Abstract
Gene therapy is a promising form of treatment for those suffering from neurological disorders or central nervous system (CNS) injury, however, obstacles remain that limit its translational potential. The CNS is protected by the blood brain barrier, and this barrier blocks genes from traversing into the CNS if administered outside of the CNS. Viral and non-viral gene delivery vehicles, commonly referred to as vectors, are modified to enhance delivery efficiency to target locations in the CNS. Still, there are few gene therapy approaches approved by the FDA for CNS disease or injury treatment. The lack of viable clinical approaches is due, in part, to the unpredictable nature of many vector systems. In particular, safety concerns exist with the use of viral vectors for CNS gene delivery. To seek some alternatives to viral vectors, development of new non-viral, biomaterial vectors is occurring at a rapid rate. This review discusses the challenges of delivering various forms of genetic material to the CNS, the use and limitations of current viral vector delivery systems, and the use of non-viral, biomaterial vectors for CNS applications.
Keywords: Biomaterials, Gene delivery, Central nervous system, Non-viral vectors
1. Introduction
Gene therapy attracted widespread interest after the first approved therapeutic gene transfer was reported in 1990 (Wirth et al., 2013). From this initial advance to present day, researchers have strived to develop new gene delivery strategies for genetic research and eventual translation into therapeutics. These strategies deliver genes to knockdown protein and gene expression for the purpose of assessing the function of a target protein (Karra and Dahm, 2010; Quraishe et al., 2018; Zuchero et al., 2015), to express tagged proteins to track protein behavior and location (Karra and Dahm, 2010; Mohr et al., 2010; Nectow et al., 2017; Pardieck and Sakiyama-Elbert, 2018), to express mutant proteins to model diseases (Jackson et al., 2016; Karra and Dahm, 2010; Pardieck and Sakiyama-Elbert, 2018; Ruiz and Déglon, 2012), and to upregulate or downregulate protein expression for regenerative and disease-mitigating applications (Biferi et al., 2017; Gower and Shea, 2013; Gwak et al., 2015; Huang et al., 2013; Pardieck and Sakiyama-Elbert, 2018; Shyam et al., 2015).
Gene delivery to the central nervous system (CNS) is being investigated for potential treatment of various neurological diseases, such as Parkinson’s disease (Bartus et al., 2014, 2013; Huang et al., 2013, 2010b; Saraiva et al., 2016), Alzheimer’s disease (Liu et al., 2016; Rafii et al., 2014), spinal muscular atrophy (Foust et al., 2010; Oliván et al., 2016), glioblastoma (Qian et al., 2014; Yao et al., 2015), and amyotrophic lateral sclerosis (Benkler et al., 2016; Biferi et al., 2017; Federici and Boulis, 2012), as well as for promoting tissue regeneration following injury (Boone et al., 2017; Gwak et al., 2015; Kwon et al., 2016; Nguyen et al., 2017; Papa et al., 2014). In subsequent sections, we present the different types of genetic material delivered via biomaterial vectors, the challenges of delivering these types of genetic material to the CNS, the advantages and disadvantages of viral vector delivery to the CNS, and the growing use of polymeric biomaterials for gene delivery to the CNS.
1.1. Forms of genetic material
There are three main forms of genetic material, illustrated in Fig. 1, delivered via non-viral biomaterial systems: plasmid DNA (pDNA), messenger RNA (mRNA), and RNA interference (RNAi) molecules such as small interfering RNA (siRNA) and micro interfering RNA (miRNA). The genetic material being transferred is commonly referred to as a transgene.
Fig. 1.
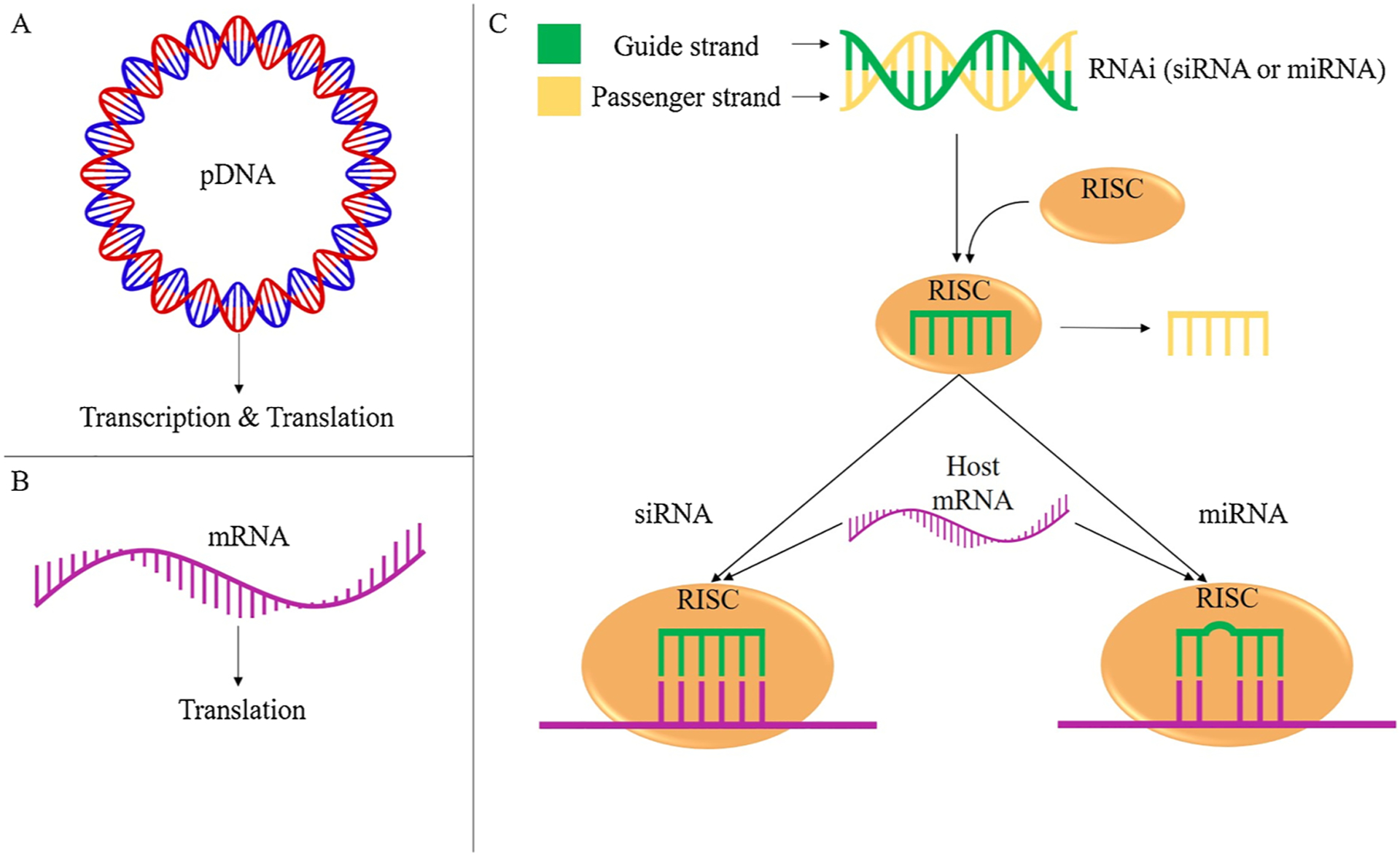
The primary forms of genetic material delivered to cells and the steps they undergo to regulate gene expression. A) pDNA is delivered and subsequently transcribed and translated via host cell machinery to upregulate target protein production. B) mRNA is delivered and subsequently translated to upregulate target protein production. C) RNAi molecules such as siRNA or miRNA are delivered as double stranded RNA. In both forms of RNAi the passenger strand (yellow) is discarded and the guide strand (green) is retained in the RISC complex. The guide strand directs the RISC complex to a target host mRNA sequence. siRNA binds to a fully complimentary target mRNA sequence to downregulate host mRNA translation. miRNA only needs to bind to a partially complementary target mRNA sequence to downregulate host mRNA translation.
pDNA delivery to cells either stably or transiently alters gene expression (Fig. 2). Stable expression occurs once the foreign pDNA enters the nucleus and is integrated into the cell’s genome (Fig. 2A). The transgene will be replicated along with the host cellular genome and passed to daughter cells, resulting in long-term expression of the transgene (Kim and Eberwine, 2010). Transient expression occurs when genetic material is taken up by the cell but not incorporated into the cell’s genome (Fig. 2B). Once in the nucleus, the pDNA transgene uses the host cell’s expression machinery, such as host RNA polymerase, to be transcribed into mRNA. The resulting mRNA is transported out of the nucleus and into the cytosol for translation by host ribosomes and tRNA. The transgene, however, is not replicated with the host genome or passed to daughter cells. Therefore, transient gene delivery results in short-term expression of the transgene (Kim and Eberwine, 2010).
Fig. 2.
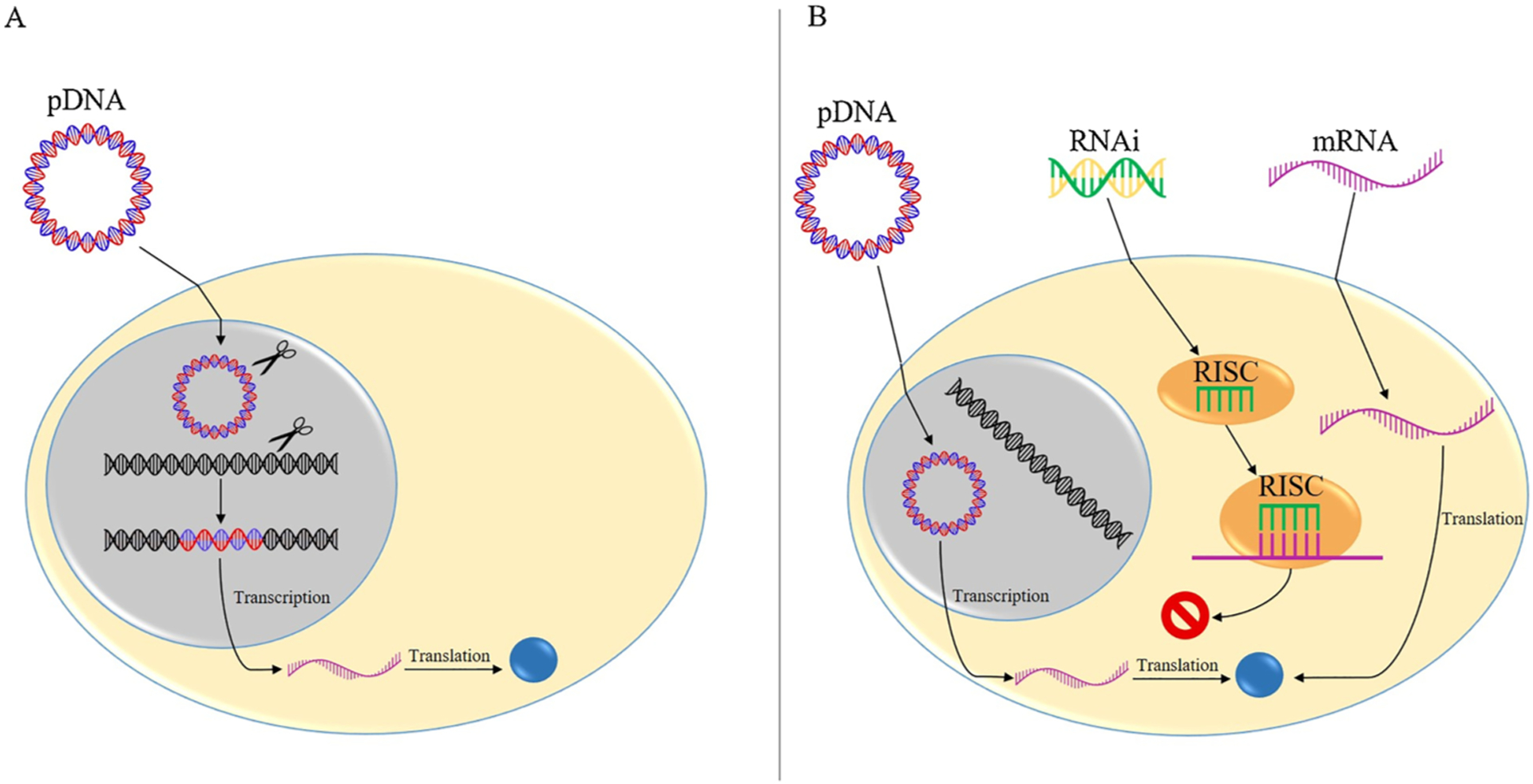
The mechanism of action of stable expression and transient expression. A) Stable expression occurs when pDNA enters the cell’s nucleus and integrates into the host genome. The integrated transgene is expressed via host cell machinery and is replicated with the remainder of the genome and passed on to new cells, resulting in long-term transgene expression. B) Transient expression occurs when either pDNA, mRNA or RNAi molecules are delivered to the cell but are not integrated into the host genome. pDNA still must enter the cell nucleus to be expressed by host cell machinery but will not integrate into the host genome. In contrast, mRNA and RNAi regulate gene expression transiently in the cytosol. Neither pDNA, mRNA, nor RNAi molecules will be replicated and passed on to new cells, so each will result in short-term gene expression.
Unlike pDNA, mRNA does not enter the nucleus to be expressed by the cell. mRNA is taken up by the cell and expressed, by host ribosomes and tRNA, in the cell’s cytosol (Fig. 2B) (Kim and Eberwine, 2010). Thus, the transgene will not be integrated into the genome and mRNA delivery will result in transient, short-term expression.
siRNA and miRNA are often delivered to cells to transiently inhibit the translation of host cell mRNA (Fig. 2B). Some RNAi molecules, however, have been developed to enter the nucleus to influence genesplicing and transcription (Gagnon et al., 2014). siRNA is delivered as an immature double-stranded RNA, which consists of an anti-sense guide strand and a passenger strand (Lam et al., 2015; Reynolds et al., 2004). The siRNA is processed by an endoribonuclease and assembled into an RNA-induced silencing complex (RISC), during which the passenger strand is removed while the guide strand remains in the complex. The anti-sense guide strand of RISC binds to a fully complementary mRNA sequence, which subsequently results in endonucleolytic cleavage, and inhibits translation of a specific gene (Lam et al., 2015).
Similar to siRNA, miRNA is delivered as an immature double stranded RNA containing a guide strand and passenger strand. Again, the RNA is processed by an endoribonuclease and the passenger strand is discarded upon assembly of the miRNA into RISC, forming miRISC. The guide strand of miRISC binds to a partially complementary mRNA sequence and inhibits mRNA expression by either blocking factors involved in translation, inducing endonucleolytic mRNA cleavage, or inducing complete degradation of the mRNA (Lam et al., 2015; Mack, 2007). siRNA inhibition is highly specific as RISC will cleave only after it is bound to a fully complementary mRNA sequence. miRNA inhibition, on the other hand, can act on multiple mRNAs as the miRISC guide strand only requires a partial complementary mRNA sequence to induce mRNA silencing.
The form of genetic material selected for each application depends on factors such as the desired time frame of gene expression or inhibition and whether the goal is to upregulate or downregulate expression.
1.2. Routes of gene delivery to the CNS
1.2.1. Peripheral administration of gene delivery systems
CNS gene delivery is challenging primarily due to the presence of the blood brain barrier (BBB). The BBB is a physical barrier that maintains homeostasis through maintenance of the CNS chemical microenvironment while protecting the CNS from bacteria and toxins (Bowers et al., 2011; Tran, 2011). The BBB consists primarily of brain microvascular endothelial cells (Tran, 2011). The vascular endothelial layer contains tight junctions between cells and performs limited vesicular transport, which is unique to the BBB (Bowers et al., 2011; Reese and Karnovsky, 1967; Saunders et al., 2016; Tran, 2011). The endothelial cells within the BBB express highly selective solute carrier proteins and efflux transporter proteins. Solute carrier proteins selectively transport nutrients, such as glucose, across the BBB and into the CNS. Larger molecules must undergo transcytosis, a form of transcellular transport, across the BBB to enter the CNS. Efflux transporter proteins rely on adenosine triphosphate metabolism to excrete waste and toxins from the CNS into the bloodstream (Chow and Gu, 2015). The BBB permits only small molecules and certain lipophilic molecules into the CNS. Due to the selectivity of the BBB, administering gene delivery systems via intravenous injection into the peripheral vasculature may limit the amount of gene delivered into the CNS. To achieve a therapeutic concentration of administered gene in the CNS, the gene delivery system must be administered at a very high dose. High amounts of a gene delivery vector in the blood stream, however, may initiate an immune or cytotoxic response (Bevan et al., 2011; Meyer et al., 2015). Still, peripheral administration through intravenous injection is a common practice within in vivo models (Fig. 3A). Joshi et al. administered modified polyethyleneimine (PEI) vectors loaded with luciferase (Luc) reporter DNA to mice via intravenous injection through the tail vein. Reporter gene expression was controlled by the glial-specific glial fibrillary acidic protein (GFAP) promoter to limit off-target expression. Astrocytes in the brain expressed Luc DNA, which demonstrated the efficacy of this gene delivery approach (Joshi et al., 2018).
