Abstract
Chromatin regulators have recently emerged as key players in the control of tissue development and tumorigenesis. One specific chromatin regulator, the Polycomb complex, has been shown to regulate the identity of embryonic stem cells, but its role in controlling fates of multipotent progenitors in developing tissues is still largely unknown. Recent findings have revealed that this complex plays a critical role in control of skin stem cell renewal and differentiation. Moreover, the expression of Polycomb complex components is often aberrant in skin diseases, including skin cancers. This review will detail recent findings on Polycomb control of skin and highlight critical unknown questions.
Keywords: Chromatin regulators, Skin cancer, Skin, Stem cells, Tissue development
Introduction
Polycomb complex architecture and mechanism of action
One of the fundamental goals of modern biology is to uncover the molecular mechanisms by which stem cells control development of tissues and organs during embryogenesis, as well as their maintenance during adulthood. Increasing evidence has pointed to the roles of chromatin regulators in these processes [1–3]. Chromatin regulators belong to a large class of proteins whose functions include DNA or histone modifications and chromatin remodeling. By altering the chromatin structure, these regulators change the accessibility to gene promoters by the transcriptional machinery, leading to either activation or silencing of a gene.
Recently, one of the key chromatin regulators, the Polycomb complex, has received a lot of attention due to its important role in the control of tissue development and cancer [4, 5]. Polycomb proteins form chromatin remodeling complexes, referred to as Polycomb-Repressive Complexes (PRC) PRC1 and PRC2 [6]. The PRC2 complex, comprised of Ezh2, Eed, and Suz12, is recruited to chromatin where the methyltransferase Ezh2 catalyzes histone H3 trimethylation on lysine 27 (H3K27me3) [7]. PRC1’s chromo-domain-containing proteins (Cbx) recognize the H3K27me3 histone mark and recruit the PRC1 complex [7, 8]. The PRC1 and PRC2 complexes inhibit transcription by compacting the chromatin and preventing the recruitment of transcriptional machinery to gene promoters [9].
How the PRC2 complex is recruited to DNA is still under investigation. In Drosophila, the Polycomb complex is present at Polycomb response elements (PRE), DNA sequences of several hundred base pairs with no simple consensus. In mammalian cells, however, the existence of PREs has only been reported in a few cases [10, 11]. Moreover, the broad distribution of PRC1 and PRC2 along mammalian genes makes it difficult to pinpoint PREs as sites of Polycomb complex binding [12].
Proteins, short stem-loop RNAs, and long non-coding RNAs (lncRNA) are all considered as good candidates to bridge PRC2 to DNA sites (Fig. 1). In Drosophila, the zinc finger protein Pleiohomeotic (Pho) binds to PRE sites, and ChIP studies have shown that disruption of PHO leads to loss of PRC1/2 binding to the PRE site of a Hox gene [13]. However, the mammalian homolog of PHO, yin and yang 1 (YY1), shows limited overlap in DNA occupancy for YY1 and PRC2 in mouse embryonic stem (ES) cells, suggesting that in mammalian cells YY1 is likely not a general recruiter of the Polycomb complex [14].
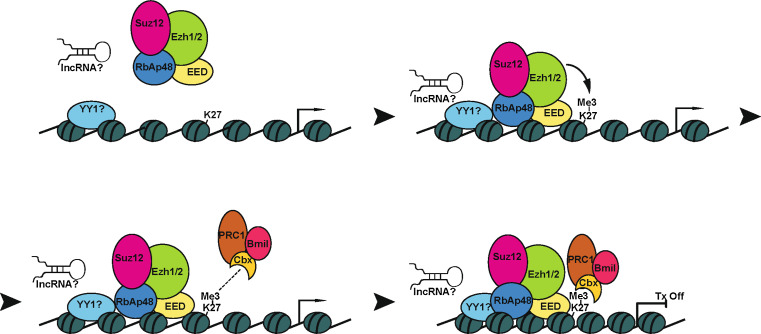
Fig. 1.
A model of gene silencing by the Polycomb complex. In mammals, PRC2 recruiters to DNA have not yet been identified. Candidates include the protein yin and yang 1 (YY1), and long non-coding RNAs (lncRNAs). EZH1/2-mediated H3K27 methylation helps to recruit the PRC1 complex through binding of the Polycomb chromodomain proteins to Me3K27. Recruitment of PRC1 helps to condense the chromatin structure by limiting the access of remodeling factors, leading to transcriptional silencing
LncRNAs gained a lot of attention in recent years as possible recruiters of the Polycomb complex to DNA sites. Xist, a 17-kb lncRNA, directly interacts with PRC2 and navigates its binding to the X chromosome to initiate X chromosome inactivation [15]. The lncRNA HOTAIR interacts with PRC2 and is required for PRC2 occupancy and repression of the HOXD locus [16]. It is still unclear whether lncRNAs directly recognize DNA sequences or serve as a scaffold molecule to mediate interactions between the Polycomb complex and DNA binding proteins. In the case of Xist, it can indeed serve as a scaffold to bridge YY1 with the PRC complex [17]. Interestingly, lncRNAs can also provide binding surfaces between different chromatin regulators. HOTAIR lncRNA can serve as a scaffold for PRC2 and the LSD1/CoREST/REST transcriptional repressor complex that establishes lysine 4 demethylation of histone H3 [18]. The ability to tether these two distinct complexes enables their coordinated recruitment to chromatin, resulting in gene repression [18].
Recent studies in ES cells have shown that short (50–200 nt) RNAs are commonly transcribed from the 5’end of Polycomb target genes [19, 20]. These short RNAs are commonly found at genes that are in the poised state, or marked with both H3K27me3 and H3K4me3, and are transcribed by RNA pol II. Upon transcription, these RNAs form stem-loop structures that interact with PRC2 through Suz12 in a mechanism similar to that of the Xist lncRNA, leading to recruitment of the Polycomb complex and gene repression [21]. One can see how these three potential Polycomb recruitment methods could interact, resulting in highly modular Polycomb repression.
It has been shown that the Polycomb complex compacts the chromatin leading to transcriptional inhibition [9, 22], but the exact transcriptional step that is inhibited by Polycomb-dependent chromatin compaction is still a subject of debate. Some reports suggest that the Polycomb complex represses the recruitment of transcription factors [23, 24] or Pol II [25–27] to gene promoters, whereas another study indicates that repression occurs early in Pol II elongation [28]. Future work should reveal whether these differences are rooted in cell type or gene specificity.
Although Polycomb activity has been mostly studied with respect to transcriptional control, in some cell types PRC2 components are found in the cytoplasm [29]. For example, in T cells, Ezh2-dependent methyltrasferase activity controls actin polymerization [29]. While it is unclear how general this phenomenon is, transcription-independent roles of the Polycomb complex do exist and should be taken into consideration in forthcoming studies.
Polycomb complex: a critical regulator of tissue development
The first evidence that the Polycomb complex controls tissue development came from Drosophila studies with the identification of the Polycomb mutations extra sex combs (esc) and Polycomb (Pc) [30]. These mutations cause the formation of additional sex combs on the second and third legs of males, instead of only on the first leg [6]. Molecular characterization of this phenotype revealed that it was caused by the loss of Hox gene repression, leading to the transformation of one body segment into the identity of another [30]. Later studies showed that the Polycomb complex represses Hox genes, and in Polycomb-mutant flies, these genes are expressed outside their normal spatial territories causing homeotic transformation [31].
