Abstract
Women with Marfan syndrome (MFS) are at high risk for pregnancy-associated aortic dissection. Pathogenic models that singularly invoke hemodynamic stress are difficult to reconcile with predominant postnatal occurrence of aortic tear, often occurring weeks to months after delivery. In consideration of events that peak at term, are sustained after delivery, and might synergize with previously defined signaling pathways implicated in aneurysm progression, we examined the hormone oxytocin, which initiates uterine contraction and milk letdown for the duration of lactation through phosphorylation of extracellular signal–regulated kinase (ERK). In a mouse model of MFS that shows highly penetrant postnatal aortic dissection, risk was strongly attenuated by preventing lactation or use of an oxytocin receptor antagonist. Survival correlated inversely with the extent of ERK activation in the aortic wall, and strong protection was observed upon attenuation of ERK phosphorylation using an inhibitor of ERK kinase (MEK) or the U.S. Food and Drug Administration–approved medication hydralazine, offering potential therapeutic strategies for pregnancy-associated vascular catastrophe in the setting of MFS.
INTRODUCTION
Marfan syndrome (MFS) is an autosomal dominant connective tissue disorder that affects about 1 in 5000 people worldwide and is caused by mutations in the fibrillin-1 gene (FBN1) encoding the extracellular matrix protein fibrillin-1. Cardinal manifestations occur in the ocular, musculoskeletal, and cardiovascular systems, including dislocation of the lens of the eye, long bone overgrowth, scoliosis, and progressive aortic root enlargement resulting in vessel tear (dissection) and death. The current recommendation is to perform aortic root replacement once the aorta reaches an absolute diameter of 5.0 cm (1). There is an increased risk of accelerated aneurysm progression and aortic dissection associated with pregnancy in MFS once the maximal aortic diameter exceeds 4.0 cm; however, precise risk counseling has been difficult because of the small number and size of studies that address this issue (2-7). Contrary to intuition and the commonly invoked mechanistic hypothesis highlighting the importance of hemodynamic stress, most of the pregnancy-associated catastrophic events do not occur during labor (8). Although the incidence of dissection rises in the third trimester, it peaks during the first few weeks postpartum. One study by Goland and Elkayam (2) estimated the overall risk of aortic dissection during pregnancy in MFS to be 3%, but only about 1% when the diameter of the aortic root was <4.0 cm around the time of conception. The risk was estimated at 10% if the aortic diameter measured ≥4.0 cm. Pacini and colleagues (6) found that the risk of aortic dissection with each pregnancy in MFS was 4.4%, and that the time of highest risk extends from the late third trimester to several weeks to months postpartum. A more recent study examined the incidence of aortic complications during pregnancy in 184 women with an established diagnosis of MFS: 10.6% experienced an aortic-related complication during pregnancy, and 75% of dissections occurred in the postpartum period (8). With the advent of valve-sparing aortic root replacement (precluding the need for chronic anticoagulation), some women choose surgery before conception with aortic dimensions below conventional surgical thresholds to mitigate pregnancy-associated risks. However, Sayama and colleagues (9) followed 14 women (17 pregnancies) with confirmed MFS and reported an increased rate of descending thoracic (type B) aortic dissections in association with pregnancy in those women who had undergone elective aortic root replacement (60%) versus those who had not (8.3%). This demonstrates the ongoing need for improved preventative treatment options for women with MFS during pregnancy.
Aspects of the pathogenesis of aneurysm growth and tear have been elucidated in MFS. A primary deficiency of fibrillin-1 in the extracellular matrix associates with increased transforming growth factor β (TGFβ) signaling in the aortic media in both patients and mouse models, as evidenced by increased activation of receptor-activated SMAD proteins and increased expression of TGFβ-responsive genes (10). Furthermore, treatment of MFS mice with a TGFβ-neutralizing antibody (TGFβNAb) after the immediate perinatal period can slow aortic root growth and diminish elastic fiber fragmentation and excessive collagen deposition in the aortic wall (11-14). Losartan, an angiotensin II type 1 (AT1) receptor blocker (ARB) that both lowers blood pressure and attenuates TGFβ expression, activation, and signaling, achieved cessation of aneurysm progression, preservation of aortic wall architecture, and delay or even prevention of aortic dissection in Marfan mice (11). More recently, activation (phosphorylation) of extracellular signal–regulated kinase 1/2 (pERK1/2) has been shown to be a prominent distal determinant of aneurysm progression in the MFS mouse model; a selective antagonist of the kinase that phosphorylates ERK1/2 (MEK) was as effective as losartan in preventing aneurysm growth. Losartan both reduces ERK activation by AT1 and augments inhibition of ERK by the type 2 angiotensin receptor (AT2) (12). Pathologic ERK1/2 activation was also shown, at least in part, to be dependent upon TGFβ signaling in MFS mice as it was diminished in the aortic wall upon treatment with TGFβNAb (12-14). ERK1/2 activation was subsequently linked to a wide variety of genetically induced presentations of aortic aneurysm (15) and to environmental provocations that accelerated aneurysm progression and dissection in MFS mice. Together, these data suggest that the predisposition for aortic aneurysm growth and tear can integrate the influence of diverse etiologies for ERK1/2 activation.
In consideration of putative pathogenic events that would initiate toward the end of pregnancy, be maintained after delivery, and might synergize with signaling events previously implicated in aortic disease, our focus turned to the hormone oxytocin. Oxytocin release by the hypothalamus increases throughout pregnancy, particularly in the late third trimester, and contributes to the initiation of uterine contraction (16). High circulating oxytocin is sustained postnatally in association with lactation to promote milk letdown (17). The expression of oxytocin receptor (OR) in the aorta is enhanced by estrogen and pregnancy (18), and oxytocin exerts its effects on peripheral tissues including the uterus and breast through ERK1/2 activation (19-21). On this basis, we hypothesized that oxytocin mediates the enhanced pregnancy-associated risk of aortic dissection in MFS and related disorders.