Fig. 3.
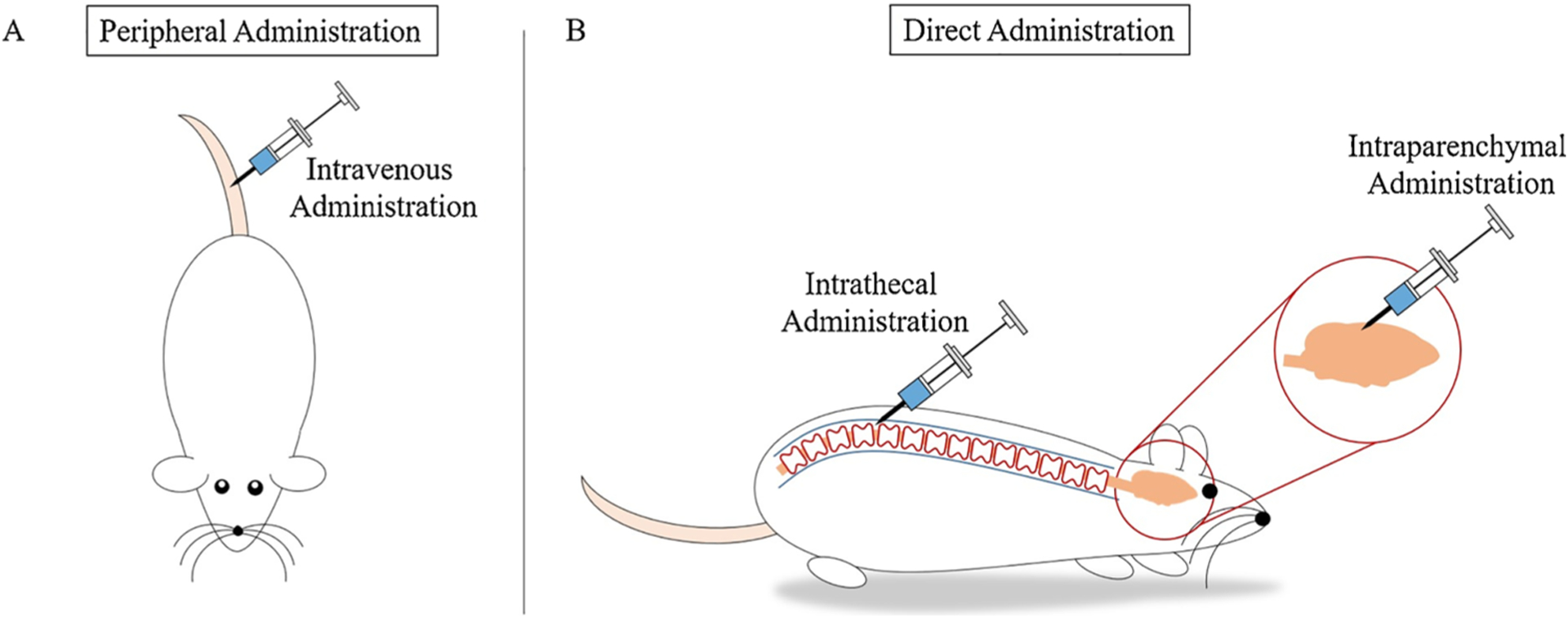
Genes are delivered through various routes of administration. A) Peripheral administration is a common route of administration in vivo and is often achieved by intravenous injection via the tail vein of a rat or mouse. Following peripheral administration, the gene delivery system must cross the BBB to enter the CNS. B) Direct administration allows researchers to bypass the BBB and directly inject the gene delivery system into the CNS. Two common routes of direct administration into the CNS are intrathecal administration and intraparenchymal administration. Following intrathecal administration, via injection into the subarachnoid space, the gene delivery system enters the cerebrospinal fluid and is delivered throughout the CNS. Following intraparenchymal administration, via injection into the brain parenchyma, the gene delivery system enters the brain parenchyma and is delivered locally in the brain.
1.2.2. Direct CNS administration of gene delivery systems
To bypass the BBB and decrease the systemic dose required for in travenous injection, several approaches inject vector systems directly into CNS, including the brain parenchyma and the cerebrospinal fluid (CSF) (Fig. 3B). Studies delivering vectors by intraparenchymal injection, show this approach being effective to target specific areas of the brain (Bergen and Pun, 2008; da Cruz et al., 2005; Gonzalez-Barrios et al., 2006). Gonzalez-Barrios et al. used this method to deliver a neurotensin-poly-L-lysine polyplex containing glial cell derived neurotrophic factor (GDNF) pDNA to a rat model of Parkinson’s disease. Parkinson’s disease is a neurodegenerative disorder that causes depletion of dopaminergic neurons and results in motor impairment. Neurotensin is capable of targeting the polyplex to dopaminergic neurons and GDNF is a suitable neurotrophic factor that supports survival of dopaminergic neurons. Gonzalez-Barrios et al. showed that local delivery of this vector system to the parenchymal striatum increased GDNF production and reversed signs of motor impairment (Gonzalez-Barrios et al., 2006).
Approaches that deliver vectors via the CSF, by either intracerebroventricular or intrathecal injection, can result in widespread delivery of the vector system throughout the CNS (Bergen and Pun, 2008; Goula et al., 1998; Gray et al., 2013; Meyer et al., 2015). This was shown in a spinal muscular atrophy preclinical study by Meyer et al.. Spinal muscular atrophy is a genetic neurodegenerative disorder where α-motor neurons are lost, leading to progressive skeletal muscle atrophy (D’Amico et al., 2011). Several researchers show that delivering genes encoding functional survival motor neuron protein (SMN) can effectively treat spinal muscular atrophy (Benkhelifa-Ziyyat et al., 2013; Dominguez et al., 2011; Foust et al., 2010; Glascock et al., 2012; Valori et al., 2010). Several of these studies administered the vector peripherally and, therefore, the injection required a high concentration of vector system to produce an efficacious effect. In the study conducted by Meyer et al., an adeno-associated virus (AAV) vector carrying the gene encoding human SMN was delivered via intracerebroventricular injection into the CSF. This delivery method resulted in widespread expression of functional SMN in motor neurons throughout the spinal cord and was effective at a dose 30 times lower than that used for intravenous injection of the same vector system (Meyer et al., 2015).
In another example, Gwak and colleagues delivered a chitosan-based vector conjugated to methylprednisolone and loaded with β-galactosidase (β-gal) reporter pDNA, to a rat model of spinal cord injury, via intrathecal injection. Methylprednisolone is an anti-inflammatory glucocorticoid commonly used to treat spinal cord injury and is also a ligand of nuclear glucocorticoid receptors. The presence of methylprednisolone on the vector promoted transport of the pDNA-loaded vector system through nuclear pores and into the nucleus where the pDNA can be expressed. Intrathecal injection of this chitosan-based vector conjugated to methylprednisolone significantly reduced inflammation and cell apoptosis throughout the injured rat spinal cord and resulted in significant expression levels of reporter pDNA in spinal cord neurons and astrocytes (Gwak et al., 2015).
The effectiveness of therapeutic gene delivery to the CNS, whether delivered via viral or biomaterial vector, is highly reliant on how the gene delivery system is administered. To reach the full potential of the therapy, it is important to select the optimal route of vector administration. When selecting the route of delivery, it is important to note how invasive the approach is and if the approach contains additional mechanisms to bring the viral or biomaterial-based vector to a specific tissue or cell type.
2. Vectors
Gene delivery vector systems for the CNS have evolved to increase the bioavailability and enhance cellular uptake of such systems in the CNS. The cellular uptake of viral gene delivery vectors is referred to as transduction, while uptake of non-viral gene delivery vectors is referred to as transfection. Vector systems protect the genetic material from harmful enzymes and guide the gene to a specific cell type to avoid unwanted off-target expression. While targeting is important, it is also crucial that the vector is not infectious or toxic to the host. Additionally, vector construction and characterization should be feasible (Goula et al., 1998). Several approaches have modified traditional vector systems to further improve targeted gene delivery and diminish possible immune responses. Such modifications include: 1) incorporating cell targeting moieties (Bouard et al., 2009; Buchholz et al., 2015; Zhi et al., 2016), 2) incorporating CNS and cell-specific promoters (Boado and Pardridge, 2011; Drinkut et al., 2012; Gray et al., 2011a,b; Griffin et al., 2019; Ren et al., 2013), and 3) fine-tuning the shape and size of the vector system (Agarwal et al., 2013; Kim and Eberwine, 2010; Kolhar et al., 2013; Qian et al., 2014). In the following sections, we introduce examples utilizing viral vectors, discuss their current challenges, and present the growing utilization of non-viral, biomaterial-based gene delivery systems.
2.1. Viral vector systems
For years, viral vectors were the favored vehicle for gene delivery due to their high transduction efficiency. There are five main classes of viruses from which viral vector systems are derived: adenoviruses, AAVs, herpes simplex-1 viruses, retroviruses, and lentiviruses. The unique characteristics that separate each vector class are explained in detail in a thorough review by Thomas et al. (Thomas et al., 2003). When fabricating a vector from a naturally occurring virus, portions of the genome that code for rapid replication and disease transmission are deleted, thus eliminating the pathogenicity of the viral vector. The transgene is then cloned into the viral genome (Thomas et al., 2003). The transgene size is limited by the packaging capacity of the viral vector genome. Space limitation is one of the major downfalls of viral vectors, in particular for adenoviral and AAV vectors. Viruses are selected for gene delivery applications due to their natural ability to successfully infect host cells. Therefore, aside from the removal of pathogenic coding regions, viruses are efficient gene delivery systems that do not require substantial modification. First-generation viral vectors, for example, have the least amount of genetic deletions from the native viral genome, compared to successive generations of viral vectors, and have successfully delivered genes without causing a severe immune response in animal models. Geddes and colleagues delivered a first-generation adenoviral vector encoding arginine vasopressin to the hypothalamus in an arginine vasopressin-deficient rat model of diabetes insipidus. Delivery of the adenoviral vector enabled recovery from the hypothalamic diabetes insipidus phenotype for approximately 4 months (Geddes et al., 1997).
Although viral vectors are successful at delivering genes, there are several critical issues that come into play when delivering genes via viral vectors. These issues include the possibility of an immune response, broad viral tropism, high cost and low production levels of viral vectors, and the general public’s view on the use of viruses as a form of medical treatment.
2.1.1. Immunogenicity
The most notable downfall of viral vector gene delivery is the possibility of a severe immune response. The previous study by Geddes and colleagues showed that gene delivery from a first-generation adenoviral vector successfully treated diabetes insipidus in a rat model (Geddes et al., 1997). Delivery of first-generation viral vectors, however, often results in an immunological response in the CNS and other tissues. This is attributed to the fact that their genomes contain the least amount of deletions from the original viral genome compared to higher generation viral vectors (Kay et al., 2001). Thomas and colleagues injected a first-generation adenoviral vector at a dose of 108 IU into the striatum of an immune competent rat model and observed increased glial cell activation. This dose significantly increased the area of astrocyte and neuronal death surrounding the injection site compared to a dose of 107 IU or less (Thomas et al., 2001). A severe immune response can ultimately lead to unintended lethal side effects. To reduce the potential of these side effects, succeeding generations of viral vectors were developed. Higher generation viral vectors contain more deletions to their viral genome to decrease the possibility of generating a severe immune response (Gándara et al., 2018; Kay et al., 2001). Nevertheless, our immune systems are designed to fight off viruses. Thus, even heavily modified viral vectors can initiate an immune response.
An immune response to viral gene delivery can also neutralize the viral vector and therefore limit gene delivery and expression (Barnes et al., 2019). Gray et al. showed that low levels of neutralizing AAV antibody titers in nonhuman primates diminished transduction of CNS cells following intravascular administration (Gray et al., 2011a,b). Although direct administration into the CNS can limit neutralization of viral vectors, this route of delivery is more invasive (Gray et al., 2013). To avoid viral vector neutralization as well as invasive procedures, researchers are currently investigating means of developing antibody-resistant viral vector systems (Barnes et al., 2019; Jose et al., 2019). In a recent study, Jose and colleagues utilized cryo-electron microscopy and 3D image reconstruction to study interactions between the antigenic regions of AAV serotype 5 and neutralizing antibodies. Characterization of such interactions will assist in development of modified AAV vectors that can evade pre-existing neutralizing antibodies (Jose et al., 2019).
Since 2012, use of AAV vectors in clinical trials, predominantly for anti-cancer applications, has more than doubled (Ginn et al., 2018, 2013). This increase is due to the overall safety of these vector systems. AAV vectors have a lower immunogenicity compared to others viral vectors, including adenoviral vectors and retroviral vectors (Mingozzi and High, 2011). Still, AAV vectors can elicit an immune response in humans and pre-existing antibodies can reduce their efficacy (Barnes et al., 2019; Jose et al., 2019). Hinderer and colleagues observed high levels of toxicity in nonhuman primates and piglets upon high-dose intravenous injection of an AAV vector encoding SMN (Hinderer et al., 2018b). To reduce the viral vector dose administered as well as the chance of off-target transduction, researchers continue to investigate direct administration of AAV vectors into the CNS (Ayers et al., 2015; Hinderer et al., 2018a). Ayers and colleagues delivered a recombinant AAV vector encoding interleukin-10 to a mouse model of amyotrophic lateral sclerosis. Mice that received a direct injection of the AAV vector into their lumbar spinal cord had a significantly prolonged life span compared to untreated mice. This method of administration resulted in significantly higher levels of CNS transduction and reduced off-target transduction compared to peripheral administration via intramuscular injection (Ayers et al., 2015).
To counteract the chance of viral vectors initiating an immune response, further modifications of viral vectors can mask these delivery vehicles from our immune system and limit the possibility of a serious immune response. Further, improved targeting of viral vectors to the CNS can diminish the possibility of an immune response by limiting off-target transduction, thus, reducing unintended side effects.
2.1.2. Vector targeting
The second critical issue associated with viral vectors involves the lack of cell-specific targeting (Bouard et al., 2009; Gray et al., 2011a,b; Kay et al., 2001). Some viruses have a broad tropism, meaning the virus can infect many cell types. As a result, some viral vectors transduce off-target cells, which may hinder transduction levels of the desired cell type(s). This off-target transduction decreases vector bioavailability and increases the risk of undesirable and potentially harmful effects. Gray and colleagues observed limited transduction into CNS cells after intravascular delivery of an AAV vector to the brain of young, non-human primates. The authors also observed increased transduction of peripheral organs, including the liver, heart, and spleen (Gray et al., 2011a,b). Off-target transduction is particularly detrimental to cells when transgene expression affects intracellular enzymatic activity or alters production of modified ion channels and cell surface receptors (Weinberg et al., 2013).
Recently developed methods have improved cell-specific targeting (de Leeuw et al., 2014; Eleftheriadou et al., 2017; Griffin et al., 2019; Körbelin et al., 2016; Passini et al., 2010; Rosario et al., 2016). Some promoters are primarily recognized by a specific cell type. Incorporating cell-specific promoters can limit transgene expression in off-target cells. Griffin et al. incorporated the astrocyte-specific GFAP promoter into the genome of an AAV vector to target transgene expression in rat spinal cord astrocytes following spinal cord injury. The GFAP promoter controlled the expression of a gene encoding yellow fluorescent protein so the resulting transduction could be easily visualized. Inclusion of the cell-specific promoter led to a significant increase in transgene expression in astrocytes and limited transgene expression in off-target cell types, including neurons, microglia, and oligodendrocytes (Griffin et al., 2019).