Early embryonic lethality of mouse embryos that are null for components of the Polycomb complex suggested an important role for this complex in early development [32–35]. In human and mouse ES cells, Polycomb proteins are present at a large cohort of developmental regulators that promote ES differentiation [25]. Consistent with a causal role in gene silencing, Polycomb-targeted differentiation genes are de-repressed in ES cells lacking the PRC2 component Eed [25]. Importantly, despite elevated expression of differentiation genes, Eed-null ES cells maintain their pluripotency [36].
Intriguingly, further studies of the Polycomb complex in ES cell control revealed that this complex is also important for proper orchestration of the differentiation process. Suz12-null ES cells are unable to differentiate to neuronal cells upon retinoid acid treatment due to the inability to repress Nanog and Oct4, key pluripotency genes [37]. Thus, in ES cells, the Polycomb complex plays a dual role in the control of differentiation: it represses differentiation genes in pluripotent ES cells and downregulates stemness genes in their differentiated progeny. Despite the important role of the Polycomb complex in the control of cultured ES cells, it is unclear whether the same mechanisms apply to in vivo regulation of lineage-committed stem cells. Thus, analysis of Polycomb functions under in vivo conditions is necessary to address these questions.
Skin architecture and skin stem cells
Skin is an excellent and well-characterized model system to uncover key regulators of tissue organogenesis. Skin is the outermost barrier of mammals that protects the body against infection and dehydration [38, 39]. During embryonic development, a single layer of embryonic skin progenitors called basal cells adheres to an underlying basement membrane that separates the epidermis from underlying dermis. These basal cells form the basal layer, which gives rise to skin lineages: the epidermis, hair follicles, and sebaceous glands (Fig. 2)[40].
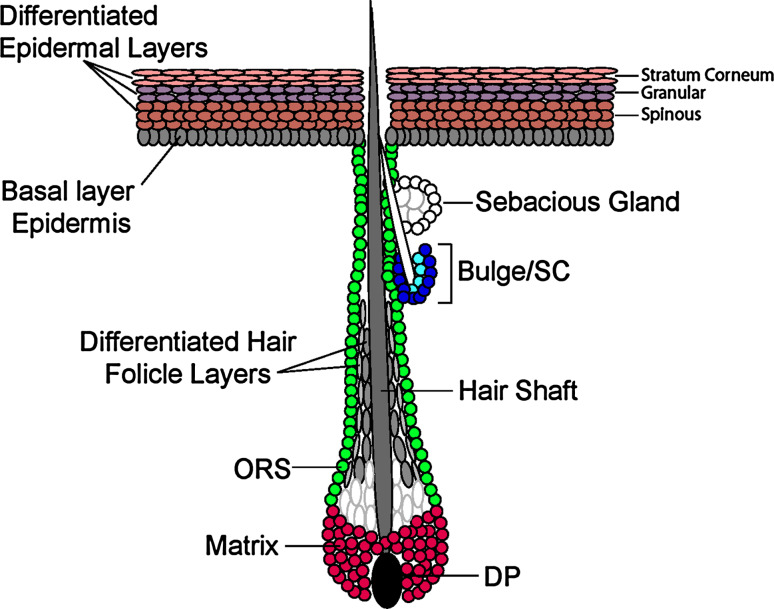
Fig. 2.
Schematic of skin lineages. Skin consists of the epidermis and hair follicles. In the hair follicle (HF), stem cells are thought to reside in the bulge, a region in the outer root sheath (ORS) just below the sebaceous gland (SG). During the growth phase of the hair cycle, stem cells become activated and initiate the downgrowth and regeneration of the ORS. During maturation, a pool of transit-amplifying matrix cells forms at the base of the HF, maintaining contact with the specialized mesenchymal cells, or dermal papilla (DP) of the follicle. Matrix cells divide rapidly several times and then commit to terminally differentiate to form the companion layer (positive for keratin 6), the inner root sheath (IRS; positive for trichohyalin), the hair shaft (HS, positive for hair keratins) and the inner core of the hair, called the medulla (positive for K6 and trichohyalin). Both the SG and the interfollicular epidermis (IFE) have their own resident population of progenitors. In the SG, these are positive for the transcriptional repressor Blimp1 and reside in the ORS at the base of the gland. SG progenitors give rise to PPARγ+ sebocytes, which then apoptose and release sebum into the hair canal. Basal epidermal progenitors reside in the innermost layer of the IFE and are K5/K14 positive. These cells terminally differentiate to first generate suprabasal spinous cells (K1/K10+) that then produce overlying granular cells (loricrin, filaggrin+), which finally undergo apoptosis to produce the flattened squamous cells that are subsequently sloughed from the skin surface
In mice, the development of the epidermis initiates at embryonic day 9 (E9) and completes shortly before birth [38]. Epidermal differentiation starts at E14 [38]. Once cells exit the basal layer, they downregulate proliferation-associated genes and execute a terminal differentiation program [41]. This process is marked by a step-wise transcriptional transition, from the early differentiation stage spinous layers to the late differentiation stage granular layers [42]. In the last step, all metabolic activity ceases as dead squames of the protective stratum corneum are formed and subsequently sloughed from the skin surface [39]. Throughout adulthood, cells that are located in the interfollicular epidermis continually fuel production of these suprabasal layers [43–45].
Hair follicle development initiates at E14.5, when some of the embryonic skin progenitors receive signals from the underlying dermis and start migrating downward [38, 40]. The initial downgrowth continues postnatally, giving rise to a mature follicle that consists of hair follicle stem cells, their transient-amplifying progeny called the matrix, and the terminally differentiating cells of the hair shaft [38, 40](Fig. 2). By postnatal day 21 (P21), the hair follicle stem cells are localized to a specific reservoir called the bulge, where they are maintained in a growth and differentiation-inhibited environment [46](Fig. 2). Multiple studies have shown that distinct pools of stem/progenitor cells maintain the epidermis and hair follicles [44, 45, 47], and even upon wounding, bulge stem cells can only transiently re-epithelialize the epidermis [44, 48–50].
The availability of genetic tools to perform in vivo loss- and gain-of-function studies, the existence of well-characterized molecular markers of stem cells and their differentiated progenies, and the ability to purify and to culture stem cells makes skin a unique model system to uncover key regulators of stemness and differentiation [51]. These advantages have recently led to great advances in understanding the role of the Polycomb complex in skin control that will be discussed next.
Polycomb repression in control of skin development
Expression of Polycomb components in developing skin
To uncover whether the Polycomb complex is involved in skin control, the expression of its components was analyzed in developing skin. At E14, the epidermis consists of a layer of proliferative and undifferentiated basal cells, and a layer of suprabasal cells that have initiated a program of terminal differentiation [39, 52]. At this stage, components of PRC2 (Ezh2, Eed) and PRC1 (Bmi1, Cbx2, Pcgf2) complexes are expressed in basal cells and are downregulated in differentiated suprabasal cells [23, 53]. By E18, epidermal development is completed and the epidermis consists of multiple layers of differentiating suprabasal cells. In fully developed epidermis, Ezh2 expression is strong in basal cells, but weak in the spinous, granular, and stratum corneum layers [23]. In vitro, when cultured basal epidermal cells are exposed to elevated calcium levels, they stop proliferating and undergo terminal differentiation [54]. Similarly to in vivo, the high levels of Polycomb proteins that are normally found in basal cells progressively decline upon calcium-induced differentiation [23]. Thus, key subunits of the Polycomb complex are expressed in epidermal basal cells and downregulated upon differentiation.
