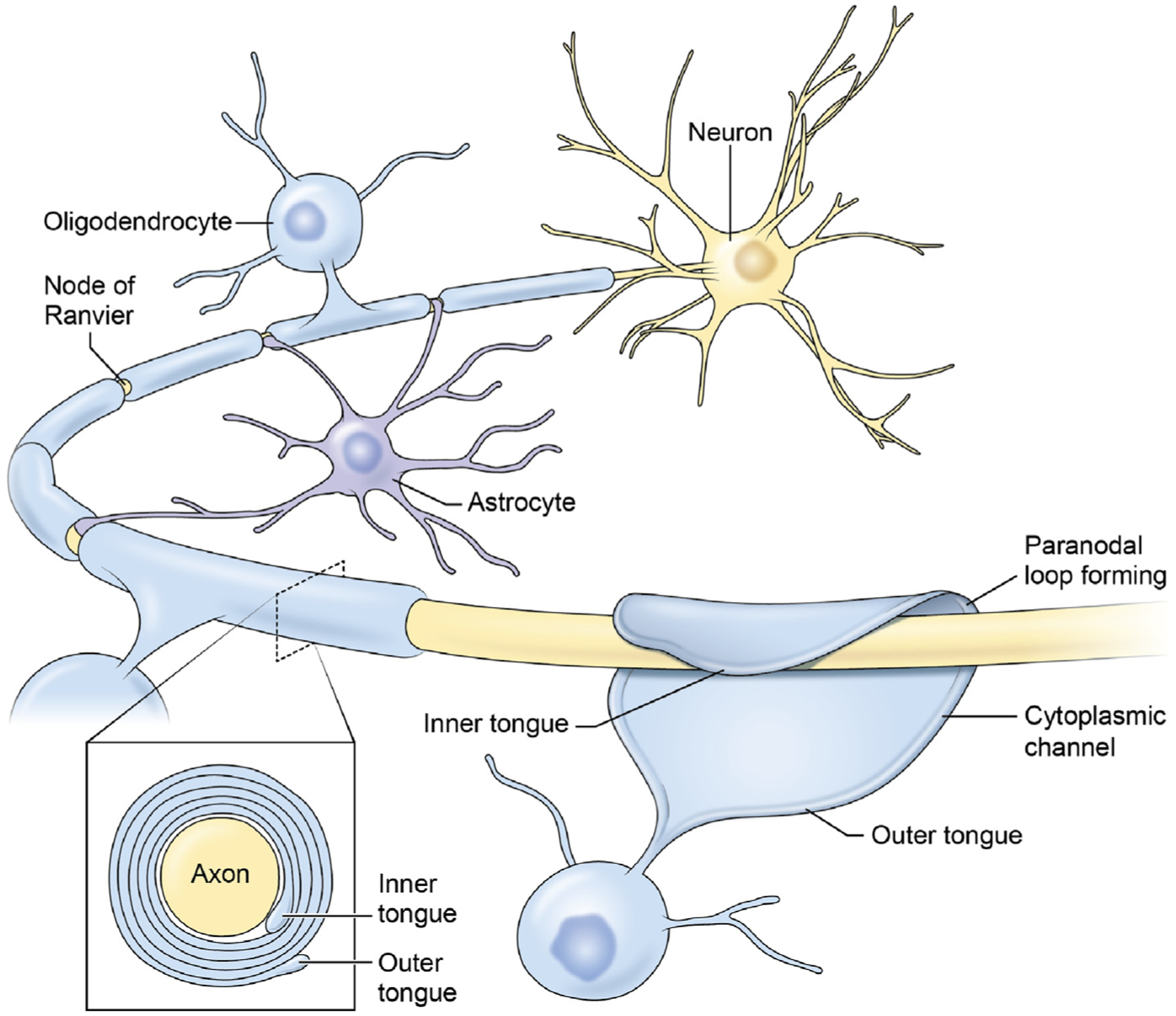
Myelin is the electrically inert membrane wrapping on large diameter vertebrate axons that enables high-speed transmission of action potentials. In the CNS, the myelin sheath is formed by oligodendrocytes, which wrap multiple layers of compacted cell membrane around the axon (inset), forcing action potentials generated at nodes of Ranvier to relay rapidly from node to node. Astrocytes contact the nodes and interact with the paranodal region. Cytoplasm is extruded from the oligodendrocyte wrapping and the apposing membranes are fused by homophilic binding of myelin basic protein, forcing cytoplasm into a narrow channel at the perimeter of the sheath (cytoplasmic channel). The inner tongue expands around the axon to deposit multiple layers of compacted membrane around the axon, whereas the lateral edges attach to the axon through cell adhesion molecules, forming a spiral bordering the node of Ranvier, with the appearance of paranodal loops when sectioned. The outer tongue is continuous with the cell body of the oligodendrocyte through a slender process. Figure modified from [6].
