Abstract
Childbirth at any age confers a transient increased risk for breast cancer in the first decade postpartum and this window of adverse-effect extends over two decades in women with late age first childbirth (>35 yoa). Cross-over to the protective effect of pregnancy is dependent on age at first pregnancy, with young mothers receiving the most benefit. Further, breast cancer diagnosis during the five-ten-year postpartum window associates with high risk for subsequent metastatic disease. Notably, lactation has been shown to be protective against breast cancer incidence overall with varying degrees of protection by race, multiparity and lifetime duration of lactation. An effect for lactation on breast cancer outcome after diagnosis has not been described. We discuss the most recent data and mechanistic insights underlying these epidemiologic findings. Post-partum involution of the breast has been identified as a key mediator of the increased risk for metastasis in women diagnosed within 5–10 years of a completed pregnancy. During breast involution, immune avoidance, increased lymphatic network, extracellular matrix remodeling and increased seeding to the liver and lymph node work as interconnected pathways, leading to the adverse effect of a postpartum diagnosis. We also discuss a novel mechanism underlying the protective effect of breastfeeding. Collectively, these mechanistic insights offer potential therapeutic avenues for the prevention and/or improved treatment of postpartum breast cancer.
Breast Cancer and Pregnancy
Cancer is an increasing complication of pregnancy worldwide, in part due to the advancing age of child-bearing women (1–3). Cancer diagnosis during pregnancy is overall a relatively rare event, affecting about 1 in 1000 pregnancies and representing 0.1% or less of all cancers (4). Globally, the most common cancers diagnosed during pregnancy follow the patterns of prevalent cancers in the underlying population. In Western countries, breast, thyroid, and gynecologic cancers, as well as melanoma and lymphomas are the most common (3,5,6). In Asia, the rate of gastric cancer is much higher, and melanoma not reported (7,8). Great progress has been made in advancing the ability to safely treat pregnant women with excellent guidelines and reviews available across many cancers (1,6–10). Of the cancers that affect woman during the childbearing life-window, only breast cancer and melanoma have been implicated as being increased in frequency, specifically among postpartum women, in comparison to age-matched peers (11). Breast cancer is globally the most frequent cancer diagnosed during pregnancy and in the postpartum years, and therefore the most studied. However, the impact of pregnancy on breast cancer is substantially more than the complexity of managing those diagnosed during pregnancy and, to date, it is only breast cancer where parity status has been shown to influence prognosis. This review will focus on the complex and varied interactions between pregnancy and breast cancer with emphasis on breast cancer metastasis, and the unique biology underlying these interactions as observed in rodent models.
Breast cancer in women of childbearing age is a significant global problem, as there is disproportionately increased mortality of young women’s breast cancer [YWBC], a problem exasperated in the lowest socioeconomic countries (11). YWBC, variously defined by patient age of ≤ 35–45 years, has increased risk for metastasis and death, with nearly twice the mortality in comparison to older women (12–15). Survival outcomes in YWBC has lagged overall improvements in the field, with the discrepancy increasing steadily since the 1970s (16). Moreover, the incidence of distant metastases is increasing by ~2% annually exclusively in YWBC (17,18). These adverse outcomes are often cited as due to delayed diagnosis and advanced disease presentation in the absence of screening for women under 40 (12,19). However, in a large recent study of early stage I-II disease, young age was an independent negative prognostic factor after adjustment for clinical, pathologic and treatment related variables (15). Another factor cited has been the greater proportion of aggressive biologic subtypes in YWBC, those that lack hormone receptor expression, including triple negative and Her 2 overexpressing cancers (TNBC and Her 2 respectively) (12,13,16,17). Yet, a large retrospective YWBC study reported significantly increased two-fold risk of death with estrogen receptor positive cancer (ER+), while risk was only modestly increased for TNBC, and was not significant in HER2+(20). In fact, the association with negative prognosis is the strongest in YWBC with early stage ER+ tumors otherwise expected to have excellent outcome (12,20,21). Overall, risk factors for YWBC are not well understood, and only 10–15% of YWBC is due to an inherited pathologic gene variant, such as the BRCA genes, p53, or PTEN (22). Lifestyle factors such as oral contraceptive use also contribute (23). Thus, while young age at diagnosis is an independent risk factor for relapse and death (12,15,16,20,21), current advances in the field find that heritable germline mutations do not account for the vast majority of YWBC cases, nor find that poor outcomes are accounted for by increases in poor prognostic tumor characteristics.
Childbearing is a natural life window that overlaps with risk for breast cancer diagnosis in women ages 20–45 and 80% of all YWBC will occur in parous women, with 50% of YWBC arising in young mothers within 10 years of their last childbirth (24). The completion of a prior childbirth is one of the more common, yet poorly recognized, risk factors for YWBC, in part because the interaction between pregnancy and breast cancer is complex. Since we and others find parity status impacts breast cancer incidence and outcomes in women diagnosed ≤45 years of age (24–26), we define YWBC as cases diagnosed at age 45 and under. Thus defined, YWBC accounts for ~13% of all breast cancer in developing countries and higher percentages in lower economic countries (27,28). Worldwide estimates of PPBC range from ~150,000 to 350,000 cases annually, based on global breast cancer (27,28) or childbirth rates (24), respectively (Text Box 1).
TEXT BOX #1.
Metrics related to reproductive history are not commonly included in breast cancer clinical data sets, such that the number of breast cancers that meet the definition of PPBC is currently derived from best estimates. One approach to estimate global burden of PPBC is to utilize data obtained from developed countries, which show that ~13% of all breast cancers are YWBC (27, 28) and that ~50% of these cases have likely completed a pregnancy within 10 years of their diagnosis (29). This approach results in a global estimation of ~130,000 PPBC cases per year. An alternative approach is to determine the incidence of PPBC cases per completed pregnancy (again relying on data from developed countries) and then determining global burden based on number of pregnancies worldwide. Best estimates for PPBC cases in the US for 2015 is ~13,000. Based on 4.5 million U.S pregnancies/year, this would predict a PPBC incidence of 0.0028% (one in every ~350 pregnancies). With ~130,000,000 live births globally per year, this estimation approach predicts 364,200 cases of PPBC worldwide (https://www.un.org/en/development/desa/population).
