Summary
Sporadic Creutzfeldt-Jakob disease (sCJD) is a fatal and potentially transmissible neurodegenerative disease caused by misfolded prion proteins (PrPSc). To date, effective therapeutics are not available and accurate diagnosis can be challenging. Clinical diagnostic criteria employ a combination of characteristic neuropsychiatric symptoms, cerebrospinal fluid (CSF) proteins 14–3-3, MRI, and EEG. Supportive biomarkers such as high CSF total Tau may aid the diagnostic process. Discordant results of studies however, have led to controversies about the clinical value of some established surrogate biomarkers. The recent development and clinical application of disease-specific protein aggregation and amplification assays such as Real-time Quaking Induced Conversion (RT-QuIC) have constituted major breakthroughs for the confident pre-mortem diagnosis of sCJD. Updated criteria for the diagnosis of sCJD including RT-QuIC will improve early clinical confirmation, surveillance, assessment of PrPSc seeding activity in different tissues, and trial monitoring. Moreover, emerging blood-based, prognostic, and potentially pre-symptomatic biomarker candidates are under current investigation.
Introduction
Sporadic Creutzfeldt-Jakob disease (sCJD) is a rapidly progressive neuropsychiatric syndrome with a fatal outcome, characterized by aggregations of misfolded prion protein Scrapie (PrPSc) in the brain. Sporadic CJD is the most common form of human prion disease (about 90% of cases) with an incidence around 1.5 to 2.0 per million person-years.1 Different phenotypes of sCJD may vary with respect to symptom evolution, biomarker profile, and neuropathological characteristics. They are associated with Methionine/Valine (M/V) polymorphism at Codon 129 of the prion gene (PRNP) and with molecular mass of PrPSc (glycotype 1 and 2).2 Definite diagnosis requires neuropathological confirmation.
The spectrum of possible symptoms is highly heterogeneous and comprises but is not restricted to rapidly progressive dementia, cerebellar ataxia, and myoclonus, making high-performing biomarkers important to a confident clinical diagnosis. In 1998, the World Health Organization (WHO) included a combination of certain symptoms, electroencephalography (EEG) and detection of cerebrospinal fluid (CSF) 14–3-3 proteins in the standard diagnostic criteria.3 Patterns of signal alteration on fluid attenuated inversion recovery (FLAIR) and/or diffusion weighted (DWI) sequences of brain magnetic resonance images (MRI) were suggested in 2009.4 Another CSF protein, total-Tau (t-Tau), is considered a valuable supportive biomarker.5 While comparative data on imaging markers for sCJD are limited, numerous studies have evaluated the diagnostic performance of CSF biomarkers, with occasional discrepancy leading to controversy about their clinical utility.6,7
The recent development and clinical application of PrPSc amplification assays such as Protein Misfolding Cyclic Amplification (PMCA) and Real-time Quaking Induced Conversion (RT-QuIC)8 have constituted major breakthroughs as aids for a more confident pre-mortem diagnosis of prion diseases. RT-QuIC has shown excellent diagnostic accuracy for sCJD in retrospective studies, ring trials (consistency between laboratories)9,10 and prospective studies11,12 also indicating its high value for an early and accurate diagnosis. Consequently, RT-QuIC (utilising CSF or other tissue such as olfactory mucosa) was included in diagnostic criteria for sCJD of some surveillance centers.12,13 However, a critical discussion of its clinical utility is needed. Another unmet need is the identification of blood-based biomarkers for early diagnosis and disease progression,14–16 especially in view of potential new therapeutic strategies.
The aim of this review is to provide an overview of the biomarker-based diagnosis of sCJD and to suggest guidelines for clinicians to utilize in the differential diagnosis of rapidly progressive dementias (RPD). Recent advances are critically discussed and put in the context of clinical relevance, established biomarkers, and epidemiology.
Search strategy and selection criteria
We searched Google Scholar and PubMed using the terms “prion” and “Creutzfeldt-Jakob disease”, each in combination with “diagnosis”, “criteria”, “biomarker”, “imaging”, “MRI”, “EEG”, and “RT-QuIC”. Articles published between January 1, 2015 and November 15, 2020 written in English or German were included based on the scientific merit and contribution to developments in biomarker research for sCJD. This means, the biomarkers have shown potential for a clinical utilization and results were independently validated. However, comprehensive lists of articles (after 2015, not mentioned in the main text) presenting altered biomarkers in sCJD are given in the appendix.). Older articles were selected based on the authors’ expertise to substantiate basic information and evidence of biomarkers in current focus.
Investigating the performance of diagnostic tests for sCJD
When estimates of diagnostic accuracy are being translated into clinical practice, potential selection biases of case and control groups should be considered. Especially the selection of control groups can be challenging. Healthy age matched controls usually do not reflect the population in which a diagnostic biomarker is utilized. On the other hand, referral centers often use CJD mimics that represent the diagnostic challenges but may not reflect the routine of a tertiary institution. An example of a biased control group was the evaluation of diagnostic criteria for sCJD reported in 2018.12 The control group included many non-CJD cases that had been further investigated because of positive CSF 14–3-3 tests, resulting in a weak specificity of this biomarker. In 2016, a study evaluating the utility of olfactory mucosa and CSF samples in RT-QuIC17 reported a rather low sensitivity compared to some other reports also employing second-generation RT-QuIC assays. Here, the case group was partially selected from samples that had prior negative first-generation RT-QuIC result, leading to a case group selection bias. Both examples underline the importance of interpreting all biomarker test results in an adequate clinical context.
Most biomarker studies report the sensitivity and the specificity of diagnostic tests. It is debatable whether these are the most useful measures of diagnostic performance as they are not easy to interpret in a clinical setting. Predictive values may be more accurate to determine the likelihood of a disease but they are associated with disease prevalence. To calculate predictive values, the rate of cases and controls in a study has to reflect the respective rate in the population, or Bayes’ rule has to be applied, which requires including disease prevalence (proportion) in the calculations.18 In the context of an extremely rare disease like sCJD, the first condition cannot be achieved and the latter would always lead to extremely low positive and extremely high negative predictive values. Thus, predictive values are not considered in this review. In case of established biomarkers with defined cut-offs, we indicate test sensitivity and specificity as measures for diagnostic accuracy. For experimental biomarkers, we indicate the area under the curve (AUC) from receiver operator characteristics.
Current state and recent advances in CJD biomarker research
Neuropathological investigation and immunostaining of PrPSc allow a definite diagnosis of prion diseases.19 For definite ante-mortem diagnosis, brain biopsy is required, but is complicated by infection control concerns, possibility of a false negative result due to sampling error in which typical pathology and PrPSc may not be present in all cortical regions (e.g. sporadic or familial fatal insomnia), and issues of tissue quality. Acknowledging these considerations and being highly invasive, brain biopsy is usually only considered when the diagnosis is not clear and potentially treatable conditions (e.g. encephalitis or lymphoma) are under strong consideration or a potential contamination of medical instruments requires a clear case definition. A less invasive procedure, tonsillar or adenoid biopsy, was established for the diagnosis of variant CJD (vCJD), but is not helpful for other forms of prion disease.20 The direct in vivo detection of PrPSc in sCJD employing routinely accessible bio-fluids appears possible but a pilot study using urine reported a poor sensitivity of 40%.21 Given the limitations of traditional methods such as biopsy and direct detection, and growing clinical evidence in support of novel PrPSc amplification/-seeded aggregation assays, a shift in clinical diagnosis criteria for sCJD is warranted. Below, we describe the evidence for these novel assays, as well as the current state of established and new diagnostic surrogate biomarkers (diagnostic tests that indirectly mark the disease process).
PrPSc-seeded aggregation assays
Protein Misfolding Cyclic Amplification (PMCA)
In 2001, PMCA was developed to reproduce and amplify PrPSc in micro-tubes. Brain homogenate provided normal prion protein (PrPC) “substrate” for the reaction and sonication fragmented growing PrPSc particles to increase their concentration.22 Subsequently, a modified protocol introduced the use of recombinant hamster PrPC as substrate to accelerate the reaction and increase its sensitivity to detect PrPSc in the CSF of scrapie-infected hamsters.23 Recent PMCA protocols showed excellent sensitivity for the detection of PrPSc in CSF (100%),24 plasma (100%)25,26 and urine (93%)27 of patients with vCJD but high sensitivity could not be demonstrated in sCJD or other prion diseases seen in current clinical practice.
Real-time Quaking Induced Conversion (RT-QuIC)
A modified multi-well plate-based PrPSc amplification technology using quaking to “energise” the misfolding of prion protein coupled to a fluorescent readout was named RT-QuIC.8,28 RT-QuIC has shown flexibility to be a potential tool not only in the diagnosis of prion diseases but also in drug screening, prion strain discrimination, and detection of other protein misfolding diseases.29,30 Different protocols concerning substrate (recombinant hamster, hamster-sheep chimeric, or bank vole PrP), reaction conditions, and the definition of test positivity have been reported.8,29,30 In general, each sample is analyzed in quadruplicates9 or triplates,29 and positivity is confirmed when at least two out of four or two out of three replicates, respectively, cross a fluorescence signal cut-off value. In 2015, the original protocol (first generation RT-QuIC) was technically modified by increasing reaction temperature and using N-terminally truncated PrPSen (second generation RT-QuIC) to shorten the assay time and to improve the sensitivity.31
CSF RT-QuIC represents a disease-specific biomarker and retrospective studies have investigated its diagnostic accuracy with test specificity of 99%−100%.8–11,17,31–37 Most studies, however, did not use control groups consisting primarily of cases with RPD in whom CJD was considered as a potential diagnosis during the disease course. Some false positive cases in retrospective studies were speculated to possibly represent unrecognized prion diseases.10 Nonetheless, two cases of autopsy-verified non-CJD showing positive CSF RT-QuIC during the diagnostic process have been reported.38,39 One of these patients had convulsions caused by steroid-responsive encephalitis, which is a potential clinical sCJD mimic.38 Prospective studies using RPDs as controls and mostly neuropathological confirmed sCJD cases, were published since 2017 and the specificity was also reported as 99%−100%,11,12,39–42 results are summarized in table 1. Due to its reliability and high diagnostic accuracy, CSF RT-QuIC was incorporated in the diagnostic criteria for sCJD of several surveillance centers.13,14
Table 1.