Selecting viruses with a specific tropism for a single cell type will further reduce off-target effects. For example, herpes virus is often used for neuronal gene delivery because of its natural neurotropism (Waehler et al., 2007; Zerboni et al., 2013). Further, a technique called pseudotyping, which incorporates an envelope protein from another viral type onto the vector surface, more specifically targets cells. For example, inclusion of glycoproteins from a neurotropic virus, such as rabies virus or herpes simplex virus, can improve viral vector targeting to the CNS (Azzouz et al., 2004; Cronin et al., 2005; Waehler et al., 2007).
For non-enveloped viruses, such as AAVs, modifications to the viral protein capsid are being investigated to improve cell-specific targeting (Barnes et al., 2019). Rosario and colleagues modified the protein capsid of a recombinant AAV vector to improve transduction of microglia in the brain of wild-type mice. A triple mutation to the gene encoding the viral capsid enhanced microglia-specific transduction. In addition, the modified AAV vector encoded the inflammatory cytokine, interleukin-6, under the control of the microglia-specific CD68 promoter. The inclusion of this promoter further improved targeted expression of the transgene. Administration of the modified viral vector into the cerebral ventricles of mice significantly increased levels of astrogliosis compared to the wild-type vector. This indicated successful targeted transduction of mouse microglia (Rosario et al., 2016).
2.1.3. Production levels, cost, and public views
Besides the limitations stated previously, there are a few external issues that also limit the potential of viral vectors. First, viral vectors cannot be mass produced. Researchers are developing new approaches to scale-up viral vector manufacturing, but production is costly and yields remain low (Bouard et al., 2009; Schaffer et al., 2008; van der Loo and Wright, 2016; Waehler et al., 2007). Although current viral vector production levels are suitable to support laboratory use, commercial viral vectors for clinical use would require a 10- to 100-fold increase in production (van der Loo and Wright, 2016). Based on production limitations, viral-based gene therapies may not be accessible to the general population due to the production levels not meeting the general public’s needs or the therapy not being affordable due to its limited supply.
Another issue limiting the widespread use of viral vectors is the general public’s willingness to accept viral agents as a viable treatment. Although virulent genes are removed from the vector system, specific viruses that are non-transmittable to humans are more likely to be widely accepted. For example, a viral vector derived from human immunodeficiency virus may not be as readily accepted by the general public as one that is derived from feline immunodeficiency virus (Bouard et al., 2009; Nayak and Herzog, 2010). However, it is important to note that a non-transmittable agent may have reduced transduction efficiency into target human cells. Together, all of these issues necessitate the development of other, equally or more efficient, non-viral gene delivery techniques.
2.1.4. Current clinical status of viral vectors
We are among a revolutionary time in the field of gene therapy. The number of gene therapies entering clinical trials continues to grow each year. Yet, very few of these therapies have made it to market. In December 2017, Voretigene neparvovec-rzyl (Luxturna) became the first FDA-approved AAV gene therapy in the US, as well as the first approved gene therapy to treat a CNS disorder (Darrow, 2019; Ginn et al., 2018; Rodrigues et al., 2018). Luxturna, marketed by Spark Therapeutics, is a treatment for those suffering from biallelic RPE65 mutation-associated retinal dystrophy. This disorder causes vision to deteriorate, which can ultimately lead to complete blindness. Luxturna consists of an AAV vector that encodes a functional version of human retinal pigment epithelial protein. Administration of the therapy via a single subretinal injection into each eye can lead to long-term transgene expression in post-mitotic cells (Darrow, 2019). However, some individuals carry a pre-existing immunity to AAV vectors, which can limit the efficacy of the treatment (Rodrigues et al., 2018). Following treatment with Luxturna, some individuals experienced improved vision. In addition, patients experienced only mild immune reactions to the viral vector. This gene therapy, although coined curative by several media sources, is unable to completely restore vision in most individuals treated (Darrow, 2019).
The approval of a second gene therapy for a CNS disorder may be on the horizon. AveXis Inc. filed for FDA-approval of AVXS-101 (Zolgensma), aimed to treat spinal muscular atrophy, in 2018. Spinal muscular atrophy is one of the most common inherited fatal genetic diseases in infants, and there are very few treatments available for those suffering (Parente and Corti, 2018; Swoboda et al., 2005). Zolgensma consists of an AAV vector encoding the fully functional version of SMN1. In clinical trials patients received a one-time intravenous injection of the AAV vector. The vector can traverse the BBB and deliver the transgene to motor neurons in the CNS. In Phase I trials, about 90% of infants treated with Zolgensma gained the ability to speak, achieved full head control, and could sit up without external support (NCT02122952) (Al-Zaidy et al., 2019). Phase III trials are ongoing and are projected to be completed by the end of 2019 (NCT03306277) (“Gene Replacement Therapy Clinical Trial for Patients With Spinal Muscular Atrophy Type 1 - ClinicalTrials.gov,” 2017).
There are several preclinical and clinical trials that are underway for other viral vector gene therapies aimed to treats CNS diseases and disorders (Jayant et al., 2016). In addition to the two viral vector gene therapies mentioned above, AAV vectors are being investigated in clinical trials for treatment of Parkinson’s disease, Batten disease, and giant axonal neuropathy (NCT01973543, NCT02725580, NCT02362438) (Ginn et al., 2018). Although results are promising, the treatments are still under investigation and there is still a chance for long-term, undesired effects to surface in individuals treated with the vectors. Further, the packaging capacity of AAV vectors is restricted to less than 5 kilobases. This may force researchers to investigate more immunogenic viral vector strategies to deliver larger transgenes. To prevent a severe immune response as well as deliver larger transgenes, researchers are investigating the use of biomaterial vectors for gene delivery.
2.2. Non-viral biomaterials systems
To circumvent the difficulties associated with translating viral gene delivery to clinical therapeutics, non-viral delivery systems are being developed. Non-viral, biomaterial delivery systems use lipid-based, synthetic polymeric, and natural polymeric biomaterials to encapsulate or immobilize genes and deliver them to target cells. Biomaterial gene delivery systems show potential to improve upon viral vector counterparts because of their reduced immunogenicity, the emerging mechanisms to target specific cell types, and the availability of simple and low cost preparation methods (Bergen and Pun, 2008). Table 1 highlights and compares the advantages and disadvantages of biomaterial vectors and viral vectors. Polymeric biomaterial vectors for gene delivery are being explored for clinical use at a rapidly growing rate. The availability of materials with improved biocompatibility and tunable biodegradability make this an even more promising area of research.
Table 1.
The Advantages and Disadvantages of Viral and Biomaterial Vectors for Gene Delivery.
| Vector Type | Advantages | Disadvantages |
|---|---|---|
| Viral | High transduction efficiency | Immunogenicity |
| Limited packaging capacity | ||
| Expensive to manufacture | ||
| Difficult to fabricate | ||
| Low production yields | ||
| Single administration | ||
| Biomaterial | Low production cost | Cytotoxicity |
| Ease of production | Low transfection efficiency | |
| High production yields | ||
| High tunability | ||
| Repeat administration capability |
Cationic biomaterials, such as PEI and poly(L-lysine) (PLL), are often used as non-viral vectors due to their positive charge (Dumitriu, 2001; Park et al., 2006). Commonly used cationic biomaterials for gene delivery to the CNS are listed in Table 2. Cationic biomaterials form electrostatic interactions with negatively charged genetic material to create vector complexes, known as polyplexes (Fig. 4) (Thomas and Klibanov, 2003). Further, positively charged vectors are drawn to negatively charged cell surface proteoglycans, and the build-up of positive charge at the cell surface induces uptake of the vector system via endocytosis, illustrated in Fig. 4 (Mislick and Baldeschwieler, 1996).
Table 2.
Commonly used Cationic Biomaterials for Gene Delivery to the CNS.
| Material Type | Key Examples | References |
|---|---|---|
| Polymers | ||
| Synthetic | ||
| Polyethyleneimine | (Abdallah et al., 1996; Gao et al., 2016; Joshi et al., 2018; Ko et al., 2009; Li et al., 2013; Park et al., 2015; Sheikh et al., 2017; Zeng et al., 2007) | |
| Poly(l-lysine) | (Gao et al., 2016; Hernandez-Chan et al., 2015; Huang et al., 2013; Liu et al., 2010; Sheikh et al., 2017) | |
| Poly(amidoamine) | (Godinho et al., 2014; Han et al., 2011; Huang et al., 2010b, 2010a, 2008, 2007) | |
| Natural | ||
| Chitosan | (Gao et al., 2014; Gu et al., 2017; Gwak et al., 2015) | |
| Lipids | ||
| Liposomes | ||
| DOTAP | (Anderson et al., 2003; Cardoso et al., 2010; da Cruz et al., 2005; Dengler et al., 2013; Sharma et al., 2013; Writer et al., 2012) | |
| DOPE | (Sharma et al., 2013; Sun et al., 2012; Writer et al., 2012) | |
| Peptides | ||
| Cell-Penetrating Peptides | ||
| Transactivating Transcriptional Activator | (Han et al., 2011; Kanazawa et al., 2013; Song et al., 2010a; Wang et al., 2010) | |
| Transportan | (Kang et al., 2016; Kwon et al., 2016) | |
Fig. 4.
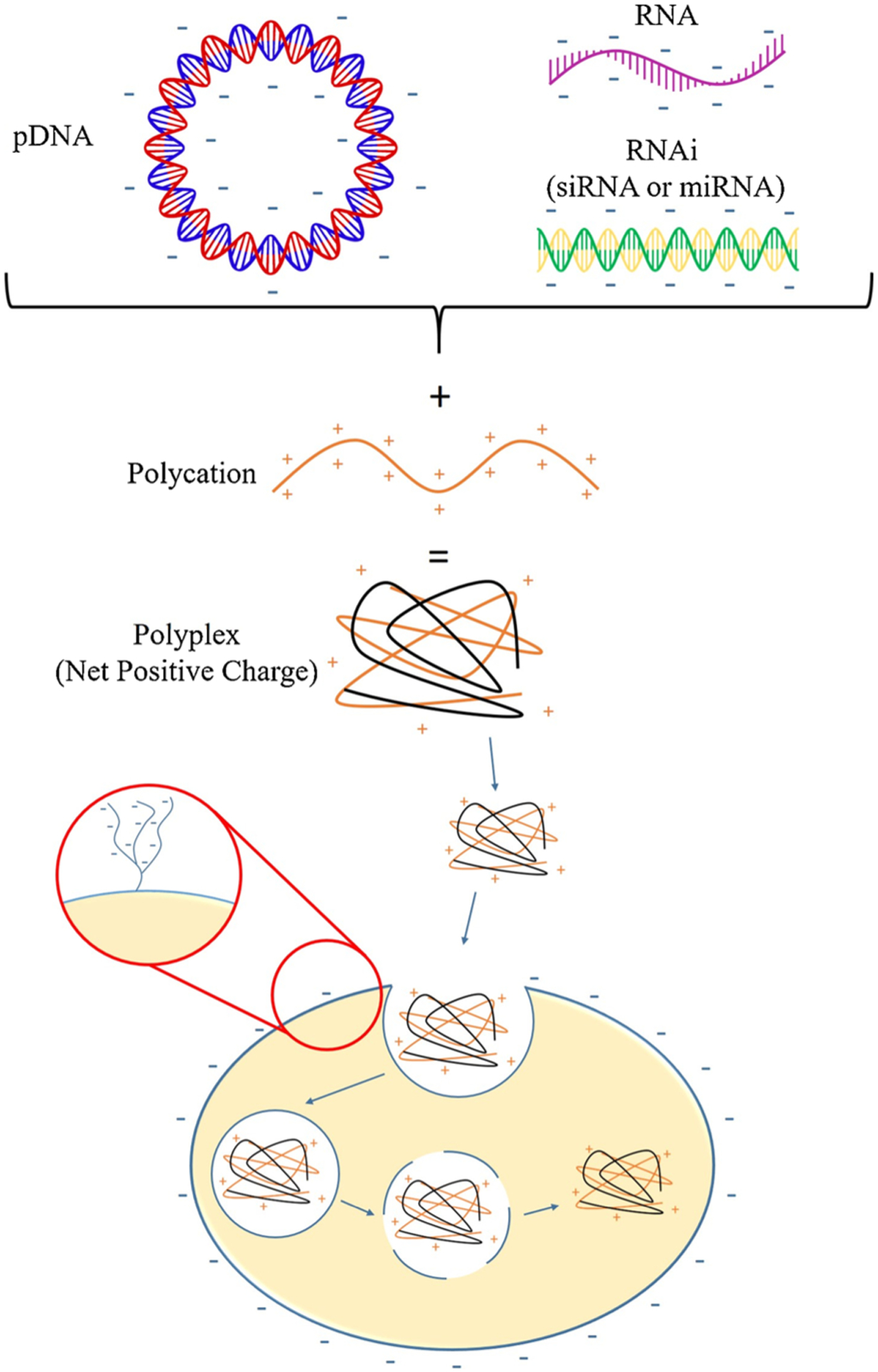
A schematic of polyplex formation and cellular uptake. Genetic material carries a negative charge due to the phosphate groups it contains. The negatively charged genetic material can be complexed with polycationic biomaterials to form a polyplex, which carries an overall positive charge. Mammalian cells carry a net negative charge due to the presence of surface proteoglycans. The positively charged polyplex is attracted to the negatively charged cell surface. The polyplex interacts with the cell surface and induces cellular uptake of the polyplex via endocytosis.
Unlike viral vectors, which are naturally occurring and modified for clinical use, polymer vectors can be finely-tuned to effectively deliver genes to the CNS. The following sections will highlight the various biomaterial vector modifications that make them safer and more effective gene delivery systems than many viral vectors.
2.2.1. Immunogenicity
Biomaterial delivery systems have low immunogenicity and are typically safer to use than viral vectors (Li et al., 2016). Due to their high immunogenicity, repeated administration of viral vectors often results in increased neutralization and, in turn, decreased transgene expression (Rivière et al., 2006; Seow and Wood, 2009). Biomaterial nanosystems, however, do not lose efficacy following repeat administration because of their low immunogenicity (Foldvari et al., 2016; Huang et al., 2013, 2010b; Mali, 2013; Xiang et al., 2017). Huang and colleagues repeatedly delivered a poly(amidoamine)-based vector loaded with pDNA encoding human GDNF to a rotenone-induced Parkinson’s disease rat model via intravenous injection. This vector was conjugated with lactoferrin to improve targeting of the gene delivery system to and across the BBB. The Parkinson’s disease rat models were injected with the human GDNF transgene-loaded polymer vectors five times every other day for either 6 or 10 days. Both treatment durations significantly improved locomotor function and decreased dopaminergic neuronal loss compared to controls not loaded with pDNA encoding human GDNF. Further, no loss in vector efficiency or significant toxicity was observed (Huang et al., 2010b). Although repeated peripheral injection is fairly safe, repeated administration directly to the CNS is invasive and damaging (Abu-Rub et al., 2016). Biomaterial vectors, however, can be manipulated to extend gene release and expression in target cells, which avoids the need for repeated vector administration to the CNS or peripherally (Xiang et al., 2017). Li and colleagues, for example, injected a cationic poly(2-aminoethyl propylene phosphate) polyplex, loaded with Luc reporter pDNA, at varying N/P ratios (nitrogen of the polymer/phosphate of the DNA) into mice brains and assessed expression of Luc. Delivery of the polyplex at an N/P ratio of 2 extended expression of Luc over the entirety of the 28-day analysis with no significant decrease in expression levels. Whereas, expression levels began to significantly decrease after 3 days following delivery of the polyplex at an N/P ratio of 0.5. Importantly, this form of delivery did not elicit an immune response in vivo (Li et al., 2004). In another study, Kang et al. fabricated self-sealing porous silicon-calcium silicate core–shell nanoparticles. The porous silicon core housed siRNA capable of silencing peptidylprolyl isomerase B. Encapsulating the core in an insoluble calcium silicate shell slowed the degradation of the core and release of siRNA. Further, siRNA-loaded core-shell nanoparticles successfully downregulated the expression of peptidylprolyl isomerase B in Neuro-2a cells by approximately 52.8% compared to untreated control Neuro-2a cells in vitro (Kang et al., 2016).