Pregnancy both promotes and protects against breast cancer (29–31). All parous women are under a 10–30% increased risk of developing breast cancer for at least a decade after childbirth in comparison to nulliparous young women, with older age at first birth increasing the magnitude of risk (31). Overtime, a cross-over effect dissipates this risk and the prior childbirth subsequently protects against breast cancer if the woman began her childbearing before age 35 (30,31). What factors other than parity also influence the incidence of breast cancer in postpartum women [postpartum breast cancer or PPBC] and YWBC remain under investigation. Increased breast density, radiographically identified as heterogeneously dense or extremely dense tissue on mammograms, elevates breast cancer risk 4-fold or higher, and equally affects risk in women of all ages (32,33). In women age 45 and under, higher breast density is seen with nulliparity and later onset of childbearing, which are life factors known to increase breast cancer risk (34,35). African-American women, who face a high incidence of YWBC, have higher breast density when multiple qualities of breast density are incorporated across age and parity group, as compared with white women (36). Further on in this review, mechanistic links between parity, lactation and active collagen deposition within the breast are described. These data offer leads to an improved understanding of the role of breast density in PPBC, and highlight the need for additional studies.
Overall, the postpartum life window is under-recognized as a time of higher risk for early onset breast cancer (Figure 1). Moreover, cancers diagnosed during this window of increased risk also carry worse prognosis (24,26,30,37–39). Women diagnosed and treated during their pregnancy have usual prognosis, based on stage and biology of the tumor (26,30). Conversely, women diagnosed postpartum have significantly increased risk for metastatic recurrence (37–39), which is highest among women diagnosed within 5 years of last childbirth but extends to ten years postpartum (37). Further, the risk for metastasis is 3–5-fold higher for postpartum stage I/II cancers, regardless of ER status (37). Recognition of this extended risk window for both ER+ and ER- cancers is critical with respect to understanding the underlying biology of postpartum breast cancer, as well as for identifying YWBC patients at highest risk of recurrence. This later point is highlighted by the fact that when outcomes are grouped as pregnancy-associated breast cancers (pregnant cases plus 1–2-year postpartum cases) and compared with an unselected group of age matched YWBC, the differences in outcome are obscured (24). Thus, the definition of PPBC matters, as it effects the interpretation of outcomes data and ultimately patient care.
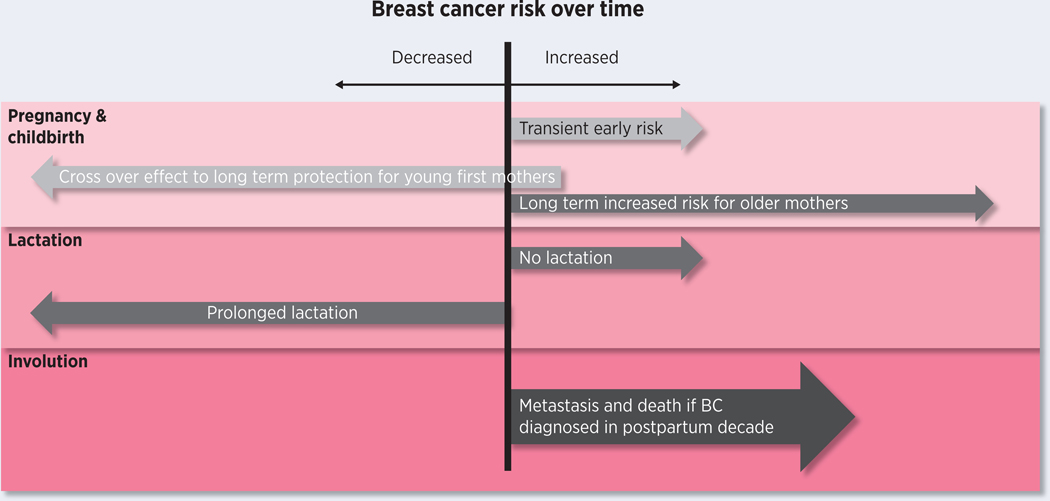
Figure 1.
Graphical presentation of the associations between pregnancy and lactation with breast cancer risk and outcome. While pregnancy confers long term protection in young first mothers, all mothers experience a transient early risk for breast cancer which extends long term in older mothers. Additionally, in those who do not lactate this risk is also increased and there is evidence that prolonged lactation can also decrease long term risk for breast cancer. Finally, if breast cancer is diagnosed in the postpartum decade there is an increased risk for metastasis and death from breast cancer compared with nulliparous or older women, which is not seen among cases diagnosed during pregnancy.
Tumor promotion during postpartum breast involution
The fact that a postpartum diagnosis in young women strongly associates with worse outcomes has led to the concept that a pro-metastatic biology is present in the postpartum breast (31). A unique breast biology specific to postpartum women that may account for the increased risk of metastasis is mammary gland involution (40). Mammary tissue expands ~10 fold during pregnancy in preparation for lactation; then, mammary gland involution occurs when milk production ends, either after birth in the absence of nursing, or after weaning (41). In adult rodents and women, postpartum mammary gland involution is reported as the most dramatic example of physiologic tissue remodeling to occur in the adult, as 80–90% of the alveolar mammary epithelium is removed by programmed cell death (42,43). Direct support for the hypothesis that postpartum mammary gland involution is tumor promotional comes from preclinical studies. Using xenograft and isogenic tumor transplant models, the normal tissue microenvironment of the actively involuting mammary gland has been demonstrated to increase breast cancer tumor take and metastasis compared to the mammary microenvironment of the nulliparous or parous host (44–46).
There are several mechanisms observed in the involuting gland that may drive metastasis. The death of the alveolar mammary epithelium is coordinated with inflammation (47,48), lymphangiogenesis (44), fibroblast activation (49), and collagen 1, fibronectin, and tenascin-C rich matrix deposition (49,50)--all stromal attributes that mirror wound-healing and are causal to cancer outcomes. Importantly, the findings of a similar pro-tumor, breast involution program being present in the breast tissue of young, recently pregnant women offers a plausible link between involution and PPBC outcomes (41,44). The potential mechanisms by which the transient event of normal mammary gland involution impacts long term outcomes in postpartum patients is likely multifactorial. Rodent models of PPBC reveal that occult tumor cells are “released” into the activated mammary stromal compartment during weaning-induced alveolar collapse, where they access the vasculature, disseminate, and set up micro-metastases in distant organs (44,45). Additionally, expansion of the lymphatic vasculature occurs during involution allowing for increased tumor cell trafficking in the lymph vessels and seeding of the lymph node (44,51). This involution-specific mammary lymphangiogenesis is consistent with the observed increased lymph node involvement in PPBC patients compared to age matched nulliparous patients (26,44).
