Abstract
Ruthenium-catalyzed cycloadditions to form 5-, 6-, and 7-membered rings are summarized, including applications in natural product total synthesis. Content is organized by ring size and reaction type. Coverage is limited to processes that involve formation of at least one C-C bond. Processes that are stoichiometric in ruthenium or exploit ruthenium as a Lewis acid (without intervention of organometallic intermediates), ring formations that occur through dehydrogenative condensation-reduction, σ-bond activation-initiated annulations that do not result in net reduction of bond multiplicity and photochemically promoted ruthenium-catalyzed cycloadditions are not covered.
Graphical Abstract

1. Introduction and Scope of Review
Metal-catalyzed cycloadditions represent an expansive, atom-efficient class of transformations that have found wide-ranging utility in chemical synthesis.1–11 The vast majority of metal-catalyzed cycloadditions exploit cobalt,12–16 nickel17–20 and rhodium;21–25 however, in the past two to three decades, significant progress on ruthenium-catalyzed cycloadditions has been made,26–34 including fundamentally new cycloaddition processes that are unknown for other metals.34 While utilized less frequently as catalysts for cycloaddition, ruthenium offers several advantages. Ruthenium is significantly less expensive than other noble metals (Figure 1), yet ruthenium complexes are generally more tractable than their low-valent base-metal counterparts. Ruthenium can adopt a wide range of oxidation levels and engages in diverse modes of reactivity, including C-C bond activation, metallacycle formation via oxidative coupling, carbene/vinylidene/allenylidene formation, alcohol-mediated hydrogen transfer and much more. Although metal-catalyzed cycloadditions have been reviewed1–11 and related ruthenium-catalyzed cycloadditions have appeared intermittently in more broadly focused monographs,26–39 the topic of ruthenium-catalyzed cycloadditions has not been exhaustively cataloged. Here, we provide a summary of ruthenium-catalyzed cycloadditions to form 5-, 6-, and 7-membered rings (Figure 2). Reactions covered in this review adhere to the IUPAC definition of a cycloaddition as “a reaction in which two or more unsaturated molecules (or parts of the same molecule) combine to form a cyclic adduct with a net reduction in bond multiplicity.”40 Consequently, previously reviewed ruthenium-catalyzed annulations that proceed through C-H bond activation fall outside the purview of the present monograph,41–43 as do certain C-C and C-N σ-bond activation-initiated annulations,44–46 and cyclocarbonylations of unsaturated alcohols or amines.47–51 Content is organized by ring size and reaction type. Coverage is limited to processes that involve formation of at least one C-C bond. Hence, the recently reviewed topic of ruthenium-catalyzed alkyne-azide “click” cycloadditions is not covered.52–55 Reactions that are stoichiometric in ruthenium or exploit ruthenium as a Lewis acid (and do not proceed by way of intermediates that possess carbon-ruthenium bonds) are not covered. This includes dehydrogenative annulations that occur through successive condensation56–58 and photochemically promoted ruthenium-catalyzed cycloadditions.59–63 Ruthenium-catalyzed cyclopropanations64–79 and (2+2) cycloadditions80–86 have been exhaustively reviewed elsewhere and are not covered.
Figure 1.

Cost of noble metals vs cost of ruthenium.
Figure 2.

Diverse products delivered by ruthenium-catalyzed cycloadditions of unsaturated substrates.
2. Five-Membered Ring Formation
2.1. (2+2+1) Cycloadditions
2.1.1. Carbonylative (2+2+1) Cycloadditions
Pursuant to the discovery of the Pauson-Khand reaction (1973)87 and its intramolecular variants,88,89 catalytic carbonylative (2+2+1) cycloadditions of alkynes and alkenes based on cobalt90–92 and titanium93–95 appeared in the 1990s. As described in the review literature, noble metal-catalyzed Pauson-Khand reactions, including enantioselective variants, soon followed.96–99 In 1997, Murai and Mitsudo independently reported the first examples of ruthenium-catalyzed Pauson-Khand-type reactions (Scheme 1).100,101 Upon exposure to substoichiometric quantities of Ru3(CO)12 under modest pressures of carbon monoxide (10−20 atm) at elevated temperatures (140−160 °C), 1,6-enynes are converted to the bicyclopentenones in high yields. Alkynoates notwithstanding, both terminal and internal alkynes participate in (2+2+1) cycloaddition, and 1,6-enynes bearing propargyl stereocenters deliver cycloadducts as single diastereomers. Whereas Murai’s conditions,100 which employ dioxane solvent, are intolerant of disubstituted alkenes, the use of DMA solvent under otherwise nearly identical conditions (as reported by Mitsudo)101 overcomes this limitation. Beller and coworkers applied this method to the formation of cycloadducts that embody the fused tricyclic ring system of the dendrobine alkaloids.102 Computational studies of the mechanism of the Ru3(CO)12-catalyzed Pauson-Khand reaction conducted by Wu in 2008 corroborate a reaction pathway in which a mononuclear alkyne-Ru(CO)4 complex is converted to a ruthenacyclobutenone, which upon alkene insertion and C-C reductive elimination delivers the cyclopentenone (not shown).103 This proposal deviates from the widely accepted mechanism for the parent Pauson-Khand reaction, which involves alkyne-alkene oxidative coupling to form a metallacyclopentene followed by CO-insertion.
Scheme 1.

Intramolecular ruthenium-catalyzed Pauson-Khand reactions reported by Murai, Mitsudo and Beller.
In 1998, Murai reported related Ru3(CO)12-catalyzed oxo-Pauson-Khand reactions of acetylenic aldehydes to form bicyclic α,β-unsaturated γ-lactones (Scheme 2).104 The reaction is applicable to both 1,6- and 1,7-ynals. Thorpe-Ingold effects105 induced by geminal substitution in the tether are not required for efficient cycloaddition. Under related cyclocarbonylation conditions, 1,6- and 1,7-acetylenic imines form bicyclic lactams in modest yield.106 Internal alkynes are required as, in the absence of terminal substitution, hydroamination-alkene isomerization occurs by way of the enamine to form dihydropyridines.
Scheme 2.

Intramolecular ruthenium-catalyzed hetero-Pauson-Khand reactions reported by Murai.
In 2000, Murai and co-workers developed the first intermolecular ruthenium-catalyzed oxo-Pauson-Khand-type cycloadditions of alkynes with a carbonyl compound (Scheme 3).107 Unlike the intramolecular processes, the intermolecular reaction requires a carbonyl partner with vicinal dicarbonyl character. In their initial report, the indicated bis-(2-pyridyl) ketone was the only carbonyl partner described. Notwithstanding this limitation, cycloaddition occurs efficiently using the ruthenium catalyst generated in situ from Ru3(CO)12 with (4-CF3Ph)3P. Murai and co-workers reported intermolecular ruthenium-catalyzed aza-Pauson-Khand-type cycloadditions of alkynes and imines the same year.108 Under identical conditions using activated imines derived from 2-picolinaldehyde, cycloaddition delivers the α,β-unsaturated lactams in good yield. As alkynes bearing Me3Si-substituents undergo partial desilylation, the crude reaction mixtures were subjected to hydrolysis to fully convert the cycloadducts to the desilylated cycloadducts. As illustrated in cycloadditions of Me3Si-propyne vs Me3Si-phenylacetylene, regioselectivity is alkyne-dependent. Later, in 2003, Chatani demonstrated these conditions also are effective in intermolecular oxo-Pauson-Khand-type cycloadditions of the indicated α-ketolactone and occur in a completely regio- and chemoselective fashion.109
Scheme 3.
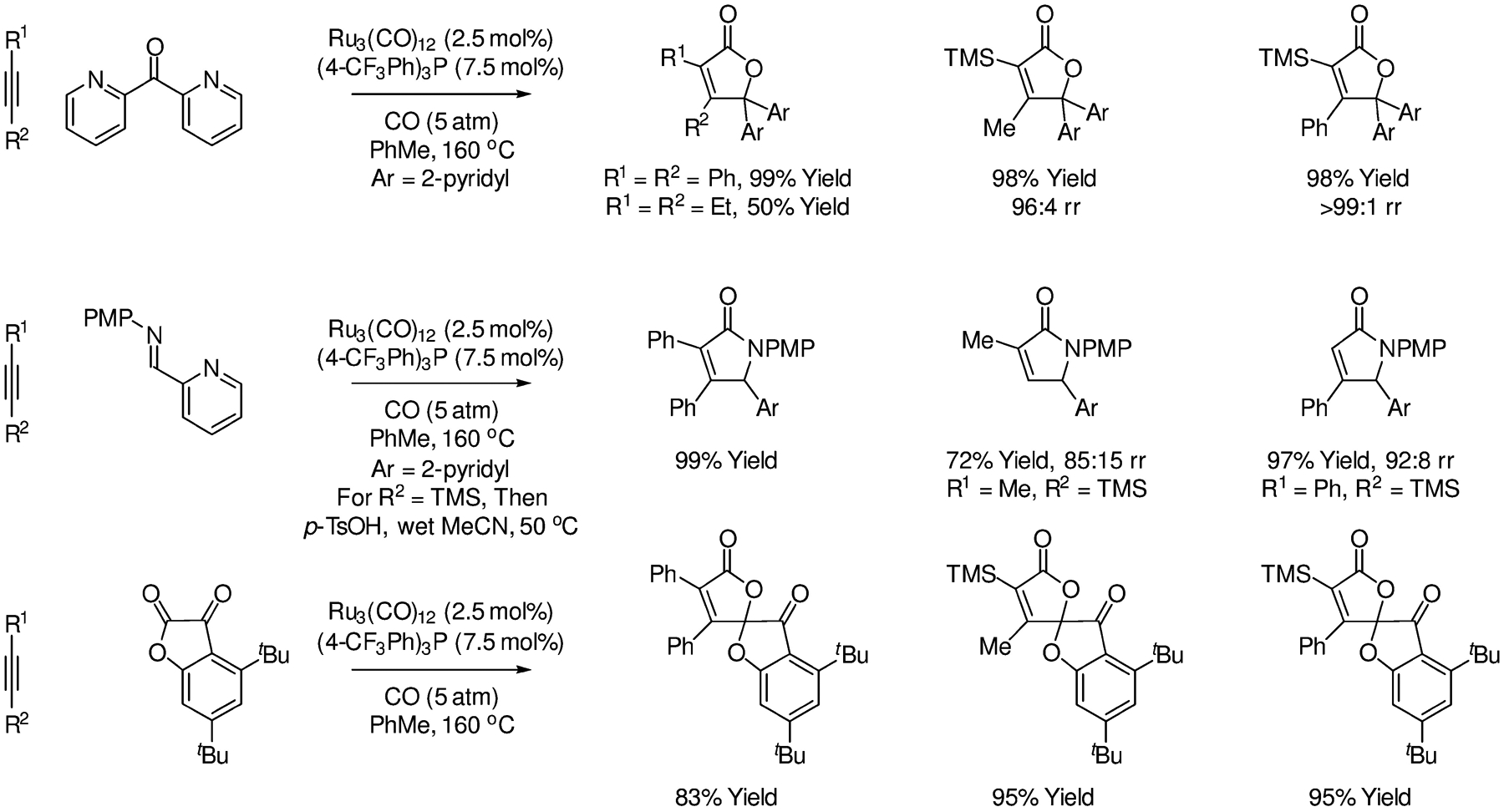
Intermolecular ruthenium-catalyzed hetero-Pauson-Khand reactions reported by Murai.
Unlike intermolecular oxo- and aza-ruthenium-catalyzed Pauson-Khand-type cycloadditions, the intermolecular reaction of alkynes with alkene partners to form carbocycles remains limited in scope. As described by Itami and Yoshida in 2002, alkenes that incorporate a silyl-tethered pyridyl directing group are viable olefinic partners (Scheme 4).110 As desilylation occurs spontaneously under the reaction conditions, the vinyl silane serves as a traceless directing group. However, the multi-step synthesis of such alkenyldimethyl(2-pyridyl)silanes,111 which requires use of organolithium reagents under cryogenic conditions, makes them a poor substitute for ethylene or higher α-olefins, which are abundant feedstocks. In subsequent mechanistic work, a ruthenacyclopentene complex (not shown) and the indicated Ru(vinylsilane)(CO)3 complex were isolated and characterized.112 Both monometallic complexes are catalytically competent. At 50 °C, the ruthenacyclopentene complex is generated quantitatively, and at 100 °C, it was converted to the cyclopentenone. These data corroborate a catalytic mechanism involving rapid ruthenacyclopentene formation followed by slow CO migratory insertion, refuting prior computational investigations.103
Scheme 4.

Intermolecular ruthenium-catalyzed Pauson-Khand reactions of alkenyldimethyl(2-pyridyl)silanes reported by Itami and Yoshida.
In 2006, Kondo reported intermolecular ruthenium-catalyzed hetero-Pauson-Khand reactions of alkynes with isocyanates (Scheme 5).113 The reactions utilize substoichiometric quantities of Ru3(CO)12 in the absence of ligand under one atmosphere of CO. Aryl and alkyl substituents are tolerated in the alkyne and isocyanate partners. The reaction displays broad scope in both the alkyne and isocyanate, enabling direct generation of structurally diverse polysubstituted maleimides from abundant precursors.
Scheme 5.

Intermolecular ruthenium-catalyzed hetero-Pauson-Khand reactions of alkynes with isocyanates reported by Kondo.
In 2007, Hua reported the Ru3(CO)12-catalyzed reductive cyclocarbonylation of internal alkynes in the presence of water to furnish 3,4-disubstituted furan-2(5H)-ones (Scheme 6).114 A high pressure of CO (39.5 atm) was required to enforce conversion to the butenolide. Non-symmetric alkyl- and aryl-substituted alkynes provided good to excellent yields of cycloadduct; however, low levels of regioselectivity were observed.
Scheme 6.

Intramolecular ruthenium-catalyzed cyclocarbonylation of internal alkynes reported by Hua.
More recently, in 2016, Shishido reported oxidative intermolecular ruthenium-catalyzed oxa-Pauson-Khand reactions of alkynes with aryl aldehydes to form γ-hydroxybutenolides (Scheme 7).115 This process exploits a CeO2-supported ruthenium catalyst modified by Xantphos under one atmosphere of CO. Exposure of the reaction mixture to air to promotes oxidation of the initially formed cycloadducts. Unlike previously reported intermolecular ruthenium-catalyzed oxa-Pauson-Khand reactions catalyzed by Ru3(CO)12,107 activated aldehydes are not required. Upon use of excess aldehyde, cycloaddition can occur in the absence of exogenous CO due to metal-catalyzed aldehyde decarbonylation.
Scheme 7.
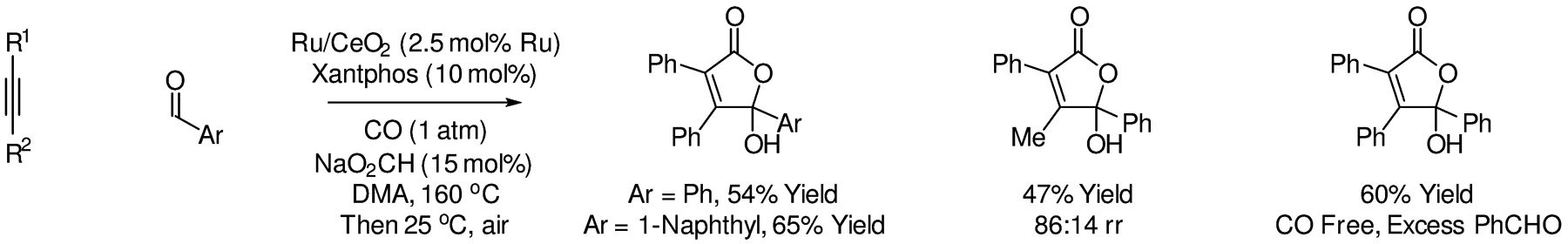
Intermolecular ruthenium-catalyzed oxidative oxo-Pauson-Khand reactions reported by Shishido.
The preceding reactions exploit at least one alkyne as a 2π-component. Carbonylative (2+2+1) cycloadditions beyond alkynes were developed in parallel. In 2000, Kondo and Mitsudo described a carbonylative cycloaddition of allyl carbonates with norbornene to form cyclopentenones (Scheme 8).116 Both [RuCl2(CO)3]2/Et3N and (η3-allyl)RuBr(CO)3/Et3N were shown to be effective catalysts. The reaction is applicable to linear or branched alkyl-substituted allylic carbonates, and the cycloadducts form with complete exo-stereoselectivity. The proposed mechanism involves cis-carboruthenation of norbornene followed by carbonyl insertion to generate an acylruthenium intermediate, which upon olefin insertion-β-hydride elimination provides an exocyclic enone to close the catalytic cycle. Isomerization of the initially formed exocyclic enone to the endocyclic enone occurs under the reaction conditions for alkyl-substituted allylic acetates but not for aryl-substituted allylic acetates.
Scheme 8.

Ruthenium-catalyzed carbonylative (2+2+1) cycloadditions of allylic carbonates with norbornene reported by Kondo and Mitsudo.
Concurrent with their work on ruthenium-catalyzed oxa-Pauson-Khand reactions (Scheme 3), Murai and co-workers developed related carbonylative (2+2+1) cycloadditions of alkenes and activated carbonyl compounds and imines to provide saturated γ-lactones and γ-lactams (Scheme 9).107–109,117 Using the ruthenium(0) catalyst derived from Ru3(CO)12 and (4-CF3Ph)3P, carbonylative cycloadditions of ethylene with α-ketoesters occurred in moderate to good yield.107 Higher olefins, for example 1-hexene and 1,2-substituted alkenes, including 5-decene, cyclohexene and cyclooctene, undergo carbonylative cycloaddition with the indicated bis-(2-pyridyl) ketone to form the corresponding γ-lactones in high yield.107 1,1-Disubstituted, conjugated and electron-deficient olefins did not provide appreciable quantities of cycloadduct under these conditions. Beyond α-ketoesters and 2-pyridyl ketones, other activating groups that confer vicinal dicarbonyl character to the ketone partner can be used, as illustrated in inter- and intramolecular carbonylative cycloadditions of 2-thiazole ketones.107 Benzofuran-2-pyridyl imines108 and benzofuran-2,3-diones (not shown)109 were subsequently explored. In the former case, γ-lactams were formed in high yield in the absence of phosphine ligand.108 The proposed mechanism for carbonylative cycloaddition involves ruthenium(0)-mediated alkene-C=X (X = O, NR) oxidative coupling to furnish a ruthenacycle, which upon carbonyl insertion-C-C reductive elimination provides the lactone.
Scheme 9.

Intermolecular carbonylative (2+2+1) cycloadditions of alkenes with activated carbonyl compounds and imines reported by Murai and Chatani.
In work closely related to that of Murai and Chatani,107–109 Imhof developed a carbonylative (2+2+1) cycloaddition of activated imidates with ethylene to form spirocyclic lactams in 2001 (Scheme 10).118 The reactants incorporate a preexisting stereocenter, yet in all cases the newly formed spirocyclic stereogenic center is formed with only low levels of substrate-directed diastereoselectivity. Internal alkenes, methyl acrylate and internal alkynes also were reported to engage in carbonylative cycloaddition with activated imidates to form the spirolactams, but in significantly lower yields (not shown).119 In a follow-up report, similar selectivity was observed using 1,3,4-oxadiazines-based diazadienes (not shown),120 and the reaction was limited to terminal olefins; no reaction took place with internal olefins or alkynes.
Scheme 10.

Intermolecular carbonylative (2+2+1) cycloadditions of ethylene with activated imidates reported by Imhof.
As demonstrated by Kang in 2002, allenyl aldehydes and ketones participate in intramolecular carbonylative (2+2+1) cycloaddition to form α-methylene-γ-butyrolactones and α-methylene-δ-butyrolactones (Scheme 11).121 The reaction exploits Ru3CO12 as a ruthenium(0) precatalyst in the absence of added ligand. Remarkably, even in the case of 6-membered ring formation, cycloadducts were generated as single diastereomers in good yield.
Scheme 11.
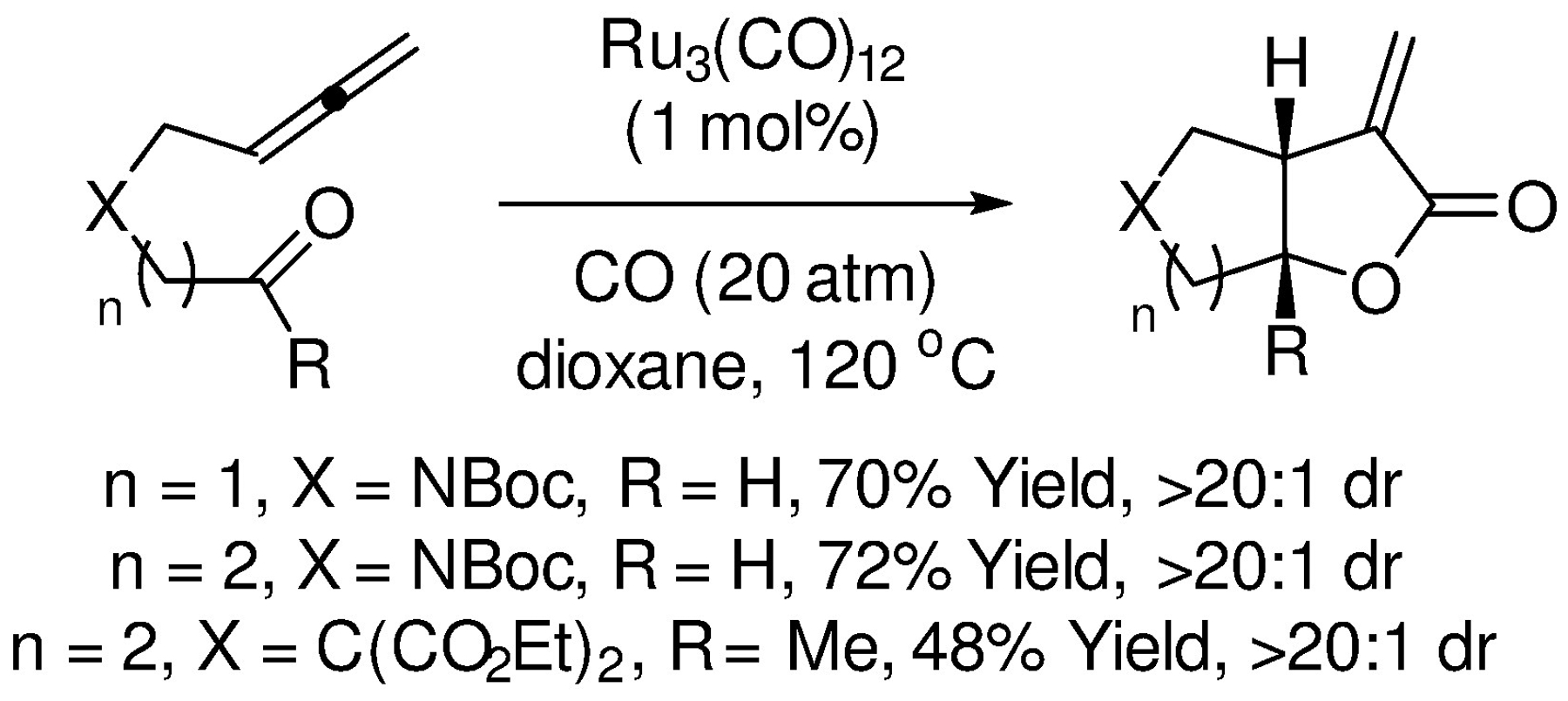
Intramolecular carbonylative (2+2+1) cycloadditions of allenyl aldehydes and ketones reported by Kang.
The ability of 2-pyridyl aldehydes and ketones to participate in carbonylative (2+2+1) cycloadditions with alkenes as initially described by Murai and Chatani107 was elegantly exploited by Snapper in 2011, who reported a tandem ring-closing metathesis-carbonylative (2+2+1) cycloaddition (Scheme 12).122 Here, ring-closing metathesis forms a cycloalkene, which is then exposed to CO and NaOMe to generate a Ru(0) catalyst that promotes cyclocarbonylation. In this way, diastereoselective formation of fused tricyclic ring systems is achieved from acyclic precursors. For reactions involving transient cycloheptenes, an inversion of diastereoselectivity is observed.
Scheme 12.

Tandem ring-closing metathesis carbonylative (2+2+1) cycloadditions with activated ketones reported by Snapper.
2.1.2. Non-Carbonylative (2+2+1) Cycloadditions
In 1987, Süss-Fink reported a ruthenium-catalyzed (2+2+1) cycloaddition that combines two isocyanates with phenylacetylene to form benzylidenehydantoins (Scheme 13).123 Although the yields of cycloadduct are not in a preparatively useful range, this process represents the first ruthenium-catalyzed cycloaddition beyond 3- and 4-membered ring formation. A control experiment involving exposure of the cycloadduct to acid resulted in equilibration of the double bond geometry, corroborating the role of ruthenium as a catalyst in the reaction.
Scheme 13.

Ruthenium-catalyzed (2+2+1) cycloaddition of alkynes with isocyanates reported by Süss-Fink.
In 2012, Yamamoto described the catalytic (2+2+1) cycloaddition of 1,6-diynes and DMSO to form bicyclic furans (Scheme 14).124 Remarkably, in these processes, DMSO serves as an O-atom transfer agent. Although the initially reported process was restricted to aryl-substituted diynes, the authors later found that the reactions could be conducted at lower temperature using nitrones as O-atom donors which, in turn, enabled use of silyl-substituted alkynes.125 Efforts to elucidate the reaction mechanism via experimental and computational studies corroborate a catalytic cycle involving rapid oxidative coupling of the 1,6-diyne to form ruthenacyclopentadiene followed by rate-determining oxygen atom transfer.126
Scheme 14.