Diagnostic accuracy of CSF RT-QuIC in retrospective and prospective studies
| Cases | Controls | Sensitivity | Specificity | Protocol | |||
|---|---|---|---|---|---|---|---|
| n | type | n | type | ||||
| Atarashi et al. 20118* | 34 | definite sCJD | 49 | OND+ | 85% | 100% | 1st Gen |
| McGuire et al. 20129 | 123 | definite sCJD | 103 | RPD | 89% | 99% | 1st Gen |
| Orrú et al. 201432 | 30 | probable + definite sCJD | 46 | non-CJD | 77% | 100% | 1st Gen |
| Orrú et al. 201531 | 48 | probable + definite sCJD | 39 | OND+ | 96% | 100% | 2nd Gen |
| Cramm et al. 201610 | 110 | definite sCJD + gCJD | 400 | OND+ | 85% | 99% | 1st Gen° |
| Groveman et al. 201633† | 113 | probable + definite sCJD | 64 | OND+ | 73% | 100% | 1st Gen |
| Groveman et al. 201633† | 113 | probable + definite sCJD | 64 | OND+ | 94% | 100% | 2nd Gen |
| Park et al. 201634 | 81 | probable + definite sCJD | 100 | non-CJD | 77% | 100% | 1st Gen |
| Franceschini et al. 201735 | 145 | probable + definite sCJD + gCJD | 42 | RPD | 97% | 100% | 2nd Gen |
| Bongianni et al. 201717† | 49 | probable + definite sCJD | 71 | OND+ | 73% | 100% | 1st Gen |
| Bongianni et al. 201717† | 22 | probable + definite sCJD | 71 | OND+ | 86% | 100% | 2nd Gen |
| Lattanzio et al. 201736 | 225 | definite sCJD | 348 | RPD | 84% | 99% | 1st Gen |
| Foutz et al. 201711 | 126 | definite sCJD + gCJD | 67 | RPD | 92% | 99% | 2nd Gen |
| Rudge et al. 201837 | 171 | definite sCJD | 47 | RPD | 89% | 100% | 1st Gen |
| Foutz et al. 201711 | 65 | definite sCJD + gCJD | 14 | RPD | 95% | 100% | 2nd Gen |
| Hermann et al. 201812 | 65 | definite sCJD | 118 | RPD | 89% | 100% | 1st Gen° |
| Abu-Rumeileh et al. 2019†40 | 65 | definite sCJD + gCJD | 62 | RPD | 82% | 100% | 1st Gen |
| Abu-Rumeileh et al. 2019†40 | 65 | definite sCJD + gCJD | 62 | RPD | 96% | 100% | 2nd Gen |
| Fiorini et al. 202042 | 102 | probable + definite sCJD | 80 | RPD | 96% | 100% | 2nd Gen |
| Mammana et al. 202043 | 24 | probable + definite sCJD | 12 | RPD | 88% | 100% | 1nd Gen |
| Rhoads et al. 202039 | 439 | definite sCJD | 69 | RPD | 93% | 99% | 2nd Gen |
1st paragraph (Atarashio et al. to Rudge et al.): retrospective studies; 2nd paragraph (Foutz et al. to Rhoads et al.): prospective studies. Abbreviations: definite sCJD: neuropathological confirmed diagnosis of sporadic Creutzfeldt-Jakob disease; probable sCJD: clinical diagnose of sporadic Creutzfeldt-Jakob disease based on syndrome and biomarkers;4 gCJD: genetic Creutzfeldt-Jakob disease; OND+: other neurological diseases including dementia syndromes; RPD: rapidly progressive dementia, clinically suspicious for CJD; non-CJD: including non-neurologic disorders, neurologic disorders and dementia syndromes; 1st Gen: first generation tests;8 2nd Gen: second generation test31
This study investigated two different cohorts. Overall sensitivity and specificity were summarized for this table.
These studies performed two different protocols and used the same control group for both investigations.
This protocol used hamster-sheep chimeric recombinant PrP as substrate (instead of hamster PrP) and test positivity was indicated by two out of three positive replicates (instead of two out of four)29
Regarding the test sensitivity, figures range from 73%19,33 to 89%9,12,37 using first generation RT-QuIC, and 92%11 to 97%35 using second generation RT-QuIC. Molecular subtypes of sCJD are defined by Codon 129 polymorphism (M and V) and PrPSc glycotype (1 and 2),2 resulting in different subtypes (MM1, MV1, etc.). The sensitivity is very high in MM1/MV1 and VV2 cases, the most common subtypes among sCJD patients, whereas it is slightly lower in MV2 cases (75% to 93%).11,35,36,39,40 Regarding rare subtypes, small reported case numbers hamper the validity of the known results, but sensitivity has been reported to be substantially lower in VV1 and MM2 cases, ranging from 0%39 to 100%37,38 (VV1) and 44%36 to 78%39 (MM2C), respectively. The MM2 subtype is further differentiated into a cortical type (MM2C) and a very rare thalamic type (MM2T) that shows a distinct clinical syndrome called sporadic fatal insomnia (sFI). Only few known cases of sFI implicate that classical sCJD biomarkers and RT-QuIC show poor sensitivity in this condition.39 CSF RT-QuIC showed high sensitivity for genetic prion diseases with E200K and V210I mutations but being low for fatal familial insomnia (FFI, D178N-129M).10,11,39,40 Once again however, supporting data are based on small case numbers. RT-QuIC might also aid in the differentiation of distinct prion diseases such as sCJD, Gerstmann-Sträussler-Scheinker syndrome (GSS), and FFI as well as sCJD subtypes.30,41
Regarding other tissues, recent promising studies that applied RT-QuIC to olfactory mucosa17,32,42 and skin biopsies43,44 showed high sensitivities of 89% to 100% suggesting even better diagnostic accuracy than using CSF. Multiple components of the eye have tested positive by RT-QuIC45 post-mortem but the diagnostic value of analysis of routinely accessible eye tissue or fluid remains to be determined.