Although biomaterial vectors do not typically induce cell death as a result of the immunogenic response, cationic biomaterials at high concentrations can be cytotoxic and induce cell death. Commonly used cationic vectors, such as 25 kD PEI, the current gold standard in bio-material-based gene delivery, are modified to reduce cytotoxicity and lower the risk of adverse reactions to the vector system. Wu and colleagues designed a functionalized PEI-based vector and compared its cytotoxicity to 25 kDa PEI. The polymer vector consisted of cell penetrating peptide functionalized polyethylene glycol (PEG) to improve vector targeting and cellular uptake. The cell penetrating peptide utilized consisted of an arginine-glycine-aspartate/twin-arginine translocation (RGD/TAT) sequence and is derived from the transactivating transcriptional protein. This functionalized PEG was conjugated to a chitosan-graft-PEI copolymer. The vector was referred to as RGD/TAT-functionalized chitosan-graft-PEI-PEG, or CPPP for short. This cationic polymer was complexed with pDNA encoding neurotrophin-3 (NT-3), a neurotrophic growth factor that supports neuron growth and function. CPPP/NT-3 treatment significantly improved cell viability in NIH3T3, 293 T, and HeLa cell cultures compared to cultures exposed to 25 kDa PEI complexed to NT-3. NT-3 transgene expression over 50 h was significantly greater in NIH3T3 and 293 T cultures transfected with CPPP/NT-3 compared to PEI/NT-3. Further, exposure of neural stem cells to 293 T/CPPP/NT-3 conditioned media improved neural stem cell differentiation and increased neurite extension. The functionalized CPPP polymer vector reduced cytotoxicity and effectively upregulated the production of NT-3 (Wu et al., 2018).
2.2.2. Vector targeting
Polymeric vectors have no pre-existing tropism. Although viral vector targeting can be improved by pseudotyping, pre-existing viral tropism can draw vectors away from their intended target to infect other cell types. Biomaterial vectors, however, will not be naturally drawn away from a targeted cell-type and there are many approaches for targeting specific cell and tissue types. The physiochemical properties of biomaterial vectors can be modified to enhance their ability to cross the BBB. For example, a build-up of cationic vectors at the surface of the negatively charged BBB disrupts vascular endothelium tight junctions, allowing the vectors to pass into the CNS (Lockman et al., 2004). Osmotic agents are also used to disrupt the BBB and increase vector uptake in the CNS. Copolymerization of the osmotic agent mannitol to PEI (poly(mannitol-co-PEI)), for example, improved transcytosis across the BBB and into the CNS compared to PEI alone (Park et al., 2015). Further, the hydrophilic polymer PEG can be conjugated to the vector surface to extend the circulation time of the vector system. This extended circulation increases the amount of vector disrupting the BBB and subsequently passing into the CNS. PEG sterically stabilizes the biomaterial vector and reduces opsonization and phagocytosis of the vector, which ultimately increases its bioavailability (Miele et al., 2012).
Targeting ligands, such as proteins, peptides, antibodies and glycosylated molecules are often incorporated on the biomaterial vector’s surface to direct them to a specific cell or tissue type (Rogers and Rush, 2012). Common strategies of targeting biomaterial vectors for gene delivery to the CNS are listed in Table 3. To improve CNS targeting, it is crucial to understand the receptors present both on cells in the CNS as well as the vascular endothelium of the BBB. Tetanus toxin, for example, is a potent neurotoxin that is studied for its targeting capabilities. This bacterial protein, which rapidly undergoes retrograde axonal transport towards the brain stem, consists of a heavy chain that recognizes and binds gangliosides on neurons and a light chain that interferes with synaptic transmission (Mochida et al., 1989). The portion of the tetanus toxin heavy chain that mediates neuron binding can be isolated and purified from the toxic protein to produce the recombinant non-toxic tetanus toxin C-fragment (TTC). TTC conjugated to a PLL vector containing the reporter genes Luc, green fluorescent protein, or β-gal guided the vectors into neuronal-derived cell lines displaying the relevant receptors (Knight et al., 1999). More recently, Liu and colleagues identified the peptide, tet1, which has a high binding affinity to the TTC ganglioside receptors (Liu et al., 2005). Park et al. showed the targeting capabilities of this peptide by conjugating tet1 to PEI (tet1-PEI) and complexing the conjugate with Luc reporter DNA. Transgene expression in PC-12 cells exposed to tet1-PEI was 7 times greater than cells exposed to a non-targeted PEI vector. The significant increase in transfection efficiency of tet1-PEI in PC-12 cells was not observed in NIH3T3 fibroblasts (Park et al., 2007). This finding was corroborated in a more recent study. Chu et al. conjugated tet1 to N-(2-Hydroxypropyl) methacrylamide-co-oligolysine copolymer for targeted neuronal gene delivery and also observed an increased transfection efficiency in PC-12 cells and not in NIH3T3 fibroblasts (Chu et al., 2013). Park et al., also saw significant levels of tet1-PEI vector transfection in cultures of dorsal root ganglia primary neurons, and only observed low levels of non-specific binding in primary cortical astrocyte cultures (Park et al., 2007).
Table 3.
Common Strategies of Targeting Biomaterial Vectors for Gene Delivery to the CNS.
| Mode of Targeting | Key Examples | Receptor | Location | References |
|---|---|---|---|---|
| Protein | ||||
| Transferrin | Transferrin receptor | BBB | (Cardoso et al., 2010; da Cruz et al., 2005; Huang et al., 2007; Sharma et al., 2013; Somani et al., 2014) | |
| Lactoferrin | Lactoferrin receptor | BBB | (Chen et al., 2010; Huang et al., 2010a, 2010b, 2009, 2008) | |
| Neurotensin | Neurotensin receptor | Neurons | (Gonzalez-Barrios et al., 2006; Hernandez-Chan et al., 2015) | |
| Angiopep | Low-density lipoprotein receptor | BBB & Glioma | (An et al., 2015; Gao et al., 2016; Huang et al., 2013; Ke et al., 2009; Sun et al., 2012) | |
| Antibody | ||||
| Anti-Transferrin Receptor Monoclonal Antibody | Transferrin receptor | BBB | (Ko et al., 2009; Kolhar et al., 2013; Zhang et al., 2003, 2004) | |
| Viral Glycoprotein | ||||
| Rabies virus Glycoprotein | Nicotinic Acetylcholine receptor | BBB & Neurons | (Gao et al., 2014; Kang et al., 2016; Kumar et al., 2007; Liu et al., 2016, 2013, 2009; Park et al., 2015) | |
| Neurotoxin | ||||
| Tetanus Toxin C Fragment | Ganglioside receptor | Neurons | (Calvo et al., 2011; Moreno-Igoa et al., 2010) | |
| Shape | ||||
| Rod-shaped | (Kolhar et al., 2013; Shyam et al., 2015) | |||
| Star-shaped | (Joshi et al., 2018; Qian et al., 2014) | |||
Rabies virus glycoprotein (RVG) is often incorporated onto vector systems to target the nicotinic acetylcholine receptor on CNS neurons (Kumar et al., 2007; Liu et al., 2009) Park and colleagues conjugated RVG to a PEG-poly(mannitol-co-PEI) system (R-PEG-PMT) to target neurons in the brain and inhibit expression of beta-secretase 1 (BACE1) using the siRNA (siBACE1). BACE1 is an enzyme involved in the production of harmful amyloid-beta plaques in Alzheimer’s disease (Murphy and LeVine, 2010). Intravenous administration of R-PEGPMT/siBACE1 into mice resulted in successful transcytosis of the vector into the CNS and a 2.32 and 3.03-fold decrease in BACE1 expression in the cortex and hippocampus, respectively. Further, delivery of R-PEG-PMT/siBACE1 also caused a significant decrease in harmful amyloid plaque production in the cortex and hippocampus. These findings show R-PEG-PMT/siBACE1 to be a promising preventative treatment for Alzheimer’s disease (Park et al., 2015). Similarly, Kwon et al. designed a tandem peptide-based biomaterial vector containing the RVG peptide, for neuron-targeting. This vector system also utilized the positively charged, membrane-interactive transportan (TP) peptide in tandem with RVG. The cationic TP-RVG peptide was complexed with siRNA against caspase-3 (CASP3). CASP3 encodes a protein involved in neuronal apoptosis following traumatic brain injury. In this study, mice underwent simulated traumatic brain injury to one hemisphere and were subsequently treated with TP-RVG/siCASP3 via intravenous administration. Delivery of TP-RVG/siCASP3 resulted in an 80% decrease in CASP3 expression in the damaged region of the injured hemisphere and no significant reduction in CASP3 expression in the uninjured hemisphere. In addition, no peptide was observed in astrocytes or microglia proving the cell-specific targeting capabilities of TP-RVG/si-CASP3 (Kwon et al., 2016).
Low-density lipoprotein receptor is a prevalent receptor on the BBB microvascular endothelium and can be used to improve gene delivery to CNS tissues. Huang and colleagues conjugated angiopep, a ligand of low-density lipoprotein receptor-related protein, to a dendrigraft PLL-based vector loaded with pDNA encoding human GDNF. Angiopep significantly improved cellular uptake of the PLL-based gene delivery system in an in vitro culture of brain capillary endothelial cells. This BBB-targeted vector was delivered every other day for 6 or 10 days, via intravenous injection, to rotenone-induced rat models of Parkinson’s disease. Repeat delivery of this angiopep-PLL vector loaded with pDNA encoding human GDNF resulted in significantly improved locomotor function and enhanced recovery of dopaminergic neurons compared to controls that were not conjugated to angiopep, not loaded with pDNA encoding human GDNF, or were only administered once. Further, no significant toxicity or loss of vector efficiency was observed upon repeat administration (Huang et al., 2013). The ability to administer this vector system long-term in a non-invasive manner makes this a promising gene delivery system for Parkinson’s disease, as well as other chronic neurodegenerative disorders.
Transferrin (Tf) receptor is another receptor present on the microvascular endothelium of BBB that is commonly used for CNS gene delivery. Tf serum protein or anti-Tf receptor monoclonal antibody (TfR-mAb) are two molecules often displayed on biomaterial vectors to help them cross the BBB (Johnsen et al., 2017). Somani et al. conjugated Tf to a diaminobutyric polypropylenimine (DAB) dendrimer and complexed the vector with Luc or β-gal reporter DNA forming DAB-Tf dendriplex. In this study, the targeting capabilities of this Tf barring dendriplex were compared to a non-targeted DAB dendriplex and naked Luc or β-gal reporter DNA. Intravenous administration of DAB-Tf/Luc, DAB/Luc, and naked Luc DNA revealed that Luc transgene expression in the brain was highest and most localized in the group treated with DAB-Tf/Luc. β-gal transgene expression in the brain of mice treated with DAB-Tf/β-gal was approximately 2-fold and 4.5-fold higher than mice treated with non-targeted DAB/β-gal or naked β-gal DNA respectively. Further, mice treated with DAB-Tf/β-gal also had very little β-gal present in the liver, lungs, and spleen compared to those treated with the DAB/β-gal or naked β-gal DNA (Somani et al., 2014).
Although the use of Tf as a targeting ligand is promising, Tf is an essential protein and is therefore naturally at mg/mL concentrations in our plasma. This high endogenous concentration will ultimately limit the potential of this targeting ligand. For this reason, the use of the TfR-mAb may be a better option (Gabathuler, 2010; Kolhar et al., 2013; Ulbrich et al., 2009). Kolhar et al., studied the ability of TfR-mAb displaying vectors to cross the BBB, and showed the importance of vector shape influencing specificity. Rod shaped vectors (nanorods) and spherical vectors (nanospheres) displayed TfR-mAb to target them to the endothelial cells of the BBB. Accumulation of nanorods displaying TfR-mAb in the brain was 7-times greater than that of TfR-mAb-coated nanospheres. The improved specificity of the nanorods was likely due to their higher area to volume ratio allowing increased interactions between the TfR-mAb and Tf receptor on the surface of the BBB endothelium (Kolhar et al., 2013).
In addition to improved transcytosis across the BBB, rod-shaped vectors also show improved transfection efficiency within the CNS. Shyam et al. fabricated worm-like, rod-like, and spherical PEI nanoparticles, grafted with increasing amounts of PEG (0.6%, 0.8%, and 1.2% PEG to PEI respectively), and loaded with siRNA against BACE1 to target Alzheimer’s disease. The nanoparticles, of varying shape, were delivered via intraventricular infusion into mice for up to 7 days. Following administration of the siRNA delivery system, no significant inflammation or cytotoxicity was observed. Further, the rod-like nanoparticles had the highest efficiency, as they knocked down BACE1 expression significantly more in the hippocampus and cortex compared to the worm-like and spherical nanoparticles (Shyam et al., 2015). The transfection efficiency of the vector in a specific cell type may also depend on the finely-tuned architecture of a particularly shaped vector system. Star-shaped vectors show promise as efficient gene delivery vectors due to their ability to hold greater amounts of genetic material (Byrne et al., 2013). Qian and colleagues designed three versions of a star-shaped vector consisting of a polylactic acid (PLA) and polydimethylaminoethyl methacrylate (PDMAEMA) copolymer, co-loaded with an RNAi miR-21 inhibitor (miR-21i) and the drug doxorubicin. The vector groups were labeled PLA-PDMAEMA3, (PLA-PDMAEMA3)2, (PLA-PDMAEMA3)3 according to their molecular architecture and were compared to a 25 kDa PEI control. The vectors were used to downregulate the expression of the onco-miRNA, miR-21, in glioma cells. (PLA-PDMAEMA3)3 exhibited the greatest increase in transfection efficiency, which was 2.5-fold higher than that of the PEI control. In addition, co-delivery of miR-21i and doxorubicin, particularly from (PLA-PDMAEMA3)3, resulted in a significant reduction in the expression of miR-21 in glioma cells as well as a decrease in glioma proliferation (Qian et al., 2014).
When modifying the shape of the vector it is important to consider the overall dimensions of the vector system. For example, it is crucial for spherical biomaterial vectors to range in size from 10 nm to 200 nm. Those that are smaller than 10 nm are rapidly cleared from circulation and those greater than 200 nm are prone to opsonization and can exhibit increased toxicity (Elsabahy and Wooley, 2012). Vector size is tunable based on the charge ratio between the often-cationic polymer system and the negatively charged genetic material. Song and colleagues varied the weight ratio of cationic PEI-capped gold nanoparticles to siRNA and monitored the resulting size and zeta potential of the different nanoparticles. The siRNA-loaded nanoparticles fabricated at a gold nanoparticle to siRNA weight ratio of 0.3:1 and 0.6:1 resulted in a particle diameter of 955mn and 712 nm respectively, both with a zeta potential of approximately 0 mV. By increasing the weight ratio to 1.2:1 and 3:1 particle diameter decreased to 122 nM and 18.2 nM respectively, both with a zeta potential of approximately +12 mV (Song et al., 2010b). This study showed that increasing positively charged material can pack the negatively charged genetic material more tightly, decreasing the size of the nanoparticle.
2.2.3. Production levels, cost, and public views
Biomaterial gene delivery vectors do not possess the same external issues as viral gene therapies. At an industrial scale, manufacturing of biomaterial vectors for gene delivery is more feasible than manufacturing of viral vectors. Large scale production of polymeric nanoparticles is easier and more cost effective than large scale production of viral vectors (Li et al., 2016; Ramalingam et al., 2012; Ramamoorth and Narvekar, 2015; Severino et al., 2015). Due to increased production and lower cost, researchers are able to create novel biomaterial-based gene delivery systems that are more translational. Biomaterial-based gene therapies can be produced at a level capable of meeting the public’s need while also being more affordable than viral-based gene therapies.
Biomaterial gene delivery is more widely accepted by the general public than viral systems. Portions of the vector systems used for targeting purposes, however, may raise concern. Fragments of toxin or viral proteins, for example TTC or RVG, can be incorporated on the vector surface as mentioned in previous sections. Recent developments, however, have resulted in the fabrication of peptides that mimic certain fragments, such as tet1 mentioned in Section 2.2.2 (Chu et al., 2013; Park et al., 2007). Such developments will help limit possible adverse reactions to vector delivery systems.