Ruthenium-catalyzed (2+2+1) cycloadditions with oxygen atom transfer reported by Yamamoto.
In 2016, Yamamoto developed related (2+2+1) cycloadditions of 1,6-diynes that occur with S-atom transfer, providing access to tetrasubstituted thiophenes (Scheme 15).127 Thionocarbamates were identified as the optimal S-atom donors. Lawesson’s reagent or elemental sulfur (S8) failed to provide cycloadducts in useful yield. Isotopic-labeling experiments implicate water-promoted sulfur-atom transfer (not shown). More recently, in 2018, Yamamoto reported N-atom-transfer cycloadditions of 1,6-diynes based on the use of sulfoximines as nitrene equivalents.128 This method delivers tetrasubstituted pyrroles that would be challenging to prepare by other means.
Scheme 15.
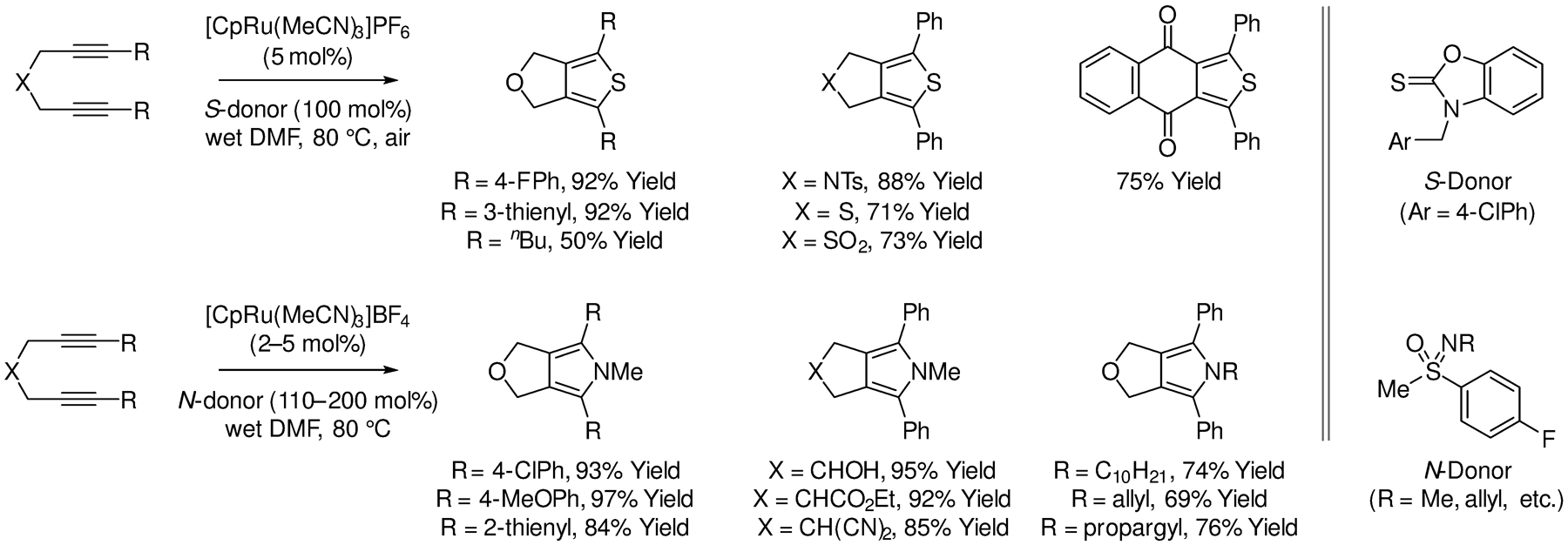
Ruthenium-catalyzed (2+2+1) cycloadditions with sulfur and nitrogen atom transfer reported by Yamamoto.
In 2003, Che reported a ruthenium porphyrin-catalyzed (2+2+1) cycloaddition of imines and α-diazoacetates with electron-deficient alkenes or alkynes to form functionalized pyrrolidines (Scheme 16).129 In these processes, the ruthenium porphyrin reacts with the α-diazoacetate to provide a ruthenium carbenoid, which is transferred to the N-aryl aldimines to afford an azomethine ylide. Subsequent 1,3-dipolar cycloaddition with α,β-unsaturated carbonyl partners provides pyrrolidines and 3,4-dehydropyrrolidines as single diastereomers in high yield. Good levels of asymmetric induction were observed using (−)-8-phenylmenthol diazoesters; however, absolute stereochemistry was undetermined (not shown).130 It was subsequently shown that use of dialkyl azodicarboxylates and nitrosoarenes as 2π-components enables formation of 1,2,4-triazolidines and isoxazolidines, respectively.131,132
Scheme 16.

Ruthenium porphyrin-catalyzed (2+2+1) cycloadditions of imines and α-diazoacetates with olefins, azodicarboxylates or alkynes reported by Che.
2.2. (3+2) Cycloadditions
2.2.1. Dipolar (3+2) Cycloadditions
In 1997, Lin described a RuH2(PPh3)4-catalyzed stereoselective cycloaddition of N-sulfonylimines with methyl isocyanoacetate to provide trans-2-imidazolines (Scheme 17).133 A mixed solvent system containing methanol was found to influence stereoselectivity and conversion more than the choice of ligand. As base-catalyzed N-sulfonylimine-isocyanoacetate cycloadditions have been described,134 it is doubtful this process involves organoruthenium intermediates as suggested by the author. Rather, in the presence of methanol, the ruthenium hydride is expected to form a ruthenium alkoxide that simply serves as a Brønsted basic catalyst.
Scheme 17.

Ruthenium-catalyzed (3+2) cycloaddition of N-sulfonylimines with methyl isocyanoacetate reported by Lin.
In 2008, Fokin reported ruthenium-catalyzed nitrile oxide-alkyne cycloadditions to form isoxazoles (Scheme 18).135 Using [Cp*RuCl(cod)] as precatalyst, nitrile oxides (formed from hydroximoyl chlorides and Et3N) react with terminal or internal alkynes to deliver 3,4-di- or 3,4,5-trisubstituted isoxazoles, respectively. The ruthenium-catalyzed reactions occur with an inversion of regioselectivity compared to the thermal and copper-catalyzed cycloadditions.136,137 The authors propose a catalytic cycle in which nitrile oxide-alkyne oxidative coupling forms a ruthenacycle, which reductively eliminates to release the oxazole. In 2014, it was demonstrated that 1-haloalkynes participate in such cycloadditions, enabling access to 4-haloisoxazoles.138 In 2018, Kniess applied the conditions reported by Fokin135 to the synthesis of 3,4-diaryl isoxazole-containing derivatives of valdecoxib, a COX-2 inhibitor (not shown).139
Scheme 18.

Ruthenium-catalyzed dipolar (3+2) cycloadditions of nitrile oxides and alkynes report by Fokin.
Ruthenium-catalyzed alkyne-azide cycloadditions52–55 and ruthenium-catalyzed dipolar cycloadditions in which the metal simply serves as a Lewis acid catalyst are beyond the scope of this monograph and the reader is referred to the review literature.140–142
2.2.2. Carbene-Mediated (3+2) Cycloadditions
In 2002, Che reported ruthenium porphyrin-catalyzed 1,3-dipolar (3+2) cycloadditions wherein carbonyl ylides are formed from α-diazo ketones (Scheme 19).143,144 In their initial studies,143 unsubstituted α-diazo ketones were shown to engage in 1,3-dipolar (3+2) cycloadditions with a range of dipolarophiles, including maleimide and dimethyl acetylenedicarboxylate, to form oxa-[3.2.1]-bicycles in good yield with complete exo-diastereoselectivity. Notably, using a soluble poly(ethylene glycol)-supported ruthenium-porphyrin, cycloaddition could be performed at 0.1 mol% catalyst loading with no apparent loss of catalytic activity after 7 cycles (not shown). In subsequent work,144 substituted α-diazo ketones were explored. However, diastereoselectivity was found to be highly substrate dependent.
Scheme 19.

Ruthenium porphyrin-catalyzed dipolar (3+2) cycloadditions of carbonyl ylides reported by Che.
In 2004, Chappellet and Müller reported enantioselective (3+2) cycloadditions of ethyl diazopyruvate with enol ethers using a PyBOX-modified ruthenium catalyst (Scheme 20).145,146 Under optimal conditions, the (3+2) cycloaddition of 2,3-dihydrofuran occurred in good yield, albeit with moderate levels of enantiocontrol.145 Absolute stereochemistry of the cycloadducts was not determined. In subsequent work, the authors developed ruthenium-catalyzed cycloadditions using phenyliodonium ylides as carbene precursors; however, low yields and negligible levels of enantioselectivity were observed (not shown).146
Scheme 20.

Ruthenium-catalyzed cycloaddition of ethyl diazopyruvate with 2,3-dihydrofuran reported by Chappellet and Müller.
In 2013, Lee reported ruthenium-catalyzed (3+2) cycloadditions of α-diazo-1,3-dicarbonyl compounds with activated olefins (Scheme 21).147 As illustrated in (3+2) cycloadditions of α-diazo-dimedone, exposure to substoichiometric quantities of [Ru(PPh3)3Cl2] in toluene solvent results in the conversion of electronically diverse alkenes to the corresponding dihydrofurans. In each case, single regioisomers were observed. The authors used this method to generate a small library of dihydrofurans that displayed potent antibacterial activity (not shown).148 In subsequent work, identical conditions were applied to the conversion of terminal alkynes to furans.149 Exposure of cyclopropene byproducts to the reaction conditions resulted in furan formation, suggesting these transformations proceed through a mechanism involving tandem cyclopropanation-cycloisomerization. Later, in 2017, Gu demonstrated that the aforementioned transformations could be conducted using ruthenium catalysts immobilized on functionalized hypercrosslinked polymers (not shown).150
Scheme 21.

Ruthenium-catalyzed dipolar (3+2) cycloadditions of α-diazo-dimedone with olefins or alkynes reported by Lee.
In 2011, Lacour reported ruthenium-catalyzed (3+2) cycloadditions of α-diazo-1,3-dicarbonyl compounds with nitriles and carbonyl compounds to form oxazoles and dioxoles, respectively (Scheme 22).151 Enantioselective (3+2) cycloadditions using ruthenium catalysts modified by PyOX ligands were attempted but low levels of enantioselectivity were observed (not shown). Later, in 2016, Lacour reported related cycloadditions of α-diazo-1,3-dicarbonyl compounds with cyclic lactones and cyclic carbonates to form spirocyclic orthoesters and orthocarbonates, respectively (Scheme 22).152 These reactions represent the first examples of intermolecular (3+2) cycloadditions of metal carbenes with esters and carbonates.
Scheme 22.

Ruthenium-catalyzed dipolar (3+2) cycloadditions of α-diazo-1,3-dicarbonyl compounds with nitriles and carbonyl compounds reported by Lacour.
2.2.3. (3+2) Cycloadditions via Hydrogen Transfer
In 2013, Krische reported oxidative (3+2) cycloadditions of 1,2-diols with acrylic esters to form γ-lactones (Scheme 23).153 The reaction mechanism involves ruthenium(0)-mediated oxidative coupling of the acrylate with a transient 1,2-dione to form an oxaruthenacycle. Protonolytic cleavage of the oxaruthenacycle by the carboxylic acid cocatalyst,154,155 1-adamantanecarboxylic acid, triggers lactonization. One equivalent of methyl acrylate is required as sacraficial hydrogen acceptor to mediate diol or ketol dehydrogenation. For acrylic esters bearing an α-hydroxymethyl-substituent, elimination of water occurs at the stage of the oxaruthenacycle, enabling formation of α-methylene-γ-butyrolactones. In the same study, redox-neutral cycloadditions of N-benzyl 3-hydroxy-2-oxindole with β-substituted acrylic esters were described (Scheme 24).153 The spiro-γ-lactones are formed in excellent yields as single diastereomers. The observed diastereoselectivity was explained on the basis of the indicated model, in which the carbomethoxy group is oriented distal with respect to C4 of the oxindole ring.
Scheme 23.

Ruthenium-catalyzed oxidative (3+2) cycloadditions of 1,2-diols with acrylic esters reported by Krische.
Scheme 24.

Ruthenium-catalyzed redox-neutral (3+2) cycloadditions of N-benzyl 3-hydroxy-2-oxindole with β-substituted acrylic esters reported by Krische.
As demonstrated by Liu in 2019, ruthenium complex [Cp*Ru(cod)Cl] catalyzes intramolecular (3+2) cycloaddition of (E)-1,6-enynes to form [3.3.0] bicycles (Scheme 25).156 Deuterium labeling and DFT calculations support a mechanism that involves initial alkyne-alkene oxidative coupling to deliver a ruthenacyclopentene, which undergoes internal hydrogen transfer via β-hydride elimination-hydroruthenation. This isomerization converts the ruthenacyclopentene to the ruthenacyclohexene, which can then undergo C-C reductive elimination to form the cycloadduct. Thorpe-Ingold effects105 induced by geminal substitution in the alkyne tether were essential for efficient cycloaddition and to prevent formation of Alder-ene byproducts.
Scheme 25.

Ruthenium-catalyzed intramolecular (3+2) cycloadditions of (E)-1,6-enynes by Liu.
2.2.4. (3+2) Cycloadditions via C-C and C-N σ-Bond Activation
A remarkable dimerization of 2,5-norbornadiene to furnish pentaquinanes was reported in 1994 by Mitsudo and Watanabe (Scheme 26).157 This process is catalyzed by the zero-valent ruthenium complex Ru(cod)(cot). Electron deficient olefinic additives, such as dimethyl fumarate (dmfm), are required.158 In 1999, further exploration of the reaction revealed that the olefin additive underwent ligand exchange to form Ru(cot)(dmfm)2, which serves as the active catalyst.159
Scheme 26.

Ruthenium-catalyzed dimerization of 2,5-norbornadiene reported by Mitsudo and Watanabe.
In 2004, Mascareñas demonstrated that the first-generation Grubbs catalyst will promote intramolecular (3+2) cycloaddition of 1,6-enynes that incorporate alkynylidenecyclopropane moieties (Scheme 27).160 The ruthenium complexes Cp*Ru(MeCN)3PF6/Et4NCl or Cl2Ru(PPh3)3 also are competent catalysts, providing the (3+2) cycloadduct in 11% and 35% yield, respectively (not shown). While the catalytic mechanism remains unclear, these data suggest non-carbene ruthenium species generated under the reaction conditions are responsible for the observed non-metathetic behavior.
Scheme 27.

Ruthenium-catalyzed intramolecular (3+2) cycloadditions of alkynylidenecyclopropanes reported by Mascareñas.
In 2007, Tam developed a ruthenium-catalyzed (3+2) cycloaddition of aza-benzonorbornadienes with conjugated alkynoates to form dihydrobenzoindoles (Scheme 28).161 The neutral ruthenium complex [Cp*Ru(cod)Cl] was an effective catalyst for cycloadditions of γ-hydroxy alkynoates. For other acetylenic esters, the cationic ruthenium catalyst [Cp*Ru(MeCN)3]PF6 was required. N-Carbamoyl protecting groups were incorporated to suppress competing formation of [2+2] cycloadducts. In contemporaneous work, Tenaglia explored reported (3+2) cycloadditions of aza-benzonorbornadienes with alkynes using a cationic ruthenium catalyst generated in situ from [CpRuCl(PPh3)2] and iodomethane (Scheme 28).162 Non-symmetric internal alkynes, including terminal alkynes, were converted to the cycloadducts with complete regiocontrol, favoring adducts in which the more polar functional group is placed proximal to the carbon atom vicinal to nitrogen. The reaction mechanism involves regioselective alkyne-alkene oxidative coupling to provide a ruthenacyclopentene, which upon β-nitrogen elimination forms a six-membered aza-ruthenacycle. Subsequent C-N reductive elimination delivers the cycloadduct. Competing C-C reductive elimination from the ruthenacyclopentene intermediate also can occur, producing cyclobutene side products (not shown). Computational studies by Chass implicate a different pathway involving isomerization of an initially formed cyclobutene [2+2] cycloadduct,163 but this hypothesis is inconsistent with experimental data reported by Cramer (not shown).164 In 2018, enantioselective variants of the ruthenium-catalyzed alkynoate-aza-benzonorbornadiene (3+2) cycloadditions were developed by Cramer using a novel class of C2-symmetric cyclopentadienyl ligands (Scheme 28).164 The addition of tetrabutylammonium iodide (TBAI) was required to enforce high levels of conversion to the cycloadduct. Diverse alkynoates and aza-benzonorbornadienes were tolerated, affording the dihydrobenzoindoles with uniformly high levels of regio- and enantioselectivity.
Scheme 28.

Ruthenium-catalyzed (3+2) cycloadditions of aza-benzonorbornadienes with conjugated alkynoates reported by Tam, Tenaglia and Cramer.
2.3. (3+1+1) Cycloadditions
In 2017, Hu reported the enantioselective (3+1+1) cycloaddition reaction of enals, anilines and α-diazoacetophenones to form trisubstituted pyrrolidines (Scheme 29).165 The authors exploit a dual catalytic system in which the aniline, the α-diazoacetophenones and ruthenium combine to form an ammonium ylide, which participates in an asymmetric conjugate addition to the iminium ion that arises upon condensation of the enal with the Hiyashi-Jørgensen secondary amine cocatalyst. Hydrolytic release of the organocatalyst followed by cyclization to form the N,O-acetal completes the catalytic cycle.
Scheme 29.
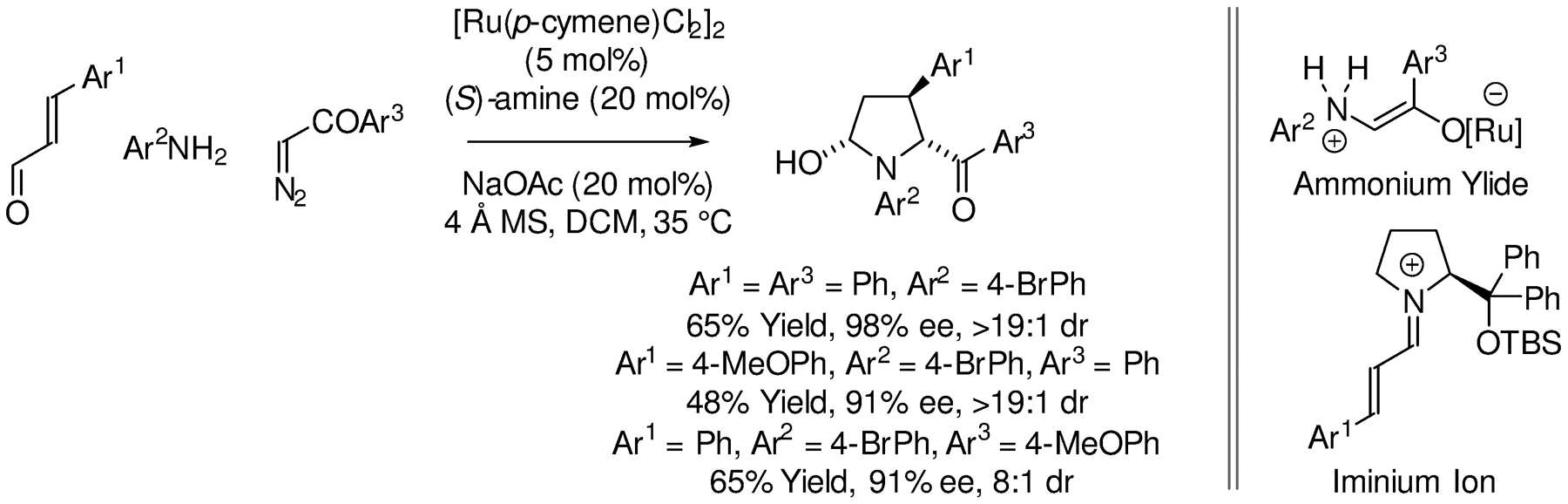
Enantioselective ruthenium-catalyzed (3+1+1) cycloaddition of enals and α-diazoacetophenones reported by Hu.
2.4. (4+1) Cycloadditions
A ruthenium-promoted (4+1) cycloaddition of an α,β-unsaturated imine with CO was reported by Murai in 1999 (Scheme 30).166 Upon exposure to Ru3(CO)12 under a CO atmosphere at elevated temperature, various conjugated imines or ketimines were converted to α,β-unsaturated γ-lactams. Aromatic imines failed to provide cyclocarbonylated products under these conditions. The authors proposed that the mononuclear ruthenium species Ru(CO)4 undergoes cyclometalation with the unsaturated imines prior to migratory insertion of CO. Subsequent reductive elimination affords β,γ-unsaturated γ-lactams which isomerize to the thermodynamic product if an α-proton is present.
Scheme 30.

Ruthenium-catalyzed (4+1) cycloadditions of α,β-unsaturated imines with CO reported by Murai.
3. Six-Membered Ring Formation
3.1. (2+2+1+1) Cycloadditions
In 1993, Murai reported a ruthenium-catalyzed (2+2+1+1) cycloaddition of 1,6-diynes that involves successive incorporation of two molecules of carbon monoxide, resulting in formation of substituted catechol derivatives (Scheme 31).167 This process is reductive and requires one equivalent of hydrosilane. The authors propose a mechanism in which oxidative addition of HSitBuMe2 to Ru(0) triggers silyl migration to provide a siloxycarbyne complex. Incorporation of a second carbon monoxide followed by tautomerization and diyne oxidative coupling delivers a ruthenacyclopentadiene complex, which upon alkyne insertion and C-C reductive elimination forms the mono-silylated cycloadduct. Further silylation under the reaction conditions provides the observed product. In 2020, Crudden, Chatani and Murai demonstrated that, in place of hydrosilane, water could be combined with carbon monoxide in a ruthenium-catalyzed water-gas shift (WGS) reaction to generate 1,2-hydroxyethyne, enabling analogous cycloadditions of 1,5-diynes to generate catechols (not shown).168
Scheme 31.

Ruthenium-catalyzed carbonylative (2+2+1+1) cycloaddition of 1,6-diynes reported by Murai.
In 1998, Mitsudo reported the ruthenium-catalyzed (2+2+1+1) cycloaddition of alkynes with norbornene, which involves successive incorporation of two molecules of carbon monoxide, resulting in formation of hydroquinones (Scheme 32).169 The proposed mechanism is initiated by double carbonylation of the alkyne to form a maleoylruthenium complex that inserts 2-norbornenes. C-C Reductive elimination followed by double tautomerization of the resulting 1,4-dione provides the hydroquinone. Later, in 2005, Ryu and Mitsudo identified reaction conditions that allowed for cycloaddition of symmetric alkynes with electron-deficient alkenes (Scheme 32).170 An analogous catalytic cycle was proposed. In 2016, Sarpong applied this doubly carbonylative ruthenium-catalyzed (2+2+1+1) cycloaddition to the formal syntheses of the indole alkaloids herbindole B and cis-trikentrin A (not shown).171
Scheme 32.

Ruthenium-catalyzed carbonylative (2+2+1+1) cycloaddition of alkynes with norbornenes or electron-deficient alkenes reported by Mitsudo.
3.2. [2+2+2] Cycloadditions to Form Carbocycles
3.2.1. [2+2+2] Cycloadditions of COD and NBD as 22π Partners
In 1993, Trost reported a remarkable transformation of 1,5-cyclooctadiene, a common spectator ligand, in a ruthenium-catalyzed bis-homo-Diels-Alder cycloaddition with alkynes to form [2.2.2] bicycles that are fused to a cyclobutane ring (Scheme 33).172 Both terminal and internal alkynes are competent partners for cycloaddition. The authors posit that the catalytic cycle is initiated by methanol-assisted dissociation of chloride from ruthenium to form a cationic Cp-ruthenium(II) complex. Alkyne coordination followed by successive olefin insertion and C-C reductive elimination provides the cycloadduct. This interpretation of the reaction mechanism is supported by computational studies,173 which suggest alkyne-alkene oxidative coupling to form the indicated ruthenacyclopentene is the rate-determining step. The catalytic competence of related cationic ruthenium complexes was subsequently demonstrated by Gimeno’s use of the indenyl ruthenium(II) precatalyst [Ru(η5-C9H7)Cl(cod)],174 Hintermann’s use of [CpRu(cod)(MeCN)]PF6175 and Tam’s application of commercial Cp*RuCl(cod) (not shown).176 In 2019, Tam further extended the scope of the reaction to include acetylenic phosphonates as 2π-components (Scheme 33).177
Scheme 33.

Ruthenium-catalyzed bis-homo-Diels-Alder cycloadditions of 1,5-cyclooctadiene with alkynes reported by Trost and Tam.
In 2004, Tenaglia reported a ruthenium-catalyzed homo-Diels-Alder reaction of internal alkynes with norbornadiene to form 8,9-disubstituted deltacyclenes (Scheme 34).178 The ruthenium catalyst [(nbd)RuCl2(PPh3)2] was found to provide optimal yields of cycloadduct. The reaction is initiated by dissociation of a phosphine ligand followed by alkyne coordination. Subsequent oxidative coupling of norbornadiene delivers a ruthenacyclobutane, which upon alkyne migratory insertion and C-C reductive elimination from the resulting ruthenacyclohexene delivers the cycloadduct. The same authors reported an intramolecular variant of this cycloaddition in 2007 (not shown).179 Additionally, in 2011 Tam further extended the scope of the reaction to include acetylenic phosphonates as 2π-components (not shown).180
Scheme 34.

Ruthenium-catalyzed homo-Diels-Alder cycloaddition of norbornadiene with alkynes reported by Tenaglia.
In 2017, Krische reported a ruthenium(0)-catalyzed transfer hydrogenative cycloaddition of norbornadiene with vicinal diols to form 8,9-cycloalkyl-substituted deltacyclene diols with complete exo-diastereoselectivity (Scheme 35).181 Remarkably, the transformation is redox-independent and can be conducted with from the diol, ketol or 1,2-dione oxidation levels (not shown). The proposed mechanism, which was corroborated by computational studies performed by Li in 2019,182 is initiated by 1,2-dione-norbornadiene oxidative coupling to form an oxaruthenacycle, which inserts the appendant ketone to form a dioxaruthenacycle. Ketol-mediated transfer hydrogenolysis of the dioxaruthenacycle delivers the exo-cycloadduct and regenerates the requisite ketone. As the overall process is oxidative, one equivalent of norbornadiene serves as hydrogen acceptor in the ruthenium-catalyzed dehydrogenation of the 1,2-diols to the 1,2-ketol.
Scheme 35.

Ruthenium-catalyzed [2+2+2] cycloaddition of norbornadiene with 1,2-diols reported by Krische.
3.2.2. [2+2+2] Cycloadditions of 3 Alkynes
Metal-catalyzed alkyne trimerization via [2+2+2] cycloaddition to form aromatic compounds has been documented in the review literature.183–188 Here, ruthenium-catalyzed alkyne [2+2+2] cycloadditions are catalogued on the basis of catalyst structure. Intermolecular alkyne [2+2+2] cycloadditions, as well as reactions of tethered diynes with alkynes and completely intramolecular reactions of acyclic triynes are all described in this section.
The first intermolecular ruthenium-catalyzed alkyne trimerization via [2+2+2] cycloaddition was reported in 1989 by Lindner (Scheme 36).189 The catalyst, which is a ruthenacyclopentadiene dimer formed from the reaction of Ru3(CO)12 with dimethyl or diethyl acetylenedicarboxylate, promotes efficient trimerization of acetylenedicarboxylates to form mellitic acid ester. As suggested by the structure of the catalyst, a mechanism involving ruthenium(0)-mediated oxidative coupling of the acetylenedicarboxylate to form ruthenacyclopentadiene followed by alkyne insertion and C-C reductive elimination was postulated.
Scheme 36.

Intermolecular ruthenium-catalyzed [2+2+2] cyclotrimerization of alkynes reported by Lindner.
As first reported by Blechert in 1997, the first-generation Grubbs catalyst is capable of promoting intramolecular [2+2+2] cycloadditions of acyclic triynes to furnish tricyclic cycloadducts that incorporate a central benzene ring (Scheme 37).190 Acyclic tetraynes also form benzene-containing cycloadducts rather than products of [2+2+2+2] cycloaddition (not shown).190 This process was most effective for the generation of benzene derivatives bearing fused 5-membered rings. The authors propose a mechanism involving a cascade of four metathesis reactions. Subsequent computational studies, however, refute this interpretation and corroborate a conventional non-metathetic pathway involving ruthenacyclopentadiene formation followed by alkyne insertion and C-C reductive elimination.191 In 2002, Undheim applied this process to the construction of the indicated bis-(pyrazine spirocycle) and, therefrom, bis-(α-amino acid) derivatives (Scheme 37).192,193 Additional examples of such alkyne [2+2+2] cycloadditions that are closely related to those initially described by Blechert were reported by Shi in 2013 (not shown).194
Scheme 37.

Use of the first-generation Grubbs catalyst in intramolecular alkyne [2+2+2] cycloadditions of acyclic triynes reported by Blechert and Undheim.
The first-generation Grubbs catalyst can also promote intermolecular alkyne [2+2+2] cycloadditions to furnish trisubstituted benzenes, as reported by Roy in 1999 (Scheme 38).195 This reaction was applied to the cyclotrimerization of propargyl alcohol derivatives, including pyranosides, silyl ethers and esters. Mixtures of 1,2,4- and 1,3,5-trisubstituted benzenes were observed with a preference for the 1,2,4-trisubstituted regioisomers. In 2011, Kotha demonstrated crossed [2+2+2] cycloadditions of phenylacetylenes with dimethyl acetylenedicarboxylate (DMAD) to form 4,5-diarylphthalic acid esters using the first-generation Grubbs’ catalyst (Scheme 38).196 Other ruthenium alkylidene complexes were evaluated for their ability to promote alkyne [2+2+2] cyclotrimerization, including those reported by Karabulut197,198 and phosphine-free ruthenium alkylidene complexes described by Czeluśniak (not shown).199,200
Scheme 38.