CSF surrogate biomarkers
14-3-3 proteins
The 14-3-3 proteins are abundantly but not solely expressed in the brain. They are located in the cytoplasm, plasma membranes, and organelles. Involvement in various functions such as cell signaling, growth, apoptosis etc. has been identified but not completely clarified.46 Since 14-3-3 protein detection by Western blot (WB) became part of commonly used clinical diagnostic criteria for sCJD,3 numerous studies evaluated its diagnostic performance. In 2012, a structured meta-analysis reported a sensitivity of 92% and a specificity of 80%47 but it was reported that the test sensitivity is lower in early disease stages and differs across the spectrum of molecular subtypes. The MV2 and MM2 subtypes displayed lower test sensitivities of around 60% to 70%.48 Reported specificity ranges between 40%49 and 92%.50 Such discrepancies might be explained, at least partially, by different characteristics of the control groups. In recent years, several studies reported a high specificity in the discrimination of sCJD and neurodegenerative diseases such Alzheimer’s disease (AD), dementia with Lewy bodies, and fronto-temporal lobar degeneration (supplementary Table 1).36,50–53 In contrast, the specificity of CSF 14-3-3 was lower when control groups included acute neuronal injury events as well as inflammatory and infiltrative neoplastic CNS diseases.36,50 Another factor possibly influencing specificity may be the execution and rating of 14-3-3 WB. Intermediate results (“weak” or “trace”) can be difficult to interpret. Comparative evaluations of a new 14-3-3γ isoform ELISA assay showed a superior diagnostic performance compared to 14-3-3 WB.40,54,55 One smaller study reported a sensitivity of 97% and a specificity of 94% with an AUC of 0·982 (optimal cut-off >14,552 AU/mL),54 whereas a larger study (including ring trials) reported a sensitivity of 88% and a specificity of 96% (cut off >20.000 AU/mL).55
Tau protein
Tau, a microtubule-associated protein, is expressed in neuronal and glial cells.56 Extremely elevated CSF t-Tau was proposed as a diagnostic biomarker for sCJD5 and most studies reported good test sensitivity and specificity, each around 90%.36,48,49,57 At present however, CSF t-Tau is not formally accepted as part of case definition criteria.4 Similar to 14-3-3, reduced sensitivity has been shown in MM2 and MV2 subtypes48,58 as well as early disease stages.59 Some studies reported rather poor specificities of 67%49 or lower than 50% at varying optimal diagnostic cut-offs.51–53 The latter was observed when cases with atypical AD were used as controls (supplementary Table 1). Some studies, however, have found t-Tau to be a better diagnostic marker than 14-3-3,49,60 leading to an ongoing controversy. Besides AD, inflammatory and neoplastic CNS diseases are important differential diagnoses of elevated t-Tau levels.61 Unfortunately, there is no general consensus regarding the best t-Tau ELISA assay or cut-off that should be used to support sCJD. Studies suggested either >1072 pg/mL62 >1250 pg/mL,35 >1300 pg/mL,5,63 or >1400 pg/mL.64 CSF t-Tau may also be a predictor of survival time.65,66 The p-Tau/t-Tau (or t-Tau/p-Tau) ratio is an important alternative biomarker for sCJD.67 It showed a very high diagnostic accuracy in the differentiation of sCJD from other neurological diseases (OND, AUC: 0·98), AD (AUC 0·99),64 and rapidly progressive AD (AUC 0·99).68 Several studies that investigated large cohorts reported a superior diagnostic performance compared to t-Tau alone.51,64,68
Neurofilaments
Neurofilaments comprise three subunits: a light (NfL), a medium, and a heavy chain. As neuron-specific cytoskeleton proteins, their presence in body fluids represents neuroaxonal damage.69 Several studies showed an excellent diagnostic accuracy in the discrimination of healthy controls and sCJD (AUCs >0·99).16,70,71 NfL however may lack sufficient specificity for sCJD.40 Concerning important differential diagnoses, reported AUCs were 0·95 versus demented and non-demented OND,15 0·77 versus AD,16 0·4516 and 0·9070 versus OND with dementia syndrome, 0·93 versus neurodegenerative dementias,53 and 0·86 to 0·89 versus RPD.72 The notable differences between these studies might be explained by different group selection criteria but this requires further clarification. In addition, different optimal cut-offs were identified, e.g. >5016 pg/ml53 or >10500 pg/ml.70 In contrast to 14-3-3 and t-Tau, NfL was shown to be markedly elevated in MV2 and VV2 compared to the MM1 sCJD subtype.53
Other CSF surrogate biomarkers
Several other CSF biomarkers for sCJD have been identified over the past two decades. Herein, only those that have a high level of supported evidence are considered. CSF S100b has been evaluated abundantly but comparative studies showed inferior diagnostic performance compared to 14-3-3 and t-Tau,48,73 and S100b has not been widely used clinically. Total prion protein (t-PrP) is decreased in the CSF of patients with sCJD, showing moderate diagnostic accuracy.51,74 A study using targeted mass spectrometry instead of the more routinely used ELISA showed that all human PrP domains were reduced in the CSF of sCJD compared to other RPD cases.75 In addition, it might have potential in trial monitoring76 and constitute a valuable part of composite biomarker profiles.51,53 Alpha-Synuclein, a synaptic protein that aggregates in synucleinopathies was observed to be massively increased in sCJD, possibly related to rapid neurodegeneration. A multi-center study showed an excellent diagnostic accuracy (AUC >0·99, 98% sensitivity, 97% specificity) in the discrimination of sCJD and OND (including dementia syndromes) at an optimal cut-off of 820 pg/mL using a commercial ELISA.77,78 Similar results were found in an inter-laboratory validation study.79 Advantages and disadvantages of common CSF biomarkers are summarized in supplementary Panel 1, supplementary Table 2 lists more potential CSF biomarker candidates evaluated in recent years.
Blood-based biomarker candidates
Several potential roles might feasibly be fulfilled by blood-based biomarkers. At present, there is no immediate prospect of a highly specific diagnostic blood test comparable to RT-QuIC in CSF. Blood assays, however, might offer an accessible triage test in primary care or first specialist assessment that flags the possibility of rapid neuronal damage and could be useful in case prioritization.
One of potential candidate is the t-Tau concentration in plasma or serum. Studies demonstrated elevated levels in sCJD compared to healthy controls and OND.15,16,80 The diagnostic accuracy ranged from an AUC of 0·94 versus healthy controls to 0·72 versus ONDs that included dementia syndromes (supplementary Table 3). Another investigation showed that the plasma t-Tau level is a better predictor of survival time in sCJD compared to CSF t-Tau levels or other fluid biomarkers.66 Another promising candidate for a blood-based biomarker is NfL, the most soluble subunit of Neurofilament. NfL was shown to be an effective therapeutic biomarker in CNS disease during trials for multiple sclerosis.81 NfL showed similar or even better diagnostic accuracy compared to t-Tau in the discrimination of sCJD from healthy controls.16,80 In contrast, a recent study that investigated a large cohort of prion diseases and used RPDs as controls showed that plasma t-Tau had better diagnostic accuracy than NfL (supplementary Table 3). Similar to the CSF counterpart, both plasma t-Tau and NfL levels were significantly associated with the sCJD subtype.82
More potential blood-based biomarkers for sCJD, such as S100b and others (supplementary Table 3) were elevated in serum or plasma, but few available data display inferior diagnostic accuracy compared to t-Tau and NfL or still have to be validated by other groups. Interestingly, PrP was reported to be decreased in the CSF of sCJD cases51,52 although it was reported in another study to be increased in plasma.83 The explanation for this dissociation has not yet been clarified.
Imaging markers
Magnetic Resonance Imaging
MRI is an essential tool in the diagnosis of sCJD. It allows the identification of important differential diagnoses such as ischemia, encephalitis, and neoplasia. CJD-typical patterns of restricted diffusion on DWI and hyperintensities in FLAIR images were suggested to be included in the diagnostic criteria of the WHO in 2009.4 Another widely used protocol recommends the use of DWI and apparent diffusion coefficient (ADC-) maps only.84,85 The CJD-typical MRI displays restricted diffusion in at least two cortical regions (“ribboning”) and/ or restricted diffusion predominantly in the caudate nucleus, followed by putamen and thalamus (Figure 1). Involvement of the subcortical white matter cannot be observed in visual assessments (DWI, ADC, FLAIR)4,84 but was detected by quantitative diffusion tensor imaging.86 Cortical ribboning and involvement of the caudate nucleus (of one or both hemispheres, rarely perfectly symmetric) is typically seen in the most common MM1 subtype. Involvement of the thalamus (aside from the caudate nucleus and putamen) is more common in VV2 and MV2 subtypes.87 High signal only on FLAIR and DWI in the posterior thalamus brighter than in anterior putamen (“pulvinar sign”) is a strong indicator of vCJD.20
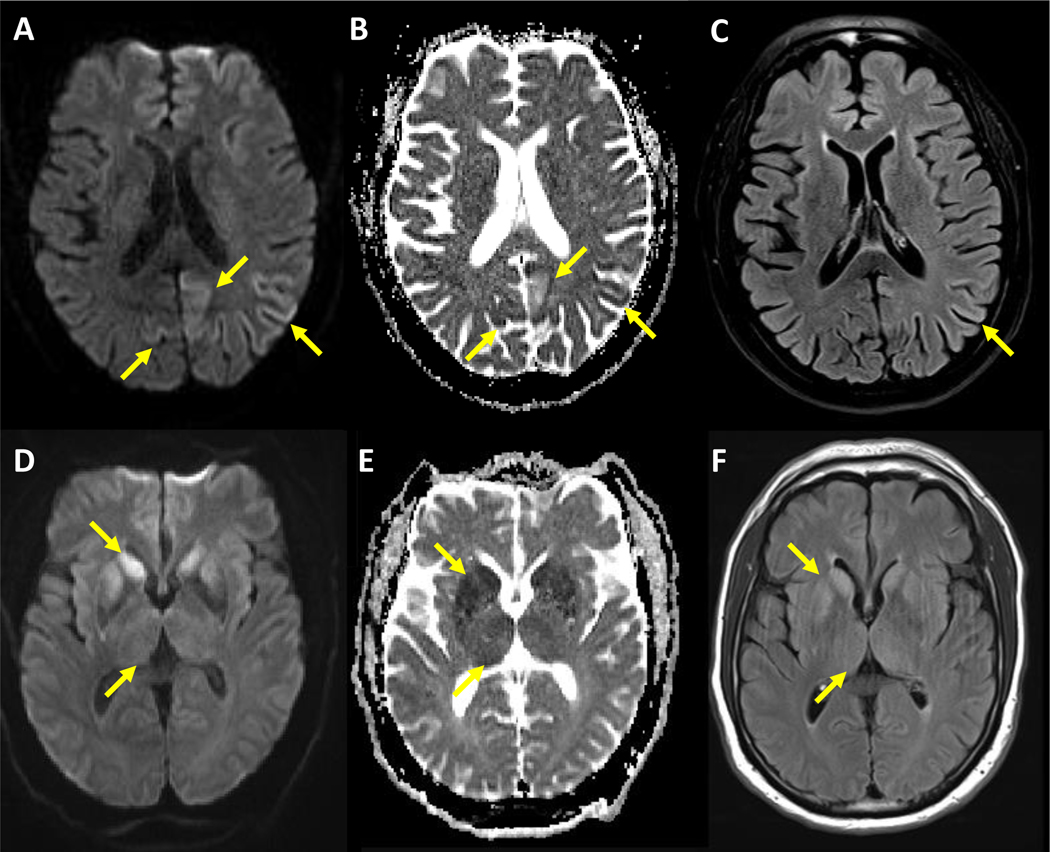
Figure 1. CJD-typical patterns of restricted diffusion on MRI.
A–C: Brain MRI of a patient with sCJD (MM1 subtype); restricted diffusion in occipital and parietal lobes, (left > right hemisphere, yellow arrows); associated hyperintensities on diffusion weighted images (DWI, A) and less impressive on fluid attenuated inversion recovery images (FLAIR, C); hypointensities on apparent diffusion coefficient maps (ADC, B); other MM1 cases may present additional restricted diffusion in caudate nucleus and putamen; a similar pattern (with caudate nucleus and putamen less likely involved) can be seen in MM2 and VV1 subtypes.