2.2.4. Current clinical status of biomaterial vectors
The number of studies that utilize biomaterial vectors for gene delivery is on the rise. Investigation into biomaterial vectors has primarily been in in vitro models. However, increasing numbers of these systems are being investigated in vivo. Despite the developing nature of the field, several biomaterial-based vector systems are being tested in preclinical and clinical trials. Systems that have reached clinical trials are mainly composed of either lipid-based or PEI-based materials and are primarily aimed at treating various forms of cancer (Li et al., 2016). In 2011, the M.D. Anderson Cancer Center completed a Phase I clinical study for a lipid-based gene delivery system that aims to treat non-small-cell lung cancer (NCT00059605). The system consists of a cationic DOTAP:cholesterol nanoparticle encapsulating pDNA that encodes the tumor suppressor gene, TUSC2. Following up to 12 cycles of intravenous administration of the gene delivery system, patients experienced minimal toxicity and did not develop antibodies to the foreign pDNA. Further, post-treatment biopsies showed 10 to 25-fold more TUSC2 protein production and increased expression of pro-apoptotic genes compared to pre-treatment biopsies (Lu et al., 2012). A Phase I/II clinical trial, sponsored by Genprex, for this gene delivery system is currently under way. In this trial the TUSC2-pDNA-loaded DOTAP:cholesterol nanoparticles are being delivered in combination with other small molecule drugs to improve the efficacy of the treatment (NCT01455389) (“TUSC2-nanoparticles and Erlotinib in Stage IV Lung Cancer - ClinicalTrials.gov,” 2011).
A Phase I clinical trial is currently recruiting for intratumoral/intralesional administration of a PEI-based vector aimed at targeting advanced/metastatic or recurrent tumors (NCT03739138). The cationic vector is complexed to an RNA oligonucleotide sequence that activates expression of retinoic acid-inducible gene 1. In addition, the PEI-based vector is delivered in combination with Pembrolizumab. Upregulated expression of retinoic acid-inducible gene 1 in tumor cells activates innate immunity, and Pembrolizumab inhibits the inactivation of T cells (“Intratumoral/Intralesional Administration of MK-4621/JetPEITM With or Without Pembrolizumab in Participants With Advanced/Metastatic or Recurrent Solid Tumors (MK-4621–002) - ClinicalTrials.gov,” 2018). This immunotherapy enhances the immune system’s ability to fight off various forms of cancer. If successful, this application has the potential to become the first synthetic polymer-based gene delivery system on the market.
Biomaterial-based vectors targeting CNS disorders have made it to preclinical trials, however they have yet to reach clinical trials (Jayant et al., 2016). Studies that have reached clinical trials, such as those explained above, will give insight to how the human body reacts to natural and synthetic biomaterial-based vectors. This will allow researchers to construct safe and efficient gene delivery systems to treat various diseases or disorders, including those affecting the CNS.
3. Advances in combinatorial biomaterial systems
Biomaterial gene delivery vectors are commonly modified to improve cell-specific targeting and extend gene delivery. However, the bioavailability of the vector in the optimal location for a sustained period of time may still be reduced by clearance or phagocytosis. The concentration of vector at the relevant location for a specific application has been improved by combining optimally designed biomaterial vectors with biomaterial scaffolds capable of sustained and localized release of therapeutic molecules. This section will review biomaterial systems that have improved gene therapy, particularly for CNS repair.
3.1. Electrospun fibers
Electrospun fibers are fabricated to resemble the fibrous proteins of the extracellular matrix and are therefore commonly used in tissue engineering applications (DöAmato et al., 2017). Highly aligned fibrous scaffolds are favorable for spinal cord injury applications, due to their ability to guide the migration of glia and regenerating axons through a lesion site and reduce glial scar formation (Hurtado et al., 2011; Wang et al., 2009). In addition, electrospun fibers are employed to provide localized and sustained release of therapeutic molecules, such as drugs, proteins, and genes, to further enhance their regenerative properties (D’Amato et al., 2017; Ji et al., 2011; Lee et al., 2014).
Yang et al. extended the release of pDNA by encapsulating the genetic material in the aqueous core of coaxial electrospun fibers. The sheath of the coaxial fibers consisted of either poly(DL-lactide)–poly (ethylene glycol) (PELA) alone or PELA conjugated to PEI (PELA-PEI). Fibers consisting of a PELA sheath released naked pDNA for 15 days, whereas fibers with a PELA-PEI sheath released pDNA complexed with PEI for 15 days. The anionic pDNA complexed with the cationic PEI as it diffused out of the core and through the sheath. The formation of this polyplex protects the genetic material from degradation after diffusion out of the fibrous scaffold (Yang et al., 2011). PEI also serves as a strong transfection agent, improving cellular uptake compared to naked genetic material (Abdallah et al., 1996; Goula et al., 1998). In an experimental group separate from those explained previously, pre-formed PEI/pDNA polyplexes were loaded into the aqueous core of the coaxial fibers. Release of the polyplexes was manipulated by incorporating PEG in the PELA sheath of the fibers. Including 10 wt.% PEG with a molecular weight of 4 kDa extended the release of polyplexes to 3 weeks. Further, inclusion of 4 kDa PEG at 10% resulted in a 10-fold increase in transfection efficiency in NIH3T3 cells compared to groups containing no PEG (Yang et al., 2011). Regeneration within the CNS is often a lengthy process so sustained release of therapeutically relevant genes over several weeks is desirable.
Strategies for incorporating therapeutic molecules into electrospun fiber scaffolds can drastically alter the release kinetics. In a recent study by Pinese and colleagues, PEI modified mesoporous silica nanoparticles (MSN-PEI) loaded with siRNA were either complexed on the surface of electrospun polycaprolactone (PCL) fibers using the mussel-inspired bioadhesive 3,4-dihydroxy-l-phenylalanin, or encapsulated in poly(caprolactone-co-ethyl ethylene phosphate) fibers. Surface-coated PCL fibers released a majority of their non-viral siRNA delivery systems over 10 days. Fibers loaded siRNA/MSN-PEI, on the other hand, showed prolonged release of the vector system over 150 days. In an in vivo model, fibers loaded with siRNA/MSN-PEI silenced gene expression over a 4-week period (Pinese et al., 2018). This scaffold holds the potential to provide sustained delivery of siRNA to suppress glioma cell growth and proliferation or downregulate expression of genes linked to neurodegenerative diseases or CNS secondary injury cascades.
In addition to providing directional guidance and sustained release of therapeutics, Li et al. showed electrospun PCL fibers can promote growth and differentiation of oligodendrocyte precursor cells and, in turn, enhance remyelination (Li et al., 2014). Diao and colleagues incorporated miRNA onto the surface of PCL fibers to further improve their regenerative properties. miR-219 and miR-338, which both silence factors that inhibit OPC differentiation and maturation, were immobilized on the surface of the fibers using bioadhesive 3,4-dihydroxyl-phenylalanin. Release of the miRNAs from the 3D fibrous PCL scaffolds lasted for 2 weeks and significantly improved OPC differentiation and maturation compared to PCL fibers alone. This scaffold can potentially promote remyelination following neurodegeneration from disease or CNS injury (Diao et al., 2015).
In addition to loading biomaterial-based vectors, researchers have also incorporated viral vectors into polymeric tissue engineering scaffolds for sustained gene delivery. Liao and colleagues fabricated adenovirus-encapsulated PCL coaxial electrospun fibers. The adenoviral vectors, localized within the core of the coaxial fibers, contained the reporter gene expressing green fluorescent protein. RAW 264.7 cells, seeded onto the fibers, expressed reduced levels of interleukin-1β, tumor necrosis factor-α, and interferon-α compared to cells directly exposed to the same viral titer. This suggested that encapsulation of the viral vectors within the fibers reduced the immunogenic properties of the scaffold. Further, HEK 293 cells cultured onto the fibers expressed detectable levels of transgene for over 30 days (Liao et al., 2009).
The studies above consisted primarily of in vitro work. However, based on the results of each, researchers can fine tune similar systems to achieve more desirable results in future in vivo studies.
3.2. Hydrogels
Although hydrogels alone do not provide the extensive cellular guidance that electrospun fibers do, they are a popular choice for CNS tissue engineering. The mechanical properties of hydrogels are highly tunable and can be matched to the soft CNS tissue. Some hydrogels can be injected as a liquid and solidify upon pH or temperature change, making them minimally invasive and space filling (Klouda and Mikos, 2008; Schmaljohann, 2006; Yang et al., 2016). Further, hydrogels can be loaded with therapeutic molecules that are released locally in a sustained manner (Li et al., 2012, 2003). Yang and colleagues fabricated an injectable thermoresponive PEG-PCL-PEG hydrogel containing a tumor-targeted folate-poly(esteramine) (FA-PEA) polymer vector. The FA-PEA vector was complexed to the anti-oncogene Wnt inhibitory factor-1 (WIF-1). Release of the FA-PEA/WIF-1 vector from the hydrogel was sustained for approximately 7 days and resulted in successful transfection and apoptosis of colorectal cancer cells. This vector delivery system was also less cytotoxic than 25 kDa PEI (Yang et al., 2016). In addition to treating colon cancer, WIF-1 is a powerful tumor suppressor for several other cancer types including glioblastoma (Wu et al., 2012). Further, the folate receptor is overexpressed on glioma cells compared to normal brain tissues and has been utilized for glioma targeted receptor-mediated vector endocytosis (Chen et al., 2014; Gao et al., 2013; Wang et al., 2015). The multicancer targeting and suppressing capabilities of Yang and colleagues’ injectable FA-PEA/WIF-1 loaded hydrogel, as well as its ease of administration, suggest this approach is promising for delivery of genes to treat cancer in the CNS.
To guide cells while maintaining the desirable mechanical characteristics of a hydrogel, the gel can be molded and solidified to contain guidance cues that will direct cellular regeneration. For example, Shepard et al. fabricated an enzymatically degradable PEG hydrogel molded into channels with varying widths to provide physical guidance for extending neurites. The gels were loaded with RGD adhesion sites and lipoplexes — cationic lipids complexed with anionic genetic material — carrying DNA expressing nerve growth factor (NGF). Dorsal root ganglia explants and fibroblasts were incorporated into the gel to test the scaffolds’ efficacy in vitro. The in vitro culture was maintained for 5 days. Secreted lipoplexes successfully transfected fibroblasts and upregulated their production of NGF for the entirety of the 5-day culture. Neurite extension increased with the delivery of increasing amounts of DNA encoding NGF. Further, the greatest extension was seen when this hydrogel delivery system was molded into a channel width of 250 μm. Topographical guidance provided by the channel walls, in combination with the biochemical cues provided by increased transgene expression of NGF in fibroblasts, resulted in robust directed neurite extension (Shepard et al., 2012). This in vitro co-culture model serves as a useful platform to investigate various materials, topographical cues, and levels of transgene expression that best support regeneration within the CNS.
Liu and colleagues performed a caudal injection of AAV vectors encoding brain derived neurotrophic factor, after implanting a Schwann cell-seeded capillary alginate hydrogel into a rat hemisection spinal cord injury model (Liu et al., 2017). Brain derived neurotrophic factor supports axonal extension and Schwann cells support regeneration of axons in the peripheral nervous system (Jessen and Mirsky, 2016; Liao et al., 2015). In addition, the aligned capillaries running through the hydrogel provided directional guidance of regenerating axons for the full length of the 8-week study. The viral vectors were not encapsulated within the hydrogel or immobilized on its surface. However, delivery of the AAV vectors in combination with the implanted capillary hydrogel significantly increased the number of regenerating axons across the lesion. Further, caudal injection of both Schwann cells and AAV vectors promoted axonal regeneration through the entirety of the capillary hydrogel and into the caudal spinal cord (Liu et al., 2017).
3.3. Composite scaffolds
To provide the guidance cues of electrospun fibers while maintaining the beneficial characteristics of a hydrogel, the two systems can be combined into a single scaffold. Nguyen and colleagues designed a fiber hydrogel composite scaffold to assess its ability to provide simultaneous non-viral drug and gene delivery and promote directed axonal regeneration following spinal cord injury. Micellar nanoparticles, consisting of poly(ε-caprolactone)-block-polyethylene glycol and poly(ε-caprolactone)-block-poly(2-aminoethyl ethylene phosphate), were fabricated and loaded with miR-222, an RNAi molecule associated with the ability of improving axon growth. The miR-222-loaded nanoparticles were spun into aligned poly(caprolactone-co-ethyl ethylene phosphate) nanofibers, which were loaded into cylindrical molds and encapsulated within a collagen hydrogel loaded with additional miR-222-loaded nanoparticles and the neurotrophic protein NT-3. Sustained release of NT-3 and miR-222 from the scaffold was observed for approximately 90 days. In an in vivo model, scaffolds were implanted into 6–8-week-old rats. Aligned regenerating axons were found within the scaffold just 1 week following scaffold implantation. Additional directed regeneration through the scaffold was observed 2 and 4 weeks following scaffold implantation. Use of the aligned fiber, hydrogel scaffold loaded with miR-222-loaded nanoparticles and NT-3 resulted in significant aligned axonal regeneration through the lesion site. The scaffold did not provoke a harmful immune response, nor did it induce glial scar formation (Nguyen et al., 2017). This scaffold shows great potential as a method of treatment following spinal cord injury.
4. Conclusion
Gene therapy is an attractive treatment option for neurodegenerative disease and injury to the brain or spinal cord. Gene delivery to the CNS, however, is a difficult task. Because of their natural ability to infect host cells, viral delivery systems are studied extensively to deliver genes to the CNS. Although they are useful gene delivery vehicles, views on the use of viral vectors have become less favorable due to their high immunogenicity, broad tropism, and high cost of production. Biomaterial vectors for gene delivery, on the other hand, are becoming increasingly popular and are promising alternatives to viral vectors. The highly tunable properties of biomaterial vectors allow for controlled delivery of genes as well as incorporation of targeting molecules for cell-specific delivery. Further, the cost of biomaterial vector production is much lower, and the production levels are much higher compared to that of viral vectors. To further enhance gene delivery to the CNS, 3D biomaterial scaffolds are being studied to provide local and sustained delivery of gene-loaded biomaterial vectors and, in some cases, also support and direct regeneration of cells. Biomaterial gene delivery to the CNS is an exciting and fast-growing field. This area of research, although still widely uncharted, has already shown the potential of leading to the discovery of effective treatments for CNS diseases and following CNS injury.
Acknowledgements
Funding for this work was provided by NIH R01 (NS092754) to R.J.G, State of New York Spinal Cord Injury Research Program Institutional Grant (C32245GG) to R.J.G, and New York Spinal Cord Injury Research Board Predoctoral Fellowship Award (C30606GG) to A.R.D. This material is also based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. (DGE-1744655) awarded to D.L.P. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Abbreviations:
- AAV
adeno-associated virus
- BACE1
beta-secretase 1
- BBB
blood brain barrier
- CASP3
caspase-3
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DAB
diaminobutyric polypropylenimine
- FA-PEA
folate-poly(esteramine)
- GDNF
glial cell derived neurotrophic factor
- GFAP
glial fibrillary acidic protein
- Luc
luciferase
- miR-21i
RNAi miR-21 inhibitor
- miRNA
micro interfering RNA
- mRNA
messenger RNA
- NGF
nerve growth factor
- NT-3
neurotrophin-3
- PCL
polycaprolactone
- PDMAEMA
polydimethylaminoethyl methacrylate
- pDNA
plasmid DNA
- PEG
polyethylene glycol
- PEI
polyethyleneimine
- PELA
poly(dl-lactide)–poly(ethylene glycol)
- PLA
polylactic acid
- PLL
poly(l-lysine)
- RGD/TAT
arginine-glycine-aspartate/twin-arginine translocation peptide
- RISC
RNA-induced silencing complex
- RNAi
RNA interference
- RVG
rabies virus glycoprotein
- siRNA
small interfering RNA
- SMN
survival motor neuron protein
- TCC
tetanus toxin C-fragment
- Tf
transferrin
- TfR-mAb
anti-transferrin receptor monoclonal antibody
- TP
transportan
- WIF-1
anti-oncogene Wnt inhibitory factor-1
- β-gal
β-galactosidase
Footnotes
Disclosure of interest
All contributing authors have no conflicts of interest to disclose.