RESULTS
Decreased survival and accelerated aortic growth in pregnant MFS mice
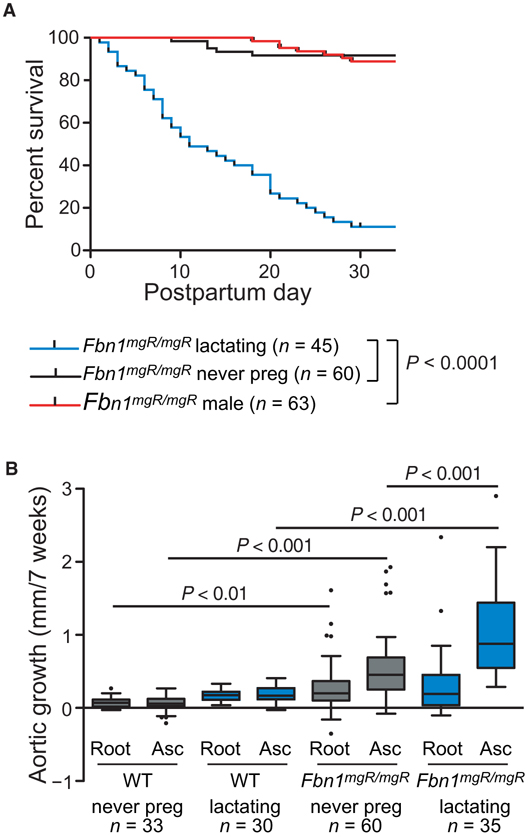
The Fbn1mgR/mgR mouse is an exaggerated homozygous model of MFS, in which there is a severe deficiency of fibrillin-1 expression (~15% of normal) early in development and throughout postnatal life due to transcriptional interference imposed by targeted insertion of a neomycin resistance cassette in the Fbn1 gene. Unlike people and mice with MFS that harbor heterozygous Fbn1 mutations, where the strongest predisposition for aneurysm is at the sinuses of Valsalva (the aortic root), Fbn1mgR/mgR mice show predominant dilatation in the more distal ascending aorta, culminating in ascending aortic dissection at ~2 to 8 months of age (22). To investigate the impact of pregnancy on survival, Fbn1mgR/mgR females were bred at 7 to 9 weeks of age and monitored for survival. All mice found dead demonstrated hemothorax, with the majority also demonstrating hemopericardium, suggesting a predominance of type A (ascending) aortic dissections. We found an extreme risk of pregnancy-associated death from dissection, with 91.1% of Fbn1mgR/mgR mice (n = 45) dying from aortic dissection within the first 4 weeks postpartum (median age of death was 11 days postpartum) as compared to 6.7% of age-matched never-pregnant Fbn1mgR/mgR females (n = 60; P ≤ 0.0001) and 9.5% of males (n = 63; P ≤ 0.0001) followed over the same age-matched time period. There was no difference in survival between never-pregnant Fbn1mgR/mgR females and corresponding males (Fig. 1A). In comparison, no wild-type (WT) females (n = 60) or lactating WT mice (n = 45) died during the same age-matched time course. We followed mice by echocardiography over the 7-week time course spanning pregnancy (3 weeks) and lactation (4 weeks) and found a significant increase in aortic root growth and ascending aortic growth in never-pregnant Fbn1mgR/mgR female mice as compared to never-pregnant WT mice (0.07 ± 0.07 versus 0.29 ± 0.32 mm/7 weeks, P ≤ 0.01, and 0.48 ± 0.48 versus 0.07 ± 0.12 mm/7 weeks, P ≤ 0.001, respectively). Whereas there was no pregnancy-associated effect on aortic root growth, there was a significant pregnancy-associated increase in ascending aortic growth in Fbn1mgR/mgR females (1.02 ± 0.61 mm/7 weeks) compared to never-pregnant Fbn1mgR/mgR females (P ≤ 0.001) and postpartum WT mice (0.19 ± 0.11 mm/7 weeks, P ≤ 0.001) (Fig. 1B and table S1).
Fig. 1. The effects of pregnancy in WT and Fbn1mgR/mgRmice.
(A) Kaplan-Meier survival curve for lactating Fbn1mgR/mgR mice (n = 45) compared to male (n = 63) or never-pregnant female (n = 60) Fbn1mgR/mgR mice. (B) Average aortic root and ascending aortic growth over the 7-week period spanning pregnancy (3 weeks) and lactation (4 weeks) in WT never-pregnant (n = 33), WT lactating (n = 30), Fbn1mgR/mgR never-pregnant (n = 60), and Fbn1mgR/mgR lactating (n = 35) mice, as measured by echocardiogram. Survival was statistically evaluated using a log-rank (Mantel-Cox) test. When data are presented as boxplots, the box extends from the 25th to 75th percentiles, median is denoted by the internal line, and whiskers indicate the range calculated using the Tukey method, with data points outside the whiskers shown as individual points. All significant P values (P < 0.05) are noted in the figure.
Survival improved by prevention of lactation
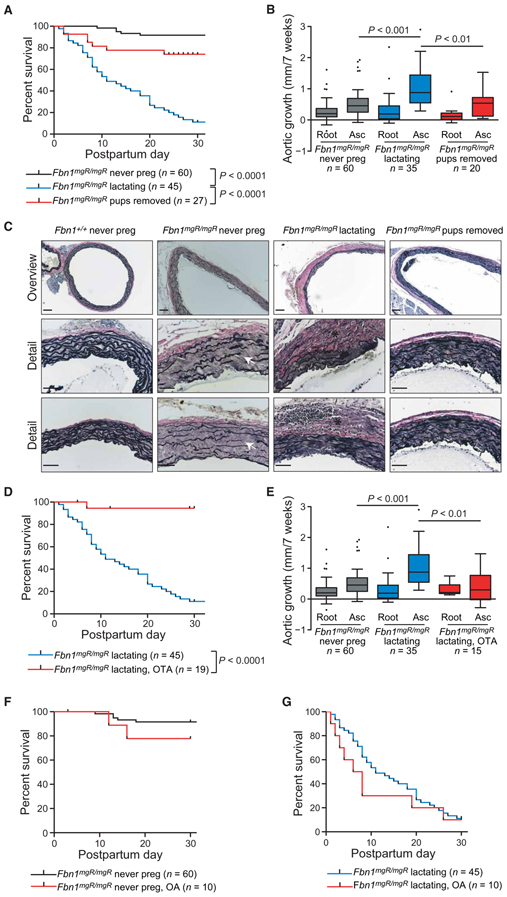
To determine whether lactation played a role in pregnancy-associated aortic dissection, we removed Fbn1mgR/mgR mothers from their pups on the day of delivery, effectively eliminating lactation-induced oxytocin release in the postpartum period. This isolated manipulation reduced the incidence of pregnancy-associated death due to aortic dissection from 91.1 to 25.9% (P ≤ 0.0001) in Fbn1mgR/mgR mice (Fig. 2A). In addition, although the growth of the aortic root (0.15 ± 0.21 mm/7 weeks) was not significantly decreased, growth of the ascending aorta (0.54 + 0.43 mm/7 weeks) was significantly less during the 7-week time period spanning pregnancy and the first 4 weeks postpartum in Fbn1mgR/mgR mice not allowed to lactate (P ≤ 0.01) (Fig. 2B and tables S1 and S2). Evaluation of the proximal ascending aorta with Verhoeff–van Gieson (VVG) staining demonstrated increased elastic fiber fragmentation in Fbn1mgR/mgR mice compared to WT controls. Marked thickening of the adventitia with an evident fibroproliferative response was observed in lactating Fbn1mgR/mgR mice and was greatly reduced by preventing lactation (Fig. 2C).
Fig. 2. Therapeutic manipulations that alter the survival and aortic growth in pregnancy.