There is also a distinct visceral pattern of metastasis in PPBC that suggests circulating tumor cells released from the involuting mammary gland have additional metastatic advantages. In postpartum patients, a ~3-fold increase in liver metastasis but not lung, brain or bone is reported (52). This metastatic pattern is consistent with a uniquely hospitable “soil” in the liver of postpartum women. This novel hypothesis is supported by rodent studies, where the postpartum liver is found to support breast cancer metastasis. The basis of this liver specific metastatic preference appears to be due to a functional link between the mammary gland and liver that is established to support lactation. Specifically, in preparation for lactation and throughout lactation, the rodent liver doubles in size and increases anabolic metabolism (52). Upon weaning, the liver undergoes a regression process that displays the hallmarks of mammary gland involution: parenchymal (hepatocyte) apoptosis, catabolic metabolism, ECM remodeling including deposition of collagen I, tenascin-C and fibronectin, and immune cell influx suggestive of immune tolerance (52). Intraportal tumor cell delivery (53) to the involuting liver leads to increased metastatic outgrowth compared to livers of nulliparous murine hosts, data consistent with the establishment of a pro-metastatic liver niche following weaning (52). While the molecular mechanisms that mediate the functional coordination between the lactating mammary gland and liver are unknown, and evidence for post-weaning liver involution in women not reported, continued investigation into site specific metastasis patterns in YWBC and PPBC is warranted.
In rodent models, involution and PPBCs are also marked by an immune phenotype typically associated with immune tolerance and T cell exhaustion. Specifically, within the lymphoid lineage, Th-17, Th-2 and Treg skewed T cells are elevated (48,54,55), with functional evidence for active suppression of T cell proliferation limited to the involution window (48). Within the myeloid lineage, there are observed enrichments in CD11b+/F480+ myeloid derived suppressor cells (MDSC) (48) and M2-skewed macrophages, which likely suppress T cell activation through secretion of IL-10, a Th1 inhibitory cytokine, and by depleting arginine from the tissue microenvironment (54,56). These macrophages exhibit characteristics that are similar to tumor associated macrophages, which have been identified to promote tumor cell invasion and extravasation (57,58). Additionally, the involuting gland is enriched for mammary macrophages similar to the recently identified tumor associated Podoplanin Expressing Macrophages or PoEMs (59). Within tumors, PoEMs promote metastasis via production of collagen and loosening of the lymphatic vasculature, allowing for tumor cells to enter the lymphatics (59). Histologic evidence confirms the presence of increased lymphatic associated macrophages in PPBC patients (51). Molecular analysis of the lymphatic vessels and macrophages during involution and in models of PPBC has also revealed expression of programmed death ligand-1 (PD-L1), similar to what is observed during peripheral tolerance (60–63). Since PD-L1 engagement of its receptor PD-1 inhibits T cell responses through activation of pro-apoptotic signaling, these data suggest that lymphatic-mediated immune avoidance (64–66) is one mechanism by which involution promotes PPBC. Consistent with this, murine PPBC tumors are particularly sensitive to anti-PD-1 therapy, which resulted in regression of the lymphatics and decreased T cell exhaustion (60). Collectively, these data provide several plausible mechanisms for increased metastases in postpartum patients that includes immune avoidance, increased tumor cell escape from the primary site, increased lymphatic network for dissemination and maintenance of immune avoidance, and increased seeding in the liver, all of which may be targetable with clinically available therapies.
In addition to involution creating favorable stromal microenvironments for the expansion and spread of tumor cells, a second mechanism by which involution might durably promote breast cancer and poor outcomes is through tumor cell imprinting. Several lines of evidence suggest that tumor cells and mammary epithelial cells are permanently altered after a pregnancy/lactation/involution cycle. Firstly, parity induced mammary stem cell populations, with similarities to tumor cells, are maintained after pregnancy and expand with multiparity in rodents (67,68). Secondly, mRNA analysis of normal mammary tissues from parous women within 10 years postpartum reveals stable changes in gene expression associated with inflammation, ECM, and hormone receptor signaling (69,70). Multiple studies have also identified programs of postpartum involution that are mirrored in tumor cells and in the TME after involution (71). Specifically, collagen, COX-2, SEMA7A, and lymphangiogenesis are described in involuting mouse mammary tissue, in normal adjacent mammary tissue from postpartum patients, and in PPBC tumors in murine models (44,46,51,56,72). Importantly, NSAID inhibition reveals that COX-2 activity is required for metastasis in murine postpartum hosts; additionally, NSAIDs reverse aspects of gland desmoplasia, lymphangiogenesis and T cell suppression, including re-establishment of Th1 cytokine production and T cell accumulation at the border of postpartum tumors (45,55). Finally, SEMA7A promotes multiple aspects of tumor progression during involution including lymphangiogenesis, mesenchymal phenotypes, as well as tumor cell invasion and survival (51,73). Moreover, knockdown of SEMA7A reduces collagen deposition and COX-2 expression in pre-clinical models of PPBC (74) and knockdown of COX-2 similarly results in downregulation of SEMA7A (73), suggesting a relationship between these two signaling molecules. Thus, both COX-2 and SEMA7A represent additional avenues that can be explored for the prevention and treatment of PPBC.
While evidence for tumor cell imprinting in PPBC is still emerging, the study of transcriptional regulators of normal mammary gland involution may also offer insights. Transcriptional regulators with known pro-and anti-tumor activities orchestrate the balance between epithelial cell survival and death during involution. For example, CEBPD, a member of the C/EBP transcription factor family, is an important regulator of involution (75,76). In CEBPD gene knockout studies in mice, CEBPD was found to support pro-apoptotic signaling of involution as well as stromal remodeling, particularly via upregulation of matrix metalloproteinases (75,76). Conversely, SIM2s, a transcription factor with known tumor suppressive activities, is required for lactogenic differentiation. Continued expression of SIM2s delays involution by decreasing Stat3 and NFKB signaling (77), two pro-apoptotic signaling pathways critical for involution (78–82). Interestingly, SIM2s was recently shown to downregulate COX-2 and loss of SIM2s promotes mammary tumorigenesis via upregulation of EMT and invasion (83,84). Importantly, inhibition and knockdown of COX-2 reduces invasion and restores SIM2 expression in breast cancer cells and xenografts (83,84). An intriguing prospect is that PPBC will be imprinted by the transcriptional programs of involution, such as CEBPD gain and SIM2s loss, which may identify additional novel pathways of vulnerability in PPBC. Indirect evidence for this hypothesis has been reported, as gene signatures associated with normal mammary gland involution (85,86) correlate with aggressive triple negative and inflammatory breast cancers and predict worse breast cancer specific survival in breast cancer overall (87,88).