Use of the first-generation Grubbs catalyst in intermolecular alkyne [2+2+2] cycloadditions reported by Roy and Kotha.
The first-generation Grubbs catalyst is also effective in promoting the chemo- and regioselective [2+2+2] cycloaddition of 1,6-diynes with alkynes to form 4,6-disubstituted isoindolines and indolines, as described by Witulski in 2000 (Scheme 39).201 The authors demonstrated that use of Wilkinson’s catalyst reversed regioselectivity to furnish the 4,5-disubstituted arenes, but was generally less tolerant of sterically demanding reactants (not shown). In work by Pérez-Castells in 2010, the second-generation Hoveyda-Grubbs catalyst was used to promote the [2+2+2] cycloaddition of 1,6-diynes with alkynes to form related ring systems (Scheme 39).202 The authors noted, however, that first-generation Grubbs catalyst delivered better results for the synthesis of more highly substituted arenes (not shown).
Scheme 39.

Use of the first-generation Grubbs catalyst and second-generation Hoveyda-Grubbs catalyst in [2+2+2] cycloadditions of 1,6-diynes with alkynes reported by Witulski and Pérez-Castells, respectively.
Although ɳ5-cyclopentadienyl complexes are used widely to catalyze a variety of transformations, an initial attempt by Kirchner to exploit [RuCp(MeCN)3]PF6 as a catalyst for alkyne [2+2+2] cycloaddition led to relatively modest results due to formation of inactive [CpRu(η6-arene)]+ complexes (not shown).203 In special cases, the parent Cp-ligand can be effective (not shown);204–206 however, corresponding ruthenium complexes bearing the ɳ5-pentamethylcyclopentadienyl or “Cp*” ligand are far more general catalysts for alkyne [2+2+2] cycloaddition. An abundance of data supports the indicated general catalytic mechanism for [2+2+2] cycloadditions catalyzed by [CpRuX] or [Cp*RuX] (Scheme 40).188 Cycloaddition is initiated by alkyne-alkyne oxidative coupling to form a ruthenacyclopentadiene207 or dicarbene ruthenacyclopentatriene species.208,209 As corroborated by single crystal X-ray diffraction data,209 the degree of ligation at ruthenium partitions formation of these structurally distinct species.188 Addition of a third alkyne to the coordinatively saturated ruthenacyclopentadiene is followed by migratory insertion to furnish a ruthenacycloheptatriene.210 In 2003, computational studies on acetylene cyclotrimerization by Kirchner and Calhorda suggest this species converts to the more stable aromatic ruthenacycloheptatetraene.210 Reductive elimination delivers CpRuCl(ɳ2-C6H6), which releases the cycloadduct to close the catalytic cycle.
Scheme 40.

Key intermediates and general mechanism of [CpRuX] and [Cp*RuX]-catalyzed [2+2+2] cycloaddition.
As demonstrated in pioneering work by Itoh and Yamamoto in 2000211 and 2003,212 exposure of non-symmetric 1,6- and 1,7- diynes to terminal alkynes in the presence of Cp*RuCl(cod) results in cycloaddition with excellent meta-selectivity, presumably due to steric influence of the Cp* ligand (Scheme 41). Reactions of homologous 1,7-diynes with 1-hexyne were inefficient, but the yield of cycloadduct could be improved by exploiting Thorpe-Ingold effects.105 In 2003, Yamamoto extended the scope of the reaction to 1,2-bis(propiolyl)benzenes, which undergo [2+2+2] cycloaddition with alkynes to form substituted anthraquinones (Scheme 41).213 Remarkably, in 2020, Tomás-Gamasa and Mascareñas performed ruthenium-catalyzed cyclotrimerizations to form anthraquinones inside live mammalian cells, demonstrating intracellular delivery of bioactive compounds that otherwise display poor cell permeability (not shown).214
Scheme 41.

Ruthenium-catalyzed [2+2+2] cycloaddition of 1,6- and 1,7-diynes with alkynes reported by Yamamoto.
In 2004, Yamamoto reported the synthesis of benzo-fused lactams and lactones through the ruthenium-catalyzed [2+2+2] cycloaddition of 1,6- or 1,7-diynes bearing amide and ester tethers with terminal alkynes (Scheme 41).215 The regioisomer in which the alkyne substituent resides para to the acyl moiety is favored due to the steric influence of the methyl group and the electron-withdrawing effect of the carbonyl.
In his 2003 study, Yamamoto also reported highly efficient Cp*RuCl(cod)-catalyzed intramolecular [2+2+2] cycloadditions of acyclic triynes to form tricyclic cycloadducts containing fused 5-, 6-, and 7-membered rings (Scheme 42).212 In 2006, the same authors applied these conditions to intramolecular [2+2+2] cycloadditions of acyclic diiodotriynes to form hexasubstituted diiodinated benzene derivatives (Scheme 42) and of diiodo-terminated 1,6-diynes and monoalkynes to form iodinated benzene derivatives bearing a wide range of functional groups (not shown).216 This capability enabled synthesis of 2,5-dihydrofuran-fused quinones from ether-tethered diiododiynes and acetylene (not shown).217 Interestingly, in a 2010 kinetic study, Yamamoto investigated the performance of a series of polymethylcyclopentadienyl ruthenium complexes in [2+2+2] cycloadditions218 and found that for reactions of diiododiyne and acetylene, 1,2,4-Me3CpRuCl(cod) and MeCpRuCl(cod) were the most efficient catalysts. Cycloaddition of the same diyne with phenylacetylene indicated steric interactions from the methyl groups on the Cp ring increase efficiency but decrease regioselectivity. A related study on the intramolecular [2+2+2] cycloadditions of diiodotriynes was reported by Aubert and Gandon in 2011 (not shown).219
Scheme 42.

Ruthenium-catalyzed intramolecular alkyne [2+2+2] cycloadditions of acyclic triynes reported by Yamamoto.
Intramolecular Cp*RuCl(cod)-catalyzed alkyne [2+2+2] cycloadditions of acyclic triynes have been utilized to great effect in the total synthesis of natural products. In 2010, Deiters reported the first total synthesis of the terpenoid cryptoacetalide using a microwave-mediated alkyne [2+2+2] cyclotrimerization to construct the central benzene ring (Scheme 43).220 Additionally, in 2011, Reisman completed the first enantioselective total synthesis of the norcaradiene-containing diterpenoid natural product (+)-salvileucalin B (Scheme 43).221
Scheme 43.

Ruthenium-catalyzed intramolecular alkyne [2+2+2] cycloadditions of acyclic triynes for the total synthesis of cryptoacetalide and (+)-salvileucalin B reported by Deiters and Reisman, respectively.
In a remarkable advance, Yamamoto demonstrated in 2004 that Cp*RuCl(cod) could catalyze the chemo- and regioselective crossed [2+2+2] cycloaddition of three different non-symmetric alkynes (Scheme 44).222–224 In this process, condensation of a propargyl alcohol with an alkynylboronate forms a 1,6-diyne-containing boronate ester, which upon oxidative coupling delivers a ruthenacyclopentadiene that inserts the terminal alkyne. The crude arylboronate underwent Suzuki-Miyaura cross-coupling with diverse aryl iodides to afford the corresponding biaryls222,224 or, as described in subsequent work, could be subjected to carbonylation conditions to form phthalides (Scheme 44).223 Independent computational studies by Kirchner210,225 and Saá226 on the mechanism of the parent Cp-ruthenium(II)-catalyzed alkyne [2+2+2] cyclotrimerization corroborate the proposed oxidative coupling-alkyne insertion pathway. Cp*RuCl(cod)-catalyzed crossed [2+2+2] cycloadditions of DMAD were studied further by Mitsudo,227,228 Teplý229 and Kotha,230 but low isomer selectivities were observed (not shown). A stunning application of Yamamoto’s crossed [2+2+2] cycloaddition of propargyl alcohols, alkynylboronate and terminal alkynes is found in Cramer’s total synthesis of the highly complex glycosylated paracyclophane natural product fijiolide A, which was reported in 2015 (Scheme 44).231 Here, steric interactions between the Cp* ligand and the TMS moiety of the propargyl alcohol at the stage of the ruthenacyclopentadiene guide the regioselectivity of terminal alkyne insertion, allowing the pentasubtituted benzene core of fijiolide A to form as a single constitutional isomer.
Scheme 44.

Chemo- and regioselective crossed [2+2+2] cycloaddition of three different non-symmetric alkynes reported by Yamamoto and Cramer’s total synthesis of fijiolide A.
In 2005, Yamamoto demonstrated that the ethynylboronate of neopentyl glycol is a competent partner for [2+2+2] cycloadditions with 1,6- and 1,7-diynes to form arylboronates (Scheme 45).232 The use of non-symmetric diynes in such cycloadditions resulted in the formation of regioisomeric mixtures (not shown). Yamamoto also showed that 1,6-diynes that incorporate the boronate moiety can react with acetylene to assemble bicyclic ortho-arylboronates (Scheme 45).233 However, when terminal alkynes were used, only a slight preference for the ortho-regioisomer was observed (not shown). In 2008, Yamamoto also showed that ethynylboronates can participate in [2+2+2] cycloadditions with diiodo-terminated 1,6-diynes to form cycloadducts that were ultimately converted to oligo(p-phenylene ethynylenes) (not shown).234
Scheme 45.

Use of alkynylboronates in ruthenium-catalyzed [2+2+2] cycloaddition of 1,6-diynes with alkynes reported by Yamamoto.
In 2006, Kotora and Hocek reported [2+2+2] cycloadditions of 1,6-diynes and 1-ethynyl-2-deoxyribose for the synthesis of C-aryldeoxyribosides (Scheme 46).235 Various transition metal complexes beyond Cp*RuCl(cod) were evaluated, including those based on Rh, Ir, Co and Ni. The most general catalyst proved to be Wilkinson’s complex, RhCl(PPh3)3. Later, Yamamoto reported the synthesis of spirocyclic C-ribosides (Scheme 46) and C-arylglycosides (Scheme 46).236,237 Use of monoiodo-terminated 1,6-diynes delivered C-(ortho-iodoaryl)ribosides and C-(ortho-iodoaryl)glycosides, which were derivatized via palladium- or copper-catalyzed C-C or C-N couplings, respectively (not shown).
Scheme 46.

Ruthenium-catalyzed [2+2+2] cycloaddition for the synthesis of C-aryldeoxyribosides, C-arylglycosides and C-ribosides reported by Kotora, Hocek, and Yamamoto.
In 2006 and 2007, Deiters explored solid-supported Cp*Ru(cod)Cl-catalyzed [2+2+2] cycloadditions of diynes and monoalkynes, enabling the generation of phthalans,238 indanones239 and, in a microwave, a variety of carbo- and heterocycles (not shown).240 In 2008, Deiters also reported regioselective microwave-mediated [2+2+2] cycloaddition of diynes and alkynes as the key step in syntheses of three cannabinoid natural products, cannabinol, cannabinol methyl ether and cannabinodiol (Scheme 47).241
Scheme 47.

Application of ruthenium-catalyzed 1,6-diyne-alkyne [2+2+2] cycloaddition for the total synthesis of cannabinol, cannabinol methyl ether and cannabinodiol reported by Deiters.
The same year, Dixneuf utilized Cp*Ru(cod)Cl for [2+2+2] cycloaddition of CF3-containing N-tethered 1,6- and 1,7-diynes with alkynes to access fluorinated bicyclic amino acid derivatives (Scheme 48).242 The cyclized products were formed in good yields over short reaction times using acetylene or terminal alkynes, but internal alkynes gave lower yields of cycloadduct. The cycloaddition could also be promoted by the Grubbs first-generation catalyst, albeit with slightly lower yields (not shown).
Scheme 48.

Cp*Ru(cod)Cl-catalyzed [2+2+2] cycloaddition of 1,6- and 1,7-diynes with alkynes reported by Dixneuf.
In 2009, Nicolaou applied a 1,6-diyne-alkyne [2+2+2] cycloaddition to the synthesis of the marine natural product sporolide B,243 and the following year, 9-epi-sporolide B (Scheme 49).244 The chemoselectivity displayed in the [2+2+2] cycloaddition is truly remarkable, as both the 1,6-diyne and alkyne partners are highly complex and incorporate numerous sensitive functional groups, including a propargyl acetate, acetylenic chloride and unprotected hydroxyl. Using Cp*RuCl(cod) as precatalyst at ambient temperature over a mere 30-minute time period, the cycloadduct in which the chloride and hydroxyalkyl side chains exist in a meta-relationship was obtained in 87% yield as a single regioisomer.
Scheme 49.

Application of ruthenium-catalyzed 1,6-diyne-alkyne [2+2+2] cycloaddition to the total synthesis of sporolide B and 9-epi-sporolide B reported by Nicolaou.
In their 2011 study,219 Aubert and Gandon also described a ruthenium-catalyzed [2+2+2] cycloaddition of 1,6-diynyl dihalides and terminal alkynes (Scheme 50). Additional reports by Sheppard and Osipov described [2+2+2] cycloadditions of various 1,6-diynes with terminal alkynes to provide polysubstituted isoindolinones245 (Scheme 50) or protected α-amino carboxylic or alkyl phosphonic acids derivatives (Scheme 50).246 In 2016, Kotora described the reaction of cyclopropyl-terminated 1,6-diynes with alkynes to form cyclopropylarenes, but yields and regioselectivities were generally poor (not shown).247
Scheme 50.

Ruthenium-catalyzed [2+2+2] cycloadditions of 1,6-diynes with alkynes reported by Aubert and Gandon, Sheppard and Osipov.
In 2018, Goswami reported ruthenium-catalyzed cycloadditions of 1,6-diynes with acetylenic nitriles (Scheme 51).248 The diyne, catalyzed by Cp*RuCl(cod), couples chemoselectively with the acetylene moiety in alkynylnitrile to deliver fused cyanoarenes as a single regioisomer. Interestingly, the chemoselectivity can be altered by adding a catalytic amount of AgOTf, resulting in the formation of 2-alkynylpyridines instead (vida infra, Scheme 62). The authors suggest that the electron-deficient cationic ruthenium complex formed by AgOTf and ruthenium chloride prefers to coordinate with the electron-rich nitrile, giving 2-alkynylpyridine as the sole cycloadduct.
Scheme 51.

Ruthenium-catalyzed [2+2+2] cycloadditions of 1,6-diynes with acetylenic nitriles reported by Goswami.
Scheme 62.

Ruthenium-catalyzed [2+2+2] cycloadditions of 1,6- and 1,7-diynes with nitriles reported by Goswami.
Reports of silacycle formation via ruthenium-catalyzed [2+2+2] cycloaddition are conspicuously absent from the literature. In 2020, in the course of experimentally and theoretically contrasting the mechanisms of cobalt and ruthenium cyclotrimerization, Kabe attempted to form silacycles from bispropargyl silanes and the homologous disilanes with alkynes (Scheme 52).249 Among the alkynes surveyed, preparatively useful yields were only observed in reactions of dimethyl acetylenedicarboxylate. Thus, general methods for silacycle formation via ruthenium-catalyzed [2+2+2] cycloaddition remain an unmet challenge.
Scheme 52.

Ruthenium-catalyzed [2+2+2] cycloadditions of silicon-containing 1,6- and 1,7-diynes with DMAD reported by Kabe.
Beyond the standard Cp*-ruthenium(II) complexes, other structurally distinct ruthenium complexes have proven to be effective catalysts for alkyne [2+2+2] cycloaddition. For example, in 2006, Cadierno and Gimeno demonstrated that the indicated bis(allyl)-ruthenium(IV) dimer could catalyze fully intermolecular alkyne [2+2+2] cycloadditions in aqueous media, albeit with poor levels of regioselectivity (Scheme 53).250 The same authors utilized microwave irradiation in lieu of thermal heating, which allowed the reactions to reach completion in only 5 minutes (not shown).251
Scheme 53.

Ruthenium(IV) and ruthenium(0) precatalysts for intermolecular alkyne [2+2+2] cycloadditions reported by Cadierno and Gimeno and Itoh and Kawatsura.
In 2007, Grigg introduced a cyclometallated N-phenylpyrazole ruthenium complex as a pre-catalyst for [2+2+2] cycloaddition of propargylic alcohols and symmetric 1,6-diynes (not shown).252 In 2011, using a ruthenium(0) precatalyst in combination with ortho-(diphenylphosphino)benzonitrile (2-DPPBN), Itoh and Kawatsura reported highly regioselective intermolecular alkyne [2+2+2] cycloadditions of CF3-substituted aryl alkynes (Scheme 53).253 Isolation of a catalytically competent ruthenacyclopentadiene, which was characterized by single crystal X-ray diffraction, corroborates a mechanism involving alkyne-alkyne oxidative coupling to form a ruthenacyclopentadiene followed by successive insertion-C-C reductive elimination of the third alkyne.188 In contrast, in 2012 Holthausen and Ghosh explored the use of arachno-[(Cp*RuCO)2B2H6] as a catalyst for intermolecular alkyne cyclotrimerization, and their computational studies implicated a mechanism in which the transient ruthenacyclopentadiene is converted to a benzene derivative through a Diels-Alder-type [4+2] cycloaddition with the third alkyne (not shown).254 Finally, in 2015 and 2016, Ratovelomanana-Vidal and Michelet developed a cost-effective intermolecular [2+2+2] cycloaddition of α,ω-diynes with alkynes to construct benzene and fluorenone derivatives, catalyzed by RuCl3·nH2O in the absence of ligand under solvent-free conditions (not shown).255,256
3.2.3. [2+2+2] Cycloadditions of Alkynes + Alkenes
Whereas the [2+2+2] cycloaddition of three alkynes provides substituted benzene derivatives, entry to cyclohexadienes and cyclohexenes is achieved via corresponding [2+2+2] cycloadditions of alkynes with alkenes. The first ruthenium-catalyzed cycloadditions of this type were described by Itoh and Yamamoto in 1998.257,258 Their initial reports were on reactions of 1,6-diynes with norbornene using CpRu(cod)Cl and the indenyl complex, (ɳ5-C9H7)Ru(PPh3)2Cl; however, the resulting cyclohexadiene was susceptible to further [4+2] cycloaddition with norbornene, which led to low isolated yields (not shown).257 Using Cp*Ru(cod)Cl as pre-catalyst, the authors later demonstrated that [2+2+2] cycloadditions of 1,6-diynes with cyclic and acyclic allylic ethers could be conducted in a highly efficient manner (Scheme 54).258,259
Scheme 54.

Ruthenium-catalyzed [2+2+2] cycloadditions of 1,6-diynes with allylic ethers and other cyclic and acyclic alkenes reported by Itoh and Yamamoto and Saá.
The authors posit that ether oxygen-assistance facilitates olefin insertion, and the steric demand of the Cp* ligand facilitates dissociation of the cycloadduct to increase turn-over number. Interestingly, cycloaddition of 1,6-heptadiyne with allyl benzyl ether refsulted in formation of the regioisomeric cyclohexadiene, which the authors postulate arises from a 1,5-hydride shift of the initially formed cycloadduct. An alternate mechanism corroborated by experimental and computational studies was proposed by Saá, in which acyclic alkenes undergo β-hydride elimination followed by C-H reductive elimination to a cyclohexatriene that forms the regioisomeric cyclohexadiene via disrotatory 6π-electrocyclization.260 In 2006, Saá demonstrated that cationic ruthenium precatalyst, [Cp*Ru(MeCN)3]PF6, promotes the [2+2+2] cycloaddition of 1,6-diynes with diverse alkenes (Scheme 54).261 In 2008, Saá expanded the scope of the reaction to include non-symmetric diynes and disubstituted alkenes, which undergo [2+2+2] cycloaddition with high levels of regio- and trans-diastereoselectivity (Scheme 54).262
In 2000, Itoh and Yamamoto demonstrated for the first time the superior performance of the dinuclear ruthenium(III) complex [Cp*RuCl2]2 as a catalyst for the [2+2+2] cycloaddition of 1,6-diynes with heterocyclic alkenes (not shown).259 In 2010, Wu reported that [Cp*RuCl2]2 was an effective catalyst for reductive [2+2+2] cycloadditions of 1,7-diaryl-1,6-heptadiynes with norbornadiene via 2-propanol-mediated transfer hydrogenation (Scheme 55).263 The cyclohexene-containing cycloadducts are formed with complete levels of diastereoselectivity. Exposure of the cycloadducts to the first-generation Grubbs catalyst promotes ring-opening metathesis polymerization to form polynorbornenes (not shown).
Scheme 55.

Ruthenium-catalyzed reductive [2+2+2] cycloaddition of 1,6-heptadiynes with norbornadiene reported by Wu.
In 2002, Mitsudo reported the [2+2+2] cycloaddition of electron-deficient 1,6-diynes or dialkyl acetylenedicarboxylates with allyl alcohol using a ruthenium complex derived from Cp*RuCl(cod) and PPh3 (Scheme 56).264 Reactions of 1,6-diynes delivered the anticipated cyclohexadiene. However, under the same conditions, dimethyl acetylenedicarboxylates react with allyl alcohol to form benzene tetracarboxylates (Scheme 56). The authors propose a mechanism involving alkyne-allyl alcohol oxidative coupling to form a ruthenacyclopentene, which upon β-hydroxy elimination and insertion of a second equivalent of alkyne provides an acyclic vinylruthenium complex. Migratory insertion of the tethered alkene and subsequent aromatization via β-hydride elimination-alkene isomerization deliver the cycloadduct.
Scheme 56.

Ruthenium-catalyzed [2+2+2] cycloaddition of electron-deficient 1,6-diynes or DMAD with allyl alcohol reported by Mitsudo.
Beyond 1,6-diynes, 1,5- and 1,6-enynes are also competent partners for [2+2+2] cycloaddition. In 2014, Esteruelas and Saá reported [2+2+2] cycloadditions of 1,5-enynes (ortho-alkenylarylacetylenes) catalyzed by the cationic ruthenium complex [Cp*Ru(MeCN)3]PF6 to form dihydrobiphenylenes (Scheme 57).265 While in the absence of exogenous alkyne the 1,5-enyne undergoes dimerization (not shown), in the presence of terminal alkynes crossed [2+2+2] cycloaddition of the 1,5-enyne with the alkyne becomes the dominant reaction pathway. In 2017, Tenaglia reported a Cp*RuCl(cod)-catalyzed [2+2+2] cycloaddition of 1,6-enynes with both internal and terminal alkynes to provide bicyclohexa-1,3-dienes (Scheme 57).266 This process displayed impressive chemoselectivity, as highlighted by compatibility with propargyl chloride and propargyl acetate functional groups. DFT calculations by Liu indicate that 1,6-enyne undergoes oxidative coupling to form a ruthenacyclopentene that undergoes regio-determining insertion of exogenous alkyne at the vinylic ruthenium-carbon bond.267
Scheme 57.

Ruthenium-catalyzed [2+2+2] cycloaddition of 1,5- and 1,6-enynes with alkynes reported by Esteruelas and Saá and Tenaglia, respectively.
Fully intermolecular crossed [2+2+2] cycloadditions are highly uncommon. In 2012, Wang, Zhao and Shi reported crossed [2+2+2] cycloadditions of internal alkynes, dimethyl acetylene dicarboxylate and ethylene to form substituted ortho-phthalates using the second-generation Grubbs catalyst (Scheme 58).268 In these processes, enyne metathesis of the internal alkyne with acetylene delivers a conjugated diene, which is subsequently treated with dimethyl acetylene dicarboxylate and then DDQ to furnish a cyclohexadiene. Notably, using CuI or AgOTf as additives, regioisomeric ortho-phthalates were formed. In 2015, Pérez-Castells also explored the use of various Grubbs-type catalysts in [2+2+2] cycloadditions of 1,6-diynes with cyclic and acyclic alkenes; however, these reactions gave mixtures of cycloadducts in low yields (not shown).269
Scheme 58.
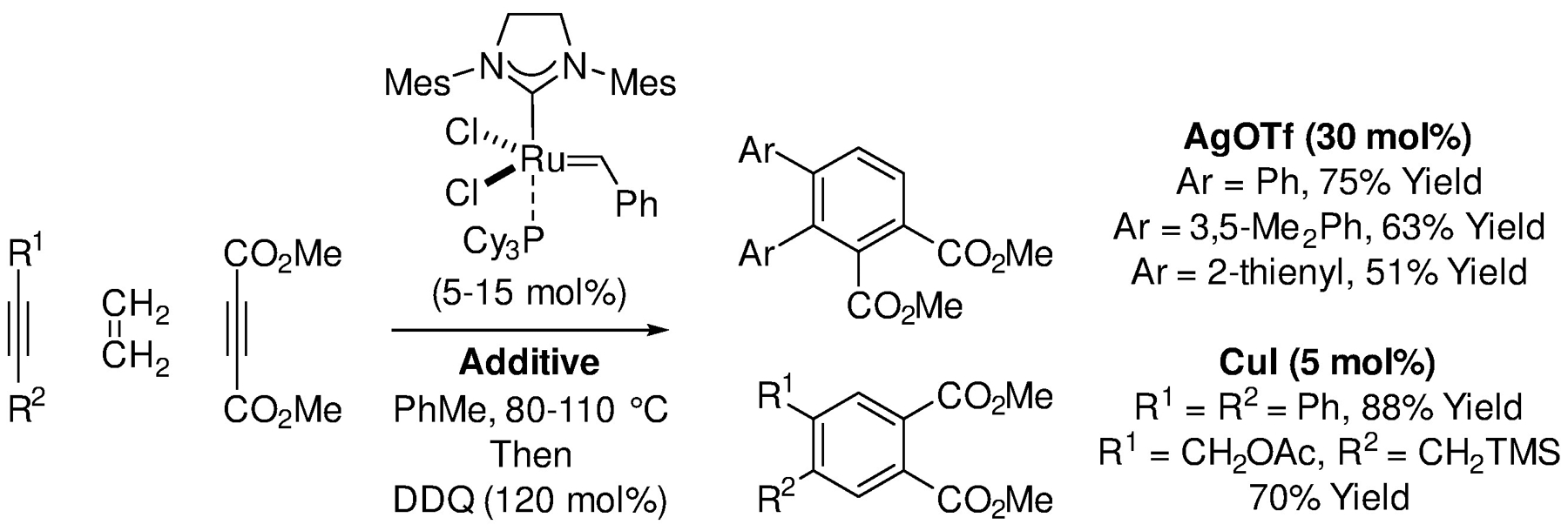
Ruthenium-catalyzed intermolecular crossed [2+2+2] cycloaddition of internal alkynes, DMAD and ethylene reported by Wang, Zhao, and Shi.
In contrast, several fully intramolecular [2+2+2] cycloadditions of alkynes with alkenes have been described. In the aforementioned 2003 study by Yamamoto, a [2+2+2] cycloaddition of a 1,6,11-enediyne was reported.212 Rather than obtaining the expected cyclohexadiene, however, a dehydroaromatization product was observed in low yield (Scheme 59). High temperatures were necessary and side reactions from competing intermolecular [2+2+2] cycloadditions were not observed. The authors isolated a cationic ruthenium complex from the reaction mixture in which the cycloadduct is bound to ruthenium as an η6-arene ligand, suggesting product inhibition may contribute to low conversion. In 2007, Sato and Mori reported a fully intramolecular [2+2+2] cycloaddition of 1,6,11-dienynes using Cp*RuCl(cod) (Scheme 59).270 This reaction proceeds via enyne oxidative coupling to form a ruthenacyclopentene followed by insertion of the second olefin. C-C Reductive elimination from the resulting ruthenacycloheptene delivers the tricyclic product. Finally, in 2012, Saito and Sato reported fully intramolecular [2+2+2] cycloadditions of allene-yne-enes to form fused tricycles (Scheme 59).271 Relative stereochemistry at the ring juncture and geometry of the (Z)-alkene were corroborated by single crystal X-ray diffraction analysis.
Scheme 59.