(A) Kaplan-Meier survival curve comparing Fbn1mgR/mgR never-pregnant (n=60) and Fbn1mgR/mgR lactating (n = 45) mice to Fbn1mgR/mgR females with pups removed on the day of delivery, thereby preventing lactation and eliminating the lactation-induced prolonged elevation of oxytocin (n = 27). (B) Average aortic root and ascending aortic growth over the 7-week period spanning pregnancy (3 weeks) and lactation (4 weeks) in Fbn1mgR/mgR never-pregnant (n = 60), Fbn1mgR/mgR lactating (n = 35), and Fbn1mgR/mgR females with pups removed (n = 20). (C) Representative proximal ascending aortic wall sections stained with VVG for elastin, demonstrating elastic fiber breaks (white arrows), cellularity, and thickness of the adventitia (black asterisks) in the Fbn1mgR/mgR and WT mice. (D) Kaplan-Meier survival curve comparing Fbn1mgR/mgR lactating mice (n = 45) to Fbn1mgR/mgR females with OTA administered via a continuous subcutaneous infusion pump implanted at the beginning of the third week of gestation and continued through the 4 weeks of lactation for a total of 5 weeks of treatment (n = 19). (E) Average aortic root and ascending aortic growth over the 7-week period spanning pregnancy and lactation in never-pregnant (n = 60), lactating (n = 35), and OTA-treated (n = 15) Fbn1mgR/mgR mice. (F) Kaplan-Meier survival curve assessing the effect of OA administration to never-pregnant Fbn1mgR/mgR mice (n = 10) via a continuous subcutaneous infusion pump implanted at 7 weeks of life and continued for a total of 5 weeks of treatment to mimic the time span of the third week of gestation and 4 weeks of lactation. (G) Kaplan-Meier survival curve assessing the effects of OA administered to Fbn1mgR/mgR mice (n = 10) via a continuous subcutaneous infusion pump implanted at the beginning of the third week of gestation and continued through the 4 weeks of lactation for a total of 5 weeks of treatment. Survival was statistically evaluated using a log-rank (Mantel-Cox) test. When data are presented as boxplots, the box extends from the 25th to 75th percentiles, median is denoted by the internal line, and whiskers indicate the range calculated using the Tukey method, with data points outside the whiskers shown as individual points. All significant P values (P < 0.05) are noted in the figure.
Survival improved by OR antagonist
To determine whether reduction of oxytocin was responsible for the improved survival resulting from prevention of lactation, we treated Fbn1mgR/mgR pregnant mice with a continuous infusion of a highly specific OR antagonist [OTA; desGly-NH2-d(CH2)5[d-Tyr2,Thr4]OVT (ornithine vasotocin)], which has 95 times greater specificity for the OR compared to the vasopressin receptor (23). Mini-osmotic pumps were implanted subcutaneously in the mice at the beginning of the third trimester and delivered OTA for the remaining 1 week of pregnancy and first 4 weeks postpartum. We observed a reduction in death from aortic dissection from 91.1 to 6.7% in treated mice (P ≤ 0.0001) (Fig. 2D). The administration of OTA resulted in an incomplete antagonism of oxytocin, as evidenced by the retained ability of the mice to deliver and to support their pups through lactation. In comparison, genetically targeted mice that are completely deficient in oxytocin are still able to deliver normally, but are not able to successfully nurse their pups due to lack of milk letdown (24). There was no significant difference in aortic root growth (0.32 ± 0.20 mm/7 weeks) in OTA-treated pregnant Fbn1mgR/mgR mice when compared to either never-pregnant or lactating Fbn1mgR/mgR animals; however, ascending aortic growth (0.44 ± 0.49 mm/7 weeks) was significantly decreased compared to lactating Fbn1mgR/mgR mice (P ≤ 0.01) and was no different than that observed in never-pregnant Fbn1mgR/mgR females (Fig. 2E and tables S1 and S2). No protection was observed in mice receiving osmotic pumps delivering vehicle alone (fig. S1), and OTA delivery was not associated with a reduction in blood pressure (fig. S2 and table S3).
Administration of oxytocin agonist not sufficient to induce dissection
To determine whether oxytocin is sufficient to induce aortic dissection, we first administered a continuous infusion of a highly specific oxytocin agonist [OA; (Thr4,Gly7)OT] (23) via mini-osmotic pumps to age-matched never-pregnant Fbn1mgR/mgR females. This did not significantly accelerate dissection (12.2 versus 8.9% died from aortic dissection, P = 0.20) (Fig. 2F). We then administered OA to pregnant Fbn1mgR/mgR mice at the beginning of the third trimester and continued for 5 weeks. There was no significant difference in the incidence of death from aortic dissection or the timing of dissection over the 5-week time period compared to vehicle-treated lactating animals (90.0 versus 91.1%, P = 0.37) (Fig. 2G). We went on to evaluate OR gene (Oxtr) expression in the aorta and observed increased Oxtr mRNA during pregnancy in both WT and Fbn1mgR/mgR mice compared to their never-pregnant counterparts (P < 0.05), with no significant difference noted between never-pregnant WT and Fbn1mgR/mgR mice and between lactating WT and Fbn1mgR/mgR mice (P = 0.69) (fig. S3 and table S4), suggesting a pregnancy-specific effect independent of genotype.
Treatment with β-blockers, the current standard of care for pregnancy in MFS
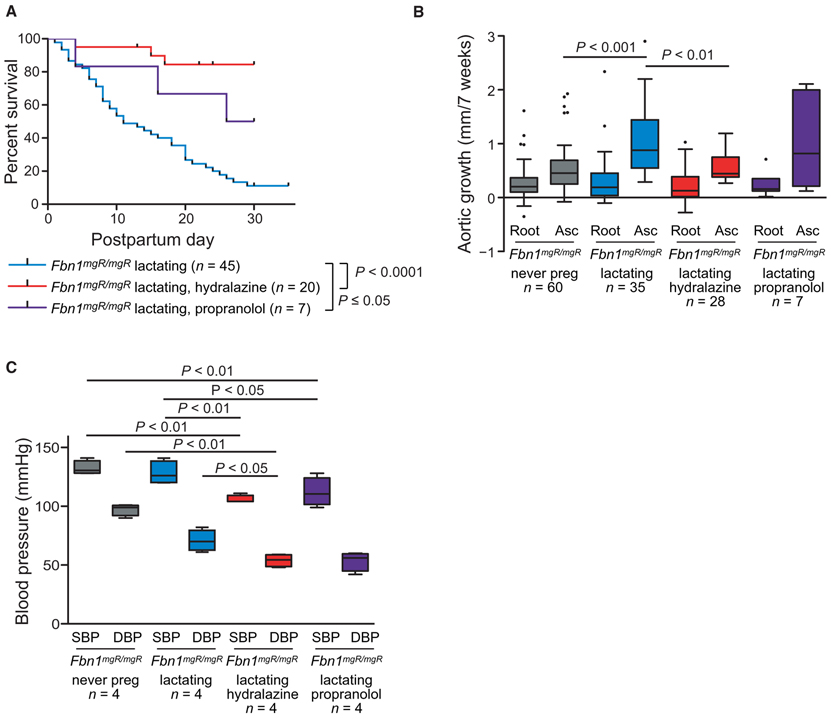
β-Adrenergic receptor blockers such as atenolol or propranolol have been shown to achieve a suppression in aortic root growth rate in a different mouse model of MFS harboring a missense mutation, Fbn1C1039G/+ (11), and are currently considered standard of care for pregnant women with MFS (2,3). Pregnant Fbn1mgR/mgR mice were treated with propranolol in their drinking water starting at the beginning of the third trimester and continuing for 5 weeks. Propranolol treatment improved survival from 9 to 57% (P ≤ 0.05) in lactating Fbn1mgR/mgR mice (Fig. 3A), but did not result in a significant reduction in either aortic root (0.24 ± 0.23 mm/7 weeks) or ascending aortic growth (0.99 ± 0.80 mm/7 weeks), as compared to placebo-treated lactating Fbn1mgR/mgR animals (Fig. 3B and tables S1 and S2).