Mechanistic insights into the protective effect of lactation
Lactation is identified as an important factor affecting incidence of breast cancer in both YWBC and postmenopausal diagnosis. Breastfeeding has a significant protective effect against triple negative breast cancers in African-American women and BRCA1 mutation carriers, and overall breastfeeding reduces risk for white women and ER+ subtypes, particularly for a postmenopausal diagnosis (23,37). A recent meta-analysis of 50,302 women from 47 independent studies revealed that extended lactation is protective against breast cancer, with risk reduction being dependent on cumulative lifetime duration, and independent of maternal age or multiparity. In this study, amongst the parous women with breast cancer, fewer had ever breastfed and for those who did, the average time of breastfeeding was shorter (9.8 months) when compared to parous women without breast cancer (15.6 months)(89). Further, from this study the authors suggested that extension of breastfeeding, by 12 months per child, may reduce the risk of breast cancer by more than half, since they reported a reduction from 6.3 breast cancer cases per 100 women to 2.7 breast cancer cases per 100 women (89). Additionally, the length of lactation was found to be reduced in high-income countries relative to low-income countries, which may partially explain the increased incidence of postmenopausal breast cancer in high-income countries (27,90) Therefore, understanding how lactation impacts risk of breast cancer, and whether it counters adverse effects of involution is critical.
Insulin-like growth factor 1 (IGF-I) protects mammary epithelial cells from apoptosis during lactation (91–93). Insulin-like growth factor binding protein-5 (IGFBP-5) prevents activation of IGF receptor by titrating away IGF-I and II (94). Thus, IGFBP5 was proposed as a mediator of involution, and IGFBP5 knockout mice exhibit delayed involution (95–98). Therefore, the effect of IGFBP-5 on IGF signaling may be especially important during involution. In agreement with the importance of limiting IGF signaling in this setting, the protective effect of pregnancy is associated with reduced expression of IGF receptor (94). IGFBP-5 is itself negatively regulated by the protease pappalysin-1 (PAPP-A)(99–103), which is overexpressed in the vast majority of breast cancers (104), and decreased expression of IGFBP-5 is associated with higher risk of developing breast cancer (105,106). Analysis of transgenic mice with mammary specific expression of PAPP-A revealed development of mammary tumors exclusively following pregnancy (107,108). Importantly, the length of lactation drastically impacted the rate of PPBC in these mice (107). Transgenic females that had an abrupt cessation after 2 days of lactation developed PPBC; in contrast, females that fed their pups for extended periods of time did not (107). This observation suggests that extended lactation is protective against the oncogenic effect of PAPP-A. Mechanistically, glycoproteins Stanniocalcin-1 (STC1) and −2 (STC2) can act as inhibitors of PAPP-A (109,110) and the protective effect of lactation is associated with the expression of STC1 and STC2 (107). Further, STCs are produced by the ovaries and can be detected at high levels in the serum only during pregnancy and lactation, but not during involution, suggesting a possible systemic role for STCs in tumor suppression during pregnancy and lactation (111).
Interestingly, a recent study reported that abrupt interruption of lactation in mice induces an increase in markers of inflammation. This finding is consistent with the role of PAPP-A in involution and the importance of the STCs to inhibit PAPP-A activity, since PAPP-A is known to be induced by inflammatory cytokines such as IL-6, TNFα, IL-1β, IL-4, and TGF-β (112). Abrupt cessation of lactation was also associated with increased cellular proliferation and deposition of collagen as well as expansion of the luminal progenitor cells in the mammary gland compared to mice where cessation of lactation was gradual (113). The latter observation in mice that abrupt cessation promoted the expression of estrogen and progesterone receptors is in agreement with the protective effect of breastfeeding against ER+ breast cancer in women (23,37). The authors also reported ductal hyperplasia four months postpartum in mice where lactation was abruptly interrupted (113). It is currently unknown whether this holds true in humans. However, in human breasts undergoing gradual weaning, lobules undergoing apoptosis and tissue inflammation are observed adjacent to non-inflamed lactational lobules (41,114,115) (Supplemental Figure 1). These observations suggest that, even with gradual weaning, tumor cells could be exposed to a pro-tumor microenvironment, and therefore by extrapolation, appears that gradual weaning would not completely protect a woman’s breast from the protumorigenic aspects of involution. Additional studies are necessary to determine how gradual and abrupt cessation of weaning interact with involution and impact the breast tissue microenvironment, and subsequent breast cancer risk and outcomes.
Of note, increased deposition of collagen during involution induces COX-2 expression, which drives PPBC metastasis (45) and the mammary glands of involuting PAPP-A transgenic mice following an abrupt cessation of lactation, exhibit an even higher level of collagen (107). Additionally, PAPP-A is dependent on collagen for its activation (107), thereby offering a mechanism by which PAPP-A transgenic mice only develop mammary tumors following pregnancy--as passage through involution and increased collagen deposition during this phase is required for its activation. In a more recent study, PAPP-A was found to activate the collagen receptor DDR2 (108), which promotes metastasis via activation of the ERK-Snail axis (116). Thus, the activation of DDR2 by PAPP-A offers an additional mechanism by which PPBCs are associated with increased metastasis and worst outcomes. Consistent with this, deletion of DDR2 by CRISPR was shown to abolish the pro-invasion effect of PAPP-A (108) and a PAPP-A/Snail/Collagen signature was found to identify patients at higher risk of metastasis (108). Collectively, these data suggest that lactation or extended lactation may be protective against breast cancer via suppression of PAPP-A and downregulation of collagen mediated pro-tumorigenic signaling in the mammary gland.
Concluding remarks
Postpartum breast cancer is a global health threat that affects ~150,000–350,000 young mothers annually, placing them at increased risk for metastasis and therefore death. Since lactation and postpartum breast involution are both predictive for breast cancer, the postpartum life window is a free, readily available “biomarker” for assessments of PPBC risk and outcomes. The insights gleaned from mechanistic studies of lactation and involution offer important avenues forward. Specifically, the roles of COX-2, SEMA7A, and PAPP-A as putative oncogenes for PPBC, and the identification of lactation-induced SIM2s, STC-1 and STC-2 as inhibitors of PPBC-associated oncogenes, provide immediate leads for targeted prevention and treatment interventions (Figure 2). To continue to advance PPBC prevention, additional programs to facilitate lactation globally are needed. Also, research efforts to identify women at high risk for PPBC will support future prevention trials. Finally, investigations into the unique molecular vulnerabilities of PPBC are anticipated to yield rich venues for targeted therapeutics. Combined, these strategies will address the unmet clinical needs of young women at risk for PPBC, a population in dire need of improved prevention and treatment strategies.
