Abstract
Adult females and males of Ixodes affinis and Ixodes scapularis are illustrated by focus stacking image photography, and morphological character states are described that reliably differentiate the two species. In conjunction with other environmental cues, such as the questing phenology of adults, these characteristics will enable the rapid identification of adults of either sex along the southern Coastal Plain of the United States, where these species are sympatric.
Keywords: morphology, tick identification, tick key, Mid-Atlantic region, sympatric
In the southeastern United States, the widespread blacklegged tick, Ixodes scapularis Say, principal vector of Borrelia burgdorferi, the causative agent of Lyme disease, has increasingly been confused with Ixodes affinis Neumann, an efficient sylvatic vector of B. burgdorferi but a species that is not known to parasitize humans and that was formerly thought to be confined to coastal Florida, Georgia, and South Carolina (Clark et al. 1998). Recent studies have demonstrated that the range of I. affinis has expanded northward to include North Carolina (Harrison et al. 2010), Virginia (Nadolny et al. 2011, Nadolny and Gaff 2018), and Maryland (H.D.G., unpublished findings). Because these two species are now regionally sympatric and superficially similar in morphology, but differ in biomedical importance, inexperienced field personnel might benefit from easily usable means to distinguish and identify them. Here, we present suites of morphological character states that, taken together, will readily differentiate adults of I. affinis and I. scapularis wherever these species co-occur.
Materials and Methods
In order to create high-resolution digital images of tick specimens, focus stacking photography was used to overcome the focal limitations of a single high-magnification image. A series of images were captured from the front to the back of each specimen and then merged using Zerene Stacker, Professional Edition v. T201106271925 (Zerene Systems LLC, Richland, WA). This software discards out-of-focus pixels and combines the in-focus pixels of each frame. The result is a fully focused high-resolution, high-magnification image, showing the entire specimen in great detail.
Ixodes affinis ticks were collected in the summer of 2011 from Virginia Beach, Virginia, by flagging. The location was a small wooded patch with a mix of hardwoods and pines. The I. scapularis photographed were collected in 2011 in Harford County, Maryland. Dead tick specimens, preserved in 70% ethanol, were first cleaned and prepared so that each specimen most closely resembled its living state. Dust and debris were cleared away under a microscope using a small brush, and then the limbs of each specimen were arranged using forceps so that no morphological features were obscured, a process that often required some time for the specimen to dry and settle in that configuration. Each specimen was then attached to an insect mounting pin and positioned to show all relevant morphological traits.
After each tick specimen was mounted and ready for imaging, but before photography began, the number of total images required was calculated. The number of images required was determined by measuring the distance between the closest point of the specimen to the camera lens and the point farthest away, and then determining a distance-per-step on the motorized rail that moves the specimen. Although most images vary, generally a distance-per-step of 75–150 μm and a camera f-stop of 5.6 allowed for enough overlap for all parts of the final image to be in focus. We used a Canon 5DS camera with a 50.6 megapixel sensor, and a Canon 65 mm MP-E f/2.8 1-5× lens, often set at a magnification level of 3 (Canon U.S.A., Inc., Melville, NY).
Once the stacking process began, the camera took a single image at each stop of movement from front to back until all frames had been captured. A flash attached to the camera was used for each frame to ensure extreme sharpness and clarity. The accumulated ‘stack’ of images was loaded into Zerene Stacker and used to produce the final, fully focused image. This 32-bit TIFF file was then opened in Photoshop v. 5.5 (Adobe, Inc., Berkeley, CA) and edited to remove the visible mounting pin and perform additional specimen cleanup that may not have been possible with a brush before photography. Each final image was saved as an 8-bit JPEG file, but the original TIFF file was stored for archival purposes.