Fig. 3. Therapeutic effect of antihypertensive therapy in pregnancy.
(A) Kaplan-Meier survival curve comparing the effects of propranolol treatment (n = 7) versus hydralazine treatment (n = 20) in Fbn1mgR/mgR mice. Treatment with propranolol or hydralazine was initiated at the beginning of the third week of pregnancy and continued through the 4 weeks of lactation. (B) Average aortic root and ascending aortic growth over the 7-week period spanning pregnancy and lactation, as measured by echocardiogram, in never-pregnant (n = 60) versus lactating Fbn1mgR/mgR mice treated with placebo (n = 35), hydralazine (n = 28), or propranolol (n = 7). (C) Average systolic and diastolic blood pressure in never-pregnant and in lactating placebo-, hydralazine-, and propranolol-treated Fbn1mgR/mgR mice (n = 4 for each treatment group). Survival was statistically evaluated using a log-rank (Mantel-Cox) test. When data are presented as boxplots, the box extends from the 25th to 75th percentiles, median is denoted by the internal line, and whiskers indicate the range calculated using the Tukey method, with data points outside the whiskers shown as individual points. All significant P values (P < 0.05) are noted in the figure.
Differential effect on survival of propranolol versus hydralazine in pregnancy
We went on to treat with hydralazine, another antihypertensive agent approved for use in pregnancy that was previously demonstrated to markedly reduce aortic root growth in the Fbn1C1039G/+ mouse model (25). Treatment of Fbn1mgR/mgR mice, again initiated at the beginning of the third trimester and continued for 5 weeks, resulted in 95% freedom from aortic dissection, which was significantly greater than in untreated pregnant Fbn1mgR/mgR mice (P ≤ 0.0001). There was no significant difference in the performance of hydralazine when compared to propranolol (P = 0.10) (Fig. 3A). In contrast to propranolol, hydralazine significantly reduced ascending aortic growth rate (0.59 ± 0.28 mm/7 weeks) as compared to untreated lactating females (P ≤ 0.01; Fig. 3B and tables S1 and S2). Both propranolol and hydralazine resulted in a decline in blood pressure, and there was no significant difference in reduction between the two (Fig. 3C and table S5).
Correlation of pregnancy outcomes with ERK1/2 activation
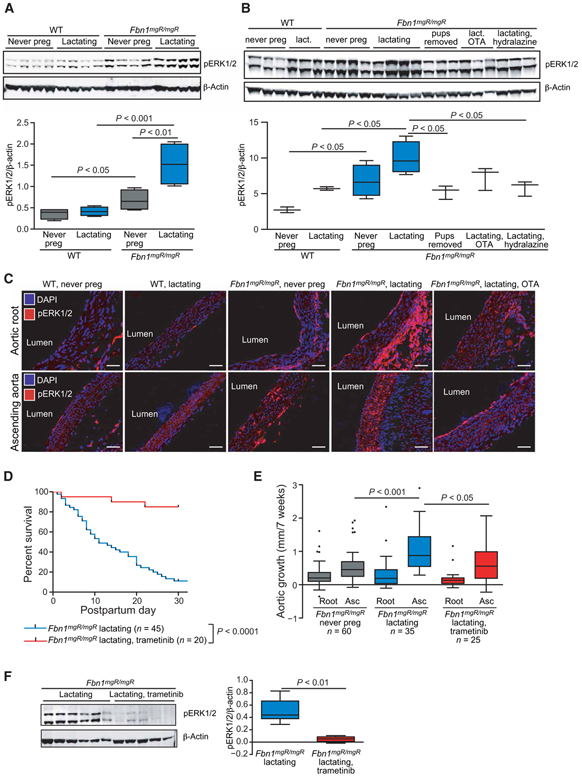
In keeping with our hypothesis that ERK1/2 signaling drives abnormal aortic growth, there was increased pERK1/2 in the aortic media of never-pregnant Fbn1mgR/mgR females as compared to never-pregnant WT females (P ≤ 0.05), with a significant further up-regulation induced by pregnancy and lactation (P ≤ 0.01). Furthermore, lactating Fbn1mgR/mgR mice had increased pERK1/2 as compared to lactating WT females (P < 0.001) (Fig. 4A and table S6). Preventing lactation by removing pups or treatment with hydralazine resulted in a marked reduction of pERK1/2 in the ascending aorta, as compared to lactating Fbn1mgR/mgR mice (P ≤ 0.05 for both); a significant reduction was not observed upon treatment with OTA (P = 0.14). Removal of pups or treatment with OTA or hydralazine resulted in ERK1/2 phosphorylation in the aortic wall of Fbn1mgR/mgR mice that was indistinguishable from that observed in lactating WT mice (Fig. 4B and table S7). Immunofluorescence (IF) staining for pERK1/2 revealed an increase in the aortic media in the aortic root and ascending aorta (Fig. 4C, most notably in the ascending aorta, in Fbn1mgR/mgR mice compared to WT animals. A further increase was noted in lactating Fbn1mgR/mgR mice, and this was reduced with OTA administration (Fig. 4C).
Fig. 4. ERK1/2 phosphorylation in the aorta and its correlation with aortic dissection and aneurysm.
(A) Western blot analysis of pERK1/2 in the aortic root and proximal ascending aorta in WT never-pregnant, WT lactating, Fbn1mgR/mgR never-pregnant, and Fbn1mgR/mgR lactating mice (n = 4 for each treatment group). (B) Western blot analysis of pERK1/2 in the aortic root and proximal ascending aorta comparing WT never-pregnant (n = 3) and lactating mice (n = 3), Fbn1mgR/mgR never-pregnant (n = 4) and lactating (n = 4) mice, Fbn1mgR/mgR mice with pups removed thereby preventing lactation (n = 3), and Fbn1mgR/mgR mice treated with OTA (n = 3) or hydralazine (n = 3). (C) pERK IF of the aortic root and ascending aorta in representative WT and Fbn1mgR/mgR never-pregnant, lactating, and lactating Fbn1mgR/mgR treated with OTA. Scale bars, 50 μm. DAPI, 4′,6-diamidino-2-phenylindole. (D) Kaplan-Meier curve demonstrating the survival of Fbn1mgR/mgR lactating mice treated with trametinib (n = 20), initiated at the start of the third week of pregnancy and continued through 4 weeks of lactation, in comparison to untreated Fbn1mgR/mgR mice (n = 45). (E) Average aortic root and proximal ascending aortic growth over the 7 weeks spanning pregnancy and lactation in Fbn1mgR/mgR never-pregnant (n = 60) or lactating Fbn1mgR/mgR mice treated with placebo (n = 35) or trametinib (n = 25). (F) Western blot analysis of pERK1/2 in the aortic root and proximal ascending aorta comparing lactating Fbn1mgR/mgR mice treated with placebo (n = 6) or trametinib (n = 5). Survival was statistically evaluated using a log-rank (Mantel-Cox) test. When data are presented as boxplots, the box extends from the 25th to 75th percentiles, median is denoted by the internal line, and whiskers indicate the range calculated using the Tukey method, with data points outside the whiskers shown as individual points. All significant P values (P < 0.05) are noted in the figure.