D–F: Brain MRI of a patient with sCJD (VV2 subtype); restricted diffusion in caudate nucleus, putamen (yellow arrows), and thalamus (less impressive, predominantly in the pulvinar, yellow arrows) in both hemispheres; associated hyperintensities on DWI (D) and FLAIR images (F); hypointensities on ADC maps (E); a similar pattern (with additional cortical involvement) can be seen in the MV2 subtype.
MR images were provided by the National Prion Disease Pathology Surveillance Center, Case Western Reserve University, Cleveland, OH, USA and processed by Peter Herman, University Medical Center Göttingen, Germany.
The overall diagnostic accuracy of MRI is possibly even superior to CSF 14-3-3 and t-Tau88,89 but extensive comparison data with CSF biomarkers is limited. Some studies showed a sensitivity of around 80%,12,35,40,42 others reported 92% to 98%.37,88,89 Similarly, specificity ranges from 74%40 to 98%.12 In 2020, a study investigating a large cohort with 770 definite sCJD cases applied an improved diagnostic index showing 92% sensitivity and 97% specificity.89 The discrepancies may be caused by different scanners, imaging and rating protocols, a study focus on other biomarkers, or the individual experience of image interpreters.90 The future possibilities of brain MRI include its application as a prognostic marker91 and as a potential marker in trial monitoring.92,93 Interestingly, some data suggest that restricted diffusion can occur in very early disease stages. Although prospective studies are not feasible in sCJD, such changes were observed more than one year before symptom onset94 in case reports.
Positron Emission Tomography
Positron emission tomography using [18F] fluoro-2-deoxy-D-glucose as tracer (FDG-PET) is able to detected decreased glucose metabolism in cortical regions of sCJD patients. The value of FDG-PET in the differential diagnosis is limited, though. No specific patterns have been identified. FDG-PET has potential as a marker of early sCJD and showed a correlation with clinical symptoms.95 In the rare MM2T subtype (sporadic fatal insomnia), an early-reduced thalamic glucose metabolism is a distinctive feature. 96
Electroencephalography
Periodic sharp-wave complexes (PSWCs) with a frequency of 1 Hz are considered as CJD-typical EEG pattern and have shown a sensitivity of 64% and a specificity of 91%.97 The non-convulsive status epilepticus is the most frequent clinical condition with CJD-like EEG.98,99 Recent CSF biomarker comparison studies reported a substantially lower sensitivity (39% to 45%) for EEG.12,34,37,40 Most likely, the decreasing sensitivity of EEG is a result of improved early recognition of sCJD cases. Typical PSWCs occur in late disease stages and are less frequent in MV2, VV2, and MM2 cases. However, the method is less invasive than CSF sampling and non-specific periodic rhythm abnormalities100 as well as quantitative analysis of frequency alterations101 may have the potential to aid the diagnosis in early stages and to predict disease progression.
Genetic markers
PRNP mutations account for about 10–15% of all human prion diseases.1 Some cause specific clinical syndromes such as GSS or FFI, others may mimic clinical presentation and biomarker profiles of sCJD (e.g. E200K).102 Thus, the sequencing of PRNP is an important biomarker that should be considered in the differential diagnosis of prion diseases and is vital in atypical cases, as well as in cases with positive or uninformed family history of RPD. In some sCJD subtypes, reduced sensitivity of surrogate biomarkers has been observed, especially in MV2 and MM2 cases.48,58 The identification of the PrPSc type is only possible in brain tissue but the analysis of codon 129 PRNP might help to interpret inconclusive biomarker results.103
Clinical value of RT-QuIC and CSF surrogate biomarkers
Over the last nine years, the evidence indicating CSF RT-QuIC as a major improvement in the clinical diagnosis of sCJD has reached a significant level. The test sensitivity is similar to the best available surrogate biomarkers and the data display superior specificity (Table 1). In contrast to all established biomarkers for sCJD as well as other neurodegenerative diseases, RT-QuIC is able to detect the agent that was consensually identified to be primarily pathogenic (PrPSc). Although different protocols and definitions of test positivity have been proposed,9,29,30 reproducibility of test results has been demonstrated in ring trials.10,104 On the other hand, RT-QuIC is rather costly regarding its substrate (recombinant PrP) and, although the test is less reliant on specialised equipment (e.g. compared to MRI and PET), the method still has to be established in more centers to provide all-encompassing availability. There is an ongoing debate on infectivity of the aggregates produced by PrPSc amplification assays. Although infectivity was shown in PMCA-replicated PrPSc from patients with vCJD,105 mouse models could not demonstrate infectivity of the RT-QuIC product from sCJD samples so far.106
Surrogate CSF biomarkers of sCJD are reliable diagnostics but the accuracy may differ with respect to the clinical context in which these markers are utilised. They are not disease specific by their very nature. Thus, physicians should interpret test results with caution. CSF 14-3-3 protein is highly sensitive and well validated, but acute brain injury events may cause false positive results. CSF 14-3-3 protein is part of a widely used clinical diagnostic gold standard3,4 and estimates of the diagnostic accuracy, especially in comparative analyses, may be influenced by verification bias.107 A problem in the utilisation of the 14-3-3 WB method is its complex interpretation and the presence of borderline results (traces). New 14-3-3 ELISAs may resolve this problem but they have not been widely established. The most commonly used alternative CSF biomarker t-Tau showed better (but still only moderate) specificity in the differentiation of sCJD and acute brain injury events or encephalitis,36,50 but there is some evidence that t-Tau may lack sufficient specificity in the discrimination of rapidly progressive or atypical AD and sCJD (supplementary Table 1). In a large cohort representing the full clinical spectrum of a non-specialised neurochemical laboratory, sCJD accounted for only 18% of patients with highly elevated (> 1200 pg/mL) CSF t-Tau levels,61 and thus, as with other biomarkers, it should not be used as a general screening tool but in the proper clinical context when suspecting prion disease. Evidence-based consensus cut-offs for CSF t-Tau, at best considering different assays, differential diagnoses, and supportive information on sCJD cases (e.g. Codon 129 PRNP polymorphism), would be most helpful and should be evaluated through a structured analysis. In conclusion, both markers (t-Tau and 14-3-3) share several characteristics, advantages, and disadvantages (supplementary Panel 1). The clinical utility has to be assessed in the light of suspected differential diagnoses and can be improved by stratification of demographic and genetic factors.103
An upcoming issue in the biomarker-based diagnosis of sCJD is the use of composites. Concerning this, the best evidence is available for the p-Tau/t-Tau ratio, which was demonstrated to be of superior diagnostic accuracy compared to t-Tau alone, especially in the differentiation of sCJD from AD.51,64,68 Proposed ratios combining t-Tau, p-Tau, 14-3-3, S100b, t-PrP, or beta amyloid showed high diagnostic accuracy48,52,73,108 but have not been established in the clinical setting.
Guidelines for the biomarker-based diagnosis of sCJD
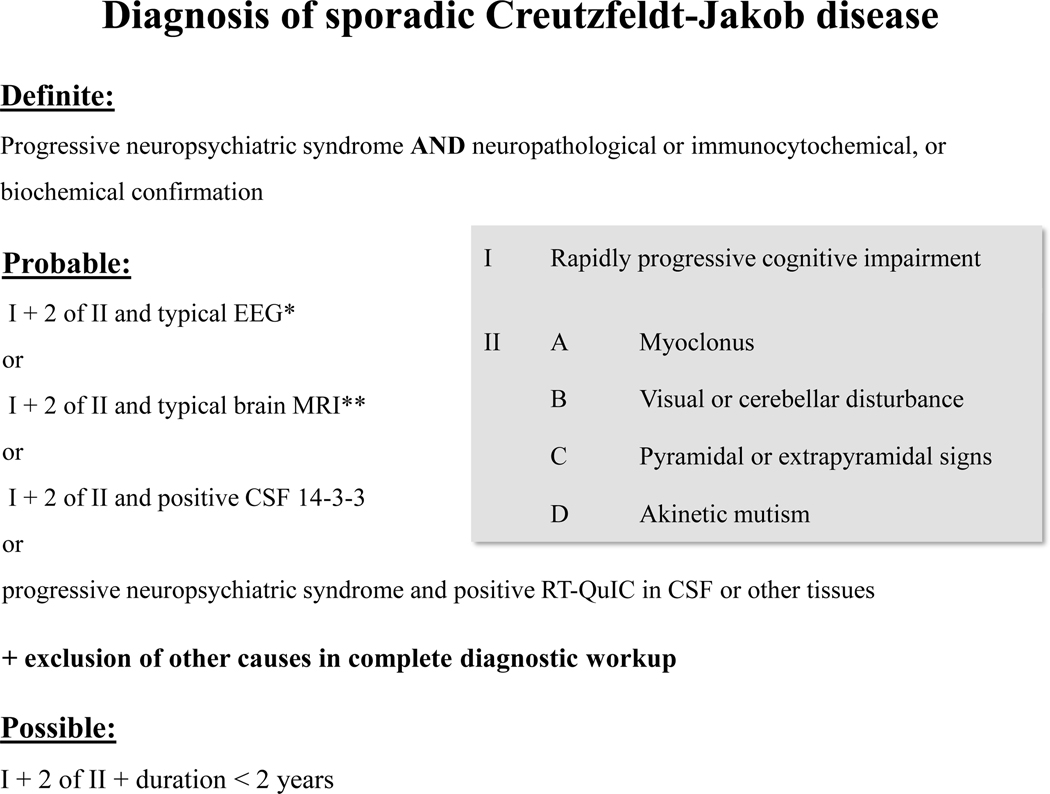
Based on the WHO criteria,3,4 the studies presented here, and previous suggestions that include RT-QuIC,12,13 the majority of the authors recommend amended criteria for the clinical diagnosis of sCJD as displayed in Figure 2. Due to the outstanding specificity of RT-QuIC, positive cases can be classified as probable sCJD in early clinical stages, even when only one cardinal symptom is present, which will improve the early identification of sCJD.12,39,108
Figure 2. Criteria for the clinical diagnosis of sporadic Creutzfeldt-Jakob disease.