Analysis of genes targeted by Polycomb repression in basal epidermal cells revealed that the Polycomb-dependent H3K27me3 histone mark was broadly associated with gene promoters [23]. Among almost 4,000 H3K27me3-targeted genes are ones that are normally expressed in muscle, neuronal, and hematopoietic cells, indicating that the Polycomb complex does not exclusively repress skin genes. Analyses of epidermal genes showed that basal or early differentiation spinous layer specific genes were not targeted by H3K27me3 [23]. On the other hand, a large number of late epidermal differentiation genes that are normally expressed in granular and stratum corneum layers contained H3K27me3 at their promoters [23]. Interestingly, many of the genes targeted by this histone mark are also Polycomb-repressed in ES cells, indicating that, whereas some Polycomb targets established in pluripotent cells are maintained in the epidermal lineage, others lose this mark during tissue development.
EZH2: a key regulator of differentiation in developing epidermis
Extensive biochemical studies showed that K27-H3 methyltransferase, Ezh2, is essential for Polycomb repression [7]. Thus, to gain insight into the significance of Polycomb repression in control of epidermal differentiation, loss-of-Ezh2 studies were performed. Conditional ablation of Ezh2 in skin (Ezh2cKO) resulted in the complete loss of Ezh2 and H3K27me3 in basal cells by E16 [23].
Interestingly, despite broad association of the H3K27me3 histone mark with gene promoters, transcriptional profiling revealed that only skin differentiation genes became upregulated in Ezh2cKO basal cells [23]. These data indicate that additional mechanisms likely maintain non-skin lineage genes in a repressed state and thus prevent their expression upon loss of Ezh2.
Comparative analysis of WT and Ezh2cKO basal cells revealed that the majority of genes upregulated in Ezh2cKO basal cells were those expressed during the normal course of terminal differentiation in the epidermis [23]. The largest differences were among genes encoding major proteins of the epidermal granular (filaggrin, loricrin, involucrin), and stratum corneum (Lce family proteins) layers [39, 55]. Notably, these genes are located within an epidermal differentiation complex (EDC), which is comprised of a large number of genes that are crucial for the maturation of the epidermis [56]. Analysis of the EDC gene cluster revealed that it was decorated by H3K27me3 in WT basal cells and that the majority of EDC genes were upregulated in Ezh2cKO cells [23]. Consistent with de-repression of late differentiation genes, the formation of granular and stratum corneum layers was tempered in Ezh2cKO embryos [23]. Indeed, in E16 Ezh2cKO embryos, a functional epidermal barrier was already formed [23], whereas in WT embryos its development was completed only by E17.
A recent study has shown that the EDC is subject to higher-order chromatin remodeling, and this process appears to be downstream of the p63/Satb1 epidermal differentiation pathway [57]. Interestingly, Polycomb has been shown to form aggregates that result in multi-looped chromatin structures, causing repression of gene regions such as the HOXA cluster in humans and BX-C in Drosophila ([58, 59; reviewed in [60]). While the EDC is both targeted by Polycomb and subject to chromatin conformation alterations, the link between these two mechanisms is still unclear.
In vivo and in vitro studies have revealed that expression of epidermal differentiation genes in the absence of Ezh2 was due to the activity of an AP1 transcription factor [23]. Biochemical studies have shown that, in basal progenitors, the Ezh2-dependent H3K27me3 mark prevented AP1 from binding to and activating late differentiation genes [23]. During the normal program of terminal differentiation, Ezh2 expression is downregulated, the H3K27me3 mark is removed, and AP1 proteins can selectively bind and activate these genes (Fig. 3). By interfering with the recruitment of transcriptional activators to differentiation genes, Polycomb complexes ensure that these genes are repressed until the appropriate stage in development and differentiation.
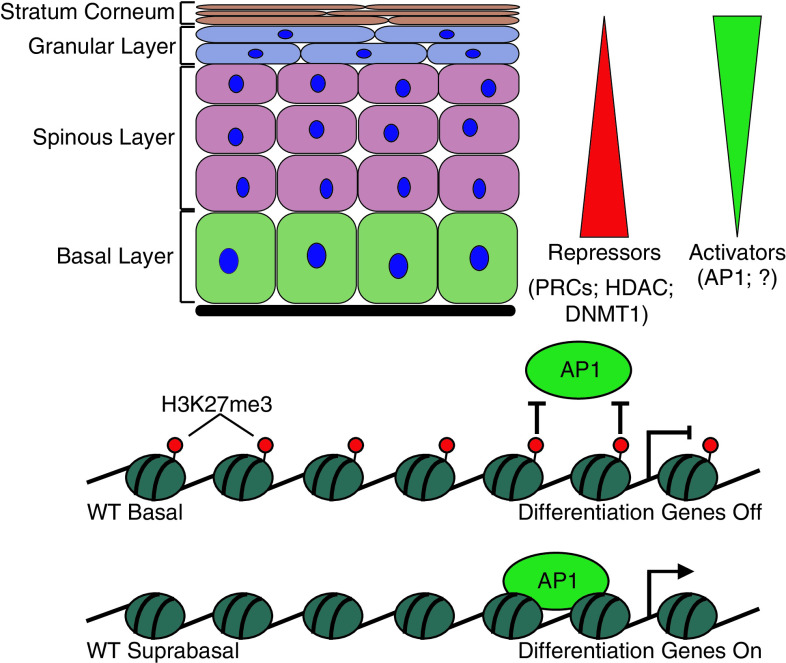
Fig. 3.
The relationship between repressive histone modifiers and transcriptional activators in skin lineage determination. Multiple studies have found a general trend showing an inverse relationship between repressors and activators during progression through terminal differentiation. Repressors are most highly active in the basal layer of the epidermis, keeping differentiation genes off and thus maintaining the stem cell state. As cells start to transition to the differentiated layers, these repressors are downregulated and activators are upregulated, allowing differentiation genes to be turned on in the suprabasal layers
Coordinated regulation of human epidermal homeostasis by the Polycomb complex, Dnmt1, and Jmjd3
Human interfollicular epidermal stem cells do not proliferate continuously, but rather transit between slow cycling and actively proliferating states during homeostasis [61]. Analysis of PRC1’s Cbx proteins has shown that CBX4 controls proliferation and differentiation of epidermal stem cells and protects them from senescence [61]. CBX4 protection from senescence in slow cycling human epidermal stem cells is achieved through its PRC1-associated Polycomb function, while its SUMOylation activity controls pathways required for terminal differentiation [61]. Thus, CBX4 plays a critical role controlling transitions between quiescent and active states.
Analysis of genes targeted by the H3K27me3 mark in in vitro cultured human keratinocytes has revealed similarities to the mouse system, as many key epidermal differentiation genes are targeted by this mark [62]. Upon differentiation, the levels of H3K27me3 at their promoters are reduced and key epidermal differentiation genes are transcribed [62].
H3K27me3 loss upon differentiation was mirrored by a marked reduction in occupancy of PRC2’s SUZ12 at some promoters of differentiation genes [62]. Interestingly, the reduction also coincides with a rise in binding of JMJD3, a member of the Jumanji C (JmjC) domain-containing proteins that are capable of removing methyl marks from lysine 27 of histone H3 [63–65]. Biochemical studies have shown that JMJD3-dependent demethylation of H3K27 leads to activation of gene expression [63].
Functional studies have revealed that, in human skin, expression of active JMJD3 leads to premature epidermal differentiation, whereas a mutant form of JMJD3 that lacks de-methylation activity fails to do so [62]. In concordance with this data, JMJD3 depletion prevents normal induction of the epidermal differentiation program with failure to induce expression of differentiation genes [62]. Consistent with mouse studies, depletion of PRC2 proteins SUZ12 and EZH2 also resulted in expression of epidermal differentiation genes in the absence of calcium [23, 62].