Treatment with MEK inhibitor
To further clarify the role of ERK activation in pregnancy-associated aortic growth and dissection, we treated pregnant Fbn1mgR/mgR mice with trametinib, a U.S. Food and Drug Administration–approved oral MEK inhibitor (MEKi), beginning at the third trimester and continuing for 5 weeks. Trametinib significantly improved freedom from aortic dissection, with 85% of mice surviving (P ≤ 0.0001) (Fig. 4D). Trametinib significantly reduced ascending aortic growth (0.64 ± 0.55 mm/7 weeks, P ≤ 0.05) as compared to lactating Fbn1mgR/mgR mice, with the result indistinguishable from never-pregnant Fbn1mgR/mgR females (Fig. 4E and tables S1 and S2). There was no significant reduction in blood pressure upon treatment with trametinib (fig. S4 and table S8). The phosphorylation of ERK1/2 in the ascending aorta was significantly decreased in lactating Fbn1mgR/mgR mice treated with MEKi (P ≤ 0.001) (Fig. 4F and table S9), demonstrating that oral trametinib was having the expected effect in this experimental system.
DISCUSSION
Although a variety of hypotheses regarding the mechanism of pregnancy-associated aortic dissection have been proposed, alteration of hemodynamic parameters is typically considered the dominant determinant of predisposition. Contributing factors could include the increased circulating volume and heart rate during pregnancy, the marked isometric strain that attends vaginal delivery, or the rapid fluid shifts and obligate diuresis in the immediate peripartum period. It is difficult to reconcile a strong predisposition for vascular catastrophe in the weeks to months after delivery with pathogenic models that singularly invoke the acute hemodynamic consequences of pregnancy. The observation of delayed risk of postpartum vascular dissection not only is restricted to MFS but also pertains to other heritable connective tissue disorders such as vascular Ehlers-Danlos syndrome or Loeys-Dietz syndrome and to more common nonsyndromic conditions without a defined genetic contribution such as pregnancy-associated spontaneous cervical or coronary artery dissection (26,27).
This study using a genetically defined and validated mouse model of MFS recapitulates a strong predisposition for postnatal aortic dissection and provides complementary and compelling evidence against a dominant hemodynamic mechanism. First, antihypertensive agents that achieved a comparable lowering of blood pressure during and after pregnancy were associated with variable outcomes, ranging from modest (propranolol) to substantial (hydralazine) protection. Second, the incidence of dissection was potently reduced by either removal of the pups or administration of a selective OTA, strongly implicating lactation as a major contributory risk factor. The suggestion of differential effect of oxytocin agonism in nonpregnant versus pregnant Fbn1mgR/mgR mice, along with the up-regulation of OR expression in the aortic wall in both WT and Fbn1mgR/mgR mice, supports the hypothesis that pregnancy induces a more vulnerable aortic environment and suggests that oxytocin is a major contributor but is not sufficient for acceleration of aneurysm or dissection and that other factors associated with pregnancy, including up-regulation of the OR, are necessary. Although in vitro studies have documented that oxytocin has vasoactive properties, with dilatation or constriction at low and high concentrations, respectively (28), this study failed to demonstrate any blood pressure differences imposed by lactation or oxytocin antagonism. Third, the marked protection afforded by inhibition of MEK-mediated ERK activation was not associated with any apparent hemodynamic consequence.
Oxytocin has been reported to be expressed in both endothelial cells and cultured vascular smooth muscle cells (VSMCs), where it can support calcium-mediated responses including ERK activation. A parsimonious explanation for the observed increase in pERK in the aortic media in lactating Fbn1mgR/mgR mice would invoke oxytocin signaling in VSMCs, but more complex paracrine interactions that involve the endothelium cannot be excluded (29-31).
The deleterious gene-by-environment interaction imposed by pregnancy and lactation in MFS mice shows apparent correlates with the acceleration of aortic aneurysm growth and tear observed in MFS mice treated with calcium channel blockers (CCBs) (25). CCBs induced maximal dilatation in the more distal ascending aorta in association with excessive ERK phosphorylation that was ultimately linked to a phospholipase C (PLC)–inositol triphosphate (IP3)/diacylglycerol (DAG)–protein kinase C (PKC) axis of activation. Normalization of both clinical and biochemical parameters was observed upon treatment with the IP3 or PKC antagonist hydralazine or enzastaurin, respectively (25). In this light, it is notable that both the OR and AT1 (the target of losartan) are G protein–coupled receptors that signal through the Gαq-PLC-IP3/DAG-PKC-ERK axis. Desensitization of the OR, and hence inhibition of uterine contraction, is dependent on β-arrestins that paradoxically enhance ERK phosphorylation (in response to either OR or AT1 stimulation) but suppress nuclear localization and transcriptional responses (32,33). Previous work has suggested that enhanced TGFβ activity can be either an upstream effector or a downstream consequence of ERK activation in the pathogenesis of MFS in a context-dependent manner (13, 34). Together, this study and previous work suggest that the timing and severity of aneurysm in MFS correlates with the extent of ERK signaling in the aortic wall. It appears that the total phosphorylation of ERK in pregnancy integrates parallel inputs, because prevention of lactation or oxytocin antagonism lowers pERK to the level seen in never-pregnant MFS mice, but not the lower level observed in WT animals.
We note that our study has several limitations. The period of observation (3 weeks of gestation and 4 weeks postpartum) provided limited time for aortic growth, which is largely restricted to the more distal ascending aorta in the Fbn1mgR/mgR mouse model. This precluded our ability to assess for differences in growth of the aortic root, an aortic segment more typically involved in people with MFS. Western blot analyses were performed on protein lysates derived from tissue extending from just above the aortic valve to the origin of the brachiocephalic artery. This practice did not allow us to distinguish between the protein expression contribution of the aortic root and the more distal ascending aorta. In addition, although careful attention was given to controlling for age and mouse background, there was variability in the absolute aortic dimensions within a given state (genotype and treatment arm) that may have resulted in underestimation of treatment effects. This was offset by using death due to aortic dissection as a discrete outcome parameter.
This study has multiple potential therapeutic implications that will require further study and validation in people. The most obvious and immediately applicable intervention would be the avoidance of lactation and/or the use of OTAs late in gestation and in the postpartum period. OTAs are currently in clinical use in Europe for the suppression of preterm labor. It is notable that OTA-treated MFS mice retained the ability to deliver spontaneously and to support their pups through lactation, suggesting that protection from pregnancy-associated aortic dissection can be achieved with incomplete OR antagonism. In contrast, Otxr-null mice support spontaneous labor and delivery, but show a critical defect in milk letdown (24). The use of Pitocin (a synthetic form of oxytocin) to induce labor or as a routine practice after delivery to suppress uterine bleeding will need to be critically evaluated in at-risk populations. Unlike ARBs, hydralazine can be used during pregnancy and could be considered as an alternative or adjunct to β-blocker therapy. Studies have documented that oxytocin concentrations in women are measurably increased at 12 weeks of gestation, rise throughout pregnancy, and peak at delivery. Concentrations return to baseline by 8 weeks postpartum in the absence of lactation, but are sustained at delivery-associated values throughout lactation (16). If epidemiologic studies confirm a role for oxytocin in pregnancy-associated vascular events in women with MFS and related disorders, there is the potential for rapid translation of therapeutic strategies that include modification of delivery and breastfeeding practices and pharmacologic interventions that include hydralazine and OTAs.