The figure has been adapted from NCJDRSU criteria13 that were based on the WHO criteria3,4 and amended by RT-QuIC as an additional biomarker. Here, imaging criteria were refined and the need for a thorough diagnostic work-up in suspected probable sCJD is emphasized.
* Generalised periodic sharp/ wave complexes (PSWCs)
** Restricted diffusion in caudate or caudate/putamen or caudate/putamen/thalamus, or at least two cortical regions (temporal, parietal, occipital) on MRI brain scan,4 no subcortical white matter involvement, no isolated restricted diffusion in the thalamus. Characteristic hyperintensities may be seen on fluid attenuated inversion recovery (FLAIR) images, but diffusion weighted (DWI) sequences are required to confirm CJD-typical restricted diffusion.84,85
As with any test, as the test becomes more widely applied, even a false positive rate below 1% will lead to some number of incorrect diagnoses. This likelihood becomes particularly concerning if treatable conditions are missed. Ability to rely solely on RT-QuIC is further compromised by the test’s inability to distinguish accurately between different forms of human prion disease, and test sensitivities that vary from 73% to 97%. In addition, RT-QuIC has limited availability in countries without major surveillance programs. In this regard, we recommend that clinicians contact national CJD surveillance units or referral centers to get information on the availability of RT-QuIC, as well as general clinical guidance for the diagnosis and management of suspected prion disease cases. See supplementary Panel 2 for more information on potential support for clinicians, patients, and their families.
Readily available, economical, and field-tested CSF biomarkers such as 14-3-3, and in some centers t-Tau, as well as EEG and MRI (preferably DWI/ADC sequences), are still of major importance and should be used as routine diagnostic tests in cases of suspected sCJD. These tools have been shown to be effective and accurate in the differential diagnosis of sCJD, when they are applied and interpreted in a reasonable context. In case of ambiguous results or uncertain differential diagnoses, the p-Tau/t-Tau (or t-Tau/p-Tau) ratio might be considered as supportive biomarker.63,64,68 Genetic analysis of PRNP should be considered in all cases of suspected CJD to determine the codon 129 polymorphism and to exclude pathogenic mutations, which might be present even in patients with a negative family history.102 Most important, routine blood, CSF, and imaging diagnostics should always be performed to rule out the most common differential diagnoses. The supplementary Table 4 gives an overview on some clinical CJD mimics; Panel 1 includes two sCJD case studies describing disease course and biomarker-based diagnoses. See Panel 2 for a guideline summary.
Panel 1.
Historical case studies from the German CJD surveillance center
| Case A (typical CJD) |
| A 63-year-old woman complained about language disturbance (mild amnestic aphasia) that had started two weeks before hospital admission. |
| Neurological and neuropsychiatric examination showed cognitive deficits and ataxia. The EEG showed continuous focal epileptiform patterns but enforced antiepileptic medication showed no clinical benefit. In the CSF, 14-3-3 proteins (64455 AU/mL, cut-off > 20000 AU/mL) and t-Tau (12460 pg/mL, cut-off >1300 pg/mL) were both highly increased and RT-QuIC was positive. No signs of CNS inflammation were present. MRI showed restricted diffusion in frontal, temporal, and parietal regions, as well as in caudate nucleus and left putamen. The clinical condition of the patient worsened within one week. Clinical examination showed severe dementia, pyramidal and extrapyramidal signs, as well as myoclonus. Follow-up EEGs showed CJD-typical PSWCs. PRNP sequencing revealed no pathogenic mutation and homozygosity for Methionine at Codon 129. The patient was diagnosed with probable sCJD according to common criteria,4 supported by positivity of CSF RT-QuIC. |
| The patient passed after 2 months of disease duration. Brain autopsy revealed PrPSc depositions with neuropathological characteristics of the most frequent MM/MV1 sCJD subtype. |
| Case B (atypical sCJD) |
| Family members recognized personality changes and mild cognitive deficits in a 54-year-old woman, and suspected an affective disorder. |
| After 5 months of symptom duration, a neurology specialist observed rapidly progressive dementia with apraxia. MRI showed restricted diffusion in parietal, occipital, and temporal regions with very subtle involvement of caudate nucleus (no other pathological findings); EEG showed sporadic triphasic complexes but no PSWCs. The CJD surveillance center was consulted and recommended further clinical investigations including CSF analyses. The CSF showed no evidence for inflammatory CNS diseases, positive 14-3-3 proteins at a rather low level (21527 AU/mL, cut-off >20000 AU/mL), and positive RT-QuIC. PRNP sequencing revealed no pathogenic mutation and homozygosity for Methionine at Codon 129. Although clinical diagnostic criteria4 for sCJD were not fulfilled at that time (the patient showed only rapidly progressive dementia), the biomarker signature was highly suggestive and no alternative diagnoses were revealed. |
| The case was classified as probable sCJD according to amended surveillance center criteria12 based on RT-QuIC positivity. Disease course (rather slow progression), MRI results (predominant cortical involvement), and Codon 129 were suggestive for the rare MM2C (“cortical”) sCJD subtype. The patient passed after 11 months of disease duration. Brain autopsy revealed PrPSc depositions with neuropathological characteristics of the MM/MV2C sCJD subtype. |
Panel 2.
Guidelines for the clinical diagnosis of sCJD
| General |
| The clinical diagnosis of sCJD requires a thorough diagnostic work-up including clinical investigation, blood sampling, lumbar puncture, neuroimaging (MRI), and EEG at minimum. Further diagnostics (e.g. body CT, PET, specific CSF analyses) can be necessary depending on suspected differential diagnoses. |
| The diagnostic criteria and its measurements |
| We recommend amending the established WHO criteria for the clinical diagnosis of “probable” sCJD (Figure 2). If available, RT-QuIC should be performed in every case of suspected prion disease. The 14-3-3 test is the primary CSF surrogate biomarker;* CSF t-Tau and the p-Tau/t-tau (or t-Tau/p-Tau) ratio are valuable supportive biomarkers. All markers have to be performed in experienced and certified laboratories. MRI and EEG are highly specific but require experienced raters. MRI sequences should include T1 weighted images with contrast agent sequences (for differential diagnosis), FLAIR, and DWI with ADC maps. CJD-typical MRI findings are clearly visible on DWI rather than other sequences. All mentioned biomarkers are less sensitive in early disease stage and in some molecular subtypes; follow-up investigations may be useful in case of negative results. The analysis of codon 129 PRNP polymorphism might assist in interpreting the results of other biomarker analyses. Diagnosis of “possible” sCJD (absence of suggestive biomarkers, figure 2) should only be made if extensive diagnostics had not revealed alternative explanations for the clinical condition. |
| Important differential diagnoses |
| Genetic analyses as well as clinical indicators of iCJD and vCJD should be considered in all cases with suspected prion disease. Rapidly progressive neurodegenerative diseases, (immune-mediated) encephalitis, status epilepticus, and cerebral ischemia, are frequent differential diagnoses. Among others, these conditions may mimic the clinical syndrome and most surrogate biomarkers of sCJD. |
| Brain biopsy |
| Brain biopsy is an invasive procedure that can be considered when non-invasive diagnostics remain inconclusive and a potentially treatable alternative diagnosis is suspected. |
In some centers, CSF t-Tau is considered as primary CSF surrogate biomarker.
Future challenges and perspectives
Despite recent improvements of diagnostic measures for sCJD, there are still plenty of challenges. The value of established and new biomarkers in the differential diagnosis of sCJD subtypes and other human prion diseases (iatrogenic CJD, vCJD, and genetic CJD) has to be clarified. RT-QuIC has to be widely distributed, protocols have to be unified, past studies on peripheral tissue have to be validated with regard to important differential diagnoses, and more candidate tissues have to be evaluated. In this context, the potential infectivity of RT-QuIC positive tissues such as olfactory mucosa106 might be reappraised.
More potential diagnostic biomarkers are currently under investigation. The authors recommend that new biomarkers may be considered in future diagnostic criteria under certain conditions. Besides strong clinical evidence (validation of cut-offs in independent cohorts, appropriate controls, etc.), such a biomarker should be able to improve the clinical diagnosis of sCJD substantially. This might be the case when a biomarker shows superior diagnostic accuracy compared to established markers or equal accuracy with reduction of test invasiveness (e.g. blood-based). Although analysis of Codon 129 PRNP polymorphism, clinical observations and biomarker profiles (especially MRI DWI lesion patterns87) already allow conclusions about the sCJD subtype, new biomarkers should be able to go beyond phenotypical variability and disease stage, or at least be evaluated in this respect.
Over the last five years, some investigations have opened the field of prodromal, prognostic, and predictive biomarkers for sCJD. One of the challenges for clinical trials in sCJD is that clinical features are highly heterogeneous, and it has been difficult to find a suitable single continuous measure as an outcome. In this circumstance, specific CJD tests such as RT-QuIC might be used at trial enrollment and blood-based biomarkers might be used repeatedly during a trial to track neuro-axonal damage in the course of experimental treatment. Further work is required to establish variability of biomarkers in the natural history of CJD and if biomarkers of neurodegeneration can contribute to prognostic or trial models. Finally, blood-based biomarkers may have a role in preventive trials as a prodromal biomarker for individuals healthy but at-risk of CJD because of iatrogenic prion exposure or PRNP mutation. Present published work suggests a prodromal biomarker window is small or rare in at risk individuals with pathogenic PRNP mutations109,110 but this is an area of active research.