Analysis of the function of the DNA methylation enzyme DNA maintenance methyltranferase (DNMT1) in human keratinocytes added yet another twist in the differentiation control program. DNMT1 is enriched in undifferentiated human keratinocytes, and genome-wide profiling of DNA methylation has revealed that a large number of epidermal differentiation genes are methylated in cultured human keratinocytes, but are demethylated upon differentiation (Fig. 3)[66]. Depletion of DNMT1 leads to exit from the progenitor cell state, premature differentiation, and eventual loss of the epidermis [66].
Integrating these studies, it is plausible to propose a model where spatial and temporal expression of PRCs, Jmjd3, Dnmt1, and AP1 coordinately establish a step-wise mechanism of a terminal differentiation program within the epidermis (Fig. 3). In basal cells, PRCs and DNMT1 target epidermal differentiation genes and prevent association of transcriptional activators with their promoters. Upon differentiation, expression of PRCs and DNMT1 decreases, which coincides with the removal of H3K27me3 and DNA methylation from gene promoters, allowing DNA binding of AP1 and transcription of differentiation genes.
Polycomb complex in control of adult skin and aging
Roles of Ezh1 and Ezh2 in adult skin control
Despite the essential role of Ezh2 for Polycomb repression, its expression is low in many adult mammalian tissues [67–69]. Analysis of the epidermis has revealed that, as in other organs, Ezh2 expression wanes after birth, and is barely detectable by P9 [70]. The decrease in Ezh2 level correlates with postnatal decline in the proliferation of basal cells. Consistent with that, in hair follicles Ezh2 expression is high in the proliferative cells of the outer root sheath (ORS) and matrix, but not in the quiescent follicle stem cells [23].
An important question that follows is how are adult tissues maintained without Ezh2 expression? Interestingly, in many tissues, as Ezh2 level declines, expression of Ezh2-paralogue, Ezh1, rises [67–69]. Ezh1 is a part of the PRC2 complex and is capable of establishing H3K27me3 [68, 71]. Studies in ES cells have shown that Ezh1 and Ezh2 are members of different PRC2 complexes [68, 71], but target the same genes and work coordinately to repress gene expression [68, 71]. Similar to other organs, expression of Ezh1 increases postnatally in skin [23, 70], and is present in basal epidermal cells as well as in bulge stem cells [23].
To gain insight into the role of the Polycomb complex in postnatal skin regulation, ablation of both Ezh1 and Ezh2 was analyzed. Loss of Ezh1 and Ezh2 results in a striking arrest in development and progressive degeneration of hair follicles and hair follicle appendages, i.e., sebaceous glands [70]. Notably, these phenotypes were not observed in singly targeted skin epithelium, indicating functional redundancy between Ezh1 and Ezh2 in postnatal skin control. Despite the striking defect in hair follicle formation, the epidermis of Ezh1/2 2KO skin is intact [70]. Analysis of Ezh1/2 2KO hair follicles revealed decreased proliferation and increased apoptosis [70]. In contrast, proliferative activities within the basal layer of double-knockout epidermis were accelerated and no apoptosis is detected [70], indicating differential roles of Ezh1/2 in control of hair follicle and epidermal lineages.
To start dissecting the molecular pathways by which loss of Polycomb repression leads to the dramatic phenotype in the hair follicle lineage, chromatin immunoprecipitation for H3K27me3 followed by sequencing (ChIP-Seq) was performed on bulge stem cells and their transient amplifying progeny, the matrix. The comparative analysis has revealed that in matrix cells H3K27me3 is acquired by stemness genes and is lost from regulators of hair follicle differentiation, suggesting that Polycomb activity controls the transition to differentiation [72]. Although the analysis of Ezh1/2-null skin revealed that bulge stem cell markers were derepressed in the matrix and matrix regulators were derepressed in bulge cells, expression of these genes was significantly lower than levels seen in normal bulge and matrix, and thus their expressions were unlikely to be responsible for the dramatic phenotype observed in Ezh1/2-null skin [70, 72]. These findings indicate that loss of H3K27me3 is not sufficient to change the fate of bulge stem cell or matrix cells and underscore the importance of additional epigenetic modifiers in maintenance of the hair follicle lineage program.
Further analysis of ChIP-seq and microarray data has revealed dramatic upregulation of Polycomb-regulated Ink4a/Arf and Ink4b genes in Ezh1/2-null cells of both hair follicle and epidermal lineages (Fig. 4). Ink4a and Ink4b genes encode the p16 and p15 inhibitors of the G1/S phase of the cell cycle, and Arf encodes the p19 inhibitor of the p53 suppressor MDM2 [73]. Despite the expression of these genes, Ezh1/2-null epidermal basal cells were proliferative, suggesting that intrinsic mechanisms likely overt the function of this locus [70]. On the other hand, the dramatic defect of the Ezh1/2 2KO hair follicle lineage was indeed rooted in ectopic activation of the Ink4a/Arf/Ink4b locus [70]. In vitro, Ezh1/2-null hair follicle progenitors also failed to proliferate and survive, features that were largely overcome by repressing the Ink4a/Arf/Ink4b locus [70]. These results emphasize the importance of Polycomb-mediated repression of the Ink4a/Arf/Ink4b locus for self-renewal and survival of hair follicle progenitor cells.
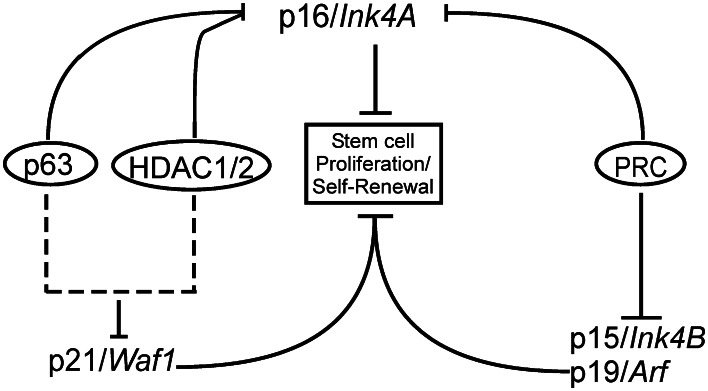
Fig. 4.
Network model of epidermal stem cell control. The proteins p16/Ink4A, p15/Ink4B, p19/Arf and p21/Waf1 are known to be involved in repressing proliferation and self-renewal of epidermal stem cells. However, these genes act through different pathways and are regulated through multiple mechanisms. PRC is responsible for repressing the Ink4a/Arf locus, though HDAC1/2 and PRC are thought to either interact to repress p16/Ink4A or do so in a redundant fashion. The p63 transcription factor is also thought to act on p16/Ink4A and another HDAC1/2 target, p21/Waf1, indicating overlap between these two pathways
HDACs and the Polycomb complex in control of the hair follicle lineage
Histone deacetylases (HDACs) remove acetylation marks from histones, resulting in compaction of chromatin structure and transcriptional repression. The HDAC family members HDAC1 and HDAC2 have been shown to interact with PRC2 components and to work coordinately to facilitate gene repression [74]. Despite physical interaction between HDACs and PRCs, mice with conditional ablation of Hdac1 and Hdac2 in skin showed a more severe phenotype than Ezh1/2-null mice. Analysis of Hdac1/2-null skin has revealed that the epidermis failed to stratify and no hair follicles developed [75]. Interestingly, these defects were reminiscent of many of the phenotypes described in embryos lacking the transcription factor p63, [76, 77] and p63-repressed targets do indeed get upregulated in Hdac1/2-deficient epidermis. The most notable of these targets were p16/Ink4a and p21, which control proliferation, consistent with the proliferation defect in Hdac1/2-null skin.