MATERIALS AND METHODS
Study design
The objective of the study was to investigate factors contributing to the increased risk of pregnancy-associated aortic aneurysm progression and dissection in MFS. We hypothesized that oxytocin played a role by increasing activation of the ERK signaling pathway and that inhibiting oxytocin or ERK directly could decrease the risk of dissection. We used an established mouse model for MFS (Fbn1mgR/mgR) and observed an increased risk of dissection in the 4 weeks postpartum, the period of lactation. All drug interventions were initiated at the beginning of the third trimester to allow for completion of organ development, and continued postnatally for the 4-week period of lactation. A minimum of seven mice were included in each treatment arm. All experiments used mice of the same age and background that were randomly assigned to treatment arms. Although each treatment trial included contemporaneous control animals, the performance of untreated mice remained constant for the full duration of this study, allowing pooling of controls for robust comparisons.
Mice
All mice were cared for under strict adherence to the guidelines of the Animal Care and Use Committee of the Johns Hopkins University School of Medicine. Heterozygous mutant mice (Fbn1mgR/+) were purchased from The Jackson Laboratory in a pure C57BL/6 background, and homozygous mutant mice (Fbn1mgR/mgR) were generated. Heterozygous mutant mice (Fbn1mgR/+) were used to surrogate nurse the pups of the homozygous females who were sacrificed or died during lactation. Control mice were pure C57BL/6 WT mice. Genotyping was performed at 3 weeks of life by polymerase chain reaction (PCR). DNA was isolated from tags using standard methods. Quantitative PCR (qPCR) was performed with four amplicons (Alb1-FOR:CTGCAATCCTGAACCGTGT, Alb1-REV:TTCCACCAGGGATCCACTAC; qNEO1-FOR:TGAATGAACTGCAGGACGAG, qNEO1-REV:AGTGACAACGTCGAGCACAG; qNEO2-FOR: TCTCCTGTCATCTCACCTTGC, qNEO2-REV:GTAGCCGGATCAAGCGTATG; and qNEO3-FOR:TCTGGATTCATCGACTGTGG, qNEO3-REV:TTCAGCAATATCACGGGTAGC) detecting the introduced neomycin cassette.
Seven- to 9-week-old female Fbn1mgR/+ and Fbn1mgR/mgR mice underwent timed mating to male Fbn1mgR/mgR mice. Survival was assessed for the 4 weeks after delivery, during which females remained with their pups for nursing. Mice were sacrificed with an inhalation overdose of 2-bromo-2-chloro-1,1,1-trifluoroethane (Sigma). Mice underwent immediate laparotomy and descending abdominal aortic transection, and phosphate-buffered saline (PBS) (pH 7.4) was infused through the right and left ventricles to flush out the blood. Mice that were used for Western blot analysis had their aortic root and ascending aortas (aortic root to right brachiocephalic trunk) immediately dissected out, flash-frozen in liquid nitrogen, and stored at −80°C until further processing. Mice that were analyzed for histology had their abdominal aorta transected before the bifurcation, and PBS, followed by latex (Ward’s Science), was infused into the left ventricle. Latex-infused aortas were then transected at the level of the aortic valve, and 3-mm transverse sections were mounted in 4% bacto-agar (BD Biosciences) and submitted for paraffin fixation. Concentric aortic rings (5 μm thick) were mounted on glass slides, stained with VVG, and imaged at ×10 and ×40 magnification using a Nikon Eclipse E400 microscope. All mice found dead were assessed for cause of death by necropsy, noting in particular hemothorax and hemopericardium.
Delivery of medication
Mice were initiated on medication at the beginning of the third week of gestation and continued for 5 weeks: 1 week of pregnancy and 4 weeks of lactation. Both the selective OTA [desGly-NH2-d(CH2)5[d-Tyr2,Thr4]OVT] and the selective OA [(Thr4,Gly7)OT] were synthesized in the Manning laboratory (23), dissolved in PBS to reach a final concentration of 0.2 mg/ml, and delivered via continuous infusion for 5 weeks with a mini Alzet pump implanted subcutaneously between the scapulae at 3 weeks of gestation for a dose of 1 μg/kg per hour. Placebo-treated animals underwent pump implantation and received continuous infusion of PBS. Propranolol (QualiTest) was dissolved in the drinking water and filtered to reach a final concentration of 0.5 g/liter, giving an estimated daily dose of 40 mg/kg per day. Hydralazine (AmerisourceBergen) was dissolved in the drinking water and filtered to reach a final concentration of 0.16 g/liter, giving an estimated daily dose of 16 mg/kg per day. Trametinib (GlaxoSmithKline) was dissolved in PBS with 5% dimethyl sulfoxide and administered by oral gavage once a day, giving an estimated daily dose of 1 mg/kg per day. Placebo-treated animals received regular drinking water.
Echocardiography
Nair hair removal cream was used on all mice the day before echocardiograms. All echocardiograms were performed on awake, unsedated mice using a VisualSonics Vevo 660 V1.3.6 imaging system and a 30-MHz transducer. Mice were imaged at baseline and weekly thereafter until death or the time of sacrifice. The aorta was imaged using a parasternal long-axis view. Three separate measurements of the maximal internal dimension at the aortic root and proximal ascending aorta were made from distinct captured images and averaged. All imaging and measurements were performed by a cardiologist (J.P.H.) who was blinded to genotype and treatment arm.
Blood pressure analysis
Blood pressures were analyzed by taking 20 tail-cuff blood pressures per day over 2 to 3 days in each mouse to habituate the mice to the tail-cuff pressure system, and then 10 to 20 blood pressure measurements were obtained on the last day and averaged. At least four mice for each treatment group were analyzed.
Antibodies and Western blot analysis
Mouse aortic root and ascending aortas (aortic root excluding the aortic valve to origin of right brachiocephalic trunk) were harvested, snap-frozen in liquid nitrogen, and stored at −80°C until processed. Protein was extracted using an automatic bead homogenizer in conjunction with reagents from the Protein Extraction Kit (Full Moon BioSystems). All protein lysis buffers contained protease and phosphatase inhibitors (Millipore). Western blotting was performed using LI-COR buffer and species-appropriate secondary antibodies conjugated to IRDye 700 (IRDye 680LT donkey anti-mouse, catalog no. 926-68022, LI-COR Biosciences) or IRDye 800 (IRDye 800CW donkey anti-rabbit, catalog no. 926-32213, LI-COR Biosciences), according to the manufacturer’s guidelines, and analyzed using LI-COR Odyssey. The following primary antibodies were used: anti-actin (Sigma-Aldrich, clone AC-74) and anti-pERK1/2 (Cell Signaling Technology, 4370).
pERK amounts were normalized to β-actin as opposed to total ERK for a variety of practical reasons. First, the functional consequences for the cell depend on the amount of pERK but not the pERK/total ERK ratio. Second, the amount of total ERK has never been shown to be limiting in in vitro contexts. Earlier work has shown the abundant recruitment of cells into the aortic wall of aneurysm models (32,33) that have high expression of total ERK but not pERK, markedly diluting the pERK signal when averaging techniques such as immunoblots are performed using protein lysates from the entire aortic wall and total ERK for normalization. This practice results in extreme intrastate variability that is minimized when a housekeeping gene is used as loading control.