Results
Differentiating Females of I. affinis and I. scapularis
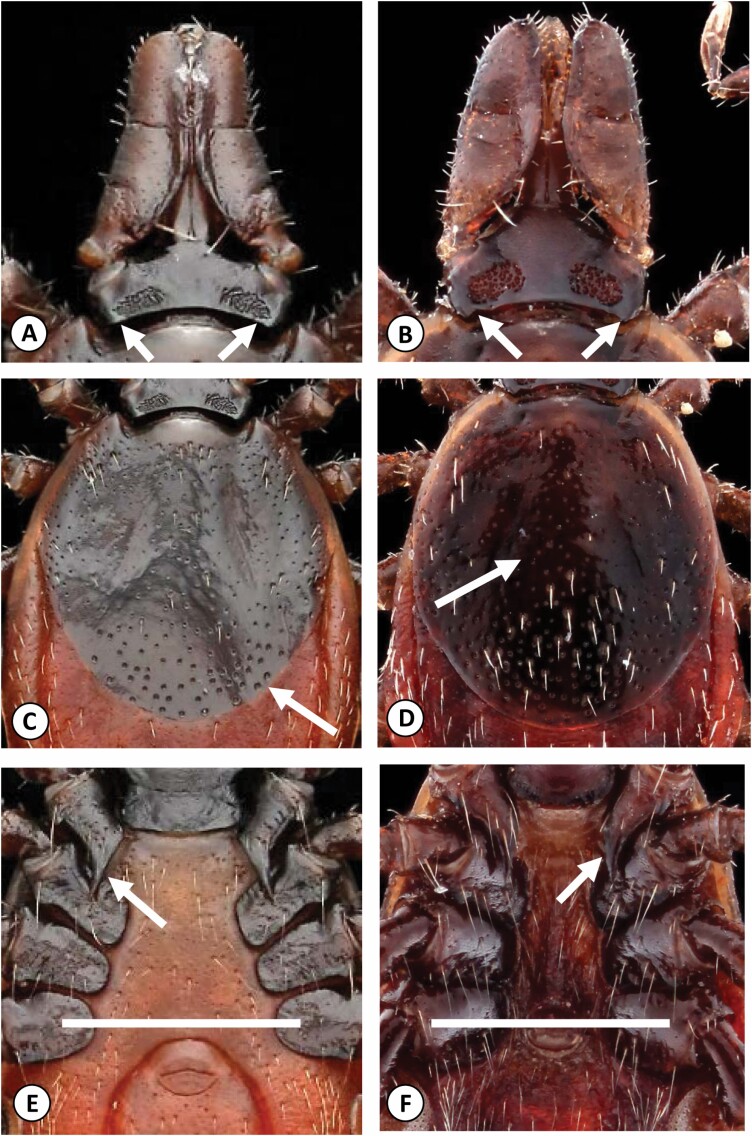
When females of I. affinis are viewed dorsally, it will be seen that the cornua—small projections from the posterolateral angles of the basis capituli—are absent, whereas cornua are present, although only moderate in size, on the basis capituli of female I. scapularis. The mouthparts (specifically the palps and hypostome) of female I. affinis are more ‘triangular’ in shape and come together in a point because of the slightly incurved external margins of the palpi, whereas the mouthparts of female I. scapularis are more rectangular with straight external margins by comparison (Fig. 1A and B). It should be noted that the palpi of specimens may not always be thus arranged, especially if the specimen being examined was found feeding, but the authors have observed that this is a useful feature in differentiating questing ticks. However, the principal morphological feature of the dorsum that distinguishes these species is the pattern of scutal punctations: in females of I. affinis, a distinct group of large, deep punctations can be seen on the posterior portion of the scutum, but in I. scapularis, the punctations are fine, more numerous, and evenly distributed over the scutal surface (Fig. 1C and D).
Fig. 1.