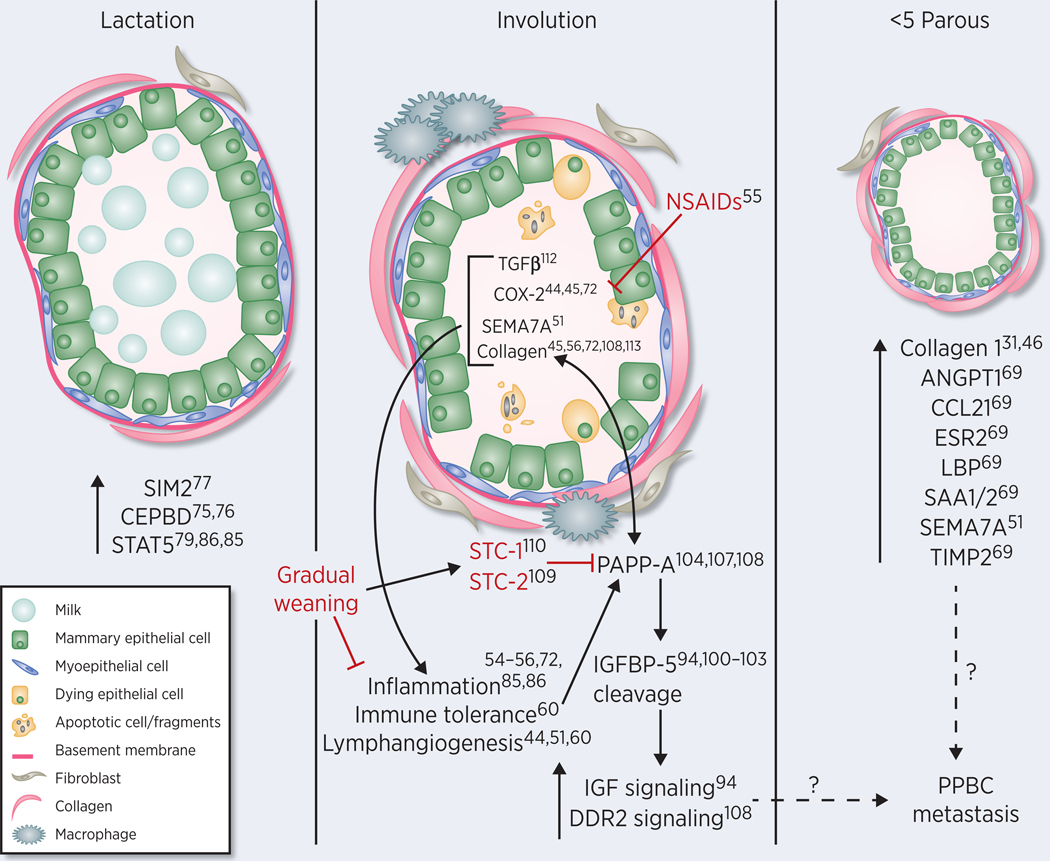
Figure 2.
Model of how lactation, postpartum involution and epithelial cell imprinting of parity intersect to impact incidence and outcomes in postpartum breast cancer (PPBC). Physiologic involution (middle panel), which is mediated in part by pro-tumorigenic TGFbeta, COX-2, SEMA7A and collagen dependent signaling, shares numerous attributes with pro-tumor wound healing, including immune cell infiltrate, immune tolerance, and lymphangiogenesis, resulting in involution being a risk window for poor prognostic breast cancer. Upregulation of molecules with known tumor suppressive functions are present during lactation (left panel) and abrupt cessation of lactation exasperates involution associated programs of inflammation, which contributes to abnormal expression of PAPP-A during involution. The increased deposition of collagen during involution acts to enhance the proteolytic activity of PAPP-A against IGFBP-5 leading to increased IGF and collagen receptor DDR2 signaling and tumor promotion. In contrast, prolonged or gradual cessation of lactation promotes the accumulation of stanniocalin 1 and 2 (STC1, 2), which act as inhibitors of PAPP-A, reducing tumor progression. Mammary epithelial cells are also imprinted by undergoing a reproductive cycle (right panel), which possibly accounts for the increased tumor risk and progression that are elevated for at least 10 years post childbirth. Image courtesy of Sarah E Tarullo, PhD (University of Colorado).
Supplementary Material
Acknowledgements
This work was supported by the following grants: PS and VB received R01CA169175; TL received R01CA211696 and RSG-16-171-010CSM from the American Cancer Society; DG received a Precision and prevention Initiative grant from the Breast Cancer Research Foundation; PS received a grant from the Willard L. Eccles Charitable Foundation.
Footnotes
Conflict of Interest Statement: The authors declare that they have no potential conflicts of interest.
References
- 1.Eastwood-Wilshere N, Turner J, Oliveira N, Morton A. Cancer in Pregnancy. Asia Pac J Clin Oncol 2019 [DOI] [PubMed] [Google Scholar]
- 2.Eibye S, Kjaer SK, Mellemkjaer L. Incidence of pregnancy-associated cancer in Denmark, 1977–2006. Obstetrics and gynecology 2013;122:608–17 [DOI] [PubMed] [Google Scholar]
- 3.Botha MH, Rajaram S, Karunaratne K. Cancer in pregnancy. Int J Gynaecol Obstet 2018;143 Suppl 2:137–42 [DOI] [PubMed] [Google Scholar]
- 4.Donegan WL. Cancer and pregnancy. CA Cancer J Clin 1983;33:194–214 [DOI] [PubMed] [Google Scholar]
- 5.Parazzini F, Franchi M, Tavani A, Negri E, Peccatori FA. Frequency of Pregnancy Related Cancer: A Population Based Linkage Study in Lombardy, Italy. Int J Gynecol Cancer 2017;27:613–9 [DOI] [PubMed] [Google Scholar]
- 6.de Haan J, Verheecke M, Van Calsteren K, Van Calster B, Shmakov RG, Mhallem Gziri M, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol 2018;19:337–46 [DOI] [PubMed] [Google Scholar]
- 7.Maggen C, Lok CA, Cardonick E, van Gerwen M, Ottevanger PB, Boere IA, et al. Gastric cancer during pregnancy: A report on 13 cases and review of the literature with focus on chemotherapy during pregnancy. Acta Obstet Gynecol Scand 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim MH, Mok CW, Chang KH, Sung JH, Choi SJ, Oh SY, et al. Clinical characteristics and outcome of cancer diagnosed during pregnancy. Obstet Gynecol Sci 2016;59:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amant F, Berveiller P, Boere I, Cardonick E, Fruscio R, Fumagalli M, et al. Gynecologic cancers in pregnancy: guidelines based on a third international consensus meeting. Ann Oncol 2019 [DOI] [PubMed] [Google Scholar]
- 10.Oga S, Hachisuga M, Hidaka N, Fujita Y, Tomonobe H, Yamamoto H, et al. Gastric cancer during pregnancy with placental involvement: case report and review of published works. Obstet Gynecol Sci 2019;62:357–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stensheim H, Moller B, van Dijk T, Fossa SD. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009;27:45–51 [DOI] [PubMed] [Google Scholar]
- 12.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008;26:3324–30 [DOI] [PubMed] [Google Scholar]
- 13.Azim HA Jr., Michiels S, Bedard PL, Singhal SK, Criscitiello C, Ignatiadis M, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res 2012;18:1341–51 [DOI] [PubMed] [Google Scholar]
- 14.Bharat A, Aft RL, Gao F, Margenthaler JA. Patient and tumor characteristics associated with increased mortality in young women (< or =40 years) with breast cancer. J Surg Oncol 2009;100:248–51 [DOI] [PubMed] [Google Scholar]
- 15.Nixon AJ, Neuberg D, Hayes DF, Gelman R, Connolly JL, Schnitt S, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1994;12:888–94 [DOI] [PubMed] [Google Scholar]
- 16.Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg 2009;208:341–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Seminars in oncology 2009;36:237–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. Jama 2013;309:800–5 [DOI] [PubMed] [Google Scholar]
- 19.Keegan TH, DeRouen MC, Press DJ, Kurian AW, Clarke CA. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast cancer research : BCR 2012;14:R55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, et al. Subtype-Dependent Relationship Between Young Age at Diagnosis and Breast Cancer Survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34:3308–14 [DOI] [PubMed] [Google Scholar]
- 21.Fredholm H, Magnusson K, Lindstrom LS, Garmo H, Falt SE, Lindman H, et al. Long-term outcome in young women with breast cancer: a population-based study. Breast cancer research and treatment 2016;160:131–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malone KE, Daling JR, Doody DR, Hsu L, Bernstein L, Coates RJ, et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer research 2006;66:8297–308 [DOI] [PubMed] [Google Scholar]
- 23.Palmer JR, Viscidi E, Troester MA, Hong CC, Schedin P, Bethea TN, et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. Journal of the National Cancer Institute 2014;106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, et al. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast cancer research and treatment 2013;138:549–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med 1994;331:5–9 [DOI] [PubMed] [Google Scholar]