References
- Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA, 1996. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum. Gene Ther 7, 1947–1954. 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- Abu-Rub MT, Newland B, Naughton M, Wang W, McMahon S, Pandit A, 2016. Non-viral xylosyltransferase-1 siRNA delivery as an effective alternative to chondroitinase in an in vitro model of reactive astrocytes. Neuroscience 339, 267–275. 10.1016/j.neuroscience.2016.10.026. [DOI] [PubMed] [Google Scholar]
- Agarwal R, Singh V, Jurney P, Shi L, Sreenivasan SV, Roy K, 2013. Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. PNAS 110, 17247–17252. 10.1073/pnas.1305000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zaidy S, Pickard AS, Kotha K, Alfano LN, Lowes L, Paul G, Church K,Lehman K, Sproule DM, Dabbous O, Maru B, Berry K, Arnold WD, Kissel JT, Mendell JR, Shell R, 2019. Health outcomes in spinal muscular atrophy type 1 following AVXS-101 gene replacement therapy. Pediatr. Pulmonol 54, 179–185. 10.1002/ppul.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, He D, Wagner E, Jiang C, 2015. Peptide-like polymers exerting effective glioma-targeted siRNA delivery and release for therapeutic application. Small 11, 5142–5150. 10.1002/smll.201501167. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Hall LL, Ayyalapu AR, Irion VR, Nantz MH, Hecker JG, 2003. Stability of mRNA/cationic lipid lipoplexes in human and rat cerebrospinal fluid: methods and evidence for nonviral mRNA gene delivery to the central nervous system. Hum. Gene Ther 14, 191–202. [DOI] [PubMed] [Google Scholar]
- Ayers JI, Fromholt S, Sinyavskaya O, Siemienski Z, Rosario AM, Li A, Crosby KW, Cruz PE, DiNunno NM, Janus C, Ceballos-Diaz C, Borchelt DR, Golde TE, Chakrabarty P, Levites Y, 2015. Widespread and efficient transduction of spinal cord and brain following neonatal AAV injection and potential disease modifying effect in ALS mice. Mol. Ther 23, 53–62. 10.1038/mt.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz M, Le T, Ralph GS, Walmsley L, Monani UR, Lee DCP, Wilkes F, Mitrophanous KA, Kingsman SM, Burghes AHM, Mazarakis ND, 2004. Lentivector-mediated SMN replacement in a mouse model of spinal muscular atrophy. J. Clin. Invest 114, 1726–1731. 10.1172/JCI200422922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C, Scheideler O, Schaffer D, 2019. Engineering the AAV capsid to evade immune responses. Curr. Opin. Biotechnol. Pharm. Biotechnol 60, 99–103. 10.1016/j.copbio.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Baumann TL, Siffert J, Herzog CD, Alterman R, Boulis N, Turner DA, Stacy M, Lang AE, Lozano AM, Olanow CW, 2013. Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology 80, 1698–1701. 10.1212/WNL.0b013e3182904faa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Weinberg MS, Samulski RJ, 2014. Parkinson’s disease gene therapy: success by design meets failure by efficacy. Mol. Ther 22, 487–497. 10.1038/mt.2013.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkhelifa-Ziyyat S, Besse A, Roda M, Duque S, Astord S, Carcenac R, Marais T, Barkats M, 2013. Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice. Mol. Ther 21, 282–290. 10.1038/mt.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkler C, Barhum Y, Ben-Zur T, Offen D, 2016. Multifactorial gene therapy enhancing the glutamate uptake system and reducing oxidative stress delays symptom onset and prolongs survival in the SOD1-G93A ALS mouse model. J. Mol. Neurosci 58, 46–58. 10.1007/s12031-015-0695-2. [DOI] [PubMed] [Google Scholar]
- Bergen JM, Pun SH, 2008. Analysis of the intracellular barriers encountered by nonviral gene carriers in a model of spatially controlled delivery to neurons. J. Gene Med 10, 187–197. 10.1002/jgm.1137. [DOI] [PubMed] [Google Scholar]
- Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L, Chan CM, McCrate M, Chicoine LG, Coley BD, Porensky PN, Kolb SJ, Mendell JR, Burghes AH, Kaspar BK, 2011. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol. Ther 19, 1971–1980. 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biferi MG, Cohen-Tannoudji M, Cappelletto A, Giroux B, Roda M, Astord S, Marais T, Bos C, Voit T, Ferry A, Barkats M, 2017. A new AAV10-U7-mediated gene therapy prolongs survival and restores function in an ALS mouse model. Mol. Ther 25, 2038–2052. 10.1016/j.ymthe.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Pardridge WM, 2011. The trojan horse liposome technology for nonviral gene transfer across the blood-brain barrier [WWW document]. J. Drug Deliv 10.1155/2011/296151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone DR, Leek JM, Falduto MT, Torres KEO, Sell SL, Parsley MA, Cowart JC, Uchida T, Micci M-A, DeWitt DS, Prough DS, Hellmich HL, 2017. Effects of AAV-mediated knockdown of nNOS and GPx-1 gene expression in rat hippocampus after traumatic brain injury. PLoS One 12, e0185943. 10.1371/journal.pone.0185943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouard D, Alazard-Dany N, Cosset F-L, 2009. Viral vectors: from virology to transgene expression: viral vectors: from virology to transgene expression. Br. J. Pharmacol 157, 153–165. 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers WJ, Breakefield XO, Sena-Esteves M, 2011. Genetic therapy for the nervous system. Hum. Mol. Genet 20, R28–R41. 10.1093/hmg/ddr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz CJ, Friedel T, Büning H, 2015. Surface-engineered viral vectors for selective and cell type-specific gene delivery. Trends Biotechnol. 33, 777–790. 10.1016/j.tibtech.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Byrne M, Victory D, Hibbitts A, Lanigan M, Heise A, Cryan S-A, 2013. Molecular weight and architectural dependence of well-defined star-shaped poly(lysine) as a gene delivery vector. Biomater. Sci 1, 1223–1234. 10.1039/C3BM60123D. [DOI] [PubMed] [Google Scholar]
- Calvo AC, Moreno-Igoa M, Mancuso R, Manzano R, Oliván S, Muñoz MJ, Penas C, Zaragoza P, Navarro X, Osta R, 2011. Lack of a synergistic effect of a non-viral ALS gene therapy based on BDNF and a TTC fusion molecule. Orphanet J. Rare Dis 6, 10. 10.1186/1750-1172-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso ALC, Costa P, de Almeida LP, Simões S, Plesnila N, Culmsee C, Wagner E, Pedroso de Lima MC, 2010. Tf-lipoplex-mediated c-Jun silencing improves neuronal survival following excitotoxic damage in vivo. J. Control. Release 142, 392–403. 10.1016/j.jconrel.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Chen H, Tang L, Qin Y, Yin Y, Tang J, Tang W, Sun X, Zhang Z, Liu J, He Q, 2010. Lactoferrin-modified procationic liposomes as a novel drug carrier for brain delivery. Eur. J. Pharm. Sci 40, 94–102. 10.1016/j.ejps.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Chen N, Shao C, Qu Y, Li S, Gu W, Zheng T, Ye L, Yu C, 2014. Folic acid-conjugated MnO nanoparticles as a T1 contrast agent for magnetic resonance imaging of tiny brain gliomas. ACS Appl. Mater. Interfaces 6, 19850–19857. 10.1021/am505223t. [DOI] [PubMed] [Google Scholar]
- Chow BW, Gu C, 2015. The molecular constituents of the blood–Brain barrier. Trends Neurosci. Neuroimmunol 38, 598–608. 10.1016/j.tins.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DSH, Schellinger JG, Bocek MJ, Johnson RN, Pun SH, 2013. Optimization of Tet1 ligand density in HPMA-co-oligolysine copolymers for targeted neuronal gene delivery. Biomaterials 34. 10.1016/j.biomaterials.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin J, Zhang X-Y, Reiser J, 2005. Altering the tropism of lentiviral vectors through pseudotyping. Curr. Gene Ther 5, 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz MTG, Cardoso ALC, de Almeida LP, Simões S, de Lima MCP, 2005. Tflipoplex-mediated NGF gene transfer to the CNS: neuronal protection and recovery in an excitotoxic model of brain injury. Gene Ther. 12, 1242–1252. 10.1038/sj.gt.3302516. [DOI] [PubMed] [Google Scholar]
- D’Amato AR, Schaub NJ, Cardenas JM, Fiumara AS, Troiano PM, Fischetti A, Gilbert RJ, 2017. Removal of retained electrospinning solvent prolongs drug release from electrospun PLLA fibers. Polymer 123, 121–127. 10.1016/j.polymer.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico A, Mercuri E, Tiziano FD, Bertini E, 2011. Spinal muscular atrophy. Orphanet J. Rare Dis 6, 71. 10.1186/1750-1172-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow JJ, 2019. Luxturna: FDA documents reveal the value of a costly gene therapy. Drug Discov. Today 10.1016/j.drudis.2019.01.019. [DOI] [PubMed] [Google Scholar]
- de Leeuw CN, Dyka FM, Boye SL, Laprise S, Zhou M, Chou AY, Borretta L, McInerny SC, Banks KG, Portales-Casamar E, Swanson MI, D’Souza CA, Boye SE, Jones SJ, Holt RA, Goldowitz D, Hauswirth WW, Wasserman WW, Simpson EM, 2014. Targeted CNS delivery using human MiniPromoters and demonstrated compatibility with adeno-associated viral vectors. Mol. Ther. – Meth. Clin. Dev 1, 5. 10.1038/mtm.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler EC, Liu J, Kerwin A, Torres S, Olcott CM, Bowman BN, Armijo L, Gentry K, Wilkerson J, Wallace J, Jiang X, Carnes EC, Brinker CJ, Milligan ED, 2013. Mesoporous silica-supported lipid bilayers (protocells) for DNA cargo delivery to the spinal cord. J. Control. Release 168, 209–224. 10.1016/j.jconrel.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao HJ, Low WC, Milbreta U, Lu QR, Chew SY, 2015. Nanofiber-mediated microRNA delivery to enhance differentiation and maturation of oligodendroglial precursor cells. J. Control. Release 208, 85–92. 10.1016/j.jconrel.2015.03.005. 12th International Nanomedicine and Drug Delivery Symposium (NanoDDS 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez E, Marais T, Chatauret N, Benkhelifa-Ziyyat S, Duque S, Ravassard P, Carcenac R, Astord S, Moura D, Pereira A, Voit T, Barkats M, 2011. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum. Mol. Genet 20, 681–693. 10.1093/hmg/ddq514. [DOI] [PubMed] [Google Scholar]
- Drinkut A, Tereshchenko Y, Schulz JB, Bähr M, Kügler S, 2012. Efficient gene therapy for Parkinson’s disease using astrocytes as hosts for localized neurotrophic factor delivery. Mol. Ther 20, 534–543. 10.1038/mt.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu S, 2001. Polymeric Biomaterials, Revised and Expanded. CRC Press. [Google Scholar]
- Eleftheriadou I, Dieringer M, Poh XY, Sanchez-Garrido J, Gao Y, Sgourou A, Simmons LE, Mazarakis ND, 2017. Selective transduction of astrocytic and neuronal CNS subpopulations by lentiviral vectors pseudotyped with Chikungunya virus envelope. Biomaterials 123, 1–14. 10.1016/j.biomaterials.2017.01.023. [DOI] [PubMed] [Google Scholar]
- Elsabahy M, Wooley KL, 2012. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev 41, 2545. 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici T, Boulis NM, 2012. Gene therapy for amyotrophic lateral sclerosis. Neurobiol. Dis 48, 236–242. 10.1016/j.nbd.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Foldvari M, Chen DW, Nafissi N, Calderon D, Narsineni L, Rafiee A, 2016. Non-viral gene therapy: gains and challenges of non-invasive administration methods. J. Control. Release 240, 165–190. 10.1016/j.jconrel.2015.12.012. SI: North America Part II. [DOI] [PubMed] [Google Scholar]
- Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, Le TT, Morales PR, Rich MM, Burghes AHM, Kaspar BK, 2010. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat. Biotechnol 28, 271–274. 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gabathuler R, 2010. Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiol. Dis. Special Issue: Blood Brain Barrier 37, 48–57. 10.1016/j.nbd.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Gagnon KT, Li L, Chu Y, Janowski BA, Corey DR, 2014. RNAi factors are present and active in human cell nuclei. Cell Rep. 6, 211–221. 10.1016/j.celrep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gándara C, Affleck V, Stoll EA, 2018. Manufacture of third-generation lentivirus for preclinical use, with process development considerations for translation to good manufacturing practice. Hum. Gene Ther. Methods 29, 1–15. 10.1089/hgtb.2017.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J-Q, Lv Q, Li L-M, Tang X-J, Li F-Z, Hu Y-L, Han M, 2013. Glioma targeting and blood–brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials 34, 5628–5639. 10.1016/j.biomaterials.2013.03.097. [DOI] [PubMed] [Google Scholar]
- Gao S, Tian H, Xing Z, Zhang D, Guo Y, Guo Z, Zhu X, Chen X, 2016. A non-viral suicide gene delivery system traversing the blood brain barrier for non-invasive glioma targeting treatment. J. Control. Release 243, 357–369. 10.1016/j.jconrel.2016.10.027. [DOI] [PubMed] [Google Scholar]
- Gao Y, Wang Z-Y, Zhang J, Zhang Y, Huo H, Wang T, Jiang T, Wang S, 2014. RVG-peptide-linked trimethylated chitosan for delivery of siRNA to the brain. Biomacromolecules 15, 1010–1018. 10.1021/bm401906p. [DOI] [PubMed] [Google Scholar]
- Geddes BJ, Harding TC, Lightman SL, Uney JB, 1997. Long-term gene therapy in the CNS: reversal of hypothalamic diabetes insipidus in the Brattleboro rat by using an adenovirus expressing arginine vasopressin. Nat. Med 3, 1402–1404. [DOI] [PubMed] [Google Scholar]
- Gene Replacement Therapy Clinical Trial for Patients With Spinal Muscular Atrophy Type 1 - ClinicalTrials.gov [WWW Document], 2017. ClinicalTrials.gov. (Accessed 16 April 2019). https://clinicaltrials.gov/ct2/show/NCT03306277.
- Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J, 2013. Gene therapy clinical trials worldwide to 2012 – an update. J. Gene Med 15, 65–77. 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR, 2018. Gene therapy clinical trials worldwide to 2017: an update. J. Gene Med 20, e3015. 10.1002/jgm.3015. [DOI] [PubMed] [Google Scholar]
- Glascock JJ, Shababi M, Wetz MJ, Krogman MM, Lorson CL, 2012. Direct central nervous system delivery provides enhanced protection following vector mediated gene replacement in a severe model of Spinal Muscular Atrophy. Biochem. Biophys. Res. Commun 417, 376–381. 10.1016/j.bbrc.2011.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho BMDC, McCarthy DJ, Torres-Fuentes C, Beltrán CJ, McCarthy J, Quinlan A, Ogier JR, Darcy R, O’Driscoll CM, Cryan JF, 2014. Differential nanotoxicological and neuroinflammatory liabilities of non-viral vectors for RNA interference in the central nervous system. Biomaterials 35, 489–499. 10.1016/j.biomaterials.2013.09.068. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Barrios JA, Lindahl M, Bannon MJ, Anaya-Martínez V, Flores G, Navarro-Quiroga I, Trudeau LE, Aceves J, Martinez-Arguelles DB, Garcia-Villegas R, Jiménez I, Segovia J, Martinez-Fong D, 2006. Neurotensin polyplex as an efficient carrier for delivering the human GDNF gene into nigral dopamine neurons of hemiparkinsonian rats. Mol. Ther 14, 857–865. 10.1016/j.ymthe.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Goula D, Remy JS, Erbacher P, Wasowicz M, Levi G, Abdallah B, Demeneix BA, 1998. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene Ther. 5, 712–717. 10.1038/sj.gt.3300635. [DOI] [PubMed] [Google Scholar]
- Gower RM, Shea LD, 2013. Biomaterial Scaffolds for controlled, localized gene delivery of regenerative factors. Adv. Wound Care (New Rochelle) 2, 100–106. 10.1089/wound.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray Steven J., Foti SB, Schwartz JW, Bachaboina L, Taylor-Blake B, Coleman J, Ehlers MD, Zylka MJ, McCown TJ, Samulski RJ, 2011a. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum. Gene Ther 22, 1143–1153. 10.1089/hum.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray Steven J., Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ, 2011b. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol. Ther 19, 1058–1069. 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R, 2013. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 20, 450–459. 10.1038/gt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JM, Fackelmeier B, Fong DM, Mouravlev A, Young D, O’Carroll SJ, 2019. Astrocyte-selective AAV gene therapy through the endogenous GFAP promoter results in robust transduction in the rat spinal cord following injury. Gene Ther 1. 10.1038/s41434-019-0075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Al-Bayati K, Ho EA, 2017. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv. Transl. Res 7, 497–506. 10.1007/s13346-017-0368-5. [DOI] [PubMed] [Google Scholar]
- Gwak S-J, Koo H, Yun Y, Yhee JY, Lee HY, Yoon DH, Kim K, Ha Y, 2015. Multifunctional nanoparticles for gene delivery and spinal cord injury. J. Biomed. Mater. Res. A 103, 3474–3482. 10.1002/jbm.a.35489. [DOI] [PubMed] [Google Scholar]
- Han L, Zhang A, Wang H, Pu P, Kang C, Chang J, 2011. Construction of novel brain-targeting gene delivery system by natural magnetic nanoparticles. J. Appl. Polym. Sci 121, 3446–3454. 10.1002/app.33995. [DOI] [Google Scholar]
- Hernandez-Chan NG, Bannon MJ, Orozco-Barrios CE, Escobedo L, Zamudio S, De la Cruz F, Gongora-Alfaro JL, Armendáriz-Borunda J, Reyes-Corona D, Espadas-Alvarez AJ, Flores-Martínez YM, Ayala-Davila J, Hernandez-Gutierrez ME, Pavón L, García-Villegas R, Nadella R, Martinez-Fong D, 2015. Neurotensinpolyplex-mediated brain-derived neurotrophic factor gene delivery into nigral dopamine neurons prevents nigrostriatal degeneration in a rat model of early Parkinson’s disease. J. Biomed. Sci 22, 59. 10.1186/s12929-015-0166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderer C, Bell P, Katz N, Vite CH, Louboutin J-P, Bote E, Yu H, Zhu Y, Casal ML, Bagel J, O’Donnell P, Wang P, Haskins ME, Goode T, Wilson JM, 2018a. Evaluation of intrathecal routes of administration for adeno-associated viral vectors in large animals. Hum. Gene Ther 29, 15–24. 10.1089/hum.2017.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderer C, Katz N, Buza EL, Dyer C, Goode T, Bell P, Richman LK, Wilson JM, 2018b. Severe toxicity in nonhuman Primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther 29, 285–298. 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Ke W, Han L, Liu Y, Shao K, Jiang C, Pei Y, 2010a. Lactoferrin-modified nanoparticles could mediate efficient gene delivery to the brain in vivo. Brain Res. Bull 81, 600–604. 10.1016/j.brainresbull.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Huang R, Ke W, Han L, Liu Y, Shao K, Ye L, Lou J, Jiang C, Pei Y, 2009. Brain-targeting mechanisms of lactoferrin-modified DNA-Loaded nanoparticles. J. Cereb. Blood Flow Metab 29, 1914–1923. 10.1038/jcbfm.2009.104. [DOI] [PubMed] [Google Scholar]
- Huang R, Ke W, Liu Y, Jiang C, Pei Y, 2008. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials 29, 238–246. 10.1016/j.biomaterials.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Huang R, Ke W, Liu Y, Wu D, Feng L, Jiang C, Pei Y, 2010b. Gene therapy using lactoferrin-modified nanoparticles in a rotenone-induced chronic Parkinson model. J. Neurol. Sci 290, 123–130. 10.1016/j.jns.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Huang R, Ma H, Guo Y, Liu S, Kuang Y, Shao K, Li J, Liu Y, Han L, Huang S, An S, Ye L, Lou J, Jiang C, 2013. Angiopep-conjugated nanoparticles for targeted long-term gene therapy of Parkinson’s disease. Pharm. Res 30, 2549–2559. 10.1007/s11095-013-1005-8. [DOI] [PubMed] [Google Scholar]
- Huang R-Q, Qu Y-H, Ke W-L, Zhu J-H, Pei Y-Y, Jiang C, 2007. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. FASEB J. 21, 1117–1125. 10.1096/fj.06-7380com. [DOI] [PubMed] [Google Scholar]
- Hurtado A, Cregg JM, Wang HB, Wendell DF, Oudega M, Gilbert RJ, McDonald JW, 2011. Robust CNS regeneration after complete spinal cord transection using aligned poly-l-lactic acid microfibers. Biomaterials 32, 6068–6079. 10.1016/j.biomaterials.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intratumoral/Intralesional Administration of MK-4621/JetPEITM With or Without Pembrolizumab in Participants With Advanced/Metastatic or Recurrent Solid Tumors (MK-4621–002) - ClinicalTrials.gov [WWW Document], 2018. ClinicalTrials.gov. (Accessed 27 April 2019). https://clinicaltrials.gov/ct2/show/NCT03739138.
- Jackson KL, Dayton RD, Deverman BE, Klein RL, 2016. Better targeting, better efficiency for wide-scale neuronal transduction with the synapsin promoter and AAVPHP.B. Front. Mol. Neurosci 9, 116. 10.3389/fnmol.2016.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayant RD, Sosa D, Kaushik A, Atluri V, Vashist A, Tomitaka A, Nair M, 2016. Current status of non-viral gene therapy for CNS disorders. Expert Opin. Drug Deliv 13, 1433–1445. 10.1080/17425247.2016.1188802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R, 2016. The repair Schwann cell and its function in regenerating nerves. J. Physiol 594, 3521–3531. 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Sun Y, Yang F, van den Beucken JJJP, Fan M, Chen Z, Jansen JA, 2011. Bioactive electrospun Scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res 28, 1259–1272. 10.1007/s11095-010-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen KB, Burkhart A, Melander F, Kempen PJ, Vejlebo JB, Siupka P, Nielsen MS, Andresen TL, Moos T, 2017. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci. Rep 7, 10396. 10.1038/s41598-017-11220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose A, Mietzsch M, Smith JK, Kurian J, Chipman P, McKenna R, Chiorini J, Agbandje-McKenna M, 2019. High-resolution structural characterization of a new adeno-associated virus serotype 5 antibody epitope toward engineering antibody-resistant recombinant gene delivery vectors. J. Virol 93, e01394–18. 10.1128/JVI.01394-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi CR, Raghavan V, Vijayaraghavalu S, Gao Y, Saraswathy M, Labhasetwar V, Ghorpade A, 2018. Reaching for the stars in the brain: polymer-mediated gene delivery to human astrocytes. Mol. Ther. - Nucleic Acids 12, 645–657. 10.1016/j.omtn.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T, Akiyama F, Kakizaki S, Takashima Y, Seta Y, 2013. Delivery of siRNA to the brain using a combination of nose-to-brain delivery and cell-penetrating peptide-modified nano-micelles. Biomaterials 34, 9220–9226. 10.1016/j.biomaterials.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Kang J, Joo J, Kwon EJ, Skalak M, Hussain S, She Z-G, Ruoslahti E, Bhatia SN, Sailor MJ, 2016. Self-sealing porous silicon-calcium silicate core–shell nanoparticles for targeted siRNA delivery to the injured brain. Adv. Mater 28, 7962–7969. 10.1002/adma.201600634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karra D, Dahm R, 2010. Transfection techniques for neuronal cells. J. Neurosci 30, 6171–6177. 10.1523/JNEUROSCI.0183-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MA, Glorioso JC, Naldini L, 2001. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat. Med 7, 33–40. 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- Ke W, Shao K, Huang R, Han L, Liu Y, Li J, Kuang Y, Ye L, Lou J, Jiang C, 2009. Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials 30, 6976–6985. 10.1016/j.biomaterials.2009.08.049. [DOI] [PubMed] [Google Scholar]
- Kim TK, Eberwine JH, 2010. Mammalian cell transfection: the present and the future. Anal. Bioanal. Chem 397, 3173–3178. 10.1007/s00216-010-3821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klouda L, Mikos AG, 2008. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. Interact. Polym. Pharmaceut. Biomed. Appl 68, 34–45. 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A, Carvajal J, Schneider H, Coutelle C, Chamberlain S, Fairweather N, 1999. Non-viral neuronal gene delivery mediated by the HC fragment of tetanus toxin. Eur. J. Biochem 259, 762–769. 10.1046/j.1432-1327.1999.00108.x. [DOI] [PubMed] [Google Scholar]
- Ko YT, Bhattacharya R, Bickel U, 2009. Liposome encapsulated polyethylenimine/ODN polyplexes for brain targeting. J. Control. Release 133, 230–237. 10.1016/j.jconrel.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S, 2013. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. PNAS 110, 10753–10758. 10.1073/pnas.1308345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körbelin J, Dogbevia G, Michelfelder S, Ridder DA, Hunger A, Wenzel J, Seismann H, Lampe M, Bannach J, Pasparakis M, Kleinschmidt JA, Schwaninger M, Trepel M, 2016. A brain microvasculature endothelial cell‐specific viral vector with the potential to treat neurovascular and neurological diseases. EMBO Mol. Med 8, 609–625. 10.15252/emmm.201506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Wu H, McBride JL, Jung K-E, Hee Kim M, Davidson BL, Kyung Lee S, Shankar P, Manjunath N, 2007. Transvascular delivery of small interfering RNA to the central nervous system. Nature 448, 39–43. 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- Kwon EJ, Skalak M, Lo Bu R, Bhatia SN, 2016. Neuron-targeted nanoparticle for siRNA delivery to traumatic brain injuries. ACS Nano 10, 7926–7933. 10.1021/acsnano.6b03858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JKW, Chow MYT, Zhang Y, Leung SWS, 2015. siRNA versus miRNA as therapeutics for gene silencing. Mol. Ther. - Nucleic Acids 4, e252. 10.1038/mtna.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Jin G, Jang J-H, 2014. Electrospun nanofibers as versatile interfaces for efficient gene delivery. J. Biol. Eng 8, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Guo Y, Kuang Y, An S, Ma H, Jiang C, 2013. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials 34, 9142–9148. 10.1016/j.biomaterials.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Li L, He Z-Y, Wei X-W, Wei Y-Q, 2016. Recent advances of biomaterials in bio-therapy. Regen. Biomater 3, 99–105. 10.1093/rb/rbw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ceylan M, Shrestha B, Wang H, Lu QR, Asmatulu R, Yao L, 2014. Nanofibers support oligodendrocyte precursor cell growth and function as a neuron-free model for myelination study. Biomacromolecules 15, 319–326. 10.1021/bm401558c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rodrigues J, Tomás H, 2012. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem. Soc. Rev 41, 2193–2221. 10.1039/C1CS15203C. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang J, Lee CGL, Wang CY, Gao SJ, Tang GP, Ma YX, Yu H, Mao H-Q, Leong KW, Wang S, 2004. CNS gene transfer mediated by a novel controlled release system based on DNA complexes of degradable polycation PPE-EA: a comparison with polyethylenimine/DNA complexes. Gene Ther 11, 109–114. 10.1038/sj.gt.3302135. [DOI] [PubMed] [Google Scholar]
- Li Z, Ning W, Wang J, Choi A, Lee P-Y, Tyagi P, Huang L, 2003. Controlled gene delivery system based on thermosensitive biodegradable hydrogel. Pharm. Res 20, 884–888. 10.1023/A:1023887203111. [DOI] [PubMed] [Google Scholar]
- Liao G-Y, Bouyer K, Kamitakahara A, Sahibzada N, Wang C-H, Rutlin M, Simerly RB, Xu B, 2015. Brain-derived neurotrophic factor is required for axonal growth of selective groups of neurons in the arcuate nucleus. Mol. Metab 4, 471–482. 10.1016/j.molmet.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao I-C, Chen S, Liu JB, Leong KW, 2009. Sustained viral gene delivery through core-shell fibers. J. Control. Release 139, 48–55. 10.1016/j.jconrel.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JK, Teng Q, Garrity-Moses M, Federici T, Tanase D, Imperiale MJ, Boulis NM, 2005. A novel peptide defined through phage display for therapeutic protein and vector neuronal targeting. Neurobiol. Dis 19, 407–418. 10.1016/j.nbd.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Liu S, Sandner B, Schackel T, Nicholson L, Chtarto A, Tenenbaum L, Puttagunta R, Müller R, Weidner N, Blesch A, 2017. Regulated viral BDNF delivery in combination with Schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury. Acta Biomater. 60, 167–180. 10.1016/j.actbio.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Liu Y, An S, Li J, Kuang Y, He X, Guo Y, Ma H, Zhang Y, Ji B, Jiang C, 2016. Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer’s disease mice. Biomaterials 80, 33–45. 10.1016/j.biomaterials.2015.11.060. [DOI] [PubMed] [Google Scholar]
- Liu Y, Guo Y, An S, Kuang Y, He X, Ma H, Li J, Lv J, Zhang N, Jiang C, 2013. Targeting caspase-3 as dual therapeutic benefits by RNAi facilitating brain-targeted nanoparticles in a rat model of parkinson’s disease. PLoS One 8, e62905. 10.1371/journal.pone.0062905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Huang R, Han L, Ke W, Shao K, Ye L, Lou J, Jiang C, 2009. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials 30, 4195–4202. 10.1016/j.biomaterials.2009.02.051. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li J, Shao K, Huang R, Ye L, Lou J, Jiang C, 2010. A leptin derived 30-amino-acid peptide modified pegylated poly-l-lysine dendrigraft for brain targeted gene delivery. Biomaterials 31, 5246–5257. 10.1016/j.biomaterials.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Lockman PR, Koziara JM, Mumper RJ, Allen DD, 2004. Nanoparticle surface charges alter blood–brain barrier integrity and permeability. J. Drug Target 12, 635–641. 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- Lu C, Stewart DJ, Lee JJ, Ji L, Ramesh R, Jayachandran G, Nunez MI, Wistuba II, Erasmus JJ, Hicks ME, Grimm EA, Reuben JM, Baladandayuthapani V, Templeton NS, McMannis JD, Roth JA, 2012. Phase I clinical trial of systemically administered TUSC2(FUS1)-nanoparticles mediating functional gene transfer in humans. PLoS One 7, e34833. 10.1371/journal.pone.0034833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack GS, 2007. MicroRNA gets down to business [WWW Document]. Nat. Biotechnol 10.1038/nbt0607-631. [DOI] [PubMed] [Google Scholar]
- Mali S, 2013. Delivery systems for gene therapy. Indian J. Hum. Genet 19, 3–8. 10.4103/0971-6866.112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Ferraiuolo L, Schmelzer L, Braun L, McGovern V, Likhite S, Michels O, Govoni A, Fitzgerald J, Morales P, Foust KD, Mendell JR, Burghes AHM, Kaspar BK, 2015. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose–response study in mice and nonhuman primates. Mol. Ther 23, 477–487. 10.1038/mt.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele Evelina, Spinelli GP, Miele Ermanno, Di Fabrizio E, Ferretti E, Tomao S, Gulino A, 2012. Nanoparticle-based delivery of small interfering RNA: challenges for cancer therapy. Int. J. Nanomed 7, 3637–3657. 10.2147/IJN.S23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, High KA, 2011. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet 12, 341–355. 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- Mislick KA, Baldeschwieler JD, 1996. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc. Natl. Acad. Sci 93, 12349–12354. 10.1073/pnas.93.22.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Poulain B, Weller U, Habermann E, Tauc L, 1989. Light chain of tetanus toxin intracellularly inhibits acetylcholine release at neuro-neuronal synapses, and its internalization is mediated by heavy chain. FEBS Lett. 253, 47–51. 10.1016/0014-5793(89)80926-3. [DOI] [PubMed] [Google Scholar]