Supplementary Material
Acknowledgements
The research of IZ and PH was funded by the German Federal Ministry of healthy through grants from the Robert-Koch-Institute, grant no. 139-341. BC was funded by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. SJC is funded in part by a NHMRC Practitioner Fellowship (ID# APP1105784). FL was supported by grant from the Institute of Health Carlos III, grant PI19/00144. MG was supported by grants from the U.S. National Institutes of Health/NIA (R01-AG AG031189, R01-AG062562; R56-AG055619) and funding from Michael J. Homer Family Fund and Alliance Biosecure KS was supported by grants for scientific research from the Ministry of Health, Labour and Welfare of Japan (KSat: No. 14507303), Research Committee of Prion Disease and Slow Virus Infection,Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health and Labour Sciences Research Grants, the Research Committee of Surveillance and Infection Control of Prion Disease, the Ministry of Health, Labour, and Welfare of Japan, and the Japan Agency for Medical Research and Development (AMED) (grant number No. 18ek0109362h0001).
The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The corresponding author (PH) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
The authors want to thank Terri Lindsay from the European Creutzfeldt-Jakob Disease Surveillance Network (EuroCJD), Suzanne Solvyns from the CJD International Support Alliance (CJDISA), and Debbie Yobs from the CJD Foundation for helping with information on international CJD referral centers and family associations.
Declaration of interest
Dr. Hermann reports grants from Robert-Koch-Institute during the conduct of the study; Dr. Appleby has nothing to disclose; Dr. Brandel has nothing to disclose; Dr. Caughey has a patent 2554996 (France, Germany, UK) issued, a patent 2179293 (Switzerland, Germany, France, UK, Ireland) issued, and a patent 8,216,788 (USA) issued; Dr. Collins has nothing to disclose; Dr. Geschwind reports grants from U.S. National Institutes of Health/NIA (R01-AG AG031189, R01-AG062562; R56-AG055619), other from Michael J. Homer Family Fund, grants from Alliance Biosecure, during the conduct of the study; personal fees from Ascel Health, LifeSci Capital LLC, ClearView Healthcare Partners, Blade Therapeutics Inc., Bioscience Pharma Partners, LLC (BPP), Teledoc Health, Inc., Best Doctors, Inc., Advance Medical Inc., Grand Rounds, Inc., and Quest Diagnostics, Inc., outside the submitted work; Dr. Green has nothing to disclose; Dr. Haïk reports grants from MedDay Pharmaceuticals, grants from Institut de Recherche Servier, grants from LFB Biomedicaments, outside the submitted work; In addition, Dr. Haïk has a patent METHOD FOR TREATING PRION DISEASES (PCT/EP2019/070457) pending; Dr. Kovacs has nothing to disclose; Dr. Ladogana has nothing to disclose; Dr. Llorens has nothing to disclose; Dr. Nishida has nothing to disclose; Dr. Mead has nothing to disclose; Dr. Parchi has nothing to disclose; Dr. Pal has nothing to disclose; Dr. Pocchiari reports personal fees from Ferring Pharmaceuticals, personal fees from Collection of National Chemical Compounds and Screening Center (CNCCS), non-financial support from Fondazione Cellule Staminali, outside the submitted work; Dr. Satoh has nothing to disclose Dr. Zanusso has nothing to disclose; Dr. Zerr reports grants from Robert-Koch-Institute, during the conduct of the study.
References
- 1.Ladogana A, Puopolo M, Croes EA, et al. Mortality from Creutzfeldt-Jakob disease and related disorders in Europe, Australia, and Canada. Neurology 2005; 64: 1586–91. [DOI] [PubMed] [Google Scholar]
- 2.Parchi P, Giese A, Capellari S, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 1999; 46: 224–33. [PubMed] [Google Scholar]
- 3.WHO. Global Surveillance, diagnosis, and Therapy of Human Transmissible spongiform Encephalopathies: Report of WHO consultation, February 9–11, 1998, Geneva, Switzerland. [Google Scholar]
- 4.Zerr I, Kallenberg K, Summers DM et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009; 132: 2659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otto M, Wiltfang J, Cepek L, et al. Tau protein and 14-3-3 protein in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology 2002; 58: 192–7 [DOI] [PubMed] [Google Scholar]
- 6.Collins SJ, Sanchez-Juan P, Masters CL, et al. Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt-Jakob-disease. Brain 2006; 129: 2278–87. [DOI] [PubMed] [Google Scholar]
- 7.Geschwind MD, Martindale J, Miller D, et al. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol 2003; 60: 813–16. [DOI] [PubMed] [Google Scholar]
- 8.Atarashi R, Satoh K, Sano K, et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med 2011; 17: 175–78. [DOI] [PubMed] [Google Scholar]
- 9.McGuire LI, Peden AH, Orrú CD et al. RT-QuIC analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol 2012; 72: 278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cramm M, Schmitz M, Karch A, et al. Stability and reproducibility underscore utility of RT-QuIC for diagnosis of Creutzfeldt-Jakob disease. Mol Neurobiol 2016; 53: 1896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foutz A, Appleby BS, Hamlin C, et al. Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Ann Neurol 2017; 81: 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermann P, Laux M, Glatzel M, et al. Validation and utilization of amended diagnostic criteria in Creutzfeldt-Jakob disease surveillance. Neurology 2018; 91: e331–e338. [DOI] [PubMed] [Google Scholar]
- 13.National Creutzfeldt-Jakob Disease Research & Surveillance Unit (NCJDRSU). Available online: https://www.cjd.ed.ac.uk/sites/default/files/criteria_0.pdf (accessed on 27 April 2020)
- 14.Noguchi-Shinohara M, Hamaguchi T, Nozaki I, Sakai K, Yamada M. Serum tau protein as a marker for the diagnosis of Creutzfeldt-Jakob disease. J Neurol 2011; 258: 1464–8. [DOI] [PubMed] [Google Scholar]
- 15.Steinacker P, Blennow K, Halbgebauer S, et al. Neurofilaments in blood and CSF for diagnosis and prediction of onset in Creutzfeldt-Jakob disease. Sci Rep 2016; 6: 38737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovacs GG, Andreasson U, Liman V, et al. Plasma and cerebrospinal fluid tau and neurofilament concentrations in rapidly progressive neurological syndromes: a neuropathology-based cohort. Eur J Neurol 2017; 24: 1326–e77. [DOI] [PubMed] [Google Scholar]
- 17.Bongianni M, Orrú C, Groveman BR, et al. Diagnosis of Human Prion Disease Using Real-Time Quaking-Induced Conversion Testing of Olfactory Mucosa and Cerebrospinal Fluid Samples. JAMA Neurol 2017; 74: 155–62. [DOI] [PubMed] [Google Scholar]
- 18.Altman DG, Bland JM. Diagnostic tests 2: Predictive values. BMJ 1994; 309: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budka H, Aguzzi A, Brown P, et al. Neuropathological diagnostic criteria for Creutzfeldt-Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases). Brain Pathol 1995; 5: 459–66. [DOI] [PubMed] [Google Scholar]
- 20.Heath CA, Cooper SA, Murray K, et al. Validation of diagnostic criteria for variant Creutzfeldt-Jakob disease. Ann Neurol 2010; 67: 761–70. [DOI] [PubMed] [Google Scholar]
- 21.Luk C, Jones S, Thomas C, et al. Diagnosing Sporadic Creutzfeldt-Jakob Disease by the Detection of Abnormal Prion Protein in Patient Urine. JAMA Neurol 2016; 73: 1454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 2001. 411: 810–3. [DOI] [PubMed] [Google Scholar]
- 23.Atarashi R, Moore RA, Sim VL, et al. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods 2007; 4: 645–50. [DOI] [PubMed] [Google Scholar]
- 24.Barria MA, Lee A, Green AJ, Knight R, Head MW. Rapid amplification of prions from variant Creutzfeldt-Jakob disease cerebrospinal fluid. J Pathol Clin Res 2018; 4: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bougard D, Brandel JP, Bélondrade M, et al. Detection of prions in the plasma of presymptomatic and symptomatic patients with variant Creutzfeldt-Jakob disease. Sci Transl Med 2016; 8: 370ra182. [DOI] [PubMed] [Google Scholar]
- 26.Concha-Marambio L, Pritzkow S, et al. Detection of prions in blood from patients with variant Creutzfeldt-Jakob disease. Sci Transl Med 2016; 8: 370ra183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moda F, Gambetti P, Notari S, et al. Prions in the urine of patients with variant Creutzfeldt-Jakob disease. N Engl J Med 2014; 371: 530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilham JM, Orrú CD, Bessen RA, et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog 2010. 6: e1001217. doi: 10.1371/journal.ppat.1001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz M, Cramm M, Llorens F. et al. The real-time quaking-induced conversion assay for detection of human prion disease and study of other protein misfolding diseases. Nat Protoc 2016; 11: 2233–42. [DOI] [PubMed] [Google Scholar]