- 26.Goddard ET, Bassale S, Schedin T, Jindal S, Johnston J, Cabral E, et al. Association Between Postpartum Breast Cancer Diagnosis and Metastasis and the Clinical Features Underlying Risk . JAMA Netw Open 2019;2:e186997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellanger M, Zeinomar N, Tehranifar P, Terry MB. Are Global Breast Cancer Incidence and Mortality Patterns Related to Country-Specific Economic Development and Prevention Strategies? J Glob Oncol 2018;4:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424 [DOI] [PubMed] [Google Scholar]
- 29.Slepicka PF, Cyrill SL, Dos Santos CO. Pregnancy and Breast Cancer: Pathways to Understand Risk and Prevention. Trends in molecular medicine 2019 [DOI] [PubMed] [Google Scholar]
- 30.Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia 2009;14:87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schedin P. Pregnancy-associated breast cancer and metastasis. Nature reviews Cancer 2006;6:281–91 [DOI] [PubMed] [Google Scholar]
- 32.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:1159–69 [DOI] [PubMed] [Google Scholar]
- 33.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007;356:227–36 [DOI] [PubMed] [Google Scholar]
- 34.El-Bastawissi AY, White E, Mandelson MT, Taplin SH. Reproductive and hormonal factors associated with mammographic breast density by age (United States). Cancer Causes Control 2000;11:955–63 [DOI] [PubMed] [Google Scholar]
- 35.Tehranifar P, Reynolds D, Flom J, Fulton L, Liao Y, Kudadjie-Gyamfi E, et al. Reproductive and menstrual factors and mammographic density in African American, Caribbean, and white women. Cancer Causes Control 2011;22:599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarthy AM, Keller BM, Pantalone LM, Hsieh MK, Synnestvedt M, Conant EF, et al. Racial Differences in Quantitative Measures of Area and Volumetric Breast Density. Journal of the National Cancer Institute 2016;108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amant F, von Minckwitz G, Han SN, Bontenbal M, Ring AE, Giermek J, et al. Prognosis of Women With Primary Breast Cancer Diagnosed During Pregnancy: Results From an International Collaborative Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2013 [DOI] [PubMed] [Google Scholar]
- 38.Johansson AL, Andersson TM, Hsieh CC, Cnattingius S, Lambe M. Increased Mortality in Women with Breast Cancer Detected during Pregnancy and Different Periods Postpartum. Cancer Epidemiol Biomarkers Prev 2011;20:1865–72 [DOI] [PubMed] [Google Scholar]
- 39.Azim HA Jr., Santoro L, Russell-Edu W, Pentheroudakis G, Pavlidis N, Peccatori FA. Prognosis of pregnancy-associated breast cancer: a meta-analysis of 30 studies. Cancer treatment reviews 2012;38:834–42 [DOI] [PubMed] [Google Scholar]
- 40.Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, et al. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development 1996;122:181–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jindal S, Gao D, Bell P, Albrektsen G, Edgerton SM, Ambrosone CB, et al. Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling. Breast cancer research : BCR 2014;16:R31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker NI, Bennett RE, Kerr JF. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat 1989;185:19–32 [DOI] [PubMed] [Google Scholar]
- 43.Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution | Development. [DOI] [PubMed] [Google Scholar]
- 44.Lyons TR, Borges VF, Betts CB, Guo Q, Kapoor P, Martinson HA, et al. Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. The Journal of clinical investigation 2014;124:3901–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med 2011;17:1109–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maller O, Hansen KC, Lyons TR, Acerbi I, Weaver VM, Prekeris R, et al. Collagen architecture in pregnancy-induced protection from breast cancer. Journal of cell science 2013;126:4108–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol 2010;176:1241–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Betts CB, Pennock ND, Caruso BP, Ruffell B, Borges VF, Schedin P. Mucosal Immunity in the Female Murine Mammary Gland. J Immunol 2018;201:734–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo Q, Minnier J, Burchard J, Chiotti K, Spellman P, Schedin P. Physiologically activated mammary fibroblasts promote postpartum mammary cancer. JCI Insight 2017;2:e89206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goddard ET, Hill RC, Barrett A, Betts C, Guo Q, Maller O, et al. Quantitative extracellular matrix proteomics to study mammary and liver tissue microenvironments. Int J Biochem Cell Biol 2016;81:223–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elder AM, Tamburini BAJ, Crump LS, Black SA, Wessells VM, Schedin PJ, et al. Semaphorin 7A Promotes Macrophage-Mediated Lymphatic Remodeling during Postpartum Mammary Gland Involution and in Breast Cancer. Cancer research 2018;78:6473–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goddard ET, Hill RC, Nemkov T, D’Alessandro A, Hansen KC, Maller O, et al. The Rodent Liver Undergoes Weaning-Induced Involution and Supports Breast Cancer Metastasis. Cancer Discov 2017;7:177–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goddard ET, Fischer J, Schedin P. A Portal Vein Injection Model to Study Liver Metastasis of Breast Cancer. J Vis Exp 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinson HA, Jindal S, Durand-Rougely C, Borges VF, Schedin P. Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. Int J Cancer 2015;136:1803–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pennock ND, Martinson HA, Guo Q, Betts CB, Jindal S, Tsujikawa T, et al. Ibuprofen supports macrophage differentiation, T cell recruitment, and tumor suppression in a model of postpartum breast cancer. J Immunother Cancer 2018;6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. The American journal of pathology 2010;176:1241–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carron EC, Homra S, Rosenberg J, Coffelt SB, Kittrell F, Zhang Y, et al. Macrophages promote the progression of premalignant mammary lesions to invasive cancer. Oncotarget 2017;8:50731–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, et al. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer discovery 2015;5:932–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bieniasz-Krzywiec P, Martin-Perez R, Ehling M, Garcia-Caballero M, Pinioti S, Pretto S, et al. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell Metab 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamburini BAJ, Elder AM, Finlon JM, Winter AB, Wessells VM, Borges VF, et al. PD-1 Blockade During Post-partum Involution Reactivates the Anti-tumor Response and Reduces Lymphatic Vessel Density. Front Immunol 2019;10:1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, Fahl SP, et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 2012;120:4772–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Humbert M, Hugues S, Dubrot J. Shaping of Peripheral T Cell Responses by Lymphatic Endothelial Cells. Front Immunol 2016;7:684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berendam SJ, Koeppel AF, Godfrey NR, Rouhani SJ, Woods AN, Rodriguez AB, et al. Comparative Transcriptomic Analysis Identifies a Range of Immunologically Related Functional Elaborations of Lymph Node Associated Lymphatic and Blood Endothelial Cells. Front Immunol 2019;10:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lund AW, Wagner M, Fankhauser M, Steinskog ES, Broggi MA, Spranger S, et al. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. The Journal of clinical investigation 2016;126:3389–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dieterich LC, Ikenberg K, Cetintas T, Kapaklikaya K, Hutmacher C, Detmar M. Tumor-Associated Lymphatic Vessels Upregulate PDL1 to Inhibit T-Cell Activation. Front Immunol 2017;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lane RS, Femel J, Breazeale AP, Loo CP, Thibault G, Kaempf A, et al. IFNgamma-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. The Journal of experimental medicine 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development 2002;129:1377–86 [DOI] [PubMed] [Google Scholar]
- 68.Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Developmental biology 2007;303:29–44 [DOI] [PubMed] [Google Scholar]
- 69.Asztalos S, Gann PH, Hayes MK, Nonn L, Beam CA, Dai Y, et al. Gene expression patterns in the human breast after pregnancy. Cancer Prev Res (Phila) 2010;3:301–11 [DOI] [PubMed] [Google Scholar]
- 70.Santucci-Pereira J, Zeleniuch-Jacquotte A, Afanasyeva Y, Zhong H, Slifker M, Peri S, et al. Genomic signature of parity in the breast of premenopausal women. Breast cancer research : BCR 2019;21:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wallace TR, Tarullo SE, Crump LS, Lyons TR. Studies of postpartum mammary gland involution reveal novel pro-metastatic mechanisms. Journal of cancer metastasis and treatment 2019;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fornetti J, Jindal S, Middleton KA, Borges VF, Schedin P. Physiological COX-2 expression in breast epithelium associates with COX-2 levels in ductal carcinoma in situ and invasive breast cancer in young women. The American journal of pathology 2014;184:1219–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Black SA, Nelson AC, Gurule NJ, Futscher BW, Lyons TR. Semaphorin 7a exerts pleiotropic effects to promote breast tumor progression. Oncogene 2016;35:5170–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tarullo SE, Hill RC, Hansen K, Behbod F, Borges VF, Nelson AC, et al. Postpartum breast cancer progression is driven by semaphorin 7a-mediated invasion and survival. Oncogene 2020; 10.1038/s41388-020-1192-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balamurugan K, Wang JM, Tsai HH, Sharan S, Anver M, Leighty R, et al. The tumour suppressor C/EBPdelta inhibits FBXW7 expression and promotes mammary tumour metastasis. EMBO J 2010;29:4106–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robinson GW, Johnson PF, Hennighausen L, Sterneck E. The C/EBPbeta transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes & development 1998;12:1907–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scribner KC, Wellberg EA, Metz RP, Porter WW. Singleminded-2s (Sim2s) promotes delayed involution of the mouse mammary gland through suppression of Stat3 and NFkappaB. Mol Endocrinol 2011;25:635–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clarkson RW, Heeley JL, Chapman R, Aillet F, Hay RT, Wyllie A, et al. NF-kappaB inhibits apoptosis in murine mammary epithelia. The Journal of biological chemistry 2000;275:12737–42 [DOI] [PubMed] [Google Scholar]
- 79.Clarkson RW, Boland MP, Kritikou EA, Lee JM, Freeman TC, Tiffen PG, et al. The genes induced by signal transducer and activators of transcription (STAT)3 and STAT5 in mammary epithelial cells define the roles of these STATs in mammary development. Mol Endocrinol 2006;20:675–85 [DOI] [PubMed] [Google Scholar]
- 80.Chapman RS, Lourenco PC, Tonner E, Flint DJ, Selbert S, Takeda K, et al. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes & development 1999;13:2604–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chapman RS, Lourenco P, Tonner E, Flint D, Selbert S, Takeda K, et al. The Role of Stat3 in Apoptosis and Mammary Gland Involution. In: Mol JA, Clegg RA, editors. Biology of the Mammary Gland. Boston, MA: Springer US; 2002. p 129–38. [DOI] [PubMed] [Google Scholar]
- 82.Connelly L, Barham W, Pigg R, Saint-Jean L, Sherrill T, Cheng DS, et al. Activation of nuclear factor kappa B in mammary epithelium promotes milk loss during mammary development and infection. J Cell Physiol 2010;222:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laffin B, Wellberg E, Kwak HI, Burghardt RC, Metz RP, Gustafson T, et al. Loss of Singleminded-2s in the mouse mammary gland induces an epithelial mesenchymal transition associated with up-regulation of Slug and MMP2. Molecular and cellular biology 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wyatt GL, Crump LS, Young CM, Wessells VM, McQueen CM, Wall SW, et al. Cross-talk between SIM2s and NFkappaB regulates cyclooxygenase 2 expression in breast cancer. Breast cancer research : BCR 2019;21:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast cancer research : BCR 2004;6:R75–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast cancer research : BCR 2004;6:R92–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bambhroliya A, Van Wyhe RD, Kumar S, Debeb BG, Reddy JP, Van Laere S, et al. Gene set analysis of post-lactational mammary gland involution gene signatures in inflammatory and triple-negative breast cancer. PloS one 2018;13:e0192689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stein T, Salomonis N, Nuyten DS, van de Vijver MJ, Gusterson BA. A mouse mammary gland involution mRNA signature identifies biological pathways potentially associated with breast cancer metastasis. J Mammary Gland Biol Neoplasia 2009;14:99–116 [DOI] [PubMed] [Google Scholar]