IF on frozen aortic tissue sections
Mice were euthanized by halothane inhalation, and the left common iliac artery was transected to allow for drainage. A total of 20 ml of PBS (pH 7.4) and then about 20 ml of PBS containing 4% paraformaldehyde were flushed through the left ventricle. The heart and thoracic aorta were then removed en bloc and fixed in fresh 4% paraformaldehyde in PBS at 4°C overnight. Tissue blocks were immersed in antigen retrieval solution (10 mM sodium citrate buffer, pH 6.0) at 4°C overnight and then in boiling antigen retrieval solution for 3 min. Immediately after that, tissue was placed in cold 30% sucrose in PBS and incubated at 4°C overnight, embedded in Tissue-Tek O.C.T. Compound, and frozen using ethanol mixed with dry ice and stored at −80°C. Frozen 10-μm long-axis view sections were obtained with a cryostat, mounted on glass slides, and dried at room temperature for at least 1 day before staining. Sections were permeabilized in staining buffer (PBS containing 0.1% Triton X-100) for 15 min and then incubated with Fc Receptor Block (Innovex Biosciences, catalog no. NB309) for 30 min at room temperature, washed in staining buffer, and then incubated again in Background Buster blocking solution (Innovex Biosciences, catalog no. NB306) for 30 min. Anti-pERK1/2 (Cell Signaling Technology, 4370) was diluted at 1:100 in staining buffer and incubated overnight at 4°C. Three consecutive washes with staining buffer were performed before incubation with goat anti-rabbit secondary antibody conjugated to Alexa Fluor 555 [goat anti-rabbit IgG (H+L) secondary antibody, Alexa Fluor 555 conjugate, #A-21428 from Thermo Fisher Scientific] at 1:200 for 1 hour. Images were acquired on Zeiss AxioExaminer with 780NLO-Meta multiphoton confocal microscope at a ×25 magnification.
OR expression
Aortas were dissected as described above, flushed in PBS, and directly stored in TRIzol (Invitrogen). RNA was extracted according to the manufacturer’s instruction and purified with RNeasy mini columns (Qiagen). An on-column deoxyribonuclease digest (Qiagen) was performed before the clean-up step to eliminate residual genomic DNA. Complementary DNA (cDNA) was generated using TaqMan High Capacity cDNA Reverse Transcription reagents, and qPCR was performed in triplicate with TaqMan Universal PCR Master Mix (Applied Biosystems). The following prevalidated TaqMan probes were used: Mm01182684_m1 (Oxtr) and Mm99999915_g1 (Gapdh) (34). Relative quantification for each transcript was obtained by normalizing against Gapdh transcript abundance according to the formula 2(−Ct)/2(−Ct Gapdh).
Statistical analysis
Quantitative data are presented as boxplots with the box extending from the 25th to 75th percentile, the internal line represents the median, and the whiskers (indicating range) are calculated using the Tukey method (the upper whisker represents 75th percentile plus 1.5 times the interquartile distance, and the lower whisker represents 25th percentile minus 1.5 times the interquartile difference); data points that fall above or below the whiskers (outliers) are shown as individual points but included in statistical analyses. One-way analysis of variance (ANOVA) and two-way ANOVA were performed to evaluate significance of comparisons between groups using Tukey’s multiple comparison test, with a P value of <0.05 considered statistically significant. Kaplan-Meier survival curves were compared using a log-rank (Mantel-Cox) test.
Supplementary Material
Fig. S1. Subcutaneous pump placement effect on survival.
Fig. S2. Blood pressure effect in Fbn1mgR/mgR mice.
Fig. S3. OR mRNA expression in the proximal ascending aorta.
Fig. S4. Blood pressure effect in Fbn1mgR/mgR mice.
Table S1. Average aortic root and ascending aortic growth over the 7-week period spanning pregnancy and lactation in untreated mice.
Table S2. Average aortic root and ascending aortic growth over the 7-week period spanning pregnancy and lactation in treated mice.
Table S3. Effect of OTA treatment on the average systolic and diastolic blood pressure.
Table S4. Average OR expression in the aorta.
Table S5. Effect of hydralazine or propranolol on the average systolic and diastolic blood pressure.
Table S6. Average ERK1/2 phosphorylation in untreated mice.
Table S7. Average ERK1/2 phosphorylation in treated mice.
Table S8. Effect of trametinib on average systolic and diastolic blood pressure.
Table S9. Average ERK1/2 phosphorylation in trametinib-treated mice.
Acknowledgments:
We thank R. Makineni, R. Tyner, F. Paulsen, and the University of Toledo College of Medicine for their generous research support (to M.M.).
Funding:
Supported by grants from the NIH (AR41135 to H.C.D.), the Howard Hughes Medical Institute (to H.C.D.), the Marfan Foundation (to J.P.H.), the Smilow Center for Marfan Syndrome Research (to H.C.D.), and the University of Toledo College of Medicine (to M.M.). E.G.M. is supported by the Loeys-Dietz Syndrome Foundation and NIH grant R00HL121287, and C.B. is supported by NIH grant GM007309.