- 30.Orrú CD, Groveman BR, Raymond LD, et al. Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains. PLoS Pathog 2015. 11: e1004983. doi: 10.1371/journal.ppat.1004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio 2015; 6: e02451–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orrú CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med 2014; 371: 519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groveman BR, Orrú CD, Hughson AG, et al. Extended and direct evaluation of RT-QuIC assays for Creutzfeldt-Jakob disease diagnosis. Ann Clin Transl Neurol 2017; 4: 139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JH, Choi YG, Lee YJ, et al. Real-Time Quaking-Induced Conversion Analysis for the Diagnosis of Sporadic Creutzfeldt-Jakob Disease in Korea. J Clin Neurol 2016; 12: 101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franceschini A, Baiardi S, Hughson AG, et al. High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci Rep 2017; 7: 10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lattanzio F, Abu-Rumeileh S, Franceschini A, et al. Prion-specific and surrogate CSF biomarkers in Creutzfeldt-Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol 2017; 133: 559–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudge P, Hyare H, Green A, Collinge J, Mead S. Imaging and CSF analyses effectively distinguish CJD from its mimics. J Neurol Neurosurg Psychiatry 2018; 89: 461–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayashi Y, Iwasaki Y, Yoshikura N, et al. An autopsy-verified case of steroid-responsive encephalopathy with convulsion and a false-positive result from the real-time quaking-induced conversion assay. Prion 2017; 11: 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhoads D, Wrona A, Foutz A, et al. Diagnosis of Prion Diseases by RT-QuIC Results in Improved Surveillance. Neurology 2020; 95: e1017–e1026. [DOI] [PubMed] [Google Scholar]
- 40.Abu-Rumeileh S, Baiardi S, Polischi B, et al. Diagnostic value of surrogate CSF biomarkers for Creutzfeldt-Jakob disease in the era of RT-QuIC. J Neurol 2019; 266: 3136–43. [DOI] [PubMed] [Google Scholar]
- 41.Zanusso G, Monaco S, Pocchiari M, Caughey B. Advanced tests for early and accurate diagnosis of Creutzfeldt-Jakob disease. Nat Rev Neurol 2016; 12: 325–33. [DOI] [PubMed] [Google Scholar]
- 42.Fiorini M, Iselle G, Perra D, et al. High Diagnostic Accuracy of RT-QuIC Assay in a Prospective Study of Patients with Suspected sCJD. Int J Mol Sci 2020; 21: pii: E880. doi: 10.3390/ijms21030880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mammana A, Baiardi S, Rossi M, et al. Detection of prions in skin punch biopsies of Creutzfeldt-Jakob disease patients. Ann Clin Transl Neurol 2020; 7: 559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orrú CD, Yuan J, Appleby BS, et al. Prion seeding activity and infectivity in skin samples from patients with sporadic Creutzfeldt-Jakob disease. Sci Transl Med 2017; 9: eaam7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orrù CD, Soldau K, Cordano C, et al. Prion Seeds Distribute throughout the Eyes of Sporadic Creutzfeldt-Jakob Disease Patients. MBio 2018. 9: e02095–18. doi: 10.1128/mBio.02095-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berg D, Holzmann C, Riess O. 14-3-3 proteins in the nervous system. Nat Rev Neurosci 2003; 4: 752–62. [DOI] [PubMed] [Google Scholar]
- 47.Muayqil T, Gronseth G, Camicioli R. Evidence-based guideline: diagnostic accuracy of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease: report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2012, 79: 1499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez-Juan P, Green A, Ladogana A, et al. CSF tests in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology 2006; 67: 637–43. [DOI] [PubMed] [Google Scholar]
- 49.Hamlin C, Puoti G, Berri S, et al. A comparison of tau and 14-3-3 protein in the diagnosis of Creutzfeldt-Jakob disease. Neurology 2012; 79: 547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoeck K, Sanchez-Juan P, Gawinecka J, et al. Cerebrospinal fluid biomarker supported diagnosis of Creutzfeldt-Jakob disease and rapid dementias: a longitudinal multicentre study over 10 years. Brain 2012; 135: 3051–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dorey A, Tholance Y, Vighetto A, et al. Association of cerebrospinal fluid prion protein levels and the distinction between Alzheimer disease and Creutzfeldt-Jakob disease. JAMA Neurol 2015; 72: 267–75. [DOI] [PubMed] [Google Scholar]
- 52.Abu Rumeileh S, Lattanzio F, Stanzani Maserati M, Rizzi R, Capellari S, Parchi P. Diagnostic Accuracy of a Combined Analysis of Cerebrospinal Fluid t-PrP, t-tau, p-tau, and Aβ42 in the Differential Diagnosis of Creutzfeldt-Jakob Disease from Alzheimer’s Disease with Emphasis on Atypical Disease Variants. J Alzheimers Dis 2017; 55: 1471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abu-Rumeileh S, Capellari S, Stanzani-Maserati M, et al. The CSF neurofilament light signature in rapidly progressive neurodegenerative dementias. Alzheimers Res Ther 2018; 10: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leitão MJ, Baldeiras I, Almeida MR, et al. Sporadic Creutzfeldt-Jakob disease diagnostic accuracy is improved by a new CSF ELISA 14-3-3γ assay. Neuroscience 2016; 322: 398–407. [DOI] [PubMed] [Google Scholar]
- 55.Schmitz M, Ebert E, Stoeck K, et al. Validation of 14-3-3 Protein as a Marker in Sporadic Creutzfeldt-Jakob Disease Diagnostic. Mol Neurobiol 2016; 53: 2189–99. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci 2016; 17: 5–21. [DOI] [PubMed] [Google Scholar]
- 57.Koscova S, Zakova Slivarichova D, Tomeckova I, et al. Cerebrospinal Fluid Biomarkers in the Diagnosis of Creutzfeldt-Jakob Disease in Slovak Patients: over 10-Year Period Review. Mol Neurobiol 2017; 54: 5919–27. [DOI] [PubMed] [Google Scholar]
- 58.Karch A, Hermann P, Ponto C, et al. Cerebrospinal fluid tau levels are a marker for molecular subtype in sporadic Creutzfeldt-Jakob disease. Neurobiol Aging 2015; 36: 1964–68. [DOI] [PubMed] [Google Scholar]
- 59.Cohen OS, Chapman J, Korczyn AD, et al. CSF tau correlates with CJD disease severity and cognitive decline. Acta Neurol Scand 2016; 133: 119–23. [DOI] [PubMed] [Google Scholar]
- 60.Coulthart MB, Jansen GH, Olsen E, et al. Diagnostic accuracy of cerebrospinal fluid protein markers for sporadic Creutzfeldt-Jakob disease in Canada: a 6-year prospective study. BMC Neurol 2011; 11: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lehmann S, Paquet C, Malaplate-Armand C, et al. Diagnosis associated with Tau higher than 1200 pg/mL: Insights from the clinical and laboratory practice. Clin Chim Acta 2019; 495: 451–56. [DOI] [PubMed] [Google Scholar]
- 62.Li QX, Varghese S, Sarros S, et al. CSF Tau supplements 14-3-3 protein detection for sporadic Creutzfeldt-Jakob disease diagnosis while transitioning to next generation diagnostics. J Clin Neurosci 2018; 50: 292–293. [DOI] [PubMed] [Google Scholar]
- 63.Llorens F, Karch A, Golanska E, et al. Cerebrospinal Fluid Biomarker-Based Diagnosis of Sporadic Creutzfeldt-Jakob Disease: A Validation Study for Previously Established Cutoffs. Dement. Geriatr Cogn Disord 2017; 43: 71–80. [DOI] [PubMed] [Google Scholar]
- 64.Skillbäck T, Rosén C, Asztely F, Mattsson N, Blennow K, Zetterberg H. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: results from the Swedish Mortality Registry. JAMA Neurol 2014; 71: 476–83. [DOI] [PubMed] [Google Scholar]
- 65.Rübsamen N, Llorens F, Hermann P, et al. A prognostic model for overall survival in sporadic Creutzfeldt-Jakob disease. [published online ahead of print, 2020 Jul 2]. Alzheimers Dement. 2020; 10.1002/alz.12133. doi: 10.1002/alz.12133 [DOI] [PubMed] [Google Scholar]
- 66.Staffaroni AM, Kramer AO, Casey M, et al. Association of Blood and Cerebrospinal Fluid Tau Level and Other Biomarkers With Survival Time in Sporadic Creutzfeldt-Jakob Disease . JAMA Neurol 2019; 76: 969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riemenschneider M, Wagenpfeil S, Vanderstichele H, et al. phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt-Jakob disease from other dementias. Mol Psychiatry 2003. 8: 343–7. [DOI] [PubMed] [Google Scholar]
- 68.Llorens F, Schmitz M, Karch A, et al. Comparative analysis of cerebrospinal fluid biomarkers in the differential diagnosis of neurodegenerative dementia. Alzheimers Dement 2016; 12: 577–89. [DOI] [PubMed] [Google Scholar]
- 69.Petzold A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci 2005; 233: 183–98. [DOI] [PubMed] [Google Scholar]
- 70.Zerr I, Schmitz M, Karch A, et al. Cerebrospinal fluid neurofilament light levels in neurodegenerative dementia: Evaluation of diagnostic accuracy in the differential diagnosis of prion diseases. Alzheimers Dement 2018; 14: 751–63. [DOI] [PubMed] [Google Scholar]
- 71.Antonell A, Tort-Merino A, Ríos J, et al. Synaptic, axonal damage and inflammatory cerebrospinal fluid biomarkers in neurodegenerative dementias. Alzheimers Dement 2020; 16: 262–72. [DOI] [PubMed] [Google Scholar]