Thus, despite shared regulation of the p16/Ink4a gene by HDACs and PRCs, strong differences between HDAC-null and Ezh1/2-null phenotypes suggest that in skin these complexes likely control different genes (Fig. 4). Alternatively, HDAC repression might persist even upon loss of Ezh1/2. Future work should focus on clarifying these interactions and reconciling these possibilities.
The Polycomb complex in control of skin aging and wound repair
Genes of the Ink4a/Arf locus are often upregulated upon aging [78]. Interestingly, expression of PRC1 subunit Bmi1 decreases in old skin cells compared to young ones, suggesting a possible decrease in Polycomb activity with aging [79, 80]. Consistent with the role of the Polycomb complex in repression of the Ink4a/Arf locus [70], p16/Ink4a is upregulated in old skin [79].
The ability to repair wounds is one of the critical characteristics of skin. Wound healing is a dramatic and complex tissue rebuilding process that requires immediate changes in expression of genes controlling epidermal proliferation, migration, and differentiation. It has been shown that PRC2 subunits EED, EZH2, and SUZ12 are downregulated during mouse skin repair, whereas H3K27-demethylases JMJD3 and UTX are upregulated [81]. Consistent with that, H3K27me3 is dramatically reduced at the site of wounded epidermis, and EED occupancy is reduced at the wound-induced genes Myc and Egfr, suggesting that loss of polycomb-mediated silencing might contribute to the induction of repair genes [81]. Since differentiation and Ink4a/b-Arf genes remain repressed in basal cells located close to wounds, other repressive mechanisms likely contribute to their repression during the wound repair process.
Polycomb complex in skin diseases and cancer
Polycomb complex in psoriasis
Psoriasis is a skin disorder that is characterized by prominent epidermal hyperplasia and a distinct inflammatory infiltrate [82]. Although psoriasis has for a long time only been considered an autoimmune disease, conditional ablation of components of the AP1 family members JunB and c-Jun in skin results in a phenotype that resembles psoriasis [83, 84]. Thus, crosstalk between immunocytes and keratinocytes, which results in the production of cytokines, chemokines, and growth factors, mediates the disease [82].
Recently, genetic linkage studies implicated mutations within the EDC cluster with two common inflammatory skin disorders, impaired barrier atopic dermatitis and psoriasis [85, 86]. The spatial and temporal expression of several genes in the EDC during epidermal differentiation, as well as in skin diseases, suggests a common genomic mechanism to coordinate their expression. Interestingly, genome-wide association studies identified association of psoriasis to a 30-kb deletion spanning the LCE3C and LCE3B genes (LCE3C_LCE3B-del) [87], where an enhancer element has been recently identified [56]. Since in vivo studies have shown that the Polycomb complex represses EDC [23], it is plausible to hypothesize that LCE3C_LCE3B-del mutation alters the recruitment and/or maintenance of the Polycomb complex to DNA leading to changes in expression of epidermal barrier genes located within this cluster.
Additionally, expression of Polycomb proteins Bmi1 and Ezh2 has been shown to be elevated in skin of psoriatic patients [88]. Future work should reveal PRC-target genes in psoriatic cells and their contribution to disease manifestation.
Polycomb complex in skin cancers
The level of EZH2 is elevated in many solid tumors including prostate, breast, lung, and skin [89–91]. Loss of microRNAs (miRNA) miR-101, or miR-26a has been shown to lead to elevated levels of EZH2 and increased PRC2 activity in tumors [92–94]. In skin, however, both these miRNAs are expressed at low levels [95], leading to the idea that other miRNAs might control Ezh2 levels in skin.
PRC1 components BMI1 and RING2 have also been shown to be elevated in tumors of different organs including skin [96]. Recently, several miRNAs have been implicated in control of BMI1, RING2, and RING1 [97]. Interestingly, expression of these miRNAs is under the control of the Polycomb complex, suggesting an autoregulatory loop in control of PRC protein levels [97].
To address cause-and-effect relationships between Ezh2 function and oncogenesis, the consequences of Ezh2 overexpression have been examined. In breast epithelial cells, Ezh2 overexpression causes anchorage-independent growth and increased cell invasiveness in vitro [98]. Moreover, Ezh2 over-expressing cells are tumorigenic when injected into the mammary fat pads of nude mice [99]. Similarly, overexpression of Ezh2 transforms non-invasive prostate cells into metastatic cells and is also essential for glioblastoma cancer stem cell maintenance [100, 101]. Analysis of immortalized/transformed skin cancer cell lines has revealed increased EZH2 and SUZ12 levels and H3K27me3 formation [102]. Moreover, downregulation of Ezh2 reduces proliferation and survival of SCC-13 skin cancer cells [102]. Together, these data indicate that the presence of H3K27me3 histone mark at genes has to be precisely controlled to prevent inappropriate activation or repression of genes, which can lead to tumorigenesis.
The mechanisms through which the Polycomb complex controls skin tumorigenesis are still largely unknown. Developmental studies have shown that this complex controls both proliferation and differentiation. Thus, it is plausible to hypothesize that misregulation of expression of PRC components transits cells to a state that is more undifferentiated and self-renewing, leading to tumor formation. Alternatively, elevated levels of Polycomb proteins could lead to promiscuous binding of PRCs to tumor suppressor genes that are not normally repressed by the Polycomb complex. For example, overexpression of Bmi1 has been shown to promote keratinocyte survival through increased levels of cyclin D1 and selected cyclin-dependent kinases [103]. BMI1 also protects keratinocytes from stress agent-mediated cell death by reducing caspase activity and poly(ADP-ribose) polymerase (PARP) cleavage [103]. In the future, comparative analysis of in vivo purified skin cancer cells and normal cells should uncover PRC targets that are critical for skin cancers.
Since Polycomb proteins are upregulated in many tumors, agents that reduce PRC protein levels are considered as potential cancer prevention agents. Sulforaphane is a biologically important isothiocyanate found in cruciferous vegetables that is a potential chemoprevention candidate [104]. Recently, treatment of skin cancer cell lines with sulforaphane showed reduction in expression of Ezh2 and Bmi1 and reduction in H3K27me3 leading to G2/M cell cycle arrest and increased apoptosis [104]. Similar results were observed with (-)-epigallocatechin-3-gallate (EGCG), an agent found in green tea [102]. It will be critical to try these agents in skin cancer models as possible anti-cancer agents.
Perspectives
Progress over the past few years has clearly determined an important role of the Polycomb complex in skin control. This knowledge now provides a foundation to tackle the next set of key questions. Here, we highlight some of them.
Molecular mechanisms of PRC recruitment and release from epidermal differentiation genes need to be uncovered. Studies in ES cells have recently implicated Jarid2 in PRCs recruitment [105–107]. However, conditional skin KO mice of Jarid2 and Ezh2 share little similarities, indicating that other proteins are likely involved in PRC recruitment in skin [108]. Analysis of the Polycomb-regulated EDC cluster might help to address this question due to coordinate expression of its genes during differentiation. The location and expression of lncRNAs within the EDC gene cluster, as well as their involvement in Polycomb recruitment, might help to pinpoint epidermal PRC recruiters.
How Polycomb-mediated gene-repression silences epidermal genes should be further studied. In basal cells, Polycomb repression prevents the recruitment of transcriptional activators to promoters of epidermal differentiation genes. It will be important to uncover how general this phenomenon is by analyzing Polycomb-repressed genes in hair follicle cells.