Intramolecular ruthenium-catalyzed [2+2+2] cycloadditions of alkynes with alkenes and allenes reported by Itoh and Yamamoto, Sato, Mori and Saito.
3.3. [2+2+2] Cycloadditions to Form Heterocycles
3.3.1. [2+2+2] Cycloadditions of 2 Alkynes + Cumulenes
In 2001, Itoh and Yamamoto reported Cp*RuCl(cod)-catalyzed [2+2+2] cycloaddition of 1,6-diynes with isocyanate to form diverse bicyclic pyridones (Scheme 60).272 Expanding the scope of the reaction to include isothiocyanates and carbon disulfide, the authors demonstrated the ability to convergently access sulfur-containing heterocycles (Scheme 60).273 As with related alkyne-mediated [2+2+2] cycloadditions, the Cp* ligand was uniquely effective for tranformations of this type, which proceed via oxidative coupling of the 1,6-diyne to form a ruthenacyclopentadiene followed by heterocumulene insertion and C-X reductive elimination (X = heteroatom).274 A theoretical study of the origins of chemoselective heterocumulene insertion in cycloadditions of 1,6-diynes with isocyanates and thioisocyanates was performed by Kirchner in 2003.275 Migratory insertion of the C=X π-bond (X = N, O, S) into the ruthanacycle is initiated by η1 attack at ruthenium by the heteroatom X, which is incorporated into the cycle. For isocyanates N attack is preferred over O, and for thioisocyanates S attack is irreversible. In 2005, Itoh and Yamamoto demonstrated that non-symmetric diynes participate in highly regioselective [2+2+2] cycloadditions with diverse cumulene partners (Scheme 60).276 Reactions of this type also can be conducted using the second-generation Hoveyda-Grubbs catalyst, as reported in 2013 by Pérez-Castells (not shown).277
Scheme 60.

Ruthenium-catalyzed cycloaddition of 1,6-diynes with heterocumulenes reported by Itoh and Yamamoto.
3.3.2. [2+2+2] Cycloadditions of 2 Alkynes + Nitriles
1,6-Diynes undergo chemoselective ruthenium-catalyzed [2+2+2] cycloaddition with nitriles to form substituted pyridines. The first ruthenium-catalyzed cycloaddition of this type was reported by Itoh and Yamamoto in 2001, who utilized Cp*RuCl(cod) to promote the [2+2+2] cycloaddition of 1,6-diynes with dicyanides to form pyridines bearing pendant nitriles (Scheme 61).278 As illustrated in corresponding reactions of electron-deficient nitriles, an inversion of regioselectivity is observed upon use of 1,6-diynes that are terminally substituted by an electron-withdrawing group. The authors posit that for alkyl- and aryl-substituted 1,6-diynes, alkyne-nitrile oxidative coupling to form an azaruthenacyclopentadiene occurs at the monosubstituted alkyne, whereas electron-deficient alkynes preferentially participate in oxidative coupling (Scheme 61).279 By introducing a carbonyl moiety at the 3-position of the 1,6-diyne, Yamamoto and Itoh were able to control regioselectivity in [2+2+2] cycloadditions to form azafluorenones and related heterocycles (Scheme 61).276 In later work, the authors demonstrated that nitriles bearing α-heteroatoms or other activating groups are essential for cycloaddition, as unactivated nitriles such as acetonitrile fail to participate (Scheme 61).280,281 Taking advantage of these electronic effects, Itoh and Yamamoto conducted [2+2+2] cycloadditions 1,6-diynes with chloroacetonitriles for the synthesis of C-arylribosides,236 and Kotora performed [2+2+2] cycloadditions of halogen-terminated 1,6-diynes with nitriles to form halopyridines (not shown).282 Additional studies demonstrate that Cp*RuCl(cod)-catalyzed [2+2+2] cycloadditions of 1,6-diynes with nitriles can be conducted in air,283 water284 and even fetal bovine serum (not shown).229
Scheme 61.

Ruthenium-catalyzed [2+2+2] cycloadditions of 1,6-diynes with nitriles reported by Itoh and Yamamoto.
In 2017, using Cp*RuCl(cod) as a precatalyst, Goswami reported [2+2+2] cycloadditions of 1,6-diynes with N-cyanoindoles to form N-(2-pyridyl)indoles (Scheme 62).285 The same year, Goswami reported related [2+2+2] cycloadditions of 1,6-diynes with 3-thiocyanatoindoles to form 3-(2-thiopyridyl)indoles (Scheme 62).286 Later in 2019, Goswami described analogous [2+2+2] cycloadditions of 1,6-diynes with selenocyanates or aryl cyanates to form selenopyridines287,288 and 2-aryloxypyridines,289 respectively (not shown). In 2018, in [2+2+2] cycloadditions of 1,6-diynes with acetylenic nitriles, Goswami found that neutral Cp*Ru catalysts provide benzonitriles (vide supra, Scheme 51), whereas cationic Cp*Ru catalysts (generated in situ from Cp*RuCl(cod) and AgOTf) provide 2-alkynylpyridines (Scheme 62).248 In 2020, the same authors were able to perform sequential cationic ruthenium-catalyzed [2+2+2] cycloadditions of alkynylthiocyanates with 1,6-diynes, chemoselectively forming aryl thiocyanates and then 2-arylthiopyridines (not shown).290
Beautiful applications of the Cp*RuCl(cod)-catalyzed [2+2+2] cycloaddition of 1,6-diynes with electron-deficient nitriles in target-oriented synthesis have been accomplished. In 2011, Witulski completed the total synthesis of the β-carboline-containing natural product eudistomin U via [2+2+2] cycloaddition of the indicated 1,6-yne-ynamide with methylcyanoformate (Scheme 63).291 In 2011, Nissen reported the formal synthesis of the antitumor antibiotic lavendamycin employing a similar [2+2+2] cycloaddition of a 1,6-yne-ynamide with methylcyanoformate (Scheme 63).292 In 2012, Mori prepared several N,N,N’,N’-tetrakis[(2-pyridylmethyl)ethylenediamine] (TPEN) ligands using the Cp*RuCl(cod)-catalyzed [2+2+2] cycloaddition of 1,6-diynes with bromoacetonitriles (not shown).293
Scheme 63.

Applications of ruthenium-catalyzed [2+2+2] cycloadditions of 1,6-diynes with nitriles in natural product synthesis reported by Witulski and Nissen.
Beyond Cp*RuCl(cod), RuCl3∙H2O has been found to catalyze the [2+2+2] cycloaddition of 1,6-diynes with cyanamides to form 2-aminopyridines (Scheme 64).294 The cationic ruthenium complex Cp*Ru(MeCN)3PF6 also catalyzes the [2+2+2] cycloaddition of 1,6- and 1,7-diynes with cyanamides295–298 or selenocyanates299 to form 2-aminopyridines or 2-selenopyridines, respectively, as illustrated in a series of reports by Michelet and Ratovelomanana-Vidal (not shown). Finally, the second-generation Hoveyda-Grubbs catalyst has been shown to be an effective and chemoselective promotor of the [2+2+2] cycloaddition of 1,6-diynes with nitriles, even generating pyridines containing highly sensitive 2-chloromethyl substituents (Scheme 64).300
Scheme 64.

Ruthenium-catalyzed [2+2+2] cycloadditions of α,ω-diynes with nitriles reported by Pérez-Castells and Michelet and Ratovelomanana-Vidal, respectively.
Fully intermolecular ruthenium-catalyzed [2+2+2] cycloadditions of alkynes with nitriles remain exceedingly uncommon, with only a single report from the laboratory of Saá appearing in the literature. Taking advantage of the superior reactivity displayed by electron-deficient alkynes and nitriles in related intramolecular processes, and using Cp*Ru(MeCN)3PF6 as precatalyst, the 2,3,6-trisubstituted pyridines could be generated with high levels of regioselectivity (Scheme 65).301
Scheme 65.

Intermolecular ruthenium-catalyzed [2+2+2] cycloadditions of alkynes with nitriles reported by Saá.
3.3.3. [2+2+2] Cycloadditions of 2 Alkynes/Alkenes + Carbonyl Compounds
Due to the weaker coordinating ability of oxygen relative to nitrogen, [2+2+2] cycloadditions of alkynes with carbonyl partners are far less developed than analogous processes involving nitriles or isocyanates. However, some progress in this area has been made. In 2002, Yamamoto and Itoh reported ruthenium-catalyzed [2+2+2] cycloadditions of 1,6-diynes with diethyl ketomalonate (Scheme 66).302 The initially formed pyran undergoes cycloreversion to form the indicated dienyl ketone. The authors proposed a mechanism involving alkyne-carbonyl oxidative coupling to form an oxaruthenacyclopentene that inserts the tethered alkyne. Subsequent computational studies by Rodríguez-Otero in 2009, however, implicate a mechanism involving oxidative cyclization of the 1,6-diyne to form a ruthenacyclopentadiene followed by carbonyl insertion and C-O reductive elimination to form the pyran.303 In 2017, in a combined experimental and computational study, Yamamoto expanded the scope of the ruthenium-catalyzed [2+2+2] cycloadditions of 1,6-diynes to aryl aldehydes (Scheme 66).304 The resulting pyrans again suffered cycloreversion to provide dienyl ketones, and the computational studies corroborated the general features of the aforesaid catalytic mechanism calculated by Rodríguez-Otero.303 Whereas aryl-substituted diynes delivered the dienyl ketones as mixtures of alkene geometrical isomers, alkyl-substituted diynes displayed increasing alkene (Z)-stereoselectivity with increasing size of the alkyl substituent (Scheme 66).304 In contrast, the same reaction catalyzed by [Rh(cod)2]BF4/H8-BINAP preferentially gives the (E)-isomer.305
Scheme 66.
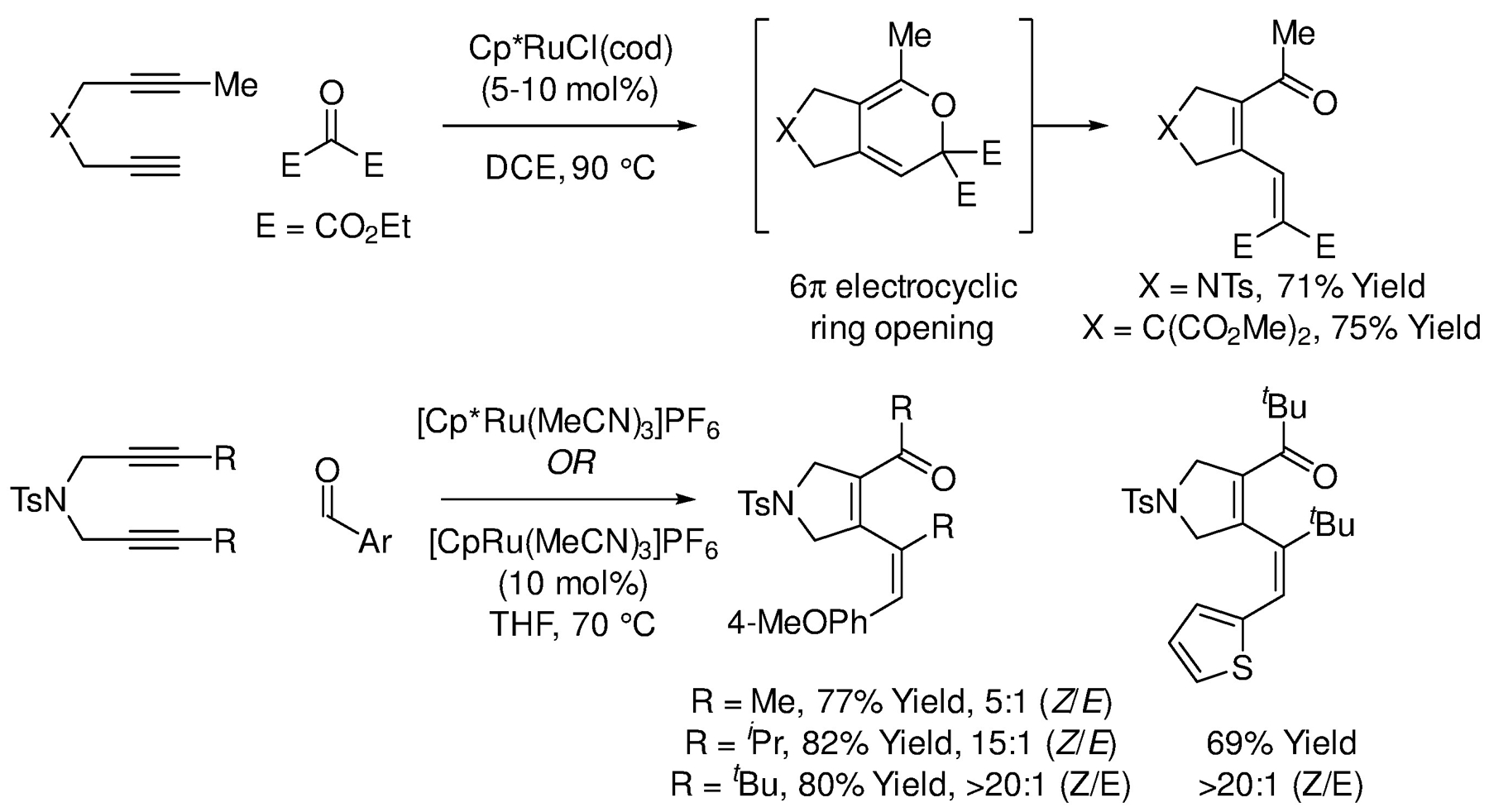
Ruthenium-catalyzed [2+2+2] cycloaddition of 1,6-diynes and carbonyls reported by Yamamoto and Itoh.
In 2016, Krische reported the first use of 1,2-diones as 22π components in transition metal-catalyzed [2+2+2] cycloadditions, as illustrated in reactions of 1,6-diynes (Scheme 67).306 A remarkable feature of these processes resides in the ability to conduct the cycloaddition using diols, ketols or diones as equivalent cycloaddition partners. Reactions of diols are oxidative processes in which one equivalent of diyne is sacrificed as a hydrogen acceptor. Reactions of α-ketols occur under identical conditions and are redox-neutral. Reactions conducted using diones are reductive processes and exploit 2-propanol as the source of hydrogen. The cycloaddition is initiated via oxidative cyclization of the 1,6-diyne to form a ruthenacyclopentadiene. Successive carbonyl insertions of the dione are followed by diol- or ketol- or 2-propanol-mediated transfer hydrogenonlysis of the resulting oxaruthenacycle to release product and return ruthenium to its zero-valent form. A carboxylic acid cocatalyst (adamantane carboxylic acid) was found to increase rate and conversion by catalyzing alcohol exchange and the transfer hydrogenolysis of transient oxaruthenacycles.154,155 In 2020, this method was applied to the synthesis of diindenoperylenes (periflanthenes) (not shown).307
Scheme 67.

Ruthenium-catalyzed [2+2+2] cycloaddition of 1,6-diynes with diones reported by Krische.
3.4. (3+2+1) Cycloadditions
Six-membered ring formation via ruthenium-catalyzed (3+2+1) cycloadditions is highly uncommon and, to our knowledge, only a single transformation of this type has been described. Following the development of carbonylative [2+2+1+1] cycloadditions by Ryu and Mitsudo that result in the generation of hydroquinones,170 in 2007 Fukuyama and Ryu discovered that enones participate in ruthenium(0)-catalyzed carbonylative (3+2+1) cycloadditions with silylacetylenes to furnish α-pyrones (Scheme 68).308 The authors propose that the addition of substoichiometric quantities of ammonium salt plays a key role in promoting formation of a ruthenium hydride. Enone hydrometalation followed by alkyne carboruthenation delivers a vinylruthenium species, which upon insertion of carbon monoxide forms an acylruthenium complex. Cyclization onto the tethered ketone with subsequent β-hydride elimination delivers the α-pyrone and regenerates the ruthenium hydride to close the catalytic cycle.
Scheme 68.

Ruthenium-catalyzed carbonylative (3+2+1) cycloaddition of silylacetylenes with enones reported by Fukuyama and Ryu.
3.5. [4+2] Cycloadditions
3.5.1. Transfer Hydrogenative Cycloadditions
In connection with their exploration of carbonyl reductive couplings via metal-catalyzed hydrogenation, transfer hydrogenation and hydrogen auto-transfer,309–315 the Krische group has developed a unique class of ruthenium(0)-catalyzed [4+2] cycloadditions in which 1,2-diones serve as 22π components.32,34 In these transfer hydrogenative cycloadditions, 1,2-diones can be used directly in combination with a reductant, or they can be generated via dehydrogenation of 1,2-diols or 1,2-ketols, which represent oxidative and redox-neutral cycloadditions, respectively. Their initial 2013 report describes a phosphine-modified ruthenium(0) catalyst for the [4+2] cycloaddition of feedstock dienes with 1,2-diones from the alcohol, ketol or dione oxidation levels (Scheme 69).316,317 Cyclic or acyclic cis- or trans-diols are all competent partners for cycloaddition, delivering the diol-containing cycloadducts as single diastereomers. The cycloaddition is initiated by diene-dione oxidative coupling to form an oxaruthenacycle. Protonolytic cleavage of the ruthenium-oxygen bond (mediated by diol or ketol) is followed by intramolecular carbonyl allylruthenation. The resulting ruthenium alkoxide undergoes β-hydride elimination and O-H reductive elimination to release the cycloadduct along with ketol or dione to be used in the next turnover of the catalytic cycle. In related mechanistic studies, the putative oxaruthenacycle was isolated and its reversible formation was demonstrated.318 In subsequent work, the diol-containing cycloadducts were subjected to iodosobenzene diacetate mediated oxidative cleavage to form 9- to 12-membered 1,6-diketones (not shown).317 Finally, a significant expansion in scope was realized through the use of carboxylic acid cocatalysts,154,155 enabling [4+2] cycloadditions of cyclohexadiene to form bridged carbocycles with complete levels of exo-selectivity (Scheme 69).181
Scheme 69.

Ruthenium-catalyzed [4+2] cycloaddition of 1,3-dienes with 1,2-diols as dione precursors reported by Krische.
The ruthenium(0)-catalyzed transfer hydrogenative diene-dione [4+2] cycloaddition delivers diol-containing cycloadducts that are readily aromatized via acid-catalyzed dehydration (or deoxydehydration), thus opening new benzannulation strategies for the synthesis of polycyclic aromatic hydrocarbons (PAH). For example, bis-1,2-diols participate in two-directional [4+2] cycloaddition-dehydration to furnish acenes and, as shown, indeno[1,2,3-cd]-fluoranthenes (Scheme 70).319 Similarly, oligo(p-phenylene ethylene glycols) are subject to triple [4+2] cycloaddition followed by exhaustive dehydration to furnish alternating oligo(o,p-phenylenes) (Scheme 70).320 The Krische laboratory has applied benzannulation strategies of this type to the construction of nanographenes,320 triple helical oligo(phenylene) cages,321 as well as all-aryl caged fac-Ir(ppy)3 analogs (not shown).322
Scheme 70.
Benzannulation via ruthenium-catalyzed [4+2] cycloaddition of 1,3-dienes with 1,2-diols as dione precursors reported by Krische.
In 2015, Krische reported a related ruthenium(0)-catalyzed [4+2] cycloaddition of 3,4-benzannulated 1,5-diynes with 1,2-ketols (Scheme 71).323 Use of diols as vicinal dicarbonyl precursors requires a sacrificial hydrogen acceptor, which led to competing transfer hydrogenation of the diyne reactant. Hence, the reaction was conducted using 1,2-ketols in a redox-neutral manner. Using a ruthenium catalyst derived from Ru3(CO)12 and RuPhos, cycloadducts bearing two trisubstituted alkenes were formed as single geometrical isomers through a mechanism involving consecutive alkyne-carbonyl oxidative coupling. To enforce head-to-tail regioselectivity, non-symmetric diynes terminally substituted by n-propyl and t-butyl groups were employed. Based on this pattern of reactivity, a mechanistically related ruthenium(0)-catalyzed [4+2] cycloaddition of ortho-acetylenic benzaldehydes was developed in which 1,2-ketols serve as latent α-ketoaldehydes (Scheme 72).324
Scheme 71.

Ruthenium-catalyzed [4+2] cycloaddition of 3,4-benzannulated 1,5-diynes with 1,2-ketols as dione precursors reported by Krische.
Scheme 72.

Ruthenium-catalyzed [4+2] cycloaddition of ortho-acetylenic benzaldehydes with 1,2-ketols as α-ketoaldehyde precursors reported by Krische.
In 2017, Krische developed a ruthenium(0)-catalyzed [4+2] cycloaddition of benzocyclobutenones with 1,2-diols to deliver cycloadducts in which adjacent diol carbon atoms undergo formal insertion into the strained C-C bond (Scheme 73).325 As in the preceding transfer hydrogenative [4+2] cycloadditions, the diol suffers dehydrogenation to form a transient dione. The benzocyclobutenone serves as a masked ortho-ketene methide that reacts with ruthenium(0) through a 1,4-oxidative addition to form a ruthenaindanone. Successive carbonyl addition to the dione is followed by transfer hydrogenolysis of the resulting oxaruthancycle to release the cycloadduct and return ruthenium to its zero-valent form. For cycloadditions that proceed through the intermediacy of non-symmetric diones, complete levels of head-to-tail regioselectivity are observed. Additionally, as illustrated in cycloadditions catalyzed by (R)-DM- SEGPHOS-modified ruthenium(0) complexes, the stereoablative nature of the cycloreversion event allows chiral racemic benzocyclobutenones to be transformed to highly enantiomerically enriched cycloadducts via dynamic kinetic resolution (Scheme 73).326
Scheme 73.

Ruthenium-catalyzed [4+2] cycloaddition of benzocyclobutanones with 1,2-diols or 1,2-ketols as 1,2-dione precursors reported by Krische.
3.5.2. Formal Diels-Alder Type [4+2] Cycloadditions
In 2000, Trost discovered an intramolecular ruthenium catalyzed [4+2] cycloaddition of an yne-enone to form a fused pyran ring (Scheme 74).327 The authors propose that the reaction proceeds through a ruthenacycle intermediate, with interconversion between five-membered C-bound and seven-membered O-bound enolates. From the O-bound enolate, reductive elimination delivers the desired cycloadduct. In aqueous conditions, the corresponding 1,5-diketone is formed (not shown). In 2015, Cramer used a chiral ruthenium catalyst developed in his laboratory to accomplish the same transformation; enantioselective ruthenacycle formation selectively gave the pyran products (Scheme 74).328 Interestingly, when a Weinreb amide was used in place of a ketone, an unstable cycloadduct formed under the reaction conditions and was directly converted into a dihydropyranone (not shown).
Scheme 74.

Ruthenium-catalyzed intramolecular [4+2] cycloaddition of acetylenic enones reported by Trost and Cramer.
In 2004, Mitsudo reported a ruthenium-catalyzed [4+2] cycloaddition of cyclobutenones that results in the formation of α-pyrones (not shown).329 The authors propose a mechanism involving cycloreversion of the cyclobutenone to form a transient vinyl ketene, which participates in a hetero-Diels-Alder type [4+2] cycloaddition to form the α-pyrone. The proposed mechanism of this process does not involve intervention of organometallic intermediates and, technically, falls outside the purview of this monograph.
3.6. (5+1) Cycloadditions
In 2000, Murai developed a ruthenium-catalyzed carbonylative (5+1) cycloaddition of cyclopropyl imines to form γ,δ-unsaturated lactams (Scheme 75).330 Ru3(CO)12 was singularly effective, as other ruthenium complexes (e.g. Cp*Ru(cod)Cl, Ru(CO)2(PPh3)3, RuH2(CO)(PPh3)3) or metal carbonyl clusters such as Co2(CO)8 did not promote cyclocarbonylation. The cycloaddition was limited to N-cyclohexyl- or N-tert-butyl-substituted cyclopropyl imines. The requisite imines could be formed in situ from the corresponding cyclopropyl ketones and alkylamines, but diminished yields were obtained. The authors propose a mechanism in which oxidative addition of the cyclopropyl imine to ruthenium(0) occurs prior to CO insertion and subsequent C-C reductive elimination to form the lactam. Moderate efficiencies were determined to arise from competing β-hydride elimination of the azaruthenacycle to form an inactive ruthenium-alkene complex (not shown).
Scheme 75.

Ruthenium-catalyzed carbonylative (5+1) cycloadditions of cyclopropyl imines by Murai.
4. Seven-Membered Ring Formation
4.1. (3+2+2) Cycloadditions
In 2016, Wang and Wan reported a Cp*RuCl(cod)-catalyzed (3+2+2) cycloaddition of 2H-azirines with 1,6-diynes for the construction of azepines (Scheme 76).331 The authors propose a mechanism involving oxidative coupling of the 1,6-diyne to form a ruthenacyclopentadiene, which upon insertion of the imino moiety of 2H-azirine and subsequent β-carbon elimination forms an 8-membered ruthenacycle. Reductive elimination followed by a 1,5-hydride shift generates the azepine ring. Although diverse aryl-substituted 2H-azirine could be employed, substitution at the alkyne termini was not tolerated. In 2018, DFT calculations on this cycloaddition were conducted by Liu, who concluded that the rate-determining step is reductive elimination, in agreement with experimental results.332
Scheme 76.

Ruthenium-catalyzed (3+2+2) cycloaddition of 1,6-diynes with 2H-azirines reported by Wang and Wan.
4.2. (5+2) Cycloadditions
As summarized in the review literature,39,333 the Trost laboratory discovered and developed intramolecular ruthenium-catalyzed (5+2) cycloadditions of alkynes with vinyl cyclopropanes to form fused seven-membered rings (Scheme 77).334–337 In an initially proposed mechanism from their 2000 report,334 which was later corroborated via DFT calculations by Houk,335 coordination of cationic ruthenium to the 1,6-enyne triggers oxidative coupling to form a ruthenacyclopentene. β-Carbon elimination cleaves the cyclopropyl C-C bond delivering an eight-membered ruthenacycle, which upon C-C reductive elimination provides the cycloadduct. Substrate-directed regio- and diastereoselectivity was observed for reactants bearing substituents at the 2-position of the cyclopropane ring. Specifically, cyclopropanes that incorporate a silyloxymethyl group are transformed to the indicated cis-cycloadduct. In contrast, the corresponding aldehyde is transformed to the regioisomeric trans-cycloadduct (Scheme 77). Further investigations into the origins of regioselectivity of (5+2) cycloaddition336 led the authors to conclude that for 1,2-cis-disubstituted cyclopropanes (notwithstanding aldehyde substituents), the less substituted C-C bond cleaves to form the eight-membered ruthenacycle, suggesting steric factors dominate regioselectivity. However, for 1,2-trans-disubstituted cyclopropanes, the more substituted C-C bond is cleaved, suggesting electronic effects dominate regioselectivity (not shown). In still deeper investigations, Trost examined the factors influencing diastereoselectivity in (5+2) cycloadditions of chiral cyclopropyl enynes to form hexahydroazulenes (Scheme 78).337 A stereochemical model based on the Stork/Houk-Jäger “inside alkoxy” effect338–340 was posited. The indicated reactive conformer avoids overlap between the C-O σ* orbital and the alkene π orbital, which would diminish the coordinating ability of the alkene. Oxidative coupling from this conformer ultimately leads to an anti-relationship between the homoallylic oxygen and the bridgehead hydrogen in the cycloadduct.
Scheme 77.