- 89.Collaborative Group on Hormonal Factors in Breast C. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 2002;360:187–95 [DOI] [PubMed] [Google Scholar]
- 90.Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016;387:475–90 [DOI] [PubMed] [Google Scholar]
- 91.Hadsell DL, Alexeenko T, Klemintidis Y, Torres D, Lee AV. Inability of overexpressed des(1–3)human insulin-like growth factor I (IGF-I) to inhibit forced mammary gland involution is associated with decreased expression of IGF signaling molecules. Endocrinology 2001;142:1479–88 [DOI] [PubMed] [Google Scholar]
- 92.Hadsell DL, Bonnette SG, Lee AV. Genetic manipulation of the IGF-I axis to regulate mammary gland development and function. J Dairy Sci 2002;85:365–77 [DOI] [PubMed] [Google Scholar]
- 93.Hadsell DL, Torres DT, Lawrence NA, George J, Parlow AF, Lee AV, et al. Overexpression of des(1–3) insulin-like growth factor 1 in the mammary glands of transgenic mice delays the loss of milk production with prolonged lactation. Biol Reprod 2005;73:1116–25 [DOI] [PubMed] [Google Scholar]
- 94.Rowzee AM, Lazzarino DA, Rota L, Sun Z, Wood TL. IGF ligand and receptor regulation of mammary development. J Mammary Gland Biol Neoplasia 2008;13:361–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akkiprik M, Feng Y, Wang H, Chen K, Hu L, Sahin A, et al. Multifunctional roles of insulin-like growth factor binding protein 5 in breast cancer. Breast Cancer Res 2008;10:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beattie J, Allan GJ, Lochrie JD, Flint DJ. Insulin-like growth factor-binding protein-5 (IGFBP-5): a critical member of the IGF axis. Biochem J 2006;395:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boutinaud M, Shand JH, Park MA, Phillips K, Beattie J, Flint DJ, et al. A quantitative RT-PCR study of the mRNA expression profile of the IGF axis during mammary gland development. J Mol Endocrinol 2004;33:195–207 [DOI] [PubMed] [Google Scholar]
- 98.Ning Y, Hoang B, Schuller AG, Cominski TP, Hsu MS, Wood TL, et al. Delayed mammary gland involution in mice with mutation of the insulin-like growth factor binding protein 5 gene. Endocrinology 2007;148:2138–47 [DOI] [PubMed] [Google Scholar]
- 99.Laursen LS, Kjaer-Sorensen K, Andersen MH, Oxvig C. Regulation of insulin-like growth factor (IGF) bioactivity by sequential proteolytic cleavage of IGF binding protein-4 and −5. Mol Endocrinol 2007;21:1246–57 [DOI] [PubMed] [Google Scholar]
- 100.Laursen LS, Overgaard MT, Soe R, Boldt HB, Sottrup-Jensen L, Giudice LC, et al. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett 2001;504:36–40 [DOI] [PubMed] [Google Scholar]
- 101.Boldt HB, Overgaard MT, Laursen LS, Weyer K, Sottrup-Jensen L, Oxvig C. Mutational analysis of the proteolytic domain of pregnancy-associated plasma protein-A (PAPP-A): classification as a metzincin. Biochem J 2001;358:359–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. The Journal of biological chemistry 2001;276:21849–53 [DOI] [PubMed] [Google Scholar]
- 103.Oxvig C The role of PAPP-A in the IGF system: location, location, location. Journal of cell communication and signaling 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mansfield AS, Visscher DW, Hart SN, Wang C, Goetz MP, Oxvig C, et al. Pregnancy-associated plasma protein-A expression in human breast cancer. Growth Horm IGF Res 2014;24:264–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wyszynski A, Hong CC, Lam K, Michailidou K, Lytle C, Yao S, et al. An intergenic risk locus containing an enhancer deletion in 2q35 modulates breast cancer risk by deregulating IGFBP5 expression. Hum Mol Genet 2016;25:3863–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghoussaini M, Edwards SL, Michailidou K, Nord S, Cowper-Sal Lari R, Desai K, et al. Evidence that breast cancer risk at the 2q35 locus is mediated through IGFBP5 regulation. Nat Commun 2014;4:4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takabatake Y, Oxvig C, Nagi C, Adelson K, Jaffer S, Schmidt H, et al. Lactation opposes pappalysin-1-driven pregnancy-associated breast cancer. EMBO Mol Med 2016;8:388–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Slocum E, Craig A, Villanueva A, Germain D. Parity predisposes breasts to the oncogenic action of PAPP-A and activation of the collagen receptor DDR2. Breast Cancer Res 2019;21:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jepsen MR, Kloverpris S, Mikkelsen JH, Pedersen JH, Fuchtbauer EM, Laursen LS, et al. Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis. The Journal of biological chemistry 2015;290:3430–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kloverpris S, Mikkelsen JH, Pedersen JH, Jepsen MR, Laursen LS, Petersen SV, et al. Stanniocalcin-1 Potently Inhibits the Proteolytic Activity of the Metalloproteinase Pregnancy-associated Plasma Protein-A. The Journal of biological chemistry 2015;290:21915–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deol HK, Varghese R, Wagner GF, Dimattia GE. Dynamic regulation of mouse ovarian stanniocalcin expression during gestation and lactation. Endocrinology 2000;141:3412–21 [DOI] [PubMed] [Google Scholar]
- 112.Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab 2012;23:242–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Basree MM, Shinde N, Koivisto C, Cuitino M, Kladney R, Zhang J, et al. Abrupt involution induces inflammation, estrogenic signaling, and hyperplasia linking lack of breastfeeding with increased risk of breast cancer. Breast cancer research : BCR 2019;21:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fornetti J, Martinson HA, Betts CB, Lyons TR, Jindal S, Guo Q, et al. Mammary gland involution as an immunotherapeutic target for postpartum breast cancer. J Mammary Gland Biol Neoplasia 2014;19:213–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O’Brien J, Schedin P. Macrophages in breast cancer: do involution macrophages account for the poor prognosis of pregnancy-associated breast cancer? J Mammary Gland Biol Neoplasia 2009;14:145–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, et al. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nature cell biology 2013;15:677–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.