- 72.Kanata E, Golanska E, Villar-Piqué A, et al. Cerebrospinal fluid neurofilament light in suspected sporadic Creutzfeldt-Jakob disease. J Clin Neurosci 2019; 60: 124–27. [DOI] [PubMed] [Google Scholar]
- 73.Chohan G, Pennington C, Mackenzie JM, et al. The role of cerebrospinal fluid 14-3-3 and other proteins in the diagnosis of sporadic Creutzfeldt-Jakob disease in the UK: a 10-year review. J Neurol Neurosurg Psychiatry 2010; 81: 1243–8. [DOI] [PubMed] [Google Scholar]
- 74.Villar-Piqué A, Schmitz M, Lachmann I, et al. Cerebrospinal Fluid Total Prion Protein in the Spectrum of Prion Diseases. Mol Neurobiol 2019; 56: 2811–21. [DOI] [PubMed] [Google Scholar]
- 75.Minikel EV, Kuhn E, Cocco A, et al. Domain-specific Quantification of Prion Protein in Cerebrospinal Fluid by Targeted Mass Spectrometry. Mol Cell Proteomics 2019; 18: 2388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vallabh SM, Nobuhara CK, Llorens F, et al. Prion protein quantification in human cerebrospinal fluid as a tool for prion disease drug development. Proc Natl Acad Sci U S A 2019; 116: 7793–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Llorens F, Kruse N, Schmitz M, et al. Evaluation of α-synuclein as a novel cerebrospinal fluid biomarker in different forms of prion diseases. Alzheimers Dement 2017; 13: 710–9. [DOI] [PubMed] [Google Scholar]
- 78.Llorens F, Kruse N, Karch A, et al. Validation of α-Synuclein as a CSF Biomarker for Sporadic Creutzfeldt-Jakob Disease. Mol Neurobiol 2018; 55: 2249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kruse N, Heslegrave A, Gupta V, et al. Interlaboratory validation of cerebrospinal fluid α-synuclein quantification in the diagnosis of sporadic Creutzfeldt-Jakob disease. Alzheimers Dement (Amst) 2018; 10: 461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thompson AGB, Luk C, Heslegrave AJ, et al. Neurofilament light chain and tau concentrations are markedly increased in the serum of patients with sporadic Creutzfeldt-Jakob disease, and tau correlates with rate of disease progression. J Neurol Neurosurg Psychiatry 2018; 89: 955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piehl F, Kockum I, Khademi M, et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler 2018; 24: 1046–54 [DOI] [PubMed] [Google Scholar]
- 82.Abu-Rumeileh S, Baiardi S, Ladogana A, et al. Comparison between plasma and cerebrospinal fluid biomarkers for the early diagnosis and association with survival in prion disease [published online ahead of print, 2020 Sep 14]. J Neurol Neurosurg Psychiatry 2020; 91:1181–88. [DOI] [PubMed] [Google Scholar]
- 83.Llorens F, Villar-Piqué A, Schmitz M, et al. Plasma total prion protein as a potential biomarker for neurodegenerative dementia: diagnostic accuracy in the spectrum of prion diseases. Neuropathol Appl Neurobiol 2020; 46: 240–54. [DOI] [PubMed] [Google Scholar]
- 84.Vitali P, Maccagnano E, Caverzasi E, et al. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology 2011; 76: 1711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Staffaroni AM, Elahi FM, McDermott D, et al. Neuroimaging in Dementia. Semin Neurol 2017; 37: 510–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caverzasi E, Mandelli ML, DeArmond SJ, et al. White matter involvement in sporadic Creutzfeldt-Jakob disease. Brain 2014; 137: 3339–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pascuzzo R, Oxtoby NP, Young AL, et al. Prion propagation estimated from brain diffusion MRI is subtype dependent in sporadic Creutzfeldt-Jakob disease. Acta Neuropathol 2020; 140: 169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forner SA, Takada LT, Bettcher BM, et al. Comparing CSF biomarkers and brain MRI in the diagnosis of sporadic Creutzfeldt-Jakob disease. Neurol Clin Pract 2015; 5: 116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bizzi A, Pascuzzo R, Blevins J, et al. Evaluation of a New Criterion for Detecting Prion Disease With Diffusion Magnetic Resonance Imaging. JAMA Neurol 2020. doi: 10.1001/jamaneurol.2020.1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carswell C, Thompson A, Lukic A, Stevens J, Rudge P, Mead S, Collinge J, Hyare H. MRI findings are often missed in the diagnosis of Creutzfeldt-Jakob disease. BMC Neurol 2012; 12: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao T, Lyu JH, Zhang JT, et al. Diffusion-weighted MRI findings and clinical correlations in sporadic Creutzfeldt-Jakob disease. J Neurol 2015; 262: 1440–6. [DOI] [PubMed] [Google Scholar]
- 92.Park SY, Wang M, Jang JW, et al. The clinical stages of Sporadic Creutzfeldt-Jakob disease with Met/Met genotype in Korean patients. Eur Neurol 2016; 75: 213–22. [DOI] [PubMed] [Google Scholar]
- 93.Eisenmenger L, Porter MC, Carswell CJ, et al. Evolution of Diffusion-Weighted Magnetic Resonance Imaging Signal Abnormality in Sporadic Creutzfeldt-Jakob Disease, With Histopathological Correlation. JAMA Neurol 2016; 73: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zanusso G, Camporese G, Ferrari S, et al. Long-term preclinical magnetic resonance imaging alterations in sporadic Creutzfeldt–Jakob disease. Ann Neurol 2016; 80: 629–32. [DOI] [PubMed] [Google Scholar]
- 95.Renard D, Castelnovo G, Collombier L, Thouvenot E, Boudousq V. FDG-PET in Creutzfeldt-Jakob disease: Analysis of clinical-PET correlation. Prion 2017; 11: 440–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abu-Rumeileh S, Redaelli V, Baiardi S, et al. Sporadic Fatal Insomnia in Europe: Phenotypic Features and Diagnostic Challenges. Ann Neurol 2018; 84: 347–60. [DOI] [PubMed] [Google Scholar]
- 97.Steinhoff BJ, Zerr I, Glatting M, et al. Diagnostic value of periodic complexes in Creutzfeldt-Jakob disease. Ann Neurol 2004; 56: 702–8. [DOI] [PubMed] [Google Scholar]
- 98.Lapergue B, Demeret S, Denys V, et al. Sporadic Creutzfeldt-Jakob disease mimicking nonconvulsive status epilepticus. Neurology 2010; 74: 1995–1999 [DOI] [PubMed] [Google Scholar]
- 99.Marquetand J, Knake S, Strzelczyk A, et al. Periodic EEG patterns in sporadic Creutzfeld-Jakob-Disease can be benzodiazepine-responsive and be difficult to distinguish from non-convulsive status epilepticus. Seizure 2017; 53: 47–50. [DOI] [PubMed] [Google Scholar]
- 100.Shin JW, Byeongsoo Y, Seung HO, Kim NK, Lee SK, Kim OJ. Redefining periodic patterns on electroencephalograms of patients with sporadic Creutzfeldt-Jakob disease. Neurophysiol Clin 2017; 128: 756–62. [DOI] [PubMed] [Google Scholar]
- 101.Franko E, Wehner T, Joly O, et al. Quantitative EEG parameters correlate with the progression of human prion diseases. J Neurol Neurosurg Psychiatry 2016; 87: 1061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ladogana A, Kovacs GG. Genetic Creutzfeldt-Jakob disease. Handb Clin Neurol 2018; 153: 219–42. [DOI] [PubMed] [Google Scholar]
- 103.Karch A, Llorens F, Schmitz M, et al. Stratification by Genetic and Demographic Characteristics Improves Diagnostic Accuracy of Cerebrospinal Fluid Biomarkers in Rapidly Progressive Dementia. J Alzheimers Dis 2016; 54: 1385–93. [DOI] [PubMed] [Google Scholar]
- 104.McGuire LI, Poleggi A, Poggiolini I, et al. Cerebrospinal fluid real-time quaking-induced conversion is a robust and reliable test for sporadic creutzfeldt-jakob disease: An international study. Ann Neurol 2016; 80: 160–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cali I, Lavrich J, Moda F, et al. PMCA-replicated PrPD in urine of vCJD patients maintains infectivity and strain characteristics of brain PrPD: Transmission study. Sci Rep 2019; 9: 5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raymond GJ, Race B, Orru CD, et al. Transmission of CJD from nasal brushings but not spinal fluid or RT-QuIC product. Ann Clin Transl Neurol 2020; 7: 932–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karch A, Koch A, Zapf A, Zerr I, Karch A. Partial verification bias and incorporation bias affected accuracy estimates of diagnostic studies for biomarkers that were part of an existing composite gold standard. J Clin Epidemiol 2016, 78: 73–82. [DOI] [PubMed] [Google Scholar]
- 108.Peckeu L, Delasnerie-Lauprètre N, Brandel JP, et al. Accuracy of diagnosis criteria in patients with suspected diagnosis of sporadic Creutzfeldt-Jakob disease and detection of 14-3-3 protein, France, 1992 to 2009. Euro Surveill 2017; 22. doi: 10.2807/1560-7917.ES.2017.22.41.16-00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vallabh SM, Minikel EV, Williams VJ, et al. Cerebrospinal fluid and plasma biomarkers in individuals at risk for genetic prion disease. BMC Med 2020; 18: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vallabh SM, Minikel EV, Schreiber SL, Lander ES. Towards a treatment for genetic prion disease: trials and biomarkers. Lancet Neurol 2020; 19: 361–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.