(A) Ixodes affinis adult female, dorsal capitulum view, noting absence of cornua. Note also the triangular shape made by the palps as they meet at the hypostome. (B) Ixodes scapularis adult female, dorsal capitulum view, noting presence of cornua. Note also the blunt mouthparts that do not make a triangular shape when they come together at the hypostome.(C) Ixodes affinis adult female, dorsal scutum view, noting cluster of large, deep punctations on the posterior portion of the scutum. (D) Ixodes scapularis adult female, dorsal scutum view, noting numerous fine punctations evenly distributed across the surface of the scutum. (E) Ixodes affinis adult female, ventral podosoma view, noting the long, thick, straight spur extending from coxa I and the position of the genital aperture, below the midline of coxa IV. (F) Ixodes scapularis adult female, ventral podosoma view, noting presence of short, curved spur extending from coxa I, and the position of the genital aperture at the midline of coxa IV.
Viewed ventrally, the genital aperture of unfed female I. affinis is located below the midline of the last coxa (illustrated with a while line, Fig. 1E and F), whereas it is positioned at or near the midline of coxa IV in I. scapularis. The internal spur of coxa I is also thick, straight, and long in I. affinis, projecting posteriorly and often extending almost to the posterior margin of coxa II, whereas in I. scapularis, this spur is relatively short and slender, the apical portion curving slightly laterad and seldom extending beyond the middle of coxa II (Fig. 1E and F).
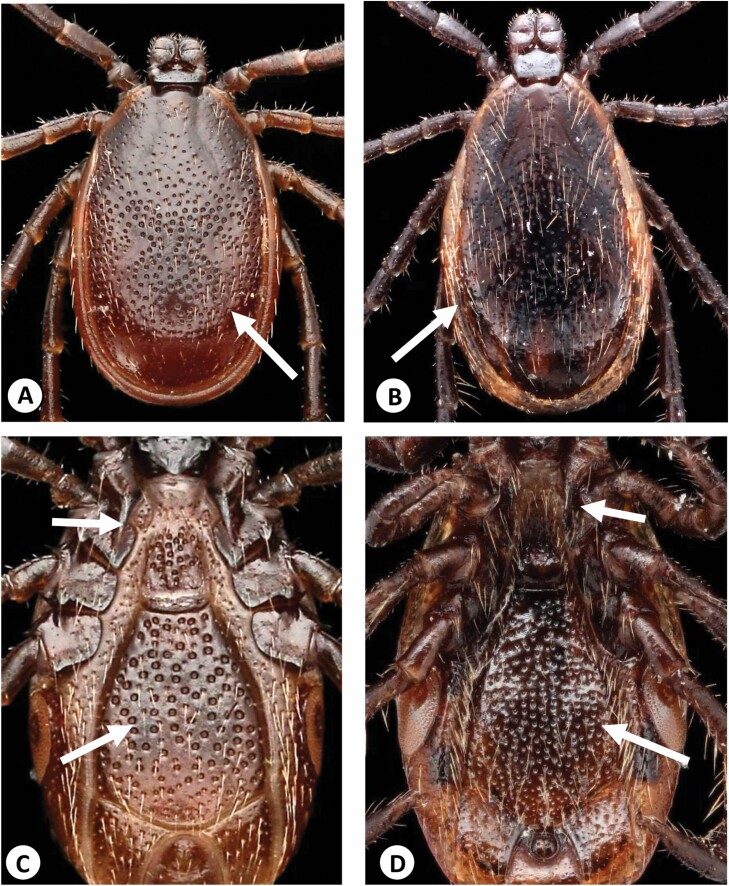
The body shape of unengorged I. affinis is rounder than that of I. scapularis, with the scutum extending posteriorly more than 50% of the way down the body to the caudal margin, while the scutum of the more oval-shaped I. scapularis covers closer to 50% of the dorsal length of the body, or idiosoma. The idiosomas of I. affinis females are also relatively shorter than those of I. scapularis females. In engorged female I. affinis, the idiosoma is usually pinched in at about the level of the spiracular plates, as illustrated by Cooley and Kohls (1945, p. 31, fig. 8G). In the authors’ observations of unengorged ticks, the body of I. affinis is often a lighter orange or even tan color, while I. scapularis can be a darker orange, which can be discerned with the naked eye. Finally, whether viewed dorsally or ventrally, the idiosomal setae of I. affinis are sparse, as are those of the scutum, but the body and scutum of I. scapularis are characterized by the presence of numerous long, slender setae (Fig. 2A–D).