Footnotes
Competing interests: H.C.D. has equity interest in Blade Therapeutics that develops drugs to treat fibrosis and consults for GlaxoSmithKline. H.C.D. and J.P.H. hold a patent entitled “Methods and compositions for the treatment of Marfan syndrome and associated disorders” (US-2010-0034806). All other authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or Supplementary Materials.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Cameron DE, Alejo DE, Patel ND, Nwakanma LU, Weiss ES, Vricella LA, Dietz HC, Spevak PJ, Williams JA, Bethea BT, Fitton TP, Gott VL, Aortic root replacement in 372 Marfan patients: Evolution of operative repair over 30 years. Ann. Thorac. Surg 87, 1344–1349 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Goland S, Elkayam U, Cardiovascular problems in pregnant women with Marfan syndrome. Circulation 119, 619–623 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Elkayam U, Ostrzega E, Shotan A, Mehra A, Cardiovascular problems in pregnant women with the Marfan syndrome. Ann. Intern. Med 123, 117–122 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Meijboom LJ, Vos FE, Timmermans J, Boers GH, Zwinderman AH, Mulder BJM, Pregnancy and aortic root growth in the Marfan syndrome: A prospective study. Eur. Heart J 26, 914–920 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Lalchandani S, Wingfield M, Pregnancy in women with Marfan’s syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol 110, 125–130 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Pacini L, Digne F, Boumendil A, Muti C, Detaint D, Boileau C, Jondeau G, Maternal complication of pregnancy in Marfan syndrome. Int. J. Cardiol 136, 156–161 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Houston L, Tuuli M, Macones G, Marfan syndrome and aortic dissection in pregnancy. Obstet. Gynecol 117, 956–960 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Roman MJ, Pugh NL, Hendershot TP, Devereux RB, Dietz H, Holmes K, Eagle KA, LeMaire SA, Milewicz DM, Morris SA, Pyeritz RE, Ravekes WJ, Shohet RV, Silberbach M; GenTAC Investigators, Aortic complications associated with pregnancy in Marfan syndrome: The NHLBI national registry of genetically triggered thoracic aortic aneurysms and cardiovascular conditions (GenTAC). J. Am. Heart Assoc 5, e004052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayama S, Takeda N, Iriyama T, Inuzuka R, Maemura S, Fujita D, Yamauchi H, Nawata K, Bougaki M, Hyodo H, Shitara R, Nakayama T, Komatsu A, Nagamatsu T, Osuga Y, Fujii T, Peripartum type B dissection in patients with Marfan syndrome who underwent aortic root replacement: A case series study. BJOG 125, 487–493 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC, Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat. Genet 33, 407–411 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC, Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312, 117–121 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC, Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 332, 361–365 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook JR, Clayton NP, Carta L, Galatioto J, Chiu E, Smaldone S, Nelson CA, Cheng SH, Wentworth BM, Ramirez F, Dimorphic effects of transforming growth factor-β signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler. Thromb. Vasc. Biol 35, 911–917 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC, Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332, 358–361 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook JR, Carta L, Galatioto J, Ramirez F, Cardiovascular manifestations in Marfan syndrome and related diseases; multiple genes causing similar phenotypes. Clin. Genet 87, 11–20 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Stock S, Bremme K, Uvnas-Moberg K, Plasma levels of oxytocin during the menstrual cycle, pregnancy and following treatment with HMG. Hum. Reprod 6, 1056–1062 (1991). [DOI] [PubMed] [Google Scholar]
- 17.Uvnäs-Moberg K, Widström A-M, Werner S, Matthiesen A-S, Winberg J, Oxytocin and prolactin levels in breast-feeding women. Acta Obstet. Gynecol. Scand 69, 301–306 (1990). [DOI] [PubMed] [Google Scholar]
- 18.Jankowski M, Wang D, Hajjar F, Mukaddam-Daher S, McCann SM, Gutkowska J, Oxytocin and its receptors are synthesized in the rat vasculature. Proc. Natl. Acad. Sci. U.S.A 97, 6207–6211 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otun HA, MacDougall MW, Bailey J, Europe-Finner GN, Robson SC, Spatial and temporal expression of the myometrial mitogen-activated protein kinase 38 and ERK1/2 in the human uterus during pregnancy and labor. J. Soc. Gynecol. Investig 12, 185–190 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Nohara A, Ohmichi M, Koike K, Masumoto N, Kobayashi M, Akahane M, Ikegami H, Hirota K, Miyake A, Murata Y, The role of mitogen-activated protein kinase in oxytocin-induced contraction of uterine smooth muscle in pregnant rat. Biochem. Biophys. Res. Commun 229, 938–944 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Je H-D, Malek S, Morgan KG, Role of ERK1/2 in uterine contractility and preterm labor in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 287, R328–R335 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, Biery NJ, Dietz HC, Sakai LY, Ramirez F, Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc. Natl. Acad. Sci. U.S.A 96, 3819–3823 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G, Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol 24, 609–628 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM, Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. U.S.A 93, 11699–11704 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle JJ, Doyle AJ, Wilson NK, Habashi JP, Bedja D, Whitworth RE, Lindsay ME, Schoenhoff F, Myers L, Huso N, Bachir S, Squires O, Rusholme B, Ehsan H, Huso D, Thomas CJ, Caulfield MJ, Van Eyk JE, Judge DP, Dietz HC; GenTAC registry consortium, MIBAVA leducq consortium, A deleterious gene-by-environment interaction imposed by calcium channel blockers in Marfan syndrome. eLife 4, e08648 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hovsepian DA, Sriram N, Kamel H, Fink ME, Navi BB, Acute cerebrovascular disease occurring after hospital discharge for labor and delivery. Stroke 45, 1947–1950 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJM, Spontaneous coronary artery dissection associated with pregnancy. J. Am. Coll. Cardiol 70, 426–435 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Barrigón S, Tamargo J, The effect of oxytocin on contractive responses and 45Ca movements in rat isolated aortic strips. Br. J. Pharmacol 87, 763–770 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grotegut CA, Feng L, Mao L, Heine RP, Murtha AP, Rockman HA, β-Arrestin mediates oxytocin receptor signaling, which regulates uterine contractility and cellular migration. Am. J. Physiol. Endocrinol. Metab 300, E468–E477 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM, β-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J. Biol. Chem 277, 9429–9436 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Rouf R, MacFarlane EG, Takimoto E, Chaudhary R, Nagpal V, Rainer PP, Bindman JG, Gerber EE, Bedja D, Schiefer C, Miller KL, Zhu G, Myers L, Amat-Alarcon N, Lee DI, Koitabashi N, Judge DP, Kass DA, Dietz HC, Nonmyocyte ERK1/2 signaling contributes to load-induced cardiomyopathy in Marfan mice. JCI Insight 15, 91588 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anidjar S, Dobrin PB, Eichorst M, Graham GP, Chejfec G, Correlation of inflammatory infiltrate with the enlargement of experimental aortic aneurysms. J. Vasc. Surg 16, 139–147 (1992). [DOI] [PubMed] [Google Scholar]
- 33.Daugherty A, Rateri DL, Charo IF, Phillip Owens A III, Howatt DA, Cassis LA, Angiotensin II infusion promotes ascending aortic aneurysms: Attenuation by CCR2 deficiency in apoE-mice. Clin. Sci 118, 681–689 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clipperton-Allen AE, Lee AW, Reyes A, Devidze N, Phan A, Pfaff DW, Choleris E, Oxytocin, vasopressin and estrogen receptor gene expression in relation to social recognition in female mice. Physiol. Behav 105, 915–924 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Subcutaneous pump placement effect on survival.
Fig. S2. Blood pressure effect in Fbn1mgR/mgR mice.
Fig. S3. OR mRNA expression in the proximal ascending aorta.
Fig. S4. Blood pressure effect in Fbn1mgR/mgR mice.
Table S1. Average aortic root and ascending aortic growth over the 7-week period spanning pregnancy and lactation in untreated mice.
Table S2. Average aortic root and ascending aortic growth over the 7-week period spanning pregnancy and lactation in treated mice.
Table S3. Effect of OTA treatment on the average systolic and diastolic blood pressure.
Table S4. Average OR expression in the aorta.
Table S5. Effect of hydralazine or propranolol on the average systolic and diastolic blood pressure.
Table S6. Average ERK1/2 phosphorylation in untreated mice.
Table S7. Average ERK1/2 phosphorylation in treated mice.
Table S8. Effect of trametinib on average systolic and diastolic blood pressure.
Table S9. Average ERK1/2 phosphorylation in trametinib-treated mice.