Ruthenium-catalyzed intramolecular (5+2) cycloadditions of alkynes with vinyl cyclopropanes reported by Trost.
Scheme 78.

Diastereoselectivity in ruthenium-catalyzed intramolecular (5+2) cycloadditions of alkynes with vinyl cyclopropanes reported by Trost.
Trost subsequently examined the (5+2) cycloaddition of substrates that have rings in the tether connecting the 1,6-enyne or rings fused to the cyclopropane moiety (Scheme 79).341,342 The tricyclic cycloadducts were formed in high yields, with excellent control of regio- and diastereoselectivity. For reactants that incorporate cyclopropropyl ketones, complexation by indium triflate was found to increase regioselectivity by rendering the C-C bond adjacent to the carbonyl more susceptible to cleavage. Notably, in a thorough survey of substrate scope, cyclization products that retain the cyclopropane ring were observed, demonstrating that oxidative coupling of the 1,6-enyne to form a ruthenacyclopentene is faster than oxidative addition to the vinyl cyclopropane to form a ruthenacyclohexene (not shown).342 The collective studies of Trost ultimately enabled total syntheses of the marine norsesquiterpenoid frondosin A (Scheme 80)343 and pseudolaric acid B (Scheme 80),344 which displays activity against multidrug resistant cancer cell lines; in the latter synthesis, however, an analogous rhodium-catalyzed cycloaddition345 proved to be optimal for the (5+2) cycloaddition to form the core seven-membered ring. Synthetic studies toward the tricyclic core of rameswaralide also were disclosed (not shown).346
Scheme 79.

Ruthenium-catalyzed intramolecular (5+2) cycloadditions of cyclic cyclopropyl enynes reported by Trost.
Scheme 80.