Fig. 2.
(A) Ixodes affinis adult female, dorsal view. Note the comparatively rounder body, the extent of the scutum, and the lack of setae. (B) Ixodes affinis adult female, ventral view. (C) Ixodes scapularis adult female, dorsal view. Note the comparatively more oval-shaped body, with less of the body surface covered by the scutum, and the dense setae. (D) Ixodes scapularis adult female, ventral view.
For the novice, the easiest characteristics to see with a simple hand lens or dissecting microscope that differentiate adult females are the position of the genital aperture, the pattern of dorsal punctations, and the density of setae.
Differentiating Males of I. affinis and I. scapularis
When males of I. affinis are viewed dorsally, the central area of the scutum is dominated by a group of large, deep punctations, whereas in I. scapularis, the scutal punctations are all similar, numerous and very small and shallow, with the result that they are often more easily seen along the lateral margins of the scutum (Fig. 3A and B).
Fig. 3.
(A) Ixodes affinis adult male, dorsal view, noting cluster of large, deep punctations in the central portion of the scutum. (B) Ixodes scapularis adult male, dorsal view, noting numerous fine punctations evenly distributed across the surface of the scutum. (C) Ixodes affinis adult male, ventral view, noting long, thick, straight spur extending from coxa I past the midline of coxa II, and pattern of deep punctations across the median plate. (D) Ixodes scapularis adult male, ventral view, noting short spur extending from coxa I that does not extend past the midline of coxa II, and numerous smaller punctations across the median plate.
Viewed ventrally, the internal spurs of coxae I in males of both I. affinis and I. scapularis are similar in structure to those seen in females, but even more pronounced, with the spur in I. scapularis seeming minute in comparison to the larger spur present in I. affinis. The pattern of median plate punctations is also noticeably different: in I. affinis, these punctations are very large and deep, seldom numbering more than 100, but in I. scapularis, the punctations of the median plate, while deep and distinct, are smaller and, consequently, usually number well over 100 (Fig. 3C and D).
Again, as in females, both the scutal and idiosomal setae of I. affinis males are few and sparse, while these areas are characterized by an abundance of long, fine setae in I. scapularis (Fig. 4A–D).
Fig. 4.
(A) Ixodes affinis adult male, dorsal view. Note the lack of setae. (B) Ixodes affinis adult male, ventral view. (C) Ixodes scapularis adult male, dorsal view. Note the comparatively more dense setae. (D) Ixodes scapularis adult male, ventral view.
For the novice, the easiest characteristics to see that differentiate adult males of the two Ixodes species are the size and shape of the internal spur on coxa I and the density of setae.
Discussion
Ixodes affinis and I. scapularis are members of the Ixodes ricinus complex, a group of 14 species within the subgenus Ixodes that occur chiefly in the Nearctic and Palearctic zoogeographic regions and that largely share similar morphological characters in all active life history stages, as described by Keirans et al. (1999). Before the northward range extension of I. affinis, Lyme borreliosis investigators sampling adults of I. scapularis on deer or on clothing after walking through tick-infested areas in the Mid-Atlantic region of the United States could be reasonably confident that all their ‘scapularis-like’ specimens belonged to this species. But as Harrison et al. (2010) observed, the presence of questing adults of I. affinis across North Carolina’s vast Coastal Plain during the warm months of the year (April–November) now demands that such collections be examined for the co-occurrence of two sibling species, and this situation has since repeated itself on the coastal plain of Virginia (Nadolny et al. 2011, Nadolny and Gaff 2018). Spring and autumn are the seasons when questing I. affinis and I. scapularis are most likely to be coincident; adult Ixodes questing in the heat of summer are probably I. affinis, and Ixodes questing on warm winter days are probably I. scapularis. Any adult Ixodes tick found questing in the spring or autumn months in the Mid-Atlantic region should be subject to enhanced scrutiny and carefully assessed for the distinguishing morphological characters we have described.