Coordinate regulation of skin genes by chromatin regulators should also be investigated. Recent findings indicate that Dnmt1, Polycomb, and HDACs all target similar genes. The roles of other chromatin regulators and non-coding RNAs in control of skin homeostasis should be evaluated. Additionally, the order of recruitment of these regulators should help to determine the exact mechanism of action of epigenetic regulators in control of skin cell fate.
How elevated PRC levels contribute to skin diseases and tumorigenesis remains unknown. It will be important to uncover PRC-target genes and understand their roles in the progression of cells from normal to disease state. There is also a need to continue exploring small molecule inhibitors of PRC1 and PRC2 as possible drugs for cancer and skin disease therapies.
Finally, the epidermal barrier does not form until shortly before birth. Prematurely born infants lack this shield, and are therefore at a high risk for infection and dehydration [109]. Therefore, accelerating barrier acquisition becomes a critical necessity for their survival. Since loss of Ezh2 accelerates epidermal barrier formation in the embryo but does not impair postnatal development [23], it offers a hitherto unanticipated target for the development of therapies that might be useful for improving infant survival rates. Testing PRC inhibitors as possible agents to accelerate epidermal barrier formation might provide novel therapies to increase the survival of preterm babies. Further mechanistic insight will clearly be needed to fuel both basic and clinically relevant advances in understanding Polycomb gene silencing in skin control.
Footnotes
J. Zhang and E. Bardot contributed equally to this work.
References
- 1.Buszczak M, Spradling AC. Searching chromatin for stem cell identity. Cell. 2006;125:233–236. doi: 10.1016/j.cell.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, Gouti M, Casanova M, Warnes G, Merkenschlager M, Fisher AG. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 3.Spivakov M, Fisher AG. Epigenetic signatures of stem-cell identity. Nat Rev Genet. 2007;8:263–271. doi: 10.1038/nrg2046. [DOI] [PubMed] [Google Scholar]
- 4.Sauvageau M, Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell . 2010;7:299–313. doi: 10.1016/j.stem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surface LE, Thornton SR, Boyer LA. Polycomb group proteins set the stage for early lineage commitment. Cell Stem Cell. 2010;7:288–298. doi: 10.1016/j.stem.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- 7.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 8.Min J, Zhang Y, Xu RM. Structural basis for specific binding of Polycomb chromodomain to histone H3 methylated at Lys 27. Genes Dev. 2003;17:1823–1828. doi: 10.1101/gad.269603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis NJ, Kingston RE, Woodcock CL. Chromatin compaction by a polycomb group protein complex. Science. 2004;306:1574–1577. doi: 10.1126/science.1100576. [DOI] [PubMed] [Google Scholar]
- 10.Sing A, Pannell D, Karaiskakis A, Sturgeon K, Djabali M, Ellis J, Lipshitz HD, Cordes SP. A vertebrate Polycomb response element governs segmentation of the posterior hindbrain. Cell. 2009;138:885–897. doi: 10.1016/j.cell.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Woo CJ, Kharchenko PV, Daheron L, Park PJ, Kingston RE. A region of the human HOXD cluster that confers polycomb-group responsiveness. Cell. 2010;140:99–110. doi: 10.1016/j.cell.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ku M, Koche RP, Rheinbay E, Mendenhall EM, Endoh M, Mikkelsen TS, Presser A, Nusbaum C, Xie X, Chi AS, Adli M, Kasif S, Ptaszek LM, Cowan CA, Lander ES, Koseki H, Bernstein BE. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet. 2008;4:e1000242. doi: 10.1371/journal.pgen.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Brown JL, Cao R, Zhang Y, Kassis JA, Jones RS. Hierarchical recruitment of polycomb group silencing complexes. Mol Cell. 2004;14:637–646. doi: 10.1016/j.molcel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Squazzo SL, O’Geen H, Komashko VM, Krig SR, Jin VX, Jang SW, Margueron R, Reinberg D, Green R, Farnham PJ. Suz12 binds to silenced regions of the genome in a cell-type-specific manner. Genome Res. 2006;16:890–900. doi: 10.1101/gr.5306606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon Y, Lee JT. YY1 tethers xist RNA to the inactive × nucleation center. Cell. 2011;146:119–133. doi: 10.1016/j.cell.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enderle D, Beisel C, Stadler MB, Gerstung M, Athri P, Paro R. Polycomb preferentially targets stalled promoters of coding and noncoding transcripts. Genome Res. 2011;21:216–226. doi: 10.1101/gr.114348.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharchenko PV, Alekseyenko AA, Schwartz YB, Minoda A, Riddle NC, Ernst J, Sabo PJ, Larschan E, Gorchakov AA, Gu T, Linder-Basso D, Plachetka A, Shanower G, Tolstorukov MY, Luquette LJ, Xi R, Jung YL, Park RW, Bishop EP, Canfield TK, Sandstrom R, Thurman RE, MacAlpine DM, Stamatoyannopoulos JA, Kellis M, Elgin SC, Kuroda MI, Pirrotta V, Karpen GH, Park PJ. Comprehensive analysis of the chromatin landscape in Drosophila melanogaster . Nature. 2011;471:480–485. doi: 10.1038/nature09725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanhere A, Viiri K, Araujo CC, Rasaiyaah J, Bouwman RD, Whyte WA, Pereira CF, Brookes E, Walker K, Bell GW, Pombo A, Fisher AG, Young RA, Jenner RG. Short RNAs are transcribed from repressed polycomb target genes and interact with polycomb repressive complex-2. Mol Cell. 2011;38:675–688. doi: 10.1016/j.molcel.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eskeland R, Leeb M, Grimes GR, Kress C, Boyle S, Sproul D, Gilbert N, Fan Y, Skoultchi AI, Wutz A, Bickmore WA. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell. 2010;38:452–464. doi: 10.1016/j.molcel.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, Tarakhovsky A, Fuchs E. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136:1122–1135. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caretti G, Di Padova M, Micales B, Lyons GE, Sartorelli V. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 2004;18:2627–2638. doi: 10.1101/gad.1241904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 26.Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, Koseki H, Fuchikami T, Abe K, Murray HL, Zucker JP, Yuan B, Bell GW, Herbolsheimer E, Hannett NM, Sun K, Odom DT, Otte AP, Volkert TL, Bartel DP, Melton DA, Gifford DK, Jaenisch R, Young RA. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juan AH, Derfoul A, Feng X, Ryall JG, Dell’Orso S, Pasut A, Zare H, Simone JM, Rudnicki MA, Sartorelli V. Polycomb EZH2 controls self-renewal and safeguards the transcriptional identity of skeletal muscle stem cells. Genes Dev. 2011;25:789–794. doi: 10.1101/gad.2027911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stock JK, Giadrossi S, Casanova M, Brookes E, Vidal M, Koseki H, Brockdorff N, Fisher AG, Pombo A. Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. Nat Cell Biol. 2007;9:1428–1435. doi: 10.1038/ncb1663. [DOI] [PubMed] [Google Scholar]
- 29.Su IH, Dobenecker MW, Dickinson E, Oser M, Basavaraj A, Marqueron R, Viale A, Reinberg D, Wulfing C, Tarakhovsky A. Polycomb group protein ezh2 controls actin polymerization and cell signaling. Cell. 2005;121:425–436. doi: 10.1016/j.cell.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Natl Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- 31.Beuchle D, Struhl G, Muller J. Polycomb group proteins and heritable silencing of Drosophila Hox genes. Development. 2001;128:993–1004. doi: 10.1242/dev.128.6.993. [DOI] [PubMed] [Google Scholar]