Application of ruthenium-catalyzed intramolecular (5+2) cycloadditions of alkynes with vinyl cyclopropanes to the total synthesis of frondosin A and pseudolaric acid B reported by Trost.
5. Conclusion and Outlook
Despite enormous advances in the area of transition metal-catalyzed cycloadditions, the vast majority of ruthenium-catalyzed cycloadditions have only appeared within the last two decades. The first ruthenium-catalyzed cycloaddition (to form a 5-, 6- or 7-membered ring) was reported in 1987,123 and in Lautens’s authoritative 1996 review entitled “Metal-Mediated Cycloaddition Reactions,”2 ruthenium-catalyzed cycloadditions to form 5-, 6-, and 7-membered rings were absent but for two reports.167,172 While reasons for the delayed exploration of ruthenium in this context remain unclear, this relatively abundant metal has since evoked an incredibly diverse range of cycloadditions, including processes that are unknown for other metals.34 Despite the diversity of cycloaddition processes, one can see that only a limited number of ruthenium complexes serve as efficient catalysts, allowing some generalizations regarding their use to be made. Zero-valent ruthenium complexes, in particular Ru3(CO)12, are most effective in cycloadditions that involve the insertion of carbon monoxide and those that proceed by way of oxaruthenacycles that arise via C=C/C=O oxidative coupling. In contrast, electron-rich [CpRuX], [Cp*RuX] and [Cp*Ru]+ catalysts are most frequently utilized in cycloadditions that involve oxidative coupling of all-carbon-containing π-unsaturated reactants (alkynes and alkenes). Additionally, ruthenium alkylidene complexes, which have famously found use in olefin metathesis, are also competent catalysts for [2+2+2] cycloaddition reactions of alkenes and alkynes. It is the authors’ hope that the present catalog of ruthenium-catalyzed cycloadditions will expedite further progress in this burgeoning field of research.
ACKNOWLEDGMENTS
The Robert A. Welch Foundation (F-0038), the National Science Foundation (CHE-1855744) and the NIH-NIGMS (RO1-GM069445) are acknowledged for financial support.
Biographies
Professor Michael J. Krische obtained a B.S. degree in Chemistry from the University of California at Berkeley (1989), where he performed research under the supervision of Professor Henry Rapoport. After a year abroad as a Fulbright Fellow, he initiated doctoral studies at Stanford University with Professor Barry Trost as a Veatch Graduate Fellow. Following receipt of his Ph.D. degree (1996), he joined the laboratory of Professor Jean-Marie Lehn at the Université Louis Pasteur as an NIH Post-Doctoral Fellow. In 1999, he joined the faculty at the University of Texas at Austin. He was promoted directly to the rank of full professor (2004) and shortly thereafter appointed the Robert A. Welch Chair in Science (2007). Professor Krische has pioneered a new class of C-C bond formations that merge the characteristics of carbonyl addition and catalytic hydrogenation. Professor Krische’s research has garnered numerous awards, including the NSF-CAREER Award (2000), Cottrell Scholar Award (2002), Eli Lilly Granteeship for Untenured Faculty (2002), Frasch Award in Chemistry (2002), Dreyfus Teacher-Scholar Award (2003), Sloan Fellowship (2003), Johnson & Johnson Focused Giving Award (2005), Solvias Ligand Prize (2006), Presidential Green Chemistry Award (2007), ACS Corey Award (2007), Dowpharma Prize (2007), Novartis Lectureship (2008), Tetrahedron Young Investigator Award (2009), Humboldt Senior Research Award (2009–2011), Mukaiyama Award (2010), Glaxo-Smith-Kline Scholar Award (2011), ACS Cope Scholar Award (2012), and JSPS Fellow (2013), Eun Lee Lectureship, Korea (2015), Royal Society of Chemistry, Pedlar Award (2015), AAAS Fellow (2017), ACS Award for Creative Work in Synthetic Organic Chemistry (2020).
Rosalie S. Doerksen obtained a B.A. in chemistry and in biochemistry from the University of Pennsylvania in 2017 where she conducted research under the supervision of Professor Amos B. Smith III. In Fall 2017, Rosalie entered the doctoral degree program at the University of Texas at Austin as a Provost’s Graduate Excellence Fellow. Rosalie is presently investigating the application of ruthenium-catalyzed transfer-hydrogenative cycloadditions to the total synthesis of type II polyketide natural products under the mentorship of Professor Michael J. Krische.
Tomáš Hodík received his MSc in Organic Chemistry from the University of Chemistry and Technology, Prague in 2015 where he conducted research with Professor Jiří Svoboda. In parallel, Tomáš earned an MSc in Physical Chemistry from the Charles University in Prague, and was trained in organometallic chemistry at the Academy of Sciences of the Czech Republic under the guidance of Dr. Jiří Pinkas. Tomáš completed his Ph.D. in the research group of Professor Christoph Schneider at Leipzig University, Germany, as a DAAD fellow. In 2019, Tomáš joined the research group of Professor Michael J. Krische as a Genentech postdoctoral research fellow. Tomáš is presently investigating the use of ruthenium complexes in site-selective protein modification.
Guanyu Hu graduated cum laude with a B.A. in chemistry from Kenyon College (2017), where he conducted research under the guidance of Professor John E. Hofferberth. In Fall 2017, Guanyu entered the doctoral degree program at the University of Texas at Austin. Guanyu is presently investigating the application of ruthenium-catalyzed transfer-hydrogenative cycloadditions to the total synthesis of type II polyketide natural products under the mentorship of Professor Michael J. Krische.
Nancy O. Huynh obtained a B.S. in chemistry with honors from the University of California, Irvine (2017), where she conducted research under the supervision of Professor David L. Van Vranken and received the ACS Division of Organic Chemistry Undergraduate Research Award. In Fall 2017, Nancy entered the doctoral degree program at the University of Texas at Austin as a Provost’s Graduate Excellence Fellow. Nancy is presently investigating the application of ruthenium-catalyzed transfer-hydrogenative cycloadditions to the total synthesis of type II polyketide natural products under the mentorship of Professor Michael J. Krische.
William G. Shuler obtained a B.S. in biochemistry from the College of Charleston (2013), where he conducted research under the supervision of the late Professor Charles F. Beam. William completed his Ph.D. in the research group of Professor Michael K. Hilinski at the University of Virginia, where he developed Lewis acid catalyzed and organocatalyzed transformations. In 2018, William initiated postdoctoral studies in the research group of Professor Michael J. Krische. William is presently investigating the development of transition metal-catalyzed reductive C-C couplings and ruthenium-catalyzed cycloadditions for the construction of PAH compounds.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Welker ME 3+2 Cycloaddition Reactions of Transition-Metal 2-Alkynyl and η1-Allyl Complexes and Their Utilization in Five-Membered-Ring Compound Syntheses. Chem. Rev 1992, 92, 97–112. [Google Scholar]
- (2).Lautens M; Klute W; Tam W Transition Metal-Mediated Cycloaddition Reactions. Chem. Rev 1996, 96, 49–92. [DOI] [PubMed] [Google Scholar]
- (3).Ojima I; Tzamarioudake M; Li Z; Donovan RJ Transition Metal-Catalyzed Carbocyclizations in Organic Synthesis. Chem. Rev 1996, 96, 635–662. [DOI] [PubMed] [Google Scholar]
- (4).Dell CP Cycloadditions in Synthesis. Contemp. Org. Synth 1997, 4, 87–117. [Google Scholar]
- (5).Frühauf H-W Metal-Assisted Cycloaddition Reactions in Organotransition Metal Chemistry. Chem. Rev 1997, 97, 523–596. [DOI] [PubMed] [Google Scholar]
- (6).Nguyen TV; Hartmann JM; Enders D Recent Synthetic Strategies to Access Seven-Membered Carbocycles in Natural Product Synthesis. Synthesis 2013, 45, 845–873. [Google Scholar]
- (7).López F; Mascareñas JL [4+2] and [4+3] Catalytic Cycloadditions of Allenes. Chem. Soc. Rev 2014, 43, 2904–2915. [DOI] [PubMed] [Google Scholar]
- (8).Deng Y; Chen Q-Q; Doyle MP Asymmetric [3+3] Cycloaddition for Heterocycle Synthesis. Synlett 2017, 28, 1695–1706. [Google Scholar]
- (9).Pellissier H Recent Developments in the [5+2] Cycloaddition. Adv. Synth. Cat 2018, 360, 1551–1583. [Google Scholar]
- (10).Cardoso AL; Soares MIL 1,3-Dipolar Cycloadditions Involving Allenes: Synthesis of Five-membered Rings. Curr. Org. Chem 2019, 23, 3064–3134. [Google Scholar]
- (11).Lam H; Lautens M Recent Advances in Transition-Metal-Catalyzed (4+3)-Cycloadditions. Synthesis 2020, 52, 2427–2449. [Google Scholar]
- (12).Vollhardt KPC Cobalt-Mediated [2+2+2]-Cycloadditions: A Maturing Synthetic Strategy. Angew. Chem. Int. Ed. Engl 1984, 23, 539–556. [Google Scholar]
- (13).Gandon V; Aubert C; Malacria M Recent Progress in Cobalt-Mediated [2+2+2] Cycloaddition Reactions. Chem. Commun 2006, 2209–2217. [DOI] [PubMed] [Google Scholar]
- (14).Hess W; Treutwein J; Hilt G Cobalt-Catalyzed Carbon-Carbon Bond-Formation Reactions. Synthesis 2008, 22, 3537–3562. [Google Scholar]
- (15).Weding N; Hapke M Preparation and Synthetic Applications of Alkene Complexes of Group 9 Transition Metals in [2+2+2] Cycloaddition Reactions. Chem. Soc. Rev 2011, 40, 4525–4538. [DOI] [PubMed] [Google Scholar]
- (16).Gandeepan P; Cheng C-H Cobalt Catalysis Involving π Components in Organic Synthesis. Acc. Chem. Res 2015, 48, 1194–1206. [DOI] [PubMed] [Google Scholar]
- (17).Montgomery J Nickel-Catalyzed Cyclizations, Couplings, and Cycloadditions Involving Three Reactive Components. Acc. Chem. Res 2000, 33, 467–473. [DOI] [PubMed] [Google Scholar]
- (18).Montgomery J; Organonickel Chemistry. In Organometallics in Synthesis; Lipshutz BH, Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 319–428; [Google Scholar]
- (19).Pellissier H Enantioselective Nickel-Catalyzed Cycloaddition Reactions. Tetrahedron 2015, 71, 8855–8869. [Google Scholar]; (d) Kurahashi T; Matsubara S Nickel-Catalyzed Reactions Directed toward the Formation of Heterocycles. Acc. Chem. Res 2015, 48, 1703–1716. [DOI] [PubMed] [Google Scholar]
- (20).Thakur A; Louie J Advances in Nickel-Catalyzed Cycloaddition Reactions to Construct Carbocycles and Heterocycles. Acc. Chem. Res 2015, 48, 2354–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Shibata Y; Tanaka K Rhodium-Catalyzed [2+2+2] Cycloaddition of Alkynes for the Synthesis of Substituted Benzenes: Catalysts, Reaction Scope, and Synthetic Applications. Synthesis 2012, 44, 323–350. [Google Scholar]
- (22).Tanaka K Rhodium-Catalyzed [2+2+2] Cycloaddition for the Synthesis of Substituted Pyridines, Pyridones, and Thiopyranimines. Heterocycles, 2012, 85, 1017–1043. [Google Scholar]
- (23).Ojima I; Athan AA; Chaterpaul SJ; Kaloko JJ; Teng Y-HG; Organorhodium Chemistry. In Organometallics in Synthesis; Lipshutz BH, Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 135–318. [Google Scholar]
- (24).Wang Y; Yu Z-X Rhodium-Catalyzed [5+2+1] Cycloaddition of Ene-Vinylcyclopropanes and CO: Reaction Design, Development, Application in Natural Product Synthesis, and Inspiration for Developing New Reactions for Synthesis of Eight-Membered Carbocycles. Acc. Chem. Res 2015, 48, 2288–2296. [DOI] [PubMed] [Google Scholar]
- (25).Amatore M; Aubert C Recent Advances in Stereoselective [2+2+2] Cycloadditions. Eur. J. Org. Chem 2015, 2015, 265–286. [Google Scholar]
- (26).Varela JA; Saá C Construction of Pyridine Rings by Metal-Mediated [2+2+2] Cycloaddition. Chem. Rev 2003, 103, 3787–3802. [DOI] [PubMed] [Google Scholar]
- (27).Domínguez G; Pérez-Castells J Recent Advances in [2+2+2] Cycloaddition Reactions. Chem. Soc. Rev 2011, 40, 3430–3444. [DOI] [PubMed] [Google Scholar]
- (28).Hua R; Abrenica MVA; Wang P Cycloaddition of Alkynes: Atom-Economic Protocols for Constructing Six-Membered Cycles. Curr. Org. Chem 2011, 15, 712–729. [Google Scholar]
- (29).Yamamoto Y; Ruthenium-Mediated [2+2+2] Cycloaddition. In Transition-Metal-Mediated Aromatic Ring Construction, First edn; Tanaka K, Ed.; John Wiley & Sons, Inc., 2013; pp. 71–125. [Google Scholar]
- (30).Ylijoki KEO; Stryker JM [5+2] Cycloaddition Reactions in Organic and Natural Product Synthesis. Chem. Rev 2013, 113, 2244–2266. [DOI] [PubMed] [Google Scholar]
- (31).Chen J-R; Hu X-Q; Lu L-Q; Xiao W-J Formal [4+1] Annulation Reactions in the Synthesis of Carbocyclic and Heterocyclic Systems. Chem. Rev 2015, 115, 5301–5365. [DOI] [PubMed] [Google Scholar]
- (32).Perez F; Oda S; Geary LM; Krische MJ Ruthenium Catalyzed Transfer Hydrogenation for C-C Bond Formation: Hydrohydroxyalkylation and Hydroaminoalkylation via Reactant Redox Pairs. Top. Curr. Chem 2016, 374, 365–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Kuila B; Kaur M; Singh P; Bhargava G Transition-Metal-Catalyzed [3+2+2] Cycloaddition Reactions. Eur. J. Org. Chem 2018, 2018, 853–868. [Google Scholar]
- (34).Sato H; Turnbull BWH; Fukaya K; Krische MJ Ruthenium(0) Catalyzed Cycloaddition of 1,2-Diols, Ketols or Diones via Alcohol-Mediated Hydrogen Transfer. Angew. Chem. Int. Ed 2018, 57, 3012–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Naota T; Takaya H; Murahashi S.-i. Ruthenium-Catalyzed Reactions for Organic Synthesis. Chem. Rev 1998, 98, 2599–2660. [DOI] [PubMed] [Google Scholar]
- (36).Toste FD; Pinkerton AB; Trost BM Non-Metathesis Ruthenium-Catalyzed C-C Bond Formation. Chem. Rev, 2001, 101, 2067–2096. [DOI] [PubMed] [Google Scholar]
- (37).Schmidt B Ruthenium-Catalyzed Cyclizations: More than Just Olefin Metathesis! Angew. Chem. Int. Ed 2003, 42, 4996–4999. [DOI] [PubMed] [Google Scholar]
- (38).Ruthenium in Catalysis. Bruneau C, Dixneuf PH, Eds.; Top. Organomet. Chem.; Springer International Publishing: Cham, Switzerland, 2014; Vol. 48, DOI: 10.1007/978-3-319-08482-4. [DOI] [Google Scholar]
- (39).Frederiksen MU; Rudd MT; Trost BM Ruthenium-Catalyzed Reactions - A Treasure Trove of Atom-Economic Transformations. Angew. Chem. Int. Ed 2005, 44, 6630–6666. [DOI] [PubMed] [Google Scholar]
- (40).Muller P Glossary of Terms Used in Physical Organic Chemistry (IUPAC Recommendations 1994). Pure Appl. Chem 1994, 66, 1077–1184. [Google Scholar]
- (41).Ackermann L Carboxylate-Assisted Transition-Metal-Catalyzed C−H Bond Functionalizations: Mechanism and Scope. Chem. Rev 2011, 111, 1315–1345. [DOI] [PubMed] [Google Scholar]
- (42).Arockiam PB; Bruneau C; Dixneuf PH Ruthenium(II)Catalyzed C-H Bond Activation and Functionalization. Chem. Rev 2012, 112, 5879–5918. [DOI] [PubMed] [Google Scholar]
- (43).Duarah G; Kaishap PP; Begum T; Gogoi S Recent Advances in Ruthenium(II)-Catalyzed C-H Bond Activation and Alkyne Annulation Reactions. Adv. Synth. Cat 2018, 361, 654–672. [Google Scholar]
- (44).Kondo T; Nakamura A; Okada T; Suzuki N; Wada K; Mitsudo T Ruthenium-Catalyzed Reconstructive Synthesis of Cyclopentenones by Unusual Coupling of Cyclobutenediones with Alkenes Involving Carbon−Carbon Bond Cleavage. J. Am. Chem. Soc 2000, 122, 6319–6320. [Google Scholar]
- (45).Kondo T; Kaneko Y; Taguchi Y; Nakamura A; Okada T; Shiotsuki M; Ura Y; Wada K; Mitsudo T Rapid Ruthenium-Catalyzed Synthesis of Pyranopyrandiones by Reconstructive Carbonylation of Cyclopropenones Involving C-C Bond Cleavage. J. Am. Chem. Soc 2002, 124, 6824–6825. [DOI] [PubMed] [Google Scholar]
- (46).Li T; Yan H; Li X; Wang C; Wan B Ruthenium-Catalyzed [3 + 2] Cycloaddition of 2H-Azirines with Alkynes: Access to Polysubstituted Pyrroles. J. Org. Chem 2016, 81, 12031–12037. [DOI] [PubMed] [Google Scholar]
- (47).Yoneda E; Kaneko T; Zhang S-W; Onitsuka K; Takahashi S Ruthenium-Catalyzed Cyclic Carbonylation of Allenyl Alcohols. Selective Synthesis of γ- and δ-Lactones. Org. Lett 2000, 2, 441–443. [DOI] [PubMed] [Google Scholar]
- (48).Kang S-K; Kim K-J; Yu C-M; Hwang J-W; Do Y-K Ru-Catalyzed Cyclocarbonylation of α- and β-Allenic Sulfonamides: Synthesis of γ- and δ-Unsaturated Lactams. Org. Lett 2001, 3, 2851–2853. [DOI] [PubMed] [Google Scholar]
- (49).Yoneda E; Zhang S-W; Onitsuka K; Takahashi S Direct synthesis of seven- and eight-membered lactones by ruthenium-catalyzed cyclocarbonylation of allenyl alcohols. Tetrahedron Lett 2001, 42, 5459–5461. [Google Scholar]
- (50).Yoneda E; Zhang S-W; Zhou D-Y; Onitsuka K; Takahashi S Ruthenium-Catalyzed Cyclocarbonylation of Allenyl Alcohols and Amines: Selective Synthesis of Lactones and Lactams. J. Org. Chem 2003, 68, 8571–8576. [DOI] [PubMed] [Google Scholar]
- (51).Grigg RD; Schomaker JM; Timokhin V C–H Amination/Cyclocarbonylation of Allene Carbamates: A Versatile Platform for the Synthesis of α,β-Unsaturated γ-Lactams. Tetrahedron 2011, 67, 4318–4326. [Google Scholar]
- (52).Kappe CO; Van der Eycken E Click Chemistry under Non-Classical Reaction Conditions. Chem. Soc. Rev 2010, 39, 1280–1290. [DOI] [PubMed] [Google Scholar]
- (53).Fehlhammer WP; Beck W Azide Chemistry - An Inorganic Perspective, Part II [3+2]-Cycloaddition Reactions of Metal Azides and Related Systems: [3+2]-Cycloaddition Reactions of Metal Azides and Related Systems. Z. Für Anorg. Allg. Chem 2015, 641, 1599–1678. [Google Scholar]
- (54).Johansson JR; Beke-Somfai T; Stålsmeden AS; Kann N Ruthenium-Catalyzed Azide Alkyne Cycloaddition Reaction: Scope, Mechanism, and Applications. Chem. Rev 2016, 116, 14726–14768. [DOI] [PubMed] [Google Scholar]
- (55).Barlow TMA; Tourwé D; Ballet S Cyclisation To Form Small, Medium and Large Rings by Use of Catalysed and Uncatalysed Azide-Alkyne Cycloadditions (AACs): Cyclisation To Form Small, Medium and Large Rings by Use of Catalysed and Uncatalysed Azide-Alkyne Cycloadditions (AACs). Eur. J. Org. Chem 2017, 2017, 4678–4694. [Google Scholar]
- (56).Yamaguchi R; Fujita K.-i.; Zhu M Recent Progress of New Catalytic Synthetic Methods for Nitrogen Heterocycles Based on Hydrogen Transfer Reactions. Heterocycles 2010, 81, 1093–1140. [Google Scholar]
- (57).Nandakumar A; Midya SP; Landge VG; Balaraman E Transition-Metal-Catalyzed Hydrogen-Transfer Annulations: Access to Heterocyclic Scaffolds. Angew. Chem. Int. Ed 2015, 54, 11022–11034. [DOI] [PubMed] [Google Scholar]
- (58).Yang Q; Wang Q; Yu Z Substitution of Alcohols by N-Nucleophiles via Transition Metal-Catalyzed Dehydrogenation. Chem. Soc. Rev 2015, 44, 2305–2329. [DOI] [PubMed] [Google Scholar]
- (59).Hardy MD; Konetski D; Bowman CN; Devaraj NK Ruthenium Photoredox-Triggered Phospholipid Membrane Formation. Org. Biomol. Chem 2016, 14, 5555–5558. [DOI] [PubMed] [Google Scholar]
- (60).Chandrasekhar D; Borra S; Nanubolu JB; Maurya RA Visible Light Driven Photocascade Catalysis: Ru(bpy)3(PF6)2/TBHP-Mediated Synthesis of Fused β-Carbolines in Batch and Flow Microreactors. Org. Lett 2016, 18, 2974–2977. [DOI] [PubMed] [Google Scholar]
- (61).Lin S; Lies SD; Gravatt CS; Yoon TP Radical Cation Cycloadditions Using Cleavable Redox Auxiliaries. Org. Lett 2017, 19, 368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Triandafillidi I; Kokotou MG; Kokotos CG Photocatalytic Synthesis of γ-Lactones from Alkenes: High-Resolution Mass Spectrometry as a Tool to Study Photoredox Reactions. Org. Lett 2018, 20, 36–39. [DOI] [PubMed] [Google Scholar]
- (63).Rozenel SS; Azpilcueta CR; Flores-Leonar MM; Rebolledo-Chávez JPF; Ortiz-Frade L; Amador-Bedolla C; Martin E Ruthenium Tris Bipyridine Derivatives and Their Photocatalytic Activity in [4 + 2] Cycloadditions. An Experimental and DFT Study. Catal. Today 2018, 310, 2–10. [Google Scholar]
- (64).Doyle MP; Protopopova MN New Aspects of Catalytic Asymmetric Cyclopropanation. Tetrahedron 1998, 54, 7919–7946. [Google Scholar]
- (65).Noels AF; Demonceau A From Olefin Cyclopropanation to Olefin Metathesis through Catalyst Engineering: Recent Applications of Olefin Metathesis to Fine Organic Synthesis and to Polymer Chemistry. J. Phys. Org. Chem 1998, 11, 602–609. [Google Scholar]
- (66).Noels AF; Demonceau A; Jan D Late Transition Metal-Based Catalysts for Olefin Cyclopropanation or Olefin Metathesis. Importance of Catalyst Unsaturation. Russ. Chem. Bull 1999, 48, 1206–1211. [Google Scholar]
- (67).Teixidor F; Núñez R; Flores M; Demonceau A; Viñas C Forced Exo-Nido Rhoda and Ruthenacarboranes as Catalyst Precursors: A Review. J. Organomet. Chem 2000, 614–615, 48–56. [Google Scholar]
- (68).Simonneaux G; Le Maux P Optically Active Ruthenium Porphyrins: Chiral Recognition and Asymmetric Catalysis. Coord. Chem. Rev 2002, 228, 43–60. [Google Scholar]
- (69).Che C-M; Huang J-S Ruthenium and Osmium Porphyrin Carbene Complexes: Synthesis, Structure, and Connection to the Metal-Mediated Cyclopropanation of Alkenes. Coord. Chem. Rev 2002, 231, 151–164. [Google Scholar]
- (70).Maas G Ruthenium-Catalysed Carbenoid Cyclopropanation Reactions with Diazo Compounds. Chem. Soc. Rev 2004, 33, 183–190. [DOI] [PubMed] [Google Scholar]
- (71).Nishiyama H Cyclopropanation with Ruthenium Catalysts. In Ruthenium Catalysts and Fine Chemistry; Bruneau C, Dixneuf PH, Eds.; Bruneau C, Dixneuf PH, Series Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2004; Vol. 11, pp. 81–92. [Google Scholar]
- (72).Nishiyama H Ruthenium-Catalyzed Cyclopropanation. In Ruthenium in Organic Synthesis; Murahashi S.-i., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, FRG, 2005; pp. 179–187. [Google Scholar]
- (73).Mezzetti A; Bonaccorsi C Ruthenium Complexes with Chiral Tetradentate PNNP Ligands in Asymmetric Catalytic Atom-Transfer Reactions. Curr. Org. Chem 2006, 10, 225–240. [Google Scholar]
- (74).Arisawa M; Terada Y; Takahashi K; Nakagawa M; Nishida A Non-Metathesis Reactions of Ruthenium Carbene Catalysts and Their Application to the Synthesis of Nitrogen-Containing Heterocycles. Chem. Rec 2007, 7, 238–253. [DOI] [PubMed] [Google Scholar]
- (75).Alcaide B; Almendros P; Luna A Grubbs’ Ruthenium-Carbenes Beyond the Metathesis Reaction: Less Conventional Non-Metathetic Utility. Chem. Rev 2009, 109, 3817–3858. [DOI] [PubMed] [Google Scholar]
- (76).Mezzetti A Ruthenium Complexes with Chiral Tetradentate PNNP Ligands: Asymmetric Catalysis from the Viewpoint of Inorganic Chemistry. Dalton Trans 2010, 39, 7851–7869. [DOI] [PubMed] [Google Scholar]
- (77).Zhou C-Y; Huang J-S; Che C-M Ruthenium-Porphyrin-Catalyzed Carbenoid Transfer Reactions. Synlett 2010, 18, 2681–2700. [Google Scholar]
- (78).Saito N; Tanaka D; Mori M; Sato Y Ruthenium-Catalyzed Cyclizations of Enynes via a Ruthenacyclopentene Intermediate: Development of Three Novel Cyclizations Controlled by a Substituent on Alkyne of Enyne. Chem. Rec 2011, 11, 186–198. [DOI] [PubMed] [Google Scholar]
- (79).Chanthamath S; Iwasa S Enantioselective Cyclopropanation of a Wide Variety of Olefins Catalyzed by Ru(II)–Pheox Complexes. Acc. Chem. Res 2016, 49, 2080–2090. [DOI] [PubMed] [Google Scholar]
- (80).Mitsudo T; Kondo T Ruthenium Complex-Catalyzed Formation and Cleavage of Carbon-Carbon σ-Bonds. On the Requirement of Highly Qualified Tuning of the Reaction Conditions. Synlett 2001, 3, 309–321. [Google Scholar]
- (81).Alcaide B; Almendros P; Aragoncillo C Exploiting [2+2] Cycloaddition Chemistry: Achievements with Allenes. Chem. Soc. Rev 2010, 39, 783–816. [DOI] [PubMed] [Google Scholar]
- (82).Tam W; Jack K; Goodreid J; Cockburn N Transition Metal-Catalyzed [2+2] Cycloaddition Reactions Between Bicyclic Alkenes and Alkynes. In Advances in Organic Synthesis; Atta-ur-Rahman, Ed.; Bentham, 2013; Vol. 6; pp. 59–114. [Google Scholar]
- (83).Dérien S C-C Bond Formation on Activation of Alkynes and Alkenes with (C5R5)Ru Catalysts. Top. Organomet. Chem 2014, 48, 289–318. [Google Scholar]
- (84).Yamamoto Y Recent Topics of Cp*RuCl-catalyzed Annulation Reactions. Tetrahedron. Lett 2017, 58, 3787–3794. [Google Scholar]
- (85).Parthasarathy K; Cheng C-H Metal-Mediated and Metal-Catalyzed [2+2] Cycloadditions. In Comprehensive Organic Synthesis, 2nd ed.; Knochel P; Molander GA; Eds.; Elsevier: Amsterdam, 2014; Vol. 5, pp. 222–272. [Google Scholar]
- (86).Boutin R; Koh S; Tam W Recent Advances in Transition Metal-Catalyzed Reactions of Oxabenzonorbornadiene. Curr. Org. Syn 2018, 16, 460–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Khand IU; Knox GR; Pauson PL; Watts WE; Foreman MI Organocobalt Complexes. Part 1. Arene Complexes Derived from Dodecacarbonyltetracobalt. J. Chem. Soc. Perkin Trans 1. 1973, 975–977. [Google Scholar]
- (88).Blanco-Urgoiti J; Añorbe L; Perez-Serrano L; Dominguez G; Perez-Castells J The Pauson-Khand Reaction, A Powerful Synthetic Tool for The Synthesis of Complex Molecules. Chem. Soc. Rev 2004, 33, 32–42. [DOI] [PubMed] [Google Scholar]
- (89).Shi L; Yang Z Cobalt and Other Metal Mediated Domino Reactions: The Pauson-Khand Reaction and Its Use in Natural Product Total Synthesis. In Science of Synthesis: Applications of Domino Transformations in Organic Synthesis; Snyder SA, Ed.; Thieme: Stuttgart, 2016; Vol. 1, pp. 601–632. [Google Scholar]
- (90).Rautenstrauch V; Mégard P; Conesa J; Küster W 2-Pentylcyclopent-2-en-1-one by Catalytic Pauson-Khand Reaction. Angew. Chem. Int. Ed 1990, 29, 1413–1416. [Google Scholar]
- (91).Jeong N; Hwang SH; Lee Y; Chung YK Catalytic version of the Intramolecular Pauson-Khand Reaction. J. Am. Chem. Soc 1994, 116, 3159–3160. [Google Scholar]
- (92).Pagenkopf BL; Livinghouse T Photochemical Promotion of the Intramolecular Pauson-Khand Reaction. A New Experimental Protocol for Cobalt-Catalyzed [2 + 2 + 1] Cycloadditions. J. Am. Chem. Soc 1996, 118, 2285–2286. [Google Scholar]
- (93).Berk SC; Grossman RB; Buchwald SL Development of a Titanocene-Catalyzed Enyne Cyclization/Isocyanide Insertion Reaction. J. Am. Chem. Soc 1994, 116, 8593–8601. [Google Scholar]
- (94).Hicks FA; Kablaoui NM; Buchwald SL Titanocene-Catalyzed Cyclocarbonylation of Enynes to Cyclopentenones. J. Am. Chem. Soc, 1996, 118, 9450–9451. [Google Scholar]
- (95).Hicks FA; Kablaoui NM; Buchwald SL Scope of the Intramolecular Titanocene-Catalyzed Pauson-Khand Type Reaction. J. Am. Chem. Soc, 1999, 121, 5881–5898. [Google Scholar]
- (96).Park JH; Chang K-M; Chung YK Catalytic Pauson-Khand-Type Reactions and Related Carbonylative Cycloaddition Reactions. Coord. Chem. Rev 2009, 253, 2461–2480. [Google Scholar]
- (97).Lee H-W; Kwong F-Y A Decade of Advancements in Pauson-Khand-Type Reactions. Eur. J. Org. Chem 2010, 2010, 789–811. [Google Scholar]
- (98).Jeong N The Pauson-Khand Reaction. In Comprehensive Organic Synthesis, 2nd ed.; Knochel P, Molander GA, Eds.; Elsevier: Amsterdam, 2014; Vol. 5, pp. 1106–1178. [Google Scholar]
- (99).Burnie AJ; Evans PA Recent Developments in Rhodium-Catalyzed Cyclocarbonylation Reactions. Top. Organomet. Chem 2018, 61, 167–230. [Google Scholar]
- (100).Morimoto TM; Chatani N; Fukumoto Y; Murai S Ru3(CO)12-Catalyzed Cyclocarbonylation of 1,6-Enynes to Bicyclo[3.3.0]octenones. J. Org. Chem 1997, 62, 3762–3765. [Google Scholar]
- (101).Kondo T; Suzuki N; Okada T; Mitsudo T First Ruthenium-Catalyzed Intramolecular Pauson-Khand Reaction. J. Am. Chem. Soc 1997, 119, 6187–6188. [Google Scholar]
- (102).Strübing D; Neumann H; Hübner S; Klaus S; Beller M Straightforward Synthesis of Di-, Tri- and Tetracyclic Lactams via Catalytic Pauson-Khand and Alder-Ene Reactions of MCR Products. Tetrahedron 2005, 61, 11345–11354. [Google Scholar]
- (103).Wang C; Wu Y-D Theoretical Studies on Ru-Catalyzed Pauson-Khand Type [2+2+1] and Related [2+2+1+1] Cycloadditions. Organometallics 2008, 27, 6152–6162. [Google Scholar]
- (104).Chatani N; Morimoto T; Fukumoto Y; Murai S Ru3(CO)12-Catalyzed Cyclocarbonylation of Yne-Aldehydes to Bicyclic α,β-Unsaturated γ-Butyrolactones J. Am. Chem. Soc 1998, 120, 5335–5336. [Google Scholar]
- (105).Jung ME; Piizzi G gem-Disubstituent Effect: Theoretical Basis and Synthetic Applications. Chem. Rev 2005, 105, 1735–1766. [DOI] [PubMed] [Google Scholar]
- (106).Chatani N; Morimoto T; Kamitani A; Fukumoto Y; Murai S Ru3(CO)12-Catalyzed Reaction of Yne–Imines with Carbon Monoxide Leading to Bicyclic α,β-Unsaturated Lactams. J. Organomet. Chem 1999, 579, 177–181. [Google Scholar]