Although keys and descriptions exist that enable the identification of I. affinis nymphs and larvae by microscopy (Oliver et al. 1987, Durden and Keirans 1996), as well as the adult stages (Cooley and Kohls 1945, Keirans and Clifford 1978), and a PCR assay has been developed to differentiate I. affinis from I. scapularis in any stage (Wright et al. 2014), the adult character states described here can usually be discerned in the field using a strong (e.g., 10×) hand lens. Although the character states described here are not new (Cooley and Kohls 1945, Keirans and Clifford 1978), the photographs and descriptions presented here can be used to easily differentiate these two commonly collected Ixodes species without the need to delve through keys that include all members of the Ixodes genus throughout the entire United States.
Finally, a question has arisen as to what species is actually represented by the name I. affinis because the original description of this tick was based on material collected in Costa Rica (Neotropical region), and in both Mexico and Central America, as well as in South America, specimens near—but not matching—I. affinis have been found, suggesting that more than one species exists under this name (Rodríguez-Vivas et al. 2016, Polsomboon et al. 2017, Saracho-Bottero et al. 2020). Studies of the population genetics of I. affinis demonstrated no connectivity between populations in Panama, Belize, and the United States, which could provide early evidence of parapatric speciation (Nadolny et al. 2016, Saracho-Bottero et al. 2020). Should further research confirm that an I. affinis species complex exists, then the Nearctic member or members of this complex may need to be renamed.
Macro imaging of arthropods using focus stacking can help distinguish between closely related species; in the case of I. affinis and I. scapularis, it can help distinguish between a human-biting vector of B. burgdorferi, and an emerging enzootic vector, which has spillover implications for human health (Nadolny et al. 2011). The extreme detail and resolution provided by this system allow viewers to tell with certainty which species they are looking at without having to rely on anatomical illustrations. In addition to the obvious utility of these images for vector identification and in taxonomic keys, the photographs can be captured in a way that provides a dynamic image for a variety of applications. Images captured in this style can be used in digital presentations, printed material, and exhibits. Focus stacking images are not only a valuable tool for entomologists seeking to better understand their subjects in fine detail, but they can also be used to inspire nonentomologists to pay closer attention to arthropods and the threats they can pose.
Acknowledgments
All material in this paper has been reviewed by the Army Public Health Center and the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the true views of the U.S. Department of the Army, the Department of Defense, or the U.S. Government. Some of the authors, as employees of the U.S. Government, conducted the work as part of their official duties. Title 17 U.S.C. 105 provides that ‘copyright protection under this title is not available for any work of the United States Government’. Title 17 U.S.C. 101 defines a U.S. Government work as work prepared by an employee of the U.S. Government as part of that person’s official duties. This work was funded in part by National Institutes of Health grant 1R01AI136035 as part of the joint NIH-NSF-USDA Ecology and Evolution of Infectious Diseases program.