- 32.Faust C, Lawson KA, Schork NJ, Thiel B, Magnuson T. The Polycomb-group gene eed is required for normal morphogenetic movements during gastrulation in the mouse embryo. Development. 1998;125:4495–4506. doi: 10.1242/dev.125.22.4495. [DOI] [PubMed] [Google Scholar]
- 33.O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21:4330–4336. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voncken JW, Roelen BA, Roefs M, de Vries S, Verhoeven E, Marino S, Deschamps J, van Lohuizen M. Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc Natl Acad Sci USA. 2003;100:2468–2473. doi: 10.1073/pnas.0434312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasini D, Bracken AP, Jensen MR, Lazzerini Denchi E, Helin K. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J. 2004;23:4061–4071. doi: 10.1038/sj.emboj.7600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamberlain SJ, Yee D, Magnuson T. Polycomb repressive complex 2 is dispensable for maintenance of embryonic stem cell pluripotency. Stem Cells. 2008;26:1496–1505. doi: 10.1634/stemcells.2008-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol. 2007;27:3769–3779. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watt FM, Lo Celso C, Silva-Vargas V. Epidermal stem cells: an update. Curr Opin Genet Dev. 2006;16:518–524. doi: 10.1016/j.gde.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- 42.Dai X, Segre JA. Transcriptional control of epidermal specification and differentiation. Curr Opin Genet Dev. 2004;14:485–491. doi: 10.1016/j.gde.2004.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 44.Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/S0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 47.Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 49.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. Faseb J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 50.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180:273–284. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reinisch CM, Uthman A, Erovic BM, Pammer J. Expression of BMI-1 in normal skin and inflammatory and neoplastic skin lesions. J Cutan Pathol. 2007;34:174–180. doi: 10.1111/j.1600-0560.2006.00587.x. [DOI] [PubMed] [Google Scholar]
- 54.Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 55.Marshall D, Hardman MJ, Nield KM, Byrne C. Differentially expressed late constituents of the epidermal cornified envelope. Proc Natl Acad Sci USA. 2001;98:13031–13036. doi: 10.1073/pnas.231489198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Guzman Strong C, Conlan S, Deming CB, Cheng J, Sears KE, Segre JA. A milieu of regulatory elements in the epidermal differentiation complex syntenic block: implications for atopic dermatitis and psoriasis. Hum Mol Genet. 2010;19:1453–1460. doi: 10.1093/hmg/ddq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fessing MY, Mardaryev AN, Gdula MR, Sharov AA, Sharova TY, Rapisarda V, Gordon KB, Smorodchenko AD, Poterlowicz K, Ferone G, Kohwi Y, Missero C, Kohwi-Shigematsu T, Botchkarev VA. p63 regulates Satb1 to control tissue-specific chromatin remodeling during development of the epidermis. J Cell Biol. 2011;194:825–839. doi: 10.1083/jcb.201101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferraiuolo MA, Rousseau M, Miyamoto C, Shenker S, Wang XQ, Nadler M, Blanchette M, Dostie J. The three-dimensional architecture of Hox cluster silencing. Nucleic Acids Res. 2011;38:7472–7484. doi: 10.1093/nar/gkq644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lanzuolo C, Roure V, Dekker J, Bantignies F, Orlando V. Polycomb response elements mediate the formation of chromosome higher-order structures in the bithorax complex. Nat Cell Biol. 2007;9:1167–1174. doi: 10.1038/ncb1637. [DOI] [PubMed] [Google Scholar]
- 60.Bantignies F, Cavalli G. Polycomb group proteins: repression in 3D. Trends Genet. 2011;27:454–464. doi: 10.1016/j.tig.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Luis NM, Morey L, Mejetta S, Pascual G, Janich P, Kuebler B, Roma G, Nascimento E, Frye M, Di Croce L, Benitah SA. Regulation of human epidermal stem cell proliferation and senescence requires polycomb- dependent and -independent functions of cbx4. Cell Stem Cell. 2011;9:233–246. doi: 10.1016/j.stem.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 62.Sen GL, Webster DE, Barragan DI, Chang HY, Khavari PA. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev. 2008;22:1865–1870. doi: 10.1101/gad.1673508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, Issaeva I, Canaani E, Salcini AE, Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- 64.De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 65.Lan F, Bayliss PE, Rinn JL, Whetstine JR, Wang JK, Chen S, Iwase S, Alpatov R, Issaeva I, Canaani E, Roberts TM, Chang HY, Shi Y. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature. 2007;449:689–694. doi: 10.1038/nature06192. [DOI] [PubMed] [Google Scholar]
- 66.Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 2010;463:563–567. doi: 10.1038/nature08683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laible G, Wolf A, Dorn R, Reuter G, Nislow C, Lebersorger A, Popkin D, Pillus L, Jenuwein T. Mammalian homologues of the Polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres. EMBO J. 1997;16:3219–3232. doi: 10.1093/emboj/16.11.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Margueron R, Li G, Sarma K, Blais A, Zavadil J, Woodcock CL, Dynlacht BD, Reinberg D. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell. 2008;32:503–518. doi: 10.1016/j.molcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su IH, Basavaraj A, Krutchinsky AN, Hobert O, Ullrich A, Chait BT, Tarakhovsky A. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4:124–131. doi: 10.1038/ni876. [DOI] [PubMed] [Google Scholar]
- 70.Ezhkova E, Lien WH, Stokes N, Pasolli HA, Silva JM, Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 2011;25:485–498. doi: 10.1101/gad.2019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen X, Liu Y, Hsu YJ, Fujiwara Y, Kim J, Mao X, Yuan GC, Orkin SH. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lien WH, Guo X, Polak L, Lawton LN, Young RA, Zheng D, Fuchs E. Genome-wide maps of histone modifications unwind in vivo chromatin states of the hair follicle lineage. Cell Stem Cell. 2011;9:219–232. doi: 10.1016/j.stem.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 74.van der Vlag J, Otte AP. Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nat Genet. 1999;23:474–478. doi: 10.1038/70602. [DOI] [PubMed] [Google Scholar]
- 75.LeBoeuf M, Terrell A, Trivedi S, Sinha S, Epstein JA, Olson EN, Morrisey EE, Millar SE. Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Dev Cell. 2010;19:807–818. doi: 10.1016/j.devcel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 77.Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 78.Chen H, Gu X, Su IH, Bottino R, Contreras JL, Tarakhovsky A, Kim SK. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23:975–985. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Durr P, Wlaschek M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5:379–389. doi: 10.1111/j.1474-9726.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- 80.Cordisco S, Maurelli R, Bondanza S, Stefanini M, Zambruno G, Guerra L, Dellambra E. Bmi-1 reduction plays a key role in physiological and premature aging of primary human keratinocytes. J Invest Dermatol. 2010;130:1048–1062. doi: 10.1038/jid.2009.355. [DOI] [PubMed] [Google Scholar]