- (107).Tobisu M; Chatani N; Asaumi T; Amako K; Le Y; Fukumoto Y; Murai S Ru3(CO)12-Catalyzed Intermolecular Cyclocoupling of Ketones, Alkenes or Alkynes, and Carbon Monoxide. [2+2+1] Cycloaddition Strategy for the Synthesis of Functionalized γ-Butyrolactones. J. Am. Chem. Soc, 2000, 122, 12663–12674. [Google Scholar]
- (108).Chatani N; Tobisu M; Asaumi T; Murai S The Ru3(CO)12-Catalyzed Intermolecular [2+2+1] Cyclocoupling of Imines, Alkenes or Alkynes, and Carbon Monoxide: A New Synthesis of Functionalized γ-Lactams. Synthesis 2000, 7, 925–928. [Google Scholar]
- (109).Chatani N; Amako K; Tobisu M; Asaumi T; Fukumoto Y; Murai S Ruthenium-Catalyzed Carbonylative Cycloaddition of α-Keto Lactones with Alkenes or Alkynes: The Participation of an Ester-Carbonyl Group in Cycloaddition Reactions as the Two-Atom Assembling Unit. J. Org. Chem 2003, 68, 1591–1593. [DOI] [PubMed] [Google Scholar]
- (110).Itami K; Mitsudo K; Yoshida J.-i A Pyridylsilyl Group Expands the Scope of Catalytic Intermolecular Pauson-Khand Reactions. Angew. Chem. Int. Ed 2002, 41, 3481–3484. [DOI] [PubMed] [Google Scholar]
- (111).Itami K; Nokami T; Yoshida J.-i. Facile Generation of [bis(2-Pyridyldimethylsilyl)methyl]lithium and Its Reaction with Carbonyl Compounds. New Method for The Stereoselective Synthesis of Vinylsilanes. Tetrahedron 2001, 57, 5045–5054. [Google Scholar]
- (112).Itami K; Mitsudo K; Fujita K; Ohashi Y; Yoshida J.-i. Catalytic Intermolecular Pauson-Khand-Type Reaction: Strong Directing Effect of Pyridylsilyl and Pyrimidylsilyl Groups and Isolation of Ru Complexes Relevant to Catalytic Reaction. J. Am. Chem. Soc 2004, 126, 11058–11066. [DOI] [PubMed] [Google Scholar]
- (113).Kondo T; Nomura M; Ura Y; Wada K; Mitsudo T Ruthenium-catalyzed [2+2+1] Cocyclization of Isocyanates, Alkynes, and CO Enables the Rapid Synthesis of Polysubstituted Maleimides. J. Am. Chem. Soc 2006, 128, 14816–14817. [DOI] [PubMed] [Google Scholar]
- (114).Huang Q; Hua R An Alternative Efficiently Ru3(CO)12-Catalyzed Reductive Cyclocarbonylation of Alkynes Affording Substituted Furan-2(5H)-ones. Catal. Commun 2007, 8, 1031–1035. [Google Scholar]
- (115).Miura H; Takeuchi K; Shishido T Intermolecular [2+2+1] Carbonylative Cycloaddition of Aldehydes with Alkynes, and Subsequent Oxidation to γ-Hydroxybutenolides by a Supported Ruthenium Catalyst. Angew. Chem. Int. Ed 2016, 55, 278–282. [DOI] [PubMed] [Google Scholar]
- (116).Morisaki Y; Kondo T; Mitsudo T A New Route to Cyclopentenones via Ruthenium-Catalyzed Carbonylative Cyclization of Allylic Carbonates with Alkenes. Org. Lett 2000, 2, 949–952. [DOI] [PubMed] [Google Scholar]
- (117).Chatani N; Tobisu M; Asaumi T; Fukumoto Y; Murai S Ruthenium Carbonyl-Catalyzed [2+2+1]-Cycloaddition of Ketones, Olefins, and Carbon Monoxide, Leading to Functionalized γ-Butyrolactones. J. Am. Chem. Soc 1999, 121, 7160–7161. [Google Scholar]
- (118).Göbel A; Imhof W One-Pot Ruthenium Catalyzed Synthesis of Spiro[pyrrolidin-2-One] Derivatives by a [2+2+1] Cycloaddition of Ketimines, Carbon Monoxide and Ethylene. Chem. Commun 2001, 7, 593–594. [Google Scholar]
- (119).Göbel A; Imhof W Transition Metal Catalyzed Cycloaddition Reactions of Chiral Ketimines with Alkenes and Carbon Monoxide: Reaction Conditions, Substrate Variations and Stereoselectivity. J. Mol. Catal. Chem 2003, 197, 15–26. [Google Scholar]
- (120).Kaleta K; Fleischhauer J; Görls H; Beckert R; Imhof W Novel Diazadienes Based on 1,3,4-Oxadiazines: Ligands in Iron Carbonyl Complexes and Substrates in Catalytic [2+2+1] Cycloaddition Reactions. J. Organomet. Chem 2009, 694, 3800–3805. [Google Scholar]
- (121).Kang S-K; Kim K-J; Hong Y-T Synthesis of α-Methylene-γ-Butyrolactones: Ru-Catalyzed Cyclocarbonylation of Allenyl Aldehydes and Allenyl Ketones. Angew. Chem. Int. Ed 2002, 41, 1584–1586. [DOI] [PubMed] [Google Scholar]
- (122).Finnegan DF; Snapper ML Formation of Polycyclic Lactones through a Ruthenium-Catalyzed Ring-Closing Metathesis/Hetero-Pauson-Khand Reaction Sequence. J. Org. Chem 2011, 76, 3644–3653. [DOI] [PubMed] [Google Scholar]
- (123).Süss-Fink G; Schmidt GF; Herrmann G Katalytische Synthese von 5-Benzylidenhydantoinen aus Alkylisocyanaten und Phenylacetylen. Chem. Ber 1987, 120, 1451–1453. [Google Scholar]
- (124).Yamashita K; Yamamoto Y; Nishiyama H Ruthenium-Catalyzed Transfer Oxygenative Cyclization of α,ω-Diynes: Unprecedented [2+2+1] Route to Bicyclic Furans via Ruthenacyclopentatriene. J. Am. Chem. Soc 2012, 134, 7660–7663. [DOI] [PubMed] [Google Scholar]
- (125).Matsui K; Shibuya M; Yamamoto Y Ruthenium-Catalyzed Transfer Oxygenative [2+2+1] Cycloaddition of Silyldiynes Using Nitrones as Adjustable Oxygen Atom Donors. Synthesis of Bicyclic 2-Silylfurans. ACS Catal 2015, 5, 6468–6472. [Google Scholar]
- (126).Yamamoto Y; Yamashita K; Nishiyama H Activation of a Water Molecule under Mild Conditions by Ruthenacyclopentatriene: Mechanism of Hydrative Cyclization of Diynes. Chem. Commun 2011, 47, 1556–1558. [DOI] [PubMed] [Google Scholar]
- (127).Matsui K; Shibuya M; Yamamoto Y Catalytic [2+2+1] Synthesis of Fused Thiophenes Using Thiocarbonyls as Sulfur Donors. Angew. Chem. Int. Ed 2016, 55, 15397–15400. [DOI] [PubMed] [Google Scholar]
- (128).Matsui K; Shibuya M; Yamamoto Y Synthesis of Pyrroles via Ruthenium-Catalyzed Nitrogen-Transfer [2+2+1] Cycloaddition of α,ω-Diynes Using Sulfoximines as Nitrene Surrogates. Commun. Chem 2018, 1, 1–8. [Google Scholar]
- (129).Li G-Y; Chen J; Yu W-Y; Hong W; Che C-M Stereoselective Synthesis of Functionalized Pyrrolidines by Ruthenium Porphyrin-Catalyzed Decomposition of α-Diazo Esters and Cascade Azomethine Ylide Formation/1,3-Dipolar Cycloaddition Reactions. Org. Lett 2003, 5, 2153–2156. [DOI] [PubMed] [Google Scholar]
- (130).Xu H-W; Li G-Y; Wong M-K; Che C-M Asymmetric Synthesis of Multifunctionalized Pyrrolines by a Ruthenium Porphyrin-Catalyzed Three-Component Coupling Reaction. Org. Lett 2005, 7, 5349–5352. [DOI] [PubMed] [Google Scholar]
- (131).Wang M-Z; Xu H-W; Liu Y; Wong M-K; Che C-M Stereoselective Synthesis of Multifunctionalized 1,2,4-Triazolidines by a Ruthenium Porphyrin-Catalyzed Three-Component Coupling Reaction. Adv. Synth. Catal 2006, 348, 2391–2396. [Google Scholar]
- (132).Reddy AR; Guo Z; Siu F-M; Lok C-N; Liu F; Yeung K-C; Zhou C-Y; Che C-M Diastereoselective Ruthenium Porphyrin-Catalyzed Tandem Nitrone Formation/1,3-Dipolar Cycloaddition for Isoxazolidines. Synthesis, in Silico Docking Study and in Vitro Biological Activities. Org. Biomol. Chem 2012, 10, 9165–9174. [DOI] [PubMed] [Google Scholar]
- (133).Lin Y-R; Zhou X-Z; Dai L-X; Sun J Ruthenium Complex-Catalyzed Reaction of Isocyanoacetate and N-Sulfonylimines: Stereoselective Synthesis of N-Sulfonyl-2-Imidazolines. J. Org. Chem 1997, 62, 1799–1803. [Google Scholar]
- (134).Zhang Z-W; Lu G; Chen M-M; Lin N; Li Y-B; Hayashi T; Chan ASC Organocatalytic Asymmetric Mannich-type Reaction of N-Sulfonylimines with Isocyanoacetate Leading to Optically Active 2-Imidazoline-4-Carboxylates. Tetrahedron: Asymmetry 2010, 21, 1715–1721. [Google Scholar]
- (135).Grecian S; Fokin VV Ruthenium-Catalyzed Cycloaddition of Nitrile Oxides and Alkynes: Practical Synthesis of Isoxazoles. Angew. Chem. Int. Ed 2008, 47, 8285–8287. [DOI] [PubMed] [Google Scholar]
- (136).Himo F; Lovell T; Hilgraf R; Rostovtsev VV; Noodleman L; Sharpless KB; Fokin VV Copper(I)-Catalyzed Synthesis of Azoles. DFT Study Predicts Unprecedented Reactivity and Intermediates. J. Am. Chem. Soc 2005, 127, 210–216. [DOI] [PubMed] [Google Scholar]
- (137).Hansen TV; Wu P; Fokin VV One-Pot Copper(I)-Catalyzed Synthesis of 3,5-Disubstituted Isoxazoles. J. Org. Chem 2005, 70, 7761–7764. [DOI] [PubMed] [Google Scholar]
- (138).Oakdale JS; Sit RK; Fokin VV Ruthenium-Catalyzed Cycloadditions of 1-Haloalkynes with Nitrile Oxides and Organic Azides: Synthesis of 4-Haloisoxazoles and 5-Halotriazoles. Chem. Eur. J 2014, 20, 11101–11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Roscales S; Bechmann N; Weiss DH; Köckerling M; Pietzsch J; Kniess T Novel Valdecoxib Derivatives by Ruthenium(II)-Promoted 1,3-Dipolar Cycloaddition of Nitrile Oxides with Alkynes – Synthesis and COX-2 Inhibition Activity. Med. Chem. Commun 2018, 9, 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Pellissier H Asymmetric 1,3-Dipolar Cycloadditions. Tetrahedron 2007, 63, 3235–3285. [Google Scholar]
- (141).Hashimoto T; Maruoka K Recent Advances of Catalytic Asymmetric 1,3-Dipolar Cycloadditions. Chem. Rev 2015, 115, 5366–5412. [DOI] [PubMed] [Google Scholar]
- (142).Singh MS; Chowdhury S; Koley S Progress in 1,3-Dipolar Cycloadditions in the Recent Decade: An Update to Strategic Development Towards the Arsenal of Organic Synthesis. Tetrahedron 2016, 72, 1603–1644. [Google Scholar]
- (143).Zhou C-Y; Yu W-Y; Che C-M Ruthenium(II) Porphyrin Catalyzed Tandem Carbonyl Ylide Formation and 1,3-Dipolar Cycloaddition Reactions of α -Diazo Ketones. Org. Lett 2002, 4, 3235–3238. [DOI] [PubMed] [Google Scholar]
- (144).Zhou C-Y; Chan PWH; Yu W-Y; Che C-M Study of Substrate Dependence on the Diastereoselectivity of the Ruthenium(II)Porphyrin Catalyzed Tandem Formation and 1,3-Dipolar Cycloaddition Reactions of Carbonyl Ylides. Synthesis 2003, 9, 1403–1412. [Google Scholar]
- (145).Chappellet S; Müller P Ru-Catalyzed Enantioselective Dipolar Cycloadditions of Ethyl Diazopyruvate. Synlett 2004, 14, 2573–2575. [Google Scholar]
- (146).Müller P; Chappellet S Asymmetric 1,3-Dipolar Cycloadditions of 2-Diazocyclohexane-1,3-Diones and Alkyl Diazopyruvates. Helv. Chim. Acta 2005, 88, 1010–1021. [Google Scholar]
- (147).Xia L; Lee YR Efficient One-Pot Synthesis of Multi-Substituted Dihydrofurans by Ruthenium(II)-Catalyzed [3+2] Cycloaddition of Cyclic or Acyclic Diazodicarbonyl Compounds with Olefins. Adv. Synth. Catal 2013, 355, 2361–2374. [Google Scholar]
- (148).Xia L; Idhayadhulla A; Lee YR; Kim SH; Wee Y-J Synthesis and Biological Evaluation of Diverse Tetrahydrobenzofuran-4-Ones as Potent Antibacterial Agents. J. Ind. Eng. Chem 2015, 22, 378–383. [Google Scholar]
- (149).Xia L; Lee YR Regioselective Synthesis of Highly Functionalized Furans Through the RuII-Catalyzed [3+2] Cycloaddition of Diazodicarbonyl Compounds. Eur. J. Org. Chem 2014, 2014, 3430–3442. [Google Scholar]
- (150).Jia Z; Wang K; Tan B; Gu Y Ruthenium Complexes Immobilized on Functionalized Knitted Hypercrosslinked Polymers as Efficient and Recyclable Catalysts for Organic Transformations. Adv. Synth. Catal 2017, 359, 78–88. [Google Scholar]
- (151).Austeri M; Rix D; Zeghida W; Lacour J CpRu-Catalyzed O-H Insertion and Condensation Reactions of α-Diazocarbonyl Compounds. Org. Lett 2011, 13, 1394–1397. [DOI] [PubMed] [Google Scholar]
- (152).Tortoreto C; Achard T; Egger L; Guénée L; Lacour J Synthesis of Spiro Ketals, Orthoesters, and Orthocarbonates by CpRu-Catalyzed Decomposition of α-Diazo-β-Ketoesters. Org. Lett 2016, 18, 240–243. [DOI] [PubMed] [Google Scholar]
- (153).McInturff EL; Mowat J; Waldeck AR; Krische MJ Ruthenium Catalyzed Hydrohydroxyalkylation of Acrylates with Diols and α-Hydroxycarbonyl Compounds to Form Spiro- and α-Methylene-γ-Butyrolactones. J. Am. Chem. Soc 2013, 135, 17230–17235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Ngai M-Y; Barchuk A; Krische MJ Iridium Catalyzed C-C Bond Forming Hydrogenation: Direct Regioselective Reductive Coupling of Alkyl-Substituted Alkynes to Activated Ketones. J. Am. Chem. Soc 2007, 129, 280–281. [DOI] [PubMed] [Google Scholar]
- (155).McInturff EL; Nguyen KD; Krische MJ Redox-Triggered C-C Coupling of Diols and Alkynes: Synthesis of β,γ-Unsaturated α-Hydroxyketones and Furans via Ruthenium Catalyzed Hydrohydroxyalkylation. Angew. Chem. Int. Ed 2014, 53, 3232–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Liu R; Chou Y; Lian B; Fang D; Gao M; Cheng T; Liu G Mechanistic Insights into the Ru(II)-Catalyzed Intramolecular Formal [3 + 2] Cycloaddition of (E)-1,6-Enynes. Org. Lett 2019, 21, 6815–6820. [DOI] [PubMed] [Google Scholar]
- (157).Mitsudo T; Zhang S-W; Watanabe Y Ruthenium Complex-Catalyzed Dimerization of Norbornadiene to Pentacyclotetradecadiene. J. Chem. Soc., Chem. Commun 1994, 4, 435–436. [Google Scholar]
- (158).Johnson JB; Rovis T More Than Bystanders: The Effect of Olefins on Transition-Metal-Catalyzed Cross-Coupling Reactions. Angew. Chem., Int. Ed 2008, 47, 840–871. [DOI] [PubMed] [Google Scholar]
- (159).Mitsudo T; Suzuki T; Zhang S-W; Imai D; Fujita K.-i.; Manabe T; Shiotsuki M; Watanabe Y; Wada K; Kondo T Novel Ruthenium Complex-Catalyzed Dimerization of 2,5-Norbornadiene to Pentacyclo[6.6.0.0.0.0]tetradeca-4,11-diene Involving Carbon-Carbon Bond Cleavage. J. Am. Chem. Soc 1999, 121, 1839–1850. [Google Scholar]
- (160).López F; Delgado A; Rodríguez JR; Castedo L; Mascareñas JL Ruthenium-Catalyzed [3 + 2] Intramolecular Cycloaddition of Alk-5-Ynylidenecyclopropanes Promoted by the “First-Generation” Grubbs Carbene Complex. J. Am. Chem. Soc 2004, 126, 10262–10263. [DOI] [PubMed] [Google Scholar]
- (161).Burton RR; Tam W Ruthenium(II)-Catalyzed Cyclization of Azabenzonorbornadienes with Alkynes. Org. Lett 2007, 9, 3287–3290. [DOI] [PubMed] [Google Scholar]
- (162).Tenaglia A; Marc S Ruthenium-Catalyzed Cross-Coupling of 7-Azabenzonorbornadienes with Alkynes. An Entry to 3a,9b-Dihydrobenzo[g]indoles. J. Org. Chem 2008, 73, 1397–1402. [DOI] [PubMed] [Google Scholar]
- (163).Mu W-H; Fang D-C; Xia S-Y; Cheng R-J; Chass GA A Multiple-Pathway Consequent Chemoselectivities of CpRuCl(PPh3)2/MeI-Catalysed Norbornadiene Alkyne Cycloadditions. Chem. Eur. J 2016, 22, 15396–15403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Wang S-G; Park SH; Cramer N A Readily Accessible Class of Chiral Cp Ligands and Their Application in RuII-Catalyzed Enantioselective Syntheses of Dihydrobenzoindoles. Angew. Chem. Int. Ed 2018, 57, 5459–5462. [DOI] [PubMed] [Google Scholar]
- (165).Li M; Chu R; Chen J; Wu X; Zhao Y; Liu S; Hu W Enantioselective Formal [3+1+1] Cycloaddition Reaction by Ru(II)/Iminium Cocatalysts for Construction of Multisubstituted Pyrrolidines. Org. Lett 2017, 19, 1290–1293. [DOI] [PubMed] [Google Scholar]
- (166).Morimoto T; Chatani N; Murai S The First Catalytic Carbonylative [4 + 1] Cycloaddition Using a 1,3-Conjugated System. A New Transformation of α,β-Unsaturated Imines to Unsaturated γ-Lactams Catalyzed by Ru3(CO)12. J. Am. Chem. Soc 1999, 121, 1758–1759. [Google Scholar]
- (167).Chatani N; Fukumoto Y; Ida T; Murai S Ruthenium-Catalyzed Reaction of 1,6-Diynes with Hydrosilanes and Carbon Monoxide: A Third Way of Incorporating CO. J. Am. Chem. Soc 1993, 115, 11614–11615. [Google Scholar]
- (168).Crudden CM; Maekawa Y; Clarke JJ; Ida T; Fukumoto Y; Chatani N; Murai S Ru3(CO)12-Catalyzed Reaction of 1,6-Diynes, Carbon Monoxide, and Water via the Reductive Coupling of Carbon Monoxide. Org. Lett 2020, 22, 8747–8751. [DOI] [PubMed] [Google Scholar]
- (169).Suzuki N; Kondo T; Mitsudo T Novel Ruthenium-Catalyzed Cross-Carbonylation of Alkynes and 2-Norbornenes to Hydroquinones. Organometallics 1998, 17, 766–769. [Google Scholar]
- (170).Fukuyama T; Yamaura R; Higashibeppu Y; Okamura T; Ryu I; Kondo T; Mitsudo T Synthesis of Functionalized Hydroquinones via [Cp*RuCl2]2-Catalyzed Cocyclization of Alkynes, α,β-Unsaturated Carbonyl Compounds, and Carbon Monoxide. Org. Lett 2005, 7, 5781–5783. [DOI] [PubMed] [Google Scholar]
- (171).Leal RA; Bischof C; Lee YV; Sawano S; McAtee CC; Latimer LN; Russ ZN; Dueber JE; Yu J-Q; Sarpong R Application of a Palladium-Catalyzed C-H Functionalization/Indolization Method to Syntheses of cis-Trikentrin A and Herbindole B. Angew. Chem. Int. Ed 2016, 55, 11824–11828. [DOI] [PubMed] [Google Scholar]
- (172).Trost BM; Imi K; Indolese AF 1,5-Cyclooctadiene as a Bis-Homodiene Partner in a Metal-Catalyzed [4 + 2] Cycloaddition. J. Am. Chem. Soc 1993, 115, 8831–8832. [Google Scholar]
- (173).Huang X; Lin Z Density Functional Theory Studies of Ruthenium-Catalyzed Bis-Diels-Alder Cycloaddition of 1,5-Cyclooctadiene with Alkynes. Organometallics 2003, 22, 5478–5484. [Google Scholar]
- (174).Alvarez P; Gimeno J; Lastra E; García-Granda S; Van der Maelen JF; Bassetti M Synthesis and Reactivity of Indenyl Ruthenium(II) Complexes Containing the Labile Ligand 1,5-Cyclooctadiene (COD): Catalytic Activity of [Ru(η5-C9H7)Cl(COD)]. Organometallics 2001, 20, 3762–3771. [Google Scholar]
- (175).Hintermann L; Xiao L; Labonne A; Englert U [CpRu(η6-naphthalene)]PF6 as Precursor in Complex Synthesis and Catalysis with the Cyclopentadienyl-Ruthenium(II) Cation. Organometallics 2009, 28, 5739–5748. [Google Scholar]
- (176).Petko D; Stratton M; Tam W Ruthenium-catalyzed Bis-Homo-Diels-Alder Reaction: Searching for Commercially Available Catalysts and Expanding the Scope of Reaction. Can. J. Org. Chem 2018, 96, 1115–1121. [Google Scholar]
- (177).Petko D; Pounder A; Tam W Ruthenium-Catalyzed [2+2+2] Bis-Homo-Diels-Alder Cycloadditions of 1,5-Cyclooctadiene with Alkynyl Phosphonates. Synthesis 2019, 51, 4271–4278. [Google Scholar]
- (178).Tenaglia A; Giordano L Ruthenium(II)-Catalyzed Homo-Diels–Alder Reactions of Disubstituted Alkynes and Norbornadiene. Tetrahedron Lett 2004, 45, 171–174. [Google Scholar]
- (179).Tenaglia A; Gaillard S Ruthenium-Catalyzed Intramolecular Homo-Diels-Alder Reaction of Alkyne-Tethered Norbornadienes. An Entry to Fused Angular Triquinanes. Org. Lett 2007, 9, 3607–3610. [DOI] [PubMed] [Google Scholar]
- (180).Kettles TJ; Cockburn N; Tam W Ruthenium-Catalyzed Homo Diels-Alder [2+2+2] Cycloadditions of Alkynyl Phosphonates with Bicyclo[2.2.1]hepta-2,5-diene. J. Org. Chem 2011, 76, 6951–6957. [DOI] [PubMed] [Google Scholar]
- (181).Sato H; Fukaya K; Poudel BS; Krische MJ Diols as Dienophiles: Bridged Carbocycles via Ruthenium(0)-Catalyzed Transfer Hydrogenative Cycloadditions of Cyclohexadiene or Norbornadiene. Angew. Chem. Int. Ed 2017, 56, 14667–14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Zhang T; Li T; Wu X; Li J Theoretical Study of Ruthenium(0)-Catalyzed Transfer Hydrogenative Cycloaddition of Cyclohexadiene and Norbornadiene with 1,2-Diols to Form Bridged Carbocycles. J. Org. Chem 2019, 84, 3377–3387. [DOI] [PubMed] [Google Scholar]
- (183).Saito S; Yamamoto Y Recent Advances in the Transition-Metal-Catalyzed Regioselective Approaches to Polysubstituted Benzene Derivatives. Chem. Rev 2000, 100, 2901–2915. [DOI] [PubMed] [Google Scholar]
- (184).Galan BR; Rovis T Beyond Reppe: Building Substituted Arenes by [2+2+2] Cycloadditions of Alkynes. Angew. Chem. Int. Ed 2009, 48, 2830–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Domínguez G; Pérez-Castells J Recent Advances in [2+2+2] Cycloaddition Reactions. Chem. Soc. Rev 2011, 40, 3430–3444. [DOI] [PubMed] [Google Scholar]
- (186).Walton JC A Valuable Upgrade to the Portfolio of Cycloaddition Reactions. Angew. Chem. Int. Ed 2016, 55, 7034–7036. [DOI] [PubMed] [Google Scholar]
- (187).Kotha S; Lahiri K; Sreevani G Design and Synthesis of Aromatics through [2+2+2] Cyclotrimerization. Synlett 2018, 29, 2342–2361. [Google Scholar]
- (188).Roglans A; Pla-Quintana A; Solà M Mechanistic Studies of Transition-Metal-Catalyzed [2+2+2] Cycloaddition Reactions. Chem. Rev 2020, DOI: 10.1021/acs.chemrev.0c00062. [DOI] [PubMed] [Google Scholar]
- (189).Lindner E; Janse RM; Mayer HA; Hiller W; Fawzi R Preparation, Properties, and Reactions of Metal-Containing Heterocycles. 65. The Behavior of Tetracarbonyl(η2-ethene)ruthenium toward Activated Alkenes and Alkynes. Organometallics 1989, 8, 2355–2360. [Google Scholar]
- (190).Peters J-U; Blechert S Ruthenium-Catalysed Conversion of Triynes to Benzene Derivatives – A Novel Metathesis Cascade. Chem. Commun 1997, 1983–1984. [Google Scholar]
- (191).Remya PR; Suresh CH Mechanistic Studies on Acetylene Cyclotrimerization Catalyzed by Grubbs First and Second Generation Catalysts. Mol. Catal 2017, 441, 63–71. [Google Scholar]
- (192).Hoven GB; Efskind J; Romming C; Undheim K Ru(II)-Catalyzed Cascade Reactions in Stereocontrolled Construction of as-Indacene-Bridged Bis(α-amino acid) Derivatives. J. Org. Chem, 2002, 67, 2459–2463. [DOI] [PubMed] [Google Scholar]
- (193).Efskind J; Undheim K High Temperature Microwave-Accelerated Ruthenium-Catalysed Domino RCM Reactions. Tetrahedron Lett 2003, 44, 2837–2839. [Google Scholar]
- (194).Yuan W; Wei Y; Shi M Ruthenium-Catalyzed Intramolecular [2+2+2] Cycloaddition and Tandem Cross-Metathesis of Triynes and Enediynes. ChemistryOpen 2013, 2, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Das SK; Roy R Mild Ruthenium-Catalyzed Intermolecular Alkyne Cyclotrimerization. Tetrahedron Lett. 1999, 40, 4015–4018. [Google Scholar]
- (196).Kotha S; Seema V; Mobin SM Synthesis of Biaryl Derivatives by Using Ruthenium-Mediated [2+2+2] Cyclotrimerization and Suzuki-Miyaura Cross-Coupling as Key Steps. Synthesis 2011, 10, 1581–1586. [Google Scholar]
- (197).Öztürk BÖ; Karabulut S; İmamoğlu Y Activity of Homobimetallic Ruthenium Alkylidene Complexes on Intermolecular [2+2+2] Cyclotrimerization Reactions of Terminal Alkynes. Inorg. Chim. Acta 2011, 378, 257–263. [Google Scholar]
- (198).Karabulut S; Sariaslan B; Öztürk BÖ A Ruthenium-Based Catalytic System with Switchable Selectivity between Cyclotrimerization and Enyne Metathesis/Diels-Alder Reactions of Terminal Alkynes. Catal. Comm 2013, 41, 12–16. [Google Scholar]
- (199).Czeluśniak I; Handzlik J; Gierada M; Szymańska-Buzar T Catalytic Transformation of Phenylacetylene Mediated by Phosphine-free Ruthenium Alkylidene Complexes. J. Organomet. Chem 2015, 786, 31–39. [Google Scholar]
- (200).Gierda M; Czeluśniak I; Handzlik J Dimerization and Cyclotrimerization of Terminal Arylalkynes Initiated by a Phosphine-Free Ruthenium Alkylidene Complex. Mol. Catal 2019, 469, 18–26. [Google Scholar]
- (201).Witulski B; Stengel T; Fernandez-Hernandez JM Chemo- and Regioselective Crossed Alkyne Cyclotrimerisation of 1,6-Diynes with Terminal Monoalkynes Mediated by Grubbs’ Catalyst or Wilkinson’s Catalyst. Chem. Commun 2000, 1965–1966. [Google Scholar]
- (202).Mallagaray Á; Medina S; Dominguez G; Pérez-Castells J Ruthenium Carbene Mediated [2+2+2] Cyclotrimerizations. Synlett 2010, 14, 2114–2118. [DOI] [PubMed] [Google Scholar]
- (203).Rüba E; Schmid R; Kirchner K; Calhorda MJ Ruthenium-Mediated Cyclotrimerization of Alkynes Utilizing the Cationic Complex [RuCp(CH3CN)3]PF6. J. Organomet. Chem 2003, 682, 204–211. [Google Scholar]
- (204).Wu C-Y; Lin Y-C; Chou P-T; Wang Y; Liu Y-H One-pot Synthesis of Substituted Benzene via Intermolecular [2+2+2] Cycloaddition Catalyzed by Air-Stable Ru(II)-Complex. Dalton Trans 2011, 40, 3748–3753. [DOI] [PubMed] [Google Scholar]
- (205).Perekalin DS; Trifonova EA; Petrovskii PV; Kudinov AR Cyclotrimerization of Alkynes Catalyzed by the Naphthalene Ruthenium Complex [CpRu(C10H8)]+. Russ. Chem. Bull 2011, 60, 2110–2113. [Google Scholar]
- (206).Silvestri AP; Oakdale J Intermolecular Cyclotrimerization of Haloketoalkynes and Internal Alkynes: Facile Access to Arenes and Phthalides. Chem. Commun 2020, 56, 13417–13420. [DOI] [PubMed] [Google Scholar]
- (207).Yi CS; Torres-Lubian JR; Liu N Selective Linear Coupling Reaction of Acetylene and Acrylonitrile Catalyzed by the Well-Defined Metallacyclopentadiene Complex C5Me5(PPh3)(Cl)RuCH = CHCH = CH. Organometallics 1998, 17, 1257–1259. [Google Scholar]
- (208).Albers MO; De Waal DJA; Liles DC; Robinson DJ; Singleton E; Wiege MB The Novel Cyclodimerization of Phenylacetylene at a Ruthenium(II) Centre. The Synthesis and X-Ray Structural Characterization of the First Metallacyclopentatriene, [(η-(C5H5)Ru(C4Ph2H2)Br], and its Facile Conversion into Metallacyclopentadienes. J. Chem. Soc., Chem. Commun 1986, 22, 1680–1682. [Google Scholar]
- (209).Ernst C; Walter O; Dinjus E Structural Characterization of Cp*Ru-Intermediates of Phenylacetylene Cyclotrimerization. J. Prakt. Chem 1999, 341, 801–804. [Google Scholar]
- (210).Kirchner K; Calhorda MJ; Schmid R; Veiros LF Mechanism for the Cyclotrimerization of Alkynes and Related Reactions Catalyzed by CpRuCl. J. Am. Chem. Soc 2003, 125, 11721–11729. [DOI] [PubMed] [Google Scholar]
- (211).Yamamoto Y; Ogawa R; Itoh K Highly Chemo- and Regio-Selective [2+2+2] Cycloaddition of Unsymmetrical 1,6-Diynes with Terminal Alkynes Catalyzed by Cp*RuCl(cod) under Mild Conditions. Chem. Commun 2000, 549–550. [Google Scholar]
- (212).Yamamoto Y; Arakawa T; Ogawa R; Itoh K Ruthenium(II)-Catalyzed Selective Intramolecular [2+2+2] Alkyne Cyclotrimerizations. J. Am. Chem. Soc 2003, 125, 12143–12160. [DOI] [PubMed] [Google Scholar]
- (213).Yamamoto Y; Hata K; Arakawa T; Itoh K Ru(II)-Catalyzed [2+2+2] Cycloaddition of 1,2-Bis(propiolyl)benzenes with Monoalkynes Leading to Substituted Anthraquinones. Chem. Commun 2003, 1290–1291. [PubMed] [Google Scholar]
- (214).Miguel‐Ávila J; Tomás‐Gamasa M; Mascareñas JL Intracellular Ruthenium‐Promoted (2+2+2) Cycloadditions. Angew. Chem. Int. Ed 2020, 59, 17628–17633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Yamamoto Y; Kinpara K; Saigoku T; Nishiyama H; Itoh K Synthesis of Benzo-Fused Lactams and Lactones via Ru(II)-catalyzed Cycloaddition of Amide- and Ester-tethered α,ω-Diynes with Terminal Alkynes: Electronic Directing Effect of Internal Conjugated Carbonyl Group. Org. Biomol. Chem 2004, 2, 1287–1294. [DOI] [PubMed] [Google Scholar]
- (216).Yamamoto Y; Hattori K; Nishiyama H Synthesis of Bicyclic p-Diiodobenzenes via Silver Catalyzed Csp-H and Ruthenium-Catalyzed Cycloaddition. J. Am. Chem. Soc 2006, 128, 8336–8340. [DOI] [PubMed] [Google Scholar]
- (217).Yamamoto Y; Takuma R; Hotta T; Yamashita K Synthesis of 2,5-Dihydrofuran-Fused Quinones from Ether-tethered Diiododiyne. J. Org. Chem 2009, 74, 4324–4328. [DOI] [PubMed] [Google Scholar]
- (218).Yamamoto Y; Yamashita K; Harada Y Systematic Evaluation of Substituted Cyclopentadienyl Ruthenium Complexes, [(η5‐C5Men H5−n)RuCl(cod)], for Catalytic Cycloadditions of Diynes. Chem. Asian J 2010, 5, 946–952. [DOI] [PubMed] [Google Scholar]
- (219).Iannazzo L; Kotera N; Malacria M; Aubert C; Gandon V Co(I)- versus Ru(II)-Catalyzed [2+2+2] Cycloadditions Involving Alkynyl Halides. J. Organomet. Chem 2011, 696, 3906–3908. [Google Scholar]
- (220).Zou Y, Deiters A Total Synthesis of Cryptoacetalide. J. Org. Chem 2010, 75, 5355–5358. [DOI] [PubMed] [Google Scholar]
- (221).Levin S; Nani RR; Reisman SE Enantioselective Total Synthesis of (+)-Salvileucalin B. J. Am. Chem. Soc 2011, 133, 774–776. [DOI] [PubMed] [Google Scholar]
- (222).Yamamoto Y; Ishii J; Nishiyama H; Itoh K Ru(II)-Catalyzed Chemo- and Regioselective Cyclotrimerization of Three Unsymmetrical Alkynes through Boron Temporary Tether. One-Pot Four-Component Coupling via Cyclotrimerization/Suzuki-Miyaura Coupling. J. Am. Chem. Soc 2004, 126, 3712–3713. [DOI] [PubMed] [Google Scholar]
- (223).Yamamoto Y; Ishii J; Nishiyama H; Itoh K Cp*RuCl-Catalyzed Formal Intermolecular Cyclotrimerization of Three Unsymmetrical Alkynes through a Boron Temporary Tether: Regioselective Four-Component Coupling Synthesis of Phthalides. J. Am. Chem. Soc 2005, 127, 9625–9631. [DOI] [PubMed] [Google Scholar]
- (224).Yamamoto Y; Ishii J; Nishiyama H; Itoh K One-Pot Sequential Four-Component Coupling via Cp*RuCl-Catalyzed Cyclotrimerization and Suzuki-Miyaura Coupling. Tetrahedron 2005, 61, 11501–11510. [Google Scholar]
- (225).Kirchner K Ruthenium-Mediated C-C Coupling Reactions of Alkynes – Mechanistic Investigations based on DFT Calculations. Monatsch Chem 2008, 139, 337–348. [Google Scholar]
- (226).Varela JA; Saá C CpRuCl- and CpCo-catalyzed or mediated Cyclotrimerizations of Alkynes and [2+2+2] Cycloadditions of Alkynes to Alkenes: A Comparative DFT Study. J. Organomet. Chem 2009, 694, 143–149. [Google Scholar]