- Mohr S, Bakal C, Perrimon N, 2010. Genomic screening with RNAi: results and challenges. Annu. Rev. Biochem 79, 37–64. 10.1146/annurevbiochem-060408-092949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Igoa M, Calvo AC, Penas C, Manzano R, Oliván S, Muñoz MJ, Mancuso R, Zaragoza P, Aguilera J, Navarro X, Osta Pinzolas R, 2010. Fragment C of tetanus toxin, more than a carrier. Novel perspectives in non-viral ALS gene therapy. J. Mol. Med 88, 297–308. 10.1007/s00109-009-0556-y. [DOI] [PubMed] [Google Scholar]
- Murphy MP, LeVine H, 2010. Alzheimer’s disease and the β-amyloid peptide. J. Alzheimers Dis 19, 311. 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S, Herzog RW, 2010. Progress and prospects: immune responses to viral vectors. Gene Ther. 17, 295–304. 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nectow AR, Moya MV, Ekstrand MI, Mousa A, McGuire KL, Sferrazza CE, Field BC, Rabinowitz GS, Sawicka K, Liang Y, Friedman JM, Heintz N, Schmidt EF, 2017. Rapid molecular profiling of defined cell types using viral TRAP. Cell Rep. 19, 655–667. 10.1016/j.celrep.2017.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Gao M, Lin J, Wu W, Wang J, Chew SY, 2017. Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci. Rep 7 10.1038/srep42212.srep42212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliván S, Calvo AC, Rando A, Herrando-Grabulosa M, Manzano R, Zaragoza P, Tizzano EF, Aquilera J, Osta R, 2016. Neuroprotective effect of non-viral gene therapy treatment based on tetanus toxin C-fragment in a severe mouse model of spinal muscular atrophy. Front. Mol. Neurosci 9. 10.3389/fnmol.2016.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S, Ferrari R, De Paola M, Rossi F, Mariani A, Caron I, Sammali E, Peviani M, Dell’Oro V, Colombo C, Morbidelli M, Forloni G, Perale G, Moscatelli D, Veglianese P, 2014. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J. Control. Release 174, 15–26. 10.1016/j.jconrel.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Pardieck J, Sakiyama-Elbert S, 2018. Genome engineering for CNS injury and disease. Curr. Opin. Biotechnol. Tissue, Cell Pathway Eng 52, 89–94. 10.1016/j.copbio.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente V, Corti S, 2018. Advances in spinal muscular atrophy therapeutics. Ther. Adv. Neurol. Disord 11, 1756285618754501. 10.1177/1756285618754501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I-K, Lasiene J, Chou S-H, Horner PJ, Pun SH, 2007. Neuron-specific delivery of nucleic acids mediated by Tet1-modified poly(ethylenimine). J. Gene Med 9, 691–702. 10.1002/jgm.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T-E, Singh B, Li H, Lee J-Y, Kang S-K, Choi Y-J, Cho C-S, 2015. Enhanced BBB permeability of osmotically active poly(mannitol-co-PEI) modified with rabies virus glycoprotein via selective stimulation of caveolar endocytosis for RNAi therapeutics in Alzheimer’s disease. Biomaterials 38, 61–71. 10.1016/j.biomaterials.2014.10.068. [DOI] [PubMed] [Google Scholar]
- Park TG, Jeong JH, Kim SW, 2006. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. Gene Deliv. Tissue Eng 58, 467–486. 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Passini MA, Bu J, Roskelley EM, Richards AM, Sardi SP, O’Riordan CR, Klinger KW, Shihabuddin LS, Cheng SH, 2010. CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J. Clin. Invest 120, 1253–1264. 10.1172/JCI41615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinese C, Lin J, Milbreta U, Li M, Wang Y, Leong KW, Chew SY, 2018. Sustained delivery of siRNA/mesoporous silica nanoparticle complexes from nanofiber scaffolds for long-term gene silencing. Acta Biomater. 76, 164–177. 10.1016/j.actbio.2018.05.054. [DOI] [PubMed] [Google Scholar]
- Qian X, Long L, Shi Z, Liu C, Qiu M, Sheng J, Pu P, Yuan X, Ren Y, Kang C, 2014. Star-branched amphiphilic PLA-b-PDMAEMA copolymers for co-delivery of miR-21 inhibitor and doxorubicin to treat glioma. Biomaterials 35, 2322–2335. 10.1016/j.biomaterials.2013.11.039. [DOI] [PubMed] [Google Scholar]
- Quraishe S, Forbes LH, Andrews MR, 2018. The extracellular environment of the CNS: influence on plasticity, sprouting, and axonal regeneration after spinal cord injury [WWW document]. Neural Plast. 10.1155/2018/2952386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii MS, Baumann TL, Bakay RAE, Ostrove JM, Siffert J, Fleisher AS, Herzog CD, Barba D, Pay M, Salmon DP, Chu Y, Kordower JH, Bishop K, Keator D, Potkin S, Bartus RT, 2014. A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer’s disease. Alzheimer’s Dementia 10, 571–581. 10.1016/j.jalz.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Ramalingam M, Tiwari A, Ramakrishna S, Kobayashi H, 2012. Integrated Biomaterials for Biomedical Technology. John Wiley & Sons. [Google Scholar]
- Ramamoorth M, Narvekar A, 2015. Non viral vectors in gene therapy- an overview. J. Clin. Diagn. Res 9, GE01–GE06. 10.7860/JCDR/2015/10443.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ, 1967. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol 34, 207–217. 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Chen X, Yang J, Kress BT, Tong J, Liu H, Takano T, Zhao Y, Nedergaard M, 2013. Improved axonal regeneration after spinal cord injury in mice with conditional deletion of ephrin B2 under the GFAP promoter. Neuroscience 241, 89–99. 10.1016/j.neuroscience.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A, 2004. Rational siRNA design for RNA interference. Nat. Biotechnol 22, 326–330. 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- Rivière C, Danos O, Douar AM, 2006. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 13, 1300–1308. 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- Rodrigues GA, Shalaev E, Karami TK, Cunningham J, Slater NKH, Rivers HM, 2018. Pharmaceutical development of AAV-based gene therapy products for the eye. Pharm. Res 36, 29. 10.1007/s11095-018-2554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M-L, Rush RA, 2012. Non-viral gene therapy for neurological diseases, with an emphasis on targeted gene delivery. J. Control. Release 157, 183–189. 10.1016/j.jconrel.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Rosario AM, Cruz PE, Ceballos-Diaz C, Strickland MR, Siemienski Z, Pardo M, Schob K-L, Li A, Aslanidi GV, Srivastava A, Golde TE, Chakrabarty P, 2016. Microglia-specific targeting by novel capsid-modified AAV6 vectors. Mol. Ther. Methods Clin. Dev 3, 16026. 10.1038/mtm.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M, Déglon N, 2012. Viral-mediated overexpression of mutant huntingtin to model HD in various species. Neurobiol. Dis 48, 202–211. 10.1016/j.nbd.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Saraiva C, Paiva J, Santos T, Ferreira L, Bernardino L, 2016. MicroRNA-124 loaded nanoparticles enhance brain repair in Parkinson’s disease. J. Control. Release 235, 291–305. 10.1016/j.jconrel.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Habgood MD, Møllgård K, Dziegielewska KM, 2016. The biological significance of brain barrier mechanisms: help or hindrance in drug delivery to the central nervous system? F1000Res 5. 10.12688/f1000research.7378.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer DV, Koerber JT, Lim K, 2008. Molecular engineering of viral gene delivery vehicles. Annu. Rev. Biomed. Eng 10, 169–194. 10.1146/annurev.bioeng.10.061807.160514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohann D, 2006. Thermo- and pH-responsive polymers in drug delivery⋆. Adv. Drug Deliv. Rev 58, 1655–1670. 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Seow Y, Wood MJ, 2009. Biological gene delivery vehicles: beyond viral vectors. Mol. Ther 17, 767–777. 10.1038/mt.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severino P, Szymanski M, Favaro M, Azzoni AR, Chaud MV, Santana MHA, Silva AM, Souto EB, 2015. Development and characterization of a cationic lipid nanocarrier as non-viral vector for gene therapy. Eur. J. Pharm. Sci 66, 78–82. 10.1016/j.ejps.2014.09.021. [DOI] [PubMed] [Google Scholar]
- Sharma G, Modgil A, Layek B, Arora K, Sun C, Law B, Singh J, 2013. Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: biodistribution and transfection. J. Control. Release 167, 1–10. 10.1016/j.jconrel.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Sheikh MA, Malik YS, Xing Z, Guo Z, Tian H, Zhu X, Chen X, 2017. Polylysinemodified polyethylenimine (PEI-PLL) mediated VEGF gene delivery protects dopaminergic neurons in cell culture and in rat models of Parkinson’s Disease (PD). Acta Biomater. 54, 58–68. 10.1016/j.actbio.2016.12.048. [DOI] [PubMed] [Google Scholar]
- Shepard JA, Stevans AC, Holland S, Wang CE, Shikanov A, Shea LD, 2012. Hydrogel design for supporting neurite outgrowth and promoting gene delivery to maximize neurite extension. Biotechnol. Bioeng 109, 830–839. 10.1002/bit.24355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyam R, Ren Y, Lee J, Braunstein KE, Mao H-Q, Wong PC, 2015. Intraventricular delivery of siRNA nanoparticles to the central nervous system. Mol. Ther. - Nucleic Acids 4, e242. 10.1038/mtna.2015.15. [DOI] [PubMed] [Google Scholar]
- Somani S, Blatchford DR, Millington O, Stevenson ML, Dufès C, 2014. Transferrin-bearing polypropylenimine dendrimer for targeted gene delivery to the brain. J. Control. Release 188, 78–86. 10.1016/j.jconrel.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Song HP, Yang JY, Lo SL, Wang Y, Fan WM, Tang XS, Xue JM, Wang S, 2010a. Gene transfer using self-assembled ternary complexes of cationic magnetic nanoparticles, plasmid DNA and cell-penetrating Tat peptide. Biomaterials 31, 769–778. 10.1016/j.biomaterials.2009.09.085. [DOI] [PubMed] [Google Scholar]
- Song W-J, Du J-Z, Sun T-M, Zhang P-Z, Wang J, 2010b. Gold nanoparticles capped with polyethyleneimine for enhanced siRNA delivery. Small 6, 239–246. 10.1002/smll.200901513. [DOI] [PubMed] [Google Scholar]
- Sun X, Pang Z, Ye H, Qiu B, Guo L, Li J, Ren J, Qian Y, Zhang Q, Chen J, Jiang X, 2012. Co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma mediated by an angiopep-conjugated liposome. Biomaterials 33, 916–924. 10.1016/j.biomaterials.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Swoboda KJ, Prior TW, Scott CB, McNaught TP, Wride MC, Reyna SP, Bromberg MB, 2005. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann. Neurol 57, 704–712. 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR, 2001. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther 3, 36–46. 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Ehrhardt A, Kay MA, 2003. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet 4, 346–358. 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- Thomas M, Klibanov AM, 2003. Non-viral gene therapy: polycation-mediated DNA delivery. Appl. Microbiol. Biotechnol 62, 27–34. 10.1007/s00253-003-1321-8. [DOI] [PubMed] [Google Scholar]
- Tran N, 2011. Blood-brain barrier. Encyclopedia of Clinical Neuropsychology. Springer, New York, NY. 10.1007/978-0-387-79948-3_299. pp. 426–426. [DOI] [Google Scholar]
- TUSC2-nanoparticles and Erlotinib in Stage IV Lung Cancer - ClinicalTrials.gov [WWW Document], 2011. ClinicalTrials.gov. (Accessed 27 April 2019). https://clinicaltrials.gov/ct2/show/NCT01455389.
- Ulbrich K, Hekmatara T, Herbert E, Kreuter J, 2009. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–-brain barrier (BBB). Eur. J. Pharm. Biopharm 71, 251–256. 10.1016/j.ejpb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Valori CF, Ning K, Wyles M, Mead RJ, Grierson AJ, Shaw PJ, Azzouz M, 2010. Systemic delivery of scAAV9 expressing SMN prolongs survival in a model of spinal muscular atrophy. Sci. Transl. Med 2 10.1126/scitranslmed.3000830.35ra42-35ra42. [DOI] [PubMed] [Google Scholar]
- van der Loo JCM, Wright JF, 2016. Progress and challenges in viral vector manufacturing. Hum. Mol. Genet 25, R42–R52. 10.1093/hmg/ddv451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waehler R, Russell SJ, Curiel DT, 2007. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet 8, 573–587. 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang S, Liao Z, Wang C, Liu Y, Feng S, Jiang X, Chang J, 2010. PEGlated magnetic polymeric liposome anchored with TAT for delivery of drugs across the blood-spinal cord barrier. Biomaterials 31, 6589–6596. 10.1016/j.biomaterials.2010.04.057. [DOI] [PubMed] [Google Scholar]
- Wang HB, Mullins ME, Cregg JM, Hurtado A, Oudega M, Trombley MT, Gilbert RJ, 2009. Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J. Neural Eng 6, 016001. 10.1088/1741-2560/6/1/016001. [DOI] [PubMed] [Google Scholar]
- Wang S, Meng Y, Li C, Qian M, Huang R, 2015. Receptor-mediated drug delivery systems targeting to Glioma. Nanomaterials (Basel) 6. 10.3390/nano6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Samulski RJ, McCown TJ, 2013. Adeno-associated virus (AAV) gene therapy for neurological disease. Neuropharmacol. New Targets Approach. Treat. Epilepsy 69, 82–88. 10.1016/j.neuropharm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth T, Parker N, Ylä-Herttuala S, 2013. History of gene therapy. Gene, 50 years of gene therapy - a contribution of Waclaw Szybalski to science and humanity. Gene 525, 162–169. 10.1016/j.gene.2013.03.137. [DOI] [PubMed] [Google Scholar]
- Writer MJ, Kyrtatos PG, Bienemann AS, Pugh JA, Lowe AS, Villegas-Llerena C, Kenny GD, White EA, Gill SS, McLeod CW, Lythgoe MF, Hart SL, 2012. Lipid peptide nanocomplexes for gene delivery and magnetic resonance imaging in the brain. J. Control. Release 162, 340–348. 10.1016/j.jconrel.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Zhang Y, Xu X, Guo T, Xie D, Zhu R, Chen S, Ramakrishna S, He L, 2018. RGD/TAT-functionalized chitosan-graft-PEI-PEG gene nanovector for sustained delivery of NT-3 for potential application in neural regeneration. Acta Biomater. 72, 266–277. 10.1016/j.actbio.2018.03.030. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang J, Yang Z, Chen F, Liu J, Wang Y, 2012. Wnt inhibitory factor-1 regulates glioblastoma cell cycle and proliferation. J. Clin. Neurosci 19, 1428–1432. 10.1016/j.jocn.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Oo NNL, Lee JP, Li Z, Loh XJ, 2017. Recent development of synthetic nonviral systems for sustained gene delivery. Drug Discov. Today, SI: Therapeutic Nanomaterials 22, 1318–1335. 10.1016/j.drudis.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li X, Cheng L, He S, Zou J, Chen F, Zhang Z, 2011. Core–sheath structured fibers with pDNA polyplex loadings for the optimal release profile and transfection efficiency as potential tissue engineering scaffolds. Acta Biomater. 7, 2533–2543. 10.1016/j.actbio.2011.02.031. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhao H, Jia Y, Guo Q, Qu Y, Su J, Lu X, Zhao Y, Qian Z, 2016. A novel gene delivery composite system based on biodegradable folate-poly (ester amine) polymer and thermosensitive hydrogel for sustained gene release. Sci. Rep 6, 21402. 10.1038/srep21402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Wang K, Wang Y, Wang S, Li J, Lou J, Ye L, Yan X, Lu W, Huang R, 2015. Enhanced blood–brain barrier penetration and glioma therapy mediated by a new peptide modified gene delivery system. Biomaterials 37, 345–352. 10.1016/j.biomaterials.2014.10.034. [DOI] [PubMed] [Google Scholar]
- Zeng J, Wang X, Wang S, 2007. Self-assembled ternary complexes of plasmid DNA, low molecular weight polyethylenimine and targeting peptide for nonviral gene delivery into neurons. Biomaterials 28, 1443–1451. 10.1016/j.biomaterials.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Zerboni L, Che X, Reichelt M, Qiao Y, Gu H, Arvin A, 2013. Herpes simplex virus 1 tropism for human sensory ganglion neurons in the severe combined immunodeficiency mouse model of neuropathogenesis. J. Virol 87, 2791–2802. 10.1128/JVI.01375-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Calon F, Zhu C, Boado RJ, Pardridge WM, 2003. Intravenous nonviral gene therapy causes normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental Parkinsonism. Hum. Gene Ther 14, 1–12. 10.1089/10430340360464660. [DOI] [PubMed] [Google Scholar]
- Zhang Yun, Zhang Yu-feng, Bryant J, Charles A, Boado RJ, Pardridge WM, 2004. Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin. Cancer Res 10, 3667–3677. 10.1158/1078-0432.CCR-03-0740. [DOI] [PubMed] [Google Scholar]
- Zhi D, Zhao Y, Cui S, Chen H, Zhang S, 2016. Conjugates of small targeting molecules to non-viral vectors for the mediation of siRNA. Acta Biomater. 36, 21–41. 10.1016/j.actbio.2016.03.048. [DOI] [PubMed] [Google Scholar]
- Zuchero JB, Fu M, Sloan SA, Ibrahim A, Olson A, Zaremba A, Dugas JC, Wienbar S, Caprariello AV, Kantor C, Leonoudakis D, Lariosa-Willingham K, Kronenberg G, Gertz K, Soderling SH, Miller RH, Barres BA, 2015. CNS myelin wrapping is driven by actin disassembly. Dev. Cell 34, 152–167. 10.1016/j.devcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