- 81.Shaw T, Martin P. Epigenetic reprogramming during wound healing: loss of polycomb-mediated silencing may enable upregulation of repair genes. EMBO Rep. 2009;10:881–886. doi: 10.1038/embor.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wagner EF, Schonthaler HB, Guinea-Viniegra J, Tschachler E. Psoriasis: what we have learned from mouse models. Nat Rev Rheumatol. 2010;6:704–714. doi: 10.1038/nrrheum.2010.157. [DOI] [PubMed] [Google Scholar]
- 83.Zenz R, Wagner EF. Jun signalling in the epidermis: From developmental defects to psoriasis and skin tumors. Int J Biochem Cell Biol. 2006;38:1043–1049. doi: 10.1016/j.biocel.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 84.Zenz R, Eferl R, Kenner L, Florin L, Hummerich L, Mehic D, Scheuch H, Angel P, Tschachler E, Wagner EF. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–375. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- 85.Esparza-Gordillo J, Weidinger S, Folster-Holst R, Bauerfeind A, Ruschendorf F, Patone G, Rohde K, Marenholz I, Schulz F, Kerscher T, Hubner N, Wahn U, Schreiber S, Franke A, Vogler R, Heath S, Baurecht H, Novak N, Rodriguez E, Illig T, Lee-Kirsch MA, Ciechanowicz A, Kurek M, Piskackova T, Macek M, Lee YA, Ruether A. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat Genet. 2009;41:596–601. doi: 10.1038/ng.347. [DOI] [PubMed] [Google Scholar]
- 86.Cookson WO, Ubhi B, Lawrence R, Abecasis GR, Walley AJ, Cox HE, Coleman R, Leaves NI, Trembath RC, Moffatt MF, Harper JI. Genetic linkage of childhood atopic dermatitis to psoriasis susceptibility loci. Nat Genet. 2001;27:372–373. doi: 10.1038/86867. [DOI] [PubMed] [Google Scholar]
- 87.de Cid R, Riveira-Munoz E, Zeeuwen PL, Robarge J, Liao W, Dannhauser EN, Giardina E, Stuart PE, Nair R, Helms C, Escaramis G, Ballana E, Martin-Ezquerra G, den Heijer M, Kamsteeg M, Joosten I, Eichler EE, Lazaro C, Pujol RM, Armengol L, Abecasis G, Elder JT, Novelli G, Armour JA, Kwok PY, Bowcock A, Schalkwijk J, Estivill X. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat Genet. 2009;41:211–215. doi: 10.1038/ng.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y, Luo W, Chen S. Comparison of gene expression profiles reveals aberrant expression of FOXO1, Aurora A/B and EZH2 in lesional psoriatic skins. Mol Biol Rep. 2011;38:4219–4224. doi: 10.1007/s11033-010-0544-x. [DOI] [PubMed] [Google Scholar]
- 89.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647:21–29. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 90.Matsukawa Y, Semba S, Kato H, Ito A, Yanagihara K, Yokozaki H. Expression of the enhancer of zeste homolog 2 is correlated with poor prognosis in human gastric cancer. Cancer Sci. 2006;97:484–491. doi: 10.1111/j.1349-7006.2006.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sudo T, Utsunomiya T, Mimori K, Nagahara H, Ogawa K, Inoue H, Wakiyama S, Fujita H, Shirouzu K, Mori M. Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma. Br J Cancer. 2005;92:1754–1758. doi: 10.1038/sj.bjc.6602531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Friedman JM, Liang G, Liu CC, Wolff EM, Tsai YC, Ye W, Zhou X, Jones PA. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69:2623–2629. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- 93.Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, Laxman B, Cao X, Jing X, Ramnarayanan K, Brenner JC, Yu J, Kim JH, Han B, Tan P, Kumar-Sinha C, Lonigro RJ, Palanisamy N, Maher CA, Chinnaiyan AM. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu J, He ML, Wang L, Chen Y, Liu X, Dong Q, Chen YC, Peng Y, Yao KT, Kung HF, Li XP. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011;71:225–233. doi: 10.1158/0008-5472.CAN-10-1850. [DOI] [PubMed] [Google Scholar]
- 95.Yi R, Pasolli HA, Landthaler M, Hafner M, Ojo T, Sheridan R, Sander C, O’Carroll D, Stoffel M, Tuschl T, Fuchs E. DGCR8-dependent microRNA biogenesis is essential for skin development. Proc Natl Acad Sci USA. 2009;106:498–502. doi: 10.1073/pnas.0810766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eckert RL, Adhikary G, Rorke EA, Chew YC, Balasubramanian S. Polycomb group proteins are key regulators of keratinocyte function. J Invest Dermatol. 2011;131:295–301. doi: 10.1038/jid.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao Q, Mani RS, Ateeq B, Dhanasekaran SM, Asangani IA, Prensner JR, Kim JH, Brenner JC, Jing X, Cao X, Wang R, Li Y, Dahiya A, Wang L, Pandhi M, Lonigro RJ, Wu YM, Tomlins SA, Palanisamy N, Qin Z, Yu J, Maher CA, Varambally S, Chinnaiyan AM. Coordinated regulation of polycomb group complexes through microRNAs in cancer. Cancer Cell. 2011;20:187–199. doi: 10.1016/j.ccr.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, Sabel MS, Livant D, Weiss SJ, Rubin MA, Chinnaiyan AM. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA. 2003;100:11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, Ping B, Otte AP, Hung MC. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310:306–310. doi: 10.1126/science.1118947. [DOI] [PubMed] [Google Scholar]
- 100.Karanikolas BD, Figueiredo ML, Wu L. Polycomb group protein enhancer of zeste 2 is an oncogene that promotes the neoplastic transformation of a benign prostatic epithelial cell line. Mol Cancer Res. 2009;7:1456–1465. doi: 10.1158/1541-7786.MCR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suva ML, Riggi N, Janiszewska M, Radovanovic I, Provero P, Stehle JC, Baumer K, Le Bitoux MA, Marino D, Cironi L, Marquez VE, Clement V, Stamenkovic I. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res. 2009;69:9211–9218. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- 102.Balasubramanian S, Adhikary G, Eckert RL. The Bmi-1 polycomb protein antagonizes the (−)-epigallocatechin-3-gallate-dependent suppression of skin cancer cell survival. Carcinogenesis. 2010;31:496–503. doi: 10.1093/carcin/bgp314. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 103.Lee K, Adhikary G, Balasubramanian S, Gopalakrishnan R, McCormick T, Dimri GP, Eckert RL, Rorke EA. Expression of Bmi-1 in epidermis enhances cell survival by altering cell cycle regulatory protein expression and inhibiting apoptosis. J Invest Dermatol. 2008;128:9–17. doi: 10.1038/sj.jid.5700949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Balasubramanain S, Chew YC, Eckert RL (2011) Sulforaphane suppresses polycomb group protein level via a proteasome-dependent mechanism in skin cancer cells. Mol Pharmacol (in press) [DOI] [PMC free article] [PubMed]
- 105.Shen X, Kim W, Fujiwara Y, Simon MD, Liu Y, Mysliwiec MR, Yuan GC, Lee Y, Orkin SH. Jumonji modulates polycomb activity and self-renewal versus differentiation of stem cells. Cell. 2009;139:1303–1314. doi: 10.1016/j.cell.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peng JC, Valouev A, Swigut T, Zhang J, Zhao Y, Sidow A, Wysocka J. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell. 2009;139:1290–1302. doi: 10.1016/j.cell.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pasini D, Cloos PA, Walfridsson J, Olsson L, Bukowski JP, Johansen JV, Bak M, Tommerup N, Rappsilber J, Helin K. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature. 2010;464:306–310. doi: 10.1038/nature08788. [DOI] [PubMed] [Google Scholar]
- 108.Mejetta S, Morey L, Pascual G, Kuebler B, Mysliwiec MR, Lee Y, Shiekhattar R, Di Croce L, Benitah SA. Jarid2 regulates mouse epidermal stem cell activation and differentiation. EMBO J. 2011;30:3635–3646. doi: 10.1038/emboj.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalia YN, Nonato LB, Lund CH, Guy RH. Development of skin barrier function in premature infants. J Invest Dermatol. 1998;111:320–326. doi: 10.1046/j.1523-1747.1998.00289.x. [DOI] [PubMed] [Google Scholar]