- (227).Ura Y; Sato Y; Shiotsuki M; Kondo T; Mitsudo T Ruthenium-Catalysed Synthesis of o-Phthalates by Highly Chemoselective Intermolecular [2+2+2] Cycloaddition of Terminal Alkynes and Dimethyl Acetylenedicarboxylate. J. Mol. Catal. A: Chem 2004, 209, 35–39. [Google Scholar]
- (228).Ura Y; Sato Y; Tsujita H; Kondo T; Imachi M; Mitsudo T Ruthenium-catalyzed [2+2+2] Cycloaddition of Three Different Alkynes. J. Mol. Catal. A: Chem 2005, 239, 166–171. [Google Scholar]
- (229).Adriaenssens L; Severa L; Vávra J; Šálová T; Hývl J; Čížková M; Pohl R; Šaman D; Teplý F Bio- and Air-Tolerant Carbon-Carbon Bond Formations via Organometallic Ruthenium Catalysis. Collect. Czech. Chem. Commun 2009, 74, 1023–1034. [Google Scholar]
- (230).Kotha S; Khedkar P A Diversity-Oriented Approach to Diphenylalkanes by Strategic Utilization of [2+2+2] Cyclotrimerization, Cross-Enyne Metathesis and Diels-Alder Reaction. Chem. Eur. J 2009, 730–738. [Google Scholar]
- (231).Heinz C; Cramer N Synthesis of Fijiolide A via an Atropselective Paracyclophane Formation. J. Am. Chem. Soc 2015, 137, 11278–11281. [DOI] [PubMed] [Google Scholar]
- (232).Yamamoto Y; Hattori K; Ishii J.-i.; Nishiyama H; Itoh K Synthesis of Bi- and Tricyclic Arylboronates via Cp*RuCl-catalyzed Cycloaddition of α,ω-Diynes with Ethynylboronate. Chem. Commun 2005, 4438–4440. [DOI] [PubMed] [Google Scholar]
- (233).Yamamoto Y; Hattori K; Ishii J.-i.; Nishiyama H Synthesis of Arylboronates via Cp*RuCl-Catalyzed Cycloaddition of Alkynylboronates. Tetrahedron 2006, 62, 4294–4305. [Google Scholar]
- (234).Yamamoto Y; Hattori K Synthesis of Multiply Functionalized Benzenes via Ruthenium-Catalyzed Cycloaddition of Diiododiynes. Tetrahedron 2008, 64, 847–855. [Google Scholar]
- (235).Novák P; Pohl R; Kotora M; Hocek M Synthesis of C-Aryldeoxyribosides by [2+2+2]-Cyclotrimerization Catalyzed by Rh, Ni, Co, and Ru Complexes. Org. Lett 2006, 8, 2051–2054. [DOI] [PubMed] [Google Scholar]
- (236).Yamamoto Y; Hashimoto T; Hattori K; Kikuchi M; Nishiyama H Synthesis of Spirocyclic C-Arylribosides via Cyclotrimerization. Org. Lett 2006, 8, 3565–3568. [DOI] [PubMed] [Google Scholar]
- (237).Yamamoto Y; Yamashita K; Hotta T; Hashimoto T; Kikuchi M; Nishiyama H Synthesis of Spirocyclic C-Arylglycosides and -Ribosides by Ruthenium-Catalyzed Cycloaddition. Chem. Asian J 2007, 2, 1388–1399. [DOI] [PubMed] [Google Scholar]
- (238).Young DD; Senaiar RS; Deiters A Solid-Supported [2+2+2] Cyclotrimerizations. Chem. Eur. J 2006, 12, 5563–5568. [DOI] [PubMed] [Google Scholar]
- (239).Senaiar RS; Teske JA; Young DD; Deiters A Synthesis of Indanones via Solid-Supported [2+2+2] Cyclotrimerization. J. Org. Chem 2007, 72, 7801–7804. [DOI] [PubMed] [Google Scholar]
- (240).Young DD; Sripada L; Deiters A Microwave-Assisted Solid-Supported Alkyne Cyclotrimerization Reactions for Combinatorial Chemistry. J. Comb. Chem 2007, 9, 735–738. [DOI] [PubMed] [Google Scholar]
- (241).Teske JA; Deiters A A Cyclotrimerization Route to Cannabinoids. Org. Lett 2008, 10, 2195–2198. [DOI] [PubMed] [Google Scholar]
- (242).Shchetnitkov GT; Osipov SN; Bruneau C; Dixneuf PH Ruthenium-Catalyzed Cyclotrimerization of 1,6- and 1,7-Azadiynes: New Access to Fluorinated Bicyclic Amino Acids. Synlett 2008, 4, 578–582. [Google Scholar]
- (243).Nicolaou KC; Tang Y; Wang J Total Synthesis of Sporolide B. Angew. Chem. Int. Ed 2009, 48, 3449–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (244).Nicolaou KC; Wang J; Tang Y; Botta L Total Synthesis of Sporolide B and 9-epi-Sporolide B. J. Am. Chem. Soc 2010, 132, 11350–11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (245).Foster RW; Tame CJ; Hailes HC; Sheppard TD Highly Regioselective Synthesis of Substituted Isoindolinones via Ruthenium-Catalyzed Alkyne Cyclotrimerization. Adv. Synth. Catal 2013, 355, 2353–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (246).Zotova MA; Vasilyeva TP; Osipov SN Ruthenium-Catalyzed Cyclotrimerization of α-Amino-α-Propargyl Carboxylates and Phosphonates with 1,6-Diynes: Synthesis of α-CF3-Containing Phenylalanine Derivatives and Their P-Analogs. Russ. Chem. Bull 2014, 63, 2455–2460. [Google Scholar]
- (247).Matoušová E; Gyepes R; Císařová I; Kotora M [2+2+2]-Cyclotrimerization of 1-Cyclopropyl-1,6-diynes with Alkynes: Formation of Cyclopropylarenes. Adv. Synth. Catal 2016, 358, 254–257. [Google Scholar]
- (248).Bhatt D; Patel N; Chowdhury H; Bharatam PV; Goswami A Additive Controlled Switchable Selectivity from Cyanobenzenes to 2-Alkynylpyridines: Ruthenium(II) Catalyzed [2+2+2] Cycloadditions of Diynes and Alkynylnitriles. Adv. Synth. Catal 2018, 360, 1876–1882. [Google Scholar]
- (249).Amakasu T; Sato K; Ohta Y; Kitazawa G; Sato H; Oumiya K; Kawakami Y; Takeuchi T; Kabe Y CpCo(I)- and Cp*Ru(II)Cl-Catalyzed [2+2+2] Cycloadditions of Siladiynes and Alkynes; A Combined Experimental and Theoretical Study. J. Organomet. Chem 2020, 905, 121006–121117. [Google Scholar]
- (250).Cadierno V; Garcia-Garrido SE; Gimeno J Efficient Intermolecular [2+2+2] Alkyne Cyclotrimerization in Aqueous Medium Using a Ruthenium(IV) Precatalyst. J. Am. Chem. Soc 2006, 128, 15094–15095. [DOI] [PubMed] [Google Scholar]
- (251).Cadierno V; Francos J; Garcia-Garrido SE; Gimeno J Ruthenium-Catalyzed Intermolecular [2+2+2] Alkyne Cyclotrimerization in Aqueous Media under Microwave Irradiation. Green Chem. Lett. Rev 2011, 4, 55–61. [Google Scholar]
- (252).Grigg R; Kilner C; Senthilnanthanan M; Seabourne CR; Sridharan V; Murrer BA Cyclometallated Ir(III), Rh(III) and Ru(II) Complexes as Catalysts for the Cyclotrimerisation of 1,6-Diynes with Monoynes. ARKIVOC 2007, xi, 145–160. [Google Scholar]
- (253).Kawatsura M; Yamamoto M; Namioka J; Kajita K; Hirakawa T; Itoh T Ruthenium-Catalyzed Regioselective [2+2+2] Cyclotrimerization of Trifluoromethyl Group Substituted Internal Alkynes. Org. Lett 2011, 13, 1001–1003. [DOI] [PubMed] [Google Scholar]
- (254).Geetharani K; Tussupbayev S; Borowka J; Holthausen MC; Ghosh S A Mechanistic Study of the Utilization of arachno-Diruthenaborane [(Cp*RuCO)2B2H6] as an Active Alkyne-Cyclotrimerization Catalyst. Chem. Eur. J 2012, 18, 8482–8489. [DOI] [PubMed] [Google Scholar]
- (255).Jaceuet J; Auvinet A; Mandadapu A,K; Haddad M; Ratovelomanana-Vidal V; Michelet V Practical Solvent-Free Ruthenium Trichloride-Mediated Benzannulation Approach to Fused Functionalized Arenes. Adv. Synth. Catal 2015, 357, 1387–1392. [Google Scholar]
- (256).Ye F; Haddad M; Michelet V; Ratovelomanana-Vidal V Access toward Fluorenone Derivatives through Solvent-Free Ruthenium Trichloride Mediated [2+2+2] Cycloadditions. Org. Lett 2016, 18, 5612–5615. [DOI] [PubMed] [Google Scholar]
- (257).Yamamoto Y; Kitahara H; Hattori R; Itoh K Ruthenium-Catalyzed Tandem [2+2+2]/[4+2] Cycloaddition of 1,6-Heptadiyne with Norbornene. Organometallics 1998, 17, 1910–1912. [Google Scholar]
- (258).Yamamoto Y; Kitahara H; Ogawa R; Itoh K Cp*Ru(cod)Cl-Catalyzed [2+2+2] Cycloaddition of 1,6-Heptadiynes with Allylic Ethers. A Decisive Role of Coordination to the Ether Oxygen Atom. J. Org. Chem 1998, 63, 9610–9611. [Google Scholar]
- (259).Yamamoto Y; Kitahara H; Ogawa R; Kawaguchi H; Tatsumi K; Itoh K Ru(II)-Catalyzed Cycloadditions of 1,6-Heptadiynes With Alkenes: New Synthetic Potential of Ruthenacyclopentatrienes as Biscarbenoids in Tandem Cyclopropanation of Bicycloalkenes and Heteroatom-Assisted Cyclocotrimerization of 1,6-Heptadiynes with Heterocyclic Alkenes. J. Am. Chem. Soc 2000, 122, 4310–4319. [Google Scholar]
- (260).Varela JA; García-Rubín S; Castedo L; Saá C “Formal” and Standard Ruthenium-Catalyzed [2+2+2] Cycloaddition Reaction of 1,6-diynes to Alkenes: A Mechanistic Density Functional Study. J. Org. Chem 2008, 73, 1320–1332. [DOI] [PubMed] [Google Scholar]
- (261).Varela JA; García-Rubín S; González-Rodríguez C; Castedo L; Saá C A New Ru-Catalyzed Cascade Reaction Forming Polycyclic Cyclohexadienes from 1,6-Diynes and Alkenes. J. Am. Chem. Soc 2006, 128, 9262–9263. [DOI] [PubMed] [Google Scholar]
- (262).García-Rubín S; Varela JA; Castedo L; Saá C Recent Advances in “Formal” Ruthenium-Catalyzed [2+2+2] Cycloaddition Reactions of Diynes to Alkenes. Chem. Eur. J 2008, 14, 9772–9778. [DOI] [PubMed] [Google Scholar]
- (263).Cheng C-C; Chang C-S; Hsu Y-L; Lee T-Y; Chang L-C; Liu S-H; Wu Y-T Ruthenium-Catalyzed Cascade Reactions of Diynes with Norbornadiene - Synthesis of Norbornene Derivatives. Eur. J. Org. Chem 2010, 2010, 672–679. [Google Scholar]
- (264).Kondo T; Kaneko Y; Tsunawaki F; Okada T; Shiotsuki M; Morisaki Y; Mitsudo T Novel Synthesis of Benzenepolycarboxylates by Ruthenium-Catalyzed Cross-Benzannulation of Acetylenedicarboxylates with Allylic Compounds. Organometallics 2002, 21, 4564–4567. [Google Scholar]
- (265).García-Rubín S; González-Rodríguez C; García-Yebra C; Varela JA; Esteruelas MA; Saá C Dihydrobiphenylenes through Ruthenium-Catalyzed [2+2+2] Cycloadditions of Ortho-Alkenylarylacetylenes with Alkynes. Angew. Chem. Int. Ed 2014, 53, 1841–1844. [DOI] [PubMed] [Google Scholar]
- (266).Liu R; Giordano L Tenaglia A Ruthenium-Catalyzed [2+2+2] Cycloaddition of 1,6-Enynes and Unactivated Alkynes: Access to Ring-Fused Cyclohexadienes. Chem. Asian J 2017, 12, 2245–2257. [DOI] [PubMed] [Google Scholar]
- (267).Liu R; Tie Y; Zhao X; Zhu J; Zou J; Liu T Mechanistic Insight into the Ruthenium-Catalyzed Cycloaddition of Enynes with Alkynes: A Theoretical Study. J. Organomet. Chem 2018, 875, 46–51. [Google Scholar]
- (268).Feng C; Wang X; Wang B-Q; Zhao K-Q; Hu P; Shi Z-J One Stone Two Birds: Construction of Polysubstituted Benzenes from the Same Starting Material and Precatalyst by Switching the Active Sites of Catalyst with Different Additives. Chem. Commun 2012, 48, 356–358. [DOI] [PubMed] [Google Scholar]
- (269).Alvarez S; Medina S; Domínguez G; Pérez-Castells J Ruthenium Alkylidene-Catalyzed Reaction of 1,6-Heptadiynes with Alkenes. J. Org. Chem 2015, 80, 2436–2442. [DOI] [PubMed] [Google Scholar]
- (270).Tanaka D; Sato Y; Mori M Ruthenium-Catalyzed [2+2+2] Cocyclization of Diene-yne. J. Am. Chem. Soc 2007, 129, 7730–7731. [DOI] [PubMed] [Google Scholar]
- (271).Saito N; Ichimaru T; Sato Y Ruthenium-Catalyzed Intramolecular [2+2+2] Cyclization of Allene-Yne-Enes: Construction of Fused-Tricyclic Skeletons. Chem. Asian. J 2012, 7, 1521–1523. [DOI] [PubMed] [Google Scholar]
- (272).Yamamoto Y; Takagishi H; Itoh K Ruthenium(II)-Catalyzed Cycloaddition of 1,6-Diynes with Isocyanates Leading to Bicyclic Pyridones. Org. Lett 2001, 3, 2117–2119. [DOI] [PubMed] [Google Scholar]
- (273).Yamamoto Y; Takagishi H; Itoh K Ruthenium-Catalyzed Cycloaddition of 1,6-Diynes with Isothiocyanates and Carbon Disulfide: First Transition-Metal Catalyzed [2+2+2] Cocyclotrimerization Involving C=S Double Bond. J. Am. Chem. Soc 2002, 124, 28–29. [DOI] [PubMed] [Google Scholar]
- (274).Dazinger G; Schmid R; Kirchner K Mechanism of the Ruthenium-Catalyzed Formation of Pyrane-2-one and their Sulfur and Selen Analogs from Acetylene and CX2 (X=O, S, Se): A Theoretical Study. New J. Chem 2004, 28, 153–155. [Google Scholar]
- (275).Schmid R; Kirchner K Theoretical Study of the Ruthenium-Catalyzed Cyclocotrimerization of Alkynes with Isocyanates and Isothiocyanates: Chemoselective Formation of Pyridine-2-one and Thiopyrane-2-imine. J. Org. Chem 2003, 68, 8339–8344. [DOI] [PubMed] [Google Scholar]
- (276).Yamamoto Y; Kinpara K; Saigoku T; Takagishi H; Okuda S; Nishiyama H; Itoh K Cp*RuCl-Catalyzed [2+2+2] Cycloadditions of α,ω-Diynes with Electron-Deficient Carbon-Heteroatom Multiple Bonds Leading to Heterocycles. J. Am. Chem. Soc 2005, 127, 605–613. [DOI] [PubMed] [Google Scholar]
- (277).Alvarez S; Medina S; Domínguez G; Pérez-Castells J [2+2+2] Cyclotrimerization of Alkynes and Isocyanates/Isothiocyanates Catalyzed by Ruthenium-Alkylidene Complexes. J. Org. Chem 2013, 78, 9995–10001. [DOI] [PubMed] [Google Scholar]
- (278).Yamamoto Y; Ogawa R; Itoh K Significant Chemo- and Regioselectivities in the Ru(II)- Catalyzed [2+2+2] Cycloaddition of 1,6-Diynes with Dicyanides. J. Am. Chem. Soc 2001, 123, 6189–6190. [DOI] [PubMed] [Google Scholar]
- (279).Yamamoto Y; Okuda S; Itoh K Ruthenium(II)-Catalyzed [2+2+2] Cycloaddition of 1,6-Diynes with Electron-Deficient Nitriles. Chem. Commun 2001, 1102–1103. [Google Scholar]
- (280).Yamamoto Y; Kinpara K; Nishiyama H; Itoh K Synthesis of 2-Haloalkylpyridines via Cp*RuCl-Catalyzed Cycloaddition of 1,6-Diynes with α-Halonitriles. Unusual Halide Effect in Catalytic Cyclocotrimerization. Adv. Synth. Catal 2005, 347, 1913–1916. [Google Scholar]
- (281).Yamamoto Y; Kinpara K; Ogawa R; Nishiyama H; Itoh K Ruthenium-Catalyzed Cycloaddition of 1,6-Diynes and Nitriles under Mild Conditions: Role of the Coordinating Group of Nitriles. Chem. Eur. J 2006, 12, 5618–5631. [DOI] [PubMed] [Google Scholar]
- (282).Bednářova E; Colacino E; Lamaty F; Kotora M A Ruthenium Complex-Catalyzed Cyclotrimerization of Halo-diynes with Nitriles. Synthesis of 2- and 3-Halopyridines. Adv. Synth. Catal 2016, 358, 1916–1923. [Google Scholar]
- (283).Severa L; Vávra J; Kohoutová A; Čížková M; Šálová T; Hývl J; Šaman D; Pohl R; Adriaenssens L; Teplý F Air-Tolerant C-C Bond Formation via Organometallic Ruthenium Catalysis: Diverse Catalytic Pathways Involving (C5Me5)Ru or (C5H5)Ru Are Robust to Molecular Oxygen. Tetrahedron Lett. 2009, 50, 4526–4528. [Google Scholar]
- (284).Xu F; Wang C; Li X; Wan B Ruthenium-Catalyzed [2+2+2] Cycloaddition of Diynes with Nitriles in Pure Water. ChemSusChem. 2012, 5, 854–857. [DOI] [PubMed] [Google Scholar]
- (285).Chowdhury H; Goswami A A Quick Access to 1-(2-Pyridyl)indoles via Solvent-Free Ruthenium(II)-Catalyzed Chemo- and Regioselective [2+2+2] Cycloaddition of α,ω-Diynes and N-Cyanoindoles. Adv. Synth. Catal 2017, 359, 314–322. [Google Scholar]
- (286).Chowdhury H; Goswami A Synthesis of 3-(2-thiopyridyl)indoles via the Ruthenium Catalyzed [2+2+2] Cycloaddition of Diynes and 3-thiocyanatoindoles. Org. Biomol. Chem 2017, 15, 5824–5830. [DOI] [PubMed] [Google Scholar]
- (287).Kalaramna P; Bhatt D; Sharma H; Goswami A An Expeditious and Environmentally-Benign Approach to 2-Aryl/Heteroaryl Selenopyridines via Ruthenium Catalyzed [2+2+2] Cycloadditions. Eur. J. Org. Chem 2019, 2019, 4694–4700. [Google Scholar]
- (288).Bhatt D; Singh PR; Kalaramna P Kumar K; Goswami A An Atom-Economical Approach to 2-Triazolyl Thio-/Selenopyridines via Ruthenium-Catalyzed One-Pot [3+2]/[2+2+2] Cycloadditions. Adv. Synth. Catal 2019, 361, 5483–5489. [Google Scholar]
- (289).Kalaramna P; Bhatt D; Sharma H; Goswami A An Atom-Economical Approach to 2-Aryloxypyridines and 2,2’/2,3’-Diaryloxybipyridines via Ruthenium-Catalyzed [2+2+2] Cycloadditions. Adv. Synth. Catal 2019, 361, 4379–4385. [Google Scholar]
- (290).Bhatt D; Kalaramna P; Kumar K; Goswami A Chemoselective Ru(II)-Catalyzed Synthesis of Aryl Thiocyanates and Step-wise Double [2+2+2] Cycloadditions to 2-Aryl Thiopyridines. Eur. J. Org. Chem 2020, 2020, 4606–4611. [Google Scholar]
- (291).Nissen F; Richard V; Alayrac C; Witulski B Synthesis of β- and γ-Carbolines via Ruthenium and Rhodium Catalyzed [2+2+2] Cycloadditions of Yne-Ynamides with Methylcyanoformate. Chem. Commun 2011, 47, 6656–6658. [DOI] [PubMed] [Google Scholar]
- (292).Nissen F; Detert H Total Synthesis of Lavendamycin by a [2+2+2] Cycloaddition. Eur. J. Org. Chem 2011, 2011, 2845–2853. [Google Scholar]
- (293).Miyazaki Y; Tanka F; Takeshita K; Mori A Synthetic Approach to N,N,N’,N’-Tetrakis[(2-pyridylmethyl)ethylenediamine] (TPEN) Derivatives through Ruthenium-Catalyzed-[2+2+2] Cycloaddition. Heterocycles 2012, 84, 527–535. [Google Scholar]
- (294).Ye F; Haddad M; Michelet V; Ratovelomanana-Vidal V Solvent-Free Ruthenium Trichloride-Mediated [2+2+2] Cycloaddition of α,ω-Diynes and Cyanamides: A Convenient Access to 2-Aminopyridines. Org. Chem. Front 2017, 4, 1063–1068. [Google Scholar]
- (295).Ye F; Haddad M; Ratovelomanana-Vidal V; Michelet V Ruthenium-Catalyzed [2+2+2] Cycloaddition Reaction Forming 2-Aminopyridine Derivatives from α,ω-Diynes and Cyanamides. Org. Lett 2017, 19, 1104–1107. [DOI] [PubMed] [Google Scholar]
- (296).Ye F; Boukattaya F; Haddad M; Ratovelomanana-Vidal V; Michelet V Synthesis of 2-aminopyridines via Ruthenium-Catalyzed [2+2+2] Cycloaddition of 1,6- and 1,7-Diynes with Cyanamides: Scope and Limitations. New. J. Chem 2018, 42, 3222–3235. [Google Scholar]
- (297).Ye F; Tran C; Jullien L; Saux TL; Haddad M; Michelet V; Ratovelomanana-Vidal V Synthesis of Fluorescent Azafluorenones and Derivatives via a Ruthenium-Catalyzed [2+2+2] Cycloaddition. Org. Lett 2018, 20, 4950–4953. [DOI] [PubMed] [Google Scholar]
- (298).Tran C; Haddad M; Ratovelomanana-Vidal V Ruthenium-Catalyzed, Microwave-Mediated [2+2+2] Cycloaddition: A Useful Combination for the Synthesis of 2-Aminopyridines. Synlett 2019, 30, 1891–1894. [Google Scholar]
- (299).Tran C; Haddad M; Ratovelomanana-Vidal V Ruthenium-Catalyzed [2+2+2] Cycloaddition of α,ω-Diynes and Selenocyanates: An Entry to Selenopyridine Derivatives. Synthesis 2019, 51, 2532–2541. [Google Scholar]
- (300).Medina S; Dominguez G; Perez-Castells J Efficient Generation of Pyridines by Ruthenium Carbene Mediated [2+2+2] Cyclotrimerization. Org. Lett 2012, 14, 4982–4985. [DOI] [PubMed] [Google Scholar]
- (301).Varela J; Castedo L; Saá C Scope of Ru(II)-Catalyzed Synthesis of Pyridines from Alkynes and Nitriles. J. Org. Chem 2003, 68, 8595–8598. [DOI] [PubMed] [Google Scholar]
- (302).Yamamoto Y; Takagishi H; Itoh K Ruthenium(II)-Catalyzed [2+2+2] Cycloaddition of 1,6-Diynes with Tricarbonyl Compounds. J. Am. Chem. Soc 2002, 124, 6844–6845. [DOI] [PubMed] [Google Scholar]
- (303).Montero-Campillo MM; Rodríguez-Otero J; Cabaleiro-Lago EM An Alternative Mechanism to Explain the Ruthenium(II)-Catalyzed [2+2+2] Cycloaddition of 1,6-Diynes and Tricarbonyl Compounds. J. Phys. Chem. A 2009, 113, 9180–9184. [DOI] [PubMed] [Google Scholar]
- (304).Yamamoto Y; Okude Y; Mori S; Shibuya M Combined Experimental and Computational Study on Ruthenium(II)-Catalyzed Reactions of Diynes with Aldehydes and N,N-Dimethylformamide. J. Org. Chem 2017, 82, 7964–7973. [DOI] [PubMed] [Google Scholar]
- (305).Otake Y; Tanaka R; Tanaka K Cationic Rhodium(I)/H8-Binap Complex Catalyzed [2+2+2] Cycloadditions of 1,6- and 1,7-Diynes with Carbonyl Compounds. Eur. J. Org. Chem 2009, 2009, 2737–2747. [Google Scholar]
- (306).Sato H; Bender M; Chen W; Krische MJ Diols, α-Ketols, and Diones as 22π Components in [2+2+2] Cycloadditions of 1,6-Diynes via Ruthenium(0)-Catalyzed Transfer Hydrogenation. J. Am. Chem. Soc 2016, 138, 16244–16247. [DOI] [PubMed] [Google Scholar]
- (307).Suravarapu SR; Parvathaneni SP; Bender JA; Roberts ST; Krische MJ Benzannulation via Ruthenium(0)-Catalyzed Transfer Hydrogenative Cycloaddition: Precision Synthesis and Photophysical Characterization of Soluble Diindenoperylenes. Chem. Eur. J 2020, 26, 7504–7510. [DOI] [PubMed] [Google Scholar]
- (308).Fukuyama T; Higashibeppu Y; Yamaura R; Ryu I Ru-Catalyzed Intermolecular [3+2+1] Cycloaddition of α,β-Unsaturated Ketones with Silylacetylenes and Carbon Monoxide Leading to α-Pyrones. Org. Lett 2007, 9, 587–589. [DOI] [PubMed] [Google Scholar]
- (309).Ngai M-Y; Kong J-R; Krische MJ Hydrogen-Mediated C-C Bond Formation - A Broad New Concept in Catalytic C-C Coupling. J. Org. Chem 2007, 72, 1063–1072. [DOI] [PubMed] [Google Scholar]
- (310).Iida H; Krische MJ Catalytic Reductive Coupling of Alkenes and Alkynes to Carbonyl Compounds and Imines Mediated by Hydrogen. Top. Curr. Chem 2007, 279, 77–104. [Google Scholar]
- (311).Hassan A; Krische MJ Unlocking Hydrogenation for C-C Bond Formation: A Brief Overview of Enantioselective Methods. Org. Proc. Res. Devel 2011, 15, 1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (312).Moran J; Krische MJ Formation of C-C Bonds via Ruthenium Catalyzed Transfer Hydrogenation. Pure Appl. Chem 2012, 84, 1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (313).Nguyen KD; Park BY; Luong T; Sato H; Garza VJ; Krische MJ Metal-catalyzed reductive coupling of olefin-derived nucleophiles: Reinventing carbonyl addition. Science 2016, 354, aah5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (314).Kim SW; Zhang W; Krische MJ Catalytic Enantioselective Carbonyl Allylation and Propargylation via Alcohol Mediated Hydrogen Transfer: Merging the Chemistry of Grignard and Sabatier. Acc. Chem. Res 2017, 50, 2371–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (315).Doerksen RS; Meyer CC; Krische MJ Feedstock Reagents in Metal-Catalyzed Carbonyl Reductive Coupling: Minimizing Preactivation for Efficiency in Target Oriented Synthesis. Angew. Chem., Int. Ed 2019, 58, 14055–14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (316).Geary LM; Glasspoole BW; Kim MM; Krische MJ Successive C-C Coupling of Dienes to Vicinally Dioxygenated Hydrocarbons: Ruthenium Catalyzed [4+2] Cycloaddition across the Diol, Hydroxycarbonyl, or Dione Oxidation Levels. J. Am. Chem. Soc 2013, 135, 3796–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (317).Kasun ZA; Geary LM, Krische MJ Ring Expansion of Cyclic 1,2-Diols to Form Medium Sized Rings via Ruthenium Catalyzed Transfer Hydrogenative [4+2] Cycloaddition. Chem. Commun 2014, 50, 7545–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (318).Park BY; Montgomery TP; Garza VJ; Krische MJ Ruthenium Catalyzed Hydrohydroxyalkylation of Isoprene Employing Heteroaromatic Secondary Alcohols: Isolation and Reversible Formation of the Putative Metallacycle Intermediate. J. Am. Chem. Soc 2013, 135, 16320–16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (319).Geary LM; Chen T-Y; Montgomery TP; Krische MJ Benzannulation via Ruthenium-Catalyzed Diol-Diene [4+2] Cycloaddition: One- and Two-Directional Syntheses of Fluoranthenes and Acenes. J. Am. Chem. Soc 2014, 136, 5920–5922. [DOI] [PubMed] [Google Scholar]
- (320).Kasun ZA; Sato H; Nie J; Mori Y; Bender JA; Roberts ST; Krische MJ Alternating Oligo(o,p-phenylenes) via Ruthenium Catalyzed Diol-Diene Benzannulation: Orthogonality to Cross-Coupling Enables de novo Nanographene and PAH Construction. Chem. Sci 2018, 9, 7866–7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (321).Sato H; Bender JA; Roberts ST; Krische MJ Helical Rod-like Phenylene Cages via Ruthenium Catalyzed Diol-Diene Benzannulation: A Cord of Three Strands. J. Am. Chem. Soc 2018, 140, 2455–2459. [DOI] [PubMed] [Google Scholar]
- (322).Sato H; Blemker MA; Hellinghausen G; Armstrong DW; Nafie JW; Roberts ST; Krische MJ Triple Helical Ir(ppy)3 Phenylene Cage Prepared by Diol-Mediated Benzannulation: Synthesis, Resolution, Absolute Stereochemistry and Photophysical Properties. Chem. Eur. J 2019, 25, 8719–8724. [DOI] [PubMed] [Google Scholar]
- (323).Saxena A; Perez F; Krische MJ Ruthenium(0) Catalyzed Endiyne-α-Ketol [4+2] Cycloaddition: Convergent Assembly of Type II Polyketide Substructures via C-C Bond Forming Transfer Hydrogenation. J. Am. Chem. Soc 2015, 137, 5883–5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (324).Saxena A; Perez F; Krische MJ Ruthenium(0)-Catalyzed [4+2] Cycloaddition of Acetylenic Aldehydes with α-Ketols: Convergent Construction of Angucycline Ring Systems. Angew. Chem. Int. Ed 2016, 55, 1493–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (325).Bender M; Turnbull BWH; Ambler BR Krische MJ Ruthenium-Catalyzed Insertion of Adjacent Diol Carbon Atoms into C-C Bonds: Entry to Type II Polyketides. Science 2017, 357, 779–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (326).Ambler BR; Turnbull BWH; Suravarapu SR; Uteuliyev MM; Huynh NO; Krische MJ Enantioselective Ruthenium-Catalyzed Benzocyclobutenone-Ketol Cycloaddition: Merging C-C Bond Activation and Transfer Hydrogenative Coupling for Type II Polyketide Construction. J. Am. Chem. Soc 2018, 140, 9091–9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (327).Brown RE; Toste FD; Trost BM A Ruthenium-Catalyzed Hydrative Cyclization and [4+2] Cycloaddition of Yne-enones. J. Am. Chem. Soc 2000, 122, 5877–5878. [Google Scholar]
- (328).Kossler D; Cramer N Chiral Cationic CpRu(II) Complexes for Enantioselective Yne-Enones Cyclizations. J. Am. Chem. Soc 2015, 137, 12478–12481. [DOI] [PubMed] [Google Scholar]
- (329).Kondo T; Taguchi Y; Kaneko Y; Niimi M; Mitsudo T Ru- and Rh-Catalyzed C-C Bond Cleavage of Cyclobutenones: Reconstructive and Selective Synthesis of 2-Pyranones, Cyclopentenes, and Cyclohexenones. Angew. Chem. Int. Ed 2004, 43, 5369–5372. [DOI] [PubMed] [Google Scholar]
- (330).Kamitani A; Chatani N; Morimoto T; Murai S Carbonylative [5 + 1] Cycloaddition of Cyclopropyl Imines Catalyzed by Ruthenium Carbonyl Complex. J. Org. Chem 2000, 65, 9230–9233. [DOI] [PubMed] [Google Scholar]
- (331).Li T; Xu F; Li X; Wang C; Wan B Ruthenium-Catalyzed C-C Bond Cleavage of 2H-Azirines: A Formal [3+2+2] Cycloaddition to Fused Azepine Skeletons. Angew. Chem. Int. Ed 2016, 55, 2861–2865. [DOI] [PubMed] [Google Scholar]
- (332).Liu R; Zhang J; Han L; Liu T Mechanistic Insight Into the Ruthenium-Catalyzed Cycloaddition of Diynes With 2,3-Diphenyl-2H-Azirines: A Theoretical Study. Comp. Theor. Chem 2018, 1127, 16–21. [Google Scholar]
- (333).Wang J; Blaszczyk SA; Li X; Tang W Transition Metal-Catalyzed Selective Carbon-Carbon Bond Cleavage of Vinylcyclopropanes in Cycloaddition Reactions. Chem. Rev 2020, DOI: 10.1021/acs.chemrev.0c00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (334).Trost BM; Toste FD; Shen H Ruthenium-Catalyzed Intramolecular [5+2] Cycloadditions. J. Am. Chem. Soc 2000, 122, 2379–2380. [Google Scholar]
- (335).Hong X; Trost BM; Houk KN Mechanism and Origins of Selectivity in Ru(II)-Catalyzed Intramolecular (5+2) Cycloadditions and Ene Reactions of Vinylcyclopropanes and Alkynes from Density Functional Theory. J. Am. Chem. Soc 2013, 135, 6588–6600. [DOI] [PubMed] [Google Scholar]
- (336).Trost BM; Shen HC On the Regioselectivity of the Ru-Catalyzed Intramolecular [5+2] Cycloaddition. Org. Lett 2000, 2, 2523–2525. [DOI] [PubMed] [Google Scholar]
- (337).Trost BM; Shen HC; Schulz T; Koradin C; Schirok H On the Diastereoselectivity of Ruthenium-Catalyzed [5+2] Cycloadditions. Org. Lett 2003, 5, 4149–4151. [DOI] [PubMed] [Google Scholar]
- (338).Haller J; Strassner T; Houk KN Models for Stereoselective Additions to Chiral Allylic Ethers: Osmium Tetroxide Bis-hydroxylations. J. Am. Chem. Soc 1997, 119, 8031–8034. [Google Scholar]
- (339).Houk KN; Moses SR; Wu Y-D; Rondan NG; Jager V; Schohe R; Fronczek FR Stereoselective Nitrile Oxide Cycloadditions to Chiral Allyl Ethers and Alcohols. The Inside Alkoxy Effect. J. Am. Chem. Soc 1984, 106, 3880–3882. [Google Scholar]
- (340).Stork G; Kahn M A Highly Stereoselective Osmium Tetroxide-Catalyzed Hydroxylation of γ-Hydroxy α,β-Unsaturated Esters. Tetrahedron Lett 1983, 24, 3951–3954. [Google Scholar]
- (341).Trost BM; Shen HC Constructing Tricyclic Compounds Containing a Seven-Membered Ring by Ruthenium-Catalyzed Intramolecular [5+2] Cycloadditions. Angew. Chem. Int. Ed 2001, 40, 2313–2316. [DOI] [PubMed] [Google Scholar]
- (342).Trost BM; Shen HC; Horne DB; Toste FD; Steinmetz BG; Koradin C Syntheses of Seven-Membered Rings: Ruthenium-Catalyzed Intramolecular [5+2] Cycloadditions. Chem. Eur. J 2005, 11, 2577–2590. [DOI] [PubMed] [Google Scholar]
- (343).Trost BM; Hu Y; Horne DB Total Synthesis of (+)-Frondosin A. Application of the Ru-Catalyzed [5+2] Cycloaddition. J. Am. Chem. Soc 2007, 129, 11781–11790. [DOI] [PubMed] [Google Scholar]
- (344).Trost BM; Waser J; Meyer A Total Synthesis of (−)-Pseudolaric Acid B. J. Am. Chem. Soc 2007, 129, 14556–14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (345).Wender PA; Williams TJ [(Arene)Rh(cod)]+ Complexes as Catalysts for [5+2] Cycloaddition Reactions. Angew. Chem. Int. Ed 2002, 41, 4550–4553. [DOI] [PubMed] [Google Scholar]
- (346).Trost BM; Nguyen HM; Koradin C Synthesis of a Tricyclic Core of Rameswaralide. Tetrahedron Lett 2010, 51, 6232–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]