Multiple modes of signaling between the axon and myelin-forming glia have been identified and many of these are responsive to functional activity in the axon [4]. Activity-dependent signaling can influence the formation of myelin, which continues throughout life, and the thickness of the myelin sheath can be increased in response to neural activity, environmental experience, and by depositing new myelin as the axon grows [4,5]. Recent findings have identified a mechanism by which the myelin sheath is thinned nondestructively [6], and this leads to the possibility of a treadmilling model of myelin plasticity described here.
Forming and Thickening the Myelin Sheath
Myelination begins when a long filopodial process from an oligodendrocyte contacts an axon and forms a specialized membrane junction that mediates intercellular communication between the axon and glial cell process, which stimulates local synthesis of myelin basic protein and initiates myelin wrapping [7]. The slender glial process then expands laterally along the axon and begins to encircle it [5]. As it wraps, the glial cell process expands laterally into a ribbon that broadens in width to wrap the entire internodal length like rolled dough in a croissant [5,8], wrapping more layers of membrane around the axon beneath the overlaying compacted sheath. Thus, myelin is a membrane system in which the layers slip over each other to expand the sheath during myelin formation and growth of the axon. However, it is unlikely that myelin is thinned nondestructively by retracting the inner tongue, because of the way the sheath is attached to the axon.
Interestingly, myelin is not attached to the axon over the extensive internodal distance. Instead, the myelin sheath attaches to the axon by forming a spiral junction in the paranodal region flanking the nodes of Ranvier (Figure 2A; Key Figure). When sectioned longitudinally for electron microscopy, this cytoplasm-filled spiral has the appearance of a series of paranodal loops that are attached to the axon via septate-like junctions. Topographically, this axoglial junction corresponds to the lateral edges of the ribbon of oligodendrocyte cell process wrapping around the axon (Figure 1). The paranodal loop that flanks the nodal gap is formed from the outermost layer of myelin, which is continuous with the plasma membrane of the oligodendrocyte through a slender process (the outer mesaxon or outer cytoplasmic tongue).
Thinning the Myelin Sheath
A mechanism of myelin thinning has been identified recently [6]. The septate-like junctions attaching the paranodal loops to the axon are comprised of a complex of three intercellular proteins, with neurofascin 155 (NF155) on the paranodal loop interacting with the Contactin1–Contactin associated protein1 (Caspr1) complex on the axon (Figure 2) [9]. New research shows that cleavage of NF155 by thrombin breaks the axoglial junction holding paranodal loops to the axon, and this proteolysis is regulated by exocytosis of thrombin inhibitors from perinodal astrocytes [6]. When exocytosis of thrombin inhibitors from astrocytes is reduced, NF155 cleavage is promoted, and the outermost paranodal loops of myelin become detached from the axon (Figure 2B). Detached paranodal loops are seen under normal conditions in wild type mice, although much less frequently than when exocytosis of thrombin protease inhibitors from astrocytes is reduced [6]. As the cytoplasm-filled wraps of uncompacted myelin (paranodal loops) detach from the axon, they withdraw away from the node, receding over the compacted myelin, thus thinning the myelin sheath (Figure 2B). In contrast to the outer tongue, the septate junctions at the inner tongue are not accessible to thrombin to detach the inner layer and permit it to retract to thin the sheath.
Remodeling of the node of Ranvier also affects impulse transmission by altering passive electrical properties and redistributing ion channels in the node. The nodal gap length is linked to changes in thickness of the myelin sheath, because as the outer layer of myelin associated with the detached loop is retracted back into the oligodendrocyte, the myelin thickness is reduced by ~30 nm and the nodal gap widens by the width of the detached paranodal loops (Figure 2). These structural changes are shown to alter conduction velocity, spike-time arrival in the CNS, and function, and the changes are reversible [6]. Three to four weeks after restoring normal levels of exocytosis from astrocytes, the myelin sheath thickness and nodal gap length return to normal, presumably by addition of new myelin by the expanding inner tongue (Figure 2A).
Thus, the treadmilling model provides a mechanism to adjust myelin thickness and nodal gap length to help achieve optimal conduction velocity in individual axons. Complementing the rapid changes in connectivity produced by synaptic plasticity, one could envision that myelin plasticity improves performance at the network level through a much slower process. This latter form of plasticity seems most relevant to behaviors involving complex neural networks in large-brained animals, where conduction latencies are substantial and where learning often requires prolonged experience.