References Cited
- Clark, K. L., J. H.Oliver, Jr, McKechnie D. B., and Williams D. C.. . 1998. Distribution, abundance, and seasonal activities of ticks collected from rodents and vegetation in South Carolina. J. Vector Ecol. 23: 89–105. [PubMed] [Google Scholar]
- Cooley, R. A., and Kohls G. M.. . 1945. The genus Ixodes in North America. National Institute of Health Bulletin No. 184, Washington, D.C. [Google Scholar]
- Durden, L. A., and Keirans J. E.. . 1996. Nymphs of the genus Ixodes (Acari: Ixodidae) of the United States: taxonomy, identification key, distribution, hosts, and medical/veterinary importance. Monograph, Thomas Say Publications in Entomology, Entomological Society of America, Lanham, MD. [Google Scholar]
- Harrison, B. A., W. H.Rayburn, Jr., Toliver M., Powell E. E., Engber B. R., Durden L. A., Robbins R. G., Prendergast B. F., and Whitt P. B.. . 2010. Recent discovery of widespread Ixodes affinis (Acari: Ixodidae) distribution in North Carolina with implications for Lyme disease studies. J. Vector Ecol. 35: 174–179. [DOI] [PubMed] [Google Scholar]
- Keirans, J. E., and Clifford C. M.. . 1978. The genus Ixodes in the United States: a scanning electron microscope study and key to the adults. J. Med. Entomol. 2: 1–149. [DOI] [PubMed] [Google Scholar]
- Keirans, J. E., Needham G. R., and J. H.Oliver, Jr. 1999. The Ixodes ricinus complex worldwide: diagnosis of the species in the complex, hosts and distribution, pp. 341–347. InNeedham G. R., Mitchell R., Horn D. J., and Welbourn W. C. (eds.), Acarology IX: volume 2, Symposia. Ohio Biological Survey, Columbus, OH. [Google Scholar]
- Nadolny, R. M., and Gaff H. D.. . 2018. Natural history of Ixodes affinis in Virginia. Ticks Tick Borne Dis. 9: 109–119. [DOI] [PubMed] [Google Scholar]
- Nadolny, R. M., Wright C. L., Hynes W. L., Sonenshine D. E., and Gaff H. D.. . 2011. Ixodes affinis (Acari: Ixodidae) in southeastern Virginia and implications for the spread of Borrelia burgdorferi, the agent of Lyme disease. J. Vector Ecol. 36: 464–467. [DOI] [PubMed] [Google Scholar]
- Nadolny, R. M., Gauthier D. T., Gaff H. D., and Bermudez S. E.. . 2016. Preliminary assessment of the population genetics of Ixodes affinis (Ixodida: Ixodidae) in North and Central America. Syst. Appl. Acarol. 21: 1300–1308. [Google Scholar]
- Oliver, J. H., Jr., Keirans J. E., Lavender D. R., and Hutcheson H. J.. . 1987. Ixodes affinis Neumann (Acari: Ixodidae): new host and distribution records, description of immatures, seasonal activities in Georgia, and laboratory rearing. J. Parasitol. 73: 646–652. [PubMed] [Google Scholar]
- Polsomboon, S., Hoel D. F., Murphy J. R., Linton Y. M., Motoki M., Robbins R. G., Bautista K., Bricen O I., Achee N. L., Grieco J. P., . et al. 2017. Molecular detection and identification of Rickettsia species in ticks (Acari: Ixodidae) collected from Belize, Central America. J. Med. Entomol. 54: 1718–1726. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Vivas, R. I., Apanaskevich D. A., Ojeda-Chi M. M., Trinidad-Martínez I., Reyes-Novelo E., Esteve-Gassent M. D., and Pérez de León A. A.. . 2016. Ticks collected from humans, domestic animals, and wildlife in Yucatan, Mexico. Vet. Parasitol. 215: 106–113. [DOI] [PubMed] [Google Scholar]
- Saracho-Bottero, M. N., Venzal J. M., Tarragona E. L., Thompson C. S., Mangold A. J., Beati L., Guglielmone A. A., and Nava S.. . 2020. The Ixodes ricinus complex (Acari: Ixodidae) in the southern cone of America: Ixodes pararicinus, Ixodes aragaoi, and Ixodes sp. cf. I. affinis. Parasitol. Res. 119: 43–54. [DOI] [PubMed] [Google Scholar]
- Wright, C. L., Hynes W. L., White B. T., Marshall M. N., Gaff H. D., and Gauthier D. T.. . 2014. Single-tube real-time PCR assay for differentiation of Ixodes affinis and Ixodes scapularis. Ticks Tick Borne Dis. 5: 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]