Activity-Dependent Myelin Plasticity
Whether thinning and thickening the myelin sheath, as proposed in the treadmilling process, are influenced by electrical activity in the axon is unknown. However, considering the Ca2+ dependence of exocytosis and the many activity-dependent signaling molecules from axons that could alter intracellular Ca2+ in perinodal astrocytes, an influence of neural impulse activity is feasible. It is noteworthy that activity may differ in how it influences addition of new myelin versus removal of the outer layer of myelin. It is further conceivable that different features of impulse firing could have differential effects on these two processes.
This mechanism of myelin sheath thinning will involve many cell biological processes related to cell motility and morphological remodeling [10] that remain to be investigated. After detachment, the cytoplasm contained in the paranodal loop must expand as the fused membranes become delaminated in the outer layer of myelin. This process could be a reversal of the cell biological mechanisms that form compact myelin during myelination, which are regulated by actin biochemistry [10] and homophilic binding of myelin basic protein [11], and which in turn are Ca2+ dependent [12].
A larger question for future research is in determining how treadmilling could be regulated to achieve optimal conduction velocity and spike-time arrival. From a theoretical perspective, a mechanism influencing myelin treadmilling according to spike-time synchrony, as in synaptic plasticity, would seem an optimal functional feedback signal.
Concluding Remarks
In summary, it is well established that action potential firing influences the formation of myelin and thickens the myelin sheath. Further, recent studies show that the myelin sheath is thinned by cleavage of NF155, breaking the attachment of myelin to the axon in the paranodal region, under regulation of exocytosis of thrombin inhibitors from perinodal astrocytes. Whether myelin thinning is influenced by electrical activity in the axon is not yet known. The cellular dynamics during myelin formation, in which a slender process extends from an oligodendrocyte to the axon, slips around it beneath a system of membranes that slide past each other, and changes its morphology by broadening into a ribbon shape, are played out in reverse after the outer layer of myelin becomes detached from the axon and withdraws.
Key Figure
Treadmilling Model for Plasticity of the Myelin Sheath.
Figure 2.

According to this model, the thickness of the myelin sheath is determined by two opposing processes, one (A) that adds additional wraps of myelin to the axon, and the other (B) that removes the outer layer. New layers of myelin are added beneath the overlaying layers by expansion of the inner tongue. Myelin is attached to the axon at the paranodal region flanking the node of Ranvier via septate-junctions, comprised of neurofascin 155 on myelin interacting with Contactin1-Caspr1 complex on the axon. Cleavage of neurofascin 155 by thrombin (red) can break this interaction, resulting in detachment of the outer paranodal loop from the axon, and withdrawal of the outer layer of myelin through the outer tongue. This increases nodal gap length and reduces myelin sheath thickness; both effects slow conduction velocity. Perinodal astrocytes at the nodes of Ranvier regulate this process by secreting thrombin inhibitors (green triangle), such as Protease Nexin1. The treadmilling process helps achieve optimal conduction velocity in individual axons. Whether action potentials influence the treadmilling process is not yet known, but if that turns out to be the case, myelin plasticity could complement synaptic plasticity in producing experience-dependent changes in neural circuits and learning.
Acknowledgments
This work is supported by an NIH intramural research grant (ZIAHD000713-22).
References
- 1.Pajevic S et al. (2014) Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276, 35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi G and Poo M (2001) Synaptic modification of correlated activity: Hebb’s postulate revisited. Annu. Rev. Neurosci 24, 139–166 [DOI] [PubMed] [Google Scholar]
- 3.Pajevic S and Basser P (2013) An optimum principle predicts the distribution of axon diameters in normal white matter. PLoS One 8, e54095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fields RD (2015) A new mechanism of nervous system plasticity: activity-dependent myelination. Nat. Rev. Neurosci 16, 756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snaidero N et al. (2014) Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156, 277–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta DJ et al. (2018) Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proc. Natl. Acad. Sci. U. S. A 115, 11832–11837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wake H et al. (2011) Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobottka B et al. (2011) CNS live imaging reveals a new mechanism of myelination: the liquid croissant model. Glia 59, 1841–1849 [DOI] [PubMed] [Google Scholar]
- 9.Poliak S and Peles E (2003) The local differentiation of myelinated axons at nodes of Ranvier. Nat. Rev. Neurosci 4, 968–980 [DOI] [PubMed] [Google Scholar]
- 10.Zuchero JB et al. (2015) CNS myelin wrapping is driven by actin disassembly. Dev. Cell 34, 152–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snaidero N et al. (2017) Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep. 18, 314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weil M-T et al. (2016) Loss of myelin basic protein function triggers myelin breakdown in models of demyelinating disease. Cell Rep. 16, 314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]


